Abstract
We tested the hypothesis that 5-HT promotes the differentiation of enteric neurons by stimulating a developmentally regulated receptor expressed by crest-derived neuronal progenitors. 5-HT and the 5-HT2 agonist (±)-2,5-dimethoxy-4-iodoamphetamine.HCl (DOI) enhanced in vitro differentiation of enteric neurons, both in dissociated cultures of mixed cells and in cultures of crest-derived cells isolated from the gut by immunoselection with antibodies to p75NTR. The promotion of in vitro neuronal differentiation by 5-HT and DOI was blocked by the 5-HT1/2 antagonist methysergide, the pan-5-HT2 antagonist ritanserin, and the 5-HT2B/2C-selective antagonist SB206553. The 5-HT2A-selective antagonist ketanserin did not completely block the developmental effects of 5-HT. 5-HT induced the nuclear translocation of mitogen-activated protein kinase. This effect was blocked by ritanserin. mRNA encoding 5-HT2A and 5-HT2B receptors was detected in the fetal bowel (stomach and small and large intestine), but that encoding the 5-HT2C receptor was not. mRNA encoding the 5-HT2B receptor and 5-HT2B immunoreactivity were found to be abundant in primordial [embryonic day 15 (E15)–E16] but not in mature myenteric ganglia. 5-HT2B-immunoreactive cells were found to be a subset of cells that expressed the neuronal marker PGP9.5. These data demonstrate for the first time that the 5-HT2B receptor is expressed in the small intestine as well as the stomach and that it is expressed by enteric neurons as well as by muscle. It is possible that by stimulating 5-HT2Breceptors, 5-HT affects the fate of the large subset of enteric neurons that arises after the development of endogenous sources of 5-HT.
Keywords: enteric nervous system, bowel, gut, neuronal development, serotonin receptors, 5-HT
The gut is the only organ that is able to display reflex activity when isolated from the CNS (Furness and Costa, 1987; Gershon et al., 1994). This activity is mediated by the intrinsic innervation of the bowel, the enteric nervous system (ENS), which structurally and chemically has more in common with the CNS than with the extraenteric peripheral nerve. Despite its unique properties, the ENS shares the neural crest origin of most of the PNS (Yntema and Hammond, 1954, 1955; Le Douarin and Teillet, 1973, 1974). At least some migrating crest-derived cells are pluripotent (Fraser and Bronner-Fraser, 1991; Ito and Sieber-Blum, 1993; Ito et al., 1993;Sieber-Blum et al., 1993) and remain so at the time they colonize the gut (Rothman et al., 1990, 1993; Sextier-Sainte-Claire Deville et al., 1994; Lo and Anderson, 1995). The enteric microenvironment, therefore, plays a role in determining the ENS-specific outcome of the differentiation of crest-derived cells within the bowel (Gershon, 1997,1998).
Signals that influence the fate of crest-derived neural and glial precursors in the enteric microenvironment are known to include glial cell line-derived neurotrophic factor (Moore et al., 1996;Pichel et al., 1996; Sánchez et al., 1996; Chalazonitis et al., 1998b; Hearn et al., 1998), neurotrophin-3 (Chalazonitis et al., 1994), neuropoietic cytokines (Gershon, 1997; Chalazonitis et al., 1998a), endothelin-3 (Baynash et al., 1994), and laminin-1 (Rothman et al., 1996; Chalazonitis et al., 1997). It has been suggested that early-developing enteric neurons, or their transmitters, might also influence the fate of later-developing cells because different types of enteric neurons arise in a reproducible sequential order (Pham et al., 1991). In fact, differential dependence on the expression of themash-1 gene has enabled early- and late-developing enteric neuronal precursor lineages to be clearly distinguished (Blaugrund et al., 1996). The mash-1-dependent cells are transiently catecholaminergic before they acquire their terminally differentiated phenotype. These cells leave the cell cycle and give rise to neurons at a time when the mash-1-independent progenitors are still proliferating (Blaugrund et al., 1996). Early neurons even form synapses on dividing neuroblasts (Gershon et al., 1981). All enteric serotonergic neurons are mash-1 dependent and develop early (Pham et al., 1991; Blaugrund et al., 1996). 5-HT could thus influence the fate of late-developing neurons. Enterochromaffin cells (EC), which are by far the largest enteric source of 5-HT, also develop before themash-1-independent lineage of enteric neurons (Branchek and Gershon, 1989). 5-HT may thus be a growth and/or survival factor, as well as a neurotransmitter or paracrine hormone (Cooke et al., 1997;Chen et al., 1998; Gershon, 1999).
Although 5-HT's ability to act as a growth factor has not yet been directly demonstrated, indirect evidence suggests that 5-HT does play a role in the development of neurons (Lauder and Krebs, 1978; Lauder, 1988, 1993; Azmitia et al., 1990; Whitaker-Azmitia et al., 1990; Nishi et al., 1996), glia (Liu and Lauder, 1992), and mesenchymal cells (Shuey et al., 1992, 1993; Choi et al., 1994, 1997). 5-HT may also affect phenotypic choice, for example, by increasing the proportion of CNS neuroblasts that develop as glutaminergic neurons (Lavdas et al., 1997). The current study was performed to test the hypothesis that 5-HT promotes enteric neuronal differentiation by stimulating a developmentally regulated subtype of the 5-HT receptor. The data show that the 5-HT2B receptor is both highly expressed and developmentally regulated in primordial enteric ganglia. 5-HT promotes the in vitro development of enteric neurons by an action that can be blocked by antagonizing 5-HT2Breceptors. 5-HT2B expression temporally follows that of sources of 5-HT and coincides with the period of terminal differentiation of mash-1-independent enteric neurons; therefore, these observations are consistent with the possibility that stimulation of 5-HT2B receptors by 5-HT influences the fates of late-developing enteric neurons.
MATERIALS AND METHODS
Animals and tissue collection. Adult Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were anesthetized with methoxyflurane (Pitman Moore) and decapitated. Guinea pigs (Kingstar Laboratories) were stunned and exsanguinated. Mice (CD-1 strain; Charles River Laboratories) were killed by asphyxiation, followed by cervical dislocation. Fetuses, obtained from timed pregnant mice, were anesthetized by cooling and exsanguinated before dissection. All procedures were approved by the Animal Care and Use Committee of Columbia University.
Cell culture. Experiments were performed with dissociated cells obtained from the intestines of 10–15 fetuses (removed from one or two dams) at embryonic day 13 (E13)–E15. The entire bowel was dissected, minced, and digested with collagenase A (5 mg/ml) in saline-G (in mm, NaCl 137, KCl 5.4, Na2HPO4 1.1, KH2PO4 1.1, and glucose 0.11%, pH 7.2–7.4) for 30 min at 37°C. After digestion, cells were dissociated by trituration in defined media (Stemple and Anderson, 1992). The dissociated cells (6.0 × 104/0.5 ml sample) were plated on laminin-coated glass coverslips, held in four-well tissue culture plates, and grown in serum-free defined media (Stemple and Anderson, 1992). Experimental compounds or vehicle (controls) was added after 24 hr in vitro. Cultured cells were fixed after 3 or 7 d of incubation. When cells were cultured for 7 d, the medium was changed at day 3. Cultures were maintained in triplicate. Cultured cells were fixed for 1 hr with 4% formaldehyde (freshly prepared from paraformaldehyde) in PBS (130 mmNaCl, 7 mmNa2HPO4, and 3 mmNaH2PO4). Compounds tested were the following: 5-HT, (±)-2,5-dimethoxy-4-iodoamphetamine.HCl (DOI), ritanserin, SB206553 (Research Biochemicals, Natick, MA), methysergide (Sandoz, Basel, Switzerland), and ketanserin (Janssen Biochimica, Berse, Belgium).
Immunoselection. Crest-derived cells of the E14 gut were separated from noncrest-derived cells by positive and negative immunoselection as described previously (Pomeranz et al., 1993;Chalazonitis et al., 1994, 1997, 1998a). Antibodies to the common neurotrophin receptor p75NTR (#9651; generously supplied by Dr. Moses Chao, Cornell University Medical College, New York, NY) (Huber and Chao, 1995) were used to immunoselect the crest-derived population.
Reverse transcription and the PCR. RNA was extracted from segments of mature or fetal bowel using the guanidinium thiocyanate method (Chomczynski and Sacchi, 1987). Reverse transcription (RT)-PCR was used to detect expression of mRNA-encoding members of the 5-HT2 receptor family in sampled regions of the gut. The set of PCR primers used for the analysis of the 5-HT2A receptor, 5′-ATGGAAATTCTCTGTGAAGACAATATCTCC-3′ and 5′-TCACACACAGCTAACCTTTTCATTCACGGT-3′, corresponded to nucleotides (nt) 1–30 and 1387–1416, respectively, of the murine receptor (Yang et al., 1992). The set of PCR primers used for the analysis of the 5-HT2B receptor, 5′-ATGGCTTCATCTTATAAAATGTCTGAAA-3′ and 5′-ATCGAGGAGGATGATTGATGAGGACTGAATGGTTGA-3′, corresponded to nt 19–45 and 1366–1401, respectively, of the murine receptor (Loric et al., 1992). The set of PCR primers used for the analysis of the 5-HT2C receptor, 5′-TAATTGGCCTATTGGTTT-3′ and 5′-ACACTACTAATCCTCT-3′, corresponded to nt 44–61 and 1361–1376, respectively, of the murine receptor (Yu et al., 1991). For first-strand cDNA synthesis, 2.5 μg of RNA was incubated for 1 hr at 42°C with 200 U of Moloney murine leukemia virus reverse transcriptase, using random primers at a concentration of 2.5 μm. This reaction and subsequent amplification withTaq polymerase was performed with a commercial kit (GeneAmp; Perkin-Elmer, Emeryville, CA) according to the manufacturer's instructions. The PCR profile of 93°C for 2 min, 55°C for 2 min, and 72°C for 4 min for 30 cycles was programmed into a model PTC-150 programmable thermal cycler (MJ Research, Watertown, MS). PCR reaction products were resolved on 1.2% agarose, 40 mm Tris-acetate, and 1 mm EDTA gels, and their size was determined using a 123 bp standard ladder.
Riboprobe biosynthesis. PCR products, amplified with primers designed on the basis of sequences found between the third and sixth transmembrane domains of the rat 5-HT2B receptor, were obtained from mouse, rat, and guinea pig tissues. These fragments were 620 bp (mouse), 606 bp (rat), and 611 bp (guinea pig). For subcloning, the PCR fragments were extracted from agarose gels (Gene-Clean; BIO 101, La Jolla, CA) and ligated into the cloning vector pCRII using the T/A cloning kit (Invitrogen, San Diego, CA). PCR fragments were sequenced using the Sanger dideoxynucleotide chain termination method (Sanger et al., 1977). cDNA fragments encoding the partial sequence of the 5-HT2B receptor for each species were used as templates for the synthesis of sense and antisense [35S]-labeled riboprobes using a commercial kit according to the manufacturer's directions (Promega, Madison, WI).
In situ hybridization. mRNA encoding the 5-HT2B receptor was located by in situhybridization in mouse, rat, and guinea pig tissues. Dissected preparations were cleaned and fixed for 4 hr (fetal) or 3 hr (adult) in 4% formaldehyde (freshly prepared from paraformaldehyde) in PBS. The fixed tissues were then cryoprotected by overnight incubation in 30% sucrose at 4°C, embedded in ornithine carbamyl transferase (OCT) compound (Tissue-Tek), frozen in liquid N2, and sectioned at −20°C with a cryostat microtome. Cross sections were cut through dissected segments of bowel or the abdominal cavities of fetal mice. The sections were thaw-mounted onto Tespa (Sigma, St. Louis, MO)-coated slides and stored at −80°C until used.
Tissue sections were post-fixed on slides with formaldehyde (4%, freshly prepared from paraformaldehyde) in PBS, containing 50 mm EDTA, for 3 min at room temperature. The sections were rinsed twice in PBS and once in water, dehydrated by passage through a graded series of ethanols, air dried for 5 min, rehydrated (2 min), and transferred to 50 mm triethanolamine. Acetic anhydride was added to yield a final concentration of 0.25%, and the sections were left for 10 min. After a 10 min rinse in 0.2× SSC (1× SSC, 150 mm NaCl and 15 mm Na citrate, pH 7), the sections were again dehydrated in a series of increasing concentrations of ethanol and air dried. Sections were prehybridized for 2 hr at 50°C in 250 μl of a solution containing 50% formamide, 600 mm NaCl, 10 mm Tris-HCl, pH 7.5, 1 mm EDTA, 1× Denhardt's solution, 0.05% boiled salmon sperm DNA, and 0.0125% yeast tRNA in a chamber humidified by a solution of 4× SSC containing 50% formamide. The sections were then hybridized overnight at 50°C with35S-antisense or35S-sense 5-HT2Briboprobes. The probes were diluted (50,000 cpm/μl) in a hybridization buffer containing 50% formamide, 600 mmNaCl, 10 mm Tris-HCl, pH 7.5, 1 mm EDTA, 1× Denhardt's solution, 10% dextran sulfate, 0.01% boiled salmon sperm DNA, 0.0125% yeast tRNA, 10 mm DTT, and 0.1% SDS. The slides were washed for 30 min at 50°C in a solution containing 50% formamide, 1× SSC, and 10 mm DTT. Formamide was removed by washing slides at room temperature in 0.5× SSC for 30 min. Sections were treated with RNase A (100 μg/ml) in buffer (10 mm Tris-HCl, pH 7.5, 1 mm EDTA, and 500 mm NaCl) for 30 min at room temperature and rinsed in the same buffer (twice for 10 min each; room temperature) and then in 0.5× SSC (twice for 60 min each; 50°C). Finally, the sections were dehydrated in ethanol containing 300 mm ammonium acetate and air dried. Slides were dipped in liquified Ilford L4 emulsion (diluted 1:1), dried, and exposed for 16–20 weeks (adult tissue) or 4–8 weeks (fetal tissue) at room temperature before development with Kodak Microdol X (Eastman Kodak, Rochester, NY). Developed slides either were viewed unstained or were counterstained with Geimsa, dehydrated, and mounted with Permount. Sections were examined with a Leica DMRB microscope (Nussloch, Germany) using bright-field and/or vertical dark-field illumination.
Immunocytochemistry. Both fresh-frozen and fixed preparations were examined. Freshly dissected and fixed tissues were infiltrated with OCT-embedding medium, frozen in liquid N2, sectioned (10 μm) with a cryostat microtome, and collected on gelatin-coated glass slides. Sections of fresh-frozen tissue were fixed on slides (1% formaldehyde; 10 min; 4°C) and washed (twice for 10 min each) with PBS containing 0.1% Triton X-100 (PBS-T). All preparations were treated for 30 min with H2O2 (0.3%) in PBS-T, washed again with PBS-T, and blocked for 30 min with 4% goat serum (GS) in PBS containing 0.3% Triton X-100. Polyclonal (Choi and Maroteaux, 1996) or monoclonal (PharMingen, San Diego, CA) antibodies to the 5-HT2B receptor were then applied (diluted 1:100 in blocking solution) to the sections for 72 hr at 4°C. Cells in culture were fixed and treated as described above. Sites of antibody binding were detected with biotinylated species-specific secondary antibodies and avidin coupled to horseradish peroxidase (ABC method; Elite Kit; Vector Laboratories, Burlingame, CA). Peroxidase activity was visualized with H2O2 and 3,3′-diaminobenzidene and nickel intensification. Alternatively, double-label fluorescence immunocytochemistry was used to identify the neuronal marker ubiquitin hydrolase (PGP9.5; diluted 1:500) (Wilkinson et al., 1989) together with 5-HT2B receptors. 5-HT2B immunoreactivity was detected with indocarbocyanine (Cy3)-labeled goat anti-mouse antibodies (diluted 1:2000) or biotinylated goat F(ab′)2anti-mouse IgG1 (γ1 chain specific; diluted 1:100; Southern Biotechnology, Birmingham, AL) and steptavidin coupled to Cy3 (diluted 1:1000; Jackson ImmunoResearch, West Grove, PA). PGP9.5 immunoreactivity was visualized with fluorescein isothiocyanate (FITC)-labeled secondary antibodies (diluted 1:1000; Jackson ImmunoResearch).
For studies of the development of neurons in vitro, the PGP9.5 immunoreactivity was again used as a neural marker. For these experiments, fixed cultures were permeabilized with 4% GS in PBS containing 0.1% Triton X-100. PGP9.5 immunoreactivity was visualized with biotinylated goat anti-rabbit IgG (diluted 1:400; Kirkegaard & Perry, Gaithersburg, MD) and streptavidin coupled to FITC (diluted 1:200; Vector Laboratories). The PGP9.5-immunoreactive cells were counted at a magnification of 100×, using a rectangle projected into the viewing oculars. Sampling errors were avoided by counting every PGP9.5-immunoreactive cell on each coverslip. To estimate the total numbers of cells in each culture, we stained the same cultures used for demonstrating PGP9.5 immunoreactivity with bisbenzamide (1.0 μg/ml for 5 min followed by two washes). Because bisbenzamide inserts into DNA, nuclei could be accurately counted, and cell numbers were determined from the nuclear counts. All conditions were analyzed in triplicate in each experiment; means were compared by ANOVA, using the STATVIEW 4.0 program for the Macintosh computer. In all instances,p < 0.05 was considered significant. No significant differences were found between conditions in total cell numbers within each experiment.
RESULTS
5-HT promotes the development of enteric neurons in vitro by stimulating a 5-HT2 receptor
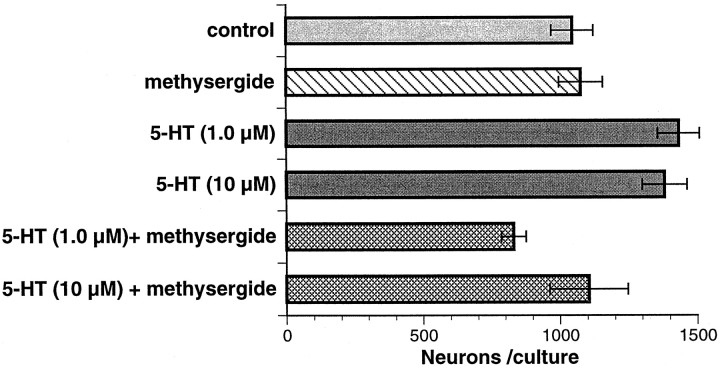
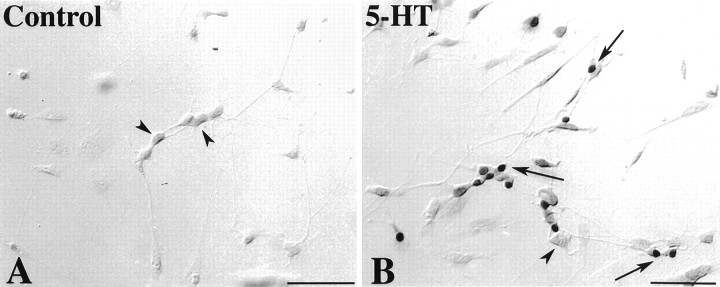
Fetal mouse gut was dissociated at E13–E16. This period precedes the differentiation of mash-1-independent neurons (Blaugrund et al., 1996) yet still encompasses both the birth of enteric serotonergic neurons (Pham et al., 1991) and the acquisition of their neurotransmitter (Rothman and Gershon, 1982). Cells were cultured in serum-free defined media. Neurons (identified as PGP9.5-immunoreactive cells) developed well under these conditions. In initial studies, cultured cells were exposed to 5-HT (1 or 10 μm) for 48 hr. 5-HT increased both the number of neurons (PGP9.5-immunoreactive cells) developing in vitro(p < 0.01 vs control, for 1 and 10 μm) and the extent of branching of their neurites (Figs. 1,2A,B). The 5-HT1/2 antagonist methysergide (10 μm), by itself, exerted no effect on neuronal development (Figs. 1, 2C); however, methysergide blocked both the 5-HT-associated promotion of neuronal development (p < 0.001 vs 1.0 μm5-HT) and the enhancement of neuritic branching (Figs. 1, compare 2D with B).
Fig. 1.
5-HT promotes the development of neurons in vitro, and this effect is blocked by methysergide. Intestinal cells (E16) were dissociated and cultured in the presence of vehicle (control) or the indicated experimental compounds for 48 hr. The concentration of methysergide was 10 μm. All of the neurons (PGP9.5-immunoreactive cells) on each cultured dish were counted. Data are presented as the actual number of neurons found in each dish. The total number of cells per culture does not differ significantly between conditions.
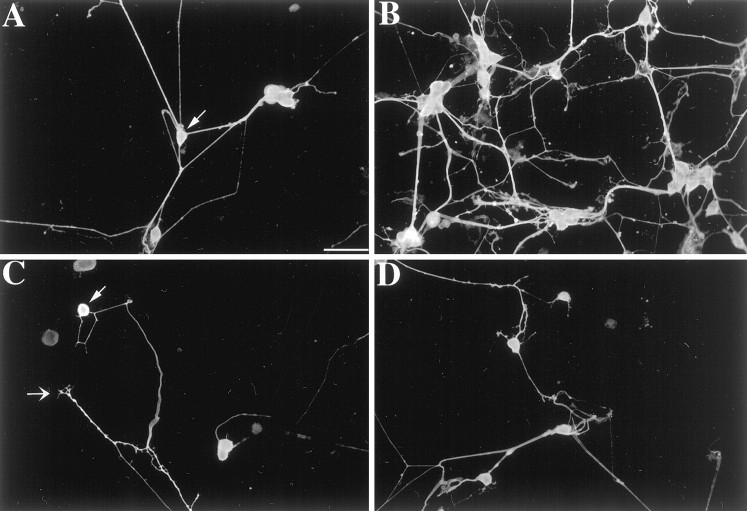
Fig. 2.
The effects of 5-HT on neuronal development were visualized by demonstrating the immunoreactivity of the neuronal marker PGP9.5. Cultures were prepared and treated as described in Figure 1. Nerve cell bodies (
 ) and a growth cone (→) are indicated. A,Control. B, 5-HT (1.0 μm).C, Methysergide (10.0 μm).D, 5-HT (1.0 μm) + methysergide (10.0 μm). 5-HT increases both the number of neurons and the complexity of the branching of their neurites. Methysergide does not itself affect the number or appearance of cultured neurons but blocks the effects of 5-HT. Scale bar, 50 μm.
) and a growth cone (→) are indicated. A,Control. B, 5-HT (1.0 μm).C, Methysergide (10.0 μm).D, 5-HT (1.0 μm) + methysergide (10.0 μm). 5-HT increases both the number of neurons and the complexity of the branching of their neurites. Methysergide does not itself affect the number or appearance of cultured neurons but blocks the effects of 5-HT. Scale bar, 50 μm.
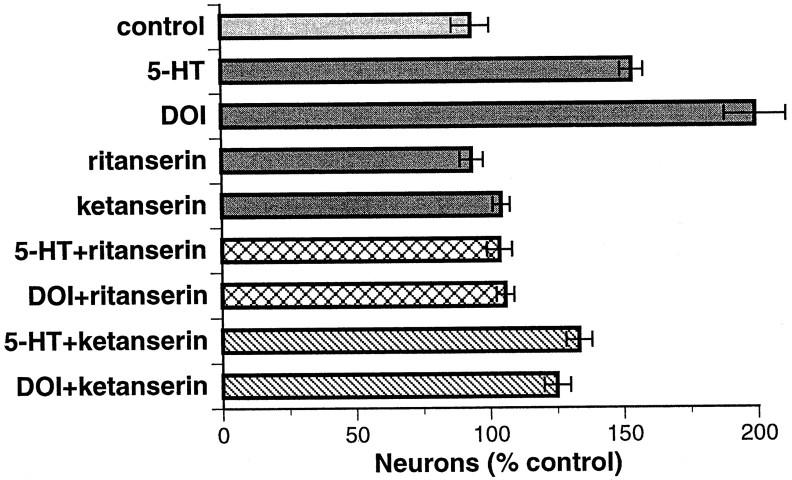
In subsequent experiments, dissociated enteric cells were exposed to 5-HT for 144 hr to provide additional time for its actions to become manifest. 5-HT (1 μm) again increased the number of neurons developing in vitro (Fig.3). The average number of neurons found in cultures of mixed cells dissociated from the fetal bowel was 1415 ± 120 neurons (n = 28), which represents ∼10% of the total number of cells per culture (14,727 ± 630). Although there were now many more neurons, the magnitude of the increase evoked by the addition of 5-HT (approximately twofold) was approximately the same as that seen in cultures exposed to 5-HT for only 48 hr. The 5-HT2 agonist DOI (1 μm) mimicked the effects of 5-HT and increased both the number of neurons developing in vitro(p < 0.001 vs control for both 5-HT and DOI) and the complexity of neuritic branching. The response to DOI was greater than that to the same concentration of 5-HT (p < 0.001 vs 5-HT). The selective 5-HT2 antagonist ritanserin (0.1 μm) abolished the effects, both of 5-HT (p < 0.001 vs 5-HT) and DOI (p < 0.001 vs DOI) (Fig. 3).
Fig. 3.
The effects of 5-HT on in vitroneuronal development are mimicked by DOI and antagonized by ritanserin more than ketanserin. Intestinal cells (E14) were dissociated and cultured in the presence of vehicle (control) or the indicated experimental compounds for 144 hr. All of the neurons (PGP9.5-immunoreactive cells) on each cultured dish were counted. Concentrations were as follows: 5-HT, 1.0 μm; DOI, 1.0 μm; ritanserin, 0.1 μm; and ketanserin, 0.1 μm.
In contrast to ritanserin, the 5-HT2A selective antagonist ketanserin (0.1 μm) did not completely prevent the stimulation of neuronal development by 5-HT or DOI (Fig. 3). Both 5-HT (1.0 μm; p < 0.001 vs control) and DOI (1.0 μm; p < 0.03 vs control) continued to promote neuronal development, despite the presence of ketanserin. Ketanserin moderately reduced the number of neurons developing in the presence of DOI (p < 0.001 vs DOI). The observation that ritanserin completely antagonizes the promotion of the in vitro development of enteric neurons by 5-HT and DOI suggests that stimulation of a 5-HT2 receptor is sufficient to account for the response. Because ketanserin (0.1 μm) did not completely block the response to 5-HT and DOI but ritanserin (0.1 μm) did, the pharmacology of the receptors that mediate the 5-HT- and DOI-induced enhancement of neuronal development seems to be more like that of a 5-HT2B and/or 5-HT2C than a 5-HT2Areceptor.
Enteric crest-derived cells respond directly to 5-HT
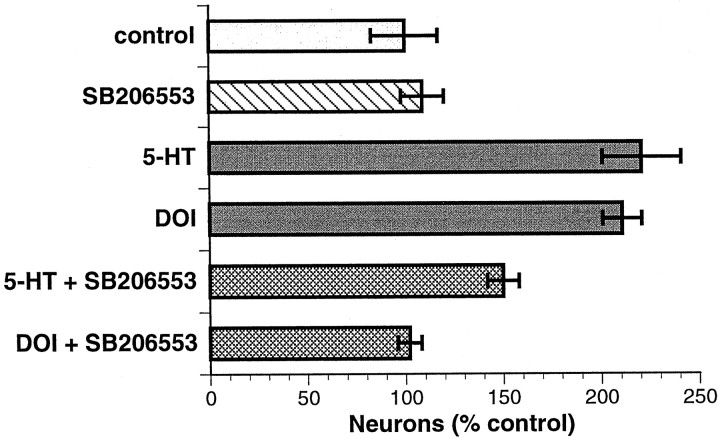
To determine whether 5-HT and/or DOI promoted the development of neurons by acting directly on their crest-derived progenitors, we cultured isolated populations (E14) of crest- and noncrest-derived cells for 3 d in the presence or absence of 5-HT or DOI. The isolated cells were obtained by a process of positive and negative immunoselection with antibodies to p75NTR. 5-HT (p < 0.001) and DOI (p < 0.001) were each found to promote the development of neurons at least as well in cultures of isolated crest-derived cells as in mixed cultures of crest- and noncrest-derived cells (Fig. 4; compare with Figs. 1, 3). The antagonist SB206553, which has an ∼500-fold greater affinity for 5-HT2B and 5-HT2C than for 5-HT2A receptors (Audia et al., 1996; Kennett et al., 1996), was used to test the premise that the growth factor-like actions of 5-HT are mediated by a 5-HT2B/2Creceptor. SB206553 (1.0 μm) antagonized both the increase in development of enteric neurons induced by 5-HT (1.0 μm; p < 0.01) and that induced by DOI (1.0 μm; p < 0.001). Virtually no neurons could be detected in the cultures of noncrest-derived cells, whether or not these cells were exposed to 5-HT or DOI (data not shown).
Fig. 4.
5-HT and DOI promote the development of neurons in cultures of isolated enteric crest-derived cells. The effects of both compounds are blocked by a selective 5-HT2B/2C antagonist, SB206553. Concentrations were as follows: SB206553, 1.0 μm; 5-HT, 1.0 μm; and DOI, 1.0 μm.
5-HT activates mitogen-activated protein kinase in developing enteric neurons in vitro
When transfected cells expressing 5-HT2B receptors are exposed to 5-HT, mitogen-activated protein kinases (MAPKs) are stimulated (Launay et al., 1996). The activation of MAPKs in enteric neuronal precursors by 5-HT might, if it occurred, foster the proliferation of cycling precursors and/or the differentiation and survival of postmitotic neurons (Marshall, 1995). We therefore tested the hypothesis that stimulation of 5-HT2receptors activates MAPKs in enteric crest-derived cells. These studies were performed by immunocytochemistry, using antibodies that specifically detect the Y204-phosphorylated forms of both p42 (Erk1) and p44 (Erk2) MAPKs (Yan and Zahradka, 1997). Because phosphorylation causes the p42 and p44 MAPKs to become catalytically active and translocated to the nucleus in stimulated cells, the detection of intranuclear immunoreactivity was taken as indicative of MAPK activation.
Cells from the fetal bowel were dissociated at E13 and cultured for 3 d. Very little background nuclear p42/44 MAP kinase immunoreactivity could be detected in control cultures (Fig.5A). Within 45 min of the addition of 5-HT (1 μm), however, many cells now displayed nuclear p42/44 MAPK immunoreactivity (Fig.5B). Most of the cells in the 5-HT-treated cultures with p42/44 MAPK-immunoreactive nuclei, moreover, were process bearing, suggesting that they were in a neuronal or glial lineage. Ritanserin (1.0 μm) abolished the response to 5-HT (data not shown). These observations are consistent with the idea that the ability of 5-HT to activate MAPKs in cells cultured from the fetal mouse gut is mediated by a 5-HT2B receptor.
Fig. 5.
5-HT activates MAPK in cultures of dissociated cells from fetal gut (E13). Phosphorylated MAPK was demonstrated immunocytochemically. Cells are visualized by interference contrast microscopy. A, Control. No nuclear immunoreactivity is demonstrable. Cells with a neuronal morphology (▸) are indicated.B, 5-HT (1 μm; 45 min). MAPK immunoreactivity (→) can be seen in the nuclei of a subset of cells with a neuronal morphology; additional cells with a neuronal morphology are not immunoreactive (▸). Scale bars, 50.0 μm.
5-HT2B receptor mRNA is expressed in the fetal mouse gut
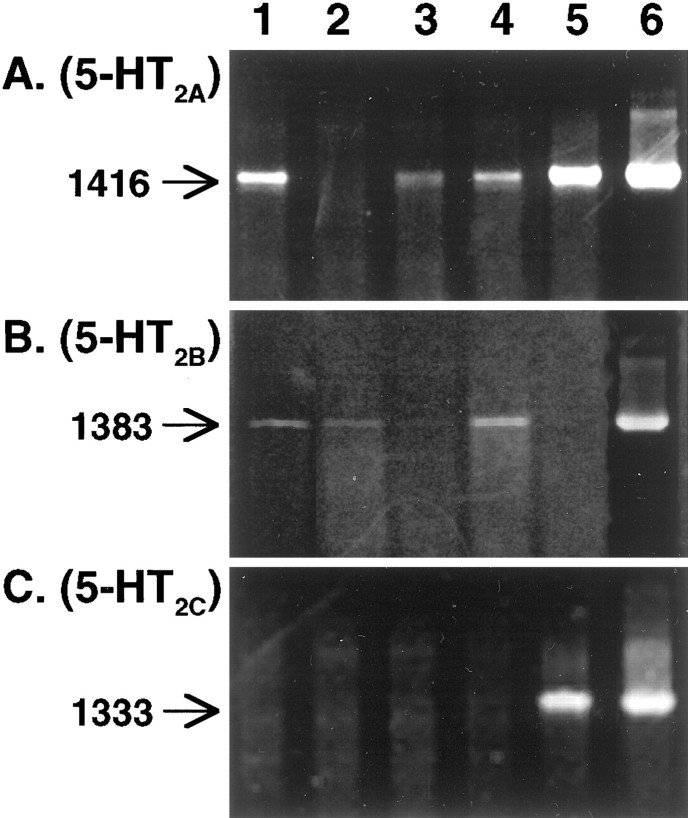
Because ritanserin and SB206553 do not adequately distinguish between 5-HT2B or 5-HT2Creceptors, studies were performed to determine which subtypes of 5-HT2 receptor are actually expressed in the fetal bowel. RNA was isolated from the E16 fetal mouse gut, and RT-PCR was used to detect transcripts encoding members of the 5-HT2 receptor family. This analysis suggested that mRNA encoding the 5-HT2A (Fig.6A) and 5-HT2B (Fig. 6B) receptors is expressed in the fetal bowel. mRNA encoding the 5-HT2A receptor was also detected in the adult mouse small intestine, stomach, and brain (Fig. 6A). mRNA encoding the 5-HT2B receptor was detected in the adult mouse colon and stomach (Fig. 6B). Little or no mRNA encoding the 5-HT2B receptor could be detected in the adult mouse small intestine or brain. The expression of the 5-HT2B receptor in the stomach was expected and probably reflects the location of this receptor in the smooth muscle of the gastric fundus (Kursar et al., 1992). No mRNA encoding the 5-HT2C receptor could be detected with RT-PCR in either the fetal or adult bowel (Fig. 6C). In contrast, mRNA encoding the 5-HT2C receptor was readily detected in the adult mouse brain with the same primers (Fig.6C). These observations suggest that 5-HT2A and 5-HT2B, but not 5-HT2C, receptors are expressed in the fetal mouse gut.
Fig. 6.
mRNAs encoding 5-HT2A(A) and 5-HT2B(B), but not 5-HT2C(C), receptors are expressed in the fetal mouse gut. Receptor expression in fetal and adult bowel was analyzed by RT-PCR. The size of the PCR products is given in base pairs.Lane 1, Fetal gut (E16); lane 2, adult colon; lane 3, adult small intestine; lane 4, stomach; lane 5, brain; lane 6, cDNA from plasmid insert.
Cells that express mRNA encoding 5-HT2B receptor were located by in situ hybridization in the fetal gut
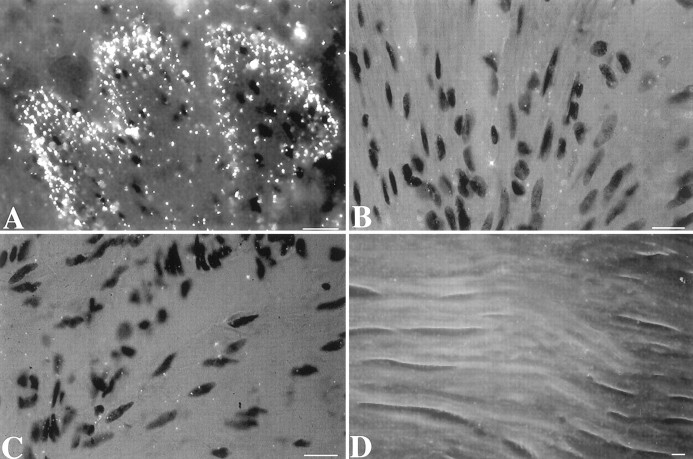
In situ hybridization was used to locate cells that express the 5-HT2B receptor in the fetal mouse gut. 35S-labeled sense and antisense riboprobes were synthesized from cDNAs encoding the region between the putative third and sixth transmembrane domains of the 5-HT2B receptor. This sequence shares very little identity with the corresponding sequences of the 5-HT2A and 5-HT2Creceptors. No hybridizing cells could be detected at E13. At E14, however, a small number of cells that hybridized weakly with the antisense but not the sense 35S-riboprobe could be detected in ganglia of the small and large intestines (Fig.7A). The intensity of labeling and the numbers of cells labeled by the antisense35S-riboprobe increased at E15 (Fig.7B) and was maximal at E16 (Fig. 7C,E), so that at this time, virtually every ganglion of the developing myenteric plexus in the fetal stomach and small and large intestine was heavily labeled. In contrast, no cells hybridized with the35S-sense riboprobe (Fig. 7D). Cells containing mRNA encoding the 5-HT2Breceptor appeared to be most abundant in the colon (Fig.7E), where developing myenteric ganglia are more numerous and closely packed than are those in the small intestine (Fig.7C) or stomach (data not shown). The numbers of cells expressing mRNA encoding the 5-HT2B receptor declined rapidly after E16, so that by E18, 5-HT2B mRNA could be detected only in occasional cells and not in every ganglion of the myenteric plexus (Fig.7F). Expression of mRNA encoding the 5-HT2B receptor could not be detected at any time in the submucosal plexus or in the smooth muscle of the fetal intestine.
Fig. 7.
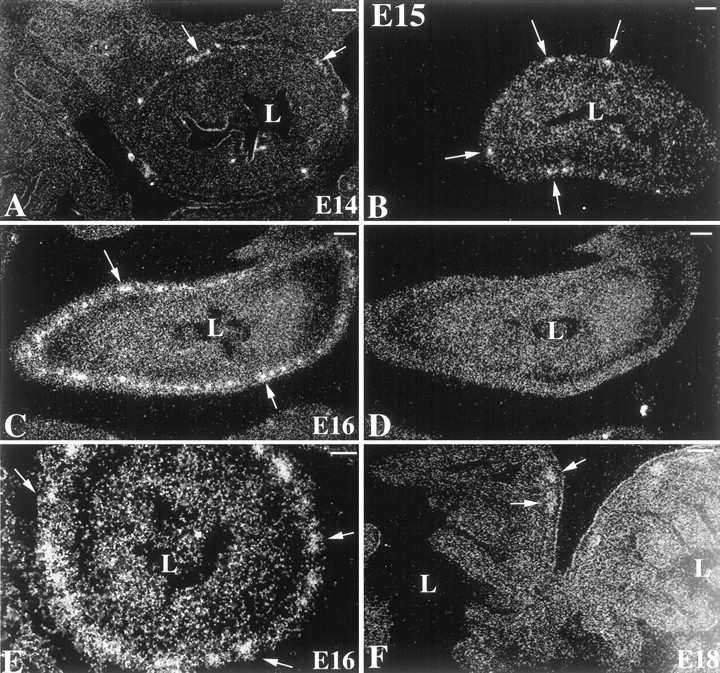
Expression of the 5-HT2B receptor is developmentally regulated in the ENS. mRNA encoding the 5-HT2B receptor was located in fetal tissue by in situ hybridization with an antisense 35S-riboprobe. As a control, alternate serial sections were hybridized with a sense35S-riboprobe. Sections are visualized by using reflected dark-field illumination. A, E14 fetal bowel (antisense riboprobe). mRNA encoding the 5-HT2B receptor is found in scattered ganglia of the primordial myenteric plexus in the outer gut mesenchyme (→). The dark-field bright material lining the lumen (L) is caused by the chemographic effects of meconium and is not specific labeling. The section was exposed for 12 weeks. B, E15 (antisense riboprobe). The degree of labeling is approximately equal to that seen at E14; however, exposure for only 8 weeks is required to detect mRNA encoding the 5-HT2B receptor in primordial myenteric ganglia (→).C, E16 small intestine (antisense riboprobe). mRNA encoding the 5-HT2B receptor is detectable in many myenteric ganglia (→) that surround the gut. D, E16 control (sense riboprobe). An adjacent section, serial to that illustrated in C, is shown. No structures are labeled.E, E16 colon (antisense riboprobe). Labeled ganglia (→) are even more numerous than in the small intestine at the same age. F, E18. Only occasional ganglia are labeled (→). Scale bars, 10 μm.
Cells that express mRNA encoding the 5-HT2B receptor were located by in situ hybridization in the adult gut
Observations made with in situ hybridization in the fetal bowel suggested that the 5-HT2B receptor might be developmentally regulated. The35S-labeled antisense riboprobe was thus used to locate cells expressing mRNA encoding the 5-HT2B receptor by in situhybridization in the adult mouse gut. To investigate the species specificity of the data, we also investigated adult bowel from rat and guinea pig. In the mouse and rat stomach, mRNA encoding the 5-HT2B receptor was confined to smooth muscle cells in the fundic area (Fig.8A,B). Neither the smooth muscle of the gastric corpus or pylorus (Fig. 8C) nor the skeletal muscle of the esophagus (Fig. 8D) of the rat or mouse was labeled. The labeled fundic smooth muscle cells were restricted to a band of cells at the periphery of muscle bundles at the junction of the muscularis externa and the connective tissue (Fig.8A). No hybridization was detected either in neurons or glia in the relatively sparse ganglia of the fundic region. No cells were labeled by the 35S-labeled antisense riboprobe in the adult guinea pig stomach (data not shown).
Fig. 8.
Smooth muscle cells express mRNA encoding the 5-HT2B receptor only in the fundus of the stomach. mRNA encoding the 5-HT2B receptor was located in the adult rat stomach by in situ hybridization with an antisense35S-riboprobe. Sections are visualized by using a combination of reflected dark-field and bright-field illumination.A, Fundus (antisense riboprobe). mRNA encoding the 5-HT2B receptor is concentrated in cells located at the periphery of muscle bundles. B, Fundus, control (sense riboprobe). An adjacent section, serial to that illustrated inA, is shown. No structures are labeled.C, Corpus (antisense riboprobe). No structures are labeled. D, Esophagus (antisense riboprobe). No structures are labeled. Scale bars, 20 μm.
In contrast to the rat and mouse gastric fundus, mRNA encoding the 5-HT2B receptor was not detected in intestinal smooth muscle. Instead, intestinal expression of the 5-HT2B receptor was confined to a small subset of myenteric neurons (compare Fig.9A with B,control). The small intestinal location of mRNA encoding the 5-HT2B receptor was essentially the same in the rat (Fig. 9A,B), mouse (Fig. 9C–F), and guinea pig (data not shown). The number of 5-HT2B-expressing neurons increased proximodistally. The expression of 5-HT2B mRNA was also observed in the crypt epithelium of the mouse small intestine (compare Fig. 9C,E with D,F, controls).
Fig. 9.
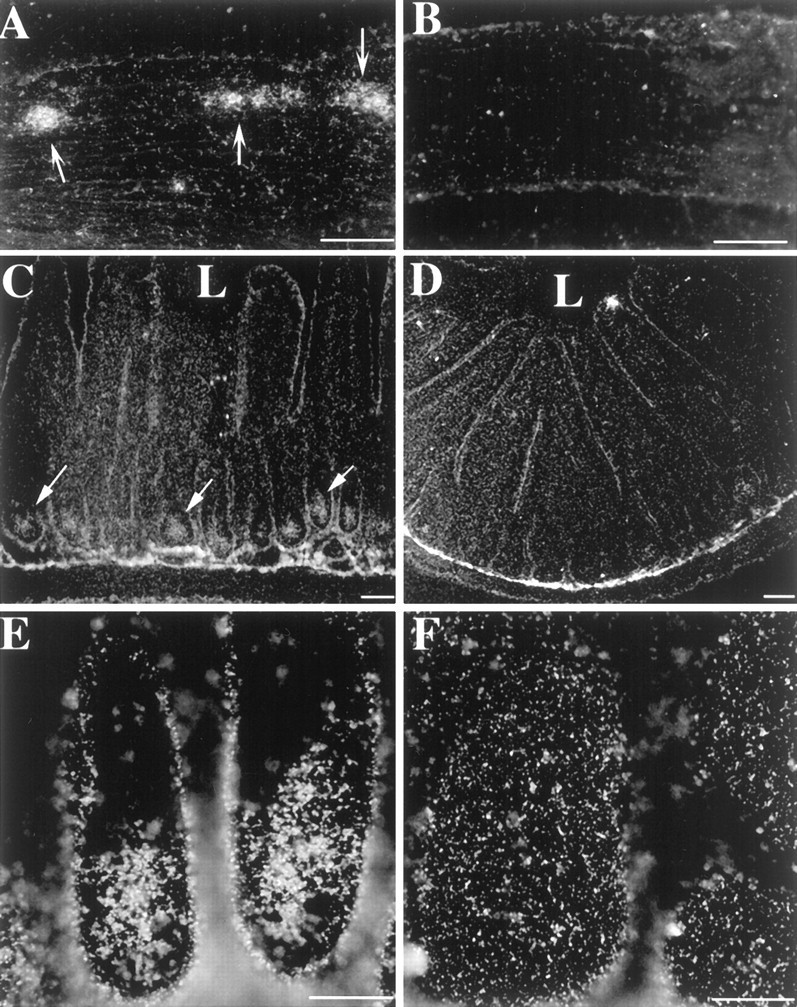
mRNA encoding the 5-HT2B receptor is expressed in intestinal neurons. In situ hybridization with an 35S-riboprobe. A, Rat colon (antisense riboprobe). mRNA encoding the 5-HT2B receptor is concentrated in cells in the myenteric ganglia. B, Rat colon, control (sense riboprobe). C, E, Mouse ileum sections (antisense riboprobe). mRNA encoding the 5-HT2Breceptor is found in crypt epithelial cells (→). D, F,Mouse ileum sections, control (sense riboprobe). L, Lumen. Scale bars: A–D, 50 μm; E, F, 20 μm.
Myenteric ganglia of the fetal and adult mouse gut contain 5-HT2B-immunoreactive cells
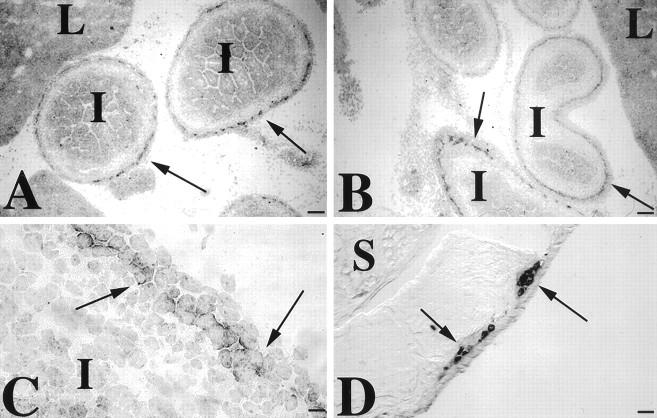
5-HT2B receptor immunoreactivity was demonstrable in neurons of the myenteric plexus of the small and large intestines of E15 fetal mice (Fig. 10). At this age, virtually every myenteric ganglion contained 5-HT2B receptor immunoreactivity (Fig.10A,B). The 5-HT2Bimmunoreactivity of most of the labeled myenteric neurons appeared to be concentrated at the periphery of the cells (Fig. 10C). 5-HT2B-immunoreactive cells were less abundant in adult than in fetal ganglia (Fig. 10D). No 5-HT2B immunoreactivity was observed in the submucosal plexus or in the smooth muscle of the mouse intestine.
Fig. 10.
5-HT2B immunoreactivity is present in neurons of the fetal and adult mouse intestine. A, B,E15 small intestinal loops (I) are shown. Note that immunoreactive myenteric ganglia (→) surround the cross sections of gut. C, 5-HT2B immunoreactivity is concentrated on ganglion cell surfaces (→). Most myenteric cells are 5-HT2B immunoreactive. D, Adult stomach is shown. 5-HT2B-immunoreactive neurons are present in myenteric ganglia. The → symbols point to some of the positive ganglia. L, Liver; S, stomach. Scale bars: A, B, 100 μm; C, 10 μm;D, 25 μm.
Neurons developing in cultures of cells dissociated from fetal mouse intestine are 5-HT2B immunoreactive
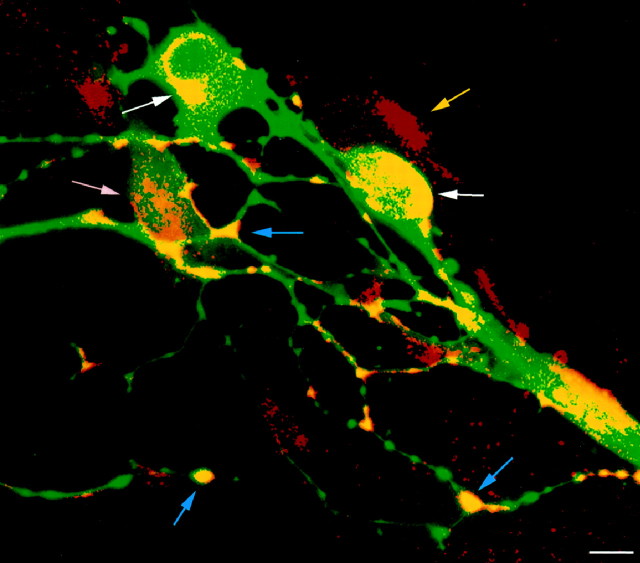
Both polyclonal and monoclonal antibodies to the 5-HT2B receptor labeled cells in cultures of dissociated E14 fetal mouse gut (Fig.11). Double-label immunocytochemistry revealed that most, but not all, of the 5-HT2B-immunoreactive cells coexpressed PGP9.5 immunoreactivity. Patches of 5-HT2Bimmunoreactivity were found on the cell bodies of these neurons and also on their varicose neurites. The perikarya of most of the doubly labeled neurons were strongly PGP9.5 immunoreactive; however, 5-HT2B receptors were also found on occasional cells with only weak PGP9.5 immunoreactivity. The cells with coincident 5-HT2B/PGP9.5 immunoreactivity represented a subset of the PGP9.5-immunoreactive cell population. The 5-HT2B-immunoreactive cells that did not express PGP9.5 immunoreactivity were located close to the neurons and thus might have been crest-derived precursors that had not yet acquired the neural marker or glia.
Fig. 11.
A subset of PGP9.5-immunoreactive neurons expresses 5-HT2B immunoreactivity in a culture of fetal bowel dissociated at E14. Laser-scanning confocal microscopic image. The immunoreactivity of PGP9.5 (FITC) appears green and that of 5-HT2B receptors appears red (Cy3); doubly labeled structures are yellow. Clusters of 5-HT2B immunoreactivity are found on PGP-immunoreactive cell bodies (white →) and the varicosities of their neurites (blue →). Occasional 5-HT2B-immunoreactive cells are weakly immunostained with antibodies to PGP9.5 (pink →). Rare cells, located close to neurons, express 5-HT2B but not PGP9.5 immunoreactivity. Scale bar, 10 μm.
DISCUSSION
The current study supports the tested hypothesis that 5-HT promotes the differentiation of enteric neurons by stimulating a developmentally regulated receptor expressed by crest-derived neuronal progenitors. First, the following observations suggest that stimulation of 5-HT2B receptors in vitro enhances the enteric neuronal development: (1) Both 5-HT and the 5-HT2 agonist DOI promote in vitrodifferentiation of enteric neurons; the effect of 5-HT is blocked by the 5-HT1/2 antagonist methysergide. (2) Ritanserin, a pan-5-HT2 antagonist, which lacks activity against 5-HT1 receptors, totally abolishes responses to 5-HT and DOI, whereas an equal concentration of the 5-HT2A-selective antagonist ketanserin does not. (3) The 5-HT2B/2C antagonist SB206553, which has little antagonistic activity at 5-HT1 or 5-HT2A sites (Audia et al., 1996; Kennett et al., 1996), blocks the promotion of neuronal differentiation by 5-HT. (4) mRNA encoding the 5-HT2B receptor is expressed in the fetal bowel, whereas that encoding the 5-HT2Creceptor, which might also have been affected by ritanserin and SB206553, is not. These data do not definitively eliminate an additional role for the 5-HT2A receptor in development.
Second, evidence that 5-HT2B receptors are expressed by crest-derived neural precursors included the following: (1) 5-HT and DOI promote neuronal development when they are added to cultures of crest-derived precursors isolated by immunoselection with antibodies to p75NTR, and the effects of both agonists are blocked by SB206553. (2) mRNA encoding the 5-HT2B receptor was detected by RT-PCR in isolated intestinal ganglia (Fiorica-Howells and Gershon, 1995). (3) mRNA encoding the 5-HT2B receptor (demonstrated by in situ hybridization) is developmentally regulated and abundant in primordial (E15–E16) myenteric ganglia. (4) 5-HT2B immunoreactivity was found in many developing enteric neurons. (5) 5-HT2B-immunoreactive cells present in cultures of fetal gut coexpress PGP9.5, a marker for cells specified as neuronal. The weak PGP9.5 immunoreactivity (which might just have been acquired) in some of the doubly labeled cells is consistent with the possibility that expression of 5-HT2B receptors precedes that of PGP9.5.
The 5-HT2B receptor was originally known as the “fundus receptor,” because it was thought to be unique to muscle cells of the fundic (rumen) region of the rat and mouse stomachs (Baez et al., 1990; Foguet et al., 1992a,b; Kursar et al., 1992; Wang et al., 1993). The 5-HT2B receptor, however, was found to be expressed, not only by the fundic muscle, but also by intestinal neurons. The muscular distribution of mRNA encoding the 5-HT2B receptor was strikingly limited to the fundic regions of the rat and mouse stomachs. The 5-HT2B-expressing muscle cells of the rat and mouse stomachs, moreover, were restricted to the periphery of muscle bundles. This localization is compatible with the idea that the 5-HT2B receptor is selectively expressed by that subset of muscle cells that is innervated. Because enteric smooth muscle cells are electrically coupled, the effects of stimulating 5-HT2B receptors on innervated cells could be transmitted to the noninnervated cells deep in muscle bundles.
It is difficult to envision a role for a receptor expressed, as is the 5-HT2B in adults, only on rare cells in a minority of enteric ganglia. Moreover, although the electrophysiological responses of enteric neurons to 5-HT have been thoroughly characterized, none have been found to be mediated by a member of the 5-HT2 receptor family (Gershon, 1995, 1999; Galligan, 1996). The timing of the developmental regulation of the 5-HT2B receptor implies that its function is likely to be more significant during the formation of the ENS, when the receptor is abundant, than in adult life, when it is rare. Conceivably, stimulation of enteric neuronal 5-HT2B receptors does not lead to a change in membrane potential but mediates a trophic function of 5-HT, even in mature animals. The rare adult ganglion cells that express the 5-HT2B receptor might represent a small set of 5-HT-responsive neuronal progenitors that persist in the adult bowel or mature neurons that retain a vestigial fetal receptor.
The 5-HT2 receptor family has been linked previously to the regulation of growth and/or differentiation. 5-HT stimulates mitosis and differentiation when it is added to transfected fibroblasts that express either the 5-HT2B (Choi et al., 1994; Launay et al., 1996) or the 5-HT2C(Julius et al., 1989) receptor. In fact, transfected fibroblasts that express the 5-HT2B receptor can be transformed by 5-HT or DOI in vitro, and the resulting foci give rise to tumors when transplanted into nude mice (Launay et al., 1996). Growth of the foci in vitro and the tumors in vivo is inhibited by ritanserin. The 5-HT2B receptor has also been shown to affect the development of cranial crest-derived ectomesenchyme (Choi et al., 1997). The developmental effects of the 5-HT2B receptor found in the present investigation, therefore, are not unprecedented. In fact, a transiently catecholaminergic cell line, 1C11, that expresses first 5-HT, then 5-HT2B, and finally 5-HT2Areceptors has been reported (Kellermann et al., 1996). This sequence resembles that found in the developing ENS.
In addition to cells in a neuronal lineage, epithelial cells in intestinal crypts were found to express the 5-HT2B receptor. Crypt epithelial cells also represent a site where 5-HT2B receptors are likely to affect growth and/or differentiation. The crypts of the gut contain a self-renewing stem cell population that continually differentiates throughout life to replace the cells that line the luminal surface of the small and large intestines (Roth et al., 1991). 5-HT stimulates the proliferation of both normal (Tutton, 1974) and neoplastic (Tutton and Barkla, 1980) intestinal crypt cells. Intestinal epithelial cells, moreover, have been shown to express a 5-HT2 receptor (Siriwardena et al., 1993). It is thus possible that the 5-HT2B receptor is linked to cell growth and/or differentiation in both neurectodermal and endodermal derivatives of the gut.
Stimulation of 5-HT2B receptors, spontaneously expressed by Mastomys tumor cells, or those expressed by transfected fibroblasts induces a rapid and transient activation of p21ras and MAPK (Launay et al., 1996). In the current study, 5-HT was also found to induce phosphorylation and nuclear translocation of MAPK in vitro. The responding cells displayed a neuronal morphology. The activation of MAPK by 5-HT, like 5-HT's ability to promote the development of enteric neurons, was blocked by ritanserin. It is thus possible that 5-HT2B-related effects on the development of enteric neurons are dependent on the activation of MAPK. Activation of MAPK could increase the number of neurons in vitro by exerting (1) a mitogenic effect on proliferating neuronal precursors, (2) a differentiating effect on postmitotic progenitors causing them to exhibit neuronal properties, and (3) a survival effect on existing neurons. Although preliminary experiments with bromodeoxyuridine did not reveal a mitogenic action of 5-HT on cultured enteric crest-derived cells, the current data do not permit a choice to be made between these alternatives.
The appearance of the 5-HT2B receptor coincides with the end of the period during which serotonergic neurons are born and with the onset of the birthdays ofmash-1-independent neurons (Pham et al., 1991). The developmental regulation of the 5-HT2B receptor is thus appropriate for it to mediate serotonergic effects on the development of the late-developing, mash-1-independent set of enteric neurons. The observation that synapses are formed between early-developing enteric neurons and still-dividing neuronal precursors in the fetal myenteric plexus (Gershon et al., 1981) is consistent with the concept that a neurotransmitter influences neuronal development. Because 5-HT-containing EC cells, as well as serotonergic neurons, are present when 5-HT2B receptors appear (Branchek and Gershon, 1989), there are two potential sources of 5-HT in the bowel during the period of time when the 5-HT2Breceptor is expressed in the gut. The gut is well equipped to inactivate 5-HT because the 5-HT transporter is expressed both in serotonergic neurons and in the mucosal epithelium (Wade et al., 1996;Chen et al., 1998).
Observations made in the present study are compatible with the hypotheses that 5-HT acts on crest-derived cells to promote the development of enteric neurons and that a developmentally regulated receptor, 5-HT2B, is responsible for this effect. The timing and the pattern of the expression of this receptor in the bowel, as well as the development of enteric sources of 5-HT, suggest that late-developing (mash-1-independent) neurons may bein situ targets of the growth factor-like actions of 5-HT. The ability of a neurotransmitter/paracrine factor, like 5-HT, to affect enteric neuronal development provides a potential mechanism by which the experience of the immature gut could influence the nature of the ENS that ultimately develops in a mature animal. By affecting the activity of enteric serotonergic neurons and/or mucosal EC cells, the luminal content might determine the number, or even the phenotypic composition (Lavdas et al., 1997), of the neurons of the adult ENS. In mice, new neurons continue to be added to the ENS for at least the first 3 postnatal weeks (Pham et al., 1991). The equivalent period has not been determined for humans, but the differences between mice and humans in size, period of gestation, and life span suggest that new neurons are probably added to the postnatal human gut for much more than 3 weeks. The development of the human ENS might thus be expected to be especially susceptible to an activity-dependent influence on its development.
Footnotes
This work was supported by National Institutes of Health Grants NS12969 and NS15547 to M.D.G. and HD35632 to E.F.-H. Confocal microscopy was supported by National Institutes of Health Grants RR10506 and CA13696. We thank Valerie Boone and Kenneth Chen for their expert technical assistance.
Correspondence should be addressed to Dr. Elena Fiorica-Howells, Department of Anatomy and Cell Biology, Columbia University, College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032. E-mail address: ef7@columbia.edu.
REFERENCES
- 1.Audia JE, Evrard DA, Murdoch GR, Droste JJ, Nissen JS, Schenk KW, Fludzinski P, Lucaites VL, Nelson DL, Cohen ML. Potent, selective, tetrahydro-β-corboline antagonists of the 5-HT2B (5-HT2B) contractile receptor in the rat stomach fundus. J Med Chem. 1996;39:2773–2780. doi: 10.1021/jm960062t. [DOI] [PubMed] [Google Scholar]
- 2.Azmitia EC, Dolan K, Whitaker-Azmitia PM. S100b, but not NGF, EGF, insulin or calmodulin is a CNS serotonergic growth factor. Brain Res. 1990;516:354–356. doi: 10.1016/0006-8993(90)90942-5. [DOI] [PubMed] [Google Scholar]
- 3.Baez M, Yu L, Cohen ML. Pharmacological and molecular evidence that the contractile response to serotonin in rat stomach fundus is not mediated by activation of the 5-hydroxytryptamine1C receptor. J Pharmacol Exp Ther. 1990;38:31–37. [PubMed] [Google Scholar]
- 4.Baynash AG, Hosoda K, Giaid A, Richardson JA, Emoto N, Hammer RE, Yanagisawa M. Interaction of endothelin-3 with endothelin-B receptor is essential for development of epidermal melanocytes and enteric neurons. Cell. 1994;79:1277–1285. doi: 10.1016/0092-8674(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 5.Blaugrund E, Pham TD, Tennyson VM, Lo L, Sommer L, Anderson DJ, Gershon MD. Distinct subpopulations of enteric neuronal progenitors defined by time of development, sympathoadrenal lineage markers, and Mash-1-dependence. Development. 1996;122:309–320. doi: 10.1242/dev.122.1.309. [DOI] [PubMed] [Google Scholar]
- 6.Branchek TA, Gershon MD. Time course of expression of neuropeptide Y, calcitonin gene related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. J Comp Neurol. 1989;285:262–273. doi: 10.1002/cne.902850208. [DOI] [PubMed] [Google Scholar]
- 7.Chalazonitis A, Rothman TP, Chen J, Lamballe F, Barbacid M, Gershon MD. Neurotrophin-3 induces neural crest-derived cells from fetal rat gut to develop in vitro as neurons or glia. J Neurosci. 1994;14:6571–6584. doi: 10.1523/JNEUROSCI.14-11-06571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalazonitis A, Tennyson VM, Kibbey MC, Rothman TP, Gershon MD. The α-1 subunit of laminin-1 promotes the development of neurons by interacting with LBP110 expressed by neural crest-derived cells immunoselected from the fetal mouse gut. J Neurobiol. 1997;33:118–138. doi: 10.1002/(sici)1097-4695(199708)33:2<118::aid-neu2>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Chalazonitis A, Rothman TP, Chen J, Vinson EN, MacLennan AJ, Gershon MD. Promotion of the development of enteric neurons and glia by neuropoietic cytokines: interactions with neurotrophin-3. Dev Biol. 1998a;198:343–365. [PubMed] [Google Scholar]
- 10.Chalazonitis A, Rothman TP, Chen J-X, Gershon MD. Age-dependent differences in the effects of GDNF and NT-3 on the development of neurons and glia from neural crest-derived precursors immunoselected from the fetal rat gut: expression of GFRa in vitro and in vivo. Dev Biol. 1998b;204:385–406. doi: 10.1006/dbio.1998.9090. [DOI] [PubMed] [Google Scholar]
- 11.Chen J-X, Pan H, Rothman TP, Wade PR, Gershon MD. Guinea pig 5-HT transporter: cloning, expression, distribution and function in intestinal sensory reception. Am J Physiol. 1998;275:G433–G448. doi: 10.1152/ajpgi.1998.275.3.G433. [DOI] [PubMed] [Google Scholar]
- 12.Choi D-S, Maroteaux L. Immunohistochemical localisation of the serotonin 5-HT2B receptor in mouse gut, cardiovascular system, and brain. FEBS Lett. 1996;391:45–51. doi: 10.1016/0014-5793(96)00695-3. [DOI] [PubMed] [Google Scholar]
- 13.Choi D-S, Colas J-F, Kellermann O, Loric S, Launay J-M, Rosay P, Maroteaux L. The mouse 5-HT2B receptor: possible involvement in trophic functions of serotonin. Cell Mol Biol. 1994;40:403–411. [PubMed] [Google Scholar]
- 14.Choi D-S, Ward SJ, Messaddeq N, Launay J-M, Maroteaux L. 5-HT2B receptor-mediated serotonin morphogenetic functions in mouse cranial neural crest and myocardiac cells. Development. 1997;124:1745–1755. doi: 10.1242/dev.124.9.1745. [DOI] [PubMed] [Google Scholar]
- 15.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 16.Cooke HJ, Sidhu M, Wang Y-Z. 5-HT activates neural reflexes regulating secretion in the guinea-pig colon. Neurogastroenterol Motil. 1997;9:181–186. doi: 10.1046/j.1365-2982.1997.d01-41.x. [DOI] [PubMed] [Google Scholar]
- 17.Fiorica-Howells E, Gershon MD. The 5-HT2B receptor: molecular cloning, identification of a splice variant, and localization of mRNA in smooth muscle and neurons of the guinea pig and rat intestines. Soc Neurosci Abstr. 1995;21:774. [Google Scholar]
- 18.Foguet M, Hoyer D, Pardo LA, Parekh A, Kluxen FW, Kalkman HO, Stümer W, Lübbert H. Cloning and functional characterization of the rat stomach fundus serotonin receptor. EMBO J. 1992a;11:3481–3487. doi: 10.1002/j.1460-2075.1992.tb05427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foguet M, Nguyen H, Le H, Lübbert H. Structure of the mouse fundus serotonin receptor genes. NeuroReport. 1992b;3:345–348. doi: 10.1097/00001756-199204000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Fraser SE, Bronner-Fraser M. Migrating neural crest cells in the trunk of the avian embryo are multipotent. Development. 1991;112:913–920. doi: 10.1242/dev.112.4.913. [DOI] [PubMed] [Google Scholar]
- 21.Furness JB, Costa M. The enteric nervous system, pp 65–69. Livingstone; New York: 1987. [Google Scholar]
- 22.Galligan JJ. Electrophysiological studies of 5-hydroxytryptamine receptors on enteric neurons. Behav Brain Res. 1996;73:199–201. doi: 10.1016/0166-4328(96)00096-4. [DOI] [PubMed] [Google Scholar]
- 23.Gershon MD. Localization and neurochemical aspects of serotonin in the gut. In: Gaginella TS, Galligan JJ, editors. Serotonin and gastrointestinal function. CRC; Boca Raton, FL: 1995. pp. 11–31. [Google Scholar]
- 24.Gershon MD. Genes and lineages in the formation of the enteric nervous system. Curr Opin Neurobiol. 1997;7:101–109. doi: 10.1016/s0959-4388(97)80127-4. [DOI] [PubMed] [Google Scholar]
- 25.Gershon MD. Genes, lineages, and tissue interactions in the development of the enteric nervous system. Am J Physiol. 1998;275:G869–G873. doi: 10.1152/ajpgi.1998.275.5.G869. [DOI] [PubMed] [Google Scholar]
- 26.Gershon MD. Roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment Pharmacol Ther. 1999;13[Suppl 2]:15–30. [PubMed] [Google Scholar]
- 27.Gershon MD, Sherman D, Gintzler A. An ultrastructural analysis of the developing enteric nervous system of the guinea pig small intestine. J Neurocytol. 1981;10:271–296. doi: 10.1007/BF01257972. [DOI] [PubMed] [Google Scholar]
- 28.Gershon MD, Kirchgessner AL, Wade PR. Functional anatomy of the enteric nervous system. In: Johnson LR, Alpers DH, Jacobson ED, Walsh JH, editors. Physiology of the gastrointestinal tract, Third Edition. Raven; New York: 1994. pp. 381–422. [Google Scholar]
- 29.Hearn CJ, Murphy M, Newgreen D. GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Dev Biol. 1998;197:93–105. doi: 10.1006/dbio.1998.8876. [DOI] [PubMed] [Google Scholar]
- 30.Huber LJ, Chao MV. Mesenchymal and neuronal cell expression of the p75 neurotrophin gene occur by different mechanisms. Dev Biol. 1995;167:237–238. doi: 10.1006/dbio.1995.1019. [DOI] [PubMed] [Google Scholar]
- 31.Ito K, Sieber-Blum M. Pluripotent and developmentally restricted neural-crest-derived cells in posterior visceral arches. Dev Biol. 1993;156:191–200. doi: 10.1006/dbio.1993.1069. [DOI] [PubMed] [Google Scholar]
- 32.Ito K, Morita T, Sieber-Blum M. In vitro analysis of mouse neural crest development. Dev Biol. 1993;157:517–525. doi: 10.1006/dbio.1993.1154. [DOI] [PubMed] [Google Scholar]
- 33.Julius D, Livelli TJ, Jessell TM, Axel R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science. 1989;244:1057–1062. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
- 34.Kellermann O, Loric S, Maroteaux L, Launay JM. Sequential onset of three 5-HT receptors during the 5-hydroxytryptaminergic differentiation of the murine 1C11 cell line. Br J Pharmacol. 1996;118:1161–1170. doi: 10.1111/j.1476-5381.1996.tb15519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennett GA, Wood MD, Bright F, Cilia J, Piper DC, Gager T, Thomas D, Baxter GS, Forbes IT, Ham P, Blackburn TP. In vitro and in vivo profile of SB 206553, a potent 5-HT2C/5-HT2B receptor antagonist with anxiolytic-like properties. Br J Pharmacol. 1996;117:427–434. doi: 10.1111/j.1476-5381.1996.tb15208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kursar JD, Nelson DJ, Wainscott DB, Cohen ML, Baez M. Molecular cloning, functional expression, and pharmacological characterization of a novel serotonin receptor (5-hydroxytryptamine2F) from rat stomach fundus. Mol Pharmacol. 1992;42:549–557. [PubMed] [Google Scholar]
- 37.Lauder JM. Neurotransmitters as morphogens. Prog Brain Res. 1988;73:365–387. doi: 10.1016/S0079-6123(08)60516-6. [DOI] [PubMed] [Google Scholar]
- 38.Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- 39.Lauder JM, Krebs H. Serotonin as a differential signal in early neurogenesis. Dev Neurosci. 1978;1:15–30. doi: 10.1159/000112549. [DOI] [PubMed] [Google Scholar]
- 40.Launay J-M, Birraux G, Bondoux D, Callebert J, Choi D-S, Loric S, Maroteaux L. Ras involvement in signal transduction by the serotonin 5-HT2B receptor. J Biol Chem. 1996;271:3141–3147. doi: 10.1074/jbc.271.6.3141. [DOI] [PubMed] [Google Scholar]
- 41.Lavdas AA, Blue ME, Lincoln J, Parnavelas JG. Serotonin promotes the differentiation of glutamate neurons in organotypic slice cultures of the developing cerebral cortex. J Neurosci. 1997;17:7872–7880. doi: 10.1523/JNEUROSCI.17-20-07872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30:31–48. [PubMed] [Google Scholar]
- 43.Le Douarin NM, Teillet MA. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol. 1974;41:162–184. doi: 10.1016/0012-1606(74)90291-7. [DOI] [PubMed] [Google Scholar]
- 44.Liu J, Lauder JM. Serotonin promotes region-specific glial influences on cultured serotonin and dopamine neurons. Glia. 1992;5:306–317. doi: 10.1002/glia.440050408. [DOI] [PubMed] [Google Scholar]
- 45.Lo L, Anderson DJ. Postmigratory neural crest cells expressing c-RET display restricted developmental and proliferative capacities. Neuron. 1995;15:527–539. doi: 10.1016/0896-6273(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 46.Loric S, Launay J-M, Colas J-F, Maroteaux L. New mouse 5-HT2-like receptor. Expression in brain, heart, and intestine. FEBS Lett. 1992;312:203–207. doi: 10.1016/0014-5793(92)80936-b. [DOI] [PubMed] [Google Scholar]
- 47.Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 48.Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 49.Nishi M, Whitaker-Azmitia PM, Azmitia EC. Enhanced synaptophysin immunoreactivity in rat hippocampal culture by 5-HT1A agonist, S100b, and corticosteroid receptor agonists. Synapse. 1996;23:1–9. doi: 10.1002/(SICI)1098-2396(199605)23:1<1::AID-SYN1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 50.Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system. J Comp Neurol. 1991;314:789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- 51.Pichel JG, Shen L, Sheng HZ, Granholm A-C, Drago J, Grinberg A, Lee EJ, Huang SB, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382:73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 52.Pomeranz HD, Rothman TP, Chalazonitis A, Tennyson VM, Gershon MD. Neural crest-derived cells isolated from the gut by immunoselection develop neuronal and glial phenotypes when cultured on laminin. Dev Biol. 1993;156:341–361. doi: 10.1006/dbio.1993.1082. [DOI] [PubMed] [Google Scholar]
- 53.Roth KA, Hermiston ML, Gordon JI. Use of transgenic mice to infer the biological properties of small intestinal stem cells and to examine the lineage relationships of their descendants. Proc Natl Acad Sci USA. 1991;88:9407–9411. doi: 10.1073/pnas.88.21.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothman TP, Gershon MD. Phenotypic expression in the developing murine enteric nervous system. J Neurosci. 1982;2:381–393. doi: 10.1523/JNEUROSCI.02-03-00381.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothman TP, Le Douarin NM, Fontaine-Pérus JC, Gershon MD. Developmental potential of neural crest-derived cells migrating from segments of developing quail bowel back-grafted into younger chick host embryos. Development. 1990;109:411–423. doi: 10.1242/dev.109.2.411. [DOI] [PubMed] [Google Scholar]
- 56.Rothman TP, Le Douarin NM, Fontaine-Pérus JC, Gershon MD. Colonization of the bowel by neural crest-derived cells re-migrating from foregut backtransplanted to vagal or sacral regions of host embryos. Dev Dyn. 1993;196:217–233. doi: 10.1002/aja.1001960308. [DOI] [PubMed] [Google Scholar]
- 57.Rothman TP, Chen J, Howard MJ, Costantini FD, Pachnis V, Gershon MD. Increased expression of laminin-1 and collagen (IV) subunits in the aganglionic bowel of ls/ls, but not c-ret −/− mice. Dev Biol. 1996;178:498–513. doi: 10.1006/dbio.1996.0234. [DOI] [PubMed] [Google Scholar]
- 58.Sánchez M, Silos-Santiago I, Frisén J, He B, Lira S, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382:70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 59.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sextier-Sainte-Claire Deville F, Ziller C, Le Douarin NM. Developmental potentials of enteric neural crest-derived cells in clonal and mass cultures. Dev Biol. 1994;163:141–151. doi: 10.1006/dbio.1994.1130. [DOI] [PubMed] [Google Scholar]
- 61.Shuey DL, Sadler TW, Lauder JM. Serotonin as a regulator of craniofacial morphogenesis: site specific malformation following exposure to serotonin uptake inhibitors. Teratology. 1992;46:367–378. doi: 10.1002/tera.1420460407. [DOI] [PubMed] [Google Scholar]
- 62.Shuey DL, Sadler TW, Tamir H, Lauder JM. Serotonin and morphogenesis. Transient expression of serotonin uptake and binding protein during craniofacial morphogenesis in the mouse. Anat Embryol (Berl) 1993;187:75–85. doi: 10.1007/BF00208198. [DOI] [PubMed] [Google Scholar]
- 63.Sieber-Blum M, Ito K, Richardson MK, Langtimm CJ, Duff RS. Distribution of pluripotent neural crest cells in the embryo and the role of brain-derived neurotrophic factor in the commitment to the primary sensory neuron lineage. J Neurobiol. 1993;24:173–184. doi: 10.1002/neu.480240205. [DOI] [PubMed] [Google Scholar]
- 64.Siriwardena AK, Smith EH, Borum EH, Kellum J., Jr Identification of a 5-hydroxytryptamine (5-HT2) receptor on guinea pig small intestinal crypt cells. Am J Physiol. 1993;265:G339–G346. doi: 10.1152/ajpgi.1993.265.2.G339. [DOI] [PubMed] [Google Scholar]
- 65.Stemple D, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 66.Tutton PJM. The influence of serotonin on crypt cell proliferation in the jejunum of rat. Virchows Arch B Cell Pathol. 1974;16:79–87. doi: 10.1007/BF02894066. [DOI] [PubMed] [Google Scholar]
- 67.Tutton PJM, Barkla DH. Neural control of colonic cell proliferation. Cancer. 1980;45:1172–1177. doi: 10.1002/1097-0142(19800315)45:5+<1172::aid-cncr2820451322>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 68.Wade PR, Chen J, Jaffe B, Kassem IS, Blakely RD, Gershon MD. Localization and function of a 5-HT transporter in crypt epithelia of the gastrointestinal tract. J Neurosci. 1996;16:2352–2364. doi: 10.1523/JNEUROSCI.16-07-02352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang H-Y, Eberle-Wang K, Simansky KJ, Friedman E. Serotonin-induced muscle contraction in rat stomach fundus is mediated by a Gαz-like guanine nucleotide binding protein. J Pharmacol Exp Ther. 1993;267:1002–1011. [PubMed] [Google Scholar]
- 70.Whitaker-Azmitia PM, Murphy R, Azmitia EC. Stimulation of astroglial 5-HT1A receptors releases the serotonergic growth factor protein, S100, and alters the astroglial morphology. Brain Res. 1990;528:155–158. doi: 10.1016/0006-8993(90)90210-3. [DOI] [PubMed] [Google Scholar]
- 71.Wilkinson LD, Lee K, Deshpande S, Duerksen-Hughes P, Boss JM, Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989;246:670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]
- 72.Yan L, Zahradka P. Immunodetection of activated mitogen-activated protein kinase in vascular tissues. Mol Cell Biochem. 1997;172:59–66. [PubMed] [Google Scholar]
- 73.Yang W, Chen K, Lan NC, Gallaher TK, Shih JC. Gene structure and expression of the mouse 5-HT2 receptor. J Neurosci Res. 1992;33:196–204. doi: 10.1002/jnr.490330203. [DOI] [PubMed] [Google Scholar]
- 74.Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J Comp Neurol. 1954;101:515–542. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- 75.Yntema CL, Hammond WS. Experiments on the origin and development of the sacral autonomic nerves in the chick embryo. J Exp Zool. 1955;129:375–414. [Google Scholar]
- 76.Yu L, Nguyen H, Le H, Bloem LJ, Kozak CA, Hoffman BJ, Snutch TP, Lester HA, Davidson N, Lübbert H. The mouse 5-HT1C receptor contains eight hydrophobic domains and is X-linked. Mol Brain Res. 1991;11:143–149. doi: 10.1016/0169-328x(91)90116-f. [DOI] [PubMed] [Google Scholar]