Abstract
Cyclosporin A (CsA) does not only exert a toxic effect on kidney parenchymal cells, but also protects them against necrotic cell death by inhibiting opening of mitochondrial permeability transition pore. However, whether CsA plays a role in hydrogen peroxide-induced kidney proximal tubular cell death is currently unclear. In the present study, treatment with CsA further increased apoptosis and necrosis in HK-2 human kidney proximal tubule epithelial cells during exposure to hydrogen peroxide. In addition, hydrogen peroxide-induced p53 activation and BH3 interacting-domain death agonist (BID) expression were higher in CsA-treated cells than those in non-treated cells, whereas hydrogen peroxide-induced activation of mitogen-activated protein kinases including p38, c-Jun N-terminal kinase, and extracellular signal-regulated kinase and activation of protein kinase B were not significantly altered by treatment with CsA. In oxidant-antioxidant system, reactive oxygen species (ROS) production induced by hydrogen peroxide was further enhanced by treatment with CsA. However, expression levels of antioxidant enzymes including manganese superoxide dismutase, copper/zinc superoxide dismutase, and catalase were not altered by treatment with hydrogen peroxide or CsA. Treatment with CsA further enhanced mitochondrial membrane potential induced by exposure to hydrogen peroxide, although it did not alter endoplasmic reticulum stress based on expression of glucose-regulated protein 78 and 94. Taken together, these data suggest that CsA can aggravate hydrogen peroxide-induced cell death through p53 activation, BID expression, and ROS production.
Keywords: Cyclosporin, Cell death, Hydrogen peroxide, p53, Reactive oxygen species
Introduction
Cyclosporins are a group of closely related cyclic undercapeptides produced as secondary metabolites in strains of fungi including Cylindrocarpon lucidum and Trichoderma polysporum isolated from soil samples [1]. Among various cyclosporins, cyclosporin A (CsA) is one of the most commonly used immunosuppressive drugs in the treatment of patients with organ transplantation and autoimmune diseases including acquired immune deficiency syndrome owing to its superior T-cell specificity and low myelotoxicity [2]. After entering into recipient cells, CsA can bind to cyclophilins known to peptidylpropyl isomerase activity through catalyzing isomerization of peptide bonds from trans form to cis form at proline residues in protein folding pathway [3]. Such binding of CsA to cyclophilins can block their peptidylpropyl isomerase activity. Thus, CsA has shown immunosuppressive effects in adipocytes [4], myocytes [5], and lymphocytes [6]. Although CsA is an extremely valuable immunosuppressive agent for organ transplant recipients, unfortunately CsA has a number of serious side effects in various tissues, including kidney damage which is the most frequent and severe side effect of CsA [7]. Moderate to severe kidney dysfunction occurs in approximately 30% of patients treated with CsA, significantly limiting its clinical application [7]. Nephrotoxicity induced by CsA is characterized by reduced glomerular filtration rates and pathological changes including kidney proximal tubular damage, macrophage infiltration, and interstitial fibrosis [8,9]. On the other hand, cyclophilin D located within the mitochondrial matrix can bind to the complex between adenine nucleotide translocator and voltage-dependent anion channel in the outer membrane of mitochondria, and form a mitochondrial permeability transition pore [10]. Mitochondrial permeability transition can induce mitochondrial swelling, rupture of mitochondrial outer membrane, and release of apoptotic stimulators, leading to apoptotic and necrotic cell death [10]. Because CsA can bind to cyclophilin D and subsequently blocks the mitochondrial permeability transition pore formation, it can inhibit mitochondria-mediated cell death [10]. These findings indicate that CsA has opposite functions as a double-edged sword. However, intracellular actions of CsA in kidneys, especially kidney parenchymal cells in vitro, remain still unclear.
Incomplete reduction of oxygen during various biological processes can generate reactive oxygen species (ROS) [11]. ROS are produced rapidly in response to extracellular stimuli followed by subsequent degradation. They mediate diverse cellular functions in the early phase of injury [11]. Compared with other members of ROS, hydrogen peroxide (H2O2) formed by either enzyme-catalyzed or spontaneous dismutation of superoxide anion is more stable and membrane-permeable. Thus, H2O2 has been used to understand the pathogenesis of various tissue damages and the induction of cell death [12]. Furthermore, exogenous and endogenous H2O2 is considered to be an important mediator of kidney tubular injury in a variety of situations including kidney ischemia reperfusion injury [13,14,15], cisplatin nephrotoxicity [16,17,18], glomerulonephritis [19], and ureteral obstruction [20,21,22]. Among kidney tubular segments, kidney proximal tubules are more susceptible to cell injury and death than kidney distal tubules during these situations [23]. Based on these findings, exogenous H2O2 has been used to induce ROS-mediated oxidant injury in kidney proximal tubule epithelial cells. However, little is known about the mechanism of actions of CsA in kidney proximal tubule epithelial cells during H2O2 injury. Therefore, the objective of this study was to determine whether exogenous CsA could affect H2O2-induced cell death in kidney proximal tubule epithelial cells and identify proteins implicated in alteration of cell death following treatment with CsA.
Materials and Methods
Cell culture and treatment
HK-2 human kidney proximal tubule epithelial cell line as purchased from the American Type Culture Collection (Rockville, MD, USA) was cultured in RPMI 1640 medium (Welgene, Daegu, Korea), supplemented with 10% fetal bovine serum (Welgene) at 37℃ with 5% CO2, as described previously [24,25,26]. After reaching 80% confluence on culture dishes, the culture medium was changed to a serum-free medium. After that, cells were treated with either CsA (1, 10, or 100 nM; Sigma, St. Louis, MO, USA) or vehicle (1% dimethyl sulfoxide [DMSO], Sigma) for 60 minutes and subsequently exposed to 1 mM H2O2 (Sigma) or distilled water (control) for 0, 30, 60, or 120 minutes. To obtain intracellular proteins, cultured cells were washed with phosphate buffered saline (PBS) and harvested in M-PER mammalian protein extraction reagent (Thermo Fisher Scientific, Waltham, MA, USA) in the presence of 1% protease inhibitor cocktail set III (Merck Millipore, Billerica, MA, USA), 0.5% phosphatase inhibitor cocktail 2 (Sigma), and 0.5% phosphatase inhibitor cocktail 3 (Sigma). Apoptotic and necrotic cells were analyzed by CytoFLEX flow cytometer (Beckman Coulter, Brea, CA, USA) with an EzWay annexin V-FITC apoptosis detection kit (Komabiotech, Seoul, Korea) [14].
Cell viability
A yellow water soluble tetrazolium dye thiazolyl blue tetrazolium bromide (MTT; Biosesang, Seongnam, Korea) was used to measure cell viability as described previously [27,28]. After removing the culture medium, cells were incubated at 37℃ with 5 mg/ml MTT in PBS for 30 minutes. After removing the MTT solution, 300 µl of DMSO was added to each well and incubated for 5 minutes. To quantifying the purplecolored formazan product, absorbance of 100 µl solution from wells incubated with DMSO was measured on a 96-well plate (SPL, Pocheon, Korea) at wavelength of 595 nm and reference wavelength of 620 nm using a VERSA max plate leader (Molecular Devices, Sunnyvale, CA, USA).
Western blot
Electrophoresis of 20 µg protein in cell lysate was performed using Any kD or 7.5% Mini-PROTEAN TGX precast gels (Bio-Rad, Hercules, CA, USA) and tris-glycine buffer systems. These proteins on gels were then blotted onto polyvinylidene fluoride membranes as described previously [29,30,31,32]. These membranes were incubated with antibodies against phosphorylated p53 (p-p53; 1:2,500 dilution, catalog No. GTX70218, GeneTex, Irvine, TX, USA), total p53 (t-p53; 1:2,500 dilution, catalog No. sc-135630, Santa Cruz Biotechnology, Santa Cruz, CA, USA), BH3 interacting-domain death agonist (BID; 1:2,500 dilution, catalog No. sc-11423, Santa Cruz Biotechnology), phosphorylated-p38 (p-p38; 1:2,500 dilution, catalog No. 9211, Cell Signaling Technology, Beverly, MA, USA), total p38 (t-p38; 1:2,500 dilution, catalog No. 9212, Cell Signaling Technology), phosphorylated c-Jun N-terminal kinase (p-JNK; 1:2,500 dilution, catalog No. 9251, Cell Signaling Technology), total JNK (t-JNK; 1:5,000 dilution, catalog No. 9252, Cell Signaling Technology), phosphorylated extracellular signal-regulated kinase (p-ERK; 1:5,000 dilution, catalog No. 4370, Cell Signaling Technology), total ERK (t-ERK; 1:5,000 dilution, catalog No. sc-93, Santa Cruz Biotechnology), phosphorylated protein kinase B (p-AKT; 1:2,500 dilution, catalog No. 3787, Cell Signaling Technology), total AKT (t-AKT; 1:2,500 dilution, catalog No. sc-8312, Santa Cruz Biotechnology), manganese superoxide dismutase (MnSOD; 1:10,000 dilution, catalog No. sc-30080, Santa Cruz Biotechnology), copper/zinc superoxide dismutase (CuZnSOD; 1:10,000 dilution, catalog No. sc-11407, Santa Cruz Biotechnology), catalase (1:5,000 dilution, catalog No. sc-271803, Santa Cruz Biotechnology), 94 kDa glucoseregulated protein (GRP94; 1:5,000 dilution, catalog No. CSBPA00109A0Rb, CUSABIO, Wuhan, China), 78 kDa glucoseregulated protein (GRP78; 1:10,000 dilution, catalog No. CSB-PA010827YA01MO, CUSABIO), and β-actin (1:5,000 dilution, catalog No. sc-47778, Santa Cruz Biotechnology) overnight at 4℃, respectively. After washing, membranes were incubated with peroxidase anti-rabbit IgG antibodies (1:5,000 dilution, catalog No. WB-1000, Vector Laboratories, Burlingame, CA, USA) against p-p53, p-p38, t-p38, p-JNK, t-JNK, p-ERK, t-ERK, p-AKT, t-AKT, MnSOD, CuZnSOD, GRP94, and GRP78 antibodies or peroxidase anti-mouse IgG antibodies (1:5,000 dilution, catalog no. WB-2000, Vector Laboratories) against t-p53, catalase, and β-actin antibodies at room temperature for 60 minutes. After that, Western Lighting chemiluminescence reagent (NEL101, PerkinElmer, Boston, MA, USA) was used to detect proteins with an Azure c300 imaging system (Azure Biosystems, Dublin, CA, USA). Anti-β-actin antibody was used as a loading control for stripped membranes. Intensities of bands were quantified using AzureSpot analysis software (Azure Biosystems).
ROS production
An oxidative sensitive dye 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA) was used to measure ROS production as described previously [33]. Briefly, HK-2 cells were seeded onto a 24-well plate at a density of 105 cells/well and treated with or without H2O2 plus/minus CsA as indicated. After that, they were incubated with 20 µM DCFDA for 45 minutes at 37℃. After washing twice with PBS, 1% Triton X-100 was added to each well. Then 2′,7′-dichlorofluorescein intensity of 200 µl of cell lysate on a Nunc 96-well black plate (Thermo Fisher Scientific) was quantified with a SpectraMax i3 plate reader (Molecular Devices) using 485 nm for excitation and 535 nm for emission.
Mitochondrial membrane potential
After cells were seeds onto a 24-well plate at a density of 105 cells/well, mitochondrial membrane potential was measured as described previously [34,35]. Briefly, at the indicated time points, cells were incubated with 200 nM tetramethylrhodamine ethyl ester perchlorate (TMRE) in 0.1% DMSO for 20 minutes at 37℃. After washing six times with PBS, 1% Triton X-100 was added to each well. TMRE intensity for 200 µl cell lysate on a Nunc 96-well black plate (Thermo Fisher Scientific) was then quantified with the SpectraMax i3 plate reader (Molecular Devices) using 549 nm for excitation and 575 nm for emission.
Statistical analysis
Results are expressed as mean±standard deviation. Analysis of variance was used to compare data among groups using Systat SigmaPlot (Systat Software Inc., San Jose, CA, USA). Differences between two groups were assessed by two-tailed unpaired Student's t tests. P-values <0.05 were considered statistically significant.
Results
CsA enhances cell death induced by H2O2 injury in kidney proximal tubule epithelial cells
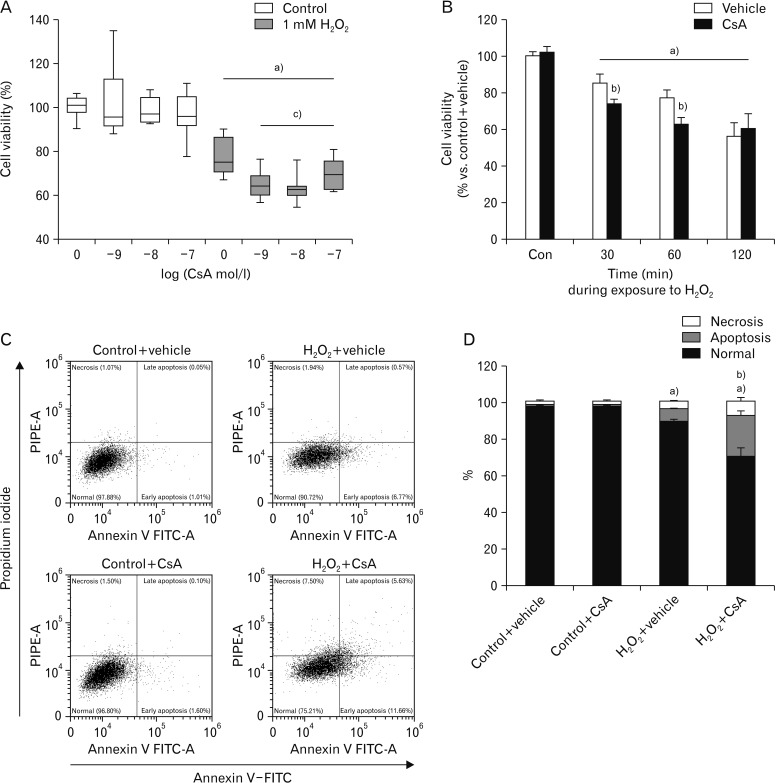
To determine whether CsA affects H2O2-induced cell death in kidney proximal tubule epithelial cells, viabilities of HK-2 cells undergoing pretreatment with CsA and subsequent exposure to H2O2 were determined. Consistent with previous results [36], 60-minute exposure to 1 mM H2O2 markedly decreased cell viability based on MTT assay results (Fig. 1A). Treatment with CsA at final concentrations of 1 nM to 100 nM did not significantly alter viabilities of control cells, but exogenous CsA further decreased viabilities of H2O2-exposed cells (Fig. 1A). The decline in viability after 30-minute exposure to H2O2 in CsA-treated cell was more severe than that in control cells (Fig. 1B). However, there was no significant difference in cell viability between CsA- and vehicle-treated groups after 120-minute exposure to H2O2 (Fig. 1B). To distinguish between apoptosis and necrosis in dead cells, flow cytometry was performed on HK-2 cells stained with FITC-conjugated annexin V and propidium iodide. Exposure to 1 mM H2O2 significantly induced apoptosis and necrosis (Fig. 1C, D). Upon H2O2 injury, treatment with 10 nM CsA further increased apoptosis and necrosis rather than vehicle-treated cells (Fig. 1C, D). However, exogenous CsA did not induce apoptosis and necrosis in control cells (Fig. 1C, D). These data suggest that CsA enhances apoptotic and necrotic cell deaths during early phase of H2O2 injury in kidney proximal tubule epithelial cells.
Fig. 1. Cyclosporin A (CsA) enhances hydrogen peroxide (H2O2) injury in human kidney proximal tubule epithelial cells. Human kidney proximal tubule epithelial HK-2 cells were cultured in RPMI 1640 until reaching 80% confluence. (A) HK-2 cells were treated with either CsA (1, 10, or 100 nM) or 1% dimethyl sulfoxide (vehicle) for 1 hour and then exposed to 1 mM H2O2 or distilled water (control) for 60 minutes. Cell viability was measured using MTT assay (n=9 wells from 3 experiments per condition). In box plots, whiskers represent the minimum and maximum; bases represent the interquartile range between the first and third quartiles; and midlines represent the median. (B) HK-2 cells were treated with either 10 nM CsA or vehicle for 60 minutes and then exposed to 1 mM H2O2 or control for 30, 60, or 120 minutes. Cell viability was measured using MTT assay (n=9 wells from 3 experiments per condition). (C, D) HK-2 cells were treated with either 10 nM CsA or vehicle for 60 minutes and then exposed to 1 mM H2O2 or control for 60 minutes. Cell death was analyzed by flow cytometry with an annexin V-FITC detection kit after treatment with FITC-conjugated annexin V and propidium iodide. The flow cytometry assay distinguishes among normal (lower left), early apoptosis (lower right), late apoptosis (upper right), and necrosis (upper left). Three experiments were performed to evaluate the cell death. In each experiment, three samples per experimental condition were included. a)P<0.05 vs. control. b)P<0.05 vs. vehicle. c)P<0.01 vs. 0 mol/l.
CsA increases p53 activation and BID expression after H2O2 injury in kidney proximal tubule epithelial cells
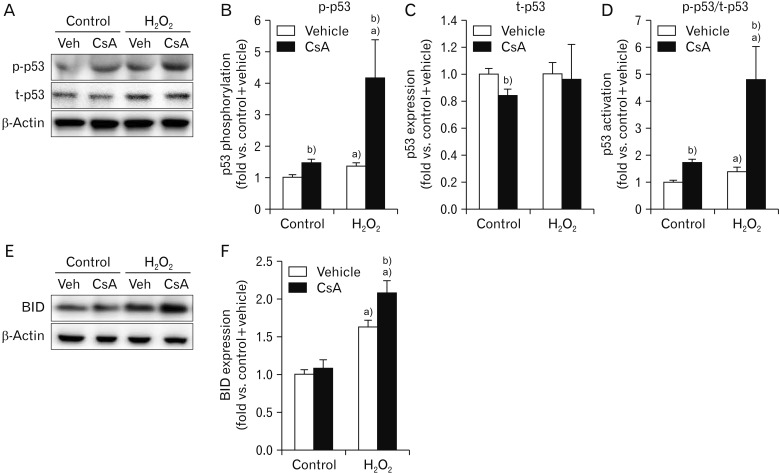
Tumor suppressor p53 is known to be upregulated and activated by phosphorylation in response to a number of cellular stresses including H2O2 injury [37]. The upregulation and activation of p53 subsequently induce cell death through apoptosis or necrosis [37]. It has been reported that treatment with CsA upregulates and activates p53 in rat C6 glioma cells and mouse embryo fibroblasts [38], while it conversely decreases the number of p53-positive cells induced by ultraviolet-B irradiation in mouse dermis [39]. To examine whether treatment with CsA might alter the activation and expression of p53 in kidney proximal tubule epithelial cells, we measured phosphorylated and total forms of p53 protein in CsA-treated HK-2 cells. Phosphorylation level of p53 was significantly increased in control cells at 60 minutes after treatment with CsA compared to that in vehicle-treated control cells (Fig. 2A, B). In contrast, total expression level of p53 was significantly decreased by CsA under the same condition (Fig. 2A, C). Although CsA decreased p53 expression in control cells, the ratio of p53 phosphorylation to its total expression was increased by treatment with CsA in control cells, indicating CsA-induced p53 activation (Fig. 2A, D). Next, we assessed CsA-induced p53 activation upon H2O2 injury. Consistent with previous data in mouse kidney mesangial cells [40], our results revealed that H2O2 significantly induced p53 phosphorylation, but not its total expression, in vehicle-treated cells (Fig. 2A–C), resulting in an increment of p53 activation based on the ratio of p53 phosphorylation to its total expression (Fig. 2A, D). Upon H2O2 injury, treatment with CsA markedly augmented the phosphorylation level of p53, but not its total expression (Fig. 2A–C). Because of this, CsA-treated cells showed striking increment in p53 activation after H2O2 injury (Fig. 2A, D), indicating that CsA increases H2O2-induced p53 activation in kidney proximal tubule epithelial cells. It is known that p53 activation results in apoptosis and that p53 transcriptionally transactivates a number of proapoptotic proteins [41]. Thus, we tested whether BID of p53 downstream genes was further upregulated by CsA after H2O2 injury in HK-2 cells. As shown in Fig. 2E and F, CsA caused further increase in BID upregulation induced by H2O2 in these cells. However, CsA did not alter BID expression in control cells (Fig. 2E, F). Taken together, these data suggest that CsA enhances H2O2-induced p53 signaling in kidney proximal tubule epithelial cells.
Fig. 2. Cyclosporin A (CsA) increases p53 activation and BH3 interacting-domain death agonist (BID) expression after H2O2 injury in human kidney proximal tubule epithelial cells. HK-2 cells were treated with either 10 nM CsA or vehicle for 60 minutes and then exposed to 1 mM H2O2 or control for 60 minutes (n=6 experiments per condition). (A) Phosphorylation level of p53 (p-p53, 53 kDa) and total expression level of p53 (t-p53, 53 kDa) were measured by western blot analysis. Antibody of β-actin (43 kDa) as a loading control was used for normalization. (B–D) Intensities of p-p53 and t-p53 protein bands were quantified using the AzureSpot software. (E) BID (22 kDa) expression was measured by western blot analysis. Antibody of β-actin (43 kDa) as a loading control was used for normalization. (F) Intensity of BID protein expression was quantified using the AzureSpot software. a)P<0.05 vs. control. b)P<0.05 vs. vehicle.
CsA does not alter activation of mitogen-activated protein kinases or AKT in kidney proximal tubule epithelial cells
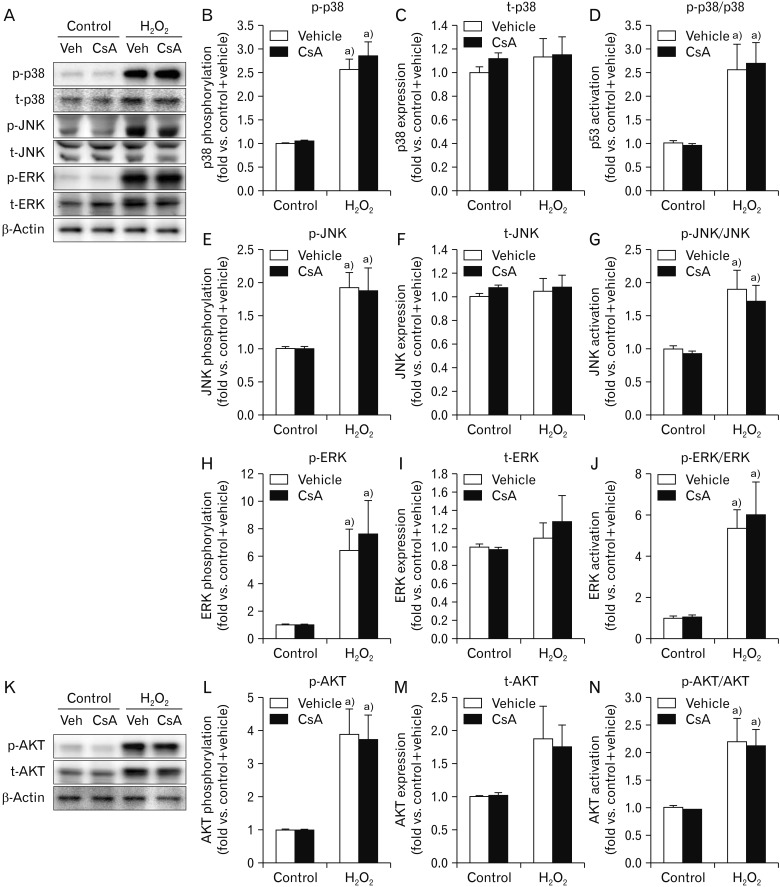
One well-documented result of H2O2 injury is mitogen-activated protein kinase (MAPK) activation in various cell types [42]. Our results showed that H2O2 significantly enhanced phosphorylation levels of p38, JNK and ERK, but did not their expression levels, resulting in significant activations of MAPKs based on the high ratio of phosphorylation level to total expression level in MAPKs (Fig. 3A–J). However, CsA did not alter their phosphorylation, total expression, or consequential activation in H2O2- or vehicle-treated cells (Fig. 3A–J). On the other hand, CsA can also activate AKT in human keratinocyte cells [43]. Thus, we determined whether CsA induced the activation of AKT in kidney proximal tubule epithelial cells. Although H2O2-induced AKT phosphorylation, expression, and consequential activation in HK-2 cells were confirmed in a previous report [44], treatment with CsA did not alter its phosphorylation, expression, or consequential activation in kidney proximal tubule epithelial cells with or without H2O2 injury (Fig. 3K–N). These data indicate that CsA does not affect the activation of MAPKs or AKT in kidney proximal tubule epithelial cells, suggesting that CsA-enhanced cell death in H2O2-exposed cells is not implicated in their activations.
Fig. 3. Cyclosporin A (CsA) does not alter activations of mitogen-activated protein kinases and protein kinase B (AKT) after H2O2 injury in human kidney proximal tubule epithelial cells. HK-2 cells were treated with either 10 nM CsA or vehicle for 60 minutes and then exposed to 1 mM H2O2 or control for 60 minutes (n=6 experiments per condition). (A) Phosphorylation levels of p38 (p-p38, 43 kDa), c-Jun N-terminal kinase (p-JNK, 46 and 55 kDa), and extracellular signal-regulated kinase (p-ERK, 42 and 44 kDa), and their total expression levels (t-p38, p-JNK, and p-ERK) were measured by western blot analysis. Antibody of β-actin (43 kDa) as a loading control was used for normalization. (B, C, E, F, H, I) Intensities of p-p38, t-p38, p-JNK, total JNK (t-JNK), p-ERK, and total ERK (t-ERK) protein bands were quantified using the AzureSpot software. (D, G, J) Activations of p38, JNK, and ERK indicated by their respective ratio of phosphorylation level to total expression level. (K) AKT phosphorylation (p-AKT) and total expression (t-AKT) with a molecular mass of 56 to 62 kDa were measured by western blot analysis. Antibody of β-actin (43 kDa) as a loading control was used for normalization. (L, M) Intensities of p-AKT and t-AKT protein bands were quantified using the AzureSpot software. (N) AKT activation based on the ratio of phosphorylation to total expression. a)P<0.05 vs. control.
CsA increases ROS production after H2O2 injury in kidney proximal tubule epithelial cells
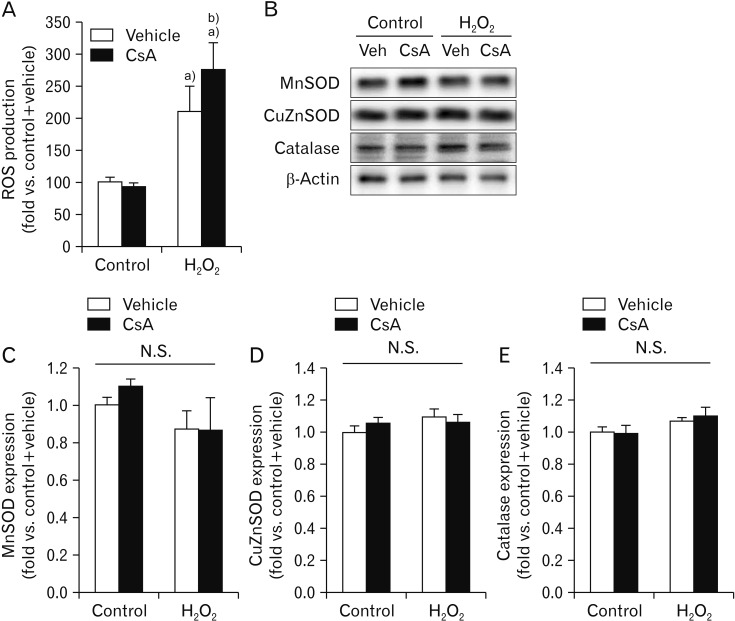
It is well-known that exogenous CsA induces oxidative stress in kidneys in vivo, leading to rapid loss of kidney function [45]. To examine whether CsA could also induce oxidative stress in kidney proximal tubule epithelial cells in vitro, we measured ROS levels in HK-2 cells with or without H2O2 injury using the oxidative sensitive DCFDA dye. The production of ROS was significantly increased by exposure to H2O2 in vehicle-treated cells (Fig. 4A). Upon H2O2 injury, CsA-treated cells had greater ROS production than vehicle-treated cells (Fig. 4A). However, treatment with CsA did not alter the level of ROS in control cells (Fig. 4A). We further determined expression levels of antioxidant enzymes using western blot analysis. Our results showed that expression levels of antioxidant enzymes were not altered by short exposure to H2O2 and/or treatment with CsA (Fig. 4B–E). Taken together, these data indicate that CsA treatment after H2O2 injury causes greater ROS production than H2O2 injury alone.
Fig. 4. Cyclosporin A (CsA) enhances reactive oxygen species (ROS) production induced by H2O2 in human kidney proximal tubule epithelial cells without altering expression level of antioxidant enzymes. HK-2 cells were treated with either 10 nM CsA or vehicle for 60 minutes and then exposed to 1 mM H2O2 or control for 60 minutes (n=3 experiments per condition). (A) ROS production was measured using oxidative sensitive dye 2′,7′-dichlorodihydrofluorescein diacetate. (B) Expression levels of manganese superoxide dismutase (MnSOD; 25 kDa), copper/zinc superoxide dismutase (CuZnSOD; 23 kDa), and catalase (64 kDa) were measured by western blot analysis. Antibody of β-actin (43 kDa) as a loading control was used for normalization. (C–E) Intensities of MnSOD, CuZnSOD, and catalase protein bands were quantified using the AzureSpot software. N.S., not significant. *P<0.05 vs. control, #P<0.05 vs. vehicle.
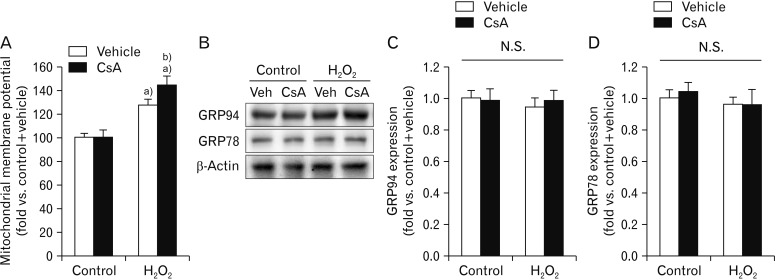
CsA increases mitochondrial depolarization induced by H2O2 injury in kidney proximal tubule epithelial cells
CsA can induce mitochondrial dysfunction by decreasing mitochondrial respiration in rat skeletal muscle cells [46] while it can conversely improve mitochondrial respiratory function by attenuating mitochondrial permeability transition in cardiomyocyte cells derived from dogs with heart failure [47]. To investigate the impact of CsA on mitochondrial function in kidney proximal tubule epithelial cells with or without H2O2 injury, we monitored mitochondrial membrane potentials using TMRE dye. It is known that exposure to H2O2 can increase the membrane potential of mitochondria during early phase in rat pulmonary microvascular endothelial cells, and then rapidly decreases the mitochondrial membrane potential after that [48]. In the present study, 60 minutes of exposure to 1 mM H2O2 significantly increased the mitochondrial membrane potential in vehicle-treated cells (Fig. 5A). The mitochondrial membrane potential was significantly increased by CsA in H2O2-exposed cells, compared to that in vehicle-treated H2O2-exposed cells (Fig. 5A). However, the mitochondrial membrane potential was not significantly different between vehicle-treated and CsA-treated cells in the control group (Fig. 5A). These data indicate that CsA worsen the mitochondrial depolarization induced by H2O2 injury in kidney proximal tubule epithelial cells. In addition to mitochondrial dysfunction, we assessed whether CsA was implicated in endoplasmic reticulum (ER) stress in kidney proximal tubule epithelial cells after H2O2 injury, as identified by alterations in GRP94 and GRP78 expressions. Expression levels of GRP94 or GRP78 were not altered by either exposure to H2O2 or treatment with CsA in HK-2 cells (Fig. 5B–D), suggesting that H2O2- or CsA-induced cell death did not involve ER stress in kidney proximal tubule epithelial cells.
Fig. 5. Cyclosporin A (CsA) increases mitochondrial depolarization induced by H2O2 in human kidney proximal tubule epithelial cells. HK-2 cells were treated with either 10 nM CsA or vehicle for 60 minutes and then exposed to 1 mM H2O2 or control for 60 minutes. (A) Percentage of mitochondrial membrane potential was measured using tetramethylrhodamine ethyl ester perchlorate dye (n=9 wells per 3 experiments per condition). The mitochondrial membrane potential in the group with control plus vehicle was taken as 100%. a)P<0.05 vs. control. b)P<0.05 vs. vehicle. (B) Expression levels of 94 kDa glucose-regulated protein (GRP94) and 78 kDa glucose-regulated protein (GRP78) were measured by western blot analysis. Antibody of β-actin (43 kDa) as a loading control was used for normalization. (C, D) Intensities of GRP94 and GRP78 protein bands were quantified using the AzureSpot software (n=6 experiments per condition). N.S., not significant.
Discussion
ROS has an important role in a variety of kidney disease models. H2O2, a diffusible reactive oxygen metabolite formed by either enzyme-catalyzed or spontaneous dismutation of superoxide anion, has been implicated in the pathogenesis of tissue damage in acute kidney injury including ischemia reperfusion injury [49,50,51] and cisplatin nephrotoxicity [16,17,18]. H2O2 inflicts oxidative stress at multiple cellular sites either directly or indirectly through generation of more reactive intermediates [12], acting as an indirect activator of p53, MAPK, and AKT [52,53], and modulating mitochondrial function [54]. Thus, H2O2 is toxic when it is administered to intact kidney or cultured kidney tubular cells [55], leading to cell death. Our present data consistently revealed H2O2-induced p53 activation, MAPK activation, AKT activation, oxidative stress, mitochondrial dysfunction, and subsequent cell death in kidney proximal tubule epithelial cells.
CsA can inhibit mitochondria-mediated cell death, since it binds to cyclophilin D and subsequently blocks the formation of mitochondrial permeability transition pore [10], but can conversely kill kidney parenchymal cells, since it induces nephrotoxicity through glomerular filtration rate reduction and immune cell infiltration [8,9]. If so, how does CsA contribute to the fate of kidney tubular cells upon H2O2 injury developed in acute kidney injury? In a previous study, exogenous H2O2 induced the permeability transition pore formation in mitochondria of subcutaneous connective tissue-derived cells while pretreatment with CsA significantly attenuated the formation of mitochondrial permeability transition pore and subsequently apoptotic cell death [56]. Contrary to our expectations, the present study revealed that pretreatment with CsA worsened mitochondrial dysfunction and cell mortality induced by exposure to H2O2 in human kidney proximal tubule epithelial cells. These data suggest that, even if CsA can efficiently bind to cyclophilin D, CsA-induced other signaling pathways may cause more severe damage and higher mortality in kidney proximal tubule epithelial cells.
The activation of p53 or the expression of its transcriptional targets including Bax and BID occurs in the response to stress-inducing agents including H2O2 injury [37,57,58]. Although one previous study has reported that CsA can protect mouse dermis against ultraviolet-B irradiation through p53 downregulation [39], several investigators have shown that CsA activates p53 in various cell types including rat C6 glioma cells [38], mouse embryo fibroblasts [38], human keratinocytes [59], and human skin fibroblasts [59]. In the present study, we found that CsA activated p53 reflected by increases in its phosphorylation and its target gene BID expression, thus triggering cell death of kidney proximal tubule epithelial cells after exposure to H2O2. The p53 protein is required for cellular apoptotic response to oxidative stress by hydrogen peroxide, supporting the idea that p53 function is critical for cell death induced by oxidative stress [60]. Oxidative stress is also an important mediator of CsA-induced cell injury. It is increased in failing transplanted kidneys from patients treated with CsA [45,61,62]. In support of these reports, the present study showed that CsA-induced p53 activation was implicated in ROS production increased by treatment with CsA during H2O2 injury. In response to oxidative stress, activated p53 does not only binds DNA to trigger apoptotic cell death by transactivating several genes [63], but also accumulates in the mitochondrial matrix to trigger necrotic cell death by opening the mitochondrial permeability transition pore [37]. CsA is a well-known cyclophilin D inhibitor which is a downstream player in cell death induced by the formation of mitochondrial permeability transition pore [10]. However, it has been reported that even in cyclophilin D-deficient cells, the mitochondrial permeability transition pore can still open in response to a strong stimulus such as oxidative stress [10,64], indicating that cyclophilin D is a regulator but not necessarily a component of the mitochondrial permeability transition pore. Based on previous reports, our data suggest that the fate of kidney proximal tubule epithelial cells is determined by CsA-induced cell death through the ROS-p53 axis rather than CsA-induced cell survival by inhibiting cyclophilin D-dependent mitochondrial permeability transition.
Antioxidants including vitamin E, melatonin, and other indolic compounds inhibit lipid peroxidation in animal models of CsA-induced nephrotoxicity [65,66,67], suggesting a role of oxidative stress in CsA-induced damage. Furthermore, it has been reported that CsA induces a dose-dependent increase in oxidants and lipid peroxidation [68]. Consistent with previous studies, the present study showed that ROS production was more enhanced by treatment with CsA than that by vehicle in H2O2-exposed kidney proximal tubule epithelial cells. Considering the well-known relationship between oxidative stress and kidney tubular cell death [69,70,71], our data suggest that CsA-induced ROS production worsen kidney proximal tubular cell damage after exposure to H2O2 through cell death.
Conclusively, the present study shows that CsA aggravates cell death induced by H2O2 injury. This phenomenon is implicated in p53 activation and ROS production stimulated by CsA. These findings may be of clinical importance for pharmacological intervention of CsA-treated organ transplant patients.
Acknowledgements
This work was supported by a research grant from the Jeju National University Hospital Research Fund of Jeju National University in 2017.
Footnotes
- Conceptualization: JK.
- Data acquisition: DM.
- Data analysis or interpretation: DM, JK.
- Critical revision of the manuscript: JK.
- Approval of the final version of the manuscript: all authors.
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Borel JF, Feurer C, Gubler HU, Stähelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6:468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 2.White DJ, Calne RY. The use of cyclosporin A immunosuppression in organ grafting. Immunol Rev. 1982;65:115–131. doi: 10.1111/j.1600-065x.1982.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi N, Hayano T, Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989;337:473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- 4.Pereira MJ, Palming J, Rizell M, Aureliano M, Carvalho E, Svensson MK, Eriksson JW. The immunosuppressive agents rapamycin, cyclosporin A and tacrolimus increase lipolysis, inhibit lipid storage and alter expression of genes involved in lipid metabolism in human adipose tissue. Mol Cell Endocrinol. 2013;365:260–269. doi: 10.1016/j.mce.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Nazareth W, Yafei N, Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J Mol Cell Cardiol. 1991;23:1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- 6.Bunjes D, Hardt C, Rollinghoff M, Wagner H. Cyclosporin A mediates immunosuppression of primary cytotoxic T cell responses by impairing the release of interleukin 1 and interleukin 2. Eur J Immunol. 1981;11:657–661. doi: 10.1002/eji.1830110812. [DOI] [PubMed] [Google Scholar]
- 7.Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984;311:699–705. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- 8.Thomas SE, Andoh TF, Pichler RH, Shankland SJ, Couser WG, Bennett WM, Johnson RJ. Accelerated apoptosis characterizes cyclosporine-associated interstitial fibrosis. Kidney Int. 1998;53:897–908. doi: 10.1111/j.1523-1755.1998.00835.x. [DOI] [PubMed] [Google Scholar]
- 9.Young BA, Burdmann EA, Johnson RJ, Alpers CE, Giachelli CM, Eng E, Andoh T, Bennett WM, Couser WG. Cellular proliferation and macrophage influx precede interstitial fibrosis in cyclosporine nephrotoxicity. Kidney Int. 1995;48:439–448. doi: 10.1038/ki.1995.312. [DOI] [PubMed] [Google Scholar]
- 10.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 11.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salahudeen AK, Clark EC, Nath KA. Hydrogen peroxideinduced renal injury: a protective role for pyruvate in vitro and in vivo. J Clin Invest. 1991;88:1886–1893. doi: 10.1172/JCI115511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Kil IS, Seok YM, Yang ES, Kim DK, Lim DG, Park JW, Bonventre JV, Park KM. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice: a role for manganese superoxide dismutase. J Biol Chem. 2006;281:20349–20356. doi: 10.1074/jbc.M512740200. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Kim KY, Jang HS, Yoshida T, Tsuchiya K, Nitta K, Park JW, Bonventre JV, Park KM. Role of cytosolic NADP+-dependent isocitrate dehydrogenase in ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2009;296:F622–F633. doi: 10.1152/ajprenal.90566.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009;297:F461–F470. doi: 10.1152/ajprenal.90735.2008. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Long KE, Tang K, Padanilam BJ. Poly(ADP-ribose) polymerase 1 activation is required for cisplatin nephrotoxicity. Kidney Int. 2012;82:193–203. doi: 10.1038/ki.2012.64. [DOI] [PubMed] [Google Scholar]
- 17.Park S, Yoon SP, Kim J. Cisplatin induces primary necrosis through poly(ADP-ribose) polymerase 1 activation in kidney proximal tubular cells. Anat Cell Biol. 2015;48:66–74. doi: 10.5115/acb.2015.48.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoon SP, Kim J. Exogenous spermidine ameliorates tubular necrosis during cisplatin nephrotoxicity. Anat Cell Biol. 2018;51:189–199. doi: 10.5115/acb.2018.51.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J, Imig JD, Yang J, Hammock BD, Padanilam BJ. Inhibition of soluble epoxide hydrolase prevents renal interstitial fibrosis and inflammation. Am J Physiol Renal Physiol. 2014;307:F971–F980. doi: 10.1152/ajprenal.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Padanilam BJ. Loss of poly(ADP-ribose) polymerase 1 attenuates renal fibrosis and inflammation during unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2011;301:F450–F459. doi: 10.1152/ajprenal.00059.2011. [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Padanilam BJ. Renal nerves drive interstitial fibrogenesis in obstructive nephropathy. J Am Soc Nephrol. 2013;24:229–242. doi: 10.1681/ASN.2012070678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J, Yoon SP, Toews ML, Imig JD, Hwang SH, Hammock BD, Padanilam BJ. Pharmacological inhibition of soluble epoxide hydrolase prevents renal interstitial fibrogenesis in obstructive nephropathy. Am J Physiol Renal Physiol. 2015;308:F131–F139. doi: 10.1152/ajprenal.00531.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 24.Yoon SP, Kim J. Exogenous CGRP upregulates profibrogenic growth factors through PKC/JNK signaling pathway in kidney proximal tubular cells. Cell Biol Toxicol. 2018;34:251–262. doi: 10.1007/s10565-017-9399-4. [DOI] [PubMed] [Google Scholar]
- 25.Kim J. Spermidine rescues proximal tubular cells from oxidative stress and necrosis after ischemic acute kidney injury. Arch Pharm Res. 2017;40:1197–1208. doi: 10.1007/s12272-017-0957-3. [DOI] [PubMed] [Google Scholar]
- 26.Kim J, Padanilam BJ. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int. 2015;87:350–358. doi: 10.1038/ki.2014.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ammar NM, El-Hawary SS, Mohamed DA, Afifi MS, Ghanem DM, Awad G. Phytochemical and biological studies of Tribulus terrestris L. growing in Egypt. Int J Pharmacol. 2018;14:248–259. [Google Scholar]
- 28.Ali A, Kim MJ, Kim MY, Lee HJ, Roh GS, Kim HJ, Cho GJ, Choi WS. Quercetin induces cell death in cervical cancer by reducing O-GlcNAcylation of adenosine monophosphate-activated protein kinase. Anat Cell Biol. 2018;51:274–283. doi: 10.5115/acb.2018.51.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim J. Spermidine is protective against kidney ischemia and reperfusion injury through inhibiting DNA nitration and PARP1 activation. Anat Cell Biol. 2017;50:200–206. doi: 10.5115/acb.2017.50.3.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J. Poly(ADP-ribose) polymerase activation induces high mobility group box 1 release from proximal tubular cells during cisplatin nephrotoxicity. Physiol Res. 2016;65:333–340. doi: 10.33549/physiolres.932948. [DOI] [PubMed] [Google Scholar]
- 31.Lee JS, Lim JY, Kim J. Mechanical stretch induces angiotensinogen expression through PARP1 activation in kidney proximal tubular cells. In Vitro Cell Dev Biol Anim. 2015;51:72–78. doi: 10.1007/s11626-014-9809-3. [DOI] [PubMed] [Google Scholar]
- 32.Park Y, Tae HJ, Cho JH, Kim IS, Ohk TG, Park CW, Moon JB, Shin MC, Lee TK, Lee JC, Park JH, Ahn JH, Kang SH, Won MH, Cho JH. The relationship between low survival and acute increase of tumor necrosis factor alpha expression in the lung in a rat model of asphyxial cardiac arrest. Anat Cell Biol. 2018;51:128–135. doi: 10.5115/acb.2018.51.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 34.Song H, Yoon SP, Kim J. Poly(ADP-ribose) polymerase regulates glycolytic activity in kidney proximal tubule epithelial cells. Anat Cell Biol. 2016;49:79–87. doi: 10.5115/acb.2016.49.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoon SP, Kim J. Poly(ADP-ribose) polymerase 1 contributes to oxidative stress through downregulation of sirtuin 3 during cisplatin nephrotoxicity. Anat Cell Biol. 2016;49:165–176. doi: 10.5115/acb.2016.49.3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye J, Li J, Yu Y, Wei Q, Deng W, Yu L. L-carnitine attenuates oxidant injury in HK-2 cells via ROS-mitochondria pathway. Regul Pept. 2010;161:58–66. doi: 10.1016/j.regpep.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 37.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pyrzynska B, Serrano M, Martínez-A C, Kaminska B. Tumor suppressor p53 mediates apoptotic cell death triggered by cyclosporin A. J Biol Chem. 2002;277:14102–14108. doi: 10.1074/jbc.M104443200. [DOI] [PubMed] [Google Scholar]
- 39.Sugie N, Fujii N, Danno K. Cyclosporin-A suppresses p53-dependent repair DNA synthesis and apoptosis following ultraviolet-B irradiation. Photodermatol Photoimmunol Photomed. 2002;18:163–168. doi: 10.1034/j.1600-0781.2002.00765.x. [DOI] [PubMed] [Google Scholar]
- 40.Kume S, Haneda M, Kanasaki K, Sugimoto T, Araki S, Isono M, Isshiki K, Uzu T, Kashiwagi A, Koya D. Silent information regulator 2 (SIRT1) attenuates oxidative stress-induced mesangial cell apoptosis via p53 deacetylation. Free Radic Biol Med. 2006;40:2175–2182. doi: 10.1016/j.freeradbiomed.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 41.Yu J, Zhang L, Hwang PM, Rago C, Kinzler KW, Vogelstein B. Identification and classification of p53-regulated genes. Proc Natl Acad Sci U S A. 1999;96:14517–14522. doi: 10.1073/pnas.96.25.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guyton KZ, Liu Y, Gorospe M, Xu Q, Holbrook NJ. Activation of mitogen-activated protein kinase by H2O2. Role in cell survival following oxidant injury. J Biol Chem. 1996;271:4138–4142. doi: 10.1074/jbc.271.8.4138. [DOI] [PubMed] [Google Scholar]
- 43.Han W, Ming M, He TC, He YY. Immunosuppressive cyclosporin A activates AKT in keratinocytes through PTEN suppression: implications in skin carcinogenesis. J Biol Chem. 2010;285:11369–11377. doi: 10.1074/jbc.M109.028142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreucci M, Fuiano G, Presta P, Lucisano G, Leone F, Fuiano L, Bisesti V, Esposito P, Russo D, Memoli B, Faga T, Michael A. Downregulation of cell survival signalling pathways and increased cell damage in hydrogen peroxide-treated human renal proximal tubular cells by alpha-erythropoietin. Cell Prolif. 2009;42:554–561. doi: 10.1111/j.1365-2184.2009.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf A, Clemann N, Frieauff W, Ryffel B, Cordier A. Role of reactive oxygen formation in the cyclosporin-A-mediated impairment of renal functions. Transplant Proc. 1994;26:2902–2907. [PubMed] [Google Scholar]
- 46.Hokanson JF, Mercier JG, Brooks GA. Cyclosporine A decreases rat skeletal muscle mitochondrial respiration in vitro. Am J Respir Crit Care Med. 1995;151:1848–1851. doi: 10.1164/ajrccm.151.6.7767529. [DOI] [PubMed] [Google Scholar]
- 47.Sharov VG, Todor A, Khanal S, Imai M, Sabbah HN. Cyclosporine A attenuates mitochondrial permeability transition and improves mitochondrial respiratory function in cardiomyocytes isolated from dogs with heart failure. J Mol Cell Cardiol. 2007;42:150–158. doi: 10.1016/j.yjmcc.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madesh M, Hawkins BJ, Milovanova T, Bhanumathy CD, Joseph SK, Ramachandrarao SP, Sharma K, Kurosaki T, Fisher AB. Selective role for superoxide in InsP3 receptor-mediated mitochondrial dysfunction and endothelial apoptosis. J Cell Biol. 2005;170:1079–1090. doi: 10.1083/jcb.200505022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon SP, Kim J. Poly(ADP-ribose) polymerase 1 activation links ischemic acute kidney injury to interstitial fibrosis. J Physiol Sci. 2015;65:105–111. doi: 10.1007/s12576-014-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim J, Kim JI, Na YK, Park KM. Intra-renal slow cell-cycle cells contribute to the restoration of kidney tubules injured by ischemia/reperfusion. Anat Cell Biol. 2011;44:186–193. doi: 10.5115/acb.2011.44.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim J, Kim JI, Jang HS, Park JW, Park KM. Protective role of cytosolic NADP(+)-dependent isocitrate dehydrogenase, IDH1, in ischemic pre-conditioned kidney in mice. Free Radic Res. 2011;45:759–766. doi: 10.3109/10715762.2011.577426. [DOI] [PubMed] [Google Scholar]
- 52.Niwa K, Inanami O, Yamamori T, Ohta T, Hamasu T, Kuwabara M. Redox regulation of PI3K/Akt and p53 in bovine aortic endothelial cells exposed to hydrogen peroxide. Antioxid Redox Signal. 2003;5:713–722. doi: 10.1089/152308603770380016. [DOI] [PubMed] [Google Scholar]
- 53.Wu GS. The functional interactions between the p53 and MAPK signaling pathways. Cancer Biol Ther. 2004;3:156–161. doi: 10.4161/cbt.3.2.614. [DOI] [PubMed] [Google Scholar]
- 54.Nulton-Persson AC, Szweda LI. Modulation of mitochondrial function by hydrogen peroxide. J Biol Chem. 2001;276:23357–23361. doi: 10.1074/jbc.M100320200. [DOI] [PubMed] [Google Scholar]
- 55.Yoshioka T, Bills T, Moore-Jarrett T, Greene HL, Burr IM, Ichikawa I. Role of intrinsic antioxidant enzymes in renal oxidant injury. Kidney Int. 1990;38:282–288. doi: 10.1038/ki.1990.197. [DOI] [PubMed] [Google Scholar]
- 56.Takeyama N, Miki S, Hirakawa A, Tanaka T. Role of the mitochondrial permeability transition and cytochrome C release in hydrogen peroxide-induced apoptosis. Exp Cell Res. 2002;274:16–24. doi: 10.1006/excr.2001.5447. [DOI] [PubMed] [Google Scholar]
- 57.Chen QM, Liu J, Merrett JB. Apoptosis or senescence-like growth arrest: influence of cell-cycle position, p53, p21 and bax in H2O2 response of normal human fibroblasts. Biochem J. 2000;347(Pt 2):543–551. doi: 10.1042/0264-6021:3470543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 59.Voskamp P, Bodmann CA, Koehl GE, Rebel HG, Van Olderen MG, Gaumann A, El Ghalbzouri A, Tensen CP, Bavinck JN, Willemze R, Geissler EK, De Gruijl FR. Dietary immunosuppressants do not enhance UV-induced skin carcinogenesis, and reveal discordance between p53-mutant early clones and carcinomas. Cancer Prev Res (Phila) 2013;6:129–138. doi: 10.1158/1940-6207.CAPR-12-0361. [DOI] [PubMed] [Google Scholar]
- 60.Yin Y, Terauchi Y, Solomon GG, Aizawa S, Rangarajan PN, Yazaki Y, Kadowaki T, Barrett JC. Involvement of p85 in p53-dependent apoptotic response to oxidative stress. Nature. 1998;391:707–710. doi: 10.1038/35648. [DOI] [PubMed] [Google Scholar]
- 61.Mohamadin AM, El-Beshbishy HA, El-Mahdy MA. Green tea extract attenuates cyclosporine A-induced oxidative stress in rats. Pharmacol Res. 2005;51:51–57. doi: 10.1016/j.phrs.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 62.Satyanarayana PS, Singh D, Chopra K. Quercetin, a bioflavonoid, protects against oxidative stress-related renal dysfunction by cyclosporine in rats. Methods Find Exp Clin Pharmacol. 2001;23:175–181. doi: 10.1358/mf.2001.23.4.634641. [DOI] [PubMed] [Google Scholar]
- 63.Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 64.Basso E, Fante L, Fowlkes J, Petronilli V, Forte MA, Bernardi P. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 65.Wang C, Salahudeen AK. Lipid peroxidation accompanies cyclosporine nephrotoxicity: effects of vitamin E. Kidney Int. 1995;47:927–934. doi: 10.1038/ki.1995.138. [DOI] [PubMed] [Google Scholar]
- 66.Longoni B, Migliori M, Ferretti A, Origlia N, Panichi V, Boggi U, Filippi C, Cuttano MG, Giovannini L, Mosca F. Melatonin prevents cyclosporine-induced nephrotoxicity in isolated and perfused rat kidney. Free Radic Res. 2002;36:357–363. doi: 10.1080/10715760290019381. [DOI] [PubMed] [Google Scholar]
- 67.Wang C, Salahudeen AK. Cyclosporine nephrotoxicity: attenuation by an antioxidant-inhibitor of lipid peroxidation in vitro and in vivo. Transplantation. 1994;58:940–946. doi: 10.1097/00007890-199410270-00014. [DOI] [PubMed] [Google Scholar]
- 68.Longoni B, Boschi E, Demontis GC, Marchiafava PL, Mosca F. Regulation of Bcl-2 protein expression during oxidative stress in neuronal and in endothelial cells. Biochem Biophys Res Commun. 1999;260:522–526. doi: 10.1006/bbrc.1999.0928. [DOI] [PubMed] [Google Scholar]
- 69.Kim J, Jung KJ, Park KM. Reactive oxygen species differently regulate renal tubular epithelial and interstitial cell proliferation after ischemia and reperfusion injury. Am J Physiol Renal Physiol. 2010;298:F1118–F1129. doi: 10.1152/ajprenal.00701.2009. [DOI] [PubMed] [Google Scholar]
- 70.Kim J, Jang HS, Park KM. Reactive oxygen species generated by renal ischemia and reperfusion trigger protection against subsequent renal ischemia and reperfusion injury in mice. Am J Physiol Renal Physiol. 2010;298:F158–F166. doi: 10.1152/ajprenal.00474.2009. [DOI] [PubMed] [Google Scholar]
- 71.Kim J, Park JW, Park KM. Increased superoxide formation induced by irradiation preconditioning triggers kidney resistance to ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2009;296:F1202–F1211. doi: 10.1152/ajprenal.90592.2008. [DOI] [PubMed] [Google Scholar]