Abstract
Purpose
This study was designed to expound the underlying mechanism of microtubule-directed chemotherapeutic drugs resistance induced by cancer-associated fibroblasts (CAFs) in breast cancer.
Materials and methods
We collected 10 microtubule-directed chemotherapeutic drugs resistant breast tumor samples and 10 normal breast tumor samples to analyze the CAFs distribution by immunohistochemistry and flow cytometry. We also detected the collagen expression in CAFs by real-time PCR. We detected the activation of PI3K/AKT signaling pathway in tumor cells by Western blotting and immunofluorescence. The subcutaneous 4T1/MCF-7 bearing mice were used to investigate the anticancer effects of integrin β1 inhibitor combined with microtubule-directed chemotherapeutic drugs.
Results
In our studies, accumulation of CAFs was observed in tumor samples from microtubule-directed chemotherapeutic drugs resistant patients. Those isolated CAFs could efficiently induce the acquisition of microtubule-directed chemotherapeutic drugs resistance in breast cancer cells. More importantly, we found that CAFs could regulate the microtubule-directed chemotherapeutic drugs resistance through the secretion of collagen to activate the integrin β1/PI3K/AKT signaling pathway. Combination of integrin α2β1 inhibitor and paclitaxel/vincristine sulfate could efficiently overcome the microtubule-directed chemotherapeutic drugs resistance induced by CAFs and enhanced the anticancer effects of chemotherapy in subcutaneous 4T1/MCF-7 bearing mice.
Conclusion
Our results demonstrated that CAFs constitute a supporting niche for cancer drug resistance acquisition. Thus, traditional microtubule-directed chemotherapeutic drugs combined with integrin β1 inhibitor may present an innovative therapeutic strategy for breast cancer therapy.
Keywords: breast cancer, cancer-associated fibroblasts, collagen, PI3K/AKT, drug resistance
Introduction
With increasing incidence and mortality, breast cancer has been the leading cause of cancer-associated death in women worldwide.1,2 Chemotherapy, one of the standard therapeutic regimens, has been proved to efficiently suppress the tumor growth and improve the prognosis in breast cancer treatment.3,4 Microtubule-directed chemotherapeutic drugs, such as paclitaxel (PTX) and vincristine sulfate (VCR), are widely used in breast cancer therapy.4 Unfortunately, a part of breast cancer patients, especially in recurrence after adjuvant treatment or triple-negative breast cancer, eventually relapse and develop resistance to chemotherapy, which results in treatment failure.3,5 Thus, there is an urgent need to expound the mechanisms of drug resistance development and establish new effective strategies to overcome the chemo-resistance for breast cancer therapy.
The development of drug resistance does not depend on sole cancer cell-autonomous defects and is controlled by various curial factors, including up-regulation of ATP-binding cassette (ABC) transporter protein, enhanced DNA damages repair, activation of pro-survival pathway induced by tumor microenvironments and so on.6 Also, the tumor microenvironment has received recent attention as an important determination of cancer cells drug resistance development.7 Increasing evidence suggest that crosstalk between cancer cells and stromal cells in tumor microenvironment is crucial for the development of drug resistance.3,7,8 As one of the most important mesenchymal cells residing within the tumor microenvironment, cancer associated fibroblasts (CAFs) are a subpopulation of stromal cells which participate in the development of tumor angiogenesis, metastasis, and chemo-resistance regulation.6,8–10 However, the potential mechanisms of CAFs involved in chemo-resistance regulation remain unclear and it is still a challenge to effectively overcome the resistance and suppress the breast cancer growth.
In our studies, enriched CAFs were observed in tumor tissues from drug resistance breast cancer patients. We further proved that those CAFs could regulate the microtubule-directed chemotherapeutic drugs resistance, which depend on a collagen/integrin β1/PI3K/AKT signaling pathway. Also, the application of integrin β1 inhibitor could efficiently reverse the microtubule-directed chemotherapeutic drugs resistance induced by CAFs. We further expounded the role of CAFs in breast cancer microtubule-directed chemotherapeutic drugs resistance development and PTX/VCR combined with integrin β1 inhibitor might be a potential strategy in breast cancer therapy.
Materials and methods
Materials and reagents
PTX and VCR were purchased from Sangon Biotech (Shanghai, China). GRGDSP was purchased from Sigma (St Louis, MO, USA). LY294002 and MK-2206 were purchased from Selleck Chemicals (MA, USA). MTT cell viability detection kit was purchased from Solarbio (Shanghai, China). Type I collagen and type I collagenase were purchased from Sigma. Anti-IGF2 neutralizing antibody was obtained from Shanghai Kirin Holdings Co., Ltd. (Shanghai, China). Other chemicals and solvents were purchased from Solarbio (Beijing, China) and all reagents were of analytical grade.
Cell lines and patients’ samples
MCF-7 (human breast cancer cell line) and 4T1 (murine breast cancer cell line) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA), which were maintained in RPMI-1640 complete medium (Gibco, Waltham, MA, USA) supplemented with 10% FBS (Gibco), at 37°C in 5% CO2 atmosphere. Murine embryonic fibroblast line NIH/3T3 was purchased from ATCC and cultured with DMEM culture medium (Gibco) with 10% FBS (Gibco). Human embryonic lung fibroblast line HLF1 was purchased from ATCC and cultured with Ham’s F-12K (Gibco) with 10% FBS (Gibco), at 37°C in 5% CO2 atmosphere.
The CAFs were collected from tumor tissues of patients or mice. Briefly, the tumor tissues (patients’ breast cancer tumor tissues or 4T1 cells derived subcutaneous tumors from mice) were cut into pieces as small as possible after washed with PBS. Then, tissues were removed to another dish with DMEM (Gibco) containing ACCUMAX™ (Sigma) medium to digest at 37°C, 5% CO2 incubator for 2 hrs, followed by filtration (BD, USA). After washed with PBS, cell precipitation was collected and seeded into 6-well plate in 2 mL RPMI-1640 medium supplemented with 10% FBS overnight at 37°C. Next day, the medium was replaced with fresh medium to remove the un-adherent cells and the remaining cells were collected. After 5–10 passages, we sorted the CD90+ cells with BD FACSAria III (USA) to collect fibroblasts and cultured for further study.11 Primary breast cancer tumor and adjacent normal samples were sterilely obtained after the surgery or the core-needle biopsy at the Second Affiliated Hospital of Zhejiang Chinese Medical University (Table 1). Samples were sent to the laboratory and treated within 2 hrs (IRB number: AF-BG-006-1.0). Samples were divided into chemo-sensitive (CS) and chemo-resistant groups(CR) according to RECIST (Response Evaluation Criteria in Solid Tumors). Complete response (ComR) was defined as disappearance of all lesions in both primary tumor and lymph nodes; partial response (ParR) was defined as at least a 30% reduction in the sum of the longest diameter of target lesions; progressive disease (ProD) was defined as at least a 20% increase in the sum of the longest diameter of target lesions; and stable disease (StaD) was defined as neither sufficient shrinkage to qualify as ParR nor sufficient increase to qualify as ProD. ComR and ParR were classified as ComS, while StaD and ProD were classified as chemo-resistant (CR). The study was approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang Chinese Medical University. All samples collection and processing were carried out respecting the Declaration of Helsinki. All patients signed informed consent prior to tumor tissues collection treatment, including allowing their data to be used for further research. All experiments were performed under the monitor of the Ethics Committee of the Second Affiliated Hospital of Zhejiang Chinese Medical University.
Table 1.
The characteristic of breast cancer patients
| Total number of samples (n=20) | ||
|---|---|---|
| Age | ≥60 | 12 (60%) |
| <60 | 8 (40%) | |
| Gender | Female | 20 (100%) |
| Male | 0 (0%) | |
| Histological grade | Well | 9 (45%) |
| Poor | 11 (55%) | |
| Clinical stage | M0 | 8 (40%) |
| M1 | 12 (60%) | |
| Chemotherapy response | Resistance | 10 (50%) |
| Sensitive | 10 (50%) | |
Cell viability analysis
All cell viability experiments were determined by MTT assay. For CAFs co-culture analysis, 4T1 cells were co-cultured with CAFs isolated from subcutaneous tumors of mice (4T1:CAFs 1:1) for 24 hrs. MCF-7 cells were co-cultured with CAFs isolated from patients’ breast tumor tissues (MCF-7:CAFs 1:1) for 24 hrs. Then, those cells were collected and separated by CD90 marker for further cell viability analysis. For collagen or CAFs cultured medium-associated analysis, 4T1 or MCF-7 were pre-treated with Type I collagen (0.5 mg/mL), CAFs culture medium (2 mL culture medium for 105 CAFs were collected after 12 hrs culture), CAFs culture medium with 0.05 mg/mL Type I collagenase for 12 hrs. After that, 3000 4T1 or MCF-7 cells were seeded into 96-well culture plates; 12 hrs later, cells were treated with PTX or VCR in different concentrations. After 24 hrs, cell growth was measured after addition of 10 μL 0.5 mg/mL MTT solution. After 4 hrs incubation at 37°C, the medium was replaced with 100 μL dimethylsulfoxide and vortexed for 10 mins. Absorbance was measured at 570 nm by a microplate reader (Bio-Rad, Hercules, CA, USA). The PBS group served as the control and Cells treated with PBS are considered as 100% viability. Each experiment was performed for at least three times.
Quantitative PCR (qPCR)
Total RNA was extracted from different samples with TRIzol reagent (Invitrogen, Waltham, MA, USA) following the manufacturer’s protocol. Briefly, total RNA were fragmented, followed by reverse transcription and cDNA synthesis with SuperScript™ III One-Step RT-PCR System (Invitrogen). qPCR was performed using the cDNA with the following primer pairs for integrin β1: 5ʹ-CCTACTTCTGCACGATGTGATG-3ʹ (forward) and 5ʹ-CCTTTGCTACGGTTGGTTACATT-3ʹ (reverse); IGFR: 5ʹ-TCGACATCCGCAACGACTATC-3ʹ (forward) and 5ʹ-CCAGGGCGTAGTTGTAGAAGAG-3ʹ (reverse); IL-6R: 5ʹ-CCCCTCAGCAATGTTGTTTGT-3ʹ (forward) and 5ʹ-CTCCGGGACTGCTAACTGG-3ʹ (reverse); HGFR: 5ʹ-AGCAATGGGGAGTGTAAAGAGG-3ʹ (forward) and 5ʹ-CCCAGTCTTGTACTCAGCAAC-3ʹ (reverse); VEGFR: 5ʹ-GGCCCAATAATCAGAGTGGCA-3ʹ (forward) and 5ʹ-CCAGTGTCATTTCCGATCACTTT-3ʹ (reverse); PDGFR: 5ʹ-AGCACCTTCGTTCTGACCTG-3ʹ (forward) and 5ʹ-TATTCTCCCGTGTCTAGCCCA-3ʹ (reverse); EGFR: 5ʹ-AGGCACGAGTAACAAGCTCAC-3ʹ (forward) and 5ʹ-ATGAGGACATAACCAGCCACC-3ʹ (reverse); SDF-1R: 5ʹ-ACTACACCGAGGAAATGGGCT-3ʹ (forward) and 5ʹ-CCCACAATGCCAGTTAAGAAGA-3ʹ (reverse); integrin β3: 5ʹ-GTGACCTGAAGGAGAATCTGC-3ʹ (forward) and 5ʹ-CCGGAGTGCAATCCTCTGG-3ʹ (reverse). Real-time PCR analyses were performed with cDNA as a template, using an SYBR Green mix (Applied Bioscience, USA) and ABI stepone plus (Applied Biosystems, Waltham, MA, USA). Values are mean ± SEM from three independent experiments which were performed in triplicate. Values of all parameters were considered statistically significant difference at a value of P<0.05.
Western blotting
Cells were lysed in cold RIPA lysis buffer supplemented with Protease Inhibitors cocktail (CST Inc., USA). The protein concentration was quantified using BCA protein assay kit (Beyotime, China) following the manufacturer’s introduction. Twenty micrograms of protein were mixed with 1× loading buffer and boiled for 5 mins at 100°C and were resolved by 8% or 10% SDS-PAGE and transferred to the nitrocellulose filter membrane (Bio-Rad). Target protein was detected using specific primary antibody, including: anti-Integrin β1 (1:1000; Abcam, Cambridge, UK), anti-PI3K (1:1000; Abcam), anti-Akt (1:1000; Abcam), anti-phospho-PI3K (1:1000; Abcam), anti-phospho-Akt (1:1000; Abcam). All experiments were repeated three independent times and the target protein level was quantified by ImageJ and normalized to internal control.
Flow cytometry
To examine the percentage of CAFs in breast cancer patient tissues, the isolated cells were incubated with CD90 (eBioscience, USA) for 30 mins at room temperature, after washed twice and then re-suspended in PBS. Flow cytometry was performed on the BD Canto II (BD Biosciences, USA). 7-AAD was used to exclude the dead cells. IgG (eBioscience) was used as the negative control.
CRISPR–Cas9
pSpCas9(BB)-2A-GFP (PX458) (a gift from F. Zhang, Addgene plasmid #48,138) was used to generate integrin β1 (human) and integrin β1 (mouse) in vitro genome editing. Two sgRNA (single guide RNAs) sequences, sg#1 5ʹ-GTGGAGAATGTATACAAGCA-3ʹ and sg#2 5ʹ-AAGCAGGGCCAAATTGTGGG-3ʹ, for integrin β1 (human) were predicted by Zhang Lab’s website (http://crispr.mit.edu/). Similarly, two sgRNA sequences, sg#1 5ʹ-AGTGACATAGAGAATCCCAG-3ʹand sg#2 5ʹ-GCCAGAAGACATTACTCAGA-3ʹ, for integrin β1 (mouse). To construct the px458-integrin β1-KO plasmid or px458-integrin β1-KO plasmid, PX458 was digested and ligated with annealed sgRNAs according to the methods provided by the relevant publications.12,13 To generate the knockout cell lines, 4 μg sg1 and sg2 plasmid were transfected into 105 MCF-7 or 4T1 with Lipofectamine® 3000 transfection reagent (Thermo, USA) according to the methods provided by the manufacturer’s instructions. After 48 hrs, high GFP expression cells were sorted by into single clones into the 96-well plate flow cytometry (BD Biosciences FACS Aria II, USA). The single clones were cultured in 96-well plates for another 14 days or a longer time dependent upon the cell growth rate. T7-endonuclease assay or anti-integrin β1 or integrin β1 immunoblotting was used to screen for the integrin β1 deficient clones, respectively. Genome type of the knockout cells was determined by DNA sequencing.
Immunofluorescence staining
Tumor tissues were kept in 4% paraformaldehyde (PFA) overnight, then processed, embedded in paraffin, and sectioned at 4 μm for further study. Then, the sections or breast cancer cells (fixed with 4% PFA and permeabilized with 0.3% Triton X-100) were blocked with 5% BSA in PBS and incubated with integrin β1 (1:300; Abcam), p-PI3K (1:200; Abcam) or p-AKT (1:300; Abcam) at 4°C overnight, followed by secondary antibodies (Thermo, USA) for 1 hr at room temperature. Nuclei was stained with the DAPI solution (1 µg/mL). Confocal microscope (Olympus, Japan) was used to visualize the sections.
Immunohistochemistry
The sections of tumors were incubated with α-SMA (1:200; Abcam), collagen (1:200; Abcam) or integrin β1 (1:100; Abcam) at 4°C overnight, followed by signal amplification using an ABC HRP Kit (Thermo, USA) and counter-staining with hematoxylin, dehydration with series of graded ethanol and cleaned with xylene. Microscope (Leica, Wetzlar, Germany) was used to visualize the sections.
Animal protocol
To investigate the drug resistance induced by collagen, female nude mice (6–8 weeks) were injected s.c. with 3×106 MCF-7 cells into the right flank. After tumor growth, up to 2×2 mm, mice were randomized into different groups based on similar tumor size and body weight. And then, these mice were treated with collagen (10 μg each mice), CAFs culture medium (10×50 μl each mice) CAFs culture medium with collagenase (1 μg in culture medium) by intratumoral injection every 2 days for a week. After that, the mice were treated with PTX (5 μg each mice) by intratumoral injection every 2 days for a week. The mice of control groups received an equal volume of saline. The incidence of tumor in mice and the survival of mice were recorded. Tumor volume was calculated according to the formula: tumor volume=length×width2/2.
To investigate the anticancer effects of GRGDSP combined with PTX or VCR, female nude mice (6–8 weeks) were injected s.c. with 3×106 MCF-7 cells into the right flank. After tumor growth, up to 2×2 mm, mice were randomized into different groups based on similar tumor size and body weight. And then, these mice were treated with 10 μg collagen (or not) by intratumoral injection. After 2 days, mice were treated with PBS, PTX (5 μg each mice), VCR (3 μg each mice), GRGDSP (10 μg each mice), and GRGDSP combined with PTX or VCR. The mice of control groups received an equal volume of saline. The incidence of tumor in mice and the survival of mice were recorded. Tumor volume was calculated according to the formula: tumor volume=length×width2/2. All our animal experiments were conducted in accordance with guidelines approved by the Institute Ethics Committee of the Second Affiliated Hospital of Zhejiang Chinese Medical University (IRB number: SYXK(ZHE)2015-0012).
Statistical analysis
Results were presented as mean ± SEM and statistical significance was examined by an unpaired Student’s t-test by the Graphpad 6.0 software. Survival analysis was performed by Kaplan–Meier method and evaluated using the log-rank test. P<0.05 was considered as statistically significant.
Results
Enriched CAFs result in the microtubule-directed chemotherapeutic drugs resistance in breast cancer
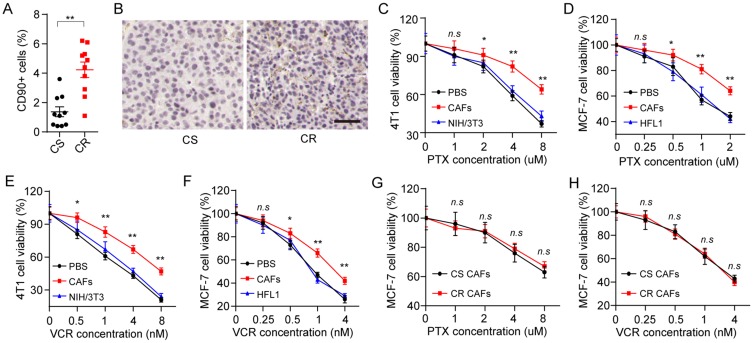
In our studies, breast cancer patients’ tumor tissues were collected and divided into CS group and chemo-resistant (CR) group. Intriguingly, increased CAFs in tumor tissues were observed in CR group compared with the CS group (Figure 1A). Accordingly, the expression of α-SMA, a marker of CAFs in tumor tissues, was significantly up-regulated in CR group, indicating the potential association between CAFs and drug-resistance development in breast cancer (Figure 1B). Next, we isolated the CAFs from the mice tumors and patients’ tumor tissues, then treated the breast cancer cells lines with PTX after co-culture with or without those CAFs. We found that the pre-treatment of CAFs resulted in the enhanced PTX resistance in 4T1 (Figure 1C) and MCF-7 (Figure 1D), whereas the normal fibroblasts cell lines NIH/3T3 and HFL1 could not induce the drug resistance of breast cancer cell lines. PTX is a diterpene alkaloid, which targets the cellular microtubules to efficiently inhibit the tumor growth. And the same results were observed in VCR (Figure 1E and F), a microtubule-directed chemotherapeutic agent in breast cancer therapy, suggesting that CAFs could facilitate the microtubule-directed chemotherapeutic drugs resistance development in breast cancer. Next, we wondered whether the CAFs from CR tumor tissues are different from the CAFs from CS tumor tissues, leading to the chemotherapy failure in clinic. We isolated the CAFs from CR and CS tumor tissues and used those CAFs to treat MCF-7 cells. MCF-7 cells treated with CAFs from CR or CS tumor tissues showed no drug resistance-associated difference to PTX (Figure 1G) or VCR (Figure 1H). Considering the enriched CAFs in CR tumor tissues (Figure 1A) and the drugs response of MCF-7 treated with CS or CR tumor tissues derived CAFs, we concluded that the CAFs from different breast tumor tissues could induce the microtubule-directed drugs resistance and the amount of CAFs is the key for the drugs resistance development.
Figure 1.
CAFs promote the microtubule-directed chemotherapeutic drugs resistance in breast cancer. (A) The percentage of CAFs (CD90+ cells) in tumor tissues from CR and CS patients. (B) The immunohistochemistry of α-SMA in tumor tissues from CR and CS patients. The scale bar is 50 μm. (C–-D) The cell viability of 4T1 (C) and MCF-7 (D) was detected after treatment of different concentrations of PTX pre-co-cultured with or without CAFs or normal fibroblasts. (E–F) The cell viability of 4T1 (E) and MCF-7 (F) was detected after treatment of different concentrations of VCR pre-co-cultured with or without CAFs or normal fibroblasts. (G–H) The cell viability of MCF-7 was detected after treatment of different concentrations of PTX (G) or VCR (H) pre-co-cultured with CAFs from CS patients or CR patients. *P<0.05 and **P<0.01.
Abbreviations: n.s, not statistically significant; CAF, cancer associated fibroblasts; CS, chemo-sensitive; CR, chemo-resistant; PTX, paclitaxel; VCR, vincristine sulfate.
CAFs regulate the drugs resistance through secretion of collagen
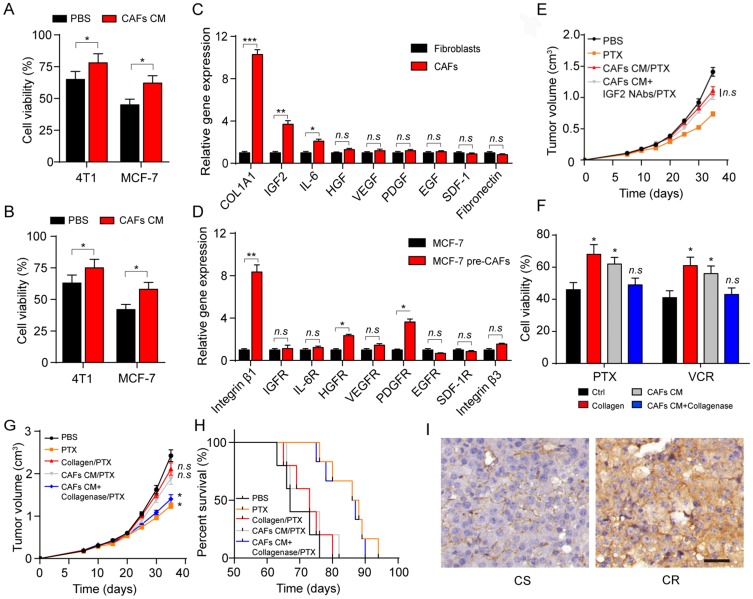
Next, we wonder how CAFs participate in the drug resistance development in breast cancer. Current thinking suggests that CAFs can remodel the surrounding cells through secreting soluble factors. So, we added the cultured medium of CAFs into the tumor cells, following by the treatment of PTX and VCR. And we found enhanced drug resistance of breast cancer cells after the pre-treatment of CAFs cultured medium (Figure 2A and B), indicating that the CAFs might regulate the tumor progression via the secretion of soluble factors. To further investigate the exact factor of CAFs induced resistance, we detected the major cytokines expression of type I collagen, IGF2, IL-6, VEGF, PDGF, EGF and SDF-1 and fibronectin in CAFs. And, we observed the increasing expression of type I collagen, IGF2 and IL-6 in CAFs compared with the normal fibroblasts (Figure 2C). Correspondingly, we also screened the receptors of those cytokines in tumor cells and the collagen receptor integrin β1 had significantly increasing expression in MCF-7 cells treated with CAFs (Figure 2D). Previous results indicated that type I collagen and IGF2 could participate in the tumor progressions and induced the tumor sustained growth or drug resistance development.14,15 Firstly, we used the CAFs cultured medium to treat the MCF-7 bearing mice to induce the PTX drug-resistant breast cancer model. Then, mice were treated with PTX or IGF2 neutralizing antibody. Intriguingly, the IGF2 neutralizing antibody did not reverse the PTX resistance induced by CAFs cultured medium (Figure 2E), suggesting that CAFs regulated breast cancer cells drug resistance through an IGF2 independent pathway. Herein, we supposed that collagen may work as a key player to mediate the microtubule-directed chemotherapeutic drugs resistance in breast cancer.
Figure 2.
Collagen produced from CAFs results in the drug resistance in breast cancer. (A–B) The cell viability of 4T1 and MCF-7 to 2 μM PTX (A) and 1 nM VCR (B) for 24 hrs was detected after treatment of CAFs cultured medium. (C) The relative mRNA level expression of type I collagen, IGF2, IL-6, HGF, VEGF, PDGF, EGF, SDF-1 and fibronectin expression in normal fibroblasts (HFL1) and CAFs (patients tissues derived). (D) The relative mRNA level expression of integrin β1, IGFR, IL-6R, HGFR, VEGFR, PDGFR, EGFR, SDF-1R and integrin β3 expression in MCF-7 and MCF-7 co-cultured with CAFs (patients tissues derived). (E) The mean tumor volume of MCF-7-bearing mice treated with PBS, PTX, CAFs cultured medium combined with PTX, IGF2 neutralizing antibodies (0.2 μg/g) combined with CAFs cultured medium and PTX. The data were presented as the mean ± SEM from three independent experiments. (F) The cell viability of MCF-7 pre-treated with collagen (0.5 mg/mL, 12 hrs), CAFs cultured medium (12 hrs) and CAFs cultured medium with collagenase (0.05 mg/mL, 12 hrs) to PTX (2 μM, 24 hrs) and VCR (1 nM, 24 hrs). (G) The tumor volumes of MCF-7-bearing mice treated with PBS, PTX, PTX and collagen, PTX and CAFs cultured medium, PTX and CAFs cultured medium with collagenase. (H) The survival time of MCF-7-bearing mice treated with PBS, PTX, PTX and collagen, PTX and CAFs cultured medium, PTX and CAFs cultured medium with collagenase. (I) The immunohistochemistry of type I collagen in tumor tissues from CR and CS patients. The scale bar is 50 μm. The data were presented as the mean ± SEM from three independent experiments. *P<0.05; **P<0.01; ***P<0.001.
Abbreviations: n.s, not statistically significant; CAF, cancer associated fibroblasts; PTX, paclitaxel.
To further verify our hypothesis, we treated MCF-7 cells with collagen, CAFs cultured medium with or without collagenase. In line with our hypothesis, MCF-7 cells treated with collagen or CAFs cultured medium showed enhanced PTX or VCR resistance while the addition of Type I collagenase in CAFs cultured medium efficiently reversed the acquired drug resistance in breast cancer cells (Figure 2F). Next, we injected collagen, CAFs cultured medium, CAFs cultured medium combined with Type I collagenase into the MCF-7 tumor-bearing mice. Then, we treated the mice with PTX by tail vein injection. Intriguingly, injection of CAFs cultured medium or collagen remarkably suppressed the PTX efficacy to inhibit the tumors growth, while the addition of Type I collagenase in CAFs culture medium reversed the phenomenon (Figure 2G). The same result was observed in the survival time of mice (Figure 2H), which is in line with our results in vitro. Furthermore, enhanced collagen expression in tumor tissues from CR breast cancer patients compared with CS breast cancer patients (Figure 2I). Together, these data suggested that collagen secreted by CAFs participates in the breast cancer cells to acquire microtubule-directed chemo-resistance.
Collagen regulates the drug resistance via the integrin β1/PI3K/AKT signaling pathway
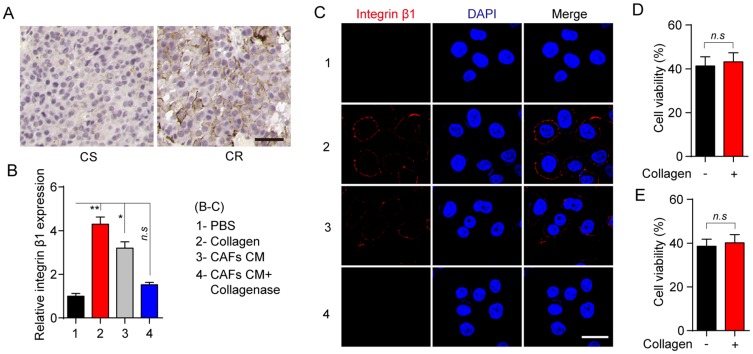
Integrin family is heterodimeric cell surface receptor, which is associated with the cellular adhesion to the extracellular matrix and signal transduction.16–18 Increasing evidence indicates that collagen could induce the tumor progressions through the integrin β1 associated signal transduction.17,19,20 Intriguingly, we found enhanced expression of integrin β1 in tumor tissues from CR patients compared with the CS patients (Figure 3A). Furthermore, increasing expression of integrin β1 was observed in MCF-7 treated with collagen or CAFs cultured medium, while the addition of collagenase efficiently reversed the inducible expression of integrin β1 (Figure 3B and C). More importantly, knockout of integrin β1 in MCF-7 cells significantly reversed the PTX resistance induced by the collagen (Figure 3D). The same result was observed in VCR treatment (Figure 3E), indicating that integrin β1 participates in the inducible microtubule-directed chemo-resistance development in breast cancer.
Figure 3.
Collagen induces the microtubule-directed chemotherapeutic drugs resistance through the integrin β1. (A) The immunohistochemistry of integrin β1 in breast tumor tissues from CR and CS patients. The scale bar is 50 μm. (B) Relative expression of integrin β1 in MCF-7 pre-treated with collagen (0.5 mg/mL, 12 hrs), CAFs cultured medium (12 hrs) and CAFs cultured medium with collagenase (0.05 mg/mL, 12 hrs). (C) The immunofluorescence of integrin β1 in MCF-7 pre-treated with collagen (0.5 mg/mL, 12 hrs), CAFs cultured medium (12 hrs) and CAFs cultured medium with collagenase (0.05 mg/mL, 12 hrs). The scale bar is 15 μm. (D, E) The cell viability of integrin β1 knockout MCF-7 treated with PBS or collagen (0.5 mg/mL, 12 hrs) to 2 μM PTX (D) and 1nM VCR (E) for 24 hrs. The data were presented as the mean ± SEM from three independent experiments. *P<0.05 and **P<0.01.
Abbreviations: n.s, not statistically significant; CAF, cancer associated fibroblasts; PTX, paclitaxel.
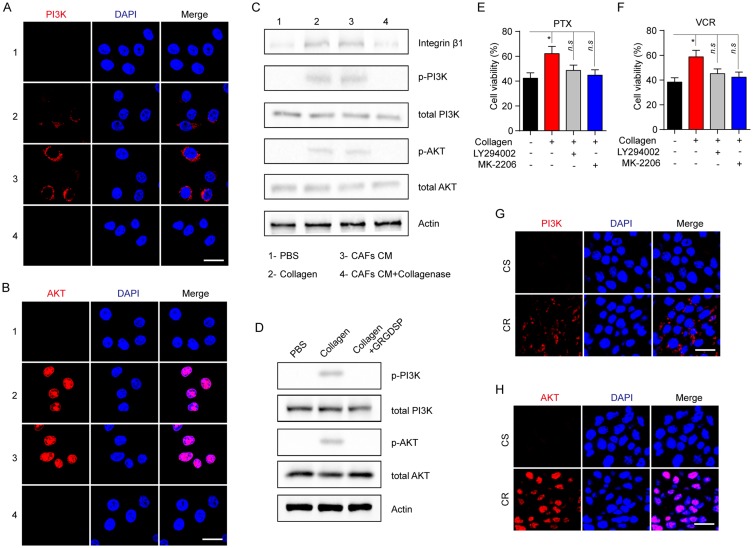
As a classic downstream molecular of the integrin β1 signaling axis, PI3K/AKT is involved in cell cycle, metabolism, sustained growth and drug resistance. Increasing evidence demonstrates PI3K/AKT signaling pathway is correlative in the acquired drug-resistance development in various solid tumors. Therefore, we evaluated the phosphorylation of PI3K and AKT in MCF-7 cells pre-cultured with collagen, CAFs cultured medium and collagenase. Increased expression of p-PI3K and p-AKT was observed in cells treated with CAFs cultured medium or collagen while the addition of collagenase in CAFs cultured medium reversed the phenomenon (Figure 4A and B), indicating that collagen might activate the PI3K/AKT pathway induced by integrin β1. To further expound the signaling pathway induced by collagen, we detected the expression of integrin β1, p-PI3K, total PI3K, p-AKT, total AKT and β-actin in MCF-7 by Western blotting and found the activation of integrin β1/PI3K/AKT signaling pathway under the condition of CAFs cultured medium or collagen treatment, while adding type I collagenase reversed the phenomenon (Figure 4C). Besides, blockade of integrin β1 also invalidated the activation of PI3K/AKT signal induced by collagen (Figure 4D).
Figure 4.
Collagen induces the drugs resistance via the activation of PI3K/AKT signaling pathway. (A, B) The immunofluorescence of p-PI3K (A) and p-AKT (B) in MCF-7 treated with collagen (0.5 mg/mL, 12 hrs), CAFs cultured medium (12 hrs) and CAFs cultured medium with collagenase (0.05mg/mL, 12 hrs). The scale bar is 15 μm. (C) The Western blotting of integrin β1, p-PI3K, total PI3K, p-AKT, total AKT and actin in MCF-7 treated with collagen (0.5 mg/mL, 12 hrs), CAFs cultured medium (12 hrs) and CAFs cultured medium with collagenase (0.05 mg/mL, 12 hrs). (D) The Western blotting of p-PI3K, total PI3K, p-AKT, total AKT and actin in MCF-7 treated with collagen (0.5 mg/mL, 12 hrs) and GRGDSP (1 μM, 12 hrs). (E, F) The cell viability of MCF-7 treated with collagen (0.5 mg/mL, 12 hrs), LY294002 (1 μM, 12 hrs) and MK-2206 (10 nM, 12 hrs) to 2 μM PTX (E) and 1nM VCR (F) for 24 hrs. (G, H) The immunofluorescence of p-PI3K (G) and p-AKT (H) in tumor tissues from CR and CS patients. The scale bar is 15 μm. The data were presented as the mean ± SEM from three independent experiments. *P<0.05.
Abbreviations: n.s, not statistically significant; CAF, cancer associated fibroblasts; PTX, paclitaxel.
As phosphorylation of PI3K/AKT reflects the active form, these data suggested that PI3K/AKT signaling may be a key player in the collagen-induced drug resistance. In support of this idea, PI3K inhibitor (LY294002) or AKT inhibitor (MK-2206) was used to reverse the acquired drug resistance induced by the collagen. And we found that the inhibition of PI3K or AKT reversed the PTX and VCR drug resistance induced by collagen (Figure 4E and F). Also, the enhanced expression of PI3K/AKT was observed in tumor tissues from the CR patients compared to the CS patients (Figure 4G and H). Collectively, these results reminded us that the integrin β1/PI3K/AKT signaling pathway activation is involved in collagen-induced microtubule-directed drug resistance in breast cancer cells.
Blockade of integrin β1 relieves microtubule-directed chemo-resistance in breast cancer
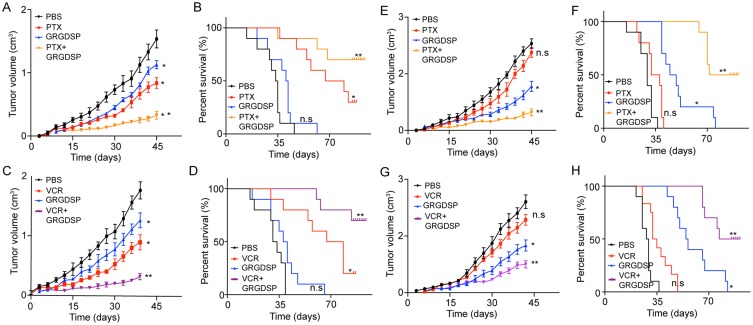
To further investigate the potential therapeutic strategies in clinic breast cancer, we generated the xenograft mouse bearing MCF-7 cells model and treated with PTX, GRGDSP (an integrin β1 inhibitor) or the combination. We found that both the PTX and GRGDSP could inhibit the tumor growth, but the combination of chemotherapeutic agent and integrin β1 inhibitor remarkably slowed the tumor growth (Figure 5A). Accordingly, the combination of PTX and GRGDSP significantly prolonged survival time compared to control group, while monotherapy showed slight anti-cancer effects (Figure 5B). Similar results were achieved when treated MCF-7-bearing mice with VCR and GRGDSP (Figure 5C and D). These results indicated that blocking integrin β1 may be helpful to enhance the chemotherapeutic effect for breast cancer.
Figure 5.
Blockade of integrin β1 reverses the microtubule-directed chemotherapeutic drugs resistance induced by collagen. (A, B) The mean tumor volume (A) and survival time (B) of MCF-7-bearing mice treated with PBS, PTX, GRGDSP and PTX combined with GRGDSP. (C, D) The mean tumor volume (C) and survival time (D) of MCF-7-bearing mice treated with PBS, VCR, GRGDSP and VCR combined with GRGDSP. (E, F) The mean tumor volume (E) and survival time (F) of MCF-7 (pre-treated with collagen) bearing mice treated with PBS, PTX, GRGDSP and PTX combined with GRGDSP. (G, H) The mean tumor volume (G) and survival time (H) of MCF-7 (pre-treated with collagen) bearing mice treated with PBS, VCR, GRGDSP and VCR combined with GRGDSP. The data were presented as the mean ± SEM from three independent experiments. *P<0.05 and **P<0.01.
Abbreviations: n.s, not statistically significant; PTX, paclitaxel.
Next, we further investigated the anticancer effects in drug-resistant breast cancer models. Here, we established the xenograft mouse bearing MCF-7 cells model and then pre-treated with collagen. Next, mice were treated with PTX, GRGDSP or the combination. The collagen treated tumors showed significant resistance to the PTX, while combining PTX with GRGDSP successfully suppressed the tumor growth (Figure 5E). Accordingly, combining PTX with GRGDSP significantly prolonged survival time (Figure 5F). Similar results were achieved in mice treated with VCR combined with GRGDSP (Figure 5G and H). Together, these results suggested the potential therapeutic value of GRGDSP combining with microtubule-directed chemotherapeutic drugs in breast cancer treatment.
Discussion
In our study, we found enriched CAFs in tumor tissues from drug-resistant breast cancer patients. We isolated those CAFs and observed enhanced microtubule-directed chemotherapeutic drugs resistance in cancer cells after co-culture with those CAFs. Increasing evidence reveals that CAFs could participate in different stages of tumor progression, including cancer stem cell maintenance, epithelial–mesenchymal transition of tumor cells, drug resistance development and so on.21 Our studies proved that CAFs could facilitate the microtubule-directed chemotherapeutic drugs resistance by secretion of collagen to activate the PI3K/AKT signaling pathway in breast cancer. Moreover, blockade of integrin β1 efficiently reverses the PTX and VCR resistance in breast cancer, which might serve as new potential target for anticancer therapy.
It has been demonstrated that several crucial features are believed to be associated with multi-drugs resistance development, including the overexpression of ABC transporter proteins, up-regulation of anti-apoptotic proteins such as BCL-2, DNA damage repair and the regulation of tumor microenvironment.22,23 Previous studies revealed types of factors involved in the tumor microenvironment, including infiltrating immune cells, extracellular matrix, hypoxia, CAFs and so on, are crucial for the tumor progression.3,10,24,25 However, traditional cancer treatments focus on suppressing the cancer cells proliferation but ignoring the tumor microenvironment elements in tumor growth. More importantly, increasing evidence indicates that the tumor microenvironment could promote the drug resistance development of normal cancer cells and tumor tissues could reacquire the drug resistance under the appropriate microenvironment even after chemotherapy.26 Herein, we revealed the function of CAFs in breast cancer cells microtubule-directed chemotherapeutic drugs resistance development. And blocking the interaction between CAFs and cancer cells could serve as the basis of the innovative treatment in drug-resistant cancer cells.
CAFs are crucial elements in tumor microenvironment, which are involved in the progressions of tumorigenesis, metastasis and drug resistance development.9,10,21,24 However, the specific mechanism of CAFs induced drug resistance remains less well understood. It has been reported that several cytokines, such as IGF2 and SDF1, are involved in the tumor-sustained growth.27 The TGF-β1 and basic fibroblast growth factor produced by CAFs could also activate important signaling pathways, including Wnt, Notch and the epithelial–mesenchymal transition in cancer cells.28 Our data further demonstrated that CAFs regulate the microtubule-directed chemotherapeutic drugs resistance in a paracrine manner through the secretion of collagen in breast cancer. Moreover, we confirmed the importance of the integrin β1 and PI3K/AKT signaling pathway in regulating the drug resistance in primary breast tumor. Hence, GRGDSP, an inhibitor of integrin β1, could serve as a potential agent to block the collagen/integrin β1/PI3K/AKT signaling pathway induced by CAFs and be combined with microtubule-directed chemotherapeutic agents to inhibit breast cancer progression.
In conclusion, we described a novel role of CAFs in microtubule-directed chemotherapeutic drugs resistance in breast cancer. The finding that CAFs facilitate the drugs resistance through the PI3K/AKT induced by collagen in breast cancer further explains the potential association between tumor development and microenvironment. And the combination of integrin inhibitors and chemotherapeutic agents might be explored as a potential therapeutic strategy to breast cancer therapy.
Acknowledgment
This work was supported by Zhejiang Provincial Medicine Health Science and Technology Program (2015KYA170, Guoqiang Huang).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–448. doi: 10.3322/caac.21412 [DOI] [PubMed] [Google Scholar]
- 3.DeMichele A, Yee D, Esserman L. Mechanisms of resistance to neoadjuvant chemotherapy in breast cancer. N Engl J Med. 2017;377(23):2287–2289. doi: 10.1056/NEJMcibr1711545 [DOI] [PubMed] [Google Scholar]
- 4.Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67(3):194–232. doi: 10.3322/caac.21397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duarte AA, Gogola E, Sachs N, et al. BRCA-deficient mouse mammary tumor organoids to study cancer-drug resistance. Nat Methods. 2018;15(2):134–140. doi: 10.1038/nmeth.4535 [DOI] [PubMed] [Google Scholar]
- 6.Nakasone ES, Askautrud HA, Kees T, et al. Imaging tumor–stroma interactions during chemotherapy reveals contributions of the microenvironment to resistance. Cancer Cell. 2012;21(4):488–503. doi: 10.1016/j.ccr.2012.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell. 2017;170(3):548–63.e16. doi: 10.1016/j.cell.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quante M, Tu SP, Tomita H, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19(2):257–272. doi: 10.1016/j.ccr.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasashima H, Yashiro M, Kinoshita H, et al. Lysyl oxidase-like 2 (LOXL2) from stromal fibroblasts stimulates the progression of gastric cancer. Cancer Lett. 2014;354(2):438–446. doi: 10.1016/j.canlet.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 10.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–598. doi: 10.1038/nrc.2016.73 [DOI] [PubMed] [Google Scholar]
- 11.Johnson P, Beswick EJ, Chao C, Powell DW, Hellmich MR, Pinchuk IV. Isolation of CD90+ fibroblast/myofibroblasts from human frozen gastrointestinal specimens. J Vis Exp. 2016;2016(107):e53691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ran FA, Hsu Patrick D, Lin CY, et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell. 2013;154(6):1380. doi: 10.1016/j.cell.2013.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott DA, Ran FA, Zhang F, Wright J, Hsu PD, Agarwala V. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;32(12):815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murayama T, Nakaoku T, Enari M, et al. Oncogenic fusion gene CD74-NRG1 confers cancer stem cell-like properties in lung cancer through a IGF2 autocrine/paracrine circuit. Cancer Res. 2016;76(4):974–983. doi: 10.1158/0008-5472.CAN-15-2135 [DOI] [PubMed] [Google Scholar]
- 15.Saladino S, Salamone M, Ghersi G. MDA-MB-231 and 8701BC breast cancer lines promote the migration and invasiveness of ECV304 cells on 2D and 3D type-I collagen matrix. Cell Biol Int. 2017;41(9):1030–1038. doi: 10.1002/cbin.10817 [DOI] [PubMed] [Google Scholar]
- 16.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15(12):802–812. doi: 10.1038/nrm3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauthier NC, Roca-Cusachs P. Mechanosensing at integrin-mediated cell-matrix adhesions: from molecular to integrated mechanisms. Curr Opin Cell Biol. 2018;50:20–26. doi: 10.1016/j.ceb.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 18.Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9 [DOI] [PubMed] [Google Scholar]
- 19.Boguslawska J, Rodzik K, Poplawski P, et al. TGF-beta1 targets a microRNA network that regulates cellular adhesion and migration in renal cancer. Cancer Lett. 2018;412:155–169. doi: 10.1016/j.canlet.2017.10.019 [DOI] [PubMed] [Google Scholar]
- 20.Mygind KJ, Schwarz J, Sahgal P, Ivaska J, Kveiborg M. Loss of ADAM9 expression impairs beta1 integrin endocytosis, focal adhesion formation and cancer cell migration. J Cell Sci. 2018;131(1). doi: 10.1242/jcs.216507 [DOI] [PubMed] [Google Scholar]
- 21.Shi Y, Du L, Lin L, Wang Y. Tumour-associated mesenchymal stem/stromal cells: emerging therapeutic targets. Nat Rev Drug Discov. 2017;16(1):35–52. doi: 10.1038/nrd.2016.193 [DOI] [PubMed] [Google Scholar]
- 22.Nigam SK. What do drug transporters really do? Nat Rev Drug Discov. 2015;14(1):29–44. doi: 10.1038/nrd4461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi YJ, Liang YJ, Huang HB, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res. 2010;70(20):7981–7991. doi: 10.1158/0008-5472.CAN-10-0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa T, Yashiro M, Nishii T, et al. Cancer-associated fibroblasts might sustain the stemness of scirrhous gastric cancer cells via transforming growth factor-beta signaling. Int J Cancer. 2014;134(8):1785–1795. doi: 10.1002/ijc.28520 [DOI] [PubMed] [Google Scholar]
- 25.Bocci G, Kerbel RS. Pharmacokinetics of metronomic chemotherapy: a neglected but crucial aspect. Nat Rev Clin Oncol. 2016;13(11):659–673. doi: 10.1038/nrclinonc.2016.64 [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Kryczek I, Dostal L, et al. Effector T cells abrogate stroma-mediated chemoresistance in ovarian cancer. Cell. 2016;165(5):1092–1105. doi: 10.1016/j.cell.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plymate SR, Bhatt RS, Balk SP. Taxane resistance in prostate cancer mediated by AR-independent GATA2 regulation of IGF2. Cancer Cell. 2015;27(2):158–159. doi: 10.1016/j.ccell.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 28.Zhu H, Gu X, Xia L, et al. A novel TGF-beta trap blocks chemotherapeutics-induced TGF-beta1 signaling and enhances their anticancer activity in gynecological cancers. Clin Cancer Res. 2018;24(12)2780–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]