Keywords: enteric nervous system, glia, IBD, inflammatory bowel disease, purines
Abstract
ATP is both an important mediator of physiological gut functions such as motility and epithelial function, and a key danger signal that mediates cell death and tissue damage. The actions of extracellular ATP are regulated through the catalytic functions extracellular nucleoside triphosphate diphosphohydrolase-1 (NTPDase1), -2, -3, and -8, which ultimately generate nucleosides. Ectonucleotidases have distinct cellular associations, but the specific locations and functional roles of individual NTPDases in the intestine are still poorly understood. Here, we tested the hypothesis that differential and cell-selective regulation of purine hydrolysis by NTPDase1 and -2 plays important roles in gut physiology and disease. We studied Entpd1 and Entpd2 null mice in health and following colitis driven by 2% dextran sulfate sodium (DSS) administration using functional readouts of gut motility, epithelial barrier function, and neuromuscular communication. NTPDase1 is expressed by immune cells, and the ablation of Entpd1 altered glial numbers in the myenteric plexus. NTPDase2 is expressed by enteric glia, and the ablation of Entpd2 altered myenteric neuron numbers. Mice lacking either NTPDase1 or -2 exhibited decreased inhibitory neuromuscular transmission and altered components of inhibitory junction potentials. Ablation of Entpd2 increased gut permeability following inflammation. In conclusion, the location- and context-dependent extracellular nucleotide phosphohydrolysis by NTPDase1 and -2 substantially impacts gut function in health and disease.
NEW & NOTEWORTHY Purines are important mediators of gastrointestinal physiology and pathophysiology. Nucleoside triphosphate diphosphohydrolases (NTPDases) regulate extracellular purines, but the roles of specific NTPDases in gut functions are poorly understood. Here, we used Entpd1- and Entpd2-deficient mice to show that the differential and cell-selective regulation of purine hydrolysis by NTPDase1 and -2 plays important roles in barrier function, gut motility, and neuromuscular communication in health and disease.
INTRODUCTION
Adenosine 5′-triphosphate (ATP) is a fundamental cellular energy source and an important mediator of intercellular communication. ATP is released into the extracellular environment via multiple mechanisms including pannexin channels, connexin hemichannels, exocytosis, and cell death or damage (54). ATP and its metabolites exert their actions through purinergic type-1 (P1) and type-2 (P2) receptors. P1 receptors (A1, A2A, A2B, and A3) transduce the effects of adenosine, while P2 (P2X and P2Y) receptors generally transduce the effects of ATP (16). Ionotropic P2X receptors (P2X1–7) are primarily activated by ATP, and metabotropic P2Y (P2Y1,2,4,6,11–14) receptors have varying affinities for specific nucleotides that can vary between species (100). For instance, P2Y1 receptors are chiefly activated by endogenous ADP regardless of species, but human P2Y4 receptors preferentially bind UTP as an agonist, while mouse and rat P2Y4 receptors are equally activated by ATP and UTP (102). Similarly, P2Y6 receptors are activated by UDP in humans and both UTP and UDP in mice (56).
Purinergic receptors are expressed throughout the gut wall and transduce the effects of purines on gut functions (reviewed in Refs. 13–15). P2Y1 receptors mediate relaxations of bowel smooth muscles (35, 36, 101) while P1 and P2 receptors on epithelial cells regulate intestinal secretion (23, 44, 60, 65) and absorption (59, 106). Nucleotides are also important transmitters in the enteric nervous system (ENS) where they mediate fast and slow synaptic transmission (reviewed in Refs. 12, 38) via P2X (3, 4, 39, 66, 108) and P2Y (3, 53, 97, 104) receptors and mediate communication between enteric neurons and glia (46–48, 50, 58, 72, 73, 87, 99). Other cell types in the gut wall express purinergic receptors including interstitial cells of Cajal (18, 22, 99) and fibroblast-like cells (62) that contribute to fine tuning of intestinal physiology such as modulation of intestinal smooth muscle cell activity (33, 96).
Purines also play important roles in pathophysiological processes involving inflammation. High extracellular concentrations of ATP are generated during the initial phase of inflammation by cell death and release from cells (54). High adenosine concentrations are generated during the later phases of inflammatory events as ATP is reduced by enzymatic degradation (reviewed in Refs. 20, 26). Extracellular ATP and ADP are danger signals that exaggerate acute tissue damage and cell death while adenosine has anti-inflammatory effects and promotes the resolution of inflammation (20, 26). Purines and their receptors regulate a variety of biological processes in all major subsets of immune cells such as chemotaxis, maturation, regulation of phagocytosis, and degranulation (reviewed in Ref. 17). Purines also contribute to neuroinflammation, and the activation of neuronal P2X7 receptors by ATP released from enteric glial cells promotes the death of enteric neurons during colitis (11, 49). Additionally, extracellular ATP disrupts epithelial barrier integrity during intestinal inflammation by promoting intestinal epithelial cell death (90). However, the role of ATP in the gut epithelium is multifaceted and the coadministration of ATP reduces indomethacin-induced increases in permeability in the human small intestine (7, 8). Approximately 60% of purinoreceptor genes are dysregulated in inflammatory bowel disease (IBD) patients, and altered purinergic signaling is considered an important contributor to IBD and other intestinal diseases involving inflammation (84).
The main pathway of extracellular ATP/UTP breakdown is ecto-enzymatic degradation by nucleoside triphosphate diphosphohydrolases (NTPDases) (82). In this family of eight enzymes, NTPDase4–7, are located on intracellular organelles where they regulate intracellular levels of nucleotides/nucleosides. Although NTPDase5 and -6 can be secreted, their low affinity and low specific activities (52, 76) render these minor contributors to the hydrolysis of extracellular ATP/UTP. Thus the major players in extracellular nucleotide breakdown are NTPDase1, -2, -3, and -8, which are expressed extracellularly on the plasma membrane. NTPDase2 preferentially causes hydrolysis of ATP to ADP, and NTPDase1, -3 and -8 hydrolyze ATP to ADP and then to AMP (61). NTPDase1 has the highest affinity for ATP (2- to 4-fold) whereas NTPDase2 has the highest maximum rate of reaction (twofold) (61). These kinetic properties suggest that NTPDase1 chiefly breaks down ATP at physiological levels of extracellular ATP. However, NTPDase1 capacity is overwhelmed during the initial phases of inflammation when extracellular ATP is abundant within tissues. Under these circumstances, extracellular and extravascular ATP degradation may shift toward NTPDase2 (89). Although it seems that these NTPDases have redundant roles, single gene knockouts of either Entpd1 or Entpd2 both exacerbate acute dextran sulfate sodium (DSS)-induced colitis and intestinal tissue damage (30, 32) as does the ablation of Entpd3, albeit to a lesser extent (30).
These results may be partially explained by the distinct expression patterns of Entpd1 and Entpd2 in the gut wall (reviewed in Ref. 69). NTPDase1 is highly expressed by the vascular endothelium and immune cells such as intraepithelial lymphocytes, regulatory T lymphocytes, and muscularis macrophages, while NTPDase2 is highly expressed by enteric glia (9). How these different compartments of ATP degradation by NTPDase1 and -2 regulate gut functions in health and disease is poorly understood and is the focus of this study. Here, we tested the hypothesis that this context-dependent and compartmentalized breakdown of ATP has specific effects on gut physiology and disease. To this end, we utilized Entpd1- and Entpd2-deficient mice to investigate their physiological roles in barrier function, gut motility, and neuromuscular communication and to assess how the lack of purine degradation by either NTPDase1 or NTPDase2 affects these roles following inflammation.
MATERIALS AND METHODS
Animals
All experimental protocols were approved by the Institutional Animal Care and Use Committees (ID PROTO201800028) at Michigan State University (MSU). Mice of both sexes were used at 8–12 wk of age. Mice were maintained in a temperature-controlled environment (Innovive, San Diego, CA; Innocage system with ALPHA-dri bedding) on a 12-h light-dark cycle (lights on at 7:00 AM) with access to water and food ad libitum. Cages typically housed two to five mice and were enriched with rodent nesting sheets. In occasional cases of single housed animals, mouse huts were provided. The derivation and characterization of Entpd1 and Entpd2 null mice have been described elsewhere (27, 40). Briefly, both strains commenced on 129Svj or 129Svj backgrounds and were backcrossed to C57BL/6 background for >10 generations (30, 32). Both lines were bred in house as homozygous knockout animals to reduce the total animal numbers generated for this study (26 and 19 litters of Entpd1−/− and Entpd2−/− mice, respectively). Genotyping was performed commercially (Transnetyx, Cordova, TN). C57BL/6 mice from The Charles River Laboratories served as control wild-type (WT) mice and were housed in the same animal facility as knockout animals for at least 1 wk before experiments. Mice were euthanized by cervical dislocation and decapitation at several time points in the day (details provided in the following sections). Groups of mice with differing genotypes and treatments were simultaneously assayed to reduce variability caused by circadian rhythms and/or day-to-day technical variability. In addition, separate animals were used for physiological assays and immunohistochemistry/histology to allow assessment of the same colonic region.
DSS-Induced Colitis
Colitis was induced by adding 2% DSS (colitis grade, molecular mass: 36–50 kDa; MP Biomedical, Solon, OH) to drinking water for 7 days. The solution was replaced with a freshly prepared 2% DSS on the third and fifth day of the 7-day treatment period. Mice were weighed in the animal facility from 10:00 AM to 12:00 PM (Zeitgeber 3–5), and experiments were performed 2 wk after the DSS treatment. Humane euthanasia was considered if weight loss was rapid and severe (>15% in 48 h) or >20% with additional signs of distress (ruffled coat, lethargy, hunched posture, etc.).
Gut Wall Paracellular Permeability
Intestinal barrier function was assessed using Ussing chambers as described previously (47). Briefly, full thickness preparations of distal colon were mounted in the Ussing chambers (aperture: 0.3 cm2; EasyMount Ussing Chamber system, Physiologic Instruments, San Diego, CA) and equilibrated for 20 min, and a cell-impermeant fluorescein-5-(and-6)-sulfonate (478.32 Da; Life Technologies, Carlsbad, CA) was added to the mucosal chamber (0.05 mg/ml). Mice were euthanized from 9:00 AM to 10:00 AM (Zeitgeber 2–3), and experiments began around 11:00 AM (Zeitgeber 4). We assessed a single preparation of distal colon per animal due to the size of this gut region in mice. We used full thickness preparations because the seromusculature layers are relatively thin in the mouse intestine and to avoid anti-inflammatory drugs used during seromuscular stripping (24). Samples from the serosal chamber were taken before the dye was added and every 20 min until 2 h (100-μL duplicates and buffer was replenished). Fluorescent intensity was measured on the Infinite M1000 PRO microplate reader (wavelength 495/520 nm; Tecan Group, Mannedorf, Switzerland) using i-control microplate reader software (Tecan, version 1.6.19.2). The paracellular permeability of the gut wall was assessed from the slope of fluorescence values of the last four time points.
Distal Colon Motility and Contractility
We performed these two tests simultaneously using an aboral part of the distal colon (~2.5 cm) to assay colon migratory motor complexes and two adjacent regions of the distal colon (each ~0.5 cm) for isometric circular muscle tension recordings. Assays were performed three times in a day: 9:00 AM, noon, and 3:00 PM (Zeitgebers 2, 5, and 8).
Colon migratory motor complex recordings.
The procedure is described in detail in a previous study (73). Briefly, colon migratory motor complexes (CMMCs) were defined as a complex in which contraction occurs first at the oral site followed by a contraction at the aboral site. CMMCs were recorded from distal colons maintained ex vivo. The tissue was secured at the ends using silk suture and affixed to force transducers (Grass Instruments, Quincy, MA), 2 cm apart, at the oral and aboral ends. The tissues were stretched to an initial tension of 5 mN and allowed to equilibrate for 1 h. CMMCs were recorded with LabChart 8 (ADInstruments, Colorado Springs, CO) for 20 min and analyzed for several parameters including frequency, amplitude, and duration. The threshold for automated detection of contractions was set to 5 mN (peak contraction – baseline > 5 mN). The cholinergic agonist carbachol (10 μM) was bath applied, and CMMCs were recorded for an additional 20-min interval after a 20-min equilibration period. Preparations that did not respond to carbachol were not analyzed. All data points for CMMC recordings (amplitude, frequency, and velocity) are averages from the contractions during the 20-min interval.
Isometric circular muscle tension recordings.
The procedure is described in detail in a previous study (72). Briefly, isometric contractions/relaxations were recorded by LabChart from circular-oriented (“ring”) segments of distal colon under 5-mN passive tension affixed to a force transducer (Grass Instruments); electrical field stimulation (EFS; 20 V, 1–30 Hz, 0.5 ms pulse duration) was generated by a GRASS stimulator (S88; GRASS Telefactor, West Warwick, RI) and applied through platinum concentric electrodes to evoke neurogenic contractions/relaxations. Neurogenic relaxations were studied in tissues precontracted with 1 μM prostaglandin F2-α (PGF2α). After EFS, maximal muscle contractions were obtained by adding carbachol (10 μM) to the bath. Preparations that did not respond to EFS or to carbachol application were not included in analysis. Data are presented from single contractions/relaxations or as averages of two contractions/relaxations, each from an individual ring preparation from adjacent regions of the same distal colon.
Sharp Electrode Electrophysiology of Circular Smooth Muscle Cells
Experiments were performed from 10:00 AM to 4:00 PM (Zeitgeber 3–9). A segment of the distal colon was used to prepare nerve muscle preparations where the longitudinal and circular muscle layers and myenteric plexus remain intact and the mucosal and submucosa layers are carefully removed in prewarmed (37°C) and oxygenated (95% O2-5% CO2) Krebs physiological solution. 0.5-cm2 sections were transferred to a recording chamber, mounted on an inverted microscope, and perfused with Krebs solution at a flow rate of 4 ml/min at 37°C. The preparations were allowed to acclimate for 30 min before the electrophysiological paradigm. Borosilicate glass microelectrodes (1.0 × 0.5 mm inner diameter w/Fiberglass; FHC, Bowdoin, ME) with a tip resistance of 60–120 MΩ were filled with 2 M KCl and used to impale circular smooth muscle cells. Transmural stimulation was performed by placing a pair of 1-mm diameter Ag/AgCl wires (A-M Systems, Seattle, WA) in the recording chamber and driven by a Grass S88 Stimulator (Grass Technologies, West Warwick, RI). The stimulation paradigm consisted of 10 Hz, 0.5-ms pulse duration, 80 V, and manipulation of the train duration (100–700 ms) to control the given number of pulses (1, 3, 5, and 7 pulses of stimulation). An Axoclamp-2A amplifier, a Digidata 1440A analog-digital converter, and Axoscope 10.6 software were used to record changes in the membrane potential (Molecular Devices, Sunnyvale, CA). The amplified signal was sampled at 2 kHz and filtered at 1 kHz. The L-type Ca2+ channel antagonist nifedipine (1 µM) was applied to the physiological solution for the duration of each experiment to limit spontaneous smooth muscle contractility. Nifedipine, MRS2179, and Nω-nitro-l-arginine (l-NNA) were dissolved in DMSO, deionized water, and HCl (1 N), respectively. Successful impalements of a circular smooth muscle cell were determined by a rapid drop in the resting membrane potential, and only cells that sustained a resting membrane potential less than or equal to −40 mV were considered for statistical analysis. On occasions where more than one cell was recorded per animal, the total number of cells were then averaged to equal the mean for one animal.
Solutions
Gut wall permeability, muscle contractility, and electrophysiology studies were performed in prewarmed (37°C) and oxygenated (95% O2-5% CO2) Krebs physiological solution consisting of the following (in mmol/L): 117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose. CMMC studies were conducted in prewarmed (37°C) DMEM/Nutrient Mixture F-12 (Life Technologies) supplemented with l-glutamine and HEPES. Solution temperature was maintained with a TC120L (Grant Instruments, Cambridge, UK), TEPS-1 (Fried Electric, Haifa, Israel), and Lauda Ecoline 003/E100 Heating Circulator (Brinkmann, Delran, NJ).
Immunohistochemistry
Both whole mount preparations of longitudinal muscle with myenteric plexus (LMMP) and frozen sections (7 to 8 µm thickness) were processed for immunohistochemistry as described elsewhere (47), and antibody details (diluted in blocking solution) are supplied in Table 1. All antibodies used have research resource identifiers. Mouse anti-NTPD1 and -2 antibodies were provided by J. Sévigny and characterized on knockout animals in prior work (5, 71), while all other antibodies were purchased from commercial sources and their specificity was validated in prior work. Briefly, mouse distal colons were fixed overnight in Zamboni’s fixative at 4°C and then rinsed three times (10 min each) in phosphate-buffered saline (PBS) or PBS containing 0.1% Triton X-100 followed by a 30- or 45-min incubation in blocking solution [containing 4% normal goat serum (or 5% normal horse serum), 0.1 or 0.4% Triton X-100, and 1% bovine serum albumin]. Primary antibodies were applied overnight at room temperature or for 48 h at 4°C before being rinsed three times with PBS. Secondary antibodies were applied for 2 h at room temperature. LMMPs were rinsed with 0.1 M phosphate buffer and mounted on slides with bicarbonate-buffered glycerol (consisting of a 1:3 mixture of 142.8 mM sodium bicarbonate and 56.6 mM carbonate to glycerol). Fluorescent labeling was imaged through the ×20 (PlanFluor, 0.75 numerical aperture) objective of an upright epifluorescence microscope (Nikon Eclipse Ni, Melville, NY) with a Retiga 2000R camera (QImaging, Surrey, BC, Canada) controlled by Qcapture Pro 7.0 (Qimaging) or by confocal imaging through the Plan-Apochromat ×40 oil immersion objective (1.3 numerical aperture) of an inverted Olympus Fluoview FV1000 microscope (Olympus, Center Valley, PA).
Table 1.
Primary and secondary antibodies used
| Antibody | Source | Catalog No. | RRID | Dilution |
|---|---|---|---|---|
| Primary antibodies | ||||
| Biotin mouse anti-HuC/D | Invitrogen, Carlsbad, CA | A21272 | AB_1500232 | 1:200 |
| Chicken anti-GFAP | Abcam, Cambridge, MA | ab4674 | AB_304558 | 1:1,000 |
| Guinea pig anti-mouse NTPDase1 | ectonucleotidases-ab.com, Quebec, QC | mN1-2C | AB_2800454 | 1:1,000 |
| Rabbit anti-mouse NTPDase2 | ectonucleotidases-ab.com | mN2-36L | AB_2800455 | 1:500 |
| Rabbit anti-S100β | Abcam, Cambridge, MA | ab52642 | AB_882426 | 1:200 |
| Rat anti-mouse CD45 | BD Biosciences, San Jose, CA | 550539 | AB_2174426 | 1:300 |
| Secondary antibodies | ||||
| Donkey anti-rabbit Alexa 594 | Jackson ImmunoResearch, West Grove, PA | 711-585-152 | AB_2340621 | 1:400 |
| Donkey anti-guinea pig Alexa 488 | Jackson ImmunoResearch | 706-545-148 | AB_2340472 | 1:400 |
| Goat anti-chicken DyLight 405 | Jackson ImmunoResearch | 103-475-155 | AB_2337389 | 1:400 |
| Goat anti-rat Cy5 | Jackson ImmunoResearch | 112-175-143 | AB_2338263 | 1:400 |
| Streptavidin DyLight 405 | Jackson ImmunoResearch | 016-470-084 | AB_2337248 | 1:400 |
RRID, research resource identifier; GFAP, glial fibrillary acidic protein.
Image Analysis
Epifluorescence images of LMMP preparations were analyzed offline using ImageJ software (ver 1.52a; National Institutes of Health, Bethesda, MD) run on Java 1.8.0_112 (64-bit). Cell counts were performed using the cell counter plug-in of ImageJ software. The preparations were imaged semirandomly, i.e., each preparation was imaged at the center and two to three peripheral regions excluding the edge of the preparations and damaged areas, while image analysis was performed in a blinded fashion. Enteric neuron and glial cell numbers are presented as ganglionic packing density, calculated by counting the number of HuC/D-immunoreactive neurons or S100β-immunoreactive glia within the defined ganglionic area. Cell counts and ganglionic expression data were performed on 7–13 ganglia per animal and averaged to obtain a value for that animal. The n values represent the number of animals in each experiment, and data are expressed as percent water control.
Chemicals and Reagents
Unless otherwise stated, chemicals and reagents for this study were purchased from Sigma-Aldrich (St. Louis, MO).
Histology
A section of intact distal colon from treated mice was fixed overnight in Zamboni’s fixative at 4°C. Tissue was washed with PBS until cleared. Hematoxylin and eosin staining were performed on paraffin-embedded cross sections (4- to 5-µm thickness) of the distal colon by the Investigative HistoPathology Laboratory at Michigan State University. Four sections per animal were scored in a blinded fashion using criteria for combined disease activity score (Table 2) that were modified from previous work (57, 103).
Table 2.
Histological disease activity scoring
| Feature Scored | Score |
|---|---|
| Epithelial damage | 0–3 |
| Immune infiltration | 0–3 |
| Crypt architecture | 0–3 |
| Abscess present | Present (1) or absent (0) |
The total score was calculated as a total of the individual scores; 0 = 0–5%, 1 = 6–25%, 2 = 26–50%, 3 = 51–100% of tissue affected.
Statistical Analysis
Data were analyzed using Prism 7 (GraphPad Software, San Diego, CA) and are shown as means ± SE. Sample sizes were determined from previous experimental data using a power of 0.8 and a significance level of 0.05. The DSS-treated group was assigned by splitting the same genotype and sex littermates into two cohorts and treating one of them with DSS. The DSS-treated and healthy control groups were equal in size if there were even number of the same genotype/sex littermates. Otherwise, the DSS-treated groups had an extra member. Male and female data were pooled and tested for normality using D'Agostino and Pearson or Shapiro-Wilk tests. Outliers were excluded using the ROUT method (Q = 5%). Data were almost exclusively analyzed by two-way ANOVA for differences between the genotypes, DSS treatment, and their interaction (unless stated otherwise) followed by a Tukey’s post hoc test. The change in mice body weights was analyzed by repeated measures two-way ANOVA. P < 0.05 was considered significant. In cases with an additional factor, such as in Fig. 8, we used Student’s t-test with Welch's correction for unequal variances or Mann-Whitney U test with adjusted level of significance to P < 0.01 using the Bonferroni correction for multiple comparisons, i.e., the critical P value (0.05) was divided by number of comparisons (5).
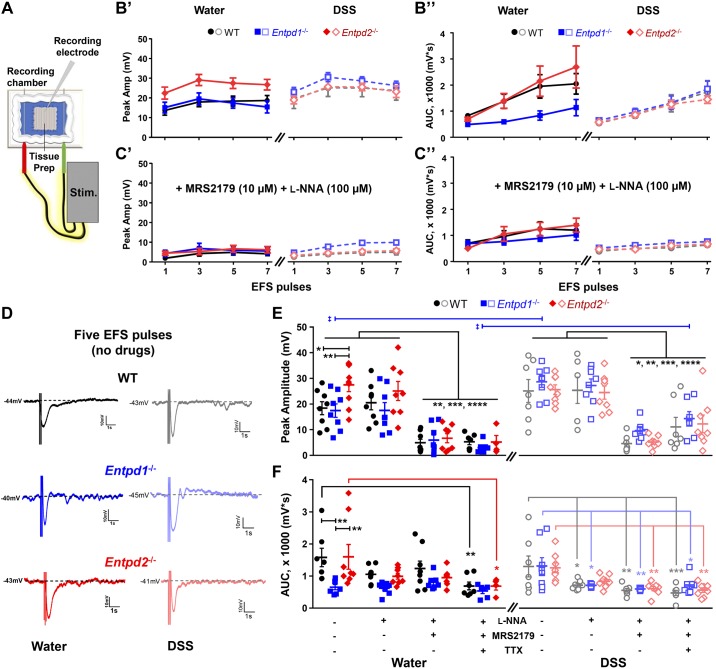
Fig. 8.
Loss of nucleoside triphosphate diphosphohydrolase-1 and -2 (NTPDase1 and -2) specifically affects the electrical field stimulation (EFS)-evoked inhibitory junction potentials (IJPs). A: schematic representation of the sharp electrode electrophysiology paradigm. B and C: line plots showing the IJP peak amplitude (B’ and C’) and area under the curve (AUC; B” and C”) following EFS of WT, Entpd1−/−, and Entpd2−/− healthy mice (left) and animal groups 2 wk after the dextran sulfate sodium (DSS) treatment (right) in the absence of drugs (B) and during co-application of Nω-nitro-l-arginine (l-NNA) and MRS2179 (C). D: representative IJP recordings following 5 electrical pulse stimulation of healthy (left) and the DSS-treated (right) WT, Entpd1−/−, and Entpd2−/− preparations. E and F: scatter plot diagrams comparing the IJP peak amplitude (E) and AUC (F) among healthy (left) and DSS-treated (right) WT, Entpd1−/−, and Entpd2−/− animals in the absence of drugs and during application of l-NNA, MRS2179, and tetrodotoxin (TTX). *P < 0.05, **P < 0.01, ***P < 0.001; ****P < 0.0001, two-way ANOVA and Tukey's post hoc test. ‡P < 0.01; Student’s t-test with Welch's correction or Mann Whitney U-test for comparisons between the healthy and DSS-treated animals in the genotype- and drug-matched groups (critical P value corrected for multiple comparisons). Animal numbers presented as control, + l-NNA, + MRS2179, and + TTX for each genotype (WT, Entpd1−/−, and Entpd2−/−), respectively: 8, 8, 8, 7–8, 8, 8, 7–8, 8, 8, 4 (in E, left); 7, 7, 7, 7–8, 8, 8, 7–8, 8, 8, 8 (in E, right); 7 (4,559 mV removed as an outlier), 8, 8, 7–7 (1,797 mV removed as an outlier), 8, 7 (1477 mV removed as an outlier), 6 (2,212 mV removed as an outlier), 8 (6,055 mV removed as an outlier), 8, 7 (3,002 mV removed as an outlier), 4 (in F, left); 7, 7, 7, 7–8, 7 (1,267 mV removed as an outlier), 7 (1,443 mV removed as an outlier), 7–8, 8, 8, 8 (in F, right). Breakdown by sex of the control groups is presented in Table 4.
RESULTS
Location of NTPDase1 and NTPDase2
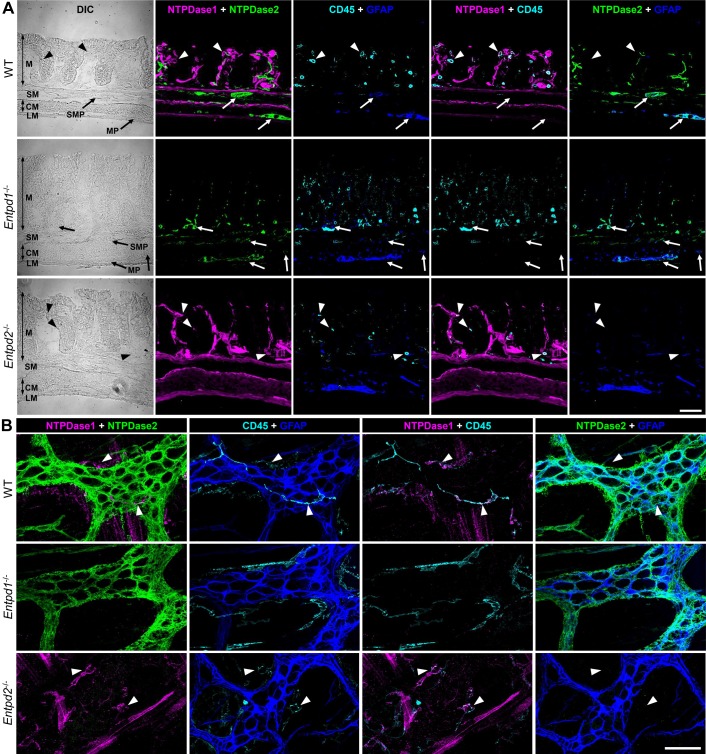
The location of these two ecto-enzymes has the potential to significantly affect local purine concentrations. We use specific antibodies to investigate the protein expression of CD39/NTPDase1 and CD39L1/NTPDase2 and found that NTPDase1 and -2 are expressed in virtually all layers of the gut wall but do not overlap (Fig. 1). Rather, NTPDase1 is chiefly expressed by immune cells and vasculature while NTPDase2 is primarily expressed by enteric glia. NTPDase2 immunoreactivity is nearly entirely colocalized with immunoreactivity for the glial marker glial fibrillary acidic protein (GFAP) at the levels of the myenteric and submucosal plexuses (Fig. 1, A and B). However, less colocalization between NTPDase2 and GFAP immunolabeling is observed at the level of the intestinal mucosa. This is likely due to the fact that GFAP labels the majority of glial cells in the myenteric and submucosal plexuses but only about a third of mucosal glia (6). However, NTPDase2 expression by other cells types such as endothelial cells and pericytes could also account for mucosal labeling that does not colocalize with GFAP (69). In addition, smooth muscle cells and platelet-derived growth factor receptor-α-positive (PDGFRα+) cells express transcripts for NTPDase1 and -2, respectively (10, 64). The qualitative intensity of NTPDase1 labeling in Entpd2−/− animals appears to increase, which could suggest that the loss of NTPDase2 increases NTPDase1 expression. However, qualitative immunohistochemical labeling intensity was not quantified. Deletion of Entpd2 did not affect the types of cells expressing NTPDase1 and vice versa (Fig. 1), allowing us to investigate the independent pathophysiological roles of NTPDase1 and -2.
Fig. 1.
Expression of nucleoside triphosphate diphosphohydrolase-1 and -2 (NTPDase1 and -2) within the gut wall. A: cross sections of distal colons from wild-type (WT; top row), Entpd1−/− (middle row), and Entpd2−/− (bottom row) mice were stained for NTPDase1 (magenta), NTPDase2 (green), pan-leukocyte marker CD45 (cyan), and glial fibrillary acidic protein (GFAP, blue), a marker of enteric glia. NTPDase1-immunoreactivity (ir) colocalized with immune cells (arrowheads) and NTPDase2-ir with enteric glia (arrows). Both the enzymes are also expressed by other cell types in the gut wall (see text). CM, circular muscle; DIC, differential interference contrast; LM, longitudinal muscle; M, mucosa; MP, myenteric plexus; SM, submucosa; SMP submucosal plexus. B: expression of NTPDase1 and NTPDase2 within the myenteric plexus. Longitudinal muscle-myenteric plexus preparations of distal colons from WT (top row), Entpd1−/− (middle row), and Entpd2−/− (bottom row) mice were stained for NTPDase1 (magenta), NTPDase2 (green), CD45 (cyan), and GFAP (blue). Scale bars = 50 µm. These are representative images from 4 to 5 mice per group.
Roles of NTPDase1 and NTPDase2 in the Resolution of Acute Intestinal Inflammation
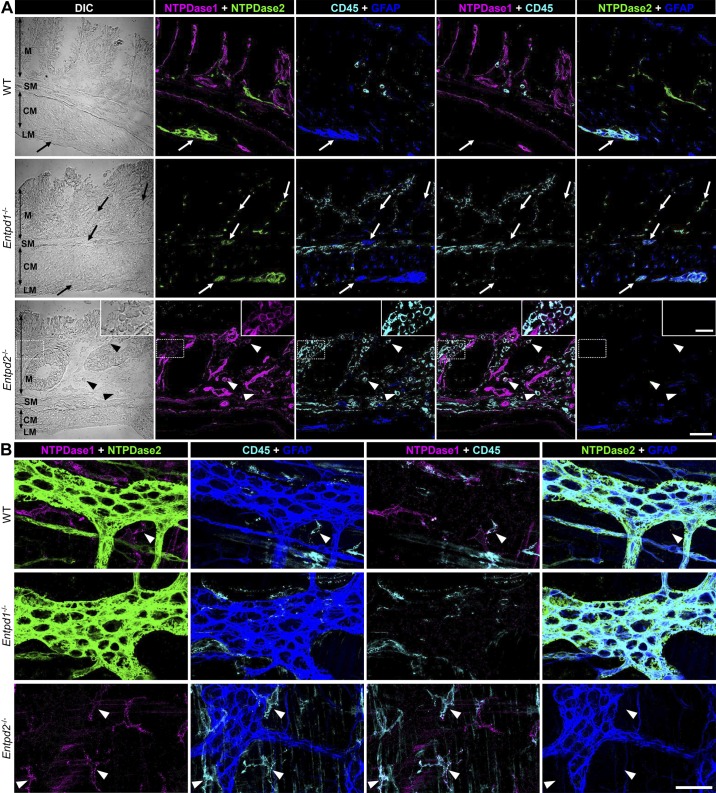
All experiments were performed 2 wk after 2% DSS treatment to assess functional recovery following inflammation (Fig. 2A). At this time point, the types of cells expressing NTPDase1 and -2 in DSS-treated mice (Fig. 3) were the same as their healthy controls (Fig. 1) where NTPDase1 is primarily expressed by immunocytes and NTPDase2 by enteric glia. However, we observed an increase in the number of CD45+ immunocytes in DSS-treated Entpd1 and Entpd2 null mice in comparison to the DSS-treated WT animals (Fig. 3A).
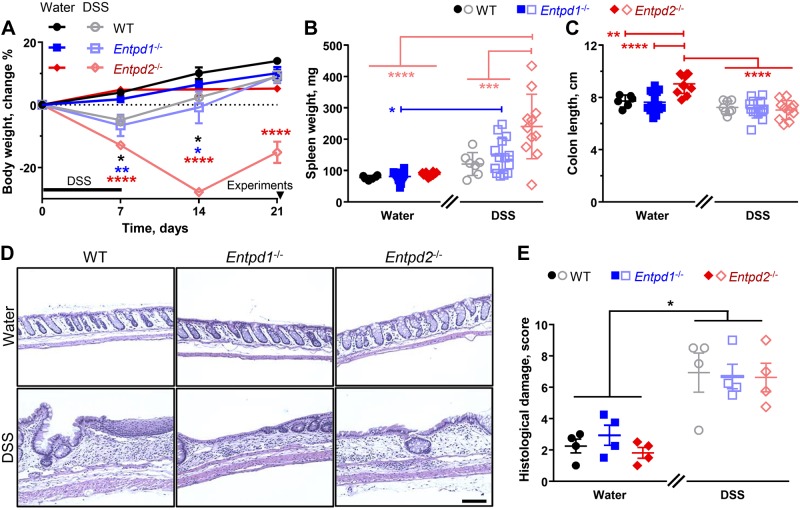
Fig. 2.
Role of nucleoside triphosphate diphosphohydrolase-1 and -2 (NTPDase1 and -2) in the resolution of acute intestinal inflammation. A: experimental timeline and body weight change. Subsets of healthy wild-type (WT), Entpd1−/−, and Entpd2−/− mice were treated with 2% dextran sulfate sodium (DSS) for 1 wk (black bar). Mouse body weight was obtained once a week and experiments were performed 2 wk after the treatment (arrowhead). *P < 0.0468, **P < 0.0088, ****P < 0.0001, two-way ANOVA and Tukey's post hoc test between the healthy and the DSS-treated mice of the same genotype (asterisks color coded: black, WT; blue, Entpd1−/−; and red, Entpd2−/−); n = 3 mice, DSS-treated Entpd2−/− males; n = 4 mice, healthy Entpd2−/− (2 males and 2 females); n = 6 mice, healthy WT (3 males and 3 females); n = 7 mice, DSS-treated Entpd1−/− (2 males and 5 females); n = 8 mice, DSS-treated WT (4 males and 4 females); n = 10 mice, healthy Entpd1−/− (2 males and 8 females). B and C: spleen weights (B) and colon lengths (C) 2 wk after the treatment. *P = 0.036, **P = 0.005, ***P < 0.001, ****P < 0.0001, two-way ANOVA and Tukey's post hoc test; n = 6 mice, healthy WT (3 males and 3 females); n = 8 mice, DSS-treated WT [4 males and 4 females (female 429 mg spleen removed as an outlier)]; n = 11 mice, DSS-treated Entpd2−/− (9 males and 2 females), healthy Entpd2−/− [6 males and 5 females (127 mg spleen removed as an outlier)]; n = 14 mice, healthy and DSS-treated Entpd1−/− [5 males (each, healthy 172.3 mg spleen removed as an outlier and an additional healthy spleen was not weighed) and 9 females (each)]. D and E: histological evaluation of distal colon tissue. D: representative images of hematoxylin and eosin stain. Scale bar = 100 µm. E: blinded scoring of tissue damage and immune infiltration. For detailed scoring information, see Table 2. *P < 0.0352, two-way ANOVA and Tukey's post hoc test; n = 2 male and 2 female mice per group.
Fig. 3.
Expression of nucleoside triphosphate diphosphohydrolase-1 and -2 (NTPDase1 and -2) within the gut wall 2 wk after the dextran sulfate sodium (DSS) treatment. A: 2 wk after the DSS treatment, cross sections of distal colons from wild-type (WT; top row), Entpd1−/− (middle row), and Entpd2−/− (bottom row) mice were stained for NTPDase1 (magenta), NTPDase2 (green), pan-leukocyte marker CD45 (cyan), and glial fibrillary acidic protein (GFAP; blue). NTPDase1 and -2 immunoreactivity (-ir) patterns were similar to the healthy controls (Fig. 1) colocalizing with immune cell marker (arrowheads) and with enteric glia (arrows), respectively. Scale bar = 50 µm. Dotted rectangle in the bottom row is enlarged in inset (scale bar = 20 µm). CM, circular muscle; DIC, differential interference contrast; LM, longitudinal muscle; M, mucosa; MP, myenteric plexus; SM, submucosa; SMP submucosal plexus. B: expression of NTPDase1 and NTPDase2 within the myenteric plexus 2 wk after the DSS treatment. Longitudinal muscle-myenteric plexus preparations of distal colons from the DSS-treated WT (top row), Entpd1−/− (middle row), and Entpd2−/− (bottom row) mice were stained for NTPDase1 (magenta), NTPDase2 (green), CD45 (cyan), and GFAP (blue). Note that NTPDase1 and NTPDase2 keep distinct cellular localization after acute intestinal inflammation, colocalizing with either immune cells or enteric glia, respectively. Scale bar = 50 µm. These are representative images from 5 Entpd1−/−, 4 Entpd2−/−, and 4 WT mice.
These data correspond well with measures of systemic inflammatory processes such as a decrease in body weight and an enlarged spleen (Fig 2, A and B). As expected, all DSS-treated mice exhibited a decrease in body weight after the treatment and all water controls mostly continued to increase in body weight. The 2% DSS-treated WT and Entpd1−/− mice exhibited rapid recovery of body weight, but Entpd2−/− animals still had significant weight loss [P < 0.0001, Tukey’s multiple comparisons test after repeated measures two-way ANOVA for time (F3,96 = 24.09), genotype/treatment (F5,32 = 16.47), and their interaction (F15,96 = 10.31)] 2 wk after the DSS treatment (−15.2 ± 3.4% of starting body weight) when compared with their respective water controls (5.2 ± 1.0%; Fig 2A).
There was no significant difference in spleen weight (P = 0.7032, two-way ANOVA with Tukey’s post hoc test) between the DSS-treated WT animals (121 ± 14 mg) and their healthy controls (78 ± 3 mg; Fig 2B). However, 2 wk after the DSS treatment, spleen masses of both Entpd1 and Entpd2 null mice (147 ± 15 mg and 241 ± 31 mg, respectively) were significantly increased [P = 0.0355 and P < 0.0001, Tukey’s multiple comparison tests after two-way ANOVA for treatment (F1,54 = 35.77), genotype (F2,54 = 7.435), and their interaction (F2,54 = 5.377)] in comparison to the respective water-treated controls (81 ± 5 mg and 88 ± 2 mg). In addition, spleen enlargement was more pronounced in Entpd2 than in Entpd1 null mice (241 ± 31 mg vs. 147 ± 15 mg; P = 0.0009, two-way ANOVA with Tukey’s post hoc test). Increased spleen mass and greater body weight loss indicate a more severe inflammatory process in Entpd2 mice. In support, about a half of the Entpd2−/− mice died during DSS colitis or had to be humanely euthanized due to health reasons while the DSS-treated WT and Entpd1−/− mice had better survivability (Table 3).
Table 3.
Mortality of the DSS-treated animals during the experiment
| Genotype (Sex) | Total, n | Survived, n | Mortality Rate, % |
|---|---|---|---|
| WT | |||
| Male | 13 | 13 | 0 |
| Female | 13 | 13 | 0 |
| Entpd1−/− | |||
| Male | 14 | 13 | 7.1 |
| Female | 21 | 15 | 28.6 |
| Entpd2−/− | |||
| Male | 33 | 21 | 36.4 |
| Female | 32 | 14 | 56.3 |
WT, wild type; DSS, dextran sulfate sodium.
We assessed colon length and histopathology of the gut wall to investigate signs of local intestinal inflammation and tissue damage. Only Entpd2−/− mice had significant colon shortening after the DSS treatment [9.0 ± 0.2 cm (water) vs. 7.0 ± 0.2 cm (DSS); P < 0.0001, Tukey’s multiple comparison tests after two-way ANOVA for treatment (F1,58 = 30.27), genotype (F2,58 = 6.064), and their interaction (F2,58 = 8.432); Fig. 2C]. However, colons from Entpd2−/− mice administered water as controls (9.0 ± 0.2 cm) were significantly longer (P ≤ 0.0051) than the other two control groups [7.7 ± 0.2 cm (WT) and 7.6 ± 0.2 cm (Entpd1−/−)]. All DSS-treated groups had comparable colon lengths [7.2 ± 0.2 cm (WT) vs. 7.1 ± 0.2 cm (Entpd1−/−) vs. 7.0 ± 0.2 cm (Entpd2−/−); P ≥ 0.9918; Fig 2C].
These findings suggest that cell and tissue damage in the colon was comparable between the groups allowing us to examine other pathophysiological parameters. In concordance with this, there were no significant differences between the DSS-treated groups when assessed by blinded histological scoring of intestinal sections [6.9 ± 1.3 (WT) vs. 6.7 ± 0.8 (Entpd1−/−) vs. 6.6 ± 0.9 (Entpd2−/−); P = 0.9997; Fig 2, D and E].
NTPDase1 and NTPDase2 Differentially Impact Gut Barrier
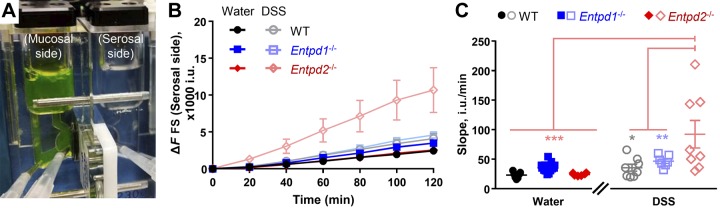
Our immunohistochemical data support the concept that NTPDase1 is expressed by mucosal immune and vascular cells and NTPDase2 is expressed by enteric glia (Fig. 1A and Fig. 3A). This strategic position allows the enzymes to control local nucleotide concentrations directly affecting intestinal epithelium. Thus we hypothesized that NTPDase1 and -2 have distinct roles in the gut barrier function. We utilized the Ussing chamber technique (Fig. 4A) to investigate the transmural flux of a cell-impermeant fluorescein-5-(and-6)-sulfonate through the distal colon (Fig. 4, B and C).
Fig. 4.
Nucleoside triphosphate diphosphohydrolase-1 and -2 (NTPDase1 and -2) differently affect the gut barrier. Paracellular permeability of the distal colon wall from healthy wild-type (WT), Entpd1−/−, and Entpd2−/− mice and 2 wk after the dextran sulfate sodium (DSS) treatment. A: schematic of Ussing chamber. B: average fluorescein-5-(and-6)-sulfonate (FS) background subtracted fluorescence (ΔF) measured in intensity units (i.u.). Note that DSS-treated Entpd2 null have a marked increase of fluorescein-5-(and-6)-sulfonate (FS) fluorescence. Permeability was determined by assessing the slope obtained during the last four measurements. C: slope of last 4 measurements (in B) as a measure of relative permeability. *P = 0.028; **P = 0.002; ***P < 0.001, two-way ANOVA and Tukey's post hoc test; n = 7 mice, healthy WT (4 males and 3 females), DSS-treated Entpd1−/− (4 males and 3 females), healthy Entpd2−/− [4 males and 3 females (37 i.u./min removed as an outlier)]; n = 8 mice, DSS-treated WT (4 males and 4 females), DSS-treated Entpd2−/− (3 males and 5 females); n = 13 mice, healthy Entpd1−/− (8 males and 5 females).
Distal colon permeability of Entpd1 and 2 null healthy controls [36 ± 2 and 16 ± 2 intensity units (i.u.)/min] was comparable to WT healthy controls (23 ± 2 i.u./min) [P ≥ 0.8961, Tukey’s multiple comparison test following two-way ANOVA for treatment (F1,43 = 13.57), genotype (F2,43 = 3.838), and their interaction (F2,43 = 4.895)]. Two weeks after DSS colitis, WT and Entpd1−/− mice (36 ± 6 and 47 ± 4 i.u./min) had similar gut wall permeability as their healthy corresponding controls (P = 0.9381 and 0.9601, respectively). However, DSS-treated Entpd2 null mice (92 ± 23 i.u./min) had significantly increased gut wall permeability in comparison to their healthy controls (P = 0.0004) and the DSS-treated WT and Entpd1−/− mice (P = 0.0021 and 0.0281, respectively; Fig. 4C). In addition, this significant increase in dye flux was driven by the DSS-treated Entpd2 null females (124 ± 29 i.u./min), while the flux in the DSS-treated Entpd2 null males (39 ± 6 i.u./min) was similar to the other two DSS-treated groups.
These results indicate that NTPDase2 has a role in protecting epithelial barrier function during and/or after acute intestinal inflammation and that the role of NTPDase2 could be female sex dependent.
Roles of NTPDase1 and NTPDase2 in Colonic Neuromuscular Function
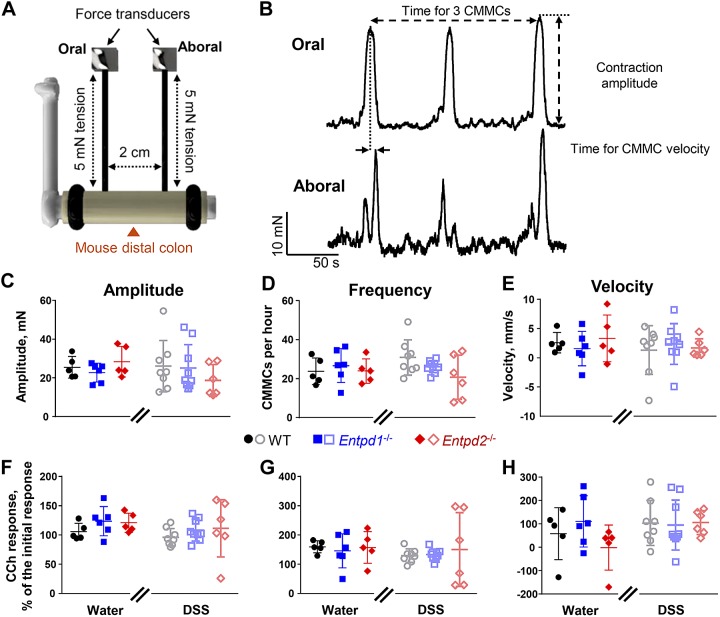
Gut motility is primary regulated by neurons in the myenteric plexus. At this level of the intestine, NTPDase1 is expressed by resident immune cells and NTPDase2 is almost exclusively expressed by glia (Fig. 1B and Fig. 3B). We began to investigate the functional roles of NTPDase1 and -2 expressed by these different cellular compartments by measuring CMMCs and neurogenic contractions and relaxations in isometric muscle tension recordings. We did not observe significant differences in CMMC frequency, propagation speed, or magnitude between Entpd1−/−, Entpd2−/−, and WT animals nor did we observed significant changes following DSS colitis [two-way ANOVA for treatment (P > 0.168, F1,33 = 0.187 to 1.983), genotype (P > 0.241, F2,33 = 0.106 to 1.485), and their interaction (P > 0. 261, F2,33 = 0.055 to 1.399); Fig. 5].
Fig. 5.
Role of nucleoside triphosphate diphosphohydrolase-1 and -2 (NTPDase1 and -2) in colonic migratory motor complexes (CMMCs). A: distal colon was mounted on a metal rod and secured at the ends with sutures. Force transducers were attached to the oral and anal ends, 2 cm apart, and the tissue was stretched to an initial tension of 5 mN at both ends. Schematic not drawn to scale. B: representative wild-type (WT) water control traces from oral (top) and aboral (bottom) force transducers. The recordings were used to obtain several CMMC parameters: 1) amplitude = (peak contraction) – (baseline); 2) frequency = (number of CMMCs)/(time elapsed from the first to last CMMC); and 3) velocity = (distance between the force transducers, i.e., 2 cm)/(time elapsed from the peak oral to peak aboral contraction in the same CMMC). C–H: amplitude (C and F), frequency (D and G), and velocity (E and H) of baseline (C–E) and carbachol (CCh; F–H)-treated preparations. CCh responses were normalized to initial/baseline responses; n = 5 mice, healthy WT (2 males and 3 females), healthy Entpd2−/− (3 males and 2 females); n = 6 mice, healthy Entpd1−/− (3 males and 3 females), dextran sulfate sodium (DSS)-treated Entpd2−/− (3 males and 3 females); n = 8 mice, DSS-treated WT (4 males and 4 females); n = 9 mice, DSS-treated Entpd1−/− (3 males and 6 females).
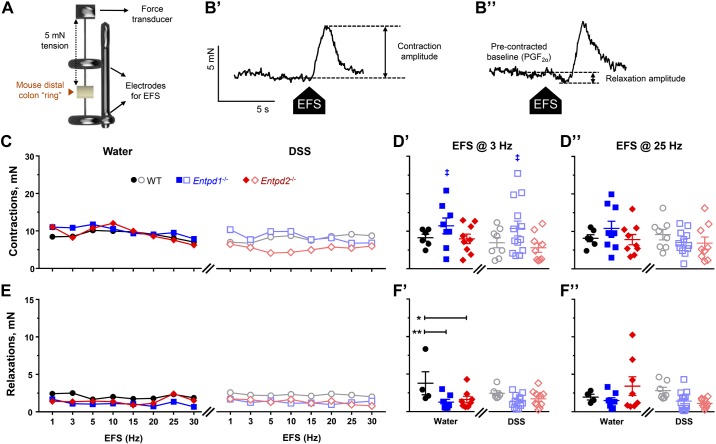
We used EFS to evoke neurogenic contractions and relaxations of the circular smooth muscle in organ bath isometric muscle tension recordings (Fig. 6, A and B). Although we did not observe significant changes in multiple groups comparisons of EFS-evoked contractions (Fig. 6, C and D), low-frequency EFS elicited stronger contractions [P = 0.03, two-way ANOVA for genotype (F2,47 = 3.783)] in NTPDase1-deficient mice [11.4 ± 2.1 mN (water) and 10.7 ± 2.0 mN (DSS)] when compared with WT [8.3 ± 0.9 mN (water) and 6.9 ± 1.4 mN (DSS)] and Entpd2 null mice [8.0 ± 1.3 mN (water) and 5.6 ± 1.2 mN (DSS); Fig. 6D’]. Higher frequency EFS evoked contractions were comparable between all the groups (Fig. 6D”).
Fig. 6.
Roles of nucleoside triphosphate diphosphohydrolase-1 and -2 (NTPDase1 and -2) in contractility of the distal colon. Isometric circular muscle tension recordings. A: circular-oriented (“ring”) segments of distal colon were affixed to a force transducer under 5-mN passive tension and between the platinum concentric electrodes to apply electrical field stimulation (EFS; 20 V, 1–30 Hz, 0.5-ms pulse duration). Brown triangle points to the lumen of the “ring” preparation. Schematic not drawn to scale. B: representative traces of neurogenic contractions (B’) and relaxations (B”) from water-treated wild-type (WT) mice. B’: neurogenic contractions were analyzed as the amplitude of the EFS-induced contractions [contraction = peak (EFS) – baseline]. B’’: neurogenic relaxations were studied in tissues precontracted with 1 μM prostaglandin F2-α (PGF2α) and analyzed as the amplitude of the EFS-induced relaxation [relaxation = baseline (PGF2α) – dip (PGF2α + EFS)]. C–F: Neurogenic contractions (C and D) and relaxations (E and F) of the distal colon from healthy wild-type (WT), Entpd1−/−, and Entpd2−/− mice and 2 wk after the dextran sulfate sodium (DSS) treatment (markings as in previous legends). Median responses to EFS 1–30 Hz (C and E; error bars omitted for clarity), individual values with mean ± SE responding to 3 Hz (D’ and F’) or 25 Hz (D’’ and F’’) EFS. ‡P = 0.03, two-way ANOVA for difference between the genotypes (see text for details). *P = 0.022, **P = 0.009, two-way ANOVA and Tukey's post hoc test; n = 4 mice, healthy WT in F [3 males and 1 female (11.5 mN removed as an outlier in F”); n = 6 mice, healthy WT in D (3 males and 3 females); n = 7 mice, DSS-treated WT in F [4 males (male 7.1 mN removed as an outlier in F’) and 3 females]; n = 8 mice, DSS-treated WT in D [4 males (male 7.1 mN removed as an outlier in F’) and 4 females], n = 8–9 mice, healthy Entpd1−/− [3 males and 5 (D’ and F’) to 6 (in D’’ and F’’) females (11.5 mN removed as an outlier in F’)]; n = 9–10 mice, healthy Entpd2−/− [5 males and 4 (in F) to 5 females (outliers removed: 30.2 mN in D’, 33.0 mN in D”, 12.9 mN in F’, and 16.0 mN in F”), DSS-treated Entpd2−/− [6 males and 4 females (outliers removed: 22.2 mN in D’ and 30.3 mN in D”)]; n = 12–13 mice, DSS-treated Entpd1−/− [4 males and 8 (in F’) to 9 females (23.2 mN removed as an outlier in D”)].
Neurogenic relaxations were reduced at lower EFS frequencies in healthy Entpd1−/− and Entpd2−/− mice [3.8 ± 1.5 mN (WT) vs. 1.3 ± 0.4 mN (Entpd1−/−) and 1.6 ± 0.4 mN (Entpd2−/−); P = 0.0087 and 0.0224, Tukey’s multiple comparison test following two-way ANOVA for treatment (F1,41 =0.886), genotype (F2,41 = 6.371), and their interaction (F2,41 = 1.19); Fig. 6F’]. The relaxations at higher frequency EFS were similar between groups (Fig. 6F”).
Collectively, our findings suggest that both NTPDase1 and -2 regulate intestinal smooth muscle relaxation.
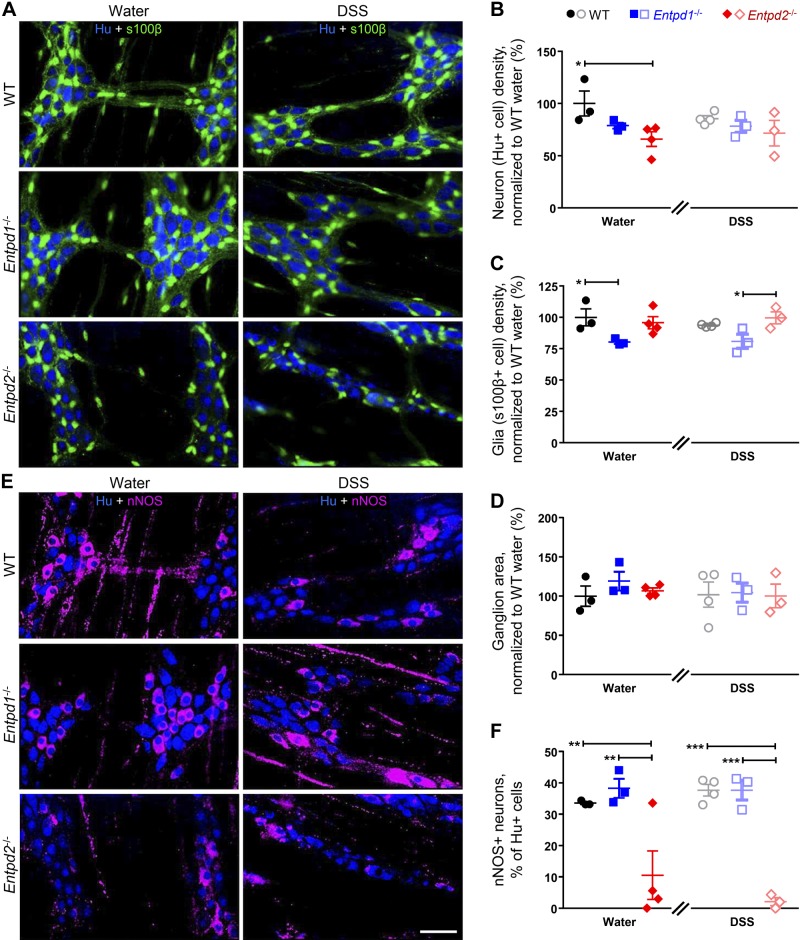
The Cellular Makeup of the Myenteric Plexus Is Affected in Entpd1−/− and Entpd2−/− Mice
The observed reduction in neurogenic relaxations in Entpd1- and Entpd2-deficient mice could potentially be explained by a global rearrangement of the ENS and/or alterations to cell-to-cell signaling. To address potential changes to the ENS, we performed blinded quantification of enteric neurons and glia. The data show that myenteric ganglia from healthy Entpd1−/− exhibited fewer glial cells, while Entpd2−/− mice had significantly fewer neurons [P = 0.0244 and 0.0161, Tukey’s multiple comparison tests after the two-way ANOVA (F2,14 =8.948 and 5.173 for genotype factor); Fig. 7, A–D]. In comparison to WT healthy controls (1,898 ± 227 neurons and 1,766 ± 121 glia per mm2), Entpd1−/− glial density was reduced by 19.8 ± 1.4% and Entpd2−/− neuronal density was reduced by 34.0 ± 7.0%. In addition, the proportion of nitrergic neurons in myenteric ganglia from Entpd2−/− mice was significantly reduced [10.5 ± 7.8%; P = 0.0053, Tukey’s multiple comparison tests after the two-way ANOVA (F2,14 = 32.77 for genotype factor)] when compared with WT controls (33.6 ± 0.4%; Fig. 7, E and F). Hence, the reduced neuronal density in Entpd2−/− mice was almost exclusively due to a loss of a nitrergic subpopulation of myenteric neurons, potentially underpinning the reduced neurogenic relaxations in NTPDase2 null mice (Fig. 6F).
Fig. 7.
Nucleoside triphosphate diphosphohydrolase-1 and -2 (NTPDase1 and -2) are required for normal density of neurons and glial cells within the myenteric plexus. A: representative overlay images of the anti-Hu (blue) and anti-s100b (green) immunoreactivity (ir), labeling neurons, and glia, respectively, from healthy (left) and dextran sulfate sodium (DSS)-treated (right) wild-type (WT; top), Entpd1−/− (middle), and Entpd2−/− (bottom) mice. B–D: neuronal (B) and glial (C) density in control (left) or DSS-treated (right) animals; Hu-ir was used for neuronal counts, while s100b-ir was used to identify glial cells and determine the area of ganglia (D). E: representative overlay images of Hu-ir (blue) and neuronal nitric oxide synthase (nNOS)-ir (magenta), labeling all enteric neurons and nitrergic neuron subpopulation, respectively, from healthy (left) and DSS-treated (right) WT (top), Entpd1−/− (middle), and Entpd2−/− (bottom) mice. F: percentage of nitrergic neurons within the myenteric neuron population. Hu-ir was used for neuronal counts, while nNOS-ir was used to identify nitrergic neurons. *P < 0.024, **P < 0.005, ***P < 0.0002, two-way ANOVA and Tukey's post hoc test; n = 3–4 mice (1–2 males and females) per group. Scale bar = 50 µm.
Purine Degradation by NTPDase1 and NTPDase2 Affects the Hyperpolarization of Smooth Muscle Cells
We performed intracellular recordings from circular smooth muscle cells to specifically assess neuromuscular transmission (Fig. 8A). Average resting membrane potentials were comparable among healthy and DSS-treated WT, Entpd1−/−, and Entpd2−/− mice (Table 4), showing that any significant differences observed in the tested animals are not due to variations in the resting membrane potential. We recorded inhibitory junction potentials (IJPs) in response to stimulation by one, three, five, and seven electrical pulses (Fig. 8, B and C). The five-pulse stimulation was selected as optimal paradigm (Fig. 8D), and we further analyzed two components of IJP recordings: the peak amplitude (resting membrane potential – minimum response) and area under the curve (AUC; measured from the stimulus to response returning to the resting membrane potential). IJP peak amplitude is mainly mediated by purinergic signaling while the slower component that dominates AUC measurements is predominantly nitrergic (43). In healthy mice, the deletion of Entpd2 increased IJP peak amplitude compared with WT controls (18.4 ± 2.5 vs. 27.5 ± 2.7 mV, P = 0.019, two-way ANOVA with Tukey’s post hoc test as described in next paragraph; Fig. 8E, left) while the deletion of Entpd1 significantly decreased the AUC (1951 ± 448 vs. 796 ± 159 mV; P = 0.002, Tukey’s multiple comparison tests after the two-way ANOVA as described below; Fig. 8F, left).
Table 4.
Average resting membrane potential of IJPs in treatment groups
| Treatment Groups | Values, mV | n |
|---|---|---|
| Healthy | ||
| WT | −46.00 ± 1.38 | 4 males, 4 females |
| Entpd1−/− | −48.99 ± 1.30 | 4 males, 3 females |
| Entpd2−/− | −48.31 ± 1.49 | 4 males, 5 females |
| DSS-treated | ||
| WT | −45.27 ± 0.99 | 4 males, 3 females |
| Entpd1−/− | −48.75 ± 2.30 | 4 males, 4 females |
| Entpd2−/− | −44.65 ± 1.40 | 4 males, 4 females |
Values are means ± SE. WT, wild type; DSS, dextran sulfate sodium; IJPs, inhibitory junction potentials. Differences between genotype, treatment, or their interaction were not significant [two-way ANOVA for genotype (F2,41 = 1.531, P = 0.359), treatment (F1,41 = 0.859, P = 0.229), and their interaction (F2,41 = 1.241, P = 0.3)].
In the distal colon, however, nitric oxide (NO) does not serve as the primary inhibitory neuromuscular transmitter (70), so we pharmacologically characterized the IJP responses by bath application of the NO synthase inhibitor l-NNA (100 µM), a specific purinergic P2Y1 receptor antagonist MRS2179 (10 µM), and a voltage-gated sodium channel blocker tetrodotoxin (TTX; 0.3 µM). IJP peak amplitudes of l-NNA-treated groups of healthy mice were similar to controls [P > 0.882, Tukey’s multiple comparison tests after the two-way ANOVA for drug treatment (F3,79 = 40.86), genotype (F2,79 = 4.382), and their interaction (F6,79 = 1.052)] but were reduced by application of MRS2179 (P < 0.006) and were not additionally inhibited by TTX (P > 0.832; Fig. 8E, left). On the other hand, l-NNA and MRS2179 did not significantly change AUC of any healthy group [P > 0.064, Tukey’s multiple comparison tests after the two-way ANOVA for drug treatment (F3,73 = 5.443), genotype (F2,73 = 7.627), and their interaction (F6,73 = 1.255)], but TTX application reduced AUC of WT and Entpd2−/− mice (689 ± 121 and 687 ± 124 mV, P = 0.007 and 0.019) and did not cause further AUC reduction in Entpd1−/− animals (774 ± 249 mV, P = 0.974; Fig. 8F, left).
After DSS colitis, all groups tended to exhibit an increase in peak amplitude when compared with their respective healthy controls, but only responses in Entpd1−/− mice reached significance [17.4 ± 2.6 mV (water) vs. 28.5 ± 2.4 mV (DSS), P = 0.0089, Student’s t-test with Welch's correction for unequal variances; Fig. 8E]. IJP peak amplitudes in l-NNA-treated samples from DSS mice were comparable to controls [P > 0.984, Tukey’s multiple comparison tests after the two-way ANOVA for drug treatment (F3,79 = 36.16), genotype (F2,79 = 1.756), and their interaction (F6,79 = 0.071)] but were abolished by application of MRS2179 (P < 0.0001) and were not additionally inhibited by TTX (P > 0.276; Fig. 8E, right).
Two weeks after DSS colitis, AUC in Entpd1−/− mice were increased to WT levels [1,302 ± 326 mV (WT) vs. 1,317 ± 260 mV (Entpd1−/−); P = 0.997, Tukey’s multiple comparison tests after the two-way ANOVA for drug treatment (F3,79 = 17.04), genotype (F2,77 = 0.287), and their interaction (F6,77 = 0.269); Fig. 8F, right]. Application of l-NNA reduced the AUC of DSS-treated WT and Entpd1−/− mice (721 ± 56 mV and 783 ± 71 mV, P = 0.030 and 0.017), and the AUC was reduced in Entpd2−/− mice after application of MRS2179 (P = 0.006; Fig. 8F, right).
Taken together, our data show that NTPDase2 regulates fast IJPs in the mouse distal colon via the generation of P2Y1 receptor agonists. On the other hand, our data suggest that slow IJPs in mouse distal colon do not require nitrergic nor P2Y1 receptor signaling and are, at least in part, physiologically regulated by NTPDase1.
DISCUSSION
Here, we investigated the role of extracellular nucleotide hydrolysis by NTPDase1 and NTPDase2 in modulating gut functions in health and after acute inflammation. We found that these enzymes have different context-dependent roles in the regulation of intestinal epithelial barrier and neuromuscular transmission (Table 5). Our data suggest that NTPDase1 promotes glial cell survival and/or turnover while NTPDase2 appears to be required for the survival or development of nitrergic neurons. Both NTPDase1 and -2 protect epithelial barrier function, neurogenic circular smooth muscle relaxations, and neuromuscular signaling, but the roles of NTPDase2 are more prominent after acute inflammation. NTPDase2 also has a female sex-specific role in the prevention of postinflammatory gut barrier dysfunction.
Table 5.
Summary table
| Feature | NTPDase1 | NTPDase2 |
|---|---|---|
| ATP hydrolysisa | ||
| ATP affinity (relative) | 4× higher than NTPDase2 | Ø |
| Rate (relative) | Ø | 2× higher than NTPDase1 |
| Specific reaction | ATP→ADP→AMP | ATP→ADP |
| Expression | ||
| Gut wall layers (water and DSS) | Throughout, high in mucosa | Throughout, high in mucosa |
| Cell types (water and DSS) | Immune cells | Enteric glial cells |
| Endothelial cells | Pericytes | |
| Smooth muscle cellsb | PDGFRα+ cellsb | |
| Barrier function | ||
| Water | Ø | Ø |
| DSS | Ø | +c |
| Circular muscle contractions | ||
| Water | −d | Ø |
| DSS | −d | Ø |
| Circular muscle relaxations | ||
| Water | +d | +d |
| DSS | Ø | Ø |
| ENS cell survival/migration | ||
| Neurons | Ø | +e |
| Glial cells | + | Ø |
| IJP peak amplitude | ||
| Water | Ø | − |
| DSS | Ø | Ø |
| IJP AUC | ||
| Water | + | Ø |
| DSS | Ø | Ø |
DSS, dextran sulfate sodium; NTPDase1 and -2, nucleoside triphosphate diphosphohydrolase-1 and -2; AUC, area under the curve; ENS, enteric nervous system; IJP, inhibitory junction potential; PDGFRα, platelet-derived growth factor receptor α; Ø, not a significant change; +/−, significant increase/decrease compared with wild-type mice (keep in mind that the functions of each enzyme are reported as opposite from our findings that came from null mice).
Based on a comparative hydrolysis study (61).
Mostly driven by females.
Only at lower frequency of electrical field stimulation.
Mostly nitrergic neurons.
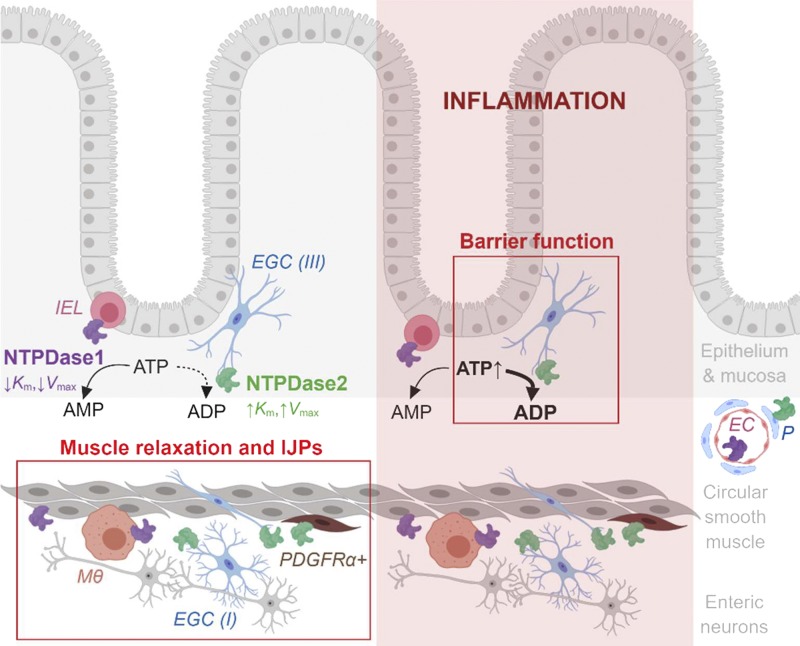
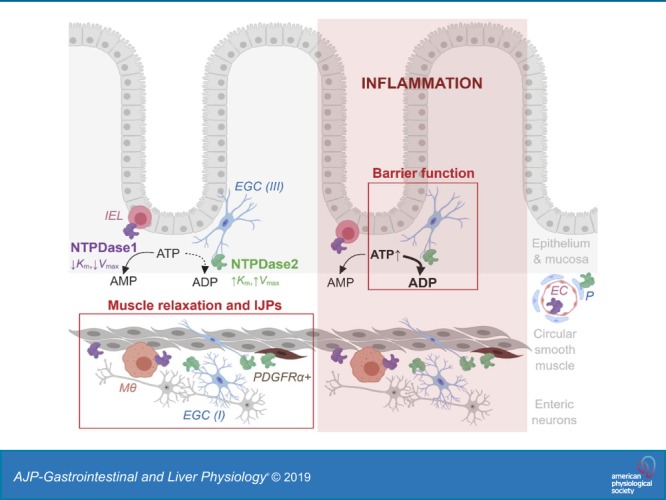
These specific effects can be explained by different enzymatic properties and distinct expression patterns of NTPDase1 and -2 (Fig. 9). While NTPDase1 breaks down ATP to AMP at physiological extracellular ATP concentration, NTPDase2 is active when extracellular ATP levels are significantly increased and hydrolyzes ATP to ADP. Our work confirmed earlier findings that NTPDase1 is expressed by immune cells, such as intraepithelial lymphocytes and muscularis macrophages (69) and NTPDase2 is abundantly expressed by enteric glia (9). Both enzymes are also expressed by the intestinal vasculature but still have distinct cell expression by endothelial cells and pericytes, respectively (69). Transcriptional data also suggest that smooth muscle cells express NTPDase1 and that PDGFRα+ cells express NTPDase2 (10).
Fig. 9.
Summary schematic. While NTPDase1 (magenta) is expressed by intraepithelial lymphocytes (IEL), muscularis macrophages (Mϴ), and smooth muscle cells, NTPDase2 (green) is expressed by all subtypes of enteric glial cells (EGC) such as mucosal (type III), intramuscular (type IV) and within myenteric ganglia (type I), and platelet-derived growth factor receptor α positive (PDGFRα+) cells. The enzymes are also expressed by vasculature, NTPDase1 by endothelial cells (EC) and NTPDase2 by pericytes (P). In basal conditions (left, unshaded side) most of the extracellular ATP is hydrolyzed into AMP by NTPDase1. This kind of ATP degradation supports physiological circular muscle relaxation and slow phase of inhibitory junction potentials (IJPs). There is also an effect of NTPDase2 on muscle relaxation and fast phase of IJPs. After inflammation (right, shaded side), however, abundant extracellular ATP is mostly degraded into ADP by NTPDase2. This process protects and/or aids recovery of intestinal barrier function. In addition, NTPDase1 is involved in regulation of circular smooth muscle contractions in health and during the recovery of inflammation (not drawn, see Table 5). The schematic is a summary of this study and previous work (10, 61, 64, 69). Created with BioRender.com and not drawn to scale.
DSS colitis causes a marked increase of NTPDase1 expression in intestinal vasculature and local macrophages leading to dysregulation of the vascular tone following intestinal inflammation (78). An increase in the expression of NTPDase1 by regulatory T cells during inflammation is also associated with therapeutic remission in IBD via increased local levels of adenosine that mediates immunosuppression (42). It is likely that similar increases in enzyme expression occurred during DSS colitis in our study and that these changes enhanced the breakdown of extracellular ATP accumulated during acute inflammation. We did not specifically assess enzyme expression levels in this study, but our immunohistochemical data suggest that DSS colitis does not cause major shifts in the types of cells that express NTPDase1 or -2. Therefore, while the relative expression levels may change, the compartmentalized breakdown of extracellular nucleotides remains relatively constant during acute DSS colitis.
Both Entpd1−/− and Entpd2−/− mice had more prominent signs of systemic inflammation 2 wk after the relatively mild 2% DSS-induced colitis. These findings are comparable to earlier studies testing the acute 3% DSS colitis (30, 32). However, we did not observe similar level of colon tissue damage. The timeline of our experiments is critical to explain the effects of Entpd1 and Entpd2 deletion on intestinal tissue damage because extracellular nucleotide concentration is dynamically regulated during inflammation. Extracellular ATP levels are particularly high during acute inflammation, and perhaps this is the reason why previous studies found significant intestinal tissue damage in Entpd1 and Entpd2 null mice during acute DSS colitis at 3% DSS and immediate evaluations (30, 32). In addition, our results with Entpd2 null mice are based on more healthy survivors because this line was more susceptible to DSS and a significant portion of these animals either died or had to be euthanized. These methodological differences were designed to probe the functional recovery after acute inflammation and may explain our observation of WT mice having a comparable level of tissue damage as Entpd1- and Entpd2-deficient mice 2 wk after colitis.
We discovered that the loss of NTPDase2 causes persistent postinflammatory gut barrier dysfunction. This finding has clinical implications as the underlying functional impairments of the intestinal barrier are linked to ongoing gastrointestinal symptoms in IBD patients without active disease, such as diarrhea and abdominal pain (21). Furthermore, we observed that Entpd2−/− female mice in particular had persistent increases in distal colon wall permeability following DSS colitis. Our findings thereby suggest that ADP could have an important role in females in the protection and/or recovery of gut barrier function. This is another important clinical aspect as female sex is a significant risk factor for irritable bowel syndrome and other functional bowel disorders (74). The observed sex difference could be due to distinct rates of ATP hydrolysis since extracellular ATP regulates gut barrier integrity and permeability (7, 8, 90). In support, stress induces unequal changes in NTPDase activity in the spinal cord of male and female rats (98). We did not observe overt differences in gut histology, so it is likely that the mechanism is due to persistent barrier dysfunction such as dysregulation of mucus secretion and/or impaired tight junctions. In support, purine signaling stimulates mucin secretion from a human goblet cell line (75) and extracellular nucleotides affect the expression and localization of the tight junction proteins (51). Future experiments are needed to uncover the underlying mechanisms and investigate if it is sex hormone dependent. In addition, NTPDase2 is primarily expressed by enteric glia, so this finding also implies that enteric glia protect the epithelial barrier in intestinal inflammation. This context-dependent role of enteric glia could help to explain some of the different outcomes of glial ablation on the health of the intestinal epithelium (19, 80).
Our results show that healthy Entpd1 and Entpd2 null mice exhibit a low density of myenteric glia and neurons, respectively. These findings indicate that NTPDase1 promotes glial cell survival, likely via mechanisms that include increased extracellular adenosine concentration and downstream immunosuppression of resident immunocytes (42) and/or the activation of protective mechanisms in glia (88). There are several potential explanations for why myenteric nitrergic neurons require NTPDase2. One possibility is that the loss of NTPDase2-mediated hydrolysis of ATP increases extracellular ATP concentration in the myenteric plexus and leads to P2X7-dependent neuronal cell death (49). Alternatively, reducing the generation of the glial P2Y1 agonist ADP by ablating NTPDase2 could impair the P2Y1-dependent release of neurotrophic factors by glia (95). Localized nucleotide hydrolysis could also control the turnover of cells in the myenteric plexus since nucleotides act as proliferation signals for neuronal progenitor cells (68) and NTPDase2 regulates nucleotide-mediated neural progenitor cell proliferation in the brain (40). Alternatively, reduced numbers of glia and neurons in constitutive null mice could be due to impaired migration of neuronal crest cells during the ENS development. In support, purine nucleotides induce migration of neuronal stem cells (45) and NTPDase1 affects migration of microglia (29). Thus new functional studies require the generation of conditional and cell-targeted gene-deleted animals for temporal and spatial control of gene ablation and look for cell-specific roles of extracellular nucleotide hydrolysis in gut functioning.
Reduced density of myenteric neurons in Entpd2−/− animals was primarily due to a decrease in neuronal NO synthase-positive neurons. This finding, at least in part, underpins the reduced relaxations of circular smooth muscle in Entpd2 null mice because nitrergic neurons mediate smooth muscle relaxation (67). The preferential death of nitrergic neurons due to high extracellular nucleotide concentration was shown earlier (49). It is also possible that the 20% reduction in myenteric glial density contributes to the reduced smooth muscle relaxations in Entpd1−/− mice because decreased activity of the enteric glial network affects both contractions and relaxations in the colon (72). Furthermore, our results corroborate previous work showing that intramuscular glia express NTPDase2 (63). Intramuscular glial cells are associated with nerve fibers interspersed between smooth muscle cells and thus are perfectly positioned to control purine nucleotide composition at neuromuscular junctions. Loss of this glial subpopulation or reduced expression of glial NTPDase2 at this location would reduce hydrolysis of motorneuron-released ATP to ADP and thereby significantly affect smooth muscle relaxations through reduced activation of P2Y1 receptors. Since smooth muscle and PDGFRα+ cells also express transcripts for NTPDase1 and -2, respectively (10, 64), it is likely that these intramuscular cells contribute to the observed change in smooth muscle relaxations.
We did not observe a significant disruption of CMMCs in tissue from mice lacking either NTPDase1 or -2 or in these animals following colitis. CMMCs are an integrative motor pattern that relies on complex communication between neurons, glia, interstitial cells of Cajal, fibroblast-like cells, and smooth muscle cells. Therefore, it is possible that the observed differences in cell density and muscle relaxations are compensated for in CMMC recordings. In support, studies on aged animals indicate that the ENS has substantial functional reserve, as evidenced by maintained functions of the aging gut despite the significant enteric neurodegeneration (85).
Changes in the cellular composition of myenteric ganglia were in agreement with the observed differences in the smooth muscle contractility. We found that both NTPDase1 and -2 modulate smooth muscle activity at lower frequency EFS. It is possible that this effect is driven by ATP overwhelming the regulation by NTPDases at high stimulation frequencies. More ATP is (co)released at higher frequency stimulations (91, 92), and high extracellular ATP could overwhelm or escape degradation by NTPDases and cause NTPDase-independent effects on muscle activity.
Most of the changes in muscle relaxation were matched by corresponding changes in IJP recordings (Table 5), suggesting a role for purine hydrolysis in neuromuscular communication. The changes in the IJP peak amplitude and area AUC reflect changes in the fast and slow IJP, respectively. Purines control the fast component of the IJP, while NO produces the slow IJP response observed in smooth muscle relaxation (37). However, NO has a very small contribution as enteric inhibitory motor neurotransmitter in the distal mouse colon at longer duration of EFS (70). Indeed, our data show that the AUC is TTX sensitive but not affected by application of l-NNA. Furthermore, a decrease in the AUC was observed in our Entpd1−/−, but not Entpd2−/−, water-treated animals. The NTPDase1 enzyme bypasses the activation of P2Y receptors by hydrolyzing both ATP and ADP to the final product AMP (83), but deletion of Entpd1 also results in P2Y1 desensitization (27). AMP is further broken down to adenosine and activates purinergic G-protein P1 receptors (93). Following activation, P1 receptors can then decrease smooth muscle contraction by inhibiting excitatory neurotransmitter release (31, 79) and by modulating nitrergic inhibitory pathways (1, 31). In addition, the activation of smooth muscle A1 and A2B receptors causes muscle relaxation (2, 55). Therefore, decreased activation of P1 receptor subtypes could explain the diminished slow IJP responses observed in water-treated NTPDase1 null mice.
NTPDase2 hydrolyzes ATP to ADP to activate ADP-sensitive purinergic receptors, such as the P2Y1 receptor (28, 82). P2Y1 receptors are the principal purinergic G protein receptors found in gastrointestinal smooth muscle and myenteric ganglia (35, 41, 107) and the principal mediator of the purinergic component of smooth muscle relaxation in the gastrointestinal tract (34). Our data show that the fast component of IJPs is increased in Entpd2 null mice and that this increase is sensitive to blockade by the P2Y1 antagonist MRS2179. This suggests that ATP is responsible for increased fast IJPs in NTPDase2-deficient mice because the concentration of MRS2179 used here effectively blocks purinergic responses (35). However, our data do not exclude the possibility of selective desensitization of other receptors since β-nicotinamide adenine dinucleotide acts as a neurotransmitter that contributes to enteric inhibitory regulation of gut smooth muscles (77). In addition, a number of purines including nicotinamide adenine dinucleotide, ADP-ribose, and uridine adenosine tetraphosphate (Up4A) are released from nerve terminals and activate P2Y1 receptors expressed on a subset of interstitial cells (86) and contribute to muscle relaxations and IJPs (25). Since Up4A is broken down to UDP and ADP in the mouse colon (25), it is possible that Up4A is a substrate for NTPDases.
After DSS treatment we observed an enhanced purinergic and decreased nitrergic IJP response in all genotype groups. Prior work has highlighted a decrease in purinergic neuromuscular transmission in the inflamed colon (81, 94). Differences in animal models of colitis (trinitrobenzene sulfonic acid versus DSS), timing of experiments after the induction of acute inflammation (1 or 2 wk), the severity of inflammation, experimental design investigating primarily purinergic transmission in the ENS or at the neuromuscular junction, and use of purinergic antagonistic drugs could partly explain these contradictory findings. For instance, the use of different transmural stimulation parameters, i.e., single 500-ms pulse of 50 V (94) or 0.5 Hz, 0.3-ms pulse duration, 50 V (81) vs. 10 Hz, 0.5-ms pulse duration, 80 V (used in this study), would activate different kinds of neuromuscular communication since (co)release of ATP varies with stimulation frequency (91, 92). Despite this, the enhanced release of ATP has been reported in the TNBS-inflamed rat colon (105). Furthermore, inhibiting the breakdown of ATP with the NTPDase1 antagonist ARL-67156 increases the frequency of action potential generation, with a more significant increase observed in TNBS-colitis animals (105). These results are similar to our IJP recordings in Entpd1−/− mice that show an increased purinergic response after DSS. Conversely, the ablation of Entpd2 does not have major impacts on neuromuscular transmission following DSS inflammation. NTPDase1 is highly expressed in immune cells (30) so it is possible that infiltrating immune cells compensate for the loss of NTPDase2 in Entpd2−/− mice. In support, the DSS-induced increase in Entpd1 null mice is not completely blocked by TTX indicating that this control over the fast IJPs response is not fully dependent on neuronal activity. In this scenario, NTPDase1 would work to break down ATP to AMP and decrease purinergic neuromuscular transmission. Therefore, NTPDase1 could also play a neuroprotective role during inflammation.
In summary, location- and context-dependent nucleotide degradation by NTPDase1 and -2 significantly affects the permeability of gut epithelial barrier, circular smooth muscle relaxations, and neuromuscular communication in mouse colon and after DSS colitis (Fig. 9). NTPDase1 is expressed by immunocytes and the vasculature and is required for the maintenance of enteric glia while glial NTPDase2 is required for the expression of nitrergic neurons. The protective roles of NTPDase2 appear more prominent after acute inflammation and particularly female sex-biased for the prevention of postinflammatory gut barrier dysfunction.
GRANTS
This work was supported by Crohn’s and Colitis Foundation Research Fellowship Award 577598 (to V. Grubišić), National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-103723 and Crohn’s and Colitis Foundation Senior Research Award 327058 (to B. D. Gulbransen), and Helmsley Charitable Trust Grant 281574.5069091.0010 (to S. C. Robson). J. Sévigny received support from Canadian Institutes of Health Research Grant PJT-156205 and was the recipient of a “Chercheur National” Scholarship from the Fonds de Recherche du Québec–Santé.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.G., A.L.P.-M., J.S., S.C.R., J.J.G., and B.D.G. conceived and designed research; V.G., A.L.P.-M., and D.E.F. performed experiments; V.G., A.L.P.-M., D.E.F., and S.C.R. analyzed data; V.G., A.L.P.-M., D.E.F., J.S., S.C.R., J.J.G., and B.D.G. interpreted results of experiments; V.G., A.L.P.-M., and D.E.F. prepared figures; V.G. and A.L.P.-M. drafted manuscript; V.G., A.L.P.-M., D.E.F., J.S., S.C.R., J.J.G., and B.D.G. edited and revised manuscript; V.G., A.L.P.-M., D.E.F., J.S., S.C.R., J.J.G., and B.D.G. approved final version of manuscript.
REFERENCES
- 1.Antonioli L, Fornai M, Colucci R, Ghisu N, Blandizzi C, Del Tacca M. A2a receptors mediate inhibitory effects of adenosine on colonic motility in the presence of experimental colitis. Inflamm Bowel Dis 12: 117–122, 2006. doi: 10.1097/01.MIB.0000198535.13822.a9. [DOI] [PubMed] [Google Scholar]
- 2.Bailey SJ, Hourani SM. Effects of purines on the longitudinal muscle of the rat colon. Br J Pharmacol 105: 885–892, 1992. doi: 10.1111/j.1476-5381.1992.tb09073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barajas-López C, Espinosa-Luna R, Christofi FL. Changes in intracellular Ca2+ by activation of P2 receptors in submucosal neurons in short-term cultures. Eur J Pharmacol 409: 243–257, 2000. doi: 10.1016/S0014-2999(00)00848-7. [DOI] [PubMed] [Google Scholar]
- 4.Barajas-López C, Huizinga JD, Collins SM, Gerzanich V, Espinosa-Luna R, Peres AL. P2x-purinoceptors of myenteric neurones from the guinea-pig ileum and their unusual pharmacological properties. Br J Pharmacol 119: 1541–1548, 1996. doi: 10.1111/j.1476-5381.1996.tb16070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DL, Sullivan SL, Lavoie EG, Sévigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. J Comp Neurol 497: 1–12, 2006. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boesmans W, Lasrado R, Vanden Berghe P, Pachnis V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 63: 229–241, 2015. doi: 10.1002/glia.22746. [DOI] [PubMed] [Google Scholar]
- 7.Bours MJ, Bos HJ, Meddings JB, Brummer RJ, van den Brandt PA, Dagnelie PC. Effects of oral adenosine 5′-triphosphate and adenosine in enteric-coated capsules on indomethacin-induced permeability changes in the human small intestine: a randomized cross-over study. BMC Gastroenterol 7: 23, 2007. doi: 10.1186/1471-230X-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bours MJ, Troost FJ, Brummer RJ, Bast A, Dagnelie PC. Local effect of adenosine 5′-triphosphate on indomethacin-induced permeability changes in the human small intestine. Eur J Gastroenterol Hepatol 19: 245–250, 2007. doi: 10.1097/MEG.0b013e328011093c. [DOI] [PubMed] [Google Scholar]
- 9.Braun N, Sévigny J, Robson SC, Hammer K, Hanani M, Zimmermann H. Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia 45: 124–132, 2004. doi: 10.1002/glia.10309. [DOI] [PubMed] [Google Scholar]
- 10.Breland A, Ha SE, Jorgensen BG, Jin B, Gardner TA, Sanders KM, Ro S. Smooth muscle transcriptome browser: offering genome-wide references and expression profiles of transcripts expressed in intestinal SMC, ICC, and PDGFRα+ cells. Sci Rep 9: 387, 2019. doi: 10.1038/s41598-018-36607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown IA, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol 2: 77–91, 2016. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 13.Burnstock G. Purinergic receptors as future targets for treatment of functional GI disorders. Gut 57: 1193–1194, 2008. doi: 10.1136/gut.2008.151134. [DOI] [PubMed] [Google Scholar]
- 14.Burnstock G. Purinergic signalling in the gastrointestinal tract and related organs in health and disease. Purinergic Signal 10: 3–50, 2014. doi: 10.1007/s11302-013-9397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnstock G. Purinergic signalling in the gut. Adv Exp Med Biol 891: 91–112, 2016. doi: 10.1007/978-3-319-27592-5_10. [DOI] [PubMed] [Google Scholar]
- 16.Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol 99: 16–34, 2014. doi: 10.1113/expphysiol.2013.071951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnstock G, Boeynaems JM. Purinergic signalling and immune cells. Purinergic Signal 10: 529–564, 2014. doi: 10.1007/s11302-014-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burnstock G, Lavin S. Interstitial cells of Cajal and purinergic signalling. Auton Neurosci 97: 68–72, 2002. doi: 10.1016/S1566-0702(02)00005-X. [DOI] [PubMed] [Google Scholar]
- 19.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93: 189–201, 1998. doi: 10.1016/S0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 20.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol 16: 177–192, 2016. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 21.Chang J, Leong RW, Wasinger VC, Ip M, Yang M, Phan TG. Impaired intestinal permeability contributes to ongoing bowel symptoms in patients with inflammatory bowel disease and mucosal healing. Gastroenterology 153: 723–731.e1, 2017. doi: 10.1053/j.gastro.2017.05.056. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Redelman D, Ro S, Ward SM, Ordög T, Sanders KM. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am J Physiol Cell Physiol 292: C497–C507, 2007. doi: 10.1152/ajpcell.00147.2006. [DOI] [PubMed] [Google Scholar]
- 23.Christofi FL. Purinergic receptors and gastrointestinal secretomotor function. Purinergic Signal 4: 213–236, 2008. doi: 10.1007/s11302-008-9104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clarke LL. A guide to Ussing chamber studies of mouse intestine. Am J Physiol Gastrointest Liver Physiol 296: G1151–G1166, 2009. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durnin L, Hwang SJ, Kurahashi M, Drumm BT, Ward SM, Sasse KC, Sanders KM, Mutafova-Yambolieva VN. Uridine adenosine tetraphosphate is a novel neurogenic P2Y1 receptor activator in the gut. Proc Natl Acad Sci USA 111: 15821–15826, 2014. doi: 10.1073/pnas.1409078111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eltzschig HK, Sitkovsky MV, Robson SC. Purinergic signaling during inflammation. N Engl J Med 367: 2322–2333, 2012. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Enjyoji K, Sévigny J, Lin Y, Frenette PS, Christie PD, Esch JS II, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med 5: 1010–1017, 1999. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 28.Erb L, Weisman GA. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip Rev Membr Transp Signal 1: 789–803, 2012. doi: 10.1002/wmts.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Färber K, Markworth S, Pannasch U, Nolte C, Prinz V, Kronenberg G, Gertz K, Endres M, Bechmann I, Enjyoji K, Robson SC, Kettenmann H. The ectonucleotidase cd39/ENTPDase1 modulates purinergic-mediated microglial migration. Glia 56: 331–341, 2008. doi: 10.1002/glia.20606. [DOI] [PubMed] [Google Scholar]
- 30.Feldbrügge L, Moss AC, Yee EU, Csizmadia E, Mitsuhashi S, Longhi MS, Sandhu B, Stephan H, Wu Y, Cheifetz AS, Müller CE, Sévigny J, Robson SC, Jiang ZG. Expression of ecto-nucleoside triphosphate diphosphohydrolases-2 and -3 in the enteric nervous system affects inflammation in experimental colitis and Crohn’s disease. J Crohn’s Colitis 11: 1113–1123, 2017. doi: 10.1093/ecco-jcc/jjx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fornai M, Antonioli L, Colucci R, Ghisu N, Buccianti P, Marioni A, Chiarugi M, Tuccori M, Blandizzi C, Del Tacca M. A1 and A2a receptors mediate inhibitory effects of adenosine on the motor activity of human colon. Neurogastroenterol Motil 21: 451–466, 2009. doi: 10.1111/j.1365-2982.2008.01213.x. [DOI] [PubMed] [Google Scholar]
- 32.Friedman DJ, Künzli BM, A-Rahim YI, Sévigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci USA 106: 16788–16793, 2009. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furuzono S, Nakayama S, Imaizumi Y. Purinergic modulation of pacemaker Ca2+ activity in interstitial cells of Cajal. Neuropharmacology 48: 264–273, 2005. doi: 10.1016/j.neuropharm.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Gallego D, Gil V, Martínez-Cutillas M, Mañé N, Martín MT, Jiménez M. Purinergic neuromuscular transmission is absent in the colon of P2Y(1) knocked out mice. J Physiol 590: 1943–1956, 2012. doi: 10.1113/jphysiol.2011.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallego D, Hernández P, Clavé P, Jiménez M. P2Y1 receptors mediate inhibitory purinergic neuromuscular transmission in the human colon. Am J Physiol Gastrointest Liver Physiol 291: G584–G594, 2006. doi: 10.1152/ajpgi.00474.2005. [DOI] [PubMed] [Google Scholar]
- 36.Gallego D, Malagelada C, Accarino A, De Giorgio R, Malagelada JR, Azpiroz F, Jimenez M. Nitrergic and purinergic mechanisms evoke inhibitory neuromuscular transmission in the human small intestine. Neurogastroenterol Motil 26: 419–429, 2014. doi: 10.1111/nmo.12293. [DOI] [PubMed] [Google Scholar]
- 37.Gallego D, Vanden Berghe P, Farré R, Tack J, Jiménez M. P2Y1 receptors mediate inhibitory neuromuscular transmission and enteric neuronal activation in small intestine. Neurogastroenterol Motil 20: 159–168, 2008. doi: 10.1111/j.1365-2982.2007.01004.x. [DOI] [PubMed] [Google Scholar]
- 38.Galligan JJ. Pharmacology of synaptic transmission in the enteric nervous system. Curr Opin Pharmacol 2: 623–629, 2002. doi: 10.1016/S1471-4892(02)00212-6. [DOI] [PubMed] [Google Scholar]
- 39.Galligan JJ, North RA. Pharmacology and function of nicotinic acetylcholine and P2X receptors in the enteric nervous system. Neurogastroenterol Motil 16, Suppl 1: 64–70, 2004. doi: 10.1111/j.1743-3150.2004.00478.x. [DOI] [PubMed] [Google Scholar]
- 40.Gampe K, Stefani J, Hammer K, Brendel P, Pötzsch A, Enikolopov G, Enjyoji K, Acker-Palmer A, Robson SC, Zimmermann H. NTPDase2 and purinergic signaling control progenitor cell proliferation in neurogenic niches of the adult mouse brain. Stem Cells 33: 253–264, 2015. doi: 10.1002/stem.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giaroni C, Knight GE, Ruan HZ, Glass R, Bardini M, Lecchini S, Frigo G, Burnstock G. P2 receptors in the murine gastrointestinal tract. Neuropharmacology 43: 1313–1323, 2002. doi: 10.1016/S0028-3908(02)00294-0. [DOI] [PubMed] [Google Scholar]
- 42.Gibson DJ, Elliott L, McDermott E, Tosetto M, Keegan D, Byrne K, Martin ST, Rispens T, Cullen G, Mulcahy HE, Cheifetz AS, Moss AC, Robson SC, Doherty GA, Ryan EJ. Heightened expression of CD39 by regulatory T lymphocytes is associated with therapeutic remission in inflammatory bowel disease. Inflamm Bowel Dis 21: 2806–2814, 2015. doi: 10.1097/MIB.0000000000000566. [DOI] [PubMed] [Google Scholar]
- 43.Goyal RK. Targets of enteric motor neurones: smooth muscle cells. Gut 47, Suppl 4: iv38–iv39, 2000. doi: 10.1136/gut.47.suppl_4.iv38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grasl M, Turnheim K. Stimulation of electrolyte secretion in rabbit colon by adenosine. J Physiol 346: 93–110, 1984. doi: 10.1113/jphysiol.1984.sp015009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grimm I, Ullsperger SN, Zimmermann H. Nucleotides and epidermal growth factor induce parallel cytoskeletal rearrangements and migration in cultured adult murine neural stem cells. Acta Physiol (Oxf) 199: 181–189, 2010. doi: 10.1111/j.1748-1716.2010.02092.x. [DOI] [PubMed] [Google Scholar]
- 46.Grubišić V, Gulbransen BD. Enteric glia: the most alimentary of all glia. J Physiol 595: 557–570, 2017. doi: 10.1113/JP271021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grubišić V, Gulbransen BD. Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. J Physiol 595: 3409–3424, 2017. doi: 10.1113/JP273492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gulbransen BD, Bains JS, Sharkey KA. Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J Neurosci 30: 6801–6809, 2010. doi: 10.1523/JNEUROSCI.0603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med 18: 600–604, 2012. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136: 1349–1358, 2009. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 51.Han X, Uchiyama T, Sappington PL, Yaguchi A, Yang R, Fink MP, Delude RL. NAD+ ameliorates inflammation-induced epithelial barrier dysfunction in cultured enterocytes and mouse ileal mucosa. J Pharmacol Exp Ther 307: 443–449, 2003. doi: 10.1124/jpet.103.056556. [DOI] [PubMed] [Google Scholar]
- 52.Hicks-Berger CA, Chadwick BP, Frischauf AM, Kirley TL. Expression and characterization of soluble and membrane-bound human nucleoside triphosphate diphosphohydrolase 6 (CD39L2). J Biol Chem 275: 34041–34045, 2000. doi: 10.1074/jbc.M004723200. [DOI] [PubMed] [Google Scholar]
- 53.Hu HZ, Gao N, Zhu MX, Liu S, Ren J, Gao C, Xia Y, Wood JD. Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol 550: 493–504, 2003. doi: 10.1113/jphysiol.2003.041731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 509: 310–317, 2014. doi: 10.1038/nature13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kadowaki M, Takeda M, Tokita K, Hanaoka K, Tomoi M. Molecular identification and pharmacological characterization of adenosine receptors in the guinea-pig colon. Br J Pharmacol 129: 871–876, 2000. doi: 10.1038/sj.bjp.0703123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kauffenstein G, Drouin A, Thorin-Trescases N, Bachelard H, Robaye B, D’Orléans-Juste P, Marceau F, Thorin E, Sévigny J. NTPDase1 (CD39) controls nucleotide-dependent vasoconstriction in mouse. Cardiovasc Res 85: 204–213, 2010. doi: 10.1093/cvr/cvp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim JJ, Shajib MS, Manocha MM, Khan WI. Investigating intestinal inflammation in DSS-induced model of IBD. J Vis Exp (60): 3678, 2012. doi: 10.3791/3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimball BC, Mulholland MW. Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C-dependent mechanism. J Neurochem 66: 604–612, 1996. doi: 10.1046/j.1471-4159.1996.66020604.x. [DOI] [PubMed] [Google Scholar]
- 59.Kinoshita N, Takahashi T, Tada S, Shinozuka K, Mizuno N, Takahashi K. Activation of P2Y receptor enhances high-molecular compound absorption from rat ileum. J Pharm Pharmacol 58: 195–200, 2006. doi: 10.1211/jpp.58.2.0006. [DOI] [PubMed] [Google Scholar]
- 60.Korman LY, Lemp GF, Jackson MJ, Gardner JD. Mechanism of action of ATP on intestinal epithelial cells. Cyclic AMP-mediated stimulation of active ion transport. Biochim Biophys Acta 721: 47–54, 1982. doi: 10.1016/0167-4889(82)90022-2. [DOI] [PubMed] [Google Scholar]
- 61.Kukulski F, Lévesque SA, Lavoie EG, Lecka J, Bigonnesse F, Knowles AF, Robson SC, Kirley TL, Sévigny J. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic Signal 1: 193–204, 2005. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurahashi M, Zheng H, Dwyer L, Ward SM, Koh SD, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol 589: 697–710, 2011. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lavoie EG, Gulbransen BD, Martín-Satué M, Aliagas E, Sharkey KA, Sévigny J. Ectonucleotidases in the digestive system: focus on NTPDase3 localization. Am J Physiol Gastrointest Liver Physiol 300: G608–G620, 2011. doi: 10.1152/ajpgi.00207.2010. [DOI] [PubMed] [Google Scholar]
- 64.Lee MY, Park C, Berent RM, Park PJ, Fuchs R, Syn H, Chin A, Townsend J, Benson CC, Redelman D, Shen TW, Park JK, Miano JM, Sanders KM, Ro S. Smooth muscle cell genome browser: enabling the identification of novel serum response factor target genes. PLoS One 10: e0133751, 2015. doi: 10.1371/journal.pone.0133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leipziger J, Kerstan D, Nitschke R, Greger R. ATP increases [Ca2+]i and ion secretion via a basolateral P2Y-receptor in rat distal colonic mucosa. Pflugers Arch 434: 77–83, 1997. doi: 10.1007/PL00008079. [DOI] [PubMed] [Google Scholar]
- 66.LePard KJ, Messori E, Galligan JJ. Purinergic fast excitatory postsynaptic potentials in myenteric neurons of guinea pig: distribution and pharmacology. Gastroenterology 113: 1522–1534, 1997. doi: 10.1053/gast.1997.v113.pm9352854. [DOI] [PubMed] [Google Scholar]
- 67.Lies B, Groneberg D, Friebe A. Toward a better understanding of gastrointestinal nitrergic neuromuscular transmission. Neurogastroenterol Motil 26: 901–912, 2014. doi: 10.1111/nmo.12367. [DOI] [PubMed] [Google Scholar]
- 68.Lin JH, Takano T, Arcuino G, Wang X, Hu F, Darzynkiewicz Z, Nunes M, Goldman SA, Nedergaard M. Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev Biol 302: 356–366, 2007. doi: 10.1016/j.ydbio.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Longhi MS, Moss A, Jiang ZG, Robson SC. Purinergic signaling during intestinal inflammation. J Mol Med (Berl) 95: 915–925, 2017. doi: 10.1007/s00109-017-1545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mañé N, Viais R, Martínez-Cutillas M, Gallego D, Correia-de-Sá P, Jiménez M. Inverse gradient of nitrergic and purinergic inhibitory cotransmission in the mouse colon. Acta Physiol (Oxf) 216: 120–131, 2016. doi: 10.1111/apha.12599. [DOI] [PubMed] [Google Scholar]
- 71.Martín-Satué M, Lavoie EG, Pelletier J, Fausther M, Csizmadia E, Guckelberger O, Robson SC, Sévigny J. Localization of plasma membrane bound NTPDases in the murine reproductive tract. Histochem Cell Biol 131: 615–628, 2009. doi: 10.1007/s00418-008-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McClain J, Grubišić V, Fried D, Gomez-Suarez RA, Leinninger GM, Sévigny J, Parpura V, Gulbransen BD. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146: 497–507.e1, 2014. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McClain JL, Fried DE, Gulbransen BD. Agonist-evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol 1: 631–645, 2015. doi: 10.1016/j.jcmgh.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology 1393–1407.e5, 2016. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- 75.Merlin D, Augeron C, Tien XY, Guo X, Laboisse CL, Hopfer U. ATP-stimulated electrolyte and mucin secretion in the human intestinal goblet cell line HT29-Cl.16E. J Membr Biol 137: 137–149, 1994. doi: 10.1007/BF00233483. [DOI] [PubMed] [Google Scholar]
- 76.Mulero JJ, Yeung G, Nelken ST, Ford JE. CD39-L4 is a secreted human apyrase, specific for the hydrolysis of nucleoside diphosphates. J Biol Chem 274: 20064–20067, 1999. doi: 10.1074/jbc.274.29.20064. [DOI] [PubMed] [Google Scholar]
- 77.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. Beta-nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci USA 104: 16359–16364, 2007. doi: 10.1073/pnas.0705510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neshat S, deVries M, Barajas-Espinosa AR, Skeith L, Chisholm SP, Lomax AE. Loss of purinergic vascular regulation in the colon during colitis is associated with upregulation of CD39. Am J Physiol Gastrointest Liver Physiol 296: G399–G405, 2009. doi: 10.1152/ajpgi.90450.2008. [DOI] [PubMed] [Google Scholar]
- 79.Nitahara K, Kittel A, Liang SD, Vizi ES. A1-receptor-mediated effect of adenosine on the release of acetylcholine from the myenteric plexus: role and localization of ecto-ATPase and 5′-nucleotidase. Neuroscience 67: 159–168, 1995. doi: 10.1016/0306-4522(94)00585-S. [DOI] [PubMed] [Google Scholar]
- 80.Rao M, Rastelli D, Dong L, Chiu S, Setlik W, Gershon MD, Corfas G. Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology 153: 1068–1081.e7, 2017. doi: 10.1053/j.gastro.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roberts JA, Durnin L, Sharkey KA, Mutafova-Yambolieva VN, Mawe GM. Oxidative stress disrupts purinergic neuromuscular transmission in the inflamed colon. J Physiol 591: 3725–3737, 2013. doi: 10.1113/jphysiol.2013.254136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Robson SC, Sévigny J, Zimmermann H. The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2: 409–430, 2006. doi: 10.1007/s11302-006-9003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Robson SC, Zimmermann H. Editorial. Purinergic Signal 2: 325–326, 2006. doi: 10.1007/s11302-006-9005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rybaczyk L, Rozmiarek A, Circle K, Grants I, Needleman B, Wunderlich JE, Huang K, Christofi FL. New bioinformatics approach to analyze gene expressions and signaling pathways reveals unique purine gene dysregulation profiles that distinguish between CD and UC. Inflamm Bowel Dis 15: 971–984, 2009. doi: 10.1002/ibd.20893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saffrey MJ. Cellular changes in the enteric nervous system during ageing. Dev Biol 382: 344–355, 2013. doi: 10.1016/j.ydbio.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 86.Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev 94: 859–907, 2014. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarosi GA, Barnhart DC, Turner DJ, Mulholland MW. Capacitative Ca2+ entry in enteric glia induced by thapsigargin and extracellular ATP. Am J Physiol Gastrointest Liver Physiol 275: G550–G555, 1998. doi: 10.1152/ajpgi.1998.275.3.G550. [DOI] [PubMed] [Google Scholar]
- 88.Schubert P, Ogata T, Marchini C, Ferroni S, Rudolphi K. Protective mechanisms of adenosine in neurons and glial cells. Ann NY Acad Sci 825, 1 Neuroprotecti: 1–10, 1997. doi: 10.1111/j.1749-6632.1997.tb48409.x. [DOI] [PubMed] [Google Scholar]
- 89.Sévigny J, Sundberg C, Braun N, Guckelberger O, Csizmadia E, Qawi I, Imai M, Zimmermann H, Robson SC. Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTPDase2 have implications for thromboregulation. Blood 99: 2801–2809, 2002. doi: 10.1182/blood.V99.8.2801. [DOI] [PubMed] [Google Scholar]
- 90.Souza CO, Santoro GF, Figliuolo VR, Nanini HF, de Souza HS, Castelo-Branco MT, Abalo AA, Paiva MM, Coutinho CM, Coutinho-Silva R. Extracellular ATP induces cell death in human intestinal epithelial cells. Biochim Biophys Acta 1820: 1867–1878, 2012. doi: 10.1016/j.bbagen.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 91.Spencer N, McCarron SL, Smith TK. Sympathetic inhibition of ascending and descending interneurones during the peristaltic reflex in the isolated guinea-pig distal colon. J Physiol 519: 539–550, 1999. doi: 10.1111/j.1469-7793.1999.0539m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stjärne L. Basic mechanisms and local modulation of nerve impulse-induced secretion of neurotransmitters from individual sympathetic nerve varicosities. Rev Physiol Biochem Pharmacol 112: 1–137, 1989. doi: 10.1007/BFb0027496. [DOI] [PubMed] [Google Scholar]
- 93.Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL. Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Invest 99: 2588–2601, 1997. doi: 10.1172/JCI119447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Strong DS, Cornbrooks CF, Roberts JA, Hoffman JM, Sharkey KA, Mawe GM. Purinergic neuromuscular transmission is selectively attenuated in ulcerated regions of inflamed guinea pig distal colon. J Physiol 588: 847–859, 2010. doi: 10.1113/jphysiol.2009.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun JJ, Liu Y, Ye ZR. Effects of P2Y1 receptor on glial fibrillary acidic protein and glial cell line-derived neurotrophic factor production of astrocytes under ischemic condition and the related signaling pathways. Neurosci Bull 24: 231–243, 2008. doi: 10.1007/s12264-008-0430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tamada H, Hashitani H. Calcium responses in subserosal interstitial cells of the guinea-pig proximal colon. Neurogastroenterol Motil 26: 115–123, 2014. doi: 10.1111/nmo.12240. [DOI] [PubMed] [Google Scholar]
- 97.Thornton PD, Gwynne RM, McMillan DJ, Bornstein JC. Transmission to interneurons is via slow excitatory synaptic potentials mediated by P2Y(1) receptors during descending inhibition in guinea-pig ileum. PLoS One 8: e40840, 2013. doi: 10.1371/journal.pone.0040840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Torres IL, Buffon A, Silveira PP, Duarte MZ, Bassani MG, Oliveira SS, Battastini AM, Sarkis JJ, Dalmaz C, Ferreira MB. Effect of chronic and acute stress on ectonucleotidase activities in spinal cord. Physiol Behav 75: 1–5, 2002. doi: 10.1016/S0031-9384(01)00605-9. [DOI] [PubMed] [Google Scholar]
- 99.Van Nassauw L, Costagliola A, Van Op den Bosch J, Cecio A, Vanderwinden JM, Burnstock G, Timmermans JP. Region-specific distribution of the P2Y4 receptor in enteric glial cells and interstitial cells of Cajal within the guinea-pig gastrointestinal tract. Auton Neurosci 126-127: 299–306, 2006. doi: 10.1016/j.autneu.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 100.von Kügelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther 110: 415–432, 2006. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 101.Wang GD, Wang XY, Hu HZ, Liu S, Gao N, Fang X, Xia Y, Wood JD. Inhibitory neuromuscular transmission mediated by the P2Y1 purinergic receptor in guinea pig small intestine. Am J Physiol Gastrointest Liver Physiol 292: G1483–G1489, 2007. doi: 10.1152/ajpgi.00450.2006. [DOI] [PubMed] [Google Scholar]
- 102.Weisman GA, Woods LT, Erb L, Seye CI. P2Y receptors in the mammalian nervous system: pharmacology, ligands and therapeutic potential. CNS Neurol Disord Drug Targets 11: 722–738, 2012. doi: 10.2174/187152712803581047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc 12: 1295–1309, 2017. doi: 10.1038/nprot.2017.044. [DOI] [PubMed] [Google Scholar]
- 104.Wunderlich JE, Needleman BJ, Chen Z, Yu JG, Wang Y, Grants I, Mikami DJ, Melvin WS, Cooke HJ, Christofi FL. Dual purinergic synaptic transmission in the human enteric nervous system. Am J Physiol Gastrointest Liver Physiol 294: G554–G566, 2008. doi: 10.1152/ajpgi.00500.2007. [DOI] [PubMed] [Google Scholar]
- 105.Wynn G, Ma B, Ruan HZ, Burnstock G. Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol 287: G647–G657, 2004. doi: 10.1152/ajpgi.00020.2004. [DOI] [PubMed] [Google Scholar]
- 106.Yamamoto T, Suzuki Y. Role of luminal ATP in regulating electrogenic Na(+) absorption in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 283: G300–G308, 2002. doi: 10.1152/ajpgi.00541.2001. [DOI] [PubMed] [Google Scholar]
- 107.Zhang Y, Lomax AE, Paterson WG. P2Y1 receptors mediate apamin-sensitive and -insensitive inhibitory junction potentials in murine colonic circular smooth muscle. J Pharmacol Exp Ther 333: 602–611, 2010. doi: 10.1124/jpet.109.160978. [DOI] [PubMed] [Google Scholar]
- 108.Zhou X, Galligan JJ. P2X purinoceptors in cultured myenteric neurons of guinea-pig small intestine. J Physiol 496: 719–729, 1996. doi: 10.1113/jphysiol.1996.sp021722. [DOI] [PMC free article] [PubMed] [Google Scholar]