Abstract
Hes1 is one of the basic helix-loop-helix transcription factors that regulate mammalian CNS development, and its loss- and gain-of-function phenotypes indicate that it negatively regulates neuronal differentiation.
Here we report that Hes1−/− mice expressed both early (TuJ1 and Hu) and late (MAP2 and Neurofilament) neuronal markers prematurely, and that there were approximately twice the normal number of neurons in theHes1−/− brain during early neural development. However, immunochemical analyses of sections and dissociated cells using neural progenitor markers, including nestin, failed to detect any changes in Hes1−/−progenitor population. Therefore, further characterization of neural progenitor cells that discriminated between multipotent and monopotent cells was performed using two culture methods, low-density culture, and a neurosphere assay. We demonstrate that the self-renewal activity of multipotent progenitor cells was reduced in theHes1−/− brain, and that their subsequent commitment to the neuronal lineage was accelerated. TheHes1−/− neuronal progenitor cells were functionally abnormal, in that they divided, on average, only once, and then generated two neurons, (instead of one progenitor cell and one neuron), whereas wild-type progenitor cells divided more. In addition, some Hes1−/− progenitors followed an apoptotic fate. The overproduction of neurons in the earlyHes1−/− brains may reflect this premature and immediate generation of neurons as well as a net increase in the number of neuronal progenitor cells.
Taken together, we conclude that Hes1 is important for maintaining the self-renewing ability of progenitors and for repressing the commitment of multipotent progenitor cells to a neuronal fate, which is critical for the correct number of neurons to be produced and for the establishment of normal neuronal function.
Keywords: Hes1, basic helix-loop-helix (bHLH) transcription factor, neuronal commitment, multipotent progenitor cell, neurosphere assay, apoptosis
The mammalian CNS is derived from a monolayer of germinal neuroepithelial cells, which is composed of self-renewing multipotent progenitor cells (CNS stem cells) in the ventricular zone (VZ) (Rakic, 1988; Bayer and Altman, 1991; McConnell, 1995; Lillien, 1998). These most immature progenitor cells generate mitotic and lineage-restricted intermediate progenitor cells, e.g., neuronal progenitor cells and glial progenitor cells (Luskin et al., 1988; Mayer-Proschel et al., 1997; Qian et al., 1998; Casarosa et al., 1999; Torii et al., 1999). Postmitotic neurons derived from neural progenitor cells subsequently migrate away from the VZ and mature. Putative multipotent progenitor cells can be isolated using the neurosphere formation assay, and successive neurosphere differentiation assays have been used to show their multipotency in giving rise to both neurons and glia (Reynolds et al., 1992; Vescovi et al., 1993; Reynolds and Weiss, 1996). Using low-density culture, an in vitroclonal analysis procedure, the developmental potential of single mitotic cells, most of which are neuronal progenitor cells, can be monitored by examining the number and morphology of their daughter cells (Temple, 1989; Sakakibara et al., 1996). In this paper, we apply these two culture systems to gain insight into developmental neurobiology, by investigating the effect of Hes1 on progenitor cells for which markers have not yet been established.
Regulatory cascades of positive- and negative basic helix-loop-helix (bHLH) transcription factors play essential roles in mammalian neurogenesis (Kageyama et al., 1995; Kageyama and Nakanishi, 1997; Lee, 1997). The bHLH genes such as Mash1 and NeuroD(Johnson et al., 1990; Guillemot and Joyner, 1993; Lee et al., 1995), are thought to positively regulate neuronal development at the level of commitment and postmitotic differentiation. Other bHLH genes, likeHes1 (Sasai et al., 1992), negatively regulate the transcription of the positive-bHLH genes. Hes1 (Akazawa et al., 1992; Sasai et al., 1992; Takebayashi et al., 1994) was originally isolated as a mammalian homolog of hairy and Enhancer of Split, which negatively regulate neurogenesis inDrosophila (Ingham et al., 1985; Klämbt et al., 1989;Nakao and Campos-Ortega, 1996). Persistent expression ofHes1 prevents migration of neural progenitor cells out of the VZ and the expression of neuronal markers (Ishibashi et al., 1994). In contrast, Hes1−/− brains prematurely express neurofilaments (Ishibashi et al., 1995). These results indicate that this gene is required for the negative regulation of neuronal differentiation. On a gross level, the neural folds ofHes1−/− embryos sometimes fail to fuse at embryonic day 8.5 (E8.5), and the cranial region is usually still open at E9.5, although no alterations in dorsoventral patterning are seen. Mutant embryos rarely survive beyond E14.5 (Ishibashi et al., 1995), which has hindered the analysis of the role of Hes1in CNS development. Because neuronal development consists of a series of processes, including neuronal commitment and postmitotic differentiation, the steps that require Hes1 action remain to be elucidated.
In this report, detailed in vivo and in vitroanalyses indicated that in addition to its effects on postmitotic neuronal differentiation, Hes1 normally functions to repress the commitment of multipotent progenitor cells to the neuronal lineage, thereby maintaining their self-renewing state (see Fig. 5).
Fig. 5.
A, Schematic model of neurogenesis in wild-type (wild) and Hes1−/−mice (Hes1−/−) based on the present results in vivo and in vitro. Because theHes1 mutation relieves the repression of neuronal commitment by MPs, the self-renewing capability of MPs is lowered (Fig. 2, represented as the thin circular arrow beside theHes1−/− MPs in Fig. 5A), and the generation of NPs is increased (Table 1, more NPs are generated from Hes1−/− MPs in Fig.5A). Prematurely committed, abnormal NPs become apoptotic (*1) or prematurely stop undergoing mitosis (*2) (accelerated apoptosis and a decreased number of daughter neurons from each NP; Figs. 3, 4). The increased number of neurons in early neurogenesis (before the lethal point ofHes1−/− mice around E14;transverse line in A) inHes1−/− mice (Fig. 1) corresponds to the premature commitment and differentiation of cells in the neuronal lineage. Dotted lines encircle the representative lineage of single NPs based on the low-density culture experiments (Fig. 3). NPs of Hes1−/− mice are likely to have ceased mitosis prematurely. There are two possible mechanisms to explain this premature cessation: (1) the accelerated transition of the developmental stages from NP to N, and from MP to NP, and (2) interruption of asymmetric cell division by the Hes1mutation. B, Diagrammatic representation of the regulation of neurogenesis by the Hes family. This diagram is based on the results of this and previous studies. Neurons (N) are derived from multipotent progenitor cells (MP); that is, a neural stem cell (MP), directly or via an intermediate progenitor state, becomes a committed neuronal progenitor cell (NP). Commitment and differentiation are two important, distinct steps in neurogenesis. Hes1 represses the commitment of MPs to the neuronal lineage, but not to glial lineages (*3; Table 1). Hes1 and Hes5 repress neuronal differentiation in a redundant manner (*4; Figs. 1,3; Ohtsuka et al., 1999).
MATERIALS AND METHODS
Animals
The Hes1−/− mice were described previously (Ishibashi et al., 1995).Hes1−/− mice and their wild-type littermates were used throughout these experiments. Successful matings were identified by the presence of vaginal plugs. Embryonic day 0.5 (E0.5) was defined as noon of the same day that vaginal plugs were observed. Timed pregnant mice were killed with an overdose of diethyl ether, and embryos were obtained by Cesarean section. Embryos were numbered, and their tails were used for genotyping. To extract DNA, tails were digested in proteinase K buffer containing 0.5% Nonidet P-40, 20 mm Tris–HCl, pH 8.4, 50 mm KCl, 2.5 mm MgCl2, and 2 mg/ml proteinase K (Life Technologies, Grand Island, NY). To determine the genotypes of embryos from heterozygous intercrosses, we performed PCR with the tail DNA, using primers described previously (Ishibashi et al., 1995). At least three Hes1−/− embryos and three wild-type littermates were used for each experiment. Embryos were examined at E10.5, E12.5, E13.5, and E14.5, but not later, because necrosis of the brains of Hes1−/−embryos starts at approximately E14, and embryos die soon thereafter (Ishibashi et al., 1995).
Immunochemistry
For cryosectioning, we selectedHes1−/− embryos that did not have an open-brain phenotype (these represented ∼30% of theHes1−/− embryos) and used their wild-type littermates for anatomical comparison. Embryos were fixed overnight in 4% paraformaldehyde in 0.1 m PBS, pH 7.4, at 4°C, cryoprotected in 30% sucrose in PBS overnight, embedded in O.C.T. compound (Tissue Tek; Miles, Elkhart, IN), and frozen on dry ice. Ten-micrometer-thick cryosections were cut and affixed to 3-aminopropyltriethoxysilane-coated glass slides (Matsunami Glass, Osaka, Japan). For immunocytochemistry, cultured cells were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature, then washed three times with PBS.
Standard procedures were used for immunostaining cryosections and cultures, and are described below. The primary antibodies used were: anti-nestin (mouse monoclonal IgM, clone RE6-96, ascitic fluid used at a dilution of 1:1000) (Miyata and Ogawa, 1994); RAT401 Developmental Studies Hybridoma Bank of Iowa University; mouse monoclonal IgG1; 1:200 dilution); anti-proliferating cell nucleus specific antigen (PCNA) (Novocastra; mouse monoclonal IgG; 1:50 dilution); anti-mouse Musashi1 (Msi1) (monoclonal rat IgG; 1:200 dilution) (Kaneko et al., 1999); Ki-67 (Novocastra; polyclonal rabbit antiserum; 1:1000 dilution); anti-microtubule-associated protein 2 (MAP2) (Sigma, St. Louis, MO; mouse monoclonal IgG1; 1:200 dilution); anti-MAP2 (a gift from Dr. Niinobe, Institute of Protein Research, Osaka University; polyclonal rabbit antiserum; 1:2000 dilution) (Niinobe et al., 1988); anti-neurofilament-M (Chemicon, Temecula, CA; polyclonal rabbit antiserum; 1:300 dilution); TuJ1 (Berkeley Antibody; mouse monoclonal IgG2a/κ; 1:200 dilution); anti-Hu (Oregon University; mouse monoclonal antibody 16A11; 1:200 dilution) (Marusich et al., 1994); anti-glial fibrillary acidic protein (GFAP) (Sigma; mouse monoclonal IgG1; 1:200 dilution); anti-GFAP (Dako, Carpinteria, CA; polyclonal antibody; 1:10 dilution); and anti-O4 (Boehringer Mannheim, Mannheim, Germany; mouse monoclonal IgM; 1:10 dilution). Secondary antibodies conjugated to rhodamine, 7-amino-4-methyl-6-sulfocoumarin-3-acetic acid (AMCA), or dichlorotriazinylaminofluorescein (DTAF) were obtained commercially from Jackson ImmunoResearch (West Grove, PA), Chemicon, or Cappel (Aurora, OH). Hoechst number 33342 (Sigma; used at 10 μm) was used for nuclear staining. Fixed cryosections and cultures were washed three times with PBS, permeabilized with 0.3% Triton X-100 in PBS for 3 min, washed three times with PBS, and blocked in 1% skim milk, 10% donkey serum, and 10% normal goat serum in PBS for 1 hr at room temperature, followed by incubation with primary antibodies diluted in the same blocking buffer for 1 hr at 37°C or overnight at 4°C. After being washed three times with PBS, they were stained with Hoechst diluted in PBS for 10 min, washed three more times with PBS, then incubated with secondary antibodies diluted in PBS for 1 hr at room temperature. After another three washes with PBS, the samples were mounted on slides and examined with a Zeiss LSM510 confocal imaging system or Zeiss Axioplan2. The cryosections were autoclaved in 0.01 m sodium citrate buffer at 110°C for 10 min before PCNA staining. The proportions of neurons and neuroepithelial cells in telencephalons (Figs. 1Q,R) were calculated based on the MAP2, nestin, and Hoechst staining of high-density cultures described below. The total number of each type of these cells could not be directly compared between wild-type andHes1−/− mice, because the number of cells in manually dissected brain tissues usually varies greatly. A minimum of 1000 cells in 10 randomly chosen microscopic fields was counted per dish, and the values obtained from at least three animals were averaged and presented as means ± SEM.
Fig. 1.
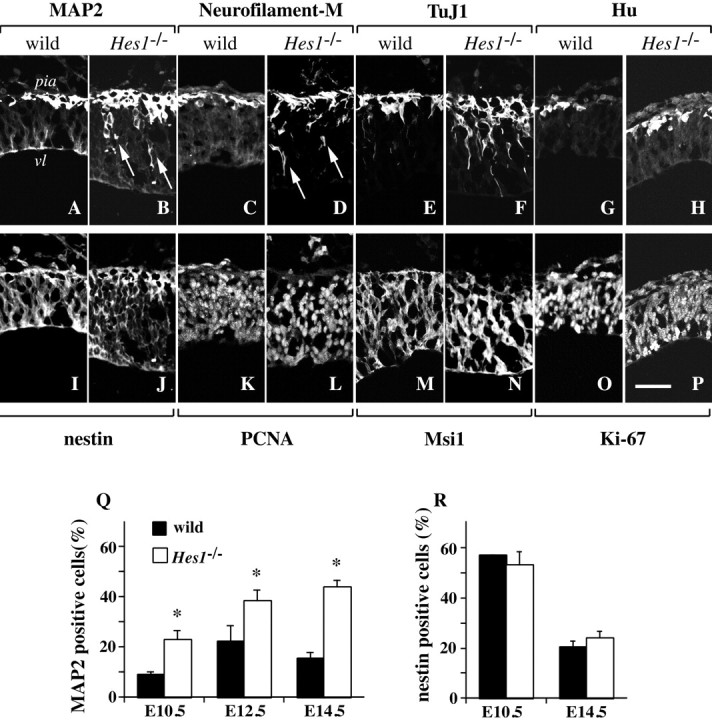
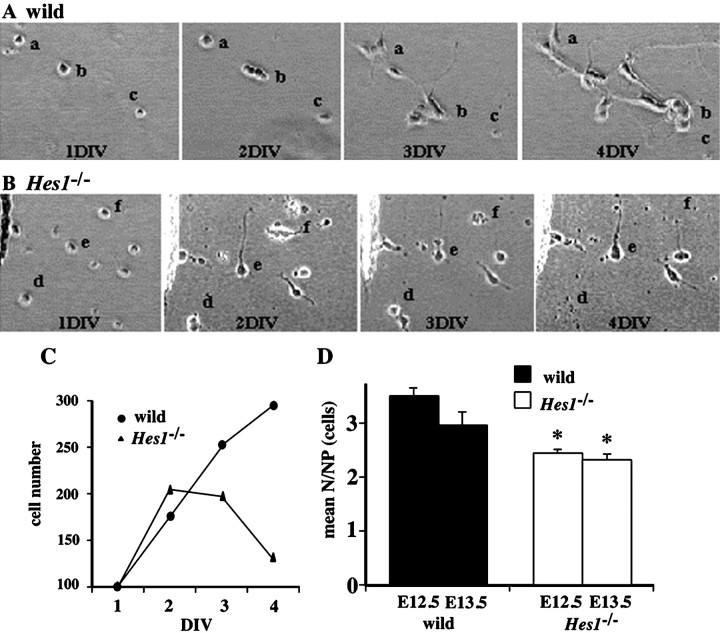
Neurons increase in theHes1−/− brain, without a detectable change in the number and distribution of neural progenitor cells.A–P, Immunohistochemical analyses of neurons and neural progenitor cells in the brain. Cryosections of wild-type andHes1−/− embryos at E10.5 were double-stained with neuronal markers (top row) and neural progenitor cell markers (bottom row): MAP2/RAT401 (A, I; B, J), neurofilament-M/PCNA (C, K; D, L), TuJ1/Msi1 (E, M; F, N), and Hu/Ki-67 (G, O; H, P). Panels aligned above and below show identical views of the lateral region of the mesencephalon. The top of each panel corresponds to the pial side. The genotype of the mouse is indicatedabove each pair of double-stained panels. vl, Ventricle; pia, pial side of the mesencephalon. Scale bar:P, 50 μm. The numbers of TuJ1+ or Hu+ newborn neurons, as well as MAP2+ or NF+ mature neurons were increased in the Hes1−/− brain compared with wild-type. However, the distribution of neural progenitor cells was not significantly changed by the Hes1 mutation. Furthermore, premature expression of late neuronal markers near the ventricle was observed in Hes1−/− mice (B, D, arrows). Q, R, Immunocytochemical analyses to quantify the fractions of MAP2+ neurons (Q) and nestin+ neural progenitor cells (R). Wild-type andHes1−/− brains were dissociated (embryonic stages are indicated below each bar) and stained for MAP2 or nestin, then the ratio of marker-positive cells to total cells was calculated and presented as the mean ± SEM(%). (*p < 0.01 in comparison to wild-type.) The fraction of Hes1−/− neurons was markedly increased, approximately twice that of wild-type, and continued to increase until a later stage (E14.5) (Q). In contrast, there was no statistical difference in the fraction of nestin+ neural progenitor cells between wild-type and Hes1−/− mice at any developmental stage (R).
Primary cultures
Fetuses were removed from the uterus of timed pregnant mice, and placed in Petri dishes containing PBS. Telencephalons were freed from meninges, and the cells were dissociated by mechanical trituration with a fire-narrowed Pasteur pipette. After centrifugation at 1000 rpm for 5 min, cells were resuspended in culture medium, seeded onto dishes specifically prepared for each experiment as described below, and then incubated at 37°C in a humidified atmosphere of 5% CO2. Culture medium was changed every 2 or 3 d. The day the cells were plated was defined as 0 d in vitro (0 DIV).
High-density culture. Cell suspensions were seeded onto polyethylenimine (Sigma)-coated cover slips in 24-well culture dishes at 5 × 105 cells/cm2. Cells were incubated until attached for immunostaining to determine the ratios of neurons and neuroepithelial cells (Figs. 1Q,R), or for 4 d, when they were examined for apoptosis (Fig.4E), in DMEM/F-12 (1:1) (Life Technologies) supplemented with 10% fetal bovine serum (FBS) (JRH Biosciences, Lenexa, KS). For the experiments assessing rescue from apoptosis (Fig. 4F), cells were incubated for 4 d in defined medium containing DMEM/F-12 (1:1) supplemented with 15 μg/ml insulin (Life Technologies), 25 μg/ml transferrin (Life Technologies), 20 nm progesterone (Sigma), 30 nm sodium selenite (Sigma), and 60 nmputrescine (Sigma). Twenty nanograms per milliliter neurotrophic factor 3 (NT-3; Amgen Inc., Thousand Oaks, CA) or 50 ng/ml brain-derived neurotrophic factor (BDNF; Amgen) was added after each medium change at 0, 1, and 3 DIV.
Fig. 4.
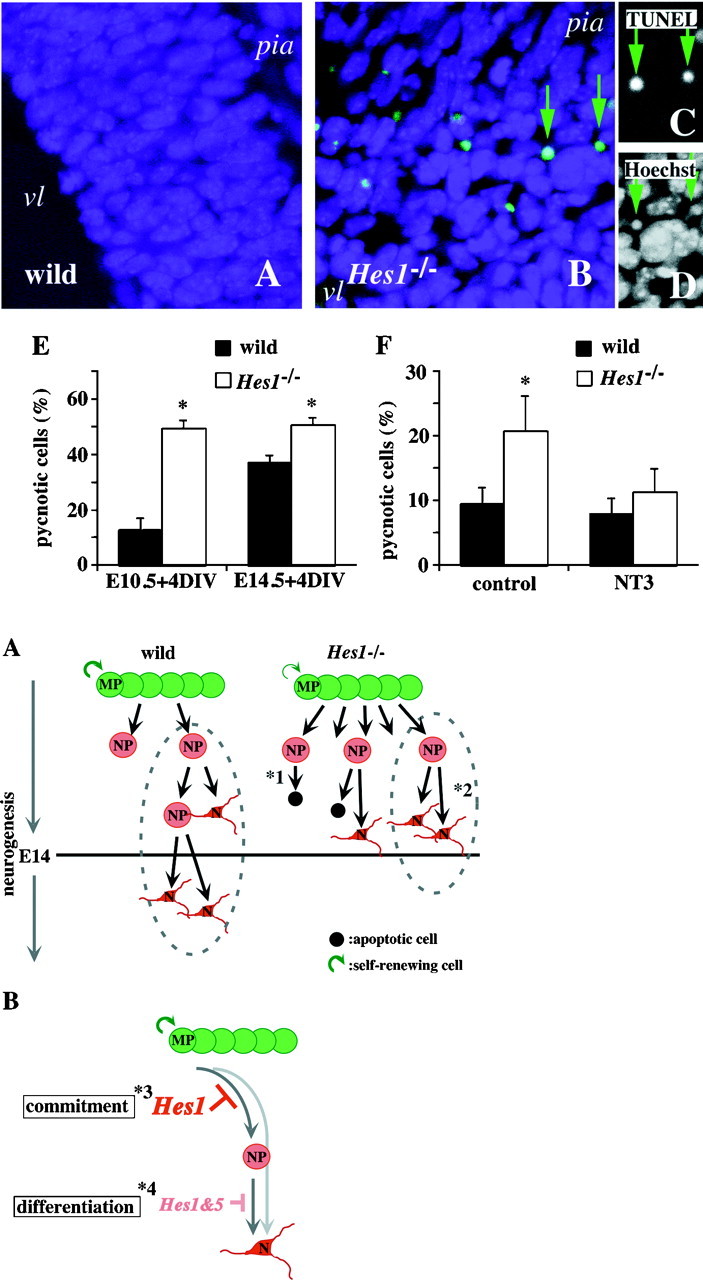
Accelerated apoptosis in theHes1−/− brain. Apoptotic cells of an E10.5 brain were stained by the TUNEL method (green; DTAF) in wild-type (wild, A) andHes1−/−(Hes1−/−; B–D) mice. Clustered apoptotic cells were observed near the ventricle (vl) in the Hes1−/− brain, whereas apoptotic cells were seldom detected in the wild-type brain. Two apoptotic cells (B, arrows) are also shown by TUNEL (C) and Hoechst staining (D), respectively. The TUNEL+ cells (C, arrows) were also pyknotic (D, arrows), suggesting they were apoptotic rather than necrotic. Blue, nuclear staining with Hoechst;pia, pial side of the telencephalon. E, The fraction of apoptotic cells in vitro. The ratios of pyknotic (apoptotic) cells to total cells were determined by examining stained nuclei and presented as the means ± SEM of at least three independent experiments. *p < 0.01 in comparison with wild-type. The developmental stages of the embryos used are indicatedbelow each bar. The apoptotic cells in the wild-type brain increased from E10.5 + 4 DIV to E14.5 + 4 DIV. In contrast, approximately half of the cells from theHes1−/− brain died within 4 DIV, independent of their original developmental stage. F, Apoptosis rescued with NT-3. Dissociated telencephalic cells from E12.5 wild-type (wild) and Hes1−/−(Hes1−/−) mice were cultured for 4 DIV in defined medium (control). Twenty nanograms per milliliter NT-3 was added to this defined medium for assessing rescue from apoptosis by NT-3. The percentage of apoptotic cells in the telencephalic cultures was estimated at 4 DIV based on nuclear staining, and presented in each bar as the means ± SEM of at least three independent experiments. *p < 0.01 in comparison with wild-type or control. A large fraction of apoptotic cells was observed in theHes1−/− cultures in the absence of NT-3 (control), which was markedly reduced by the administration of NT-3. In contrast, NT-3 had only a small effect on the level of apoptosis in the wild-type cultures.
Low-density culture. For clonal analyses of single neuronal progenitor cells (Fig. 3), we used a low-density culture system with a feeder layer of astrocytes, which supports the proliferation and long-term survival of isolated immature cells (Temple, 1989; Sakakibara et al., 1996). Astrocytes harvested from the telencephalons of perinatal ICR mice (Charles River) were maintained for ∼3 weeks until subcultured as follows (Cohen and Wilkin, 1995; Sakakibara et al., 1996). Silicon rubber rings (0.5-mm-thick, 8 mm inner diameter) were attached to the base of polyethylenimine-coated 35 mm plastic culture dishes, to create separate culture spaces. Astrocytes were plated outside the ring. The inner space was coated again with fibronectin (10 μg/ml; Sigma), and the telencephalic cells to be tested were seeded at a density of 1 × 103cells/cm2. After the cells had attached to the substratum, the rings were removed, and fresh medium was added [DMEM/F-12 (1:1) containing 10% FBS, 1.7 ng/ml basic fibroblast growth factor (bFGF; Wako), 3.3 ng/ml epidermal growth factor (EGF; Wako), 10 ng/ml 2.5 S nerve growth factor (NGF; Wako), 20 nm progesterone, 30 nm sodium selenite, 15 μg/ml insulin, and 25 μg/ml transferrin]. We followed the fates of single progenitor cells for 4 d using a phase-contrast microscope (Olympus, Tokyo, Japan; IX70), identifying these cells from their positions relative to scratches made on the culture dishes with tungsten needles. Images of cells were recorded using a laser videodisc recorder LVR-3000AN (Sony, Tokyo, Japan) or Image Grabber/PCI (Neotech) equipped with a 3 CCD color video camera DXC930 (Sony). Long-term low-density culture experiments using wild-type mice demonstrated that 99% of the proliferating cells generated only neurons (data not shown).
Fig. 3.
The developmental profiles of neuronal progenitor cells was followed in low-density culture. A, B, The dissociated cells derived from E13.5 telencephalons of wild-type (A) and Hes1−/− mice (Hes1−/−, B), were clonally cultured, and phase-contrast photos of identical fields were obtained at 1, 2, 3, and 4 DIV. The letters a–f in each panel are placed beside the colony derived from the same single progenitor cell. For instance, in colony b in the wild-type culture (A), the single progenitor cell at 1 DIV underwent several cell divisions, and four cells were observed at 2 DIV. They extended their processes toward the neighboring colony a and appeared to form network-like connections by 3 DIV. This network of cells became better established, and cells showed a more differentiated neuronal morphology with long and branched processes by 4 DIV. These cells also appeared to keep dividing until 4 DIV. In contrast, in coloniesd and f in theHes1−/− culture (B), single progenitor cells divided once by 2 DIV, but the resulting cells soon fell into apoptosis (d at 4 DIV; f at 3 DIV). The Hes1−/− neuron ein (B) extended thick processes rapidly by 2 DIV, but network-like connections were not formed as in the case of the wild-type culture at 4 DIV. C, Proliferating profiles of neuronal progenitor cells. The number of cells that originated from 100 neuronal progenitor cells in the low-density culture are summarized at the indicated day below the line graph. In the wild-type culture, progenitor cells kept increasing in number during the observation period. In contrast, the initial proliferation of progenitor cells was not disturbed by the Hes1mutation, but the cells stopped proliferating after 2 DIV and decreased in number (by apoptosis) in the Hes1−/−culture. D, The average number of neurons derived from a single neuronal progenitor cell. Numbers of neurons are represented in each bar as the means ± SEM of ∼100 NPs of three independent experiments. *p < 0.05 in comparison with the wild-type control of the same stage. The embryonic stages of the mice are indicated below each bar. The mean N/NP was reduced inHes1−/− mice, irrespective of the developmental stage.
Neurosphere assay. Standard procedures were followed, as described in previous papers (Vescovi et al., 1993; Reynolds and Weiss, 1996; Tropepe et al., 1999). The basic culture medium for the neurosphere assay was composed of DMEM/F-12 (1:1) (Life Technologies) supplemented with 25 μg/ml insulin, 100 μg/ml transferrin, 20 nm progesterone, 60 μm putrescine, and 30 nm sodium selenite. Twenty nanograms per milliliter EGF and 20 ng/ml bFGF (R and D, MN), for the proliferation medium, and 1% FBS, for the differentiation medium (EGF and bFGF-free medium), were added to the basic culture medium for the neurosphere assay. It is known that virtually all spheres are clonally derived, when cells are plated at a density <5 × 104 cells/ml (Hulspas et al., 1997).
Primary sphere formation assay. Mechanically dissociated telencephalic cells were plated at 1 × 105cells/3 ml proliferation medium in each well of a 6 well plate (Corning), with no substrate pretreatment.
Secondary sphere formation assay. Primary spheres were collected and digested with 0.25% trypsin-EDTA (Life Technologies) for 5 min at 37°C. They were then gently triturated with a fire-narrowed Pasteur pipette, spun down at 400 rpm for 3 min, resuspended in proliferation medium, and plated at 500 cells/200 μl in each well of a 96 well ultra-low cluster plate (Corning). The numbers of primary and secondary spheres were counted at 10–14 DIV.
Sphere differentiation assay. Assay slides (hydrophobic coated HT slides; Weaton) with 24 wells, 4 mm diameter, were coated with polyethylenimine and used for the sphere differentiation assay. Primary spheres were collected and transferred from the proliferation medium to the differentiation medium described above. Single primary spheres in 20 μl of differentiation medium were then transferred to each well of a precoated HT slide in a humidified 15 cm culture dish within a CO2 incubator. The medium was not changed for the remainder of the experiment. These slides were processed 10 d later for triple-labeled indirect immunocytochemistry to detect three major cell types: neurons, oligodendrocytes, and astrocytes, using TuJ1, anti-O4, and anti-GFAP antibodies, respectively. The phenotype of the progenitor was determined based on the cell types (neuron, astrocyte, and/or oligodendrocyte) that were present in the clone, regardless of the cell number. Approximately 24 spheres per each animal were examined in three independent experiments.
Detection of apoptosis
To detect apoptosis in vivo, cryosections were stained with the ApopTag Plus in situ apoptosis detection kit (Oncor), according to the manufacturer's instructions, with minor modifications. Before immunodetection of digoxigenin-labeled nicked DNA ends, sections were stained with Hoechst nuclear dye. DTAF-conjugated anti-digoxigenin antibody (Boehringer Mannheim; used at a dilution of 1:500) was used for the immunodetection of apoptotic cells.
Statistical analysis
The statistical significance of the variations was evaluated using an unpaired two-group t test (either Student's or Welch's t test was used depending on the F test;p > 0.01 for the Student's t test). Statistically significant differences between mutants and wild-type controls are indicated with an asterisk in the figures.
RESULTS
There are several developmental stages in neuronal life. Here, we define the most immature self-renewing stem-like cell as the “multipotent progenitor cell” (MP), which gives rise to both neurons and glia; and the unipotent and mitotic cell as the “committed neuronal progenitor cell” (NP), which only generates neurons. “Immature neurons” and “mature neurons”, before and after neuronal maturation, respectively, are discriminated by neuronal markers and morphological features. The term “neuronal differentiation” only refers to the postmitotic maturation of neurons in this paper, unless otherwise noted.
Hes1 functions before the expression of early neuronal markers
To find out at what point the Hes1 mutation started to affect cellular development, the expression patterns of early and late neuronal markers were examined. The late neuronal markers, MAP2 and neurofilament, are primarily expressed in postmigratory mature neurons (Izant and McIntosh, 1980; Debus et al., 1983; Crandall et al., 1986;Johnson and Jope, 1992). More MAP2+ mature neurons were observed in the Hes1−/− than in the wild-type brain, and MAP2 was prematurely expressed in migrating immature neurons (Fig. 1A,B, arrows), consistent with a recent report (Ohtsuka et al., 1999). Neurofilament-M was also prematurely expressed in the Hes1−/− brain (Fig.1C,D). The expression of the earliest markers for postmitotic immature neurons, TuJ1 and Hu (Lee et al., 1990; Menezes and Luskin, 1994; Sakakibara et al., 1996; Okano and Darnell, 1997;Wakamatsu and Weston, 1997), begins just after the final cell division. As shown in Figure 1E–H, TuJ1 and Hu seemed to label more newborn neurons in Hes1−/− mice and were expressed earlier in development near the ventricle. This premature expression of early and late neuronal markers was observed both in the telencephalon and mesencephalon of the mutant embryos (Fig.1 shows the lateral region of the mesencephalon). The number ofHes1−/− neurons in the brains was then quantified by counting immunostained dissociated telencephalic cells (Fig. 1Q). The fraction of MAP2+ neurons in the Hes1−/− telencephalon was approximately twice the number in wild-type (Fig. 1Q) (wild-type: E10.5, 9.0 ± 1.0%; E12.5, 22.1 ± 6.3%; E14.5, 15.5 ± 2.3%; Hes1−/−: E10.5, 22.8 ± 3.7%; E12.5, 38.3 ± 4.5%; E14.5, 43.9 ± 2.8%, *p < 0.01 in comparison with wild-type at the same developmental stages).
Thus, even at an early stage when immature postmitotic neurons were still migrating, the neurons were increased in theHes1−/− brain more than in wild-type. These facts suggest that Hes1 is likely to function in mitotic progenitor cells.
Neural progenitor cells of Hes1−/−mice were indistinguishable from wild-type by immunochemical analyses
To further investigate whether the overproduction of neurons in the Hes1−/− brain was attributable to an abnormality in the neural progenitor cells, the expression patterns of various markers for neural progenitor cells (MP + NP) were examined. The antibodies used in this study for the detection of neural progenitor cells were: (1) RAT401 (nestin), an intermediate filament specific to neuroepithelial cells, which correspond to neural progenitor cells (Hockfield and McKay, 1985; Frederiksen and McKay, 1988; Lendahl et al., 1990), (2) anti-PCNA, proliferating cell nucleus specific antigen (Galand and Degraef, 1989), (3) anti-mouse Musashi1 (Msi1), an RNA-binding protein highly enriched in neural progenitor cells (Sakakibara et al., 1996; Sakakibara and Okano, 1997; Kaneko et al., 1999), and (4) Ki-67, an antibody to cell proliferation-associated nuclear antigen (Gerdes et al., 1983; Schlüter et al., 1993;Thomson et al., 1998). Unfortunately, to date, there is no antibody that clearly distinguishes between MPs and NPs.
At E10.5, cells that were positive for these neural progenitor cell markers resided throughout the neural tube from the ventricle to the pia both in Hes1−/− and wild-type mice (Fig. 1I–P). Brains from E12.5 embryos also failed to show differences in the distribution of neural progenitor cells between the two genotypes (data not shown). Because nestin is expressed on neural fibers, instead of in cell bodies (which are readily counted), it remains unclear whether the number of nestin+ neural progenitor cells was actually unchanged. To quantify the number of neural progenitor cells, telencephalons were dissociated and immunostained. The ratio ofHes1−/− nestin+cells to the total number of cells counted was not statistically different from wild-type (Fig. 1R). Taken together, the immunochemical examinations failed to detect any difference in the distribution and number of neural progenitor cells between theHes1−/− and wild-type embryonic brain.
Thus, immunochemical analyses demonstrated that the premature differentiation and overproduction ofHes1−/− neurons were not accompanied by any apparent change in the total population of neural progenitor cells (MP + NP).
Self-renewing ability of Hes1−/−MPs was decreased
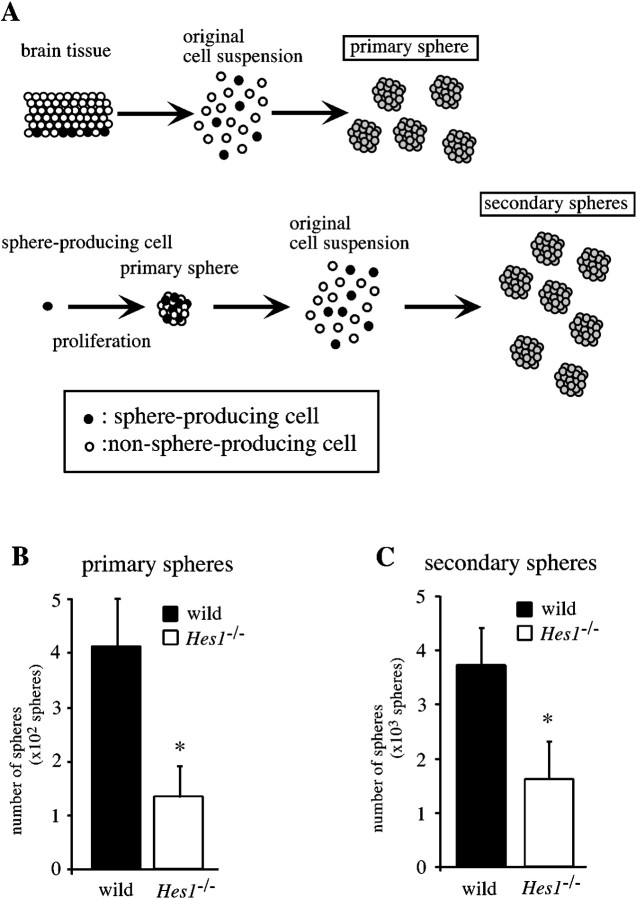
Next, to determine whether Hes1−/−neural progenitor cells were functionally affected, putative MPs were studied using a neurosphere-formation assay (Reynolds et al., 1992;Reynolds and Weiss, 1996; Tropepe et al., 1999). In the presence of mitogens such as EGF and bFGF, neural progenitor cells proliferate and form clonally derived neurospheres. Most of the sphere-producing progenitor cells are self-renewing and multipotent progenitor cells (Reynolds and Weiss, 1996). In the present study, putative MPs (sphere-producing cells) in the brain were isolated by primary neurosphere formation, and their self-renewing capacity was demonstrated by observing the number of secondary neurospheres formed (Fig. 2A).
Fig. 2.
Self-renewing capability of multipotent progenitor cells is lower in Hes1−/− mice (neurosphere formation assay). A, Schematic representation of the experimental approaches used to investigate the self-renewing capability of MPs. The number of neurospheres retrospectively indicates the number of sphere-producing cells in the original cell suspension. Because most spheres are clonally derived from self-renewing and multipotent progenitor cells, the number of primary spheres indicates the MP population in the brain, and that of the secondary spheres indicates the MP population in primary spheres. Considering that the primary spheres are the products of the proliferation of the sphere-producing cells, the number of secondary spheres is likely to represent the self-renewing capability of MPs. B, Telencephalic cells of E10.5 wild-type andHes1−/− brains were dissociated and plated at 1 × 105 cells per well of a 6-well plate, and the resultant primary spheres in each well were counted. Note that the number of Hes1−/− primary spheres was smaller, revealing the decreased MP-population in theHes1−/− brain. C, These primary spheres were collected, dissociated, and replated at 500 cells per well of a 96-well plate. The number of resultant secondary spheres on each plate was counted (derived from the originally plated 500 × 96 = 4.8 × 104 cells). The decreased number of secondary spheres represents the lowered self-renewal activity of Hes1−/− MPs. All data are presented as the mean ± SEM of three independent culture experiments. *p < 0.01 in comparison with the wild-type control.
Telencephalic cells were dissociated and plated at 1 × 105 cells per well in 6-well plates, cultured for 10 d in proliferation medium containing EGF and bFGF, and the number of primary spheres per well was counted (Fig.2B). The mean number ofHes1−/− primary spheres was one-third of that in wild-type cultures (wild-type, 409 ± 89 spheres;n = 15; Hes1−/−, 133 ± 54 spheres; n = 8 per 1 × 105 cells; *p < 0.01). To examine whether this decrease in the number ofHes1−/− primary spheres was merely subsequent to the accelerated neurogenesis, the number of primary spheres for wild-type brains at E12.5 versusHes1−/− at E10.5 were compared, because the fraction of MAP2+ neurons in the E10.5Hes1−/− brain corresponded to that of the E12.5 wild-type brain (Fig. 1Q). We generated 319 ± 31 spheres (n = 3 per 1 × 105 cells; *p < 0.01) from E12.5 wild-type telencephalic cells, a number that is still 2.5 times that for Hes1−/− brains at E10.5. Thus, the decrease in the number of primary spheres indicates that there are fewer sphere-producing cells in theHes1−/− brain.
In the secondary sphere formation assay, primary spheres were dissociated to form a suspension of single cells, then cultured in the proliferation medium at a density of 500 cells/0.2 ml per well of a 96-well plate, and the secondary spheres that formed were counted 2 weeks later (Fig. 2C). As schematically shown in Figure2A, the number of secondary spheres corresponds to the number of sphere-producing cells within the primary sphere, which is itself the product of the proliferation of a single sphere-producing cell. Therefore, the number of secondary spheres represents the frequency of self-renewing cell divisions arising from the original sphere-producing cell. The size of the primary neurospheres was not reduced in Hes1−/− mice, indicating that the ability of the sphere-forming cells to proliferate was not affected by the Hes1 mutation. There were, however, fewerHes1−/− secondary spheres than wild-type secondary spheres [wild-type, 3.7 × 103 ± 0.7 spheres, n = 5;Hes1−/−, 1.6 × 103 ± 0.7 spheres, n = 4, per each plate (500 cells × 96 wells = 4.8 × 104 original cells), *p < 0.01), indicating that the loss of the Hes1 gene lowered the self-renewing capability of the sphere-producing cells.
These results indicate that Hes1 functions to keep MPs in a self-renewing state. The next question we asked was whetherHes1 affected the commitment of MPs to a specific cell lineage.
Hes1 represses the commitment of MPs to the neuronal lineage
We examined whether the Hes1 mutation affected the multipotency of MPs using a neurosphere differentiation assay. Most neurospheres are derived from multipotent and self-renewing progenitor cells (Reynolds and Weiss, 1996). However, it has also been demonstrated that a certain percentage of neurospheres is derived from committed progenitor cells, which differentiate only into a specific cell type (Reynolds and Weiss, 1996). Here, MPs and NPs, which are indistinguishable by immunochemical analyses, were distinguished retrospectively using a neurosphere differentiation assay. Because one neurosphere is clonally derived from a single progenitor cell, cell types contained within a neurosphere reveal the lineage potency of the original progenitor.
We prepared primary neurospheres derived from E10.5 telencephalons of wild-type and Hes1−/− embryos. These were clonally transferred onto polyethylenimine-coated glass slides for culturing (HT slide), one sphere per well, then were cultured in the absence of EGF and bFGF and in the presence of 1% fetal bovine serum (differentiation medium) and processed for triple-labeled indirect immunocytochemistry after 10 d to detect three major cell types. Neurons, oligodendrocytes, and astrocytes were detected using TuJ1, anti-O4, and anti-GFAP antibodies, respectively. The clone type composition of the primary neurospheres is summarized in Table1. More than 90% of wild-type neurospheres differentiated into clones containing neurons and glia, and thus were originally multipotential. Less than 2% of the wild-type spheres were neuronal clones. In contrast, ∼20% of theHes1−/− neurospheres were neuronal clones, and the fraction of multipotential clones was 73%. The fraction of glial clones was not significantly affected by theHes1 mutation (Table 1).
Table 1.
Phenotype composition of clonally derived primary spheres
| N | NG | G | |||||
|---|---|---|---|---|---|---|---|
| Experimental paradigm | N | NA | NAO | NA + NAO | AO | A | AO + A |
| Wild-type (n = 11) | 1.9 ± 1.4 | 40.5 ± 5.1 | 50.6 ± 8.1 | 91.0 ± 4.0 | 0.0 ± 0.0 | 7.1 ± 3.6 | 7.1 ± 3.6 |
| Hes1−/− (n = 7) | 20.8 ± 5.1* | 31.3 ± 5.7* | 39.5 ± 8.7* | 73.2 ± 2.9* | 3.7 ± 1.9 | 3.3 ± 2.2 | 7.0 ± 2.9 |
Accelerated commitment to the neuronal lineage in theHes1−/− brain. The phenotype of each primary sphere was determined using the neurosphere differentiation assay and was based on the cell types contained in the clone. The clone type composition in each animal was averaged and presented as mean ± SEM. *p < 0.01 in comparison with wild-type control (unpaired two group t test). The phenotypes of the clones are represented by capital letters: N, neurons; A, astrocytes; O, oligodendrocytes; and G, glia. The numbers of neuronal clones were significantly increased, and in turn, the numbers of neuronal/glial clones (multipotent clones) were decreased inHes1−/− mice. Glial clone composition was not significantly different between wild-type andHes1−/− mice.
These results directly show that Hes1 plays a role in repressing the commitment of MPs to the neuronal lineage, but not to the glial lineage, at least in the telencephalon.
Reduced neuronal production by singleHes1−/− NPs
To characterize the NPs that were overproduced by the accelerated neuronal commitment of MPs in Hes1−/−mice, we took advantage of a low-density culture technique (Sakakibara et al., 1996) as a clonal analysis procedure for demonstrating the developmental potential of NPs (i.e., the number of daughter neurons generated from single NPs). Under our low-density culture conditions, most proliferating cells were NPs, generating only neurons in long-term culture (data not shown). Given this information, the fates of clonally plated proliferating cells (putative NPs) were recorded, and their developmental potentials were retrospectively identified based on the numbers and morphologies of the daughter cells.
Figure 3,A and B, shows the representative patterns of neuronal production from NPs that were prepared from E13.5 telencephalons of wild-type (A) and Hes1−/−(B) mice. As shown in Figure 3A, the wild-type NPs (Fig. 3Aa, Ab) slowly divided several times, generating a cluster of cells with long processes. In contrast, mostHes1−/− NPs (Fig. 3Bd, Bf) divided once, the daughter cells extended short processes within 2 d, and they eventually became apoptotic (Fig.3Bd, Bf, 3-4 DIV). The relatively short processes of theHes1−/− neurons did not form network-like connections among neighboring cells, as seen in the wild-type cultures. However, the maturation of theHes1−/− neurons, which was recognized by the extension of their processes (Fig. 3Be, Bf, 2 DIV), appeared to be accelerated, consistent with the premature expression of late neuronal markers in vivo (Fig. 1B–D, arrows). Some of the Hes1−/−neurons also survived beyond the observation period (Fig.3Be), as did most wild-type neurons.
Figure 3C represents the proliferation pattern of wild-type and NPs Hes1−/−, showing the number of daughter cells derived from 100 NPs in the low-density cultures. Although the wild-type NPs showed a continuously increasing number of daughter cells during 4 DIV, Hes1−/−NPs increased to twice their original number until 2 DIV (indicating they quickly divided once), then stopped dividing or fell into apoptosis. As a result, the average number of total neurons generated from single NPs was reduced in Hes1−/−mice (Fig. 3D). The number of neurons was reduced irrespective of the developmental stage at which the culture was started, indicating that it did not simply result from an advanced developmental stage (wild-type: E12.5, 3.5 ± 0.2 cells; E13.5, 2.9 ± 0.2 cells; Hes1−/−: E12.5, 2.4 ± 0.1 cells; E13.5, 2.3 ± 0.1 cells; ∼100 NPs from each of three Hes1−/− and five wild-type mice were observed in three independent experiments; *p < 0.05). The cell cycle length ofHes1−/− NPs was not prolonged, as indicated by the steep rise in the line graph during first 2 d (Fig. 3C).
Thus, it seems that the Hes1−/− NPs escaped from their mitotically active state much earlier than wild-type NPs (without reducing their proliferation rate), so the resultant number of their daughter neurons was reduced after 4 d in culture. The poor survival of Hes1−/− cells was the next issue we examined (Fig. 4).
Accelerated apoptosis of theHes1−/− NPs
As described above, Hes1−/−cultured cells seldom survived beyond 5 DIV, and mutant embryos sometimes exhibited anencephaly, suggesting accelerated apoptosis in the Hes1−/− brain (Ishibashi et al., 1995). To explore this possibility and investigate the cell types undergoing apoptosis, we examined the distribution of apoptotic cells in the brain. Figure4A–D shows TUNEL+ apoptotic cells, and their nuclear morphology is revealed by staining with Hoechst in the E10.5 brains. In the wild-type brains, dying cells were rare at E10.5, in agreement with previous observations (Blaschke et al., 1996). In contrast, there were TUNEL+ apoptotic cells clustered near the ventricle in the E10.5 Hes1−/− brains. Figure4B shows one of the clusters of TUNEL+ cells in theHes1−/− telencephalon. These TUNEL+ cells appeared to be apoptotic rather than necrotic, based on the pyknotic nuclei (Fig. 4C,D). These apoptotic cells were likely to be neural progenitor cells and/or immature neurons, but not mature neurons, given their location (Bayer and Altman, 1991). This accelerated apoptosis was quantified by nuclear staining of dissociated telencephalic cells at 4 DIV (Fig.4E). In the wild-type cultures, the fraction of apoptotic cells increased as neurogenesis progressed (from E10.5 to E14.5), consistent with the in vivo time course (Blaschke et al., 1996) (wild-type: E10.5 + 4 DIV, 12.8 ± 4.3%; E14.5 + 4 DIV, 36.9 ± 2.8%). However, approximately half of the cultured Hes1−/− cells underwent apoptosis (Hes1−/−: E10.5 + 4 DIV, 49.4 ± 3.0%; E14.5 + 4 DIV, 50.8 ± 2.4%; *p < 0.01 compared to wild-type), independent of their original developmental stages.
To further investigate the cell types undergoing accelerated apoptosis in the Hes1−/− brains, we examined the effects of neurotrophic factors on cultured cells. NT-3 is a neurotrophic factor that acts on mitotic neuronal progenitor cells to promote their withdrawal from the cell cycle (DiCicco-Bloom et al., 1993; Ghosh and Greenberg, 1995), rather than supporting the survival of postmitotic neurons (Ghosh and Greenberg, 1995). BDNF is thought to support the survival of mature neurons (Ghosh et al., 1994; Jones et al., 1994; Nawa et al., 1994; McAllister et al., 1995). Sister cultures were prepared from E12.5 Hes1−/− and wild-type telencephalons and cultured for 4 DIV in defined medium with or without one of the neurotrophic factors, then fixed and stained with Hoechst to estimate the ratio of apoptotic to total cells. In the absence of NT-3, the percentage of apoptotic cells in theHes1−/− culture was approximately twice that in the wild-type culture (Fig. 4F) (wild-type, 9.4 ± 1.6%; Hes1−/−, 20.7 ± 5.3%) (*p < 0.01). Administration of NT-3 greatly reduced the percentage of apoptotic cells in theHes1−/− culture, whereas it had only a small effect on the level of apoptosis in the wild-type culture (Fig.4F) (wild-type, 7.9 ± 2.3%;Hes1−/−, 11.2 ± 3.6%). Similar experiments were performed using BDNF, but a significant reduction of apoptotic cells was not seen for eitherHes1−/− or wild-type cultures (data not shown). Thus, it was suggested that NT-3-responsive NPs, which were abnormally committed to the neuronal lineage by the Hes1mutation, were undergoing apoptosis. However, it is also possible that some populations of immature neurons became apoptotic as a secondary effect of premature neuronal differentiation in theHes1−/− brains.
Our present results can be summarized as follows (Fig. 5A). The Hes1 mutation reduces the self-renewing ability of neural progenitor cells, and in turn accelerates the commitment of MPs to the neuronal lineage. The resultant NPs are increased in number, are functionally abnormal (i.e., they generate fewer daughter neurons, Fig.3), and they follow an apoptotic fate.
DISCUSSION
Neuronal determination, differentiation, or both?
Neuronal development encompasses various events that occur throughout neuronal life, including the cell-fate decision (neuronal commitment), and postmitotic maturation (a narrow definition of neuronal differentiation). Hes1 is expressed in the VZ at a high level (Sasai et al., 1992), and neurons are overproduced in theHes1−/− brain (Fig.1A–H; Ohtsuka et al., 1999), raising the possibility that Hes1 mainly represses commitment to the neuronal fate. However, because there was no evidence specifying the function ofHes1 in neuronal commitment, the role of Hes1 was identified—rather broadly—as a repressor of neuronal differentiation. To clarify the exact role of Hes1 in neuronal development, the cellular development of the Hes1−/−brain was examined here in detail.
Hes1 is known to negatively regulate neuronal differentiation: persistent expression of Hes1 in neural progenitor cells in the VZ prevents expression of late neuronal markers (Ishibashi et al., 1994). The post-translational inhibition ofHes1 is required for the neuronal differentiation of PC12 cells induced by NGF signaling (Ström et al., 1997). The present study demonstrated that the Hes1−/−immature neurons prematurely expressed late neuronal markers while they were still migrating (Fig. 1B,D, arrows). The low-density culture experiments demonstrated a quicker extension of neuronal processes in Hes1−/− than in wild-type cultures (Fig. 3Be). Thus, the role ofHes1 in negatively regulating neuronal differentiation (maturation) is well supported.
Furthermore, we showed in this paper that Hes1 is necessary for neural progenitor cells to maintain their capacity for self-renewal and to repress the neuronal commitment of MPs, as follows.
First, we investigated whether or not Hes1 plays its role in progenitor cells. TuJ1 and Hu are very early markers for postmitotic neurons that have just finished their final cell division (Lee et al., 1990; Menezes and Luskin, 1994; Sakakibara et al., 1996; Okano and Darnell, 1997; Wakamatsu and Weston, 1997), whereas MAP2 and neurofilament are late neuronal markers for postmigratory, mature neurons (Izant and McIntosh, 1980; Niinobe et al., 1988). Considering that even TuJ1 and Hu are prematurely expressed in vivo in the Hes1−/− brain (Fig.1F,H), it is most likely that the absence ofHes1 affects the mitotic progenitor cells, (which do not express these markers), that is, MPs and/or NPs. In addition, the accelerated apoptosis that was observed near the ventricle in theHes1−/− brain responded to NT-3, but not to BDNF, indicating that it was the NPs, rather than mature neurons, that underwent apoptosis. On the other hand, many mature MAP2+ neurons were observed in theHes1−/− brain in vivo (Fig.1B,D), and someHes1−/− neurons in the low-density cultures survived beyond the observation period in vitro, as did wild-type neurons (Fig. 2A,B). Thus, mitotic progenitor cells (MPs and/or NPs), rather than mature neurons, are likely to be the target of Hes1 action.
Second, our experiments using neurospheres, floating cell clusters derived from single sphere-producing cells (putative MPs) (Reynolds et al., 1992; Vescovi et al., 1993; Reynolds and Weiss, 1996), demonstrated that the self-renewing capability ofHes1−/− MPs is lower than that of wild-type MPs (Fig. 2). This weaker self-renewing activity indicates that the Hes1 mutation converted the proliferation mode of MPs from self-renewal to non-self-renewing. This conversion further suggests that Hes1 acts on the MPs and maintains their capacity for self-renewal.
Finally, the neurosphere differentiation assay showed thatHes1 functions to repress the neuronal commitment. The neurosphere differentiation assay is capable of discriminating, retrospectively, the multipotency and unipotency of the original sphere-producing cells. In wild-type mice, >90% of the sphere-producing cells were multipotent, meaning they proliferate and differentiate into neurons and glia, and <2% generated only neurons (Table 1). In Hes1−/− mice, however, >20% of the sphere-producing cells generated only neurons, and ∼70% were multipotent. The increased number of neuronal clones in the sphere differentiation assays, together with the increased responsiveness of Hes1−/− cells to NT-3 (Fig. 4F), indicate that the number of NPs is increased in the Hes1−/− brain. These results suggest that Hes1 normally prevents the MPs from being committed to the neuronal lineage.
These observations strongly suggest that Hes1 functions to maintain the self-renewing state of MPs by repressing their commitment to the neuronal lineage. The premature differentiation ofHes1−/− neurons might be a consequence of the accelerated neuronal commitment of the MPs, from which an increased number of abnormal NPs are generated inHes1−/− mice. These points, together with the fact that Hes1 is normally expressed in the VZ, lead to the conclusion that Hes1 mainly functions to repress neuronal commitment (Fig. 5B). However, our present experiments do not rule out the possibility that Hes1 is involved in both neuronal commitment and differentiation at various steps in mammalian CNS development, as is another bHLH gene,NeuroD, in Xenopus (Lee et al., 1995) and murine retina (Morrow et al., 1999).
Regulatory cascades in neurogenesis
The negative bHLH transcription gene Hes1 was originally identified as a mammalian homolog of hairy, which is a downstream target of the Drosophila Notch signaling pathway (Akazawa et al., 1992; Sasai et al., 1992). Here, our observations extend our knowledge of mammalian neurogenesis by examining the molecular basis of the action of Hes1.
It is known that the cascades of positive and negative bHLH transcription factors play important roles in mammalian neurogenesis (for review, see Kageyama et al., 1995; Lee, 1997). Recently, several groups proposed that Mash1, a positive bHLH gene, is required to specify intermediate progenitors (Lo et al., 1991; Casarosa et al, 1999; Torii et al., 1999). Mash1 mutant mice exhibit a severe loss of neuronal progenitor cells in the subventricular zone (Casarosa et al., 1999), and the onset of Mash1 expression coincides with the induction of differentiation (broad definition) of self-renewing stem cells in vitro (Torii et al., 1999). Considering that the transcription of Mash1 is negatively regulated by Hes1, these proposals support our conclusion that Hes1 represses the first step of MP differentiation (neuronal commitment).
Notch signaling is known to be evolutionarily conserved, andHes1 appears to function as an effector gene of mammalian Notch signaling together with Hes5, in a redundant manner (Ohtsuka et al., 1999). Mammalian Notch signaling has pleiotropic functions. First, it negatively controls the rate at which stem cells differentiate, to avoid exhausting the stock of stem cells (Artavanis-Tsakonas et al., 1995; Tanabe and Jessell, 1996). Second, the proper regulation of Notch signaling is also essential for asymmetric cell division (Guo et al., 1996; Jan and Jan, 1998). Third, activation of Notch signaling inhibits apoptosis (Artavanis-Tsakonas et al., 1999). These functions of Notch signaling are consistent with our results. First, Hes1−/− MPs failed to maintain their self-renewing progenitor state and prematurely moved to the next developmental state (NP) (Fig. 2, Table 1). Second, the asymmetric cell division of the Hes1−/−neural progenitor cells may be altered, resulting in two neurons, instead of one progenitor cell and one neuron in vitro(Figs. 3, 5A). Third, interrupted Notch signaling may have accelerated the apoptosis in theHes1−/− brain (Fig. 4).
The number of neurons
The present study provided two seemingly inconsistent observations about the number of neurons: Hes1−/−neurons were overproduced in vivo (Fig. 1), whereas each of the Hes1−/− NPs produced fewer neurons after 4 d in culture (reduced N/NP) (Fig. 3). These results can be interpreted as follows: In Hes1−/−mice, the increased number of NPs that results from the accelerated neuronal commitment of the MPs may dominate over the effects of the reduced number of progenitor divisions and of apoptosis, leading to an increase in the total number of neurons. The reduced number of N/NPs appeared to result from the Hes1−/− NPs becoming prematurely postmitotic, consistent with another aspect of the function of Hes1, in that alleviating the normalHes1 repression changes the developmental state of the neuronal lineage—from multipotent to unipotent (neuronal commitment), and from mitotic to postmitotic. Furthermore, the increased number of neurons in the Hes1−/− brain (before E14.5, Fig. 1Q) may correspond with only the earlier stage of neuronal generation, as represented in the first 2 d of low-density culture started at E12.5 or E13.5, because the mutant embryos die after E14 (Figs. 3, 5A). The simple model of neuronal lineage as seen in low-density culture is that the wild-type progenitor cell produces one self-renewing progenitor cell and one postmitotic neuron, whereas the Hes1−/−progenitor cell generates two postmitotic neurons in the first 2 din vitro (Fig. 5A). Thus, a greater number ofHes1−/− than wild-type neurons were counted at the earlier stage of culture.
Hes1 and gliogenesis
Our observations suggest that Hes1 is not essential as a negative regulator of glial commitment (Table 1), at least in the telencephalon. However, persistent overexpression of Hes1prevents the expression of both neuronal and glial markers in the mouse cortex (Ishibashi et al., 1994). TheHes1−/− telencephalic cells in vitro started to express the glial marker GFAP slightly earlier than did the wild-type control (data not shown). Thus, it cannot be totally excluded that Hes1 negatively regulates glial differentiation, although Hes1 appeared not to be essential in repressing glial commitment in the mouse cortex. It is also possible that other members of the Hes family regulate glial development. For example, Hes5, not Hes1, is known to be involved in oligodendroglial differentiation (Wang et al., 1998).
Footnotes
This work was supported by grants from the Japanese Ministry of Education, Science, and Technology Corporation. S.W. is an Alberta Heritage Foundation for Medical Research Scientist. We are grateful to Amgen for providing NT-3 and BDNF. We thank Drs. Sally Temple, Masato Nakafuku, Tetsuichiro Saito, and Freda Miller for their valuable comments.
Correspondence should be addressed to Dr. Hideyuki Okano, Department of Neuroanatomy, Biomedical Research Center, Osaka University, Suita, Osaka 565-0871, Japan. E-mail: okano@nana.med.osaka-u.ac.jp.
REFERENCES
- 1.Akazawa C, Sasai Y, Nakanishi S, Kageyama R. Molecular characterization of a rat negative regulator with a basic helix-loop-helix structure predominantly expressed in the developing nervous system. J Biol Chem. 1992;267:21879–21885. [PubMed] [Google Scholar]
- 2.Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 3.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 4.Bayer SA, Altman J. Neocortical development. Raven; New York: 1991. [Google Scholar]
- 5.Blaschke AJ, Staley K, Chun J. Widespread programmed cell death in proliferative and postmitotic regions of the fetal cerebral cortex. Development. 1996;122:1165–1174. doi: 10.1242/dev.122.4.1165. [DOI] [PubMed] [Google Scholar]
- 6.Casarosa S, Fode C, Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J, Wilkin GP. Neural cell culture. Oxford UP; New York: 1995. [Google Scholar]
- 8.Crandall JE, Jacobson M, Kosik KS. Ontogenesis of microtubule-associated protein 2 (MAP2) in embryonic mouse cortex. Dev Brain Res. 1986;28:127–133. doi: 10.1016/0165-3806(86)90072-6. [DOI] [PubMed] [Google Scholar]
- 9.Debus E, Weber K, Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25:193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 10.DiCicco-Bloom E, Friedman WJ, Black IB. NT-3 stimulates sympathetic neuroblast proliferation by promoting precursor survival. Neuron. 1993;11:1101–1111. doi: 10.1016/0896-6273(93)90223-e. [DOI] [PubMed] [Google Scholar]
- 11.Frederiksen K, McKay RD. Proliferation and differentiation of rat neuroepithelial precursor cells in vivo. J Neurosci. 1988;8:1144–1151. doi: 10.1523/JNEUROSCI.08-04-01144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galand P, Degraef C. Cyclin/PCNA immunostaining as an alternative to tritiated thymidine pulse labeling for marking S phase cells in paraffin sections from animal and human tissues. Cell Tissue Kinet. 1989;22:383–392. doi: 10.1111/j.1365-2184.1989.tb00223.x. [DOI] [PubMed] [Google Scholar]
- 13.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh A, Greenberg ME. Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron. 1995;15:89–103. doi: 10.1016/0896-6273(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 16.Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17:27–41. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- 17.Guillemot F, Joyner AL. Dynamic expression of the murine Achaete-scute homolog Mash1 in the developing nervous system. Mech Dev. 1993;42:171–185. doi: 10.1016/0925-4773(93)90006-j. [DOI] [PubMed] [Google Scholar]
- 18.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulspas R, Tiarks C, Reilly J, Hsieh CC, Recht L, Quesenberry PJ. In vitro cell density-dependent clonal growth of EGF-responsive murine neural progenitor cells under serum-free conditions. Exp Neurol. 1997;148:147–156. doi: 10.1006/exnr.1997.6672. [DOI] [PubMed] [Google Scholar]
- 20.Ingham PW, Howard KR, Ish-Horowicz D. Transcription pattern of the Drosophila segmentation gene hairy. Nature. 1985;318:439–445. [Google Scholar]
- 21.Ishibashi M, Moriyoshi K, Sasai Y, Shiota K, Nakanishi S, Kageyama R. Persistent expression of helix-loop-helix factor HES-1 prevents mammalian neural differentiation in the central nervous system. EMBO J. 1994;13:1799–1805. doi: 10.1002/j.1460-2075.1994.tb06448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishibashi M, Siew-Lan A, Shiota K, Nakanishi S, Kageyama R, Guillemot F. Targeted disruption of mammalian hairy and Enhancer of split homolog-1 (HES-1) leads to up-regulation of neural helix-loop-helix factors, premature neurogenesis, and severe neural tube defects. Genes Dev. 1995;9:3136–3148. doi: 10.1101/gad.9.24.3136. [DOI] [PubMed] [Google Scholar]
- 23.Izant JG, McIntosh JR. Microtubule-associated proteins: a monoclonal antibody to MAP2 binds to differentiated neurons. Proc Natl Acad Sci USA. 1980;77:4741–4745. doi: 10.1073/pnas.77.8.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jan YN, Jan LY. Asymmetric cell division. Nature. 1998;392:775–778. doi: 10.1038/33854. [DOI] [PubMed] [Google Scholar]
- 25.Johnson GVW, Jope RS. The role of microtubule-associated protein 2 (MAP2) in neuronal growth, plasticity, and degeneration. J Neurosci Res. 1992;33:505–512. doi: 10.1002/jnr.490330402. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JE, Birren SJ, Anderson DJ. Two rat homologues of Drosophila achaete-scute specifically expressed in neuronal precursors. Nature. 1990;346:858–861. doi: 10.1038/346858a0. [DOI] [PubMed] [Google Scholar]
- 27.Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kageyama R, Nakanishi S. Helix-loop-helix factors in growth and differentiation of the vertebrate nervous system. Curr Opin Genet Dev. 1997;7:659–665. doi: 10.1016/s0959-437x(97)80014-7. [DOI] [PubMed] [Google Scholar]
- 29.Kageyama R, Sasai Y, Akazawa C, Ishibashi M, Takebayashi K, Shimizu C, Tomita K, Nakanishi S. Regulation of mammalian neural development by helix-loop-helix transcription factors. Crit Rev Neurobiol. 1995;9:177–188. [PubMed] [Google Scholar]
- 30.Kaneko Y, Sakakibara S, Imai T, Suzuki A, Nakamura Y, Sawamoto K, Ogawa Y, Toyama Y, Miyata T, Okano H. Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev Neurosci. 2000;22:138–152. doi: 10.1159/000017435. [DOI] [PubMed] [Google Scholar]
- 31.Klämbt C, Knust E, Tietze K, Campos-Ortega JA. Closely related transcripts encoded by the neurogenic gene complex Enhancer of split of Drosophila melanogaster. EMBO J. 1989;8:203–210. doi: 10.1002/j.1460-2075.1989.tb03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JE. Basic helix-loop-helix genes in neural development. Curr Opin Neurobiol. 1997;7:13–20. doi: 10.1016/s0959-4388(97)80115-8. [DOI] [PubMed] [Google Scholar]
- 33.Lee JE, Hollenberg SM, Snider L, Turner D, Lipnick N, Weintraub H. Conservation of Xenopus Ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- 34.Lee MK, Tittle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific β-tubulin isotype during chick embryogenesis. Cell Motil Cytoskel. 1990;17:117–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- 35.Lendahl U, Zimmerman LB, McKay RDG. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 36.Lillien L. Neural progenitors and stem cells: mechanisms of progenitor heterogeneity. Curr Opin Neurobiol. 1998;8:37–44. doi: 10.1016/s0959-4388(98)80006-8. [DOI] [PubMed] [Google Scholar]
- 37.Lo LC, Johnson JE, Wuenschell CW, Saito T, Anderson DJ. Mammalian achaete-scute homolog 1 is transiently expressed by spatially-restricted subsets of early neuroepithelial and neural crest cells. Genes Dev. 1991;5:1524–1537. doi: 10.1101/gad.5.9.1524. [DOI] [PubMed] [Google Scholar]
- 38.Luskin MB, Pearlman AL, Sanes JR. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988;1:635–647. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- 39.Marusich MF, Furneasx HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25:143–155. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- 40.Mayer-Proschel M, Kalyani AJ, Mujtaba T, Rao MS. Isolation of lineage-restricted neuronal precursors from multipotent neuroepithelial stem cells. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- 41.McAllister AK, Lo DC, Kats LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 42.McConnell SK. Constructing the cerebral cortex: neurogenesis and fate determination. Neuron. 1995;15:761–768. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- 43.Menezes JR, Luskin MB. Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J Neurosci. 1994;14:5399–5416. doi: 10.1523/JNEUROSCI.14-09-05399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyata T, Ogawa M. Developmental potentials of early telencephalic neuroepithelial cells: a study with microexplant culture. Dev Growth Differ. 1994;36:319–331. doi: 10.1111/j.1440-169X.1994.00319.x. [DOI] [PubMed] [Google Scholar]
- 45.Morrow EM, Furukawa T, Lee JE, Cepko CL. NeuroD regulates multiple functions in the developing neural retina in rodent. Development. 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- 46.Nakao K, Campos-Ortega JA. Persistent expression of genes of the Enhancer of Split complex suppresses neural development in Drosophila. Neuron. 1996;16:275–286. doi: 10.1016/s0896-6273(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 47.Nawa H, Pelleymounter MA, Carnahan J. Intraventricular administration of BDNF increases neuropeptide expression in newborn rat brain. J Neurosci. 1994;14:3751–3765. doi: 10.1523/JNEUROSCI.14-06-03751.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niinobe M, Maeda N, Ino H, Mikoshiba K. Characterization of microtubule-associated protein 2 from mouse brain and its localization in the cerebellar cortex. J Neurochem. 1988;51:1132–1139. doi: 10.1111/j.1471-4159.1988.tb03078.x. [DOI] [PubMed] [Google Scholar]
- 49.Ohtsuka T, Ishibashi M, Gradwohl G, Nakanishi S, Guillemot F, Kageyama R. Hes1 and Hes5 as Notch effectors in mammalian neuronal differentiation. EMBO J. 1999;18:2196–2207. doi: 10.1093/emboj/18.8.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okano HJ, Darnell RB. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J Neurosci. 1997;17:3024–3037. doi: 10.1523/JNEUROSCI.17-09-03024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qian X, Goderie SK, Shen Q, Stern JH, Temple S. Intrinsic programs of patterned cell lineages in isolated vertebrate CNS ventricular zone cells. Development. 1998;125:3143–3152. doi: 10.1242/dev.125.16.3143. [DOI] [PubMed] [Google Scholar]
- 52.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakakibara S, Okano H. Expression of neural RNA-binding proteins in the postnatal CNS: implications of their roles in neuronal and glial cell development. J Neurosci. 1997;17:8300–8312. doi: 10.1523/JNEUROSCI.17-21-08300.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sakakibara S, Imai T, Hamaguchi K, Okabe M, Aruga J, Nakajima K, Yasutomi D, Nagata T, Kurihara Y, Uesugi S, Miyata T, Ogawa M, Mikoshiba K, Okano H. Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev Biol. 1996;176:230–242. doi: 10.1006/dbio.1996.0130. [DOI] [PubMed] [Google Scholar]
- 57.Sasai Y, Kageyama R, Tagawa Y, Shigemoto R, Nakanishi S. Two mammalian helix-loop-helix factors structurally related to Drosophila hairy and Enhancer of split. Genes Dev. 1992;6:2620–2634. doi: 10.1101/gad.6.12b.2620. [DOI] [PubMed] [Google Scholar]
- 58.Schlüter C, Duchrow M, Wohlenberg C, Becker MHG, Key G, Flad HD, Gerdes J. The cell proliferation-associated antigen of antibody Ki-67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J Cell Biol. 1993;123:513–522. doi: 10.1083/jcb.123.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ström A, Castella P, Rockwood J, Wagner J, Caudy M. Mediation of NGF signaling by post-translational inhibition of HES-1, a basic helix-loop-helix repressor of neuronal differentiation. Genes Dev. 1997;11:3168–3181. doi: 10.1101/gad.11.23.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takebayashi K, Sasai Y, Sakai Y, Watanabe T, Nakanishi S, Kageyama R. Structure, chromosomal locus, and promoter analysis of the gene encoding the mouse helix-loop-helix factor HES1: negative autoregulation through the multiple N box elements. J Biol Chem. 1994;269:5150–5156. [PubMed] [Google Scholar]
- 61.Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274:1115–1123. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- 62.Temple S. Division and differentiation of isolated CNS blast cells in microculture. Nature. 1989;340:471–473. doi: 10.1038/340471a0. [DOI] [PubMed] [Google Scholar]
- 63.Thomson JA, Marshall VS, Trojanowski JQ. Neural differentiation of rhesus embryonic stem cells. APMIS. 1998;106:149–157. doi: 10.1111/j.1699-0463.1998.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 64.Torii M, Matsuzaki F, Osumi N, Kaibuchi K, Nakamura S, Casarosa S, Guillemot F, Nakafuku M. Transcription factors Mash-1 and Prox-1 delineate early steps in differentiation of neural stem cells in the developing central nervous system. Development. 1999;126:443–456. doi: 10.1242/dev.126.3.443. [DOI] [PubMed] [Google Scholar]
- 65.Tropepe V, Sibilia M, Ciruna BG, Rossant J, Wagner EF, van der Kooy D. Distinct neural stem cells proliferate in response to EGF and FGF in the developing mouse telencephalon. Dev Biol. 1999;208:166–188. doi: 10.1006/dbio.1998.9192. [DOI] [PubMed] [Google Scholar]
- 66.Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- 67.Wakamatsu Y, Weston JA. Sequential expression and role of Hu RNA-binding proteins during neurogenesis. Development. 1997;124:3449–3460. doi: 10.1242/dev.124.17.3449. [DOI] [PubMed] [Google Scholar]
- 68.Wang S, Sdrulla AD, duSibio G, Bush G, Nofziger D, Hicks C, Weinmaster G, Barres BA. Notch recepter activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]