Abstract
Blubber, a specialized hyperdermic adipose tissue found in marine mammals, has been identified as a useful tissue for the assessment of steroid hormone homeostasis in cetaceans. However, blubber cortisol measurements are not quantitatively predictive of circulating cortisol concentrations in bottlenose dolphins. In other mammals, adipose tissue metabolizes steroid hormones. Thus, it is proposed that the disagreement between blubber and blood cortisol in bottlenose dolphins could be due in part to metabolism of corticosteroids in blubber. The purpose of this study is to characterize the ability of blubber to interconvert cortisol and cortisone using an in vitro design. Results demonstrate that bottlenose dolphin blubber microsomes interconvert cortisol and cortisone, an effect that is abated by denaturing the microsomes, indicating this is an enzymatic process. These findings lead to the conclusion that blubber is likely a site of active steroid metabolism, which should be considered in future studies utilizing blubber as a matrix for endocrine assessment.
Introduction
Blood is the traditional matrix used to assess endocrine status in bottlenose dolphins (Tursiops truncatus), but collection of blood from free-ranging cetaceans requires capture and handling, which induces the stress response and makes it very difficult to measure baseline stress physiology/endocrinology using blood (Thomson and Geraci 1986; Schroeder and Keller 1989; St. Aubin et al. 1996). Therefore, an alternative matrix that can be collected remotely would help provide better assessments of baseline physiology. Blubber is a form of subcutaneous adipose tissue found in marine mammals, and presents one possible alternative matrix because blubber biopsies can be collected remotely (Struntz et al. 2004). Although remote biopsy collection may be somewhat stress-inducing, due to need for close proximity for sampling, the stress should be minimal compared to blood collection. In cetaceans, blubber steroid hormones can qualitatively reflect systemic endocrine status, as physiological conditions that cause changes in circulating steroid hormone profiles produce similar changes in blubber steroid profiles (e.g., blubber cortisol values are elevated following exposure to stress stimuli) (Mansour et al. 2002; Kellar et al. 2006, 2009; Pérez et al. 2011; Trego et al. 2013; Kellar et al. 2015). However, blubber steroid measurements are purportedly poor quantitative predictors of circulating steroid hormone concentrations—blubber cortisol measurements only explained 57% of the variance in circulating cortisol concentrations in bottlenose dolphins—calling into question the utility of blubber for quantitative assessments of endocrine function (Champagne et al. 2017). There are two likely hypotheses as to why blood cortisol concentrations are not well-described by blubber cortisol concentrations. First, if blubber cortisol is entirely of central origin, then the rate of change in blubber cortisol concentration could potentially differ from rates of change in blood, owing to the need for cortisol to diffuse from blood into the blubber, leading to poor temporal matching. Second, blubber could be an endocrine-active tissue that actively metabolizes or synthesizes cortisol. This study investigates the latter hypothesis.
Adipose tissue in terrestrial mammals metabolizes steroids, ostensibly to control local hormone concentrations (Bleau et al. 1974; Folkerd and James 1983; Deslypere et al. 1985; Newton et al. 1986; Livingstone et al. 2000; Rask et al. 2001; Lindsay et al. 2003; MacKenzie et al. 2008; Tchernof et al. 2015; Li et al. 2015) (reviews: Labrie et al. 2000, 2003; Luu-The and Labrie 2010). The bidirectional metabolism of cortisol and cortisone is mediated by the enzyme 11β-hydroxysteroid dehydrogenase (11βHSD). The dehydrogenase reaction converts cortisol to cortisone, while the reductase reaction converts cortisone to cortisol. The dehydrogenase reaction has been observed and characterized in human and rat adipose tissues in vitro using either whole tissue homogenate or microsomes (fragments of the endoplasmic reticulum, to which 11βHSD is localized) (Náray-Fejes-Tóth and Fejes-Tóth 1996; Livingstone et al. 2000; Rask et al. 2001; Odermatt et al. 2001; Lindsay et al. 2003). However, these studies did not explore the reductase reaction, claiming that the dehydrogenase direction predominates in the in vitro environment, making the reductase reaction difficult to observe (Livingstone et al. 2000; Rask et al. 2001; Lindsay et al. 2003). Considering that blubber is adipose tissue and cortisone has been quantified in bottlenose dolphin blubber (Boggs et al. 2017), this study investigates whether bottlenose dolphin blubber has the ability to enzymatically interconvert cortisol and cortisone (i.e., dehydrogenase and reductase directions) in vitro. Identification of steroid hormone metabolism in blubber has the potential to aid in the interpretation of blubber hormone measurements in relation to circulating values and physiological state.
Methods
Sample collection and microsome preparation
Full-depth blubber samples were collected from three male stranded (code 1—stranded alive) bottlenose dolphins (one juvenile, one subadult, and one adult) from the southeastern United States, and were stored at −80 °C until analysis. Samples were collected under NOAA’s authority to collect samples under Section 109(h) of the Marine Mammal Protection Act. Blubber microsomes were prepared via methods adapted from Huderson et al. (2010). Skin was removed, and approximately 4 g of blubber were minced in glass beakers on dry ice using razors and forceps. Prior to mincing, all glassware and utensils were rinsed three times with acetone (purity ≥ 99.5%, GC Resolv™, Fisher Scientific, Waltham, MA, USA) followed by three rinses with hexane (purity ≥ 65%, GC Resolv™, Fisher Scientific, Waltham, MA, USA). Minced tissue was homogenized in 4–5 mL of sucrose-TKM buffer (sucrose 0.25 mol mL−1, Tris 80 mmol mL−1, KCl 25 mmol mL−1, MgCl2 5 mmol mL−1, pH 7.4) on ice with a Polytron homogenizer (VWR, Radnor, PA, USA). Homogenates were centrifuged at 10,000×gravity (gn) for 10 min at 4 °C, and the supernatant was transferred to a new 2 mL ultracentrifuge tube and centrifuged again at 15,000×gn for 15 min at 4 °C. The supernatant was transferred to a new 2 mL ultracentrifuge tube and centrifuged at 100,000×gn for 60 min at 4 °C. The microsomal pellet was washed three times with sucrose-TKM buffer before being reconstituted in 1 mL of the same buffer. Protein concentrations in the microsomal preparations were measured with a microplate-based Bradford assay, using a kit and following the manufacturer’s instructions (GeneCopoeia, Rockville, MD, USA).
Cortisol–cortisone metabolism assays
Cortisol–cortisone metabolism was assayed using methods adapted from Livingstone et al. (2000). Calibration and isotopically labeled internal standards were acquired from various manufacturers (Table 1). Calibration and internal standard (IS) mixture solutions were diluted in methanol (purity ≥ 99.9%, Optima™, Fisher Scientific, Waltham, MA, USA), with the concentration of each compound calculated gravimetrically (ng compound g−1 mixture). Cortisol or cortisone standards in methanol were gravimetrically added to clean borosilicate culture tubes, and solvent was evaporated to dryness under purified N2 (100–130 kPa) in a water bath at 40 °C. Krebs–Ringer buffer (NaCl 118 mmol mL−1, KCl 3.8 mmol mL−1, KH2PO4 1.19 mmol mL−1, CaCl2 2.54 mmol mL−1, MgSO4 1.19 mmol mL−1, and NaHCO3 25 mmol mL−1, pH 7.4), cofactor (NADP+ or NADPH, for dehydrogenase and reductase reactions, respectively; purity ≥ 97% [dry weight]; Sigma-Aldrich, St. Louis, MO, USA), and microsomes were added to the dried hormone, bringing the total reaction volume to 250 μL, where final substrate (cortisol or cortisone) concentration was approximately 1 μmol mL−1, cofactor concentration was 2 mmol mL−1, and protein concentration was 60 μg protein mL−1. Reaction mixtures were briefly vortexed before being incubated at 37 °C for 90 min.
Table 1.
Calibration and internal standard compound manufacturer, purity information, and monitored transitions
| Compound | Manufacturer | Stated purity (%) | Precursor ion (m/z) | Primary transition (m/z) | Secondary transition (m/z) |
|---|---|---|---|---|---|
| Cortisol | Sigma-Aldrich | ≥ 98 | 363.2 | 121.3 | 267.3 |
| Cortisone | Sigma-Aldrich | ≥ 98 | 361.1 | 163.3 | 121.3 |
| Cortisol-d4 | Cerilliant | 99.99 | 367.3 | 121.2 | 271.5 |
| Cortisone-13C3 | Sigma-Aldrich | 98 | 364.2 | 166.5 | 124.1 |
Negative controls included reaction mixtures containing only hormone, cofactor, and buffer (hereafter referred to as “No Protein Controls” or NPCs), and reaction mixtures containing only microsomes, cofactor, and buffer (hereafter referred to as “No Hormone Control” or NHC). An NHC was utilized for each individual sample. Mouse liver microsomes (male CD-1 mice; Sigma-Aldrich, St Louis, MO, USA) were utilized as a preliminary positive control; this control experiment was performed before proceeding with blubber microsomes to ensure that assay conditions were acceptable. All treatments, except mouse liver positive controls, were performed in at least triplicate.
Following the 90 min incubation, 150 μL of internal standard mixture (containing cortisol-d4 and cortisone-13C3 diluted in methanol) was gravimetrically amended to each reaction mixture, providing internal standards for the extraction process (Supplemental Table 1). An ethyl acetate liquid:liquid extraction was used to extract hormones. Ethyl acetate (2 mL) was added to each reaction tube. These mixtures were vortexed for 2 min, then allowed to rest undisturbed for 5 min to allow phase separation. The upper ethyl acetate layer was transferred to a clean borosilicate tube, and was evaporated to dryness under N2 (100–130 kPa) in a water bath at 40 °C. Dried extracts were reconstituted in 50:50 methanol:water (volume fraction), and transferred into amber autosampler vials containing glass inserts. Cortisol and cortisone were measured using an Agilent (Santa Clara, CA, USA) 1200 Series HPLC system with a binary pump and an autosampler linked to an AB Sciex (Framingham, MA, USA) API4000 QTRAP hybrid triple quadrupole/linear ion trap mass spectrometer. Corticosteroids were separated with an Agilent ZORBAX Eclipse Plus C18 column (21 mm × 150 mm, 5.0 μm particle size) and a gradient of methanol and deionized water (Millipore, Billerica, MA, USA) (both with 0.1% acetic acid) held at 46% methanol (volume fraction) for 10 min, increased to 82.5% methanol over 10 min, then increased to 83.3% methanol over 5 min. The column was then washed with 100% methanol for 5 min, and re-equilibrated to 46:54 methanol:water (volume fraction) for 10 min. Scheduled multiple reaction monitoring (sMRM) was used to quantify both endogenous compounds and internal standards. Two transitions were monitored per compound in all separations—the transition with the largest signal (hereafter designated the primary transition) was used for quantification, while the other (secondary transition) was used for qualitative identity confirmation (Table 1). Chromatographic peaks for target compounds and internal standard compounds were integrated using Sciex Analyst software (version 1.5; Framingham, MA). Target compound peak areas were divided by the peak area of the matched isotopically labeled IS. These area ratios were interpolated on regressions calculated from extracted calibration standards (Supplemental Table 2). Observed reporting limits (RLobs) are defined as the lowest calibration standard used in the calibration curve; calculated reporting limits (RLcalc) were calculated as three times the standard deviation of the mean plus the mean of the extracted blanks (Supplemental Table 2).
For both assays (dehydrogenase and reductase), we established a threshold above which a result would be classified as positive. For the dehydrogenase reaction, the NPCs contained quantifiable cortisone, therefore the threshold for this experiment was calculated as three times the standard deviation of the NPCs plus the mean of the NPCs. For the reductase reaction, neither negative controls (NPC or NHC) exhibited cortisol peaks, thus the presence of any cortisol signal indicated a positive result. If a positive result was detected, the experiment was repeated with microsomes that had been denatured by boiling for 20 min, and additional NHCs were performed with denatured microsomes to ensure that denaturing did not produce additional/different interferences compared to normal microsomes.
Statistics
Statistical analyses were performed with International Business Machines Corporation (IBM) SPSS Statistics 23 or 24 (IBM, North Castle, NY, USA). A mixed-effects model utilizing treatment (endogenous or denatured) as a fixed factor and individual as a random factor was used to determine whether denaturation of microsomes significantly reduced relative hormone signal compared to endogenous microsomes.
Results
Blubber 11βHSD dehydrogenase activity assay: metabolism of cortisol to cortisone
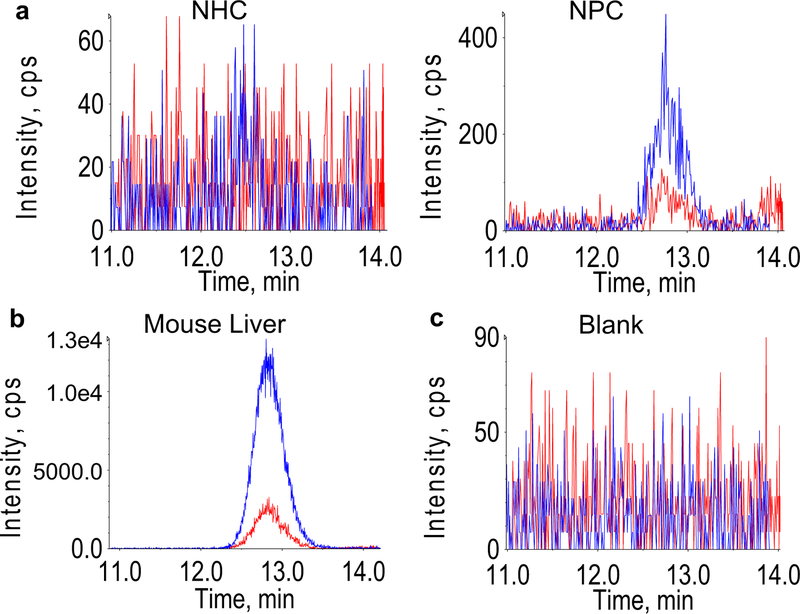
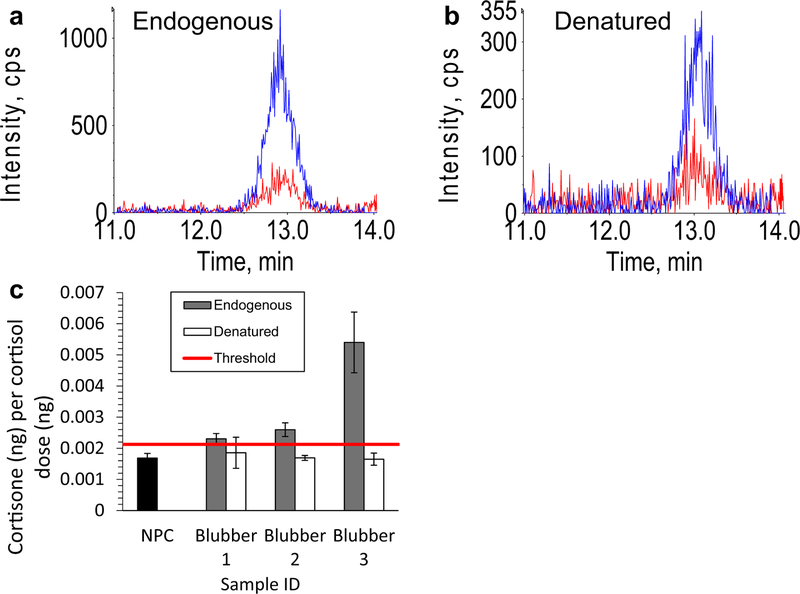
No cortisone was detected in any NHCs, but NPCs had detectable cortisone, which was caused by impurities in the neat cortisol standard that was used for dosing (Fig. 1a). Mouse liver microsomes treated with cortisol produced a cortisone signal several orders of magnitude greater than baseline cortisone contamination observed in NPCs, indicating assay conditions were acceptable (Fig. 1b). Blanks run after positive results exhibited a lack of cortisone, indicating that positive results are not a result of cortisone carry-over (Fig. 1c). Microsomal preparations from each blubber sample exhibited cortisone signals greater than the threshold (three times the standard deviation of NPCs plus mean of NPCs), while denatured microsomes failed to exceed the threshold (with the exception of one replicate from one sample); mean relative cortisone signal is significantly reduced in denatured (0.001731 ± 0.000657 ng cortisone ng−1 cortisol dosed) compared to endogenous microsomes (0.00356 ± 0.000629 ng cortisone ng−1 cortisol dosed) as indicated by mixed-effects model (P = 0.00017; Fig. 2).
Fig. 1.
Dehydrogenase reaction controls. Representative chromatograms of no hormone control (NHC) and no protein control (NPC) (a), mouse liver microsomes (b), and blank run immediately after a positive sample indicating lack of carry-over (c) where blue and red chromatograms are the primary and secondary transitions of cortisone, and intensity (y axes) units are counts per second (CPS)
Fig. 2.
Blubber dehydrogenase reaction. Representative cortisone chromatograms for endogenous (non-denatured) (a) and denatured microsomes (b) where blue and red chromatograms are the primary and secondary transitions of cortisone, and intensity (y axes) units are counts per second (CPS); mean measured cortisone mass relative to the dose of cortisol in endogenous and denatured blubber microsomes (c). Horizontal red line indicates threshold for positive result and error bars are standard deviations
Blubber 11βHSD reductase activity assay: metabolism of cortisone to cortisol
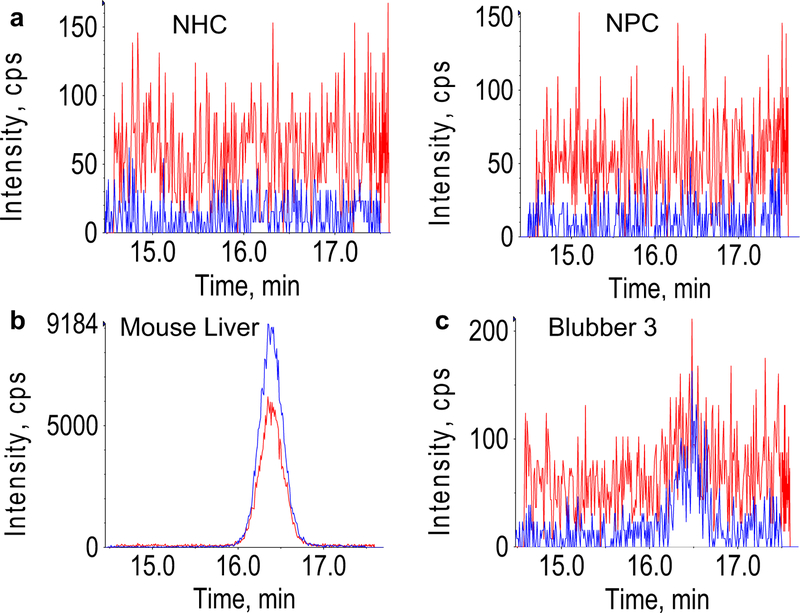
Cortisol was not detected in NHCs or NPCs in the reductase experiment (Fig. 3a). Cortisol was detected above baseline (three times the standard deviation of NPCs plus mean of NPCs) both in mouse liver microsomes (Fig. 3b) and one blubber sample (mean: 1.365 × 10−4 ± 1.034 × 10−4 ng cortisol ng−1 cortisone dosed) (Fig. 3c). No cortisol was detected in denatured microsomes from this sample.
Fig. 3.
Blubber reductase reaction. Representative chromatograms for no hormone control (NHC) and no protein control (NPC) (a), mouse liver microsomes (b), and “Blubber 3” (c), where blue and red chromatograms are the primary and secondary transitions of cortisone, and intensity (y axes) units are counts per second (CPS)
Discussion
Blubber microsomes exhibited the ability to metabolize cortisol to cortisone, and potentially cortisone to cortisol, ostensibly through the activity of 11βHSD. The experimental design for this study was based on that used in Livingstone et al. (2000), in which the ability of rat adipose to metabolize corticosteroids was demonstrated. However, whereas Livingstone et al. used whole adipose tissue homogenate in their study, we used blubber microsomes, which provides an important improvement over the use of whole tissue homogenate. Blubber contains endogenous cortisol and cortisone, which would make it very difficult to interpret results from this experiment if whole blubber homogenate was used (Boggs et al. 2017). Conversely, blubber microsomal isolates do not contain endogenous cortisol or cortisone, as demonstrated by the lack of hormone in the NHCs, which simplifies interpretation compared to whole blubber homogenate.
Whereas NHCs did not contain cortisol or cortisone, the NPCs for the dehydrogenase experiment did contain cortisone. Thus, in the dehydrogenase (cortisol to cortisone) direction, the threshold for a positive result was based on the baseline signal of the NPCs (three times the standard deviation of the NPCs plus the mean of the NPCs). This indicates that there is cortisone in the cortisol standard used to dose the microsomes. Thus, the amount of cortisone present in any given replicate will be partially dependent upon the quantity of cortisol standard used; while each replicate should have received the same dose, slight variation could potentially impact the results. Therefore, cortisone values are reported relative to the mass of cortisol dosed. Furthermore, we ensured that there was no cortisone carry-over between samples in the chromatography, which would produce false positives, by confirming that no cortisone was evident in blanks run immediately after positive samples.
The positive control (mouse liver microsomes) exhibited production of cortisone and cortisol in the dehydrogenase and reductase reactions, respectively. Notably, we did not expect to observe a reductase reaction (cortisone to cortisol), because previous studies state (but do not demonstrate) that the reductase reaction is not observable in vitro (Livingstone et al. 2000; Rask et al. 2001; Lindsay et al. 2003). We do not report the quantity of cortisone or cortisol produced by mouse liver microsomes, because this experiment was a qualitative positive control to verify suitability of assay conditions before proceeding with blubber microsome experiments.
In the blubber dehydrogenase reaction experiment, the relative cortisone value measured in treated blubber microsomes exceeded the threshold for a positive result. Thus, the amount of cortisone measured cannot be attributed to impurities in the standard or other external contamination, and we conclude that T. truncatus blubber microsomes metabolize cortisol to cortisone in vitro. This is further supported by the demonstration that denaturing these microsomal preparations by heating significantly reduced mean relative cortisone signal compared to endogenous microsomes. While all three blubber samples produced cortisone, one sample (the subadult) exhibited greater production than the other two. Microsome (i.e., protein) yield did vary by sample, but final protein concentration in the reaction mixtures were standardized (60 μg protein mL−1 per reaction); thus, variation in yield should not influence the results. Rather, this could be driven by differences in 11βHSD expression between the samples, though protein abundance or gene expression data would be needed to support this hypothesis. Furthermore, without knowing the physiological factors that influence 11βHSD expression and activity in bottlenose dolphin blubber, it is impossible to establish any sort of causal mechanism for this apparent variation. Future studies should repeat this experiment with a larger sample size and include measures of 11βHSD gene expression and protein abundance in the analysis.
In the blubber 11βHSD reductase experiment, only one blubber sample (“Blubber 3”) exhibited detectable cortisol in all three of its replicates, while the other two did not have detectable cortisol. Furthermore, denatured Blubber 3 microsomal preparations did not have quantifiable cortisol, suggesting that the cortisol detected in the endogenous microsomes was produced enzymatically. Notably, this is the sample that displayed a higher signal in the dehydrogenase direction, which further supports the hypothesis that 11βHSD expression is higher in this individual. With a positive result in only one of three individuals, we cautiously conclude that blubber microsomes can potentially metabolize cortisone to cortisol in vitro. Future studies should repeat this experiment with expanded sample size.
These conclusions provide preliminary evidence that blubber possesses the ability to metabolize steroid hormones, in keeping with what has previously been observed in the adipose tissue of other mammals. With only three bottlenose dolphin samples, all of which came from stranded (i.e., stressed) animals, the generalization of these results to other physiological states or species requires more investigation. However, the overall hypothesis is dichotomous, and power analysis indicates that two samples provide sufficient power (α = 0.05, β = 0.8) to determine whether blubber does or does not metabolize cortisol or cortisone. It should also be noted that these experiments were performed with isolated microsomes. While the use of microsomes simplifies study of enzymatic activity in blubber, repetition of this study with blubber explants ex vivo would be more conclusive in indicating whether corticosteroid metabolism occurs in living blubber. Nevertheless, the results presented here are a compelling first step and should prompt broader investigation of blubber as a site of steroid hormone metabolism.
Ultimately, the identification of blubber as a steroidmetabolizing tissue could complicate the interpretation of blubber steroid hormone measurements. That is, it may prove difficult to differentiate between hormones of central origin and those produced in the blubber, potentially limiting the utility of blubber as matrix for characterizing central endocrine function. Blubber cortisol measurements have been used to examine the effects of anthropogenic stressors, such as contaminant exposure and gillnet fishery bycatch, on the adrenal stress response in cetaceans (Kellar et al. 2015; Galligan et al. in preparation), but providing a quantitative diagnosis of adrenal stress response using blubber cortisol measurements may be challenging if long-term storage of cortisol in the blubber results in conversion to cortisone. Considering this potential conversion, measuring cortisol alone may underestimate the magnitude of stress; perhaps measuring cortisol and cortisone in tandem could provide a more complete assessment. The findings presented herein suggest that investigators using blubber to study stress should be cautious in directly linking blubber cortisol measurements to central adrenal function until the metabolic activities of blubber are better understood. Overall, this study serves as the first evidence of steroid metabolism by blubber, which should be further investigated to fully understand the mechanisms and implications of these findings.
Supplementary Material
Acknowledgements
This research was made possible through a grant from The Gulf of Mexico Research Initiative and funding provided by the National Institute of Standards and Technology. Data are publicly available through the Gulf of Mexico Research Initiative Information & Data Cooperative (GRIIDC) at https://data.gulfresearchinitiative.org (https://doi.org/10.7266/n74b2zsr). We would like to thank Michael Janech, the Medical University of South Carolina (MUSC) Nephrology Proteomics Laboratory, and Sang-Ho Kwon for assistance in experimental design and/or the use of laboratory space and equipment for microsome isolations. We thank the South Carolina Marine Mammal Stranding Network for their efforts in sample collection. Finally, we thank the reviewers for their time and effort dedicated to reviewing this manuscript.
Footnotes
Ethical approval Samples were collected under NOAA’s authority to collect samples under Section 109(h) of the MMPA. All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. Commercial equipment, instruments, or materials are identified to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology nor the National Oceanographic and Atmospheric Administration, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00227-018-3373-4) contains supplementary material, which is available to authorized users.
Conflict of interest The authors declare that they have no conflict of interest in the publication of this manuscript.
References
- Bleau G, Roberts KD, Chapdelaine A (1974) The in vitro and in vivo uptake and metabolism of steroids in human adipose tissue. J Clin Endocrinol Metabol 39:236–246 [DOI] [PubMed] [Google Scholar]
- Boggs ASP, Schock TB, Schwacke LH, Galligan TM, Morey JS, McFee WE, Kucklick JR (2017) Rapid and reliable steroid hormone profiling in Tursiops truncatus blubber using liquid chromatography tandem mass spectrometry (LC-MS/MS). Anal bioanal 409(21):5019–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne CD, Kellar NM, Crocker DE, Wasser SK, Booth RK, Trego ML, Houser DS (2017) Blubber cortisol qualitatively reflects circulating cortisol concentrations in bottlenose dolphins. Mar Mamm Sci 33:134–153 [Google Scholar]
- Deslypere J, Verdonck L, Vermeulen A (1985) Fat tissue: a steroid reservoir and site of steroid metabolism. J Clin Endocrinol Metabol 61:564–570 [DOI] [PubMed] [Google Scholar]
- Folkerd E, James V (1983) Aromatization of steroids in peripheral tissues. J Steroid Biochem 19:687–690 [DOI] [PubMed] [Google Scholar]
- Huderson AC, Harris DL, Niaz MS, Ramesh A (2010) Effect of benzo(a)pyrene exposure on fluoranthene metabolism by mouse adipose tissue microsomes. Toxicol Mech Methods 20:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellar NM, Trego ML, Marks CI, Dizon AE (2006) Determining pregnancy from blubber in three species of delphinids. Mar Mamm Sci 22:1–16 [Google Scholar]
- Kellar NM, Trego ML, Marks CI, Chivers SJ, Danil K, Archer FI (2009) Blubber testosterone: a potential marker of male reproductive status in short-beaked common dolphins. Mar Mamm Sci 25:507–522 [Google Scholar]
- Kellar NM, Catelani KN, Robbins MN, Trego ML, Allen CD, Danil K, Chivers SJ (2015) Blubber cortisol: a potential tool for assessing stress response in free-ranging dolphins without effects due to sampling. PLoS One 10:e0115257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Lin S-X, Simard J, Labrie C (2000) Role of 17β-hydroxysteroid dehydrogenases in sex steroid formation in peripheral intracrine tissues. Trends Endocrinol Metab 11:421–427 [DOI] [PubMed] [Google Scholar]
- Labrie F, Luu-The V, Labrie C, Bélanger A, Simard J, Lin S-X, Pelletier G (2003) Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev 24:152–182 [DOI] [PubMed] [Google Scholar]
- Li J, Papadopoulos V, Vihma V (2015) Steroid biosynthesis in adipose tissue. Steroids 103:89–104 [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Wake DJ, Nair S, Bunt J, Livingstone DEW, Permana PA, Tataranni PA, Walker BR (2003) Subcutaneous adipose 11β-hydroxysteroid dehydrogenase type 1 activity and messenger ribonucleic acid levels are associated with adiposity and insulinemia in Pima Indians and Caucasians. J Clin Endocrinol Metabol 88:2738–2744 [DOI] [PubMed] [Google Scholar]
- Livingstone DEW, Jones GC, Smith K, Jamieson PM, Andrew R, Kenyon CJ, Walker BR (2000) Understanding the role of glucocorticoids in obesity: tissue-specific alterations of corticosterone metabolism in obese zucker rats. Endocrinology 141:560–563 [DOI] [PubMed] [Google Scholar]
- Luu-The V, Labrie F (2010) Chapter 10—the intracrine sex steroid biosynthesis pathways. In: Luciano M (ed) Progress in brain research Elsevier, New York: [DOI] [PubMed] [Google Scholar]
- Mackenzie SM, Huda SS, Sattar N, Fraser R, Connell JM, Davies E (2008) Depot-specific steroidogenic gene transcription in human adipose tissue. Clin Endocrinol (Oxf) 69:848–854 [DOI] [PubMed] [Google Scholar]
- Mansour AA, Mkay DW, Lien J, Orr JC, Banoub JH, Øien N, Stenson G (2002) Determination of pregnancy status from blubber samples in minke whales (Balaenoptera acutorostrata). Mar Mamm Sci 18:112–120 [Google Scholar]
- Náray-Fejes-tóth A, Fejes-Tóth G (1996) Subcellular localization of the type 2 11β-hydroxysteroid dehydrogenase: a green fluorescent protein studY. J Biol Chem 271:15436–15442 [DOI] [PubMed] [Google Scholar]
- Newton CJ, Samuel DL, James VHT (1986) Aromatase activity and concentrations of cortisol, progesterone and testosterone in breast and abdominal adipose tissue. J Steroid Biochem 24:1033–1039 [DOI] [PubMed] [Google Scholar]
- Odermatt A, Arnold P, Frey FJ (2001) The intracellular localization of the mineralocorticoid receptor is regulated by 11β-hydroxysteroid dehydrogenase type 2. J Biol Chem 276:28484–28492 [DOI] [PubMed] [Google Scholar]
- Pérez S, García-López Á, de Stephanis R, Giménez J, García-Tiscar S, Verborgh P, Mancera J, Martínez-Rodriguez G (2011) Use of blubber levels of progesterone to determine pregnancy in free-ranging live cetaceans. Mar Biol 158:1677–1680 [Google Scholar]
- Rask E, Olsson T, Soderberg S, Andrew R, Livingstone DEW, Johnson O, Walker BR (2001) Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metabol 86:1418–1421 [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Keller KV (1989) Seasonality of serum testosterone levels and sperm density in Tursiops truncatus. J Exp Zool 249:316–321 [DOI] [PubMed] [Google Scholar]
- Aubin DJ, Ridgway SH, Wells RS, Rhinehart H (1996) Dolphin thyroid and adrenal hormones: circulating levels in wild and semidomesticated Tursiops truncatus, and influence of sex, age, and season. Mar Mamm Sci 12:1–13 [Google Scholar]
- Struntz D, McLellan W, Dillaman R, Blum J, Kucklick J, Pabst D (2004) Blubber development in bottlenose dolphins (Tursiops truncatus). J Morphol 259:7–20 [DOI] [PubMed] [Google Scholar]
- Tchernof A, Mansour MF, Pelletier M, Boulet M-M, Nadeau M, Luu-The V (2015) Updated survey of the steroid-converting enzymes in human adipose tissues. J Steroid Biochem Mol Biol 147:56–69 [DOI] [PubMed] [Google Scholar]
- Thomson C, Geraci J (1986) Cortisol, aldosterone, and leucocytes in the stress response of bottlenose dolphins, Tursiops truncatus. Can J Fish Aquat Sci 43:1010–1016 [Google Scholar]
- Trego ML, Kellar NM, Danil K (2013) Validation of blubber progesterone concentrations for pregnancy determination in three dolphin species and a porpoise. PLoS One 8:e69709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.