Abstract
Background.
In the United States, 1 in 10 infants and 1 in 20 older children die on the liver transplant waiting list. Increasing split liver transplantation could increase organ availability for these children, without decreasing transplants in adults.
Methods.
Using United Network for Organ Sharing Standard Transplant Analysis and Research data, we identified livers transplanted 2010 to 2015 that could potentially have been used for split transplant, based on strict criteria. Livers not suitable for pediatric patients or allocated to high-risk recipients were excluded. Number and distribution of potentially “split-able” livers were compared to pediatric waitlist deaths in each region.
Results.
Of 37 333 deceased donor livers transplanted, 6.3% met our strict criteria for utilization in split liver transplant. Only 3.8% of these were actually utilized for split liver transplantation. 96% were used for a single adult recipient. Of the 2253 transplanted as whole livers, 82% of their recipients were listed as willing to accept a segmental liver, and only 3% were listed as requiring a cold ischemia time less than 6 hours. Over the same 5 years, 299 children died on the waitlist. In every United Network for Organ Sharing region, there were more potentially “split-able” livers than pediatric waitlist deaths. Thirty-seven percent of pediatric waitlist deaths occurred at transplant centers that averaged 1 or less pediatric split liver transplantation annually during the study period.
Conclusions.
This comparison, although not conclusive, suggests that we might be missing opportunities to reduce pediatric waitlist mortality without decreasing access for adults—using split liver transplant. Barriers are significant, but further work on strategies to increase split liver transplant is warranted.
Among children listed for liver transplant in the US, more than 1 in 10 who are younger than 2 years die while waiting, as do more than 1 in 20 older children. For children who reach transplant, more than 40% spend over a year on the waitlist1—taxing their organs, growth, and development. Increasing the number of livers available to children could reduce waitlist mortality, waiting time, and potentially improve long-term post-transplant outcomes.
Split liver transplantation is 1 strategy for increasing availability, without having to increase the number of deceased donors or the number of livers available for adults. In the United States, splits are used primarily to increase organ availability for children.2 Recent analyses suggest that graft and patient survivals after transplant with a deceased donor liver—for both children and adults—have become comparable for split and whole liver recipients.3–5
Recent experience at 1 high volume adult and pediatric transplant center in the United Kingdom offers a real-world demonstration of the potential for increased split liver transplantation to reduce pediatric waitlist mortality.6 Under their policy, if a donor meets established criteria, the liver is primarily allocated as a split, for transplantation into 2 patients. In the 4 years after institution of an “Intention to Split” policy, 0 children died on their liver transplant waiting list.
In 2007, the Organ Procurement and Transplantation Network (OPTN) elucidated criteria for deceased donor livers with the potential to be split: nonobese donors younger than 40 years, on a single vasopressor medication, with serum transaminase levels 3 times or less normal. Preliminary analyses since then suggest that more than 10% of all donors meet these criteria. However, these livers are not offered preferentially to children or to those listed as willing to accept a split. Over the last decade, fewer than 2% of all US liver transplants have been split—and children continue to die on the waiting list.2 Detailed analysis of additional risk factors in these potential donors, and in their primary recipients, has not been previously undertaken.
To explore the possible impact of increased split liver transplantation on children awaiting liver transplant in the United States, we sought to identify deceased donor livers that might have been “split-able” and acceptable for pediatric donors, without increasing risk to the adult recipient. We compared the number of potentially “split-able” livers to pediatric waitlist deaths in each United Network for Organ Sharing (UNOS) region. We hypothesized that, even using our conservative criteria, potentially “split-able” livers would outnumber pediatric waitlist deaths.
MATERIALS AND METHODS
We used the UNOS Standard Transplant Analysis and Research files, through 12/2/2016. The Standard Transplant Analysis and Research files include deidentified data on all US donors, waitlisted candidates, and transplant recipients, as submitted by all OPTN member centers. The Health Resources and Services Administration, US Department of Health and Human Services, oversees activities of the OPTN and SRTR contractors. Institutional review board approval from the University of California, San Francisco, was obtained before analysis (CHR 14–15024).
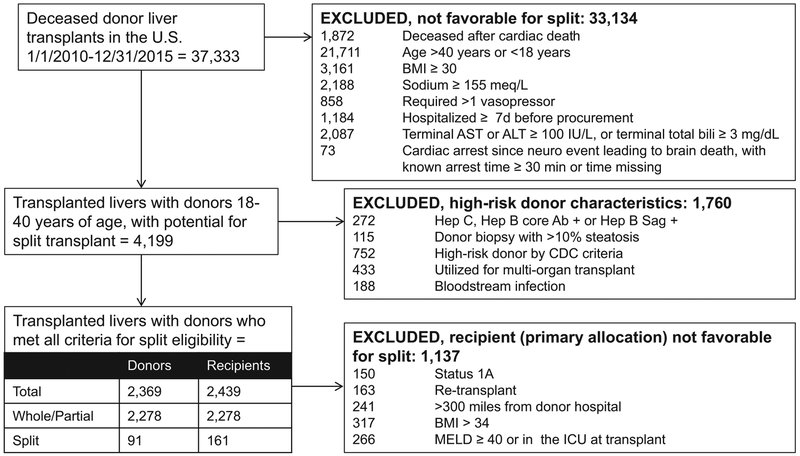
For the analysis of deceased donors with potential for utilization in split liver transplantation, we included liver from donors aged 18 to 40 years that were recovered January 1, 2010, to December 31, 2015, within the United States and were transplanted. Discarded organs were excluded. Criteria for subsequent exclusions are detailed in Figure 1. We excluded donor livers that would not have been favorable for split liver transplantation based on criteria recommended by UNOS, and/or used clinically at UCSF and other centers, based on published data and author review.2,6,7 To avoid overestimating the number of split-able livers, we then excluded donor livers with public health service (PHS) high-risk characteristics. Finally, to identify favorable livers that could have been utilized for split transplant under the current allocation scheme, we excluded livers primarily allocated to recipients that might have been considered high risk for split liver transplant (Figure 1).
FIGURE 1.
Selection of potentially “split-able” livers, from all deceased donor livers transplants 2010 to 2015 in the United States.
For the analysis of pediatric waitlist mortality, we included patients 0 to 18 years of age at listing who were removed from the waitlist because they either died or were deemed too sick for transplant. We identified 8 wait-list candidates who were removed from the waitlist for being too sick but were later relisted and transplanted, we excluded these children.
Statistical Analysis
Descriptive statistics were performed with χ2 testing for categorical variables, using exact methods as appropriate. Median and interquartile range (IQR, 25th-75th percentile) were reported for continuous variables because of skewed distributions. Kruskal-Wallis and Wilcoxon rank sum tests for between-group differences were used for continuous variables. Competing risks regression was used to evaluate whether center utilization of split liver transplant was associated with pediatric waitlist mortality. Data analysis was completed using SAS version 9.4 (SAS Institute, Cary, NC) and Stata/IC 14 (StataCorp, College Station, TX).
RESULTS
Distribution and Utilization of Livers That Met Donor Criteria for Split Liver Transplantation
Of 37 333 deceased donor livers transplanted 2010 to 2015, 11% (n = 4199) met our criteria for potential utilization in split liver transplantation. Of those meeting initial criteria, 42% had PHS high-risk characteristics that made it less likely they would be accepted for pediatric donors. This left 2369 livers—6.3% of transplanted livers—that met all stipulated donor criteria (Figure 1).
Of these potentially “split-able” livers (n = 2369), only 3.8% (n = 91) were actually used for split liver transplantation (Table 1). Among donors deemed eligible for splitting and pediatric transplant, the percentage transplanted as split ranged from less than 1% in regions 3, 6, and 11% to 11% in region 1, and 15% in region 9. Those transplanted as split or partial were more likely regional shares, and they had slightly longer cold ischemia times, but they were otherwise comparable in characteristics associated with donor risk (Table 1).
TABLE 1.
Deceased donor liver transplants that met donor criteria for utilization in split liver transplantation, n (%) or median (IQR)a
| Total | Whole liver | Split liver | Partial liver | ||
|---|---|---|---|---|---|
| Donor characteristics | N = 2369 | n = 2253 | n = 91 | n = 25 | P |
| Age, y | 25 (21–32) | 25 (21–32) | 24 (20–30) | 25 (19–29) | 0.02 |
| Weight, kg | 73.5 (65.0–82.0) | 73.7 (65.2–82.0) | 71.9 (65.0–80.0) | 70.0 (63.3–76.3) | 0.21 |
| Height, cm | 175 (168–183) | 175 (168–183) | 175 (168–180) | 175 (170–178) | 0.70 |
| BMI, kg/m2 | 24.2 (21.9–26.6) | 24.2 (21.9–26.6) | 23.5 (22.1–25.8) | 23.2 (21.2–24.9) | 0.18 |
| Cause of death | |||||
| Head trauma | 1564 (66.0) | 1479 (65.6) | 67 (73.6) | 18 (72.0) | 0.55* |
| Stroke | 428 (18.1) | 408 (18.1) | 16 (17.6) | 4 (16.0) | |
| Anoxia | 311 (13.1) | 302 (13.4) | 7 (7.7) | 2 (8.0) | |
| Other | 66 (2.8) | 64 (2.8) | 1 (1.1) | 1 (4.0) | |
| Share type | |||||
| Local | 1724 (72.8) | 1656 (73.5) | 55 (60.4) | 13 (52.0) | 0.02* |
| Regional | 627 (26.5) | 579 (25.7) | 36 (39.6) | 12 (48.0) | |
| National | 18 (0.8) | 18 (0.8) | 0 (0.0) | 0 (0.0) | |
| Cold ischemia time (n = 2360), h | 6.08 (4.81–8.00) | 6.05 (4.80–7.95) | 6.33 (4.56–8.69) | 7.00 (6.30–8.26) | 0.06 |
| Days hospitalized | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 2.0 (2.0–3.0) | 0.64 |
| Donor Risk lndexc | 1.08 (0.98–1.22) | 1.07 (0.98–1.19) | 1.65 (1.52–1.81) | 1.69 (1.60–1.89) | <0.001 |
| Donor Risk Index (excluding factor for split liver)c | 1.08 (0.98–1.19) | 1.07 (0.98–1.19) | 1.08 (1.00–1.19) | 1.11 (1.05–1.24) | 0.35 |
| Recipient characteristicsd | Total | Whole liver | Split liver | Partial liver | P |
| N = 2439 | n = 2253 | n = 161 | n = 25 | ||
| Female | 904 (37.1) | 812 (36.0) | 83 (51.6) | 9 (36.0) | <0.001 |
| Pediatric | 169 (6.9) | 58 (2.6) | 87 (54.0) | 24 (96.0) | <0.001 |
| Age, adult recipients, y | 56 (48–61) | 56 (48–61) | 58 (52–63) | 33 (33–33) | 0.04 |
| Age, pediatric recipients, y | 6.0 (1.0–13.0) | 14.0 (13.0–15.0) | 1.0 (1.0–6.0) | 3.0 (1.0–8.5) | <0.001 |
| Indication for liver transplant | |||||
| Tumor | 557 (22.8) | 501 (22.2) | 45 (28.0) | 11 (44.0) | e |
| Hepatitis C | 544 (22.3) | 532 (23.6) | 12 (7.5) | 0 (0.0) | |
| Other liver diseasef | 370 (15.2) | 345 (15.3) | 21 (13.0) | 4 (16.0) | |
| Alcoholic cirrhosis | 249 (10.2) | 240 (10.7) | 9 (5.6) | 0 (0.0) | |
| Cholestatic liver diseaseb | 245 (10.0) | 228 (10.1) | 15 (9.3) | 2 (8.0) | |
| Nonalcoholic steatohepatitis | 172 (7.1) | 170 (7.5) | 2 (1.2) | 0 (0.0) | |
| Acute liver failure | 161 (6.6) | 146 (6.5) | 12 (7.5) | 3 (12.0) | |
| Metabolic liver diseaseg | 105 (4.3) | 84 (3.7) | 18 (11.2) | 3 (12.0) | |
| Biliary atresia | 36 (1.5) | 7 (0.3) | 27 (16.8) | 2 (8.0) | |
| At waitlist removal | |||||
| Status | <0.001* | ||||
| 1A | 170 (7.0) | 150 (6.7) | 12 (7.5) | 8 (32.0) | |
| 1B | 54 (2.2) | 11 (0.5) | 31 (19.3) | 12 (48.0) | |
| MELD/PELD | 2,215 (90.8) | 2,092 (92.9) | 118 (73.3) | 5 (20.0) | |
| MELD > 40 or ICU | 607 (24.9) | 570 (25.3) | 27 (16.8) | 10 (40.0) | 0.01 |
| MELD/PELD laboratory score | 24 (14–34) | 24 (15–35) | 13 (6–24) | 10 (–5–15) | <0.001 |
| Allocated MELD/PELD score | 29.0 (24.0–37.0) | 29.0 (24.0–37.0) | 30.0 (25.0–35.0) | 40.0 (39.0–40.0) | 0.04 |
| Active exception | 794 (32.6) | 710 (31.5) | 80 (49.7) | 4 (16.0) | <0.001* |
| Total days on waiting list | 65 (11–251) | 64 (10–249) | 105 (26–301) | 25 (7–40) | <0.001 |
| Graft survival at 3 y | 82.7% (80.9–84.3) | 82.7% (80.9–84.4) | 79.4% (47.6–93.1) | 81.8% (70.7–88.9) | 0.93 |
| Patient survival at 3 y | 84.9% (83.2–86.4) | 84.6% (82.8–86.2) | 89.3% (82.0–93.8) | 79.4% (47.6–93.1) | 0.25 |
Continuous variables reported as median (IQR). Categorical variables reported as n (%). P value calculated using χ2 testing for categorical variables, using exact methods where appropriate (marked with “*”), and using Kruskal-Wallis test for continuous variables.
Cholestatic liver disease includes Alagille syndrome, Byler disease, progressive intrahepatic cholestatic syndromes, total parenteral nutrition cholestasis, sclerosing cholangitis, and idiopathic cholestasis.
Donor risk index calculated per Feng S, Goodrich NP, Bragg-Gresham JL et al. Am J Transpl 2006;6:783–790.
12 recipients of multiorgan transplant excluded from this analysis.
P value not calculated due to insufficient n in each cell.
Other liver disease includes congenital hepatic fibrosis, Budd-Chiari syndrome, autoimmune hepatitis cirrhosis, drug toxicity, and unknown cirrhosis.
Metabolic liver disease includes alpha-1-antitrypsin deficiency, Crigler-Najjar syndrome, cystic fibrosis, glycogen storage disease, inborn errors in bile acid metabolism, neonatal hemochromatosis, primary hyperoxaluria, tyrosinemia, urea cycle defects, and Wilson disease.
ICU, intensive care unit; MELD, Medical End-stage Liver Disease; PELD, pediatric end-stage liver disease.
Ninety-three percent of all the “split-able” livers went to adults, 3.3% as split livers. Only 6.9% (n = 169) went to pediatric recipients, with just over half transplanted as split livers (Table 1)
Of “split-able” livers transplanted whole (n = 2253), 97% went to adults. The minority of these whole liver recipients were status 1A at waitlist removal, but 25% did have Medical End-stage Liver Disease greater than 40 or were in the intensive care unit. (Table 1) 82% were listed as willing to accept a segmental liver, and only 3% were listed as requiring a cold ischemia time <6 hours (Figure 2). For those with a maximum cold ischemia time specified (n = 697), median was 12 hours (IQR, 10–15 hours).
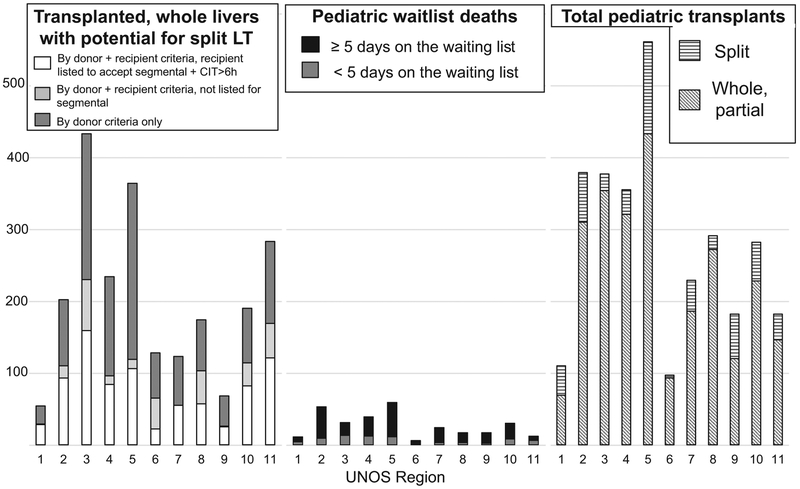
FIGURE 2.
By United Network for Organ Sharing (UNOS) region for the study period 2010 to 2015, comparison of (1) deceased donor livers that were transplanted as whole livers but were potentially “split-able” (ie, met criteria for potential split based on each category), (2) pediatric deaths on the liver transplant waiting list, and (3) number of pediatric whole and split liver transplants. We separated children listed ≥5 days before death/waitlist removal from the small percentage who died after less than 5 days on the waitlist, as a proxy for sufficient waitlist time to actually receive an offer and survive to transplant.
Of the whole liver recipients (n = 2253), we deemed 50% to be potentially high-risk for split liver transplantation (Figure 1). Of the 1116 lower risk patients, 77% were listed as willing to accept a segmental liver and only 3% were listed as requiring a cold ischemia time less than 6 hours.
Livers Actually Used for Split Liver Transplant
The 91 livers that met our criteria and were actually used for split liver transplant were transplanted. Of the 182 liver segments from these donors, only 4.9% were recovered but not transplanted. These segments went into 173 recipients and accounted for 18.6% of all deceased donor livers used for split liver transplant 2010 to 2015 (total, n = 490). Organs outside our criteria that were split appeared to be slightly higher risk than organs meeting our criteria; they were from smaller donors—including pediatric donors—that had been hospitalized longer, had a higher donor risk index, and higher than ideal transaminases and sodium (Table S1, SDC, http://links.lww.com/TP/B566). Only 9% were high risk by PHS criteria; 0.6% were hepatitis B (surface antigen or core antibody) or C (antibody) positive.
The recipients of split livers outside our criteria did not differ significantly from those that received splits meeting our criteria—by demographics, measures of illness severity, or transplant indication—or by graft or patient survival at 3 years posttransplant. Recipients of a split liver outside our criteria were more likely to be at a transplant center more than 300 miles from the donor hospital (Table S1, SDC, http://links.lww.com/TP/B566).
Pediatric Waitlist Mortality
Over the same 5 years, 299 pediatric patients died on the waitlist (n = 172) or were removed for being too sick (n = 127) at 60 transplant centers (Figure 2). Fifty-six percent were younger than 2 years at listing, 23% were 2 to 11 years, and 20% were 12 to 17 years. Median weight at listing was 9.4 kg (IQR, 6–30 kg), and 68% weighed 20 kg or less. Median days on the waitlist was 39 (IQR, 6–146 days).
Thirty-seven percent of pediatric waitlist deaths occurred at transplant centers that reported doing no pediatric split liver transplants (15%) or performed a mean of (averaged) 1 or less per year (22%) during the study period. However, 96% of these patients were listed as willing to accept a segmental liver at their final waitlist update. Of the 14 centers that had pediatric waitlist deaths and did pediatric transplants but no splits, 12 performed a mean of (averaged) 6 or less pediatric transplants per year. Of the remaining, 17 centers averaged 1 or less pediatric split liver transplant per year; 24 reported a median 16 pediatric split liver transplants over the 5-year study period (IQR, 9–24; range, 6–48).
Children who died at centers that averaged 0 to 1 pediatric split transplants annually were slightly older (median, 1.5 years; IQR, 0–11 years) and heavier (median, 10.4 kg; IQR, 6.4–36.9 kg) than those who died at centers that averaged >1 pediatric split transplant annually (median age, 0 year; IQR, 0–8 years; P = 0.04; median weight, 8.8 kg; IQR, 5.9–28 kg; P = 0.09). Median days on the waitlist did not differ significantly (P = 0.54). Children listed as centers that averaged 6 or less total pediatric transplants per year had an increased risk of waitlist mortality (subhazard ratio, 1.68; 95% CI, 1.25–2.26; P = 0.001), as has been previously demonstrated. After controlling for total center size and age at listing (<2, 2–11, 12–18 years), neither volume of pediatric split transplants per year or averaging more than 1 per year was associated with pediatric waitlist mortality (data not shown).
DISCUSSION
Of the 6.3% of transplanted, deceased donor livers that met our strict criteria for potential split transplant into pediatric recipients, the vast majority of these livers were transplanted into a single adult recipient. In every UNOS region, the number of potentially “split-able” livers—including having a primary recipient listed as willing to accept a segmental liver—was larger than the number of children that died waiting for a liver transplant. This comparison, although not conclusive, suggests that we might be missing opportunities to reduce pediatric waitlist mortality without decreasing access to liver transplants for adults.
Although the majority of adults who received a “split-able” liver were listed as willing to accept a segmental liver, very few received a split liver. Currently, the decision to split a liver is made at organ allocation by an individual provider for an individual patient. Almost all split transplants in the US occur when the primary recipient is too small for the entire allocated liver. Previous policy attempts to incentivize split liver activity has had minimal, if any, impact on practice. For example, a 2012 UNOS policy variance allowing transplant programs who split the graft into a right lobe for use in an adult to retain the left lobe for use in another patient at their center was used only once in the 2 years after implementation.8
One center in the United Kingdom addressed this gap between potential and practice with an “Intention to Split” policy; livers meeting their criteria are allocated to 2 recipients. This essentially increased the priority of children awaiting transplant—as has been done recently in the US renal and lung transplant allocation systems. From 2008 to 2014 in Birmingham, more than 65% of children received a split liver transplant, with 5- and 10-year patient survivals (89%–90%) and graft survivals (86%–87%).5 In the United States, 5- and 10-year posttransplant survivals for pediatric recipients Are very similar,9,10 but the percentage of children receiving split liver transplants is only 15%.1 For Birmingham’s right lobe recipients, 5-year patient and graft survival were 81% to 82%.
A 2016 UNOS Ethics Committee White Paper, approved by all 11 Regional Committees, supported policy changes in a similar direction. They advocated for policy “such that when 2 patients…are likely to have favorable outcomes with a split liver, then split liver transplantation should be offered….as the only transplantation option with that organ.”2 Another option would be to further prioritize children so that they more often receive the primary offer of a “split-able” liver. Among adult donors that meet UNOS criteria for split eligibility, 4.1% of organs primarily allocated to children are used for split transplant versus 0.6% of those primarily allocated to adults.11 Versions of this are used in some European countries—as are policies in which “split-able” livers are first offered to pediatric liver centers.12
Although early analyses suggested worse outcomes with split liver transplant,13,14 recent studies demonstrate equivalent graft and patient posttransplant survival for US pediatric recipients of split liver transplants, after adjusting for other factors, both in highly experienced single centers7,15 and in the comprehensive UNOS database.3,16
Splitting a liver does add technical complexity and ischemic time. An increased risk of posttransplant complications—most notably bile leaks and strictures—remains in most studies.6 These complications are largely treatable, but require hospitalization and invasive procedures.
Additional barriers to increasing split liver transplant in the United States include logistical challenges and surgeon availability and experience. These challenges are compounded by the geographic size of our country. Uncertainty remains about best technical practices, but a standardized approach might be helpful. For example, allocating the proper hepatic artery with the left lateral segment can more reliably yield an appropriately sized orifice for arterial reconstruction in both recipients. Using this strategy, Birmingham’s hepatic artery thrombosis prevalence fell to 6%.6
Our analysis supports that split liver transplantation may not currently be a realistically accessible option for all waitlisted children. Almost all children who died on the waitlist were listed as willing to accept a segmental liver; two thirds were 20 kg or less—suggesting a left lateral segment might be sufficient. However, more than one third that died were at centers averaging 1 or less pediatric split liver transplants per year. Further inquiry into offers and factors impacting a center’s decision to accept a liver for split transplantation would be helpful next steps in considering policy adjustments. Multi-institution collaboratives to increase split availability/utilization17 and increased use of ex vivo normothermic machine perfusion to preserve graft quality during split or transfer18 might also help protect these vulnerable children.
Our strict criteria for “split-able” livers provide a conservative estimate of the potential impact for increased split liver transplant. In fact, 80% of deceased donor livers actually used for split liver transplantation were slightly outside our criteria. This suggests that even small or conservative adjustments in split utilization could have a significant impact on waitlisted children.
This is not a conclusive analysis of the impact that increasing split liver transplant would have on pediatric outcomes. Limitations include those of our retrospective data set and our descriptive statistical approach. We did not have access to data on organ offers, to evaluate whether children that died had feasible organ offers for split transplant. UNOS data do not include detailed data on why surgeons accept or reject specific organs for individual patients, limiting our ability to predict or model their behavior. Fully describing the impact that greater access to “split-able” livers would require additional simulation modeling—perhaps estimating the impact of offering livers that meet strict criteria as potential splits if not accepted by any status 1 candidate.
Increasing utilization of split liver transplant is a complex endeavor. However, over the past 30 years, almost 1500 children have died on the liver transplant waiting list. In contrast, children that are transplanted have excellent long-term outcomes. Increasing split liver transplantation would likely reduce waitlist mortality in children without significantly decreasing the number of organs available for adults. Further research into barriers—and efforts to overcome them—is a worthwhile next step.
Supplementary Material
Acknowledgments
This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C (UNOS Data), the NIH-NIDDK (Dr. Perito, K23 DK0990253-A101), the UCSF Liver Center (P30 DK026743), and the UCSF Department of Pediatrics (Clinical/Translational Pilot Study Grant). The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and should not be seen as an official policy of or interpretation by the SRTR or the US Government. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the NIH or the Department of Health and Human Services, nor does mention of trades names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
The authors declare no conflicts of interest.
E.R.P. led the study design, IRB approval, data analysis and interpretation, writing and revision of the article. G.R. participated in study design, data interpretation, writing and revision of the article. J.L.D. participated in study design, led data analysis and interpretation, participated in writing and revision of the article. S.R. participated in study design, data interpretation, writing and revision of the article. J.P.R. participated in study design, data interpretation, writing and revision of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2015 annual data report: liver. Am J Transplant. 2017;17(Suppl 1):174–251. [DOI] [PubMed] [Google Scholar]
- 2.OPTN/UNOS Ethics Committee. Split versus whole liver transplantation. https://optn.transplant.hrsa.gov/resources/ethics/split-versus-whole-liver-transplantation/. Updated December 2016.
- 3.Cauley RP, Vakili K, Potanos K, et al. Deceased donor liver transplantation in infants and small children: are partial grafts riskier than whole organs? Liver Transpl. 2013;19:721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan P, Li Q, Zhang J, et al. Right lobe split liver transplantation versus whole liver transplantation in adult recipients: a systematic review and meta-analysis. Liver Transpl. 2015;21:928–943. [DOI] [PubMed] [Google Scholar]
- 5.Moussaoui D, Toso C, Nowacka A, et al. Early complications after liver transplantation in children and adults: are split grafts equal to each other and equal to whole livers? Pediatr Transplant. 2017;21. [DOI] [PubMed] [Google Scholar]
- 6.Battula NR, Platto M, Anbarasan R, et al. Intention to split policy: a successful strategy in a combined pediatric and adult liver transplant center. Ann Surg. 2017;265:1009–1015. [DOI] [PubMed] [Google Scholar]
- 7.Vagefi PA, Parekh J, Ascher NL, et al. Outcomes with split liver transplantation in 106 recipients: The University of California, San Francisco, experience from 1993 to 2010. Arch Surg. 2011;146:1052–1059. [DOI] [PubMed] [Google Scholar]
- 8.OPTN/UNOS Pediatric Transplantation Committee. OPTN/UNOS Pediatric Transplantation Committee Report to the board of directors. https://optn.transplant.hrsa.gov/media/1324/pediatric_boardreport_20150603.pdf. Accessed Oct 31, 2017 Updated June 1–2, 2015.
- 9.Ng VL, Fecteau A, Shepherd R, et al. Studies of Pediatric Liver Transplantation Research Group. Outcomes of 5-year survivors of pediatric liver transplantation: report on 461 children from a North American multicenter registry. Pediatrics. 2008;122:e1128–e1135. [DOI] [PubMed] [Google Scholar]
- 10.Ng VL, Alonso EM, Bucuvalas JC, et al. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: report of the studies of pediatric liver transplantation experience. J Pediatr. 2012;160:820–826.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu EK, Shaffer ML, Gao L, et al. Analysis of liver offers to pediatric candidates on the transplant wait list. Gastroenterology. 2017;153:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu EK, Mazariegos GV. Global lessons in graft type and pediatric liver allocation: a path toward improving outcomes and eliminating wait-list mortality. Liver Transpl. 2017;23:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDiarmid SV, Anand R, Martz K, et al. A multivariate analysis of pre-, peri-, and post-transplant factors affecting outcome after pediatric liver transplantation. Ann Surg. 2011;254:145–154. [DOI] [PubMed] [Google Scholar]
- 14.Diamond IR, Fecteau A, Millis JM, et al. Impact of graft type on outcome in pediatric liver transplantation: a report from studies of pediatric liver transplantation (SPLIT). Ann Surg. 2007;246:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hashimoto K, Quintini C, Aucejo FN, et al. Split liver transplantation using hemiliver graft in the MELD era: a single center experience in the united states. Am J Transplant. 2014;14:2072–2080. [DOI] [PubMed] [Google Scholar]
- 16.Mogul DB, Luo X, Bowring MG, et al. Fifteen-year trends in pediatric liver transplants: split, whole deceased, and living donor grafts. J Pediatr. 2018;pii:S0022–3476:31508–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spada M, Gridelli B, Colledan M, et al. Extensive use of split liver for pediatric liver transplantation: a single-center experience. Liver Transpl. 2000;6:415–428. [DOI] [PubMed] [Google Scholar]
- 18.Ceresa CDL, Nasralla D, Coussios CC, et al. The case for normothermic machine perfusion in liver transplantation. Liver Transpl. 2018;24:269–275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.