Abstract
The purpose of this pictorial review was to describe various echocardiographic techniques that can be used for the functional assessment of the fetal heart. The systolic and diastolic assessments of the fetal heart are presented separately, with an emphasis on 2-dimensional Doppler methods and an overview of new technologies. The aim of this summary was to review the tools that can be used by the echocardiographer, and on that basis, to systematize the process of performing a functional assessment.
Keywords: Fetal heart, Function, Echocardiography, Color Doppler
Introduction
A fetal cardiac evaluation is composed of two stages: an anatomic evaluation, in which the presence or absence of congenital heart disease (CHD) is detected by means of the sequential segmental analysis of structures, and a study of cardiac function, in which the effective contraction and relaxation of the myocardial fibers, their capacity to maintain fetal cardiac output (CO), and heart rate and rhythm are evaluated.
Currently, the procedure for anatomical evaluations of the fetal heart is well established and routinely performed by specialists in fetal cardiology. However, there is still no consensus on the technique to be used in the evaluation of cardiac function, despite its unquestionable importance [1,2]. There are some difficulties in determining the most appropriate method capable of detecting early fetal cardiac dysfunction, which is why many researchers are studying the various methods that are available [1,3]. Challenges include the movement and positioning of the fetus, the high fetal heart rate, the different architecture of fetal myocardial fibers [3] and the parallel blood circulation characteristic of fetuses, which may interfere with assessments of the heart [4].
The functional assessment of the fetal heart conveys important information regarding the hemodynamic status and cardiovascular adaptation of a fetus in the face of several perinatal complications. Numerous studies have evaluated existing echocardiographic techniques in order to improve functional assessments [2]. Information from functional assessments can be very useful for the clinical management of pregnant women with fetuses with growth restriction, hydrops fetalis, and fetal arrhythmias, as well as pregnant women with monochorionic twins or diabetes.
The cardiac cycle comprises a period of systole and diastole. With regard to fetal cardiac function, it is necessary to determine the quality of systolic and diastolic function, as well as which ventricle is more important for maintaining a good hemodynamic status in the fetus, bearing in mind that the right ventricle shows some dominance in relation to CO.
Evaluation of Systolic Function in the Fetal Heart
Systolic function in the fetal heart can be evaluated primarily through the ejection fraction (EF), shortening fraction, CO, cardiac volume, maximal displacement of the valve ring (tricuspid or mitral), myocardial performance index (MPI), and parameters derived from cardiac strain [3,5]. Systolic evaluation of the fetal heart can be performed using 1- (M-mode), 2- (2D), and 3-dimensional (3D) techniques.
EF and Shortening Fraction
The EF and shortening fraction are criteria traditionally used in adult and child echocardiography to evaluate global radial/circumferential cardiac function [5,6]. These values are usually obtained using M-mode (1-dimensional technique) from a 2D or 3D image on the parasternal plane of the long axis (Fig. 1) or the short axis of the heart at the height of the papillary muscles of the mitral valve (MV). Measurements of the ventricle at diastole and systole are obtained, and using the relationship between these measurements, calculations are performed to determine the EF ([diastole measurement-systole measurement]/diastole) measurement and shortening fraction [3-5].
Fig. 1. Axial section of the fetal thorax showing the parasternal long axis of the left ventricle.
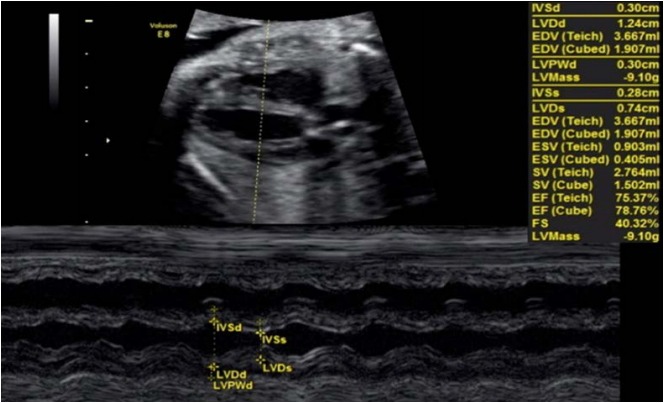
The M-mode interrogation line is perpendicular to the ventricular wall at the level of the papillary muscles. Measurements of the ejection fraction and shortening fraction of the left ventricle are shown.
The shortening fraction is a percentage-based relationship between the dimensions of the ventricle cavity. The EF represents the ratio between ventricular volumes expressed as a percentage; therefore, when its determination requires the measurement of volume from 2D measurements, the margin of error increases. Thus, we have observed that dimension measurements may be subject to less error than measurements of the ventricular volume; this is one of the main reasons why the shortening fraction is considered to be more valuable for fetuses than the EF [6].
However, using the shortening fraction to assess the systolic function of the ventricles presents some technical difficulties in fetuses, due to difficulties in the proper alignment of the plane, higher heart rate, and fetal body movements [5,7,8]. M-mode assessment of cardiac function has the disadvantage of evaluating a single line of the cardiac plane, resulting in limitations in cases of myocardial asymmetry [3]. In addition to this technical difficulty, it should also be kept in mind that this parameter is altered only in the later phase of cardiac dysfunction, and it is necessary to use other echocardiographic techniques, such as the MPI, for the earlier diagnosis and treatment of fetuses with growth restriction, hydrops fetalis, as well as pregnant women with monochorionic twins or diabetes [2,9].
Cardiac Output and Stroke Volume
CO and cardiac volume can be evaluated by 2D and 3D methods. CO is the product of the heart rate and the stroke volume (SV) ejected by the left ventricle. The SV is the product of the valvular cross-sectional area and the velocity-time integral (VTI) (Figs. 2, 3) [4,5,10]. Mathematically, CO=FHR×πr2 ×VTI, where CO refers to the cardiac output, FHR is the fetal heart rate, r2 equals half the diameter of the ventricular outflow tract, VTI is the velocity-time integral of the flow out of the assessed ventricle, and π is a constant with a value of 3.14.
Fig. 2. Assessment of left ventricular cardiac output using pulsed-wave Doppler.
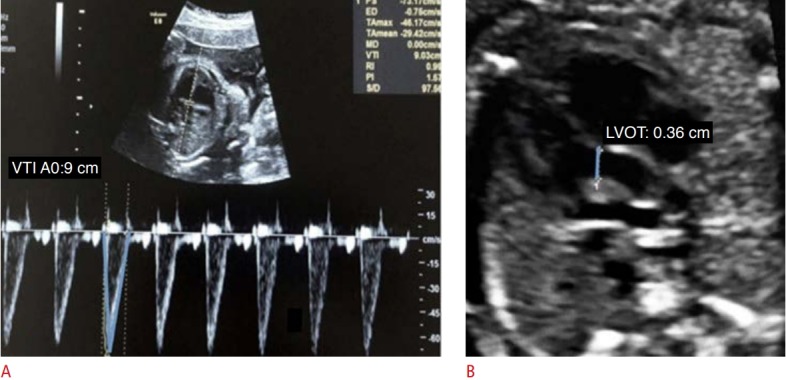
A. The velocity-time integral (VTI) of the left ventricular outflow tract (LVOT) was obtained. B. The parasternal long axis incidence shows the LVOT.
Fig. 3. Assessment of right ventricular cardiac output using pulsed-wave Doppler.
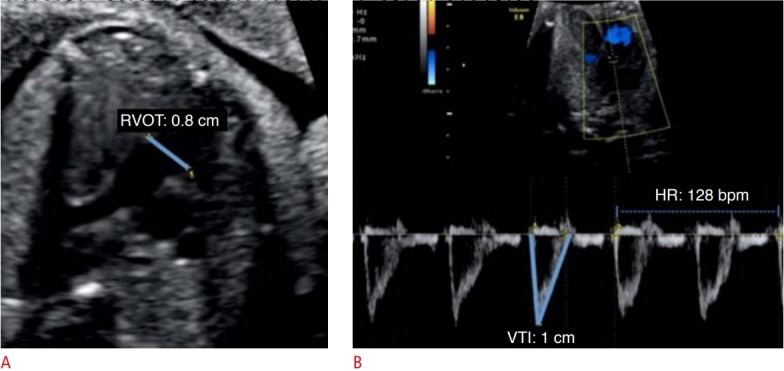
A. The parasternal long-axis incidence with mild anteriorization of the plane shows the right ventricle outflow tract (RVOT). B. The velocitytime integral (VTI) of the RVOT was obtained. HR, heart rate.
In the evaluation of CO by the 2D method, it is necessary to acquire the values of the VTI, ventricular outflow tract diameter, and fetal heart rate. The VTI is obtained through the pulsed Doppler sample volume positioned at the ventricular outflow tract. The VTI is the area under the velocity spectral curve, which indicates the sum of all individual velocities throughout the ventricular ejection period (Figs. 2, 3). The measurement of the ventricular outflow tract at the end of the systolic period of the cardiac cycle using the 2D method can be an important challenge for this aspect of the functional evaluation, as it is difficult to make exact measurements because the valve has elliptical characteristics. The measurement should be made with extreme care because the square of the value of this radius will be used (Figs. 2, 3) [4,5,10]. Measurement of the ventricular outflow tract is contraindicated in cases of valve injury (obstructive or not).
Using the 3D method to evaluate the CO and SV, it is possible to perform a volumetric acquisition of the fetal heart [11]. Subsequently, the image is processed through a spatio-temporal image correlation, using the volume mode to determine the cardiac volume and, consequently, the CO by multiplying it by the fetal heart rate [11].
CO in fetuses may increase in cases of active CHD [4], that is, in situations in which high CO causes hemodynamic repercussions even in the intrauterine period, such as agenesis of the ductus venosus, arteriovenous fistulas, teratomas, and placental transfusion syndrome. In such cases, the calculation of CO may assist in clinical follow-up and more careful management of the fetus [5]. CO in fetuses may decrease in cases where there is a deficit of cardiac contractility, such as fetal myocarditis or cardiomyopathy secondary to a viral infection in the gestational period [5].
Maximum Displacement of the Valvar Ring
The evaluation of the maximum displacement of the valvar ring emphasizes the concept of longitudinal movement of the left ventricle during the cardiac cycle. During systole, the valvar ring nears the apical region, and the greater the displacement of the valvar ring, the better the longitudinal systolic function of that ventricle. Thus, we can evaluate this shift using M-mode aligned in the direction of the lateral portion of the valvar ring. The M-mode trace demonstrates the excursion movement of the ring during the cardiac cycle, and this distance is the displacement of the annulus. This parameter can be assessed for both the left ventricle and the right ventricle through measurements of the mitral annular plane systolic excursion (MAPSE) and the tricuspid annular plane systolic excursion, respectively (Fig. 4) [12].
Fig. 4. M-mode cursor applied on the tricuspid annual myocardium on an apical 4-chamber view to evaluate long-axis displacement of the tricuspid valve annulus.
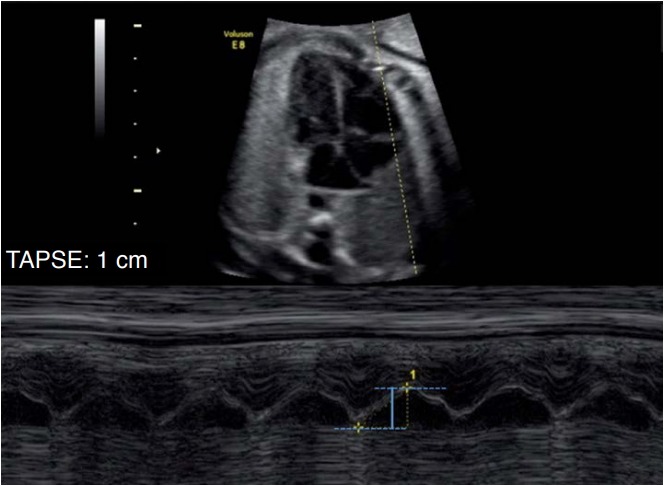
The maximal longitudinal annular displacement, also referred to tricuspid annular plane systolic excursion (TAPSE), is shown in blue.
The evaluation of longitudinal systolic cardiac function through the MAPSE in adults demonstrated a good correlation with scintigraphy. However, these measures may be underestimated or overestimated, because they are 1-dimensional measurements that are dependent on the position of the transducer [13,14].
Care should be taken regarding values of maximum displacement of the valvar ring, as there is already an estimated normal value in the adult population [12]. Although reference curves exist for infants and fetuses, we should not take into account isolated measurements only. Monitoring the left ventricle while performing serial measurements of valvar displacement is preferable for evaluating the performance of the myocardium [12,14].
Other Techniques
In addition to the parameters used for systolic evaluation, there are also parameters used to assess the velocity of contractility of myocardial fibers derived from Doppler tissue imaging [5]. These parameters are well documented for fetuses, and deformities of the myocardial fibers identified based on "speckle tracking" should receive further evaluation, as these techniques must still be validated in the fetal heart [4,14].
Evaluation of Diastolic Function in the Fetal Heart
The diastolic phase occupies approximately two-thirds of the cardiac cycle, comprising four steps: isovolumetric relaxation, the rapid filling phase, the slow filling phase or diastasis, and atrial systole. Considering these four steps, we can understand why the diastolic blood volume of the ventricle can be transmitted to the atrium and veins (pulmonary veins and superior and inferior venae cavae) over a relatively long period.
The diastolic evaluation of the fetal heart can utilize the blood flow of the atrioventricular valves, pulmonary veins, and inferior vena cava, as well as evaluations of the isovolumetric relaxation and velocity of the myocardial fibers using the tissue Doppler technique.
Blood Flow of the Atrioventricular Valves, Pulmonary Vein, and Inferior Vena Cava
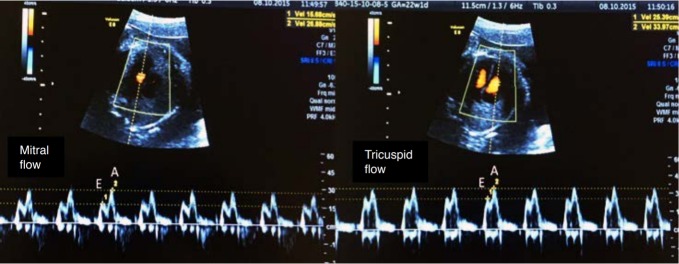
To characterize the blood flow from the atrioventricular valves and pulmonary veins, it is necessary to use the 4-chamber view (4C) with color Doppler. The sample volume of the pulsed Doppler should have a 2-mm aperture [3]. To obtain the spectral curve of the blood flows of the MV and tricuspid valve (TV), the pulsed Doppler sample is positioned inside the ventricular cavity, immediately below the valve (Fig. 5) [4,5]. To obtain the spectral curve of pulmonary vein blood flow, the pulsed Doppler sample is positioned at the connection of the pulmonary vein to the left atrium (Fig. 6) [4,5,15].
Fig. 5. Spectral Doppler waveforms across the atrioventricular valves (mitral and tricuspid).
Note that the Doppler waveforms are quantified by the E/A ratio, representing fetal diastolic function. The E wave represents passive ventricular filling, while the A wave represents active ventricular filling associated with atrial contraction.
Fig. 6. Spectral Doppler waveforms across the pulmonary vein (PV).
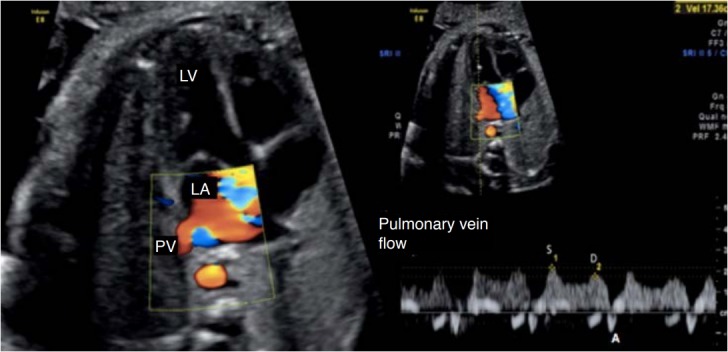
The Doppler waveforms show a characteristic triphasic pattern. LV, left ventricle; LA, left atrium; S, peak systolic wave velocity; D, peak diastolic wave velocity; A, atrial contraction at the end of diastole.
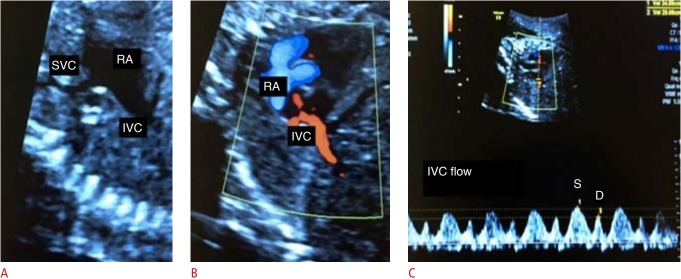
To obtain the spectral curve of the inferior vena cava, we should highlight the arrival of the two venae cavae into the right atrium through the bicaval plane, in the sagittal plane of the fetus. The pulsed Doppler sample is positioned at the inferior vena cava, with care to avoid interference with the vascular bed of the proximities and maintaining a maximum angle of 30° (Fig. 7) [4,5].
Fig. 7. Assessment of blood flow from the inferior vena cava (IVC).
A. Two-dimensional image demonstrates the bicaval plane. B. Two-dimensional color Doppler image demonstrates the IVC. C. Spectral Doppler waveforms of the IVC are shown. SVC, superior vena cava; RA, right atrium; S, peak systolic wave velocity; D, peak diastolic wave velocity.
The normal blood inflow into the ventricles consists of a biphasic waveform, in which the initial flow coincides with the E wave, which is the passive filling period of the ventricle, and the late flow coincides with the A wave, the phase of atrial systole. Due to the lower ventricular compliance characteristic of the fetal heart, the magnitude of the A wave normally exceeds that of the E wave, with an E/A wave ratio<1 (Fig. 5) [5].
The normal blood flow in the veins in adults and children presents two anterograde waves (above the baseline, a peak systolic velocity [S wave] and a diastolic peak velocity [D wave]) and a retrograde wave (below the baseline [A wave]) (Fig. 6) [5]. The S wave comprises the systolic period of the ventricle and coincides with the period of atrial relaxation. The D wave occurs during the rapid filling phase of the ventricle during diastole (Figs. 6, 7). The A wave, which is usually not very evident, arises during atrial contraction at the end of diastole and corresponds to retrograde flow in the vein [4,5].
The normal blood flow in the pulmonary veins in fetuses may present three anterograde waves [15,16]. Because some of the blood in the ductus venosus, while passing through the inferior vena cava and right atrium, is directed to the left atrium through the foramen ovale, the waveform in the pulmonary vein is very similar to that of the inferior vena cava and ductus venosus [17]. Accordingly, during systole and rapid filling of the left ventricle during diastole, through a patent foramen ovale, the flows of the inferior vena cava and ductus venosus may be influenced by the pressure of the left atrium, which in turn may undergo changes according to the pressure of the left ventricle [17].
Few studies have been conducted on normal blood flow in the inferior vena cava of fetuses. The findings are similar to those of adults and children, with a triphasic pattern: S wave, period of ventricular systole; D wave, period of early diastole; and A wave, reverse flow of atrial contraction. The indexes studied in the analysis of the flow of the inferior vena cava include the preload index (A wave-to-S wave ratio), the index of peak velocity, and the pulsatility index [18].
Isovolumetric Relaxation Time
The isovolumetric relaxation time (IVRT) of the left ventricle corresponds to the initial period of diastole. This measurement can be obtained through pulsed Doppler (Fig. 8) or tissue Doppler (Fig. 9), with the sample positioned between the outflow and inflow [3]. The basic idea of this measurement is to record the time between the closing of the MV outlet and the opening of the atrioventricular valve. Diastolic dysfunction may be present when the IVRT is abnormally high [4,19].
Fig. 8. Assessment of fetal heart function using the myocardial performance index (MPI) on spectral Doppler.
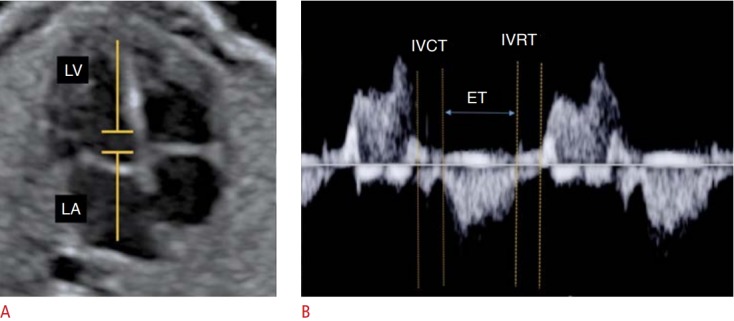
A, B. From the Doppler sample in the 4-chamber plane (A). MPI (isovolumetric contraction time [IVCT]+ isovolumetric relaxation time [IVRT]/ ejection time [ET]) is calculated on the spectral Doppler wave form of the mitral valve (B). LA, left atrium; LV, left ventricle.
Fig. 9. Assessment of fetal heart function using the myocardial performance index (MPI) on tissue Doppler.
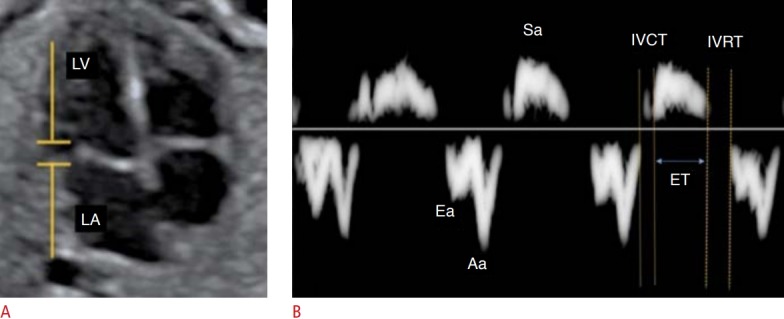
A, B. From the spectral tissue Doppler in the lateral portion of the mitral valve ring (A), MPI (isovolumetric contraction time [IVCT]+isovolumetric relaxation time [IVRT]/ejection time [ET]) is calculated on the spectral tissue Doppler waveform of the mitral valve (B). LA, left atrium; LV, left ventricle; Sa,annular velocity during ventricular systole; Ea, early diastolic annular relaxation velocity; Aa, annular velocity during the atrial contraction.
Tissue Doppler measures the velocity of myocardial fiber movement. The sample volume can be positioned in the lateral portion of the MV ring, the TV ring, and the septal wall of the valve ring. The spectral curve obtained shows the systolic movement of the ring (Sa) and the diastolic movement of the ring (Ea and Aa) [3]. From this curve, data on the segmental function of the fetal heart can be obtained (Fig. 9).
Although this technique is more sensitive than classic spectral Doppler and allows the assessment of segmental diastolic function, some practical difficulties exist, such as making measurements of these values and the availability of this tool on ultrasound equipment.
Other Techniques
Other techniques to assess diastolic function in fetuses include the systolic-to-diastolic time index in the fetal heart cycle, measurement of the atrial volume, the pulsatility index of the pulmonary vein, and measurement of the excursion of the foramen ovale. All these techniques are worthy of consideration and are being researched worldwide, but they must still be validated to be fully incorporated into the daily routine of the echocardiographer [20,21].
Myocardial Performance Index
Additionally, the MPI, also known as the Tei index, can be used. The MPI was developed in the mid-1990s as a measure of global ventricular performance, including both systolic and diastolic parameters, and can be applied to the left or right ventricle. The MPI encompasses the following time intervals derived from spectral or tissue Doppler: ejection time (ET; the entire ejection period observed with Doppler), isovolumetric contraction time (IVCT; end of the A wave and the beginning of systole), and IVRT (end of the systole and beginning of the E wave) (Figs. 8, 9). With these values, we obtain the following equation: MPI=IVCT+IVRT/ET. Systolic dysfunction is indicated by abnormally high values of IVCT or abnormally low values of ET. Diastolic dysfunction corresponds to high values of IVRT. Thus, the MPI increases in both systolic and diastolic dysfunction. Several studies have sought to validate the Tei index for the functional evaluation of the fetal heart [3]. The MPI determined using tissue Doppler is one of the most sensitive and reproducible methods for evaluating fetal cardiac function of the right ventricle [22].
Cardiovascular Profile Score
The fetal cardiovascular failure score was first reported by Huhta [23], and has been validated as a predictor of perinatal morbidity and mortality [24,25]. The score combines the following direct and indirect markers of fetal cardiovascular function: fetal hydrops, venous and arterial Doppler findings, heart size, and cardiac function using parameters such as the presence of regurgitation in the atrioventricular valves, the biphasic pattern of the filling flow in diastole, and the relationship between the shortening fraction of the ventricles [23]. The normal score is 10, and scores of less than 5 points are predictive of perinatal mortality [25,26].
Footnotes
Author Contributions
Conceptualization: Rocha LA. Data acquisition: Rocha LA. Data analysis or interpretation: Rocha LA. Drafting of the manuscript: Rocha LA. Critical revision of the manuscript: Rolo LC, Araujo Júnior E. Approval of the final version of the manuscript: all authors.
No potential conflict of interest relevant to this article was reported.
References
- 1.Donofrio MT, Moon-Grady AJ, Hornberger LK, Copel JA, Sklansky MS, Abuhamad A, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;129:2183–2242. doi: 10.1161/01.cir.0000437597.44550.5d. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez-Andrade E, Benavides-Serralde JA, Cruz-Martinez R, Welsh A, Mancilla-Ramirez J. Evaluation of conventional Doppler fetal cardiac function parameters: E/A ratios, outflow tracts, and myocardial performance index. Fetal Diagn Ther. 2012;32:22–29. doi: 10.1159/000330792. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey ME, Messing B, Cohen SM, Valsky DV, Yagel S. Functional assessment of the fetal heart: a review. Ultrasound Obstet Gynecol. 2012;39:131–144. doi: 10.1002/uog.9064. [DOI] [PubMed] [Google Scholar]
- 4.Rudolph A. Congenital diseases of the heart: clinical-physiological considerations. 3rd ed. Hoboken, NJ: Wiley-Blackwell; 2009. [Google Scholar]
- 5.Allan L, Hornberger L, Sharland G. Textbook of fetal cardiology. New York: Greenwich Medical Media; 2000. pp. 3–13. [Google Scholar]
- 6.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 7.Huhta JC. Fetal congestive heart failure. Semin Fetal Neonatal Med. 2005;10:542–552. doi: 10.1016/j.siny.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Tongsong T, Wanapirak C, Piyamongkol W, Sirichotiyakul S, Tongprasert F, Srisupundit K, et al. Fetal ventricular shortening fraction in hydrops fetalis. Obstet Gynecol. 2011;117:84–91. doi: 10.1097/AOG.0b013e3181fc3887. [DOI] [PubMed] [Google Scholar]
- 9.Bravo-Valenzuela NJ, Peixoto AB, Nardozza LM, Souza AS, Araujo Junior E. Applicability and technical aspects of two-dimensional ultrasonography for assessment of fetal heart function. Med Ultrason. 2017;19:94–101. doi: 10.11152/mu-934. [DOI] [PubMed] [Google Scholar]
- 10.Mielke G, Benda N. Cardiac output and central distribution of blood flow in the human fetus. Circulation. 2001;103:1662–1668. doi: 10.1161/01.cir.103.12.1662. [DOI] [PubMed] [Google Scholar]
- 11.Araujo Junior E, Novoa Y Novoa VA, Barros FS, Rocha LA, Peixoto AB, Martins WP, et al. Reference values for the volumes of foetal heart atrial wall by three-dimensional ultrasound using STIC and VOCAL methods between 20w0d and 33w6d weeks of gestation. J Matern Fetal Neonatal Med. 2016;29:3076–3083. doi: 10.3109/14767058.2015.1118454. [DOI] [PubMed] [Google Scholar]
- 12.Eriksen BH, Nestaas E, Hole T, Liestol K, Stoylen A, Fugelseth D. Longitudinal assessment of atrioventricular annulus excursion by grey-scale m-mode and colour tissue Doppler imaging in premature infants. Early Hum Dev. 2013;89:977–982. doi: 10.1016/j.earlhumdev.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Giusca S, Dambrauskaite V, Scheurwegs C, D'Hooge J, Claus P, Herbots L, et al. Deformation imaging describes right ventricular function better than longitudinal displacement of the tricuspid ring. Heart. 2010;96:281–288. doi: 10.1136/hrt.2009.171728. [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Zielinsky P, Piccoli A Jr, Gus E, Manica JL, Satler F, Nicoloso LH, et al. Dynamics of the pulmonary venous flow in the fetus and its association with vascular diameter. Circulation. 2003;108:2377–2380. doi: 10.1161/01.CIR.0000093195.73667.52. [DOI] [PubMed] [Google Scholar]
- 16.Lenz F, Chaoui R. Reference ranges for Doppler-assessed pulmonary venous blood flow velocities and pulsatility indices in normal human fetuses. Prenat Diagn. 2002;22:786–791. doi: 10.1002/pd.410. [DOI] [PubMed] [Google Scholar]
- 17.Kiserud T, Eik-Nes SH, Blaas HG, Hellevik LR. Foramen ovale: an ultrasonographic study of its relation to the inferior vena cava, ductus venosus and hepatic veins. Ultrasound Obstet Gynecol. 1992;2:389–396. doi: 10.1046/j.1469-0705.1992.02060389.x. [DOI] [PubMed] [Google Scholar]
- 18.Luewan S, Srisupundit K, Tongprasert F, Tongsong T. Normal reference ranges of inferior vena cava doppler indices from 14 to 40 weeks of gestation. J Clin Ultrasound. 2012;40:214–218. doi: 10.1002/jcu.21878. [DOI] [PubMed] [Google Scholar]
- 19.Van Mieghem T, Gucciardo L, Lewi P, Lewi L, Van Schoubroeck D, Devlieger R, et al. Validation of the fetal myocardial performance index in the second and third trimesters of gestation. Ultrasound Obstet Gynecol. 2009;33:58–63. doi: 10.1002/uog.6238. [DOI] [PubMed] [Google Scholar]
- 20.Nawaytou HM, Peyvandi S, Brook MM, Silverman N, Moon-Grady AJ. Right ventricular systolic-to-diastolic time index: hypoplastic left heart fetuses differ significantly from normal fetuses. J Am Soc Echocardiogr. 2016;29:143–149. doi: 10.1016/j.echo.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Zielinsky P, Nicoloso LH, Firpo C, Marcantonio S, Scheid M, Gus EI, et al. Alternative parameters for echocardiographic assessment of fetal diastolic function. Braz J Med Biol Res. 2004;37:31–36. doi: 10.1590/s0100-879x2004000100005. [DOI] [PubMed] [Google Scholar]
- 22.Bui YK, Kipps AK, Brook MM, Moon-Grady AJ. Tissue Doppler is more sensitive and reproducible than spectral pulsed-wave Doppler for fetal right ventricle myocardial performance index determination in normal and diabetic pregnancies. J Am Soc Echocardiogr. 2013;26:507–514. doi: 10.1016/j.echo.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Huhta JC. Right ventricular function in the human fetus. J Perinat Med. 2001;29:381–389. doi: 10.1515/JPM.2001.054. [DOI] [PubMed] [Google Scholar]
- 24.Falkensammer CB, Paul J, Huhta JC. Fetal congestive heart failure: correlation of Tei-index and Cardiovascular-score. J Perinat Med. 2001;29:390–398. doi: 10.1515/JPM.2001.055. [DOI] [PubMed] [Google Scholar]
- 25.Hofstaetter C, Hansmann M, Eik-Nes SH, Huhta JC, Luther SL. A cardiovascular profile score in the surveillance of fetal hydrops. J Matern Fetal Neonatal Med. 2006;19:407–413. doi: 10.1080/14767050600682446. [DOI] [PubMed] [Google Scholar]
- 26.Makikallio K, Rasanen J, Makikallio T, Vuolteenaho O, Huhta JC. Human fetal cardiovascular profile score and neonatal outcome in intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;31:48–54. doi: 10.1002/uog.5210. [DOI] [PubMed] [Google Scholar]