Abstract
This study investigated whether positive modulators of AMPA-type glutamate receptors influence neurotrophin expression by forebrain neurons. Treatments with the ampakine CX614 markedly and reversibly increased brain-derived neurotrophic factor (BDNF) mRNA and protein levels in cultured rat entorhinal/hippocampal slices. Acute effects of CX614 were dose dependent over the range in which the drug increased synchronous neuronal discharges; threshold concentrations for acute responses had large effects on mRNA content when applied for 3 d. Comparable results were obtained with a second, structurally distinct ampakine CX546. Ampakine-induced upregulation was broadly suppressed by AMPA, but not NMDA, receptor antagonists and by reducing transmitter release. Antagonism of L-type voltage-sensitive calcium channels blocked induction in entorhinal cortex but not hippocampus. Prolonged infusions of suprathreshold ampakine concentrations produced peak BDNF mRNA levels at 12 hr and a return to baseline levels by 48 hr. In contrast, BDNF protein remained elevated throughout a 48 hr incubation with the drug. Nerve growth factor mRNA levels also were increased by ampakines but with a much more rapid return to control levels during chronic administration. Finally, intraperitoneal injections of CX546 increased hippocampal BDNF mRNA levels in aged rats and middle-aged mice. The present results provide evidence of regional differences in mechanisms via which activity regulates neurotrophin expression. Moreover, these data establish that changes in synaptic potency produce sufficient network level physiological effects for inducing neurotrophin genes, indicate that the response becomes refractory during prolonged ampakine exposure, and raise the possibility of using positive AMPA modulators to regulate neurotrophin levels in aged brain.
Keywords: brain-derived neurotrophic factor, nerve growth factor, ampakine, hippocampus, gene regulation, trkB, aging
Neurotrophins protect neurons from a variety of pathogenic conditions (Lindvall et al., 1994; Mattson and Scheff, 1994). Consequently, there is considerable therapeutic interest in finding means to increase their availability in adult brain.In vivo the neurotrophins brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) are positively regulated by neuronal activity across a broad intensity range as demonstrated by the effects of seizures (Gall and Isackson, 1989; Bengzon et al., 1993;Lauterborn et al., 1995), afferent (Patterson et al., 1992;Castrén et al., 1993) and sensory (Castrén et al., 1992;Rocamora et al., 1996) stimulation, physical exercise (Neeper et al., 1996), and behavior in complex environments (Torasdotter et al., 1996;Gall et al., 1998; Kesslak et al., 1998). Although there is no direct evidence that variations in patterns of activity associated with everyday behaviors are sufficient to alter expression, the above observations suggest that elevations in neurotrophin production might be achieved by enhancing normally occurring physiological events.
Current evidence suggests that activity-dependent expression is primarily mediated by non-NMDA glutamate receptors. Kainic acid is a potent inducer of BDNF and NGF in hippocampal neurons, whereas NMDA is not (Zafra et al., 1990; Ghosh et al., 1994; Wetmore et al., 1994). The degree to which kainate's effects on expression are caused by stimulation of kainate- as opposed to AMPA-type glutamate receptors has not been established. The excitotoxin causes seizures, the concomitants of which undoubtedly include AMPA receptor activation. AMPA receptors bind kainate with moderate affinity (Hall et al., 1994) and do not desensitize as rapidly as kainate receptors (Patneau and Mayer, 1991;Lerma et al., 1993). Because the former receptors are orders of magnitude more numerous than the latter (Simon et al., 1976; Olsen et al., 1987), slowly desensitizing binding to them is likely to be a significant contributor to the effects induced by kainate. If AMPA-gated ion currents regulate neurotrophin gene regulation, then compounds that specifically enhance these currents should promote expression. The present studies tested this prediction using cultured hippocampal slices.
Ampakines are a group of recently introduced, small compounds (Arai et al., 1994; Staubli et al., 1994a) that slow deactivation of AMPA receptors and thereby increase fast excitatory synaptic currents (Arai et al., 1996). The drugs do not have agonistic or antagonistic properties but instead modulate the receptor rate constants for transmitter binding, channel opening, and desensitization (Arai et al., 1996). Ampakines are of particular interest with regard to neurotrophin regulation because they freely cross the blood–brain barrier (Staubli et al., 1994b) and have subtle effects on behavior at dosages sufficient to affect unit activity in the hippocampus (Hampson et al., 1998b). They enhance the encoding of several variants of memory in rats (Staubli et al., 1994a; Rogan et al., 1997) and possibly humans (Ingvar et al., 1997) without detectably affecting performance or mood. Moreover, repeated administration produces lasting improvements in learned behaviors without causing evident side effects (Hampson et al., 1998a). If positive modulation of AMPA receptors is an adequate stimulus for upregulating neurotrophins, then ampakines could provide a means to exploit this for therapeutic purposes. Accordingly, the present studies included tests of whether systemic ampakine treatment increases neurotrophin gene expression in brain.
MATERIALS AND METHODS
In vitro experiments. Cultured hippocampal slices were prepared from Sprague Dawley rat pups (9–10 d after birth; Simonsen Labs, Gilroy, CA; n = 70) as described previously (Stoppini et al., 1991; Rivera et al., 1993). In most cases, the cultured slices contained the hippocampus, subiculum, and portions of entorhinal cortex. Slices were explanted onto Millicel-CM biomembrane inserts (Millipore, Bedford, MA) in a six-well culture cluster plate (Corning, Cambridge, MA) containing sterile media (1 ml/well) consisting of minimum essential media, 30 mm dextrose, 30 mm HEPES, 5 mmNa2HCO3, 3 mm glutamine, 0.5 mmascorbic acid, 2 mm CaCl2, 2.5 mm MgSO4, 1 mg/l insulin, and 20% horse serum, pH 7.2 (all reagents from Sigma, St. Louis, MO). For each rat, slices from both hippocampi were explanted onto four biomembranes (four slices per membrane group). The tissue was maintained for 12–18 d in a humidified incubator at 37°C in 5% CO2; the medium was changed every other day.
Treatment was as follows. All experiments were begun on days 11–12 in culture. Two AMPA receptor-modulating drugs (ampakines) were used: CX614 (LiD37 or BDP-37) (Arai et al., 1997; Hennegriff et al., 1997; Kessler et al., 1998) and CX546 (GR87 or BDP-17) (Rogers et al., 1988; Holst et al., 1998), both gifts from Cortex Pharmaceuticals (Irvine, CA). The ampakines were dissolved in 100% dimethylsulfoxide (DMSO; Sigma) and stored at −20°C. Doses and treatment schedules are presented in Results with each experiment. For controls, cultures were either untreated or treated with equivalent concentrations of vehicle (i.e., DMSO at final dilutions of 1:2000–1:10,000). For experiments in which multiple drug doses were used, the DMSO concentration used for the vehicle control was the same as that used for the highest ampakine dose. Preliminary control experiments demonstrated that treatment with DMSO vehicle alone had no significant effect on BDNF mRNA content over the treatment intervals used here. Specifically, explants were treated with the highest dose of DMSO (1:2000) and fixed after exposure intervals of 12 hr (n = 8), 24 hr (n = 7), 48 hr (n = 15), and 96 hr (n = 7); mRNA levels were compared with those in untreated explants (n = 16). Evaluation of BDNF cRNA-labeling densities within the principal cell layers of the hippocampus and in layers II/III of the entorhinal cortex revealed no significant differences between untreated and DMSO-treated explants at any time point (p = 0.233 for stratum granulosum andp = 0.1 for entorhinal cortex, Kruskal–Wallis;p = 0.294 for CA1 stratum pyramidale andp = 0.164 for CA3 stratum pyramidale, ANOVA). Because DMSO had no effect on mRNA expression, control tissue from each culture plate was generally fixed to correspond to the longest time point tested. However, to verify further that there were no effects of the vehicle on mRNA content, in some sets DMSO-treated control tissue also was fixed at additional time points as noted in the figure captions. Because there were no significant differences in labeling densities across control groups in time course experiments, the control data were grouped for statistical comparisons with measures from experimental tissue. For all experiments, the ampakines were maintained in the culture media throughout the full treatment interval unless otherwise indicated. For experiments using CNQX (20 μm;Tocris Cookson, Ballwin, MO), APV (100 μm;Tocris Cookson), nimodipine (20 μm; Alomone Labs, Jerusalem, Israel), or CoCl2 (5 mm; Sigma), cultured slices were first pretreated (1 hr for CNQX and APV, 30 min for nimodipine, and 10 min for CoCl2) with either the blocker or vehicle in media and then treated for 3 hr with either blocker alone, blocker + 50 μm CX614, 50 μm CX614 alone, or vehicle.
For in situ hybridization analyses, treatments were terminated by fixation with 4% paraformaldehyde in 0.1m phosphate buffer, pH 7.2 (PPB). After overnight fixation the slices were cryoprotected with 20% sucrose in PPB for 1 hr and then sectioned (20 μm) parallel to the broad explant surface using a freezing microtome. Sections were mounted onto Superfrost/Plus slides (Fisher Scientific, Houston, TX) and processed for in situ hybridization as described below. For BDNF protein assay, tissue was collected as described below.
In vivo experiments. Aged Long–Evans rats (Charles River Laboratories, Wilmington, MA) ranging in age from 18 to 21 months were used (n = 10). Because stress has been reported to inhibit BDNF mRNA expression (Smith et al., 1995), rats were first acclimated to procedures by handling (10 min/d) for 5 d followed by handling with daily intraperitoneal injections of 0.9% saline for 5 additional days. The rats were then injected once daily for 5 d either with 30 mg/kg CX546 dissolved in 16.5% cyclodextrin [2-hydroxypropyl-β-cyclodextrin (CDX); Aldrich, Milwaukee, WI] in 0.9% saline (n = 6) or with a comparable volume of vehicle (16.5% CDX in 0.9% saline;n = 3). One additional rat was injected daily with 0.9% saline. Animals were killed 24 hr after the last injection by overdose with sodium pentobarbital and intracardial perfusion with PPB.
Middle-aged C57/blk mice (The Jackson Laboratory, Bar Harbor, ME) from 9 to 11 months of age were used (n = 9). Mice were injected intraperitoneally once with 40 mg/kg CX546 in 16.5% CDX in 0.9% saline (n = 3) or with vehicle (n= 3). Mice were killed 24 hr after injection, with paired untreated controls (n = 3), by overdose with sodium pentobarbital and perfusion with PPB.
Brains were post-fixed for 24 hr at 4°C in perfusate, cryoprotected in 20% sucrose in PPB for 48–72 hr at 4°C, sectioned (25 μm, coronal) through the hippocampus using a freezing microtome, and collected into cold PPB.
cRNA probe preparation. All cRNA probes were transcribed in the presence of 35S-labeled UTP (DuPont NEN, Boston, MA). The cRNA to BDNF exon V was generated fromPvuII-digested recombinant plasmid pR1112-8 (Isackson et al., 1991), yielding a 540 base length probe with 384 bases complementary to BDNF exon V-containing mRNA (Timmusk et al., 1993). The rat NGF cRNA transcribed from PvuII-digested genomic clone pBSNGF (Stratagene, La Jolla, CA) was 970 bases long with a span complementary to 750 bases of the coding region of rat NGF mRNA (Whittemore et al., 1988). The trkB cRNA was transcribed fromEcoRI-digested pSKTrkBPCR; the insert includes 338 bp specific for the kinase (+) form of the receptor (Dixon and McKinnon, 1994).
In situ hybridization. In situ hybridization procedures for slide-mounted and free-floating tissue were as described in detail elsewhere (Lauterborn et al., 1994) with hybridization incubation times of 16–20 hr for slide-mounted tissue or 30–36 hr for free-floating tissue at 60°C and the35S-labeled cRNA probe at a concentration of 1 × 107 cpm/ml. After a final posthybridization wash in 0.1× SSC buffer (1× SSC = 0.15m NaCl and 0.015 m Na citrate, pH 7.0) at 60°C, the free-floating tissue was mounted onto gelatin-coated slides. All tissue was processed for both film (β-max; Amersham, Arlington Heights, IL) and emulsion (NTB2; Kodak Eastman, Rochester, NY) autoradiography with exposure times of 2–4 d and 3–5 weeks, respectively. After emulsion development, the tissue was stained with cresyl violet or hematoxylin and coverslipped with Permount (Fisher Scientific).
Quantification of in situ hybridization.Hybridization densities were measured from film autoradiograms, with labeling densities calibrated relative to film images of14C-labeled standards (American Radiolabeled Chemicals, St. Louis, MO), using the Microcomputer Imaging Device (Imaging Research, St. Catherines, Ontario, Canada). The standards were rated by micro-Curies per gram; therefore these same units of measure were applied to tissue hybridization densities reported herein. For in vitro experiments, measurements were taken from the internal leaf of the dentate gyrus stratum granulosum, CA3b stratum pyramidale, and CA1b stratum pyramidale. Where possible, hybridization densities also were measured in layers II/III of the entorhinal cortex. Measurements from the dentate gyrus molecular layer were collected to estimate tissue background levels. For all experiments, multiple measurements were taken from three to four tissue sections per explant using a square sample-field template that spanned the depth of the target cell layer. Ten adjacent samples were collected from each cell layer per section and averaged. The section averages were then totaled and averaged to generate an explant mean for each field. For NGF cRNA hybridization, density measures were analyzed for the stratum granulosum alone because this neurotrophin is not expressed by the principal neurons within CA3–CA1 stratum pyramidale (Lauterborn et al., 1993). For most in vitro experiments, the significance of effect of treatment was determined by the one-way ANOVA followed by the Student–Newman–Keuls (SNK) post hoc test or Student's t test for individual comparisons. In circumstances in which the SDs were significantly different between treatment groups (as determined by Bartlett's test for homogeneity of variances), significance was determined using the Kruskal–Wallis nonparametric ANOVA followed by Mann–Whitney U or Student's t tests for individual comparisons. For experiments in which blocking agents and ampakines were used together, a two-way ANOVA was performed followed by either Student–Newman–Keuls, Student's t, or Mann–WhitneyU tests for planned comparisons. For in vivoexperiments, measurements were taken from the midseptotemporal hippocampus within the internal leaf of the stratum granulosum, CA3b stratum pyramidale, and CA1b stratum pyramidale; 10–15 measurements per section were made for each field as described above. For each animal, measurements were taken from three to four sections; section means were averaged to generate a mean value for each field. The significance of differences between drug-treated and control groups was determined using Student's t test. In all instances statistical analyses were conducted using the Instat 2.03 program (Graph Pad, San Diego, CA) with reference to Motulsky (1995), and the 95% confidence level was considered significant. Unless otherwise stated, statistical results presented in the text are for comparison with control values.
BDNF immunoassay. Cultures were collected into 100 μl of cold lysis buffer (137 mm NaCl, 20 mm Tris, 10% glycerol, 1 mm PMSF, 10 μg/ml aprotinin, 1 μg/ml leupeptin, 0.5 mm Na vanadate, and 1% NP-40). Four hippocampal slices from one insert were pooled for each “sample” assayed; each time point included three to four separate samples. Tissue was manually homogenized in lysis buffer, acidified to pH 2.5 with 1N HCl, and incubated for 15 min on ice. The pH was neutralized to pH 8.0 with 1N NaOH, and samples were frozen (−70°C) until assayed. Total BDNF protein content for each sample was measured using the BDNF Emax Immunassay System (Promega, Madison, WI) according to kit instructions, with the absorbance at 450 nm determined using a plate reader. Data from two separate immunoassay experiments were pooled for statistical analyses using ANOVA followed by the Student–Newman–Keuls test for individual comparisons.
Electrophysiology. Culture inserts containing three to four hippocampal slices were transferred to a recording chamber 10–20 min before recording. Slices were maintained at the interface between a moist atmosphere containing 5% CO2 (flow rate, 1 l/min) and serum-free culture media at 33°C. Extracellular recordings from the stratum radiatum of field CA1 and the granule cell layer were made using a glass pipette filled with 2 m NaCl. Explants exhibiting high basal activity, i.e., massive spike events followed by spreading depression, were discarded. For acute effects of drug, basal activity was monitored for 30 min, in normal serum-free medium, and then culture medium containing CX614 was infused into the chamber; a stock solution of CX614 (50 mm) was prepared in DMSO and diluted 1:1000–1:5000 for experimental use. Preliminary experiments demonstrated that DMSO at concentrations up to 0.2% (i.e., double the highest concentration used in the present experiments) had no effect on synaptic responses. Spontaneous field potentials were recorded during sweeps of 1 sec duration; recording blocks consisted of 200 consecutive sweeps separated by a 1 sec interval and thus extended over a period of 6–7 min. Recording blocks were collected during baseline monitoring and 10 min after switching the media to one containing CX614. Field potentials were acquired and digitized at 10 kHz using the Neuronal Activity Acquisition Program (Eclectek Enterprise, Irvine, CA). Statistical significance was determined using the one-way ANOVA followed by Student's t or Mann–Whitney U tests for planned comparisons.
RESULTS
In vitro studies
Ampakines increase BDNF expression in a dose-dependent manner
Cultured hippocampal slices were incubated with the ampakine CX614 at various concentrations and for different durations, and effects on BDNF mRNA content were assessed by35S-cRNA in situ hybridization and quantification of autoradiographic labeling. As shown in Figure1B, treatment with 50 μm for 3 hr caused a striking increase in BDNF cRNA labeling in all subdivisions of the hippocampus. This effect was fully reversed after switching to drug-free media for 15 or 21 hr (Fig.1C). Group results for the dentate gyrus stratum granulosum are summarized in Figure 1D. BDNF cRNA labeling at the 3 hr time point was elevated above control values (p < 0.01, SNK) and had declined to control levels in the 18 and 24 hr washout groups.
Fig. 1.
The ampakine CX614 increases BDNF mRNA expression in hippocampal explant cultures. A–C, Bright-field photomicrographs of film autoradiograms showing the in situ hybridization localization of BDNF cRNA labeling in sections from a vehicle-control explant (A), an explant treated for 3 hr with 50 μm CX614 (B), and an explant treated for 3 hr with CX614 (50 μm) and then maintained in drug-free media for an additional 15 hr (C). Shown in B, the 3 hr CX614 treatment increased BDNF cRNA labeling in the stratum granulosum (sg) and stratum pyramidale (sp) (regions CA3–CA1). This effect was reversed by 18 hr after drug washout (C). Scale bar, 300 μm.D, Bar graph showing group mean-labeling density values (± SEM; n > 4/group) within the stratum (str.) granulosum of control explants treated for 3 hr with DMSO (con), explants treated for 3 hr with CX614 (50 μm) alone, and explants treated for 3 hr with CX614 (50 μm), followed by drug washout for an additional 15 or 21 hr (for 18 and 24 hr time points, respectively). As shown, CX614 significantly increased BDNF cRNA labeling in the granule cells by 3 hr (p < 0.01 vs control, SNK; overall effect of treatment, p < 0.0005, ANOVA). This increase was eliminated by subsequent 15–21 hr drug washout.
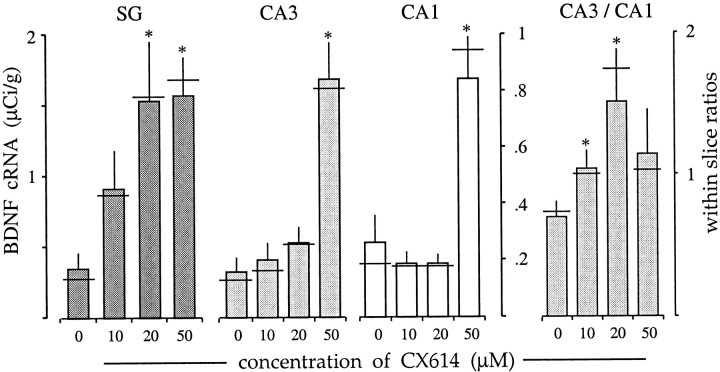
Dose–response data for 3 hr incubations with CX614 are summarized in Figure 2. The threshold dose for the granule cells appeared to be ∼10 μm, with 20 μm causing a robust and reliable increase relative to that in control slices (p < 0.04). BDNF mRNA levels in CA3 stratum pyramidale tended to be greater than control values with CX614 treatment at 10 and 20 μm but not significantly so, whereas the effects at 50 μm were robust. Measures from CA1 stratum pyramidale gave no evidence of a response to 10 or 20 μm; as shown in Figure 2, median scores (horizontal lines) were virtually identical for the 0, 10, and 20 μm ampakine treatment groups (n = 4 each). However, as in the other hippocampal subfields, 50 μm CX614 produced a significant (p < 0.001) elevation. Densitometric values for CA3 and CA1 were highly correlated (r = 0.986 for controls), making it possible to use within-slice comparisons to test for effects at low drug concentrations. As shown in Figure 2(right), the within-slice CA3/CA1 ratio increased significantly from 0.70 in controls to 1.04 at 10 μm and to 1.51 at 20 μm, thereby demonstrating that there was indeed a significant response to the lower dose. These latter observations indicate that 3 hr exposures to threshold concentrations of ampakine are sufficient to modify the balance of neurotrophin expression across pyramidal cell subfields.
Fig. 2.
Ampakine-induced increases in BDNF mRNA content are dose-dependent. Bar graphs show the effect of a 3 hr treatment with various concentrations of CX614 on BDNF cRNA labeling in the dentate gyrus stratum granulosum (SG), CA3 stratum pyramidale, and CA1 stratum pyramidale. Left, Graphs show mean density values for each subfield (± SEM; n = 4/group; lefty-axis applies to the stratum granulosum; righty-axis applies to CA3 and CA1). In all fields there was a significant effect of treatment (p = 0.0336 for SG;p = 0.003 for CA3; p < 0.0001 for CA1). For the granule cells, a modest increase in labeling was seen with 10 μm, and more dramatic increases were apparent with the higher doses (p < 0.05 vs control, SNK). For the pyramidal cells, only 50 μm elicited significant increases (p < 0.001 for CA3;p < 0.001 for CA1 vs control, SNK).Right, The graph shows the within-slice values for the CA3/CA1-labeling ratio (± SEM). As shown, there were significant increases in the CA3/CA1 ratio at both 10 and 20 μm(p < 0.05 and 0.03, respectively, Student's t test for comparison with controls).
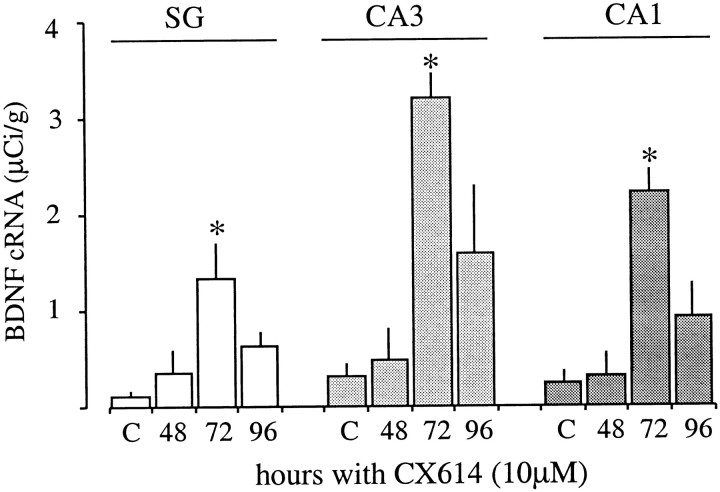
The possibility that ampakine concentrations that were near threshold at 3 hr would increase BDNF expression with prolonged application was tested using 10 μm CX614. The drug was reapplied every 24 hr for 2, 3, or 4 d. ANOVA indicated that the treatment significantly increased BDNF mRNA content in each of the three hippocampal subdivisions. As shown in Figure3, dramatic increases were evident after 72 hr and, in marked contrast to the results obtained with acute administration of this dose, were of approximately the same magnitude (∼10-fold) in pyramidal and granule neurons. Thus, when applied at a relatively low dose the ampakine progressively increases BDNF expression over time and can sustain BDNF mRNA content at elevated levels for days.
Fig. 3.
Chronic treatment with near-threshold doses of CX614 increases BDNF expression with time in vitro. The bar graph shows the effect of maintaining cultured hippocampal explants in 10 μm CX614 for 48, 72, or 96 hr; control cultures (C) were treated with DMSO vehicle (1:10,000) for 96 hr. Group mean densitometric measures (± SEM; n= 8/group) of BDNF cRNA labeling in the stratum granulosum (SG), CA3 stratum pyramidale, and CA1 stratum pyramidale are presented. For all three regions, there was a significant effect of treatment (p < 0.0152 forSG, 0.0012 for CA3, and 0.0003 for CA1, ANOVA), with the greatest increase in labeling density occurring at 72 hr as compared with controls (p < 0.05, 0.01, and 0.001 for SG, CA3, and CA1, respectively, SNK).
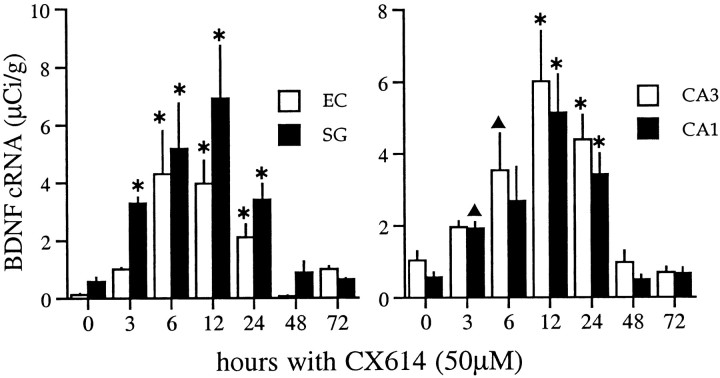
The time course of ampakine-induced increases in BDNF mRNA content was further evaluated using 50 μm CX614, shown above to influence mRNA content in all subfields after a 3 hr exposure. Results obtained with treatments of 12, 24, and 48 hr are illustrated in Figure4. As shown, a 12 hr exposure (Fig.4B) increased labeling above that in control slices (Fig. 4A) in all subdivisions of the hippocampus and retrohippocampal cortex. With a 24 hr treatment (Fig. 4C), BDNF mRNA levels were also increased but not to the degree seen after 12 hr. The falloff in labeling was still more evident in slices continuously exposed to the ampakine for 48 hr (Fig.4D), at which time expression approached control levels. Figure 5 summarizes time course results with CX614 at 50 μm for seven experiments involving 85 explants. Differences in labeling densities across time points were highly significant for each region sampled (stratum granulosum, CA3 and CA1 stratum pyramidale, and entorhinal cortex layer II; p < 0.0001, ANOVA). Peak values for the hippocampus were attained by 12 hr; increases in the entorhinal cortex may have reached their maximum previously, but variance between experiments precludes a strong conclusion. The size of the increase differed across regions, ranging from 5-fold for the pyramidal cell layers to 30-fold for the superficial entorhinal cortex. The quantitative results in Figure 5 also confirm that the elevation in BDNF mRNA declines between 12 and 24 hr (p < 0.05 for CA1; p < 0.01 for the entorhinal cortex;p > 0.05 for the stratum granulosum and CA3, SNK) and is gone by 48 hr despite continuous exposure to the ampakine. Still longer incubations at this dose (i.e., 72 hr) did not result in further increases or decreases from control values.
Fig. 4.
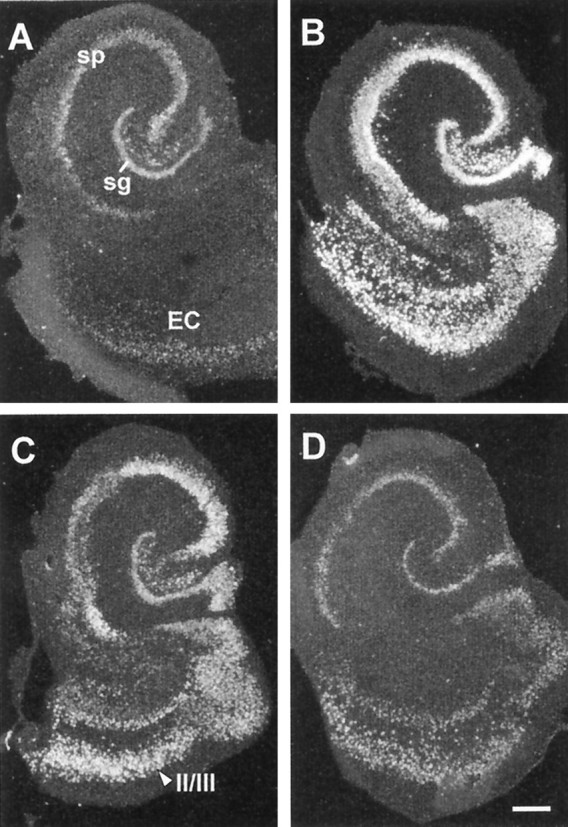
Suprathreshold CX614 treatment induces a transient increase in BDNF mRNA expression in hippocampal/entorhinal cortex explant cultures. Dark-field photomicrographs of tissue autoradiograms show BDNF cRNA labeling in tissue sections through a control explant (A) and explants treated continuously with 50 μm CX614 for 12 hr (B), 24 hr (C), or 48 hr (D). As shown in B, a 12 hr treatment markedly increased BDNF mRNA levels throughout the strata granulosum (sg) and pyramidale (sp) of the hippocampus and in the entorhinal cortex (EC). At 24 hr (C), labeling had decreased in each of these fields, as compared with the 12 hr time point (layers II/III entorhinal cortex indicated). At 48 hr (D), labeling densities had returned to near-control levels. Scale bar, 300 μm.
Fig. 5.
Time course of changes in BDNF mRNA content in hippocampal and entorhinal cortex explants treated continuously with 50 μm CX614. Bar graphs show group mean densitometric measures of BDNF cRNA labeling (μCi/gm) within the stratum granulosum (SG) and the superficial entorhinal cortex (EC) (left) and the stratum pyramidale of regions CA3 and CA1 (right). Graphs show the cumulative data from seven experiments (n = 85 explants; group mean values ± SEM) measured from film autoradiograms. Values from vehicle-control slices are presented at the 0 time point; control values represent combined measures from 3, 24, and 72 hr DMSO-treated explants (n = 31). In all fields, there was a significant effect of treatment (p < 0.0001, ANOVA). BDNF cRNA labeling was elevated by 3 hr, was maximal at 12 hr, and was still significantly elevated through 24 hr of treatment. Hybridization densities were not significantly different from control values in slices treated for 48 or 72 hr (*p < 0.001; ▴p < 0.05, for comparison with controls, SNK).
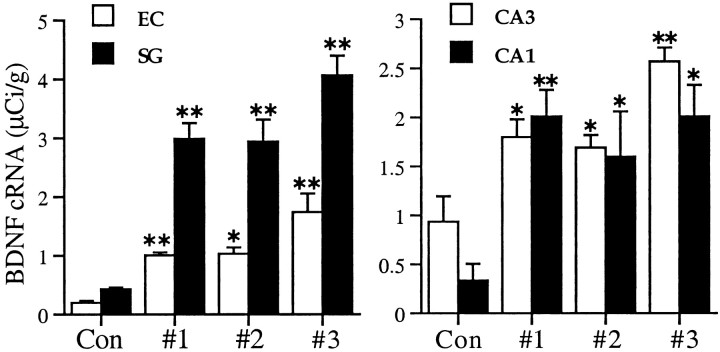
Among other possibilities, the inverted U curve for duration versus BDNF expression observed with suprathreshold CX614 treatment could indicate that the ampakine is in some way altered by exposure to the tissue or that slices so treated generate diffusible materials that interfere with the drug's actions. As a first test of these possibilities, media from slices that had been exposed to CX614 at 50 μm for 3 hr were collected and applied to a second set of previously untreated slices for 3 hr. The media from this group were then collected and applied to a third set of previously untreated slices for 3 hr. Figure 6 shows that the ampakine was as potent on second and third applications as it was on the first in inducing BDNF mRNA levels across the hippocampal subfields and entorhinal cortex.
Fig. 6.
CX614 retains the ability to induce BDNF mRNA expression over time in vitro. Bar graphs show group mean BDNF cRNA-labeling densities (μCi/gm; ± SEM) within the stratum granulosum (SG) and the superficial entorhinal cortex (EC) (left) and the stratum pyramidale of regions CA3 and CA1 (right). Group #1 explants were treated with fresh 50 μm CX614 for 3 hr. The media were then transferred to group #2 for 3 hr and then collected and transferred to group #3 for another 3 hr. Each group of slices was fixed at the end of its 3 hr ampakine treatment interval. Values from untreated control slices are presented as Con;n = 4–5 explants per group. In all fields, there was a significant overall effect of treatment (ANOVA,p < 0.0001 for the stratum granulosum;p = 0.0005 for CA3; p = 0.0084 for CA1; p = 0.0002 for EC). In comparison with the control group, hybridization was significantly elevated in all drug-treated groups (*p < 0.05; **p < 0.01 for CA1 and the entorhinal cortex; **p < 0.001 for the stratum granulosum and CA3, SNK). Notably, as compared with group #1 values, hybridization densities were not reduced in later-treated groups.
A second and less potent ampakine, CX546, was also tested for its ability to induce BDNF expression. In an acute study, explants were treated for 3 hr with CX546 at a range of concentrations (50, 100, and 250 μm; n = 4–5 explants/drug group;n = 7 for 3 hr DMSO controls). With the 3 hr treatment, BDNF mRNA levels in the stratum granulosum were modestly increased (190% of control values) by a 50 μm dose (p < 0.001, ANOVA for overall effect of treatment; p < 0.006 for 50 μmvs control, Student's t test), a concentration that is close to the threshold for enhancing field EPSPs in hippocampal slices (A. Arai and G. Lynch, unpublished observations). The highest concentration tested (250 μm) generated a 5.5-fold increase in expression (p < 0.006 vs control, Mann–Whitney U test) in this cell layer; this is close to the approximate sixfold increase elicited over 3 hr by a functionally equivalent concentration (50 μm) of CX614. Expression in the CA3 stratum pyramidale was unaffected by the threshold CX546 concentration, tended to be elevated at 100 μm, and was robustly increased 3.8-fold above control values at 250 μm(p < 0.001, ANOVA; p = 0.0031, Mann–Whitney U test for 250 μm vs control). Labeling within CA1 was only elevated after treatment with 250 μm (1.6-fold above control values;p < 0.001, ANOVA; p = 0.0154, Student's t test, for 250 μm vs control). In all, acute treatment with CX546 produced the same quantitative results as CX614 and with a similar regional distribution of sensitivities (dentate > CA3 > CA1).
Treatment with CX546 for 24 hr (n = 6–10 explants for drug-treated and 24 hr DMSO-control groups) increased BDNF mRNA content over a far lower dose range (25, 50, and 100 μm). There was a significant effect of treatment with the drug for all three hippocampal subfields (p < 0.02, Kruskal–Wallis nonparametric ANOVA for all fields). Labeling was elevated 2.2- and 1.9-fold in the CA1 stratum pyramidale of slices treated with CX546 at 50 and 100 μm, respectively, as compared with DMSO-treated controls (p < 0.02, Mann–Whitney Utest); these concentrations had no clear effect on BDNF mRNA by 3 hr (above). All three doses significantly increased mRNA content in the granule cell layer [2.4-fold for 25 μm(p < 0.05, Student's t test); 5.9-fold for 50 μm and 4.6-fold for 100 μm (p < 0.011, Mann–Whitney U test)] and the CA3 stratum pyramidale [1.3-fold for 25 μm (p< 0.027, Student's t test); 5.3-fold for 50 μm and 3.8-fold for 100 μm (p < 0.027, Mann–Whitney U test)], with the 50 and 100 μm concentrations having much larger effects with longer incubations.
Ampakine treatment increases BDNF protein content
To determine whether induced increases in BDNF mRNA content were associated with increases in BDNF protein, immunoassays were performed on slices treated with CX614 at 50 μm for 6, 24, and 48 hr. Six hours were chosen as the earliest time point because previous work demonstrated a 3–4 hr delay between activity-induced increases in BDNF mRNA and immunoreactivity (IR) in vivo (Nawa et al., 1995). For analysis, the four hippocampal slices within a single culture well were pooled to create an individual sample; three to four samples were measured per group. CX614 induced a significant increase in BDNF-IR in all treatment groups, and in contrast to mRNA measures (Fig. 5), protein levels were comparably elevated above control values after 6, 24, and 48 hr exposures [control, 3.92 ± 1.63 ng/μg (± SEM); 6 hr, 9.76 ± 1.07 ng/μg; 24 hr, 11.82 ± 1.21 ng/μg; 48 hr, 11.99 ± 2.00 ng/μg; p < 0.05, for all control vs experimental comparisons]. Values between ampakine-treated groups were not significantly different.
Receptor and ion channel dependencies
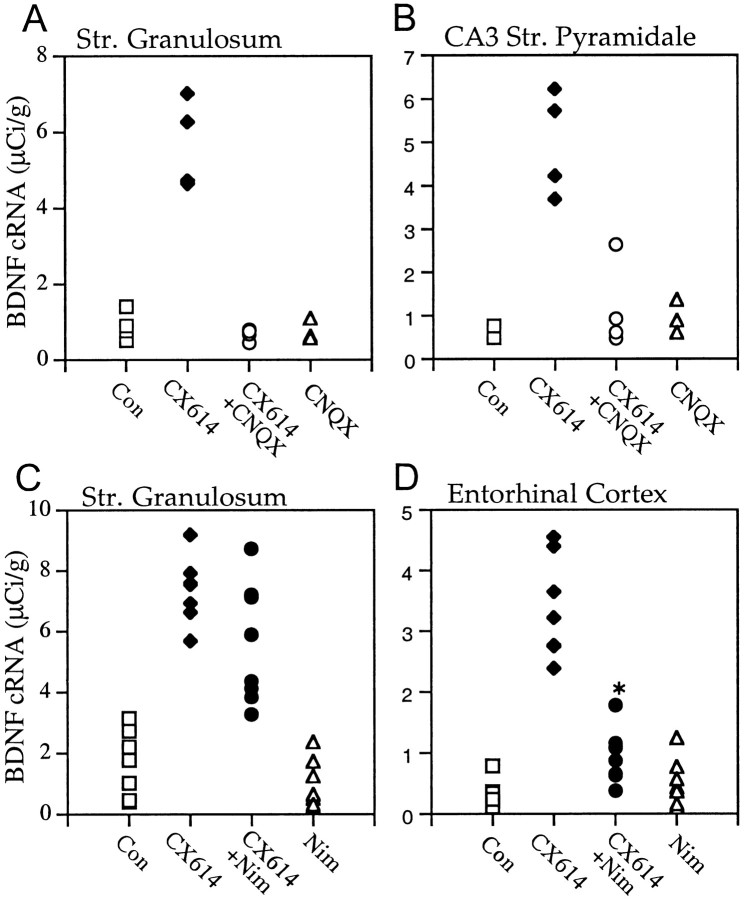
Cultured slices were treated with CX614 in combination with receptor or ion channel blockers. Figure7, A and B, shows a control slice and one treated with 50 μm CX614 for 3 hr, respectively; drug-induced increases in BDNF cRNA hybridization in the dentate gyrus granule cells, hippocampal pyramidal cells, and retrohippocampal cortex are evident. As shown in Figure7C, this effect was essentially eliminated in paired slices cotreated with the ampakine and the selective AMPA receptor antagonist CNQX at 20 μm, a dose shown in other work to block fast excitatory transmission in slices. In contrast, the NMDA receptor antagonist APV (100 μm) had no obvious effect on CX614-induced increases in BDNF mRNA content at the 3 hr time point (Fig. 7D). This impression was reinforced by densitometric analyses that revealed no measurable difference between slices treated for 3 or 6 hr with 50 μm CX614 + 100 μm APV and those treated with 50 μm CX614 alone (data not shown).
Fig. 7.
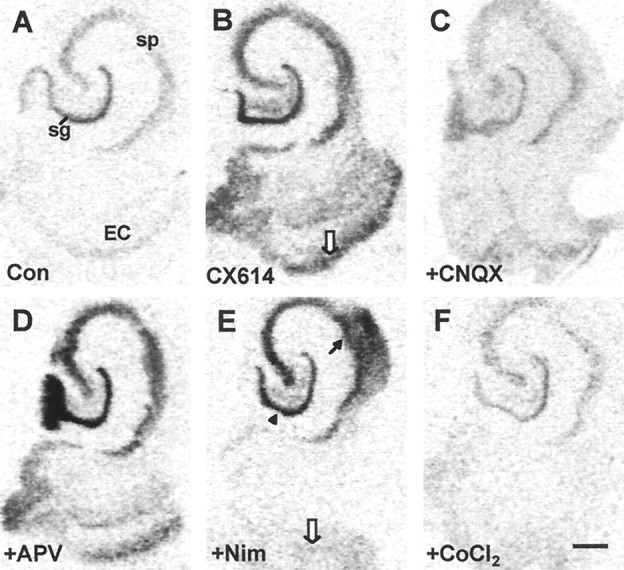
Effects of various glutamate receptor and voltage-sensitive calcium channel blockers on CX614-induced increases in BDNF mRNA expression. Light-field photomicrographs of film autoradiograms show BDNF cRNA labeling in sections from a control (Con) culture (A) and cultures treated for 3 hr with CX614 (50 μm) alone (B) or in combination with CNQX (20 μm;C), APV (100 μm;D), nimodipine (Nim; 20 μm;E), or CoCl2 (5 mm;F). In comparison with cultures treated with CX614 alone, cultures treated with CX614 + CNQX (C) have BDNF cRNA labeling that was not elevated in the hippocampus or entorhinal cortex (EC). By contrast, in cultures treated with CX614 + APV (D), labeling was elevated in all fields. In cultures treated with CX614 + nimodipine (E), BDNF cRNA labeling was elevated in the strata granulosum (sg;arrowhead) and pyramidale (sp;blackarrow) of the hippocampus but not in the entorhinal cortex (whitearrow). Cotreatment with CX614 + CoCl2(F) resulted in complete blockade of ampakine-induced increases in BDNF mRNA in all fields. Scale bar, 500 μm.
Further evidence that the ampakine-induced increase in BDNF expression is dependent on transmission was obtained with 5 mmCoCl2, a treatment that blocks calcium fluxes and thus glutamate release. Figure 7F shows that the effects of CX614 are altogether absent in a CoCl2-cotreated slice. In contrast to this broad effect, cotreatment with the L-type voltage-sensitive calcium channel blocker nimodipine (20 μm) reduced CX614-induced increases in BDNF mRNA in layers II and III of the entorhinal cortex (Fig. 7E, white arrow) but did not block effects of the ampakine in the hippocampus proper or the dentate gyrus.
Figure 8 summarizes group results for nimodipine and CNQX applied alone or with CX614 for 3 hr. Note that there was no overlap in labeling densities in the dentate gyrus (Fig.8A) and field CA3 (Fig. 8B) for slices given CX614 alone versus those cotreated with CNQX (p < 0.001) and that the latter group values were not detectably different from control. Similar results were obtained in field CA1 stratum pyramidale and superficial entorhinal cortex (data not shown). The regionally differentiated effects of nimodipine were confirmed by quantitative analyses; within the same slice the channel blocker significantly attenuated the ampakine effect on BDNF expression in the entorhinal cortex but not in the stratum granulosum (Fig. 8C,D).
Fig. 8.
CNQX and nimodipine significantly attenuate CX614-induced increases in BDNF mRNA content. Scatter graphs show BDNF cRNA-labeling densities (μCi/gm) in the stratum granulosum (A, C), the CA3 stratum pyramidale (B), and the entorhinal cortex layers II and III (D) within individual explants treated 3 hr with vehicle (Con) or with CX614 (50 μm), CX614 (50 μm) + CNQX (20 μm), CNQX (20 μm) alone, CX614 (50 μm) + nimodipine (Nim; 20 μm), or nimodipine (20 μm) alone. Solidsymbolsindicate significant differences between experimental and control group mean values (n ≥ 4/group for A, B;n = 7/group for C, D).A,B, CX614 alone increased BDNF cRNA labeling over control values in both hippocampal fields (p < 0.001 for both, SNK), whereas cotreatment with CNQX markedly blocked this effect. C, D, In measures from a separate set of slices, CX614 alone increased BDNF cRNA labeling in the stratum (str.) granulosum and the entorhinal cortex (p ≤ 0.0003, Mann–Whitney U test). Moreover, in explants treated with CX614 + Nim, labeling was increased above both control and nimodipine-alone values in both fields (CX614 + Nim vs Nim,p = 0.0002 for the str. granulosum, Mann–WhitneyU test; p = 0.0307 for the entorhinal cortex, Student's t test; CX614 + Nim vs Con,p = 0.0002 for the str. granulosum, Student's t test; p = 0.0014 for the entorhinal cortex, Student's t test). However, cotreatment with nimodipine significantly attenuated the effect of CX614 on BDNF expression in the entorhinal cortex (*p < 0.0001 vs the CX614 group, Student'st test) but not in the str. granulosum.
Effects on NGF and trkB mRNA levels
Previous studies have demonstrated that, in addition to effects on BDNF, seizures and/or intense glutamate receptor activation increase the expression of NGF, specifically within the dentate gyrus granule cells (Lauterborn et al., 1993), and of the BDNF receptor trkB (Bengzon et al., 1993; Merlio et al., 1993). To test the generality of ampakine effects on activity-regulated neurotrophin genes, we examined the influence of CX614 on NGF and trkB mRNA levels in cultured hippocampal slices. As the photomicrographs in Figure9, A and B, illustrate, a 6 hr incubation with CX614 at 50 μm caused a marked increase in NGF cRNA labeling within the stratum granulosum but did not appear to affect labeling densities or numbers of the NGF mRNA-positive interneurons. The increase in granule cell labeling was maximal after 3 hr, the earliest time point tested, and declined thereafter (3 vs 6 hr incubation; p < 0.01, SNK). Labeling densities were not different from control values in slices exposed to CX614 for 24 hr (Fig. 9C).
Fig. 9.
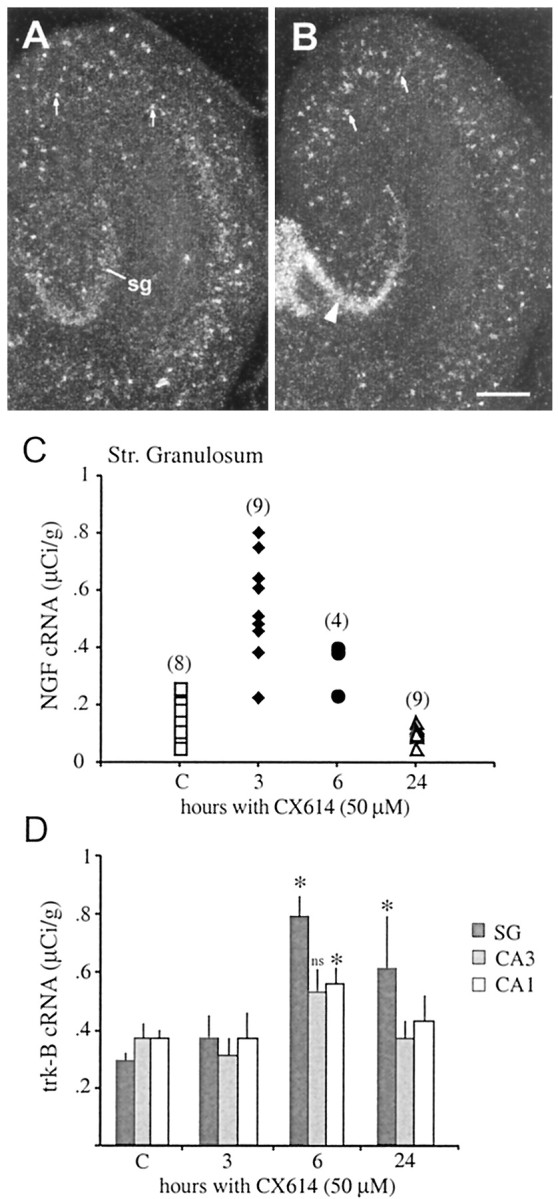
CX614 increases NGF and trkB mRNA contentin vitro. PanelsA, B, Dark-field photomicrographs showing in situ hybridization localization of NGF cRNA labeling in cultured hippocampal slices treated with vehicle (panelA) or with 50 μm CX614 (panelB) for 6 hr. As shown in panelB, CX614 treatment increased NGF cRNA labeling of the stratum granulosum (sg;arrowhead) but not of scattered NGF cRNA-positive interneurons (arrows inpanelsA, B). Scale bar, 300 μm.PanelC, Scatter graph showing the effect of different CX614 treatment intervals on NGF cRNA labeling in the stratum (str.) granulosum. As shown, labeling was significantly increased above control (C) values in slices treated with 50 μm CX614 for 3 and 6 hr but not for 24 hr (p < 0.0001, ANOVA;solidsymbols indicate significant differences between experimental and control group mean values,p < 0.01, SNK; the number of explants per group is given in parentheses). Control values represent combined measures from 3 and 24 hr DMSO-treated explants; there was no difference in mean densities between the two groups.PanelD, Bar graph showing the effect of different CX614 treatment intervals on trkB cRNA labeling in the stratum granulosum (SG), the CA3 stratum pyramidale, and the CA1 stratum pyramidale (group mean values ± SEM;C, values from 24 hr DMSO controls;n = 6 per group). CX614 significantly increased trkB mRNA levels in all fields (p < 0.008, Kruskal–Wallis nonparametric ANOVA). In the stratum granulosum, labeling was elevated at both 6 and 24 hr (p= 0.0011 and 0.03, respectively; Mann–Whitney U test). Labeling was increased in stratum pyramidale cells at 6 hr, but the data only reached significance for region CA1 (p = 0.013, Mann–Whitney Utest). ns, Not significant.
Treatment with CX614 at 50 μm had smaller effects on mRNA levels for trkB than for the two neurotrophins (Fig. 9D). Three hour incubations had no detectable influence on trkB cRNA labeling within the strata granulosum or pyramidale. Treatment for 6 hr yielded modest but significant increases in labeling within the stratum granulosum (172% elevation; p = 0.0001 for comparison with control values) and CA1 stratum pyramidale (51% elevation;p < 0.02) with no reliable change in region CA3. After 24 hr, labeling was still elevated in the stratum granulosum but had returned to control values in the pyramidal cell layer.
Ampakine effects on hippocampal slice electrical activity
Previous studies have demonstrated that the ampakines positively modulate AMPA receptor function without direct agonist activity (Arai et al., 1994). In particular, they enhance monosynaptic potentials, cause much larger increases in polysynaptic responses (Arai et al., 1994, 1996; Sirvio et al., 1996), and facilitate the induction of long-term potentiation (Staubli et al., 1994b). However, the influence of the ampakine CX614 on evoked and spontaneous activity in cultured hippocampal slices has not been well characterized. To address this, extracellular recordings were collected to identify synaptic events associated with the changes in gene expression reported here.
Bouts of synchronized activity and spontaneous field potentials in the absence of stimulation were uncommon in untreated hippocampal explants (Fig. 10A,D). Stimulation (1–3 μA; 0.1 msec) of Schaffer-commissural fibers in the CA1 stratum radiatum evoked synaptic responses with a slightly different time course than that typically obtained in acute hippocampal slices; i.e., responses had onset latencies of ∼4 msec (vs 2 msec in acute slices) and durations of 40–50 msec (vs 15–20 msec) (Fig.10C). Activation of Schaffer-commissural fibers in untreated slices often triggered repetitive synaptic activity (five to eight events at 5–10 Hz), presumably originating from CA3 recurrent circuitry (Granger et al., 1996). As shown in Figure 10C, this repetitive synaptic activity was characterized by a single or complex population spike followed by a series of EPSPs (only the initial 200 msec segment is shown).
Fig. 10.
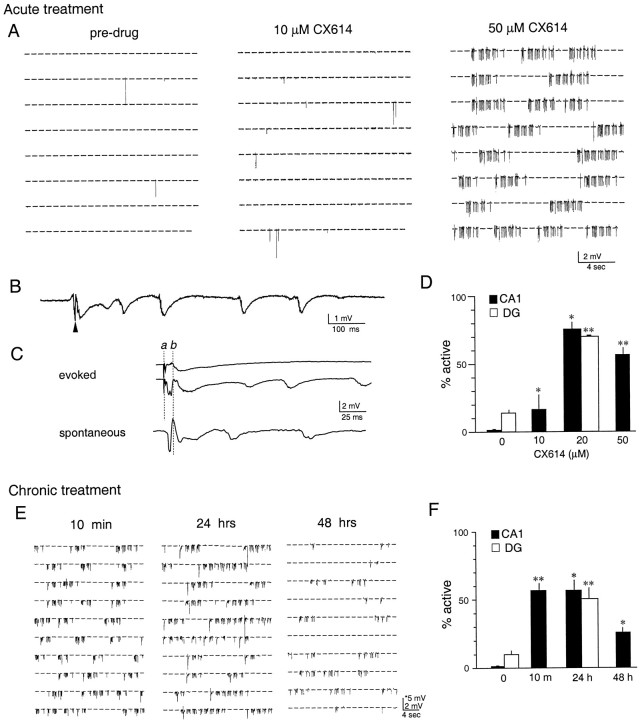
Effects of acute and chronic application of CX614 on electrical activity within hippocampal explants.A–D, Acute treatment. A, Representative field recordings from the CA1 stratum radiatum of a single slice. Each block shows 199 recordings of 1 sec duration collected over a 400 sec period. The three blocks were recorded consecutively during an initial 30 min period of monitoring predrug baseline activity (left), 10 min after adding 10 μm CX614 into the recording chamber (middle), and 10 min after increasing the drug concentration to 50 μm(right). As shown, there was a modest increase in the incidence of spontaneous potentials with the 10 μm dose. At 50 μm there was a striking increase in spontaneous potentials that appeared in clusters of 4–7 sec duration. Prolonged recording in the absence of CX614 did not lead to an increase in activity (data not shown). B, A spontaneous event taken from A (50 μm CX614) shown at a faster sweep speed. The arrowhead indicates the population spike. C, Representative traces from another experiment. Evoked synaptic responses within the CA1 were recorded in the presence of 50 μm CX614 (top trace). The bottom trace shows a spontaneous event recorded from the same explant before drug infusion.Lowercaseletters indicate the time point of the stimulation artifact (a) and the onset of the synaptic potential (b). D, Bar graph showing a summary of the data for recordings from the CA1 (black bars) and the dentate gyrus granule cell layer (DG; white bars) with the measure of activity being the percentage of total trials that exhibited spontaneous synaptic events. Values plotted are percentage means ± SEM from recordings of predrug (n = 5; 5 experiments for CA1; n = 3; 3 experiments for DG), 10 μm-treated (n = 3; 3 experiments), 20 μm-treated (n = 3; 3 experiments for CA1;n = 3; 3 experiments for DG), and 50 μm-treated (n = 9; 3 experiments) hippocampal slices (*p = 0.0357; **p = 0.001 vs predrug group, Mann–WhitneyU test; for CA1, there was no significant difference in spontaneous activity between the 20 μm- and 50 μm-treated slices, p > 0.05, SNK).E, F, Chronic treatment. E,Representative recordings from three cultured slices exposed to 50 μm CX614 for 10 min, 24 hr, and 48 hr. Field recordings were made in the presence of the drug. F, Bar graph showing the summarized activity levels for the CA1 and theDG in slices treated for 24 hr (n = 13; 4 experiments for CA1;n = 7; 3 experiments for DG) and 48 hr (n = 7; 3 experiments) with 50 μmCX614. For the sake of comparison, the data for CA1 baseline activity (indicated as 0) and the 10 min exposure to CX614 (50 μm) from D are included here. For DG, only predrug activity (indicated as 0; n = 10) and spontaneous activity after the 24 hr drug exposure are presented. Values plotted are percentage means ± SEM (*p= 0.0043 vs control; **p = 0.001 vs control for CA1; **p = 0.0001 vs control for DG, Mann–Whitney U test).
Figure 10 illustrates the effects of CX614 on spontaneous synaptic activity in the CA1 stratum radiatum and stratum granulosum in vitro. As shown in Figure 10A, at 10 μm, a concentration near the threshold for modifying field EPSPs in acute slices (Arai and Lynch, unpublished observations), CX614 caused a small but detectable increase in the activity level within CA1 of all slices tested, but the magnitude of this effect was highly variable (1 sec recording epochs with spontaneous synaptic events ranged from 4 to 39%; average, 16 ± 11 vs 1.5 ± 0.5% before drug application;p = 0.0357, Mann–Whitney U test, two-tail;n = 3). At 20 μm, a dose that increased BDNF mRNA in the granule cells but not the CA1, acute application of CX614 resulted in a large and consistent increase in activity within both fields. Spontaneous synaptic events in the granule cells occurred in 70 ± 0.5% of the trials with CX614 as compared with 13.8 ± 2.5% before drug (p < 0.0001, two-tail Student's t test; n = 3). There was a corresponding increase in spontaneous activity within field CA1 (Fig. 10D; average, 75 ± 5.7%;p = 0.0357 vs control, Mann–Whitney U test, two-tail; n = 3). With CX614 applied at 50 μm, a dramatic increase in spontaneous activity was reliably seen in all slices (Fig. 10A,D;n = 9 slices; 3 experiments). The field EPSPs in the CA1 occurred in trains usually lasting for 4–7 sec and at a frequency of 5–10 Hz. Examples of the repetitive potentials are shown at a faster sweep speed in Figure 10, B and C. Notably, the time course of the spontaneous activity was similar to that of evoked responses. Although high- and low-activity phases alternated approximately every 10–20 sec, they did not have the high voltage, sharp spikes, or postevent refractoriness of evoked field EPSPs that are associated with epileptiform discharges.
Recordings were also collected from cultured slices treated continuously for 24 or 48 hr with 50 μm CX614. Activity in CA1 after 24 hr (Fig. 10E) was nearly the same as after 10 min infusions with 57 ± 5.4% of the trials showing spontaneous synaptic events (Fig. 10F;n = 13 slices; 4 experiments). As shown in Figure10F, spontaneous activity also was elevated in the granule cells after 24 hr treatments (average, 50 ± 7.8 vs 9 ± 1.9% before CX614; n = 8; p < 0.0001, Mann–Whitney U test, two-tail). The frequency of spontaneous activity (25.9 ± 3.8%) in CA1 decreased by approximately one-half between 24 and 48 hr (Fig. 10F; p = 0.002, 48 vs 24 hr, U test); recordings were not made for the granule cells at this time point. In all cases, short episodes of repetitive field EPSPs punctuated by complex spikes were frequent and unaccompanied by evident disturbances in the size or shape of monosynaptic responses elicited by single-pulse stimulation of the Schaffer-commissural fibers. Accordingly, the above described reductions in ampakine-induced BDNF expression occurring after 12 hr incubations with 50 μm CX614 cannot be ascribed to drug desensitization or a gradually developing depression of neuronal activity.
Neurocytology
Chronic effects of the ampakine were also evaluated using conventional histological procedures. Sections from slices treated with CX614 (50 μm) for 24 hr were Nissl-stained and evaluated with regard to neuronal morphology and numbers of pyknotic cell bodies. Darkly stained cells were commonplace in the extreme lower portion of the explants (adjacent to the interface with the supporting membrane). These seemingly pyknotic bodies were present in all laminae within deeper levels of both vehicle-control and ampakine-treated slices. However, as shown in Figure 11, they were primarily absent in sections taken from central and superficial levels. Neuronal somata in vehicle-control and drug-treated slices appeared to be of similar size, number, and distribution with little evidence of pyknotic cells in the principal cell layers. These qualitative data suggest that a 24 hr treatment with CX614 at 50 μm does not induce overt neuropathology.
Fig. 11.
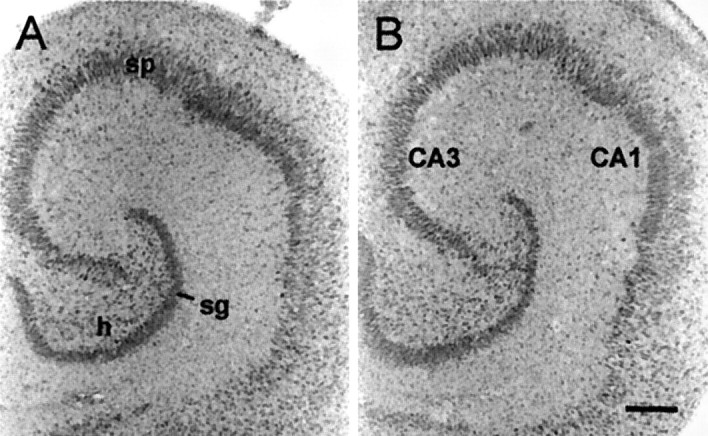
Treatment with CX614 does not induce overt cell death. Light-field photomicrographs showing Nissl-stained tissue sections from a vehicle-treated control culture (A) and a culture treated with 50 μm CX614 (B) for 24 hr. As shown inB, after a 24 hr exposure to CX614 the granule (sg) and pyramidal (sp) cell layers appear normal with neuronal morphology and distribution comparable with that in control tissue. h, Hilus. Scale bar, 300 μm.
In vivo studies
Although BDNF mRNA levels are reduced in the hippocampus with Alzheimer's disease (Phillips et al., 1991; Murray et al., 1994), decreases in BDNF gene expression have not been observed with aging in the rat (Lapchak et al., 1993; Narisawa-Saito and Nawa, 1996; Croll et al., 1998; Sugaya et al., 1998). Nevertheless, protective effects of BDNF in vitro (Alderson et al., 1990; Spina et al., 1992;Kokaia et al., 1994) and in vivo (Ferrer et al., 1998; Hagg, 1998) suggest that increases in BDNF gene expression and/or local availability may have therapeutic value for counteracting degenerative processes associated with age and age-related disease in brain. With an interest in this possibility, effects of AMPA receptor modulators on BDNF mRNA expression in vivo were tested in aged (18- to 21-month-old) rats and middle-aged (9- to 11-month-old) mice using CX546. The ampakine CX546 was chosen for in vivo studies because, unlike CX614, effective in vivo doses have been identified in behavioral studies (Hess et al., 1999). Figure12A illustrates the quantitative results obtained in rats with five daily intraperitoneal injections of CX546 and 24 hr survival after the last injection. As shown, BDNF cRNA labeling was markedly increased in the granule cells with this ampakine treatment regimen. Labeling was 49% greater in the stratum granulosum of ampakine-treated aged rats as compared with controls (p < 0.03; because there were no meaningful differences between CDX and saline controls, their values were combined in the control group). There were no group differences in hybridization densities within the pyramidal cell fields.
Fig. 12.
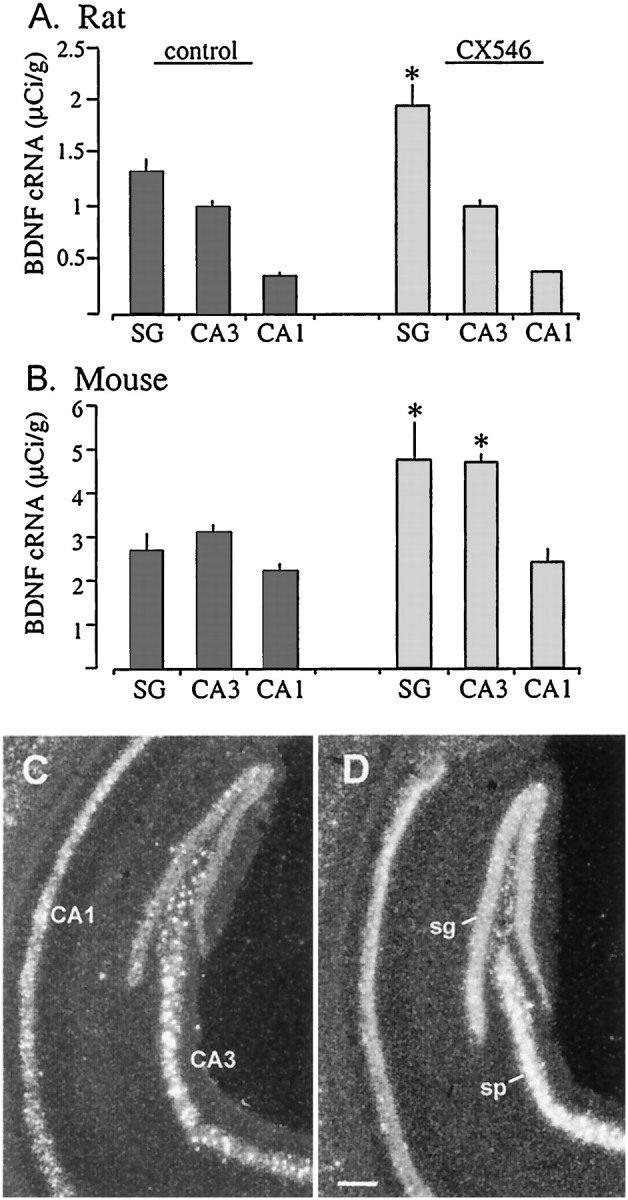
Systemic treatment with the ampakine CX546 increases BDNF mRNA content in rat and mouse hippocampus in vivo. A, B, Bar graphs show the densitometric quantification of BDNF cRNA labeling in hippocampal stratum granulosum (SG), CA3 stratum pyramidale, and CA1 stratum pyramidale of control (darkbars) and CX546-treated (lightbars) aged rats (A) and middle-aged mice (B). A, Data from one naive and three vehicle-control rats were combined for analysis, and aged rats were treated for 5 d with CX546 (30 mg/kg, i.p., once a day;n = 6) and killed 24 hr after the last injection. Error bars represent group mean values (± SD) for theSG and stratum pyramidale of fields CA3 and CA1. As shown, in vivo treatment with CX546 resulted in increased cRNA labeling in the granule cells as compared with control values (p < 0.03, Student'st test). No change was seen in the pyramidal cells.B, The bar graph shows BDNF cRNA hybridization levels in sections from control (naive and cyclodextrin vehicle;n = 6 total) middle-aged mice and middle-aged mice treated with CX546 (40 mg/kg, i.p.; n = 3) and killed 24 hr after injection. Error bars show group means (± SEM). Treatment with CX546 resulted in significantly elevated levels of BDNF cRNA labeling in the stratum granulosum (p = 0.036, Student's t test) and CA3 stratum pyramidale (p = 0.0025, Student's ttest) as compared with controls. As seen for the aged rat, there was no difference in hybridization within CA1 in the middle-aged mice.C, D, Dark-field photomicrographs show examples of BDNF cRNA hybridization in sections through the hippocampus from a control (naive) middle-aged mouse (C) and a middle-aged mouse killed 24 hr after a single injection of CX546 (40 mg/kg, i.p.;D). As shown in D, CX546 resulted in elevated BDNF cRNA labeling within the stratum granulosum (sg) and the CA3 stratum pyramidale (sp) that is restricted to the cell layers with no obvious change in hybridization within CA1. Scale bar, 200 μm.
To determine whether the ampakine effects on BDNF expression can be generalized to other mammalian species, we tested for the influence of CX546 in middle-aged (9- to 11-month-old) mice. As shown in the photomicrographs of Figure 12, C and D, after a single injection of CX546 and 24 hr survival, BDNF cRNA labeling was clearly increased in the granule cells and CA3 pyramidal cells relative to densities in the same regions in yoked vehicle controls. In contrast, labeling in field CA1 was not obviously different between the two animals (Fig. 12C). Quantitative analyses confirmed these impressions (Fig. 12B). Labeling was 76% more dense in the stratum granulosum of ampakine-treated as compared with control mice (p < 0.04). Measures from the stratum pyramidale demonstrated a 51% increase in CA3 (p < 0.003) but no effect in CA1 after ampakine treatment.
DISCUSSION
The present results demonstrate that positive modulation of AMPA receptor function causes rapid and substantial increases in neurotrophin expression in the hippocampus and allied cortex. Like effects of elevated neuronal activity in vivo (Gall, 1992), in ampakine-treated explants BDNF mRNA levels were increased throughout granule and pyramidal cell layers, NGF mRNA was increased within the stratum granulosum alone, and trkB mRNA levels were more modestly affected. Ampakine concentrations that were suprathreshold for enhancing synaptic responses increased BDNF mRNA by 5- to 10-fold within 3 hr, and this effect was reversed with drug washout. Drug-induced activity patterns in the CA1 consisted of 5–10 sec episodes of spontaneous field EPSPs with individual potentials separated by 100–200 msec. The periods of activity were numerous and sometimes accompanied by synchronized cell discharges (population spikes). The relatively brief duration of the episodes, the short intervals between them, and their lack of effect on evoked field EPSPs distinguish these events from classic epileptiform discharges. It is of interest that the frequency of potentials within an episode approximated that found within the theta rhythm (Vanderwolf, 1969;Vertes, 1986). Repetitive, endogenously generated activity with known relationships to complex behaviors thus appears to be sufficient to trigger reasonably rapid and pronounced changes in neurotrophin expression. This AMPA receptor-mediated effect is clearly independent of recently described BDNF induction via AMPA receptor-linked tyrosine kinase signaling that requires high-ligand concentrations and occurs in the absence of transmembrane calcium and sodium currents (Hayashi et al., 1999).
The dose dependency for ampakine effects on expression paralleled that for physiology. Three hour treatments with CX614 at near-threshold concentrations for enhancing monosynaptic responses increased BDNF mRNA levels in the dentate gyrus but were without effect in field CA1. Physiological recordings from the dentate gyrus and CA1 showed that spontaneous synaptic events in both regions were significantly enhanced at near-threshold concentrations. The close correspondence between dose dependencies for induction and synaptic facilitation, as well as the greater responsivity of the dentate gyrus for BDNF expression, was confirmed with a second ampakine. Why neurotrophin expression should be more responsive in the granule cells than in the pyramidal neurons is not apparent. The two classes of cells are distinct with regard to the genes they express and the responsivity of gene expression to peripheral stimuli (Gall et al., 1991a,b; Hess et al., 1995; Lauterborn et al., 1995; Link et al., 1995). Firing rate is also notably different for pyramidal and granule neurons, with pyramidal cells typically firing in triplet bursts and granule cells occasionally firing in extended high-frequency trains (Deadwyler et al., 1975; Rose et al., 1983). Recent studies have demonstrated that temporal features of neuronal firing determine the degree to which activity regulates intracellular-signaling pathways and neuronal gene expression in primary sensory neurons (Fields et al., 1997; Itoh et al., 1997). Similar temporal specificities may account for regional differences in the magnitude of activity-induced changes in gene expression within the hippocampus.
Although threshold ampakine concentrations did not produce sizeable changes in pyramidal cell BDNF expression, they generated impressive effects when applied for 24–72 hr. The fact that time can be substituted for concentration is important with regard to the possible use of ampakines for increasing neurotrophin levels in vivo. The results obtained with prolonged treatment with low dosages also provide clues about which aspects of physiological activity stimulate neurotrophin genes. Threshold concentrations of CX614 increased (1) the frequency of endogenous field EPSPs and complex spikes as well as (2) the likelihood that these events would occur in clusters. Both effects were relatively subtle (drug-induced changes became apparent only after several minutes of recording) and indeed constitute the minimal physiological conditions described to date for upregulating neuronal gene expression. Although the amount of activity over extended periods can reasonably be assumed to influence expression, burst activity might more effectively link excitatory drive with regulatory cascades. Long-term potentiation studies have shown that two short bursts of afferent stimulation trigger postsynaptic enzymes (Vanderklish et al., 1995) when applied in a pattern similar to that initiated by ampakines in the present study. It remains to be tested whether increasing synchronized activity (as opposed to overall activity level) with manipulations other than ampakines will affect hippocampal neurotrophin expression.
Blocking the release of transmitter or its binding to AMPA-type glutamate receptors suppressed the effects of ampakines throughout the hippocampus and retrohippocampal cortex. This is as expected for a positive modulator of AMPA receptors. Increases in the strength of excitatory input presumably affect gene expression by potentiating dendritic depolarization and/or cell spiking. Both processes promote the opening of voltage-sensitive calcium channels (VSCCs) and NMDA receptors, events shown to stimulate BDNF gene expression in embryonic cortical neurons, with VSCCs having the larger effect (Ghosh et al., 1994). BDNF induction by ampakines was unaffected by an NMDA receptor antagonist, leaving the VSCCs as likely mediators. This was confirmed for the entorhinal cortex with the L-type calcium channel blocker nimodipine. The absence of nimodipine effects on induction in the hippocampus indicates regional specificity in mechanisms via which activity regulates BDNF expression. This was suggested previously by the finding that the calcium/calmodulin-dependent protein kinase (CamK) II/IV inhibitor KN-62 attenuates BDNF induction by seizure in the neocortex but not in the dentate gyrus (Murray et al., 1998). Regional differences could arise at levels of calcium influx (e.g., nimodipine-sensitive vs -insensitive VSCCs), intracellular signaling, and/or BDNF promoter use. There are four major BDNF transcript forms; each contains a distinct promoter-associated 5′ exon (I–IV) and a common 3′ exon (V) that encodes BDNF protein (Timmusk et al., 1993). The BDNF cRNA used here recognizes BDNF exon V and, therefore, all transcripts. Within cultured cortical neurons KCl depolarization specifically induces BDNF exon III-containing mRNA via L-type VSCCs and CamK IV/CRE-binding protein-dependent signaling (Ghosh et al., 1994;Shieh et al., 1998; Tao et al., 1998). This specific mechanism, which would be blocked by nimodipine as well as by KN-62, is likely to account for ampakine effects on BDNF mRNA in entorhinal cortex. By contrast, basal and activity-induced expression of transcripts containing exons I and II is reportedly negatively regulated via a neuron-restrictive silencer element in promoter II (Timmusk et al., 1999). Major goals of future research will be to determine the regional transcript profiles and signaling pathways that differentiate ampakine-induced BDNF expression in the hippocampus and cortex.
Although BDNF mRNA levels initially increased over the time of ampakine exposure, suprathreshold doses set in motion an effect that gradually offset drug-induced upregulation. The ampakines did not lose potency when incubated with cultured slices, as demonstrated with media transfer experiments, and continued to exert electrophysiological effects, whereas BDNF mRNA content returned toward control levels. Recent in vivo studies indicate that NGF becomes refractory to induction after transient upregulation (C. M. Gall and J. C. Lauterborn, unpublished observations); reduced BDNF mRNA levels with continued ampakine exposure may represent a similar effect. BDNF protein levels did not decrease when drug incubations were extended beyond 12 hr. This raises the possibility that reduced gene expression with longer treatments may be caused by end-product inhibition; i.e., elevated levels of neurotrophin protein (or another gene product upregulated by ampakine exposure) may depress gene expression and/or block activating cascades. Refractoriness to chronic ampakine exposure is likely to be a general phenomenon because induced NGF expression reversed even more rapidly than did induced BDNF expression. In this case, the fall off occurs in a sufficiently brief period that protein synthesis inhibitors might be used to test the idea of end-product inhibition. Whatever its mechanism, refractoriness will be a consideration when devising pharmacological regimens for manipulating neurotrophin expression and signaling.
The possibility of using ampakines to increase neurotrophin expression in aged and middle-aged animals was tested using peripheral injections of CX546 and a 24 hr delay. Doses were selected that generate a characteristic ampakine effect on behavior, namely, suppression of methamphetamine-induced hyperactivity (Hess et al., 1999). Five daily injections in aged rats resulted in a marked increase in BDNF mRNA levels in the dentate gyrus granule cells. Similarly, a single treatment in middle-aged mice increased BDNF mRNA content in the dentate gyrus and field CA3. For both species there was no change in BDNF expression within field CA1. Because CA1 also was less responsive in slices, these results suggest that the in vivo results involve the same processes initiated by ampakines in vitro. Optimal treatment regimens and effects on BDNF protein are not known, but the present results strongly suggest that systemic treatment with doses that do not perturb behavior can be used to upregulate at least one aspect of neurotrophism in the middle-aged and aged brain. Important goals for future research will be to determine whether systemic ampakine treatment can sustain elevated brain neurotrophin levels and to test the general idea that increasing endogenous neurotrophin expression can be used to offset impairments in brain functioning that arise with age.
Footnotes
This work was supported by Cortex Pharmaceuticals Grant CP22357 and the National Institute of Neurological Disorders and Stroke Grant NS26748 to C.M.G. We wish to thank Yilu Xie and Fiesal Yamani for valuable technical assistance and Dr. David McKinnon (State University of New York, Stony Brook, NY) for generously providing the cDNA for trkB.
Correspondence should be addressed to Dr. Julie C. Lauterborn, Gillespie Neuroscience Research Facility, Room 3226, University of California, Irvine, CA 92697-4292. E-mail address: jclauter@uci.edu.
REFERENCES
- 1.Alderson RF, Alterman AL, Barde Y-A, Lindsay RM. Brain-derived neurotrophic factor increases survival and differentiated functions of rat septal cholinergic neurons in culture. Neuron. 1990;5:297–306. doi: 10.1016/0896-6273(90)90166-d. [DOI] [PubMed] [Google Scholar]
- 2.Arai A, Kessler M, Xiao P, Ambros-Ingerson J, Rogers G, Lynch G. A centrally active drug that modulates AMPA receptor gated currents. Brain Res. 1994;638:343–346. doi: 10.1016/0006-8993(94)90669-6. [DOI] [PubMed] [Google Scholar]
- 3.Arai A, Kessler M, Roger G, Lynch G. Effects of a memory-enhancing drug on dl-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionin acid receptor currents and synaptic transmission in hippocampus. J Pharmacol Exp Ther. 1996;278:627–638. [PubMed] [Google Scholar]
- 4.Arai A, Ly J, Kessler M, Hennegriff M, Rogers G, Lynch G. Effects of the AMPA receptor modulator CX614 on synaptic responses and AMPA receptor kinetics. Soc Neurosci Abstr. 1997;23:213. [Google Scholar]
- 5.Bengzon J, Kokaia Z, Ernfors P, Kokaia M, Leanza G, Nilsson OG, Persson H, Lindvall O. Regulation of neurotrophin and trkA, trkB and trkC tyrosine kinase receptor messenger RNA expression in kindling. Neuroscience. 1993;53:433–446. doi: 10.1016/0306-4522(93)90207-v. [DOI] [PubMed] [Google Scholar]
- 6.Castrén E, Zafra F, Thoenen H, Lindholm D. Light regulates expression of brain-derived neurotrophic factor mRNA in rat visual cortex. Proc Natl Acad Sci USA. 1992;89:9444–9448. doi: 10.1073/pnas.89.20.9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castrén E, PitkΣnen M, Sirvi÷ J, Parsadanian A, Lindholm D, Thoenen H, Riekkinen PJ. The induction of LTP increases BDNF and NGF mRNA but decreases NT-3 mRNA in the dentate gyrus. NeuroReport. 1993;4:895–898. doi: 10.1097/00001756-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Croll SD, Ip NY, Lindsay RM, Wiegand SJ. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Res. 1998;812:200–208. doi: 10.1016/s0006-8993(98)00993-7. [DOI] [PubMed] [Google Scholar]
- 9.Deadwyler S, West J, Cotman C, Lynch G. Physiological studies of the reciprocal connection between the hippocampus and entorhinal cortex. Exp Neurol. 1975;49:35–37. doi: 10.1016/0014-4886(75)90194-6. [DOI] [PubMed] [Google Scholar]
- 10.Dixon J, McKinnon D. Expression of the trk gene family of neurotrophin receptors in prevertebral sympathetic ganglia. Dev Brain Res. 1994;77:177–182. doi: 10.1016/0165-3806(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 11.Ferrer I, Ballabriga J, Marti E, Perez E, Alberch J, Arenas E. BDNF up-regulates TrkB protein and prevents the death of CA1 neurons following transient forebrain ischemia. Brain Pathol. 1998;8:253–261. doi: 10.1111/j.1750-3639.1998.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields RD, Eshete F, Stevens B, Itoh K. Action potential-dependent regulation of gene expression: temporal specificity in Ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. J Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gall C. Regulation of brain neurotrophin expression by physiological activity. Trends Pharmacol Sci. 1992;13:401–403. doi: 10.1016/0165-6147(92)90123-n. [DOI] [PubMed] [Google Scholar]
- 14.Gall C, Isackson PJ. Limbic seizures increase neuronal production of mRNA for nerve growth factor. Science. 1989;245:758–760. doi: 10.1126/science.2549634. [DOI] [PubMed] [Google Scholar]
- 15.Gall C, Lauterborn J, Bundman M, Murray K, Isackson PJ. Seizures and the regulation of growth factor and neuropeptide gene expression in brain. In: Anderson VE, Hauser WA, Leppik TE, Noebels JL, Rich SS, editors. Epilepsy research, Suppl 4. Elsevier; Amsterdam: 1991a. pp. 219–239. [PubMed] [Google Scholar]
- 16.Gall C, Murray K, Isackson PJ. Kainic acid-induced seizures stimulate increased expression of nerve growth factor in adult rat hippocampus. Mol Brain Res. 1991b;9:113–123. doi: 10.1016/0169-328x(91)90136-l. [DOI] [PubMed] [Google Scholar]
- 17.Gall CM, Hess US, Lynch G. Mapping brain networks engaged by—and changed by—learning. Neurobiol Learn Mem. 1998;70:14–36. doi: 10.1006/nlme.1998.3835. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 19.Granger R, Wiebe SP, Taketani M, Lynch G. Distinct memory circuits composing the hippocampal region. Hippocampus. 1996;6:567–578. doi: 10.1002/(SICI)1098-1063(1996)6:6<567::AID-HIPO2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Hagg T. Neurotrophins prevent death and differentially affect tyrosine hydroxylase of adult rat nigrostriatal neurons in vivo. Exp Neurol. 1998;149:183–192. doi: 10.1006/exnr.1997.6684. [DOI] [PubMed] [Google Scholar]
- 21.Hall R, Kessler M, Lynch G. Kainate binding to the AMPA receptor in rat brain. Neurochem Res. 1994;19:777–782. doi: 10.1007/BF00967719. [DOI] [PubMed] [Google Scholar]
- 22.Hampson R, Rogers G, Lynch G, Deadwyler S. Facilitative effects of the ampakine CX516 on short-term memory in rats: enhancement of delayed-nonmatch-to-sample performance. J Neurosci. 1998a;18:2740–2747. doi: 10.1523/JNEUROSCI.18-07-02740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampson R, Rogers G, Lynch G, Deadwyler S. Facilitative effects of the ampakine CX516 on short-term memory in rats: correlations with hippocampal neuronal activity. J Neurosci. 1998b;18:2748–2763. doi: 10.1523/JNEUROSCI.18-07-02748.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi T, Umemori H, Mishina M, Yamamoto T. The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature. 1999;397:72–76. doi: 10.1038/16269. [DOI] [PubMed] [Google Scholar]
- 25.Hennegriff M, Arai A, Kessler M, Vanderklish P, Mutneja MS, Rogers G, Neve RL, Lynch G. Stable expression of recombinant AMPA receptor subunits: binding affinities and effects of allosteric modulators. J Neurchem. 1997;68:2424–2434. doi: 10.1046/j.1471-4159.1997.68062424.x. [DOI] [PubMed] [Google Scholar]
- 26.Hess U, Whalen S, Sandoval L, Lynch G, Gall C. Ampakine suppression of dopamine-mediated rotation: implication for treatment of schizophrenia. Soc Neurosci Abstr. 1999;25:2047. [Google Scholar]
- 27.Hess US, Lynch G, Gall CM. Regional patterns of c-fos mRNA expression in rat hippocampus following exploration of a novel environment versus performance of a well-learned discrimination. J Neurosci. 1995;15:7796–7809. doi: 10.1523/JNEUROSCI.15-12-07796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holst BD, Vanderklish PW, Krushel LA, Zhou W, Langdon RB, McWhirter JR, Edelman GM, Crossin KL. Allosteric modulation of AMPA-type glutamate receptors increases activity of the promoter for the neural cell adhesion molecule, N-CAM. Proc Natl Acad Sci USA. 1998;95:2597–2602. doi: 10.1073/pnas.95.5.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ingvar M, Ambros-Ingerson J, Davis M, Granger R, Kessler M, Rogers G, Schehr R, Lynch G. Enhancement by an ampakine of memory encoding in humans. Exp Neurol. 1997;146:553–559. doi: 10.1006/exnr.1997.6581. [DOI] [PubMed] [Google Scholar]
- 30.Isackson PJ, Murray K, Huntsman M, Gall CM. BDNF mRNA expression is increased in adult rat forebrain after limbic seizures: temporal patterns of induction distinct from NGF. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- 31.Itoh K, Ozaki M, Stevens B, Fields RD. Activity-dependent regulation of N-cadherin in DRG neurons: differential regulation of N-cadherin, NCAM, and L1 by distinct patterns of action potentials. J Neurobiol. 1997;33:735–748. doi: 10.1002/(sici)1097-4695(19971120)33:6<735::aid-neu3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 32.Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- 33.Kessler M, Mutneja MS, Rogers G, Lynch G. Regional preferences of AMPA receptor modulators determined through agonist binding autoradiography. Brain Res. 1998;783:121–126. doi: 10.1016/s0006-8993(97)01315-2. [DOI] [PubMed] [Google Scholar]
- 34.Kokaia Z, Othberg A, Kokaia M, Lindvall O. BDNF makes cultured dentate granule cells more resistant to hypoglycemic damage. NeuroReport. 1994;5:1241–1244. doi: 10.1097/00001756-199406020-00021. [DOI] [PubMed] [Google Scholar]
- 35.Lapchak PA, Araujo DM, Beck KD, Finch CE, Johnson SA, Hefti F. BDNF and trkB mRNA expression in the hippocampal formation of aging rats. Neurobiol Aging. 1993;14:121–126. doi: 10.1016/0197-4580(93)90087-r. [DOI] [PubMed] [Google Scholar]
- 36.Lauterborn JC, Tran T, Isackson P, Gall CM. NGF mRNA is expressed by GABAergic neurons in rat hippocampus. NeuroReport. 1993;5:273–276. doi: 10.1097/00001756-199312000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Lauterborn JC, Isackson PJ, Gall CM. Cellular localization of NGF and NT-3 mRNAs in postnatal rat forebrain. Mol Cell Neurosci. 1994;5:46–62. doi: 10.1006/mcne.1994.1005. [DOI] [PubMed] [Google Scholar]
- 38.Lauterborn JC, Berschauer R, Gall CM. Cell-specific modulation of basal and seizure-induced neurotrophin expression by adrenalectomy. Neuroscience. 1995;68:363–378. doi: 10.1016/0306-4522(95)00150-h. [DOI] [PubMed] [Google Scholar]
- 39.Lerma J, Paternain AV, Naranjo JR, Mellstrom B. Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci USA. 1993;90:11688–11692. doi: 10.1073/pnas.90.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindvall O, Kokaia Z, Bengzon J, Elmθr E, Kokaia M. Neurotrophins and brain insults. Trends Neurosci. 1994;17:490–496. doi: 10.1016/0166-2236(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 41.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mattson M, Scheff S. Endogenous neuroprotection factors and traumatic brain injury: mechanisms of action and implications for therapy. J Neurotrauma. 1994;11:3–33. doi: 10.1089/neu.1994.11.3. [DOI] [PubMed] [Google Scholar]
- 43.Merlio J-P, Ernfors P, Kokaia Z, Middlemas DS, Bengzon J, Kokaia M, Smith M-J, SiesjÜ BK, Hunter T, Lindvall O, Persson H. Increased production of the TrkB protein tyrosine kinase receptor after brain insults. Neuron. 1993;10:151–164. doi: 10.1016/0896-6273(93)90307-d. [DOI] [PubMed] [Google Scholar]
- 44.Motulsky H. Intuitive biostatistics. Oxford UP; New York: 1995. [Google Scholar]
- 45.Murray KD, Gall CM, Jones EG, Isackson PJ. Differential regulation of brain-derived neurotrophic factor and type II calcium/calmodulin-dependent protein kinase messenger RNA expression in Alzheimer's disease. Neuroscience. 1994;60:37–48. doi: 10.1016/0306-4522(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 46.Murray KD, Hayes VY, Gall CM, Isackson PJ. Attenuation of the seizure-induced expression of BDNF mRNA in adult rat brain by an inhibitor of calcium/calmodulin-dependent protein kinases. Eur J Neurosci. 1998;10:377–387. doi: 10.1046/j.1460-9568.1998.00019.x. [DOI] [PubMed] [Google Scholar]
- 47.Narisawa-Saito M, Nawa H. Differential regulation of hippocampal neurotrophins during aging in rats. J Neurochem. 1996;67:1124–1131. doi: 10.1046/j.1471-4159.1996.67031124.x. [DOI] [PubMed] [Google Scholar]
- 48.Nawa H, Carnahan J, Gall C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur J Neurosci. 1995;7:1527–1535. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 49.Neeper S, G≤mez-Pinilla F, Choi J, Cotman C. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res. 1996;726:49–56. [PubMed] [Google Scholar]
- 50.Olsen RW, Szamraj O, Houser CR. [3H]AMPA binding to glutamate receptor subpopulations in rat brain. Brain Res. 1987;402:243–254. doi: 10.1016/0006-8993(87)90030-8. [DOI] [PubMed] [Google Scholar]
- 51.Patneau DK, Mayer ML. Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron. 1991;6:785–798. doi: 10.1016/0896-6273(91)90175-y. [DOI] [PubMed] [Google Scholar]
- 52.Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M. Neurotrophin expression in rat hippocampal slices—a stimulus paradigm inducing LTP in CA1 evokes increases in BDNF and NT-3 messenger RNAs. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 53.Phillips HS, Heins JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- 54.Rivera S, Vanderklish PW, Lynch G, Gall CM. Glutamate receptor activation increases brain-derived neurotrophic factor mRNA in organotypic hippocampal cultures. Soc Neurosci Abstr. 1993;19:258. [Google Scholar]
- 55.Rocamora N, Welker E, Pascual M, Soriano E. Upregulation of BDNF mRNA expression in the barrel cortex of adult mice after sensory stimulation. J Neurosci. 1996;16:4411–4419. doi: 10.1523/JNEUROSCI.16-14-04411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rogan MT, Staubli UV, LeDoux JE. AMPA receptor facilitation accelerates fear learning without altering the level of conditioned fear acquired. J Neurosci. 1997;17:5928–5935. doi: 10.1523/JNEUROSCI.17-15-05928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- 58.Rose G, Diamond D, Lynch GS. Dentate granule cells in the rat hippocampal formation have the behavioral characteristics of theta neurons. Brain Res. 1983;266:29–37. doi: 10.1016/0006-8993(83)91306-9. [DOI] [PubMed] [Google Scholar]
- 59.Shieh PB, Hu S-C, Bobb K, Timmusk T, Ghosh A. Identification of a signaling pathway involved in calcium regulation of BDNF expression. Neuron. 1998;20:727–740. doi: 10.1016/s0896-6273(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 60.Simon JR, Contrera JF, Kuhar MJ. Binding of [3H]kainic acid, an analogue of l-glutamate, to brain membranes. J Neurochem. 1976;26:141–147. [PubMed] [Google Scholar]
- 61.Sirvio J, Larson J, Quach CN, Rogers GA, Lynch G. Effects of pharmacologically facilitating glutamatergic transmission in the trisynaptic intrahippocampal circuit. Neuroscience. 1996;74:1025–1035. doi: 10.1016/0306-4522(96)00170-4. [DOI] [PubMed] [Google Scholar]
- 62.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spina MB, Squinto SP, Miller J, Lindsay R. Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity—involvement of the glutathione system. J Neurochem. 1992;59:99–106. doi: 10.1111/j.1471-4159.1992.tb08880.x. [DOI] [PubMed] [Google Scholar]
- 64.Staubli U, Rogers G, Lynch G. Facilitation of glutamate receptors enhances memory. Proc Natl Acad Sci USA. 1994a;91:777–781. doi: 10.1073/pnas.91.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Staubli U, Perez Y, Xu F-B, Ingvar M, Stone-Erlender S, Lynch G. Centrally active modulators of glutamate (AMPA) receptors facilitate the induction of LTP in vivo. Proc Natl Acad Sci USA. 1994b;91:11158–11162. doi: 10.1073/pnas.91.23.11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stoppini S, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 67.Sugaya K, Greene R, Personett D, Robbins M, Kent C, Bryan D, Skiba E, Gallagher M, McKinney M. Septo-hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiol Aging. 1998;19:351–361. doi: 10.1016/s0197-4580(98)00072-4. [DOI] [PubMed] [Google Scholar]
- 68.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron. 1998;20:709–726. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 69.Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, Persson H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10:475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- 70.Timmusk T, Palm K, Lendahl U, Metsis M. Brain-derived neurotrophic factor expression in vivo is under the control of neuron-restrictive silencer element. J Biol Chem. 1999;274:1078–1084. [PubMed] [Google Scholar]
- 71.Torasdotter M, Metsis M, Henriksson B, Winblad B, Mohammed A. Expression of neurotrophin-3 mRNA in the rat visual cortex and hippocampus is influenced by environmental conditions. Neurosci Lett. 1996;218:107–110. doi: 10.1016/s0304-3940(96)13127-x. [DOI] [PubMed] [Google Scholar]
- 72.Vanderklish P, Saido T, Gall C, Arai A, Lynch G. Proteolysis of spectrin by calpain accompanies theta-burst stimulation in cultured hippocampal slices. Mol Brain Res. 1995;32:25–35. doi: 10.1016/0169-328x(95)00057-y. [DOI] [PubMed] [Google Scholar]
- 73.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 74.Vertes RP. Brainstem modulation of the hippocampus: anatomy, physiology, and significance. In: Isaacson RL, Pribram KH, editors. The hippocampus. Plenum; New York: 1986. pp. 41–75. [Google Scholar]
- 75.Wetmore C, Olson L, Bean A. Regulation of brain-derived neurotrophic factor (BDNF) expression and release from hippocampal neurons is mediated by non-NMDA type glutamate receptors. J Neurosci. 1994;14:1688–1700. doi: 10.1523/JNEUROSCI.14-03-01688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Whittemore SR, Friedman PL, Larhammar D, Persson H, Gonzales-Carvajal M, Holets VR. Rat §-nerve growth factor sequence and site of synthesis in the adult hippocampus. J Neurosci Res. 1988;20:403–410. doi: 10.1002/jnr.490200402. [DOI] [PubMed] [Google Scholar]
- 77.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]