Abstract
Formaldehyde (FA) is an environmentally-available pollutant. Since the liver acts as a detoxifier in the human body, it is the first and most affected organ in individuals exposed to higher-than-normal amounts of FA. FA mainly alters oxidant/antioxidant status and initiates oxidative stress, and by means, causes functional damage to the liver. Thus, it is important to identify natural bioactive compounds with antioxidant properties in order to be used as food additives. Cinnamon (Cinnamomum zeylanicum) is a popular flavor and also a medicinal plant with a variety of beneficial effects. In the present original study, cinnamon essential oil (CEO) has been administrated at doses of 10, 20, and 100 mg/kg, orally, to hepatotoxicity rat models caused by FA (10 mg/kg, intraperitoneally). Liver enzymes and its histology were assessed and oxidative stress biomarkers in the liver tissue were also examined. CEO administration caused a significant increase in superoxide dismutase, glutathione peroxidase, and catalase and a prominent decrease in nitric oxide levels in the liver tissue. Also, in serum samples, CEO significantly reduced the elevated amounts of alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase. When assessed histologically, portal area and central vein fibrosis alongside with the hepatocytes' hypereosinophilia and swelling, focal inflammation, and necrotic areas were found to be prominently decreased in the CEO group. In conclusion, our study suggested that the CEO may have the potential for being used against FA-induced hepatotoxicity.
Keywords: Formaldehyde, Oxidative stress, Liver, Cinnamomum zeylanicum, Antioxidants
Introduction
The liver is the largest internal organ in the body, weighing around 968–1,860 g in normal men and 603 to 1,767 g in normal women [1,2]. It plays a pivotal role in the maintenance of body homeostasis by regulation of various physiological processes such as metabolism of carbohydrates and fats, bile acids synthesis, production of proteins, glycogen storage, etc. [3]. Owing to its continuous exposure to various drugs, chemical agents, and environmental pollutants as well as xenobiotics, the liver is highly susceptible to damage [4].
Among all environmental pollutants, formaldehyde, being produced more than 21 million pounds annually, is a major industrial chemical threatening public health worldwide. It is widely used as a tissue preservative and disinfectant in anatomy and pathology laboratories [5], as an antimicrobial agent in cosmetics [6], as a fumigant in agriculture [7], and surprisingly, in the production of crease-resistant garments and textiles [8]. Although most of the exposures to formaldehyde (FA) occur in workplaces (occupational exposure) [9], it can also occur in public areas, for the fact that it is present in the indoor and outdoor [10]. NAD-dependent aldehyde dehydrogenase and glutathione-dependent FA dehydrogenase are two major enzymes involved in the metabolism of FA, localized mostly in the liver [11]. After being metabolized, it leaves the body as formic acid and CO2+H2O through the urinary system and respiratory system, respectively [12]. Studies have shown that FA initiates oxidative stress by causing oxidant/antioxidant imbalance [13]. Under physiological conditions, there is a certain amount of reactive oxygen species (ROSs) production, as normal metabolism byproducts but in the case of pathology, the excess amount of ROSs production would adversely affect whole cell's functions, which may lead to cell death. Since liver malfunction in most cases is caused by toxicity during the process of clearing blood from reactive chemical agents, the use of natural antioxidants with the ability to neutralize these agents or enhance liver cells' strength through involving in a variety of pathways, is of utmost importance. Herbal medicine is cheaper, more available, and have shown fewer side effects than chemical drugs, and for that, they are now of great interest to researchers.
Cinnamon is the inner bark of the plant Cinnamomum zeylanicum, with many approved therapeutic properties. It has antioxidant and anti-inflammatory activities, makes it able to controlling glucose tolerance and lowering blood sugar in diabetic patients [14]. Cinnamon showed antiproliferative activity against tumor cells [15]. Being able to decrease blood pressure and blood cholesterol, cinnamon can positively affect heart and circulation system [16] and studies showed that it has also hepatoprotective effects [17]. The essential oil of cinnamon bark is composed of about 50% cinnamaldehyde. Cinnamaldehyde is an unsaturated aldehyde naturally synthesized in the shikimate pathway. It has been shown that cinnamaldehyde in cinnamon is responsible for most of the inflammatory activity of this plant [18].
According to numerous papers highlighting the anti-inflammatory and antioxidant activity of cinnamon, the present study was conducted to investigate the potential effect of cinnamon essential oil (CEO) on FA-induced liver damage in male adult rats.
Materials and Methods
CEO extraction
Dried bark of Cinnamomum zeylanicum was purchased from a local store. After authentication by the Department of Pharmacognosy (School of Pharmacy, Guilan University of Medical Sciences) with the herbarium number of GUMSC17, it was grounded into powder. A quantity of 100 g of the powder was combined with 300 ml of distilled water and essential oil was extracted by hydrodistillation in a Clevenger type apparatus for 4 hours, according to the European Pharmacopoeia (1975) [19]. The yellowish essential oil was dried using anhydrous sodium sulfate then kept at 4℃ until needed.
Experimental design
In this original study, 42 male Wistar rats (6–8 weeks, 200–250 g) were purchased from the Pasteur Institute of Iran. Animals were acclimatized for 2 weeks in a temperature-controlled room (23–25℃) with 12 hours light/dark cycling, having free access to food and water during acclimatization and throughout the experiment. They were then randomly divided into six groups: control group (C group) no intervention (n=8), formaldehyde group (FA group) received FA at the dose of 10 mg/kg intraperitoneally for 30 days (n=8), three experimental groups (n=8 in each group) which received doses of 10 (CEO10), 20 (CEO20) and 100 (CEO100) mg/kg cinnamon essential oil [20,21] by syringe feeding [22] an hour after FA treatment, and a vehicle group (V group) which received the solvent of cinnamon essential oil (10% dimethyl sulfoxide/10% sucrose) by syringe feeding (n=8) after FA treatment. Afterward, animals were sacrificed by cervical dislocation under deep anesthesia with ketamine (87 mg/kg) and xylazine (13 mg/kg) [23]. Blood samples were collected by cardiac puncture and then centrifuged at 10,000 rpm for 20 minutes and serums were collected and stored at −80℃ for analysis of liver enzymes. Liver tissues were carefully dissected and weighed. Samples needed for histological studies were fixed in 10% neutral buffered formalin and after undergoing tissue processing, were blocked in paraffin (Bio-Optica, Milan, Italy). Later, 4-µm-thick sections were obtained via the aid of a microtome (Microtec, Walldorf, Germany) for histological evaluations. Liver samples needed for analysis of antioxidant enzymes were also stored in liquid nitrogen.
Body and liver weights' assessments
Body weights were recorded weekly for calculation of required doses of FA and CEO, and one last time before sacrificing animals. Then, the first and last week's data were used to calculate the weight changes (ΔWeight) in experimental groups. Liver weights were also recorded after sacrificing animals.
Liver index calculation
Liver indices were calculated by dividing liver weights (g) by body weights (g) and multiplying the results by 100.
Biochemical analyses of serum samples
Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) were measured using commercially available photometry kits according to manufacturer's instructions (Pars Azmun Co., Karaj, Iran). ALT and AST levels have been measured and absorbance was read at 340 nm, data are represented as unit per liter (U/l). ALP levels were measured and absorbance was read at 405 nm, data are represented as U/l.
Biochemical analyses of oxidative stress biomarkers in liver
Samples preparations
Briefly, frozen liver samples were homogenized in 100 mM/l Tris-HCl, pH 7.4 at 4℃. After centrifuging at 12,000 rpm for 15 minutes, the supernatants were collected and stored at −80℃ until needed for enzymatic measurements.
Superoxide dismutase determination
Levels of superoxide dismutase (SOD) were measured according to Kakkar et al. [24] and by inhibition of nitroblue tetrazolium formation, which optical density was read at 520 nm. Each of the SOD units was defined as the enzyme amount required for decreasing the production rate of chromogen by 50%. Data are represented as unit per milligram protein (U/mg protein).
Malondialdehyde (MDA) determination
Levels of MDA were measured as described by Wasowicz et al. [25] in which amounts of MDA reacting with thiobarbituric acid were read at 532 nm. Data are represented as nanomole per milligram protein (nM/mg protein).
Glutathione peroxidase determination
Levels of glutathione peroxidase (GPx) were estimated as described by Paglia and Valentine [26] in which the enzyme activity was read at 340 nm while oxidizing NADPH into NADP+ in reaction environment. Data are represented as U/mg protein.
Catalase determination
Levels of catalase (CAT) were determined according to Sinha [27] by measuring the amount of hydrogen peroxide (H2O2) reacting with dichromate-acetic acid at 590 nm. Data are represented as the rate of micromole H2O2 consumption per milligram protein (µM/mg protein).
NO determination
Levels of total nitrate (NO3−)/nitrite (NO2−) were estimated according to Peralta et al. [28], which used the method earlier explained by Hortelano et al. [29] with a minor modification and via the aid of nitrate/nitrite commercial colorimetric assay kit (Cayman Chemical Co., Ann Arbor, MI, USA). Amounts of nitrite were read at 540 nm in the presence of Griess reagent after nitrates were reduced to nitrites by nitrate reductase enzyme while incubated with NADPH. Data are represented as nM/mg protein.
Histological evaluations
Sections of 4 µm from tissue specimens in each group were cleared in three changes of xylene and rehydrated through 100%, 96%, 90%, and 70% ethanol. To evaluate morphological alterations (hepatocytes' hyper-eosinophilia and swelling, inflammation, and necrosis), sections were stained with hematoxylin and eosin and scored according to French et al. [30]. Scores used to evaluate the liver pathology was as follows.
- Score 0: no visible cell damage
- Score 1: focal hepatocyte damage on less than 25% of tissue
- Score 2: focal hepatocyte damage on 25–50% of tissue
- Score 3: extensive but focal hepatocyte lesions
- Score 4: global hepatocyte necrosis
Masson's trichrome was another staining method used to evaluate portal area and central veins' fibrosis. Three sections of each animal were stained as described and five microscopic fields were randomly examined microscopically. All histological evaluations were performed under Olympus light microscopy (Olympus, Tokyo, Japan).
Statistical analyses
Quantitative data were reported as mean±SD. Multiple comparisons were performed using one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test and the difference was considered significant when the P-value was <0.05. Data analyses were performed using GraphPad PRISM version 6.0e (GraphPad Software Inc., San Diego, CA, USA).
Ethical considerations
All experiments were performed in accordance with the guidelines for experimental ethics and laboratory animal handling of Guilan University of Medical Sciences parallel to the National Institutes of Health's guide for the care and use of laboratory animals [31].
Results
Body and liver weights assessments
ΔWeights were calculated using the first and the last recorded weights. FA treatment significantly inhibited weight gain (P<0.001) (Table 1). Administration of CEO at the dose of 100 mg/kg reversed this adverse effect of FA (P<0.001). FA also caused a significant increase in liver weight (P<0.001), which have been ameliorated by 100 mg/kg CEO administration (P=0.001).
Table 1. Changes in body weights, liver weights, liver indices, and total scores gained based on the severity of liver pathology among experimental groups.
| C | FA | V | CEO10 | CEO20 | CEO100 | |
|---|---|---|---|---|---|---|
| Primary body weight (g) | 255.50±23.43 | 251.62±16.81 | 251.87±15.08 | 254.75±27.88 | 253.62±22.60 | 253.75±25.08 |
| Final body weight (g) | 294.37±12.53 | 230.75±16.49a) | 233.75±21.71a) | 239.87±17.62a) | 243.87±21.75a) | 284.75±23.10e) |
| ∆Weight (g) | 41.87±17.38 | −0.25±6.90a) | −0.12±3.97a) | 3.37±7.40a) | 10.62±3.70a) | 35.50±18.52e) |
| Liver weight (g) | 5.73±0.31 | 7.07±0.55a) | 7.05±0.38a) | 6.66±0.40c) | 6.42±0.58 | 6.01±0.65f) |
| Liver index | 1.93±0.19 | 2.83±0.37a) | 2.81±0.24a) | 2.59±0.21b) | 2.43±0.22d,g) | 2.08±0.28e) |
| Scoreh) | ||||||
| Fatty degeneration | 0 | 0 | 0 | 0 | 0 | 0 |
| Cell swelling | 0 | 3 | 3 | 3 | 2 | 2 |
| Inflammation | 0 | 4 | 4 | 3 | 3 | 1 |
| Necrosis | 0 | 4 | 4 | 4 | 2 | 1 |
| Total scores | 0 | 11 | 11 | 10 | 7 | 4 |
Values are expressed as mean±SD. C, control; FA, formaldehyde 10 mg/kg; V, FA 10 mg/kg+solvent; CEO10,20,100, FA 10 mg/kg+cinnamon essential oil 10, 20, 100 mg/kg. a)P<0.0001 when compared to control. b)P<0.001 when compared to control. c)P<0.01 when compared to control. d)P<0.05 when compared to control. e)P<0.0001 when compared to formaldehyde. f)P<0.01 when compared to formaldehyde. g)P<0.05 when compared to formaldehyde). h)Scores used to evaluate the liver pathology was as follows: score 0, no visible cell damage; score 1, focal hepatocyte damage on less than 25% of tissue; score 2, focal hepatocyte damage on 25%–50% of tissue; score 3, extensive but focal hepatocyte lesions; score 4, global hepatocyte necrosis.
Liver index calculation
Liver indices were calculated using liver weights (g) and body weights (g) (Table 1). FA group have shown a significant increase in the liver weights (P<0.001). CEO at the dose of 20 and 100 mg/kg (P=0.0479 and P<0.001, respectively) could reverse the FA-induced hepatomegaly.
Biochemical analyses of serum samples
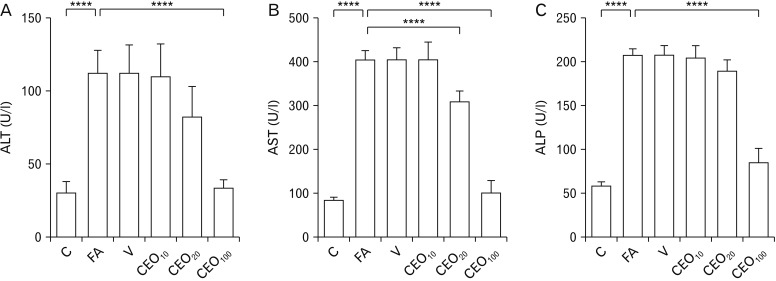
Serum ALT (Fig. 1A), AST (Fig. 1B), and ALP (Fig. 1C) levels significantly increased in response to FA treatment (P<0.001). CEO at the dose of 100 mg/kg ameliorated the effect of FA on all three liver enzymes (P<0.001). It also reduced the elevated AST (P<0.001) and ALT (P=0.0143) when administered at the dose of 20 mg/kg.
Fig. 1. The effect of cinnamon essential oil administration on liver enzymes in serum among groups. FA treatment significantly increased the serum levels of ALT (A), AST (B), and ALP (C) as a sign of tissue damage. CEO administration caused an increase in serum levels of all three liver hormones, indicating the protection CEO administration caused against FA-induced hepatotoxicity. C, control; FA, formaldehyde 10 mg/kg; V, FA 10 mg/kg+solvent; CEO10,20,100, FA 10 mg/kg+cinnamon essential oil 10,20,100 mg/kg; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase. ****P<0.0001.
Biochemical analyses of oxidative stress biomarkers in liver
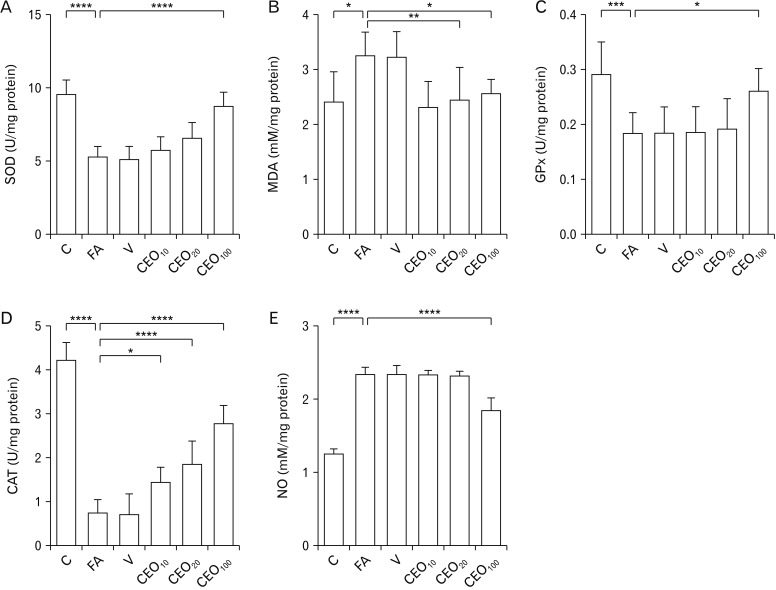
FA treatment caused a significant decrease in the levels of SOD (P<0.001) (Fig. 2A), GPx (P=0.001) (Fig. 2B), and CAT (P<0.001) (Fig. 2D) in the liver. Administration of CEO at the dose of 100 mg/kg inhibited the increment of SOD (P<0.001) and GPx (P=0.034) and all three doses of 10 (P=0.025), 20, and 100 mg/kg (P<0.001 for both) CEO abolished the change made by FA in level of CAT in the liver. MDA (Fig. 2B) and NO (Fig. 2E) activities were found to be significantly higher in the FA group (P=0.019 and P<0.001, respectively). CEO at the dose 100 mg/kg decreased the elevation of NO levels (P<0.001). Two lower doses of 10 and 20 mg/kg caused a significant decrease in levels of MDA in liver (P=0.005 and P=0.029, respectively).
Fig. 2. Effect of cinnamon essential oil administration on oxidative stress markers in liver among groups. Levels of SOD (A), GPx (C), and CAT (D) were found to be decreased due to FA treatment, as a result of oxidative stress. CEO administration at the dose of 100 mg/kg ameliorated these abnormal elevations. MDA (B) and NO (E) activities which were increased in response to FA treatment, were significantly decreased following 100 mg/kg CEO administration. C, control; FA, formaldehyde 10 mg/kg; V, FA 10 mg/kg+solvent; CEO10,20,100, FA 10 mg/kg+cinnamon essential oil 10,20,100 mg/kg; SOD, superoxide dismutase; GPx, glutathione peroxidase; CAT, catalase; MDA, malondialdehyde; NO, nitric oxide. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Histological evaluations
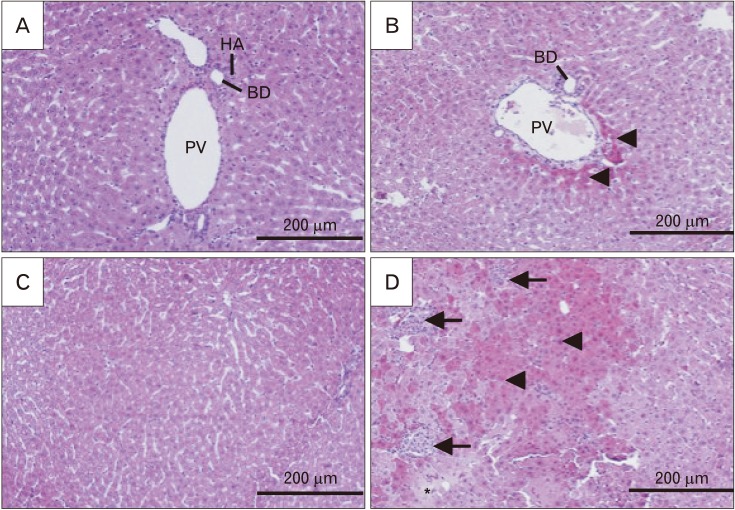
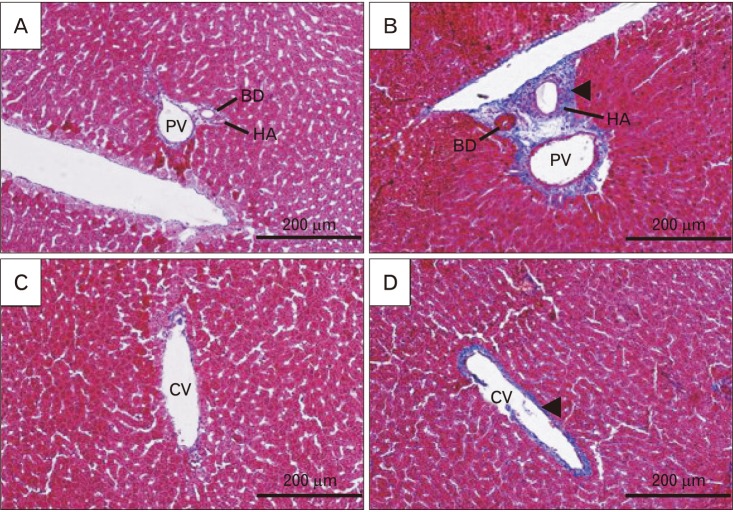
Results of histological alterations are summarized in Table 1. FA treatment clearly elevated the hepatocytes with swelling and hyper-eosinophilic cytoplasm, inflammation, and necrotic areas (Fig. 3B, D). CEO, when administered at the dose of 100 mg/kg, prominently lowered the damaged hepatocytes with swelled and hyper-eosinophil phenotype and inhibited necrosis (Fig. 4A, C). It also successfully limited the parenchymal infiltration of inflammatory cells, so that in most of the sections, there were only small populations of inflammatory cells around portal areas and/or central veins and focal parenchymal inflammation, if present, were plainly small in size (Fig. 4A, C). Portal area and central vein fibrosis were occurred in response to FA treatment and administration of CEO at the dose of 100 mg/kg prominently decreased the fibrous tissue in both areas (Figs. 4B, D, 5).
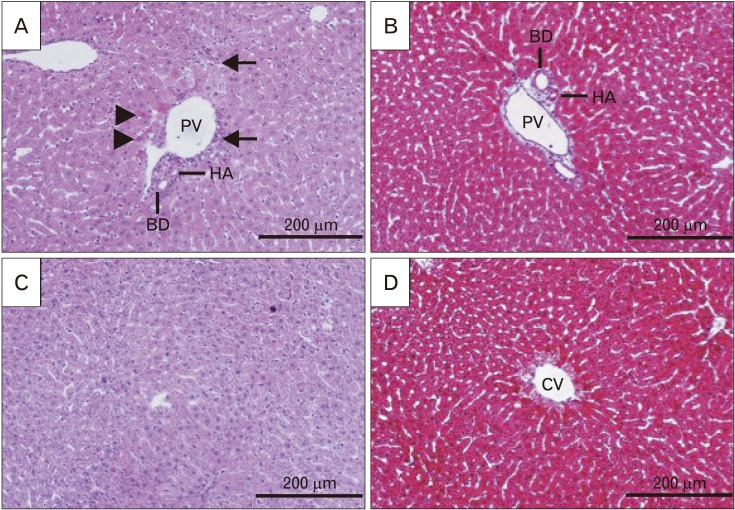
Fig. 3. Histological alterations of liver following formaldehyde (FA) administration in comparison with control in H&E stained sections (×200). Samples from FA group (B, D) showed an increase in hyper-eosinophilic and swelled hepatocytes (arrowheads), focal inflammation (arrows) and necrotic areas (asterisk) when compared to control group (A, C). This shows how FA caused damage to the cellular architecture of portal area and liver parenchyma. PV, portal vein; HA, hepatic artery; BD, bile duct.
Fig. 4. Histological alterations and fibrotic changes of liver following co-treatment of formaldehyde (FA) and cinnamon essential oil (CEO; 100 mg/kg) in H&E and Masson's trichrome stained sections (×200). CEO administration ameliorated FA-induced hepatocytes hyper-eosinophilia and swelling (arrowheads), inflammation (arrows), and necrosis (not seen in the picture) in H&E stained sections (A, C). It also inhibited fibrous tissue formation around portal area and central vein in Masson's trichrome stained sections (B, D). PV, portal vein; HA, hepatic artery; BD, bile duct; CV, central vein.
Fig. 5. Fibrotic changes of liver following formaldehyde (FA) administration in comparison with control in Masson's trichrome stained sections (×200). Samples from FA group (B, D) showed an increase in fibrous tissue around portal area and central vein (arrowheads) when compared with control group (A, C). This figure shows that FA prominently disrupts portal area network by causing fibrotic tissue. PV, portal vein; HA, hepatic artery; BD, bile duct; CV, central vein.
Discussion
The liver is the main organ involved in the detoxification process of drugs and chemicals as FA. It has been shown that the histology and biochemistry of the liver can be affected by FA exposure. The liver weights were found to be significantly increased in FA-treated rats (10 mg/kg for four weeks), regardless of their general weight loss (Table 1). This leads to a significant increase in the liver index in the FA group when compared with the control group (Table 1). Administration of CEO in all three doses of 10, 20, and 100 mg/kg helped animals with weight gain and decreased the weight of the liver. However, only in the group administered with 100 mg/kg of CEO changes were significant. Studies have shown that FA inhibits energy production by causing damage to the mitochondria [32]. That might be a good explanation for the FA-induced growth inhibition we observed in the present study. In a similar research done by Eidi et al. [17], the beneficial effect of cinnamon alcoholic extract against carbon tetrachloride (CCl4) toxicity was assessed and results showed that co-treatment of cinnamon alcoholic extract at doses of 0.01, 0.05, and 0.1 g/kg significantly increased the body weight and decreased the liver weights, and by that mean, improved the liver index.
A series of histological changes would also take place in response to FA-induced ROS elevation, including the increase in the number of hyper-eosinophilic and swelled hepatocytes, parenchymal inflammation and necrosis, alongside with disturbed cellular architecture of the portal area [33]. Our results suggested that FA treatment caused significant damage to the liver. The increment in hyper-eosinophilic and swelled hepatocyte was clearly visible in tissue samples from the FA group and infiltration of inflammatory cells caused distributed focal inflammation. Necrotic areas were also seen in almost all microscopic fields (Fig. 3C, D). Excess fibrous tissue formation in portal areas and around central veins was another sign confirming the toxic effects of FA on the liver tissue (Fig. 5B, D). Administration of 100 mg/kg CEO effectively prevented FA-induced changes in the cellular architecture of the liver. Tissue sections from group CEO100 have shown to have clearly less hyper-eosinophilic hepatocytes with swelling. Focal parenchymal inflammation was eliminated and there were no massive amounts of fibrous tissue in the portal area and around portal veins (Fig. 4B, D). In accordance with our results, a survey conducted by Eidi et al. [17] showed that cinnamon alcoholic extract effectively reduced the toxic effects of CCl4 on liver histology.
It is reported that FA causes DNA-protein crosslinks [34]. The mechanism of action of FA is thought to be the enhancement of ROS production [35], resulting in damage to important molecules in the tissue, including proteins, nucleic acids, and membrane lipids. Membrane lipids are easily harmed by ROS leading to lipid peroxidation, in which the disruption of membranes in cell and its organelles may occur, and the damaged cell would be subjected to cell lysis. As a result of cell lysis, a significant increase in serum levels of liver enzymes will occur. Therefore, these enzymes can be used as an indicator for functional evaluation of the liver. Our results suggested that serum amounts of ALT, AST, and ALP in the FA group is significantly higher compared to the control group (Fig. 1A–C). With a careful look at histological evaluations, it seems reasonable. In the group CEO100, the total recovery in liver enzymes' activity was seen. There are other studies supporting these results and the idea that herbal bioactive compounds might improve the enzymatic function of the liver [36].
Previous studies have shown that FA initiates oxidative stress in liver [13]. MDA is one of the products of lipid oxidation that has shown to be affected in FA toxicity [12]. We observed a significant increase in tissue amounts of MDA in the FA group. CEO administration lowered the activity of MDA when administered at low doses (Fig. 2B). Interestingly, our results showed that with increment in the dose of CEO, levels of MDA in tissue would also increase in a dose-dependent manner. So, the results from the group received 100 mg/kg CEO found to be not significantly different when compared to the FA group. This may suggest that there might be a relationship between CEO and lipid peroxidation and/or there may be an unwanted substance in the essential oil of cinnamon that needs to be omitted in order to gain better results.
NO production and release appeared to be enhanced in the presence of free radicals. Studies showed that Kupffer cells and hepatocytes release NO in response to macrophage-released free radicals during liver injuries and hepatocellular necrosis [37]. NO is synthesized by inducible nitric oxide synthesized [38] enzyme in the liver. We observed that 100 mg/kg CEO administration significantly reduced the tissue amounts of NO, which was elevated in response to FA treatment (Fig. 2E). This may due to cinnamon antioxidant activity.
In the present study, activities of SOD, GPx, and CAT were found to be significantly higher than the control group (Fig. 2A, C, D). SOD is one of the antioxidant enzymes which actively scavenge superoxide anions; its mechanism of action is to join every two free radicals and make oxygen and hydrogen peroxide [39]. CAT continues this process by breaking H2O2 to CO2 and H2O [39]. GPx is another oxidative damage indicator. It serves to join two glutathione molecules in the presence of ROS [40]. CEO administration at the dose of 100 mg/kg improved the activity of SOD and GPx. It also lowered the activity of CAT at all three doses (Fig. 2). In agreement with our study, Gerin et al. [39] reported that the disturbed balance of liver CAT, MDA, GPx, and SOD significantly improved following treatment with Ferulic acid. Another study confirming the important role of antioxidants against FA-induced hepatotoxicity have done by Bakar et al. [41], in which they showed that vitamin E and proanthocyanidins may have beneficial effects against FA-induced hepatotoxicity.
In conclusion, our study indicated that cinnamon essential oil might prevent oxidative damage caused by FA in liver tissue through rebalancing antioxidant enzymes which by the way eliminates cellular damage and therefore the enzymatic function of this organ.
Acknowledgements
This research was fully supported by the Research and Technology Chancellor of Guilan University of Medical Sciences (Grant number: IR.GUMS.REC.1395.377).
Footnotes
- Conceptualization: EN, SSF.
- Data acquisition: FY, FN, AK, MGA.
- Data analysis or interpretation: EN, SSF.
- Drafting of the manuscript: EN, SSF.
- Critical revision of the manuscript: EN, FY.
- Approval of the final version of the manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Molina DK, DiMaio VJ. Normal organ weights in men: part II-the brain, lungs, liver, spleen, and kidneys. Am J Forensic Med Pathol. 2012;33:368–372. doi: 10.1097/PAF.0b013e31823d29ad. [DOI] [PubMed] [Google Scholar]
- 2.Molina DK, DiMaio VJ. Normal organ weights in women: part II. the brain, lungs, liver, spleen, and kidneys. Am J Forensic Med Pathol. 2015;36:182–187. doi: 10.1097/PAF.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 3.Gil MN, Choi DR, Yu KS, Jeong JH, Bak DH, Kim DK, Lee NS, Lee JH, Jeong YG, Na CS, Na DS, Ryu KH, Han SY. Rhus verniciflua stokes attenuates cholestatic liver cirrhosis-induced interstitial fibrosis via Smad3 down-regulation and Smad7 up-regulation. Anat Cell Biol. 2016;49:189–198. doi: 10.5115/acb.2016.49.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya A, Dhar P, Mehra RD. Preliminary morphological and biochemical changes in rat liver following postnatal exposure to sodium arsenite. Anat Cell Biol. 2012;45:229–240. doi: 10.5115/acb.2012.45.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saowakon N, Ngernsoungnern P, Watcharavitoon P, Ngernsoungnern A, Kosanlavit R. Formaldehyde exposure in gross anatomy laboratory of Suranaree University of Technology: a comparison of area and personal sampling. Environ Sci Pollut Res Int. 2015;22:19002–19012. doi: 10.1007/s11356-015-5078-2. [DOI] [PubMed] [Google Scholar]
- 6.Bunyavaree M, Kasemsarn P, Boonchai W. Cosmetic preservative labelling on the Thai market. Contact Dermatitis. 2016;74:217–221. doi: 10.1111/cod.12520. [DOI] [PubMed] [Google Scholar]
- 7.Ou LT. Enhanced degradation of the volatile fumigant-nematicides 1,3-d and methyl bromide in soil. J Nematol. 1998;30:56–64. [PMC free article] [PubMed] [Google Scholar]
- 8.O'Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593–596. [PubMed] [Google Scholar]
- 9.Viegas S, Ladeira C, Nunes C, Malta-Vacas J, Gomes M, Brito M, Mendonca P, Prista J. Genotoxic effects in occupational exposure to formaldehyde: a study in anatomy and pathology laboratories and formaldehyde-resins production. J Occup Med Toxicol. 2010;5:25. doi: 10.1186/1745-6673-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotzias D, Geiss O, Tirendi S, Barrero-Moreno J, Reina V, Gotti A, Cimino-Reale G, Casati B, Marafante E, Sarigiannis D. Exposure to multiple air contaminants in public buildings, schools and kindergartens: The European Indoor Air Monitoring and Exposure Assessment Study (AIRMEX) Study. Fresen Environ Bull. 2009;18:670–681. [Google Scholar]
- 11.Teng S, Beard K, Pourahmad J, Moridani M, Easson E, Poon R, O'Brien PJ. The formaldehyde metabolic detoxification enzyme systems and molecular cytotoxic mechanism in isolated rat hepatocytes. Chem Biol Interact. 2001;130-132:285–296. doi: 10.1016/s0009-2797(00)00272-6. [DOI] [PubMed] [Google Scholar]
- 12.Beall JR, Ulsamer AG. Formaldehyde and hepatotoxicity: a review. J Toxicol Environ Health. 1984;14:1–21. doi: 10.1080/15287398409530560. [DOI] [PubMed] [Google Scholar]
- 13.Matsuoka T, Takaki A, Ohtaki H, Shioda S. Early changes to oxidative stress levels following exposure to formaldehyde in ICR mice. J Toxicol Sci. 2010;35:721–730. doi: 10.2131/jts.35.721. [DOI] [PubMed] [Google Scholar]
- 14.Talaei B, Amouzegar A, Sahranavard S, Hedayati M, Mirmiran P, Azizi F. Effects of cinnamon consumption on glycemic indicators, advanced glycation end products, and antioxidant status in type 2 diabetic patients. Nutrients. 2017;9:E991. doi: 10.3390/nu9090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noudeh GD, Sharififar F, Noodeh AD, Moshafi MH, Afzadi MA, Behravan E, Aref M, Sakhtianchi R. Antitumor and antibacterial activity of four fractions from Heracleum persicum Desf. and Cinnamomum zeylanicum Blume. J Med Plants Res. 2010;4:2176–2180. [Google Scholar]
- 16.Sambaiah K, Srinivasan K. Effect of cumin, cinnamon, ginger, mustard and tamarind in induced hypercholesterolemic rats. Nahrung. 1991;35:47–51. doi: 10.1002/food.19910350112. [DOI] [PubMed] [Google Scholar]
- 17.Eidi A, Mortazavi P, Bazargan M, Zaringhalam J. Hepatoprotective activity of cinnamon ethanolic extract against CCI4-induced liver injury in rats. EXCLI J. 2012;11:495–507. [PMC free article] [PubMed] [Google Scholar]
- 18.Gunawardena D, Karunaweera N, Lee S, van Der Kooy F, Harman DG, Raju R, Bennett L, Gyengesi E, Sucher NJ, Münch G. Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts: identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct. 2015;6:910–919. doi: 10.1039/c4fo00680a. [DOI] [PubMed] [Google Scholar]
- 19.Maisonneune SA. European Pharmacopoeia, Vol. 3. Saint-Ruffine: Maisonneuve; 1975. [Google Scholar]
- 20.Malik J, Munjal K, Deshmukh R. Attenuating effect of standardized lyophilized Cinnamomum zeylanicum bark extract against streptozotocin-induced experimental dementia of Alzheimer's type. J Basic Clin Physiol Pharmacol. 2015;26:275–285. doi: 10.1515/jbcpp-2014-0012. [DOI] [PubMed] [Google Scholar]
- 21.Jain S, Sangma T, Shukla SK, Mediratta PK. Effect of Cinnamomum zeylanicum extract on scopolamine-induced cognitive impairment and oxidative stress in rats. Nutr Neurosci. 2015;18:210–216. doi: 10.1179/1476830514Y.0000000113. [DOI] [PubMed] [Google Scholar]
- 22.Atcha Z, Rourke C, Neo AH, Goh CW, Lim JS, Aw CC, Browne ER, Pemberton DJ. Alternative method of oral dosing for rats. J Am Assoc Lab Anim Sci. 2010;49:335–343. [PMC free article] [PubMed] [Google Scholar]
- 23.Van Pelt LF. Ketamine and xylazine for surgical anesthesia in rats. J Am Vet Med Assoc. 1977;171:842–844. [PubMed] [Google Scholar]
- 24.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 25.Wasowicz W, Neve J, Peretz A. Optimized steps in fluorometric determination of thiobarbituric acid-reactive substances in serum: importance of extraction pH and influence of sample preservation and storage. Clin Chem. 1993;39:2522–2526. [PubMed] [Google Scholar]
- 26.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 27.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 28.Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 1999;29:126–132. doi: 10.1002/hep.510290104. [DOI] [PubMed] [Google Scholar]
- 29.Hortelano S, Genaro AM, Bosca L. Phorbol esters induce nitric oxide synthase activity in rat hepatocytes. Antagonism with the induction elicited by lipopolysaccharide. J Biol Chem. 1992;267:24937–24940. [PubMed] [Google Scholar]
- 30.French SW, Miyamoto K, Ohta Y, Geoffrion Y. Pathogenesis of experimental alcoholic liver disease in the rat. Methods Achiev Exp Pathol. 1988;13:181–207. [PubMed] [Google Scholar]
- 31.Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, Hengartner M, Knight RA, Kumar S, Lipton SA, Malorni W, Nuñez G, Peter ME, Tschopp J, Yuan J, Piacentini M, Zhivotovsky B, Melino G Nomenclature Committee on Cell Death 2009. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strubelt O, Younes M, Pentz R, Kuhnel W. Mechanistic study on formaldehyde-induced hepatotoxicity. J Toxicol Environ Health. 1989;27:351–366. doi: 10.1080/15287398909531306. [DOI] [PubMed] [Google Scholar]
- 33.Nasiri E, Naserirad S, Pasdaran Lashgari A, Gazor R, Mohammadghasemi F, Atrkar Roushan Z. Hepatoprotective effect of Acantholimon bracteatum (Girard) Boiss. on formaldehyde-induced liver injury in adult male mice. Res J Pharmacogn. 2016;3:55–61. [Google Scholar]
- 34.Lovschall H, Eiskjaer M, Arenholt-Bindslev D. Formaldehyde cytotoxicity in three human cell types assessed in three different assays. Toxicol In Vitro. 2002;16:63–69. doi: 10.1016/s0887-2333(01)00093-5. [DOI] [PubMed] [Google Scholar]
- 35.Saito Y, Nishio K, Yoshida Y, Niki E. Cytotoxic effect of formaldehyde with free radicals via increment of cellular reactive oxygen species. Toxicology. 2005;210:235–245. doi: 10.1016/j.tox.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh N, Ghosh R, Mandal V, Mandal SC. Recent advances in herbal medicine for treatment of liver diseases. Pharm Biol. 2011;49:970–988. doi: 10.3109/13880209.2011.558515. [DOI] [PubMed] [Google Scholar]
- 37.Kolios G, Valatas V, Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol. 2006;12:7413–7420. doi: 10.3748/wjg.v12.i46.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego, CA: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- 39.Gerin F, Erman H, Erboga M, Sener U, Yilmaz A, Seyhan H, Gurel A. The effects of ferulic acid against oxidative stress and inflammation in formaldehyde-induced hepatotoxicity. Inflammation. 2016;39:1377–1386. doi: 10.1007/s10753-016-0369-4. [DOI] [PubMed] [Google Scholar]
- 40.Yang MS, Chan HW, Yu LC. Glutathione peroxidase and glutathione reductase activities are partially responsible for determining the susceptibility of cells to oxidative stress. Toxicology. 2006;226:126–130. doi: 10.1016/j.tox.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Bakar E, Ulucam E, Cerkezkayabekir A. Protective effects of proanthocyanidin and vitamin E against toxic effects of formaldehyde in kidney tissue. Biotech Histochem. 2015;90:69–78. doi: 10.3109/10520295.2014.954620. [DOI] [PubMed] [Google Scholar]