Abstract
Diabetes mellitus (DM) during pregnancy can result in fetal overgrowth, likely due to placental dysfunction, which has health consequences for the infant. Here we test our prediction from previous work using a placental cell line that high glucose concentrations affect placental lipid metabolism. Placentas from women with type 1 (n = 13), type 2 (n = 6) or gestational (n = 12) DM, BMI-matched to mothers without DM (n = 18), were analysed for lipase and fatty acid transport proteins and fatty acid and triglyceride content. Explants from uncomplicated pregnancies (n = 6) cultured in physiological or high glucose were similarly analysed. High glucose levels did not alter placental lipase or transporter expression or the profile and abundance of fatty acids, but triglyceride levels were higher (p < 0.05), suggesting reduced β- oxidation. DM did not affect placental protein expression or fatty acid profile. Triglyceride levels of placentas from mothers with pre-existing DM were similar to controls, but higher in obese women with gestational DM. Maternal hyperglycemia may not affect placental fatty acid uptake and transport. However, placental β-oxidation is affected by high glucose and reduced in a subset of women with DM. Abnormal placental lipid metabolism could contribute to increased maternal-fetal lipid transfer and excess fetal growth in some DM pregnancies.
Subject terms: Endocrine system and metabolic diseases, Reproductive disorders
Introduction
Pregnancies complicated by diabetes mellitus (DM) are associated with adverse perinatal outcomes, most commonly relating to fetal overgrowth (macrosomia). Macrosomic infants can suffer from stillbirth, birth injuries and post-natal metabolic disturbances and are more likely to develop obesity, DM and cardiovascular disease in later life1. Maternal hyperglycemia is associated with fetal macrosomia2, yet the role of the placenta, the organ responsible for transferring oxygen and nutrients from mother to fetus, in mediating this effect is not well understood. Our previous work using an unbiased systems biology approach to study a placental cell line (BeWo) response to high glucose levels highlighted aberrant trophoblast lipid metabolism and β-fatty acid oxidation3.
Maternal plasma free fatty acids are elevated in pregnancies complicated by DM4. It is thought that these fatty acids are transferred across the placenta to fetal adipose tissue where they are esterified into triglycerides using glycerol derived from excess maternal glucose, thereby contributing to macrosomia5. However, it is possible that maternal glucose may also affect placental lipid metabolism and the transfer of triglycerides from mother to fetus.
In order to enter the placenta, maternal circulating triglycerides carried in lipoproteins must be hydrolysed at the placental membrane by placental lipases (endothelial lipase (EL) or lipoprotein lipase (LPL))6,7. While some of the resulting free fatty acids can passively diffuse into the placental trophoblast cells, most require facilitated transport by membrane-bound and intracellular proteins6, such as fatty acid translocase (FAT) and fatty acid transporter family proteins (FATP1–6)6. Once inside the placenta, fatty acids can be directly transported into the fetal circulation, metabolised via β-oxidation8 or stored as triglycerides for subsequent transfer to the fetal circulation. The rate-limiting step for β-oxidation is the conversion of fatty acyl CoA to acylcarnitine in a reaction that is catalysed by carnitine palmityltransferase (CPT1)8. This study assessed how high glucose levels affect fatty acid transport and metabolism by placental trophoblast and whether these are disrupted in placentas from women with type 1 and type 2 DM.
Results
Effect of high glucose levels on lipid metabolism in placental explants from uncomplicated pregnancies
Initially, we sought to confirm our prediction from previous work3 – that lipid metabolism is altered in a choriocarcinoma cell-line (BeWo) exposed to high (25 mM) glucose levels - by assessing the effect of such glucose concentrations on the triglyceride content of placental explants isolated from uncomplicated pregnancies at term (Table 1). The data presented in Fig. 1 show that glucose promotes placental triglyceride accumulation; levels were 2.7-fold greater (p < 0.05) in placental explants exposed to high glucose versus explants maintained at physiological glucose concentrations.
Table 1.
Demographic, obstetric and biophysical data for patient participants with type 1 diabetes (T1DM), type 2 diabetes (T2DM), gestational diabetes and a BMI ≤ 30 (GDM BMI ≤ 30), gestational diabetes and a BMI ≥ 30 (GDM BMI ≥ 30) and BMI matched controls.
| T1DM (n = 13) | T2DM (n = 6) | GDM (BMI < 30) (n = 6) | GDM (BMI ≥ 30) (n = 6) | Control (BMI < 30) (n = 9) | Control (BMI ≥ 30) (n = 9) | p | |
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 25 (20–33) | 33 (22–41) | 26.1 (22–29) | 37.9 (32–47) | 22 (22–27) | 31 (30–32) | ns |
| Maternal Age (years) | 32 (19–43) | 38 (26–42) | 33 (26–41) | 35 (29–41) | 34 (29–39) | 33 (21–42) | ns |
| Parity | 1 (0–5) | 2 (0–5) | 1 (0–3) | 4 (1–9) | 1 (0–2) | 1 (1–3) | ns |
| Caucasian (%) | 80 | 83 | 50 | 67 | 77 | 67 | ns |
| Smoker (%) | 0 | 0 | 0 | 0 | 0 | 0 | ns |
| Gestation (week + days) | 37 + 1 (36 + 1–39 + 0) | 37 + 3 (34 + 0–39 + 0) | 38 + 4 (36 + 0–40 + 4) | 38 + 4 (38 + 0–40 + 3) | 39 + 0 (36 + 5–41 + 4) | 39 + 1 (38 + 3–41 + 6) | ns |
| Male (%) | 40 | 83 | 50 | 33 | 33 | 55 | ns |
| Caesarean (%) | 87 | 50 | 50 | 83 | 77 | 73 | ns |
| Birth Weight (g) | 3400 (2660–4340) | 3209 (2990–3867) | 3338 (3035–3720) | 4204 (3580–4518) | 3080 (2580–3800) | 3640 (3180–4200) | ns |
| Birthweight centile§ | 77.5 (43–100) | 67.0 (35–95) | 62.8 (30–89) | 96.7 (94–100) | 43 (12–80) | 52 (30–88) | a) <0.05 |
| Large (>90th) for gestational age (%) | 5/13 (42.8) | 2/6 (33.3) | 0/6 (0) | 6/6 (100) | 0/9 (0) | 0/9 (0) | b) <0.05 |
| HbA1C (3rd trimester) (mmol/mmol) | 49 (37–62) | 46 (23–72) |
Data are median (range). Abbreviations: BMI, body mass index; §customised birthweight centile42 a) - T1 DM compared to controls with a BMI ≤ 30; Kruskal-Wallis test with Dunn’s post-hoc test; b) – Chi-square test.
Figure 1.
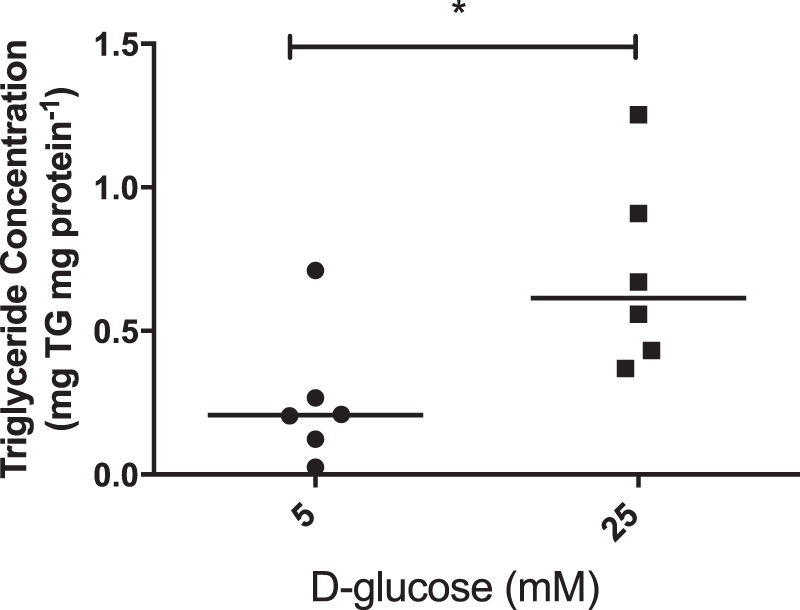
Explants of placenta from uncomplicated pregnancies (n = 6) were cultured for 18 hours in medium containing either 5 mM or 25 mM D-glucose. Explant triglyceride content was measured, in duplicate, using a commercially available kit and concentrations, normalised to protein content, are presented as median and range. *p < 0.05; Mann-Whitney test.
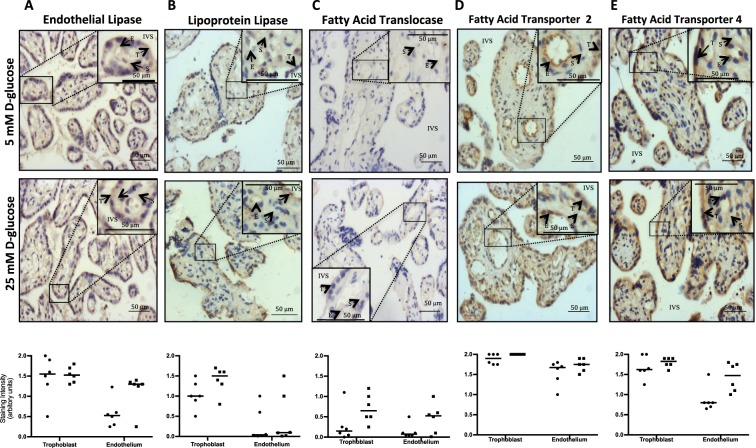
Next we investigated whether the glucose-mediated increase in placental triglyceride levels was a consequence of altered uptake or transport of fatty acids by determining if glucose affects the expression of EL, LPL, FATP2, FATP4 and FAT, key proteins required for these processes, and/or the level of individual fatty acids accumulated in the placenta9–12. Immunohistochemical analyses (shown in Fig. 2) revealed that trophoblast express both EL and LPL in abundance, while endothelium mainly expresses EL (Fig. 2A,B). FAT (Fig. 2C) FATP2 (Fig. 2D) and FATP4 (Fig. 2E) were expressed by all cellular compartments of the placenta, though FATP2 was the most abundant. Treatment of placental explants with high glucose did not alter the localisation or intensity of staining for the lipases or FA transporters (Fig. 2A–E; p > 0.05).
Figure 2.
Immunohistochemical analysis of placental tissue from uncomplicated term pregnancies (maternal BMI < 30) cultured in 5 mM (circle) or 25 mM (square) D-glucose for (A) endothelial lipase, (B) lipoprotein lipase, (C) fatty acid translocase, (D) fatty acid transporter protein (FATP) 2 and (E) FATP4. T – trophoblast, E – endothelium, S - stroma, IVS – intervillous space. Images are representative of data obtained from 6 different placentas. The intensity of staining in the trophoblast and endothelium of explants from each placenta was scored as low (0), medium (1) or high (2) from a mean of ten images; bar represents the median value of 6 placentas. Data were compared using Wilcoxon matched-pairs analysis; no differences in staining for either of the lipases or the fatty acid transporters was observed following explant culture in 5 mM Vs 25 mM D-glucose (p > 0.05).
Analysis of the total level of each fatty acid present in placental explants cultured in physiological concentration of glucose (5 mM) (Supplementary Table 1) showed that palmitic acid, stearic acid, oleic acid and arachidonic acid were present in greatest abundance. Neither the profile, nor relative abundance of the fatty acids present in placental explants were affected by exposing tissue to high levels of glucose (Supplementary Table 1; p > 0.05).
We therefore explored the possibility that altered placental fatty acid metabolism, specifically their reduced catabolism via β-oxidation, might provide an explanation for the glucose-induced increase in triglyceride levels. The observation that glucose-stimulated accumulation of triglyceride is attenuated in explants co-cultured with all doses of clofibrate (10–40 μM), a PPARα agonist that enhances the expression of CPT1, the rate limiting step for β-oxidation, provides indirect support for this hypothesis (Fig. 3).
Figure 3.
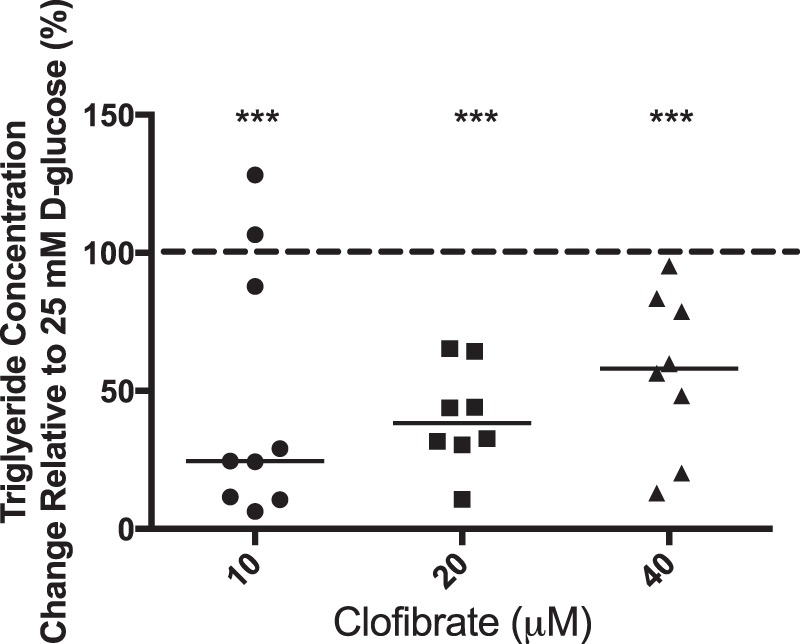
Placental explants from uncomplicated pregnancies (n = 9) were cultured overnight in 25 mM D-glucose and then switched to media containing 25 mM D-glucose in the absence (control) or presence of Clofibrate (10, 20 or 40 μM) for a further 18 hours. Explant triglyceride content was measured, (technical duplicates), using a commercially available colorimetric kit and concentrations were normalised to protein content. Data are presented as % change (median and range) relative to culture in 25 mM D-glucose alone (shown as line at 100%). ***p < 0.001 versus culture in 25 mM D-glucose alone; Wilcoxon-signed rank test.
Characterisation of fatty acid uptake and metabolism in placentas from pregnancies complicated by pre-existing maternal DM
Our assessment of the expression of EL, LPL and fatty acid transporters in placentas obtained from women with T1DM or T2DM (Table 1) suggested that both the location and level of each of these proteins are similar to that observed in placentas from BMI-matched uncomplicated pregnancies (p > 0.05; Fig. 4). Similarly, the profile and level of fatty acids found in placental tissue was not affected by the presence of maternal DM (Supplementary Table 2; p > 0.05; Kruskal-Wallis).
Figure 4.
Placental tissue from pregnancies complicated by T1DM (n = 6) or T2DM (n = 6) and uncomplicated term pregnancies (maternal BMI < 30 (n = 6) and >30 (n = 6)) were stained using antibodies that recognise (A) endothelial lipase, (B) lipoprotein lipase, (C) fatty acid translocase, (D) fatty acid transporter protein (FATP) 2 or (E) FATP4 then analysed by scoring the intensity of staining in the trophoblast and endothelium as low (0), medium (1) or high (2). Data points represent the mean score from ten images, with the median value plotted as a bar. Circle - T1DM; square - T2DM; triangle - control BMI < 30; inverted triangle - control BMI > 30. Data were compared using the Kruskal-Wallis test; no differences in staining for the lipases or fatty acid transporters were observed, in either compartment, between the subject groups (p > 0.05).
Surprisingly, analysis of the triglyceride content of explants from mothers with DM revealed that neither T1DM nor T2DM affected placental triglyceride accumulation when compared to their respective BMI-matched controls (Fig. 5). These data contrast with the observations made in explants exposed to high (25 mM) glucose levels ex vivo and therefore, as a control, we also assessed the level of triglycerides in placentas obtained from pregnancies complicated by gestational DM as a previous study reported elevated levels in such tissue13. We also found the level of triglycerides in placentas from mothers with gestational diabetes to be significantly higher but only in those with concurrent obesity (Fig. 5).
Figure 5.
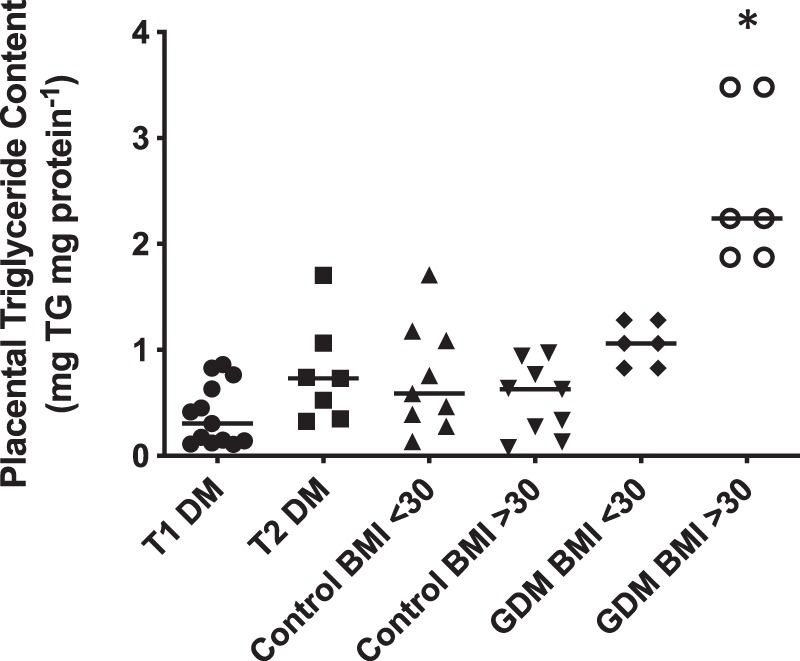
Placental tissue was collected from women with type 1 DM (T1 DM; n = 13), type 2 DM (T2 DM; n = 6), GDM (BMI < 30 (n = 6), BMI > 30 (n = 6)) and BMI matched controls (BMI < 30; n = 9) and (BMI ≥ 30; n = 9). Triglyceride content of each placental homogenate was measured using a commercially available kit and concentrations, normalised to protein content; bar – median. *p < 0.05 Vs control BMI > 30, Kruskal-Wallis with Dunn’s post-hoc test.
Discussion
Our previous systems biology analysis of a trophoblast cell line (BeWo) exposed to high glucose levels predicted alterations in placental lipid metabolism3 that could contribute to the enhanced fetal growth often observed in pregnancies complicated by maternal DM. Our current analysis of human placental explants suggests that placental lipid metabolism, but not uptake or transport, is affected by supraphysiological glucose levels; however, this aspect of placental function appears to be maintained in women who have received prolonged hypoglycaemic treatment during pregnancy.
Our immunohistochemical analysis of placental lipase expression revealed that both EL and LPL are expressed by trophoblast. These data suggest that, in contrast to previous hypotheses7, EL also has an important role to play in the hydrolysis of maternal lipoproteins as the lysophospholipids generated by EL activity can be further hydrolysed (by EL) to provide a source of fatty acids for the placenta14; this suggestion is further supported by reports of an adequate supply of FA to the fetus in women with LPL deficiency15, and, importantly, unlike LPL, EL expression by trophoblast is maintained through to term11.
We found no evidence of altered lipase expression in placentas exposed, albeit for relatively short time periods, to high glucose ex vivo, though our data on in vivo exposure (from placentas from women with DM) support this finding. Our data on placental lipase expression corroborate previous reports relating to LPL expression in placentas from women with type 1 DM16,17, but others have found higher EL expression in placentas from such pregnancies, particularly in women with poor glycemic control16. Therefore, it is possible that satisfactory glycaemic control during pregnancy, which was evident amongst the women in our study, enables maintenance of appropriate EL expression.
The expression of fatty acid transporter proteins FAT, FATP2 and FATP4 in placentas from pregnancies complicated by DM has not been assessed previously, though others have demonstrated increased expression of FATP2 at the basal (fetal-facing) membrane in pregnancies complicated by obesity (defined in that study as BMI > 25)18. Our study did not corroborate this finding as the expression of FATP2 in the trophoblast of placentas from women with a BMI ≥ 30 was similar to that observed in tissue from women with a normal BMI, although we did not specifically quantify expression at the basal membrane. In fact, none of the fatty acid transporter proteins demonstrated differential abundance as a consequence of ex vivo high glucose or in vivo exposure. Although we acknowledge that a limitation of our study is that we did not assess whether the activity of the lipases and transporter proteins, which also influences the availability of fatty acids for metabolism, is altered as a consequence of exposure to altered glucose conditions or maternal DM, our data on protein expression coupled with our finding that the profile and concentrations of fatty acids present in the placenta was unaffected by ex vivo exposure to high glucose, or by the presence of maternal DM, suggest that changes solely to the glucose environment are unlikely to alter fatty acid transport into and across the placenta. Rather, any such alterations are likely to be the consequence of composite changes to the metabolic milieu19, including hyperinsulinaemia and the altered cytokine/chemokine profile reflective of systemic inflammation20 in DM. Nonetheless, it may be beneficial to study the content of the syncytial microvillous (maternal-facing) and basal membranes separately as well as whole tissue homogenates, as others have reported altered levels of oleic acid and linoleic acid in the basal membrane of placentas from other pregnancies complications, including fetal growth restriction21. However, our study identified palmitic acid, arachidonic acid, a long chain polyunsaturated fatty acid required for fetal neural development22, oleic acid and stearic acid to be the most abundant fatty acids in term placental tissue, which is in keeping with previous reports21.
Whilst exposure to aberrant glucose levels appears to have no effect on placental fatty acid uptake and/or transport, our data are indicative of an effect on β-oxidation, as reported by others13. Term placental explants cultured in high glucose conditions had higher levels of triglycerides, suggesting that under such conditions, cellular energy is provided by glycolysis rather than lipid oxidation, enabling fatty acid esterification for storage in the form of triglycerides23. This putative mechanism is supported by data showing that treatment with the PPARα agonist, clofibrate resulted in attenuation of the glucose-stimulated increase in placental triglyceride levels. PPARα up-regulates CPT124, the rate-limiting enzyme for fatty acid β-oxidation by catalysing the conversion of fatty acyl CoA to acetylcarnitine. Similarly, Martinez et al., demonstrated reduced triglyceride levels in explants of placenta from control and diabetic rats following culture with clofibrate23. Interestingly, PPARα levels are reduced in placentas from streptozotocin-induced diabetic rats 25, however activation of the receptor using the specific ligand, leukotriene B4, led to a decrease in placental and fetal weight in such animals 25, suggesting that reducing the potential for glucose stimulated storage of fatty acids as triglycerides could reduce the availability of fatty acids for transfer to the fetus, thereby preventing excessive fetal growth. Nonetheless, further studies to directly assess the effect of PPARα on CPT1 expression in placenta, as well as the other mechanisms potentially involved in glucose-mediated regulation of CPT1 activity and β-oxidation, e.g., enhanced acetyl CoA carboxylase production of malonyl CoA, are required to confirm our observations.
Contrary to our initial hypothesis, we did not observe increased triglyceride levels in placental explants from women with either type 1 or type 2 DM. This finding was unexpected, as others have reported elevated levels of triglycerides in placentas from women with gestational DM13. We therefore assessed the triglyceride content of such placentas as a control for our assay and also found increased levels, though only in tissue from mothers who had GDM and were also obese (BMI > 30). It is possible that our findings in women with pre-existing DM reflect the fact that these women have intensive monitoring and glucose-lowering treatment throughout their pregnancy with the majority achieving at least satisfactory blood glucose control in the third trimester (only two were admitted and delivered before 37 weeks secondary to concerns regarding glyacemic control). It is also likely that in vivo, abnormal glucose metabolism is not the sole cause of aberrant lipid metabolism within the placenta and that maternal insulin levels and dyslipidaemia may also be an important determinant of placental fatty acid metabolism. Previous studies have suggested that the balance between lipogenesis and lipolysis in pregnancies complicated by diabetes may be affected by maternal insulin concentrations26,27. Consequently, it is possible that lipid metabolism and triglyceride storage within the placentas of pregnancies complicated by type 1 and 2 diabetes may change following prolonged exposure to an altered maternal metabolic environment associated with prolonged hypoglycaemic treatment (insulin ± metformin).
The increase in triglycerides in women with gestational DM who are also obese suggests that obesity is a potentially important confounding factor present in many women with DM. Critically, the mean BMI in women with gestational DM investigated by Visiedo et al.13 was 25.9 (n = 8) meaning any effect of obesity would not have been evident. In our study, the women with high BMI and GDM, who all had macrosomic babies, may have had longer (unrecognized) exposure to elevated glucose levels without pharmacological treatment, and/or they may represent a severe phenotype within the GDM population. Furthermore, we acknowledge that the glucose concentration used in our ex vivo studies, chosen to reflect previous similar studies13,28,29 and our initial work indicating aberrant lipid metabolism in response to exposure to high glucose concentrations3, is likely higher than levels typically observed in pregnant women. Further studies to elicit the concentration of glucose needed to drive placental fatty acid metabolism towards storage as triglycerides are required to determine the level of hyperglycemia likely to provoke this effect in women with DM. Future studies should also consider fetal sex as data from pigs30 and rabbits31 suggest that the transfer and storage of fatty acids is higher in female placentas.
In conclusion, we have confirmed that high glucose leads to increased placental triglyceride levels, indicating decreased placental β-oxidation in a subset of women with DM and obesity, which could lead to an increase in lipid transfer and potentially excessive fetal growth. The effect of high glucose on placental β-oxidation can be attenuated using the PPARα agonist clofibrate. It is possible that targeting CPT1 using PPARα agonists could provide a novel therapeutic intervention to prevent excess placental esterification of fatty acids to triglycerides and attenuate the increased fetal growth observed in pregnancies complicated by maternal DM.
Methods
Human placental tissue collection and culture
Human placentas were collected following delivery of a singleton infant between 37 to 42 weeks gestation (Table 1) with informed, written consent in accordance with Research Ethics Committee approval (08/H1010/55 + 5, Manchester UK). As women with type 2 DM are often obese and obesity is associated with altered placental structure and function32–35 and altered fetal growth36,37, placentas were obtained from two groups of women without DM – those with a BMI < 30 and women that were obese (BMI ≥ 30) – resulting in two groups of ‘control’ placentas. Placentas from two groups of women with GDM were also collected (BMI < 30 and ≥30). Women were diagnosed with GDM following an oral glucose tolerance test performed after 24 weeks gestation or following abnormal home blood glucose monitoring in women with a history of prior GDM (n = 3).
In order to obtain an overall assessment of placental function, a full thickness sample (≈1 cm3) was cut from the centre, middle and edge of each placenta (along a ‘line’, chosen at random, radiating from the centre) then the three samples were pooled, washed with sterile, cold phosphate buffered saline and processed for subsequent immunohistochemistry or lipid analyses or dissected into explants (as described previously38) for immediate culture.
Explants were cultured in 1:1 DMEM:F12, containing 10% FBS and physiological (5 mM) or high (25 mM) levels of D-glucose for 18 h then fixed for immunohistochemistry analysis or processed for triglyceride analysis. In some experiments, explants cultured in high (25 mM) D-glucose were incubated for a further 18 h in the absence or presence of the PPARα agonist (which can upregulate CPT1 expression24), Clofibrate (10–40 μM; Sigma Aldrich), then analysed for triglyceride content. Culture for 18 h was used as this time point had previously been used in similar studies to assess the effects of high glucose concentration on placental explants13.
Immunostaining
Immunohistochemical analysis was performed on 5 µm sections of formalin-fixed wax-embedded villous tissue from the three regions of the placenta (centre, middle and edge) using colorimetric detection as described39 and the primary antibodies detailed in Table 2. Ten random images per section were captured and staining in trophoblast and endothelium separately quantified using a semi-quantitative scale (0 – no staining; 1 – some staining and 2 – heavy staining) by two scorers blinded to sample identity. The mean score for each placenta was calculated and tissue from different treatment and patient groups were analysed by Wilcoxon matched-pairs and Kruskal-Wallis with Dunn’s post-hoc tests respectively. p < 0.05 was considered to represent a statistically significant difference. Representative images of data obtained when using non-immune mouse or rabbit IgG as a negative control are presented in Supplementary Fig. 1.
Table 2.
Antibodies used in immunohistochemical analysis of explants of human placenta obtained at term.
| Primary Antibody | Secondary Antibody | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | Target | Gene ID | Supplier | Working Concentration | Species | Target | Supplier | Concentration |
| Mouse | Lipoprotein Lipase | LPL | Millipore (#MABC674) | 5 µg/ml | Goat | Mouse IgG | DAKO | 7.5 mg/ml |
| Mouse | Fatty Acid Translocase | FAT | Santa Cruz (sc-21772)43 | 1 µg/ml | ||||
| Rabbit | Endothelial Lipase | EL | Pierce (#PA1-16799) | 5 µg/ml | Swine | Rabbit IgG | DAKO | 4.4 mg/ml |
| Rabbit | Fatty Acid Transporter 4 | FATP4 | Santa Cruz (#sc-25670)44 | 1 µg/ml | ||||
| Goat | Fatty Acid Transporter 2 | FATP2 | Santa Cruz (#sc-161311)45 | 0.27 µg/ml | Rabbit | Goat IgG | DAKO | 3.9 mg/ml |
| Mouse | Non-immune IgG | Sigma | At same concentration as primary antibody of interest | Goat | Mouse IgG | DAKO | 7.5 mg/ml | |
| Rabbit | Non-immune IgG | Sigma | At same concentration as primary antibody of interest | Swine | Rabbit IgG | DAKO | 4.4 mg/ml | |
| Goat | Non-immune IgG | Sigma | At same concentration as primary antibody of interest | Rabbit | Goat IgG | DAKO | 3.9 mg/ml | |
Analysis of placental fatty acid composition
The profile and relative abundance of individual fatty acids in placental lipid extracts was investigated using gas chromatography as described before40; briefly: 100 mg of tissue (made up of tissue from the centre, middle and edge of the placenta) was homogenised in ice-cold chloroform:methanol (2:1 v/v) containing the anti-oxidant butylated hydroxytoluene (BHT) (0.01%) using a blade homogeniser (Ystral; 3 × 15 s). The lipid extracts were trans-esterified using a BF3-methanol solution (14%) with C21:0 (Supelco) as internal standard. Fatty acid methyl esters (FAME) were analysed on an Agilent 6850 GC-FID gas chromatograph (Agilent Technologies) with a BPX70 capillary column (0.25 μm film, 60 m × 0.25 mm; SGE Europe Ltd) using helium as the carrier gas and a flame ionization detector. Fatty acids were identified by comparison of retention times to those obtained by analysis of FAME standards (Sulpeco, UK). Data reported as relative percentage weight of total fatty acids.
The effect of glucose on the percentage weight of individual fatty acids was assessed using the Wilcoxon-signed rank test. Differences in the percentage weight of individual fatty acids in placental tissue from T1DM and T2DM compared to their BMI-matched controls were measured using a Kruskal-Wallis test with a Dunn’s post-hoc test. p < 0.05 was considered to be statistically significant.
Analysis of placental triglyceride concentration
Triglycerides were extracted by homogenising placental tissue (20 mg, containing tissue from the centre, middle and edge of the placenta) using an electronic blade homogeniser (LTF Labortechnick, Germany) in HPLC- grade acetone then rotating samples overnight at 4 °C and centrifuging at 15,000 × g for 15 min. Triglyceride concentrations were measured using a commercial colorimetric assay based upon enzymatic hydrolysis of the triglycerides by lipase to produce glycerol and free fatty acids, following manufacturers’ instructions (Caymann Chemical, Cambridge) and are presented as per mg of protein (determined using the Lowry method41). The effect of ex vivo glucose exposure on placental triglyceride content was assessed using a Mann-Whitney test whereas the Wilcoxon-signed rank test was used to analyse experiments including clofibrate and the levels of triglyceride in placentas from the different patient groups were compared by Kruskal-Wallis. p < 0.05 was considered to represent a significant difference.
Supplementary information
Acknowledgements
CHH was funded through a University of Manchester Research Impact Scholarship award.
Author Contributions
C.H.H., A.N., A.E.P.H., J.E.M. and M.W. - conception and design of research; C.H.H. and S.A.M. - performed experiments; C.H.H., A.N. and S.A.M. - analysed data; C.H.H., A.N., A.E.P.H., J.E.M. and M.W. - interpreted results of experiments; C.H.H. - prepared figures and drafted manuscript; C.H.H., A.N., A.E.P.H., J.E.M. and M.W. - edited and revised manuscript; C.H.H., A.N., S.A.M., A.E.P.H., J.E.M. and M.W. - approved final version of manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-50626-x.
References
- 1.Catalano PM. Obesity, insulin resistance, and pregnancy outcome. Reproduction. 2010;140:365–371. doi: 10.1530/REP-10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen J. Diabetes and pregnancy; blood sugar of newborn infants during fasting and glucose administration. Nord Med. 1952;47:1049. [PubMed] [Google Scholar]
- 3.Hulme CH, et al. Identification of the functional pathways altered by placental cell exposure to high glucose: lessons from the transcript and metabolite interactome. Scientific reports. 2018;8:5270. doi: 10.1038/s41598-018-22535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montelongo A, Lasuncion MA, Pallardo LF, Herrera E. Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes. 1992;41:1651–1659. doi: 10.2337/diab.41.12.1651. [DOI] [PubMed] [Google Scholar]
- 5.Szabo AJ, Szabo O. Placental free-fatty-acid transfer and fetal adipose-tissue development: an explantation of fetal adiposity in infants of diabetic mothers. Lancet. 1974;2:498–499. doi: 10.1016/S0140-6736(74)92020-0. [DOI] [PubMed] [Google Scholar]
- 6.Duttaroy AK. Transport of fatty acids across the human placenta: a review. Prog Lipid Res. 2009;48:52–61. doi: 10.1016/j.plipres.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Lindegaard ML, et al. Endothelial and lipoprotein lipases in human and mouse placenta. J Lipid Res. 2005;46:2339–2346. doi: 10.1194/jlr.M500277-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Xu Y, Wang Q, Cook TJ, Knipp GT. Effect of placental fatty acid metabolism and regulation by peroxisome proliferator activated receptor on pregnancy and fetal outcomes. J Pharm Sci. 2007;96:2582–2606. doi: 10.1002/jps.20973. [DOI] [PubMed] [Google Scholar]
- 9.Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26(Suppl A):S70–75. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Black PN, Sandoval A, Arias-Barrau E, DiRusso CC. Targeting the fatty acid transport proteins (FATP) to understand the mechanisms linking fatty acid transport to metabolism. Immunol Endocr Metab Agents Med Chem. 2009;9:11–17. doi: 10.2174/187152209788009850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gauster M, et al. Dysregulation of placental endothelial lipase and lipoprotein lipase in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab. 2007;92:2256–2263. doi: 10.1210/jc.2006-2403. [DOI] [PubMed] [Google Scholar]
- 12.Mishima T, Miner JH, Morizane M, Stahl A, Sadovsky Y. The expression and function of fatty acid transport protein-2 and -4 in the murine placenta. PloS one. 2011;6:e25865. doi: 10.1371/journal.pone.0025865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Visiedo F, et al. High glucose levels reduce fatty acid oxidation and increase triglyceride accumulation in human placenta. Am.J.Physiol Endocrinol.Metab. 2013;305:E205–E212. doi: 10.1152/ajpendo.00032.2013. [DOI] [PubMed] [Google Scholar]
- 14.Lagarde Michel, Bernoud Nathalie, Brossard Nicole, Lemaitre-Delaunay Dominique, Thiès Frank, Croset Martine, Lecerf Jean. Lysophosphatidylcholine as a Preferred Carrier Form of Docosahexaenoic Acid to the Brain. Journal of Molecular Neuroscience. 2001;16(2-3):201–204. doi: 10.1385/JMN:16:2-3:201. [DOI] [PubMed] [Google Scholar]
- 15.Magnusson-Olsson AL, Lager S, Jacobsson B, Jansson T, Powell TL. Effect of maternal triglycerides and free fatty acids on placental LPL in cultured primary trophoblast cells and in a case of maternal LPL deficiency. Am J Physiol Endocrinol Metab. 2007;293:E24–30. doi: 10.1152/ajpendo.00571.2006. [DOI] [PubMed] [Google Scholar]
- 16.Lindegaard ML, Damm P, Mathiesen ER, Nielsen LB. Placental triglyceride accumulation in maternal type 1 diabetes is associated with increased lipase gene expression. J Lipid Res. 2006;47:2581–2588. doi: 10.1194/jlr.M600236-JLR200. [DOI] [PubMed] [Google Scholar]
- 17.Gauster M, et al. Endothelial lipase releases saturated and unsaturated fatty acids of high density lipoprotein phosphatidylcholine. J Lipid Res. 2005;46:1517–1525. doi: 10.1194/jlr.M500054-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Lager SGF, Ramirez VI, Jansson T, Powell TL. High maternal BMI is associated with inhobition of PPAR gamma signaling and increased basal plasma membrane FATP2 expression. Placenta. 2013;34:A47. doi: 10.1016/j.placenta.2013.06.143. [DOI] [Google Scholar]
- 19.Buchanan TA, Kitzmiller JL. Metabolic interactions of diabetes and pregnancy. Annu Rev Med. 1994;45:245–260. doi: 10.1146/annurev.med.45.1.245. [DOI] [PubMed] [Google Scholar]
- 20.Jawerbaum A, Gonzalez E. Diabetic pregnancies: the challenge of developing in a pro-inflammatory environment. Curr Med Chem. 2006;13:2127–2138. doi: 10.2174/092986706777935302. [DOI] [PubMed] [Google Scholar]
- 21.Powell TL, et al. Composition and permeability of syncytiotrophoblast plasma membranes in pregnancies complicated by intrauterine growth restriction. Biochim Biophys Acta. 1999;1420:86–94. doi: 10.1016/S0005-2736(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 22.Gil-Sanchez A, et al. Maternal-fetal in vivo transfer of [13C]docosahexaenoic and other fatty acids across the human placenta 12 h after maternal oral intake. Am J Clin Nutr. 2010;92:115–122. doi: 10.3945/ajcn.2010.29589. [DOI] [PubMed] [Google Scholar]
- 23.Thompson, G. A. The regulation of membrane lipid metabolism. 2nd edn, (CRC Press Inc, 1992).
- 24.Song S, et al. Peroxisome proliferator activated receptor alpha (PPARalpha) and PPAR gamma coactivator (PGC-1alpha) induce carnitine palmitoyltransferase IA (CPT-1A) via independent gene elements. Mol Cell Endocrinol. 2010;325:54–63. doi: 10.1016/j.mce.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez N, et al. Activation of the nuclear receptor PPARalpha regulates lipid metabolism in foetal liver from diabetic rats: implications in diabetes-induced foetal overgrowth. Diabetes/metabolism research and reviews. 2011;27:35–46. doi: 10.1002/dmrr.1151. [DOI] [PubMed] [Google Scholar]
- 26.Das SK, et al. Micro RNA-124a regulates lipolysis via adipose triglyceride lipase and comparative gene identification 58. Int J Mol Sci. 2015;16:8555–8568. doi: 10.3390/ijms16048555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis RM, Desoye G. Placental Lipid and Fatty Acid Transfer in Maternal Overnutrition. Annals of nutrition &. metabolism. 2017;70:228–231. doi: 10.1159/000463397. [DOI] [PubMed] [Google Scholar]
- 28.Hahn T, et al. Hyperglycaemia-induced subcellular redistribution of GLUT1 glucose transporters in cultured human term placental trophoblast cells. Diabetologia. 2000;43:173–180. doi: 10.1007/s001250050026. [DOI] [PubMed] [Google Scholar]
- 29.Weiss U, et al. Hyperglycaemia in vitro alters the proliferation and mitochondrial activity of the choriocarcinoma cell lines BeWo, JAR and JEG-3 as models for human first-trimester trophoblast. Diabetologia. 2001;44:209–219. doi: 10.1007/s001250051601. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Contreras Consolacion, Vazquez-Gomez Marta, Astiz Susana, Torres-Rovira Laura, Sanchez-Sanchez Raul, Gomez-Fidalgo Ernesto, Gonzalez Jorge, Isabel Beatriz, Rey Ana, Ovilo Cristina, Gonzalez-Bulnes Antonio. Ontogeny of Sex-Related Differences in Foetal Developmental Features, Lipid Availability and Fatty Acid Composition. International Journal of Molecular Sciences. 2017;18(6):1171. doi: 10.3390/ijms18061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tarrade A, et al. Sexual dimorphism of the feto-placental phenotype in response to a high fat and control maternal diets in a rabbit model. PloS one. 2013;8:e83458. doi: 10.1371/journal.pone.0083458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desforges M, et al. Reduced placental taurine transporter (TauT) activity in pregnancies complicated by pre-eclampsia and maternal obesity. Advances in experimental medicine and biology. 2013;776:81–91. doi: 10.1007/978-1-4614-6093-0_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins L, et al. Maternal obesity and its effect on placental cell turnover. J Matern Fetal Neonatal Med. 2013;26:783–788. doi: 10.3109/14767058.2012.760539. [DOI] [PubMed] [Google Scholar]
- 34.Lager S, et al. Diet-induced obesity in mice reduces placental efficiency and inhibits placental mTOR signaling. Physiol Rep. 2014;2:e00242. doi: 10.1002/phy2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lassance L, Haghiac M, Minium J, Catalano P, Hauguel-de Mouzon S. Obesity-induced down-regulation of the mitochondrial translocator protein (TSPO) impairs placental steroid production. J Clin Endocrinol Metab. 2015;100:E11–18. doi: 10.1210/jc.2014-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstet Gynecol. 2004;103:219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 37.Jain D, Khuteta R, Chaturvedi V, Khuteta S. Effect of body mass index on pregnancy outcomes in nulliparous women delivering singleton babies: observational study. J Obstet Gynaecol India. 2012;62:429–431. doi: 10.1007/s13224-012-0225-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siman CM, Sibley CP, Jones CJ, Turner MA, Greenwood SL. The functional regeneration of syncytiotrophoblast in cultured explants of term placenta. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1116–1122. doi: 10.1152/ajpregu.2001.280.4.R1116. [DOI] [PubMed] [Google Scholar]
- 39.Hayward CE, Greenwood SL, Sibley CP, Baker PN, Jones RL. Effect of young maternal age and skeletal growth on placental growth and development. Placenta. 2011;32:990–998. doi: 10.1016/j.placenta.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 40.Green P, Anyakoha N, Yadid G, Gispan-Herman I, Nicolaou A. Arachidonic acid-containing phosphatidylcholine species are increased in selected brain regions of a depressive animal model: implications for pathophysiology. Prostaglandins Leukot Essent Fatty Acids. 2009;80:213–220. doi: 10.1016/j.plefa.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 42.Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. Lancet. 1992;339:283–287. doi: 10.1016/0140-6736(92)91342-6. [DOI] [PubMed] [Google Scholar]
- 43.Tetu B, et al. Immunohistochemical analysis of possible chemoresistance markers identified by micro-arrays on serous ovarian carcinomas. Mod Pathol. 2008;21:1002–1010. doi: 10.1038/modpathol.2008.80. [DOI] [PubMed] [Google Scholar]
- 44.Zhu MJ, Ma Y, Long NM, Du M, Ford SP. Maternal obesity markedly increases placental fatty acid transporter expression and fetal blood triglycerides at midgestation in the ewe. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1224–1231. doi: 10.1152/ajpregu.00309.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klipsic D, et al. Impact of SCP-2/SCP-x gene ablation and dietary cholesterol on hepatic lipid accumulation. Am J Physiol Gastrointest Liver Physiol. 2015;309:G387–399. doi: 10.1152/ajpgi.00460.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.