Abstract
Background:
Tivozanib is a selective inhibitor of vascular endothelial growth factor receptors 1, 2 and 3 tyrosine kinases. This open-label, crossover clinical study (AV-951–09-902) provided access to tivozanib for patients who progressed on sorafenib in TIVO-1, comparing tivozanib with sorafenib in patients with advanced renal cell carcinoma (RCC).
Methods:
Patients enrolled in this single-arm, phase 2 crossover study were previously randomised to sorafenib on TIVO-1, progressed and then crossed over to tivozanib. Patients received tivozanib (1.5 mg/day orally; 3 weeks on/1 week off) within 4 weeks after their last sorafenib dose.
Findings:
Crossover patients were exposed to tivozanib for a median of eight cycles. From the start of tivozanib treatment, median progression-free survival was 11.0 months (95% confidence interval [CI]: 7.3–12.7) and median overall survival was 21.6 months (95% CI: 17.0–27.6). Best overall response was partial response in 29 (18%) patients and stable disease in 83 (52%) patients, with a median duration of response of 15.2 and 12.7 months, respectively. About 77% of patients experienced adverse events, most frequently hypertension (26%), followed by diarrhoea (14%) and fatigue (13%); 53% of patients had treatment-related adverse events, including 24% grade ≥3. About 9% and 16% of patients had dose reductions and dose interruptions due to adverse events, respectively. A total of 30% of patients had serious adverse events, and 4% had treatment-related serious adverse events.
Interpretation:
This crossover study of patients with advanced RCC demonstrated potent tivozanib anti-tumour activity. Safety and tolerability profiles were acceptable and consistent with the established adverse event profile of tivozanib.
Keywords: Tivozanib, Sorafenib, First-line therapy, Metastatic renal cell, carcinoma
1. Introduction
The National Cancer Institute (NCI) estimates that in 2018, approximately 63,300 new cases of renal cell carcinoma (RCC) and 14,900 RCC disease–related deaths will be reported [1,2]. Recent advances in the understanding of the pathophysiologic mechanisms underlying RCC have facilitated the development of therapies that target signalling pathways, in particular, the selective inhibition of vascular endothelial growth factor (VEGF), revolutionising treatment for this disease [3,4]. Small-molecule tyrosine kinase inhibitors (TKIs) including sorafenib, sunitinib, pazopanib and cabozantinib, as well as antibodies like bevacizumab (in combination with interferon alpha), are commonly used for first-line and advanced treatment of RCC [4–8]. However, sorafenib, sunitinib and pazopanib, in particular, demonstrate limitations, including broad-spectrum kinase inhibitory activity and subsequent adverse events (AEs), such as fatigue, diarrhoea, skin rash, hand-foot skin reaction, myelosuppression, liver toxicity and transaminitis [9–12]. Two inhibitors of mammalian target of rapamycin (mTOR), temsirolimus and everolimus, are also approved for treatment of this intractable disease [13,14], as well as a TKI/mTOR combination (lenvatinib and everolimus) [15]. In addition, second-generation TKIs such as axitinib [16] and a monoclonal antibody against the programmed death receptor-1 (PD-1), nivolumab, are approved and used commonly for second-line treatment in patients with RCC [15].
Tivozanib is a TKI recently approved by the European Commission for the treatment of untreated RCC and is currently in further development for the treatment of advanced RCC [17]. Tivozanib has demonstrated a favourable pharmacodynamic profile characterised by highly potent and selective inhibition of VEGF receptors (VEGFRs) 1, 2 and 3 tyrosine kinases, requiring an eight-fold increase in concentration to inhibit other tyrosine kinases, therefore optimising blockade of VEGFRs with minimal toxicities [18–21]. Tivozanib inhibits angiogenesis and vascular permeability in tumour tissues, leading indirectly to inhibition of tumour growth [18]. Furthermore, the half-life (t1/2) of tivozanib is approximately 4 days, allowing convenient once-daily dosing at 1.5 mg to maintain effective serum concentrations [19,22]. Clinical study data in the tivozanib programme repeatedly demonstrated the efficacy of tivozanib in the treatment of patients with advanced RCC. In a phase 2 trial of tivozanib in 272 patients with advanced RCC (untreated or following one prior line of therapy), the median progression-free survival (PFS) was 11.7 months, and the objective response rate was 24%, supporting further investigation in a phase 3 study [23].
TIVO-1, a phase 3 trial comparing tivozanib with sorafenib in 517 patients with advanced RCC, either untreated or following progression on a cytokine, met its primary end-point with an improved median PFS of 11.9 months in the tivozanib arm, compared with 9.1 months in the sorafenib arm (P = 0.042) [17]. Tivozanib was well tolerated, and patients randomised to tivozanib received 94% of scheduled chemotherapy, compared with 80% in the sorafenib arm. In the protocol-specified final analysis, the median overall survival was 28.8 months (95% confidence interval [CI]: 22.5, NA) in tivozanib-treated patients and 29.3 months (95% CI: 29.3, NA) in sorafenib-treated patients (hazard ratio [HR] 1.245; P = 0.105). Of note, 74% of patients randomised to sorafenib on TIVO-1 were treated with next-line therapy (mostly tivozanib) after progressive disease, whereas only 35% of patients in the tivozanib arm received next-line therapy [11,24]. This discrepancy was due to the lack of available second-line therapies in the Eastern European countries, such that many patients randomised to tivozanib had no salvage therapy. The results of overall survival in TIVO-1 were likely confounded by the differential use of subsequent cancer therapy in the two treatment arms, as well as the activity of tivozanib in second line. The current report will describe the AV-951–09-902 crossover study (hereafter, designated 902) of the patients in TIVO-1 who progressed on sorafenib and were subsequently treated with tivozanib.
2. Methods
2.1. Study design and participants
In this single-arm, open-label, multicenter extension study, we recruited a total of 277 patients from the three reporting groups in TIVO-1, the trial details of which have been previously reported [17]. The current analysis included 161 patients initially treated with sorafenib, including patients who crossed over to tivozanib after the first documented disease progression (n = 147) and patients who continued on sorafenib on entering this study and subsequently progressed and started tivozanib (n = 14). This study also provided continued access to tivozanib or sorafenib for patients who were receiving those therapies and had clinical benefit and acceptable toxicity at the time of TIVO-1 closure; however, those patients are not characterised in the current report. Other key inclusion criteria included documented negative pregnancy test (if female and of childbearing potential) and an Eastern Cooperative Oncology Group performance status ≤2. Key exclusion criteria were ≥4 weeks since the last dose of sorafenib (for patients with disease progression during treatment with sorafenib), newly identified central nervous system (CNS) malignancies or documented progression of CNS metastases, haematologic or serum chemistry abnormalities, unhealed wounds (including active peptic ulcers), uncontrolled hypertension, serious/active infections or infections requiring parenteral antibiotics, inadequate recovery from surgery within 4 weeks and current receipt of treatment with another oncology therapy. Patients provided written informed consent to participate in this study. The protocol, amendments and patient consent forms were approved by the institutional review board or independent ethics committee before the start of the trial.
2.2. Procedures
Tivozanib was administered on day 1 at a dose of 1.5 mg orally once daily. All patients followed the dosing schedule of 3 weeks on treatment, followed by 1 week off treatment for as long as the patient tolerated treatment in the absence of disease progression or unacceptable toxicity; one cycle was defined as 4 weeks of treatment. Patients who experienced unacceptable toxicities or clinical or documented progressive disease were discontinued from the study. Patients who developed early progressive disease before response evaluation were considered to have progressed. The duration of any objective response was measured from the date the initial response was observed to the date that disease progression was observed. Changes in tumour measurements had to be confirmed by repeat studies performed ≥4 weeks after the criteria for response were first met to be assigned a status of partial response or complete response. Patients receiving ≥2 cycles of tivozanib were considered evaluable for response using Response Evaluation Criteria in Solid Tumours (RECIST), version 1.0. Disease assessment by the investigator included response assessment and diagnostic imaging and measuring of target lesions. All patients had computed tomography and/or magnetic resonance imaging scans for assessing disease status. Once discontinued, all patients were to have an end-of-treatment visit within 5 days of discontinuation and a follow-up visit 30 days after the last dose of the study drug was received. All patients were followed up until death from any cause.
2.3. Outcomes
The measures of efficacy in this study were patients’ best overall responses (RECIST, version 1.0), PFS (defined as the time from first tivozanib dose date to first documentation of tumour progression or any-cause death), overall survival (defined as the time from the first dose date to date of any-cause death) and duration of response (defined as the time from the first documentation of objective tumour response, according to RECIST, version 1.0, to the first documentation of tumour progression or any-cause death). All efficacy assessments were based on investigator assessment.
Treatment-emergent AEs were defined as events occurring or increasing in severity or relationship to the study drug. Toxicities were graded using the NCI Common Terminology Criteria for Adverse Events, version 3.0. Patients were monitored throughout the treatment and follow-up periods for AEs, treatment compliance, concomitant medications, clinical status, vital sign measurements (including blood pressure) and laboratory data (haematology, serum chemistry, urinalysis, coagulation, thyroid function and pregnancy).
2.4. Statistical analysis
Distribution of all end-points was estimated using the Kaplan–Meier method. LIFETEST procedures in SAS software (SAS Institute Inc., Cary, NC) were used to produce estimates of quartiles and 95% CIs via the Brookmeyer and Crowley method. The standard error of the Kaplan–Meier quartile estimates was estimated using Greenwood’s formula. Kaplan–Meier plots of the survival distribution function were presented for all time-to-event analyses. The efficacy (distribution of all time-to-event end-points) and safety (treatment-emergent AEs) populations included all 161 crossover patients. This single-arm study was not designed to test a specific hypothesis. Only descriptive statistics were used in the analysis.
This study is registered with ClinicalTrials.gov, number NCT01076010.
2.5. Role of the funding source
This study was designed by the sponsor. Data collected by the investigators were interpreted jointly with all authors. The study funder paid for writing and editorial support. The authors had full access to the data, and the corresponding author had final responsibility for the decision to submit for publication.
3. Results
Between May 24, 2010 and July 4, 2014, we enrolled 161 patients initially treated with sorafenib in TIVO-1, including 147 patients who crossed over to tivozanib after the first documented progressive disease and 14 patients who continued on sorafenib on entering this study and subsequently progressed and crossed over to tivozanib. Baseline characteristics were generally balanced between the study groups (Table 1). The majority of patients were male and white, had a median age of 59 years (range: 23–86) and were from Central/Eastern Europe, as shown in the baseline characteristics. At the time of analysis, 125 (78%) patients discontinued the study and 36 (22%) patients were ongoing (Table 2). The most common reason for treatment discontinuation was progressive disease (56%). The median number of cycles in crossover patients exposed to tivozanib was eight cycles (range: 1–35) over a median of 225 days (range: 15–172). The mean total dose administered was 318.84 mg, and the relative dose intensity was 95%, with over half of the patients receiving the full dose of tivozanib throughout the course of therapy. Treatment interruptions due to AEs were observed in 25 (16%) patients and dose reductions due to AEs in 15 (9%) patients.
Table 1.
Baseline characteristics of crossover patients from study TIVO-1 to study 902 (N = 161).
| Baseline characteristics | Evaluable population (N = 161) |
|---|---|
| Gender | |
| Female | 46 (20) |
| Male | 115 (71) |
| Age | |
| Median | 59.0 |
| Range | 23–85 |
| Age group | |
| <65 years | 127 (79) |
| ≥65 years | 34 (21) |
| Race | |
| White | 156 (97) |
| Asian | 5 (3) |
| ECOG PS | |
| 0 | 92 (57) |
| 1 | 69 (43) |
| Geographic region | |
| Central/Eastern Europe | 143 (89) |
| North America/Western Europe | 10 (6) |
| Rest of world | 8 (5) |
ECOG PS, Eastern Cooperative Oncology Group performance status. Data are n (%), unless otherwise specified.
Table 2.
Summary of patient disposition and compliance of crossover patients from study TIVO-1 to study 902 (N = 161).
| Patient disposition and compliance | Evaluable population (N = 161) |
|---|---|
| Treated | 161 (100) |
| Ongoing | 36 (22) |
| Discontinued treatmenta | 125 (78) |
| Progressive disease | 90 (56) |
| Deathb | 15 (9) |
| Adverse event | 7 (4) |
| Otherc | 5 (3) |
| Lack of efficacy | 4 (3) |
| Consent withdrawn | 3 (2) |
| Non-compliance | 1 (1) |
| Treatment interruption >2 weeks | 0 (0) |
| Dose reduction | 16 (10) |
| Adverse event | 15 (9) |
| Other | 1 (1) |
| Dose interruption | 50 (31) |
| Adverse event | 25 (16) |
| Other | 31 (19) |
Data are n (%), unless otherwise specified.
Reasons for discontinuation were based on the end-of-treatment electronic case report form page.
Eight patients had death as the reason for discontinuation.
Four patients discontinued but agreed to continue on study in the follow-up, and one patient was withdrawn because of an investigator’s mistake in calculation of the sum of the longest diameters.
Anti-tumour activity with tivozanib was demonstrated with a median PFS of 11 months (95% CI: 7.3–12.7 months; Fig. 1A). Median overall survival from the start of the first tivozanib dose in this study was 22 months (95% CI: 17.0–27.6 months; Fig. 1B). None of the crossover patients had a complete response; 29 (18%) had a partial response, 83 (52%) had stable disease and 34 (21%) had progressive disease (Table 3). The median duration of response for the 29 responders was 15 months (95% CI: ≥11.1 months). Among these evaluable patients, the confirmed overall response rate was 18% (95% CI: 12.4–28.8). There were an additional 15 (9%) unevaluable patients. A total of 149 of 161 crossover patients had measurable disease post baseline. Of these patients, 138 (92.6%) had a reduction in target lesion diameter (Fig. 2).
Fig. 1.
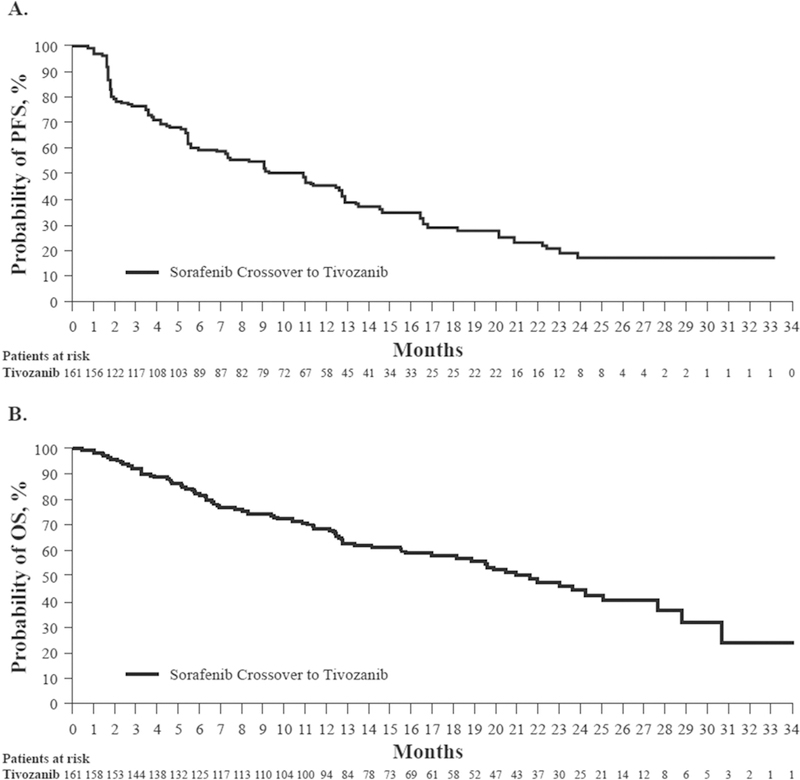
Kaplan–Meier curves of investigator-associated disease progression (A) and overall survival (B). PFS, progression-free survival; OS, overall survival.
Table 3.
Summary of best overall response (N = 161).
| Best overall confirmed response | Evaluable population (N = 161) |
|---|---|
| Complete response | 0 (0) |
| Partial response | 29 (18) |
| Stable disease | 83 (52) |
| Progressive disease | 34 (21) |
| Not evaluablea | 15 (9) |
| Confirmed ORR (CR + PR) | 29 (18 [12.4–24.8]) |
| Progression-free survival, months (95% CI) | 11.0 (7.3–12.7) |
| Overall survival, months (95% CI) | 21.6 (17.0–27.6) |
| Median duration of response, months (95% CI) | 15.2 (≥11.1) |
CI, confidence interval; CR, complete response; ORR, objective response rate; PR, partial response.
Data are n (% [95% CI]), n (%) or n/N (%), unless otherwise specified.
Patients who did not have lesion imaging and ≥1 subsequent scan at baseline.
Fig. 2.
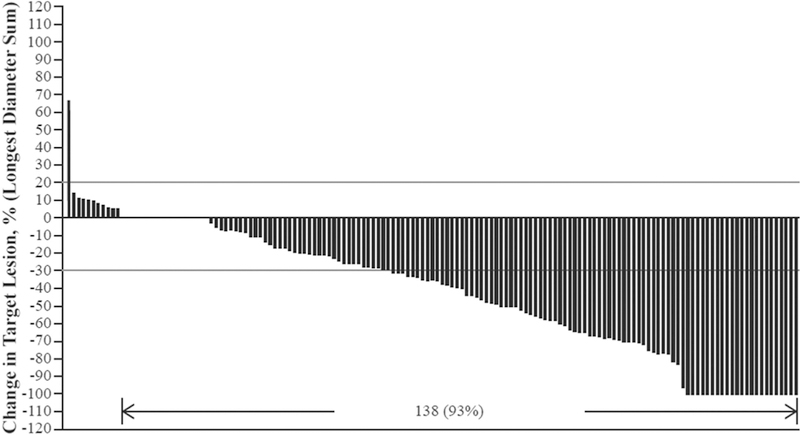
Maximum percentage change in target lesion diameter from baseline of crossover study; evaluable population (N = 149).
A total of 124 of 161 (77%) crossover patients experienced AEs; 86 (53%) had treatment-related AEs, and 12 (13%) had AEs leading to death. grade ≥3 AEs were reported by 48% of patients, including 24% that were treatment related. A total of 30% of patients had serious AEs, 4% of which were treatment related. About 4% of patients discontinued treatment because of AEs.
The most common treatment-related AEs observed in the crossover population were hypertension (26%), diarrhoea (14%), fatigue (13%), asthenia (12%) and palmar-plantar erythrodysesthesia syndrome (10%; Table 4). Elevations in transaminases were also reported in less than 5% of patients. The most commonly reported grade 3 or 4 AEs were hypertension (11%), increased amylase (5%), fatigue (4%), asthenia (4%) and increased lipase (4%). Hypertension was the most commonly reported AE among patients on tivozanib and was the most frequently reported ≥ grade 3 AE. There were 21 (13%) patients who had AEs leading to death while on the study or within 30 days after completing treatment; one case of myocardial infarction was assessed by the investigator as possibly related to study drug. The other deaths on the study were considered unrelated or likely to be unrelated to study drug. Nine deaths were due to disease progression categorised as an AE or serious AE.
Table 4.
Most frequently reported AEs occurring in >5% of patients by preferred term in crossover evaluable population (N = 161).a
| AE, n (%) | Grade 1–2 |
Grade 3 |
Grade 4 |
Total |
|---|---|---|---|---|
| Hypertension | 23 (14) | 18(11) | 0 (0) | 41 (26) |
| Diarrhoea | 18 (11) | 4 (2) | 0 (0) | 22 (13) |
| Fatigue | 14 (9) | 7 (4) | 0 (0) | 21 (13) |
| Asthenia | 14 (9) | 6 (4) | 0 (0) | 20 (12) |
| Palmar-plantar erythrodysesthesia syndrome | 14 (9) | 2 (1) | 0 (0) | 16 (10) |
| Cough | 9 (6) | 0 (0) | 0 (0) | 9 (6) |
| Dysphonia | 9 (6) | 0 (0) | 0 (0) | 9 (6) |
| Decreased appetite | 9 (6) | 0 (0) | 0 (0) | 9 (6) |
| Dyspnoea | 7 (4) | 2 (1) | 0 (0) | 9 (6) |
AE, adverse event; MedDRA, Medical Dictionary for Regulatory Activities.
Data are n (%), unless otherwise specified.
AEs are presented by MedDRA (version 17.0)-coded preferred terms in decreasing frequency by total patients.
No patient reported grade 5 adverse events.
4. Discussion
In TIVO-1, tivozanib was associated with a significant improvement in PFS compared with sorafenib (11.9 months versus 9.1 months) in the treatment of first-line advanced RCC. Furthermore, the confirmed overall response rate was significantly higher for tivozanib-treated patients than for those in the sorafenib group (33% versus 23%; P = 0.014). However, the median overall survival was 28.8 months (95% CI: 22.5, NA) for tivozanib and 29.3 months (95% CI: 29.3, NA) for sorafenib (HR 1.245; P = 0.015), due to a one-way crossover from sorafenib to tivozanib [17]. Owing to the lack of available second-line therapies at the time, many patients in the TIVO-1 study randomised to tivozanib in the Eastern European countries had no salvage therapy. In the current analysis, anti-tumour activity of tivozanib in the 161 patients who crossed over after radiographic progression on sorafenib was demonstrated with a median PFS of 11 months (95% CI: 7.3–12.7 months), a median overall survival of 22 months (95% CI: 17.0–27.6 months) and 18% of patients experiencing a partial response. These data compare favourably with other second-line therapies for RCC, including both small molecules and antibodies to VEGFR or PD-1.
Tivozanib had a favourable safety profile compared with other VEGFR TKIs, with a low incidence of class-related AEs, including diarrhoea, asthenia and palmar-plantar erythrodysesthesia syndrome. Although it is difficult to compare across the different studies, the frequencies of all-grade diarrhoea and fatigue/asthenia were both over 50% for sunitinib, pazopanib, axitinib and cabozantinib [10,16,25,26] and less than 25% for tivozanib in TIVO-1 [17]. Additionally, the frequency of fatigue was greater than 30% for sunitinib, pazopanib, axitinib and cabozantinib [10,16,25,26] and less than 20% for tivozanib, and the frequency of palmar-plantar erythrodysesthesia was 54% for sorafenib, compared with 13% for tivozanib in TIVO-1 [17]. The AE profile of tivozanib in this study was consistent with the AE profile in the TIVO-1 study, with the exception of hypertension. Although hypertension was the most frequently reported AE on tivozanib in this study, the incidence was lower in the present study (26%) than for patients in TIVO-1 treated with tivozanib (44% [17]). The lower incidence of hypertension in this study may be secondary to a selection bias, or perhaps to closer monitoring and management of this class effect, particularly as patients came off sorafenib. In addition, more than half of the patients enrolled in this study completed treatment without dose reductions or interruptions, with a relative dose intensity of 95%.
At the time of the TIVO-1 study, several TKIs (sunitinib, sorafenib and pazopanib) were approved as first-line therapies for metastatic or relapsed RCC, although sunitinib and pazopanib had been the drugs of choice [2,27]. There are now many agents available for the treatment of RCC, including six anti-angiogenic agents, two mTOR inhibitors and an antibody targeting PD-1. Current research is focussed on combinations of active agents in first and subsequent lines of therapy. Overlapping toxicities are likely to limit the number of combinations that are feasible. Tivozanib, with its very favourable AE profile, is an ideal candidate for studies in combination. The demonstration of efficacy in the first line, based on the head-to-head comparison to an active targeted agent, sorafenib [17], as well as considerable activity in the second line demonstrated in the current study, reveals that tivozanib is a novel anti-VEGFR TKI for the treatment of advanced RCC. In addition, based on its excellent toxicity profile, there is further opportunity for tivozanib in combination with other agents. Tivozanib as a single agent has demonstrated significant activity and a favourable toxicity profile, likely due to the high level of selectivity for VEGFRs 1, 2 and 3, and therefore, should be considered as an attractive option for treatment-naive or refractory RCC patients who may prefer a well-tolerated treatment.
Tivozanib is currently being evaluated in third-line RCC in a comparative study to sorafenib, as well as in early combination with nivolumab. Overall, data from this crossover study of patients with advanced RCC demonstrated the anti-tumour activity of tivozanib, with a median PFS of 11 months and a median overall survival of 22 months after progressive disease with sorafenib treatment on TIVO-1. Safety and tolerability profiles were found to be acceptable and consistent with the established AE profile of tivozanib.
The nature of the study design introduced the study limitations common to all single-arm studies, namely the fact that there was no control group for comparison. In addition, a specific limitation is that the study included only patients with Eastern Cooperative Oncology Group performance status of 0 or 1 and therefore, did not include poor prognosis patients. This may limit the ability to extend these results to the general patient population.
5. Conclusions
In this study reporting crossover data from a randomised, phase 3 clinical trial investigating tivozanib and sorafenib as initial targeted therapy in patients with advanced RCC (TIVO-1), tivozanib provided favourable survival outcomes, particularly progression-free and overall survival, in comparison to sorafenib. This study also provided clarity of the TIVO-1 trial, in which patient crossover was thought to have confounded the overall survival results, as well as evidence of activity in patients with recurrent disease. Collectively, these data provide evidence of the anti-tumour activity of tivozanib and may be used to help frame future studies in patients with recurrent disease.
Acknowledgments
Funding/support
This study was supported by AVEO Oncology and Astellas Pharma US, Inc. Editorial assistance was provided by Scientific Connexions and was funded by AVEO Oncology.
A.M.M. has received honoraria from AVEO, Novartis and Eisai. R.M. has been a consultant for and received honoraria from Pfizer, Novartis and Exelixis. D.N. has received honoraria from Pfizer, Novartis and Bristol-Myers Squibb. C.N.S. has been a consultant for Novartis, Eisai and Bristol-Myers Squibb and has received honoraria from Pfizer and Ipsen. T.E. has received grant support from AstraZeneca and Pfizer.
Footnotes
Conflict of interest statement
The other authors have no conflict of interest to disclose.
References
- [1].American Cancer Society. Cancer facts and figures 2018. Atlanta, GA: American Cancer Society; 2017. https://www.cancer.gov/types/kidney/hp/kidney-treatment-pdq. [Accessed 16 January 2018]. [Google Scholar]
- [2].National Comprehensive Cancer Network (NCCN) guidelines for kidney cancer, version 2.2018. Fort Washington, PA: NCCN; 2018. https://www.nccn.org/professional/physician_gls/PDF/kidney. [Accessed 16 January 2018]. [Google Scholar]
- [3].Cohen RB, Oudard S. Antiangiogenic therapy for advanced renal cell carcinoma: management of treatment-related toxicities. Invest New Drugs 2012;30:2066–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Escudier B, Albiges L, Sonpavde G. Optimal management of metastatic renal cell carcinoma:current status. Drugs 2013;73:427–38. [DOI] [PubMed] [Google Scholar]
- [5].Cabometyx (cabozantinib) [Prescribing Information]. San Francisco, CA: Exelixis Inc; 2017. [Google Scholar]
- [6].Mihaly Z, Sztupinszki Z, Surowiak P, Gyorffy B. A comprehensive overview of targeted therapy in metastatic renal cell carcinoma. Curr Cancer Drug Targets 2012;12:857–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sun M, Shariat SF, Trinh Q-D, Meskawi M, Bianchi M, Hansen J, et al. An evidence-based guide to the selection of sequential therapies in metastatic renal cell carcinoma. Ther Adv Urol 2013;5:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal cell carcinoma. N Engl J Med 2007;356:125–34. [DOI] [PubMed] [Google Scholar]
- [9].Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24. [DOI] [PubMed] [Google Scholar]
- [10].Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Oudard S, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009;27:3584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res 2013;9:327–37. [PubMed] [Google Scholar]
- [12].Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–109. [DOI] [PubMed] [Google Scholar]
- [13].Torisel (temsirolimus) injection [Prescribing Information]. Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2017. [Google Scholar]
- [14].Afinitor (everolimus) [Prescribing Information]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2017. [Google Scholar]
- [15].Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal cell carcinoma. N Engl J Med 2015;373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 2013;14:552–62. [DOI] [PubMed] [Google Scholar]
- [17].Motzer R, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, et al. Tivozanib versus sorafenib as initial targeted therapy for patients with metastatic renal cell carcinoma: results from a phase III trial. J Clin Oncol 2013;31:3791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nakamura K, Taguchi E, Miura T, Yamamoto A, Takahashi K, Bichat F, et al. KRN951, a highly potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, has antitumor activities and affects functional vascular properties. Cancer Res 2006;66:9134–42. [DOI] [PubMed] [Google Scholar]
- [19].Eskens FA, de Jonge MJ, Bhargava P, Isoe T, Cotreau MM, Esteves B, et al. Biologic and clinical activity of tivozanib (AV-951, KRN-951), a selective inhibitor of VEGF receptor-1, −2, and −3 tyrosine kinases, in a 4-week-on, 2-week-off schedule in patients with advanced solid tumors. Clin Cancer Res 2011;17:7156–63. [DOI] [PubMed] [Google Scholar]
- [20].Hepgur M, Sadeghi S, Dorff TB, Quinn DI. Tivozanib in the treatment of renal cell carcinoma. Biologics 2013;7:139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Haberkorn BC, Eskens FA. Structure, development, preclinical and clinical efficacy of tivozanib (KRN-951, AV-951). Future Oncol 2013;1:13–20. [DOI] [PubMed] [Google Scholar]
- [22].Cotreau M, King T, Massmanian L, Strahs A, Slichenmyer Vargo D. The effect of food on the pharmacokinetics of tivozanib. Cancer Res 2012;72(8 suppl). Abstract 752. [Google Scholar]
- [23].Nosov DA, Esteves B, Lipatov BN, Lyulko AA, Anischenko AA, Chacko RT, et al. Antitumor activity and safety of tivozanib (AV-951) in a phase II randomized discontinuation trial in patients with renal cell carcinoma. J Clin Oncol 2012;30:1678–85. [DOI] [PubMed] [Google Scholar]
- [24].Motzer RJ, Nosov D, Eisen T, Bondarenko IN, Lesovoy V, Lipatov ON, et al. Tivozanib hydrochloride versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: results from a phase III randomized, open-label, multicenter trial. J Clin Oncol 2012;30(15s). abstract 4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol 2016;17:917–27. [DOI] [PubMed] [Google Scholar]
- [26].Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28(6):1061–8. [DOI] [PubMed] [Google Scholar]
- [27].Escudier B, Eisen T, Porta C, Rioux-Leclercq N, Bex A, Khoo V, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii 65–71. [DOI] [PubMed] [Google Scholar]


