Abstract
Several signaling proteins clustered at the postsynaptic density specialization in neurons harbor a conserved C-terminal PDZ domain recognition sequence (X-S/T-X-V/I) that mediates binding to members of the PSD-95/SAP90 protein family. This motif is also present in the C termini of some inwardly rectifying K+ (Kir) channels. Constitutively active Kir2 channels as well as G protein-gated Kir3 channels, which are fundamental for neuronal excitability, were analyzed as candidates for binding to PSD-95/SAP90 family members. Therefore C termini of Kir2.1(+), Kir2.3(+), Kir2.4(−), Kir3.1(−), Kir3.2(+), Kir3.3(+) and Kir3.4(−) subunits (+, motif present; −, motif absent) were used as baits in the yeast two-hybrid assay to screen for in vivo interaction with PDZ domains 1–3 of PSD-95/SAP90. In contrast to Kir2.1 and Kir2.3, all Kir3 fragments failed to bind PSD-95 in this assay, which was supported by the lack of coimmunoprecipitation and colocalization of the entire proteins in mammalian cells. A detailed analysis of interaction domains demonstrated that the C-terminal motif in Kir3 channels is insufficient for binding PDZ domains. Kir2.1 and Kir2.3 subunits on the other hand coprecipitate with PSD-95. When coexpressed in a bicistronic internal ribosome entry site expression vector in HEK-293 cells macroscopic and elementary current analysis revealed that PSD-95 suppressed the activity of Kir2.3 channels by >50%. This inhibitory action of PSD-95, which predominantly affects the single-channel conductance, is likely attributable to a molecular association with additional internal interaction sites in the Kir2.3 protein.
Keywords: inwardly rectifying, Kir channel, GIRK, postsynaptic density, chapsyns, MAGUK, yeast two-hybrid
Ion channels, receptors, signaling enzymes, and adhesion molecules in neurons are typically localized at specific subcellular specializations such as axon terminals, presynaptic and postsynaptic sites, or nodes of Ranvier. A series of anchor and adapter elements have now been identified to coordinate the targeted distribution of these proteins on the cell surface for proper signaling within and between neurons (Sheng, 1996; Sheng and Kim, 1996; Kornau et al., 1997; Craven and Bredt, 1998). Well known examples constitute rapsyn, which clusters nicotinic acetylcholine receptors at the neuromuscular junction (Colledge and Froehner, 1998), the 93 kDa protein gephyrin, which aggregates glycine and GABAA receptors in the postsynaptic membrane of spinal cord neurons (Kirsch et al., 1993), and syntaxin/solubleN-ethylmaleimide-sensitive factor attachment protein 25, which complexes presynaptic N-type Ca2+ channels and possibly other components of the exocytotic machinery (Sheng et al., 1994). Protein targeting to the postsynaptic density (PSD; Kennedy, 1993), the electron-dense cytoskeletal specialization of excitatory synapses in CNS neurons, is typically associated with members of the PSD-95/SAP90 (synapse-associated proteins) protein family. They are alternatively referred to as chapsyns (channel-associated proteins of the synapse) or MAGUK proteins (membrane-associated guanylate kinases; Ehlers et al., 1996; Sheng and Kim, 1996). In mammals this family includes four members, PSD-95/SAP-90, PSD-93/chapsyn110, SAP102, and SAP97/hdlg. In their N termini these proteins are characterized by the presence of three, 90 amino acid long PDZ domains, initially recognized as repeats in PSD-95, discs large protein and the tight junction protein ZO-1. In the C terminus they contain additional modules for interaction with cellular proteins, such as thesrc homology 3 domain and an enzymatically inactive guanylate kinase-like domain. Together with other proteins PSD-95/SAP90 proteins have now been shown to organize a complex PSD signaling matrix that may contain kainate and NMDA receptors (Kornau et al., 1995;Niethammer et al., 1996; Garcia et al., 1998), neuronal nitric oxide synthase (nNOS; Brenman et al., 1996), voltage-gated K+(Kv; Kim et al., 1995), and inwardly rectifying K+ (Kir) channels (Cohen et al., 1996;Horio et al., 1997), fasciclin II (Tejedor et al., 1997; Thomas et al., 1997; Zito et al., 1997), calmodulin (Masuko et al., 1999), Ca2+ ATPase 4β (Kim et al., 1998a); regulator of G protein signaling 12 (Snow et al., 1998), synaptic rasGAP (Kim et al., 1998b), guanylate-kinase-associated protein (Kim et al., 1997; Takeuchi et al., 1997), the putative cell adhesion molecule neuroligin (Irie et al., 1997), and cysteine-rich interactor of PDZ three (CRIPT; Niethammer et al., 1998).
It was originally demonstrated that the C-terminal tetrapeptide of both Kv1.4 channels and NMDA 2B subunits (NR2B) interact with PDZ domains I and II of PSD-95. Sequence comparison of the proteins listed above and supported by random peptide library screening (Songyang et al., 1997), as well as x-ray crystallography (Doyle et al., 1996) suggested the sequence X-S/T-X-I/V as the conserved recognition motif for PDZ domains in PSD-95/SAP90 proteins. In the mammalian CNS, the abundantly expressed Kir2 and Kir3 channels are synaptically regulated by protein phosphorylation and direct G protein activation, respectively, and thus play a role in the fine tuning of neuronal excitability after synaptic input (Doupnik et al., 1995; Isomoto et al., 1997). Because Kir2.1, Kir2.2, Kir2.3, Kir3.2, and Kir3.3 subunits harbor the PDZ binding consensus in their C termini, whereas others (Kir2.4, Kir3.1, and Kir3.4) do not, we investigated their possible differential association with PSD-95.
MATERIALS AND METHODS
Plasmids and clones. cDNAs encoding the rat Kv1.4 (Stühmer et al., 1989), rat Kir2.1 (Wischmeyer et al., 1995), rat Kir2.3 (Falk et al., 1995), rat Kir3.1 (Wischmeyer et al., 1997), human Kir3.2 (Wischmeyer et al., 1997), rat Kir3.3 (Dissmann et al., 1996), human Kir3.4 (Spauschus et al., 1996), and rat PSD-95 (Cho et al., 1992) were subcloned into the mammalian expression vector pcDNA3 (Invitrogen, Leek, The Netherlands). For biochemical and functional analysis cDNAs of Kir subunits were alternatively cloned together with PSD-95 into a modified pcDNA3-internal ribosome entry site (IRES) vector, which contained a single promoter and an additional IRES of the poliovirus to allow translation of two open reading frames from one mRNA (LaMonica et al., 1986). For immunodetection PSD-95, Kir2.3, Kv1.4, and Kir2.1 were epitope-tagged at the N terminus with the c-myc sequence MEQKLISEEDLN. The assembly of concatemeric Kir3.1/3.2 constructs has been described elsewhere (Wischmeyer et al., 1997).
Yeast two-hybrid assay. The entire C-terminal domains of Kir2.1 [amino acids (aa) 179–427], Kir2.3 (aa 171–446), Kir2.4 (aa 195–435; Töpert et al., 1998), Kir3.1 (aa 180–501), Kir3.2 (aa 191–425), Kir3.3 (aa 157–393), and Kir3.4 (aa 186–419) were amplified by PCR and subcloned into the yeast DNA-binding domain vector pGBT9 (Clontech, Palo Alto, CA). As positive controls the C-terminal domains of the voltage-gated rat Kv1.4 channel (aa 568–655;Stühmer et al., 1989) and the rat NR2B (aa 1371–1482; Monyer et al., 1992) were also cloned into pGBT9. PDZ domains 1–3 of PSD-95/SAP90 (aa 1–401) were inserted into the activation domain vector pGAD-GH (Clontech). Deletion constructs of Kir2.1, Kir2.3, and Kir3.2 were generated by PCR with appropriate oligonucleotides, and a Kir2.1/Kir3.2 chimera was constructed as described (Higuchi et al., 1988). Yeast strain HF7c was cotransformed with 100 ng each of bait and prey vector and was streaked out on agar plates lacking tryptophan, leucine, and histidine. Colony growth showing activation of the nutritional reporter gene HIS3 was controlled for the individual constructs after 4 d.
Coimmunoprecipitation. COS-7 cells were transiently transfected with Kir channel subunits and PSD-95 individually in pcDNA3 and together in pcDNA3-IRES, respectively, by calcium phosphate precipitation. In brief, transfected cells were washed with PBS 60 hr after transfection, harvested, and homogenized in ice-cold lysis buffer [50 mm Tris, pH 7.4, 150 mm NaCl, 5 mm EGTA, protease inhibitors (1 μg/ml leupeptin and pepstatin A, 2 μg/ml aprotenin, and 1 mm PMSF), and 1% Triton X-100]. Nonsoluble components were removed by centrifugation at 4°C, and extracts were preincubated with 50 μl of protein A- or protein G-agarose beads (Roche Diagnostics, Mannheim, Germany) to avoid unspecific binding. For immunoprecipitation preadsorbed extracts were incubated for 1 hr with mouse anti-myc (Santa Cruz Biotechnology, Santa Cruz, CA) or rabbit anti-myc (Upstate Biotechnologies, Lake Placid, NY) to precipitate tagged proteins. After overnight incubation on a rotating disk, bound proteins were pulled down with either protein A- or protein G-agarose beads and washed four times for 20 min each with lysis buffer. Pull-down experiments in the absence of specific antibodies were performed to verify the specificity of the immunoprecipitations (data not shown). Cell extracts and precipitated proteins were analyzed by Western immunoblots probed with monoclonal mouse α-PSD-95 antibodies (1:1000, clone K28/43; Upstate Biotechnology), polyclonal rabbit αKir3.2 and αKir2.1 antibodies (1:100 and 1:1000, respectively), and α-myc antibody (mouse, 1:100; rabbit, 1:1000). Blots were developed using horseradish peroxidase-conjugated goat α-rabbit and goat α-mouse Igs (Jackson ImmunoResearch Laboratories, West Grove, PA), respectively, and an enhanced chemoluminescence detection system (ECL; Amersham, Buckinghamshire, UK).
Immunocytochemistry. COS-7 cells were plated on poly-l-lysine-coated coverslips 1 d before transfection. Plasmids were transfected using LipofectAMINE (Life Technologies, Gaithersburg, MD). After 2 d cells were fixed with 2% paraformaldehyde and 0.1% Triton X-100 and blocked with 2% normal goat serum. For double staining cells were incubated overnight with the primary rabbit α-myc (1:1000) or α-Kir3.2 antisera (1:100), together with the monoclonal mouse α-PSD-95 antibody (1:1000). After washing with PBS cells were incubated 1 hr with Cy3-conjugated goat secondary IgG (1:1000; Jackson ImmunoResearch) and FITC-conjugated goat secondary IgG (1:200, Jackson ImmunoResearch). Coverslips were washed again with PBS, mounted on slides, and analyzed on an LSM410 laser scanning microscope equipped with an argon-crypton laser (Zeiss, Oberkochen, Germany).
Electrophysiology. Semiconfluent HEK-293 cells, grown on glass coverslips, were transfected with 0.8 μg/ml cDNAs using LipofectAMINE and Opti-MEM I (Life Technologies) following the manufacturer's protocol. Whole-cell recordings were performed at room temperature 48–72 hr after transfection in a bath solution consisting of 135 mm NaCl, 5.4 mm KCl, 1.8 mmCaCl2, 1 mmMgCl2, 10 mm glucose, and 5 mm HEPES, pH 7.4. Patch pipettes were pulled from borosilicate glass capillaries (Kimble Products, Sussex, UK), and heat-polished to give input resistances of 4–6 mΩ. The pipette recording solution contained 140 mm KCl, 2 mm MgCl2, 1 mm EGTA, 1 mm Na2ATP, 100 μm cAMP, 100 μm GTP, and 5 mm HEPES, pH 7.3. Currents were recorded with an EPC9 (Heka Electronics, Lamprecht, Germany) patch-clamp amplifier and low-pass-filtered at 1–2 kHz. Stimulation and data acquisition were controlled by the Pulse/Pulsefit software package (Heka) on a Macintosh computer (Apple, Cupertino, CA), and data analysis was performed with Igor software (WaveMetrics, Lake Oswego, OR). Data are presented as mean ± SD (number of cells).
RESULTS
Yeast two-hybrid assay
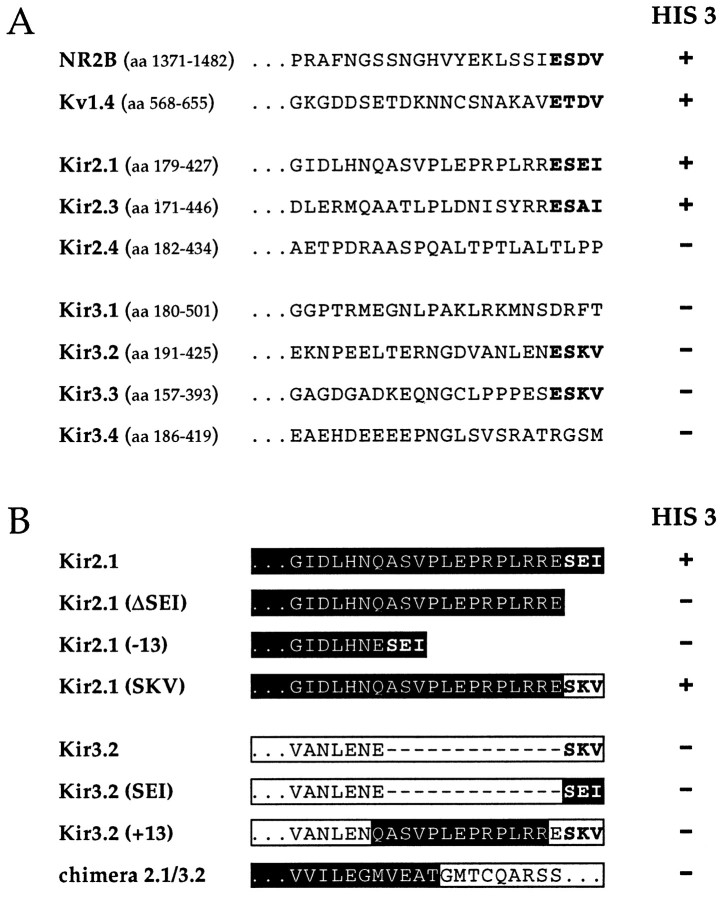
The presence of the C-terminal PDZ domain recognition motif X-S/T-X-V/I prompted us to investigate on the association of neuronal Kir2 and Kir3 channel members with proteins of the PSD-95/SAP90 family. In a first assay the complete C termini of Kir2.1, Kir2.3, Kir3.2, Kir3.3 (harboring the motif), as well as Kir2.4, Kir3.1, and Kir3.4 (in which the motif is absent) were used as bait in the yeast two-hybrid (Y2H) system (Fields and Song, 1989) for interaction with PDZ domains 1–3 (aa 1–401) of PSD-95/SAP90 (Fig.1A). The C termini of voltage-dependent Kv1.4 channels (Kim et al., 1995) as well as NR2B subunits (Kornau et al., 1995), for which PSD-95 interaction had been originally demonstrated, were used as positive control baits for strong interaction. As reported by the yeast HIS3 selection marker, we found that only Kir2.1, Kir2.3, Kv1.4, and NR2B but not Kir2.4 and none of the Kir3 channel subunits associated with PDZ domains 1–3 in the Y2H assay (Fig. 1A). Whereas lack of interaction was expected for Kir2.4, Kir3.1, and Kir3.4 in which the PDZ recognition motif is not conserved, we sought to analyze in more detail why the presence of the X-S/T-X-V/I sequence in Kir3.2 and Kir3.3 subunits did not confer the capability to bind to PDZ domains 1–3. A series of C-terminal deletion constructs and chimera between Kir2.1 and Kir3.2 were generated and used as bait in a more precise interaction domain analysis (Fig. 1B). We first demonstrated that in Kir2.1 subunits indeed removal of the last three residues (Kir2.1ΔSEI) completely disrupted PDZ binding. Exchange of these residues by the terminal triplet present in Kir3.2 subunits, however, restored its proper binding function Kir2.1(SKV). Moreover, yeast transformed with the Kir2.1 construct in which the triplet SEI was maintained but 13 amino acids were deleted further upstream of the C terminus was HIS3-negative, indicative of a contribution of this region in binding PDZ1–3. An interesting phenomenon was observed in equivalent experiments using Kir2.3 subunits. When the isoleucine at position 0, the serine at position −2, or the complete C-terminal triplet SAI was removed, a few transformants still contained the prey, suggesting an additional interaction site in Kir2.3 for PSD-95 (see below).
Fig. 1.
Kir2 channels but not Kir3 channels interact with PDZ domains 1–3 in the Y2H system. A, C termini of the Kir channels listed were used as bait in pGBT9 to test the interaction with PDZ1–3 of PSD-95 in pGAD-GH. The NR2B subunit and the Kv1.4 C termini were used as positive controls. The consensus sequence X-S/T-X-I/V is shown in bold letters.Numbers on the left denote amino acid positions of C-terminal fragments. B, Cross-mutational analysis of Kir2.1 and Kir3.2 mutants. See Results for details. Colony growth was controlled after 4 d.
Conversely, all manipulations performed on Kir3.2 subunits in which (1) the functional SEI motif of Kir2.1 was introduced and (2) 13 amino acids upstream of this motif in Kir2.1 were inserted or (3) the first half of the C terminus of Kir3.2 was replaced by the equivalent region in Kir2.1 (aa 179–305; aa 318–425 derive from Kir3.2), failed to transfer PDZ binding affinity to Kir3.2 channels. For demonstrating a putative interaction with other PSD-95/SAP90 family members, various fragments including the complete C termini of all Kir3 subunits were used as bait in a Y2H screen of two (E16 and P5) rat brain cDNA libraries constructed in the activation domain vector pAD-GAL4. In these experiments no interaction partner was identified for Kir3.1 (amino acids 1–85, 180–363, 321–501, and 180–501) or any other Kir3 subunit. As test for the quality of our assay, the full-length cDNA of PSD-95/SAP90 was isolated when the NR2B C terminus was used as bait (data not shown). From these results it is concluded that at least in the Y2H system the PDZ-binding motif X-S/T-X-I/V in Kir3.2 or Kir3.3 subunits is insufficient for binding PDZ domains 1–3 of PSD-95/SAP90.
Coimmunoprecipitations
The apparent differential association of PSD-95/SAP90 with Kir subunits in the yeast assay was also analyzed for the full-length Kir proteins biochemically by immunoprecipitation and Western immunoblotting in membranes of transfected COS-7 cells. Whole-cell extracts were prepared from COS-7 cells that had been cotransfected with myc-tagged Kir2.1 and PSD-95 (Fig.2A, left panel) or alternatively a pcDNA3IRES vector that contained both Kir2.1 and myc-tagged PSD-95 (Fig. 2A, right panel). In both experiments α-myc antibodies were used to precipitate tagged Kir2.1 and PSD-95, respectively. Western blot analysis of immunoprecipitates demonstrated that each protein could be coprecipitated with the tagged interaction partner, showing that the association is revealed independent of which protein is initially pulled down. A corresponding analysis for Kir2.3 subunits showed a similarly strong bonding to PSD-95 (Fig. 2B). The construct mycKir2.3(ΔSAI) in which the terminal amino acid triplet was removed did not precipitate significantly with PSD-95 in this assay (Fig. 2B, right panel).
Fig. 2.
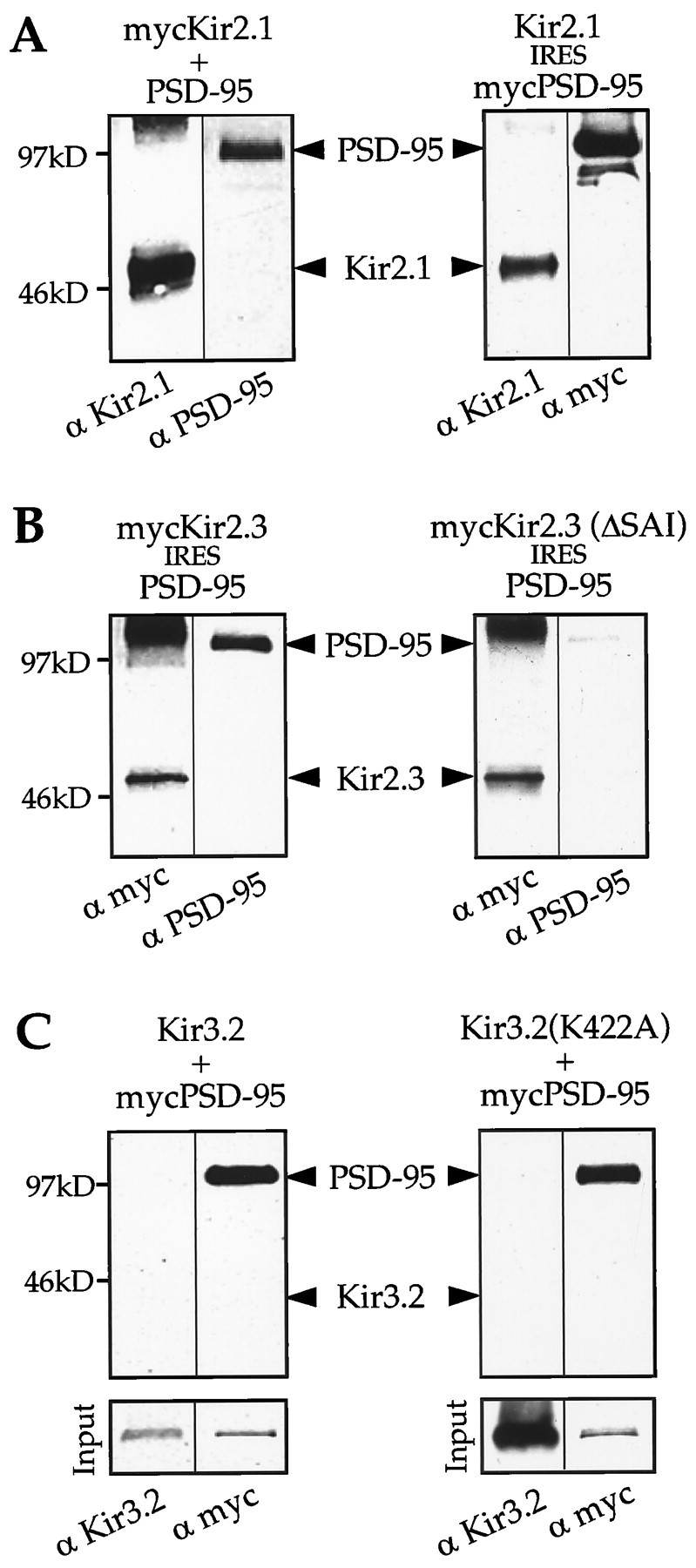
PSD-95 coprecipitates with Kir2.3 but not with Kir3.2 channels. Proteins from whole-cell extracts of COS-7 cells, cotransfected with mycKir2.1 and PSD-95 (or Kir2.1IRESmycPSD-95) (A), mycKir2.3IRESPSD-95 and mycKir2.3(ΔSAI)IRESPSD-95 (B), as well as Kir3.2 (Kir3.2K422A) and mycPSD-95 (C), were immunoprecipitated with α-myc antibodies. Immunoprecipitates were separated on 10% SDS gels, blotted onto polyvinylidene difluoride membranes, and probed with specific antibodies as indicated. Note that in A when either interaction partner is myc-tagged, the corresponding protein can be detected in the α-myc precipitates.Bottom panels in C show the presence of Kir3.2 and mycPSD-95 in whole-cell extracts of cotransfected COS-7 cells. In all other blots marker bands are shown on theleft.
Under the same conditions we precipitated mycPSD from extracts of COS-7 cells cotransfected with mycPSD-95/Kir3.2. However, no signal was detected with αKir3.2 antibodies in the precipitates (Fig.2C). This experiment was also performed with mutant Kir3.2K422A subunits in which the lysine at position −1 had been changed to an alanine residue (SAV), which approximates the C terminus to that of Kir2.3 (SAI) and optimizes the motif for recognizing PDZ domains. Yet, using α-Kir3.2 antibodies we were again unable to detect the appropriate band in the precipitates of PSD-95. Thus our data verify in mammalian cells that entire Kir2 subunits interact with PSD-95/SAP90 proteins and support the failure of strong complexing between Kir3.2 and PSD-95, as suggested by the yeast assay.
Coclustering
Myc-tagged Kir2.3 and Kir3.2 channels were also probed for colocalization with PSD-95 when cotransfected in COS-7 cells. Using confocal microscopy we confirmed earlier results (Kim et al., 1995) that voltage-gated Kv1.4 channels and PSD-95 are distributed homogeneously when transfected individually (data not shown) but colocalize and cluster with a typical punctuate staining after coexpression (Fig. 3A,B). A less homogenous distribution and moderate clustering were observed for transfected Kir2.3 channels. In the presence of PSD-95 both proteins were found to overlap completely (Fig. 3C,D). Contrasting these results, PSD-95 remains homogeneously distributed in COS-7 cells in the presence of Kir3.2 subunits, which demonstrate punctate distribution and profound perinuclear localization (Fig.3E,F).
Fig. 3.
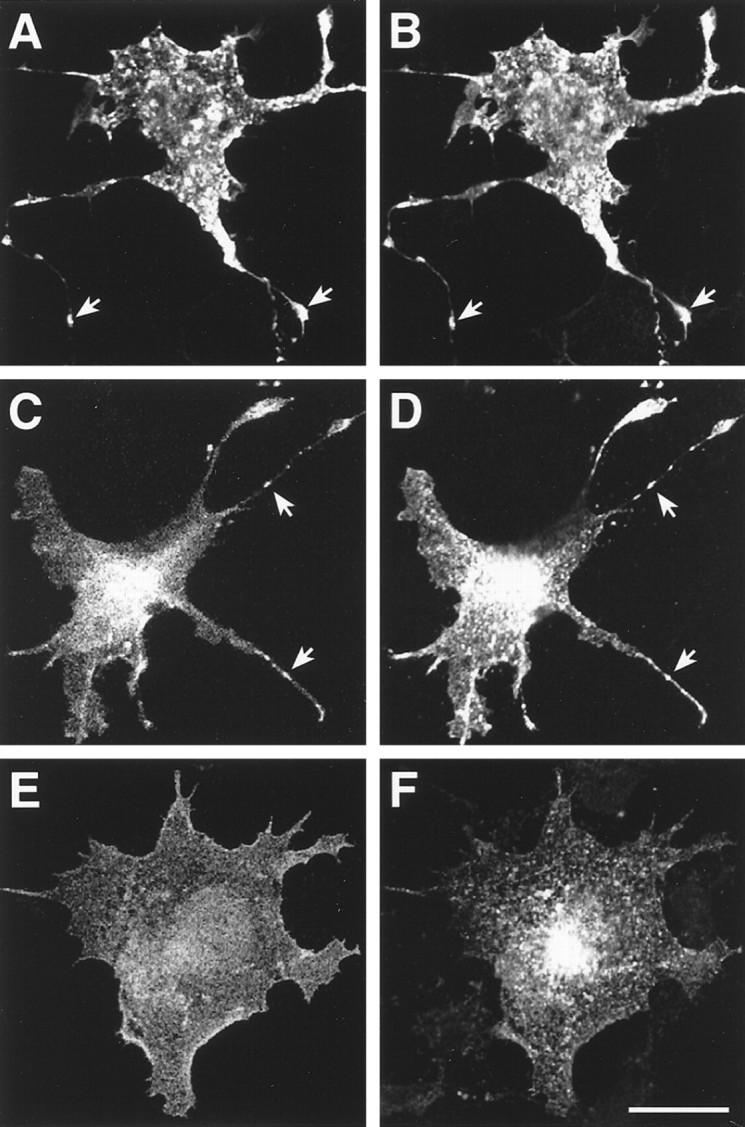
Kir2.3 channels are coclustered with PSD-95 after transfection in COS-7 cells. Confocal images are shown of COS-7 cells cotransfected with PSD-95 and mycKv1.4 (A, B), PSD-95 and myc Kir2.3 (C, D), and PSD-95 and Kir3.2 (E, F), respectively. The distribution of FITC-labeled PSD-95 is shown in A, C, and E; distribution of Cy3-labeled channel subunits is shown in B, D, and F using two different sets of filters. Sites of PSD-95/channel clustering are marked witharrowheads. Scale bar, 20 μm.
Functional consequences
The question was addressed of whether the association of Kir2 channels with PSD-95/SAP90 affects (1) the expression and (2) acute or long-term functional channel properties. For a quantitative analysis of Kir channel activity in the presence and absence of PSD-95, we compared Kir channel expression in mammalian cells from pcDNA3 containing just the cDNA of Kir2.3 and the bicistronic pcDNA3IRES vector that permits translation of both Kir and PSD-95 from one mRNA. In these experiments Kir2 subunits were expressed individually, and Kir3 subunits were expressed as concatemers from Kir3.1 and Kir3.2 subunits. The latter were found to generate currents indistinguishable from those that originated from a mixture of individual Kir3.1 and Kir3.2 subunits with respect to single-channel characteristics, activation potential and kinetics, dependence on extracellular [K+], as well as block by extracellular Ba2+ and Cs+ (Wischmeyer et al., 1997; data not shown). For both Kir2.3 and Kir3.1/3.2, macroscopic basal (in 25 mm external K+) currents were measured in transfected HEK-293 cells using step and ramp voltage-clamp whole-cell recordings. Kir2.3 current density (49 ± 24 pA/pF;n = 32; amplitude range, 0.33–7.18 nA) was reduced by 61% in the presence of PSD-95 (20 ± 15 pA/pF; n= 34; amplitude range, 0.17–2.61 nA; p < 0.01, Student's t test). Swapping the insertion site for Kir2.3 and PSD-95 in the pcDNA3IRES vector did not affect current suppression by PSD-95. In contrast, agonist (5-HT)-induced Kir3.1/3.2 inward current density at −100 mV averaged to 39 ± 19 pA/pF (n = 10) and was not significantly altered after coexpression of PSD-95 (37 ± 16 pA; n = 10; Fig.4A).
Fig. 4.
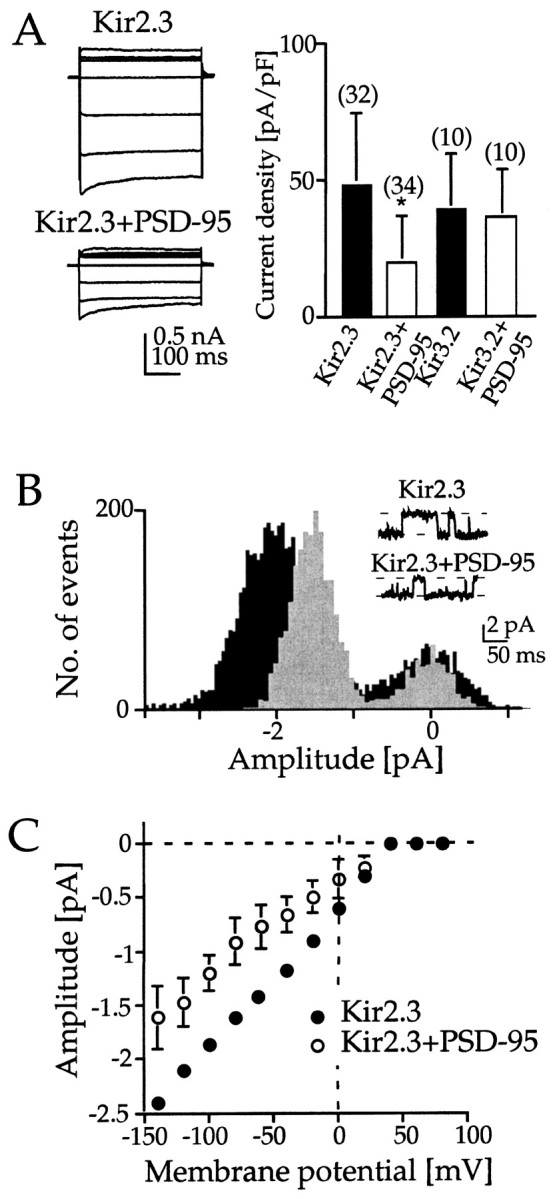
Coexpression of Kir2.3 channels with PSD-95 causes reduction of macroscopic current amplitude and unitary channel conductance. A, Whole-cell current responses to voltage pulses from +20 to −140 mV from a holding potential of −60 mV of HEK-293 cells transfected with Kir2.3 and Kir2.3 plus PSD-95 in the expression vector pcDNA3IRES, respectively. Currents are digitized at 5 kHz and filtered at 2.9 kHz. The bar graph shows current densities of Kir2.3 and Kir3.2 channels measured at −100 mV in elevated 25 mm extracellular K+ in the absence and presence of PSD-95. B, Amplitude histogram of cell-attached single-channel recordings from Kir2.3 channels (seeinset) with the number of data points plotted against mean current flow per 0.75 msec bin for single-channel events recorded at −100 mV. Kir2.3 channels are shown in black, and Kir2.3 channels plus PSD-95 are overlayed in gray. Currents were digitized and filtered at 1 kHz. C,I–V plot (pipette potential on theabscissa) of the single-channel currents inB reveals strong inward rectification and a slope conductance of 14 pS for Kir2.3 and 8 pS for Kir2.3 plus PSD-95, respectively.
To clarify whether the PSD-95 interaction in HEK-293 cells affected the efficiency of channel expression or altered single-channel properties, elementary currents were analyzed in the cell-attached configuration with 140 K+ in the pipette (Fig.4B). Kir2.3 currents were measured with a typically small unitary conductances of 14 pS (n = 2) that occurred at a frequency of po = 0.72 and is in accordance with previously published values (Morishige et al., 1993; Makhina et al., 1994; Périer et al., 1994). The frequency of Kir2.3 channel events was unaltered in the presence of PSD-95 (po = 0.73), but the elementary conductance was decreased to 8 ± 1.2 pS (n = 13; 10 different experiments; Fig.4B,C). The product γ ·po (as a measure of the average current flux through a single channel) for Kir2.3 and Kir2.3/PSD-95 was calculated to differ by a factor of 1.85, indicating that the approximately twofold reduction by PSD-95 in macroscopic currents is primarily caused by a change in single-channel properties.
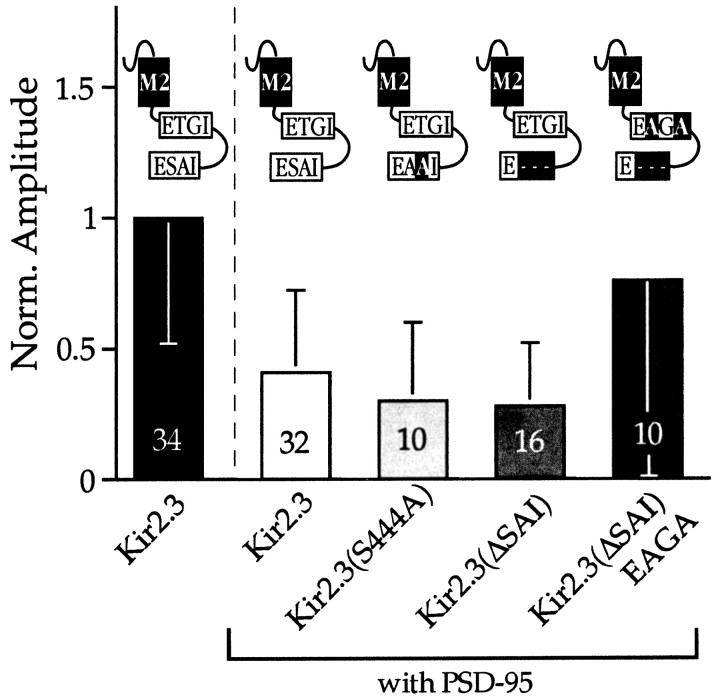
Using this assay we finally investigated the observation that Kir2.3 mutants lacking the C-terminal PDZ domain recognition motif may still weakly interact with PSD-95. Two mutant channels with the serine at position −2 exchanged to an alanine (AAI) and the last triplet removed completely (ΔSAI) generated current densities that were reduced in the presence of PSD-95 by 69, and 72%, respectively (Fig.5). PDZ-mediated interactions have been recently found to occur through modes other than by recognition of a C-terminal motif (Hillier et al., 1999). Thus, in a mutant channel lacking the C terminus, the sequence of a putative alternative interaction site at aa position 409–412 (ETGI) was changed to EAGA. In the presence of PSD-95 macroscopic currents generated by these mutant Kir2.3 channels were reduced by only 23%, suggesting that interaction with the C-terminal motif in Kir2.3 channels may not be the sole determinant in the functional association with PSD-95.
Fig. 5.
Analysis of C-terminal and internal PDZ domain recognition motifs in Kir2.3 subunits. The bar graphsummarizes relative current amplitudes (measured at −100 mV) from HEK-293 cells cotransfected with PSD-95 and wild-type or mutated Kir2.3 subunits as depicted in the diagrams. In Kir2.3(444A) a serine residue has been replaced by an alanine at the −2 position; in Kir2.3(ΔSAI) the terminal triplet has been removed; and in Kir2.3(ΔSAI)EAGA a threonine (aa 420) and an isoleucine (aa 422) in putative internal PDZ domain recognition motif (E-T-G-I) have been replaced by alanine residues. Amplitudes in each barhave been normalized to currents revealed in the absence of PSD-95.
DISCUSSION
As representative members of two different Kir subfamilies both Kir2.3 and Kir3.2 subtypes carry a conserved C-terminal recognition motif for PDZ domains of chapsyns. Yet in our hands the Y2H assay as well as biochemical and functional properties provide consistent evidence that Kir2.3 but not Kir3.2 subunits strongly interact with these proteins (but see Inanobe et al., 1999). Consequently, it is concluded that the presence of the canonical C-terminal X-S/T-X-V/I sequence alone does not necessarily predict strong bonding of a candidate protein to PSD-95, even if closely related to another stable interaction partner. Instead, other structural determinants of binding, e.g., in Kir channels the tertiary structure of the complete cytoplasmic tail, are likely to affect the specificity of coupling. Binding of G protein-gated Kir3.2 channels to PSD-95 may be impaired because in the heterotetrameric channel protein the C termini are engaged in coupling to Gβγ subunits, phosphatidylinositol-4,5 phosphate Na+, and possibly other modulators of channel activity (Dascal, 1997). The crystal structure of PDZ domains complexed to their ligands (Doyle et al., 1996; Hillier et al., 1999) predicts that specific side chain interactions of residues upstream to the tetrapeptide also affect the specificity of binding. As an example, these nearby residues in CRIPT determine the specific preference for interaction with PDZ3 (Niethammer et al., 1998), even though the same C-terminal consensus site applies to all three PDZ domains. Also, the multivalent “channel-interacting PDZ domain protein” CIPP whose PDZ domains show high homology to PDZ1–3 of PSD-95 selectively associates with Kir4.1/Kir4.2 subunits (SNV) but not with Kir2.1 and Kir2.3 subunits (Kurschner et al., 1998). Most recently, a specific internal PDZ domain recognition site, which mediates head-to-tail heterodimerization with PSD-95 and syntrophin, has been described for nNOS (Brenman et al., 1996; Hillier et al., 1999). There, a two stranded β-finger in nNOS, of which the first strand mimics the C-terminal PDZ motif, acts as a PDZ ligand to interact with an antiparallel β-sheet in syntrophin. In this interaction the normally required terminal carboxylate is replaced by a sharp β-turn at the tip of the β-finger. Our electrophysiological data in HEK-293 cells may point to a similar mode of PDZ domain scaffolding in Kir2.3 subunits. At least two pseudopeptide motifs, ESKI (aa 355–358) and ETGI (aa 409–412), including several nearby β-turns, are present in the Kir2.3 tail region in addition to the conserved terminal ESAI sequence (aa 442–445). Although direct PDZ domain interaction with an internal Kir2.3 binding site has yet to be proven biochemically, the inhibitory action of PSD-95 on Kir2.3 currents was alleviated when one of these sites was disrupted. Both internal motifs are not present in Kir2.1 subunits (or Kir2.2 and Kir2.4), which may explain the slightly different results for the Kir2.1 and Kir2.3 truncated mutants in the Y2H assay.
Kir2 channels, even though their activity is subject to modulation by phosphorylation, are constitutively active and thus balance the postsynaptic potential in the PSD. We have demonstrated colocalization of Kir2.3 and PSD-95 in the heterologous system, and both proteins have been shown earlier to occur together in forebrain PSD fractions (Cohen et al., 1996). However, ultrastructural evidence for Kir localization at the PSD is still missing. The exact mechanism by which members of the PSD-95/SAP90 family mediate clustering of Kir2.3 or any other channel protein is still uncertain. The channel protein may be initially targeted uniformly to the membrane and then immobilized and clustered at the synapse by monomeric or oligomerized PSD-95 that has already aggregated at the synapse (Craven and Bredt, 1998; Rao et al., 1998; Burke et al., 1999). There is also evidence that chapsyns must be palmitoylated (Topinka and Bredt, 1998) and interact with a membrane protein to be localized to the PSD and that targeting of K+ channels is dependent both on permissive signals from chapsyns as well as an intrinsic signal that specifies location (Arnold and Clapham, 1999). Previous findings suggested that as a functional consequence of this interaction the number of Kir channels in the membrane is increased, possibly by slowing channel turnover or promoting tetramer assembly. When Kir4.1 channels were expressed together with PSD-95, SAP97 (Horio et al., 1997), or CIPP (Kurschner et al., 1998), current densities were approximately doubled with no apparent effect on the channel properties. The association of Kir2.3 and PSD-95 predominantly affects the Kir2.3 elementary conductance and reduces current density by half, possibly because of a more complex PDZ domain scaffolding with PSD-95. It is interesting to note that in the presence of PSD-95 Kir2.3 channels on a time scale of 2–3 min are still subject to inhibition by PKA phosphorylation. The phosphorylated consensus serine overlaps with the C-terminal PDZ-binding motif, and an earlier report by Cohen et al. (1996) suggested that PKA stimulation interferes with the channel binding to PSD-95. As predicted, the free phosphorylated C terminus in Kir2.1 channels acts as a channel-closing gate (Wischmeyer and Karschin, 1996).
Heteromeric Kir3 channels in the mammalian brain are directly controlled by receptor-released Gβγ subunits, and the majority are constituted from Kir3.1, Kir3.2, and Kir3.3 subunits. All three subunits are expressed at high levels throughout the brain with a large degree of overlap (Karschin and Karschin, 1999). At the cellular resolution level Kir3.1 and Kir3.2 immunoreactivity has been found in various potentially postsynaptic sites on neuronal somata and dendritic spines but also on distal axon terminals (Murer et al., 1997). Although PSD-95/SAP90 in most neurons has a punctate distribution (Kornau et al., 1995) and is mostly associated with postsynaptic densities (Hunt et al., 1996), there is no obligate colocalization with Kir3 channels. A recent study showed that postsynaptically on dendrites of dopaminergic substantia nigra neurons neither PSD-95 nor SAP97 coaggregates with the abundant Kir3.2 channels (Inanobe et al., 1999). It should be considered that Kir3 channels are directly associated with a series of heptahelical receptors via G proteins in a mandatory manner (Dascal, 1997), underlying many forms of slow, inhibitory synaptic transmission. Thus there is no strict requirement for Kir3 channels to be localized and tightly clustered at the postsynaptic density typical of fast excitatory synapses. In fact, there is plenty of evidence that both G protein-coupled receptors and target channels are not concentrated opposite of the nerve terminals but are rather expressed uniformly. The time constraints of these slower signaling systems are such that Kir channels are in the proximity of their receptors, but they do not have to be near the transmitter release sites (Hille, 1992). Based on the unsuccessful Y2H screens of rat brain libraries with Kir3 channel termini, we tend to believe that Kir3 localization and assembly in the signaling cascade may not be crucially dependent on PDZ domain interactions but rather are controlled through other intrinsic targeting signals.
Footnotes
This work was funded by Grants Ka1175/1-2 and Ve187/1-2 from the Deutsche Forschungsgemeinschaft. We thank D. Reuter for excellent technical help, S. Voigt for preparing the oocytes, and M. Stocker and T. Falk for supplying cDNAs. We also thank M. Niethammer for stimulating the collaboration between Göttingen and Boston and S. Stamm for invaluable advice on the Y2H assay.
Correspondence should be addressed to Dr. Andreas Karschin, Max-Planck-Institut for Biophysical Chemistry, Molecular Neurobiology of Signal Transduction, Am Fassberg 11, 37070 Göttingen, Germany. E-mail: akarsch@gwdg.de.
REFERENCES
- 1.Arnold DB, Clapham DE. Molecular determinants for subcellular localization of PSD-95 with an interacting K+ channel. Neuron. 1999;23:149–157. doi: 10.1016/s0896-6273(00)80761-8. [DOI] [PubMed] [Google Scholar]
- 2.Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and α1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 3.Burke NA, Takimoto K, Li D, Han W, Watkins SC, Levitan ES. Distinct structural requirements for clustering and immobilization of K+ channels by PSD-95. J Gen Physiol. 1999;113:71–80. doi: 10.1085/jgp.113.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- 5.Cohen NA, Brenman JE, Snyder SH, Bredt DS. Binding of the inward rectifier K+ channel Kir 2.3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996;17:759–767. doi: 10.1016/s0896-6273(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 6.Colledge M, Froehner SC. Signals mediating ion channel clustering at the neuromuscular junction. Curr Opin Neurobiol. 1998;8:357–363. doi: 10.1016/s0959-4388(98)80061-5. [DOI] [PubMed] [Google Scholar]
- 7.Craven SE, Bredt DS. PDZ proteins organize synaptic signaling pathways. Cell. 1998;93:495–498. doi: 10.1016/s0092-8674(00)81179-4. [DOI] [PubMed] [Google Scholar]
- 8.Dascal N. Signalling via the G protein-activated K+ channels. Cell Signal. 1997;9:551–573. doi: 10.1016/s0898-6568(97)00095-8. [DOI] [PubMed] [Google Scholar]
- 9.Dissmann E, Wischmeyer E, Spauschus A, von Pfeil D, Karschin C, Karschin A. Functional expression and cellular mRNA localization of a G protein-activated K+ inward rectifier isolated from rat brain. Biochem Biophys Res Commun. 1996;223:474–479. doi: 10.1006/bbrc.1996.0918. [DOI] [PubMed] [Google Scholar]
- 10.Doupnik CA, Davidson N, Lester HA. The inward rectifier potassium channel family. Curr Opin Neurobiol. 1995;5:268–277. doi: 10.1016/0959-4388(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 11.Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: Molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- 12.Ehlers MD, Mammen AL, Lau L-F, Huganir RL. Synaptic targeting of glutamate receptors. Curr Opin Cell Biol. 1996;8:484–489. doi: 10.1016/s0955-0674(96)80024-x. [DOI] [PubMed] [Google Scholar]
- 13.Falk T, Meyerhof W, Corrette BJ, Schäfer J, Bauer CK, Schwarz JR, Richter D. Cloning, functional expression and mRNA distribution of an inwardly rectifying potassium channel protein. FEBS Lett. 1995;367:127–131. doi: 10.1016/0014-5793(95)00527-g. [DOI] [PubMed] [Google Scholar]
- 14.Fields S, Song O-K. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 15.Garcia EP, Mehta S, Blair LAC, Wells DG, Shang J, Fukushima T, Fallon JR, Garner CC, Marshall J. SAP90 binds and clusters kainate receptors causing incomplete desensitization. Neuron. 1998;21:727–739. doi: 10.1016/s0896-6273(00)80590-5. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi R, Krummel B, Saiki RK. A general method of in vivo preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hille B. G protein-coupled mechanisms and nervous signaling. Neuron. 1992;9:187–195. doi: 10.1016/0896-6273(92)90158-a. [DOI] [PubMed] [Google Scholar]
- 18.Hillier BJ, Christopherson KS, Prehoda KE, Bredt DS, Lim WA. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science. 1999;284:812–815. [PubMed] [Google Scholar]
- 19.Horio Y, Hibino H, Inanobe A, Yamada M, Ishii M, Tada Y, Satoh E, Hata Y, Takai Y, Kurachi Y. Clustering and enhanced activity of an inwardly rectifying potassium channel, Kir4.1, by an anchoring protein, PSD-95/SAP90. J Biol Chem. 1997;272:12885–12888. doi: 10.1074/jbc.272.20.12885. [DOI] [PubMed] [Google Scholar]
- 20.Hunt CA, Schenker LJ, Kennedy MB. PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane at forebrain synapses. J Neurosci. 1996;16:1380–1388. doi: 10.1523/JNEUROSCI.16-04-01380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inanobe A, Yoshimoto Y, Horio Y, Morishige K-I, Hibino H, Matsumoto S, Tokunaga Y, Maeda T, Hata Y, Takai Y, Kurachi Y. Characterization of G-protein-gated K+ channels composed of Kir3.2 subunits in dopaminergic neurons of the substantia nigra. J Neurosci. 1999;19:1006–1017. doi: 10.1523/JNEUROSCI.19-03-01006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Südhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–1515. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- 23.Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and function. Jpn J Physiol. 1997;47:11–39. doi: 10.2170/jjphysiol.47.11. [DOI] [PubMed] [Google Scholar]
- 24.Karschin C, Karschin A. Distribution of inwardly rectifying potassium channels in the brain. In: Kurachi Y, Jan LY, Lazdunski M, editors. Potassium ion channels. Molecular structure, function, and diseases. Academic; San Diego: 1999. pp. 273–292. [Google Scholar]
- 25.Kennedy MB. The postsynaptic density. Curr Opin Neurobiol. 1993;3:732–737. doi: 10.1016/0959-4388(93)90145-o. [DOI] [PubMed] [Google Scholar]
- 26.Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- 27.Kim E, Naisbitt S, Hsueh Y-P, Rao A, Rothschild A, Craig AM, Sheng M. GKAP, a novel synaptic protein that interacts with the guanylate kinase-like domain of the PSD-95/SAP90 family of channel clustering molecules. J Cell Biol. 1997;136:669–678. doi: 10.1083/jcb.136.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim E, DeMarco SJ, Marfatia SM, Chishti AH, Sheng M, Strehler EE. Plasma membrane Ca2+ ATPase isoform 4b binds to membrane-associated guanylate kinase (MAGUK) proteins via their PDZ (PSD-95/Dlg/ZO-1) domains. J Biol Chem. 1998a;273:1591–1595. doi: 10.1074/jbc.273.3.1591. [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Liao D, Lau L-F, Huganir RL. SynGAP: a synaptic RasGAP that associates with the PSD-95/SAP90 protein family. Neuron. 1998b;20:683–691. doi: 10.1016/s0896-6273(00)81008-9. [DOI] [PubMed] [Google Scholar]
- 30.Kirsch J, Wolters I, Triller A, Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993;366:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- 31.Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 32.Kornau H-C, Seeburg PH, Kennedy MB. Interaction of ion channels and receptors with PDZ domain proteins. Curr Opin Neurobiol. 1997;7:368–373. doi: 10.1016/s0959-4388(97)80064-5. [DOI] [PubMed] [Google Scholar]
- 33.Kurschner C, Mermelstein PG, Holden WT, Surmeier JD. CIPP, a novel multivalent PDZ domain protein, selectively interacts with Kir4.0 family members, NMDA receptor subunits, neurexins, and neuroligins. Mol Cell Neurosci. 1998;11:161–172. doi: 10.1006/mcne.1998.0679. [DOI] [PubMed] [Google Scholar]
- 34.LaMonica N, Meriam C, Racaniello VR. Mapping of sequences required for mouse neurovirulence of poliovirus type 2 Lansing. J Virol. 1986;57:515–525. doi: 10.1128/jvi.57.2.515-525.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Makhina EN, Kelly AJ, Lopatin AN, Mercer RW, Nichols CG. Cloning and expression of a novel human brain inward rectifier potassium channel. J Biol Chem. 1994;269:20468–20474. [PubMed] [Google Scholar]
- 36.Masuko N, Makino K, Kuwahara H, Fukunaga K, Sudo T, Araki N, Yamamoto H, Yamamoto Y, Miyamoto E, Saya H. Interaction of NE-dlg/SAP102, a neuronal and endocrine tissue-specific membrane-associated guanylate kinase protein, with calmodulin and PSD-95/SAP90. J Biol Chem. 1999;274:5782–5790. doi: 10.1074/jbc.274.9.5782. [DOI] [PubMed] [Google Scholar]
- 37.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: Molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 38.Morishige K-I, Takahashi N, Findlay I, Koyama H, Zanelli JS, Peterson C, Jenkins NA, Copeland NG, Mori Y, Kurachi Y. Molecular cloning, functional expression and localization of an inward rectifier potassium channel in the mouse brain. FEBS Lett. 1993;336:375–380. doi: 10.1016/0014-5793(93)80840-q. [DOI] [PubMed] [Google Scholar]
- 39.Murer G, Adelbrecht C, Lauritzen I, Lesage F, Lazdunski M, Agid Y, Raisman-Vozari R. An immunocytochemical study on the distribution of two G-protein-gated inward rectifier potassium channels (GIRK2 and GIRK 4) in the adult rat brain. Neuroscience. 1997;80:345–357. doi: 10.1016/s0306-4522(97)00001-8. [DOI] [PubMed] [Google Scholar]
- 40.Niethammer M, Kim E, Sheng M. Interaction between the C terminus of NMDA receptor subunits and multiple members of the PSD-95 family of membrane-associated guanylate kinases. J Neurosci. 1996;16:2157–2163. doi: 10.1523/JNEUROSCI.16-07-02157.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niethammer M, Valtschanoff JG, Kapoor TM, Allison DW, Weinberg RJ, Craig AM, Sheng M. CRIPT, a novel postsynaptic protein that binds to the third PDZ domain of PSD-95/SAP90. Neuron. 1998;20:693–707. doi: 10.1016/s0896-6273(00)81009-0. [DOI] [PubMed] [Google Scholar]
- 42.Périer F, Radeke CM, Vandenberg CA. Primary structure and characterization of a small-conductance inwardly rectifying potassium channel from human hippocampus. Proc Natl Acad Sci USA. 1994;91:6240–6244. doi: 10.1073/pnas.91.13.6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao A, Kim E, Sheng M, Craig AM. Heterogeneity in the molecular composition of excitatory postsynaptic sites during development of hippocampal neurons in culture. J Neurosci. 1998;18:1217–1229. doi: 10.1523/JNEUROSCI.18-04-01217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sheng M. PDZs and receptor/channel clustering: rounding up the latest suspects. Neuron. 1996;17:575–578. doi: 10.1016/s0896-6273(00)80190-7. [DOI] [PubMed] [Google Scholar]
- 45.Sheng M, Kim E. Ion channel associated proteins. Curr Opin Neurobiol. 1996;6:602–608. doi: 10.1016/s0959-4388(96)80091-2. [DOI] [PubMed] [Google Scholar]
- 46.Sheng ZH, Rettig J, Takahashi M, Catterall WA. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 1994;13:1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 47.Snow BE, Hall RA, Krumins AM, Brothers GM, Bouchard D, Brothers CA, Chung S, Mangion J, Gilman AG, Lefkowitz RJ, Siderovski DP. GTPase activating specificity of RGS12 and binding specificity of an alternatively spliced PDZ (PSD-95/Dlg/ZO-1) domain. J Biol Chem. 1998;273:17749–17555. doi: 10.1074/jbc.273.28.17749. [DOI] [PubMed] [Google Scholar]
- 48.Songyang Z, Fanning As, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- 49.Spauschus A, Lentes K-U, Wischmeyer E, Dissmann E, Karschin C, Karschin A. A G-protein-activated inwardly rectifying K+ channel (GIRK4) from human hippocampus associates with other GIRK channels. J Neurosci. 1996;16:930–938. doi: 10.1523/JNEUROSCI.16-03-00930.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stühmer W, Ruppersberg JP, Schröter KH, Sakmann B, Stocker M, Giese KP, Perschke A, Baumann A, Pongs O. Molecular basis of functional diversity of voltage-gated potassium channels in mammalian brain. EMBO J. 1989;8:3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeuchi M, Hata Y, Hirao K, Toyoda A, Irie M, Takai Y. SAPAPS. A family of PSD-95/SAP90-associated proteins localized at postsynaptic density. J Biol Chem. 1997;272:11943–11951. doi: 10.1074/jbc.272.18.11943. [DOI] [PubMed] [Google Scholar]
- 52.Tejedor FJ, Bokhari A, Rogero O, Gorczyca M, Zhang J, Kim E, Sheng M, Budnik V. Essential role for dig in synaptic clustering of Shaker K+ channels in vivo. J Neurosci. 1997;17:152–159. doi: 10.1523/JNEUROSCI.17-01-00152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas U, Kim E, Uhlendahl S, Ho Koh Y, Gundelfinger ED, Sheng M, Garner CC, Budnik V. Synaptic clustering of the cell adhesion molecule fasciclin II by discs-large and its role in the regulation of presynaptic structure. Neuron. 1997;19:787–799. doi: 10.1016/s0896-6273(00)80961-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Töpert C, Döring F, Wischmeyer E, Karschin C, Brockhaus J, Ballanyi K, Derst C, Karschin A. Kir2.4: a novel K+ inward rectifier channel associated with motoneurons of cranial nerve nuclei. J Neurosci. 1998;18:4096–4105. doi: 10.1523/JNEUROSCI.18-11-04096.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Topinka JR, Bredt DS. N-terminal palmitoylation of PSD-95 regulates association with cell membranes and interaction with K+ channel KV1.4. Neuron. 1998;20:125–134. doi: 10.1016/s0896-6273(00)80440-7. [DOI] [PubMed] [Google Scholar]
- 56.Wischmeyer E, Karschin A. Receptor stimulation causes slow inhibition of IRK1 inwardly rectifying K+ channels by direct protein kinase A-mediated phosphorylation. Proc Natl Acad Sci USA. 1996;93:5819–5823. doi: 10.1073/pnas.93.12.5819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wischmeyer E, Lentes K-U, Karschin A. Physiological and molecular characterization of an IRK-type inward rectifier K+ channel in a tumour mast cell line. Pflügers Arch. 1995;429:809–819. doi: 10.1007/BF00374805. [DOI] [PubMed] [Google Scholar]
- 58.Wischmeyer E, Döring F, Wischmeyer E, Spauschus A, Thomzig A, Veh R, Karschin A. Subunit interactions in the assembly of neuronal Kir3.0 inwardly rectifying K+ channels. Mol Cell Neurosci. 1997;9:194–206. doi: 10.1006/mcne.1997.0614. [DOI] [PubMed] [Google Scholar]
- 59.Zito K, Fetter RD, Goodman CS, Isacoff EY. Synaptic clustering of fasciclin II and shaker: essential targeting sequences and role of Dlg. Neuron. 1997;19:1007–1016. doi: 10.1016/s0896-6273(00)80393-1. [DOI] [PubMed] [Google Scholar]