Abstract
We measured stimulation of c-fos and oxytocin gene expression during excitation of oxytocin cells induced by systemic or local morphine withdrawal. Female rats were made morphine-dependent by intracerebroventricular morphine infusion over 5 d. Morphine withdrawal, induced by systemic injection of the opioid antagonist naloxone (5 mg/kg) in conscious or anesthetized rats, increased the density of c-fos messenger RNA and of oxytocin heterogeneous nuclear RNA in supraoptic nucleus cells compared with those of nonwithdrawn rats; c-fos messenger RNA was also increased in the magnocellular and parvocellular paraventricular nuclei of withdrawn rats. Morphine withdrawal increased the number of Fos-immunoreactive cells in the supraoptic and magnocellular paraventricular nuclei of conscious or pentobarbitone-anesthetized rats. Morphine withdrawal also increased Fos-immunoreactive cell numbers in the parvocellular paraventricular nucleus of conscious but not anesthetized rats. Central administration of the α1-adrenoreceptor antagonist benoxathian (5 μg/min) did not prevent morphine withdrawal-induced increases in the numbers of Fos-immunoreactive neurons in the supraoptic or magnocellular paraventricular nucleus. Unilateral microdialysis administration of naloxone (10−5m) into the supraoptic nucleus of anesthetized morphine-dependent rats increased Fos-immunoreactive cell numbers compared with the contralateral nucleus. Finally, we investigated whether dependence could be induced by chronic unilateral infusion of morphine into a supraoptic nucleus; systemic naloxone (5 mg/kg) increased Fos-immunoreactive cell numbers in the morphine-infused nucleus compared with the contralateral nucleus. Thus, morphine withdrawal excitation increases c-fos and oxytocin gene expression in supraoptic nucleus neurons. This occurs independently from excitation of their ascending noradrenergic inputs, and both dependence and withdrawal can be induced within the supraoptic nucleus.
Keywords: adrenoreceptors, benoxathian, dependence, hypothalamic paraventricular nucleus, microdialysis, naloxone, opioid, supraoptic nucleus
Magnocellular neurosecretory cells of the hypothalamic supraoptic nucleus (SON) and paraventricular nucleus (PVN) project to the neurohypophysis (posterior pituitary gland) in which they secrete oxytocin or vasopressin into the systemic circulation (Hatton, 1990). The μ-opioid agonist morphine inhibits oxytocin cells in vivo, an effect that is reversed by the opioid antagonist naloxone (Ludwig et al., 1997a). Oxytocin cells develop tolerance to, and dependence on, morphine during prolonged central administration of this opioid (Pumford et al., 1991). Tolerance involves downregulation of μ-receptors within the supraoptic nucleus (Sumner et al., 1990), but the mechanisms underlying morphine dependence in oxytocin (or any other) neurons have yet to be fully elucidated.
Naloxone-precipitated morphine withdrawal causes a robust hyperexcitation of oxytocin cells, evident as a marked (approximate threefold) increase in firing rate (Leng et al., 1989). In combination with naloxone antagonism of endogenous opioid actions at κ-opioid receptors on the neurohypophysial neurosecretory terminals (Russell et al., 1993) and frequency facilitation of hormone release (Bicknell, 1988), this excitation causes a 100-fold increase in systemic oxytocin release (Bicknell et al., 1988). Vasopressin cells are little affected by morphine and do not show morphine withdrawal excitation (Bicknell et al., 1988). Direct application of naloxone into the supraoptic nucleus of morphine-dependent rats elicits electrical excitation of oxytocin cells typical of morphine withdrawal (Ludwig et al., 1997a). Thus, the mechanisms that underlie morphine dependence–withdrawal of oxytocin neurons may reside within these cells or at local synapses.
Fos protein (the product of the immediate early gene c-fos) expression is a reliable marker of activation in magnocellular neurosecretory neurons (Hoffman et al., 1993). Electrical excitation of oxytocin cells during withdrawal is accompanied by increased Fos expression in the supraoptic and paraventricular nuclei (Stornetta et al., 1993; Jhamandas et al., 1996; Murphy et al., 1997). Currently, the consequences of increased Fos expression in magnocellular neurosecretory cells are uncertain. Fos has been proposed to be involved in oxytocin gene regulation (Ivell and Richter, 1984; Walther et al., 1991), but it is not known whether increased Fos expression is followed by oxytocin gene stimulation.
Morphine withdrawal also induces Fos expression in brainstem sites that project to the supraoptic nucleus (Aghajanian, 1978; Stornetta et al., 1993; Murphy et al., 1997), including the A2 noradrenergic cell group in the nucleus tractus solitarii (NTS). Such evidence, and that from other studies, has led to the proposal that other non-noradrenergic neurons are activated by their excited noradrenergic input during withdrawal (Redmond and Krystal, 1984). Although oxytocin cell withdrawal excitation is temporarily interrupted by acute α1-adrenoreceptor blockade, it is unaffected by chronic neurotoxin-induced hypothalamic noradrenaline depletion (Brown et al., 1998).
Here, we measured the expression of c-fos messenger RNA (mRNA), Fos protein, and oxytocin heterogeneous nuclear RNA (hnRNA) in magnocellular neurosecretory cells during naloxone-precipitated morphine withdrawal to determine whether changes in activity of the c-fos gene are accompanied by altered expression of the oxytocin gene. Subsequently, we used Fos protein expression to test whether dependence and withdrawal excitation could be induced by local actions of morphine and naloxone within the supraoptic nucleus.
MATERIALS AND METHODS
Induction of tolerance–dependence by intracerebroventricular morphine. Morphine tolerance–dependence was induced as described previously (Rayner et al., 1988). Briefly, virgin female rats (250–350 gm) were implanted, under ether (in some experiments) or halothane anesthesia, with a cannula in a lateral cerebral ventricle connected via polyethylene tubing to a subcutaneous osmotic minipump (1 μl/hr; Alzet 2001; Alza Corporation, Palo Alta, CA), filled with morphine sulfate (50 μg/μl). The tubing contained 40 μl of 10 μg/μl morphine solution, followed by 40 μl of 20 μg/μl morphine solution. After surgery, the rats were housed singly for 5 d.
In situ hybridization for c-fos mRNA.Morphine-dependent rats were anesthetized with urethane (ethyl carbamate, 1.25 gm/kg, i.p.), and a cannula was inserted into a femoral vein for removal of blood samples. These are conditions identical to those in which we have studied previously oxytocin neuron tolerance and dependence and withdrawal excitation with electrophysiological techniques (Russell et al., 1995; Ludwig et al., 1997a; Brown et al., 1998). The rats were injected with naloxone (5 mg/kg, 10 mg/ml, s.c.;n = 4) or 0.15 m saline (subcutaneously; n = 4) at least 2 hr after the completion of the surgery and decapitated an additional 45 min later. The brains were rapidly removed, frozen on crushed dry ice, and stored at −70°C until processed for c-fos mRNA in situ hybridization. Blood samples (0.3 ml) were removed 5 min before and 5 and 30 min after naloxone–vehicle administration, and the separated plasma was stored at −20°C until assayed for oxytocin content.
The in situ hybridization for c-fos mRNA was performed as described previously (Hamamura et al., 1991). Briefly, 10 μm coronal cryostat sections were cut through the hypothalamus and through a series of brain paste 35S standards and mounted onto RNase-free, gelatin–chrome alum-coated slides, air-dried, and fixed in 3% paraformaldehyde and 0.03% diethyl pyrocarbonate in 0.1 m phosphate buffer. After dehydration, the sections were stored desiccated at −70°C. Representative brain paste standard sections were counted in a scintillation counter. The sections were prehybridized and hybridized overnight at 37°C using a35S-dATP-labeled oligonucleotide probe corresponding to base pairs 138–185 of the rat c-fos gene (Curran et al., 1987). After washing, the sections were exposed to autoradiographic film for 2 weeks, dipped in K5 emulsion (Ilford Imaging, Mobberley, UK), and exposed for an additional 9 weeks before development and counterstaining with methylene blue.
Autoradiographs of the supraoptic nucleus were quantified with a computer-based analysis system (μMagiscan; Joyce-Loebl Ltd., Gateshead, UK) with images magnified in a microscope (10× objective) and collected by a CCD video camera (Cohu Inc., San Diego, CA). The analyzer was set to measure silver grain area per supraoptic nucleus profile, and hence grain density per unit area of supraoptic nucleus. Background density was measured over an adjacent tissue-free area of film and subtracted. From the standards, the tissue measurements were on the linear part of the relationship between natural log[radioactivity] and silver grain density. Animal means were calculated from the individual supraoptic nucleus measurements in each animal. The emulsion-dipped autoradiographs showed that the hybridization signal was over neurons and not glia (Ludwig et al., 1997b).
Fos protein immunocytochemistry. Conscious morphine-dependent rats and morphine-naïve rats (implanted with a vehicle-containing minipump attached via polyethylene tubing to the intracerebroventricular cannula) were injected on day 6 with naloxone (5 mg/kg, s.c.) or 0.15 m saline (all four groupsn = 7) and decapitated 90 min later. The brains were rapidly removed, frozen on crushed dry ice, and stored at −70°C until processed to detect Fos-immunoreactive (IR) cells.
Coronal 15 μm cryostat sections were cut through the supraoptic and paraventricular nuclei, mounted on gelatin-coated slides, and fixed in 4% paraformaldehyde in phosphate buffer. The sections were then processed immunocytochemically using a rabbit polyclonal antibody raised against the N terminal of rat Fos protein (1:1000 dilution; Oncogene Science, Uniondale, NY), with incubation for 24 hr in a humidified chamber at 4°C. Sections were incubated for an additional 24 hr at 4°C with peroxidase-labeled goat anti-rabbit IgG (1:500 dilution; Vector Laboratories, Burlingame, CA). Then, Fos protein immunoreactivity was visualized as a black precipitate using a glucose oxidase-based, Ni-intensified, 3,3′-diaminobenzidine (DAB) procedure (Shu et al., 1988). The slides were then dehydrated through an alcohol series, dewaxed in xylene, and coverslipped in DePeX mountant. Immunocytochemically processed sections from each rat containing the supraoptic nucleus [1.3–1.4 mm posterior to bregma (Paxinos and Watson, 1996)] and the magnocellular (1.8 mm) and parvocellular paraventricular nucleus (1.8–2.12 mm) were selected for analysis. The number of Fos-IR cells within the supraoptic and paraventricular nuclei were counted on coded sections (n = 6–8 per region per rat) under a light microscope (10× objective). The animal mean number of Fos-IR cells per section for each region and finally the group mean ± SEM was then calculated. Because activation may enlarge magnocellular neurons and the section thickness was constant, any activation-induced enlargement of the neurons would decrease the number of neurons in each section; no correction was made for this possibility. The results show large increases in number of Fos-positive neurons per SON–PVN section with morphine withdrawal so any contribution from this counting error is not important for interpretation in this study.
Some supraoptic nucleus sections were processed further after the Fos immunocytochemical procedure to identify oxytocin neurons immunocytochemically. Rabbit anti-oxytocin antibody (Higuchi et al., 1985) (1:50,000 dilution) was applied and visualized after incubation for 24 hr at 4°C with goat anti-rabbit IgG–peroxidase complex (24 hr, 4°C) and DAB.
In situ hybridization for oxytocin hnRNA. In this experiment, conscious rats were given an injection of either naloxone (5 mg/kg, 10 mg/ml, s.c.; n = 6) or vehicle (0.15m NaCl; n = 5) on the sixth day of intracerebroventricular morphine infusion. The rats were decapitated 2 hr later, and the brains were removed and frozen on crushed dry ice before storage at −70°C until processed for oxytocin hnRNA in situ hybridization, as described previously (Douglas et al., 1998). Briefly, 10 μm coronal sections were cut through the supraoptic nucleus at −14°C and mounted onto RNase-free, gelatin-subbed slides. Oxytocin hnRNA was detected using a single-stranded 3H-cDNA probe directed against intron 1 of the primary transcript of the rat preprooxytocin gene (Brooks et al., 1993). Slides containing supraoptic nucleus sections (between 0.92 and 1.30 mm posterior to bregma) were incubated in prehybridization buffer at 42°C for 2 hr, rinsed, air dried, and then incubated overnight at 42°C in hybridization buffer containing 100,000 counts per minute per section. Sections were washed, incubated overnight with gentle agitation, and finally washed for 30 min at 50°C. Slides were dipped in G5 photographic emulsion and exposed for 50 d at 4°C. The sections were then counterstained with hematoxylin and eosin and mounted in DePeX. Sections (10 μm) of autoradiographic [3H] microscales cut from polymer blocks (Amersham, Arlington Heights, IL) were mounted onto subbed slides, dipped in photographic emulsion, exposed, and developed simultaneously with the brain sections. Control sections (RNase A pretreated before hybridization or incubated in hybridization buffer without labeled probe) showed no specific hybridization over the hypothalamic magnocellular nuclei.
Four sections per animal were viewed on a computer monitor using a CCD video camera (Cohu) connected to a Vickers M17 microscope. Measurements were made on coded sections containing the SON at a level of 1.30 mm posterior to bregma (Paxinos and Watson, 1996) using an image analyzer. Cells were considered positive when an accumulation of silver grains (at least three times greater than background) could be discerned over the nucleus (Brooks et al., 1993; Xing et al., 1993). The area of the supraoptic nucleus profile was measured using a calibrated image analyzing system, and the number of positive cells was calculated per supraoptic nucleus area (in square micrometers) for each section.
Silver grain area, representing hybridization of oxytocin hnRNA, over the cell nucleus was measured (50× oil immersion objective). All positive cells in four supraoptic nucleus profiles were measured for each animal, at least five background measurements were obtained from adjacent tissue dorsolateral to the supraoptic nucleus, and the mean background was subtracted from each supraoptic nucleus cell silver grain measurement. The mean silver grain area per magnocellular cell nucleus was then calculated for each profile.
Grain area was measured for each level of the calibrated radioactive microscale, and corresponding backgrounds were subtracted. For each experiment, a semilog curve was plotted for the average grain area per3H standard (corresponding to an area equivalent to a neuronal nucleus or cytoplasm or supraoptic nucleus) against the tissue equivalent radioactivity. All detected grain areas in tissue lay on the linear part of the relationship between radioactivity and grain area.
Oxytocin radioimmunoassay. Plasma oxytocin concentrations were measured in duplicate aliquots by specific radioimmunoassay as described previously (Leng et al., 1988) using antiserum kindly provided by Dr. T. Higuchi (Fukui Medical University, Fukui, Japan) (Higuchi et al., 1985). The oxytocin concentrations in all samples were determined in a single assay. The assay sensitivity was <2.4 pg/ml, and the intra-assay coefficient of variation was <12%.
Central α1-antagonist administration to morphine-dependent rats. Rats were prepared for chronic intracerebroventricular morphine (n = 12) or vehicle (n = 12) infusion as described above, with the addition that a 28 gauge stainless steel cannula (Plastics One, Roanoke, VA) was placed into the left lateral cerebral ventricle (0.6 mm caudal, 1.6 mm lateral to bregma, and 4.5 mm below the surface of the skull) at the time of implantation of the morphine-containing minipump for subsequent intracerebroventricular infusion of the potent and selective α1-adrenoreceptor antagonist benoxathian (Melchiorre et al., 1984). On the sixth day after surgery, the rats were anesthetized with pentobarbitone (60 mg/kg, i.p.), and an intracerebroventricular infusion of benoxathian (5 μg/min; 0.5 μl/min in 0.9% saline) or vehicle from a slow infusion pump was started at least 2 hr later. Naloxone was administered (5 mg/kg, s.c.) 30 min into the benoxathian–vehicle infusion, and the infusion was continued until the rats were decapitated 90 min later. Again, the brains were removed, frozen on crushed dry ice, and stored at −70°C until processed for Fos-IR as above.
We used barbiturate anesthesia in this and the next experiments to avoid the stimulation of Fos expression that can be seen with urethane anesthesia (Takayama et al., 1994). A time course study in barbiturate-anesthetized morphine-dependent rats showed no Fos expression in the supraoptic nucleus or magnocellular paraventricular nucleus after subcutaneous vehicle injection (at 30, 45, 60, 90, and 120 min after injection; four rats per time point), but there was a significant increase in Fos expression in both nuclei after naloxone injection at all time points except 30 min (four rats per time point). There was no significant difference in the number of Fos-positive neurons between 45 and 120 min after naloxone, but the variance was least at 90 min, and so this was selected as the sampling point in the main experiments.
Fos immunocytochemistry was performed on coronal brainstem sections through the A1 and A2 groups of noradrenergic neurons from benoxathian-infused rats and their controls.
Microdialysis administration of naloxone into the supraoptic nucleus of morphine-dependent rats. A U-shaped microdialysis probe [molecular weight cutoff, 5 kDa; 3.0 mm long × 1.0 mm outer diameter; 0.2 mm inner diameter (i.d.)] was placed adjacent to the left supraoptic nucleus (0.9 mm caudal, 1.7 mm lateral, and 9.1 mm below bregma) at the time of implantation of the intracerebroventricular chronic infusion cannula and morphine-containing minipump. Five days later, the probe was used for microdialysis administration (retrodialysis) of naloxone under sodium pentobarbitone anesthesia (60 mg/kg, i.p.). Identical probes, when inserted into the brain parenchyma, have been shown to effectively dialyze a brain volume with a radius of ∼1 mm after infusion through the probe for 30 min (Ludwig and Leng, 1997). A 1 ml Hamilton syringe was mounted on a slow infusion pump (flow rate set at 2.84 μl/min) and connected to the microdialysis probe via polyethylene tubing (0.4 mm i.d.). This was filled with artificial CSF (aCSF) (in mm: NaCl 138, KCl 3.36, NaHCO3 9.52, Na2HPO4 0.49, urea 2.16, CaCl2 1.26, and MgCl2 1.18, pH 7.2). The rats were left for ∼120 min before the dialysate was switched to aCSF containing 10−5m naloxone (n = 10) or aCSF alone (n = 9). The rats were killed by decapitation 90 min later, and their brains were processed for Fos-IR as described above.
Chronic intrasupraoptic nucleus morphine infusion. Rats were anesthetized with 5% halothane. A 28 gauge stainless steel cannula filled with morphine or Ringer's solution (in mm: 147 NaCl, 4 KCl, and 2.5 CaCl2) was inserted immediately dorsal to the right supraoptic nucleus (0.9 mm caudal and 1.7 mm lateral to bregma and 9.1 mm below the surface of the skull). The cannula was attached via silicone tubing (0.25 mm i.d.; 0.91 mm wall thickness) to a subcutaneous Alzet 2002 miniosmotic pump set to deliver morphine at 0.25 μg/hr or Ringer's solution at 0.5 μl/hr. The cannula was secured using dental acrylic bonded to stainless steel screws inserted in the skull. After surgery, the rats were housed individually with access to food and water ad libitum.
On the sixth day after implantation of the minipump, the rats were anesthetized with pentobarbitone (60 mg/kg, i.p.) and injected (intraperitoneally) with naloxone (5 mg/kg; n = 7) or 0.15 m saline (n = 5) at least 2 hr later. The rats were decapitated an additional 90 min later, and the brains were rapidly removed, frozen on crushed dry ice, and stored at −70°C until processed for Fos-IR.
Statistics. Statistical tests were completed using the SigmaStat software package (Jandel Scientific GmbH, Erkrath, Germany). Data from each experiment were analyzed by using Student'st test or, where appropriate, two-way repeated measures ANOVA or one-way ANOVA on ranks. Where the F ratio was significant, post hoc analyses were performed between groups using Student–Newman–Keuls test or Dunn's method depending on group numbers.
RESULTS
c-fos mRNA expression in the supraoptic and paraventricular nuclei after morphine withdrawal
Plasma oxytocin concentrations were determined to confirm the expression of morphine withdrawal excitation by oxytocin neurons in each rat under urethane anesthesia. The mean initial plasma oxytocin concentrations in the two groups of morphine-dependent rats subsequently administered vehicle or systemic naloxone were 58.4 ± 25.6 and 29.0 ± 12.8 pg/ml, respectively (bothn = 4). Five and 30 min after vehicle administration (0.15 m saline, s.c.), the plasma oxytocin concentrations were 36.6 ± 15.0 and 32.0 ± 15.8 pg/ml, respectively. In the rats administered naloxone (5 mg/kg, s.c.), the plasma oxytocin concentrations were 1315.6 ± 260.7 and 365.6 ± 53.2 pg/ml at 5 and 30 min after the naloxone injection (bothp < 0.05; two-way repeated measures ANOVA, followed by Student–Newman–Keuls test), indicative of morphine withdrawal excitation.
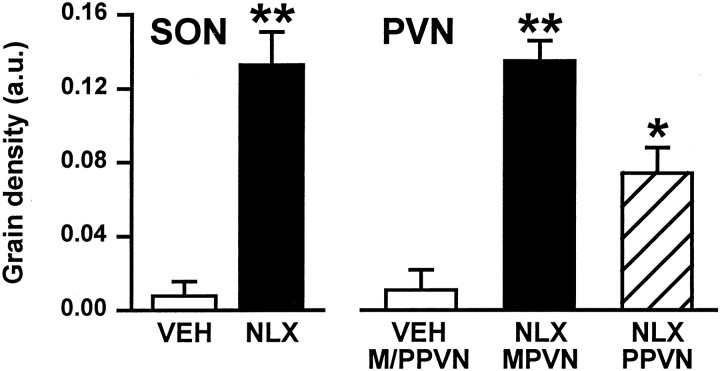
Forty-five minutes after naloxone administration (5 mg/kg, s.c.), c-fos gene expression was increased with respect to vehicle-injected (0.15 m saline, s.c.) controls in both the supraoptic nucleus (p < 0.01; Student's t test) and in the magnocellular (p < 0.01) and parvocellular (p < 0.05) portions of the paraventricular nucleus (Fig. 1).
Fig. 1.
Changes in expression of c-fos mRNA in the supraoptic and paraventricular nuclei during morphine withdrawal. Mean silver grain density [arbitrary units (a.u.) + SEM] in c-fos-labeled sections through the SON and PVN (magnocellular and parvocellular divisions, MPVN and PPVN, respectively) of morphine-dependent rats 45 min after vehicle (VEH; 0.15 m saline, s.c.; n = 4, magnocellular and parvocellular paraventricular nuclei combined) or naloxone (NLX; 5 mg/kg, s.c.; n = 4) administration. *p < 0.05, **p < 0.01 versus vehicle; Student'st test.
Hypothalamic Fos protein expression after morphine withdrawal excitation
We used immunocytochemistry to investigate whether the increased expression of the c-fos gene induced by morphine withdrawal results in increased translation into Fos protein. In conscious morphine-dependent rats, systemic naloxone induced the behavioral signs of withdrawal (e.g., wet dog shakes, teeth-chattering, defecation), and Fos-IR nuclei were prominent in magnocellular neurosecretory cells of the supraoptic (Fig.2) and paraventricular nuclei, as well as in parvocellular cells of the paraventricular nucleus. The expression of Fos-IR cells in the forebrain was discrete, with substantial numbers consistently found in the anterior hypothalamus, primary olfactory cortex, medial amygdaloid nucleus, anterior paraventricular thalamic nucleus, endopiriform nucleus, and the cerebral cortex surrounding the cingulate gyrus. There were only a few Fos-IR cells in the perinuclear zone adjacent to the supraoptic nucleus in all groups.
Fig. 2.
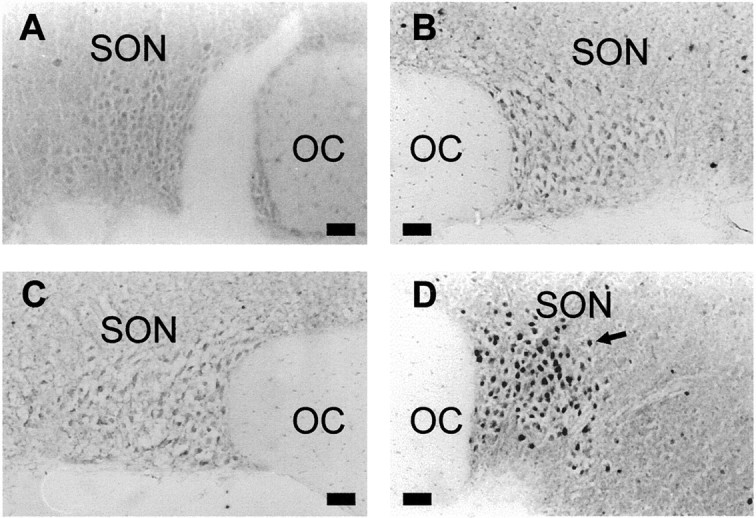
Fos protein in the supraoptic nuclei of morphine-naïve and -dependent rats. Photomicrographs of coronal sections through the SON processed for Fos immunohistochemistry from a morphine-naïve rat given vehicle (0.9% saline, 0.5 ml/kg, s.c.) (A), a morphine-naïve rat given naloxone (5 mg/kg, s.c.) (B), a morphine-dependent rat given vehicle (C), and a morphine-dependent rat given naloxone (D). Nuclei immunoreactive for Fos protein appear as intense black staining (arrow). OC, Optic chiasma. Scale bars, 50 μm.
The number of Fos-IR cells in the supraoptic nucleus of the morphine-dependent rats given naloxone (5 mg/kg, s.c.) was significantly greater (p < 0.05) than that of all other groups (Fig. 3). The number of Fos-IR cells in the supraoptic nucleus of morphine-naïve rats given 0.15 m saline (subcutaneously;n = 6) was not different from that found in the supraoptic nuclei of morphine-naïve rats given naloxone (n = 6) or that of morphine-dependent rats given saline (subcutaneously; n = 6) (Fig. 3).
Fig. 3.
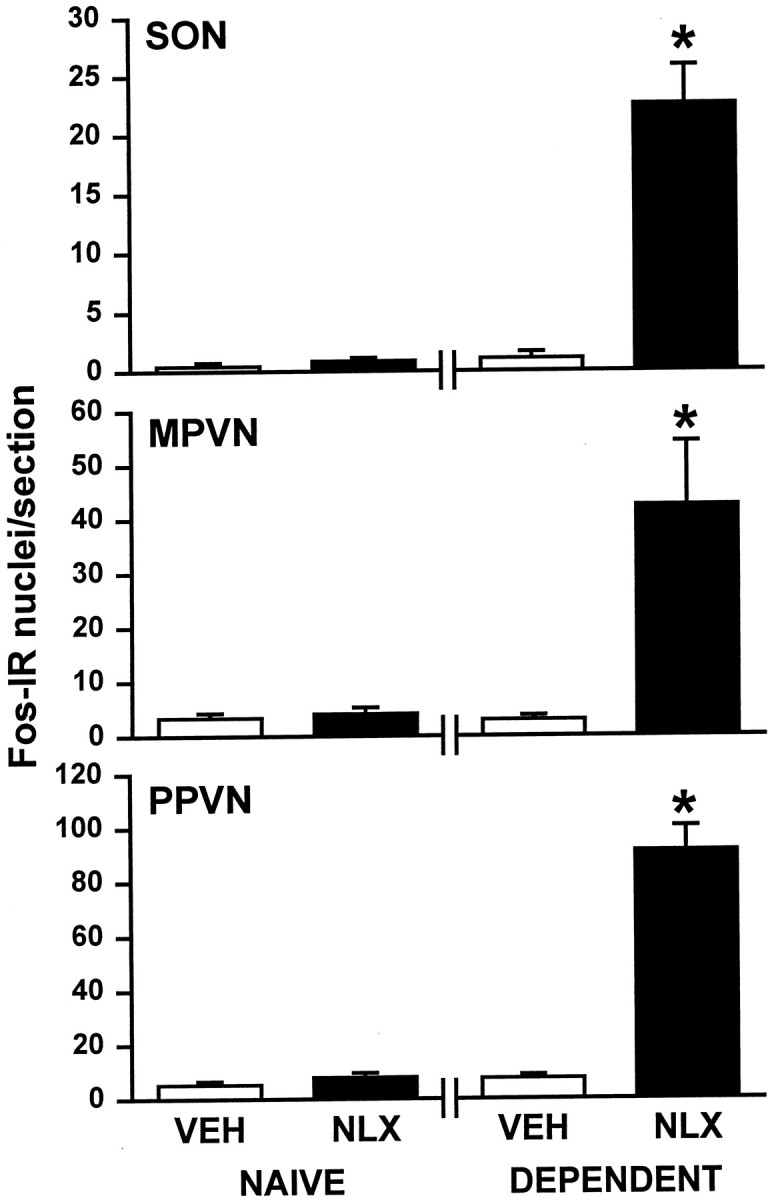
Fos protein in the supraoptic and paraventricular nuclei of morphine-naïve and -dependent rats. The mean + SEM number of Fos-IR cells in the SON, the magnocellular paraventricular nucleus (MPVN), the parvocellular paraventricular nucleus (PPVN), morphine-naïve rats (NAIVE) given 0.15 m saline (VEH; subcutaneously; n = 6 or 7) or naloxone (NLX; 5 mg/kg, s.c.; n = 6) and morphine-dependent rats (DEPENDENT) given saline (VEH; subcutaneously; n = 6 or 7) or naloxone (NLX). *p< 0.05 versus morphine-naïve vehicle or naloxone-injected rats, and morphine-dependent vehicle-injected rats; one-way ANOVA on ranks, followed by Student–Newman–Keuls test.
Similarly to its effects in the supraoptic nucleus, naloxone (5 mg/kg, s.c.) significantly increased the number of Fos-IR cells in the magnocellular and parvocellular paraventricular nuclei of morphine-dependent rats (both p < 0.05;n = 7) (Fig. 3). The number of Fos-IR cells in the magnocellular and parvocellular paraventricular nuclei of morphine-naïve rats given 0.15 m saline (subcutaneously) were similar to those in these nuclei after administration of naloxone (5 mg/kg, s.c.) to morphine-naïve rats or of vehicle to morphine-dependent rats (all n = 5) (Fig. 3).
In supraoptic nucleus sections processed for both Fos and oxytocin immunocytochemistry, the great majority of oxytocin neurons contained Fos-positive nuclei. Some supraoptic nucleus neurons that did not show oxytocin immunoreactivity and were presumptive vasopressin neurons also contained Fos-positive nuclei.
Effects of morphine withdrawal excitation on supraoptic nucleus oxytocin hnRNA expression
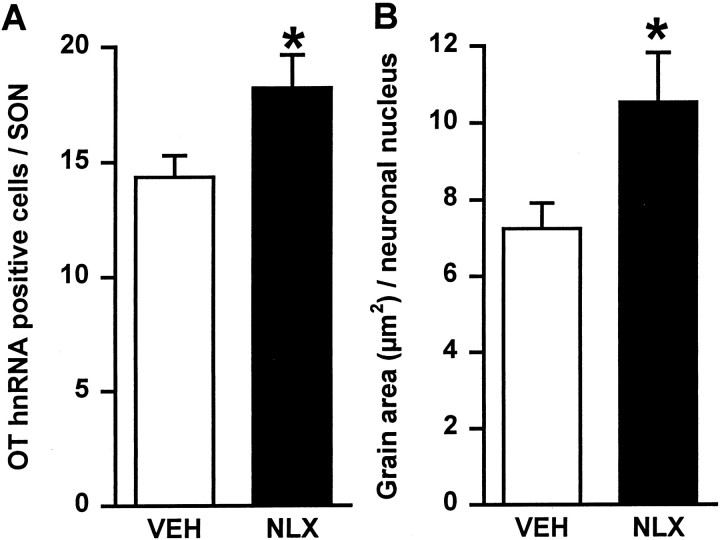
After morphine withdrawal excitation in conscious rats, labeling with the intronic oxytocin hnRNA probe was restricted predominantly to the dorsal oxytocin cell-rich region of the supraoptic nucleus. For oxytocin hnRNA, hybridization was confined to the nuclei of magnocellular cells and was evident as discrete accumulations of silver grains, as described previously (Brooks et al., 1993) (Fig.4). The number of oxytocin hnRNA-labeled neurons in the supraoptic nucleus (Fig.5A) and the area of silver grain per labeled supraoptic neuronal nucleus (Fig. 5B) were significantly greater (p < 0.05; Student'st test) in the morphine-dependent group given naloxone (n = 6) compared with the vehicle-treated morphine-dependent group (n = 5). In untreated virgin rat brains (n = 6) processed with the morphine-treated rat brains, the number of oxytocin hnRNA-labeled cells (12.2 ± 2.0) and the area of silver grain per labeled neuronal nucleus (6.9 ± 0.6 μm2) in the supraoptic nucleus were similar to those in morphine-dependent vehicle-treated rats.
Fig. 4.
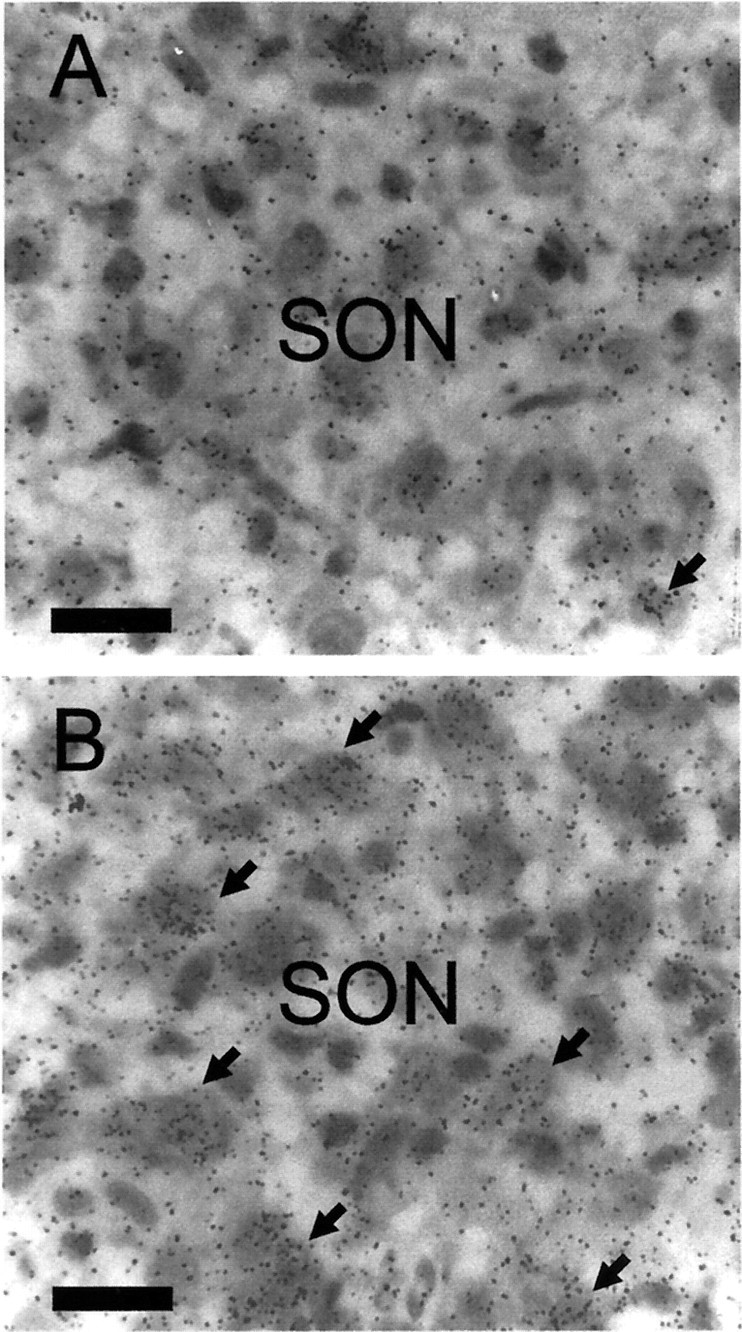
Oxytocin hnRNA expression in the supraoptic nucleus of morphine-dependent and morphine-withdrawn rats. Hypothalamic sections containing the SON were pretreated and hybridized for oxytocin hnRNA (with a 3H-labeled cDNA probe complementary to 210 bases of intron 1). The sections were exposed to gel emulsion for 50 d. Autoradiographs are of oxytocin hnRNA labeling in supraoptic nucleus neurons in morphine-dependent (A) and morphine-withdrawn (B) rats. Note foci of labeling over the neuronal nuclei (arrows). Scale bars, 20 μm.
Fig. 5.
Changes in expression of oxytocin hnRNA in supraoptic nucleus cells during morphine withdrawal. Coronal sections containing the supraoptic nucleus from rats treated with either chronic morphine and acute subcutaneous 0.15 m saline (VEH; n = 5) or chronic morphine and acute naloxone (NLX; 5 mg/kg, s.c; withdrawn,n = 6) were hybridized with a3H-labeled cDNA probe complementary to 210 bases of intron 1 of rat oxytocin hnRNA. A, Mean + SEM number of oxytocin hnRNA-positive cells per supraoptic nucleus section.B, Mean + SEM silver grain area per supraoptic nucleus neuronal nucleus. *p < 0.05; Student'st test.
Effects of central α1-adrenoreceptor antagonism on Fos protein expression in magnocellular neurosecretory cells during morphine withdrawal
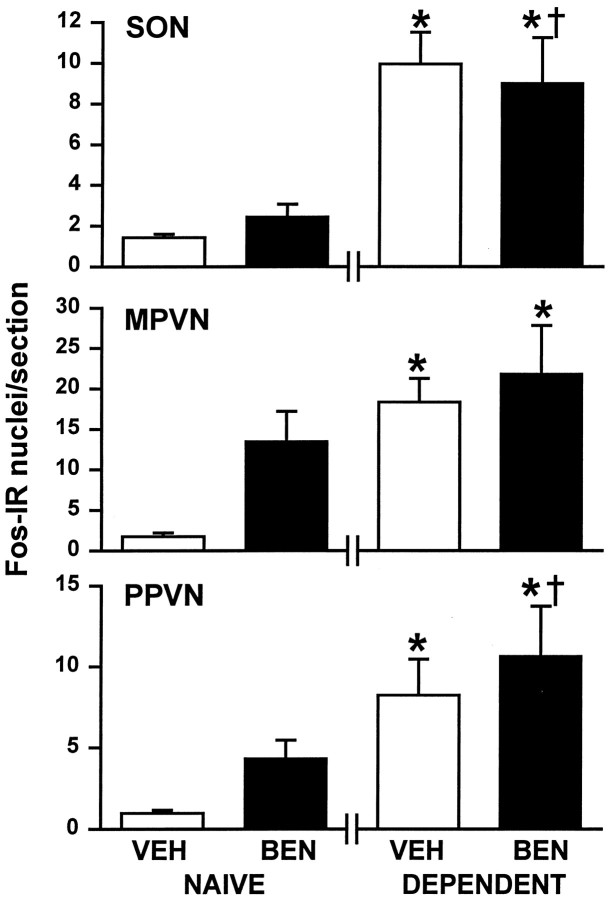
Because noradrenergic systems also undergo morphine withdrawal excitation and project to magnocellular neurosecretory cells, we induced withdrawal during intracerebroventricular infusion of the α1-adrenoreceptor antagonist benoxathian. First, Fos expression was increased in supraoptic and magnocellular paraventricular nucleus neurons after morphine withdrawal under barbiturate anesthesia (Fig. 6), and this was quantitatively similar to the expression in conscious withdrawn rats (Fig. 3). Fos expression in parvocellular paraventricular nucleus neurons was barely, although significantly, increased by morphine withdrawal in barbiturate-anesthetized rats compared with controls (Fig. 6). However, this expression was significantly less than that seen in conscious withdrawn rats (Fig. 3). Second, benoxathian infusion (5 μg/min over 2 hr) did not affect the number of Fos-IR cells in the supraoptic nucleus, magnocellular paraventricular nucleus, or the parvocellular paraventricular nucleus in morphine-naïve or morphine-dependent rats administered naloxone (5 mg/kg, i.p.) 30 min into the benoxathian–vehicle infusion (Fig. 6). In addition, the number of Fos-IR cells in the A2 cell group of the NTS and the A6 cell group of the locus ceruleus of benoxathian-infused morphine-withdrawn rats (at 6.94 ± 2.37 and 3.30 ± 1.97 Fos-IR cells per section, respectively) were similar to those of vehicle-infused morphine-withdrawn rats (at 8.43 ± 2.43 and 2.26 ± 0.42 Fos-IR cells per section, respectively).
Fig. 6.
Effects of α1-adrenoreceptor antagonism on Fos protein expression in magnocellular and parvocellular cells in morphine-dependent and morphine-withdrawn rats. Mean + SEM number of Fos-IR cells in the SON, the magnocellular paraventricular nucleus (MPVN), and the parvocellular paraventricular nucleus (PPVN). Morphine-naïve (NAIVE) and morphine-dependent (DEPENDENT) rats were pentobarbitone-anesthetized, infused for 2 hr with 0.15 msaline (intracerebroventricularly; VEH) or benoxathian (5 μg/min, i.c.v.; BEN), and decapitated 90 min after administration of naloxone (5 mg/kg, i.p., 30 min into the intracerebroventricular infusion; n = 5–7). *p < 0.05 versus morphine-naïve vehicle-infused rats; †p < 0.05 versus morphine-naïve benoxathian-infused rats; one-way ANOVA on ranks, followed by Dunn's method.
Fos protein expression after unilateral administration of naloxone into a supraoptic nucleus of morphine-dependent rats
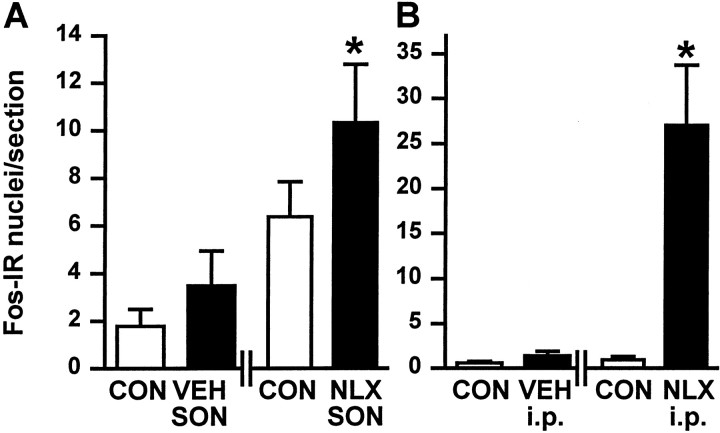
To determine whether the mechanisms that generate excitation of oxytocin cells upon morphine withdrawal reside within the supraoptic nucleus, we applied naloxone (10−5m) directly into a supraoptic nucleus unilaterally using microdialysis in barbiturate-anesthetized morphine-dependent rats. The resultant increase in cells that expressed Fos-IR in the naloxone-dialyzed supraoptic nucleus was significantly greater than that found in the contralateral nucleus in the same rats or the ipsilateral supraoptic nucleus in vehicle-dialyzed morphine-dependent rats (both p < 0.05) (Fig.7A).
Fig. 7.
Fos protein expression in the supraoptic nucleus after unilateral naloxone or morphine infusion. A, Intracerebroventricular infusion of morphine for 5 d. Mean + SEM number of Fos-IR cells in the SON of morphine-dependent rats dialyzed with aCSF (VEH) or naloxone (NLX; 10 −5m) and in the respective contralateral (CON) supraoptic nucleus. *p< 0.05 versus aCSF-dialyzed SON, the SON contralateral (CON) to naloxone SON, and the SON contralateral (CON) to aCSF SON (one-way ANOVA on ranks, followed by Dunn's method). B, Unilateral infusion of morphine (0.25 μg/hr, for 5 d) into a supraoptic nucleus. Mean + SEM number of Fos-IR cells in the morphine-infused (filled bars) and contralateral (CON; open bars) supraoptic nuclei of rats given 0.15 m saline (VEH; intraperitoneally) or naloxone (NLX; 5 mg/kg, i.p.). *p < 0.05 versus the SON contralateral to morphine infusion, the morphine-treated nucleus in vehicle-injected rats (VEH; intraperitoneally), and the contralateral (CON) nucleus in vehicle-injected rats (one-way ANOVA on ranks, followed by Dunn's method).
Systemic naloxone-induced Fos protein expression after chronic unilateral morphine infusion into a supraoptic nucleus
Similarly, to investigate whether the mechanisms that induce morphine dependence in oxytocin cells also reside within the supraoptic nucleus, we unilaterally infused morphine into the supraoptic nucleus for 5 d. We then injected naloxone (5 mg/kg, i.p.) under barbiturate anesthesia to induce morphine withdrawal. The naloxone-induced increase in number of neurons expressing Fos-IR in the morphine-infused supraoptic nucleus was significantly greater than that found in the contralateral nucleus in the same rats or in the ipsilateral vehicle-infused supraoptic nucleus (both p< 0.05) (Fig. 7B).
DISCUSSION
Here, we have shown that morphine withdrawal elicits a rapid increase in oxytocin gene transcription in magnocellular supraoptic neurons. This increase in the activity of the oxytocin gene is preceded by an increase in c-fos gene transcription in magnocellular neurosecretory cells and by increased Fos protein expression in oxytocin neurons in the supraoptic nucleus. We have shown that morphine withdrawal-induced increases in supraoptic nucleus Fos protein expression result from actions of morphine and naloxone exerted within the supraoptic nucleus itself that do not require the ascending noradrenergic input to oxytocin cells.
Activation of the c-fos and oxytocin genes by morphine withdrawal
In the rat, naloxone-precipitated morphine withdrawal excitation of oxytocin cells is a highly robust phenomenon, causing a threefold increase in the firing rate of the cells (Leng et al., 1989) and consequently a 100-fold rise in plasma oxytocin concentrations (Bicknell et al., 1988). The augmentation of the secretory response is partly a consequence of frequency facilitation of hormone release (Bicknell, 1988) and of antagonism of κ-opioid activity at the posterior pituitary (Russell et al., 1993). This withdrawal excitation of oxytocin secretion is seen in conscious, as well as urethane- and barbiturate-anesthetized, rats (Bicknell et al., 1988; Rayner et al., 1988; Brown et al., 1998). Here, we have shown that these alterations in the activity of oxytocin cells are accompanied by increases in the activity of the c-fos and oxytocin genes in these neurons, after only 45 min and by 2 hr after morphine withdrawal, respectively. The rat oxytocin gene promoter contains an activator protein-1-like site (Ivell and Richter, 1984), and Fos protein expression is elevated in the supraoptic nucleus between 30 and 45 min after morphine withdrawal (data not shown). Thus, Fos protein expression in oxytocin cells may be involved in the increased oxytocin gene transcription observed 2 hr after withdrawal. The rapid stimulation of oxytocin gene expression in supraoptic neurons by morphine withdrawal is comparable with the stimulation by parturition (Douglas et al., 1998), which is also accompanied by Fos expression (Luckman et al., 1993). However, other transcription factors are also likely to be involved in the regulation of oxytocin gene expression (Burbach et al., 1994; Luckman et al., 1996), but their expression during withdrawal has not been characterized.
The withdrawal-induced increase in c-fos gene activity in the supraoptic and paraventricular nuclei is more rapid than has been reported for locus ceruleus neurons that also undergo withdrawal excitation (Nye and Nestler, 1996). The increased electrical activity in oxytocin cells occurs within the first few minutes after morphine withdrawal (Leng et al., 1989), as it does in locus ceruleus neurons (Rasmussen et al., 1990). It appears that increased activity of the c-fos gene (and consequently of the oxytocin gene) occurs in parallel with the other manifestations of withdrawal excitation rather than driving these events. The induction of Fos protein expression in magnocellular neurosecretory cells does not simply reflect increased electrical activity and is not a direct consequence of increased secretory activity (Luckman et al., 1994; Ludwig et al., 1997b). It follows that the increased c-fos gene activity induced by morphine withdrawal excitation involves receptor-mediated events or mechanisms that converge on the second messenger systems that normally activate the c-fos gene (Sheng and Greenberg, 1990).
Fos protein expression and the mechanism of excitation of oxytocin neurons by morphine withdrawal
It follows from the above that Fos protein expression in oxytocin neurons is a marker of the activation of intracellular regulatory mechanisms during withdrawal that is independent of excitation of electrical or secretory activity of the neurons. We used this principle to explore the role of the noradrenergic input to the oxytocin neurons in morphine withdrawal excitation and whether the mechanisms leading to dependence are likely to reside in the oxytocin neurons themselves.
Noradrenergic inputs to the supraoptic nucleus are activated during morphine withdrawal (Murphy et al., 1997) and may contribute to the excitation of oxytocin cells at this time (Brown et al., 1998). However, lesioning of the noradrenergic inputs with 6-hydroxydopamine does not prevent morphine withdrawal-induced oxytocin secretion, thus it is clear that the noradrenergic input is not essential for withdrawal to occur (Brown et al., 1998). The α2-adrenoreceptor agonist clonidine reduces withdrawal-induced Fos protein expression in the C1 adrenergic cell group in the rostral ventrolateral medulla, as well as in neurons in the locus ceruleus (Baraban et al., 1995). Clonidine also reduces the withdrawal excitation of oxytocin secretion in dependent rats (Brown et al., 1998). We used the specific α1-antagonist benoxathian to eliminate synaptic activation of oxytocin cells by noradrenergic inputs during morphine withdrawal because benoxathian has been shown previously to block excitation of oxytocin cells in response to intravenous cholecystokinin, a response established to arise from activation of A2 cells of the NTS (Brown et al., 1998). The dose of benoxathian used reverses morphine withdrawal-induced increases in electrical and secretory activity of oxytocin cells (Brown et al., 1998), but Fos protein expression in the supraoptic and magnocellular paraventricular nuclei was unaffected. This indicates that the induction of Fos protein in these neurons by withdrawal does not require activation through noradrenergic synapses. It can be inferred also that it does not require excitation of the firing rate or secretory activity of the neurons.
This argument is further supported by considering the observation that both the A2 noradrenergic cell group in the NTS and the A1 noradrenergic cell group in the ventrolateral medulla are activated by morphine withdrawal (Murphy et al., 1997). The A2 cell group projects preferentially to oxytocin cells and the A1 cell group preferentially to vasopressin cells (Cunningham and Sawchenko, 1988). Nevertheless, only oxytocin cells are electrically activated upon morphine withdrawal (Bicknell et al., 1988). Furthermore, there is little increase in noradrenaline release in the supraoptic nucleus during withdrawal (Murphy et al., 1997), and systemic cholecystokinin, which activates A2 neurons and thus oxytocin neurons, neither triggers withdrawal nor has an attenuated effect during withdrawal (Brown et al., 1996). Thus, morphine withdrawal phenomena, including Fos expression, in oxytocin cells are unlikely to result solely from an increase in the activity of noradrenergic inputs to these cells. Similarly, other well characterized inputs to the supraoptic nucleus are not extensively activated during or essential for morphine withdrawal (Russell et al., 1992; Murphy et al., 1997). Rather, the withdrawal phenomena in oxytocin neurons may follow the triggering of intracellular mechanisms when naloxone displaces morphine from chronic occupancy of μ-opioid receptors on these neurons.
Direct application of naloxone into the supraoptic nucleus of morphine-dependent rats increases intranuclear oxytocin release and the firing rate of oxytocin cells (Brown et al., 1997; Ludwig et al., 1997a), indicating that the mechanisms underlying withdrawal excitation reside in the nucleus itself. The present study further shows that, in morphine-dependent rats, activation of the c-fos gene (increased Fos protein expression) in supraoptic neurons can be induced by local application of naloxone within the nucleus. Furthermore, direct chronic application of morphine into the nucleus also induced morphine dependence in oxytocin cells. Given the above conclusion that neither the A2 noradrenergic input nor other inputs drive withdrawal excitation in oxytocin cells (Brown et al., 1998), it appears likely that these mechanisms are located within the oxytocin neurons themselves.
Excitation of parvocellular paraventricular nucleus cells by morphine withdrawal
In conscious rats, the stimulation of c-fos and Fos expression in parvocellular paraventricular nucleus neurons by morphine withdrawal was evident in conscious and urethane-anesthetized rats but was essentially suppressed by barbiturate anesthesia. These neurons express corticotrophin-releasing factor and met-enkephalin, and some also express vasopressin. These genes are activated by stressors, including naloxone-precipitated morphine withdrawal in conscious or urethane-anesthetized rats (Lightman and Young, 1987; Harbuz et al., 1991). Fos may have a role in regulating the vasopressin gene in these neurons (Kovacs and Sawchenko, 1996). The persistence of withdrawal excitation of oxytocin neurons under barbiturate anesthesia and the suppression of Fos induction in parvocellular paraventricular nucleus neurons points to activation of the latter by the stressful nature of withdrawal in the conscious state. In particular, this also indicates that, unlike oxytocin neurons, the parvocellular paraventricular nucleus neurons do not themselves develop morphine dependence.
Conclusions
Morphine withdrawal elicits a rapid increase in oxytocin gene transcription that is preceded by, and perhaps results from, an increase in the activity of the c-fos gene in magnocellular neurosecretory cells. The induction of Fos protein expression in oxytocin neurons by naloxone-precipitated morphine withdrawal results from actions of morphine and naloxone exerted within the supraoptic nucleus that do not require ascending noradrenergic inputs to oxytocin cells. Thus, it appears that oxytocin neurons develop morphine dependence and express morphine withdrawal excitation separately from the afferent inputs. Therefore, the mechanisms that generate morphine dependence, as well as those that generate withdrawal excitation, in oxytocin cells reside within the oxytocin cells themselves. However, exactly what these mechanisms are has still to be defined.
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council (L.E.J. and C.H.B.). H.K.M.M. and C.L.V. were Erasmus Exchange students, partially funded by the European Commission. We thank Dr. M. Hamamura, Dr. B. E. H. Sumner, and I. Murray for expert assistance with hybridization, K. Opstad for technical assistance, and Dr. G. Blackburn-Munro for expert assistance in the preparation of the figures.
Correspondence should be addressed to Dr. J. A. Russell, Department of Biomedical Sciences, University Medical School, Edinburgh, EH8 9XD, UK. E-mail: j.a.russell@ed.ac.uk.
REFERENCES
- 1.Aghajanian GK. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276:186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- 2.Baraban SC, Stornetta RL, Guyenet PG. Effects of morphine and morphine withdrawal on adrenergic neurons of the rat rostral ventrolateral medulla. Brain Res. 1995;676:245–257. doi: 10.1016/0006-8993(95)00097-a. [DOI] [PubMed] [Google Scholar]
- 3.Bicknell RJ. Optimizing release from peptide hormone secretory nerve terminals. J Exp Biol. 1988;139:51–65. doi: 10.1242/jeb.139.1.51. [DOI] [PubMed] [Google Scholar]
- 4.Bicknell RJ, Leng G, Lincoln DW, Russell JA. Naloxone excites oxytocin neurones in the supraoptic nucleus of lactating rats after chronic morphine treatment. J Physiol (Lond) 1988;396:297–317. doi: 10.1113/jphysiol.1988.sp016963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks PJ, Kaplitt MG, Kleopoulos SP, Funabashi T, Mobbs CV, Pfaff DW. Detection of messenger RNA and low-abundance heteronuclear RNA with single-stranded DNA probes produced by amplified primer extension labeling. J Histochem Cytochem. 1993;41:1761–1766. doi: 10.1177/41.12.8245424. [DOI] [PubMed] [Google Scholar]
- 6.Brown CH, Munro G, Murphy NP, Leng G, Russell JA. Activation of oxytocin neurones by systemic cholecystokinin is unchanged by morphine dependence or withdrawal excitation in the rat. J Physiol (Lond) 1996;496:787–794. doi: 10.1113/jphysiol.1996.sp021727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CH, Munro G, Johnstone LE, Robson AC, Landgraf R, Russell JA. Oxytocin neurone autoexcitation during morphine withdrawal in anaesthetized rats. NeuroReport. 1997;8:951–955. doi: 10.1097/00001756-199703030-00027. [DOI] [PubMed] [Google Scholar]
- 8.Brown CH, Murphy NP, Munro G, Ludwig M, Bull PM, Leng G, Russell JA. Interruption of central noradrenergic pathways and morphine withdrawal excitation of oxytocin neurones in the rat. J Physiol (Lond) 1998;507:831–842. doi: 10.1111/j.1469-7793.1998.831bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burbach JP, Lopes da Silva S, Cox JJ, Adan RA, Cooney AJ, Tsai MJ, Tsai SY. Repression of estrogen-dependent stimulation of the oxytocin gene by chicken ovalbumin upstream promoter transcription factor I. J Biol Chem. 1994;269:15046–15053. [PubMed] [Google Scholar]
- 10.Cunningham ETJ, Sawchenko PE. Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol. 1988;274:60–76. doi: 10.1002/cne.902740107. [DOI] [PubMed] [Google Scholar]
- 11.Curran TC, Gordon MB, Rubino KL, Sambucetti LC. Isolation and characterization of the c-fos (rat) cDNA and analysis of post-translational modification in vitro. Oncogene. 1987;2:79–84. [PubMed] [Google Scholar]
- 12.Douglas AJ, Meeren HK, Johnstone LE, Pfaff DW, Russell JA, Brooks PJ. Stimulation of expression of the oxytocin gene in rat supraoptic neurons at parturition. Brain Res. 1998;782:167–174. doi: 10.1016/s0006-8993(97)01275-4. [DOI] [PubMed] [Google Scholar]
- 13.Hamamura M, Leng G, Emson PC, Kiyama H. Electrical activation and c-fos mRNA expression in rat neurosecretory neurones after systemic administration of cholecystokinin. J Physiol (Lond) 1991;444:51–63. doi: 10.1113/jphysiol.1991.sp018865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harbuz M, Russell JA, Sumner BE, Kawata M, Lightman SL. Rapid changes in the content of proenkephalin A and corticotrophin releasing hormone mRNAs in the paraventricular nucleus during morphine withdrawal in urethane-anaesthetized rats. Mol Brain Res. 1991;9:285–291. doi: 10.1016/0169-328x(91)90074-8. [DOI] [PubMed] [Google Scholar]
- 15.Hatton GI. Emerging concepts of structure-function dynamics in adult brain: the hypothalamo-neurohypophysial system. Prog Neurobiol. 1990;34:437–504. doi: 10.1016/0301-0082(90)90017-b. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi T, Honda K, Fukuoka T, Negoro H, Wakabayashi K. Release of oxytocin during suckling and parturition in the rat. J Endocrinol. 1985;105:339–346. doi: 10.1677/joe.0.1050339. [DOI] [PubMed] [Google Scholar]
- 17.Hoffman GE, Smith MS, Verbalis JG. c-fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol. 1993;14:173–213. doi: 10.1006/frne.1993.1006. [DOI] [PubMed] [Google Scholar]
- 18.Ivell R, Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci USA. 1984;81:2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jhamandas JH, Harris KH, Petrov T, Jhamandas KH. Activation of nitric oxide-synthesizing neurones during precipitated morphine withdrawal. NeuroReport. 1996;7:2843–2846. doi: 10.1097/00001756-199611250-00006. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs KJ, Sawchenko PE. Sequence of stress-induced alterations in indices of synaptic and transcriptional activation in parvocellular neurosecretory neurons. J Neurosci. 1996;16:262–273. doi: 10.1523/JNEUROSCI.16-01-00262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leng G, Mansfield S, Bicknell RJ, Blackburn RE, Brown D, Chapman C, Dyer RG, Hollingsworth S, Shibuki K, Yates JO. Endogenous opioid actions and effects of environmental disturbance on parturition and oxytocin secretion in rats. J Reprod Fertil. 1988;84:345–356. doi: 10.1530/jrf.0.0840345. [DOI] [PubMed] [Google Scholar]
- 22.Leng G, Russell JA, Grossmann R. Sensitivity of magnocellular oxytocin neurones to opioid antagonists in rats treated chronically with intracerebroventricular (i.c.v.) morphine. Brain Res. 1989;484:290–296. doi: 10.1016/0006-8993(89)90372-7. [DOI] [PubMed] [Google Scholar]
- 23.Lightman SL, Young WS. Vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. J Physiol (Lond) 1987;394:23–39. doi: 10.1113/jphysiol.1987.sp016858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luckman SM, Antonijevic I, Leng G, Dye S, Douglas AJ, Russell JA, Bicknell RJ. The maintenance of normal parturition in the rat requires neurohypophysial oxytocin. J Neuroendocrinol. 1993;5:7–12. doi: 10.1111/j.1365-2826.1993.tb00358.x. [DOI] [PubMed] [Google Scholar]
- 25.Luckman SM, Dyball RE, Leng G. Induction of c-fos expression in hypothalamic magnocellular neurons requires synaptic activation and not simply increased spike activity. J Neurosci. 1994;14:4825–4830. doi: 10.1523/JNEUROSCI.14-08-04825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luckman SM, Dye S, Cox HJ. Induction of members of the Fos/Jun family of immediate-early genes in identified hypothalamic neurons: in vivo evidence for differential regulation. Neuroscience. 1996;73:473–483. doi: 10.1016/0306-4522(96)00076-0. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo: a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig M, Brown CH, Russell JA, Leng G. Local opioid inhibition and morphine dependence of supraoptic nucleus oxytocin neurones in the rat in vivo. J Physiol (Lond) 1997a;505:145–152. doi: 10.1111/j.1469-7793.1997.145bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludwig M, Johnstone LE, Neumann I, Landgraf R, Russell JA. Direct hypertonic stimulation of the rat supraoptic nucleus increases c-fos expression in glial cells rather than magnocellular neurones. Cell Tissue Res. 1997b;287:79–90. doi: 10.1007/s004410050733. [DOI] [PubMed] [Google Scholar]
- 30.Melchiorre C, Brasili L, Giardina D, Pigini M, Strappaghetti G. 2-[[[2-(2,6-Dimethoxyphenoxy)ethyl]amino]-methyl]-1,4-benzoxathian: a new antagonist with high potency and selectivity toward alpha 1-adrenoreceptors. J Med Chem. 1984;27:1535–1536. doi: 10.1021/jm00378a001. [DOI] [PubMed] [Google Scholar]
- 31.Murphy NP, Onaka T, Brown CH, Leng G. The role of afferent inputs to supraoptic nucleus oxytocin neurons during naloxone-precipitated morphine withdrawal in the rat. Neuroscience. 1997;80:567–577. doi: 10.1016/s0306-4522(97)00142-5. [DOI] [PubMed] [Google Scholar]
- 32.Nye HE, Nestler EJ. Induction of chronic Fos-related antigens in rat brain by chronic morphine administration. Mol Pharmacol. 1996;49:636–645. [PubMed] [Google Scholar]
- 33.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Ed 3. Academic; London: 1996. [DOI] [PubMed] [Google Scholar]
- 34.Pumford KM, Leng G, Russell JA. Morphine actions on supraoptic oxytocin neurones in anaesthetized rats: tolerance after i.c.v. morphine infusion. J Physiol (Lond) 1991;440:437–454. doi: 10.1113/jphysiol.1991.sp018717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rasmussen K, Beitner-Johnson DB, Krystal JH, Aghajanian GK, Nestler EJ. Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J Neurosci. 1990;10:2308–2317. doi: 10.1523/JNEUROSCI.10-07-02308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rayner VC, Robinson IC, Russell JA. Chronic intracerebroventricular morphine and lactation in rats: dependence and tolerance in relation to oxytocin neurones. J Physiol (Lond) 1988;396:319–347. doi: 10.1113/jphysiol.1988.sp016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redmond DE, Jr, Krystal JH. Multiple mechanisms of withdrawal from opioid drugs. Annu Rev Neurosci. 1984;7:443–478. doi: 10.1146/annurev.ne.07.030184.002303. [DOI] [PubMed] [Google Scholar]
- 38.Russell JA, Pumford KM, Bicknell RJ. Contribution of the region anterior and ventral to the third ventricle to opiate withdrawal excitation of oxytocin secretion. Neuroendocrinology. 1992;55:183–192. doi: 10.1159/000126113. [DOI] [PubMed] [Google Scholar]
- 39.Russell JA, Coombes JE, Leng G, Bicknell RJ. Morphine tolerance and inhibition of oxytocin secretion by kappa-opioids acting on the rat neurohypophysis. J Physiol (Lond) 1993;469:365–386. doi: 10.1113/jphysiol.1993.sp019818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell JA, Leng G, Bicknell RJ. Opioid tolerance and dependence in the magnocellular oxytocin system: a physiological mechanism? Exp Physiol. 1995;80:307–340. doi: 10.1113/expphysiol.1995.sp003850. [DOI] [PubMed] [Google Scholar]
- 41.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 42.Shu SY, Ju G, Fan LZ. The glucose oxidase–DAB–nickel method in peroxidase histochemistry of the nervous system. Neurosci Lett. 1988;85:169–171. doi: 10.1016/0304-3940(88)90346-1. [DOI] [PubMed] [Google Scholar]
- 43.Stornetta RL, Norton FE, Guyenet PG. Autonomic areas of rat brain exhibit increased Fos-like immunoreactivity during opiate withdrawal in rats. Brain Res. 1993;624:19–28. doi: 10.1016/0006-8993(93)90055-r. [DOI] [PubMed] [Google Scholar]
- 44.Sumner BE, Coombes JE, Pumford KM, Russell JA. Opioid receptor subtypes in the supraoptic nucleus and posterior pituitary gland of morphine-tolerant rats. Neuroscience. 1990;37:635–645. doi: 10.1016/0306-4522(90)90095-l. [DOI] [PubMed] [Google Scholar]
- 45.Takayama K, Suzuki T, Miura M. The comparison of effects of various anesthetics on expression of Fos protein in the rat brain. Neurosci Lett. 1994;176:59–62. doi: 10.1016/0304-3940(94)90871-0. [DOI] [PubMed] [Google Scholar]
- 46.Walther N, Wehrenberg U, Brackmann B, Ivell R. Mapping of the bovine oxytocin gene control region: Identification of binding sites for luteal nuclear proteins in the 5′ non-coding region of the gene. J Neuroendocrinol. 1991;3:539–550. doi: 10.1111/j.1365-2826.1991.tb00315.x. [DOI] [PubMed] [Google Scholar]
- 47.Xing Y, Johnson CV, Dobner PR, Lawrence JB. Higher level organization of individual gene transcription and RNA splicing. Science. 1993;259:1326–1330. doi: 10.1126/science.8446901. [DOI] [PubMed] [Google Scholar]