Abstract
Glial cell line-derived neurotrophic factor (GDNF) has potent trophic effects on adult sensory neurons after nerve injury and is one of a family of proteins that includes neurturin, persephin, and artemin. Sensitivity to these factors is conferred by a receptor complex consisting of a ligand binding domain (GFRα1–GFRα4) and a signal transducing domain RET. We have investigated the normal expression of GDNF family receptor components within sensory neurons and the response to nerve injury.
In normal rats, RET and GFRα1 were expressed in a subpopulation of both small- and large-diameter afferents projecting through the sciatic nerve [60 and 40% of FluoroGold (FG)-labeled cells, respectively]. GFRα2 and GFRα3 were both expressed principally within small-diameter DRG cells (30 and 40% of FG-labeled cells, respectively). Two weeks after sciatic axotomy, the expression of GFRα2 was markedly reduced (to 12% of sciatic afferents). In contrast, the proportion of sciatic afferents that expressed GFRα1 increased (to 66% of sciatic afferents) so that virtually all large-diameter afferents expressed this receptor component, and the expression of GFRα3 also increased (to 66% of sciatic afferents) so that almost all of the small-diameter afferents expressed this receptor component after axotomy. There was little change in RET expression.
The changes in the proportions of DRG cells expressing different receptor components were mirrored by alterations in the total RNA levels within the DRG. The changes in GFRα1 and GFRα2 expression after axotomy could be largely reversed by treatment with GDNF.
Keywords: GDNF receptor expression, GFRα, RET, axotomy, DRG, rat
Glial cell line-derived neurotrophic factor (GDNF) was the first described member of a novel family of trophic factors that also includes neurturin (NTN), persephin (PSP), and artemin (Lin et al., 1993; Kotzbauer et al., 1996; Milbrandt et al., 1998; Baloh et al., 1999). In addition to effects in the CNS (Henderson et al., 1993), GDNF, NTN, and artemin promote thein vitro survival of many peripheral neurons, including enteric, sympathetic, and sensory neurons (Buj-Bello et al., 1995;Ebendal et al., 1995; Trupp et al., 1995; Kotzbauer et al., 1996; Baloh et al., 1999). PSP does not promote survival of these populations (Milbrandt et al., 1998).
GDNF has important trophic effects on sensory neurons both during development and in the adult. Mice lacking GDNF show a significant reduction in the number of DRG neurons (Moore et al., 1996). GDNF can also prevent the death of axotomized neonatal sensory neurons in vivo (Matheson et al., 1997). In the first two weeks after birth, a population of small-diameter nonpeptidergic DRG cells (identified by binding of the lectin IB4) lose their NGF sensitivity and become GDNF-sensitive (Bennett et al., 1996; Molliver et al., 1997). These IB4-binding DRG cells remain profoundly sensitive to GDNF in adulthood. Many effects of nerve injury within these cells and within a subpopulation of large-diameter DRG cells can be reversed by administration of GDNF (Bennett et al., 1998; Munson and McMahon, 1997).
Members of the GDNF family exert these dramatic effects via a multicomponent receptor complex consisting of RET, a tyrosine kinase receptor acting as a signal transducing domain, in combination with a member of the GFRα family of GPI-linked receptors (GFRα1–GFRα4) acting as ligand binding domains (Jing et al., 1996, 1997; Treanor et al., 1996; Baloh et al., 1997, 1998;Buj-Bello et al., 1997; Creedon et al., 1997; Klein et al., 1997;Naveilhan et al., 1997, 1998; Sanicola et al., 1997; Worby et al., 1998). Either GFRα1 or GFRα2 in conjunction with RET can mediate GDNF or NTN signaling (Schuchardt et al., 1994; Moore et al., 1996;Pichel et al., 1996; Sanchez et al., 1996; Sanicola et al., 1997;Cacalano et al., 1998; Enomoto et al., 1998), although GDNF is thought to bind preferentially to GFRα1, and NTN to GFRα2. Artemin is thought to signal preferentially via GFRα3, although in a similar manner to GDNF and NTN alternative receptor interactions may also occur (Baloh et al., 1999). PSP cannot signal via GFRα1 or GFRα2 (Milbrandt et al., 1998) but does bind to GFRα4, a receptor currently identified only in chicken (Enokido et al., 1998).
We have used labeled afferents that project through the sciatic nerve as a means of identifying a defined population of sensory neurons. This has been combined with in situ hybridization and quantitative analysis of mRNA to study GDNF family receptor component expression within normal and injured sensory neurons. The response to treatment with exogenous GDNF after nerve injury has also been investigated.
MATERIALS AND METHODS
Animal surgery and FluoroGold labeling. Adult male Wistar rats weighing 200–250 gm were used in all experiments. The sciatic nerve was labeled in a number of different experimental groups of animals. In a control group (n = 5), there was no manipulation of the sciatic nerve before the labeling procedure. In another group of animals, the sciatic nerve was exposed under pentobarbitone anesthesia (40 mg/kg, i.p., with sterile precautions) 13 d before labeling and was ligated and cut 20 mm distal to the obturator tendon; this was combined with an intrathecal infusion. Intrathecal cannulae were implanted as described previously (Bennett et al., 1998). A small laminectomy was performed between the T7 and T8 vertebrae, and the dura was cut. A SILASTIC tube with an external diameter of 0.6 mm was then passed intrathecally so that its tip lay over the lumbar enlargement of the spinal cord. The other end of this tube was connected to a mini-osmotic pump (Alzet; Alza, Palo Alto, CA). The pumps were filled with rat serum albumin (1 mg/ml in saline; n = 4) or this vehicle in combination with rhGDNF (12 μg/d; n = 4). This dose of GDNF was chosen because we have demonstrated previously that this has maximal effects on sensory neurons after nerve injury (Bennett et al., 1998). Another group of animals (n = 6) underwent 2 week axotomy but did not receive an intrathecal infusion and were used for RNA quantitation (described below).
To label afferents projecting through the sciatic nerve of normal and previously axotomized animals, the nerve was re-exposed at the level of the midthigh using sterile precautions and under pentobarbitone anesthesia (40 mg/kg, i.p.). The nerve was injected with 4 μl of 4% FluoroGold (FG) (in distilled water; Fluorochrome Inc., Engelwood, CO) using a glass micropipette glued to a Hamilton syringe. The animals were allowed to recover (which in all cases was uneventful) for 20–24 hr to allow retrograde transport of FG. There was also another group of animals that did not undergo the sciatic labeling procedure (n = 4).
After the appropriate recovery period, experimental animals were deeply anesthetized with pentobarbitone (60 mg/kg, i.p.) and then perfused transcardially with 250 ml of cold saline, followed by 500 ml of cold, freshly prepared 4% paraformaldehyde. The L4 and L5 DRGs were removed, as well as the L3–L6 segments of spinal cord. Tissues were post-fixed in 4% paraformaldehyde for 2 hr, after which they were transferred to a solution of 15% sucrose that also contained diethyl pyrocarbonate (1 ml/l) to inhibit the action of RNases. Tissue was then processed as described below.
In situ hybridization. Cryostat sections of ganglia were cut at a thickness of 15 μm. Sections of DRG were cut serially onto slides so that each slide contained an ordered series of sections throughout the ganglia at a separation of at least 120 μm between sections. A series of consecutive slides were hybridized against different probes so that sections for each receptor component represented a systematic random sampling through the DRG. Control and experimental tissue was processed simultaneously to try and ensure consistency. Sections were processed for in situhybridization by a method described previously (Phillips et al., 1990).33P-UTP-labeled RNA probes were generated as described previously (Melton et al., 1984). Sense and antisense probes were synthesized using T7 RNA polymerase. Probes were generated corresponding to the following areas of published sequences: for GFRα1, the probe was between bases 309 and 795 of GenBank accession number U59486; for GFRα2, the probe was between bases 660 and 1344 of GenBank accession number U97143; and for GFRα3, a probe to the mouse sequence was used between bases 543 and 868 of GenBank accession numberAF020305. For RET, expressed sequence tags (ESTs) corresponding to rat RET (GenBank accession numbers U22513 and U22514) were aligned with the mouse sequence (GenBank accession number X67812), and primers were designed to amplify a rat RET probe using the PCR and an embryonic rat brain cDNA as template. The final probe corresponds to bases 141–427 of the mouse sequence (base 13 of GenBank accession numberU22414 to base 326 of accession number U22513).
Combined N52 immunostaining with GFRα1 in situhybridization was performed as described previously (Bennett et al., 1998). This was performed on animals in which the normal (n = 4) or axotomized (n = 4) sciatic nerve had been labeled previously with FG as described earlier. Sections were incubated 40–48 hr at room temperature with N52 monoclonal antibody to phosphorylated heavy chain neurofilament (1:400; Sigma, St. Louis, MO) diluted in diethylpyrocarbonate (DEPC)-treated PBS containing 0.2% Triton X-100, 0.1% sodium azide, 0.5 mm dithiothreitol, and 100 U/ml RNasin (Promega, Madison, WI). Sections were washed in DEPC PBS and incubated for 4 hr in tetramethyl rhodamine isothiocyanate-conjugated secondary antibodies (1:200; Jackson ImmunoResearch, West Grove, PA). After further washes in DEPC PBS, sections were processed through prehybidization steps, hybridized to35S-dATP end-labeled oligonucleotides, and washed as described previously. Slides were dipped in autoradiographic emulsion (Amersham, Arlington Heights, IL) and developed after 4–6 weeks. After coverslipping with PBS glycerol (1:3 containing 2.5% 1,4-diazobicyclo-(2,2,2)-octane), fluorescent labeling and silver grains were visualized using epifluorescence microscopy combined with either epipolarized illumination or dark-field illumination. The oligonucleotide used for the GFRα1 probe was complementary to nucleotides 996–1029 of the rat GFRα1 sequence.
IB4 staining. Cryostat sections of L4–L5 spinal cord were cut at a thickness of 20 μm, and every fifth section was mounted serially onto slides. Sections were then stained for IB4 (10 μg/ml, biotinylated Griffonia Simplicifolicia IB4 lectin; Sigma); the secondary reagent used was ExtrAvidin-FITC (1:100, for IB4 localization; Sigma). IB4 was diluted in a buffer of PBS containing 0.1 mm CaCl2, 0.1 mm MgCl2, and 0.1 mm MnCl2. After incubation in the secondary reagent, sections were washed briefly in PBS and then mounted in PBS/glycerol (1:3) containing 2.5% 1,4 diazobicyclo-(2,2,2)-octane (antifading agent; Sigma).
mRNA quantitation. Messenger RNA encoding RET, GFRα1, GFRα2, GFRα3, and glyceraldehyde phosphate dehydrogenase (GAPDH) were quantified by using the “TaqMan” technique (Heid et al., 1996). This method allows real-time quantitation by monitoring fluorescence continuously during the PCR. RNA was prepared from rapidly dissected L4–L5 DRG from rats treated identically to those used for in situ hybridization studies, except that FG was not injected and they were perfused with only saline. RNA was purified (RNeasy; Qiagen, Hilden, Germany), quantified by fluorescence with RiboGreen (Molecular Probes, Eugene, OR) and added to reaction mixes with primers and probes specific to the indicated messages shown in Table 1. Primers and probes were designed from published sequences. For GFRα3, there is no full cDNA published, and so primers and probes were designed from GenBank accession numberAI179473, a rat EST that corresponds to the 3′ region of GFRα3. The Ct was determined in duplicate for each sample, and the mean was compared with a standard curve of serially diluted RNA obtained from rat DRG dissected from all axial levels. Results are expressed as nanograms of standard RNA equivalent to the Ct obtained. For all primer and probe sets, controls consisted of verification of a single reaction product of the correct molecular weight (as determined by PAGE) and no more than 1% of signal in samples that received no reverse transcriptase. No attempt was made to quantitate absolute levels of any of the messenger RNAs.
Table 1.
Oligonucleotide sequences used in the quantitative RT-PCR reactions
| mRNA | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| RET | CATCGATGCGGGCACTG | CTGAGCTGCTCCCAGGAACT | TCCTCTACCTCAATCAGAGCCTGGACCA |
| GFRα1 | GCAGGGTCTGAGAATGAGATCC | TCAGCTTCTGAGCCTGCAAA | CACACACGTTTTACCACCCTGTGCGA |
| GFRα2 | GGCAGATTTCCACGCCAA | GTAGTTGTCCGCAGGACAGCT | TGTCGAGCCTCCTACCGGACAATCA |
| GFRα3 | TCATGTCTGCCAGGCCATC | ATGGAGACAGTGCTAGGAGTTAAGC | ACTCAAAGGCTTTTAGCTCTTCTTGCCCAA |
| GAPDH | CAGTGGCAAAGTGGAGATTGT | AATTTGCCGTGAGTGGAGTC | CCATCAACGACCCCTTCATTGACCTC |
All are listed 5′ to 3′; probes are phosphorylated on the 3′ end and derivatized with FAM on the 3′ end and TAMARA on the 5′ end.
Data analysis. Initial experiments demonstrated that significant fading of FG occurred after the in situhybridization procedure, and this phenomenon was apparent particularly in large-diameter neurons. Furthermore, the dense deposition of silver grains over some cells obscured visualization of the fluorescent marker. Therefore, before hybridization, FG-labeled sections of DRG were photographed using 10× magnification and fluorescence illumination. After hybridization, the appropriate portions of each section were identified, and images were captured directly off the microscope using a Grundig FA87 digital camera with integrating frame store. This was done using both fluorescence illumination to demonstrate the morphology of the ganglion (and N52 immunostaining) and dark-field illumination to reveal the hybridization signal. These images could be directly compared with the prehybridization image showing FG-labeled profiles. Individual FG-labeled profiles were outlined, and hence cell profile area and diameter were calculated. Compared with the posthybridization images, FG-labeled profiles positively stained for the relevant marker were identified. Profiles that had silver grains over the cell cytoplasm at least five times background were counted as positive. This process was performed on four randomly selected DRG sections for each marker in each animal. The beginning slide of each series was selected with a random number generator. All the FG-labeled profiles were drawn in each section, and thus there was an unbiased sampling of cells from each animal. An average of 500 profiles were drawn for each marker in each animal.
Because large DRG neurons are sectioned into more profiles than small ones during histological preparation (and some of these are smaller than the true diameter of the neuron), an accurate estimation of the proportion of positively marked neurons and their size distribution requires a correction of the raw data derived from observing cell profiles. The method of recursive translation (Rose and Rohrlich, 1987) was used to convert sizes and numbers of FG-stained profiles into estimates of cell diameters and counts. This method does make the assumption that neurons are spherical, although, in practice, it does not seem to be very sensitive to this assumption. The FG-labeled profiles that were positive or negative for a particular probe were drawn separately to calculate profile areas, and these were then computer analyzed by the program of Rose and Rohrlich (1987) to reveal an estimation of the size distribution from which the profiles derived. The overall percentage of positively labeled neurons and the size distribution of positively and negatively labeled neurons could then be calculated, and these were used for all analyses.
To determine overall hybridization intensity in different experimental groups, grain density was estimated over sections of DRG as described previously (McMahon et al., 1994). Images of DRG sections were viewed with dark-field illumination and were captured directly from the microscope using 5× magnification as described above. The image was then thresholded to a set level to reveal the labeling. Boxes (each 2500 μm2) were then placed over the section, and the area occupied by silver grains within the box was calculated. All the sections used for a particular comparison were analyzed in one session using constant illumination. Four randomly selected sections were analyzed for each probe in each animal.
RESULTS
The expression of GDNF family receptor components in normal uninjured sensory neurons
In sections of normal L4–L5 DRG, GFRα1 and RET were expressed in subpopulations of both small- and large-diameter DRG cells, whereas the expression of GFRα2 and GFRα3 was restricted primarily to small-diameter DRG cells (Fig. 1). There appeared to be greater background labeling over white matter tracts when using the GFRα1 probe compared with other probes, and this may be attributable to the fact that this receptor is known to be expressed in glial cells, as well as neurons (Trupp et al., 1997). No labeling was apparent when sense probes for GFRα1, GFRα2, GFRα3, or RET were used (Fig. 1).
Fig. 1.
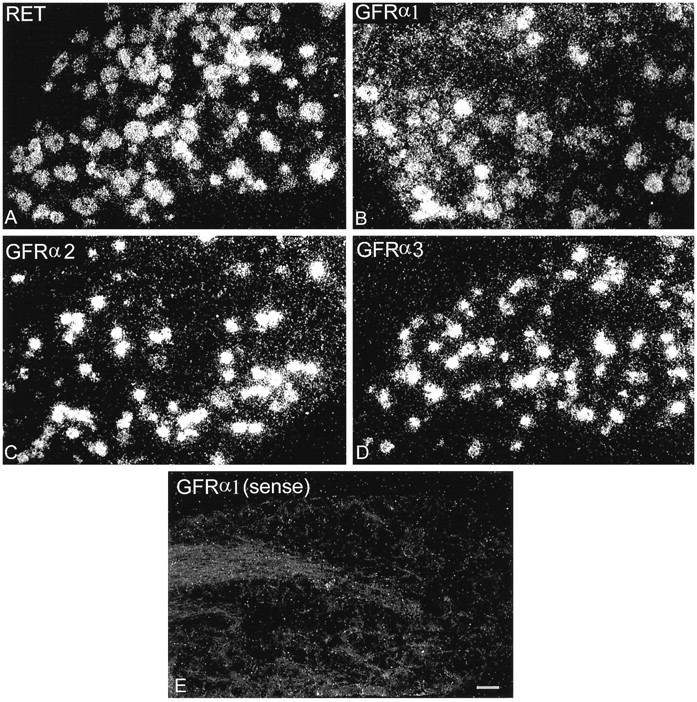
Dark-field photomicrographs of sections of L4–L5 DRG after hybridization with probes for RET (A), GFRα1 (B), GFRα2 (C), GFRα3 (D), or a sense strand control probe (E). Scale bar, 50 μm.
To study GDNF receptor component expression in an identified population of DRG neurons, in situ hybridization was performed on DRG tissue after labeling of the sciatic nerve with FG. Injection of the sciatic nerve with FG resulted in robust labeling of DRG cell bodies within L4–L5 ganglia 24 hr later (Fig.2A). Several hundred FG-labeled cells were present within each DRG section. Labeled cells were observed only in the relevant ganglia (L4 and L5 ganglia ipsilateral to the label). The prehybridization photographs of the FG image could be readily matched with posthybridization dark-field images (Fig. 2) so that retrogradely identified cells could easily be scored for the presence or absence of hybridization signal. We quantified thein situ hybridization signal over sections to confirm that the labeling procedure that we used did not itself alter GDNF family receptor component expression within sensory neurons. We found no significant difference for any of the receptors in silver grain density over sections labeled with FG compared with the contralateral unlabeled side (the ratio of grain density measured in arbitrary units for the labeled vs unlabeled normal sections was 0.98, 1.06, 0.9, and 0.87 for GFRα1, GFRα2, GFRα3, and RET respectively; p > 0.1; unpaired t test).
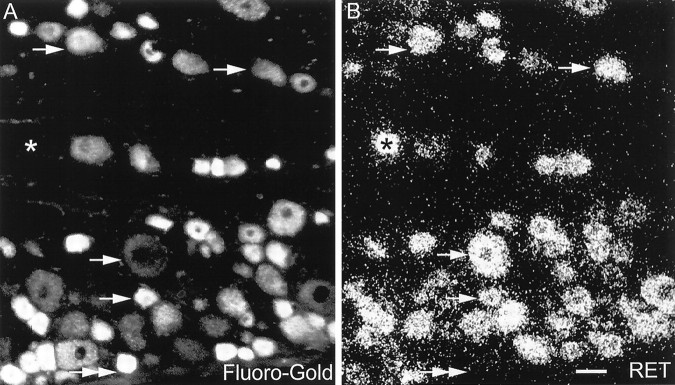
Fig. 2.
Determination of GDNF family receptor component expression in afferents projecting through the sciatic nerve.A, FluoroGold image of L5 after labeling of the sciatic nerve. B, The same section after hybridization with the probe to RET. Single arrows indicate cells that are labeled by both retrograde tracer and the in situhybridization. A double-headed arrow indicates a cell that is FG-labeled but that does not express RET. Anasterisk demonstrates a cell that is not FG-labeled but that does express RET. Scale bar, 50 μm.
DRG cells in L4–L5 projecting through the sciatic nerve, identified by retrograde transport of FG, were hybridized with probes for GFRα1, GFRα2, GFRα3, and RET. GFRα1 was expressed in 42% of sciatic afferents and was expressed by DRG neurons of both large and small cell diameter (Figs. 3A,4). GFRα2 was expressed by 32% of sciatic afferents, being found selectively within small-diameter DRG neurons (Figs. 3B, 4). GFRα3 was expressed in 42% of sciatic afferents, again present principally within small-diameter DRG cells (Fig. 4). RET was expressed in 60% of sciatic afferents, similarly to GFRα1 by DRG neurons of both large and small cell diameter (Fig. 4)
Fig. 3.
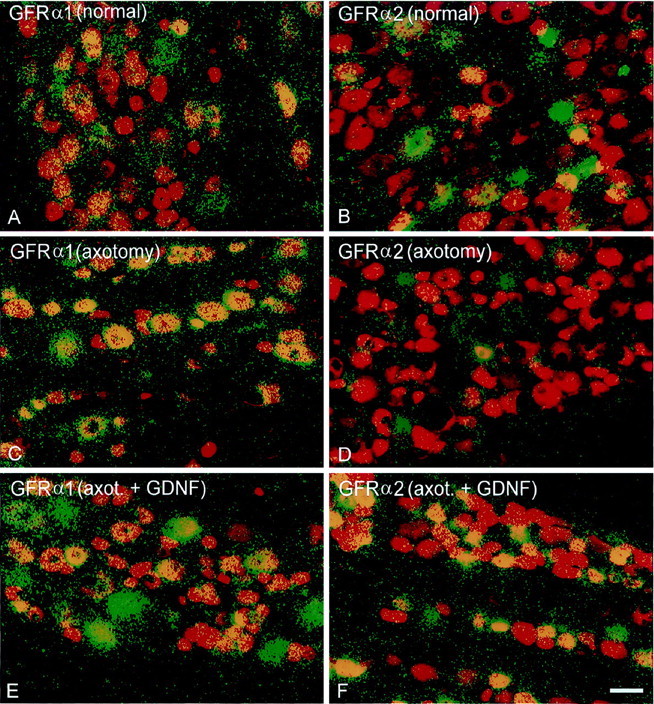
Dual-color images showing FG labeling of DRG cells projecting through the sciatic nerve before hybridization (red) and silver grain deposition (green) after hybridization using probes for GFRα1 (A, C, E) or GFRα2 (B, D, F).Yellow indicates regions in which silver grains are deposited over FG-labeled cells. A subpopulation of sciatic afferents express GFRα1 (A) and GFRα2 (B) normally. After axotomy, the proportion of sciatic afferents that express GFRα1 increases (C), whereas the proportion that express GFRα2 declines (D). Administration of GDNF after axotomy can partially reverse the increased expression of GFRα1 (E) and can restore the expression of GFRα2 to normal (F). Scale bar, 50 μm.
Fig. 4.
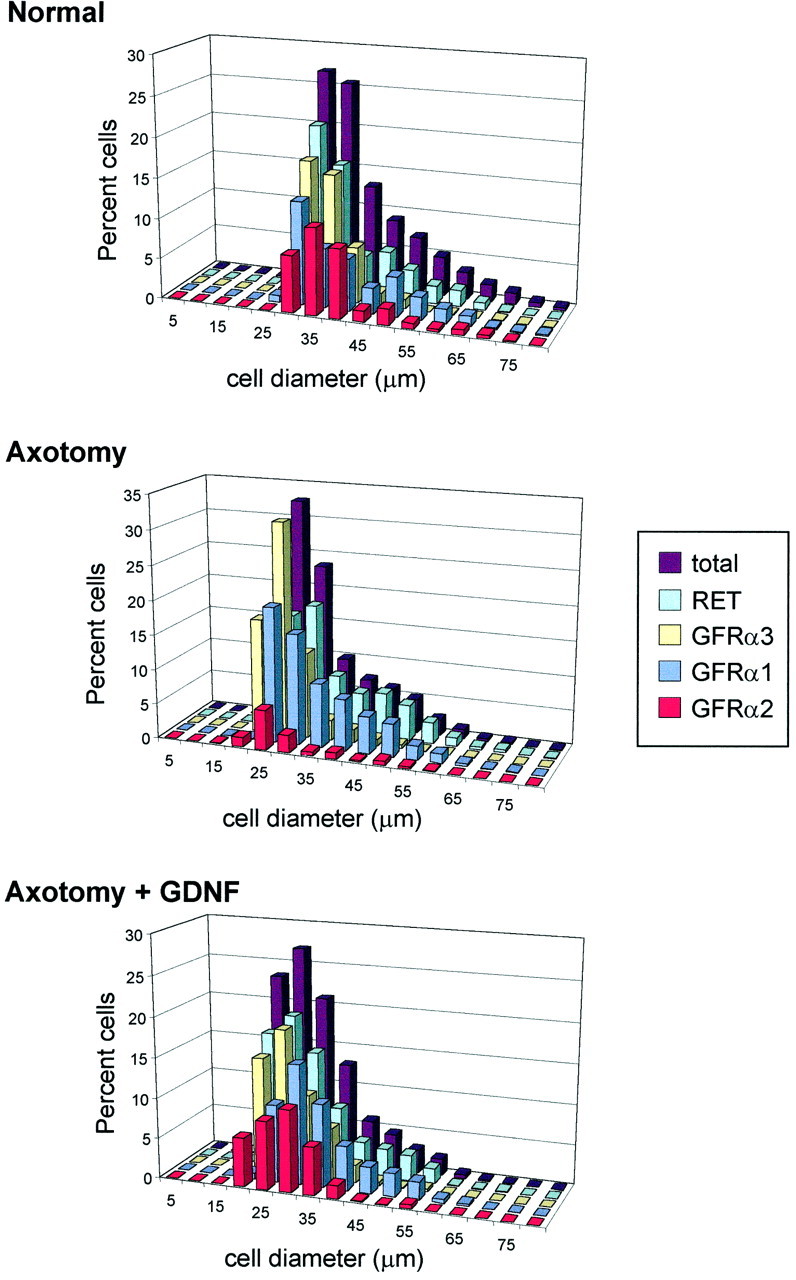
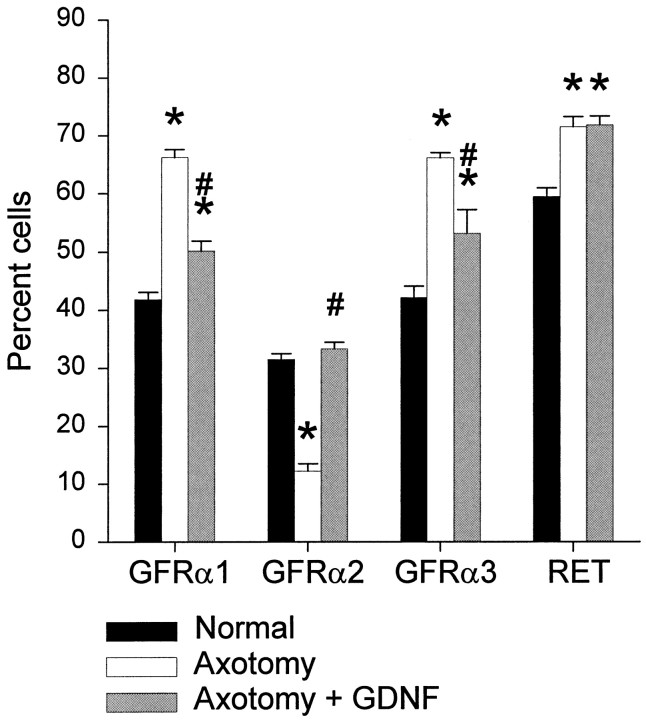
Cell size distributions of all L4–L5 sciatic afferents and of those expressing GFRα1, GFRα2, GFRα3, or RET. Note that Total represents the combined cell size distribution of all the cells analyzed in each group. Distributions are shown for the normal animal (n = 5) and those that have undergone axotomy (n = 4) or axotomy in combination with an intrathecal infusion of GDNF at a dose of 12 μg/d (n = 4). Note that, in the normal L4–L5 DRG, GFRα1 and RET are expressed by neurons of both large and small cell diameter, whereas GFRα2 and GFRα3 are present principally within small-diameter cells. After axotomy, the expression of GFRα1 and RET is upregulated, and the majority of large-diameter cells express these receptor components; axotomy also induces an upregulation in GFRα3 expression, but this remains primarily confined to small-diameter cells. Provision of exogenous GDNF partially reverses the axotomy-induced changes in receptor distribution.
The expression of GDNF family receptor components within injured sensory neurons and after treatment with exogenous GDNF
To study the effects of nerve injury on GDNF family receptor component expression, the sciatic nerve was labeled in a group of animals 2 weeks after axotomy. There was a significant decline in the mean cell diameter of FG-labeled sciatic afferents after axotomy compared with normal unaxotomized afferents (p< 0.05; Kolmogorov–Smirnov; data not shown). It is for this reason that we used the method of recursive translation (Rose and Rohrlich, 1987) to provide an estimate of cell numbers and to try to reduce the bias caused by alterations in cell size after axotomy.
As measured by in situ hybridization, dramatic changes occurred in the expression of GDNF family receptor components within sensory neurons after axotomy. The expression of GFRα1 increased markedly after nerve injury such that GFRα1 was expressed by 66% of sciatic afferents after axotomy (Figs. 3C, 4,5). This increased expression occurred principally in large-diameter DRG cells, and it should be noted that there was still a significant population of small-diameter DRG cells that did not express GFRα1 after axotomy. To confirm the increased expression of GFRα1 in large-diameter DRG cells after axotomy, we combined FG labeling of the sciatic nerve with immunostaining for phosphorylated neurofilament heavy chain (a marker for large-diameter DRG cells) and in situ hybridization for GFRα1. The proportion of sciatic afferents that expressed N52 and GFRα1 increased from 43 ± 5% in control animals to 80 ± 2% in axotomized animals (Fig. 6). This increase was highly significant (p < 0.001; unpaired t test).
Fig. 5.
The percentage of FG-labeled cells in the L4–L5 DRG that also express mRNA for the GDNF receptor family components GFRα1, GFRα2, GFRα3, or RET. Values are shown (±SEM) for the normal animal (n = 5) and those that have undergone axotomy (n = 4) or axotomy in combination with an intrathecal infusion of GDNF at a dose of 12 μg/d (n = 4). Axotomy induced a significant increase in the proportion of cells expressing message for GFRα1, GFRα3, or RET, and a significant decrease in those expressing GFRα2 (*p < 0.05, significant difference from normal values; Tukey post hoc analysis; one-way ANOVA). The infusion of GDNF partially prevented these changes; the upregulation of GFRα1 and GFRα3 was partly reversed, whereas the level of GFRα2 expression returned to normal (#p < 0.05, significant difference between values in the axotomy and axotomy plus GDNF animals; Tukey post hoc analysis; one-way ANOVA). Intrathecal GDNF had no effect on the proportion of FG cells expressing RET after axotomy.
Fig. 6.
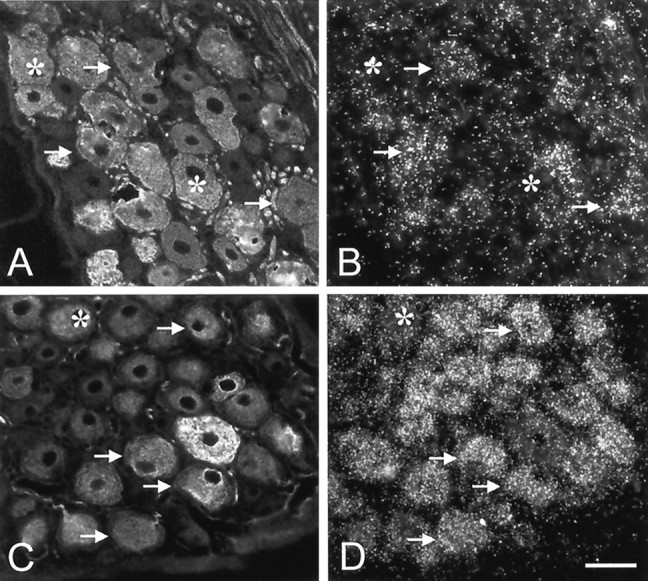
Double labeling for N52 (phosphorylated neurofilament heavy chain; A, C) and GFRα1 mRNA (B, D) in normal (A, B) and axotomized (C,D) animals. N52 is a marker for large-diameter DRG cells. Arrows denote cells that express both N52 and GFRα1, and asterisks denote cells that are N52-positive but that do not express GFRα1. After axotomy, the proportion of N52-positive cells that express GFRα1 increases. Scale bar, 50 μm.
In contrast to GFRα1, the expression of GFRα2 was reduced 2 weeks after axotomy so that only 12% of sciatic afferents expressed this receptor component (Figs. 3D, 4, 5). Like GFRα1, the expression of GFRα3 significantly increased after axotomy so that 66% of sciatic afferents expressed this receptor component, but unlike GFRα1, this increase occurred principally within small-diameter DRG cells. After axotomy, virtually all small-diameter profiles express GFRα3. The proportion of sciatic afferents that expressed RET increased a small but significant degree after nerve injury (72% of sciatic afferents expressed this receptor component after nerve injury) (Fig. 5). This increased expression of RET occurred principally in large-diameter DRG cells (Fig. 4).
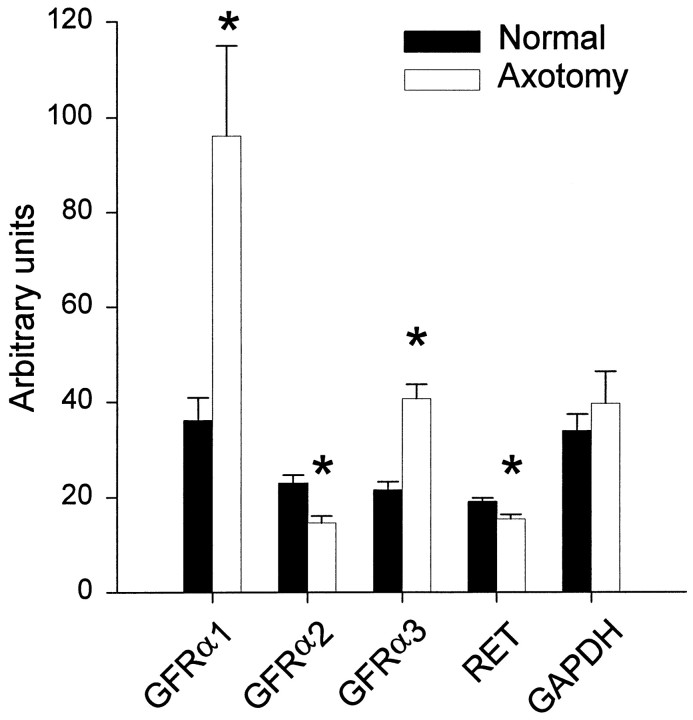
The changes in numbers of cells expressing the GDNF receptor family components after axotomy were primarily mirrored by changes observed in the level of expression of the mRNAs encoding these proteins. The levels of GFRα1 and GFRα3 mRNAs increased 250 and 200%, respectively, after axotomy (p < 0.05; Mann–Whitney rank sum test). There was a 40% reduction in GFRα2 expression (p < 0.05; Mann–Whitney rank sum test) at this time point (Fig. 7). The changes seen with RET expression, however, did not parallel those seen when examined by counting the numbers of expressing cells. Although the number of cells expressing detectable RET increased after axotomy, the total amount of RET mRNA slightly, but significantly, decreased (p < 0.05; Mann–Whitney rank sum test).
Fig. 7.
RNA levels of RET, GFRα1–3, and GAPDH measured using the TaqMan technique in normal and axotomized L4–L5 DRGs. Axotomy resulted in a significant increase in GFRα1 and GFRα3 expression and a significant reduction in GFRα2 expression. There was also a small but significant reduction in RET RNA levels after axotomy. *p < 0.05, comparing normal with axotomized; Mann–Whitney rank sum test.
We investigated whether provision of intrathecal GDNF (12 μg/d) could reverse the changes in GDNF-family receptor component expression after nerve injury. We have demonstrated previously that this dose of GDNF has powerful neuroprotective actions on IB4-binding small-diameter DRG cells after nerve injury (Bennett et al., 1998) and that this dose of GDNF has maximal effects in vivo. To ensure that the GDNF was effective in the animals that we used in this study, we stained for IB4 in the dorsal horn of the spinal cord of these animals. In the group of animals that underwent sciatic axotomy with no treatment, there was an almost complete loss of IB4 binding within the dorsal horn at the level of L4–L5. In comparison, the animals treated with intrathecal GDNF had essentially normal IB4 binding within the dorsal horn (Fig. 8). This indicates that the dose of GDNF used was having maximal effects by this outcome measure.
Fig. 8.
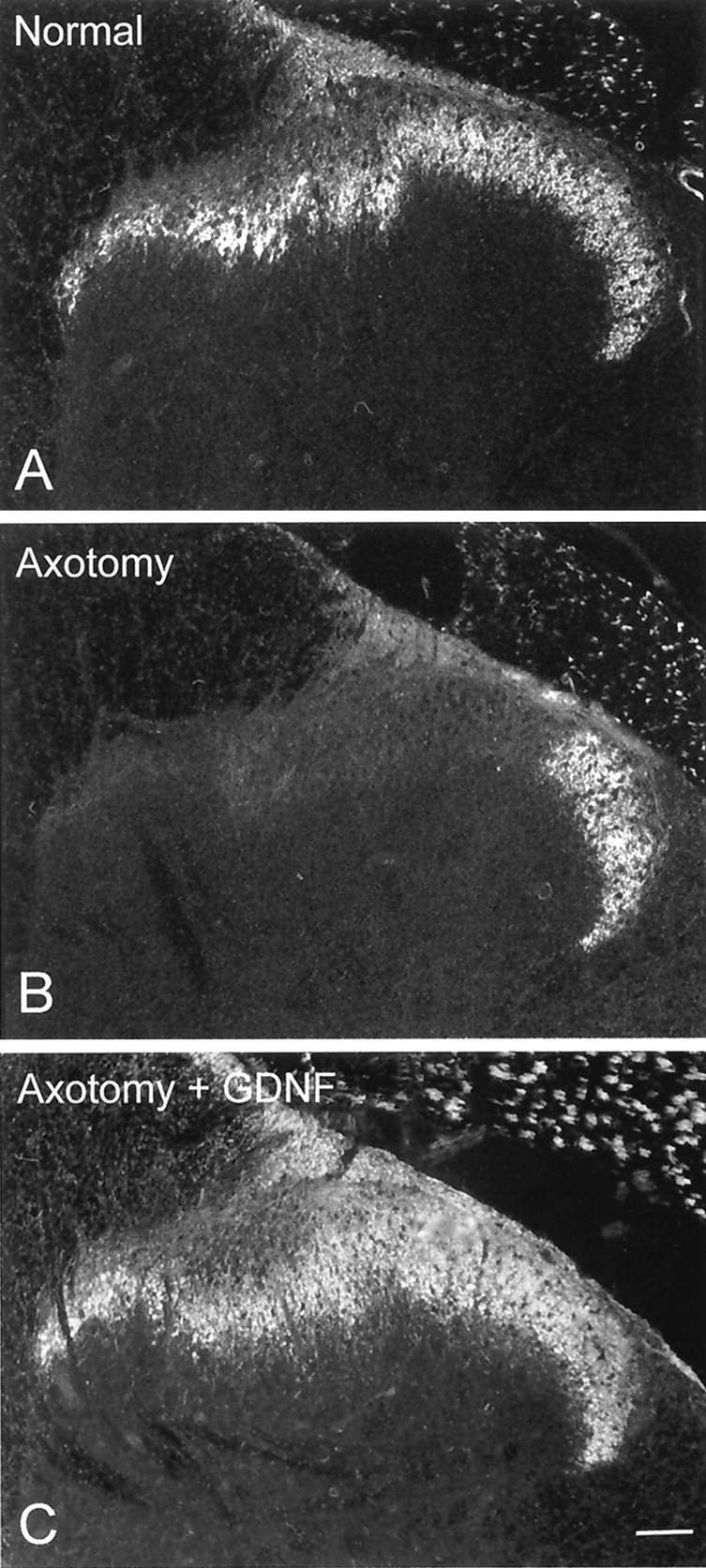
IB4 labeling within the dorsal horn of the spinal cord in a normal animal (A), an animal that has undergone sciatic axotomy (B), and an animal that has undergone sciatic axotomy combined with intrathecal treatment with GDNF (C; 12 μg/d). Axotomy results in a marked reduction in IB4 binding within the dorsal horn (B), which can be prevented by treatment with GDNF (C). Scale bar, 100 μm.
Treatment with GDNF could partially prevent the upregulation in the proportion of sciatic afferents that expressed GFRα1 and GFRα3 after axotomy, and this was a significant effect (Figs. 3E,4, 5). GDNF could also completely prevent the reduction in the proportion of sciatic afferents that expressed GFRα2 after axotomy. The proportion of sciatic afferents that expressed this receptor component returned to normal after axotomy when animals were treated with GDNF (Figs. 3F, 4, 5). GDNF, however, had no effect on the expression of RET within sensory neurons after axotomy (Figs. 4,5).
DISCUSSION
We found that GDNF family receptor components are expressed in distinct subsets of primary sensory neurons and that nerve injury results in their differential regulation. This results in increased expression of GFRα1 and GFRα3 and reduced expression of GFRα2. These changes could be partially reversed by treatment with exogenous GDNF.
GDNF family receptor components are expressed by subpopulations of normal sciatic afferents
We have found that GFRα1, GFRα2, GFRα3, and RET are expressed within 40, 30, 40, and 60% of sciatic afferents, respectively. These results agree closely with previous published findings on the expression of GFRα1, GFRα2, and RET within DRG cells (Molliver et al., 1997; Bennett et al., 1998). We have used FG labeling as a means to identify those neurons that project through the sciatic nerve, allowing unambiguous identification of axotomized neurons. We do not think this technique has altered the baseline expression of the GDNF family receptor components for two reasons. First, there is close agreement between our own and previous findings on receptor expression in normal uninjured sensory neurons. Second, we have found no difference in overall silver grain intensity between sections of FG-labeled and unlabeled DRG cells.
We found that RET and GFRα1 were expressed in a subpopulation of both small- and large-diameter DRG cells, whereas GFRα2 and GFRα3 were expressed selectively in small-diameter DRG cells. These cell size distributions are consistent with previous studies relating GDNF family receptor component expression to the different histochemical subpopulations of sensory neurons (Molliver et al., 1997; Bennett et al., 1998). Virtually all nonpeptidergic (IB4-binding) small-diameter DRG cells express RET and many also coexpress GFRα1 and/or GFRα2. Few of the peptidergic (calcitonin gene-related peptide-expressing) small-diameter DRG cells express these receptor components. A significant population of “large light” DRG cells also express RET and GFRα1. The expression of GFRα3 has not yet been related to these different subgroups.
There is differential regulation of GDNF family receptor components within sciatic afferents after axotomy
After axotomy, there was a large increase in the proportion of sciatic afferents that expressed GFRα1 (to 66% of labeled neurons), and total GFRα1 RNA also increased markedly. Interestingly, the upregulation in GFRα1 expression occurred principally in large-diameter DRG cells. Consistent with these findings, we found that the proportion of GFRα1-positive N52 labeled cells (a marker for large-diameter DRG cells) also increased. There is clearly a population of small-diameter DRG cells that do not express GFRα1 after axotomy. One previous study has also described an increase in expression of GFRα1 after axotomy (Kashiba et al., 1998).
The expression of GFRα3, although remaining restricted to small-diameter DRG cells, increased to 66% of labeled neurons, and this was also reflected in an increase in GFRα3 RNA content. It is interesting to note that, after axotomy, the majority of the small-diameter profiles express detectable levels of GFRα3 and that more of these small profiles express GFRα3 than RET. It is unclear what this means for functional signal transduction. It has been shown that GFRα components can cooperate with RET when present in their soluble form (Yu et al., 1998), but it is not know whether this system can function with its two receptor components expressed on two adjacent cells.
In contrast to GFRα1 and GFRα3, the expression of GFRα2 fell to 12% of labeled neurons after axotomy, and there was also a reduction in the total level of GFRα2 RNA.
Changes in RET expression after axotomy were less clear, because we found a small increase in the proportion of neurons that expressed RET (principally in large-diameter DRG cells), with a small reduction in total RET RNA. Previous studies have demonstrated either an increase (Naveilhan et al., 1997; Bar et al., 1998) or no change (Kashiba et al., 1998) in RET expression by DRG cells after nerve injury. These inconsistencies are probably caused by methodological differences (Swett et al., 1991). It is important to note that, when studying changes in GDNF receptor component expression, we have examined changes in RNA levels, and we do not as yet know how these reflect alterations in protein levels.
One interesting question concerns the signal that leads to these changes in expression. One possible source of signal would be the decreased availability of GDNF family ligands caused by separation from the periphery. After nerve injury, there is an increased expression of GDNF and GFRα1 in the distal nerve (Naveilhan et al., 1997; Trupp et al., 1997). This increased expression of GDNF in the distal nerve, however, may be insufficient to compensate for the lack of GDNF from peripheral targets. It is possible that alterations in availability of other members of the GDNF family might be responsible for the observed changes in receptor expression.
We have begun to examine this hypothesis by determining whether exogenous GDNF can reverse any of these postaxotomy changes. We found that GDNF treatment does, in fact, modulate the expression of its own receptor components. Treatment with GDNF could partially prevent the upregulation in the expression of GFRα1 and could completely prevent the downregulation in GFRα2 expression. In contrast, GDNF had no effect on the expression of RET. Interestingly, it had a small but significant effect in reducing the upregulation in GFRα3 expression that normally occurs after axotomy. These results are consistent with lack of GDNF being responsible for at least some of the observed changes accompanying axotomy.
Functional implications of the alterations in GDNF family receptor component expression
RET and GFRα1 are thought to be the principal mediators of GDNF action in vivo. The increased expression GFRα1 after axotomy therefore implies that the proportion of sensory neurons that are GDNF-sensitive will increase after nerve injury. In particular, large-diameter DRG cells are likely to become more GDNF-sensitive; indeed, GDNF has been shown previously to partially reverse the conduction velocity slowing that occurs in large-diameter DRG cells after axotomy (Munson and McMahon, 1997). Increased expression of RET and GFRα1 have also been described in motoneurons and hippocampal neurons after nerve injury (Colucci-D'Amato et al., 1996; Nakamura et al., 1996; Naveilhan et al., 1997; Trupp et al., 1997).
A receptor complex consisting of RET and GFRα2 is thought to mediate the actions of NTN. The marked reduction in GFRα2 expression in sensory neurons implies therefore, a reduced sensitivity of injured sensory neurons to NTN. It has been demonstrated recently that artemin, a novel member of the GDNF ligand family, can signal via GFRα3. Our results would therefore suggest that artemin may have important trophic actions on small-diameter DRG cells, particularly after nerve injury.
The other major finding from this study was that GDNF can modulate the expression of its own receptor components after axotomy. There is a precedent for this in that the neurotrophins (NGF and NT-3) have been demonstrated to reverse the changes that occur in their respective receptors after nerve injury (Verge et al., 1992, 1996). There appears therefore to be complex feedback mechanisms whereby trophic factors can regulate the expression of their receptors.
The coexpression of GDNF family receptor components by sensory neurons adds a high level of complexity to this signaling system. In the normal DRG, there is a high level of coexpression between the ligand binding domain GFRα1 and the signal transducing domain RET (Molliver et al., 1997; Bennett et al., 1998). There is also a degree of coexpression of different ligand binding domains, such as GFRα1 and GFRα2, within sensory neurons (for instance, approximately one-third of IB4-binding DRG cells coexpress these receptor components). We do not yet know how these patterns of coexpression change after nerve injury.
Interestingly, not only did GDNF partially prevent the upregulation of GFRα1 after axotomy, but it could also prevent the downregulation of GFRα2. GDNF can signal via GFRα2 in vitro (Baloh et al., 1997); however, it is still unclear as to what extent GDNF acts via GFRα2 in sensory neurons in vivo. Results from gene deletion experiments indicate that it can act via GFRα2 during development (Cacalano et al., 1998; Enomoto et al., 1998). GDNF could also partially prevent the upregulation of GFRα3 after axotomy. GDNF may be exerting its action on GFRα3 via signaling through GFRα1 or GFRα2 in neurons that coexpress these receptor components. However, there is some evidence that GDNF can bind to GFRα3 in vitro, although with somewhat lower affinity than to GFRα1 or GFRα2 (Trupp et al., 1998). GDNF may also be exerting its effects on receptor expression indirectly, for instance, via paracrine effects within the DRG.
Damaged sensory neurons are likely to become more sensitive to GDNF and artemin and less sensitive to NTN. Treatment with these ligands may also alter the expression of their receptors within sensory neurons, leading to complex interactions. For instance, the fact that exogenous GDNF can increase the expression of GFRα2 after axotomy indicates that GDNF may increase the efficacy of NTN on sensory neurons in this condition. Changes in the expression of GDNF family receptor components after nerve injury may be of clinical relevance if these molecules are to be used therapeutically in the treatment of peripheral neuropathy.
Footnotes
This work was supported by the Medical Research Council of Great Britain and by a grant from Genentech to S.B.M. We acknowledge the technical assistance of Vivien Cheah.
Drs. Bennett and Boucher contributed equally to this work.
Correspondence should be addressed to David L. H. Bennett, Neuroscience Research Centre, St. Thomas' Hospital Campus, GKT, King's College London, Lambeth Palace Road, London SE1 7EH, United Kingdom. E-mail: db70@umds.ac.uk.
REFERENCES
- 1.Baloh RH, Tansey MG, Golden JP, Creedon DJ, Heuckeroth RO, Keck CL, Zimonjic DB, Popescu NC, Johnson EMJ, Milbrandt J. TrnR2, a novel receptor that mediates neurturin and GDNF signaling through Ret. Neuron. 1997;18:793–802. doi: 10.1016/s0896-6273(00)80318-9. [DOI] [PubMed] [Google Scholar]
- 2.Baloh RH, Gorodinsky A, Golden JP, Tansey MG, Keck CL, Popescu NC, Johnson EMJ, Milbrandt J. GFRα3 is an orphan member of the GDNF/neurturin/persephin receptor family. Proc Natl Acad Sci USA. 1998;95:5801–5806. doi: 10.1073/pnas.95.10.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EMJ, Milbrandt J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRα3-RET receptor complex. Neuron. 1999;21:1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- 4.Bar KJ, Saldanha GJ, Kennedy AJ, Facer P, Birch R, Carlstedt T, Anand P. GDNF and its receptor component Ret in injured human nerves and dorsal root ganglia. NeuroReport. 1998;9:43–47. doi: 10.1097/00001756-199801050-00009. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DLH, Averill S, Clary DO, Priestley JV, McMahon SB. Postnatal changes in the expression of the trkA high affinity NGF receptor in primary sensory neurons. Eur J Neurosci. 1996;8:2204–2208. doi: 10.1111/j.1460-9568.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 6.Bennett DLH, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buj-Bello A, Buchman VL, Horton A, Rosenthal A, Davies AM. GDNF is an age-specific survival factor for sensory and autonomic neurons. Neuron. 1995;15:821–828. doi: 10.1016/0896-6273(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 8.Buj-Bello A, Adu J, Pinon LG, Horton A, Thompson J, Rosenthal A, Chinchetru M, Buchman VL, Davies AM. Neurturin responsiveness requires a GPI-linked receptor and the Ret receptor tyrosine kinase. Nature. 1997;387:721–724. doi: 10.1038/42729. [DOI] [PubMed] [Google Scholar]
- 9.Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies, Rosenthal A. GFRα1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colucci-D'Amato GL, D'Alessio A, Filliatreau G, Florio T, Di Giamberardino L, Chiappetta G, Vecchio G, Fusco A, Santoro M, de, Franciscis V. Presence of physiologically stimulated RET in adult rat brain: induction of RET expression during nerve regeneration. Cell Growth Differ. 1996;7:1081–1086. [PubMed] [Google Scholar]
- 11.Creedon DJ, Tansey MG, Baloh RH, Osborne PA, Lampe PA, Fahrner TJ, Heuckeroth RO, Milbrandt J, Johnson EM., Jr Neurturin shares receptors and signal transduction pathways with glial cell line-derived neurotrophic factor in sympathetic neurons. Proc Natl Acad Sci USA. 1997;94:7018–7023. doi: 10.1073/pnas.94.13.7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebendal T, Tomac A, Hoffer BJ, Olson L. Glial cell line-derived neurotrophic factor stimulates fiber formation and survival in cultured neurons from peripheral autonomic ganglia. J Neurosci Res. 1995;40:276–284. doi: 10.1002/jnr.490400217. [DOI] [PubMed] [Google Scholar]
- 13.Enokido Y, de Sauvage F, Hongo J-A, Ninkina N, Rosenthal A, Buchman VL, Davies AM. GFRα4 and the tyrosine kinase RET form a functional receptor complex for Persephin. Curr Biol. 1998;8:1019–1022. doi: 10.1016/s0960-9822(07)00422-8. [DOI] [PubMed] [Google Scholar]
- 14.Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EMJ, Milbrandt J. GFRα1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- 15.Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. PCR Methods Appl. 1996;6:986–994. doi: 10.1101/gr.6.10.986. [DOI] [PubMed] [Google Scholar]
- 16.Henderson CE, Camu W, Mettling C, Gouin A, Poulsen K, Karihaloo M, Rullamas J, Evans T, McMahon SB, Armanini MP. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363:266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- 17.Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, Louis JC, Hu S, Altrock BW, Fox GM. GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-alpha, a novel receptor for GDNF. Cell. 1996;85:1113–1124. doi: 10.1016/s0092-8674(00)81311-2. [DOI] [PubMed] [Google Scholar]
- 18.Jing S, Yu Y, Fang M, Hu Z, Holst PL, Boone T, Delaney, Schultz H, Zhou R, Fox GM. GFRα-2 and GFRα-3 are two new receptors for ligands of the GDNF family. J Biol Chem. 1997;272:33111–33117. doi: 10.1074/jbc.272.52.33111. [DOI] [PubMed] [Google Scholar]
- 19.Kashiba H, Hyon B, Senba E. Glial cell line-derived neurotrophic factor and nerve growth factor receptor mRNAs are expressed in distinct subgroups of dorsal root ganglion neurons and are differentially regulated by peripheral axotomy in the rat. Neurosci Lett. 1998;252:107–110. doi: 10.1016/s0304-3940(98)00558-8. [DOI] [PubMed] [Google Scholar]
- 20.Klein RD, Sherman D, Ho WH, Stone D, Bennett GL, Moffat B, Vandlen R, Simmons L, Gu Q, Hongo JA, Devaux B, Poulsen K, Armanini M, Nozaki C, Asai N, Goddard A, Phillips H, Henderson CE, Takahashi M, Rosenthal A. A GPI-linked protein that interacts with Ret to form a candidate neurturin receptor. Nature. 1997;387:717–721. doi: 10.1038/42722. [DOI] [PubMed] [Google Scholar]
- 21.Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EMJ, Milbrandt J. Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature. 1996;384:467–470. doi: 10.1038/384467a0. [DOI] [PubMed] [Google Scholar]
- 22.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 23.Matheson CR, Carnahan J, Urich JL, Bocangel D, Zhang TJ, Yan Q. Glial cell line-derived neurotrophic factor (GDNF) is a neurotrophic factor for sensory neurons: comparison with the effects of the neurotrophins. J Neurobiol. 1997;32:22–32. [PubMed] [Google Scholar]
- 24.McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 25.Melton DA, Krieg PA, Rebagliati MR, Maniatis T, Zinn K, Green MR. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984;12:7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milbrandt J, de Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MG, Lampe PA, Heuckeroth RO, Kotzbauer PT, Simburger KS, Golden JP, Davies JA, Vejsada R, Kato AC, Hynes M, Sherman D, Nishimura M, Wang LC, Vandlen R, Moffat B, Klein RD, Poulsen K, Gray C, Garces A, Johnson EMJ. Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron. 1998;20:245–253. doi: 10.1016/s0896-6273(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 27.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 28.Moore MW, Klein RD, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 29.Munson JB, McMahon SB. Effects of GDNF on axotomized sensory and motor neurons in adult rats. Eur J Neurosci. 1997;9:1126–1129. doi: 10.1111/j.1460-9568.1997.tb01465.x. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura M, Ohta K, Hirokawa K, Fukushima M, Uchino M, Ando M, Tanaka H. Developmental and denervation changes in c-ret proto-oncogene expression in chick motoneurons. Brain Res Mol Brain Res. 1996;39:1–11. doi: 10.1016/0169-328x(95)00347-u. [DOI] [PubMed] [Google Scholar]
- 31.Naveilhan P, ElShamy WM, Ernfors P. Differential regulation of mRNAs for GDNF and its receptors Ret and GDNFR alpha after sciatic nerve lesion in the mouse. Eur J Neurosci. 1997;9:1450–1460. doi: 10.1111/j.1460-9568.1997.tb01499.x. [DOI] [PubMed] [Google Scholar]
- 32.Naveilhan P, Baudet C, Mikaels A, Shen L, Westphal H, Ernfors P. Expression and regulation of GFRα3, a glial cell line-derived neurotrophic factor family receptor. Proc Natl Acad Sci USA. 1998;95:1295–1300. doi: 10.1073/pnas.95.3.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips HS, Hains JM, Laramee GR, Rosenthal A, Winslow JW. Widespread expression of BDNF but not NT3 by target areas of basal forebrain cholinergic neurons. Science. 1990;250:290–294. doi: 10.1126/science.1688328. [DOI] [PubMed] [Google Scholar]
- 34.Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 35.Rose RD, Rohrlich D. Counting sectioned cells via mathematical reconstruction. J Comp Neurol. 1987;263:365–386. doi: 10.1002/cne.902630305. [DOI] [PubMed] [Google Scholar]
- 36.Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 37.Sanicola M, Hession C, Worley D, Carmillo P, Ehrenfels C, Walus L, Robinson S, Jaworski G, Wei H, Tizard R, Whitty A, Pepinsky RB, Cate RL. Glial cell line-derived neurotrophic factor-dependent RET activation can be mediated by two different cell-surface accessory proteins. Proc Natl Acad Sci USA. 1997;94:6238–6243. doi: 10.1073/pnas.94.12.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuchardt A, D'Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994;367:380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 39.Swett JE, Torigoe Y, Elie VR, Bourassa CM, Miller PG. Sensory neurons of the rat sciatic nerve. Exp Neurol. 1991;114:82–103. doi: 10.1016/0014-4886(91)90087-s. [DOI] [PubMed] [Google Scholar]
- 40.Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F, Phillips HS, Goddard A, Moore MW, Buj-Bello A, Davies AM, Asai N, Takahashi M, Vandlen R, Henderson CE, Rosenthal A. Characterization of a multicomponent receptor for GDNF. Nature. 1996;382:80–83. doi: 10.1038/382080a0. [DOI] [PubMed] [Google Scholar]
- 41.Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez CF. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trupp M, Belluardo N, Funakoshi H, Ibanez CF. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-α indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trupp M, Raynoschek C, Belluardo N, Ibanez CF. Multiple GPI-anchored receptors control GDNF-dependent and independent activation of the c-Ret receptor tyrosine kinase. Mol Cell Neurosci. 1998;11:47–63. doi: 10.1006/mcne.1998.0667. [DOI] [PubMed] [Google Scholar]
- 44.Verge VM, Merlio JP, Grondin J, Ernfors P, Persson H, Riopelle RJ, Hokfelt T, Richardson PM. Colocalization of NGF binding sites, trk mRNA, and low-affinity NGF receptor mRNA in primary sensory neurons: responses to injury and infusion of NGF. J Neurosci. 1992;12:4011–4022. doi: 10.1523/JNEUROSCI.12-10-04011.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verge VM, Gratto KA, Karchewski LA, Richardson PM. Neurotrophins and nerve injury in the adult. Phil Trans R Soc Lond B Biol Sci [Review] 1996;351:423–430. doi: 10.1098/rstb.1996.0038. [DOI] [PubMed] [Google Scholar]
- 46.Worby CA, Vega QC, Chao HH, Seasholtz AF, Thompson RC, Dixon JE. Identification and characterization of GFRα-3, a novel co-receptor belonging to the glial cell line-derived neurotrophic receptor family. J Biol Chem. 1998;273:3502–3508. doi: 10.1074/jbc.273.6.3502. [DOI] [PubMed] [Google Scholar]
- 47.Yu T, Scully S, Yu Y, Fox GM, Jing S, Zhou R. Expression of GDNF family receptor components during development: implications in the mechanisms of interaction. J Neurosci. 1998;18:4684–4696. doi: 10.1523/JNEUROSCI.18-12-04684.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]