Abstract
One of the key components for environmental risk assessment of engineered nanomaterials (ENMs) is data on bioaccumulation potential. Accurately measuring bioaccumulation can be critical for regulatory decision making regarding material hazard and risk, and for understanding the mechanism of toxicity. This perspective provides expert guidance for performing ENM bioaccumulation measurements across a broad range of test organisms and species. To accomplish this aim, we critically evaluated ENM bioaccumulation within three categories of organisms: single-celled species, multicellular species excluding plants, and multicellular plants. For aqueous exposures of suspended single-celled and small multicellular species, it is critical to perform a robust procedure to separate suspended ENMs and small organisms to avoid overestimating bioaccumulation. For many multicellular organisms, it is essential to differentiate between the ENMs adsorbed to external surfaces or in the digestive tract and the amount absorbed across epithelial tissues. For multicellular plants, key considerations include how exposure route and the role of the rhizosphere may affect the quantitative measurement of uptake, and that the efficiency of washing procedures to remove loosely attached ENMs to the roots is not well understood. Within each organism category, case studies are provided to illustrate key methodological considerations for conducting robust bioaccumulation experiments for different species within each major group. The full scope of ENM bioaccumulation measurements and interpretations are discussed including conducting the organism exposure, separating organisms from the ENMs in the test media after exposure, analytical methods to quantify ENMs in the tissues or cells, and modeling the ENM bioaccumulation results. One key finding to improve bioaccumulation measurements was the critical need for further analytical method development to identify and quantify ENMs in complex matrices. Overall, the discussion, suggestions, and case studies described herein will help improve the robustness of ENM bioaccumulation studies.
Graphical Abstract
Strategies, discussion, and case studies are provided for making robust and accurate measurements of engineered nanomaterial bioaccumulation by single-cell organisms, multicellular organisms, and plants.
Introduction
There is a broad range of potential applications of engineered nanomaterials (ENMs), materials with at least one dimension between 1 nm and 100 nm,1,2 stemming from their novel or enhanced properties as compared to equivalent materials of larger sizes or conventional chemical form. Thus, it is anticipated that ENMs will be increasingly used in consumer products and for commercial applications in the future.3,5 To responsibly develop ENM-enabled products, it is critical to develop a comprehensive understanding of the potential environmental and human health risks that ENMs may pose during a product’s life cycle (i.e., manufacturing, usage, and disposal).6,9
Regulatory decision making on potential environmental risks focus on the extent to which substances such as ENMs exhibit persistent, bioaccumulative, and toxic (PBT) behaviors. This highlights the importance of understanding the capacity for ENMs to bioaccumulate in organisms and subsequently transfer through and biomagnify within food chains. In addition, fundamentally understanding the target organs and absorption, distribution, metabolism and excretion (ADME) processes that together determine bioaccumulation extent and dynamics are important to identifying the hazards of ENMs to whole organisms, as well as to specific target organs, systems (e.g., digestive system), or organelles.
As for conventional chemicals, it is recognized that an understanding of the toxicokinetics of ENM uptake is important for determining their behavior and risk. There is a broad range of studies in the nanotoxicological literature evaluating the bioaccumulation and biomagnification of various ENMs including carbon nanotubes (CNTs),10, 11 fullerenes,12, 13 graphene family nanomaterials (GFNs),14, 15 Au ENMs,16–18 Ag ENMs,19, 20 CuO ENMs21 and cadmium selenide quantum dots.22, 23 Results from these studies have often shown that ENMs behave differently from conventional bioaccumulative substances such as hydrophobic organic chemicals. For example, ingested ENMs may accumulate on or in gut tissues of organisms and are often not readily absorbed across epithelial surfaces for systemic circulation.11, 15, 24 Further, ENMs are likely absorbed by vesicular transport across cell membranes, rather than passive diffusion or facilitated uptake on solute transporters. Thus, the typical assumption for organic chemicals and metals of rapid absorption across the tissues and distribution into specific tissues or organelles (e.g., lipids for hydrophobic organic substances; inorganic biominerals for some metals) may not generally be applicable for ENMs. While it is possible for terrestrial wildlife to be exposed through inhalation, there have not been studies on this topic to our knowledge relating to environmental exposure, except for the extensive literature in which rodents are exposed through inhalation to assess potential worker safety or consumer health risks.24–27 Therefore, this paper will mainly focus on ENM exposure in soil, sediments, or water. Further complicating our understanding of ENM bioaccumulation is the dynamic nature of ENM fate, with some ENMs releasing dissolved constituents21, 28, 29 and with some biota capable of reducing dissolved elements to an ENM form.
While a large number of ENM bioaccumulation studies have been conducted, differences in the experimental methods used such as quantification method, exposure time, ENM physicochemical characteristics and associated transformation during exposure, and ENM dispersion methods, make comparisons difficult, even when the same taxa and same type of ENM were tested. In addition, the terminology used among studies to describe bioaccumulation-related results is neither consistent nor standardized, which can lead to confusion when comparing the results of different studies. There may also be artifacts or biases when quantifying concentrations in organisms such as different gut voidance approaches or methods to remove gut contents from consideration, incomplete separation of the test species from suspended ENMs, and variations in methods for the removal of loosely attached ENMs from the outer surface by washing. Therefore, the value of many studies is to demonstrate the potential for bioaccumulation or biomagnification based on individual study conditions; extrapolating to real-world conditions outside of the laboratory depends on environmental measurements that can confirm that such potentials manifest in field conditions.
In this perspective, the overall aim is to assess the current literature on ENM bioaccumulation methods and describe best practices for making measurements to support comparability across ENM bioaccumulation studies. To accomplish this aim, we propose bioaccumulation terminology, describe relevant analytical methods, and offer guidance for conducting bioaccumulation studies for a number of different groups of test organisms. In addition, we describe key considerations for associated measurements, such as approaches to differentiate between ENMs remaining in the gut tracts of organisms and those absorbed by multicellular organisms after oral exposure. When available, we also describe strategies using the unique physiologies and behaviors of the organisms to provide additional insights into ENM bioaccumulation quantification.
Bioaccumulation terminology, metrics, and considerations for ENM bioaccumulation test design
There are several issues to be considered in the vocabulary and quantification of ENM bioaccumulation. First, terminology from studying the bioaccumulation of other chemicals should be scrutinized for applicability, as common terms relating to physicochemical characteristics and transport processes differ for ENMs. Second, testing guidelines30–32 may recommend modeling approaches and bioaccumulation metrics without stating modeling assumptions. Before use, models should be evaluated to identify assumptions and their validity for ENMs. Issues related to ENM bioaccumulation measurements and metrics have been addressed before in the context of a specific type of ENMs10 and a specific organism33 but are discussed more generally here covering all types of ENMs and several organism groups.
A non-exhaustive list of common terms used in the general subject of bioavailability and bioaccumulation is provided, and critically adapted for application to ENMs (Box 1). There are many other terms that are potentially of interest but not listed herein, including “bioaccessibility” and “bioactivity” which have been used in discussing ENMs in soils although they can also be applied to all environmental organisms and humans.34 In our listing of terms, we do not aim to be exhaustive, but rather to make suggestions based on synthesis across relevant sources, when and how common terms can apply to ENM bioaccumulation considerations.
Box 1. Definitions of key terms used in the current review.30,35,329.
(The term “ENM” includes ENMs and its transformation products.)
Assimilation efficiency – a measure of the proportion of ingested ENMs assimilated into (initially) the alimentary epithelium of the feeding animal; the amount absorbed per amount ingested from the diet.
Bioaccumulation – the process and phenomenon of ENM accumulation in or on an organism, regardless of exposure regime (i.e. whether ingesting or otherwise taking up ENMs via water, food, sediment, soil, or air).
Bioaccumulation factor (BAF) – (1) the ratio of the ENM concentration associated with the organism exposed through all possible routes (CB, g ENM/kg dry mass) and the concentration in the exposure medium (air, water, soil or sediment) or food (Cs, g ENM/kg wet mass or volume), or (2) the ratio between the uptake rate coefficient (k1) and elimination rate coefficient (k2), termed “kinetic BAF” or BAFk. Note that steady state is not assumed here, unlike in conventional BAF definitions, because steady state is likely not reached in ENM exposures, particularly in field studies.
Bioavailability – the ability of ENMs to interact with organism biosystems.
Bioconcentration – the process and phenomenon of ENM accumulation in an organism from the ambient environment via uptake through all routes excluding diet.330
Bioconcentration factor (BCF) – for aqueous ENM exposures in the absence of food, (1) the ratio of the ENM concentration associated with the exposed organism (CB, g ENM/kg dry mass) and the concentration in water or (2) the ratio between the uptake rate coefficient (k1) and elimination rate coefficient (k2), termed “kinetic BCF” or BCFk.
Biomagnification – the increase in whole-body ENM concentration from one trophic level to the next resulting from ENM accumulation in food.
Biomagnification factor (BMF) – the ratio of ENM concentration in an organism (trophic level n, CB, g ENM/kg dry mass) to that of the diet (trophic level n-1, CD, g ENM/kg dry mass), using organisms of known or assumed trophic status.
Biodistribution – ENM distribution within an organism.331,332
Body burden – the ENM concentration in, or on, an organism at a given time.
Elimination rate coefficient (k2) – the numerical value defining the rate of decrease in the ENM concentration in the test organism, or specified tissues thereof, following the test organism transfer from a medium containing the ENM to an ENM-free medium.
Elimination – the combined process of metabolism, excretion, and degradation which results in ENM removal from an organism.
Growth dilution – the decrease in ENM concentration in a growing organism because the amount of tissue in which the ENM is distributed is increasing at a faster rate than the increase in ENM amount in the organism.
Gut voidance – ENM loss from the gut lumen when an organism is removed from ENM-contaminated media and placed into clean media free of ENMs or is fed an ENM-free diet.
Toxicokinetics – the study of organismal rates of ENM uptake, transfer between biological compartments, biotransformation and elimination.
Trophic level – a conceptual level in a food web such as primary producer, primary consumer or secondary consumer, recognizing that omnivorous organisms do not have discrete trophic levels.
Uptake – that part of the bioaccumulation or bioconcentration process(es) involving ENM movement from the external environment into an organism, either through direct exposure to an ENM-contaminated medium or by consumption of food (including prey) containing the ENM. This can be defined as an uptake rate (e.g., mass of ENM per day), an uptake rate coefficient or, particularly for plants, as the total uptake over the course of an exposure.
Uptake rate coefficient (k1) – the numerical value defining the rate of increase in ENM concentration in or on the organisms, or specified tissues thereof, when the organisms are exposed to ENMs.
In general, bioaccumulation is defined as the accumulation of a chemical in, or on, an organism from all sources including water, air, soil, sediment and food (Box 1).35 Bioconcentration (i.e., chemical accumulation in an organism from water only) is a process that contributes to chemical bioaccumulation but can only be measured using controlled laboratory conditions.36 The concept of “bioconcentration” is based on lipid-water partitioning properties of hydrophobic organic chemicals. The applicability of equilibrium partitioning theory has been rejected for ENMs for multiple reasons.37, 38 For ENMs, organismal uptake routes and biotransformation are either unknown or occur via multiple pathways. As such, the use of the term “bioconcentration” for ENMs would be recommended only in limited occasions where, in well-controlled laboratory conditions, organisms are exposed to ENMs in the test medium without added food and active uptake of ENMs by ingestion does not occur. The term “bioaccumulation” is preferred, as it captures all potential ENM associations with organisms, including sorption to external surfaces and uptake via ingestion. As will be discussed in additional detail below, differentiating between internalized ENMs and those adsorbed to external surfaces is analytically challenging. Sorption to organisms as a specific ENM bioaccumulation mode is included since membrane-adsorbed ENMs have been shown to exert toxicity via released metal ions.39
The calculation of a bioaccumulation parameter, such as either the bioaccumulation factor (BAF), bioconcentration factor (BCF) or the biomagnification factor (BMF), is useful for expressing the bioaccumulative potential of ENMs for the purposes of hazard assessment. Considering the possible ENM exposure routes and association modes with cells, tissues, and organisms described above, we recommend using two approaches for deriving bioaccumulation parameters in ENM studies: biodynamic models for representing ENM bioaccumulation in laboratory studies (“kinetic BAF” or BAFk) and the ratio of tissue or organism-associated ENM concentration to the concentration of ENM in the surrounding media (BAF) in laboratory, mesocosm, or field studies. Note that BAF is ideally measured under steady state conditions when ENM uptake and elimination rates are constant and steady state can be achieved within the lifetime of an organism.40 However, we are intentionally not constraining the definition to steady state conditions here, as such conditions may be observable under laboratory conditions but may not occur in environmental systems that are open and inherently dynamic. In contrast, in depositional sediment systems, steady-state conditions may occur.
In designing and interpreting bioaccumulation tests, both ENM and test organism characteristics need to be considered (Figure 1). For instance, different test organism sizes and ventilation rates, exposure duration (hours to months), exposure type (flow-through, static, or semi-static), feeding regimes, and elimination periods are several of the many variables that influence the outcome and interpretation of ENM bioaccumulation tests. Additionally, ENM physico-chemical factors and environmental variables affecting ENM fate determine the potential for ENM exposure, uptake and bioaccumulation in biota, as well as biotransformation in the environment and organisms,41 and thus should be considered when designing and interpreting bioaccumulation tests (Figure 1).
Figure 1.
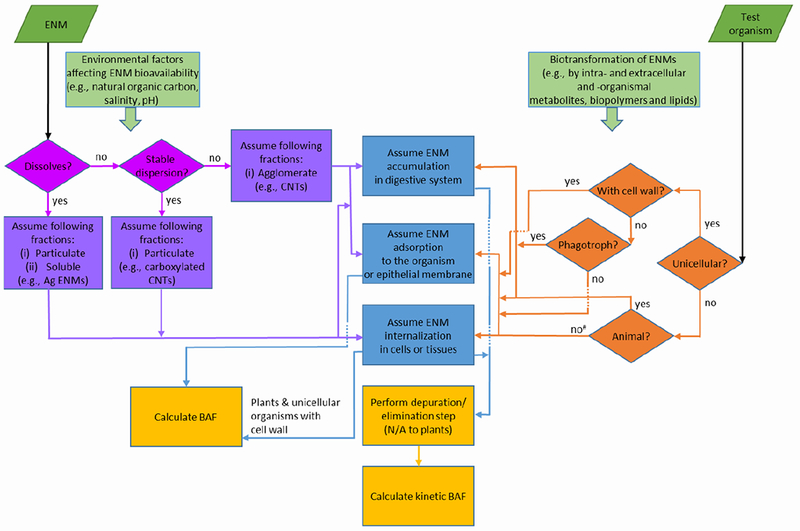
Scheme of decision steps, processes and factors important to consider in designing engineered nanomaterial (ENM) bioaccumulation tests and calculating bioaccumulation factors. The scheme depicts how the physicochemical properties of ENMs (purple boxes and violet diamonds) and the physiology of the test organism (orange diamonds) influence ENM internalization or adsorption to organisms or cell membranes (blue boxes) and the consequent steps for calculation of single metrics of ENM bioaccumulation (yellow boxes).
ENM interactions with cells and organisms (blue boxes) have been grouped based on the potential of ENMs to adsorb or become internalized into cells or tissues. Accumulation into the digestive system has been presented as a special case because ingestion is a significant uptake pathway of ENMs for certain types of organisms (e.g., filter feeders, phagotrophs, and fish). Whether or not ENMs are assimilated into the tissues or cells, or merely adsorbed on the epithelial membrane of the digestive system depends on the ENM physico-chemical properties and biotransformations in the digestive system. Regardless of their fate in the digestive system, ingested ENMs contribute to the total body burden of ENMs that can be transferred to subsequent trophic levels, and should be taken into account in bioaccumulation measurements. Based on the potential of ENMs to either dissolve or form stable aqueous dispersions (purple diamonds), ENMs can be divided into (1) water-soluble ENMs, such as ZnO, Cu, CuO, and Ag, with particulate and dissolved fractions interacting with organisms, (2) insoluble ENMs, such as carbon nanotubes (CNTs), graphene, boron nitride nanotubes or flakes, and TiO2, which are not water-dispersible and tend to agglomerate in environmental matrices and thus are less likely to be internalized into cells and tissues but may be adsorbed to organisms or cell membranes, and (3) insoluble ENMs that form stable aqueous dispersions, such as functionalized carbon or boron nitride nanotubes, graphene oxide, and TiO2 with hydrophilic coatings, and may interact in nanoparticulate forms (violet boxes) with organisms. In addition to intrinsic ENM properties, environmental factors affecting ENM bioavailability and ENM biotransformations need to be considered in the test design (light green boxes). Conversely, the ENM interaction with organisms depends on the structure and physiology of the latter (orange diamonds). For example, ENMs can accumulate in multicellular animals by entering the digestive system, adsorption to the organism, and internalization in the tissues (blue boxes). The pathway of ENM accumulation in the digestive system is excluded for multicellular plants (non-unicellular organisms which are not animals), unicellular organisms with cell walls (bacteria, fungi and green algae) and non-phagotrophic unicellular organisms without cell walls (some protists and mixotrophic algae). If no internalization of ENMs in organisms is assumed (e.g., in the case of insoluble poorly dispersed ENMs interacting with bacteria) or in case of plants and unicellular organisms with cell wall, an elimination step may not be necessary before quantifying bioaccumulated ENMs (yellow boxes). In this case, a bioaccumulation factor (BAF) can be calculated. If accumulation in the digestive system or internalization of ENMs is assumed, it is advisable to perform an elimination step for calculating a kinetic BAF.
Organism exposure and ENM transformations in different media
The form of a given ENM, which can change in different environmental media and over time, is critical to understanding its potential bioaccumulation by organisms (Figure 1). The transformations that ENMs undergo in different environment media have been thoroughly described.42–51 As a summary of the field, Lowry et al.45 discussed four broad types of transformations including chemical, physical, biological and macromolecular interactions. From the perspective of transformations having the greatest impact on bioaccumulation, the three main processes affecting the transformations ENMs experience during exposure are agglomeration, dissolution, and chemical transformation (e.g., oxidation or reduction). While homoagglomeration and heteroagglomeration affect most ENMs in environmental media, dissolution is primarily relevant for ENMs composed of metals (e.g., quantum dots,52 CuO ENMs,21, 53, 54 and Ag ENMs19, 55, 56). The impact of these processes on bioaccumulation remains unclear but in general larger contaminants or agglomerated ENMs are considered less bioavailable than individual contaminant molecules/ions or individual ENMs.57 Furthermore, agglomeration generally leads to gravitational settling of particles,44 increasing their interactions with sedimentary and soil surfaces and associated organisms while reducing their bioavailability to pelagic organisms.58–61 Disagglomeration may also occur in the environmental matrix or in the gut environment after intake, although these mechanisms are poorly understood.62 Dissolution also complicates our understanding of ENM bioaccumulation. For example, for metal ENMs, if bioaccumulation is observed by an organism, it is often unclear if the metal accumulated was delivered in the form of ENM or ionic metal.
Like most particles in environmental media, ENMs are likely to agglomerate, especially at higher ENM or background particle concentrations and under saline conditions, leading to sedimentation of ENMs from aqueous solution to the benthos. At higher concentrations, ENMs are more apt to collide and agglomerate, while high saline (i.e., ionic strength) conditions reduce the electrophoretic mobility of ENMs and also promote agglomeration.46, 63 Other variables influencing agglomeration include the ENMs’ surface charge, shape and size along with the pH and temperature of the aqueous media. For metal ENMs, coatings such as citrate and polyvinylpyrrolidone (PVP) are used to stabilize ENMs against agglomeration; for carbon, boron nitride and other hydrophobic ENMs, surfactants, synthetic polymers, and natural organic matter have been used as dispersing agents.64 However, the environmental stability of these coatings may vary as they can be lost due to environmental degradation (e.g., microbial or photodegradation) or replaced by other natural organic ligands.65–67 When ENMs undergo agglomeration, the exposed surface area of the particles declines, potentially resulting in decreased ENM-cell contact and thus bioavailability. Agglomeration can also reduce the dissolution rate for ENMs that have dissolvable components.
Many metal ENMs will undergo some degree of dissolution that involves the release of ionic forms of the metal into the aqueous phase.52–54 The degree of dissolution is driven by the type of ENM including the elemental composition and the ENM size, shape, and surface coating as well as the media characteristics. For example, media pH, temperature, natural organic matter (NOM) concentration, availability of anions such as chloride or sulfide, and salinity will influence dissolution and also the fate of the released metal (e.g., ionic silver will often be sequestered by the chloride ions in seawater to form insoluble AgCl).19, 55 As suggested above, because of the composition and manner in which they were synthesized, carbonaceous ENMs such as single- and multi-walled carbon nanotubes (SWCNT, MWCNT), GFNs and fullerenes do not undergo dissolution in the same way as metal ENMs although there can be release of ions from metal catalysts if used in the ENM synthesis process.63, 68
Chemical transformations of ENMs can occur in the natural environment and during ENM bioaccumulation experiments. For example, graphene oxide can be reduced to form reduced graphene oxide (rGO) by microorganisms,69, 70 and other GFNs can also be oxidized and degraded under certain environmentally relevant conditions, which can decrease their bioaccumulation and also result in organismal exposure to degradation products 71 Carbon nanotubes can also be oxidized or degraded by environmental processes,72–75 although the molecular stability of CNTs often means that degradation requires relatively extreme conditions or is slow.75, 76 It is also broadly known that metal and metal oxide ENMs can be chemically transformed through oxidation and reduction processes.28, 77, 78
Relevant analytical methods
This brief overview of methods for ENM detection and quantification provides context for subsequent discussions of bioaccumulation measurement strategies for different types of organisms. It is essential during bioaccumulation experiments to make accurate quantitative measurements of the ENM concentration in the biota and also the matrix of exposure. This will enable the calculation of bioaccumulation metrics such as BAF values. More extensive reviews of quantification procedures have been recently published for carbon and metal-based ENMs.63, 79–81 Since many of the methods differ between ENM types (carbonaceous ENMs (CNMs) or metal-based ENMs), the relevant methods will be discussed separately. While some techniques can quantitatively detect various types of ENMs in organisms within certain parameters (e.g., above a certain concentration in organism tissue), they typically do not provide information about the ENM size distribution in the tissue. Also, many techniques do not distinguish between ENMs versus ions in the case of metal ENMs. Other techniques, such as many microscopic methods, can provide definitive identification of ENMs in tissues, but they are typically qualitative or semi-quantitative.
Bioaccumulation of CNMs is often detected using their unique characteristics such as their thermal or spectroscopic properties. In laboratory studies, isotope labeling is a frequently used approach to quantify bioaccumulation of CNTs, GFNs, and fullerenes.14, 15, 60, 82–86 Unlike CNTs or GFNs which are typically highly polydisperse, fullerenes can be quantified using mass spectroscopic techniques such as high-performance liquid chromatography (HPLC) or liquid chromatography-mass spectrometry (LC-MS).87, 88 In the absence of isotopically labeled samples, it is often necessary to use extraction or separation steps to isolate CNMs from the sample matrix prior to analysis.59, 89–92 However, few studies have been conducted to develop these methods for CNMs other than for fullerenes and SWCNTs.79 This remains an important area for future research. There are some methods that can be used for CNT quantification in organisms without extraction, such as a microwave method93–96 and near-infrared fluorescence for SWCNTs.97, 98
Bioaccumulation of metal-based ENMs (e.g., Ag ENMs,99–103 ZnO ENMs,104 CuO ENMs21, 62, 105) is most often assessed using total elemental analysis after digestion (e.g. acid assisted) with mass spectrometry or spectroscopy techniques. These measured concentrations include the original ENMs and various aged and decomposition products, such as released ions and biogenic/transformed structures. A major challenge with this approach is that these techniques do not distinguish between the background concentration of the main element (except for isotopically enriched ENMs), bioaccumulation of dissolved ions released from the ENMs, and bioaccumulation of the ENMs themselves. Thus, also testing the bioaccumulation of the dissolved metal is usually needed.
For complex matrices such as soils and sediments, it is important to assess the relative availability of the different forms of metal or metal oxide ENMs (e.g., intact ENMs or dissolved ions) in soil or sediment porewater or associated with soil or sediment particles, because ENMs in the porewater may be more bioavailable or easily transported in the environment.106 For plant exposures, a water-only (hydroponic) design enables the most straightforward ENM characterization, while characterization of ENMs in soils is more challenging as a result of the dynamic nature of ENM behavior in soil,107 particularly in the rhizosphere due to microbial processes and root exudation (although these processes would still occur to some degree in water-only (i.e., hydroponic) exposures), and the complexity and heterogeneity of the soil matrix.108 Information on the different forms that contribute to the total metal levels in soils or organisms can be obtained by analyzing the soils using a range of different pore water and weak extraction techniques such as sequential extraction105, 109 coupled with the use of filtration and/or centrifugation methods to separate particulate and dissolved species. However, the separation approach needs to be evaluated to determine if the procedure would unintentionally remove ENMs located in the pore water, confirm that specific steps can fully remove ENMs if desired, and to assess adsorption of ions or ENMs onto the sidewalls of the containers or to the membrane used for filtration. The resulting fractions can then be analyzed for metal content and possible speciation. Overall, filtering of extracts from more complex matrices (soil, sediment, tissues) may be difficult, because ions, ENMs, and other materials (e.g., NOM) may adsorb to the filter-membrane. This may result in the capturing of smaller materials than expected based on the pore size cut-off of the filter used, and therefore may bias the characterization of the relative concentrations of the different forms of the ENM. Separation of ENMs from soils or sediments using field flow fractionation (FFF) has also been shown to be effective in certain situations.110, 111 Additional discussion regarding quantification approaches for ENMs in soils, sediments, and organisms and discussion related to spiking ENMs in soils are provided in the Supporting Information.
Stable isotope-enriched metal ENMs have proven useful for assessing the fate and biological uptake of ENMs, especially those based on elements that have high background levels in soil and biota. Studies with isotope-enriched ENMs can be conducted at environmentally relevant concentrations, because elements sourced from such ENMs can be readily separated from the natural background.112 For example, nominal concentrations up to 6400 mg/kg soil were used in one bioaccumulation study with typical ZnO ENMs,113 while isotopically enriched Zn allowed for detection of differences compared to the background Zn in soils at a concentrations of only 5 mg/kg to 10 mg/kg soil.114 However, use of isotope-enriched ENMs does have some limitations. For example, by itself isotope-based discrimination cannot provide information on the ENM form, since, for example, it will not be known whether the isotopes remain present in particles or have formed free ionic species.114 In some cases, isotopic labelling approaches may be used to distinguish between intact ENMs and dissolved ions released from ENMs through constraining the isotopic compositions of elements taken up in dissolved form where there is a dissolved background of that element with natural isotopic abundance.115 Dual labelling strategies may provide possible insights into ENM fate and bioavailability when used in different forms.116 Prior to the use of stable isotope-enriched ENMs, it should be confirmed that uptake kinetics of the different forms of the ENM are similar for the different isotopes.
Another promising approach to characterize metal-based ENMs in organisms is single particle inductively coupled plasma-mass spectrometry (spICP-MS), a technique that can provide size distributions, mass concentration, and number concentration of ENMs in suspensions and distinguish between ENMs and ions.80, 117–122 However, this technique has only been used in a limited number of ENM bioaccumulation studies and additional research is needed to assess potential biases from ENM extraction processes.121, 123–127 Additionally, this technique determines particle size based on assumed stoichiometry and crystal structure of particles, and the ENM size detection limit is relatively high for some elements.29, 128 Recently, the use of spICP-MS has also been optimized to characterize and quantify metal ENMs (concentrations and size distributions) in soil129 and soil organisms.20 A key component of this approach is to distinguish ENMs from ionic background concentrations, which requires an optimized dilution of the extracts.129 Employing spICP-MS for the detection of ENMs in biota may be complicated by the fact that organisms may form biogenic nanostructures of the metals released from ENMs, a finding recently shown using transmission electron microscopy (TEM) and energy dispersive X-ray spectroscopy (EDS) for earthworms exposed to silver ENMs.20 The assumptions of the assumed stoichiometry and crystal structure for spICP-MS data interpretation are likely not met in such cases. Therefore, particles detected in the organisms may not be the same particles to which the organisms were exposed. In this case, it is essential to also perform spICP-MS analyses on control organisms exposed to ions, which can also contain nano-sized particles of biogenic origin.20
Microscopic approaches can provide an alternative or additional methodology to verify the bioaccumulation of ENMs in tissues and cells. However, there are challenges related to providing quantitative information about the mass, particle number, or concentration in the biological sample from microscopic images. Also, microscopy in general can be limited by the ability to locate ENMs within the matrices when the concentrations are low. Nevertheless, EDS can be used for some ENMs to provide elemental information about the particles observed when using scanning electron microscopy (SEM) or TEM. The confidence in microscopic measurements of ENM bioaccumulation can be strengthened by comparing results to those obtained using mature orthogonal measurements such as total elemental analysis when applicable. Additional limitations for analysis using EM are time and labor-consuming sample preparation, and the potential for introduction of artifacts in the samples. In addition to common artifacts like osmium-containing deposit formation in the cells after osmium tetroxide post-fixation, ENM-specific artifacts have been reported in studies with Ag, ZnO, and MgO ENMs.130 Ag ENMs were shown to react with osmium tetroxide, while staining with uranyl acetate and lead citrate resulted in dissolution of ZnO and MgO ENMs. Thus, it was recommended to test the reactivity between the ENMs and the staining reagents, confirm observed particles by EDS, and use SEM in addition to TEM to confirm the position of ENMs in the sample.130 Nevertheless, EM methods have been extensively used to uniquely provide visual evidence of bioaccumulation for a wide range of ENMs such as cerium oxide,131 ZnO,131 TiO2,132 carbon nanotubes,11, 133–135 graphene family nanomaterials,14, 24 and Au ENMs136, 137 in a range of species. EM methods can also provide key information about the distribution of ENMs within cells such as intact CdSe QDs that have been biomagnified,23 information that can be challenging to obtain using other approaches.
X-ray absorption spectroscopy (XAS) is a technique that can obtain definitive information about the chemical form of metals in biological samples and can differentiate between the dissolved ions, metal or metal oxide ENMs in the initial form used to dose cells or organisms, and transformed ENMs that may have been produced.138–140 Overall, XAS is perhaps the most frequently used technique to characterize transformations of ENMs in complex matrices such as soils141–143 and biological matrices136, 140, 144, 145 and to characterize certain types of transformations in aqueous media such as sulfidation.146–149 XAS is available at synchrotron user facilities and thus not for routine analysis, yet there are many synchrotron facilities worldwide. XAS measures the local coordination environment of metal centers and the presence of an ENM is inferred from this. The smallest probe size for beamlines capable of performing XAS is ~ 30 nm, which can enable localization of particles within tissues and provide information about the states of those particles such as if they have been transformed; for example, ENM dissolution can be inferred in cells from the oxidation state of a released component metalloid and its NP form.150 Assumptions that particles are in nanoparticulate form based on local coordination environment of metal atoms determined by XAS must be justified using deductions based on the XAS spectra or orthogonal measurements136 such as EM and EDS.150
Given that artifacts and biases can impact some measurements, orthogonal approaches are needed wherever possible to provide multiple lines of evidence for quantification and visualization of accumulated ENMs.29,151 For example, three orthogonal techniques (scanning TEM (STEM) with EDS, spICP-MS, and ICP-optical emission spectroscopy (OES)) were utilized to assess bioaccumulation of TiO2 ENMs by hydroponically grown plants.123 STEM was coupled with EDS analysis to visualize the distribution and confirm the elemental composition of TiO2 ENMs inside the plants tissues; a similar approach was used for analysis of TiO2 ENMs in protozoans.132 ICP-OES analysis was performed to determine the bulk elemental concentration of Ti, while spICP-MS was used to analyze ENM size distribution inside plant tissues.123 Two plant digestion procedures (i.e. acid vs. enzymatic digestion) were also compared regarding their effects on the spICP-MS analysis. A similar approach was applied to quantify earthworm uptake kinetics of different forms of Ag-nanomaterials (including those biogenically formed from accumulated ions).20
Evaluation of detection limits for different analytical methods
The detection limit of a quantification method impacts bioaccumulation methods because lower concentration detection limits will improve quantification of the exposure dose and concentration in the biota, enabling testing at lower and more environmentally relevant ENM concentrations. Decreasing the detection limit will also enable better differentiation between ENMs in biota versus the background from other potentially interfering compounds. This is especially important for ENMs composed of elements which are present at a high concentration in the environment, for example Cu, and for some CNMs.
The lowest achievable mass detection limit when quantifying ENMs in environmental matrices—for many analytical techniques—will be similar to that achieved when using the same technique to quantify the element comprising the ENM. For example, elemental techniques based on measuring carbon to quantify CNMs (e.g., total organic carbon analysis or thermal optical transmittance) will have a lowest achievable detection limit at the concentration for detecting total carbon.63, 79,152–154 A similar relationship exists for techniques based on elemental concentration measurements of metal-based ENMs (e.g., ICP-MS). An exception is spICP-MS, which can detect individual ENMs as a result of the substantially shorter dwell times (50 μs to 10 ms) compared to total elemental analysis (approximately 300 ms). Since a spike in the intensity signal is detected in this shorter dwell time windows, spICP-MS has far lower mass detection limits than those for total elemental analysis.117, 120 In general, the ENM size and concentration detection limits need to be determined on a case-by-case basis for each ENM and matrix combination and depend upon the sensitivity of the instrument to distinguish the ENM from the matrix among other considerations. To further investigate the recovery and detection limit for a particular ENM in a test organism, it is possible to spike a known mass (often applied as a volume of an ENM suspension with a known concentration) or range of masses directly to a mass of organism tissue similar to the mass that will be used in the experiments, and then perform the analytical procedure including any sample digestion steps.91, 121, 124 However, it is possible that this approach may overestimate the recovery and detection limit if internalization of the ENM within the tissue or cells would lower the recovery of or otherwise bias the analytical method. Furthermore, dissolution of metal ENMs in organisms would increase the ionic background concentration, potentially increasing the smallest ENM size that can be detected.
Theoretically, microscopic techniques such as EM could be used to detect a single ENM particle in an organism. However, detection is not the same as quantification since the latter requires understanding the detection limit if comparative analysis is a goal. In practice, the detection limit (particle concentration of an ENM in a volume of tissue or number of cells) in a specific matrix depends on several factors such as the capacity of a particular microscopic technique to differentiate the ENM of interest from other natural or incidental particles and other materials in the matrix including avoiding false-positive or false-negative results, the number of cells or area of tissue analyzed, and the acquisition of enough visual information in two dimensions such that a three dimensional impression of ENM distribution in tissue can be acquired. The first two challenges are also present for other scenarios where TEM is used quantitatively such as for the standard method for determining asbestos concentrations in air samples155 or for counting the nanoparticle number concentration in a suspension.156 In studies assessing whether an ENM can be detected in a biological matrix after exposure, it is not possible to determine the detection limit from the information provided unless the area of tissue analyzed is reported. For the asbestos quantification method, a known area (determined by the number of grids viewed) are analyzed, allowing for calculating the detection limit. Without a similar approach to ENM quantification, it is infeasible to statistically relate the lack of observing an ENM in the tissue to the ENM concentration in that tissue. Thus, a recommendation for EM, if it is to be used quantitatively, is to attend to establishing the NP detection limit. Further, attention to the three-dimensional nature of biological specimens with their bioaccumulated ENMs would be needed, such as by imaging numerous sections representative of the tissue and arriving at a statistically defensible scheme for assembling data across sections into a model of the whole tissue specimen.
Subcellular separation approaches
One approach that can be used to better understand ENM bioaccumulation at the subcellular level (e.g., concentration of an ENM associated with organelles or metallothionein-like proteins) is to perform a subcellular separation technique.127 This data can improve the potential for toxicokinetic modelling by supporting the selection of appropriate multi-compartment models. Multiple subcellular fractionation techniques have been published for plants and other multicellular organisms.127, 157 This information may be informative in understanding toxicity mechanisms and the potential for the ENMs to exert toxicity through different adverse outcome pathways. For example, internalization of metals in biota reveals the internal distribution processes that occur during metal accumulation, and may, therefore, provide information on metal toxicity and tolerance after exposure to ions or metal-based ENMs.157–160 When applying subcellular fractionation for metal-based ENMs, measuring the metal concentration both as the total body burden and in subcellular fractions as a means to assess methodological losses (i.e., comparing the total body burden and the sum of the metal in each of the subcellular fractions) can reveal if an acceptable recovery is obtained. Similar measurements should be performed for CNMs.
There are a number of steps needed for the analysis of tissue compartmentalization. First, the organisms or tissues need to be homogenized, and then the homogenate is subjected to a fractionation procedure such as differential centrifugation. One significant potential complication is if the homogenization process resuspends ENMs, such as those located in the cytosol. These suspended ENMs could then potentially adsorb to other cellular components during the separation steps or be removed from the supernatant by differential centrifugation steps especially if ENM agglomeration occurs. Therefore, appropriate control measurements need to be included such as performing the separation steps with dispersed ENMs added directly to the extraction buffer. In addition, one should conduct the homogenization process on an unexposed organism, spiking in dispersed ENMs, and then perform the extraction process.158 There is a possibility that the adsorption of a large number of dense ENMs could influence the separation of different organelles if there is a sufficiently large change in density of an organelle to cause it to be removed in a sequential differential centrifugation procedure at a different step. It may be possible to perform calculations using Stokes’ Law to theoretically estimate the potential for this to occur using a worst-case scenario such as by estimating the maximum potential loading of the ENMs onto each cellular fraction. However, performing this calculation would require information about the buoyant density and diameter of the organelles and of the ENMs. In addition, ENMs in cells may have their buoyant density decreased as a result of interactions with biomolecules.161 It is possible to compare results obtained from a subcellular separation process with orthogonal methods such as microscopic analysis using EM13,158 or Raman spectroscopy.162 One approach to avoid some of the issues with sequential differential centrifugation approaches would be to use density gradient centrifugation since only a single centrifugation step is typically performed. Density gradient centrifugation separations rely on the use of centrifugal force to separate particles of different sizes, densities, and masses; larger and denser particles sediment at faster rates than less dense, smaller particles.163 It is possible to estimate the conditions that should be used for density gradient centrifugation using Stokes’ Law as described above if the relevant information is available.164 To facilitate identification of the ENM-containing subcellular fraction using density gradient centrifugation, using dye-labeled ENMs has been proposed.165 More information about density gradient centrifugation (e.g., density of ENMs and commonly used media) is provided in the following section when discussing the separation of single-celled organisms and ENMs.
Case studies
Given the different considerations related to making accurate and robust bioaccumulation measurements for various species (Figure 1), multiple case studies will be discussed. Single-celled organisms will be evaluated separately from multi-cellular species given that there are some important considerations for bioaccumulation measurements based on the size and complexity of the organism. In addition, plant species will be discussed separately from other multi-cellular organisms, reflecting differences in their physiology and also specific exposure considerations for studies between multicellular plants and other species. Descriptions of how to prepare and characterize the ENM exposure media (water and soil as examples) are provided in the Supporting Information.
Single-celled organisms
To examine bioaccumulation in single-celled organisms, it is important to consider overarching topics that are relevant for multiple species such as separating them from suspended ENMs and considerations related to bioaccumulation by individual cells or cell populations. To provide more specific examples about how this information can be utilized, case studies are also provided for single-celled organisms without a cell wall and for biofilms.
Separation of single-celled organisms from suspended ENMs
For analytical techniques such as confocal microscopy,166, 167 coherent anti-Stokes Raman scattering microscopy,168 hyperspectral imaging,169–171 X-ray fluorescence,172, 173 or secondary ion mass spectrometry,174 separation steps may not be critical or necessary as the detection capabilities of these instruments allow for penetration past the cell surface without destruction of the organism prior to analysis and may allow for distinguishing between particles on the cell surface versus those that are internalized. On the other hand, many techniques that provide quantitative information on bioaccumulation such as the total elemental analysis methods described above require separation of the cells from suspended ENMs prior to analysis. This is critical because insufficient separation of cells and suspended ENMs can lead to biased bioaccumulation measurements since suspended ENMs will be mistakenly interpreted as being associated with the cells.
When separating ENMs from suspended cells using filtration or centrifugation, the primary focus is separation, while a secondary purpose can be to dislodge surface-attached but not internalized ENMs.121, 169, 172, 175 Repetitive rinsing and differential centrifugation steps have often been applied to algae and bacteria before quantification of the cell-associated ENMs.39, 150, 176 In studies with protists and algae, repetitive centrifugation, washing with clean medium and filtration though a > 1-μm pore size filter have been applied with similar aims. Some authors have shown that the filtering and rinsing approach is efficient in removing the loosely bound ENMs from cells by confirming that additional washes do not reduce cell-associated ENM concentrations,177 especially when the ENMs are well dispersed.178 However, these simple rinsing procedures may not be sufficient to remove suspended particles or their agglomerates from single-celled organisms that could be in the same size range as ENM agglomerates. To further assess ENM removal using these approaches, it may be helpful to perform experiments where the cells and ENMs are mixed, and then the separation step immediately performed to assess the extent to which ENMs are fully removed. This control experiment revealed a lack of full ENM removal with several rinsing steps of multicellular nematode Caenorhabditis elegans,121 although it is unclear if a similar result would be obtained for suspended cells. For larger or agglomerated ENMs, alternative approaches may be required. For example, the mobility of ciliated protozoa can be utilized in separating unicellular organisms from the pellets of CNTs: after pelleting the samples by centrifugation, Tetrahymena thermophila were allowed to swim out of the pellet into the supernatant prior to collection.179 If it is critical to determine if surface-attached ENMs have been removed, it is possible to evaluate the outer surface of a statistically sufficient number of exposed organisms using SEM or TEM to assess the presence of ENMs.
Recently, alternative separation strategies such as the use of density gradient centrifugation, a technique commonly used to achieve size separation and selectivity of ENMs in the post-synthesis and purification steps,180–184 have been implemented to separate unassociated ENMs from organisms in cases where water or media rinses and differential centrifugation were found to be insufficient.82, 164, 185 Media of particular densities can be selected to enable separation of the ENMs and organisms based on either their size and mass (rate-zonal centrifugation) or solely on density (isopycnic centrifugation).164 Rate-zonal centrifugation is similar to differential centrifugation in the sense that the sedimentation speed of the particles depends on their size and mass. The advantage of this approach is that it allows for complete separation of smaller from larger particles121 unlike in differential centrifugation where cross-contamination of particles of different sedimentation rates may occur.186 In rate-zonal centrifugation, the cells and ENMs form distinct zones when moving down the density medium as the faster sedimenting larger and heavier particles move ahead of the slower ones.121 Since the density of the gradient medium is lower than the density of the cells and ENMs, the sample components will pellet if centrifuged for a sufficiently long period. Thus, selecting the centrifugation time and force is crucial for optimal separation.164 In isopycnic separation, the density of the medium must be in the range of equal to or greater than the density of the sample components so that the cells and ENMs remain in the media layer equal to their buoyant density.187 Important factors to consider in choosing a suitable density gradient medium include the following: (i) biocompatibility to avoid adverse impacts on cell physiology, behaviors, and viability; (ii) sufficient solubility to produce the range of desired densities; and (iii) easy removability from the purified cells. To optimize this procedure, certain organisms may require gentle centrifugations speeds, while others do not. The density ranges for the most prevalently used gradient media, species that are suitable for use with this separation technique, and the density ranges reported for ENMs are highlighted in Figure 2. If purified organisms are intended to be used in further experiments, such as trophic transfer tests, optimization of the centrifugation time is especially important to ensure complete separation while keeping the centrifugation time short enough not to compromise the viability of the organism. Theoretical approaches based on Stokes’ Law have proved useful in optimizing centrifugal times and assessing the likelihood of effective separations in density gradient centrifugations.164 Calculating the theoretical minimum diameters of the particles that would sediment can guide the optimization of both differential and density gradient centrifugation procedures. However, it must be noted that possible discrepancies between the theoretical and experimental results should be considered in cases where the density gradient medium is expected to interact with cell surfaces or permeate the cell membrane, such as with sucrose,164 or when coating with biomolecules may change the buoyant density of ENMs.161 Depending on the size, mass and buoyant density of the particles to be separated, a sequential separation approach that combines differential, size- and buoyant density-based centrifugation may be needed.
Figure 2:
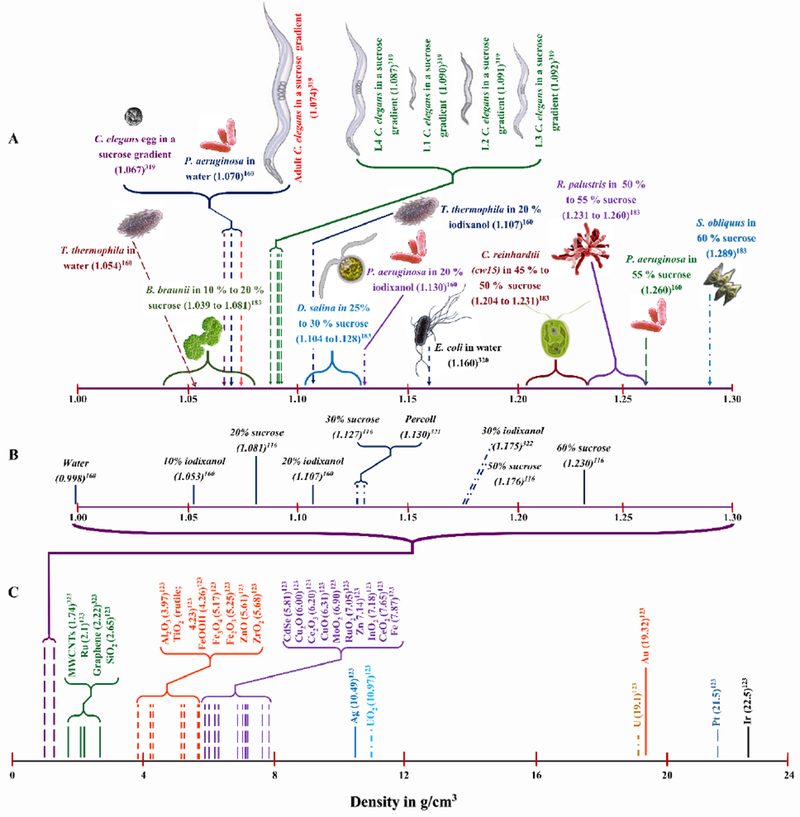
Comparison of densities among (A) biological organisms in density media, (B) media used for density gradient centrifugation separations, and (C) ENMs (bulk). Densities for gradient density media are represented in percentages of weight by volume (w/v; 10 % iodixanol, 20 % iodixanol, 30 % iodixanol, Percoll (23 % coated silica spheres in water), 20 % sucrose, 30 % sucrose, 50 % sucrose, and 60 %sucrose). T. thermophila: Tetrahymena thermophila; B. braunii: Botryococcus braunii var. Showa; C. elegans: Caenorhabditis elegans; P. aeruginosa: Pseudomonas aeruginosa; D. salina: Dunaliella salina; E. coli: Escherichia coli; C. reinhardtii (cw15): Chlamydomonas reinhardtii (cw15); R. palustris: Rhodobacter palustris (CGA009); S. obliquus: Scenedemus obliquus 128, 324–328
Considerations regarding bioaccumulation measurements of individual cells and cell populations
The bioaccumulation assessment of ENMs in microorganisms usually involves planktonic cultures composed of hundreds of thousands to millions of single cells. Unlike tests with larger organisms, such assays enable population-level measurements. Microbial studies offer a unique opportunity of evaluating ENM bioaccumulation across thousands of individuals as well as multiple generations.188, 189 ENM bioaccumulation measurements using growth assays, sampled at different time points, can provide valuable information on the ENM content associated with the cells at different population growth stages. It has been reported that uptake of ENMs by eukaryotic cells can be influenced by their cell cycle phase.190 ENMs that are internalized by cells or associated with the cell membrane are split between daughter cells when the parent cell divides. Consequently, in a cell population, the concentration of ENM in each cell varies depending on the cell cycle phase. Similarly, association of ENMs with prokaryotic cells in a growing culture varies depending on the growth phase: in the phase of fast division the bioaccumulation rate of ENMs could be overpowered by the rate of cell division such that the concentration of ENMs in or on individual cells could be diluted in a manner similar to the growth dilution that can occur in plants. Therefore, it is important to consider cell cycle phase (eukaryotic microbes), growth phase (prokaryotic microbes), and thus growth rate, when interpreting the bioaccumulation of ENMs in single-celled organisms.
Often, the addition of ENMs to single-celled organism cultures results in heteroagglomeration. For example, cell agglomeration has been noted when co-incubating CNTs164 or positively charged ENMs191 with bacteria, or CNTs192 or alumina-coated SiO2 ENMs193 with algae. Such heteroagglomeration complicates bioaccumulation measurements because (i) determination of cell numbers by direct counting is typically not possible and other approaches, such as ATP concentration of the cells194 or photosynthetic activity of the algae193 instead need to be employed, although the potential for artifacts in cell viability assays is well known and appropriate controls should be used;28, 195, 196 (ii) separation of cells and ENMs not tightly associated with the cells is challenging as described above; and (iii) heteroagglomeration becomes an issue in single-cell analysis methods such as flow cytometry and single cell analysis by ICP-MS. Application of the latter methods for quantification of ENMs associated with cells is discussed in more detail below.
Conventional analytical methods used for quantification of ENMs associated with cells (e.g., ICP-MS, ICP-OES, liquid chromatography/mass spectrometry, fluorimetry, ultraviolet-visible (UV-Vis) spectroscopy) require harvesting at least several hundred micrograms of biological material to provide a sufficient mass for analysis. These analyses yield an average ENM concentration in the cell population. While some of these methods (ICP-MS and ICP-OES) enable detection of trace metal concentrations, they typically do not provide information on ENM distribution among the cells in the population. However, flow cytometry and single cell cytometry by time of flight (TOF) ICP-MS can provide information on the distribution of ENMs in hundreds or thousands of individual cells.197, 198 Techniques used for ENM quantification at the single-cell level, including flow cytometry, have been recently reviewed from a nanomedicine viewpoint, focusing on ENM bioaccumulation in mammalian cell lines.199
In flow cytometry, ENM bioaccumulation is quantified either based on fluorescence (in the case of fluorescent or fluorescently-labeled ENMs) or other optical properties of ENMs. Measurement of non-fluorescent ENMs is achieved based on side scattering (SSC) intensity that correlates with changes in cellular granularity due to the uptake of ENMs. Flow cytometry as a semi-quantitative technique has been successfully used for measuring uptake kinetics of quantum dots (QDs) in protozoa T. thermophila200 and algae Ochromonas danica167 and of TiO2 ENMs in Paramecium caudatum 201 One of the challenges in using flow cytometry for measurements of single-celled species exposed to ENMs is avoiding misinterpreting signals from ENM agglomerates as those from ENM-coated cells. The latter is especially important with bacteria or small protists. It may be possible to minimize this impact if separations are performed first as described above. Aggregated cells, heteroagglomerates of cells and ENMs, and ENM association with cell debris can also complicate analysis and signal interpretation. It is also important to note that some ENMs have been shown to cause false-positive or false-negative results in a viability assay to test for apoptosis or necrosis using flow cytometry and thus careful control experiments also need to be included for bioaccumulation measurements to avoid artifacts.202
More recently, ICP-MS has been developed and commercialized for the analysis of single cells.203–205 Similar to spICP-MS, in single-cell ICP-MS (SC-ICP-MS) the cell suspension is nebulized through an ICP-MS sample introduction system, each cell is ionized, and the metal ions originating from a single cell are detected. Considering that SC-ICP-MS is a new technique, it is not surprising that the applications for ENM quantification are still in the development phase and relevant literature is limited. SC-ICP-MS has been successfully applied for the detection of QDs in mouse cells206 and Au ENMs in algae,204 and laser ablation ICP-MS (LA-ICP-MS) has been used for measurement of Au and Ag ENM bioaccumulation by and within mouse cell lines.207, 208 Considering that concentrations of trace elements in various other environmental single-celled species have been studied using SC-ICP-MS,209–211 there is substantial promise for the use of this technique to assess cellular ENM bioaccumulation. Important considerations when using this method include a careful separation of non-associated ENMs from the cells prior to analysis so as to ensure that the measured signal originates from within the cells, and adjusting the cell concentration in the sample and instrument dwell time so that only one cell is detected at a time. Similar to flow cytometry, one of the limitations of SC-ICP-MS is that no distinction can be made between internalized and cell surface-attached ENMs. Coupling ICP-MS with laser ablation provides information about the spatial distribution of ENMs in cells, although resolution at the nanometer scale remains a limiting factor.205
Microscopic methods that can resolve ENMs associated with the cells are often used for confirming ENM localization within cells.23, 167, 200, 212 Intracellular ENM quantification methods that are particularly suitable for protist model organisms that are relatively large (e.g., Tetrahymena sp., Euglena sp., and Ochromonas sp.) include optical microscopy (i.e., bright field, phase contrast, and darkfield microscopy with hyperspectral analysis)82, 200 and EM.132 Such techniques can also be used semi-quantitatively or quantitatively for ENM bioaccumulation measurements. Semi-quantitative approaches include measurements of ENM area or fluorescence per cell. In quantitative microscopy, ENMs are counted in cells or the measured ENM area per cell is converted to mass or number concentration based on the size, shape and density of the ENM. In ENM research, high-resolution techniques are desired for the visualization of single ENMs in cells. In addition to being a valuable tool for characterizing ENM-cell interactions, EM can be used quantitatively. For instance, TiO2 ENM accumulation in the food vacuoles of T. thermophila was quantified from the scanning transmission electron microscopy (STEM) images of T. thermophila thin-sections.132 Based on the geometries of T. thermophila food vacuoles with accumulated TiO2, the ENM concentration per cell volume was calculated using the volume and number of food vacuoles per cell and the density of TiO2. Similar to making quantitative microscopic measurements of cells for other purposes, there are a number of sources of uncertainty in microscopic imaging relevant to understanding the precision of these measurements for ENM bioaccumulation: (i) the impact of microscopic imaging parameters (e.g., focus),213 (ii) image quality such as the signal to noise ratio for the ENM area compared to the background, (iii) determining the adequate number of cells to analyze to sufficiently reflect the behavior in the larger population; and (iv) the precision and reproducibility of image processing algorithms to calculate the ENM area;214–217 assessing the image processing algorithms could be performed by comparing manual measurements of the ENM area for a certain number of images to those calculated by the computer program to assess the accuracy of the algorithm.
Although light microscopy cannot resolve single ENMs, it is suitable for visualizing ENM agglomerates when these are larger than the resolution limit of light microscopes with a conventional lens, i.e., approximately 200 nm. This may occur if ENMs are packed into agglomerates in the food vacuoles of particle feeding (phagocytosing) single-celled species.82 This phenomenon provides a good opportunity for using quantitative optical microscopy for ENM uptake and elimination kinetics measurements. Dark field microscopy coupled with hyperspectral analysis also enables identification of ENMs in cells, confirming that only the intracellular agglomerates composed of ENMs are measured.171 Since single-celled species vary in physiology and ENM uptake mechanisms, it is advisable to validate microscopic image-based quantification with another analytical method. For example, uptake of carbonaceous nanomaterials in the protozoan T. thermophila was quantified in parallel by image analysis and measuring 14C labelled MWCNTs, and the two methods were found to correlate well.82
Single-Celled Species Case Study #1: Species without a cell wall (protozoa)
The lack of a cell wall makes the membrane of single-celled species such as protists and some mixotrophic algae directly accessible to ENMs. ENMs can adsorb onto and associate with the cell membrane and subsequently be internalized by endocytosis.167, 177 In addition to endocytosis, some protists and mixotrophic algae acquire nutrients by phagocytosis, a mechanism by which particulate materials (organic particles, bacterial, yeast and small algal cells) are internalized by the formation of food vacuoles. Thus, in contrast to microorganisms with cell walls that cannot internalize particulate matter, protists and some algae are expected to take up ENMs and their agglomerates at sizes larger than 50 nm218 by natural feeding mechanisms, as reported for various species and different types of ENMs.82, 132, 167, 171, 200, 219 Food vacuoles containing ENMs are trafficked through the cell similarly to those containing nutrients. For inert ENMs or non-toxic ENM exposure concentrations, the contents may be subsequently expelled through the cell membrane. Therefore, from the perspective of bioaccumulation assessment, food vacuoles in protists function similarly to the digestive system of multicellular organisms and thus, the experimental design warrants the inclusion of an elimination phase before quantification of bioaccumulated ENMs (Figure 1). So far, only a few studies have measured elimination of ENMs in single-celled species, including those without a cell wall.167,171,200
Single-Celled Species Case Study #2: Biofilms
Biofilms (Figure 3) comprise surface associations of microbial cells embedded in hydrated extracellular polymeric substances (EPS).220 Biofilms are prevalent forms of microbial growth in all compartments of natural and built environments.221 Yet they are less studied in the realm of microbial-ENM interactions, including assessments of ENM bioaccumulation, than free living microorganisms.222 EPS appears to trap ENMs, as demonstrated for ZnO ENMs in activated sludge floes,223 and Ag ENMs in bacterial monocultures under laboratory conditions.224 Because EPS is a physical structure surrounding the cells, the association of ENMs with EPS influences exposure of biofilm cells to ENMs, and may affect direct ENM bioaccumulation. For example, Au ENMs in estuarine mesocosms16 and TiO2 in paddy microcosms225 were shown to accumulate in biofilms with subsequent transfer to higher, predating organisms such as grazing snails. The quantification of such ENM bioaccumulation within biofilms is currently largely unresolved; this may be significant if ENMs are compartmentalized in biofilms with preferential association either on cells or in the EPS. As shown in Figure 3, ENMs associated with EPS or cells would be quantified in a total biofilm mass-based accounting of prey in a grazing experiment. However, trophic transfer and biomagnification may hinge on ENMs being firmly associated with cells, especially in cases where a predator’s digestion of EPS and prey differ. In environmental microbiology, it is an established convention to separate biofilm cells from EPS and to quantify toxicant association with each of these two broad biofilm components separately, such that increased EPS production—a common stress response in biofilm bacteria—can be assessed along with toxicant accumulation.226 A future recommendation in the assessment of ENM bioaccumulation for biofilms would be to adopt a similar approach. This would allow the normalization of ENM accumulation in the biofilm to total cell count and also to EPS dry mass, rather than wet-mass which can be system- and condition-dependent. This approach, coupled with ENM quantification for each biofilm component (EPS and cells), would allow determining overall biofilm bioaccumulation assessments in terms of ENM distribution. Furthermore, it would allow trophic transfer or biomagnification factors to be better expressed according to either the whole biofilm (in the event that ENMs are evenly distributed across EPS and cell components), EPS (if ENMs are mainly concentrated there), or cells (if ENMs are preferentially adsorbed to their external surfaces).
Figure 3:
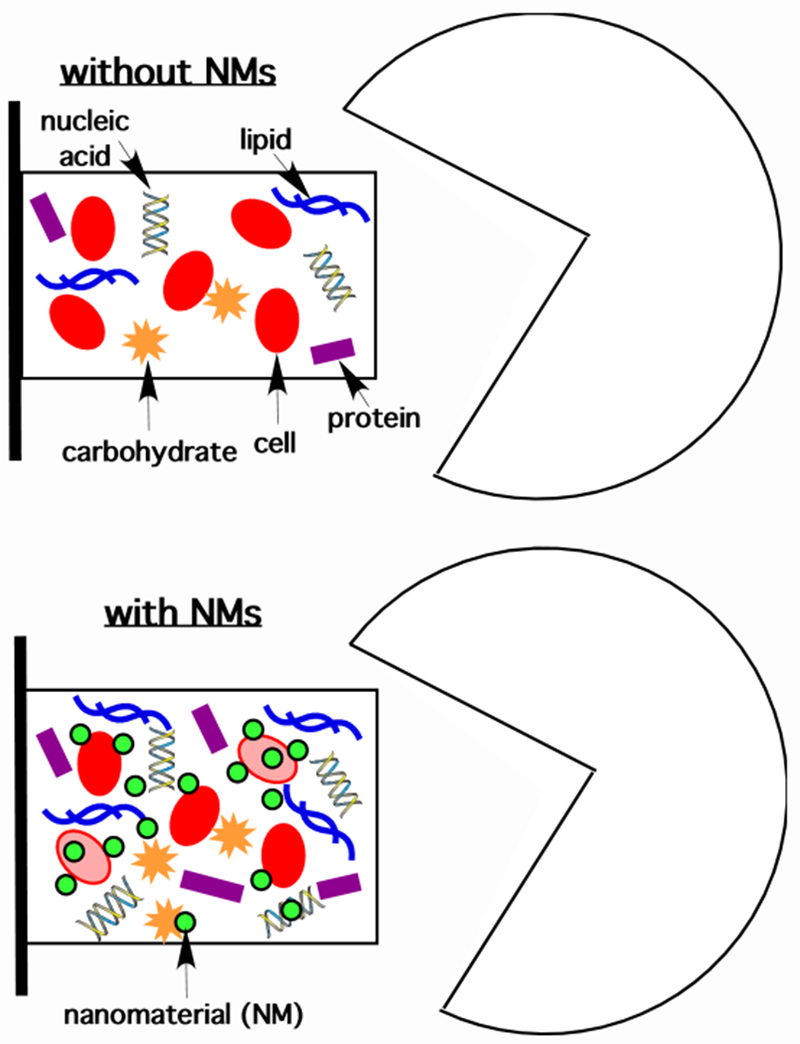
Conceptual representation of microbial biofilms (left) subject to predation by grazing (right) without (top) or with (bottom) ENMs accumulated in the biofilms. Note that the extracellular polymeric substances (EPSs) are depicted as macromolecules (lipids, nucleic acids, carbohydrates, and proteins) that are hydrated, surrounding biofilm cells. In the presence of ENMs that impose cellular stress, EPS accumulations may increase (bottom) which could increase the overall abundance of retained ENMs in the vicinity of prey (biofilm cells) and predator (grazer or similar).
Multicellular organisms (excluding plants)
For multicellular organisms, it may be important to distinguish between the total body burden in the absence of voiding the gut (as the ENM concentration in the gut tract can readily be voided), the ENM concentration adhering to an epithelial surface (e.g., gut microvilli), and the ENM concentration that has been truly adsorbed through an epithelial surface, for example in daphnids (Figure 4). Which of these fractions is relevant for an individual assessment may be context dependent (Figure 1). For example, trophic transfer studies may consider all fractions, while toxicokinetic and mechanistic toxicology studies may focus only on the absorbed fraction. However, even in the latter case it is important to bear in mind that it is entirely possible that the ENMs may cause adverse effects during simple passage through the gut tract (or while in contact with gills), and thus concentrations in the gut tract and in other tissues may be important to measure, depending upon the other endpoints that are measured and the ultimate purpose of the experiment. The importance of such considerations is illustrated through a set of relevant case studies provided for fish, soil invertebrates, Daphnia, and marine bivalves.
Figure 4:
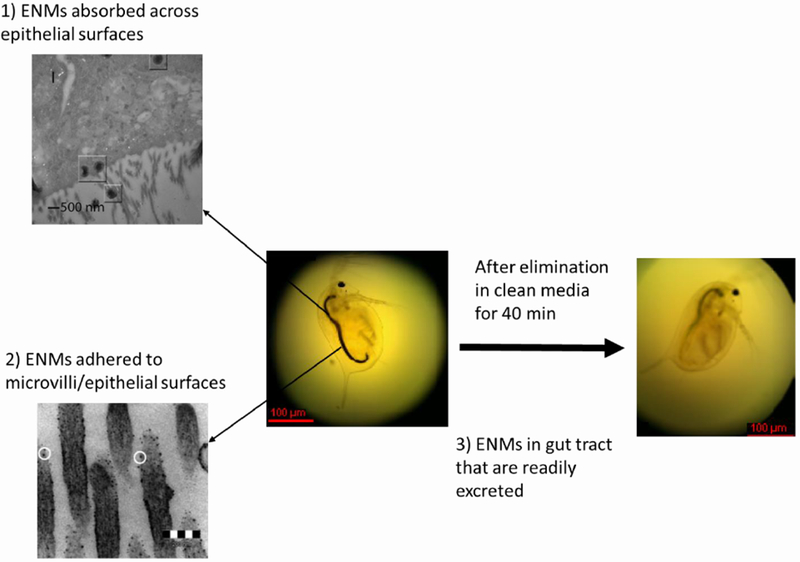
Fractions of engineered nanomaterials (ENMs) that can be detected in organisms with a digestive tract: 1) ENMs absorbed across epithelial surfaces; this figure (upper left) shows carbon nanotubes (CNTs) that had been absorbed by microvilli (see squares) although additional analysis using high resolution transmission electron microscopy (HRTEM) revealed that these particles were amorphous carbon and not CNTs.11 2) ENMs adhered to microvilli; this figure (bottom left) shows apparent fullerene particles adhered to the microvilli.12 3) ENMs in gut tract that are readily excreted; this figure (far right) shows that the gut tract of the Daphnia magna turned from black (as a result of uptake of few layer graphene for 24 h) to transparent or green after an elimination period of 40 min with algae feeding;256 adapted with permission from 256 2013 American Chemical Society.
Another key approach that can be used to elucidate the bioaccumulation of ENMs is to evaluate the toxicokinetics of uptake and elimination behaviors of whole organisms or specific organs or tissues. With regards to the elimination rates, one key difference between ENMs and dissolved organic chemicals or metals for multicellular organisms with a digestive tract is that the majority of the ENMs can be loosely associated with the digestive tract and, therefore, potentially subject to rapid egestion within the early part of an elimination phase. Therefore, taking additional time points close to the conclusion of the elimination period may be valuable for discerning if all of the ENMs associated with the organism after the uptake period can be eliminated by voiding the gut tract. Depending upon the organism’s physiology, feeding during the elimination period may be needed for voiding the gut tract. For some species, the time period needed to void the gut tract has been measured (e.g., Lumbriculus variegatus227 and earthworms or enchytreaids228) or visually inspected in semi-transparent organisms (e.g., Capitella teleta229) and is, hence, relatively well understood. However, such information is not always readily available for other species. If the gut voiding kinetics are unknown for a species, it is possible to assess this for soil and sediment organisms by measuring the rate of soil/sediment elimination by the organism. This can be measured during a depuration experiment by determining the ash content after combustion of organisms to determine the quantity of soil or sediment remaining,227 or by measuring the amount of a non-bioaccumulating rare earth metals such as lanthanides in the test species and comparing that concentration to the amount in the soil or sediment to determine the soil or sediment mass remaining in the organism.230 For smaller species, such measurements may require population cohorts rather than individuals to meet detection limit thresholds. One important consideration is the need to balance gut voidance time with the potential for elimination of ENMs from the tissues being investigated. Hence, longer elimination periods are not necessarily better, because there can be rapid elimination in the time period shortly after the cessation of exposure. The initial kinetics of elimination may be overlooked if longer elimination periods to void gut contents are used.231 Thus, it is recommended to make measurements during the elimination time series to initially include smaller steps (hours to days) to assess gut voiding and then longer steps (days or weeks) toward the end of the elimination period.
For ENMs that dissolve (e.g., Ag ENMs) or for ENMs composed of an element that is present in the exposure matrix (e.g., Zn in a sediment experiment), measuring the elimination rate at additional time points may be important to assess if there is a biphasic elimination process such as rapid elimination of the ENMs followed by a slower release of the accumulated dissolved ions or indeed the reverse case of fast eliminating labile and slower released particulate pools in cells. As described above, these measurements can potentially be refined by evaluating the ENMs associated with the organism such as by conducting spICP-MS analysis after digestion, or by measuring isotopically labeled ENMs for metal or metal oxide ENMs using isotope specific mass spectrometry. For ENMs that dissolve, it can be informative to compare the toxicokinetic rates obtained to those for a metal ion exposure using similar conditions. This can allow differences in toxicokinetic rates to be identified based on model fits and parameters values for different single compartment and multiple compartment kinetic models. These quantitative methods could be coupled with imaging techniques to obtain a better estimation of actual particles versus dissolved fractions in the organism tissues.
Multicellular Species Case Study #1: Fish
Measurement of the bioaccumulation potential for ENMs in fish requires special attention because the principle regulatory bioaccumulation test is a fish bioaccumulation assay (OECD TG 30530). Fish are a group of organisms that are large enough to facilitate dissection of the internal organs to identify the ‘target organs’ and the ENM biodistribution.49 However, there remains a substantial problem: the relationship between the exposure concentration and the internal dose leading to adverse effects remains unclear. The absence of routine measurement methods for ENMs in tissues has prevented unequivocal demonstration of cause and effect.
The initial step in the case of waterborne exposure after the exposure period is the removal of any excess water containing the ENM from the body surface. Experience so far suggests that there are no special or additional steps needed to do this for ENMs compared to traditional chemicals. For trout, netting the fish into a closed bucket of clean water with dilute anaesthetic to calm the animal and facilitate handling is needed. Typically, the fish is rinsed for about a minute in one bucket, and then transferred to another bucket of water containing a more sufficient level of anaesthetic to enable terminal anaesthesia (i.e. euthanasia in preparation for later dissection). Once the fish is euthanized, larger fish can be further triple rinsed in ultrapure water or completely immersed in a series of beakers of ultrapure water for smaller fish. This procedure will remove loosely bound material and dilute away any residual water from the tank. However, this procedure may not fully remove ENMs trapped in the mucus layers on the gill, skin or gut.
Fortunately, there are methods available to quantify the surface-associated ENMs in the mucus of the gill microenvironment and for the gut mucosa. These ‘ Surface Binding Experiments’ have been well established for metals and other solutes232 and are the experimental basis for the biotic ligand models (BLM233, 234). The technique involves a separate short experiment with previously unexposed fish tissue. The tissue (e.g., gill filaments or piece of intestine) is allowed to instantaneously adsorb the ENM onto the surface of the epithelium over a few seconds (i.e., before true uptake can occur). Then the total metal concentration in the tissue is determined. This method has been used successfully to measure the surface-bound TiO2 ENMs, for example, on the mucosa of the mid and hind intestine of rainbow trout.235 This study revealed that surface adsorption can be significant and, when exposure concentrations of 1 mg/L or less are used, it is likely that approximately 20 % of the apparent total tissue Ti is adhered to the surface of, not within, the tissue. Instantaneous adsorption measurements therefore become a vital consideration when interpreting data on ENM uptake by the gill, skin, gut or other external barriers of organisms (Figure 1).
Multicellular Species Case Study #2: Marine Bivalves
Marine bivalves (e.g., clams, mussels and oysters) are ideal candidates for the study of ENM fate and effects and have been exposed to a wide range of ENMs.236–241 Their physiology is well studied, and they are generally tolerant to varying environmental conditions and therefore relatively easy to culture and test. These species are commonly used as monitoring organisms because of their sessile and widespread nature. In addition, they serve as a food source for many higher trophic level aquatic and non-aquatic organisms including humans. Bivalves are unique in that their internal organs are often bathed in external or environmental media. In addition to direct exposure of external media, their capacity to filter large volumes of water ensures their exposure to large quantities of contaminants present in the water column, and for burrowing bivalves exposure at the sediment-water interface and in sediment interstitial water.
Assessing the biodistribution in these organisms via dissection enables a better understanding of what organism tissues are exposed to ENMs and if absorption of ENMs across epithelial surfaces has occurred. The gills are often the first organ to be exposed due to their filtering role, and studies have shown that bivalve gills have the capacity to differentiate among particles as a result of particle sizes and surface characteristics,242–244 although ENMs are subsequently translocated into the digestive system. For example, Mytilus edulis had a progressive uptake and transport of SiO2 particles from the gills to the digestive gland and then to hemocytes.245 Similarly, Au ENMs accumulated primarily in the digestive gland (93 %) of M. edulis with smaller amounts in the gills (3.9 %) and mantle (1.5 %) 246 Similar findings have been observed for TiCE ENMs247 and Ag ENMs (although Ag ions were not distinguished from Ag ENMs241), while a study on ZnO ENMs showed higher Zn concentrations in the gill compared to the digestive gland.248 Once ENMs enter the organism, they have been shown to transfer across cell membranes and interact with key internal cell organelles causing cellular damage 49, 249, 250 In addition, while pristine ENMs may be smaller than the preferred size for uptake by bivalves, either homo- or heteroagglomeration may change the bioavailability of the ENM based upon the filtering capacity of the gills or particle capturing apparati. Therefore, a number of researchers point out the importance, particularly in high ionic strength marine waters, of characterizing the ENM agglomerates to which organisms are exposed.244, 251, 252
There are some important considerations for both laboratory procedures and data interpretation when working with bivalves. Bivalve organs typically dissected include the gills, digestive gland as well as the gonad tissue in mature animals. The hemolymph can be collected via a syringe from the adductor muscle.234 There is a concern that these invertebrate animals have an open circulation system and any ENM will bathe all the internal organs in an undirected manner (i.e., not via a blood vessel253). Direct contact with the organs in an open circulatory system may change the interpretation of both the internalized dose and the notion of a true target organ. Practically, at the bench, it becomes even more important to ensure that all of the internal organs are suitably washed, as without this step the hemolymph may contaminate all tissues and lead to erroneous estimate of actual tissue burdens. In bivalves, because of this, there is also a concern that excretory products may incidentally contaminate the tissue sample. Special attention needs to be given to the pseudofeces or biodeposits produced by bivalves. In the animal’s normal biology, biodeposits are an efficient way of preventing the accumulation of unwanted naturally occurring particulates and insoluble metal deposits. These biodeposits alter the ENM form when it reenters the environment, as the ENMs will be packaged in a carbon rich, dense, mucous bundle that most likely enters the sediments and will be reprocessed by deposit feeders or organisms that filter larger particles. During bivalve bioaccumulation experiments, only a minute contamination of bivalve tissue with such biodeposits can lead to overestimation of the tissue metal concentration. There is also concern about particles settling onto the external surfaces of the body organs in the elevated ionic strength conditions of the hemolymph or in seawater 254 However, surface-binding experiments such as those conducted on trout tissue have not been performed with shellfish. Careful dissection and detailed washing procedures are needed to avoid this contamination, and such methodological details should be reported for ENM studies with bivalves.
Multicellular Species Case Study #3: Daphnia
Daphnia species have been widely used in bioaccumulation studies, as they represent a key level in trophic chains while feeding on unicellular organisms and serving as prey for second consumers. Uptake, elimination and bioaccumulation studies with Daphnia magna have been described in the literature for a broad range of metal-based ENMs and CNMs.11,12,15, 71, 255–258 Bioaccumulation experiments with D. magna have been conducted using experimental designs that include an uptake followed by an elimination phase in clean media, or by independent experiments evaluating both processes. Exposure through media only or via contaminated food (e.g. algae) are also experimental setups available in the literature.257 Uptake phase durations range between 1 h to 48 h, while elimination phases last similar periods or can be extended up to 10 d.259
The organism age varies substantially among studies of ENM bioaccumulation (<1 d256 to 14 d260) which impact ENM bioaccumulation results as a result of different body morphometries; similar findings were observed for bivalves as described in the Supporting Information. It has been suggested that differences in body burden that result after MWCNT exposure may stem from differences in the sizes of the organisms: smaller organisms, for which the gut tract is a larger fraction of the total organism, may have higher body burdens than larger organisms if the gut tract is not voided.255 Within this variability regarding age, the organism’s growth and reproductive status should be considered in ENM bioaccumulation experiments, avoiding as much as possible different life-cycle stages within sampling times. Before the uptake phase, some studies also report the need to void daphnids’ guts,96, 258 while other studies report a short feeding period prior to ENM exposure.261 These practical details can complicate comparing data, as differences in age, exposure time and gut status (voided or not) can cause substantial differences in bioaccumulation patterns among studies. There is also a relationship between ENM uptake, size of the organism, and volume of the ENM test media as described in more depth in the Supporting Information.
Daphnids sampled for analysis are expected to adsorb ENMs to their carapace. Several studies have already identified the presence of attached ENMs in moult samples.96,262 Therefore, several procedures have been described for sampling daphnids for chemical analysis. These methodologies range from a gentle wash96 to a vigorous agitation by pipetting daphnids in and out of the water,261 to collecting daphnids with a small sieve and rinsing them with Milli-Q water 257 or with the exposure media.12, 258 Although different procedures are described, little evidence is provided on method effectiveness. While adsorption onto the carapace can be seen as an external accumulation that will typically not directly harm the organisms (unless by impacting molting), external accumulation can be important to trophic transfer.
Multicellular Species Case Study #4: Soil invertebrates
Soil is considered a major sink for chemicals and also for ENMs, which may reach this compartment through direct ENM application as an agrochemical (e.g, fertilizer pesticide, or biocide), or from solid waste including sewage sludge.106 Soil is an extremely complex matrix, and the transformation and fate of ENMs in soils are similarly complex.106, 263, 264
Soil invertebrates can accumulate ENMs or dissolved, or otherwise transformed, materials from the soil or soil porewater both through direct dermal contact or orally via ingestion with food.114, 265 Key soil properties such as pH, organic matter content, clay mineralogy and cation exchange capacity, as well as the specific physiology of the species, can all potentially influence ENM bioaccumulation potential. For assessment of bioaccumulation of ENMs in these species, ENM characterization and quantification both in soil and organisms can help to understand routes of uptake and modes of action and also to gauge the potential for trophic transfer. Similar to fish and bivalves, key tissues that are recognized as key sites of ENM accumulation can be readily dissected including tissue associated with the posterior gut and surrounding chlorogogenous tissue of earthworms and mid-gut gland of snails.265 Many soil-dwelling organisms, similar to bivalves, may produce inorganic biominerals in response to ENM exposure either directly for accumulated intact particles or, more often secondarily after initial dissolution. The production of the metal rich granules has been investigated for species including earthworms, soil arthropods and molluscs 266–269 Results have shown that the specific routes of metal ion trafficking may vary between metals, with some forming inorganic mineral deposits (e.g. phosphates ligands) and others associating into metal ion clusters with sulfur rich ligands. The biogenic production of nano-structures has also been shown for Ag ENMs and Ag ions in earthworms.20 The potential toxicological availability and potential for trophic transfer can vary between these different forms.
Soil invertebrates can be hard bodied or soft bodied, depending also on their life stage. These differences are important with respect to bioaccumulation, as the presence of a hard integument can greatly affect the balance between the two major routes of uptake across the dermal and oral pathways.270 Soft bodied organisms may accumulate chemicals through skin (dermal uptake),271 which is less likely for hard bodied organisms. Furthermore, hard bodied organisms that shed their integument during growth have this additional and potentially efficient route of excretion that may not be available to soft bodies species.
It has been shown that in (soft bodied) earthworms uptake of Ag ENMs is both dermal as well as through the gut, and that the distribution of the Ag within the organisms differed for Ag ENMs and Ag ions.265 In contrast, earthworm uptake of stable isotope labelled ZnO ENMs was dominated by uptake from the gut, as earthworms precluded from feeding only accumulated approximately 5 % of the Zn assimilated by feeding individuals.114 The two metals used differ with respect to their physiological requirement, with Zn being an important essential nutrient, and thus potentially subject to efficient gut assimilation, while Ag has no known physiological function. Hence, earthworms may be particularly efficient at assimilating Zn from their diet to meet physiological requirements, which may also contribute to the apparent differences between the two studies of these ENMs with different compositions. Another study of the uptake of different forms of Ag (ionic, pristine and sulfidized nanomaterials) has shown that uptake was primarily related to ionic Ag.20 Uptake of non-dissolving Ag2S-ENMs was minimal, while uptake kinetics of Ag-ions and pristine, rapidly dissolving, ENMs were more or less similar.
For hard bodied organisms, studies with isopods have indicated that uptake can occur both via food, by direct contact of the body integument with the soil, and by soil ingestion.100 Establishing the dominance of these two exposure routes under environmentally relevant scenarios is difficult as it can be influenced by the release form and environmental fate of the tested ENMs. Some studies have shown that metals derived from ENMs can be accumulated in the hepatopancreas of isopods in the S-cells, along with S and Cu granules.100, 272 Hence physiological mechanisms may play an important role in determining ENM partitioning and intracellular fate that ultimately govern bioaccumulation potential.
Multicellular plants
The potential bioaccumulation of ENMs in plants is of obvious concern for trophic transfer in the food chain and risks to food safety. One important consideration for plant bioaccumulation studies is the accumulation metrics (Figure 1). In the literature, BAF values for plants have been estimated by calculating the ratios of ENM concentrations in plants to ENM concentrations in the exposure media (e.g., hydroponic solution or soil).41 For plants, it is important to provide accumulation metrics using both the ENM concentration and the total EMN mass in the tissue of concern. By plotting the data using both metrics, one can address the potential for growth dilution, as well as physiological changes as the plant moves from vegetative to reproductive growth stages. In addition, one should measure the dry mass of the plants given that some ENMs such as MWCNTs can alter water accumulation.273 To assess ENM bioaccumulation, either root (through hydroponic or soil exposure) or foliar exposures have been studied. The following case studies address the major considerations for measuring ENM bioaccumulation in plants under each exposure scenario.
Plant case study #1: Hydroponic exposure
Hydroponic (growing plants in liquid culture media274) exposure is often used in nanotoxicology research, since its less complex but defined exposure medium composition facilitates ENM characterization. Hydroponic exposures ensure a relatively greater bioavailability of ENMs to plants, in comparison to exposures via the soil matrix which can sorb or otherwise change ENM bioavailability.
To conduct a hydroponic exposure, the test medium can either be reagent water123 or a defined nutrient medium for plant growth such as Hoagland’s solution of different strengths.275 Water has been commonly used in short-term exposure (e.g. < 7 d), although nutrient media is more often used for longer experiments.151 The medium selected should be fully characterized, as its properties (e.g. pH and ionic strength) can affect ENMs behavior and bioavailability. For example, TiO2 ENMs may undergo significant agglomeration (measured as hydrodynamic diameter increase with time) in plant growth media.276 This may result in ENM settling and heterogeneous ENM exposure concentration within the test medium. Although TiO2 agglomeration has been found to decrease linearly with the dilution of the plant growth medium,276 solutions with low ionic strength may physiologically stress the test plant species.277 Therefore, the choice of the specific test medium may depend on the purpose of study and the requirements of the plant species. In some cases, assessing ENM bioaccumulation using a series of test media with different composition and characteristics may allow investigating the effects of environmental conditions on ENM behavior, bioavailability, and bioaccumulation.108
The quantification and characterization of ENMs during exposure may raise another issue: how to maintain a constant ENM exposure for plant bioaccumulation measurements. The U.S. EPA guideline OCSPP 850.4800 for testing plant uptake and translocation specifies that during exposure, the chemical concentration in the test medium should not change by over 20 % as compared to the initial (or nominal) dose.278 This is in accordance with the OECD guidelines for aquatic toxicity testing.279 However, this may be challenging to implement and perhaps not even environmentally relevant for ENM testing, given the dynamic transformations that may occur for many ENMs (e.g. dissolution and agglomeration) in aqueous exposure media.279 In addition, plants continue to take up water from the medium and evapotranspire during exposure,277 which may gradually concentrate the ENMs within the test medium. In some hydroponic studies, water or nutrient solution was added to the system to compensate for water loss due to evapotranspiration.280 In other studies, the test medium was periodically renewed during a relatively long period of exposure (e.g., 15 d275 and 4 weeks281). In any case, the specific procedure used during exposure should be appropriate for the questions being asked and should be clearly described. It is worth noting that ENM behavior and bioavailability may be significantly modified in the presence of plants, due to the influence of root exudates (including amino acids, organic acids, and sugars) and a microbial community that develops in the solution.282, 283 Therefore, one should quantify and characterize ENMs in the medium during and after plant exposure,123, 277 which may enable a better understanding of the actual exposure conditions and may assist in the possible interpretation of bioaccumulation results relative to ENM concentrations and speciation.
During hydroponic exposure, ENMs are in immediate contact with plant roots, and may attach extensively to the root surfaces prior to accumulation.151 Therefore, one major consideration in assessing ENM bioaccumulation in plants is to distinguish absorbed ENMs from that adsorbed on the surfaces of root tissue. If the purpose of the study is to visualize the interactions between ENMs and root surfaces, then no washing may be needed.284 If, however, the ENM concentration within the roots is of interest, then proper washing to remove surface associated ENMs before analysis is necessary to avoid overestimating bioaccumulation. Washing has been conducted using distilled or deionized water,123, 275, 281, 285 phosphate buffer,286 dilute acid (e.g. 0.01 M HNO3),287 and complexing agents,288; notably, few studies actually investigated the removal efficiency of the washing steps. For example, nearly 80 % and 10 % of ceria initially measured in unwashed cucumber roots was removed in the first and second round of washing by deionized water, respectively, with negligible removal in the subsequent three rinses.285 Metal complexing agents (NaOAc and Na4EDTA) have been found to be more effective than water, as they compete for metal ions. Similarly, a surfactant desorbed CuO ENMs from wheat root surfaces, with the mode of action being acceleration of CuO ENM dissolution and subsequent efficient complexation with dissolved Cu ions.288 Even after washing, it is possible that there may be some ENMs fraction that is strongly adsorbed on the external root surface.123, 151, 288 When measuring ENM bioaccumulation in aboveground tissues, washing may not be necessary, given that these tissues were not in direct contact with ENMs during exposure.151
Plant case study #2: Soil exposure
Although hydroponic studies have advantages such as simple and defined exposure media which allow for increased bioavailability, this design does lack a certain degree of environmental relevance.151 Soil matrices can affect ENM fate and bioavailability57 due to the interactions with complex soil components including microorganisms.107 In addition, some plant species may develop different root morphologies (e.g. a lack of root hairs) when grown under hydroponic conditions,289 and may have different ENM accumulation patterns in soil than for experiments using hydroponic exposures. Therefore, it is necessary to assess ENM accumulation in plants grown to maturity in soil to full characterize potential risk to food safety. Some of the considerations in hydroponic exposure are also applicable to soil; therefore, those specific to soil will be emphasized here. The choice of a particular soil type needs to be fit for the purpose of the experiment. Both the OECD Test No. 208290 and the U.S. EPA guideline OCSPP 850.4100291 describe that either natural or artificial soil (with a high sand content and up to 1.5% organic carbon) may be used in the terrestrial plant seedling emergence and growth tests. Additionally, the OECD standard artificial soil (10% sphagnum peat, 20% kaolin clay, 69.5% sand, 0.5% CaCO3) specified for earthworm acute toxicity testing292 has also been used in assessing ENM uptake in soil-grown plants 293 Since standard artificial soil is of known and less complex composition than natural soils, its use may better allow interpretation and reproducibility of the bioaccumulation tests, as well as benchmarking across different studies.108 However, artificial soil not only lacks the physicochemical composition and complex structure of natural soil, but it is also biologically limited with regard to natural soil microbial communities that are known to interact with plants and to affect ENM behavior.57, 107 Thus, natural soil would be a more environmentally relevant exposure matrix for assessing ENM bioaccumulation. In either case, the soil used should be sufficiently characterized for parameters including texture, pH, organic matter, major nutrients, cation exchange capacity, moisture content, and redox potential.108, 294 This is necessary because soil characteristics affect both plant growth and ENM behavior,295 including uptake by plants 296 Standard natural soils such as the LUFA soils (http://www.lufa-speyer.de/) are available and have been used in ecotoxicity tests.101, 297, 298
In natural soils, there are a large number of plant-root symbioses, such as mycorrhizae. Rhizosphere microbial communities, including populations that form symbioses with plants, can affect local geochemical characteristics relevant to ENM dissolution or similar physicochemical processes that in turn affect exposure at the plant root and therefore plant uptake of ENMs. Notably, this applies to the leaf surface as well, where a phyllosphere community exists. Plants may respond to rhizosphere plant-microbe interactions by changing their exudate chemistry, which can in turn further alter ENM bioavailability and uptake.299 Conditions of the rhizosphere or phyllosphere microbial communities—including changes from sampling and storing (e.g. refrigeration) of field soil, or including growing plants under variable conditions that would change phyllosphere physiochemistry—could alter ENM fate and distribution to plants, which in turn affects bioaccumulation. Given these complex interactions, investigations should ideally acknowledge such complexities in study designs by carefully designing exposures and sampling practices. It is also important to archive samples (e.g. of soil) that can be analyzed to reflect the realistic conditions of the plant and matrix (and therefore associated microbial communities) in situ so that changes leading up to the actual exposure can be considered when interpreting results. For example, Chen et al.300 showed that a significant reduction of microbial biomass and a shift in microbial community composition occurred during storage of soil plus biosolids mixtures for six months at 4 °C.
During long term soil exposure, irrigation using either water57 or nutrient solution (e.g. Hoagland’s solution)295 will be necessary. When quantifying uptake of metal or metal oxide ENMs, it is important to quantify the background concentration of elements of the same composition as the ENMs in both the irrigation water or other irrigating solution and soil;301 it should be noted that there is a potential for loss of sensitive tissues during washing which may decrease the biomass. It is also useful to place a tray under the pot to collect any leachate from irrigation, so that any potential leaching of ENMs can be monitored quantitatively.302
The overall sample preparation procedures and analytical techniques for ENM quantification and visualization in soil-grown plants are similar to those used in hydroponic studies. One specific consideration for soil exposure is that additional care is needed to fully recover the root system from the soil with minimal root system disturbance; this can be particularly difficult with species that have fibrous root systems.57, 281, 301 If a significant amount of belowground biomass is lost, ENM bioaccumulation (based on total mass) might be underestimated. Washing belowground harvested biomass using tap or deionized water is commonly used to remove the surface associated soil particles and ENMs.57, 281, 301, 302 After exposure, it is important to dissect the plants to obtain the different tissue types so as to fully characterize inplanta translocation processes (e.g., stem, leaves, pods, roots, seeds, and nodules).
Plant case study #3: Foliar exposure
While most work conducted thus far on plant-ENM interactions has focused on root exposure through soil or hydroponic media, foliar exposure is another significant pathway by which terrestrial plant species may interact with ENMs. This pathway encompasses a wide range of exposure routes, including aerial deposition of industrially derived materials such as nanoceria from vehicle combustion, airborne particles from tire or paint weathering, resuspension of contaminated soils, and direct application of nano-enabled agrichemicals such as nanopesticides to suppress pathogens and pests and nanofertilizers to enhance growth yield. In the foliar exposure literature, a limited number of studies have a toxicity focus but a larger body of work has addressed issues of intentional application, largely through nano-enabled agrichemicals. Importantly, within a given experimental design, the precise nature of the exposure (dose, concentration, application regime, etc.) will vary with the questions being investigated and the overall goal of the study.
In studies seeking to evaluate toxic response, isolating the exposure route is recommended. For example, one study compared the in planta accumulation and distribution of TiO2 ENMs in rapeseed and wheat after both separate foliar and root exposures.303 The authors noted that particles accumulated in the plants through both pathways, although toxicity was negligible by both routes. Studying both routes of uptake simultaneously is possible but would require ENM exposure in one pathway using an isotopically enriched or labeled material. Care may also be needed to prevent, or at least be aware of, stem exposure; many species have stomata on stem tissue and contamination there could confound attempts to mechanistically describe in planta movement of particles from exposed leaves to other tissues. Although some work has been done on ENM transformation in soils and within plants (see above), reactions on the plant leaf surface remain almost completely unexplored. In certain studies, it may be important to differentiate between surface adsorbed materials (on or within the cuticle, attached to the outer epidermis) and that fraction which has been truly absorbed into the tissue by diffusion through the cuticle and epidermis or through the stomata. In such cases, a number of techniques for the removal of the surface adsorbed particles could be used, including mild acid rinsing or washing with specific organic solvents (given the hydrophobic nature of the cuticle). Importantly, the use of any such removal technique would first require validation of the method through the appropriate quality assurance and quality control checks. This could include injecting materials into the tissue to ensure that the rinsing procedures do not impact the absorbed particles or using labeled particles on the surface only to ensure complete or near complete recovery. Separately, in an experiment involving foliar exposure of TiO2 ENMs to lettuce in pristine form or from a weathered paint product, both particles were found in exposed plants.304 Alternatively, lettuce exposed to foliar treatment of Ag ENMs exhibited ENM entrapment within the cuticle, followed by entry through the stomata.305 Importantly, either ex planta or in planta oxidation resulted in significant complexation of Ag ENMs to thiol-containing biomolecules by a potentially significant series of biotransformation reactions. Additional important considerations for this type of work include possible physical or oxidative damage to leaf structures or morphology, as well as the role of the phyllosphere in potential ENM transformations and the impact of ENM exposure on the associated microbial community. It should also be noted that species-specific properties such as cuticle thickness and stomatal distribution on shoot tissues will significantly impact the uptake and accumulation of ENMs. In studies where determining the mechanism of uptake is of interest, being able to determine the distribution of ENM across the leaf surface could be important. EM with EDS can be used for this purpose, although labelled or fluorescently-tagged ENMs facilitate use of other analytical and visualization methods. Laser ablation ICP-MS may also be a useful technique in these studies.
For foliar exposure studies designed to exploit nanoscale size properties, environmental conditions such as moisture status, water potential, or UV light impacts may be important as they will influence leaf physiology. Importantly, these factors are dynamic during growth and exposure. For example, in an early study, leaf stomata were shown to readily permit entry of materials as large as 50 nm, although not all stomata were functionally equivalent, with only some structures allowing particle entry.306 The authors speculated that the wettability of the guard cell cuticle was the key factor controlling activity. Alternatively, ENM exposure may alter stomatal function. Foliar Fe2O3 ENM application increased stomatal opening, with subsequent increases in soybean photosynthesis and growth.307 Both particle size and particle number were key factors impacting uptake and translocation of ENMs upon delivery to watermelon leaves with an optimized aerosol platform.308 Again, understanding species-specific properties of the plant such as stomatal distribution on the leaves, stems, and other tissues plus cuticle thickness, will be important.
One other area of interest is the use of foliar applications of nano-enabled agrichemicals in response to infection or disease. It is also important to note that the majority of commercial agrichemicals intended for foliar application have additional materials in the formulation, including surfactants or “stickers” to promote retention on the leaf surface.309 The activity of these potentially complex formulation materials will also influence the nature of the exposure under realistic conditions, and their activity must be taken into consideration. A final consideration is the role of pathogens in affecting uptake as these may affect leaf or stem tissue leading to necrotic damage. These changes can result in the loss of the cuticle barrier, and ENM entry through those tissues may change the amount of ENM bioaccumulation in comparison to plants not impacted by pathogens.
Trophic transfer
Laboratory trophic transfer studies
Many of the considerations in trophic transfer studies are similar to those which have been described in feeding studies, yet there are also a number of specific considerations. Trophic transfer studies involve exposing one population of organisms to an ENM and then feeding the prey with bioaccumulated ENMs to a predator type of organism, for example in a simulated laboratory food chain. Because synchronization of the exposures of the populations of two or more species is challenging, researchers may be tempted to simply “spike” the organisms from the lower tropic level with ENMs. An example of this could be spraying an ENM onto a leaf and then feeding it to an insect, or growing algae and then simply spiking a suspension of the algae with an ENM. Two studies have demonstrated that this approach can underestimate the bioavailable fraction of ENMs for the predator species. For example, the assimilation of Au ENMs by tobacco horn worms from tobacco plants which had taken up the ENMs hydroponically was significantly higher than assimilation from leaves onto which Au ENMs had been sprayed.18 Similarly, bullfrogs accumulate Au ENMs more efficiently from consuming earthworms raised in Au ENM contaminated soil than when they were exposed to pristine Au ENMs via oral gavage.40 There are many possible explanations for this behavior including biological modifications of the particles, such as acquisition of a protein corona, that favor their cellular uptake. In a third study with SWCNTs, ambiguous results were reported when algae were amended with a SWCNT suspension and then fed to bivalves which were then fed to polychaetes.310 No evidence of trophic transfer was detected. As noted in the previous studies with Au ENMs, there are several possible explanations for these results such as analytical interferences and poor uptake of SWCNTs by the algae.310
Numerous challenges exist in preparing ENMs for inclusion in trophic transfer studies via food consumption. Researchers must balance loading prey items with ENM concentrations high enough to observe an effect at the next level and keeping ENM concentrations low enough to avoid unwanted toxicity to the prey organisms and to stay environmentally relevant. Exposure time of prey to the ENMs must also be balanced to maximize the uptake concentration before elimination occurs and decreases the concentration. It should be noted that, in the case of food web accumulation, ENMs that are attached to organisms or in their gut but not fully assimilated in the tissues are still of importance. Hence, decision about the preparation of plant and animal food items for the consumers species should be sensitive to such considerations depending on the aims of the study.
Algae or bacteria are often starting points in trophic transfer studies as they are relatively easily cultured and are common food items for many invertebrates. Sorption to or uptake by unicellular organisms is affected by surface charge of both the ENM and the organism, as well as by the presence or absence of cell walls and membranes which may serve as a barrier to ENMs.311 Coatings on ENMs such as citrate or other organic compounds increase the stability of the ENMs in aquatic environments and play a critical role in the interaction of ENMs with an algal or bacterial cell.191 Sorption to the outside of single-celled organisms is another mechanism to move ENMs through the food chain; however, care should be taken through multiple washing steps and analysis of the prey media to ensure that the ENM is thoroughly bound to the prey organism and not easily dislodged to prevent exposure to the next trophic level through direct contact with ENMs rather than by food uptake. Collection of ENM-exposed prey can be performed using procedures that include various methods of filtration, centrifugation and rinsing steps. Density gradient separation is described in detail in the single cell species section and is a robust method for separating single-celled organisms from suspended ENMs.
For uptake at the next trophic level(s), the same concerns exist with respect to determining the length of exposure to reach maximal uptake with a minimum of elimination and toxicity to the prey organism. Using an elimination period for prey organisms is not generally recommended, because many consumers will usually eat prey whole and as such exposure will be both to prey tissue and also via the gut load. However, consumption of the gut content does not occur for some organisms such as the European mole (Talpa europaea), which will often squeeze the gut contents from earthworm prey before consuming them.312 The timing of introducing ENMs to prey and subsequent transfer of the ENM through a food web must also be considered. Researchers have generally exposed protozoans and crustaceans used as secondary trophic level prey for periods of 1 d to 7 d. While most researchers rinsed the prey, the decision could be based upon the objective of the exposure. It can be argued that rinsing the organisms may represent the ENM that is truly incorporated within the prey while, conversely, not rinsing the organisms may be more representative of the body burden that the organisms may experience in the field. Generally, some rinsing is necessary to ensure that ENMs are transferred via the food and not via exposure media. Additionally, when composite ENMs, such as QDs, are being transferred, it is important to assess if the composite ENM has decomposed inside the prey organism or between transfers.
Mesocosm and Field Studies
Inherently, quantifying bioaccumulation is a step towards understanding the potential for ENM trophic transfer and biomagnification, both of which are important concerns in ecotoxicology. Although many controlled, multiple-population based, trophic transfer studies regarding ENM biomagnification have been performed for food chains of microbial23, 82, 132 and higher17, 40, 313 organisms, the assessment of ENM distribution in complex food webs consisting of many biotic trophic levels with multidirectional nutrient flows is more rare. In some studies, ENMs are isotopically labeled to allow for specific quantification of low ENM bioaccumulation abundances, as would occur with initially low exposure concentrations,82, 314 although the use of stable isotopes does not necessarily indicate that the bioaccumulated material is still nano-sized. However, use of isotopically-labeled ENMs in large scale mesocosm studies is unrealistic as the synthesis of labeled ENMs is specialized and typically expensive, and radioactive isotope use is more safely conducted at small scales under highly controlled conditions.
Determination of trophic status in mesocosm or field studies can be challenging, a challenge not restricted to studies on ENMs.270 Furthermore, many organisms feed from multiple food chains and trophic levels during their lifespans or even simultaneously in the case of omnivory. Stable isotope (e.g. 13C and 15N) and ENM bioaccumulation measurements of organisms at various trophic levels in a food web may be used to infer predator-prey interactions that may influence final ENM distributions, such as has been utilized in a study of TiO2 in a paddy mesocosm.315 However, stable isotope methods need to be used with caution as they can only be used to determine trophic structure of relatively simple food webs. For example, only two sources of coupled nitrogen and carbon administered into a food chain can be traced with conventional 15N and 13C studies.316 If more sources exist at the base of food chain or if nitrogen and carbon cycling are decoupled, then erroneous determinations of trophic status result.317 In such cases, traditional methods, such as the examination of stomach contents, may provide more reliable information.
Study designs would ideally be well-informed by an existing understanding of the system ecology. For example, CeO2 ENMs were traced through an aquatic food web by using temporally and spatially dense sampling, since ENMs quickly compartmentalized by settling into sediments, then redistributed within food webs starting from the benthos.318 In this case, understanding the dynamics of physicochemical processes affecting ENM compartmentalization, relative to feeding and organismal reproductive rates, allowed for judiciously designing a biotic sampling program that revealed ENM distribution across multiple trophic levels.318
Future work and next steps
The recommendations discussed here are intended to inform the design (Figure 1) and interpretation of studies examining ENM bioaccumulation. While the best practices for conducting nanomaterial bioaccumulation assays have been described for a broad range of ecological receptors, additional research described throughout this manuscript can further refine these methods. One key factor is the further development of analytical methods to quantify ENMs in the test species. Different methods can be refined to quantify ENMs in individual single-celled organisms, populations of these organisms, or multicellular species. These include a range of different analytical and microscopy methods that can be used for assessment ranging from determination of overall concentrations to assessments of localization and chemical form.81 This is especially important for ENMs that may be transformed in which case it is valuable to quantify the different forms. One promising approach that is increasingly being utilized for the detection and quantification of ENMs in biological samples is spICP-MS. The value of this method is that it can distinguish between dissolved ions and ENMs and for directly measuring particle number concentrations. In addition to continued refinement of this technique to improve its robustness, research is needed to develop effective extraction techniques, which minimally change the ENMs for different types of organisms. One challenge with these measurements though is that there typically are not readily available orthogonal techniques to evaluate the size distribution of ENMs in the organisms for comparison.
Separation of ENMs from suspended particles is another critical consideration for research on ENM bioaccumulation by single-celled organisms, small multicellular organisms, and in subcellular fractionation studies using cells or tissue samples from larger species. The need for more effective and complex separation procedures such as density gradient centrifugation is among the main differences in the analytical methods for bioaccumulation of ENMs by these species as compared to studies with dissolved chemicals. Additional research is needed to evaluate the conditions under which sequential differential centrifugation is sufficient for separating ENMs from the test species or different cellular fractions and when density gradient centrifugation is needed. In addition, the application of density gradient centrifugation to separate freely dispersed ENMs from ENMs associated with different cellular fractions as compared to sequential differential centrifugation procedures need thorough evaluation. This will require the development and testing of density gradient centrifugation procedures to separate organelles for different types of tissues or cells and determining how interactions with ENMs affect the buoyant density of organelles and cells. This can result in a set of clear recommendations on the application of this approach in ENM bioaccumulation studies.
One of the challenges with providing guidance on bioaccumulation studies with ENMs is that the recommended protocol depends to a large degree on the purpose of the measurements. In some instances, a fit for purpose method would include voiding of the gut tract while for other situations, it would be helpful to measure the body burden without voiding the gut tract. Even when the aim is to assess the exposure of consumer in trophic transfer studies it may be necessary to treat samples in a different way depending on, for example, whether the predator consumes or avoids eating the prey gut content. Quantifying the kinetics of the uptake and elimination processes can provide key insights into the bioaccumulation processes and is recommended as opposed to measuring a bioaccumulation-related factor (e.g., BAF) at a single time point. For comparison to results with dissolved species, voiding the gut tract of multicellular organisms is an appropriate step. Results from plant ENM bioaccumulation studies should be reported both in terms of ENM concentration and the total mass of ENM in the plant tissue. When testing ENM bioaccumulation in soils and sediments, it is important to assess how bioaccumulation factors and bioaccumulation kinetics relate to the soil or sediment porewater concentrations as compared to the total soil or sediment concentration, because the porewater concentrations may be more bioavailable.
The robustness of ENM bioaccumulation methods in general can be improved. Given that the methods among studies vary regarding how to conduct these experiments, it would be helpful to know the sensitivity of bioaccumulation methods to changes in the protocol. For example, it has been shown that organism size can impact ENM bioaccumulation studies with bivalves, and it has been proposed that the daphnid size can impact bioaccumulation measurements in the absence of gut voiding. However, to date there have not been systematic studies to specifically evaluate how the age of the daphnid used in bioaccumulation studies impacts on the results. Hence, it remains unclear whether the use of standard age and size organisms is needed and the extent to which studies conducted with different age cohorts can be directly compared. In plant bioaccumulation studies, a step of the assay protocol that often varies is the washing procedure used to separate weakly-attached ENMs from the roots. However, the impact of these different washes procedures on ENM bioaccumulation results and their comparability across studies is unclear. It is likely that no one method can be the requirement to fully remove all loosely attached ENMs, while fully retaining root fine tissue structure integrity. The reproducibility of results (e.g., to what degree would a similar result be obtained if the experiment was repeated) is unclear and often not reported. If a bioaccumulation experiment is repeated within a single laboratory, it would be helpful if these results were reported, such as in the Supporting Information which typically do not have length limits. Another important topic within each study is to ensure that there is an adequate number of replicates to make robust statistical comparisons among conditions tested. It is also important that sufficient detail is provided about if each replicate within a measurement is from a single organism or the average of multiple organisms.
The practices and discussion described here will enable researchers to make more accurate ENM bioaccumulation measurements using a broad range of species. This will help advance the field of environmental nanotoxicology through supporting regulatory decision making and elucidating interactions of ENMs with organisms. Careful attention to the key topics discussed throughout this paper will facilitate researchers making results that are comparable across studies and reproducible, a key issue in science in general319, 320 and also especially in nanotoxicology.321–323 Overall, these measurements will support the sustainable commercialization of nanotechnology.
Supplementary Material
Environmental Significance Statement.
While the potential for engineered nanomaterials (ENMs) to bioaccumulate has been the focus of substantial research attention, how best to conduct needed measurements has yet to be comprehensively evaluated for the broad range of organisms present in the environment. This analysis develops key recommendations for improving the quality of ENM bioaccumulation measurements during different steps of the measurement procedure, such as how to avoid artifacts in the analytical measurements in the organism tissue and environmental media, and unique considerations for different types of test organisms. The suggested strategies and discussion described herein will help to improve the robustness of ENM bioaccumulation measurements and promote the sustainable development of products utilizing ENMs.
Acknowledgements
This research was funded by the National Science Foundation (NSF) and the Environmental Protection Agency (EPA) under Cooperative Agreement DBI-0830117. Any opinions, findings, and conclusions expressed in this material are those of the author(s) and do not necessarily reflect those of either the NSF or EPA. This work has not been subjected to EPA review, and no official endorsement should be inferred. This work was also supported by NSF CBET 1437451, UKRI/NERC Research Grant NE/N006224/1, USDA grant 2016-67021-24985, USDA Hatch CONH00147, project NanoFASE through the European Union’s Horizon 2020 research and innovation programme under grant agreement number 646002, and financial support to CESAM (ETD/AMB/50017/2019), by FCT/MCTES through national funds. This research was initiated at the 2016 U.S-EU: Bridging NanoEHS Research Efforts workshop in the Ecotoxicology Community of Research. We thank Rhema Bjorkland of the National Nanotechnology Coordination Office for assistance with this project.
Footnotes
Conflict of interest
There are no conflicts to declare.
NIST disclaimer
Certain commercial products or equipment are described in this paper in order to specify adequately the experimental procedure. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that it is necessarily the best available for the purpose.
FDA Disclaimer
Although an author is currently an FDA/CTP employee, this work was not done as part of his official duties. This publication reflects the views of the authors and should not be construed to reflect the FDA/CTP’s views or policies.
References
- 1.ISO (International Organization for Standardization), TS 80004–1: nanotechnologies — vocabulary — Part 1: Core terms. 2010.
- 2.ASTM (American Society for Testing Materials) International, E2456–06: standard terminology relating to nanotechnology. 2006.
- 3.Petersen EJ, Pinto RA, Shi XY and Huang QG, Impact of size and sorption on degradation of trichloroethylene and polychlorinated biphenyls by nano-scale zerovalent iron, J. Hazard. Mater, 2012, 243, 73–79. [DOI] [PubMed] [Google Scholar]
- 4.Mao HY, Laurent S, Chen W, Akhavan O, Imani M and Ashkarran AA, Graphene: promises, facts, opportunities, and challenges in nanomedicine, Chem. Rev, 2013, 113. [DOI] [PubMed] [Google Scholar]
- 5.Sun J, Petersen EJ, Watson SS, Sims CM, Kassman A, Frukhtbeyn S, Skrtic D, Ok MT, Jacobs DS, Reipa V, Ye Q and Nelson BC, Biophysical characterization of functionalized titania nanoparticles and their application in dental adhesives, Acta Biomaterialia, 2017, 53, 585–597. [DOI] [PubMed] [Google Scholar]
- 6.Froggett SJ, Clancy SF, Boverhof DR and Canady RA, A review and perspective of existing research on the release of nanomaterials from solid nanocomposites, Part. Fibre Toxicol, 2014, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nowack B, David RM, Fissan H, Morris H, Shatkin JA, Stintz M, Zepp R and Brouwer D, Potential release scenarios for carbon nanotubes used in composites, Environ. Intl, 2013, 59, 1–11. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen T, Petersen EJ, Pellegrin B, Gorham JM, Lam T, Zhao M and Sung L, Impact of UV irradiation on multiwall carbon nanotubes in nanocomposites: Formation of entangled surface layer and mechanisms of release resistance, Carbon, 2017, 116, 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuhlbusch TAJ, Wijnhoven SWP and Haase A, Nanomaterial exposures for worker, consumer and the general public, Nanoimpact, 2018, 10, 11–25. [Google Scholar]
- 10.Bjorkland R, Tobias DA and Petersen EJ, Increasing evidence indicates low bioaccumulation of carbon nanotubes, Environ. Scl.: Nano, 2017, 4, 747–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edgington AJ, Petersen EJ, Herzing AA, Podila R, Rao A and Klaine SJ, Microscopic investigation of single-wall carbon nanotube uptake by Daphnia magna, Nanotoxicology, 2014, 8, 2–10. [DOI] [PubMed] [Google Scholar]
- 12.Tervonen K, Waissi G, Petersen EJ, Akkanen J and Kukkonen JVK, Analysis of fullerene-C60 and kinetic measurements for its accumulation and depuration in Daphnia magna, Environ. Toxicol. Chem, 2010, 29, 1072–1078. [DOI] [PubMed] [Google Scholar]
- 13.Waissi-Leinonen GC, Petersen EJ, Pakarinen K, Akkanen J, Leppanen MT and Kukkonen JVK, Toxicity of fullerene (C60) to sediment-dwelling invertebrate Chironomus riparius larvae, Environ. Toxicol. Chem, 2012, 31, 2108–2116. [DOI] [PubMed] [Google Scholar]
- 14.Lu K, Dong S, Petersen EJ, Niu J, Chang X, Wang P, Lin S, Gao S and Mao L, Biological Uptake, Distribution, and Depuration of Radio-Labeled Graphene in Adult Zebrafish: Effects of Graphene Size and Natural Organic Matter, ACS Nano, 2017, 11, 2872–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao L, Liu C, Lu K, Su Y, Gu C, Huang Q and Petersen EJ, Exposure of few layer graphene to Limnodrilus hoffmeisteri modifies the graphene and changes its bioaccumulation by other organisms, Carbon, 2016, 109, 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferry JL, Craig P, Hexel C, Sisco P, Frey R, Pennington PL, Fulton MH, Scott IG, Decho AW, Kashiwada S, Murphy CJ and Shaw TJ, Transfer of gold nanoparticles from the water column to the estuarine food web, Nat. Nanotechnol, 2009, 4, 441–444. [DOI] [PubMed] [Google Scholar]
- 17.Judy JD, Unrine JM and Bertsch PM, Evidence for Biomagnification of Gold Nanoparticles within a Terrestrial Food Chain, Environ. Sci. Technol, 2011, 45, 776–781. [DOI] [PubMed] [Google Scholar]
- 18.Judy JD, Unrine JM, Rao W and Bertsch PM, Bioaccumulation of Gold Nanomaterials by Manduca sexta through Dietary Uptake of Surface Contaminated Plant Tissue, Environ. Sci. Technol, 2012, 46, 12672–12678. [DOI] [PubMed] [Google Scholar]
- 19.Cleveland D, Long SE, Pennington PL, Cooper E, Fulton MH, Scott GI, Brewer T, Davis J, Petersen EJ and Wood L, Pilot estuarine mesocosm study on the environmental fate of silver nanomaterials leached from consumer products, Sci. Tot. Environ, 2012, 421, 267–272. [DOI] [PubMed] [Google Scholar]
- 20.Baccaro M, Undas AK, de Vriendt J, van den Berg JHJ, Peters RJB and van den Brink NW, Ageing, dissolution and biogenic formation of nanoparticles: how do these factors affect the uptake kinetics of silver nanoparticles in earthworms?, Environ. Sci.: Nano, 2018, 5, 1107–1116. [Google Scholar]
- 21.Atha DH, Wang HH, Petersen EJ, Cleveland D, Holbrook RD, Jaruga P, Dizdaroglu M, Xing BS and Nelson BC, Copper Oxide Nanoparticle Mediated DNA Damage in Terrestrial Plant Models, Environ. Sci. Technol, 2012, 46, 1819–1827. [DOI] [PubMed] [Google Scholar]
- 22.Holbrook RD, Murphy KE, Morrow JB and Cole KD, Trophic transfer of nanoparticles in a simplified invertebrate food web, Nat. Nanotech, 2008, 3, 352–355. [DOI] [PubMed] [Google Scholar]
- 23.Werlin R, Priester JH, Mielke RE, Kramer S, Jackson S, Stoimenov PK, Stucky GD, Cherr GN, Orias E and Holden PA, Biomagnification of cadmium selenide quantum dots in a simple experimental microbial food chain, Nat. Nanotech, 2011, 6, 65–71. [DOI] [PubMed] [Google Scholar]
- 24.Mao L, Hu M, Pan B, Xie Y and Petersen EJ, Biodistribution and toxicity of radio-labeled few layer graphene in mice after intratracheal instillation, Part. Fibre Toxicol, 2016, 13, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aalapati S, Ganapathy S, Manapuram S, Anumolu G and Prakya BM, Toxicity and bioaccumulation of inhaled cerium oxide nanoparticles in CD1 mice, Nanotoxicology, 2014, 8, 786–798. [DOI] [PubMed] [Google Scholar]
- 26.Silva RM, Doudrick K, Franzi LM, TeeSy C, Anderson DS, Wu Z, Mitra S, Vu V, Dutrow G, Evans JE, Westerhoff P, Van Winkle LS, Raabe OG and Pinkerton KE, Instillation versus Inhalation of Multiwalled Carbon Nanotubes: Exposure-Related Health Effects, Clearance, and the Role of Particle Characteristics, ACS Nano, 2014, 8, 8911–8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Yang JZ, Huang Q, Zhang Y, Peng C and Zhang YJ, Biodistribution and pulmonary toxicity of intratracheally instilled graphene oxide in mice, NPG Asia Mater, 2013, 5. [Google Scholar]
- 28.Petersen EJ, Henry TB, Zhao J, MacCuspie RI, Kirschling TL, Dobrovolskaia MA, Hackley V, Xing B and White JC, Identification and Avoidance of Potential Artifacts and Misinterpretations in Nanomaterial Ecotoxicity Measurements, Environ. Sci. Technol, 2014, 48, 4226–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von der Kammer F, Ferguson PL, Holden PA, Masion A, Rogers KR, Klaine SJ, Koelmans AA, Horne N and Unrine JM, Analysis of engineered nanomaterials in complex matrices (environment and biota): General considerations and conceptual case studies, Environ. Toxicol. Chem, 2012, 31, 32–49. [DOI] [PubMed] [Google Scholar]
- 30.Organization for Economic Cooperation and Development. 2012. Bioaccumulation in fish: Aqueous and Dietary Exposure. OECD Guideline 305; Paris, France. [Google Scholar]
- 31.Organization for Economic Cooperation and Development. 2008. Bioaccumulation in Sedimentdwelling Benthic Oligochaetes. OECD Guideline 315; Paris, France [Google Scholar]
- 32.Organization for Economic Cooperation and Development. 2010. Bioaccumulation in Terrestrial Oligochaetes. Test 317; Paris, France. [Google Scholar]
- 33.Handy RD, Ahtiainen J, Navas JM, Goss G, Bleeker EAJ and von der Kammer F, Proposal for a tiered dietary bioaccumulation testing strategy for engineered nanomaterials using fish, Environ. Sci.: Nano, 2018, 5, 2030–2046. [Google Scholar]
- 34.Loureiro S, Tourinho PS, Cornelis G, Van Den Brink NW, Díez-Ortiz M, Vázquez-Campos S, Pomar-Portillo V, Svendsen C and Van Gestel CAM, in Soil Pollution, eds. Duarte AC, Cachada A and Rocha-Santos T, Academic Press, 2018, DOI: 10.1016/B978-0-12-849873-6.00007-8, pp. 161–190. [DOI] [Google Scholar]
- 35.Newman MC, Fundamentals of ecotoxicology: the science of pollution, CRC Press, Taylor & Francis Group, Boca Raton, FL, 2015. [Google Scholar]
- 36.Gobas FA, de Wolf W, Burkhard LP, Verbruggen E and Plotzke K, Revisiting Bioaccumulation Criteria for POPs and PBT Assessments, Integ. Environ. Ass. Manag, 2009, 5, 624–637. [DOI] [PubMed] [Google Scholar]
- 37.Petersen EJ, Huang QG and Weber WJ Jr., Relevance of octanol-water distribution measurements to the potential ecological uptake of multi-walled carbon nanotubes, Environ. Toxicol. Chem, 2010, 29, 1106–1112. [DOI] [PubMed] [Google Scholar]
- 38.Praetorius A, Tufenkji N, Goss KU, Scheringer M, von der Kammer F and Elimelech M, The road to nowhere: equilibrium partition coefficients for nanoparticles, Environ. Sci.: Nano, 2014, 1, 317–323. [Google Scholar]
- 39.Li XM, Schirmer K, Bernard L, Sigg L, Pillai S and Behra R, Silver nanoparticle toxicity and association with the alga Euglena gracilis, Environ. Sci.: Nano, 2015, 2, 594–602. [Google Scholar]
- 40.Unrine JM, Shoults-Wilson WA, Zhurbich O, Bertsch PM and Tsyusko OV, Trophic transfer of Au nanoparticles from soil along a simulated terrestrial food chain, Environ. Sci. & Technol, 2012, 46, 9753–9760. [DOI] [PubMed] [Google Scholar]
- 41.Ma CX, White JC, Dhankher OP and Xing BS, Metal-Based Nanotoxicity and Detoxification Pathways in Higher Plants, Environ. Sci. Technol, 2015, 49, 7109–7122. [DOI] [PubMed] [Google Scholar]
- 42.Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RD, Lyon DY, Mahendra S, McLaughlin MJ and Lead JR, Nanomaterials in the environment: Behavior, fate, bioavailability, and effects, Environ. Toxicol. Chem, 2008, 27, 1825–1851. [DOI] [PubMed] [Google Scholar]
- 43.Keller AA, Wang H, Zhou D, Lenihan HS, Cherr G, Cardinale BJ, Miller R and Ji Z, Stability and Aggregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices, Environ. Sci. Technol, 2010, 44, 1962–1967. [DOI] [PubMed] [Google Scholar]
- 44.Petosa AR, Jaisi DP, Quevedo IR, Elimelech M and Tufenkji N, Aggregation and deposition of engineered nanomaterials in aquatic environments: Role of physicochemical interactions, Environ. Sci. Technol, 2010, 44, 6532–6549. [DOI] [PubMed] [Google Scholar]
- 45.Lowry GV, Gregory KB, Apte SC and Lead JR, Transformations of Nanomaterials in the Environment, Environ. Sci. Technol, 2012, 46, 6893–6899. [DOI] [PubMed] [Google Scholar]
- 46.Baker TJ, Tyler CR and Galloway TS, Impacts of metal and metal oxide nanoparticles on marine organisms, Environ. Pollut, 2014, 186, 257–271. [DOI] [PubMed] [Google Scholar]
- 47.Chowdhury I, Duch MC, Mansukhani ND, Hersam MC and Bouchard D, Interactions of Graphene Oxide Nanomaterials with Natural Organic Matter and Metal Oxide Surfaces, Environ. Sci. Technol, 2014, 48, 9382–9390. [DOI] [PubMed] [Google Scholar]
- 48.Chang X, Henderson WM and Bouchard DC, Multiwalled Carbon Nanotube Dispersion Methods Affect Their Aggregation, Deposition, and Biomarker Response, Environ. Sci. Technol, 2015, 49, 6645–6653. [DOI] [PubMed] [Google Scholar]
- 49.Rocha TL, Gomes T, Sousa VS, Mestre NC and Bebianno MJ, Ecotoxicological impact of engineered nanomaterials in bivalve molluscs: An overview, Mar. Environ. Res, 2015, 111, 74–88. [DOI] [PubMed] [Google Scholar]
- 50.Rathnayake S, Unrine JM, Judy J, Miller AF, Rao W and Bertsch PM, Multitechnique Investigation of the pH Dependence of Phosphate Induced Transformations of ZnO Nanoparticles, Environ. Sci. Technol, 2014, 48, 4757–4764. [DOI] [PubMed] [Google Scholar]
- 51.Lead JR, Batley GE, Alvarez PJJ, Croteau M-N, Handy RD, McLaughlin MJ, Judy JD and Schirmer K, Nanomaterials in the environment: Behavior, fate, bioavailability, and effectsAn updated review, Environ. Toxicol. Chem, 2018, 37, 2029–2063. [DOI] [PubMed] [Google Scholar]
- 52.Xiao Y, Ho RM, Burgess RM and Cashman M, Aggregation, Sedimentation, Dissolution, and Bioavailability of Quantum Dots in Estuarine Systems, Environ. Sci. Technol, 2017, 51, 1357–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parks AN, Cantwell MG, Katz DR, Cashman MA, Luxton TP, Clar JG, Perron MM, Portis L, Ho KT and Burgess RM, Assessing the release of copper from nanocopper-treated and conventional copper-treated lumber into marine waters II: Forms and bioavailability, Environ. Toxicol. Chem, 2018, 37, 1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parks AN, Cantwell MG, Katz DR, Cashman MA, Luxton TP, Ho KT and Burgess RM, Assessing the release of copper from nanocopper-treated and conventional copper-treated lumber into marine waters I: Concentrations and rates, Environ. Toxicol. Chem, 2018, 37, 1956–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu JY and Hurt RH, Ion Release Kinetics and Particle Persistence in Aqueous Nano-Silver Colloids, Environ. Sci. Technol, 2010, 44, 2169–2175. [DOI] [PubMed] [Google Scholar]
- 56.Liu JY, Sonshine DA, Shervani S and Hurt RH, Controlled Release of Biologically Active Silver from Nanosilver Surfaces, ACS Nano, 2010, 4, 6903–6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y, Chang CH, Ji Z, Bouchard DC, Nisbet RM, Schimel JP, Gardea-Torresdey JL and Holden PA, Agglomeration Determines Effects of Carbonaceous Nanomaterials on Soybean Nodulation, Dinitrogen Fixation Potential, and Growth in Soil, ACS Nano, 2017, 11, 5753–5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kennedy AJH, M. S.; Steevens JA; Dontsova KM; Chappell MA; Gunter JC; Weiss CA Jr, Factors influencing the partitioning and toxicity of nanotubes in the aquatic environment, Environ. Toxicol. Chem, 2008, 27, 1932–1941. [DOI] [PubMed] [Google Scholar]
- 59.Parks AN, Portis LM, Schierz PA, Washburn KM, Perron MM, Burgess RM, Ho KT, Chandler GT and Ferguson PL, Bioaccumulation and toxicity of single-walled carbon nanotubes to benthic organisms at the base of the marine food chain, Environ. Toxicol. Chem, 2013, 32, 1270–1277. [DOI] [PubMed] [Google Scholar]
- 60.Zhao Q, Petersen EJ, Cornells G, Wang X, Guo X, Tao S and Xing B, Retention of 14C-labeled multiwall carbon nanotubes by humic acid and polymers: Roles of macromolecule properties, Carbon, 2016, 99, 229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang LW, Petersen EJ, Zhang W, Chen YS, Cabrera M and Huang QG, Interactions of C-14-labeled multi-walled carbon nanotubes with soil minerals in water, Environ. Pollut, 2012, 166, 75–81. [DOI] [PubMed] [Google Scholar]
- 62.Golobič M, Jemec A, Drobne D, Romih T, Kasemets K and Kahru A, Upon Exposure to Cu Nanoparticles, Accumulation of Copper in the Isopod Porcellio scaber Is Due to the Dissolved Cu Ions Inside the Digestive Tract, Environ. Sci. Technol, 2012, 46, 12112–12119. [DOI] [PubMed] [Google Scholar]
- 63.Goodwin DG, Adeleye AS, Sung L, Ho KT, Burgess RM and Petersen EJ, Detection and Quantification of Graphene-Family Nanomaterials in the Environment, Environ. Sci. Technol, 2018, 52, 4491–4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Mortimer M, Chang CH and Holden PA, Alginic Acid-Aided Dispersion of Carbon Nanotubes, Graphene, and Boron Nitride Nanomaterials for Microbial Toxicity Testing, Nanomaterials, 2018, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kirschling TL, Golas PL, Unrine JM, Matyjaszewski K, Gregory KB, Lowry GV and Tilton RD, Microbial Bioavailability of Covalently Bound Polymer Coatings on Model Engineered Nanomaterials, Environ. Sci. Technol, 2011, 45, 5253–5259. [DOI] [PubMed] [Google Scholar]
- 66.Louie SM, Gorham JM, McGivney EA, Liu JY, Gregory KB and Hackley VA, Photochemical transformations of thiolated polyethylene glycol coatings on gold nanoparticles, Environ. Sci.: Nano, 2016, 3, 1090–1102. [Google Scholar]
- 67.Louie SM, Gorham JM, Tan JJ and Hackley VA, Ultraviolet photo-oxidation of polyvinylpyrrolidone (PVP) coatings on gold nanoparticles, Environ. Sci.: Nano, 2017, 4, 1866–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petersen EJ and Henry TB, Methodological considerations for testing the ecotoxicity of carbon nanotubes and fullerenes: Review, Environ. Toxicol. Chem, 2012, 31, 60–72. [DOI] [PubMed] [Google Scholar]
- 69.Wang G, Qian F, Saltikov CW, Jiao Y and Li Y, Microbial reduction of graphene oxide by Shewanella, Nano Research, 2011, 4, 563–570. [Google Scholar]
- 70.Salas EC, Sun Z, LUttge A and Tour JM, Reduction of Graphene Oxide via Bacterial Respiration, ACS Nano, 2010, 4, 4852–4856. [DOI] [PubMed] [Google Scholar]
- 71.Feng YP, Lu K, Mao L, Guo XK, Gao SX and Petersen EJ, Degradation of C-14-labeled few layer graphene via Fenton reaction: Reaction rates, characterization of reaction products, and potential ecological effects, Water Res, 2015, 84, 49–57. [DOI] [PubMed] [Google Scholar]
- 72.Zhang LW, Petersen EJ, Habteselassie MY, Mao L and Huang QG, Degradation of multiwall carbon nanotubes by bacteria, Environ. Pollut, 2013, 181, 335–339. [DOI] [PubMed] [Google Scholar]
- 73.Hou WC, BeigzadehMilani S, Jafvert CT and Zepp RG, Photoreactivity of Unfunctionalized Single-Wall Carbon Nanotubes Involving Hydroxyl Radical: Chiral Dependency and Surface Coating Effect, Environ. Sci. Technol, 2014, 48, 3875–3882. [DOI] [PubMed] [Google Scholar]
- 74.Hou WC, He CJ, Wang YS, Wang DK and Zepp RG, Phototransformation-Induced Aggregation of Functionalized Single-Walled Carbon Nanotubes: The Importance of Amorphous Carbon, Environ. Sci. Technol, 2016, 50, 3494–3502. [DOI] [PubMed] [Google Scholar]
- 75.Flores-Cervantes DX, Maes HM, Schaffer A, Hollender J and Kohler HP, Slow biotransformation of carbon nanotubes by horseradish peroxidase, Environ. Sci. Technol, 2014, 48, 4826–4834. [DOI] [PubMed] [Google Scholar]
- 76.Parks AN, Chandler GT, Ho KT, Burgess RM and Ferguson PL, Environmental biodegradability of [C14] single-walled carbon nanotubes by Trametes versicolor and natural microbial cultures found in New Bedford Harbor sediment and aerated wastewater treatment plant sludge, Environ. Toxicol. Chem, 2015, 34, 247–251. [DOI] [PubMed] [Google Scholar]
- 77.Levard C, Hotze EM, Colman BP, Dale AL, Truong L, Yang XY, Bone AJ, Brown GE, Tanguay RL, Di Giulio RT, Bernhardt ES, Meyer JN, Wiesner MR and Lowry GV, Sulfidation of Silver Nanoparticles: Natural Antidote to Their Toxicity, Environ. Sci. Technol, 2013, 47, 13440–13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gao X, Spielman-Sun E, Rodrigues SM, Casman EA and Lowry GV, Time and Nanoparticle Concentration Affect the Extractability of Cu from CuO NP-Amended Soil, Environ. Sci. Technol, 2017, 51, 2226–2234. [DOI] [PubMed] [Google Scholar]
- 79.Petersen EJ, Flores-Cervantes DX, Bucheli TD, Elliott LCC, Fagan JA, Gogos A, Hanna S, Kagi R, Mansfield E, Bustos ARM, Plata DL, Reipa V, Westerhoff P and Winchester MR, Quantification of Carbon Nanotubes in Environmental Matrices: Current Capabilities, Case Studies, and Future Prospects, Environ. Sci. Technol, 2016, 50, 4587–4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laborda F, Bolea E, Cepria G, Gomez MT, Jimenez MS, Perez-Arantegui J and Castillo JR, Detection, characterization and quantification of inorganic engineered nanomaterials: A review of techniques and methodological approaches for the analysis of complex samples, Anal. Chem. Acta, 2016, 904, 10–32. [DOI] [PubMed] [Google Scholar]
- 81.Schultz C, Powell K, Crossley A, Jurkschat K, Kille P, Morgan AJ, Read D, Tyne W, Lahive E, Svendsen C and Spurgeon DJ, Analytical approaches to support current understanding of exposure, uptake and distributions of engineered nanoparticles by aquatic and terrestrial organisms, Ecotoxicology, 2015, 24, 239–261. [DOI] [PubMed] [Google Scholar]
- 82.Mortimer M, Petersen EJ, Buchholz BA, Orias E and Holden PA, Bioaccumulation of Multiwall Carbon Nanotubes in Tetrahymena thermophila by Direct Feeding or Trophic Transfer, Environ. Sci. Technol, 2016, 50, 8876–8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Su Y, Yang G, Lu K, Petersen EJ and Mao L, Colloidal properties and stability of aqueous suspensions of few-layer graphene: Importance of graphene concentration, Environ. Pollut, 2017, 220, Part A, 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Navarro DA, Kookana RS, McLaughlin MJ and Kirby JK, Fate of radiolabeled C-60 fullerenes in aged soils, Environ. Pollut, 2017, 221, 293–300. [DOI] [PubMed] [Google Scholar]
- 85.Avanasi R, Jackson WA, Sherwin B, Mudge JF and Anderson TA, C60 Fullerene Soil Sorption, Biodegradation, and Plant Uptake, Environ. Sci. Technol, 2014, 48, 2792–2797. [DOI] [PubMed] [Google Scholar]
- 86.Li D, Fortner JD, Johnson DR, Chen C, Li QL and Alvarez PJJ, Bioaccumulation of 14C60 by the Earthworm Eisenia fetida, Environ. Sci. Technol, 2010, 44, 9170–9175. [DOI] [PubMed] [Google Scholar]
- 87.Chen Z, Westerhoff P and Herckes P, Quantification of C60 fullerene concentrations in water, Environ. Toxicol. Chem, 2008, 27, 1852–1859. [DOI] [PubMed] [Google Scholar]
- 88.Isaacson CW, Kleber M and Field JA, Quantitative analysis of fullerene nanomaterials in environmental systems: A critical review, Environ. Sci. Technol, 2009, 43, 6463–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schierz A, Espinasse B, Wiesner MR, Bisesi JH, Sabo-Attwood T and Ferguson PL, Fate of single walled carbon nanotubes in wetland ecosystems, Environ. Sci.: Nano, 2014, 1, 574–583. [Google Scholar]
- 90.Schierz A, Parks AN, Washburn KM, Chandler GT and Ferguson PL, Characterization and Quantitative Analysis of Single-Walled Carbon Nanotubes in the Aquatic Environment Using Near-Infrared Fluorescence Spectroscopy, Environ. Sci. Technol, 2012, 46, 12262–12271. [DOI] [PubMed] [Google Scholar]
- 91.Waissi GC, Bold S, Pakarinen K, Akkanen J, Leppanen MT, Petersen EJ and Kukkonen JVK, Chironomus riparius exposure to fullerene-contaminated sediment results in oxidative stress and may impact life cycle parameters, J. Hazard. Mater, 2017, 322, 301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pakarinen K, Petersen EJ, Leppanen MT, Akkanen J and Kukkonen JVK, Adverse effects of fullerenes (nC(60)) spiked to sediments on Lumbriculus variegatus (Oligochaeta), Environ. Pollut, 2011, 159, 3750–3756. [DOI] [PubMed] [Google Scholar]
- 93.Cano AM, Kohl K, Deleon S, Payton P, Irin F, Saed M, Shah SA, Green MJ and Cañas-Carrell JE, Determination of uptake, accumulation, and stress effects in corn (Zea mays L.) grown in single-wall carbon nanotube contaminated soil, Chemosphere, 2016, 152, 117–122. [DOI] [PubMed] [Google Scholar]
- 94.Irin F, Shrestha B, Canas JE, Saed MA and Green MJ, Detection of carbon nanotubes in biological samples through microwave-induced heating, Carbon, 2012, 50, 4441–4449. [Google Scholar]
- 95.Li SB, Irin F, Atore FO, Green MJ and Canas-Carrell JE, Determination of multi-walled carbon nanotube bioaccumulation in earthworms measured by a microwave-based detection technique, Sci. Tot. Environ, 2013, 445, 9–13. [DOI] [PubMed] [Google Scholar]
- 96.Cano AM, Maul JD, Saed M, Shah SA, Green MJ and Canas-Carrell JE, Bioacccumulation, stress, and swimming impairment in Daphnia magna exposed to multiwalled carbon nanotubes, graphene, and graphne oxide, Environ. Toxicol. Chem, 2017, 36, 2199–2204. [DOI] [PubMed] [Google Scholar]
- 97.Bisesi JH, Merten J, Liu K, Parks AN, Afrooz A, Glenn JB, Klaine SJ, Kane AS, Saleh NB, Ferguson PL and Sabo-Attwood T, Tracking and Quantification of Single-Walled Carbon Nanotubes in Fish Using Near Infrared Fluorescence, Environ. Sci. Technol, 2014, 48, 1973–1983. [DOI] [PubMed] [Google Scholar]
- 98.Bisesi JH Jr., Thuy N, Ponnavolu S, Liu K, Lavelle CM, Afrooz ARMN, Saleh NB, Ferguson PL, Denslow ND and Sabo-Attwood T, Examination of Single-Walled Carbon Nanotubes Uptake and Toxicity from Dietary Exposure: Tracking Movement and Impacts in the Gastrointestinal System, Nanomaterials, 2015, 5, 1066–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Diez-Ortiz M, Lahive E, George S, Ter Schure A, Van Gestel CAM, Jurkschat K, Svendsen C and Spurgeon DJ, Short-term soil bioassays may not reveal the full toxicity potential for nanomaterials; bioavailability and toxicity of silver ions (AgNO3) and silver nanoparticles to earthworm Eisenia fetida in long-term aged soils, Environ. Pollut, 2015, 203, 191–198. [DOI] [PubMed] [Google Scholar]
- 100.Tourinho PS, van Gestel CAM, Morgan AJ, Kille P, Svendsen C, Jurkschat K, Mosselmans JFW, Soares AMVM and Loureiro S, Toxicokinetics of Ag in the terrestrial isopod Porcellionides pruinosus exposed to Ag NPs and AgNO3 via soil and food, Ecotoxicology, 2016, 25, 267–278. [DOI] [PubMed] [Google Scholar]
- 101.Waalewijn-Kool PL, Klein K, Fornies RM and van Gestel CAM, Bioaccumulation and toxicity of silver nanoparticles and silver nitrate to the soil arthropod Folsomia candida, Ecotoxicology, 2014, 23, 1629–1637. [DOI] [PubMed] [Google Scholar]
- 102.Starnes DL, Unrine JM, Starnes CP, Collin BE, Oostveen EK, Ma R, Lowry GV, Bertsch PM and Tsyusko OV, Impact of sulfidation on the bioavailability and toxicity of silver nanoparticles to Caenorhabditis elegans, Environ. Pollut, 2015, 196, 239–246. [DOI] [PubMed] [Google Scholar]
- 103.van der Ploeg MJC, Handy RD, Waalewijn-Kool PL, van den Berg JHJ, Rivera ZEH, Bovenschen J, Molleman B, Baveco JM, Tromp P, Peters RJB, Koopmans GF, Rietjens I and van den Brink NW, Effects of silver nanoparticles (NM-300K) on Lumbricus rubellus earthworms and particle characterization in relevant test matrices including soil, Environ. Toxicol. Chem, 2014, 33, 743–752. [DOI] [PubMed] [Google Scholar]
- 104.Heggelund LR, Diez-Ortiz M, Lofts S, Lahive E, Jurkschat K, Wojnarowicz J, Cedergreen N, Spurgeon D and Svendsen C, Soil pH effects on the comparative toxicity of dissolved zinc, non-nano and nano ZnO to the earthworm Eisenia fetida, Nanotoxicology, 2014, 8, 559–572. [DOI] [PubMed] [Google Scholar]
- 105.Gomes SIL, Murphy M, Nielsen MT, Kristiansen SM, Amorim MJB and Scott-Fordsmand JJ, Cu-nanoparticles ecotoxicity - Explored and explained?, Chemosphere, 2015, 139, 240–245. [DOI] [PubMed] [Google Scholar]
- 106.Cornelis G, Hund-Rinke K, Kuhlbusch T, van den Brink N and Nickel C, Fate and Bioavailability of Engineered Nanoparticles in Soils: A Review, Crit. Rev. Environ. Sci. Technol, 2014, 44, 2720–2764. [Google Scholar]
- 107.de Santiago-Martín A, Constantin B, Guesdon G, Kagambega N, Raymond S and Cloutier RG, Bioavailability of engineered nanoparticles in soil systems, J. Hazard., Toxic Radioact. Waste, 2016, 20, B4015001. [Google Scholar]
- 108.Handy RD, van den Brink N, Chappell M, Muhling M, Behra R, Dusinska M, Simpson P, Ahtiainen J, Jha AN, Seiter J, Bednar A, Kennedy A, Fernandes TF and Riediker M, Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials: what have we learnt so far?, Ecotoxicology, 2012, 21, 933–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coutris C, Hertel-Aas T, Lapied E, Joner EJ and Oughton DH, Bioavailability of cobalt and silver nanoparticles to the earthworm Eisenia fetida, Nanotoxicology, 2012, 6, 186–195. [DOI] [PubMed] [Google Scholar]
- 110.Gogos A, Kaegi R, Zenobi R and Bucheli TD, Capabilities of asymmetric flow field-flow fractionation coupled to multi-angle light scattering to detect carbon nanotubes in soot and soil, Environ. Sci.: Nano, 2014, 1, 584–594. [Google Scholar]
- 111.von der Kammer F, Legros S, Larsen EH, Loeschner K and Hofmann T, Separation and characterization of nanoparticles in complex food and environmental samples by field-flow fractionation, Trends Anal. Chem, 2011, 30, 425–436. [Google Scholar]
- 112.Misra SK, Dybowska A, Berhanu D, Croteau MN, Luoma SN, Boccaccini AR and Valsami-Jones E, Isotopically Modified Nanoparticles for Enhanced Detection in Bioaccumulation Studies, Environ. Sci. Technol, 2012, 46, 1216–1222. [DOI] [PubMed] [Google Scholar]
- 113.Waalewijn-Kool PL, Ortiz MD, Lofts S and van Gestel CAM, The effect of pH on the toxicity of zinc oxide nanoparticles to Folsomia candida in amended field soil, Environ.Toxicol. Chem, 2013, 32, 2349–2355. [DOI] [PubMed] [Google Scholar]
- 114.Laycock A, Diez-Ortiz M, Larner F, Dybowska A, Spurgeon D, Valsami-Jones E, Rehkamper M and Svendsen C, Earthworm Uptake Routes and Rates of Ionic Zn and ZnO Nanoparticles at Realistic Concentrations, Traced Using Stable Isotope Labeling, Environ. Sci. Technol, 2016, 50, 412–419. [DOI] [PubMed] [Google Scholar]
- 115.Larner F and Rehkamper M, Evaluation of Stable Isotope Tracing for ZnO Nanomaterials-New Constraints from High Precision Isotope Analyses and Modeling, Environ. Sci. Technol, 2012, 46, 4149–4158. [DOI] [PubMed] [Google Scholar]
- 116.Laycock A, Romero-Freire A, Najorka J, Svendsen C, van Gestel CAM and Rehkamper M, Novel Multi-isotope Tracer Approach To Test ZnO Nanoparticle and Soluble Zn Bioavailability in Joint Soil Exposures, Environ. Sci. Technol, 2017, 51, 12756–12763. [DOI] [PubMed] [Google Scholar]
- 117.Montoro Bustos AR, Petersen EJ, Possolo A and Winchester MR, Post hoc Interlaboratory Comparison of Single Particle ICP-MS Size Measurements of NIST Gold Nanoparticle Reference Materials, Anal. Chem, 2015, 87, 8809–8817. [DOI] [PubMed] [Google Scholar]
- 118.Mitrano DM, Barber A, Bednar A, Westerhoff P, Higgins CP and Ranville JF, Silver nanoparticle characterization using single particle ICP-MS (SP-ICP-MS) and asymmetrical flow field flow fractionation ICP-MS (AF4-ICP-MS), J. Anal. At. Spectrom, 2012, 27, 1131–1142. [Google Scholar]
- 119.Mitrano DM, Lesher EK, Bednar A, Monserud J, Higgins CP and Ranville JF, Detecting nanoparticulate silver using single-particle inductively coupled plasma-mass spectrometry, Environ. Toxicol. Chem, 2012, 31, 115–121. [DOI] [PubMed] [Google Scholar]
- 120.Montano MD, Badiei HR, Bazargan S and Ranville JF, Improvements in the detection and characterization of engineered nanoparticles using spICP-MS with microsecond dwell times, Environ. Sci.: Nano, 2014, 1, 338–346. [Google Scholar]
- 121.Johnson ME, Hanna SK, Bustos ARM, Sims CM, Elliott LCC, Lingayat A, Johnston AC, Nikoobakht B, Elliott JT, Holbrook RD, Scoto KCK, Murphy KE, Petersen EJ, Yu LL and Nelson BC, Separation, Sizing, and Quantitation of Engineered Nanoparticles in an Organism Model Using Inductively Coupled Plasma Mass Spectrometry and Image Analysis, ACS Nano, 2017, 11, 526–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.El Hadri H, Petersen EJ and Winchester MR, Impact of and correction for instrument sensitivity drift on nanoparticle size measurements by single-particle ICP-MS, Anal. Bioanal. Chem, 2016, 408, 5099–5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Deng Y, Petersen EJ, Challis KE, Rabb SA, Holbrook RD, Ranville JF, Nelson BC and Xing B, Multiple Method Analysis of TiO2 Nanoparticle Uptake in Rice (Oryza sativa L.) Plants, Environ. Sci. Technol, 2017, 51, 10615–10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gray EP, Coleman JG, Bednar AJ, Kennedy AJ, Ranville JF and Higgins CP, Extraction and Analysis of Silver and Gold Nanoparticles from Biological Tissues Using Single Particle Inductively Coupled Plasma Mass Spectrometry, Environ. Sci. Technol, 2013, 47, 14315–14323. [DOI] [PubMed] [Google Scholar]
- 125.Dan Y, Ma X, Zhang W, Liu K, Stephan C and Shi H, Single particle ICP-MS method development for the determination of plant uptake and accumulation of CeO2 nanoparticles, Anal. Bioanal. Chem, 2016, 408, 5157–5167. [DOI] [PubMed] [Google Scholar]
- 126.Dan YB, Zhang WL, Xue RM, Ma XM, Stephan C and Shi HL, Characterization of Gold Nanoparticle Uptake by Tomato Plants Using Enzymatic Extraction Followed by Single-Particle Inductively Coupled Plasma-Mass Spectrometry Analysis, Environ. Sci. Technol, 2015, 49, 3007–3014. [DOI] [PubMed] [Google Scholar]
- 127.Makama S, Peters R, Undas A and van den Brink NW, A novel method for the quantification, characterisation and speciation of silver nanoparticles in earthworms exposed in soil, Environ. Chem, 2015, 12, 643–651. [Google Scholar]
- 128.Lee S, Bi X, Reed RB, Ranville JF, Herckes P and Westerhoff P, Nanoparticle Size Detection Limits by Single Particle ICP-MS for 40 Elements, Environ. Sci. Technol, 2014, 48, 10291–10300. [DOI] [PubMed] [Google Scholar]
- 129.Schwertfeger DM, Velicogna JR, Jesmer AH, Scroggins RP and Princz JI, Single Particle-Inductively Coupled Plasma Mass Spectroscopy Analysis of Metallic Nanoparticles in Environmental Samples with Large Dissolved Analyte Fractions, Anal. Chem, 2016, 88, 9908–9914. [DOI] [PubMed] [Google Scholar]
- 130.Leung YH, Guo MY, Ma APY, Ng AMC, Djurisic AB, Degger N and Leung FCC, Transmission electron microscopy artifacts in characterization of the nanomaterial-cell interactions, Appl. Microbiol. Biotechnol, 2017, 101, 5469–5479. [DOI] [PubMed] [Google Scholar]
- 131.Priester JH, Ge Y, Mielke RE, Horst AM, Moritz SC, Espinosa K, Gelb J, Walker SL, Nisbet RM, An YJ, Schimel JP, Palmer RG, Hernandez-Viezcas JA, Zhao LJ, Gardea-Torresdey JL and Holden PA, Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption, Proc. Natl. Acad. Sci. U.S.A, 2012, 109, E2451–E2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mielke RE, Priester JH, Werlin RA, Gelb J, Horst AM, Orias E and Holden PA, Differential Growth of and Nanoscale TiO2 Accumulation in Tetrahymena thermophila by Direct Feeding versus Trophic Transfer from Pseudomonas aeruginosa, Appl. Environ. Microbiol, 2013, 79, 5616–5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Edgington AJ, Roberts AP, Taylor LM, Alloy MM, Reppert J, Rao AM, Ma JD and Klaine SJ, The influence of natural organic matter on the toxicity of multiwalled carbon nanotubes, Environ. Toxicol. Chem, 2010, 29, 2511–2518. [DOI] [PubMed] [Google Scholar]
- 134.Mouchet F, Landois P, Puech P, Pinelli E, Flahaut E and Gauthier L, Carbon nanotube ecotoxicity in amphibians: assessment of multiwalled carbon nanotubes and comparison with double-walled carbon nanotubes, Nanomedicine, 2010, 5, 963–974. [DOI] [PubMed] [Google Scholar]
- 135.Mouchet F, Landois P, Sarremejean E, Bernard G, Puech P, Pinelli E, Flahaut E and Gauthier L, Characterisation and in vivo ecotoxicity evaluation of double-wall carbon nanotubes in larvae of the amphibian Xenopus laevis, Aquat. Toxicol, 2008, 87, 127–137. [DOI] [PubMed] [Google Scholar]
- 136.Unrine JM, Hunyadi SE, Tsyusko OV, Rao W, Shoults-Wilson WA and Bertsch PM, Evidence for Bioavailability of Au Nanoparticles from Soil and Biodistribution within Earthworms (Eisenia fetida), Environ. Sci. Technol, 2010, 44, 8308–8313. [DOI] [PubMed] [Google Scholar]
- 137.Lovern SB, Owen HA and Klaper R, Electron microscopy of gold nanoparticle intake in the gut of Daphnia magna, Nanotoxicology, 2008, 2, 43–48. [Google Scholar]
- 138.Jackson BP, Pace H, Lanzirotti A, Smith R and Ranville JF, Synchrotron X-ray 2D and 3D elemental imaging of CdSe/ZnS quantum dot nanoparticles in Daphnia magna, Anal. Bioanal. Chem, 2009, 394, 911–917. [DOI] [PubMed] [Google Scholar]
- 139.Judy JD, McNear DH Jr., Chen C, Lewis RW, Tsyusko OV, Bertsch PM, Rao W, Stegemeier J, Lowry GV, McGrath SP, Durenkamp M and Unrine JM, Nanomaterials in Biosolids Inhibit Nodulation, Shift Microbial Community Composition, and Result in Increased Metal Uptake Relative to Bulk/Dissolved Metals, Environ. Sci. Technol, 2015, 49, 8751–8758. [DOI] [PubMed] [Google Scholar]
- 140.Ma R, Levard C, Judy JD, Unrine JM, Durenkamp M, Martin B, Jefferson B and Lowry GV, Fate of Zinc Oxide and Silver Nanoparticles in a Pilot Wastewater Treatment Plant and in Processed Biosolids, Environ. Sci. Technol, 2014, 48, 104–112. [DOI] [PubMed] [Google Scholar]
- 141.Shoults-Wilson WA, Reinsch BC, Tsyusko OV, Bertsch PM, Lowry GV and Unrine JM, Effect of silver nanoparticle surface coating on bioaccumulation and reproductive toxicity in earthworms (Eisenia fetida), Nanotoxicology, 2011, 5, 432–444. [DOI] [PubMed] [Google Scholar]
- 142.Whitley AR, Levard C, Oostveen E, Bertsch PM, Matocha CJ, von der Kammer F and Unrine JM, Behavior of Ag nanoparticles in soil: Effects of particle surface coating, aging and sewage sludge amendment, Environ. Pollut, 2013, 182, 141–149. [DOI] [PubMed] [Google Scholar]
- 143.Shi J, Ye J, Fang H, Zhang S and Xu C, Effects of Copper Oxide Nanoparticles on Paddy Soil Properties and Components, Nanomaterials, 2018, 8, 839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hernandez-Viezcas JA, Castillo-Michel H, Andrews JC, Cotte M, Rico C, Peralta-Videa JR, Ge Y, Priester JH, Holden PA and Gardea-Torresdey JL, In Situ Synchrotron X-ray Fluorescence Mapping and Speciation of CeO2 and ZnO Nanoparticles in Soil Cultivated Soybean (Glycine max), ACS Nano, 2013, 7, 1415–1423. [DOI] [PubMed] [Google Scholar]
- 145.Marie T, Melanie A, Lenka B, Julien I, Isabelle K, Christine P, Elise M, Catherine S, Bernard A, Ester A, Jerome R, Alain T and Jean-Yves B, Transfer, Transformation, and Impacts of Ceria Nanomaterials in Aquatic Mesocosms Simulating a Pond Ecosystem, Environ. Sci. Technol, 2014, 48, 9004–9013. [DOI] [PubMed] [Google Scholar]
- 146.Thalmann B, Voegelin A, Sinnet B, Morgenroth E and Kaegi R, Sulfidation Kinetics of Silver Nanoparticles Reacted with Metal Sulfides, Environ. Sci. Technol, 2014, 48, 4885–4892. [DOI] [PubMed] [Google Scholar]
- 147.Thalmann B, Voegelin A, Morgenroth E and Kaegi R, Effect of humic acid on the kinetics of silver nanoparticle sulfidation, Environ. Sci.: Nano, 2016, 3, 203–212. [Google Scholar]
- 148.Reinsch BC, Levard C, Li Z, Ma R, Wise A, Gregory KB, Brown GE Jr. and Lowry GV, Sulfidation of Silver Nanoparticles Decreases Escherichia coli Growth Inhibition, Environ. Sci. Technol, 2012, 46, 6992–7000. [DOI] [PubMed] [Google Scholar]
- 149.Gogos A, Thalmann B, Voegelin A and Kaegi R, Sulfidation kinetics of copper oxide nanoparticles, Environ. Sci.: Nano, 2017, 4, 1733–1741. [Google Scholar]
- 150.Priester JH, Stoimenov PK, Mielke RE, Webb SM, Ehrhardt C, Zhang JP, Stucky GD and Holden PA, Effects of Soluble Cadmium Salts Versus CdSe Quantum Dots on the Growth of Planktonic Pseudomonas aeruginosa, Environ. Sci. Technol, 2009, 43, 2589–2594. [DOI] [PubMed] [Google Scholar]
- 151.Deng Y, White JC and Xing B, Interactions between engineered nanomaterials and agricultural crops: implications for food safety, J. Zhejiang Univ. Sci. A, 2014, 15, 552–572. [Google Scholar]
- 152.Reipa V, Hanna SK, Urbas A, Sander L, Elliott J, Conny J and Petersen EJ, Efficient electrochemical degradation of multiwall carbon nanotubes, J. Hazard. Mater, 2018, 354, 275–282. [DOI] [PubMed] [Google Scholar]
- 153.Doudrick K, Herckes P and Westerhoff P, Detection of Carbon Nanotubes in Environmental Matrices Using Programmed Thermal Analysis, Environ. Sci. Technol, 2012, 46, 12246–12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Saheli PT, Rowe RK, Petersen EJ and O’Carroll DM, Diffusion of multiwall carbon nanotubes through a high-density polyethylene geomembrane, Geosynth. Internat., 2017, 24, 184–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.ASTM (American Society for Testing Materials) International, D6281-15, Standard Test Method for Airborne Asbestos Concentration in Ambient and Indoor Atmospheres as Determined by Transmission Electron Microscopy Direct Transfer (TEM). 2015.
- 156.Prasad A, Lead JR and Baalousha M, An electron microscopy based method for the detection and quantification of nanomaterial number concentration in environmentally relevant media, Sci. Tot. Environ, 2015, 537, 479–486. [DOI] [PubMed] [Google Scholar]
- 157.Wallace WG, Lee BG and Luoma SN, Subcellular compartmentalization of Cd and Zn in two bivalves. I. Significance of metal-sensitive fractions (MSF) and biologically detoxified metal (BDM), Mar. Ecol. Prog. Ser, 2003, 249, 183–197. [Google Scholar]
- 158.Garcia-Aonso J, Khan FR, Misra SK, Turmaine M, Smith BD, Rainbow PS, Luoma SN and Valsami-Jones E, Cellular Internalization of Silver Nanoparticles in Gut Epithelia of the Estuarine Polychaete Nereis diversicolor, Environ. Sci. Technol, 2011, 45, 4630–4636. [DOI] [PubMed] [Google Scholar]
- 159.Thit A, Banta GT and Selck H, Bioaccumulation, subcellular distribution and toxicity of sediment-associated copper in the ragworm Nereis diversicolor: The relative importance of aqueous copper, copper oxide nanoparticles and microparticles, Environ. Pollut, 2015, 202, 50–57. [DOI] [PubMed] [Google Scholar]
- 160.Thit A, Ramskov T, Croteau MN and Selck H, Biodynamics of copper oxide nanoparticles and copper ions in an oligochaete - Part II: Subcellular distribution following sediment exposure, Aquat. Toxicol, 2016, 180, 25–35. [DOI] [PubMed] [Google Scholar]
- 161.Arnold MS, Stupp SI and Hersam MC, Enrichment of single-walled carbon nanotubes by diameter in density gradients, Nano Lett, 2005, 5, 713–718. [DOI] [PubMed] [Google Scholar]
- 162.Efeoglu E, Keating M, McIntyre J, Casey A and Byrne HJ, Determination of nanoparticle localisation within subcellular organelles in vitro using Raman spectroscopy, Anal. Methods, 2015, 7, 10000–10017. [Google Scholar]
- 163.Brakke M, 1961, Density gradient centrifugation and its application to plant viruses, Adv. Virus Res, 7, 193–224. [Google Scholar]
- 164.Mortimer M, Petersen EJ, Buchholz BA and Holden PA, Separation of Bacteria, Protozoa and Carbon Nanotubes by Density Gradient Centrifugation, Nanomaterials, 2016, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nowacek A, Kadiu I, McMillan J and Gendelman HE, in Cellular and Subcellular Nanotechnology: Methods and Protocols, eds. Weissig V, Elbayoumi T and Olsen M, Humana Press, Totowa, NJ, 2013, DOI: 10.1007/978-1-62703-336-7_6, pp. 47–55. [DOI] [Google Scholar]
- 166.Hunt PR, Marquis BJ, Tyner KM, Conklin S, Olejnik N, Nelson BC and Sprando RL, Nanosilver suppresses growth and induces oxidative damage to DNA in Caenorhabditis elegans, J. Appl. Toxicol, 2013, 33, 1131–1142. [DOI] [PubMed] [Google Scholar]
- 167.Wang Y, Miao AJ, Luo J, Wei ZB, Zhu JJ and Yang LY, Bioaccumulation of CdTe Quantum Dots in a Freshwater Alga Ochromonas danica: A Kinetics Study, Environ. Sci. Technol, 2013, 47, 10601–10610. [DOI] [PubMed] [Google Scholar]
- 168.Ribeiro F, Gallego-Urrea JA, Goodhead RM, Van Gestel CAM, Moger J, Soares AMVM and Loureiro S, Uptake and elimination kinetics of silver nanoparticles and silver nitrate by Raphidocelis subcapitata: The influence of silver behaviour in solution, Nanotoxicology, 2015, 9, 686–695. [DOI] [PubMed] [Google Scholar]
- 169.Arnold MC, Badireddy AR, Wiesner MR, Di Giulio RT and Meyer JN, Cerium Oxide Nanoparticles are More Toxic than Equimolar Bulk Cerium Oxide in Caenorhabditis elegans, Arch. Environ. Contam. Toxicol, 2013, 65, 224–233. [DOI] [PubMed] [Google Scholar]
- 170.Meyer JN, Lord CA, Yang XY, Turner EA, Badireddy AR, Marinakos SM, Chilkoti A, Wiesner MR and Auffan M, Intracellular uptake and associated toxicity of silver nanoparticles in Caenorhabditis elegans, Aquat. Toxicol, 2010, 100, 140–150. [DOI] [PubMed] [Google Scholar]
- 171.Mortimer M, Gogos A, Bartolome N, Kahru A, Bucheli TD and Slaveykova VI, Potential of Hyperspectral Imaging Microscopy for Semi-quantitative Analysis of Nanoparticle Uptake by Protozoa, Environ. Sci. Technol, 2014, 48, 8760–8767. [DOI] [PubMed] [Google Scholar]
- 172.Gao Y, Liu N, Chen C, Luo Y, Li Y, Zhang Z, Zhao Y, Zhao B, Iida A and Chai Z, Mapping technique for biodistribution of elements in a model organism, Caenorhabditis elegans, after exposure to copper nanoparticles with microbeam synchrotron radiation X-ray fluorescence, J. Anal. At. Spectrom, 2008, 23, 1121–1124. [Google Scholar]
- 173.Collin B, Oostveen E, Tsyusko OV and Unrine JM, Influence of Natural Organic Matter and Surface Charge on the Toxicity and Bioaccumulation of Functionalized Ceria Nanoparticles in Caenorhabditis elegans, Environ. Sci. Technol, 2014, 48, 1280–1289. [DOI] [PubMed] [Google Scholar]
- 174.Geier FM, Fearn S, Bundy JG and McPhail DS, ToF-SIMS analysis of biomolecules in the model organism Caenorhabditis elegans, Surf. Interface Anal, 2013, 45, 234–236. [Google Scholar]
- 175.Kim SW, Nam SH and An YJ, Interaction of Silver Nanoparticles with Biological Surfaces of Caenorhabditis elegans, Ecotox. Environ. Saf., 2012, 77, 64–70. [DOI] [PubMed] [Google Scholar]
- 176.Hu XG, Ouyang SH, Mu L, An J and Zhou Q, Effects of Graphene Oxide and Oxidized Carbon Nanotubes on the Cellular Division, Microstructure, Uptake, Oxidative Stress, and Metabolic Profiles, Environ. Sci. Technol, 2015, 49, 10825–10833. [DOI] [PubMed] [Google Scholar]
- 177.Huang B, Miao A-J, Xiao L and Yang L-Y, Influence of nitrogen limitation on the bioaccumulation kinetics of hematite nanoparticles in the freshwater alga Euglena intermedia, Environ. Sci.: Nano, 2017, 4, 1840–1850. [Google Scholar]
- 178.Sousa C, Sequeira D, Kolen’ko YV, Pinto IM and Petrovykh DY, Analytical Protocols for Separation and Electron Microscopy of Nanoparticles Interacting with Bacterial Cells, Anal. Chem, 2015, 87, 4641–4648. [DOI] [PubMed] [Google Scholar]
- 179.Chan TSY, Nasser F, St-Denis CH, Mandal HS, Ghafari P, Hadjout-Rabi N, Bols NC and Tang X, Carbon nanotube compared with carbon black: effects on bacterial survival against grazing by ciliates and antimicrobial treatments, Nanotoxicology, 2013, 7, 251–258. [DOI] [PubMed] [Google Scholar]
- 180.Xiong B, Cheng J, Qiao YX, Zhou R, He Y and Yeung ES, Separation of nanorods by density gradient centrifugation, J. Chromat. A, 2011, 1218, 3823–3829. [DOI] [PubMed] [Google Scholar]
- 181.Chen G, Wang Y, Tan LH, Yang M, Tan LS, Chen Y and Chen H, High-Purity Separation of Gold Nanoparticle Dimers and Trimers, J. Am. Chem. Soc, 2009, 131, 4218–4219. [DOI] [PubMed] [Google Scholar]
- 182.Yanagi K, litsuka T, Fujii S and Kataura H, Separations of Metallic and Semiconducting Carbon Nanotubes by Using Sucrose as a Gradient Medium, J. Phys. Chem. C, 2008, 112, 18889–18894. [Google Scholar]
- 183.Zhang Y, Shi Y, Liou Y-H, Sawvel AM, Sun X, Cai Y, Holden PA and Stucky GD, High performance separation of aerosol sprayed mesoporous TiO2 sub-microspheres from aggregates via density gradient centrifugation, J. Mat. Chem, 2010, 20, 4162–4167. [Google Scholar]
- 184.Lee SH, Salunke BK and Kim BS, Sucrose density gradient centrifugation separation of gold and silver nanoparticles synthesized using Magnolia kobus plant leaf extracts, Biotechnol. Bioproc. Eng, 2014, 19, 169–174. [Google Scholar]
- 185.Rhiem S, Riding MJ, Baumgartner W, Martin FL, Semple KT, Jones KC, Schaffer A and Maes HM, Interactions of multiwalled carbon nanotubes with algal cells: Quantification of association, visualization of uptake, and measurement of alterations in the composition of cells, Environ. Pollut, 2015, 196, 431–439. [DOI] [PubMed] [Google Scholar]
- 186.Hopkins DW, Macnaughton SJ and Odonnell AG, A dispersion and differential centrifugation technique for representatively sampling microorganisms from soil, Soil Biol. Biochem, 1991, 23, 217–225. [Google Scholar]
- 187.Eroglu E and Melis A, “Density Equilibrium” Method for the Quantitative and Rapid In Situ Determination of Lipid, Hydrocarbon, or Biopolymer Content in Microorganisms, Biotechnology and Bioengineering, 2009, 102, 1406–1415. [DOI] [PubMed] [Google Scholar]
- 188.Holden PA, Nisbet RM, Lenihan HS, Miller RJ, Cherr GN, Schimel JP and Gardea-Torresdey JL, Ecological nanotoxicology: integrating nanomaterial hazard considerations across the subcellular, population, community, and ecosystems levels, Acc. Chem. Res, 2013, 46, 813–822. [DOI] [PubMed] [Google Scholar]
- 189.Holden PA, Schimel JP and Godwin HA, Five reasons to use bacteria when assessing manufactured nanomaterial environmental hazards and fates, Curr. Opin. Biotech, 2014, 27, 73–78. [DOI] [PubMed] [Google Scholar]
- 190.Kim JA, Aberg C, Salvati A and Dawson KA, Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population, Nat Nanotechnol, 2011, 7, 62–68. [DOI] [PubMed] [Google Scholar]
- 191.Hanna SK, Montoro Bustos AR, Peterson AW, Reipa V, Scanlan LD, Hosbas Coskun S, Cho TJ, Johnson ME, Hackley VA, Nelson BC, Winchester MR, Elliott JT and Petersen EJ, Agglomeration of Escherichia coli with Positively Charged Nanoparticles Can Lead to Artifacts in a Standard Caenorhabditis elegans Toxicity Assay, Environ. Sci. Technol, 2018, 52, 5968–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schwab F, Bucheli TD, Lukhele LP, Magrez A, Nowack B, Sigg L and Knauer K, Are Carbon Nanotube Effects on Green Algae Caused by Shading and Agglomeration?, Environ. Sci. Technol, 2011, 45, 6136–6144. [DOI] [PubMed] [Google Scholar]
- 193.Van Hoecke K, De Schamphelaere KAC, Ramirez-Garcia S, Van der Meeren P, Smagghe G and Janssen CR, Influence of alumina coating on characteristics and effects of SiO2 nanoparticles in algal growth inhibition assays at various pH and organic matter contents, Environ Int, 2011, 37, 1118–1125. [DOI] [PubMed] [Google Scholar]
- 194.Mortimer M, Devarajan N, Li D and Holden PA, Multiwall Carbon Nanotubes Induce More Pronounced Transcriptomic Responses in Pseudomonas aeruginosa PG201 than Graphene, Exfoliated Boron Nitride, or Carbon Black, ACS Nano, 2018, 12, 2728–2740. [DOI] [PubMed] [Google Scholar]
- 195.Ong KJ, MacCormack TJ, Clark RJ, Ede JD, Ortega VA, Felix LC, Dang MKM, Ma GB, Fenniri H, Veinot JGC and Goss GG, Widespread Nanoparticle-Assay Interference: Implications for Nanotoxicity Testing, Plos One, 2014, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Elliott JT, Rosslein M, Song NW, Toman B, Kinsner-Ovaskainen A, Maniratanachote R, Salit ML, Petersen EJ, Sequeira F, Romsos EL, Kim SJ, Lee J, von Moos NR, Rossi F, Hirsch C, Krug HF, Suchaoin W and Wick P, Toward Achieving Harmonization in a Nanocytotoxicity Assay Measurement Through an Interlaboratory Comparison Study, Altex-Alt. Anim. Exper, 2017, 34, 201–218. [DOI] [PubMed] [Google Scholar]
- 197.Collins AR, Annangi B, Rubio L, Marcos R, Dorn M, Merker C, Estrela-Lopis I, Cimpan MR, Ibrahim M, Cimpan E, Ostermann M, Sauter A, El Yamani N, Shaposhnikov S, Chevillard S, Paget V, Grall R, Delic J, Goni-de-Cerio F, Suarez-Merino B, Fessard V, Hogeveen KN, Fjellsbo LM, Pran ER, Brzicova T, Topinka J, Silva MJ, Leite PE, Ribeiro AR, Granjeiro JM, Grafstrom R, Prina-Mello A and Dusinska M, High throughput toxicity screening and intracellular detection of nanomaterials, Wiley Interdisc. Rev.-Nanomed. Nanobiotechnol., 2017, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yang YSS, Atukorale PU, Moynihan KD, Bekdemir A, Rakhra K, Tang L, Stellacci F and Irvine DJ, High-throughput quantitation of inorganic nanoparticle biodistribution at the single-cell level using mass cytometry, Nat. Comm, 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Vanhecke D R-LL, Clift MJ, Blank F, Petri-Fink A, Rothen-Rutishauser B, Quantification of nanoparticles at the single-cell level: an overview about state-of-the-art techniques and their limitations, Nanomedicine, 2014, 9, 1885–1900. [DOI] [PubMed] [Google Scholar]
- 200.Mortimer M, Kahru A and Slaveykova VI, Uptake, localization and clearance of quantum dots in ciliated protozoa Tetrahymena thermophila, Environ. Pollut, 2014, 190, 58–64. [DOI] [PubMed] [Google Scholar]
- 201.Gupta GS, Kumar A, Shanker R and Dhawan A, Assessment of agglomeration, co-sedimentation and trophic transfer of titanium dioxide nanoparticles in a laboratory-scale predator-prey model system, Sci. Rep, 2016, 6, 31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bohmer N, Rippl A, May S, Walter A, Heo MB, Kwak M, Roesslein M, Song NW, Wick P and Hirsch C, Interference of engineered nanomaterials in flow cytometry: A case study, Colloids Surf., B, 2018, 172, 635–645. [DOI] [PubMed] [Google Scholar]
- 203.Corte Rodríguez M, Álvarez-Fernández García R, Blanco E, Bettmer J and Montes-Bayón M, Quantitative Evaluation of Cisplatin Uptake in Sensitive and Resistant Individual Cells by Single-Cell ICP-MS (SC-ICP-MS), Anal. Chem, 2017, 89, 11491–11497. [DOI] [PubMed] [Google Scholar]
- 204.Merrifield RC, Stephan C and Lead JR, Quantification of Au Nanoparticle Biouptake and Freshwater Algae Using Single Cell - ICP-MS, Environ. Sci. Technol, 2018, 52, 2271–2277. [DOI] [PubMed] [Google Scholar]
- 205.Mueller L, Traub H, Jakubowski N, Drescher D, Baranov VI and Kneipp J, Trends in single-cell analysis by use of ICP-MS, Anal. Bioanal. Chem, 2014, 406, 6963–6977. [DOI] [PubMed] [Google Scholar]
- 206.Zheng LN, Wang M, Wang B, Chen HQ, Ouyang H, Zhao YL, Chai ZF and Feng WY, Determination of quantum dots in single cells by inductively coupled plasma mass spectrometry, Talanta, 2013, 116, 782–787. [DOI] [PubMed] [Google Scholar]
- 207.Wang M, Zheng LN, Wang B, Chen HQ, Zhao YL, Chai ZF, Reid HJ, Sharp BL and Feng WY, Quantitative Analysis of Gold Nanoparticles in Single Cells by Laser Ablation Inductively Coupled Plasma-Mass Spectrometry, Anal Chem, 2014, 86, 10252–10256. [DOI] [PubMed] [Google Scholar]
- 208.Drescher D, Giesen C, Traub H, Panne U, Kneipp J and Jakubowski N, Quantitative imaging of gold and silver nanoparticles in single eukaryotic cells by laser ablation ICP-MS, Anal Chem, 2012, 84, 9684–9688. [DOI] [PubMed] [Google Scholar]
- 209.Groombridge AS, Miyashita S, Fujii S, Nagasawa K, Okahashi T, Ohata M, Umemura T, Takatsu A, Inagaki K and Chiba K, High Sensitive Elemental Analysis of Single Yeast Cells (Saccharomyces cerevisiae) by Time-Resolved Inductively-Coupled Plasma Mass Spectrometry Using a High Efficiency Cell Introduction System, AnalSci, 2013, 29, 597–603. [DOI] [PubMed] [Google Scholar]
- 210.Badiei HR, Rutzke MA and Karanassios V, Calcium content of individual, microscopic, (sub) nanoliter volume Paramecium sp. cells using rhenium-cup in-torch vaporization (ITV) sample introduction and axially viewed ICP-AES, J. Anal. At. Spectrom, 2002, 17, 1007–1010. [Google Scholar]
- 211.Ho K-S and Chan W-T, Time-resolved ICP-MS measurement for single-cell analysis and on-line cytometry, J. Anal. At. Spectrom, 2010, 25, 1114–1122. [Google Scholar]
- 212.Leclerc S and Wilkinson KJ, Bioaccumulation of nanosilver by Chlamydomonas reinhardtii-nanoparticle or the free ion?, Environ. Sci. Technol, 2014, 48, 358–364. [DOI] [PubMed] [Google Scholar]
- 213.Halter M, Bier E, DeRose PC, Cooksey GA, Choquette SJ, Plant AL and Elliott JT, An Automated Protocol for Performance Benchmarking a Widefield Fluorescence Microscope, Cytometry Part A, 2014, 85A, 978–985. [DOI] [PubMed] [Google Scholar]
- 214.Hagwood C, Bernal J, Halter M and Elliott J, Evaluation of Segmentation Algorithms on Cell Populations Using CDF Curves, IEEE Trans. Med. Imag,, 2012, 31, 380–390. [DOI] [PubMed] [Google Scholar]
- 215.Chalfoun J, Kociolek M, Dima A, Halter M, Cardone A, Peskin A, Bajcsy P and Brady M, Segmenting time-lapse phase contrast images of adjacent NIH 3T3 cells, J. Microsc. 2013, 249, 41–52. [DOI] [PubMed] [Google Scholar]
- 216.Dima AA, Elliott JT, Filliben JJ, Halter M, Peskin A, Bernal J, Kociolek M, Brady MC, Tang HC and Plant AL, Comparison of Segmentation Algorithms For Fluorescence Microscopy Images of Cells, Cytometry Part A, 2011, 79A, 545–559. [DOI] [PubMed] [Google Scholar]
- 217.Bajcsy P, Cardone A, Chalfoun J, Halter M, Juba D, Kociolek M, Majurski M, Peskin A, Simon C, Simon M, Vandecreme A and Brady M, Survey statistics of automated segmentations applied to optical imaging of mammalian cells, BMC Blolnformatlcs, 2015, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kettler K, Veltman K, van de Meent D, van Wezel A and Hendriks AJ, Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type, Environ Toxicol Chem, 2014, 33, 481–492. [DOI] [PubMed] [Google Scholar]
- 219.Miao AJ, Luo ZP, Chen CS, Chin WC, Santschi PH and Quigg A, Intracellular Uptake: A Possible Mechanism for Silver Engineered Nanoparticle Toxicity to a Freshwater Alga Ochromonas danica, Plos One, 2010, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Characklis WG and Marshall KC, in Biofilms, eds. Characklis WG and Marshall KC, John Wiley & Sons, Inc., New York, 1990, pp. 3–15. [Google Scholar]
- 221.Hall-Stoodley L, Costerton JW and Stoodley P, Bacterial biofilms: from the natural environment to infectious diseases, Nat. rev. Microbiol, 2004, 2, 95–108. [DOI] [PubMed] [Google Scholar]
- 222.Ikuma K, Decho AW and Lau BLT, When nanoparticles meet biofilms—interactions guiding the environmental fate and accumulation of nanoparticles, Front. Microbiol, 2015, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cervantes-Avilés P and Cuevas-Rodríguez G, Changes in nutrient removal and floes characteristics generated by presence of ZnO nanoparticles in activated sludge process, Chemosphere, 2017, 182, 672–680. [DOI] [PubMed] [Google Scholar]
- 224.Fabrega J, Luoma SN, Tyler CR, Galloway TS and Lead JR, Silver nanoparticles: Behaviour and effects in the aquatic environment, Environ. Intl, 2011, 37, 517–531. [DOI] [PubMed] [Google Scholar]
- 225.Yeo MK and Nam DH, Influence of different types of nanomaterials on their bioaccumulation in a paddy microcosm: a comparison of TiO2 nanoparticles and nanotubes, Environ. Pollut, 2013, 178, 166–172. [DOI] [PubMed] [Google Scholar]
- 226.Priester JH, Olson SG, Webb SM, Neu MP, Hersman LE and Holden PA, Enhanced exopolymer production and chromium stabilization in Pseudomonas putida unsaturated biofilms, Appl Environ Microbiol, 2006, 72, 1988–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mount DR, Dawson TD and Burkhard LP, Implications of gut purging for tissue residues determined in bioaccumulation testing of sediment with Lumbriculus variegatus, Environ. Toxicol. Chem, 1999, 18, 1244–1249. [Google Scholar]
- 228.ASTM (American Society for Testing Materials) International, 2004. E1676-04: Standard Guide for Conducting Laboratory Soil Toxicity or Bioaccumulation Tests with the Lumbricid Earthworm Eisenia Fetida and the Enchytraeid Potworm Enchytraeus albidus.
- 229.Ramskov T, Forbes VE, Gilliland D and Selck H, Accumulation and effects of sediment-associated silver nanoparticles to sediment-dwelling invertebrates, Aquat. Toxicol, 2015, 166, 96–105. [DOI] [PubMed] [Google Scholar]
- 230.Jager T, Fleuren R, Roelofs W and de Groot AC, Feeding activity of the earthworm Eisenia andrei in artificial soil, Soil Biol. Biochem, 2003, 35, 313–322. [Google Scholar]
- 231.Spurgeon DJ and Hopkin SP, Comparisons of metal accumulation and excretion kinetics in earthworms (Eisenia fetida) exposed to contaminated field and laboratory soils, Appl. Soil Ecol, 1999, 11, 227–243. [Google Scholar]
- 232.Handy RD and Eddy FB, in Physicochemical Kinetics and Transport at Chemical-Biological Interphases, ed. Koster H. P. v. L. a. W, John Wiley, Chichester, United Kingdon, 2004, pp. 337–356. [Google Scholar]
- 233.Paquin PR, Gorsuch JW, Apte S, Batley GE, Bowles KC, Campbell PGC, Delos CG, Di Toro DM, Dwyer RL, Galvez F, Gensemer RW, Goss GG, Hogstrand C, Janssen CR, McGeer JC, Naddy RB, Playle RC, Santore RC, Schneider U, Stubblefield WA, Wood CM and Wu KB, The biotic ligand model: a historical overview, Compar. Biochem. Physiol. C-Toxicol. Pharmacol., 2002, 133, 3–35. [DOI] [PubMed] [Google Scholar]
- 234.Sheir SK and Handy RD, Tissue Injury and Cellular Immune Responses to Cadmium Chloride Exposure in the Common Mussel Mytilus edulis: Modulation by Lipopolysaccharide, Arch. Environ. Contam. Toxicol, 2010, 59, 602–613. [DOI] [PubMed] [Google Scholar]
- 235.Al-Jubory AR and Handy RD, Uptake of titanium from TiO2 nanoparticle exposure in the isolated perfused intestine of rainbow trout: nystatin, vanadate and novel CO2-sensitive components, Nanotoxicology, 2013, 7, 1282–1301. [DOI] [PubMed] [Google Scholar]
- 236.Peyrot C, Gagnon C, Gagne F, Willkinson KJ, Turcotte P and Sauve S, Effects of cadmium telluride quantum dots on cadmium bioaccumulation and metallothionein production to the freshwater mussel, Elliptio complanara, Compar. Biochem. Physiol. C-Toxicol. Pharmacol, 2009, 150, 246–251. [DOI] [PubMed] [Google Scholar]
- 237.Pan J-F, Buffet P-E, Poirier L, Amiard-Triquet C, Gilliland D, Joubert Y, Pilet P, Guibbolini M, de Faverney CR, Romeo M, Valsami-Jones E and Mouneyrac C, Size dependent bioaccumulation and ecotoxicity of gold nanoparticles in an endobenthic invertebrate: The Tellinid clam Scrobicularia plana, Environ. Poll, 2012, 168, 37–43. [DOI] [PubMed] [Google Scholar]
- 238.Hanna SK, Miller RJ and Lenihan HS, Accumulation and Toxicity of Copper Oxide Engineered Nanoparticles in a Marine Mussel, Nanomaterials, 2014, 4, 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Hull MS, Vikesland PJ and Schultz IR, Uptake and retention of metallic nanoparticles in the Mediterranean mussel (Mytilus galloprovincialis), Aquat. Toxicol, 2013, 140, 89–97. [DOI] [PubMed] [Google Scholar]
- 240.Conway JR, Hanna SK, Lenihan HS and Keller AA, Effects and Implications of Trophic Transfer and Accumulation of CeO2 Nanoparticles in a Marine Mussel, Environ. Sci. Technol, 2014, 48, 1517–1524. [DOI] [PubMed] [Google Scholar]
- 241.Zuykov M, Pelletier E and Demers S, Colloidal complexed silver and silver nanoparticles in extrapallial fluid of Mytilus edulis, Mar. Environ. Res, 2011, 71, 17–21. [DOI] [PubMed] [Google Scholar]
- 242.Rosa M, Ward JE, Shumway SE, Wikfors GH, Pales-Espinosa E and Allam B, Effects of particle surface properties on feeding selectivity in the eastern oyster Crassostrea virginica and the blue mussel Mytilus edulis, J. Exper. Mar. Biol. Ecol, 2013, 446, 320–327. [Google Scholar]
- 243.Espinosa EP, Perrigault M, Ward JE, Shumway SE and Allam B, Microalgal Cell Surface Carbohydrates as Recognition Sites for Particle Sorting in Suspension-Feeding Bivalves, The Biol.Bull, 2010, 218, 75–86. [DOI] [PubMed] [Google Scholar]
- 244.Doyle JJ, Ward JE and Mason R, An examination of the ingestion, bioaccumulation, and depuration of titanium dioxide nanoparticles by the blue mussel (Mytilus edulis) and the eastern oyster (Crassostrea virginica), Mar. Environ. Res, 2015, 110, 45–52. [DOI] [PubMed] [Google Scholar]
- 245.Koehler A, Marx U, Broeg K, Bahns S and Bressling J, Effects of nanoparticles in Mytilus edulis gills and hepatopancreas - a new threat to marine life?, Mar. Environ. Res, 2008, 66, 12–14. [DOI] [PubMed] [Google Scholar]
- 246.Tedesco S, Doyle H, Redmond G and Sheehan D, Gold nanoparticles and oxidative stress in Mytilus edulis, Mar. Environ. Res, 2008, 66, 131–133. [DOI] [PubMed] [Google Scholar]
- 247.D’Agata A, Fasulo S, Dallas LJ, Fisher AS, Maisano M, Readman JW and Jha AN, Enhanced toxicity of ‘bulk’ titanium dioxide compared to ‘fresh’ and ‘aged’ nano-TiO2 in marine mussels (Mytilus galloprovincialis), Nanotoxicology, 2014, 8, 549–558. [DOI] [PubMed] [Google Scholar]
- 248.Marisa I, Matozzo V, Munari M, Binelli A, Parolini M, Martucci A, Franceschinis E, Brianese N and Marin MG, In vivo exposure of the marine clam Ruditapes philippinarum to zinc oxide nanoparticles: responses in gills, digestive gland and haemolymph, Env Sci Poll Res Int, 2016, 23, 15275–15293. [DOI] [PubMed] [Google Scholar]
- 249.Rocha TL, Gomes T, Durigon EG and Bebianno MJ, Subcellular partitioning kinetics, metallothionein response and oxidative damage in the marine mussel Mytilus galloprovincialis exposed to cadmium-based quantum dots, Sci. Tot. Environ, 2016, 554, 130–141. [DOI] [PubMed] [Google Scholar]
- 250.Rocha TL, Gomes T, Pinheiro JP, Sousa VS, Nunes LM, Teixeira MR and Bebianno MJ, Toxicokinetics and tissue distribution of cadmium-based Quantum Dots in the marine mussel Mytilus galloprovincialis, Environ. Pollut, 2015, 204, 207–214. [DOI] [PubMed] [Google Scholar]
- 251.Balbi T, Smerilli A, Fabbri R, Ciacci C, Montagna M, Grasselli E, Brunelli A, Pojana G, Marcomini A, Gallo G and Canesi L, Co-exposure to n-TiO2 and Cd2+ results in interactive effects on biomarker responses but not in increased toxicity in the marine bivalve M. galloprovincialis, Sci. Tot. Environ, 2014, 493, 355–364. [DOI] [PubMed] [Google Scholar]
- 252.Trevisan R, Delapedra G, Mello DF, Arl M, Schmidt EC, Meder F, Monopoli M, Cargnin-Ferreira E, Bouzon ZL, Fisher AS, Sheehan D and Dafre AL, Gills are an initial target of zinc oxide nanoparticles in oysters Crassostrea gigas, leading to mitochondrial disruption and oxidative stress, Aquat. Toxicol, 2014, 153, 27–38. [DOI] [PubMed] [Google Scholar]
- 253.Rossbach LM, Shaw BJ, Piegza D, Vevers WF, Atfield AJ and Handy RD, Sub-lethal effects of waterborne exposure to copper nanoparticles compared to copper sulphate on the shore crab (Carcinus maenas), Aquat. Toxicol, 2017, 191, 245–255. [DOI] [PubMed] [Google Scholar]
- 254.Windeatt KM and Handy RD, Effect of nanomaterials on the compound action potential of the shore crab, Carcinus maenas, Nanotoxicology, 2013, 7, 378–388. [DOI] [PubMed] [Google Scholar]
- 255.Petersen EJ, Pinto RA, Mai DJ, Landrum PF and Weber WJ Jr., Influence of polyethyleneimine graftings of multi-walled carbon nanotubes on their accumulation and elimination by and toxicity to Daphnia magna, Environ. Sci. Technol, 2011, 45, 1133–1138. [DOI] [PubMed] [Google Scholar]
- 256.Guo X, Dong S, Petersen EJ, Gao S, Huang Q and Mao L, Biological Uptake and Depuration of Radio-labeled Graphene by Daphnia magna, Environ. Sci. Technol, 2013, 47, 12524–12531. [DOI] [PubMed] [Google Scholar]
- 257.Ribeiro F, Van Gestel CAM, Pavlaki MD, Azevedo S, Soares AMVM and Loureiro S, Bioaccumulation of silver in Daphnia magna: Waterborne and dietary exposure to nanoparticles and dissolved silver, Sci. Tot. Environ, 2017, 574, 1633–1639. [DOI] [PubMed] [Google Scholar]
- 258.Li WM and Wang WX, Distinct biokinetic behavior of ZnO nanoparticles in Daphnia magna quantified by synthesizing 65Zn tracer, Water Res, 2013, 47, 895–902. [DOI] [PubMed] [Google Scholar]
- 259.Khan FR, Paul KB, Dybowska AD, Valsami-Jones E, Lead JR, Stone V and Fernandes TF, Accumulation Dynamics and Acute Toxicity of Silver Nanoparticles to Daphnia magna and Lumbriculus variegatus: Implications for Metal Modeling Approaches, Environ. Sci. Technol, 2015, 49, 4389–4397. [DOI] [PubMed] [Google Scholar]
- 260.Fan W, Liu L, Peng R and Wang WX, High bioconcentration of titanium dioxide nanoparticles in Daphnia magna determined by kinetic approach, Sci. Tot. Environ, 2016, 569–570, 1224–1231. [DOI] [PubMed] [Google Scholar]
- 261.Lee B-T, Kim H-A, Williamson JL and Ranville JF, Bioaccumulation and in-vivo dissolution of CdSe/ZnS with three different surface coatings by Daphnia magna, Chemosphere, 2016, 143, 115–122. [DOI] [PubMed] [Google Scholar]
- 262.Botha TL, Boodhia K and Wepener V, Adsorption, uptake and distribution of gold nanoparticles in Daphnia magna following longterm exposure, Aquat. Toxicol, 2016, 170, 104–111. [DOI] [PubMed] [Google Scholar]
- 263.Scott-Fordsmand JJ, Peijnenburg W, Semenzin E, Nowack B, Hunt N, Hristozov D, Marcomini A, Irfan MA, Jimenez AS, Landsiedel R, Tran L, Oomen AG, Bos PMJ and Hund-Rinke K, Environmental Risk Assessment Strategy for Nanomaterials, Int.J. Environ. Res. Pub. Health, 2017, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tourinho PS, van Gestel CAM, Lofts S, Svendsen C, Soares AMVM and Loureiro S, Metal-based nanoparticles in soil: Fate, behavior, and effects on soil invertebrates, Environ. Toxicol. Chem, 2012, 31, 1679–1692. [DOI] [PubMed] [Google Scholar]
- 265.Diez-Ortiz M, Lahive E, Kille P, Powell K, Morgan AJ, Jurkschat K, Van Gestel CAM, Mosselmans JFW, Svendsen C and Spurgeon DJ, Uptake routes and toxicokinetics of silver nanoparticles and silver ions in the earthworm Lumbricus rubellus, Environ. Toxicol. Chem,, 2015, 34, 2263–2270. [DOI] [PubMed] [Google Scholar]
- 266.Vijver MG, Gestel C. A. M. v, Straalen N. M. v., Lanno RP and Peijnenburg WJGM , Biological significance of metals partitioned to subcellular fractions within earthworms (Aporrectodea caliginosa), Environ. Toxicol. Chem, 2006, 25, 807–814. [DOI] [PubMed] [Google Scholar]
- 267.Schill RO and Kohler HR, Energy reserves and metal-storage granules in the hepatopancreas of Oniscus asellus and Porcellio scaber (Isopoda) from a metal gradient at Avonmouth, UK, Ecotoxicology, 2004, 13, 787–796. [DOI] [PubMed] [Google Scholar]
- 268.Gimbert F, Vijver MG, Coeurdassier M, Scheifler R, Peijnenburg WJ, Badot PM and de Vaufleury A, How subcellular partitioning can help to understand heavy metal accumulation and elimination kinetics in snails, Environ Toxicol Chem, 2008, 27, 1284–1292. [DOI] [PubMed] [Google Scholar]
- 269.Bednarska AJ and Swiqtek Z, Subcellular partitioning of cadmium and zinc in mealworm beetle (Tenebrio molitor) larvae exposed to metal-contaminated flour, Ecotox. Environ. Saf, 2016, 133, 82–89. [DOI] [PubMed] [Google Scholar]
- 270.van den Brink NW, Arblaster JA, Bowman SR, Conder JM, Elliott JE, Johnson MS, Muir DCG, Natal-da-Luz T, Rattner BA, Sample BE and Shore RF, Use of terrestrial field studies in the derivation of bioaccumulation potential of chemicals, Integrat. Environ. Assess. Manag, 2016, 12, 135–145. [DOI] [PubMed] [Google Scholar]
- 271.Vijver MG, Vink JPM, Miermans CJH and van Gestel CAM, Oral sealing using glue: A new method to distinguish between intestinal and dermal uptake of metals in earthworms, Soil Biol. Blochem. 2003, 35, 125–132. [Google Scholar]
- 272.Romih T, Jemec A, Kos M, Hocevar SB, Kralj S, Makovec D and Drobne D, The role of PVP in the bioavailability of Ag from the PVP-stabilized Ag nanoparticle suspension, Environ. Pollut, 2016, 218, 957–964. [DOI] [PubMed] [Google Scholar]
- 273.Khodakovskaya M, Dervishi Ε, Mahmood M, Xu Υ, Li ΖR, Watanabe F and Biris AS, Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth, ACS Nano, 2009, 3, 3221–3227. [DOI] [PubMed] [Google Scholar]
- 274.Gericke WF, Hydroponics—Crop production in liquid culture media, Science, 1937, 85, 177–178. [DOI] [PubMed] [Google Scholar]
- 275.Wang Z, Xie X, Zhao J, Liu X, Feng W, White JC and Xing B, Xylem- and phloem-based transport of CuO nanoparticles in maize (Zea mays L.), Environ Sci Technol, 2012, 46, 4434–4441. [DOI] [PubMed] [Google Scholar]
- 276.Nur Y, Lead JR and Baalousha M, Evaluation of charge and agglomeration behavior of TiO2 nanoparticles in ecotoxicological media, Sci Total Environ, 2015, 535, 45–53. [DOI] [PubMed] [Google Scholar]
- 277.Schwabe F, Schulin R, Limbach LK, Stark W, Burge D and Nowack B, Influence of two types of organic matter on interaction of CeO2 nanoparticles with plants in hydroponic culture, Chemosphere, 2013, 91, 512–520. [DOI] [PubMed] [Google Scholar]
- 278.U.S. Environmental Protection Agency, Ecological effects test guidelines OCSPP 850.4800: Plant uptake and translocation test. EPA 712-C-002, Office of Chemical Safety and Pollution Prevention, Washington, DC, 2012. [Google Scholar]
- 279.Petersen EJ, Diamond SA, Kennedy AJ, Goss GG, Ho K, Lead J, Hanna SK, Hartmann NB, Hund-Rinke K, Mader B, Manier N, Pandard P, Salinas ER and Sayre P, Adapting OECD Aquatic Toxicity Tests for Use with Manufactured Nanomaterials: Key Issues and Consensus Recommendations, Environ Sci Technol, 2015, 49, 9532–9547. [DOI] [PubMed] [Google Scholar]
- 280.Zhai G, Gutowski SM, Walters KS, Yan B and Schnoor JL, Charge, size, and cellular selectivity for multiwall carbon nanotubes by maize and soybean, Environ Sci Technol, 2015, 49, 7380–7390. [DOI] [PubMed] [Google Scholar]
- 281.Wang P, Menzies NW, Lombi E, McKenna BA, Johannessen B, Glover CJ, Kappen P and Kopittke PM, Fate of ZnO nanoparticles in soils and cowpea (Vigna unguiculata), Environ Sci Technol, 2013, 47, 13822–13830. [DOI] [PubMed] [Google Scholar]
- 282.Huang Y, Zhao L and Keller AA, Interactions, transformations, and bioavailability of nano-copper exposed to root exudates, Environ Sci Technol, 2017, DOI: 10.1021/acs.est.7b02523. [DOI] [PubMed] [Google Scholar]
- 283.Judy JD, Unrine JM, Rao W, Wirick S and Bertsch PM, Bioavailability of Gold Nanomaterials to Plants: Importance of Particle Size and Surface Coating, Environ. Sci. Technol, 2012, 46, 8467–8474. [DOI] [PubMed] [Google Scholar]
- 284.Wild E and Jones KC, Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants, Environ. Sci. Technol, 2009, 43, 5290–5294. [DOI] [PubMed] [Google Scholar]
- 285.Zhang Z, He X, Zhang H, Ma Y, Zhang P, Ding Y and Zhao Y, Uptake and distribution of ceria nanoparticles in cucumber plants, Metallomics : integrated biometal science, 2011, 3, 816–822. [DOI] [PubMed] [Google Scholar]
- 286.Khodakovskaya MV, de Silva K, Nedosekin DA, Dervishi E, Biris AS, Shashkov ΕV, Galanzha ΕI and Zharov VP, Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions, Proc. Natl. Acad. Sci. U.S.A, 2011, 108, 1028–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Lopez-Moreno ML, de la Rosa G, Hernandez-Viezcas JA, Castillo-Michel H, Botez CE, Peralta-Videa JR and Gardea-Torresdey JL, Evidence of the differential biotransformation and genotoxicity of ZnO and CeO2 nanoparticles on soybean (Glycine max) plants, Environ Sci Technol, 2010, 44, 7315–7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhou D, Jin S, Li L, Wang Y and Weng N, Quantifying the adsorption and uptake of CuO nanoparticles by wheat root based on chemical extractions, J. Environ. Sci, 2011, 23, 1852–1857. [DOI] [PubMed] [Google Scholar]
- 289.Ralston EJ and Imsande J, Nodulation of Hydroponically Grown Soybean Plants and Inhibition of Nodule Development by Nitratel, J Exp Bot, 1983, 34, 1371–1378. [Google Scholar]
- 290.Organization for Economic Cooperation and Development, 2006. Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test, Paris, France. [Google Scholar]
- 291.U.S. Environmental Protection Agency, Ecological effects test guidelines OCSPP 850.4100: Seedling emergence and seedling growth. EPA 712-C-012, Office of Chemical Safety and Pollution Prevention, Washington, DC, 2012. [Google Scholar]
- 292.Organization for Economic Cooperation and Development, 1984. Test No. 207: Earthworm, Acute Toxicity Tests, Paris, France. [Google Scholar]
- 293.Yoon SJ, Kwak JI, Lee WM, Holden PA and An YJ, Zinc oxide nanoparticles delay soybean development: a standard soil microcosm study, Ecotoxicology and environmental safety, 2014, 100, 131–137. [DOI] [PubMed] [Google Scholar]
- 294.Holden PA, Gardea-Torresdey JL, Klaessig F, Turco RF, Mortimer M, Hund-Rinke K, Hubal EAC, Avery D, Barcelo D, Behra R, Cohen Y, Deydier-Stephan L, Ferguson PL, Fernandes TF, Harthorn BH, Henderson WM, Hoke RA, Hristozov D, Johnston JM, Kane AB, Kapustka L, Keller AA, Lenihan HS, Lovell W, Murphy CJ, Nisbet RM, Petersen EJ, Salinas ER, Scheringer M, Sharma M, Speed DE, Sultan Y, Westerhoff P, White JC, Wiesner MR, Wong EM, Xing B, Horan MS, Godwin HA and Nel AE, Considerations of Environmentally Relevant Test Conditions for Improved Evaluation of Ecological Hazards of Engineered Nanomaterials, Environ. Sci. Technol, 2016, 50, 6124–6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhang W, Musante C, White JC, Schwab P, Wang Q, Ebbs SD and Ma X, Bioavailability of cerium oxide nanoparticles to Raphanus sativus L. in two soils, Plant Physiol. Biochem, 2017, 110, 185–193. [DOI] [PubMed] [Google Scholar]
- 296.Layet C, Auffan M, Santaella C, Chevassus-Rosset C, Montes M, Ortet P, Barakat M, Collin B, Legros S, Bravin MN, Angeletti B, Kieffer I, Proux O, Hazemann J-L and Doelsch E, Evidence that soil properties and organic coating drive the phytoavailability of cerium oxide nanoparticles, Environ Sci Technol, 2017, DOI: 10.1021/acs.est.7b02397. [DOI] [PubMed] [Google Scholar]
- 297.Garcia-Velasco N, Pena-Cearra A, Bilbao E, Zaldibar B and Soto M, Integrative assessment of the effects produced by Ag nanoparticles at different levels of biological complexity in Eisenia fetida maintained in two standard soils (OECD and LUFA 2.3), Chemosphere, 2017, 181, 747–758. [DOI] [PubMed] [Google Scholar]
- 298.Tourinho PS, van Gestel CAM, Lofts S, Soares AMVM and Loureiro S, Influence of soil pH on the toxicity of zinc oxide nanoparticles to the terrestrial isopod Porcellionides pruinosus, Environ. Toxicol. Chem, 2013, 32, 2808–2815. [DOI] [PubMed] [Google Scholar]
- 299.Ge Y, Priester JH, Van De Werfhorst LC, Walker SL, Nisbet RM, An Y-J, Schimel JP, Gardea-Torresdey JL and Holden PA, Soybean plants modify metal oxide nanoparticle effects on soil bacterial communities, Environ Sci Technol, 2014, 48, 13489–13496. [DOI] [PubMed] [Google Scholar]
- 300.Chen C, Tsyusko OV, McNear DH, Judy J, Lewis RW and Unrine JM, Effects of biosolids from a wastewater treatment plant receiving manufactured nanomaterials on Medicago truncatula and associated soil microbial communities at low nanomaterial concentrations, Sci. Tot. Environ, 2017, 609, 799–806. [DOI] [PubMed] [Google Scholar]
- 301.Priester JH, Ge Y, Mielke RE, Horst AM, Moritz SC, Espinosa K, Gelb J, Walker SL, Nisbet RM, An YJ, Schimel JP, Palmer RG, Hernandez-Viezcas JA, Zhao L, Gardea-Torresdey JL and Holden PA, Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption, Proc. Natl. Acad. Sci. U. S. A, 2012, 109, E2451–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gogos A, Moll J, Klingenfuss F, van der Heijden M, Irin F, Green MJ, Zenobi R and Bucheli TD, Vertical transport and plant uptake of nanoparticles in a soil mesocosm experiment, J. Nanobiotechnol, 2016, 14, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Larue C, Veronesi G, Flank A-M, Surble S, Herlin-Boime N and Carriere M, Comparative uptake and impact of TiO2 nanoparticles in wheat and rapeseed, J. Toxicol. Environ. Health-Part A-Curr. Iss, 2012, 75, 722–734. [DOI] [PubMed] [Google Scholar]
- 304.Larue C, Castillo-Michel H, Sobanska S, Trcera N, Sorieul S, Cecillon L, Ouerdane L, Legros S and Sarret G, Fate of pristine TiO2 nanoparticles and aged paint-containing TiO2 nanoparticles in lettuce crop after foliar exposure, J. Hazard. Mater, 2014, 273, 17–26. [DOI] [PubMed] [Google Scholar]
- 305.Larue C, Castillo-Michel H, Sobanska S, Cecillon L, Bureau S, Barthes V, Ouerdane L, Carriere M and Sarret G, Foliar exposure of the crop Lactuca sativa to silver nanoparticles: Evidence for internalization and changes in Ag speciation, J. Hazard. Mater, 2014, 264, 98–106. [DOI] [PubMed] [Google Scholar]
- 306.Eichert T, Kurtz A, Steiner U and Goldbach HE, Size exclusion limits and lateral heterogeneity of the stomatal foliar uptake pathway for aqueous solutes and water-suspended nanoparticles, Physiologia Plantarum, 2008, 134, 151–160. [DOI] [PubMed] [Google Scholar]
- 307.Alidoust D and Isoda A, Effect of gamma Fe2O3 nanoparticles on photosynthetic characteristic of soybean (Glycine max (L.) Merr.): foliar spray versus soil amendment, Acta Physiologiae Plantarum, 2013, 35, 3365–3375. [Google Scholar]
- 308.Wang W-N, Tarafdar JC and Biswas P, Nanoparticle synthesis and delivery by an aerosol route for watermelon plant foliar uptake, J. Nano. Res., 2013, 15. [Google Scholar]
- 309.Steurbaut W, Adjuvants for use with foliar fungicides, Pesticide Science, 1993, 38, 85–91. [Google Scholar]
- 310.Parks AN, Burgess RM, Ho KT and Ferguson PL, On the likelihood of single-walled carbon nanotubes causing adverse marine ecological effects, Integ. Environ. Ass. Manag, 2014, 10, 472–474. [Google Scholar]
- 311.Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao A-J, Quigg A, Santschi PH and Sigg L, Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi, Ecotoxicology, 2008, 17, 372–386. [DOI] [PubMed] [Google Scholar]
- 312.Atkinson R and Atkinson B, Moles, Whittet Books, 2013. [Google Scholar]
- 313.Majumdar S, Trujillo-Reyes J, Hernandez-Viezcas JA, White JC, Peralta-Videa JR and Gardea-Torresdey JL, Cerium biomagnification in a terrestrial food chain: influence of particle size and growth stage, Environ Sci Technol, 2016, 50, 6782–6792. [DOI] [PubMed] [Google Scholar]
- 314.Larner F, Dogra Y, Dybowska A, Fabrega J, Stolpe B, Bridgestock LJ, Goodhead R, Weiss DJ, Moger J, Lead JR, Valsami-Jones E, Tyler CR, Galloway TS and Rehkamper M, Tracing bioavailability of ZnO nanoparticles using stable isotope labeling, Environ Sci Technol, 2012, 46, 12137–12145. [DOI] [PubMed] [Google Scholar]
- 315.Kim JI, Park HG, Chang KH, Nam DH and Yeo MK, Trophic transfer of nano-TiO2 in a paddy microcosm: A comparison of single-dose versus sequential multi-dose exposures, Environ. Poll, 2016, 212, 316–324. [DOI] [PubMed] [Google Scholar]
- 316.Post DM, Using stable isotopes to estimate trophic position: Models, methods, and assumptions, Ecology, 2002, 83, 703–718. [Google Scholar]
- 317.Unrine JM, Hopkins WA, Romanek CS and Jackson BP, Bioaccumulation of trace elements in omnivorous amphibian larvae: Implications for amphibian health and contaminant transport, Environ. Pollut, 2007, 149, 182–192. [DOI] [PubMed] [Google Scholar]
- 318.Tella M, Auffan M, Brousset L, Issartel J, Kieffer I, Pailles C, Morel E, Santaella C, Angeletti B, Artells E, Rose J, Thiery A and Bottero JY, Transfer, transformation, and impacts of ceria nanomaterials in aquatic mesocosms simulating a pond ecosystem, Environ Sci Technol, 2014, 48, 9004–9013. [DOI] [PubMed] [Google Scholar]
- 319.Plant AL, Locascio LE, May WE and Gallagher PD, Improved reproducibility by assuring confidence in measurements in biomedical research, Nat Meth, 2014, 11, 895–898. [DOI] [PubMed] [Google Scholar]
- 320.Schmidt CW, Research Wranglers: Initiatives to improve reproducibility of study findings, Environ. Health Perspect, 2014, 122, A188–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Rosslein M, Elliott JT, Salit M, Petersen EJ, Hirsch C, Krug HF and Wick P, Use of Cause-and-Effect Analysis to Design a High-Quality Nanocytotoxicology Assay, Chem. Res. Toxicol, 2015, 28, 21–30. [DOI] [PubMed] [Google Scholar]
- 322.Haase A and Lynch I, Quality in nanosafety - Towards reliable nanomaterial safety assessment, Nanoimpact, 2018, 11, 67–68. [Google Scholar]
- 323.Poland C, Miller M, Duffin R and Cassee F, The elephant in the room: reproducibility in toxicology, Part. Fibre Toxicol, 2014, 11, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Reina A, Subramaniam AB, Laromaine A, Samuel ADT and Whitesides GM, Shifts in the Distribution of Mass Densities Is a Signature of Caloric Restriction in Caenorhabditis elegans, Plos One, 2013, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Godin M, Bryan AK, Burg TP, Babcock K and Manalis SR, Measuring the mass, density, and size of particles and cells using a suspended microchannel resonator, Applied Physics Letters, 2007, 91. [Google Scholar]
- 326.Vanierland ET and Peperzak L, Separation of marine seston and density determination of marine diatoms by density gradient centrifugation, J. Plankton Res, 1984, 6, 29–44. [Google Scholar]
- 327.Van Veldhoven PP, Baumgart E and Mannaerts GP, Iodixanol (Optiprep), an improved density gradient medium for the iso-osmotic isolation of rat liver peroxisomes, Anal Biochem, 1996, 237, 17–23. [DOI] [PubMed] [Google Scholar]
- 328.Kim SH, Mulholland GW and Zachariah MR, Density measurement of size selected multiwalled carbon nanotubes by mobility-mass characterization, Carbon, 2009, 47, 1297–1302. [Google Scholar]
- 329.van Leeuwen CJ and Hermens JLM, Risk Assessment of Chemicals, Springer, Dordrecht, Netherlands, 1995. [Google Scholar]
- 330.Arnot JA and Gobas F, A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms, Environ. Rev, 2006, 14, 257–297. [Google Scholar]
- 331.Leeuw TK, Reith RM, Simonette RA, Harden ME, Cherukuri P, Tsyboulski DA, Beckingham KM and Weisman RB, Single-walled carbon nanotubes in the intact organism: Near-IR imaging and biocompatibility studies in Drosophila, Nano Lett, 2007, 7, 2650–2654. [DOI] [PubMed] [Google Scholar]
- 332.Petersen EJ, Zhang LW, Mattison NT, O’Carroll DM, Whelton AJ, Uddin N, Nguyen T, Huang QG, Henry TB, Holbrook RD and Chen KL, Potential release pathways, environmental fate, and ecological risks of carbon nanotubes, Environ. Sci. Technol, 2011, 45, 9837–9856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


