Abstract
Introduction:
The extraction of salient information from the environment is impaired by the activation of dopamine receptors. Using rodent models, we previously reported that this perceptual deficit, as measured by the prepulse inhibition (PPI) of startle, is effectively opposed by inhibitors of the steroidogenic enzyme 5α-reductase (5αR). The specific 5αR isoenzyme and steroids implicated in these effects, however, remain unknown.
Methods:
The effects of the selective D1 dopamine receptor agonist SKF-82958 (SKF, 0.3 mg/kg, IP) and D2 receptor agonist quinpirole (QUIN, 0.5 mg/kg, IP) were tested in the startle reflex and PPI of knockout (KO) mice for either 5αR type 1 (5αR1) or type 2 (5αR2). Furthermore, we established whether these effects may be modified by the 5α-reduced steroids dihydroprogesterone (DHP), allopregnanolone (AP), dihydrotestosterone (DHT), 5α-androstane-3α,17β-diol (3α-diol), or androsterone. To test the mechanisms whereby 5αR products may alter the PPI-disrupting properties of D1 agonists, we studied the involvement of GABA-A and PXR, two receptors targeted by neuroactive steroids. Specifically, we tested the effects of SKF in combination with the GABA-A antagonist bicuculline, as well as in KO mice for the GABA-A δ subunit and PXR.
Results:
5αR1, but not 5αR2 knockout (KO) mice, were insensitive to the PPI-disrupting effects of SKF. This sensitivity was reinstated by AP (3 mg/kg, IP), but not other 5α-reduced steroids. The PPI deficits induced by SKF were not modified by bicuculline, δ-subunit KO mice and PXR KO mice.
Conclusions:
These results collectively suggest that 5αR1 enables the negative effects of D1 dopamine receptor activation on information processing via production of AP. The contribution of AP to the PPI-disrupting mechanisms of D1 receptor agonists, however, do not appear to be mediated by either GABA-A or PXR receptors.
Keywords: Allopregnanolone, dopamine, sensorimotor gating, D1 receptors, 5α-reductase, behavioral studies
1. INTRODUCTION
The efficiency of information processing is contingent on the ability to filter out irrelevant stimuli. Impairments of this function – termed sensorimotor gating - have been observed across a broad range of neuropsychiatric conditions, including schizophrenia, obsessive-compulsive disorder (OCD) and Tourette syndrome (TS) (Geyer et al., 2006). The best-validated operational index to measure sensorimotor gating integrity in humans and animal models is the prepulse inhibition (PPI) of the acoustic startle reflex, a cross-species phenotype corresponding to the reduction of the startle response following a weak pre-stimulus (Hoffman and Ison, 1980).
Due to its high cross-species translational validity, PPI has been extensively used in rodent models to screen putative antipsychotic agents and investigate the molecular and neurobiological underpinnings of schizophrenia and other gating disorders. A widely used rodent model to screen antipsychotic efficacy is based on PPI deficits induced by dopaminergic agonists (Geyer et al., 2001). In most mouse strains, D1-like receptor agonists disrupt PPI in an antipsychotic-sensitive fashion (Ralph-Williams et al., 2003); conversely, the effects of selective D2 receptor agonists are generally poor in this species, with the only exception of a few strains (Ralph and Caine, 2005).
Our group has shown that the PPI deficits induced by dopaminergic agonists is blocked by inhibitors of 5α-reductase (5αR) (Bortolato et al., 2008). This family of steroidogenic enzymes catalyze the saturation of the 4,5-double bond of the A ring of Δ4-3-ketosteroid substrates, including progesterone, androstenedione, and testosterone (Paba et al., 2011). This reaction leads to the synthesis of 5α-dihydrosteroids, including dihydroprogesterone (DHP), androstanedione (ASD), and dihydrotestosterone (DHT). In turn, these steroids are metabolized by 3α-hydroxysteroid oxidoreductase (3α HSOR) into 5α-pregnan-3α-ol-20-one (allopregnanolone; AP), 5α-androstan-3α-ol-17-one (androsterone), and 5α-androstane-3α,17β-diol (3α-diol) (Paba et al., 2011). Although the two main 5αR isoenzymes, termed 1 (5αR1) and 2 (5αR2), serve overlapping functional roles, they diverge with respect to several key characteristics, including localization throughout the organism, substrate affinity and pH optima (Paba et al., 2011).
We recently documented that, in mice and rats, the antipsychotic-like effects of the prototypical 5αR inhibitor finasteride is based on the opposition of the PPI-disrupting effects of D1 receptor agonists (Frau et al., 2013; 2016). However, as finasteride is not isoenzyme-specific in rodents (Thigpen and Russell, 1992), the specific implication of 5αR1 and 5αR2 with respect to the dopaminergic modulation of PPI remains elusive. Capitalizing on these premises, we aimed the present study at studying which 5αR isoenzyme is directly implicated in the modulation of dopaminergic responses in PPI, by testing the effects of D1- and D2-like receptor agonists in 5αR1 and 5αR2 knockout (KO) mice. Furthermore, we examined the specific steroid-based mechanisms involved in the observed mechanisms, by testing whether the effects of 5αR inactivation on PPI were reversed by the implementation of different 5αR products, including 5α-dihydro- and 3α,5α-tetrahydro steroids.
2. MATERIALS AND METHODS
2.1. Animals.
The experiments included in this study were performed on adult (3-month old) male mice. The following mutant lines were used: 5αR1, 5αR2, GABA-A subunit δ, and pregnane X receptor (PXR) knockout (KO). These mice and their wild-type (WT) littermates were generated by mating heterozygous sires and dams. Both 5αR1 and 5αR2 KO mouse progenitors were a kind donation from Dr. Mala Mahendroo (Southwestern University). Unless stated otherwise for specific experimental purposes, all mice were housed in groups of 4–5/cage, with at least 1 mouse/genotype, and had ad libitum access to food and water. Housing facilities were maintained at 22°C with a 12 h light/dark cycle (lights on at 06:00 AM hours and on at 06:00 PM). Experimental manipulations were carried out in the animals’ light cycle between 8:00 AM and 4:00 PM. All handling and experimental procedures were performed in compliance with the National Institute of Health guidelines and approved by the local Institutional Animal Care and Use Committees. To avoid potential confounds due to carry-over effects, each mouse was only used once throughout the study, with the only exception of the experiments aimed at assessing the behavioral effects of 3α-diol and androsterone in combination with SKF, for which animals were tested in counterbalanced order. Group sizes were defined based on power analyses run on preliminary studies.
2.2. Genotyping.
Mouse genotyping was performed by PCR. Samples of genomic DNA were extracted from tail biopsies acquired from mice at weaning (postnatal day 21). The following primers were used to identify 5αR1 KO mice: 1) GAT TGG GAA GAC AAT AGC AGG CAT GC 2) CCA GAC ACG AAC TTC CAC GCT TCT G 3) ATG GAG TTG GAT GAG TTG TGC. Reaction conditions were: 94°C × 1.5 min, 94°C × 20s, 55°C × 30s, 72°C × 2 min, 4°C × ∞, as previously described (Mahendroo et al., 2001). The following primers were used to identify 5αR2 KO mice: 1) GAT GAC CTC TCC GGG CTT CC 2) GAA TGT TCC AAG TCA CAG GC 3) CGC TTC TGA GGA GAG AAC TGA CTG A. Reaction conditions were: 94°C × 2 min, 94°C × 40s, 55°C × 40s, 72°C × 5 min, 4°C × ∞, as previously described (Mahendroo et al., 2001). The following primers were used to identify δ KO mice: 1) GCA GGC TGT CCT ACA ACC AT; 2) ATG CCA CTC CTG ATG CCT AC; 3) GGG GTT TGC TCG ACA TTG. Reaction conditions were: 94°C × 2 min, 94°C × 20s, 65°C × 15s, 68°C × 10s. The following primers were used to identify PXR KO: 1) CTG GTC ATC ACT GTT GCT GTA CCA; 2) GCA GCA TAG GAC AAG TTA TTC TAG AG; 3) CTA AAG CGC ATG CTC CAG ACT GC. Reaction conditions were: 95°C × 3 min, 95°C × 45s, 60°C × 30s, 72°C × 60s.
2.3. Drugs.
The following drugs were used in this study: the D1 receptor agonist SKF-82958 (SKF; Sigma Aldrich, St. Louis, MO, USA; 0.3 mg/kg, IP; 10 min before behavioral testing); the D2 receptor agonist quinpirole (QUIN) (Sigma Aldrich; 0.5 mg/kg, IP; 15 min before testing); the GABA-A receptor bicuculline (Sigma Aldrich; 1 mg/kg, IP; 15 min before testing); DHP (Sigma Aldrich; 3–10 mg/kg, IP; 15 min before testing); AP (Tocris Bioscience, Bristol, UK; 1–3 mg/kg, IP; 30 min before testing); DHT (Sigma; 3 mg/kg, IP; 15 min before testing); 3α-diol (Tocris; 3 mg/kg, IP; 15 min before testing); and androsterone (Tocris; 3 mg/kg, IP; 15 min before testing). SKF and QUIN were dissolved in saline. Bicuculline was dissolved in saline with few drops of concentrated acetic acid. DHP, AP, DHT, androsterone, and 3α-diol were dissolved in 2% DMSO, 3% Tween 80 and saline. Doses were based on previous studies on the effects of these compounds in PPI in mice (Yeomans et al., 2010; Frau et al., 2016) as well as pilot studies on the behavioral effects of steroids.
2.4. Acoustic startle reflex and PPI.
Startle testing was conducted as previously described (Godar et al., 2016). Briefly, the apparatus used for detection of startle reflexes (SR-LAB; San Diego Instruments, San Diego, CA, USA) consisted of five Plexiglas cages (diameter: 5 cm) in sound attenuated chambers with fan ventilation. Each cage was mounted on a piezoelectric accelerometric platform connected to an analogue digital converter. The response to each stimulus was recorded as 65 consecutive 1 ms readings. A dynamic calibration system was used to ensure comparable sensitivities across chambers. The startle testing protocol featured a 70-dB background white noise, and consisted of a 5 min acclimatization period, followed by three consecutive blocks of pulse, prepulse + pulse and ‘no stimulus’ trials. During the first and the third block, mice received only five pulse-alone trials of 115 dB. Conversely, in the second block mice were exposed to a pseudorandom sequence of 50 trials, consisting of 12 pulse-alone trials, 30 trials of pulse preceded by 73, 76 or 82 dB pre-pulses intensities (10 for each level of prepulse loudness) and eight no stimulus trials, where only the background noise was delivered. Intertrial intervals were selected randomly between 10 and 15 s. Sound levels were assessed using an A-scale setting. Percent PPI was calculated with the following formula:
The first five pulse-alone bursts were excluded from the calculation. As no interactions between prepulse levels and treatment were found in the statistical analysis, %PPI values were collapsed across prepulse intensity to represent average %PPI.
2.5. Locomotor Activity.
Locomotor behaviors were measured in a square force-plate actometer (28cm × 28cm) as previously described (Fowler et al., 2001). In brief, mice (n=8/genotype) were placed in the center and their behavior was monitored for 20 min for the baseline locomotor activity studies. In a subsequent study 5αR1 KO, HZ, and WT mice (n=8/genotype) were placed in the center and their behavior was monitored for 60 min before they were removed, treated with SKF, QUIN or vehicle and placed back into the actometer for an additional 120 min. Each force plate actometer consisted of four force transducers placed at the corners of each load plate. Transducers were sampled 100 times s−1, yielding a 0.01 s temporal resolution, a 0.2 g force resolution and a 2-mm spatial resolution. Custom software directed the timing and data-logging processes via a LabMaster interface (Scientific Solutions Inc., Mentor, OH, USA). Distance travelled was calculated as the sum of the distances between coordinates of the location of center of force recorded every 0.50 s over the recording session.
2.6. Statistical Analyses.
Data were tested for normality by the Kolmogorov-Smirnov test. Parametric statistical analyses were performed by multiway ANOVAs, followed by Tukey’s test for post-hoc comparisons. Significance was set at P = 0.05.
3. RESULTS
3.1. Genetic 5αR1 deficiency renders mice insensitive to the PPI-disruptive effects of D1 receptor activation.
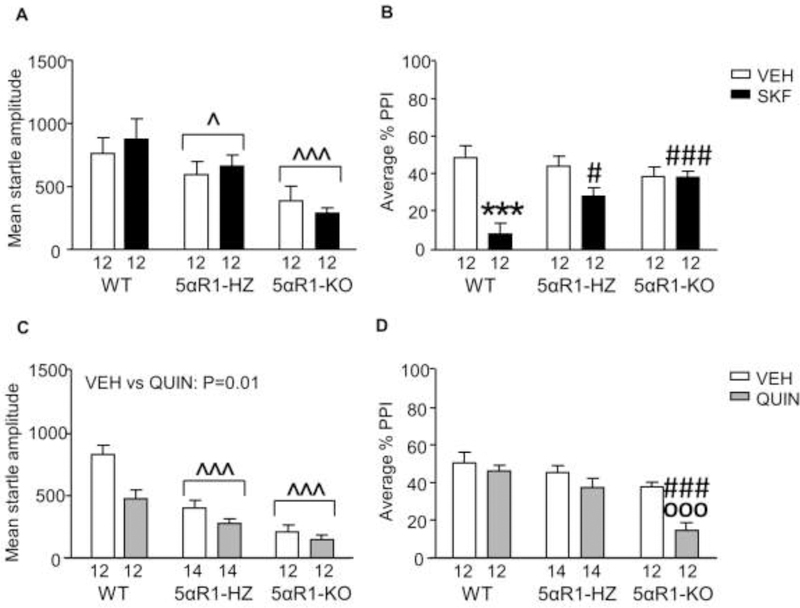
We first analyzed the effects of SKF (0.3 mg/kg, SC) on startle amplitude and PPI in 5αR1 KO and HZ mice, as compared to WT littermates (n=12/group). As described in Fig. 1A, WT mice exhibited higher startle values [Main effect for genotype: F(2,66)=8.00, P=0.0008] than HZ (P=0.02) and KO (P=0.0008; Tukey’s). However, SKF did not modify this parameter in either genotype. The analyses of PPI values (Fig. 1B) revealed that SKF reduced PPI in WT [genotype x treatment interaction: F(2,66) = 5.47, P=0.006; SKF vs vehicle: P=0.0003; Tukey’s), but not in either HZ or KO mice. The effects of SKF were significantly greater in WT mice than their HZ (P=0.03) and KO littermates (P=0.0003).
Fig. 1.
Responsiveness of 5αR1-deficient mice to the effects of D1 and D2 receptor agonists on startle reflex and prepulse inhibition (PPI). Data are shown as means ± SEM. ^P<0.05, ^^^P<0.001 in comparison with wildtype (WT) mice (Main effect of genotype). ***P<0.001 vs vehicle (VEH)-treated WT mice (genotype x treatment interaction). #,P<0.05, ###P<0.001 vs WT mice treated with dopaminergic agonists (genotype x treatment interaction); ooo,P<0.001 vs KO mice treated with VEH (genotype x treatment interaction). The number of mice in each group is indicated. Abbreviations: HZ, 5αR1 heterozygous; KO, 5αR1 knockout; SKF, SKF 82958 (0.3 mg/kg, IP); QUIN, QUIN (0.5 mg/kg, IP).
We then analyzed the effects of the D2 receptor agonist QUIN in 5αR1-deficient mice (n=12–14/group). Startle amplitude was reduced by both 5αR1 deficiency [Main effect of genotype: F(2,74) = 15.59, P < 0.0001; WT vs HZ: P=0.0009; WT vs KO: P=0.0001, Tukey’s] (Fig. 1C) and QUIN [Main effect of treatment: F(1,74) = 6.65, P = 0.01]. However, no interaction between these two factors was found. PPI analyses (Fig. 1D) surprisingly revealed that QUIN lowered PPI levels in KO mice [Genotype x treatment interaction: F(2,74)=3.48, P=0.04; QUIN- vs saline- treated KO mice: P=0.001; Tukey’s], but not in WT (QUIN-treated WT vs QUIN-treated KO: P=0.0002) or HZ (QUIN-treated HZ vs QUIN-treated KO: P=0.004) counterparts.
3.2. Genetic 5αR1 deficiency does not alter the locomotor effects of D1 or D2 receptor activation.
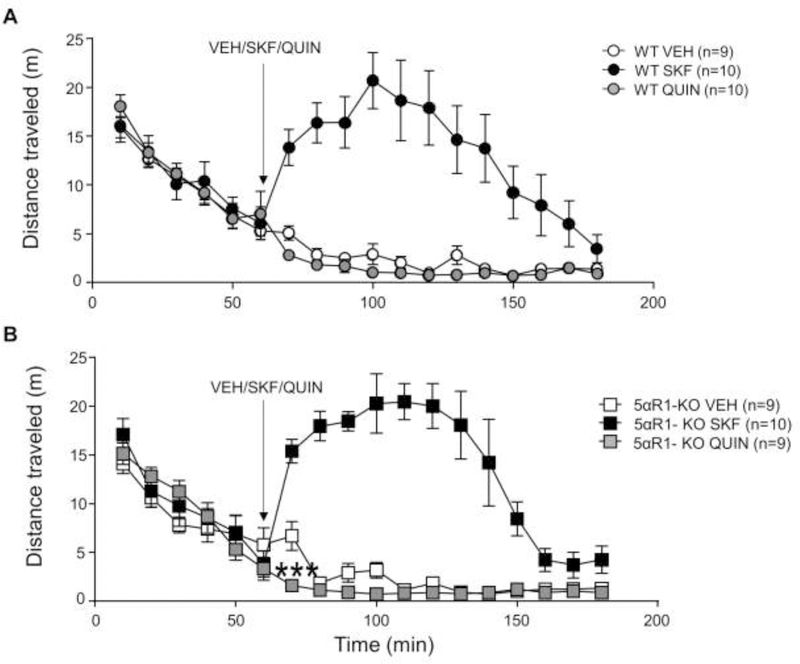
We next tested the locomotor effects of D1 and D2 receptor agonists on 5αR1 KO mice (Fig 2). As expected, the D1 receptor agonist SKF increased locomotor activity in both WT and KO mice for the 90-min period following injection (Fig 2A) [treatment x time interaction: F(12,624)=32.04, P<0.0001; Ps<0.001 for all comparisons with 0’; Tukey’s]. The locomotor effects of SKF, however, were comparable across genotypes. In contrast with SKF, QUIN was found to significantly reduce locomotor activity in both genotypes [treatment x time interaction: F(12,552)=3.69, P<0.0001]. In addition, unlike WT mice, KO littermates exhibited a significant hypolocomotor response of QUIN in the 10-min bin after injection [genotype x treatment x time interaction: F(24,552)=1.91, P=0.006; P=0.0001 for WT vs KO mice; Tukey’s]
Fig. 2.
Locomotor responses to dopamine D1 and D2 agonists in 5αR1 knockout (KO) mice. Mice were allowed to habituate to the force plate actometer for 60 minutes. At 60 minutes the mice were briefly removed and injected with vehicle (VEH), SKF 82958 (SKF, 0.3mg/kg, IP) or QUIN (QUIN, 0.5mg/kg, IP) and returned to the actometer. The responses of wild type (WT) mice (A) and 5αR1 KO littermates (B) are described. Data are shown as mean ± SEM. ***P<0.001 for comparisons between KO mice treated with either QUIN or vehicle at 10 min after injection. The number of mice in each group is indicated.
3.3. Genetic 5αR2 deficiency does not alter the sensitivity of mice to the PPI-disruptive effects of D1 receptor activation.
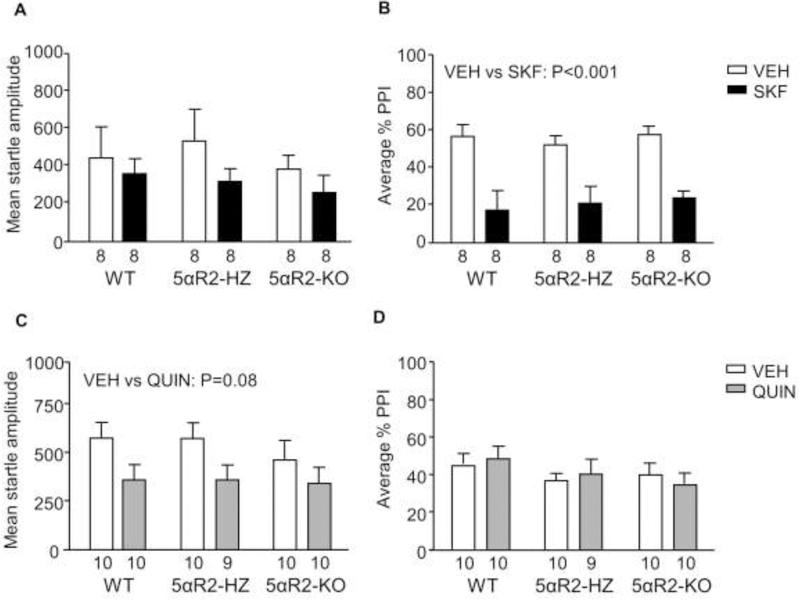
Contrary to our findings on 5αR1 KO mice, SKF did not affect startle amplitude in 5αR2-deficient mice (n=8/group; Fig. 3A); furthermore, 5αR2 KO and HZ mice were equally sensitive as their WT littermates to the PPI-disrupting effects of SKF (Fig 3B) [Main effect of treatment: F(1,41) = 38.19, P < 0.001]. Conversely, QUIN elicited a statistical trend towards a significant reduction in startle amplitude in 5αR2 KO mice and their littermates [Main effect of treatment (F(1,53) = 3.52, P = 0.08) (Fig. 3C)] (n=9–10/group); however, this drug did not elicit any significant PPI alterations in any genotype (Fig. 3D).
Fig. 3.
Responsiveness of 5αR2-deficient mice to the effects of D1 and D2 receptor agonists on startle reflex and prepulse inhibition (PPI). Data are shown as means ± SEM. The number of mice in each group is indicated. Abbreviations: WT, wildtype; HZ, 5αR1 heterozygous; KO, 5αR1 knockout; VEH, vehicle; SKF 82958 (SKF, 0.3 mg/kg, IP). QUIN, QUIN (0.5 mg/kg, IP).
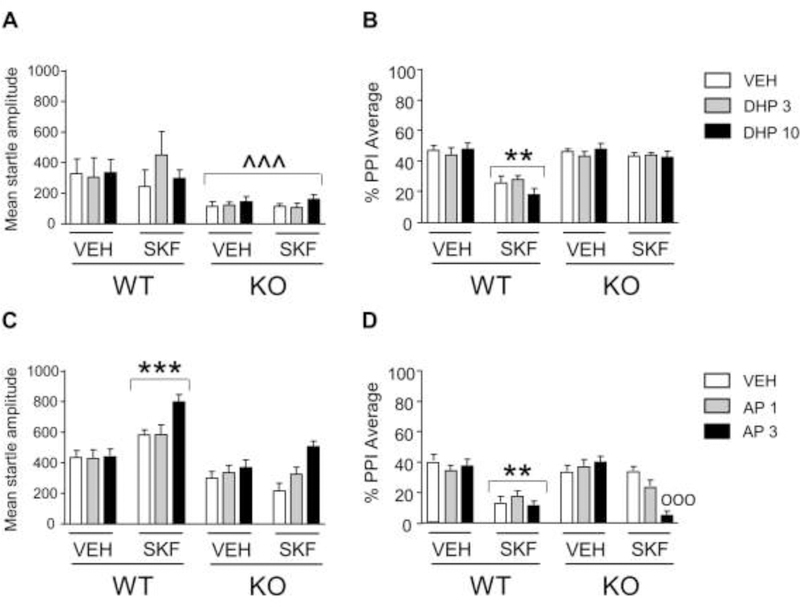
3.4. The insensitivity of 5αR1 KO mice to the PPI-disrupting effects of the D1 receptor agonist is restored by AP, but not by DHP.
We next tested whether the effects of 5αR1 deficiency on the PPI-disrupting properties of SKF could be reversed by administration of two of its pregnane products, namely DHP and its metabolite AP. Analyses were run by 3-way ANOVAs, with genotype, steroid treatment, and SKF as factors. In conformity with our previous results, the analysis of the effects of DHP (3 mg/kg, IP) (Fig. 4A) showed that KO mice exhibited lower startle values than those of WT [main effect of genotype: F(1,84)=17.08, P= 0.0001]. As expected, SKF significantly reduced PPI (Figs. 4B) in WT, but not KO mice [genotype x SKF interactions: DHP: F(1,84)=29.35, P= 0.00001; Ps<0.001 for comparison between SKF-treated KO and vehicle-treated KO as well as SKF-treated WT; Tukey’s test]; however, DHP did not modify the effects of SKF in either WT or KO mice. The analysis of the effects of AP on startle magnitude (Fig. 4C) revealed that SKF-treated WT mice exhibited an increased startle amplitude in comparison with both vehicle-treated WT and SKF-treated KO mice [genotype x SKF interaction: F(1,132)=13.60, P=0.0003; Ps =0.0008; Tukey’s test]. Furthermore, the combination of SKF and the 3 mg/kg of AP produced a significant enhancement of startle magnitude in comparison with each of all other treatment combinations [AP x SKF interaction: F(2,132)=5.77, P=0.004; Ps<0.001, Tukey’s test], irrespective of genotypes. As expected, the combination of SKF and vehicle in KO mice failed to cause PPI deficits, but the dose of 3 mg/kg of AP restored the sensitivity of these animals to the PPI-disrupting effects of SKF [genotype x AP x SKF interaction: F(2,132)=5.00, P=0.008; P=0.0002 for comparison between SKF-treated KO mice treated with AP or its vehicle; Fig. 4D].
Fig. 4.
Responsiveness of 5αR1 knockout (KO) mice to the effects of the D1 receptor agonist SKF 82958 (SKF; 0.3 mg/kg, IP) in combination with dihydroprogesterone (DHP, 3–10 mg/kg, IP; A-B; n=8/group) and allopregnanolone (AP, 1–3 mg/kg, IP; C-D; n=12/group), on startle reflex and prepulse inhibition (PPI). Data are shown as means ± SEM. ^^^, P<0.001 in comparison with wild-type (WT) mice (Main effect of genotype); **, P<0.01, ***, P<0.001 in comparison with VEH-treated WT mice (genotype x SKF interaction). ooo, P<0.001 in comparison with KO mice treated with SKF and vehicle (VEH) (genotype x SKF x AP interaction).
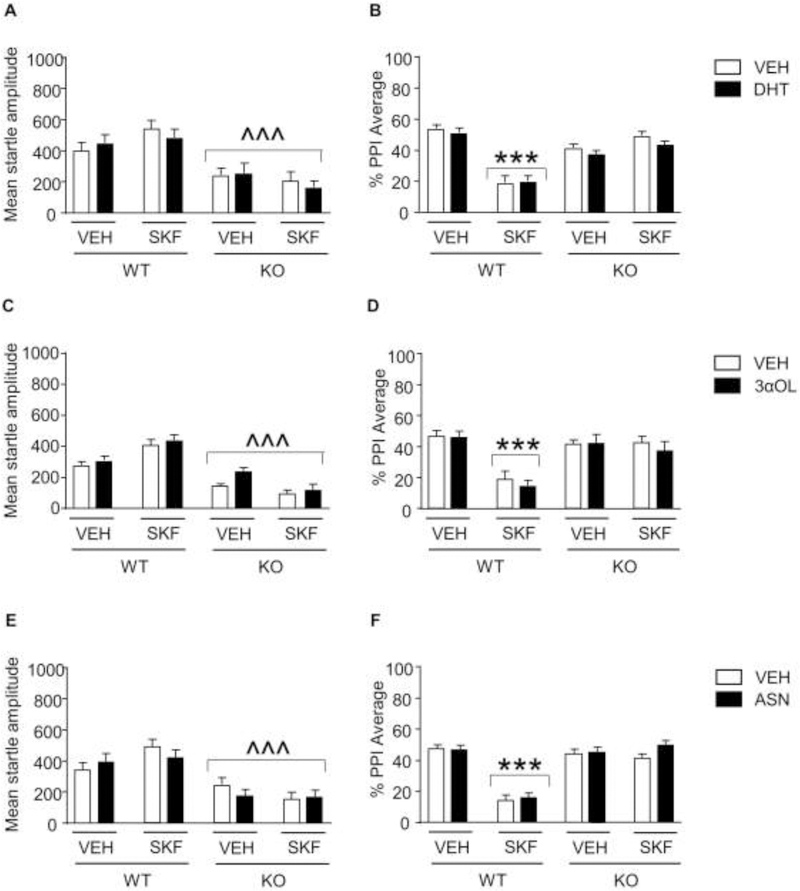
3.5. Androgen products of 5αR1 do not reinstate the PPI-disrupting effect of D1 receptor agonists in 5αR1 KO mice.
We next tested whether the effects of 5αR1 deficiency on the PPI-disrupting properties of SKF could be prevented by administration of 5α-reduced androgens, including DHT, androsterone, and 3α-diol. Analyses were run by 3-way ANOVAs, with genotype, steroid, and SKF treatments as factors. The analysis of the effects of DHT (3 mg/kg, IP) (Fig. 5A), 3α-diol (3 mg/kg, IP) (Fig. 5C), and androsterone (3 mg/kg, IP) (Fig. 5E) on startle magnitude showed a significant reduction in startle values in KO mice, as compared with their WT littermates [Main genotype effect: DHT: F(1,88)=57.00, P<0.0001; 3α-diol: F(1,88)=171.45, P<0.0001; androsterone: F(1,88)=63.32, P<0.0001]. As expected, SKF significantly reduced PPI (Figs. 5B, 5D, and 5F) in WT, but not KO mice [genotype x SKF interactions: DHT: F(1,88)= 159.65 P<0.0001; 3α-diol: F(1,88)=79.43, P<0.0001; Androsterone: F(1,88)=121.98, P<0.0001; Ps<0.001 for comparisons between SKF-treated KO and WT, Tukey’s test]; however, the PPI disruption induced by the D1 receptor agonist was not modified by any of these steroids.
Fig. 5.
Responsiveness of 5αR1 knockout (KO) mice to the effects of the D1 receptor agonist SKF 82958 (SKF; 0.3 mg/kg, IP) in combination with dihydrotestosterone (DHT; A-B), 5α-androstane-3α,17β-diol (3αOL; C-D), and androsterone (ASN, 3 mg/kg, IP; E-F) on startle reflex and prepulse inhibition (PPI). N=12/group for all experiments. Data are shown as means ± SEM. ^^^, P<0.001 in comparison with wild-type (WT) mice (Main effect of genotype); ***, P<0.001 in comparison with VEH-treated WT mice (genotype x SKF interaction).
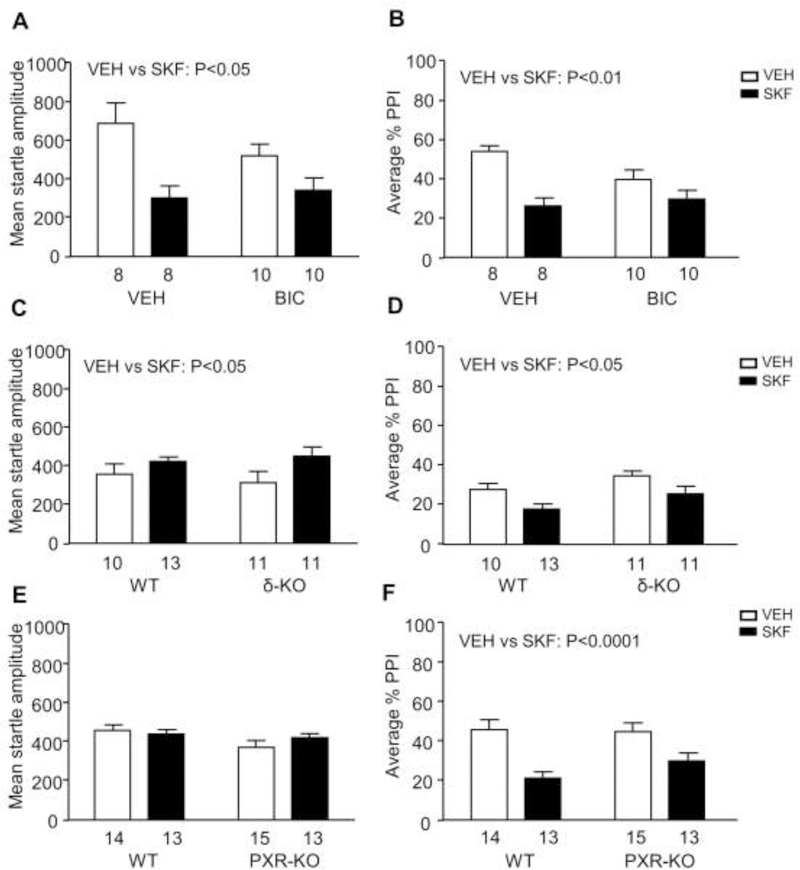
3.6. The sensitivity to the PPI-disrupting effects of D1 receptor activation is not moderated by either GABA-A or PXR.
Given that our results indicated that AP enables D1-mediated PPI disruption, we next tested whether the latter may be moderated by two of the best-characterized molecular targets implicated in the function of this neurosteroid, GABA-A and PXR receptors. We first began testing the effects of the GABA-A receptor bicuculline (1 mg/kg, IP; n=8–10/group) on the startle effects of SKF in mice (Fig. 6A–B). The analysis of PPI values showed that SKF reduced this index [Main effect of treatment: F(1,32)=24.16, P=0.0001] (Fig. 6B), but this effect was not affected by bicuculline.
Fig. 6.
Effects of the D1 receptor agonist SKF 82958 (SKF, 0.3 mg/kg, IP) on startle reflex and prepulse inhibition (PPI), in combination with the GABA-A receptor antagonist bicuculline (1 mg/kg, IP) (A-B), and in knockout (KO) mice for GABA-A δ-subunit (C-D) and pregnane X receptor (PXR) (E-F). Data are shown as means ± SEM. The number of mice in each group is indicated. Abbreviations: WT, wildtype; VEH, vehicle.
We then tested the effects of SKF in δ KO mice (Fig. 6C–D) (n=10–13/ group), Unlike our previous results, SKF (0.3 mg/kg, IP) significantly enhanced startle [Main effect of treatment: F(1,41)=5.03, P=0.03], likely reflecting differences in genetic background. PPI was reduced by both KO [Main effect of genotype: F(1,41)=5.18, P=0.03] and SKF [Main effect of treatment: F(1,41)=9.50, P=0.003]; however, no interactions between these two factors were found.
The analysis of the effects of SKF (0.3 mg/kg, IP) in PXR KO mice (Fig. 6E–F) (n=13–15/ group) showed that this drug increased startle amplitude in both genotypes [F(1,51)=4.09, P=0.04]. The analysis of PPI showed that SKF significantly disrupted PP [F(1,51)=33.46, P<0.0001], irrespective of genotypes, suggesting no involvement of PXR in this effect.
4. DISCUSSION
The results of this study document that 5αR1 genetic deficiency renders mice insensitive to the sensorimotor gating deficits, but not hyperactivity, induced by the potent dopamine D1 receptor agonist SKF. These findings complement previous data from our group on the ability of non-selective 5αR inhibitors, such as finasteride, to counter the PPI reduction induced by D1 receptor activation in both mice and rats, without producing specific effects on locomotor activity (Frau et al., 2013; 2016). Furthermore, we found that, in substantial agreement with our previous data on finasteride (Bortolato et al., 2008; Frau et al., 2016), 5αR1 deficiency produced a significant decrement in startle magnitude without affecting baseline PPI values, pointing to a role of this enzyme in the modulation of acoustic startle exerted by several neuroactive steroids.
Our findings also highlighted that 5αR1 KO mice are sensitive to the PPI-disrupting effects of the D2 receptor agonist QUIN. In alignment with these results, previous data from our group showed that finasteride also renders mice sensitive to PPI deficits induced by QUIN (Frau et al., 2013). Taken together, these results suggest that changes in neuroactive steroid profiles may be critical to modulate the sensitivity to the activation of either D1 or D2 receptors with respect to sensorimotor gating. As previous studies have shown that the genetic background plays a critical role in defining the sensitivity of rats and mice to dopaminergic agonists (Swerdlow et al., 2000; 2002; Ralph and Caine, 2005; Mosher et al., 2016), these data raise the interesting question as to whether variations in gene expression of 5αR1 or other key neurosteroidogenic enzymes may contribute to interindividual differences in the sensitivity to D1 and D2 receptor activation with respect to startle modulation.
In contrast with 5αR1 KO mice, 5αR2-deficient animals were not found to exhibit alterations in startle reflex, PPI values, or their dopaminergic modulation. These data confirm previous findings on the lack of startle and PPI modifications in 5αR2 KO mice (Mosher et al., 2018). Furthermore, they point to a functional divergence between 5αR isoenzymes with respect to the modulation of dopaminergic neurotransmission, as well as perception and information processing. While both 5αR1 and 5αR2 are predicted to serve overlapping enzymatic functions, they diverge with respect to substrate affinity as well as brain distribution pattern (Paba et al., 2011; Castelli et al., 2013). The selective role of 5αR1 in the responsiveness of mice to D1 receptor agonists in PPI may particularly reflect the predominance of this isoenzyme in the brain (Normington and Russell, 1992). From this perspective, it is worth noting that our previous results pointed to a key role of the medial prefrontal cortex and the nucleus accumbens – two regions with abundant expression of 5αR1 - in the PPI-ameliorating effects of finasteride (Devoto et al., 2012).
Another major finding of this study was that acute treatment with AP, but not other 5α-reduced steroids (including DHP, DHT, 3α-diol, and androsterone) restored the sensitivity of 5αR1 KO mice to the PPI-disrupting effects of SKF. This finding is in line with recent findings from our group, indicating that AP triggers PPI deficits in mouse models of TS (Mosher et al. 2017) and sleep-deprived rats (Frau et al., 2018). Given the well-known role of 5αR1 in the regulation of the biosynthetic pathway of AP, these data suggest that the inactivation of this enzyme may impair the synthesis of AP in the brain. The baseline AP brain and plasma levels of 5αR1 KO mice have been shown to be equivalent to that of their WT counterparts (Osborne and Frye, 2009; Koonce and Frye, 2013; Tanchuck-Nipper et al., 2015). However, specific brain-regional differences in baseline AP levels may occur between WT and KO mice; alternatively, 5αR1 KO mice may exhibit an impaired synthesis of AP in response to startle-associated stress and/or activation of D1 receptors. Accordingly, these mutants display a deficit in the conversion of progesterone into AP (Koonce and Frye, 2003). Unfortunately, as the limited small size of mouse brain regions represent a key limitation for steroid detection, our analyses in the present study did not include the measurement of AP levels. Future analyses, however, are warranted to verify whether brain-regional steroid profiles can be modified by either D1 receptor activation or startle testing.
Our next experimental step focused on the identification of the molecular mechanisms whereby AP enables susceptibility to D1 receptor-mediated PPI disruption. The best-characterized receptor of AP is GABA-A, although its mechanisms of interaction with this neurosteroid remain partially elusive. AP is a positive allosteric modulator of this receptor (Majewska et al., 1986; Paul and Purdy, 1992; Rupprecht, 2003), even though this steroid may also act as its agonist at micromolar concentrations (Belelli and Lambert, 2005). Recent evidence points to the presence of two distinct AP-binding sites within the GABA-A receptor, which are posited to be localized in the α subunit and the interface between α and β subunits, respectively (Hosie et al., 2006; 2009). However, the actions of AP on GABA-A receptors have been shown to be influenced by subunit composition (Lambert et al., 2001). In particular, AP is a powerful modulator of GABA-A receptors containing δ subunits (Adkins et al., 2001; Brown et al., 2002; Wohlfahrth et al., 2002), likely due to a primary influence of this subunit in the transduction of neurosteroid signals (Hosie et al., 2009). Accordingly, the lack of δ subunits has been shown to greatly reduce the sensitivity of GABA-A receptor to neurosteroids (Mihalek et al., 1999). Our data showed that neither the GABA-A receptor antagonist bicuculline nor the genetic deficiency of the δ subunit modified the sensitivity of mice to the PPI-disrupting effects of D1 receptor stimulation. Although these data challenge the idea that GABA-A receptors may play a key role in the observed effects of AP, we cannot exclude that some specific families of these receptors and other neurosteroids may still contribute to the dopaminergic modulation of PPI; in particular, future studies will be needed to evaluate whether the role of AP in sensorimotor gating may be modified by fluctuations in the levels of its 3β-epimer isoallopregnanolone (3β,5α, tetrahydroprogesterone), which acts as an endogenous antagonist for the AP site within GABA-A receptors.
We also studied the potential implications of PXR in the role of AP on PPI deficits induced by D1 receptor agonists. PXR is a promiscuous nuclear receptor involved in xenobiotic metabolism and elimination (di Masi et al., 2009). While most studies on PXR have focused on its role in the liver, this target is also present in the brain (Lamba et al., 2004) and is implicated in the metabolism of cholesterol. AP is a well-known ligand of PXR, and some of its biological actions can be attenuated by reducing the expression of this receptor (Frye et al., 2014). It should be noted that PXR is also likely involved in the biosynthesis of AP (Frye et al., 2014). However, PXR-deficient mice did not show any significant modification of the PPI-disrupting effects of D1 receptor agonists.
These results suggest that other downstream mechanisms of AP are likely linked to the sensitivity of mice to the effects of D1 receptor agonist on PPI. For example, the effects of AP may be mediated by other receptors, such as NMDA glutamate receptors. AP sulfate has been shown to exert a modest negative modulation of these receptors (Weaver et al., 2000). Interestingly, previous data in our lab have shown that the actions of D1 agonists in decreasing PPI is facilitated and potentiated by low doses of NMDA antagonists (Bortolato et al., 2005).
Several limitations to this study should be acknowledged. First, we did not test female mice. Prior testing in rats showed that the antidopaminergic effects of finasteride were preserved in both males and females, as well as castrated males (Paba et al., 2011; Devoto et al., 2012). Thus, we speculate that females may display the same effects as those observed in males; however, this conclusion will need to be confirmed by future studies. Second, while our analyses were limited to a few steroids, it is possible that other neuroactive steroids may participate to some of the observed effects. Extensive research has indicated that neuroactive steroids extensively interact with dopamine signaling to regulate a broad array of physiological functions (Sanchez et al, 2010). For example, both progesterone and testosterone alter dopamine release and turnover in rodents (Sanchez et al, 2010). Further research is warranted to address how these interactions may contribute to the pathophysiology of gating disorders.
These limitations notwithstanding, the results of these studies strongly support a role for AP in the mediation of dopamine induced PPI deficits and raise important questions for future research on the balance between D1 and D2 receptors in the regulation of sensorimotor gating mechanisms. As mentioned in the introduction PPI is impaired in several disorders, including schizophrenia and TS and OCD; notably, the ability of drugs to restore PPI in rodents treated with dopaminergic agonists has been shown to predict antipsychotic potency (Swerdlow et al, 1994) or validate therapeutic efficacy for TS (Godar et al., 2014). In line with this idea, it is worth noting that finasteride may have therapeutic effects for TS (Bortolato et al., 2007; Muroni et al., 2011) and schizophrenia (Koethe et al., 2008). If translationally valuable, our results may support the possibility that selective 5αR1 inhibitors could have therapeutic effects for these conditions, without some of the adverse effects of finasteride, including low libido and depression (Traish et al., 2015). A highly selective 5αR1 inhibitor, MK386, has been developed for clinical use (Schwartz et al., 1992; Ellsworth et al., 1996) and shown to be safe and well-tolerated in Phase I and II clinical trials. Future studies will be needed to evaluate whether selective 5αR1 inhibitors may have effects akin to those elicited by finasteride in animal models of schizophrenia and TS.
Highlights.
5α-reductases (5αRs) catalyze the rate-limiting step of neurosteroid synthesis
In mice, D1 receptor agonists reduce the prepulse inhibition (PPI) of the startle
5αR1 knockout mice are insensitive to PPI deficits caused by D1 receptor activation
The PPI-disrupting properties of D1 agonists are reinstated by allopregnanolone
Acknowledgments
We are grateful to Claudia Collu and Eva Vigato for their valuable assistance with the execution of the study. The present manuscript was supported by the following grants from the National Institute of Health: R21 NS108722 (to M.B.) and F31 NS093939 (to L.J.M).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
No conflicts declared.
References
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. alpha4beta3delta GABA(A) receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem 2001. October 19;276(42):38934–9. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci 2005. July;6(7):565–75. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Aru GN, Fà M, Frau R, Orrù M, Salis P, Casti A, Luckey GC, Mereu G, Gessa GL. Activation of D1, but not D2 receptors potentiates dizocilpine-mediated disruption of prepulse inhibition of the startle. Neuropsychopharmacology 2005. March;30(3):561–74. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orrù M, Bourov Y, Marrosu F, Mereu G, Devoto P, Gessa GL. Antipsychotic-like properties of 5-alpha-reductase inhibitors. Neuropsychopharmacology 2008. December;33(13):3146–56. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Muroni A, Marrosu F. Treatment of Tourette’s syndrome with finasteride. Am J Psychiatry 2007. December;164(12):1914–5. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol 2002. August;136(7):965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli MP, Casti A, Casu A, Frau R, Bortolato M, Spiga S, Ennas MG. Regional distribution of 5α-reductase type 2 in the adult rat brain: an immunohistochemical analysis. Psychoneuroendocrinology 2013. February;38(2):281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto P, Frau R, Bini V, Pillolla G, Saba P, Flore G, Corona M, Marrosu F, Bortolato M. Inhibition of 5α-reductase in the nucleus accumbens counters sensorimotor gating deficits induced by dopaminergic activation. Psychoneuroendocrinology 2012. October;37(10):1630 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Masi A, De Marinis E, Ascenzi P, Marino M. Nuclear receptors CAR and PXR: Molecular, functional, and biomedical aspects. Mol Aspects Med 2009. October;30(5):297–343. [DOI] [PubMed] [Google Scholar]
- Ellsworth K, Azzolina B, Baginsky W, Bull H, Chang B, Cimis G, Mitra S, Toney J, Bakshi RK, Rasmusson GR, Tolman RL, Harris GS. MK386: a potent, selective inhibitor of the human type 1 5alpha-reductase. J Steroid Biochem Mol Biol 1996. July;58(4):377–84. [DOI] [PubMed] [Google Scholar]
- Fowler SC, Birkestrand BR, Chen R, Moss SJ, Vorontsova E, Wang G, Zarcone TJ. A force-plate actometer for quantitating rodent behaviors: illustrative data on locomotion, rotation, spatial patterning, stereotypies, and tremor. J Neurosci Methods 2001. May 30;107(1–2):107–24. [DOI] [PubMed] [Google Scholar]
- Frau R, Bortolato M. Repurposing steroidogenesis inhibitors for the therapy of neuropsychiatric disorders: Promises and caveats. Neuropharmacology 2018. May 11 pii: S0028–3908(18)30232–6. [DOI] [PubMed]
- Frau R, Mosher LJ, Bini V, Pillolla G, Pes R, Saba P, Fanni S, Devoto P, Bortolato M. The neurosteroidogenic enzyme 5α-reductase modulates the role of D1 dopamine receptors in rat sensorimotor gating. Psychoneuroendocrinology 2016. January;63:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frau R, Pillolla G, Bini V, Tambaro S, Devoto P, Bortolato M. Inhibition of 5α-reductase attenuates behavioral effects of D1-, but not D2-like receptor agonists in C57BL/6 mice. Psychoneuroendocrinology 2013. April;38(4):542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Walf AA. Novel receptor targets for production and action of allopregnanolone in the central nervous system: a focus on pregnane xenobiotic receptor. Front Cell Neurosci 2014. April 9;8:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001. July;156(2–3):117–54. [DOI] [PubMed] [Google Scholar]
- Geyer MA. The family of sensorimotor gating disorders: comorbidities or diagnostic overlaps? Neurotox Res 2006. December;10(3–4):211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SC, Mosher LJ, Di Giovanni G, Bortolato M. Animal models of tic disorders: a translational perspective. J Neurosci Methods 2014. December; 238:54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godar SC, Mosher LJ, Strathman HJ, Gochi AM, Jones CM, Fowler SC, Bortolato M.The D1CT-7 mouse model of Tourette syndrome displays sensorimotor gating deficits in response to spatial confinement. Br J Pharmacol 2016. July;173(13):2111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev 1980. March;87(2):175–89. [PubMed] [Google Scholar]
- Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of GABA A receptors. Neuropharmacology 2009. January;56(1):149–54. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 2006. November 23;444(7118):486–9. [DOI] [PubMed] [Google Scholar]
- Koethe D, Bortolato M, Piomelli D, Leweke FM. Improvement of general symptoms in a chronic psychotic patient treated with finasteride: case report. Pharmacopsychiatry 2008 2008. May;41(3):115–6. [DOI] [PubMed] [Google Scholar]
- Koonce CJ, Frye CA. Progesterone facilitates exploration, affective and social behaviors among wildtype, but not 5α-reductase Type 1 mutant, mice. Behav Brain Res 2013. September 15;253:232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba V, Yasuda K, Lamba JK, Assem M, Davila J, Strom S, Schuetz EG. PXR NR1I2): splice variants in human tissues, including brain, and identification of neurosteroids and nicotine as PXR activators. Toxicol Appl Pharmacol 2004. September 15;199(3):251–65. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Harney SC, Belelli D, Peters JA. Neurosteroid modulation of recombinant and synaptic GABAA receptors. Int Rev Neurobiol 2001;46:177–205. [DOI] [PubMed] [Google Scholar]
- Mahendroo MS, Cala KM, Hess DL, Russell DW. Unexpected virilization in male mice lacking steroid 5 alpha-reductase enzymes. Endocrinology 2001. November;142(11):4652–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 1986. May 23;232(4753):1004–7. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci U S A 1999. October 26;96(22):12905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher LJ, Frau R, Pardu A, Pes R, Devoto P, Bortolato M. Selective activation of D1 dopamine receptors impairs sensorimotor gating in Long-Evans rats. Br J Pharmacol 2016. July;173(13):2122–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher LJ, Godar SC, Morissette M, McFarlin KM, Scheggi S, Gambarana C, Fowler SC, Di Paolo T, Bortolato M. Steroid 5α-reductase 2 deficiency leads to reduced dominance-related and impulse-control behaviors. Psychoneuroendocrinology 2018. May;91:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher LJ, Godar SC, Nelson M, Fowler SC, Pinna G, Bortolato M. Allopregnanolone mediates the exacerbation of Tourette-like responses by acute stress in mouse models. Sci Rep 2017. June 13;7(1):3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroni A, Paba S, Puligheddu M, Marrosu F, Bortolato M. A preliminary study of finasteride in Tourette syndrome. Mov Disord 2011. September;26(11):2146–7. [DOI] [PubMed] [Google Scholar]
- Normington K, Russell DW. Tissue distribution and kinetic characteristics of rat steroid 5 alpha-reductase isozymes. Evidence for distinct physiological functions. J Biol Chem 1992. September 25;267(27):19548–54. [PubMed] [Google Scholar]
- Osborne DM, Frye CA. Estrogen increases latencies to seizures and levels of 5alpha-pregnan-3alpha-ol-20-one in hippocampus of wild-type, but not 5alpha-reductase knockout, mice. Epilepsy Behav 2009. November;16(3):411–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paba S, Frau R, Godar SC, Devoto P, Marrosu F, Bortolato M. Steroid 5α-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders. Curr Pharm Des 2011;17(2):151–67 [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J 1992. March;6(6):2311–22. [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague-Dawley rats to Swiss-Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. J Pharmacol Exp Ther 2005. February;312(2):733–41. [DOI] [PubMed] [Google Scholar]
- Ralph-Williams RJ, Lehmann-Masten V, Geyer MA. Dopamine D1 rather than D2 receptor agonists disrupt prepulse inhibition of startl.e in mice. Neuropsychopharmacology 2003. January;28(1):108–18. [DOI] [PubMed] [Google Scholar]
- Rupprecht R Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology 2003. February;28(2):139–68. [DOI] [PubMed] [Google Scholar]
- Sánchez MG, Bourque M, Morissette M, Di Paolo T. Steroids-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 2010. June;16(3):e43–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JI, Tanaka WK, Wang DZ, Ebel DL, Geissler LA, Dallob A, Hafkin B, Gertz BJ. MK-386, an inhibitor of 5alpha-reductase type 1, reduces dihydrotestosterone concentrations in serum and sebum without affecting dihydrotestosterone concentrations in semen. J Clin Endocrinol Metab 1997. May;82(5):1373–7. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Martinez ZA, Hanlon FM, Platten A, Farid M, Auerbach P, Braff DL, Geyer MA. Toward understanding the biology of a complex phenotype: rat strain and substrain differences in the sensorimotor gating-disruptive effects of dopamine agonists. J Neurosci 2000. June 1;20(11):4325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Pitcher L, Platten A, Kuczenski R, Eleey CC, Auerbach P. Genetic differences in startle gating-disruptive effects of apomorphine: evidence for central mediation. Behav Neurosci 2002. August;116(4):682–90. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Zisook D, Taaid N. Seroquel (ICI 204,636) restores prepulse inhibition of acoustic startle in apomorphine-treated rats: Similarities to clozapine. Psychopharmacology (Berl) 1994. May;114(4):675–8. [DOI] [PubMed] [Google Scholar]
- Tanchuck-Nipper MA, Ford MM, Hertzberg A, Beadles-Bohling A, Cozzoli DK, Finn DA. Sex Differences in Ethanol’s Anxiolytic Effect and Chronic Ethanol Withdrawal Severity in Mice with a Null Mutation of the 5α-Reductase Type 1 Gene. Behav Genet 2015. May;45(3):354–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen AE, Russell DW. Four-amino acid segment in steroid 5 alpha-reductase 1 confers sensitivity to finasteride, a competitive inhibitor. J Biol Chem 1992. April 25;267(12):8577–83. [PubMed] [Google Scholar]
- Traish AM, Melcangi RC, Bortolato M, Garcia-Segura LM, Zitzmann M. Adverse effects of 5α-reductase inhibitors: What do we know, don’t know, and need to know? Rev Endocr Metab Disord 2015. September;16(3):177–98. [DOI] [PubMed] [Google Scholar]
- Weaver CE, Land MB, Purdy RH, Richards KG, Gibbs TT, Farb DH. Geometry and charge determine pharmacological effects of steroids on N-methyl-D-aspartate receptor-induced Ca(2+) accumulation and cell death. J Pharmacol Exp Ther 2000. June;293(3):747–54. [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci 2002. March 1;22(5):1541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS, Bosch D, Alves N, Daros A, Ure RJ, Schmid S. GABA receptors and prepulse inhibition of acoustic startle in mice and rats. Eur J Neurosci 2010. June;31(11):2053–61. [DOI] [PubMed] [Google Scholar]