Abstract
GABAA-mediated IPSCs typically decay more rapidly than receptors in excised patches in response to brief pulses of applied GABA. We have investigated the source of this discrepancy in CA1 pyramidal neurons. IPSCs in these cells decayed rapidly, with a weighted time constant τDecay of ∼18 msec (24°C), whereas excised and nucleated patch responses to brief pulses of GABA (2 msec, 1 mm) decayed more than three times as slowly (τDecay, ∼63 msec). This discrepancy was not caused by differences between synaptic and exogenous transmitter transients because (1) there was no dependence of τDecayon pulse duration for pulses of 0.6–4 msec, (2) responses to GABA at concentrations as low as 10 μm were still slower to decay (τDecay, ∼41 msec) than IPSCs, and (3) responses of excised patches to synaptically released GABA had decay times similar to brief pulse responses. These data indicate that the receptors mediating synaptic versus brief pulse responses have different intrinsic properties. However, synaptic receptors were not altered by the patch excision process, because fast, spontaneous IPSCs could still be recorded in nucleated patches. Elevated calcium selectively modulated patch responses to GABA pulses, with no effect on IPSCs recorded in nucleated patches, demonstrating the presence of two receptor populations that are differentially regulated by intracellular second messengers. We conclude that two receptor populations with distinct kinetics coexist in CA1 pyramidal cells: slow extrasynaptic receptors that dominate the responses of excised patches to exogenous GABA applications and fast synaptic receptors that generate rapid IPSCs.
Keywords: GABAA receptors, hippocampus, receptor kinetics, IPSCs, patch clamp, extrasynaptic receptors
The time course of synaptic transmission at synapses that use “fast” ligand-gated ion channels is determined primarily by the kinetics of the receptor/ionophore in response to a brief, high concentration of neurotransmitter (Clements et al., 1992; Maconochie et al., 1994; Jones and Westbrook, 1995;Legendre, 1998; but see, Frerking and Wilson, 1996). For glutamate receptors, the currents generated by AMPA and NMDA receptors in response to rapidly applied pulses of ligand have onset and decay kinetics similar to synaptic currents mediated by these receptors (Trussell and Fischbach, 1989; Lester et al., 1990; Clements et al., 1992; Colquhoun et al., 1992; Dudel et al., 1992; Hestrin, 1992). For GABAA receptors, however, responses of receptors in excised patches to brief (∼2 msec), exogenous GABA applications typically decay more slowly than GABAAreceptor-mediated synaptic currents (Galarreta and Hestrin, 1997; Jones and Westbrook, 1997; Mellor and Randall, 1997; Mozrzymas et al., 1999;Perrais and Ropert, 1999). Although patch currents, or “simulated IPSCs”, are often described as being similar to synaptic GABA currents, the decay time constants of patch responses exceeded those of synaptic responses in these studies by 70 to >400%. This discrepancy is comparable to the effects of benzodiazepines and general anesthetics on GABAA receptor-mediated IPSCs (Vicini et al., 1986; Tanelian et al., 1993), and thus may have significant functional consequences.
This discrepancy in kinetics could arise because excised receptors differ in some way from synaptic receptors or because the transmitter application differs from the synaptic transient. How might excised receptors differ from synaptic receptors? Synaptic receptors are regulated by cytoskeletal elements (Rosenmund and Westbrook, 1993) and phosphorylation systems (Jones and Westbrook, 1997). It is possible that the process of patch excision disrupts such interactions to alter the intrinsic kinetics of the receptors. Alternatively, excised patches may contain primarily extrasynaptic receptors with different kinetic properties (Tia et al., 1996a), possibly because of differences in subunit composition between extrasynaptic and synaptic receptors (Nusser et al., 1998; Brickley et al., 1999). Differences attributable to agonist application include time course and concentration, the identity of the transmitter itself, the presence of synaptically released cofactors, or the presence of extracellular modulatory agents.
To investigate these issues, we have compared the responses of receptors in patches excised from the somata of CA1 pyramidal cells with spontaneous GABAA receptor-mediated IPSCs recorded in these cells. We found that under our experimental conditions, the deactivation kinetics of receptors in excised patches never matched the decay kinetics of fast IPSCs, regardless of the concentration or time course of transmitter, or the presence of cofactors such as H+ or Zn2+ ions. Rather, our data suggest that excised receptors represent primarily extrasynaptic receptors and that these receptors have intrinsic kinetics that differ substantially from those of synaptic receptors.
MATERIALS AND METHODS
Slice preparation. Young rats (8 to 24-d-old) were decapitated under halothane anesthesia, and the head was immediately immersed in cold (4°C) artificial CSF (ACSF) (in mm: NaCl 127, KH2PO4 1.21, KCl 1.87, NaHCO3 26, CaCl2 2.17, MgSO4 1.44, and glucose 10) saturated with 95% O2 and 5% CO2. A block of tissue containing both hippocampi was dissected out with the brain immersed in ACSF, and the tissue was glued to a vibratome tray with cyanoacrylate glue. Slices (400 μm) were cut and then held submerged at 35°C for 1 hr before transfer to the recording chamber, which was perfused at 3 ml/min with ACSF saturated with 95% O2 and 5%CO2 at 24°C.
Patch-clamp electrophysiology. Cells in stratum pyramidale of CA1 were visualized using a video camera (Hamamatsu C2400; Hamamatsu, Tokyo, Japan) connected to an upright microscope (Axioskop;Carl Zeiss, Thornwood, NY) equipped with an infrared bandpass filter (model D775/220; Chroma Technology, Brattleboro, VT), a long working-distance water-immersion objective (Achroplan 40×; 0.75 numerical aperture; Carl Zeiss) and differential interference contrast optics. Whole-cell recordings were obtained at room temperature (24°C), using an Axopatch 1D (Axon Instruments, Foster City, CA) patch-clamp amplifier. All data were recorded using pClamp software (Axon Instruments). Data were filtered at 5 kHz, then sampled at 10–20 kHz (Digidata 1200; Axon Instruments) and stored on a Pentium-based personal computer (PC). Patch pipettes were fabricated from borosilicate glass (1.7 mm outer diameter, 1.1 mm inner diameter; KG-33; Garner Glass, Claremont, CA) using a two-stage puller (Flaming–Brown model P-87; Sutter Instruments, Novato, CA), fire-polished and coated with Sylgard (Dow-Corning Company, Midland, MI) to reduce electrode capacitance. Tight-seal whole-cell recordings were obtained using standard techniques (Hamill et al., 1981; Edwards et al., 1989). Patch pipettes had open-tip resistances of 2–4 MΩ when filled with the recording solution (in mm: CsCl 140, Na-HEPES 10, BAPTA 10, MgATP 2, and QX-314 5, pH 7.3). Access resistances were typically 10–20 MΩ and were then compensated at 60–80%. All data were recorded at a holding potential of −60 mV. GABAA IPSCs were isolated by bath application of 20 μm CNQX and 40 μmd,l-APV to block AMPA- and NMDA-mediated currents and by the inclusion of CsCl and QX-314 in the patch pipette to block GABAB-mediated currents. The remaining currents were completely blocked by bath application of 10 μmbicuculline (data not shown). Miniature, i.e., action potential-independent, spontaneous IPSCs were recorded in the presence of 1 μm tetrodotoxin (TTX).
APV, CNQX, TTX, and bicuculline were prepared at 50–100× stock solutions in 0.9% saline and applied using syringe pumps (model 55–1111; Harvard Apparatus, Natick, MA) set to flow at 1–2% of the ACSF flow rate to achieve the desired bath concentrations.
Rapid agonist application. Excised or nucleated (Sather et al., 1992) outside-out patches were obtained from the somata of CA1 pyramidal cells and exposed to ligand using a rapid application system consisting of a two-barrel “theta” application pipette (fashioned from Thin Theta; Sutter Instruments) connected to a piezoelectric stacked translator (model P-245.50; Physik Instrumente, Costa Mesa, CA). Using gravity feed, solutions flowing through the application pipette could be exchanged in approximately 10 seconds via a series of low-volume, zero unswept volume, manually controlled Teflon valves (model 1126; Omnifit Limited, Cambridge, UK). The flow rate out of the tip of the theta pipette was 4.8 μl/sec out of a barrel with a diameter of 300 μm, giving a forward velocity of ∼70 μm/msec. The voltage input to the high-voltage amplifier (model P-270; Physik Instrumente) used to drive the stacked translator was filtered (300 Hz) using an 8-pole Bessel filter (model 902LPF; Frequency Devices, Haverhill, MA) to reduce oscillations arising from rapid acceleration of the pipette.
Solution exchange rates (10–90% in 500 μsec) were estimated by measuring open tip junction currents with dilute perfusion solution at the conclusion of each experiment. The duration of the agonist pulse was defined by measuring the time between the points at 10% of peak amplitude of the junction current. Previous studies have demonstrated that this estimate is adequate for excised patches (Trussell and Fischbach, 1989). However, because of the large amount of membrane at the tip of the pipette, the exchange time around a nucleated patch is likely to be substantially slower than indicated by the open tip exchange time. We tested the solution exchange time directly by activating voltage-gated K currents in the nucleated patches and altering [K+] during the voltage step. Using this technique, we found that the solution exchange proceeded monoexponentially with τExchange = 1.7 ± 0.2 msec (n = 4; in these patches, the open tip exchange time constant was 0.2 ± 0.1 msec). This exchange rate is further corroborated by experiments using the low-affinity agonist taurine, which unbinds rapidly from the GABAAreceptor (Zhu and Vicini, 1997), similar to β-alanine (Jones et al., 1998). We found that brief pulses of 20 mmtaurine elicited currents that deactivated monoexponentially, with τDecay = 1.8 ± 0.1 msec (n = 19), similar to τExchangemeasured using K currents. Finally, the similarity in decay kinetics between nucleated patches and excised patches (Fig.1), which are likely to have much faster exchange times, suggests that the deactivation kinetics were not grossly distorted by slow exchange in nucleated patches. Access resistance and capacitance of nucleated patches were measured using the amplifier circuitry. Series resistance was compensated 80–90%, yielding errors in VHold caused by uncompensated series resistance of 4.4 ± 0.6 mV.
Fig. 1.
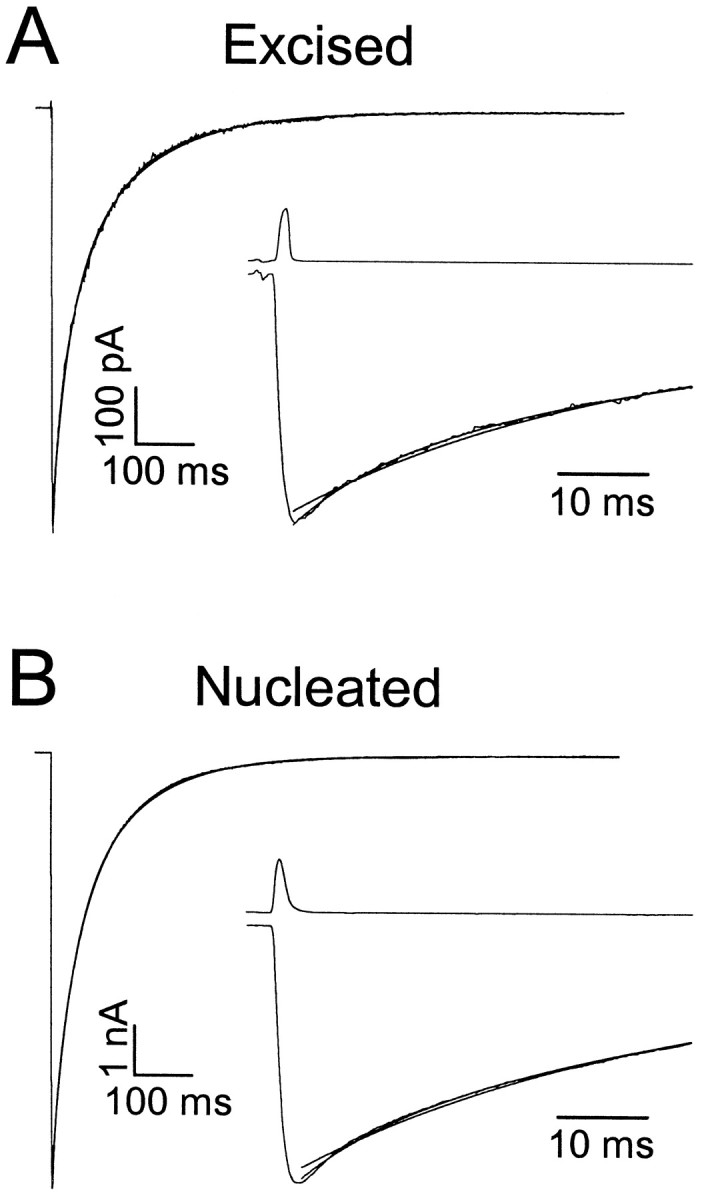
Kinetics of excised and nucleated patch responses.A, Averaged responses to brief exogenous pulses of GABA (2.0 msec, 1 mm) recorded in an excised outside-out patch taken from the soma of a CA1 pyramidal cell. Top traceshows the open-tip junction current recorded immediately after terminating the recording. Inset shows data plotted on an expanded time scale, to illustrate superior fit obtained with three versus two exponential components. Biexponential fit parameters were τDec1,2 = 29.4 (50%) and 129.8 msec. Triexponential fit parameters were τDec1,2,3 = 10.4 (23%), 61.7 (52%), and 173.3 msec. B, Same as A, but for a nucleated patch obtained from a different cell. Note difference in vertical calibration. Biexponential fit parameters were τDec1,2 = 32.9 (50%) and 112.7 msec. Triexponential fit parameters were τDec1,2,3 = 9.88 (17%), 56.7 (59%), and 145.2 msec.
Sniffer patch experiments. To expose excised receptors to synaptically released GABA, “sniffer patches” (Isaacson et al., 1993) were formed by excising an outside-out patch from the soma of a CA1 pyramidal cell and then reinserting the patch back in the slice. Synaptic responses in sniffer patches were elicited under identical conditions as for whole-cell synaptic responses, i.e., in the presence of CNQX and APV, and the magnitudes of the electrical stimuli applied (typically ∼10 μA) were the same as in whole-cell before patch excision. For each patch, the position at which responses with the most rapid rise times were measured was assumed to be the closest to synaptic release sites, and the data at this position was averaged to yield rise times, decay times, and peak amplitude for that patch.
Surface area measurements. The somatic surface area was measured in two ways. In the first method, direct anatomical measurements were made of the width and length of the somata of pyramidal cells in our slices from video photomicrographs taken of cells in slices before patching. For the length measurements, the transition from soma to apical dendrite was defined as the point at which process diameter was one-half maximal somatic diameter. In 27 cells, the length and width were 20.0 ± 0.3 and 10.0 ± 0.5 μm, respectively. Somatic surface area was then estimated by assuming that the soma was a prolate spheroid, yielding an average surface area of 520 ± 16 μm2. In the second method, the fast component of the capacitative transient was assumed to originate from purely somatic capacitance (Jackson, 1992). In 34 cells, this component of the capacitance averaged 11.6 ± 0.8 pF. Assuming a specific membrane capacitance of 10−2pF/μm2 (Jack et al., 1983), these measurements yield a somatic surface area of 1160 ± 80 μm2. The discrepancy between these two values could arise because of assumptions about the exact shape of the soma, contributions of proximal dendrites to the fast capacitative transient, and small deviations in specific capacitance. We chose to use the lower estimate of 520 μm2 in our calculations so that we would give conservative estimates of the contribution of extrasynaptic receptors to patch responses.
Nucleated patches invariably appeared spherical and typically had diameters of 5–10 μm, yielding surface areas of ∼80 to 320 μm2. In these patches, capacitative transients consisted of a single exponential component, with capacitance values of 1.9 ± 0.1 pF. Assuming a specific capacitance of 10−2pF/μm2, these values correspond to patch surface areas of 190 ± 10 μm2, consistent with direct anatomical observations. Thus, the ratio of surface area in a nucleated patch to that of the intact soma was 190/520 = 0.36.
Data analysis. Data were analyzed on a Pentium-based PC using ClampFit (Axon Instruments), Origin (MicroCal, Northampton, MA) and StatMost (DataMost, Salt Lake City, UT). Data were filtered off-line at 2 kHz. Spontaneous events were analyzed using an automated event detection algorithm (Banks and Pearce, 1999). In this algorithm, two windows were moved along the data, a “peak” window and a “baseline” window. At each time point, the data within the two windows was averaged, and the baseline was subtracted from the peak. This yielded a “pseudodifferentiated” form of the data that was characterized by large, rapid peaks at the onset of fast GABAA IPSCs. Threshold-level crossings were determined from this pseudodifferentiated data, with threshold set as 3*ςNoise, where ςNoisewas measured during periods of no visually detectable events, and was typically 2–4 pA. Because the baseline value was constantly updated during the analysis, slow changes in baseline had no effect on the accuracy of the algorithm. Analysis was confined to GABAA,fast IPSCs, which were identified based on rise times of <2 msec (Banks et al., 1998). The algorithm successfully detected >99% of spontaneous IPSCs (sIPSCs) and miniature IPSCs (mIPSCs) with these fast rise times.
To analyze the decay kinetics of fast IPSCs, a subset of events was selected for exponential curve fitting as well. Events were selected only if no other event occurred within 250 msec of the peak. The decay kinetics of patch responses and synaptic currents was characterized by multiexponential fit parameters. For all responses to 10 and 30 μm GABA, which were obtained from nucleated patches, a three exponential fit was clearly superior to two exponentials by visual inspection. For responses to 1 mm GABA, for 22 of 25 nucleated patches, a three exponential fit was clearly superior to two exponentials (Fig. 1), and for the remaining three patches the fast decay component was absent. In 18 of 27 excised patch responses to 1 mm GABA, the decays were best fit by three decay components. In four of the remaining nine patches, the fast decay component was absent, whereas in the other five patches the slow component was absent. To facilitate comparisons between data with disparate numbers of decay components, we used the weighted time constant τDecay = ΣAiτi/ΣAi, where Ai is the amplitude of theith component. Statistical comparisons of decay times and peak amplitudes were made using paired or unpaired Student's t tests, as indicated. All data are presented as mean ± SE.
RESULTS
Kinetic differences between patch responses and IPSCs
The synaptic transmitter transient at GABAergic synapses is believed to consist of a brief, high-concentration component, possibly followed by an extended, low-concentration tail (Jones and Westbrook, 1995, 1996; Hill et al., 1998). As an approximation to this transient, we tested the responses of GABAA receptors in excised and nucleated outside-out patches to brief (0.5–4 msec) square pulses of 1 or 10 mm GABA. In both excised and nucleated patches, currents in response to applications of 1 mm GABA had rapid rates of rise (t10–90% = 1.2 ± 0.1 msec) and had decay phases that were typically best described by three exponential components (Fig. 1, Table1). Weighted time constants (τDecay) of the responses of excised and nucleated patches to 1 mm GABA were indistinguishable (Student's t test; p > 0.3), and were grouped for subsequent analyses. Decay kinetics of responses to 1 versus 10 mm GABA were also not significantly different (1 mm: τDecay = 63.9 ± 2.6 msec,n = 52; 10 mm: 66.6 ± 6.3 msec, n = 14; p > 0.3, Student'st test).
Table 1.
Kinetic parameters for excised and nucleated patch responses to four different GABA concentrations and for whole-cell IPSCs
| tRise(msec) | τDec,1 (msec) | A1(%) | τDec,2 (msec) | A2(%) | τDec,3 (msec) | A3(%) | τDecay (msec) | n | |
|---|---|---|---|---|---|---|---|---|---|
| 1 mm GABA | 1.2 ± 0.1 | 13.9 ± 1.0 | 25 ± 3 | 56.8 ± 1.9 | 60 ± 2 | 199 ± 12 | 15 ± 1 | 63.9 ± 2.6 | 52 |
| 30 μm GABA | 1.6 ± 0.1 | 3.2 ± 0.9 | 37 ± 10 | 37.3 ± 11.6 | 36 ± 10 | 166 ± 35 | 29 ± 10 | 57.4 ± 5.7 | 4 |
| 10 μm GABA | 1.9 ± 0.1 | 5.0 ± 0.5 | 34 ± 4 | 31.7 ± 4.9 | 50 ± 6 | 173 ± 33 | 17 ± 5 | 41.3 ± 2.9 | 6 |
| Whole-cell IPSC | 0.9 ± 0.1 | 10.9 ± 0.5 | 62 ± 4 | 31.3 ± 1.2 | 38 ± 4 | — | — | 18.4 ± 0.5 | 34 |
tRise represents the 10–90% rise time. τDec,i and Ai represent the time constant and amplitude of the ith component of multiexponential fits. τDecay = ΣAiτi/ΣAi. n is the number of cells or patches. Data are presented as mean ± SEM.
These “simulated IPSCs” had substantially different time courses from whole-cell spontaneous IPSCs. Rise times of IPSCs (0.9 ± 0.1 msec) were shorter than those of patch responses (p < 10−5; Student's t test). Decays of IPSCs were typically best described by a sum of two exponential components (Table 1), with rates comparable to the two fastest decay components in the patch responses (Fig. 2A). In 12 cells, we directly compared these rates by recording spontaneous IPSCs in the intact cell and rapid application responses after patch excision. Decay kinetics were approximately fourfold slower for patch responses than for whole-cell (WC) IPSCs in these cells (WC IPSC, τDecay = 17.9 ± 1.1 msec; patch response: τDecay = 70.4 ± 3.9 msec;p < 10−7, paired Student's t test) (Fig. 2B). The fastest decay component (τDec,1 ∼ 10 msec) was not significantly different between whole-cell and patch responses (p > 0.5; paired Student's t test), whereas the amplitude of this component and the rate and amplitude of the second component were significantly different (p < 0.01). We will present evidence below that the rapid decay component of the patch responses likely represents the contribution of synaptic receptors to this response, whereas the second decay component of the patch responses represents the comingling of fast synaptic and slow extrasynaptic receptors. Contrary to the population as a whole, rise times in these twelve cells were not significantly different for synaptic versus patch responses (WC, 0.9 ± 0.1 msec; patch, 1.1 ± 0.1 msec; p > 0.2).
Fig. 2.
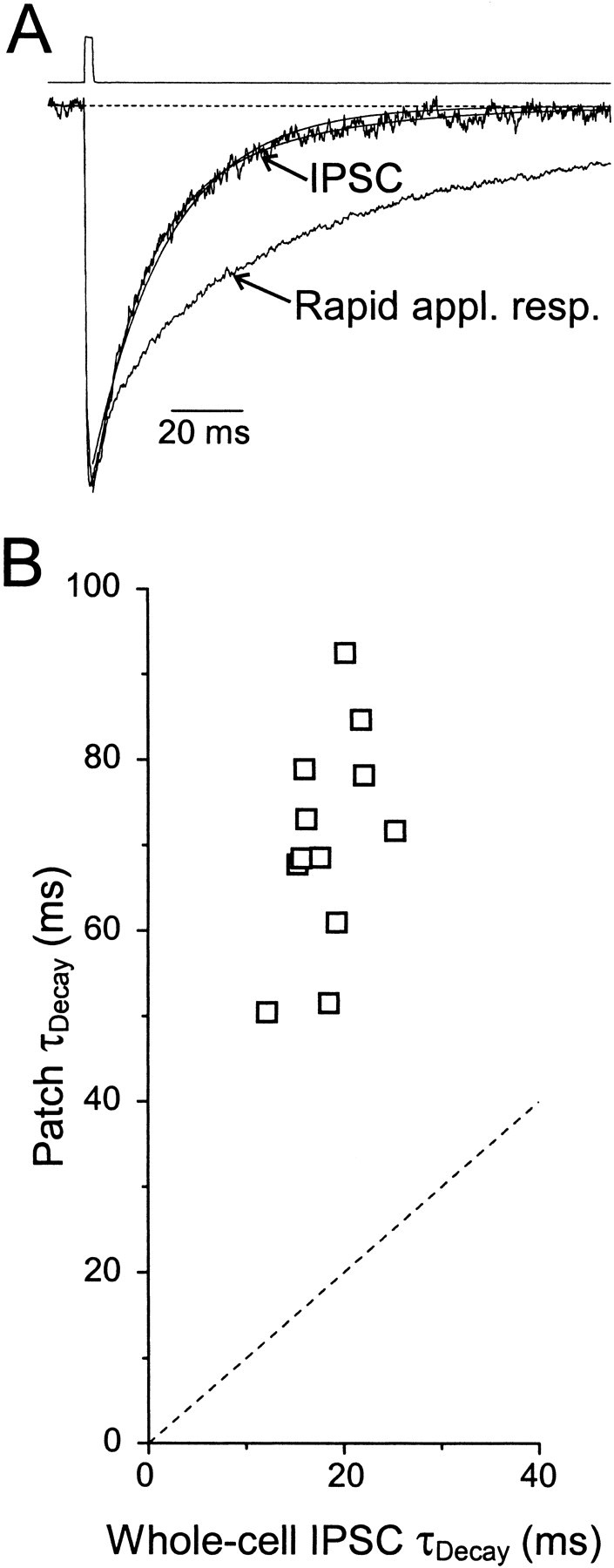
IPSCs and patch responses have different decay kinetics. A, Normalized average spontaneous IPSC and rapid application response to 1 mm GABA in an excised patch recorded from the same cell before and after patch excision. For the IPSC, biexponential fit parameters were τDec1,2 = 13.1 (68%) and 41.4 msec; monoexponential fit parameter was τDec = 22.0 msec. For the patch response, triexponential fit parameters were τDec1,2,3 = 11.6 (30%), 69.3 (55%), and 244 msec. Top trace shows the open-tip junction current recorded immediately after terminating the recording. B, Decay kinetics of rapid application responses plotted versus decay kinetics of IPSCs recorded in the same cells before patch formation. Dotted line has unity slope. In these 12 cells, fit parameters for the whole-cell IPSCs were τDec1,2 = 11.7 ± 1 (63 ± 1%) and 31.6 ± 2.6 msec. Fit parameters for the rapid application responses were τDec1,2,3 = 9.7 ± 1.6 (16 ± 3%), 54.9 ± 2.8 (63 ± 4%), and 168 ± 11 msec.
Possible reasons for the observed difference in kinetics fall into two general categories: (1) factors external to the patch, such as the transmitter transient and extracellular modulatory agents, and (2) factors intrinsic to the patch, such as the isomerization kinetics of the receptors.
Influence of transmitter concentration and time course on τdecay
The deactivation of GABAA receptors after brief exposure to ligand is a complex process involving entry and exit from open, bound, and desensitized states of both singly and doubly liganded receptors (Jones and Westbrook, 1995). The rates of transitions between these states, together with the duration and peak concentration of the transmitter transient, determine to what extent each of these states is visited during, and after, ligand exposure. Monoliganded receptors generate much briefer and less frequent openings than doubly liganded receptors, presumably because of slow entry into and rapid exit from the monoliganded open state (Macdonald et al., 1989). Thus, brief transients and/or low agonist concentrations may result in smaller responses and more rapid return of receptors to their resting state after agonist exposure if the binding rate is slow enough to produce a high percentage of monoliganded receptors compared to doubly liganded receptors. This may be relevant to synaptic GABAA responses, because some studies have shown that IPSCs are generated by subsaturating transmitter transients (Frerking et al., 1995; Nusser et al., 1997).
We saw no correlation between decay time course and pulse duration for pulses between 0.6 and 3.7 msec (r = 0.08;p > 0.6) (Fig.3A), indicating that for 1 mm GABA, the binding rate remains large compared to even the briefest pulses that we delivered, so that doubly liganded receptor responses must still dominate the decay. This is consistent with estimates of the binding rate of GABA to the GABAA receptor of 5.38 mm−1msec−1 (Jones et al., 1998), which yields a binding time constant of 186 μsec for 1 mm GABA. Although we were unable to reliably deliver pulses briefer than several hundred microseconds, we could equivalently test the decay kinetics of monoliganded receptors by using 2 msec pulses of 10 μm GABA. At this concentration, the pulse duration is approximately nine times briefer than the binding time constant and three times briefer than the unbinding time constant, and thus the vast majority of bound receptors will be monoliganded. Responses of nucleated patches to 2 msec pulses of 10 μm GABA had amplitudes that were 4.2% of the responses to 1 mm GABA (10 μm: 287 ± 97 pA, n = 6; 1 mm: 6850 ± 532 pA, n = 25). These responses decayed more rapidly than responses to 1 mm GABA (Fig. 3B, Table 1), primarily because of a prominent, rapid initial decay phase. However, this initial decay component was approximately twice as fast as the initial IPSC decay component, and these responses were still substantially slower than the decays of IPSCs (p < 0.005) because of a prolonged tail with time constant >100 msec. Responses to 2 msec pulses of 30 μm GABA had amplitudes that were 10.7% of the response to 1 mm GABA (731 ± 267 pA; n = 4) and intermediate decay kinetics (Table 1). These results indicate that differences between synaptic and exogenous transmitter transients in time course and concentration are unable to account for the observed differences in τDecay.
Fig. 3.
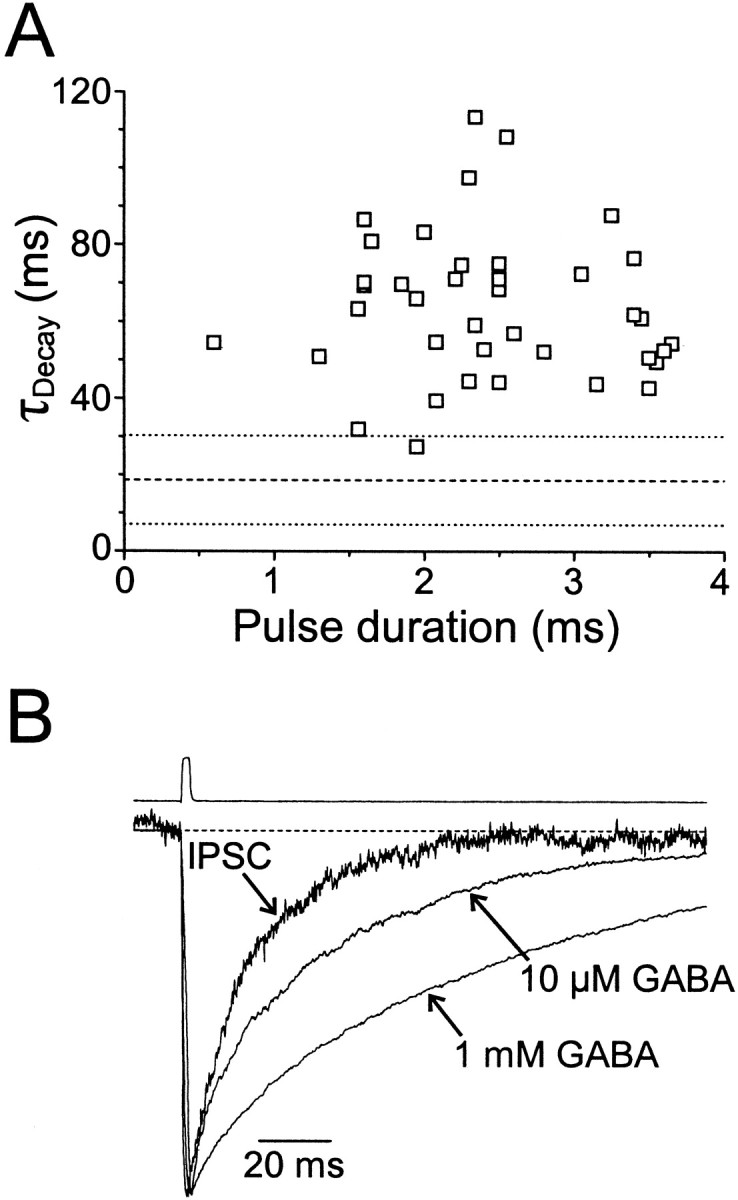
Effect of transmitter duration and concentration on decay kinetics. A, Decay time constants of rapid application responses of excised patches versus the duration of the GABA pulses (1 mm). Dashed and dotted lines represent the mean ± 2 SD τDecay for whole-cell IPSCs. B, Normalized average spontaneous IPSC and rapid application response to 10 μm and 1 mm GABA recorded from the same cell before and after formation of a nucleated patch. For the IPSC, τDec1,2 = 13.4 (74%) and 39.4 msec. For the 10 μm patch response, τDec1,2,3 = 4.67 (20%), 34.7 (61%), and 115 msec. For the 1 mm patch response, τDec1,2,3 = 9.8 (12%), 66.1 (57%), and 173 msec. Top trace shows the open-tip junction current recorded immediately after terminating the recording.
Effects of Zn2+, pH, and pre-equilibration with GABA on patch deactivation kinetics
The deactivation time course of synaptic GABAA receptors could also be affected by cofactors released with GABA or continuously present in the extracellular milieu. Both Zn2+ and H+ ions have been shown to modulate the amplitude and kinetics of GABAA receptor-mediated currents (Westbrook and Mayer, 1987; Krishek et al., 1996; Berger et al., 1998; Gingrich and Burkat, 1998). If these cofactors are released with GABA at the synaptic cleft (Assaf and Chung, 1984; Miesenbock et al., 1998), it is possible that they could contribute to the rapid decay kinetics of synaptic responses. We tested this hypothesis by coapplying either H+ (1 μm, i.e., 16-fold higher concentration than control) or Zn2+ (300 μm) with 1 mm GABA to outside-out patches. (By “coapplication”, we mean that the cofactors were present only when GABA was present.) Raising [H+] to 1 μmresulted in only a small (∼11%) decrease in τDecay and an increase in peak amplitude by ∼12% (n = 3; p < 0.05, paired Student's t test). Coapplication of Zn2+ had no effect on either deactivation kinetics or peak amplitude (n = 3; p > 0.5; Student's t test), suggesting that corelease of Zn2+ or H+ is unlikely to explain the approximately threefold difference in decay kinetics between patch and synaptic responses. We also continuously bathed outside-out patches in 10 μmZn2+ to assess the effect of low levels of extracellular Zn2+ on receptor kinetics. In two patches, τDecay was reduced by 20 and 30%, less than the 70% reduction required to explain the kinetic difference between synaptic and rapid application responses. Peak amplitudes were reduced by 15 and 25%, respectively, in these two patches.
The time course of relaxation of the receptor population back to its resting state after exposure to ligand will depend in part on the distribution of receptors in the available states immediately before the agonist pulse. GABA is present in the extracellular space of the hippocampus at submicromolar concentrations (Lerma et al., 1986;Tossman et al., 1986) and, thus, some receptors will be bound by GABA at any given moment in time. We tested the effects of pre-equilibration of synaptic receptors with micromolar levels of GABA by exposing excised and nucleated receptors to low concentrations of GABA for several seconds to several minutes before applying 1 mmGABA for 2 msec. GABA applied at a concentration of 1 μmhad no measurable effect on τDecay of the brief pulse responses (n = 3; p > 0.4; paired Student's t test), although it did reduce slightly the amplitude of the response by <10%. Thus, pre-equilibration cannot account for the difference between patch and synaptic responses.
Responses of excised patches to synaptically released transmitter
Although none of the presynaptic or extracellular factors that we tested caused the decay times of brief-pulse rapid application responses to be comparable to IPSCs, it is possible that some other untested modulator, cofactor, or transmitter is present in the slice. To test the responses of excised receptors in their native environment, we exposed excised patches to synaptically released transmitter, with any attendant cofactors and extracellular modulatory agents, by reinserting the excised patch back into stratum pyramidale to act as a sniffer patch (Isaacson et al., 1993) and electrically evoking synaptic release in the presence of CNQX and APV. We then compared these responses with whole-cell evoked synaptic responses recorded immediately before patch excision and to rapid application responses in other patches.
Sniffer patch responses evoked by stimuli in stratum pyramidale were variable in amplitude and time course (Fig.4). When patches were held above the slice, out of the tissue, no response could be measured (Fig.4A). When inserted into the slice, response amplitude was a sensitive function of position, presumably reflecting variable proximity to release sites. Small amplitude responses typically had slower rise times and longer latencies (Fig. 4A), consistent with diffusion of GABA from a remote release site to the sniffer patch. Small changes in the position of the sniffer patch could result in much larger amplitude responses with faster rise times (Fig.4A–C). Unlike the effect on rise time and amplitude, proximity to the release site had little impact on current decay (Fig.4B), suggesting that details of the time course or peak concentration of the transmitter transient were not the primary factors in determining decay kinetics.
Fig. 4.
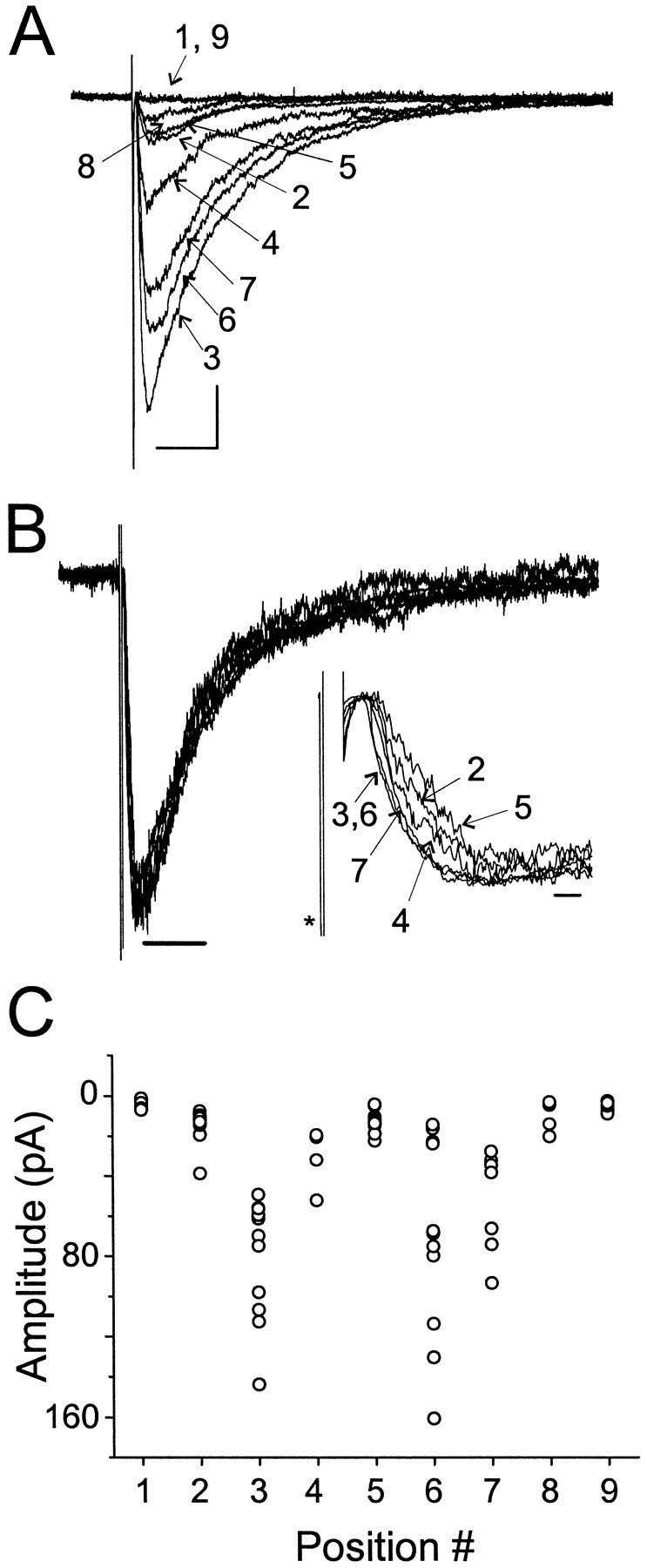
Responses of excised sniffer patches to synaptically released transmitter. A, Sniffer patch responses to stimuli in stratum pyramidale. Shown are the averaged responses at nine different positions, indicated bynumbers, above and in the slice. Positions 1 and 9 were above the slice. Positions 3 and 6 were the same location and yielded the largest amplitude responses. Position 2 was 10 μm from 3, whereas positions 4, 5, 7, and 8 were 3, 6, 10, and 20 μm from position 3, respectively, in the opposite direction as position 2. Calibration: 15 pA, 20 msec. B, Normalized data from A. Note that the decay kinetics exhibit little dependence on position. Calibration: 50 msec. Inset shows the normalized traces. Note the change in latency and rise time as position was changed.Asterisk marks the stimulus artifact. Trace from position 8 had a high noise level and is not shown. Calibration: 2 msec. C, Peak amplitude as a function of position for individual responses from the positions shown in A andB. Note that although the response amplitudes were variable, there were abrupt changes in the amplitude range as a function of position.
In four cases, we were able to position the sniffer patches close enough to the presynaptic terminals to generate responses with rise times <2 msec (1.6 ± 0.1 msec), i.e., within the range of the rapid application responses observed with other patches (Fig.5). These rapid rise times most likely reflect the brief duration of the transmitter transient eliciting the sniffer patch response. The amplitudes of these responses averaged >250 pA (range, 48.1–695 pA). Decay times of these sniffer patch responses were significantly slower than those of IPSC decays (67.0 ± 2.7 vs 19.4 ± 1.3 msec; p < 10−3, paired Student's ttest), but were not significantly different from rapid application responses of excised receptors to brief pulses of GABA (p > 0.4; Fig. 5A,B).
Fig. 5.
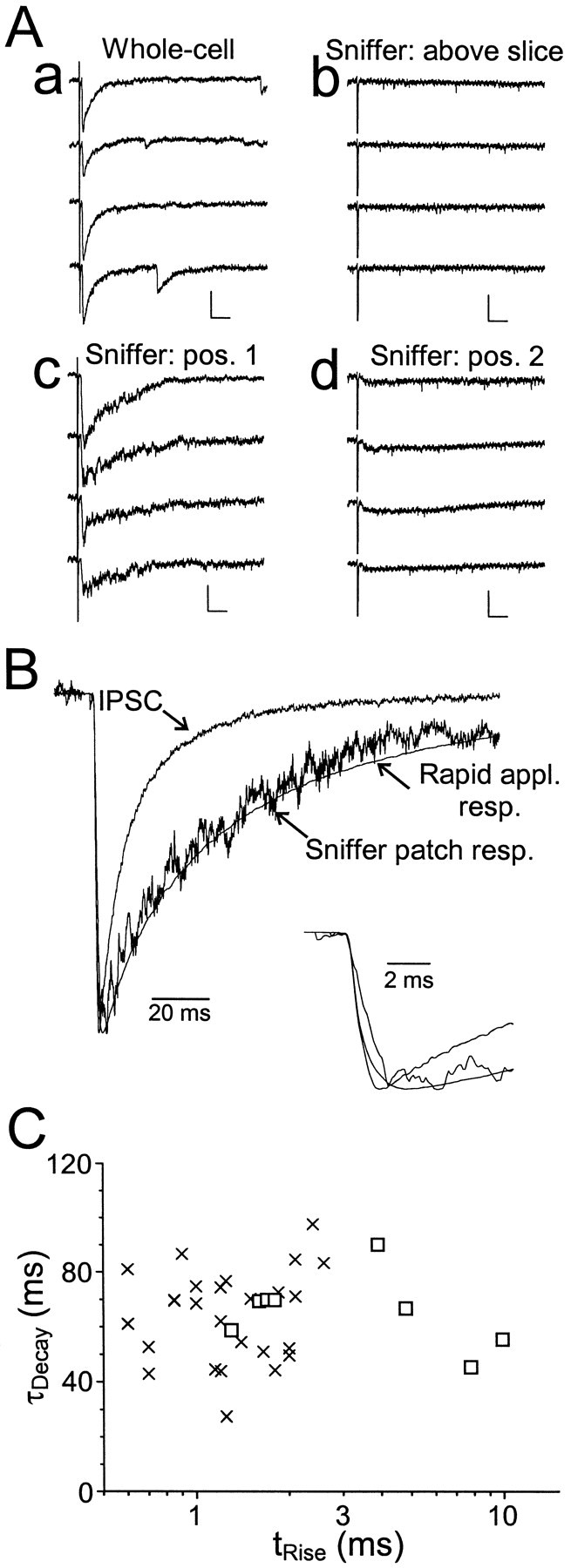
Comparison of sniffer patch and whole-cell synaptic responses. A, Raw traces recorded in response to stimuli applied to stratum pyramidale. a, Whole-cell IPSCs. b, Sniffer patch data recorded from an excised patch placed above the slice. Note that in this position, no response was evoked by the stratum pyramidale stimulus. c,Response of the same patch to the same stimulus, but recorded after placing the recording electrode tip on a pyramidal cell body.d, Response to the same stimulus after moving the recording electrode ∼5 μm away from the cell body. Distance was gauged using the tip diameter of the patch pipette, which was typically 2–3 μm. Calibration bars: a, 100 pA, 20 msec;b, 25 pA, 20 msec. B, Normalized averaged traces from the data shown in Aa and Ac.Inset shows the same data on an expanded time scale. For the IPSC, tRise = 0.7 msec, τDecay = 16.0 msec. For the sniffer patch response, tRise = 1.7 msec, τDecay = 69.9 msec. Also shown is the averaged rapid application response from all excised patches. C,Weighted decay time constant versus rise time plotted for eight sniffer patch responses (squares) and for excised patch responses (crosses). Note that there is no correlation between rise time and decay time for the sniffer patch data. Note also that the responses from four of the sniffer patches fall within the range of rise times recorded from excised patches.
Although the rapid rise times of these sniffer patch responses are indicative that they were exposed to a brief, high concentration pulse of transmitter, we considered the possibility that the prolonged decay of the response is attributable to the extended presence of a low concentration of transmitter in the extrasynaptic space and that the rapid decay kinetics of IPSCs represents the intrinsic deactivation kinetics of the receptors in the absence of a prolonged, low-concentration GABA “tail”. In this case, the farther the sniffer patch is from the synaptic cleft, the slower its decay kinetics should be. However, when the data from sniffer patch responses with rapid rise times were compared with those from slowly rising responses (range, 3.9–9.9 msec), we found no correlation between rise time and decay time, even though the proximity to the release site (and thus the transmitter transient) was undoubtedly quite variable between patches (Fig. 5C). Thus, the sniffer patch data indicate that the slow decay rate of patch responses is governed primarily by the intrinsic properties of the receptors in the patch, rather than presynaptic or extracellular factors such as the time course of the transmitter transient, the presence of cotransmitters, or of modulatory factors.
Properties of IPSCs recorded in nucleated patches
There are two scenarios in which postsynaptic factors could explain the kinetic differences between IPSCs and patch responses: (1) the receptors mediating patch responses to exogenous GABA pulses are synaptic receptors whose properties have been altered following patch excision; (2) synaptic receptors compose only a subset of the receptors that mediate patch responses, with the remainder comprised of extrasynaptic receptors with slower kinetics. Although it might seem likely that the process of excising GABAAreceptors from their native environment might itself alter their kinetic properties, this did not appear to be the case. We observed that after forming nucleated patches and lifting them 2–3 mm above the slice, spontaneous IPSCs could still be recorded in ∼20% of the patches (Fig. 6A,B). These events most likely arose from spontaneous release of transmitter from synaptic terminals that remained anchored to the postsynaptic membrane. These “nucleated patch IPSCs” had kinetics that were similar to IPSCs recorded in the intact cells [patch:t10–90% = 0.8 ± 0.1 msec, τDec1,2 = 10.3 ± 1.3 (60 ± 10%) and 36.6 ± 2.9 msec; whole-cell:t10–90% = 0.9 ± 0.1 msec, τDec1,2 = 11.9 ± 0.8 (70 ± 10%) and 30.6 ± 2.2 msec; p > 0.1 for all fit parameters by paired Student's t test] (Fig.6C,D, Table 2), suggesting that the process of establishing a nucleated patch does not alter the determinants of deactivation kinetics of synaptic receptors. The amplitudes of nucleated patch IPSCs were also similar to the amplitudes of spontaneous IPSCs recorded in intact cells (whole-cell, 71.7 ± 15.2 pA; patch, 71.1 ± 29.2 pA; n = 10 cells;p > 0.9, paired Student's t test), providing evidence that the peak open probability of synaptic receptors is also not altered by patch excision. In four cells, sufficient numbers of spontaneous IPSCs were recorded in nucleated patches to analyze the variability of IPSC amplitude and τDecay (Fig. 6E,F, Table 2). There were no consistent differences in the coefficient of variation of amplitudes or the SD of τDecay, indicating that nucleated patch IPSCs do not consist of responses of a mixed population of damaged and undamaged postsynaptic receptors.
Fig. 6.
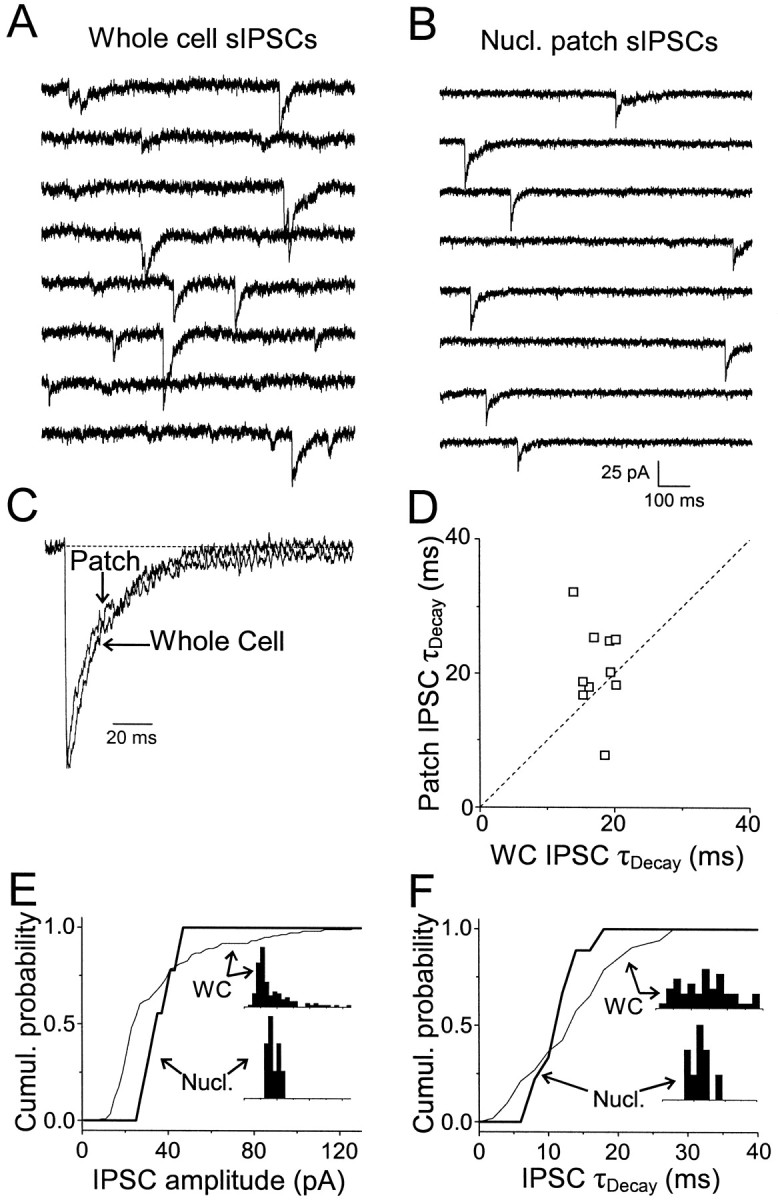
Properties of spontaneous IPSCs recorded in nucleated patches. A, Whole-cell sIPSCs recorded before patch excision. Traces are consecutive. This cell corresponds to cell 1 in Table 2. B, sIPSCs recorded from a nucleated patch after removal from the slice. Traces are not consecutive and represent all of the spontaneous events recorded over an 80 sec period.C, Normalized averaged sIPSCs from the cell inA and B. Biexponential fits were: whole-cell, τDec1,2 = 9.9 (47%) and 27.8 msec; patch, τDec1,2 = 6.1 (47%) and 32.8 msec.D, Patch IPSC weighted time constant versus whole-cell IPSC weighted time constant for 10 cells. There was no significant difference in mean τDecay between the patch and whole-cell data (p = 0.2, paired Student'st test). E, Cumulative amplitude distribution for spontaneous IPSCs recorded in the same cell as inA-C in whole-cell (thin line) and nucleated patch (thick line). Inset shows normalized amplitude distributions. F, Same as E, but for τDecay.
Table 2.
Amplitude and kinetic parameters for whole-cell and nucleated patch spontaneous IPSCs
| Whole-cell IPSCs | Nucleated patch IPSCs | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amp (pA) | CVAmp | τDecay(msec) | ςτ | tRise(msec) | n | Amp (pA) | CVAmp | τDecay(msec) | ςτ | tRise(msec) | n | |
| Cell#1 | 33.9 | 0.66 | 19.4 | 6.9 | 0.7 | 121 | 36.2 | 0.19 | 20.3 | 3.2 | 0.6 | 9 |
| Cell#2 | 54.3 | 1.11 | 14.7 | 5.1 | 0.6 | 369 | 144.8 | 1.26 | 17.1 | 7.6 | 0.8 | 39 |
| Cell#3 | 56.3 | 0.88 | 11.4 | 3.9 | 1.2 | 1711 | 93.2 | 1.01 | 14.4 | 7.1 | 1.0 | 27 |
| Cell#4 | 41.3 | 0.65 | 17.2 | 6.9 | 0.7 | 129 | 27.8 | 0.44 | 17.1 | 6.8 | 0.7 | 28 |
Shown is the mean amplitude and its coefficient of variation, the mean τDecay and its SD, and the mean 10–90% rise time for spontaneous IPSCs in four cells before and after the formation of a nucleated patch and its removal from the slice. n is the number of IPSCs.
Coexistence of two receptor classes in nucleated patches
Although nucleated patch IPSCs show that the kinetics of synaptic receptors are not altered by patch excision, we sought direct evidence that two kinetic classes of GABAA receptors coexist in the same nucleated patch by subsequently exposing these same nucleated patches to exogenous GABA pulses. Rapid application responses were markedly prolonged relative to nucleated patch IPSCs [nucleated patch IPSC: τDec = 15.2 ± 2.5 msec; rapid application response (1 mm, 2 msec GABA): τDec = 76.6 ± 10.3 msec;n = 4 patches; p < 0.005 paired Student's t test] (Fig.7A). These results demonstrate directly the presence of two kinetic classes of receptors in these patches.
Fig. 7.
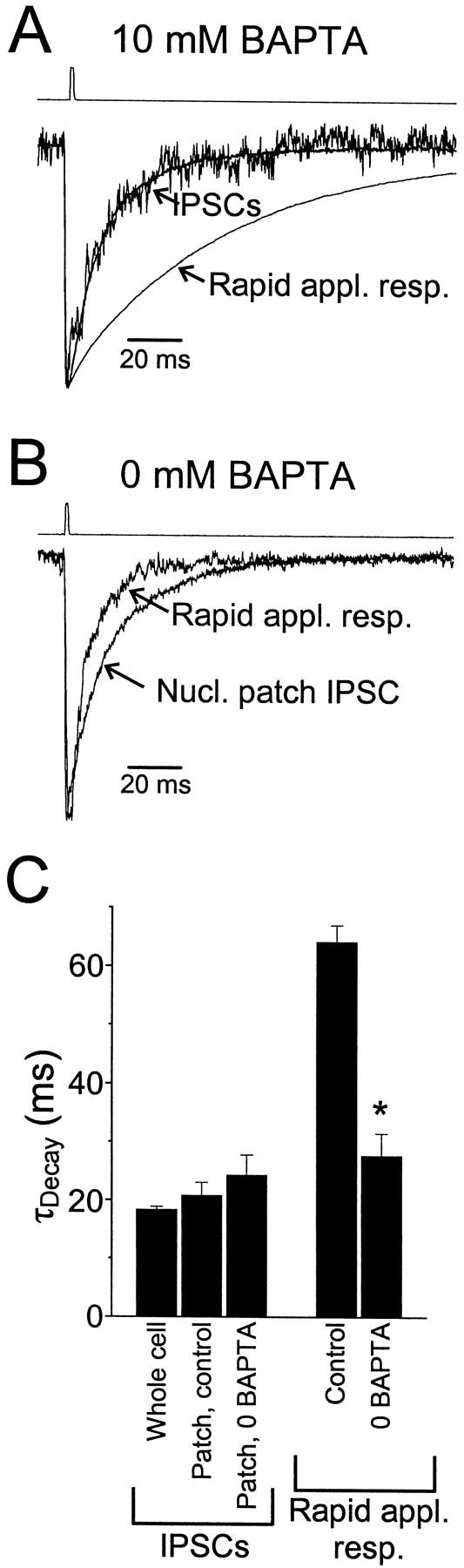
Selective modulation of rapid application responses by elevated calcium. A, Normalized whole-cell (smooth trace) and nucleated patch (noisy trace) sIPSCs and rapid application response of the nucleated patch to 1 mm, 2 msec pulse of GABA recorded with a pipette containing 10 mm BAPTA. All data were recorded from the same cell. τDecay values were as follows: whole-cell IPSC, 16.8 msec; patch IPSC, 15.3 msec; rapid application response, 67.8 msec. B, Normalized nucleated patch IPSC and response in the same patch to a 1 mm, 2 msec GABA pulse recorded in a different patch from A with a pipette containing 0 BAPTA. Note that in 0 BAPTA, the rapid application response is faster than in control, but the IPSC decay is similar. τDecay values were as follows: patch IPSC, 18.6 msec; rapid application response, 7.7 msec. C, Average weighted time constants of whole-cell and patch sIPSCs under control conditions and with 0 BAPTA, and rapid application responses to 1 mm, 2 msec GABA pulses under control conditions and with 0 BAPTA. There was no significant difference between τDecayfor the patch IPSC data (10 mm BAPTA vs 0 BAPTA:p > 0.4, Student's t test), but rapid application responses were significantly faster in the absence of BAPTA (*p < 10−6, Student'st test).
The similarity between whole-cell and nucleated sIPSCs (Fig. 6) demonstrates that the kinetic properties of synaptic receptors are unchanged in patches. It follows then that a large proportion of receptors mediating responses to exogenous GABA pulses must arise from receptors whose kinetics are slow relative to synaptic receptors (i.e., extrasynaptic), and that the amplitude of responses to exogenous GABA pulses should exceed the amplitude that would be generated by synaptic receptors alone. To determine whether this is the case, we compared the responses of nucleated patches to exogenously applied GABA with the amplitude that would be expected if these responses were mediated only by synaptic receptors. We used miniature (action-potential independent) IPSC (mIPSC) amplitude as a measure of the response amplitude at a single synapse and found that mIPSC amplitudes averaged 39.1 ± 2.0 pA at −60 mV (n = 32 cells). By multiplying by the density of inhibitory synapses on the somata of CA1 pyramidal cells (0.2 ± 0.02 synapses/μm2;n = 5 cells; M. Megías, Z. Emri, T. F. Freund, and A. I. Gulyás, personal communication), by the somatic surface area (520 μm2; see Materials and Methods), and by the ratio of surface area in the nucleated patch to that in the intact soma (0.36; see Materials and Methods), the maximal response amplitude of synaptic receptors can then be calculated to be 1464 pA if saturation of synaptic receptors is achieved. This response amplitude is indeed smaller than what we observed in response to 1 mm GABA (6850 pA).
Differences in calcium modulation between synaptic and extrasynaptic receptors
In addition to differences in deactivation kinetics, we found that synaptic and extrasynaptic receptors also differ in their modulation by elevated cytoplasmic levels of calcium. The pipette solution used for all of the experiments described above contained 10 mmBAPTA. Using a calcium-sensitive electrode, we measured the free calcium concentration in this pipette solution to be ∼20 nm. When BAPTA was omitted from the pipette solution, free calcium levels were >2 μm with 0 added calcium, presumably because of contaminants present in other salts. Elevated calcium levels significantly decreased the decay times of rapid application responses (τDecay = 27.7 ± 3.8 msec; n = 11) (Fig. 7) when compared with 10 mm BAPTA responses (p < 10−6, Student's t test). These data demonstrate that it is possible to record rapid responses to GABA in outside-out patches and thus that the slow decay kinetics of rapid application responses under control conditions is not the result of some unidentified artifact of the technique.
In three patches recorded with 0 BAPTA, patch IPSCs could also be observed. In contrast to the rapid application responses of these patches, the decay times of these synaptic responses (24.3 ± 3.5 msec) appeared to be unaffected by elevated calcium, because they were not significantly different from patch IPSCs recorded in the presence of 10 mm BAPTA (p > 0.4; Student'st test) (Fig. 7B,C). The selective modulation of patch responses to exogenous transmitter application by elevated cytoplasmic calcium is further evidence that the sets of receptors that mediate synaptic responses and rapid application responses are not the same and is consistent with the hypothesis that rapid application responses are dominated by extrasynaptic receptors. This selective modulation may also be a manifestation of the regulatory mechanism that is the basis of the difference in receptor kinetics in the intact cells.
DISCUSSION
The main findings of this study are that neither presynaptic factors (transmitter transient, the presence of extracellular modulatory agents), nor alteration of receptors by patch excision can account for kinetic differences between synaptic and excised patch responses. We conclude that extrasynaptic receptors dominate patch responses and that these receptors have slower intrinsic kinetics than synaptic receptors.
Sniffer patch responses
To assess whether an untested cotransmitter or extracellular modulator underlies the rapid decays of IPSCs, we measured the responses of GABAA receptors in excised patches to synaptically released transmitter. Decay kinetics of these sniffer patch responses were similar to those in response to exogenous GABA pulses, indicating that the kinetic differences between IPSCs and exogenous application responses lie in the receptors themselves. One caveat to this conclusion is that low concentrations of low-affinity agonists such as taurine or β-alanine, which produce rapid deactivation (Jones et al., 1998), may be difficult to detect with sniffer patches outside the synaptic cleft. However, for a low-affinity cotransmitter to produce rapidly decaying IPSCs, it would have to prevent nearly all synaptic receptors from binding two GABA molecules, as we have shown that receptors fully liganded by GABA have slow deactivation kinetics. Because a low-affinity agonist such as β-alanine has a binding rate that is >200-fold slower than GABA (Jones et al., 1998), in this scenario it would have to be present at >1000-fold higher concentration than GABA. The possibility that a cotransmitter would be released at such high concentrations and would remain undetected by the sniffer patches is remote.
Kinetics of synaptic versus extrasynaptic receptors
One explanation for kinetic differences between synaptic and extrasynaptic receptors is subunit composition (Verdoorn, 1994;Gingrich et al., 1995; Tia et al., 1996b; McClellan and Twyman, 1999). Extrasynaptic receptors in immature cerebellar granule cells are composed solely of α and β subunits, whereas the composition of synaptic receptors is αβγ (Brickley et al., 1999). Consistent with this, γ subunits are required for clustering at synapses, and receptors composed exclusively of α and β subunits are diffusely distributed in the plasma membrane (Essrich et al., 1998). It may be possible that the subunit compositions of synaptic versus extrasynaptic receptors are similarly distinguished in hippocampus. However, the presence or absence of γ2 subunits in α1β2- and α6β2-containing receptors has little effect on deactivation kinetics (Tia et al., 1996b), and so differences in α or β subunits may better explain the slower kinetics of extrasynaptic receptors. For example, in human embryonic kidney cells expressing αxβ1γ2receptors, substituting α2- for α1-containing receptors changes the τDecay of patch responses from 18 to 50–80 msec (McClellan and Twyman, 1999), similar to difference between IPSCs and rapid application responses in our data.
An alternative possibility is that synaptic and extrasynaptic receptors are regulated differently by second messenger systems, as suggested by our results with 0 BAPTA. Elevated [Ca]imodulates affinity and kinetics of GABAAreceptors (Inoue et al., 1986; Chen et al., 1990; Martina et al., 1994;Stelzer and Shi, 1994; Mozrzymas and Cherubini, 1998), often by activation of kinases or phosphatases. In cultured hippocampal neurons, τDecay of synaptic and exogenous GABA responses is regulated by the calcium-dependent phosphatase calcineurin and by unknown kinases (Jones and Westbrook, 1997). In these cells, maximally phosphorylated GABAA receptors produced responses that were approximately twice as fast as under control conditions, although IPSCs were still much slower than in hippocampal slices. Despite these quantitative differences, this suggests that in our experiments synaptic receptors may be more fully phosphorylated than extrasynaptic receptors. In this scenario, elevated [Ca] in nucleated patches from slices may activate calcium-dependent kinases that phosphorylate extrasynaptic receptors but have no effect on synaptic receptors because they are already maximally phosphorylated. This suggestion is consistent with a recent study that found that activation of kinases had little effect on the overall decay kinetics of IPSCs in CA1 pyramidal cells (Poisbeau et al., 1999).
Relative density of synaptic versus extrasynaptic receptors
We calculated that synaptic receptors contributed ∼25% of the rapid application response, assuming that synaptic receptors are saturated. Their contribution will be higher, however, if the peak cleft concentration is lower. We know from the sniffer patch data that the concentration is at least high enough to generate ∼45% of the response to 1 mm GABA. (Note that this comparison is between different groups of patches, albeit pulled with the same size pipettes and same technique. Sniffer patches were not calibrated using rapid application of GABA.) If the peak transmitter concentration in the cleft is this low, the contribution of synaptic receptors in nucleated patches to 1 mm GABA may be as high as 3250 pA. Assuming that peak open probability and single-channel conductance of synaptic and extrasynaptic receptors are the same, synaptic receptors account for 21–47% of the patch response (i.e., 1464–3250 of 6850 pA).
We can corroborate this estimate using the deactivation kinetics of patch responses. The triphasic responses of excised and nucleated patches to exogenous GABA pulses most likely reflect the commingling of synaptic receptors (with time constants of ∼10 and 30 msec) with slower extrasynaptic receptors. The fastest decay component (∼14 msec) in patch responses accounted for 25% of the total response amplitude. If this component represents only synaptic receptors, then their maximum contribution to the patch response can be calculated by dividing this amplitude by the amplitude of the fast decay component in IPSCs. This estimate indicates that >60% of the patch response is generated by extrasynaptic receptors, within the range calculated above. The calculated percentages of synaptic versus extrasynaptic receptor contributions will only translate into relative numbers of receptors if peak open probabilities and the single-channel conductances of the two receptor populations are the same.
Although extrasynaptic GABAA receptors have been observed previously using immunocytochemical techniques (Richards et al., 1987; Houser et al., 1988; Fritschy et al., 1994; Nusser et al., 1998), quantitative measurements of relative densities of synaptic versus extrasynaptic receptors have not been made in CA1. In cerebellar granule cells, extrasynaptic receptors occur at much lower density than synaptic receptors (Nusser et al., 1995). In cultured hippocampal neurons, however, only half of GABAA receptor clusters were apposed by presynaptic specializations, the remaining 50% presumably corresponding to clustered extrasynaptic receptors (Kannenberg et al., 1999). The observation that extrasynaptic receptors may outnumber synaptic receptors in patches is still consistent with enrichment of receptors at synaptic junctions. These areas of high receptor density likely represent <0.2 μm2/synapse (Harris et al., 1985; Halasy and Somogyi, 1993) or <25 μm2 for all of the synapses on the cell body, corresponding to <5% of the total amount of membrane. Thus, there is at least 5- to 10-fold enrichment of receptors in the synaptic zone.
Kinetic classes of IPSCs in CA1
In CA1 pyramidal neurons, two distinct IPSCs are observed (Pearce, 1993; Banks et al., 1998). GABAA,fast is the rapidly decaying, somatic IPSC measured in this study, whereas GABAA,slow is mediated by dendritic synapses and has decay kinetics several fold slower than GABAA,fast. Although the basis for this difference in kinetics is unclear, the different pharmacological properties of these two IPSCs suggests that the receptors mediating GABAA,fast and GABAA,sloware distinct (Banks et al., 1998). The observation that two kinetic classes of GABAA receptors exist in these cells raises the possibility that GABAA,slow IPSCs are mediated by the same type of receptors that are extrasynaptic at the soma. It may be that proteins responsible for clustering these receptors are targeted only to dendritic regions and that these slow receptors are distributed diffusely in the somatic membrane, but are clustered in the dendritic membrane.
Functional significance of extrasynaptic receptors
One possible function of extrasynaptic receptors is that they set the resting conductance of the postsynaptic cell by way of tonic activation by ambient GABA or by GABA that spills over from the synaptic clefts of nearby terminals (Brickley et al., 1996; Hausser and Clark, 1997). In cerebellar granule cells, extrasynaptic, δ subunit-containing receptors have a high affinity for GABA and do not desensitize, making them particularly well-suited to this task (Saxena and Macdonald, 1994, 1996). The relatively slow deactivation kinetics of extrasynaptic receptors in CA1 may similarly be a specialization appropriate for mediating tonic inhibition.
This role for extrasynaptic receptors would allow endogenous modulators of GABAA receptors and GABA uptake to regulate the excitability of the hippocampal network. In addition, some pharmacological agents may act via this mechanism. A number of general anesthetics are able to gate GABAA receptors directly (Yang et al., 1992; Belelli et al., 1997; Ueno et al., 1997) and may also increase the sensitivity of these receptors to ambient GABA (Banks and Pearce, 1999). Thus, modulation of extrasynaptic receptors may mediate some of the behavioral effects of general anesthetics. The observation that the volatile anesthetic isoflurane has different effects on synaptic and rapid application responses (Banks et al., 1997) raises the possibility that general anesthetics may differentially target transient versus tonic inhibition, and this could contribute to the complexity of the behavioral effects of these agents.
Footnotes
This work was supported by National Institutes of Health Grant GM55719 (R.A.P.), University of Wisconsin–Howard Hughes Medical Institute Research Resources Program, and the Department of Anesthesiology, University of Wisconsin-Madison. Thanks to Donna Cole and Philippe Shils for technical support.
Dr. Pearce is the Betty J. Bamforth Research Professor of Anesthesiology.
Correspondence should be addressed to Dr. Matthew I. Banks, Department of Anesthesiology, University of Wisconsin, 43 Bardeen Laboratories, 1300 University Avenue, Madison, WI 53706. E-mail:mibanks@facstaff.wisc.edu.
REFERENCES
- 1.Assaf SY, Chung SH. Release of endogenous Zn 2+ from brain tissue during activity. Nature. 1984;308:734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- 2.Banks MI, Pearce RA. Dual actions of volatile anesthetics on GABAA IPSCs: dissociation of blocking and prolonging effects. Anesthesiology. 1999;90:120–134. doi: 10.1097/00000542-199901000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Banks MI, Li T-B, Pearce RA. Effects of isoflurane on mIPSCs and excised neuronal GABAA receptors. Soc Neurosci Abstr. 1997;23:104. [Google Scholar]
- 4.Banks MI, Li T-B, Pearce RA. The synaptic basis of GABAA,slow. J Neurosci. 1998;18:1305–1317. doi: 10.1523/JNEUROSCI.18-04-01305.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci USA. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger T, Schwarz C, Kraushaar U, Monyer H. Dentate gyrus basket cell GABAA receptors are blocked by Zn2+ via changes of their desensitization kinetics: an in situ patch-clamp and single-cell PCR study. J Neurosci. 1998;18:2437–2448. doi: 10.1523/JNEUROSCI.18-07-02437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol (Lond) 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen QX, Stelzer A, Kay AR, Wong RK. GABAA receptor function is regulated by phosphorylation in acutely dissociated guinea-pig hippocampal neurones. J Physiol (Lond) 1990;420:207–221. doi: 10.1113/jphysiol.1990.sp017908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clements JD, Lester RA, Tong G, Jahr CE, Westbrook GL. The time course of glutamate in the synaptic cleft. Science. 1992;258:1498–1501. doi: 10.1126/science.1359647. [DOI] [PubMed] [Google Scholar]
- 11.Colquhoun D, Jonas P, Sakmann B. Action of brief pulses of glutamate on AMPA/kainate receptors in patches from different neurones of rat hippocampal slices. J Physiol (Lond) 1992;458:261–287. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudel J, Franke C, Hatt H. Rapid activation and desensitization of transmitter-liganded receptor channels by pulses of agonists. Ion Channels. 1992;3:207–260. doi: 10.1007/978-1-4615-3328-3_8. [DOI] [PubMed] [Google Scholar]
- 13.Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflügers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- 14.Essrich C, Lorez M, Benson JA, Fritschy JM, Luscher B. Postsynaptic clustering of major GABAA receptor subtypes requires the γ2 subunit and gephyrin. Nat Neurosci. 1998;1:563–571. doi: 10.1038/2798. [DOI] [PubMed] [Google Scholar]
- 15.Frerking M, Wilson M. Saturation of postsynaptic receptors at central synapses? Curr Opin Neurobiol. 1996;6:395–403. doi: 10.1016/s0959-4388(96)80125-5. [DOI] [PubMed] [Google Scholar]
- 16.Frerking M, Borges S, Wilson M. Variation in GABA mini amplitude is the consequence of variation in transmitter concentration. Neuron. 1995;15:885–895. doi: 10.1016/0896-6273(95)90179-5. [DOI] [PubMed] [Google Scholar]
- 17.Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galarreta M, Hestrin S. Properties of GABAA receptors underlying inhibitory synaptic currents in neocortical pyramidal neurons. J Neurosci. 1997;17:7220–7227. doi: 10.1523/JNEUROSCI.17-19-07220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gingrich KJ, Burkat PM. Zn2+ inhibition of recombinant GABAA Receptors - an allosteric, state-dependent mechanism determined by the gamma-subunit. J Physiol (Lond) 1998;506:609–625. doi: 10.1111/j.1469-7793.1998.609bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gingrich KJ, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol (Lond) 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halasy K, Somogyi P. Distribution of GABAergic synapses and their targets in the dentate gyrus of rat: a quantitative immunoelectron microscopic analysis. J Hirnforsch. 1993;34:299–308. [PubMed] [Google Scholar]
- 22.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 23.Harris KM, Marshall PE, Landis DM. Ultrastructural study of cholecystokinin-immunoreactive cells and processes in area CA1 of the rat hippocampus. J Comp Neurol. 1985;233:147–158. doi: 10.1002/cne.902330202. [DOI] [PubMed] [Google Scholar]
- 24.Hausser M, Clark BA. Tonic synaptic inhibition modulates neuronal output pattern and spatiotemporal synaptic integration. Neuron. 1997;19:665–678. doi: 10.1016/s0896-6273(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 25.Hestrin S. Activation and desensitization of glutamate-activated channels mediating fast excitatory synaptic currents in the visual cortex. Neuron. 1992;9:991–999. doi: 10.1016/0896-6273(92)90250-h. [DOI] [PubMed] [Google Scholar]
- 26.Hill MW, Reddy PA, Covey DF, Rothman SM. Contribution of subsaturating GABA concentrations to IPSCs in cultured hippocampal neurons. J Neurosci. 1998;18:5103–5111. doi: 10.1523/JNEUROSCI.18-14-05103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houser CR, Olsen RW, Richards JG, Mohler H. Immunohistochemical localization of benzodiazepine/GABAA receptors in the human hippocampal formation. J Neurosci. 1988;8:1370–1383. doi: 10.1523/JNEUROSCI.08-04-01370.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue M, Oomura Y, Yakushiji T, Akaike N. Intracellular calcium ions decrease the affinity of the GABA receptor. Nature. 1986;324:156–158. doi: 10.1038/324156a0. [DOI] [PubMed] [Google Scholar]
- 29.Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 30.Jack JJ, Noble D, Tsien RW. Electric current flow in excitable cells. Oxford UP; Oxford: 1983. [Google Scholar]
- 31.Jackson MB. Cable analysis with the whole-cell patch clamp. Theory and experiment. Biophys J. 1992;61:756–766. doi: 10.1016/S0006-3495(92)81880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 33.Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- 34.Jones MV, Westbrook GL. Shaping of IPSCs by endogenous calcineurin activity. J Neurosci. 1997;17:7626–7633. doi: 10.1523/JNEUROSCI.17-20-07626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones MV, Sahara Y, Dzubay JA, Westbrook GL. Defining affinity with the GABAA receptor. J Neurosci. 1998;18:8590–8604. doi: 10.1523/JNEUROSCI.18-21-08590.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kannenberg K, Sieghart W, Reuter H. Clusters of GABAA receptors on cultured hippocampal cells correlate only partially with functional synapses. Eur J Neurosci. 1999;11:1256–1264. doi: 10.1046/j.1460-9568.1999.00533.x. [DOI] [PubMed] [Google Scholar]
- 37.Krishek BJ, Amato A, Connolly CN, Moss SJ, Smart TG. Proton sensitivity of the GABAA receptor is associated with the receptor subunit composition. J Physiol (Lond) 1996;492:431–443. doi: 10.1113/jphysiol.1996.sp021319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legendre P. A reluctant gating mode of glycine receptor channels determines the time course of inhibitory miniature synaptic events in zebrafish hindbrain neurons. J Neurosci. 1998;18:2856–2870. doi: 10.1523/JNEUROSCI.18-08-02856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lerma J, Herranz AS, Herreras O, Abraira V, Martin DR. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- 40.Lester RA, Clements JD, Westbrook GL, Jahr CE. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990;346:565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- 41.Macdonald RL, Rogers CJ, Twyman RE. Kinetic properties of the GABAA receptor main conductance state of mouse spinal cord neurones in culture. J Physiol (Lond) 1989;410:479–499. doi: 10.1113/jphysiol.1989.sp017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maconochie DJ, Zempel JM, Steinbach JH. How quickly can GABAA receptors open? Neuron. 1994;12:61–71. doi: 10.1016/0896-6273(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 43.Martina M, Kilic G, Cherubini E. The effect of intracellular Ca2+ on GABA-activated currents in cerebellar granule cells in culture. J Membr Biol. 1994;142:209–216. doi: 10.1007/BF00234942. [DOI] [PubMed] [Google Scholar]
- 44.McClellan AL, Twyman RE. Receptor system response kinetics reveal functional subtypes of native murine and recombinant human GABAA receptors. J Physiol (Lond) 1999;515:711–727. doi: 10.1111/j.1469-7793.1999.711ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mellor JR, Randall AD. Frequency-dependent actions of benzodiazepines on GABAA receptors in cultured murine cerebellar granule cells. J Physiol (Lond) 1997;503:353–369. doi: 10.1111/j.1469-7793.1997.353bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miesenbock G, Deangelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 47.Mozrzymas JW, Cherubini E. Changes in intracellular calcium concentration affect desensitization of GABAA receptors in acutely dissociated P2–P6 rat hippocampal neurons. J Neurophysiol. 1998;79:1321–1328. doi: 10.1152/jn.1998.79.3.1321. [DOI] [PubMed] [Google Scholar]
- 48.Mozrzymas JW, Barberis A, Michalak K, Cherubini E. Chlorpromazine inhibits miniature GABAergic currents by reducing the binding and by increasing the unbinding rate of GABAA receptors. J Neurosci. 1999;19:2474–2488. doi: 10.1523/JNEUROSCI.19-07-02474.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nusser Z, Roberts JD, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J Neurosci. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nusser Z, Cull-Candy SG, Farrant M. Differences in synaptic GABAA receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- 51.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearce RA. Physiological evidence for two distinct GABAA responses in rat hippocampus. Neuron. 1993;10:189–200. doi: 10.1016/0896-6273(93)90310-n. [DOI] [PubMed] [Google Scholar]
- 53.Perrais D, Ropert N. Effect of zolpidem on miniature IPSCs and occupancy of postsynaptic GABAA receptors in central synapses. J Neurosci. 1999;19:578–588. doi: 10.1523/JNEUROSCI.19-02-00578.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poisbeau P, Cheney MC, Browning MD, Mody I. Modulation of synaptic GABAA receptor function by PKA and PKC in adult hippocampal neurons. J Neurosci. 1999;19:674–683. doi: 10.1523/JNEUROSCI.19-02-00674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richards JG, Schoch P, Haring P, Takacs B, Mohler H. Resolving GABAA/benzodiazepine receptors: cellular and subcellular localization in the CNS with monoclonal antibodies. J Neurosci. 1987;7:1866–1886. doi: 10.1523/JNEUROSCI.07-06-01866.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenmund C, Westbrook GL. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- 57.Sather W, Dieudonne S, MacDonald JF, Ascher P. Activation and desensitization of N-methyl-d-aspartate receptors in nucleated outside-out patches from mouse neurones. J Physiol (Lond) 1992;450:643–672. doi: 10.1113/jphysiol.1992.sp019148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saxena NC, Macdonald RL. Properties of putative cerebellar gamma-aminobutyric acidA receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- 60.Stelzer A, Shi H. Impairment of GABAA receptor function by N-methyl-d-aspartate-mediated calcium influx in isolated CA1 pyramidal cells. Neuroscience. 1994;62:813–828. doi: 10.1016/0306-4522(94)90479-0. [DOI] [PubMed] [Google Scholar]
- 61.Tanelian DL, Kosek P, Mody I, MacIver MB. The role of the GABAA receptor/chloride channel complex in anesthesia. Anesthesiology. 1993;78:757–776. doi: 10.1097/00000542-199304000-00020. [DOI] [PubMed] [Google Scholar]
- 62.Tia S, Wang JF, Kotchabhakdi N, Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons–role of GABAA receptor α6 subunit. J Neurosci. 1996a;16:3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tia S, Wang JF, Kotchabhakdi N, Vicini S. Distinct deactivation and desensitization kinetics of recombinant GABAA receptors. Neuropharmacology. 1996b;35:1375–1382. doi: 10.1016/s0028-3908(96)00018-4. [DOI] [PubMed] [Google Scholar]
- 64.Tossman U, Jonsson G, Ungerstedt U. Regional distribution and extracellular levels of amino acids in rat central nervous system. Acta Physiol Scand. 1986;127:533–545. doi: 10.1111/j.1748-1716.1986.tb07938.x. [DOI] [PubMed] [Google Scholar]
- 65.Trussell LO, Fischbach GD. Glutamate receptor desensitization and its role in synaptic transmission. Neuron. 1989;3:209–218. doi: 10.1016/0896-6273(89)90034-2. [DOI] [PubMed] [Google Scholar]
- 66.Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Verdoorn TA. Formation of heteromeric gamma-aminobutyric acid type A receptors containing two different alpha subunits. Mol Pharmacol. 1994;45:475–480. [PubMed] [Google Scholar]
- 68.Vicini S, Alho H, Costa E, Mienville JM, Santi MR, Vaccarino FM. Modulation of gamma-aminobutyric acid-mediated inhibitory synaptic currents in dissociated cortical cell cultures. Proc Natl Acad Sci USA. 1986;83:9269–9273. doi: 10.1073/pnas.83.23.9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westbrook GL, Mayer ML. Micromolar concentrations of Zn2+ antagonize NMDA and GABA responses of hippocampal neurons. Nature. 1987;328:640–643. doi: 10.1038/328640a0. [DOI] [PubMed] [Google Scholar]
- 70.Yang J, Isenberg KE, Zorumski CF. Volatile anesthetics gate a chloride current in postnatal rat hippocampal neurons. FASEB J. 1992;6:914–918. doi: 10.1096/fasebj.6.3.1740240. [DOI] [PubMed] [Google Scholar]
- 71.Zhu WJ, Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci. 1997;17:4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


