Abstract
Adults with short stature have been previously reported to have increased risk of cardiovascular events and hyper-LDL-cholesterolemia. We aimed to assess the association between height and lipid profiles among Korean adolescents and adults. We analyzed data from the Korea National Health and Nutrition Examination Survey from 2007 to 2015, from 37,889 individuals (aged 12–59 years). In adolescents, total cholesterol (TC) and low density lipoprotein-cholesterol (LDL-C) levels had profound associations with height in both boys and girls, while high density lipoprotein-cholesterol (HDL-C) levels had an inverse association with height only in boys. Height was inversely associated with TC, triglycerides (TG), and LDL-C concentrations in men and women and positively correlated with HDL-C concentration in women. In boys, the odds ratios (ORs) for hypercholesterolemia, hypertriglyceridemia, hyper-LDL-cholesterolemia were higher for shorter subjects (ORs = 2.38~7.01), while only the OR of hyper-LDL-cholesterolemia was significantly higher in girls with short stature (OR = 3.12). In adults, the ORs for hypercholesterolemia, hypo-HDL-cholesterolemia, and hyper-LDL-cholesterolemia were significantly higher in short subjects than in tall subjects after controlling for covariates (ORs = 1.50~2.61). Also, short men showed significantly higher ORs for hypertriglyceridemia (OR = 1.85) than tall men. Short stature was significantly associated with adverse lipid profiles in both adolescents and adults.
Subject terms: Dyslipidaemias, Growth disorders
Introduction
Height is recognized as an important indicator that reflects genetic and environmental factors, including nutrition, hormonal regulation, pubertal development, and chronic disease during the fetal-to-adolescent period. Following the first report in 1951 of the increased risk of coronary heart disease (CHD) with short stature in adults, this inverse association between adult height and cardiovascular disease (CVD) risk has been supported by the results of several prospective large-scale studies and meta-analyses. A meta-analysis of the relationship between height and CHD revealed that short adults had ~1.5 times higher risk for CHD morbidity and mortality than adults with tall stature1. Another meta-analysis with 1 million subjects reported that the risk of fatal or non-fatal coronary artery disease (CAD) decreases by 8% per every 6.5 cm increase in height2. Similarly, previous studies from Korea have reported a negative association between height and all-cause mortality3,4.
Dyslipidemia is one of the major established risk factors for CVD; treatment of dyslipidemia ameliorates the risk of CVD, and dyslipidemia has been reported to be inversely associated with adult height5–7. Lipid levels in childhood often track to adulthood. Although CVD usually develops in later life, it is well known that the accumulation of intimal fatty streaks, which are early atherosclerotic lesions, develops from childhood in association with dyslipidemia. Furthermore, dyslipidemia often accompanies other CVD risk factors including insulin resistance and hypertension, which exacerbate atherosclerosis. The identification of risk factors for dyslipidemia in children and adolescents is therefore essential for its early detection and management and the consequent prevention of CVD in later life8. In Korea, the overall prevalence of dyslipidemia in adolescents aged 10–18 years is 21.7~25.2%; prevalence is reported to be two times higher (53.1~56.1%) in obese adolescents9. However, few studies have been conducted on the relationship between height and blood lipid concentrations in children and adolescents10–15. Specifically, there have been no studies on the association of dyslipidemia and height in Korean adolescents and adults. In the present study, we therefore aimed to assess the association between height and serum lipid profile in Korean adolescents and adults using data from the Korean National Health and Nutrition Examination Survey (KNHANES).
Results
General characteristics and lipid profiles of the study population
The mean age of the adolescents was 15.1 years, and the mean age of adult men and women were 38.9 and 39.3 years, respectively (Supplementary Table S1). Tables 1 and 2 shows the general characteristics of the study population according to the height percentile. No significant differences in age, fasting glucose, alcohol consumption, physical activity, and household income were found in adolescents by height percentile (P > 0.05). Significantly higher body mass index (BMI) (P < 0.001) in boys with higher height percentile was noted, while such association was not noted in girls. Also, significant elevation of systolic and diastolic blood pressures was found in boys (P < 0.001), while only a modest but significant elevation of diastolic pressure was found in girls (P = 0.002). No significant differences in age, BMI, blood pressures were found according to the height percentile in men (P > 0.05), while a modest but significant decreases in age, BMI, and systolic blood pressure according to the elevation of height percentile were noted in women (P < 0.05). Adults with higher height percentile showed significantly higher proportions of alcohol consumption, active physical activity, and higher household income than those with lower height percentile in both men and women (P < 0.01). The prevalence of postmenopausal women was not significantly different by height percentile (P = 0.114).
Table 1.
General characteristics of the subjects according to the height percentile in 5,207 adolescents.
| Height percentile | P-value | |||||
|---|---|---|---|---|---|---|
| <10th | 10–29th | 30–69th | 70–89th | ≥90th | ||
| Boys | ||||||
| No. | 267 | 557 | 1102 | 571 | 279 | |
| Age (year) | 15.1 (14.8–15.3) | 15.0 (14.8–15.2) | 15.2 (15.0–15.3) | 15.0 (14.9–15.2) | 15.1 (14.8–15.3) | 0.768 |
| Height (cm) | 158.4 (157.2–159.5) | 163.9 (163.2–164.5) | 169.5 (169.1–169.9) | 174.6 (174.1–175.1) | 180.3 (179.6–181.0) | <0.001 |
| WC (cm) | 69.1 (67.6–70.5) | 71.1 (70.1–72.2) | 73.1 (72.3–73.9) | 76.2 (75.2–77.3) | 77.4 (75.9–78.9) | <0.001 |
| BMI(kg/m2) | 20.4 (19.9–21.0) | 21.0 (20.6–21.4) | 21.4 (21.1–21.7) | 22.1 (21.7–22.5) | 22.1 (21.6–22.7) | <0.001 |
| Systolic BP (mmHg) | 107.9 (106.0–109.8) | 109.6 (108.6–110.6) | 110.4 (109.6–111.2) | 112.5 (111.5–113.5) | 111.2 (109.8–112.6) | <0.001 |
| Diastolic BP (mmHg) | 66.2 (64.8–67.6) | 67.8 (67.0–68.7) | 68.5 (67.8–69.2) | 69.6 (68.8–70.4) | 69.2 (68.1–70.4) | <0.001 |
| Fasting glucose (mg/dL) | 90.1 (89.0–91.2) | 89.5 (88.8–90.1) | 89.2 (88.8–89.7) | 90.2 (89.5–90.9) | 89.7 (88.7–90.7) | 0.184 |
| Alcohol consumption | 0.376 | |||||
| No | 226 (79.0%) | 491 (86.4%) | 949 (83.2%) | 497 (83.5%) | 233 (82.2%) | |
| Occasionally | 34 (19.3%) | 56 (12.7%) | 128 (14.8%) | 64 (15.2%) | 36 (14.8%) | |
| Excessively | 3 (1.7%) | 5 (0.9%) | 17 (2.0%) | 6 (1.3%) | 6 (3.0%) | |
| Physical activity | 0.787 | |||||
| No | 160 (59.0%) | 333 (58.9%) | 634 (57.1%) | 325 (57.4%) | 165 (61.7%) | |
| Yes | 102 (41.0%) | 217 (41.1%) | 457 (42.9%) | 241 (42.6%) | 109 (38.3%) | |
| Household income | 0.399 | |||||
| Quartile 1 | 37 (14.4%) | 65 (13.7%) | 130 (12.9%) | 59 (11.8%) | 28 (12.7%) | |
| Quartile 2 | 73 (32.2%) | 127 (26.5%) | 241 (25.9%) | 139 (26.4%) | 66 (24.7%) | |
| Quartile 3 | 77 (32.2%) | 185 (26.0%) | 341 (25.9%) | 184 (26.4%) | 85 (24.7%) | |
| Quartile 4 | 80 (27.3%) | 170 (28.0%) | 371 (32.9%) | 186 (30.0%) | 96 (31.6%) | |
| Girls | ||||||
| No. | 236 | 487 | 976 | 484 | 248 | |
| Age (year) | 15.2 (14.9–15.5) | 15.0 (14.8–15.2) | 15.05 (14.9–15.2) | 15.1 (14.9–15.3) | 15.3 (15.0–15.6) | 0.574 |
| Height (cm) | 150.0 (149.4–150.7) | 155.2 (155.0–155.5) | 159.9 (15938–160.1) | 164.5 (164.3–164.7) | 169.2 (168.9–169.5) | <0.001 |
| WC (cm) | 65.7 (64.6–66.9) | 67.6 (66.7–68.5) | 68.7 (69.6–71.6) | 70.6 (69.6–71.6) | 71.5 (70.5–72.5) | <0.001 |
| BMI(kg/m2) | 20.5 (20.0–21.0) | 20.9 (20.6–21.4) | 20.8 (20.6–21.1) | 21.0 (20.6–21.4) | 21.1 (20.7–21.6) | 0.390 |
| Systolic BP (mmHg) | 103.1 (101.6–104.8) | 103.8 (102.9–104.7) | 104.3 (103.6–105.1) | 105.3 (104.3–106.3) | 105.1 (103.9–106.3) | 0.093 |
| Diastolic BP (mmHg) | 65.8 (64.3–67.3) | 65.8 (65.0–66.7) | 66.3 (65.6–66.9) | 67.6 (66.8–68.5) | 67.9 (66.8–69.1) | 0.002 |
| Fasting glucose (mg/dL) | 89.4 (86.8–92.0) | 88.2 (87.4–88.9) | 88.2 (87.7–88.7) | 88.6 (87.9–89.3) | 89.5 (88.6–90.4) | 0.150 |
| Alcohol consumption | 0.481 | |||||
| No | 212 (88.9%) | 441 (90.3%) | 887 (88.0%) | 439 (88.9%) | 219 (87.4%) | |
| Occasionally | 17 (9.1%) | 33 (7.8%) | 79 (11.0%) | 36 (9.4%) | 24 (12.4%) | |
| Excessively | 4 (2.0%) | 5 (1.9%) | 7 (1.0%) | 6 (1.7%) | 1 (0.2%) | |
| Physical activity | 0.342 | |||||
| No | 162 (70.2%) | 320 (64.1%) | 664 (67.2%) | 329 (69.1%) | 162 (62.1%) | |
| Yes | 70 (29.8%) | 158 (35.9%) | 308 (32.8%) | 148 (30.9%) | 82 (37.9%) | |
| Household income | 0.647 | |||||
| Quartile 1 | 33 (15.9%) | 66 (15.4%) | 119 (14.2%) | 55 (13.4%) | 25 (11.3%) | |
| Quartile 2 | 71 (32.3%) | 136 (29.2%) | 233 (24.6%) | 109 (25.4%) | 64 (28.8%) | |
| Quartile 3 | 76 (29.1%) | 144 (28.7%) | 305 (32.2%) | 161 (32.3%) | 79 (31.3%) | |
| Quartile 4 | 53 (22.7%) | 135 (26.7%) | 309 (29.0%) | 150 (28.9%) | 76 (28.6%) | |
The data were present as mean (95% confidence interval) or number (%).
BMI, body mass index; BP, blood pressure; WC, waist circumference.
Table 2.
General characteristics of the subjects according to the height percentile in 32,682 adults.
| Height percentile | P-value | |||||
|---|---|---|---|---|---|---|
| <10th | 10–29th | 30–69th | 70–89th | ≥90th | ||
| Men | ||||||
| No. | 1375 | 2696 | 5463 | 2727 | 1383 | |
| Age (yr) | 39.4 (38.7–40.1) | 39.0 (38.5–39.5) | 38.9 (38.5–39.3) | 38.6 (38.1–39.1) | 38.5 (37.8–39.2) | 0.306 |
| Height (cm) | 161.6 (161.4–161.8) | 167.2 (167.2–167.3) | 171.9 (171.9–172.0) | 176.8 (176.7–176.9) | 182.1 (181.9–182.3) | <0.001 |
| Weight(kg) | 62.9 (62.4–63.5) | 67.6 (67.2–68.0) | 71.6 (71.3–71.9) | 75.8 (75.3–76.2) | 81.2 (80.4–81.9) | <0.001 |
| WC (cm) | 80.9 (80.3–81.4) | 82.4 (83.7–84.2) | 83.9 (83.7–84.2) | 85.4 (84.9–85.8) | 87.4 (86.8–88.0) | <0.001 |
| BMI(kg/m2) | 24.0 (23.8–24.2) | 24.1 (24.0–24.3) | 24.2 (24.1–24.3) | 24.3 (24.1–24.4) | 24.5 (24.3–24.7) | 0.169 |
| Systolic BP (mmHg) | 118.2 (117.3–119.1) | 118.2 (117.5–118.8) | 117.6 (117.2–118.1) | 117.7 (117.1–118.3) | 117.6 (116.7–118.4) | 0.480 |
| Diastolic BP (mmHg) | 78.6 (77.9–79.3) | 79.4 (78.9–79.8) | 79.3 (78.9–79.6) | 79.4 (78.9–79.8) | 79.5 (78.9–80.1) | 0.339 |
| Fasting glucose (mg/dL) | 96.7 (95.4–98.9) | 96.3 (95.4–97.1) | 96.9 (96.3–97.6) | 96.6 (95.7–97.4) | 98.3 (96.9–99.8) | 0.159 |
| Alcohol consumption | 0.017 | |||||
| No | 364 (36.5%) | 590 (22.5%) | 1104 (20.6%) | 558 (21.1%) | 272 (21.2%) | |
| Occasionally | 701 (52.4%) | 1398 (54.1%) | 2955 (56.4%) | 1465 (55.0%) | 727 (55.3%) | |
| Excessively | 272 (21.0%) | 622 (23.4%) | 1248 (23.0%) | 623 (23.9%) | 335 (23.4%) | |
| Physical activity | 0.001 | |||||
| No | 936 (69.7%) | 1764 (67.9%) | 3595 (67.5%) | 1778 (66.9%) | 844 (61.1%) | |
| Yes | 392 (30.3%) | 819 (32.1%) | 1662 (32.5%) | 834 (33.1%) | 473 (38.9%) | |
| Household income | <0.001 | |||||
| Quartile 1 | 160 (12.4%) | 251 (9.3%) | 458 (9.1%) | 149 (6.5%) | 87 (6.4%) | |
| Quartile 2 | 395 (29.7%) | 678 (26.9%) | 1217 (23.6%) | 584 (22.6%) | 288 (21.2%) | |
| Quartile 3 | 424 (31.0%) | 829 (31.6%) | 1772 (32.5%) | 910 (34.8%) | 452 (33.8%) | |
| Quartile 4 | 376 (26.9%) | 896 (32.1%) | 1948 (34.8%) | 1049 (36.2%) | 544 (38.7%) | |
| Women | ||||||
| No. | 1932 | 3736 | 7616 | 3853 | 1901 | |
| Age (yr) | 39.5 (38.9–40.2) | 39.5(39.1–40.0) | 39.4 (39.1–39.8) | 39.0 (18.6–39.5) | 38.6 (38.0–38.2) | 0.038 |
| Height (cm) | 149.6 (149.5–149.8) | 154.4 (154.3–154.5) | 158.9 (158.8–159.0) | 163.5 (163.4–163.6) | 168.4 (168.2–168.6) | <0.001 |
| WC (cm) | 75.4 (74.9–75.9) | 75.7 (75.3–76.1) | 76.2 (75.9–76.4) | 77.2 (76.8–77.6) | 77.8 (77.3–78.4) | <0.001 |
| BMI(kg/m2) | 23.5 (23.3–23.7) | 23.1 (22.9–23.3) | 22.8 (22.7–22.9) | 22.7 (22.6–22.8) | 22.4 (22.2–22.6) | <0.001 |
| Systolic BP (mmHg) | 111.3 (110.4–112.1) | 110.2 (109.7–110.8) | 110.2 (109.7–110.8) | 109.7 (1109.2–110.3) | 109.0 (108.3–109.8) | 0.001 |
| Diastolic BP (mmHg) | 72.5 (71.9–73.1) | 72.4 (71.9–72.8) | 72.7 (72.4–73.0) | 72.8 (72.4–73.2) | 72.8 (72.3–73.3) | 0.465 |
| Fasting glucose (mg/dL) | 92.7 (91.8–93.6) | 93.1 (92.5–93.9) | 92.9 (92.4–93.4) | 92.6 (91.9–93.1) | 92.6 (91.9–93.4) | 0.687 |
| Alcohol consumption | <0.001 | |||||
| No | 1080 (55.5%) | 1977 (52.3%) | 3913 (50.6%) | 1942 (49.3%) | 979 (49.6%) | |
| Occasionally | 728 (39.7%) | 1508 (42.5%) | 3149 (43.6%) | 1600 (43.9%) | 770 (43.1%) | |
| Excessively | 87 (4.7%) | 173 (5.2%) | 422 (5.9%) | 228 (6.8%) | 116 (7.3%) | |
| Physical activity | <0.001 | |||||
| No | 1442 (76.4%) | 2697 (73.8%) | 5464 (72.9%) | 2652 (69.8%) | 1301 (70.5%) | |
| Yes | 443 (23.6%) | 942 (26.2%) | 1942 (27.1%) | 1095 (30.2%) | 539 (29.5%) | |
| Household income | <0.001 | |||||
| Quartile 1 | 253 (13.2%) | 354 (9.3%) | 595 (8.2%) | 312 (8.7%) | 136 (7.3%) | |
| Quartile 2 | 571 (30.5%) | 988 (37.2%) | 1884 (26.4%) | 872 (23.1%) | 415 (23.2%) | |
| Quartile 3 | 592 (30.5%) | 1216 (27.2%) | 2406 (26.4%) | 1226 (23.1%) | 583 (23.2%) | |
| Quartile 4 | 493 (24.8%) | 1145 (30.4%) | 2638 (34.0%) | 1392 (35.1%) | 738 (38.0%) | |
| Menopause | 0.114 | |||||
| No | 1426 (77.6%) | 2796 (78.8%) | 5770 (79.5%) | 2943 (79.9%) | 1496 (81.3%) | |
| Yes | 506 (22.4%) | 940 (21.2%) | 1846 (20.5%) | 910 (20.1%) | 405 (18.7%) | |
The data were present as mean (95% confidence interval) or number (%). All continuous variables were adjusted for age in women.
BMI, body mass index; BP, blood pressure; WC, waist circumference.
Relationship between lipid profile and height
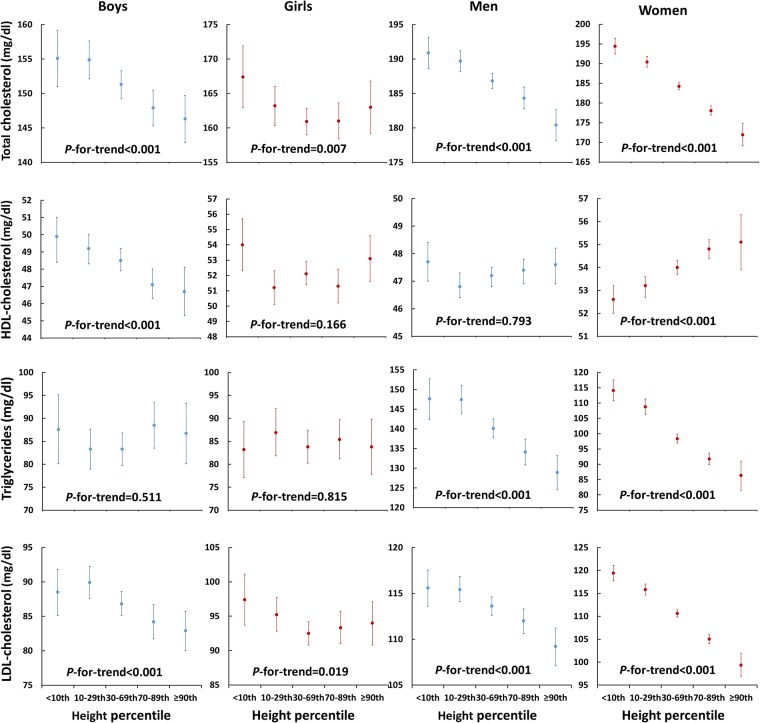
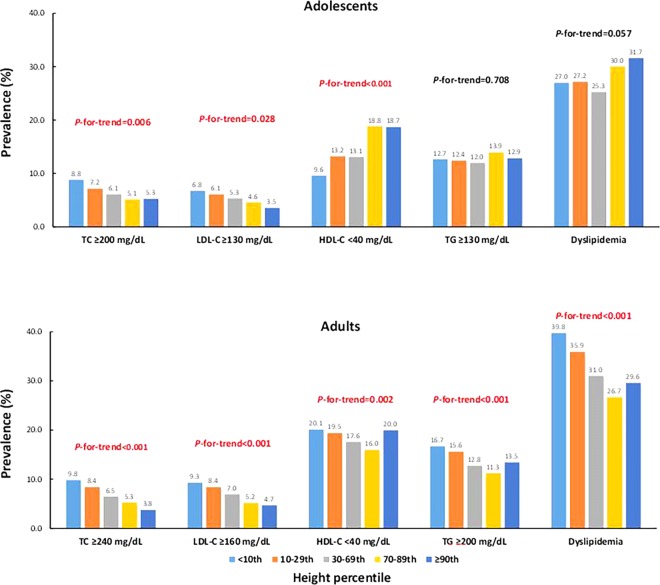
Figure 1 shows the relationship between lipid profile and height percentile. In adolescents, total cholesterol (TC) and low density lipoprotein-cholesterol (LDL-C) levels showed inverse associations with height in boys and girls (P-for-trend < 0.05), while high density lipoprotein-cholesterol (HDL-C) levels had an inverse association with height only in boys (P-for-trend < 0.001). TC, triglycerides (TG), and LDL-C levels were inversely associated with height in both men and women. HDL-C levels did not significantly associate with height in men, while they positively associated in women (P-for-trend < 0.001). The prevalence of dyslipidemia according to height is shown in Fig. 2 and Supplementary Table S2. In adolescents, the prevalence of hypercholesterolemia and hyper-LDL-cholesterolemia was significantly higher in shorter subjects (P-for-trend < 0.05), while that of hypo-HDL-cholesterolemia was significantly lower in shorter subjects (P-for-trend < 0.001). No significant difference was observed in the prevalence of hypertriglyceridemia and dyslipidemia according to the height percentiles among adolescents. In adults, the prevalence of hypercholesterolemia, hyper-LDL-cholesterolemia, hypo-HDL-cholesterolemia, hypertriglyceridemia, and dyslipidemia was significantly higher in shorter subjects (P-for-trend < 0.01).
Figure 1.
Relationship between the lipid profile and height percentile. The data were presented as mean and 95% confidence intervals (error bars).
Figure 2.
Prevalence of adverse lipid profile according to the height percentile.
ORs for dyslipidemia according to the height percentile
The odds ratios (ORs) and 95% confidence intervals for dyslipidemia according to the height percentile are presented in Tables 3 and 4. In boys, the ORs for hypercholesterolemia, hypertriglyceridemia, and hyper-LDL-cholesterolemia were significantly increased in shorter subjects than in taller subjects (ORs = 2.38~7.01, P-for-trend < 0.05), while the OR for hypo-HDL-cholesterolemia and dyslipidemia did not show any significant difference according to the height percentile (Table 3). In girls, only the OR for hyper-LDL-cholesterolemia was significantly increased in shorter subjects than in taller subjects (OR = 3.12, P-for-trend = 0.026). In both men and women, the ORs for hypercholesterolemia, hyper-LDL-cholesterolemia, and dyslipidemia were significantly increased in shorter subjects (ORs = 1.50~2.61, P < 0.01) than in taller subjects (height ≥90 percentile). Of note, a linear trend but an inverted J-shaped association was observed in the relationship between height and hypo-HDL-cholesterolemia in women. While the OR (95% confidence intervals) for hypo-HDL-cholesterolemia in women with heights between 10 and 29th percentile was 1.54 (1.02–2.33), the OR in women with heights less than 10th percentile was 1.33 (0.86–2.04). In men, the OR for hypertriglyceridemia (OR = 1.85) was significantly increased in shorter subjects than in taller subjects in men, while such an increase in OR was not statistically significant in women (Table 4).
Table 3.
Adjusted odds ratios (95% confidence interval) for dyslipidemia in adolescents according to the height percentile.
| Height percentile | P-for-trend | |||||
|---|---|---|---|---|---|---|
| <10th | 10–29th | 30–69th | 70–89th | ≥90th | ||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Boys | ||||||
| TC ≥ 200 mg/dL | ||||||
| Model 1 | 3.06 (1.01–9.24) | 3.86 (1.42–10.5) | 3.01 (1.13–8.01) | 2.05 (0.71–5.93) | ref | 0.006 |
| Model 2 | 7.01 (2.26–21.7) | 6.60 (2.39–18.3) | 3.72 (1.38–10.1) | 2.18 (0.75–6.29) | ref | <0.001 |
| HDL-C < 40 mg/dL | ||||||
| Model 1 | 0.42 (0.24–0.74) | 0.52 (0.34–0.78) | 0.58 (0.38–0.87) | 0.90 (0.60–1.35) | ref | <0.001 |
| Model 2 | 0.69 (0.37–1.27) | 0.68 (0.43–1.06) | 0.72 (0.47–1.11) | 0.99 (0.63–1.54) | ref | 0.271 |
| TG ≥ 130 mg/dL | ||||||
| Model 1 | 1.10 (0.11–0.25) | 0.87 (0.62–1.96) | 0.91 (0.59–1.40) | 1.09 (0.67–1.78) | ref | 0.997 |
| Model 2 | 2.38 (1.21–4.66) | 1.55 (0.87–2.12) | 1.31 (0.81–2.12) | 1.20 (0.68–2.08) | ref | 0.048 |
| LDL-C ≥ 130 mg/dL | ||||||
| Model 1 | 1.56 (0.57–4.28) | 2.94 (1.25–6.89) | 2.17 (0.94–5.01) | 1.28 (0.49–3.34) | ref | 0.050 |
| Model 2 | 4.35 (1.48–12.8) | 5.48 (2.23–13.6) | 3.05 (1.28–7.23) | 1.34 (0.51–3.51) | ref | <0.001 |
| Dyslipidemia | ||||||
| Model 1 | 0.73 (0.46–1.14) | 0.72 (0.49–1.04) | 0.66 (0.46–0.94) | 0.90 (0.62–1.30) | ref | 0.074 |
| Model 2 | 1.36 (0.81–2.27) | 1.11 (0.74–1.66) | 0.86 (0.59–1.26) | 1.00 (0.67–1.50) | ref | 0.092 |
| Girls | ||||||
| TC ≥ 200 mg/dL | ||||||
| Model 1 | 1.48 (0.75–2.92) | 0.88 (0.47–1.65) | 0.78 (0.44–1.39) | 0.75 (0.40–1.40) | ref | 0.237 |
| Model 2 | 1.92 (0.96–3.82) | 1.07 (0.56–2.04) | 0.83 (0.46–1.49) | 0.80 (0.42–1.51) | ref | 0.063 |
| HDL-C < 40 mg/dL | ||||||
| Model 1 | 0.56 (0.25–1.24) | 1.05 (0.60–1.83) | 0.86 (0.53–1.39) | 1.27 (0.74–2.17) | ref | 0.061 |
| Model 2 | 0.81 (0.32–2.03) | 1.30 (0.74–2.30) | 1.05 (0.63–1.76) | 1.35 (0.78–2.37) | ref | 0.396 |
| TG ≥ 130 mg/dL | ||||||
| Model 1 | 1.23 (0.58–2.58) | 1.40 (0.74–2.66) | 1.09 (0.63–1.88) | 1.13 (0.61–2.08) | ref | 0.571 |
| Model 2 | 1.19 (0.56–2.53) | 1.42 (0.75–2.70) | 1.10 (0.64–1.89) | 1.15 (0.62–2.13) | ref | 0.749 |
| LDL-C ≥ 130 mg/dL | ||||||
| Model 1 | 0.45 (0.20–1.01) | 0.82 (0.39–1.72) | 0.82 (0.41–1.63) | 0.75 (0.37–1.53) | ref | 0.225 |
| Model 2 | 3.12 (1.38–7.09) | 1.71 (0.81–3.64) | 1.37 (0.68–2.75) | 1.46 (0.70–3.04) | ref | 0.026 |
| Dyslipidemia | ||||||
| Model 1 | 0.91 (0.57–1.45) | 0.94 (0.63–1.41) | 0.83 (0.58–1.20) | 0.96 (0.64–1.43) | ref | 0.400 |
| Model 2 | 1.33 (0.80–2.21) | 1.20 (0.78–1.84) | 0.96 (0.65–1.43) | 1.03 (0.67–1.57) | ref | 0.253 |
Model 1: Unadjusted.
Model 2: Adjusted for age, waist circumference, systolic/diastolic blood pressure, fasting glucose levels, household income, physical activity, and alcohol consumption.
CI, confidence interval; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; OR, odd ratio; TC, total cholesterol; TG, triglycerides.
Table 4.
Adjusted odds ratios (95% confidence interval) for dyslipidemia in adults according to the height percentile.
| Height percentile | P-for-trend | |||||
|---|---|---|---|---|---|---|
| <10th | 10–29th | 30–69th | 70–89th | ≥90th | ||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Men | ||||||
| TC ≥ 240 mg/dL | ||||||
| Model 1 | 1.91 (1.32–2.74) | 2.01 (1.44–2.81) | 1.54 (1.13–2.12) | 1.53 (1.11–2.12) | ref | <0.001 |
| Model 2 | 1.84 (1.23–2.74) | 1.87 (1.31–2.69) | 1.51 (1.09–2.11) | 1.51 (1.09–2.11) | ref | 0.004 |
| HDL-C < 40 mg/dL | ||||||
| Model 1 | 1.27 (1.04–1.56) | 1.17 (0.98–1.40) | 1.14 (0.97–1.34) | 1.04 (0.87–1.23) | ref | 0.045 |
| Model 2 | 1.50 (1.19–1.89) | 1.29 (1.05–1.58) | 1.26 (1.04–1.51) | 1.12 (0.93–1.36) | ref | 0.003 |
| TG ≥ 200 mg/dL | ||||||
| Model 1 | 1.58 (1.27–1.96) | 1.50 (1.24–1.82) | 1.27 (1.07–1.52) | 1.12 (0.92–1.36) | ref | <0.001 |
| Model 2 | 1.85 (1.44–2.38) | 1.64 (1.32–2.05) | 1.37 (1.12–1.68) | 1.18 (0.94–1.47) | ref | <0.001 |
| LDL-C ≥ 160 mg/dL | ||||||
| Model 1 | 1.52 (1.05–2.21) | 1.61 (1.17–2.21) | 1.47 (1.08–1.99) | 1.25 (0.90–1.73) | ref | 0.001 |
| Model 2 | 1.56 (1.05–2.32) | 1.60 (1.14–2.27) | 1.51 (1.10–2.07) | 1.28 (0.92–1.78) | ref | 0.026 |
| Dyslipidemia | ||||||
| Model 1 | 1.69 (1.41–2.02) | 1.51 (1.29–1.76) | 1.33 (1.15–1.54) | 1.16 (0.99–1.36) | ref | <0.001 |
| Model 2 | 1.93 (1.57–2.39) | 1.62 (1.35–1.93) | 1.44 (1.22–1.70) | 1.26 (1.05–1.47) | ref | <0.001 |
| Women | ||||||
| LDL-C ≥ 160 mg/dL | ||||||
| Model 1 | 6.76 (3.75–12.2) | 4.75 (2.67–8.45) | 3.50 (1.96–6.25) | 2.22 (1.22–4.05) | ref | <0.001 |
| Model 2 | 2.61 (1.41–4.81) | 2.12 (1.17–3.84) | 2.01 (1.11–3.63) | 1.64 (0.89–3.02) | ref | <0.001 |
| HDL-C < 40 mg/dL | ||||||
| Model 1 | 1.48 (0.99–2.21) | 1.56 (1.05–2.33) | 1.22 (0.83–1.79) | 0.99 (0.66–1.46) | ref | <0.001 |
| Model 2 | 1.33 (0.86–2.04) | 1.54 (1.02–2.33) | 1.28 (0.86–1.91) | 1.12 (0.74–1.68) | ref | 0.003 |
| TG ≥ 200 mg/dL | ||||||
| Model 1 | 2.24 (1.40–3.60) | 1.99 (1.26–3.13) | 1.39 (0.89–2.19) | 1.11 (0.69–1.77) | ref | <0.001 |
| Model 2 | 1.34 (0.78–2.31) | 1.35 (0.81–2.29) | 1.11 (0.67–1.85) | 1.06 (0.63–1.79) | ref | 0.087 |
| LDL-C ≥ 160 mg/dL | ||||||
| Model 1 | 4.70 (2.64–8.33) | 3.62 (2.06–6.35) | 2.64 (1.50–4.64) | 1.57 (0.87–2.83) | ref | <0.001 |
| Model 2 | 1.84 (1.03–3.30) | 1.71 (0.97–3.01) | 1.52 (0.87–2.67) | 1.19 (0.66–2.13) | ref | 0.002 |
| Dyslipidemia | ||||||
| Model 1 | 3.09 (2.27–4.20) | 2.49 (1.85–3.36) | 1.80 (1.34–2.40) | 1.24 (0.92–1.68) | ref | <0.001 |
| Model 2 | 1.88 (1.35–2.61) | 1.79 (1.31–2.45) | 1.52 (1.12–2.06) | 1.27 (0.93–1.73) | ref | <0.001 |
Model 1: Unadjusted.
Model 2: Adjusted for age, waist circumference, systolic/diastolic blood pressure, fasting glucose levels, household income, physical activity, alcohol consumption, and menopause status (for women).
CI, confidence interval; HDL-C, high density lipoprotein-cholesterol; LDL-C, low density lipoprotein-cholesterol; OR, odd ratio; TC, total cholesterol; TG, triglycerides.
Discussion
The present study found that short stature has a significant inversely association with dyslipidemia in both Korean adolescents and adults. In adolescents, this inverse relationship between height and dyslipidemia showed sex-related differences. The risk of hypercholesterolemia, hypertriglyceridemia, and hyper-LDL-cholesterolemia was linked with height in boys, while only hyper-LDL-cholesterolemia was significantly related to height in girls. In adults, all four types of dyslipidemia were confirmed to be negatively affected by short stature in both men and women.
Since a small cross-sectional study from the 1980s reported a negative correlation between height and TC in boys10, a few prospective studies have analyzed the association between height gain and LDL-C levels in adolescents. The Bogalusa Heart Study reported that changes in height for five years in boys were inversely correlated with TC, LDL-C, and HDL-C levels12 (9). This negative correlation between the LDL-C concentration and growth rate in both boys and girls was also supported by the findings of large-scale prospective study conducted in Japan14. Finally, a recent study of 6,300 adolescents conducted by the West Virginia Coronary Artery Risk Detection in Appalachian Communities (CARDIAC) project reported that the risk of hyper-LDL-cholesterolemia increases significantly in the first (shortest) quartile stature compared with that in the fourth (tallest) quartile stature (OR = 1.3~3)15. Consistent with previous studies, the present study also found that shorter adolescents had higher TC and LDL-C levels, with significantly increased OR for hyper-LDL-cholesterolemia (OR = 3.12~4.35). Unlike those on LDL-C, studies on the association of TG with height are rare. A previous cross-sectional study in children and adolescents aged 13 to 18 years from the UK demonstrated no significant association between height and TG concentration10. Similarly, a longitudinal study among boys in the USA found no association between changes in TG levels and rapid height increase12. In contrast to this previous study, the present study found statistically higher OR for hypertriglyceridemia in shorter boys, and this association was not observed in girls. It is also important to note that obesity indices were significantly higher in boys with taller heights than in boys with shorter heights in this study population. These results suggest that the adverse effect of short stature on CVD risk in adolescents might be comparable or even stronger than obesity indices, especially for boys.
Unlike the negative correlation between height and LDL-C in both boys and girls, which is reported by most studies, reports on the relationship between height and HDL-C are inconsistent. In previous studies from the UK and USA, HDL-C levels were negatively associated with height increase in boys, but not in girls, aged 8 to 18 years10,12. Another study from Japan reported a negative correlation between HDL-C levels and height in both sexes in the age of 10–14 years13. In the present study, height was negatively correlated with HDL-C levels, only in boys, but its association with hypo-HDL-cholesterolemia was not statistically significant after adjusting for relevant covariates. In contrast with adolescents, adults with shorter height percentile showed a significantly higher risk of hypo-HDL-cholesterolemia, which might be explained by the effects of aging on HDL metabolism. Increased insulin resistance and systemic inflammation, a decline of sex hormone, and impaired lecithin cholesterol acyltransferase activity due to cellular senescence16 may enforce the inverse relationship between height and HDL-cholesterol in adults. The results of studies on the relationship between height and dyslipidemia/lipid concentration in adults have been inconsistent. Two prospective cohort studies from Europe reported an association between greater leg length and favorable levels of TC, LDL-C, and HDL-C at 53 years in UK adults6 and between taller stature and lower non-fasting TC and non-HDL-C levels in middle-aged men5. These two studies did not include TG concentration. In a study of individuals aged 50 years and older in China, height was negatively correlated with both LDL-C and HDL-C levels and positively correlated with TG concentration7. In a hospital-based study of 3,016 patients aged 30–59 years in Japan, no significant correlation was found between height and individual lipid profiles, but the OR for overall dyslipidemia in non-obese male patients was significantly decreased in taller subjects17. The present findings suggest that height in adulthood is favorably associated with not only LDL-C and HDL-C levels, but also TG levels. This finding is consistent with that of a recent genome-wide association study, which reported that increased height-raising allele was associated with decreased LDL-C and TG levels18.
An interesting fact in this study population was that taller adults tend to have higher alcohol consumption and physical activity compared with shorter adults. Higher alcohol consumption had been related to higher HDL-cholesterol in previous studies, but its beneficial effect on CVD risk is known to be alleviated by TG-elevating effect of alcohol19. Unlike alcohol consumption with conflicting effects on CVD risk, beneficial effects of physical activity on the lipid profiles, including lowering LDL-cholesterol and triglycerides, and increasing HDL-cholesterol have been consistently reported by previous studies20. To eliminate the possible favorable impact of these lifestyle factors on lipid profiles, we included these confounding factors for final adjustment. Another thing that we have to note is an inverted J-shaped association between OR for hyper-HDL-cholesterolemia and height in women. We could not find any difference in age and confounding factors between the shortest women (height <10th percentile) and the next shortest women (height 10~29th percentile). There has been no report of an inverted J-shaped association between height and HDL-cholesterol similar to our result. In overall, a favorable trend for hypo-HDL-cholesterolemia according to the height increase was found in women. Therefore, further research is needed to determine whether the risk of hypo-HDL-cholesterolemia is rather alleviated in severe short stature compared with mild short stature in women.
There were gender-related differences in the relationships between height and lipid profiles. In both adults and adolescents, lipid profiles in males tended to be more clearly affected by short stature. Boys with short stature showed a remarkably increased risk of both hyper-LDL-cholesterolemia (OR = 4.35) and hypertriglyceridemia (OR = 2.38), while girls with short stature showed an increased risk of hyper-LDL-cholesterolemia only (OR = 3.12). Likewise, the unfavorable effects of short stature in hypertriglyceridemia and hypo-HDL-cholesterolemia were more evident in men than in women. This gender difference can be explained by the beneficial effects of estrogen on lipid metabolism. Estrogen increases the accumulation of subcutaneous fat and restrains the increase of visceral fat, consequently allows a metabolically favorable distribution of adipose tissue21. Also, estrogen promotes reverse cholesterol transport process, such as hepatic secretion of cholesterol into bile, and reduces hepatic TG content through hepatic estrogen receptor α21.
The mechanisms supporting the link between height and dyslipidemia are not currently clear. Two hormones that play a decisive role in bone growth are growth hormone (GH) and thyroid hormone, both of which contribute to the improvement of lipid profiles. The lipolytic action of GH has been well described, particularly on visceral fat, and the administration of recombinant human GH in children and adults with GH deficiency or obesity has been reported to improve lipid metabolism by reducing TC and LDL-C levels22,23. In addition, it is well known that thyroid hormone plays an important role in hepatic synthesis and metabolism of fatty acids and cholesterol, and hypothyroidism leads to hypercholesterolemia and hypertriglyceridemia24. Therefore, it can be inferred that adolescents with adequate GH and thyroid hormone secretion are likely to have optimal growth and lipid profiles. In addition, since cholesterol is an essential component of cell membranes and steroid hormones, serum cholesterol may be lowered in individuals with a high growth velocity due to increased cholesterol consumption12. The skeletal system is now recognized as an important endocrine organ, which is involved in lipid metabolism. Several studies have shown that blood fatty acids are taken up by the skeletal system and are used as an energy source by the osteoblasts, thereby clearing circulating lipoproteins and non-esterified fatty acids25. In addition, osteocalcin, an osteoblast-derived hormone, increases the expression of the adiponectin gene in adipocytes26. Adiponectin causes an increase in serum HDL via inducing an increase in the hepatic production of apolipoprotein A-1 and the ATP-binding cassette transporter A127. Additionally, adiponectin lowers serum TG by enhancing VLDL catabolism in skeletal muscle adipose tissue27. Therefore, high osteocalcin levels in adolescents with high growth rates28 may result in favorable lipid profiles29. Finally, as noted above, a recent genome-wide association study reported a correlation between genetically determined short height and increased risk of coronary artery disease, which is partly explained by an association between height-related single nucleotide polymorphisms and lipid profiles, especially those of LDL-C and TG18.
Our study does have some limitations. As this was a cross-sectional study, causality could not be inferred between short stature and dyslipidemia. In addition, we did not examine some confounding factors, such as pubertal stage, dietary intakes and family history of dyslipidemia. Nonetheless, to the best of our knowledge, this is the first study that reports the association of the profiles of all lipids, including TG, with height in adults as well as in adolescents, using nationally representative data. We tried to minimize the age-associated bias by using age or age-group specific height percentile. We also included various covariates including age, waist circumference, blood pressures, fasting glucose, and lifestyle factors, that are known to be potentially related to lipid profiles in the statistical analysis.
In conclusion, this study presents the evidence of short stature as associated with adverse lipid profiles in both adolescents and adults, and also suggests possible mechanisms to explain this association. Our results may help partly explain the association between short height and increased risk of CVD.
Methods
Study population
We analyzed data from the Korean National Health and Nutrition Examination Survey (KNHANES), conducted by the Korean Centers for Disease Control and Prevention between 2007 and 2015. This nationwide cross-sectional survey includes demographic and anthropometric information, lipid profiles and information on health-related behaviors, including alcohol intake, physical activity and household income. We limited the analyses to individuals aged 12–59 years. Among the potentially eligible 44,448 individuals, we excluded participants for whom incomplete data were available for lipid profiles and anthropometric measurements (n = 5463). Other exclusion criteria included serum triglycerides ≥400 mg/dL (n = 784); medical history of liver cirrhosis, nephrotic syndrome, or endocrine disorders; and use of medications affecting blood lipid concentrations (n = 312). Eventually, 37,889 individuals aged 12–59 years who completed blood tests for lipid profiles after an overnight fast were enrolled in this study, comprising 5,207 adolescents (2,776 boys and 2,431 girls, aged 12–18 years) and 32,682 adults (13,644 men and 19,038 women, aged 19–59 years). The KNHANES protocol was approved by an Institutional Review Board of the Korean Centers for Disease Control and Prevention (IRB No. 2007-02CON-04-P, 2008-04EXP-01-C, 2009-01CON-03-2C, 2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01-2C, 2013-07CON-03-4C, and 2013-12EXP-03-5C), and conducted according to the Declaration of Helsinki. All participants or their parents/guardians provided written informed consent form. This study was approved by the institutional review board of Inje University, Sanggye Paik Hospital (IRB No. SGPAIK 2019-02-005), which waived the requirement for informed consent due to the anonymity of the data obtained from the KNHANES dataset.
Data collection and study variables
Participant height and body weight were measured to the nearest 0.1 cm using a stadiometer and 0.1 kg using a digital weight scale, respectively. BMI was calculated as the weight (kg) divided by the square of the height (m). Waist circumference was measured following a full expiration at the narrowest point between the bottom of the 10th rib and the superior border of the iliac crest. Systolic and diastolic blood pressure were measured using a standard mercury sphygmomanometer on the right arm on three occasions while the participants in a sitting position after they rested for more than 10 minutes. The final blood pressure values used for analysis were the average values of three measurements.
Blood collections were performed via venipunctures from the antecubital fossa after a fasting period of at least 8 h. In 2007, serum concentrations of TC, HDL-C, TG and fasting glucose were measured enzymatically using an ADIVIA 1650 (Siemens, New York, USA). Serum LDL-C levels were calculated using Friedewald’s formula30. In 2008–2015, serum fasting glucose and lipid profiles, including LDL-C, were determined enzymatically using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan). Participants were categorized as five groups according to the height percentile (<10th, 10–29th, 30–69th, 70–89th, ≥90th percentile). Sex and age-specific height percentile was applied for adolescents, and sex and age group-specific (under the 30 s, 30 s, 40 s, and 50 s) height percentile was applied for adults. The sex and age-specific height percentile values were presented Supplementary Table S3. Household income was categorized into quartiles based on the equalization of income, calculated as monthly family income divided by the square root of the number of family members. Alcohol consumption was marked as “occasionally” for a subject consuming at least one drink per month in the previous year and “excessively” when the subject consumed seven drinks or more (for males) or five drinks or more (for females) on a single occasion more than 2 times a week. Alcohol consumption less than once per month in the past year was classified as “none”. Physically active persons were defined as subjects performing regular vigorous activity at least 20 minutes per session for 3 or more days per week.
Definition of dyslipidemia
Dyslipidemia was defined as the presence of at least one of the following: hypercholesterolemia, hypertriglyceridemia, hyper-LDL-cholesterolemia, and hypo-HDL-cholesterolemia. In children and adolescents, hypercholesterolemia (≥200 mg/dL), hypertriglyceridemia (≥130 mg/dL); hyper-LDL-cholesterolemia (≥130 mg/dL), and hypo-HDL-cholesterolemia (<40 mg/dL) were defined per the National Heart, Lung, and Blood Institute’s (NHLBI) Expert Panel Guidelines31. In adults, hypercholesterolemia (≥240 mg/dL), hypertriglyceridemia (≥200 mg/dL), hyper-LDL-cholesterolemia (≥160 mg/dL), and hypo-HDL-cholesterolemia (<40 mg/dL) were determined according to the Korean Society of Lipid and Atherosclerosis guidelines for adults32.
Statistical analysis
Statistical analyses were performed using SPSS version 21.0 (SPSS, Inc., Chicago, IL, USA). The complex samples descriptive procedure was used to evaluate the mean and standard error of scale variables. The complex samples crosstabs procedure was used for categorical or ordinal variables. The complex samples general linear model was used to compare serum concentrations of lipid profiles according to the height percentile and to calculate P values for trend. Multiple logistic regression analyses were used to calculate the ORs for dyslipidemia according to the height percentile after adjusting for covariates including age, waist circumference, systolic/diastolic blood pressure, fasting glucose, household income (quartile 1, 2, 3, 4), physical activity (yes, no), alcohol consumption (no, occasionally, excessively), and menopausal status (yes, no). TG levels were log-transformed owing to their skewed distribution. For all analyses, P-values were two-tailed and P < 0.05 was considered statistically significant.
Supplementary information
Acknowledgements
We thank the Korea Centers for Disease Control and Prevention for providing the data.
Author Contributions
M.J. Park and S.-H. Kim designed the study concept. S.-H. Kim constructed the database set and assisted statistical analysis. N.-K. Oh and Y.-M. Song wrote the manuscript. All authors discussed data and reviewed manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shin-Hye Kim and Mi Jung Park jointly supervised this work.
Contributor Information
Shin-Hye Kim, Email: s2635@paik.ac.kr.
Mi Jung Park, Email: pmj@paik.ac.kr.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-50524-2.
References
- 1.Paajanen TA, Oksala NK, Kuukasjarvi P, Karhunen PJ. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. Eur Heart J. 2010;31:1802–1809. doi: 10.1093/eurheartj/ehq155. [DOI] [PubMed] [Google Scholar]
- 2.Emerging Risk Factors C. Adult height and the risk of cause-specific death and vascular morbidity in 1 million people: individual participant meta-analysis. Int J Epidemiol. 2012;41:1419–1433. doi: 10.1093/ije/dys086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song YM, Smith GD, Sung J. Adult height and cause-specific mortality: a large prospective study of South Korean men. Am J Epidemiol. 2003;158:479–485. doi: 10.1093/aje/kwg173. [DOI] [PubMed] [Google Scholar]
- 4.Song YM, Sung J. Adult height and the risk of mortality in South Korean women. Am J Epidemiol. 2008;168:497–505. doi: 10.1093/aje/kwn187. [DOI] [PubMed] [Google Scholar]
- 5.Henriksson KM, Lindblad U, Agren B, Nilsson-Ehle P, Rastam L. Associations between body height, body composition and cholesterol levels in middle-aged men. the coronary risk factor study in southern Sweden (CRISS) Eur J Epidemiol. 2001;17:521–526. doi: 10.1023/A:1014508422504. [DOI] [PubMed] [Google Scholar]
- 6.Skidmore PM, Hardy RJ, Kuh DJ, Langenberg C, Wadsworth ME. Life course body size and lipid levels at 53 years in a British birth cohort. J Epidemiol Community Health. 2007;61:215–220. doi: 10.1136/jech.2006.047571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schooling CM, et al. Height, its components, and cardiovascular risk among older Chinese: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Am J Public Health. 2007;97:1834–1841. doi: 10.2105/AJPH.2006.088096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berenson GS, et al. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Ahn BC, Joung H, Park MJ. Lipid Profiles and Prevalence of Dyslipidemia in Korean Adolescents. Endocrinol Metab. 2012;27:208–216. doi: 10.3803/EnM.2012.27.3.208. [DOI] [Google Scholar]
- 10.Orchard TJ, Rodgers M, Hedley AJ, Mitchell JR. Changes in blood lipids and blood pressure during adolescence. Br Med J. 1980;280:1563–1567. doi: 10.1136/bmj.280.6231.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu NF, Rimm EB, Wang DJ, Liou HS, Shieh SM. Relationship between anthropometric variables and lipid levels among school children: The Taipei Children Heart Study. Int J Obes Relat Metab Disord. 1998;22:66–72. doi: 10.1038/sj.ijo.0800546. [DOI] [PubMed] [Google Scholar]
- 12.Chiang YK, Srinivasan SR, Webber LS, Berenson GS. Relationship between change in height and changes in serum lipid and lipoprotein levels in adolescent males: the Bogalusa Heart Study. J Clin Epidemiol. 1989;42:409–415. doi: 10.1016/0895-4356(89)90129-7. [DOI] [PubMed] [Google Scholar]
- 13.Kouda K, Nakamura H, Fan W, Takeuchi H. Negative relationships between growth in height and levels of cholesterol in puberty: a 3-year follow-up study. Int J Epidemiol. 2003;32:1105–1110. doi: 10.1093/ije/dyg207. [DOI] [PubMed] [Google Scholar]
- 14.Fujita Y, Kouda K, Nakamura H, Iki M. Inverse association between height increase and LDL cholesterol during puberty: A 3-year follow-up study of the Fukuroi City. Am J Hum Biol. 2016;28:330–334. doi: 10.1002/ajhb.22784. [DOI] [PubMed] [Google Scholar]
- 15.Pyles LA, et al. LDL cholesterol level in fifth-grade schoolchildren associates with stature. J Lipid Res. 2017;58:2197–2201. doi: 10.1194/jlr.P078816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walter M. Interrelationships among HDL metabolism, aging, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1244–1250. doi: 10.1161/ATVBAHA.108.181438. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu Y, et al. Height correlates with dyslipidemia in non-overweight middle-aged Japanese men. J Physiol Anthropol. 2016;35:29. doi: 10.1186/s40101-016-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson CP, et al. Genetically determined height and coronary artery disease. N Engl J Med. 2015;372:1608–1618. doi: 10.1056/NEJMoa1404881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. BMJ. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SH, Song YH, Park S, Park MJ. Impact of lifestyle factors on trends in lipid profiles among Korean adolescents: the Korea National Health and Nutrition Examination Surveys study, 1998 and 2010. Korean J Pediatr. 2016;59:65–73. doi: 10.3345/kjp.2016.59.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmisano BT, Zhu L, Eckel RH, Stafford JM. Sex differences in lipid and lipoprotein metabolism. Mol Metab. 2018;15:45–55. doi: 10.1016/j.molmet.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang S, Xue J, Li G. Effects of recombinant human growth hormone administration on cardiovascular risk factors in obese children with relative growth hormone deficiency. Lipids Health Dis. 2018;17:66. doi: 10.1186/s12944-018-0721-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Marco S, et al. Circulating asymmetric dimethylarginine and lipid profile in pre-pubertal children with growth hormone deficiency: effect of 12-month growth hormone replacement therapy. Growth Horm IGF Res. 2014;24:216–220. doi: 10.1016/j.ghir.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Sinha RA, Singh BK, Yen PM. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat Rev Endocrinol. 2018;14:259–269. doi: 10.1038/nrendo.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kushwaha P, Wolfgang MJ, Riddle RC. Fatty acid metabolism by the osteoblast. Bone. 2018;115:8–14. doi: 10.1016/j.bone.2017.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee NK, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanai Hidekatsu, Yoshida Hiroshi. Beneficial Effects of Adiponectin on Glucose and Lipid Metabolism and Atherosclerotic Progression: Mechanisms and Perspectives. International Journal of Molecular Sciences. 2019;20(5):1190. doi: 10.3390/ijms20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanbur NO, Derman O, Sen TA, Kinik E. Osteocalcin. A biochemical marker of bone turnover during puberty. Int J Adolesc Med Health. 2002;14:235–244. doi: 10.1515/ijamh.2002.14.3.235. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Zhao Q, Du G, Xu Y. Association between serum osteocalcin and glucose/lipid metabolism in Chinese Han and Uygur populations with type 2 diabetes mellitus in Xinjiang: two cross-sectional studies. Lipids Health Dis. 2017;16:139. doi: 10.1186/s12944-017-0512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 31.Expert Panel on Integrated Guidelines for Cardiovascular, H., Risk Reduction in, C., Adolescents, National Heart, L. & Blood, I. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(Suppl 5):S213–256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Expert Panel on Detection, E. & Treatment of High Blood Cholesterol in, A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.