Abstract
Innovations in ophthalmic imaging have made a profound impact on the diagnosis and treatment of ophthalmic disease. In ocular oncology, the development of optical coherence tomography with enhanced depth imaging and swept source technologies has made it possible to visualize the anatomical characteristics of retinoblastoma and uveal melanoma with a level of detail previously unobtainable on clinical exam alone. As a result, our understanding of the pathophysiology of vision loss in choroidal melanoma in particular has improved. These modalities have also helped identify fundoscopically “invisible” tumors and risk stratify pre-malignant choroidal lesions, making a strong case for their inclusion in all screening evaluations. Optical coherence tomography angiography, on the other hand, has allowed non-invasive imaging of the retinal and uveal vasculatures, providing insight into vascular changes associated with malignant transformation and vision loss following exposure to radiation. While the impact of new imaging technologies on clinical outcomes and overall survival in ocular oncology has yet to be determined, several reports cited herein offer promising results.
Keywords: Ocular oncology, melanoma, retinoblastoma, radiation retinopathy, ocular surface squamous neoplasia, optical coherence tomography, angiography
Introduction
Recent advances in imaging have made a profound impact on the diagnosis and treatment of both anterior and posterior segment ophthalmic disease. The field of ocular oncology is no exception to this trend; novel imaging modalities are enhancing our understanding of intraocular tumors, their impact on ocular physiology, and their response to treatment. The utility of fundus photography and ultrasound (US) imaging has been magnified over the past few years by the adjunctive use of newer modalities – in particular, new forms of ocular coherence tomography (OCT) and OCT angiography (OCTA). The present review serves as a brief update on these imaging modalities and their current role in the characterization of choroidal melanoma (CM), retinoblastoma, anterior segment tumors, and radiation retinopathy.
Choroidal melanoma
CM is the most common primary intraocular malignancy in adults. Historically, choroidal nevi and melanomas have been diagnosed and monitored with fundoscopic examination, fundus photography, and ocular US. Invasive methods, such as fluorescein angiography (FA) and indocyanine green angiography (ICGA), have been used where necessary to help differentiate melanoma from masquerading pathology, to assess for secondary neovascularization or ischemia, or to assist in visualizing lesions obscured by media opacity 1. More recently, OCT and OCTA have made it possible to non-invasively image superficial and deep structures of the retina and choroid with a level of detail previously unobtainable by clinical exam alone.
Enhanced depth imaging (EDI) OCT using spectral domain (SD) technology and swept source (SS) OCT, with faster scanning engines, are technologies employed to image deep intraocular structures from the choroid to the inner sclera. While EDI SD-OCT is an adaptation of prior technology, SS-OCT is a newer technology that uses a longer wavelength (1,050 nm compared to 840 nm), reduced sensitivity roll-off, and faster scanning speeds, allowing for better light penetration through the RPE, higher likelihood of detecting subtle signals from deeper layers, and denser scan patterns over larger scan areas 2. Both imaging technologies offer a superior level of detail of the deep intraocular structures when compared to ocular US. One recent study highlighting the power of EDI-OCT brings to light five distinct imaging patterns of flat choroidal nevi that were previously not appreciated with US alone 3. Of 94 fundoscopically and ultrasonographically flat nevi included in this review, nearly one-third were found to be elevated on EDI-OCT 3. Interestingly, only nevi demonstrating elevation on initial EDI-OCT were found to show growth or change over a 22 month follow-up time period 3. Furthermore, the posterior lesional border was visible in a majority of cases (approximately 80%), making it possible to monitor for multi-dimensional change 3. In a second recent study on EDI-OCT, Shield et al. found that the thickness of small CM was more accurately determined by EDI-OCT as compared to US, which may overestimate size by up to 55% 4. Both EDI-OCT and SS-OCT have been used to characterize CM and associated changes in the overlying neurosensory retina. Characteristic features of melanoma include deep optical shadowing, thinning or compaction of the choriocapillaris, disruption of the adjacent retinal photoreceptor layer, presence of subretinal fluid with or without lipofuscin deposition, and presence of intraretinal fluid 4.
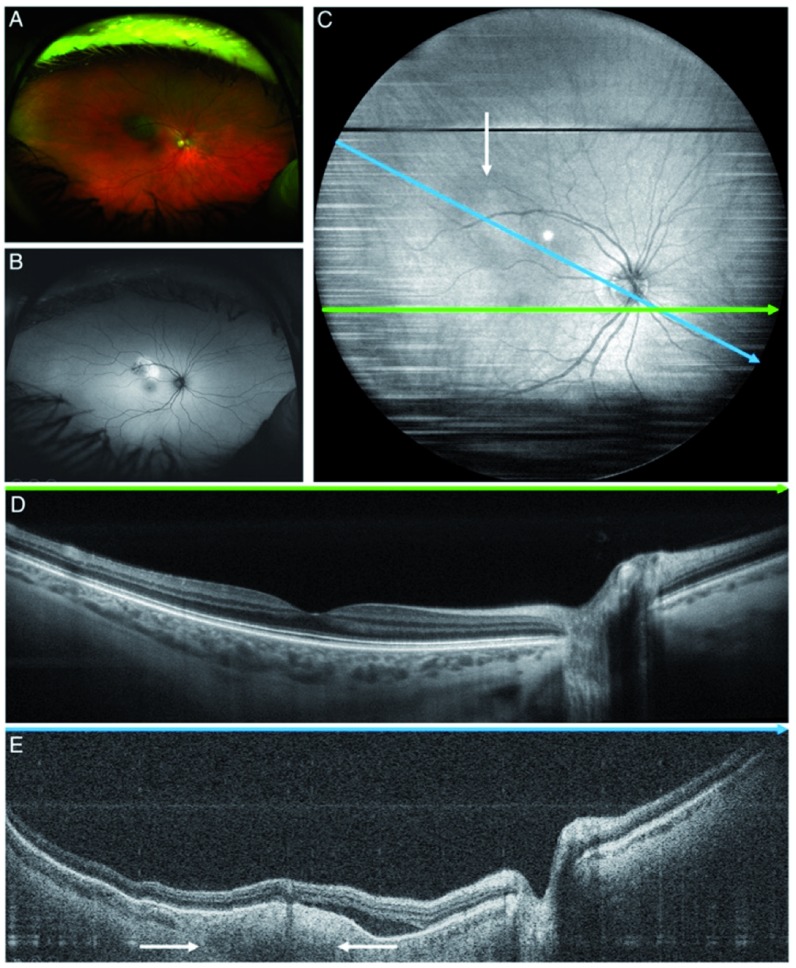
Recently, SS-OCT has been modified algorithmically to produce “speckle noise-free” images, which are meant to reduce the granular noise signal inherent to several imaging methods including OCT, computerized tomography, and magnetic resonance 5, 6. OCT technology has also been adapted to view peripheral tumors. In one report, SS-OCT was modified with wide-angle, 20 and 40 diopter indirect ophthalmoscopy lenses to capture wide field-of-view images of peripheral CM in a single imaging session ( Figure 1) 7. The field of view was a mean of 4.6 optic disc-to-fovea distance units compared to the mean 2.1 disc-to-fovea units of SD-OCT and 9.4 disc-to-fovea units of widefield scanning laser ophthalmoscopy 7. Furthermore, microscope-integrated OCT (MIOCT) has been used intra-operatively to guide real-time (4D-MIOCT) 27-gauge transvitreal biopsy of CM ( Figure 2) 8. The reported benefit of intra-operative 4D-MIOCT is to achieve adequate biopsy depth with the vitrectomy cutter, avoid unnecessary damage to surrounding neurosensory retina, and avoid the risk of deep penetration and subsequent choroidal hemorrhage.
Figure 1. Wide field of view image of choroidal nevus (white arrows).
Images used with permission from Mcnabb et al. 7. A) Red/green and B) autofluorescence scanning laser ophthalmoscopy. C) Wide field of view swept-source optical coherence tomography (OCT) B-scan projection, blue line corresponding to position of image D), which demonstrates normal optic nerve, fovea, and retinal layers. E) OCT corresponding to green arrow demonstrating choroidal nevus with overlying drusen, loss of choriocapillaris, and associated subretinal fluid.
Figure 2. Intraoperative 4D-microscope-integrated optical coherence tomography (4D-MIOCT).
Images used with permission from Grewal et al. 8. A) Elevated choroidal lesion superonasal to optic nerve without surrounding hyperautofluorescence ( B), consistent with choroidal melanoma. C) OCT showing choroidal tumor without subretinal fluid. D) Ultrasound showing low internal reflectivity. E– H) sequential images from intraoperative 4D-MIOCT with three-dimensional volumes showing cutter tip making contact with and penetrating the retinal surface at nearly perpendicular orientation with adequate depth ( H) (white and yellow arrow show cutter tip).
The advent of OCTA has given further insight into retinal vascular abnormalities induced by CM. Specifically, OCTA has been able to non-invasively demonstrate a significant enlargement of the deep foveal avascular zone and a decrease in superficial and deep parafoveal capillary vascular densities in eyes with CM as compared to their fellow healthy eyes 9. These changes appear to be correlated with the presence of subretinal fluid and larger tumor size, bringing to light the possible role of tumor-related pro-inflammatory factors, such as vascular endothelial growth factor (VEGF), in the pathogenesis of vision loss in CM 9. One proposed mechanism behind the association between CM size and presence of subretinal fluid is a VEGF-induced microvascular compromise that precedes macular edema in affected eyes 9. Furthermore, these features appear to be absent in eyes with choroidal nevi, giving them a potential role in differentiating choroidal nevi from melanoma 10.
While retinal vascular imaging is well suited to OCTA, imaging of the deeper choroidal vessels remains a challenge, both in the posterior pole and in the choroidal periphery. ICGA, which involves injection of intravenous dye, is the standard for choroidal vascular imaging. More recently, a novel method for OCTA, SS-OCTA, has shown the capacity to non-invasively visualize deeper choroidal vessels with a level of detail akin to that of ICGA 11. In one case report, it was used to diagnose choroidal neovascularization arising from a choroidal nevus masquerading as neovascular age-related macular degeneration 12. While not a usual part of the pre- or post-treatment evaluation of CM, routine, non-invasive imaging of the choroidal vasculature may reveal microvascular patterns that have previously been implicated in higher degrees of malignancy, metastasis, and successful eradication after radiation therapy 13, 14. Choroidal vascular features observed to potentially differentiate nevus from melanoma include irregularity of lesion margins, heterogeneous hyporeflective choriocapillary plexus with avascular areas, hyperreflective choriocapillary rings, thick choroidal vascular networks, and choroidal vascular loops 15, 16. In a study by Fuste et al. comparing OCTA findings of 18 cases of choroidal nevus to 18 cases of choroidal melanoma, border irregularity, choriocapillaris hyporeflectivity, presence of avascular areas, and vascular anomalies, such as thick networks or vascular loops, were found to be present in a majority of cases of CM but only a minority of choroidal nevi 15. Interestingly, one of the 18 cases of CM treated with brachytherapy demonstrated complete regression of vascular anomalies seen prior to treatment 15.
The malignant potential of a presumed choroidal nevus is most accurately assessed by considering key features from several different imaging modalities. In a recent study by Shields et al. 17, over 2,000 cases of choroidal nevus with multimodal imaging were reviewed to assess for features imparting a higher risk of development of malignant features. Significant risk factors included US thickness >2 mm, subretinal fluid on OCT, central visual acuity loss, orange pigment on autofluorescence, lesion hollowness (US), and diameter >5 mm on photography. The presence of a greater number of risk factors was found to correlate with a greater risk of the development of malignant features. At 5-year follow-up, the percentage of lesions that transformed into melanoma were 1% (hazard ratio [HR] 0.8) for no features, 11% (HR 3.09) for one feature, 22% (HR 10.6) for two features, 34% (HR 15.1) for three features, 51% (HR 15.2) for four features, and 55% (HR 26.4) for five features. It will be interesting to see the role of newer imaging modalities, such as OCTA, in the evaluation of high-risk choroidal nevi.
Retinoblastoma
Retinoblastoma is the most common primary intraocular malignancy in children worldwide, with an estimated incidence of one in 15,000 to 18,000 live births 17. Recent advances in ophthalmic imaging have improved our understanding of the anatomy of retinoblastoma, its relationship to the fovea, and, thus, implications on visual prognosis in vivo. SD-OCT and hand-held SD-OCT (HHSD-OCT), the latter performed bedside during examination under anesthesia, have made it possible to evaluate the anatomical position of tumors with a new level of precision 18– 21. Retinoblastoma has been characterized on OCT as a retinal tumor arising from inner, middle, or outer retinal layers with disorganization of surrounding retina and with occasional intralesional cavities 22. “Retinal draping” is a term used to refer to the outer retinal tumor with overlying normal-appearing inner retinal layers 23. Using OCT, it is possible to visualize the border between anatomically normal- and abnormal-appearing retina, and to evaluate the structural integrity of the fovea, and thus to estimate the possible impact on visual potential 24, 25.
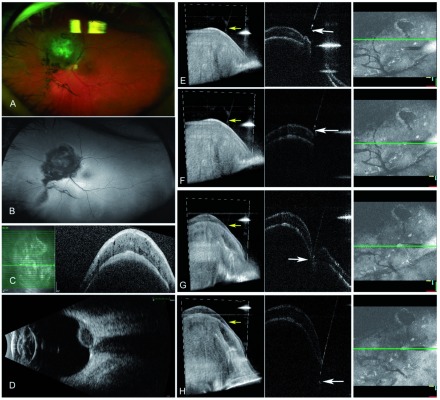
As in other retinal diseases, OCT has the potential for improving diagnostic accuracy, clinical staging, and treatment outcomes in retinoblastoma. In a recent retrospective review of 63 eyes from 44 children diagnosed with retinoblastoma and with a median of five HHSD-OCT sessions per eye, Soliman et al. found that OCT changed the diagnosis (excluding tumor in clinically suspicious areas, changing staging group, or detecting clinically invisible tumor) in seven out of 293 (3%) OCT sessions, changed treatment (detection of unsuspected tumor within scar) in 27 out of 293 (11%) cases, and changed follow-up (showing anatomical details excluding activity and thereby changing treatment plan) in 16 out of 293 (7%) OCT sessions 26. There are now several reports of HHSD-OCT being used to detect submillimeter and fundoscopically “invisible” tumors ( Figure 3). Specifically, OCT has been used to detect and monitor response to treatment in cases of parafoveolar submillimeter retinoblastoma and retinoblastoma resistant to chemotherapy 27– 30. In cases of “invisible” tumors, HHSD-OCT has been able to detect tumor activity in the contralateral eye of patients with known unilateral retinoblastoma and in a patient with strong family history of retinoblastoma undergoing screening evaluation 31– 33. The long-term impact of these new imaging modalities on life expectancy and visual outcome is yet to be discovered.
Figure 3. Fundoscopically invisible retinoblastoma.
Written informed consent was obtained from the patient's legal guardian in Figure 3, for the use and publication of these images. A) Fundus photograph of a right eye taken during examination under anesthesia, arrowheads pointing location of transparent retinal mass seen centrally in image C. B) Fundus photograph of left eye taken during examination under anesthesia, with large, elevated retinoblastoma inferotemporal to fovea. C) Optical coherence tomography (OCT) of right eye demonstrating retinal mass not visualized on fundoscopy (located between arrows in image A). D) OCT of left eye demonstrating large, elevated hyperreflective retinal mass with loss of normal retinal layers, corresponding to mass visualized in image B.
Anterior segment tumors
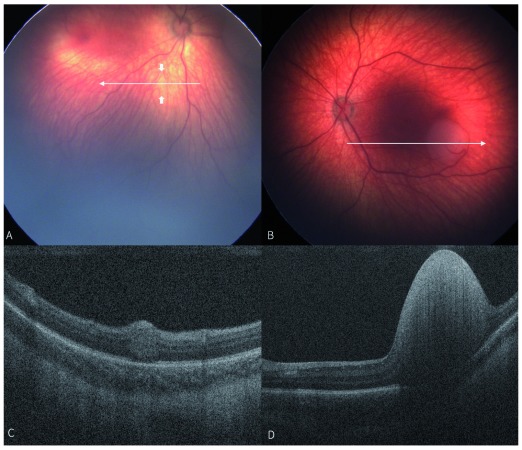
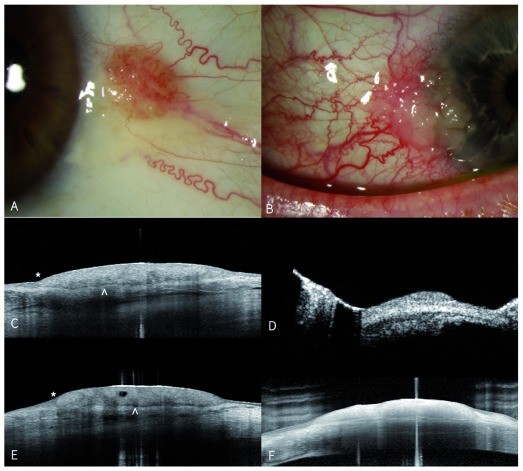
Ocular surface squamous neoplasia (OSSN) refers to several distinct histopathologic entities of the conjunctiva and cornea, ranging from dysplasia to invasive carcinoma 34. OSSN has traditionally been diagnosed and monitored by slit lamp examination, serial slit lamp photography, and US biomicroscopy (UBM). The advent of high-resolution OCT (HR-OCT) and ultra-HR OCT (UHR-OCT) of the anterior segment, attaining a resolution of less than 5 microns, has introduced the possibility of evaluating lesions obscured from external evaluation due to overlying ocular surface disease 35. In one study by Atallah et al., biopsy-confirmed OSSN obscured by overlying ocular surface degeneration and/or scarring (due to rosacea, pterygia, Salzmann nodular degeneration, or limbal cell deficiency with scarring) was detected reliably with HR-OCT 36. The primary lesional characteristics seen in cases of OSSN were thickening of the epithelial layer and an abrupt transition of epithelial reflectivity between normal and abnormal tissue 36. A separate study using time domain anterior segment OCT found 90% of OSSN cases to exhibit a clear hyporeflective plane of separation between thickened epithelium and underlying tissue ( Figure 4) 37. Still, OCT has limitations. In a direct comparison between UBM and OCT imaging of conjunctival nevi, UBM demonstrated superiority in visualizing lesions that were highly pigmented and remarkably elevated. OCT, on the other hand, was more sensitive for visualizing the internal structures of nevi, such as intralesional cysts 38.
Figure 4. Ocular surface squamous neoplasia (OSSN).
Written informed consent was obtained from the patient in Figure 4, for the use and publication of these images. A) External photograph of left eye showing OSSN with large feeder vessel and surrounding pinguecula temporal to limbus. C) Horizontal and E) vertical anterior segment (AS) OCT scans demonstrating abrupt transition to elevated epithelial lesion (asterisk) and clear plane of separation from underlying tissue (white arrow). B) External photograph of a right eye with OSSN lesion extending from temporal limbus onto cornea. AS-OCT of the lesion ( F) demonstrates significantly higher resolution than ultrasound biomicroscopy of the same lesion ( D).
OCTA has been applied to visualize malignant iris melanomas in comparison to benign iris lesions, such as iris freckles, nevi, and pigment epithelial cysts. In one cross-sectional study, OCTA of iris melanomas demonstrated vascular tortuosity and disorganization with increased intralesional vascular density as compared to benign iris lesions and normal controls 39. These findings were previously described using FA 40. Interestingly, lesions treated with radiation therapy demonstrated a subsequent reduction in intralesional vascular density 39.
Radiation retinopathy
Radiation retinopathy is a chronic vascular retinopathy that develops after exposure to ionizing radiation from radioactive plaque use for the treatment of intraocular tumors or external beam radiation for head and neck cancers. The underlying pathophysiology shares similarities with rapidly progressive diabetic retinopathy. Certain patients appear to have a higher likelihood of developing radiation retinopathy. Specifically, those with pre-radiation subclinical macular edema, with large tumors, with tumors close to the foveola, and with tumors associated with overlying hemorrhage 41. As demonstrated by Rose et al. using prototype doppler SD-OCT and hyperspectral retinal camera, total retinal blood flow and retinal blood oxygen saturation were significantly lower in patients with unilateral ischemic radiation retinopathy as compared to their healthy fellow eye 42. Early vascular changes may be first detected with OCTA, as irregular widening of the foveal avascular zone, discontinuity of retinal vasculature, and microaneurysms of the deep or superficial retina 43. These features, in combination with SD-OCT findings, including cystic macular edema and increased macular thickness, have been used to generate a new grading scheme for radiation retinopathy as well as to guide treatment with intravitreal anti-VEGF agents 43, 44.
Conclusions
Recent innovations in ophthalmic imaging have made a significant impact on our understanding of ocular oncology and its effect on ocular physiology. Given their potential for detecting early disease with high sensitivity, it will be interesting to see the impact they may have on visual prognosis and life expectancy.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Bertil E. Damato, Department of Ophthalmology, University of California, San Francisco, CA, USA
Jill Wells, Department of Ophthalmology, Emory University School of Medicine, Atlanta, Georgia, USA
Alison Skalet, Casey Eye Institute, Oregon Health & Science University, Portland, OR, USA
Funding Statement
JD and PM: Funding from the National Eye Institute (P30-026877) and Research to Prevent Blindness, Inc to Byers Eye Institute.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; peer review: 3 approved]
References
- 1. Motiani MV, McCannel CA, Almanzor R, et al. : Diagnosis of Choroidal Melanoma in Dense Asteroid Hyalosis. Semin Ophthalmol. 2017;32(2):257–9. 10.3109/08820538.2015.1053627 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Potsaid B, Baumann B, Huang D, et al. : Ultrahigh speed 1050nm swept source/Fourier domain OCT retinal and anterior segment imaging at 100,000 to 400,000 axial scans per second. Opt Express. 2010;18(19):20029–48. 10.1364/OE.18.020029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jonna G, Daniels AB: Enhanced Depth Imaging OCT of Ultrasonographically Flat Choroidal Nevi Demonstrates 5 Distinct Patterns. Ophthalmol Retina. 2019;3(3):270–7. 10.1016/j.oret.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Shields CL, Kaliki S, Rojanaporn D, et al. : Enhanced depth imaging optical coherence tomography of small choroidal melanoma: Comparison with choroidal nevus. Arch Ophthalmol. 2012;130(7):850–6. 10.1001/archophthalmol.2012.1135 [DOI] [PubMed] [Google Scholar]
- 5. Maloca P, Gyger C, Schoetzau A, et al. : Ultra-Short-Term Reproducibility of Speckle-Noise Freed Fluid and Tissue Compartmentalization of the Choroid Analyzed by Standard OCT. Transl Vis Sci Technol. 2015;4(6):3. 10.1167/tvst.4.6.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maloca P, Gyger C, Hasler PW: A pilot study to image the vascular network of small melanocytic choroidal tumors with speckle noise-free 1050-nm swept source optical coherence tomography (OCT choroidal angiography). Graefes Arch Clin Exp Ophthalmol. 2016;254(6):1201–10. 10.1007/s00417-015-3259-9 [DOI] [PubMed] [Google Scholar]
- 7. McNabb RP, Grewal DS, Mehta R, et al. : Wide field of view swept-source optical coherence tomography for peripheral retinal disease. Br J Ophthalmol. 2016;100(10):1377–82. 10.1136/bjophthalmol-2015-307480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grewal DS, Bhullar PK, Pasricha ND, et al. : Intraoperative 4-Dimensional Microscope-Integrated Optical Coherence Tomography-Guided 27-Gauge Transvitreal Choroidal Biopsy for Choroidal Melanoma. Retina. 2017;37(4):796–9. 10.1097/IAE.0000000000001326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li Y, Say EA, Ferenczy S, et al. : Altered parafoveal microvasculature in treatment-naive choroidal melanoma eyes detected by optical coherence tomography angiography. Retina. 2017;37(1):32–40. 10.1097/IAE.0000000000001242 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Valverde-Megías A, Say EA, Ferenczy SR, et al. : Differential macular features on optical coherence tomography angiography in eyes with choroidal nevus and melanoma. Retina. 2017;37(4):731–40. 10.1097/IAE.0000000000001233 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Pellegrini M, Corvi F, Invernizzi A, et al. : Swept-source optical coherence tomography angiography in choroidal melanoma: An Analysis of 22 Consecutive Cases. Retina. 2019;39(8):1510–9. 10.1097/IAE.0000000000002205 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Namavari A, Zheng F, Motulsky EH, et al. : Swept-Source OCT Angiography Identifies Choroidal Neovascularization Arising From a Choroidal Nevus. Ophthalmic Surg Lasers Imaging Retina. 2018;49(5):360–3. 10.3928/23258160-20180501-10 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Folberg R, Pe'er J, Gruman LM, et al. : The morphologic characteristics of tumor blood vessels as a marker of tumor progression in primary human uveal melanoma: A matched case-control study. Hum Pathol. 1992;23(11):1298–305. 10.1016/0046-8177(92)90299-i [DOI] [PubMed] [Google Scholar]
- 14. Folberg R, Rummelt V, Parys-Van Ginderdeuren R, et al. : The Prognostic Value of Tumor Blood Vessel Morphology in Primary Uveal Melanoma. Ophthalmology. 1993;100(9):1389–98. 10.1016/s0161-6420(93)31470-3 [DOI] [PubMed] [Google Scholar]
- 15. Garcia-Arumi Fuste C, Peralta Iturburu F, Garcia-Arumi J: Is optical coherence tomography angiography helpful in the differential diagnosis of choroidal nevus versus melanoma? Eur J Ophthalmol. 2019;18:1120672119851768. 10.1177/1120672119851768 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Toledo JJ, Asencio M, García JR, et al. : OCT Angiography: Imaging of Choroidal and Retinal Tumors. Ophthalmol Retina. 2018;2(6):613–22. 10.1016/j.oret.2017.10.006 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Skalet AH, Gombos DS, Gallie BL, et al. : Screening Children at Risk for Retinoblastoma: Consensus Report from the American Association of Ophthalmic Oncologists and Pathologists. Ophthalmology. 2018;125(3):453–8. 10.1016/j.ophtha.2017.09.001 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Rootman DB, Gonzalez E, Mallipatna A, et al. : Hand-held high-resolution spectral domain optical coherence tomography in retinoblastoma: Clinical and morphologic considerations. Br J Ophthalmol. 2013;97(1):59–65. 10.1136/bjophthalmol-2012-302133 [DOI] [PubMed] [Google Scholar]
- 19. House RJ, Hsu ST, Thomas AS, et al. : Vascular Findings in a Small Retinoblastoma Tumor Using OCT Angiography. Ophthalmol Retina. 2019;3(2):194–5. 10.1016/j.oret.2018.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 20. Hsu ST, Chen X, Ngo HT, et al. : Imaging Infant Retinal Vasculature with OCT Angiography. Ophthalmol Retina. 2019;3(1):95–6. 10.1016/j.oret.2018.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Finn AP, House RJ, Hsu ST, et al. : Hyperreflective Vitreous Opacities on Optical Coherence Tomography in a Patient With Bilateral Retinoblastoma. Ophthalmic Surg Lasers Imaging Retina. 2019;50(1):50–2. 10.3928/23258160-20181212-08 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Shields CL, Manalac J, Das C, et al. : Review of spectral domain-enhanced depth imaging optical coherence tomography of tumors of the retina and retinal pigment epithelium in children and adults. Indian J Ophthalmol. 2015;63(2):128–32. 10.4103/0301-4738.154384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shields CL, Pellegrini M, Ferenczy SR, et al. : Enhanced depth imaging optical coherence tomography of intraocular tumors: From placid to seasick to rock and rolling topography--the 2013 Francesco Orzalesi Lecture. Retina. 2014;34(8):1495–512. 10.1097/IAE.0000000000000288 [DOI] [PubMed] [Google Scholar]
- 24. Samara WA, Pointdujour-Lim R, Say EA, et al. : Foveal microanatomy documented by SD-OCT following treatment of advanced retinoblastoma. J AAPOS. 2015;19(4):368–72. 10.1016/j.jaapos.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 25. Hasanreisoglu M, Dolz-Marco R, Ferenczy SR, et al. : Spectral Domain Optical Coherence Tomography Reveals Hidden Fovea Beneath Extensive Vitreous Seeding From Retinoblastoma. Retina. 2015;35(7):1486–7. 10.1097/IAE.0000000000000477 [DOI] [PubMed] [Google Scholar]
- 26. Soliman SE, Vandenhoven C, MacKeen LD, et al. : Optical Coherence Tomography-Guided Decisions in Retinoblastoma Management. Ophthalmology. 2017;124(6):859–72. 10.1016/j.ophtha.2017.01.052 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Welch RJ, Rao R, Gordon PS, et al. : Optical Coherence Tomography of Small Retinoblastoma. Asia Pac J Ophthalmol (Phila). 2018;7(5):301–6. 10.22608/APO.2018189 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Malik K, Welch RJ, Shields CL: Hand-Held Optical Coherence Tomography Monitoring Of Chemoresistant Retinoblastoma. Retin Cases Brief Rep. 2018. 10.1097/ICB.0000000000000714 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Yarovaya V, Sioufi K, Shields CL: Parafoveolar retinoblastoma regression with foveal preservation following intra-arterial chemotherapy documented on hand-held optical coherence tomography in a newborn. Int J Retina Vitreous. 2017;3:43. 10.1186/s40942-017-0098-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spencer MA, Welch RJ, Shields CL: Hand-held Optical Coherence Tomography Monitoring of Submillimeter Retinoblastoma Treated with Indocyanine Green-enhanced Transpupillary Therapy. Middle East Afr J Ophthalmol. 2018;25(2):108–10. 10.4103/meajo.MEAJO_280_17 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Seider MI, Grewal DS, Mruthyunjaya P: Portable Optical Coherence Tomography Detection or Confirmation of Ophthalmoscopically Invisible or Indeterminate Active Retinoblastoma. Ophthalmic Surg Lasers Imaging Retina. 2016;47(10):965–8. 10.3928/23258160-20161004-12 [DOI] [PubMed] [Google Scholar]
- 32. Berry JL, Cobrinik D, Kim JW: Detection and Intraretinal Localization of an ‘Invisible' Retinoblastoma Using Optical Coherence Tomography. Ocul Oncol Pathol. 2016;2(3):148–52. 10.1159/000442167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saktanasate J, Vongkulsiri S, Khoo CT: Invisible Retinoblastoma. JAMA Ophthalmol. 2015;133(7):e151123. 10.1001/jamaophthalmol.2015.1123 [DOI] [PubMed] [Google Scholar]
- 34. Lee GA, Hirst LW: Ocular surface squamous neoplasia. Surv Ophthalmol. 1995;39(6):429–50. 10.1016/s0039-6257(05)80054-2 [DOI] [PubMed] [Google Scholar]
- 35. Thomas BJ, Galor A, Nanji AA, et al. : Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul Surf. 2014;12(1):46–58. 10.1016/j.jtos.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Atallah M, Joag M, Galor A, et al. : Role of high resolution optical coherence tomography in diagnosing ocular surface squamous neoplasia with coexisting ocular surface diseases. Ocul Surf. 2017;15(4):688–95. 10.1016/j.jtos.2017.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Singh S, Mittal R, Ghosh A, et al. : High-Resolution Anterior Segment Optical Coherence Tomography in Intraepithelial Versus Invasive Ocular Surface Squamous Neoplasia. Cornea. 2018;37(10):1292–8. 10.1097/ICO.0000000000001680 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Vizvári E, Skribek Á, Polgár N, et al. : Conjunctival melanocytic naevus: Diagnostic value of anterior segment optical coherence tomography and ultrasound biomicroscopy. PLoS One. 2018;13(2):e0192908. 10.1371/journal.pone.0192908 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Skalet AH, Li Y, Lu CD, et al. : Optical Coherence Tomography Angiography Characteristics of Iris Melanocytic Tumors. Ophthalmology. 2017;124(2):197–204. 10.1016/j.ophtha.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Dart JK, Marsh RJ, Garner A, et al. : Fluorescein angiography of anterior uveal melanocytic tumours. Br J Ophthalmol. 1988;72(5):326–37. 10.1136/bjo.72.5.326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mashayekhi A, Schönbach E, Shields CL, et al. : Early subclinical macular edema in eyes with uveal melanoma: association with future cystoid macular edema. Ophthalmology. 2015;122(5):1023–9. 10.1016/j.ophtha.2014.12.034 [DOI] [PubMed] [Google Scholar]
- 42. Rose K, Krema H, Durairaj P, et al. : Retinal perfusion changes in radiation retinopathy. Acta Ophthalmol. 2018;96(6):e727–e731. 10.1111/aos.13797 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Veverka KK, AbouChehade JE, Iezzi R, Jr, et al. : Noninvasive grading of radiation retinopathy: The use of optical coherence tomography angiography. Retina. 2015;35(11):2400–10. 10.1097/IAE.0000000000000844 [DOI] [PubMed] [Google Scholar]
- 44. Reichstein D: Current treatments and preventive strategies for radiation retinopathy. Curr Opin Ophthalmol. 2015;26(3):157–66. 10.1097/ICU.0000000000000141 [DOI] [PubMed] [Google Scholar]