Abstract
Background
With antiretroviral therapy (ART), AIDS-defining cancer incidence has declined and non-AIDS–defining cancers (NADCs) are now more frequent among human immunodeficiency virus (HIV)–infected populations in high-income countries. In sub-Saharan Africa, limited epidemiological data describe cancer burden among ART users.
Methods
We used probabilistic algorithms to link cases from the population-based cancer registry with electronic medical records supporting ART delivery in Malawi’s 2 largest HIV cohorts from 2000–2010. Age-adjusted cancer incidence rates (IRs) and 95% confidence intervals were estimated by cancer site, early vs late incidence periods (4–24 and >24 months after ART start), and World Health Organization (WHO) stage among naive ART initiators enrolled for at least 90 days.
Results
We identified 4346 cancers among 28 576 persons. Most people initiated ART at advanced WHO stages 3 or 4 (60%); 12% of patients had prevalent malignancies at ART initiation, which were predominantly AIDS-defining eligibility criteria for initiating ART. Kaposi sarcoma (KS) had the highest IR (634.7 per 100 000 person-years) followed by cervical cancer (36.6). KS incidence was highest during the early period 4–24 months after ART initiation. NADCs accounted for 6% of new cancers.
Conclusions
Under historical ART guidelines, NADCs were observed at low rates and were eclipsed by high KS and cervical cancer burden. Cancer burden among Malawian ART users does not yet mirror that in high-income countries. Integrated cancer screening and management in HIV clinics, especially for KS and cervical cancer, remain important priorities in the current Malawi context.
Keywords: HIV, antiretroviral therapy, cancer, Africa
In sub-Saharan Africa, limited epidemiological data describe cancer burden among antiretroviral therapy users. Integrated cancer screening and management in human immunodeficiency virus clinics, especially for Kaposi sarcoma and cervical cancer, remain important priorities in the current Malawi context.
Three AIDS-defining malignancies, Kaposi sarcoma (KS), cervical cancer, and non-Hodgkin lymphoma (NHL), are among the top 10 cancers in sub-Saharan Africa (SSA), where 70% of global human immunodeficiency virus (HIV) burden is concentrated [1, 2]. Rapid scale-up of antiretroviral therapy (ART) availability over the past decade [1] is likely to affect regional cancer burden. In high-income countries, cancer risk among HIV populations is evolving, with notable declines in risk of KS, NHL, and some non-AIDS–defining cancers (NADCs) over the course of ART expansion since 1996 [3]. The burden of certain NADCs is now projected to increase and surpass that of AIDS-defining cancers (ADCs) due to growth and aging of the population living with HIV [4]. However, extrapolations from high-income countries may not apply to SSA, where delays in accessing HIV care are substantial, advanced immunosuppression at ART initiation is common [5], and prevalence of oncogenic viral infections is high [6]. Epidemiological evidence is therefore needed to understand cancer trends specifically in SSA.
In the Malawi HIV-Cancer Match Study, we aim to characterize cancer incidence among ART initiators. In Malawi, HIV prevalence is 9% and ART coverage has reached 67%, using a threshold for ART eligibility of 500 CD4 cells/µL or World Health Organization (WHO) clinical stages 3 and 4 [7]. Our work differs from previous studies in the region [8–10], in that we used 2 of the largest, actively traced cohorts of ART users in the country over a period spanning national ART scale-up from 2000 through 2010. We also conducted cross-sectional linkage of cancers using the population-based national cancer registry.
METHODS
Populations
Study participants were HIV-infected people receiving ART at Lighthouse Trust (LT) and the Queen Elizabeth Central Hospital (QECH) HIV clinics. In the central region, LT in the capital, Lilongwe, is Malawi’s largest public ART provider. In the south, the QECH HIV clinic is situated in Blantyre, Malawi’s second largest city. Clinics use electronic monitoring systems to routinely collect demographic information, WHO stage at clinic enrollment, ADC clinical diagnoses (KS and cervical cancer only), drug regimens, and patient outcomes [11, 12]. Active tracing is used for patient follow-up and ascertainment of vital status. In Malawi, CD4 count measurement was historically restricted to stage 1 and 2 patients who were not clinically eligible for ART (eg, stages 3 and 4). For QECH prior to 2011, CD4 counts were not captured in the electronic monitoring system and therefore were not available for analysis. Routine HIV RNA monitoring in Malawi did not begin until 2011. Therefore, limited CD4 count data and no HIV RNA data were available during the time period of our study, reflecting practice within the Malawi national HIV program.
The population-based Malawi Cancer Registry (henceforth, the registry) is a founding member of the African Cancer Registry Network and 1 of only 5 cancer registries from SSA included in WHO international estimates of cancer [2]. Active case-finding procedures have been described previously [13]. During the study period, QECH housed the sole pathology laboratory for the entire country; a second laboratory opened at Kamuzu Central Hospital, Lilongwe, in 2011 [14]. Thus, historically only 18% of cases were pathologically confirmed, with most cancer diagnoses supported by clinical, radiological, and/or laboratory data [13]. Pathology confirmation rate is comparable to that of other cancer registries from SSA [15, 16].
Electronic Medical Record Linkage
In the absence of unique personal identifiers, we used probabilistic algorithms to link electronic medical records (EMRs) from ART cohorts with cancer records. All HIV-infected people who initiated ART at QECH from 2000 to 2010 or at LT from 2007 to 2010 were eligible based on years of registry coverage in Blantyre and Lilongwe, respectively. EMRs were matched on first and last name, year of birth, sex, and district of residence as these identifiers were shared across datasets. Consistent with other SSA settings, date of birth was estimated for a substantial proportion of records. Therefore, we allowed for 5-year age discrepancies between record pairs during subsequent iterations of the linkage [10]. Highest-weight classification was used to classify record pairs as matches, potential matches, or nonmatches, followed by manual adjudication of potential matches (Supplementary Materials, p. 3) [10]. Potential matches were manually reviewed and validated through clinical review by 3 senior Malawian investigators who used additional oncology treatment data collected by the registry and National Oncology Review Board, when available. Data preprocessing and probabilistic record linkage were performed in KNIME Analytics Platform, version 2.12.1 (Konstanz, Germany). Data pre- and post-processing was performed using Stata 14 (Stata Corporation, College Station, TX). Analyses were conducted in SAS software 9.4 for Windows (Cary, NC). The University of North Carolina Institutional Review Board and the University of Malawi College of Medicine Research Ethics Committee approved the study.
Study Design and Statistical Analyses
We used an observational multicohort design. ART-naive individuals were eligible for analysis if they had a first clinic date that occurred between 1 January 2000 and 30 August 2010 at QECH and 1 January 2007 and 30 August 2010 at LT and were followed for at least 90 days. The event of interest was the first incident primary cancer diagnosed either clinically or pathologically more than 90 days after ART start. As in other record linkage studies in the United States [17] and Africa [10], the 90-day window accounts for clinical workup of prevalent cancers as patients enter the medical system. Cancers were identified either through registry linkage or EMR. We further classified new cancer as early incident (4 to 24 months after ART start) and late incident (>24 months). Person-years at risk for incident cancer was calculated from 90 days after ART enrollment to the earliest of cancer diagnosis or censor due to ART cessation, clinic transfer, last contact, death, or 1 October 2015 administrative censor. For those lost to follow-up, person-time at risk included a 180-day window past the missed appointment date. Cancer diagnoses that were linked beyond the last date of contact or the 180-day window were excluded from primary analyses (n = 36). Subsequent multiple primaries of different anatomical site or histology (n = 32) and prevalent malignancies defined as a diagnosis prior to enrollment or within 90 days of ART start (n = 3463) were excluded from incidence rate analysis (Supplementary Figures 1–2). Cancer data were coded using the International Classification of Disease for Oncology (Supplementary Table 1) [18].
Incidence rates (IRs) and 95% confidence intervals (CIs) were calculated for individual cancer sites, early vs late incidence periods, and WHO stage at clinic enrollment. Rates were age-adjusted to the 2007 Malawi standard population (ages 0–14, 15–24, 25–34, 35–44, 45–54, 55+ years) [19]. We also conducted a sensitivity analysis using only cancer matches with the highest linkage weights to estimate a conservative lower bound on cancer-specific IR (Supplementary Table 3). Age-adjusted rates to the World Standard are also provided (Supplementary Table 4).
RESULTS
Our study included 28 576 new ART users who initiated care at LT (n = 15 920) and QECH (n = 12 656). Median age at enrollment was 33 years (Table 1). New patients tended to initiate ART at an advanced WHO stage (LT stage 3: 41%, stage 4: 14%; QECH stage 3: 50%, stage 4: 16%).
Table 1.
Characteristics of New Antiretroviral Therapy Initiators Enrolled at Queen Elizabeth Hospital Human Immunodeficiency Virus (HIV) Clinic, Blantyre, and Lighthouse Trust HIV Clinic, Lilongwe, Malawi
| Characteristic | Lighthouse Trust (2007–2010) | Queen Elizabeth Central Hospital (2000–2010) | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Cohort | Person-Years at Risk | Total Cohort | Person-Years at Risk | |||||
| N | % | N | % | N | % | N | % | |
| Total | 15 920 | … | 49 981 | … | 12 656 | … | 50 834 | … |
| Sex | ||||||||
| Male | 6713 | 42.2 | 19 558 | 39.1 | 5529 | 43.7 | 20 591 | 40.5 |
| Female | 9207 | 57.8 | 30 423 | 60.9 | 7127 | 56.3 | 30 243 | 59.5 |
| Age category (years) | ||||||||
| <16 | 706 | 4.6 | 2181 | 4.5 | 1730 | 13.7 | 6183 | 12.2 |
| 16–25 | 1641 | 10.7 | 4881 | 10.1 | 1203 | 9.5 | 4049 | 8.0 |
| 26–35 | 6273 | 40.8 | 19 980 | 41.5 | 4628 | 36.6 | 18 641 | 36.7 |
| 36–45 | 4527 | 29.5 | 14 422 | 30.0 | 3283 | 25.9 | 14 217 | 28.0 |
| 46–55 | 1624 | 10.6 | 5013 | 10.4 | 1321 | 10.4 | 5799 | 11.4 |
| 56+ | 595 | 3.9 | 1652 | 3.4 | 491 | 3.9 | 1945 | 3.8 |
| Missing | 554 | … | 1851 | … | … | … | … | … |
| Age at enrollment, years, median (interquartile range) | 33.3 (27.9, 39.8) | 33.5 (16.7, 40.9) | ||||||
| World Health Organization stage | ||||||||
| 1 or 2 | 4852 | 30.5 | 17 693 | 35.4 | 4181 | 33.0 | 18 793 | 37.0 |
| 3 | 6499 | 40.8 | 19 362 | 38.7 | 6261 | 49.5 | 26 123 | 51.4 |
| 4 | 2207 | 13.9 | 4855 | 9.7 | 2010 | 15.9 | 5477 | 10.8 |
| Not applicable/unknown | 2362 | 14.8 | 8070 | 16.1 | 204 | 1.6 | 441 | 0.4 |
Overall, 4346 cancers were identified; 16% were identified through record linkage (LT, n = 202; QECH, n = 477) and 84% through the EMR (LT, n = 3351; QECH, n = 528). The majority of diagnoses did not overlap across data sources (<4%; Supplementary Figures 1–2); information on NHL and NADC was derived exclusively from the match with the registry. Pathological confirmation of cancer diagnosis was low and varied by clinic site; it was 3% at LT and 19% at QECH, reflecting diagnostic pathology availability in Lilongwe and Blantyre, respectively, during the study period. Prevalence of cancer at time of enrollment was 12%, with prevalent cancers being predominantly ADCs.
A total of 23 655 cancer-free individuals at cohort entry were followed for 100 815 person-years at risk (Table 1). Median time on ART was 3.6 years (interquartile range, 0.4, 6.0 years). Most incident cancers occurred within 4 to 24 months after starting ART (early incident, n = 618; late incident, n = 265); 21% of early-incident and 26.4% of late-incident diagnoses were identified through linkage to the registry Overall age-adjusted cancer incidence rate for all sites combined was 689.2 (95% CI, 610.2, 768.0; Figure 1). Incidence was greatest during the early period of 4–24 months following ART initiation (Table 2).
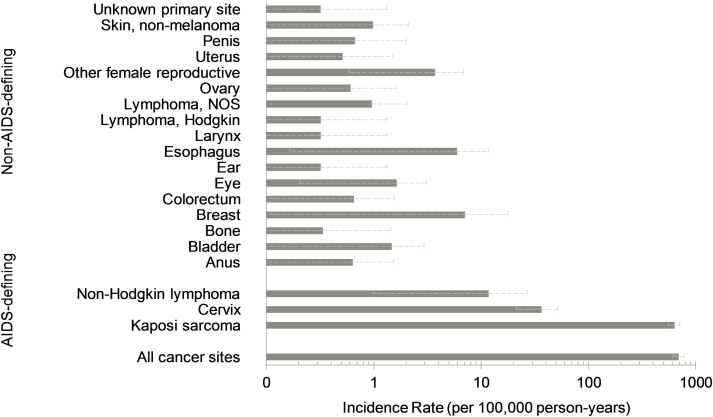
Figure 1.
Age-adjusted cancer incidence rates among new antiretroviral therapy users in Malawi, 2000–2010. Abbreviation: NOS, not otherwise specified.
Table 2.
Cancer Incidence Rates by Timing of Diagnosis After Antiretroviral Therapy Initiation
| Timing of Diagnosis | Men | Women | ||
|---|---|---|---|---|
| Incidence Rate | (95% CI) | Incidence Rate | (95% CI) | |
| All sites, total | 765.5 | (621.7–909.3) | 643.3 | (543.6–743.0) |
| Early incidence | 534.7 | (417.5–651.9) | 399.1 | (324.5–473.7) |
| Late incidence | 230.8 | (147.5–314.2) | 244.2 | (178.0–310.4) |
| Kaposi sarcoma | ||||
| Early incidence | 525.0 | (407.9–642.1) | 371.7 | (298.2–445.2) |
| Late incidence | 203.4 | (126.3–280.5) | 202.8 | (140.0–265.6) |
| Cervical cancer | ||||
| Early incidence | … | … | 13.7 | (7.7–19.7) |
| Late incidence | … | … | 22.9 | (9.0–36.8) |
Incidence rates are per 100 000 person-years on antiretroviral therapy (ART) and are age-adjusted to the 2007 Malawi standard population. Cancer sites include invasive cases only. Early incidence is defined as 4 to 24 months after start of ART; late incidence is defined as >24 months after start of ART.
Abbreviation: CI, confidence interval.
AIDS-defining Cancers
KS, cervical cancer, and NHL accounted for 94% of new malignancies among new ART users (Supplementary Table 1). The majority of KS and cervical cancer cases were diagnosed clinically; 10% and 27% received a pathological confirmation of diagnosis at LT and QECH, respectively. Squamous cell carcinoma was the most common type of cervical cancer (42%), followed by nonspecified histological types based on clinical diagnosis. All NHL cases were pathologically confirmed.
Overall KS incidence was 634.6 per 100 000 person-years on ART (95% CI, 558.3, 711.0; Figure 1). KS occurred most frequently in the early incidence period of 4–24 months after starting ART for both men and women (Table 2). Early-incident KS was greater among men (IR, 525.0; 95% CI, 417.5, 615.9) than among women (IR, 399.1; 95% CI, 324.5, 473.7; Table 2). Men and women experienced 40% increased incidence of KS at advanced WHO stage relative to early stage (Table 3); however, this association was not observed among clinical stage 4 cases.
Table 3.
Cancer Incidence Rates by World Health Organization Clinical Stage at Time of Cohort Enrollment
| Timing of diagnosis | Incidence Rate | (95% CI) | Incidence Rate Ratio | (95% CI) |
|---|---|---|---|---|
| Kaposi sarcoma | ||||
| Stage 1 or 2 | 145.3 | (109.0–181.6) | 1 | … |
| Stage 3 | 202.8 | (150.3–255.4) | 1.4 | (1.1–1.7) |
| Stage 4 | 150.2 | (106.5–193.9) | 1.0 | (0.8–1.3) |
| Cervical cancer | ||||
| Stage 1 or 2 | 12.6 | (6.0–19.1) | 1 | … |
| Stage 3 | 21.3 | (8.0–34.7) | 1.7 | (0.9–3.2) |
| Stage 4 | 1.0 | (0.0–2.4) | 0.1 | (0.0–0.3) |
Incidence rates are per 100 000 person-years on antiretroviral therapy (ART) and are age-adjusted to the 2007 Malawi standard population. Cancer sites include invasive cases only. Cases with missing World Health Organization stage at enrollment were excluded (Kaposi sarcoma, n = 229; cervical cancer, n = 2).
Abbreviation: CI, confidence interval.
Cervical cancer was the second most commonly occurring cancer, with an incidence rate of 36.6 (95% CI, 21.4, 51.7; Figure 1). No discernible pattern was observed in early vs late incidence of cervical cancer (Table 2). Women with advanced WHO stage had a 70% higher rate of cervical cancer than women with early stage HIV, but this association was not significant (Table 3). NHL was detected at a low rate in our study (IR, 11.7; 95% CI, 0, 27.0).
Non-AIDS–defining Cancers
NADCs accounted for 6% of the total cancer burden among 2000–2010 ART users (Supplementary Table 1). The highest IRs were for cancers of the breast (7.1; 95% CI, 0, 17.8), esophagus (6.0; 95% CI, 0.2, 11.8), female reproductive cancers (3.7; 95% CI, 0.6, 6.9), eye and conjunctiva (1.6; 95% CI, 0.2, 3.1), and bladder (1.5; 95% CI, 0, 2.9; Figure 1; Supplementary Table 2).
Pathological confirmation varied across NADC sites as follows: esophagus (19%), cervix (27%), breast (53%), anus (75%), lymphomas (100%), oral cavity/pharynx (100%), and eye/conjunctiva (100%). Squamous cell carcinoma was the most common type of lip/oral cavity (40%), anus (75%), and eye/conjunctiva (85%) malignancy. Squamous cell carcinoma of the esophagus was also predominant (89%). Among breast cancers, 71% were infiltrating ductal carcinoma. Infection-associated cancers linked to Helicobacter pylori (stomach), hepatitis B and C virus (liver), schistosomiasis (bladder), and Epstein-Barr virus (Hodgkin lymphoma subtypes) were rarely detected in our study (Figure 1, Supplementary Table 2).
DISCUSSION
Our goal in the Malawi HIV-Cancer Match Study was to characterize the burden and spectrum of cancer among ART users in Malawi, where HIV prevalence is 9% and 1 in 20 Malawian adults is now on ART [7]. In our study of nearly 29 000 ART users, the overall cancer burden was high and predominantly driven by ADCs, even during the early era of improving access to ART. KS was the most common cancer, and ADCs were a reason for presenting to care for 1 in 8 patients. The incidence of new KS was most pronounced during the first 2 years of ART but remained high over long-term follow-up. Our KS incidence estimates in Malawi are among the highest for ART users in SSA, with other reported rates including 77 per 100 000 person-years in Zimbabwe, 169 in Zambia [20], 270 in Kenya, 340 in Uganda [21], and 432 in South Africa [9]. High incidence of KS at HIV centers of excellence in Malawi in 2000–2010 are similar in magnitude to ART users with CD4 count of 51–100 (IR, 716) in East African ART populations [21]. High KS burden in Malawi is likely attributable to the 35%–88% prevalence of the causative virus, human herpesvirus-8, in Southern Africa [22] and typically advanced HIV stage with late presentation to care among ART initiators. This is especially true during the relatively early period of ART scale-up analyzed, which began in Malawi in 2004. KS burden may therefore still decline as the national ART program matures [23] with earlier and more widespread application of ART under “treat-all” guidelines [24].
Reflecting late presentation to care, advanced WHO stage was associated with increased KS incidence, although this association was not observed for those who presented to care with stage 4 disease, perhaps owing to differences in competing risk of death and prevalence of individuals who already had KS at ART initiation. Advanced HIV stage was associated with a 40% increase in KS incidence. Cervical cancer was the second most common cancer, and more advanced WHO stage was not associated with increased incidence of cervical cancer in our study. These findings might suggest KS burden will be more immediately impacted by earlier ART application than cervical cancer in SSA, as also suggested by early epidemiologic data from Uganda and Botswana that show modest incidence declines for KS but not cervical cancer [25, 26].
We also observed a range of NADCs even among Malawians with relatively advanced HIV prior to ART initiation. While NADC incidence rates were low overall, our findings highlight the heterogeneous cancer burden among Malawian ART initiators beyond KS and cervical cancer, as also suggested by other regional studies [25–27]. The highest incidence rates observed were for breast, esophageal, other female reproductive, eye and conjunctiva, and bladder cancers. Of these, other female reproductive cancers and bladder cancer have confirmed etiologic associations with infectious pathogens (human papilloma virus and schistosomiasis, respectively), but associations with HIV in SSA are uncertain. For esophageal cancer, there is no known infectious etiology [28], although a Zambia case-control study suggested possible association with HIV [29]. For breast cancer, large studies from high-income countries have repeatedly shown reduced risk among HIV-infected individuals [3]. However, HIV prevalence among breast cancer patients in Botswana is substantially higher than in the general population [26]. Our work and that of others thus highlight the need for larger and more definitive epidemiologic studies to define relationships between HIV and cancer that may be unique to SSA in order to inform comprehensive, holistic cancer screening and prevention programs in regional ART clinics.
The lower-than-expected incidence of lymphomas and NADCs in our study is likely due to underdiagnosis. Geographical differences in screening and diagnostic confirmation likely contributed to overall completeness of cancer ascertainment by the registry and, therefore, record linkage performance. This observation is similar to the overall context of cancer ascertainment in SSA, for which regional limitations have been extensively described [30]. Cancer awareness is often low but improving among patients and clinicians in Malawi, and the ability to obtain diagnostic tissue from deep visceral sites is more challenging than from more accessible sites. In addition, a single pathology center in Blantyre provided services to the entire country during the study period, and only approximately one-fifth of all cancer cases were thus pathologically confirmed. Lymphomas may be particularly susceptible to misdiagnosis, and studies from Uganda and Malawi have shown that lymphomas are commonly clinically misdiagnosed as tuberculosis [31, 32]. Our study may also be subject to underreporting of cancer incidence during late patient follow-up. Cancer surveillance after 2010 was limited to central hospitals; therefore, diagnoses that occurred during follow-up beyond 2010 may be less completely captured by the registry.
The Malawi HIV-Cancer Match Study used probabilistic record linkage algorithms to ascertain cancer outcomes at centers of excellence for HIV care. This is one of the largest epidemiological studies of its kind in SSA to provide a comprehensive overview of the cancer burden among individuals receiving ART. We used information on active patient follow-up to construct a retrospective cohort of approximately 29 000 new ART initiators. Our study leveraged probabilistic methods and extensive clinical review to link data from Malawi’s national cancer registry with existing electronic medical systems that support ART delivery within large HIV clinics. We performed validation of cancer outcomes through extensive clerical and clinical review of matched cases.
Our study has downstream implications for strengthening health systems in Malawi. Registry data matches are an important mechanism for providing HIV clinics with NADC and other noncommunicable disease outcomes. Routine queries of HIV point-of-care clinics and further validation studies may improve cancer registration among HIV-infected populations in SSA. While our study used high-quality data from HIV clinics participating in the International epidemiologic Databases to Evaluate AIDS (IeDEA) consortium, improving data quality and completeness of cancer diagnoses outside of HIV centers of excellence is a long-term priority. Interdisciplinary collaboration between HIV and cancer systems is of continued importance to build efficient and complete public health data resources in low-income settings [33].
In conclusion, we provide the first comprehensive baseline description of cancer burden against which to monitor cancer control efforts for HIV-infected populations in Malawi. Our findings demonstrate an ongoing high burden of KS and cervical cancer in a young, urban patient population and the importance of integrated screening and management of KS and cervical cancer in ART programs. Longer follow-up and additional linkages may be needed to monitor these populations as they age into a demographic group where NADCs are most common. Nearly 2.3 million people with HIV over 50 years are African, greater than half the global burden, and demographic shifts are expected to continue under expanded ART guidelines for earlier treatment and the growing number of people accessing ART [34]. In high-income countries, the burden and incidence of KS and NHL declined substantially since the introduction of ART [35–37], but the NADC burden has actually increased with growth and aging of HIV-infected populations, as well as declines in competing causes of death [36, 38]. Whether cancer trends in high-income countries will be replicated among HIV populations in Africa remains uncertain. Validation of our findings through companion studies in other parts of SSA is needed, as well as longer-term studies to monitor potential shifts in cancer distribution under expanded ART guidelines. Continued investment in high-quality cancer surveillance will be essential to inform evidence-based national cancer control efforts in SSA. Preparing African healthcare systems for the impending challenges of caring for aging HIV populations and forthcoming noncommunicable disease burden is essential [39, 40].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors acknowledge the support of clinicians at the Lighthouse Trust and Queen Elizabeth Central Hospital human immunodeficiency virus clinics. The authors also thank Ande Salima and Chrissie Chilima of the Kamuzu Central Hospital Cancer Registry for their technical assistance with data cleaning.
Author contributions. M. J. H. and S. G. wrote the first draft of the report. M. J. H., S. C., and S. G. acquired the data. M. J. H., S. C., and A. S. performed statistical analysis. S. G. is the principal investigator in the Malawi human immunodeficiency virus (HIV)-Cancer Match Study and supervised the study; S. G. and J. B. obtained funding. C. D., S. P., and K. M. are co-investigators. All authors contributed to the study design and the interpretation of the data, critically reviewed the manuscript for intellectual content, and approved the final version of the report.
Financial support. This research was supported by the Malawi Cancer Consortium, funded by the National Cancer Institute (U54CA190152), the Malawi Regional Center of Research Excellence for Non-Communicable Diseases, funded by the National Cancer Institute (P20CA210285), and the Swiss Cancer League (KLS-3399-02-2014). HIV cohorts were supported by International epidemiology Databases to Evaluate AIDS Southern Africa, funded by the National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, National Cancer Institute (U01AI069924). Other support for Malawi cancer registration and pathology came from the National Cancer Institute (P30CA016086), Center for AIDS Research funded by the National Institute of Allergy and Infectious Diseases (P30AI50410), and Medical Education Partnership Initiative funded by the Fogarty International Center (R24TW008927). M. J. H. was supported by UNC Lineberger Cancer Control Education Program Fellowship, funded by the National Cancer Institute (R25CA57726-25) and UJMT Fogarty Global Health Fellowship, Fogarty International Center and National Cancer Institute (R25TW009340).
Potential conflicts of interest. All authors: no reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.UNAIDS. Global AIDS update 2016 http://www.unaids.org/sites/default/files/media_asset/global-AIDS-update-2016_en.pdf Accessed 28 January 2018.
- 2. Forman D, Bray F, Brewster DH, et al. , eds. Cancer Incidence in Five Continents, Vol. X (electronic version). Lyon: International Agency for Research on Cancer; 2013. Available at: http://ci5.iarc.fr. Accessed 7 September 2017. [Google Scholar]
- 3. Hernandez-Ramirez RU, Shiels MS, Dubrow R, Engels EA. Cancer risk in HIV-infected people in the USA from 1996 to 2012: a population-based, registry-linkage study. Lancet HIV 2017; 4:e495–e504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Islam JY, Rosenberg PS, Hall HI, Jacobson EU, Engels EA, Shiels MS. Projections of cancer incidence and burden among the HIV-positive population in the United States through 2030. Cancer Res 2017; 77:5302. [Google Scholar]
- 5. Tweya H, Feldacker C, Heller T, et al. . Characteristics and outcomes of older HIV-infected patients receiving antiretroviral therapy in Malawi: a retrospective observation cohort study. PLoS One 2017; 12:e0180232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Martel C, Ferlay J, Franceschi S, et al. . Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13:607–15. [DOI] [PubMed] [Google Scholar]
- 7.Government of Malawi. Malawi AIDS Response Progress Report April 2015. Accessible at: http://www.unaids.org/sites/default/files/country/documents/MWI_narrative_report_2015.pdf [Accessed on 31 October 2015].
- 8. Akarolo-Anthony SN, Maso LD, Igbinoba F, Mbulaiteye SM, Adebamowo CA. Cancer burden among HIV-positive persons in Nigeria: preliminary findings from the Nigerian AIDS-Cancer Match study. Infect Agent Cancer 2014; 9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sengayi M, Spoerri A, Egger M, et al. . Record linkage to correct under-ascertainment of cancers in HIV cohorts: the Sinikithemba HIV Clinic Linkage project. Int J Cancer 2016; 139:1209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mbulaiteye SM, Katabira ET, Wabinga H, et al. . Spectrum of cancers among HIV-infected persons in Africa: the Uganda AIDS-Cancer Registry Match study. Int J Cancer 2006; 118:985–90. [DOI] [PubMed] [Google Scholar]
- 11. Tweya H, Gareta D, Chagwera F, et al. . Early active follow-up of patients on antiretroviral therapy (ART) who are lost to follow-up: the “Back-to-Care” project in Lilongwe, Malawi. Trop Med Int Health 2010; 15 Suppl 1:82–9. [DOI] [PubMed] [Google Scholar]
- 12. Sloan DJ, van Oosterhout JJ, Malisita K, et al. . Evidence of improving antiretroviral therapy treatment delays: an analysis of eight years of programmatic outcomes in Blantyre, Malawi. BMC Public Health 2013; 13:490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Msyamboza KP, Dzamalala C, Mdokwe C, et al. . Burden of cancer in Malawi; common types, incidence and trends: national population-based cancer registry. BMC Res Notes 2012; 5:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gopal S, Krysiak R, Liomba NG, et al. . Early experience after developing a pathology laboratory in Malawi, with emphasis on cancer diagnoses. PLoS One 2013; 8:e70361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chokunonga E, Borok MZ, Chirenje ZM, Nyakabau AM, Parkin DM. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. Int J Cancer 2013; 133:721–9. [DOI] [PubMed] [Google Scholar]
- 16. Jedy-Agba E, Curado MP, Ogunbiyi O, et al. . Cancer incidence in Nigeria: a report from population-based cancer registries. Cancer Epidemiol 2012; 36:e271–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Engels EA, Biggar RJ, Hall HI, et al. . Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer 2008; 123:187–94. [DOI] [PubMed] [Google Scholar]
- 18. Percy CL, Van Holten V, Muir CS, eds. International Classification of Diseases for Oncology, 2nd edition (ICD-O-2). Geneva: World Health Organization; 1990. [Google Scholar]
- 19.National Statistical Office of Malawi (NSO). 2008 Population and Housing Census Accessible at: http://www.nsomalawi.mw Accessed 02 June 2016.
- 20. Rohner E, Valeri F, Maskew M, et al. . Incidence rate of Kaposi sarcoma in HIV-infected patients on antiretroviral therapy in Southern Africa: a prospective multicohort study. J Acquir Immune Defic Syndr 2014; 67:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin J, Wenger M, Busakhala N, et al. . Prospective evaluation of the impact of potent antiretroviral therapy on the incidence of Kaposi’s sarcoma in East Africa: findings from the International Epidemiologic Databases to Evaluate AIDS (IeDEA) Consortium. Infectious Agents Cancer 2012; 7:O19. [Google Scholar]
- 22. Begré L, Rohner E, Mbulaiteye SM, Egger M, Bohlius J. Is human herpesvirus 8 infection more common in men than in women? Systematic review and meta-analysis. Int J Cancer 2016; 139:776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.2014 Clinical Management of HIV in Children and Adults. Malawi Integrated Guidelines for Providing HIV Services in Antenatal Care, Maternity Care, Under 5 Clinics, Family Planning Clinics, HIV Exposed Child/pre-ART Clinics, ART Clinics. Second Edition. Malawi: Ministry of Health; Available at www.hivunitmohmw.org, Department for HIV and AIDS of the Ministry of Health. [Google Scholar]
- 24. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy, July 2017. Geneva: World Health Organization; 2017. [PubMed] [Google Scholar]
- 25. Mutyaba I, Phipps W, Krantz EM, et al. . A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015; 69:481–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dryden-Peterson S, Medhin H, Kebabonye-Pusoentsi M, et al. . Cancer incidence following expansion of HIV treatment in Botswana. PLoS One 2015; 10:e0135602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sengayi MM, Spoerri A, Egger M, et al. . Risk of Cancer in HIV-Positive Adults on ART in South Africa: A Record Linkage Study. (Presented at CROIFebruary 22-23 2016, Boston, MA Conference abstract 613). [Google Scholar]
- 28. Liu W, Snell JM, Jeck WR, et al. . Subtyping sub-Saharan esophageal squamous cell carcinoma by comprehensive molecular analysis. JCI Insight 2016; 1:e88755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kayamba V, Bateman AC, Asombang AW, et al. . HIV infection and domestic smoke exposure, but not human papillomavirus, are risk factors for esophageal squamous cell carcinoma in Zambia: a case-control study. Cancer Med 2015; 4:588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morhason-Bello IO, Odedina F, Rebbeck TR, et al. . Challenges and opportunities in cancer control in Africa: a perspective from the African Organisation for Research and Training in Cancer. Lancet Oncol 2013; 14:e142–51. [DOI] [PubMed] [Google Scholar]
- 31. Masamba LPL, Jere Y, Brown ERS, Gorman DR. Tuberculosis diagnosis delaying treatment of cancer: experience from a new oncology unit in Blantyre, Malawi. J Glob Oncol 2016; 2:26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buyego P, Nakiyingi L, Ddungu H, et al. . Possible misdiagnosis of HIV associated lymphoma as tuberculosis among patients attending Uganda Cancer Institute. AIDS Res Ther 2017; 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mbulaiteye SM, Bhatia K, Adebamowo C, Sasco AJ. HIV and cancer in Africa: mutual collaboration between HIV and cancer programs may provide timely research and public health data. Infect Agent Cancer 2011; 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mahy M, Autenrieth CS, Stanecki K, Wynd S. Increasing trends in HIV prevalence among people aged 50 years and older: evidence from estimates and survey data. AIDS 2014; 28 Suppl 4:S453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shiels MS, Cole SR, Wegner S, et al. . Effect of HAART on incident cancer and noncancer AIDS events among male HIV seroconverters. J Acquir Immune Defic Syndr 2008; 48:485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Franceschi S, Lise M, Clifford GM, et al. ; Swiss HIV Cohort Study. Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer 2010; 103:416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pipkin S, Scheer S, Okeigwe I, Schwarcz S, Harris DH, Hessol NA. The effect of HAART and calendar period on Kaposi’s sarcoma and non-Hodgkin lymphoma: results of a match between an AIDS and cancer registry. AIDS 2011; 25:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shiels MS, Pfeiffer RM, Gail MH, et al. . Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 2011; 103:753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Negin J, Bärnighausen T, Lundgren JD, Mills EJ. Aging with HIV in Africa: the challenges of living longer. AIDS 2012; 26 Suppl 1:S1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mills EJ, Bärnighausen T, Negin J. HIV and aging–preparing for the challenges ahead. N Engl J Med 2012; 366:1270–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.