Abstract
BACKGROUND:
We investigated whether visual augmentation (3D, real time, color visualization) of a procedural simulator improved performance during training in the supraclavicular approach to the subclavian vein, not as widely known or used as its infraclavicular counterpart.
METHODS:
To train anesthesiology residents to access a central vein, a mixed reality simulator with emulated ultrasound imaging was created using an anatomically authentic, 3D-printed, physical mannequin based on a computed tomographic scan of an actual human. The simulator has a corresponding 3D virtual model of the neck and upper chest anatomy. Hand-held instruments such as a needle, an ultrasound probe, and a virtual camera controller are directly manipulated by the trainee and tracked and recorded with sub-millimeter resolution via miniature, 6 degrees of freedom magnetic sensors.
After IRB approval, 69 anesthesiology residents and faculty were enrolled and received scripted instructions on how to perform subclavian venous access using the supraclavicular approach based on anatomic landmarks. The volunteers were randomized into two cohorts. The first used real-time 3D visualization concurrently with trial 1, but not during trial 2. The second did not use real-time 3D visualization concurrently with trial 1 or 2. However, after trial 2, they observed a 3D visualization playback of trial 2 prior to performing trial 3 without visualization. An automated scoring system based on time, success, and errors/complications generated objective performance scores. Nonparametric statistical methods were used to compare the scores between subsequent trials, differences between groups (real-time visualization vs. no visualization vs. delayed visualization) and improvement in scores between trials within groups.
RESULTS:
Although the real-time visualization group demonstrated significantly better performance than the delayed visualization group on trial 1 (P = 0.01), there was no difference in gain scores, between performance on the first trial and performance on the final trial, that were dependent on group (P = 0.13). In the delayed visualization group, the difference in performance between trial 1 and trial 2 was not significant (P = 0.09); reviewing performance on trial 2 before trial 3 resulted in improved performance when compared to trial 1 (P < 0.0001). There was no significant difference in median scores (P = 0.13) between the real-time visualization and delayed visualization groups for the last trial after both groups had received visualization. Participants reported a significant improvement in confidence in performing supraclavicular access to the subclavian vein. Standard deviations of scores, a measure of performance variability, decreased in the delayed visualization group after viewing the visualization.
CONCLUSIONS:
Real-time visual augmentation (3D visualization) in the mixed reality simulator improved performance during supraclavicular access to the subclavian vein. No difference was seen in the final trial of the group that received real-time visualization compared to the group that had delayed visualization playback of their prior attempt. Training with the mixed reality simulator improved participant confidence in performing an unfamiliar technique.
The supraclavicular approach to the subclavian vein (first described by Yoffa et al. in 19651) has a shorter distance and less tissue to traverse before access to the vein compared to the more familiar infraclavicular technique. The supraclavicular approach provides a larger cross-sectional area of the vein as a target, a larger margin of safety from accidental violation of the pleura, a lower rate of malpositioning, and some evidence of fewer complications.2–4 Complications include arterial puncture and pneumothorax.2 The largest barrier to adoption of the supraclavicular approach in clinical practice is likely the lack of familiarity compared to the internal jugular, femoral vein, or infraclavicular access to the subclavian.
The UF Central Venous Access Mixed Reality (CVA) simulator facilitates practice in learning to access the internal jugular and subclavian central veins.5 This mixed reality simulator allows real-time or delayed 3D visualization of the needle’s location inside soft tissue structures within a mannequin’s virtual anatomy displayed on a computer screen while also providing tactile feedback for non-deformable (bony) structures on a modular anatomical block (Fig. 1). The CVA simulator has a built-in automated scoring algorithm based on contact with or proximity to the lung/arteries/trachea, time to venous access, number of attempts, and number of skin punctures. Training with a task trainer enhanced acquiring and retaining clinical skills related to aseptic placement of central venous catheters.6–9 Using a mixed reality simulator may not only provide a risk-free environment for training, but also facilitate successful training for accessing the subclavian vein via the supraclavicular approach.
Figure 1.
The mixed reality simulator of central venous access used in the study. The three-dimensional visualization is displayed on the laptop screen.
The hypotheses were: (1) 3D visualization of internal structures improves the efficacy of simulation for trainees learning how to access a central vein and (2) real-time 3D visualization during a trainee’s central venous access attempt is superior to delayed 3D visualization playback.
METHODS
Participants were randomized into either the Real-Time Visualization Group (RTVG), with concurrent 3D visualization with their first attempt, or the Delayed Visualization Group (DVG), with 3D visualization play back of their second attempt. Comparisons were made between the two group’s scores for each attempt to investigate potential relationships.
The simulator used for the study is a turnkey, mixed reality simulator for practicing, learning, teaching, and debriefing central venous access by the internal jugular, infraclavicular, supraclavicular, and axillary approaches with and without ultrasound (US) guidance. For more information, see Figure 1 and videos: http://simulation.health.ufl.edu/research/cva_sim.mp4 and https://www.youtube.com/watch?v=0lTIFbiiwRs.
The simulator uses mixed reality via an anatomically authentic, 3D-printed physical mannequin based on a computed tomographic scan of an actual human and a corresponding 3D virtual model of the anatomy of the neck and upper chest. Hand-held instruments such as a needle, an US probe, and a virtual camera controller are directly manipulated by the trainee and tracked and recorded with sub-millimeter resolution via miniature, six degrees of freedom magnetic sensors.10 An automated scoring algorithm and a replay system allow self-debriefing.10,11 Furthermore, the user can practice US-guided central venous access by orientating the needle and the US probe out-of-plane or in-plane with reference to one another.
Study Protocol
After Institutional Review Board approval and informed consent, volunteers (anesthesia trainees, emergency medicine trainees, and attending anesthesiologists) at the University of Florida took part in a study with the CVA simulator in performing the supraclavicular approach to the subclavian vein. Enrollment of participants was combined with two other studies evaluating participants’ ability to access the internal jugular vein with simulated US and to access the subclavian vein using an infraclavicular approach. Individuals were free to refuse to take part in the study and were also excluded from analysis if they only wanted to receive training for central venous access.
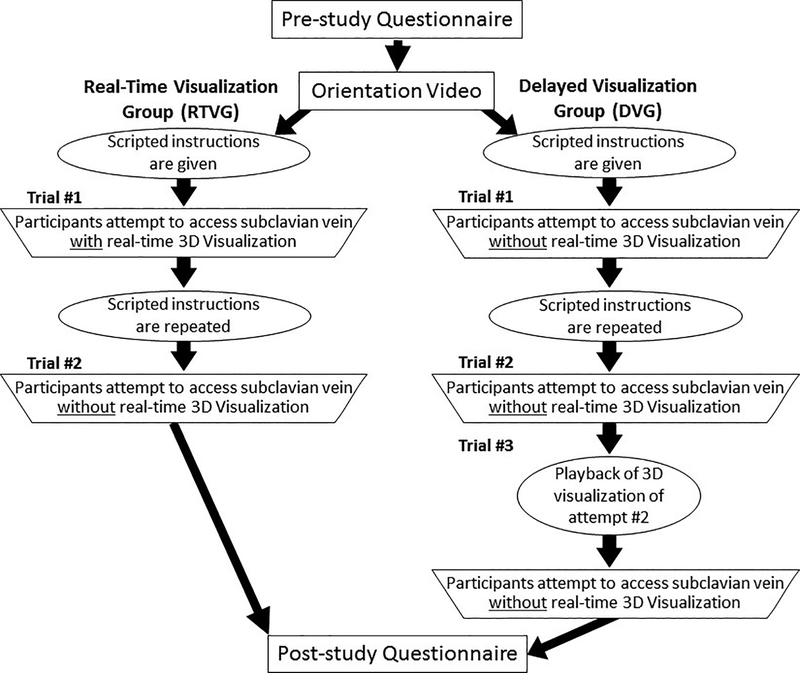
Participants completed a pre-study questionnaire to provide demographic data, including level of training, prior experience, and self-rated confidence in the ability to perform the supraclavicular approach. This was followed by a brief video that oriented them to the features of the CVA simulator and provided instructions on how to use it. All participants also received scripted instructions (video and text, but not verbal) on how to perform a supraclavicular approach. In addition, all participants had prior experience with the CVA simulator’s elements while attempting to access the subclavian vein using the infraclavicular approach and the internal jugular vein with US prior to their first attempt at accessing the subclavian vein using the supraclavicular approach. Participants were then randomly assigned to one of two groups, the real-time visualization group (RTVG) or the delayed visualization group (DVG). Neither group used US imaging for the supraclavicular approach to the subclavian vein. As shown in Figure 2, the RTVG was allowed to concurrently use real-time 3D visualization of soft tissue structures inside the simulator while performing venous access only for their first attempt (trial 1); the DVG performed the procedure three times, all without real-time 3D visualization. Participants were denied access to the real-time 3D visualization (displayed on the screen of a generic laptop computer running the simulator) by using the virtual camera controller to change the perspective of the virtual camera away from the virtual patient.
Figure 2.
Supraclavicular access study design flow chart for Groups A and B.
Participants in the DVG performed their initial attempt (trial 1) without real-time 3D visualization, which ended either with successful access or after 5 minutes had elapsed. This attempt was scored by the simulator’s automated scoring algorithm. Participants were then allowed to re-watch the scripted video or re-read the written instructions. Participants in the DVG were then given a second attempt (without real-time 3D visualization). Afterward, delayed 3D visualization of the DVG participant’s second attempt (trial 2) was played back to the participant with an instructor present. Afterward, participants in the DVG were given a third attempt without real-time 3D visualization, which was also scored. The third attempt (trial 3) was to test our second hypothesis (see Fig. 2) on whether real-time 3D visualization was superior to delayed 3D visualization playback after an attempt. The comparison between the final attempt in the DVG (trial 3) and RTVG (trial 2) evaluates each group’s attempt immediately after their use of the 3D visualization and the participant’s ability to access the vein based on information acquired from the 3D visualization.
Participants randomized to the RTVG watched the same instructional video and received the same written instructions as the DVG after the video orientation to the simulator. The participants from the RTVG were asked to access the subclavian vein via the supraclavicular approach while using the real-time 3D visualization of the simulator. Participants were then allowed to re-watch the video or re-read the written instructions on how to access the subclavian vein using the supraclavicular approach. Afterward, RTVG participants performed a second attempt but without real-time 3D visualization.
An automated scoring algorithm is integrated into the simulator. The algorithm generates an objective numerical score by applying penalties associated with errors and potential complications such as pneumothorax, arterial puncture, back-walling the vein, too many needle passes, and taking too much time. Incidents that cause significant injury, such as arterial punctures and pneumothoraces, incur larger penalties, whereas lesser penalties are applied to less serious events such as taking too much time, back-walling the vein, or taking extra attempts. Unsafe near-misses are also penalized, i.e., coming dangerously close to the artery or lung incur penalties scaled to how close the needle tip came to causing injury. Penalties may be applied to needle trajectories outside pre-defined safe and acceptable zones (“cones of safety”) that are specific to the type of approach for attaining central venous access. Recorded central venous access attempts from experts as well as substandard performance examples were used to tune the scoring algorithm. All attempts for both groups were scored. All participants completed a post-study questionnaire and were debriefed.
Statistical Analysis
SAS, version 9.4 (SAS Institute, Cary, NC), was used for data analysis. Descriptive statistics, reported in Table 1, were calculated for baseline characteristics for the RTVG, DVG, and for all participants. Because the score distributions for the RTVG and DVG were negatively skewed and non-normal, as assessed by the Shapiro-Wilk test (W; P < 0.0001), non-parametric statistical methods were used for analysis. The difference in algorithm scores for each participant between trial 1 and the final trial (trial 2 for RTVG and trial 3 for DVG) was calculated, and the Wilcoxon two-sample rank sum test (equivalent to the Mann-Whitney U statistic) was used to test whether improvement in performance was dependent on group. Between-groups differences were tested on the algorithm score on trial 1 (RTVG1 vs DVG1) and trial 2 (RTVG2 vs DVG2), and for final attempts (trial 2 for RTVG vs trial 3 for DVG). The Wilcoxon rank sum test was also used to test group differences on the computed difference variables (RTV1 − RTV2) vs. (DV1 − DV2) and (RTV1 − RTV2) vs. (DV1 − DV3). The Wilcoxon rank sum test does not assume that the difference between two independent samples is normally distributed or that the population variances are equal.13 The Hodges-Lehman estimator was computed for the difference in medians and a 95% confidence intervals for the median difference.13,14 The Wilcoxon signed rank test was used to determine if there was a significant improvement in scores between trials within each group. All statistical tests were two-tailed and the significance level was set at α = 0.05.
Table 1.
Baseline Demographic Characteristics for Real-Time Visualization group (RTVG), Delayed Visualization group (DVG), and all participants.
| Real-time visualization | Delayed visualization | All participants | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Experience | ||||||
| Expert | 3 | 11% | 11 | 26% | 14 | 20% |
| Intermediate | 18 | 67% | 20 | 48% | 38 | 55% |
| Novice | 6 | 22% | 11 | 26% | 17 | 25% |
| Handedness | ||||||
| Right | 21 | 81% | 33 | 85% | 54 | 83% |
| Left | 5 | 19% | 6 | 15% | 11 | 17% |
| Specialty | ||||||
| Anesthesiology | 24 | 89% | 28 | 67% | 52 | 75% |
| Other (EM, FM, MS) | 2 | 7% | 7 | 17% | 9 | 13% |
| Unidentified | 1 | 4% | 7 | 17% | 8 | 12% |
| M (SD) | M (SD) | M (SD) | ||||
| Age | 32.1 (6.7) | 33.2 (7.8) | 32.7 (7.3) | |||
The data collected on the supraclavicular approach was part of a larger study investigating additional approaches to central venous access where in addition to group (RTVG vs. DVG) a second between-subjects factor, level of training with three levels, was being studied. Based on our previous work with simulation for central venous access, this study was planned for power = 0.80, assuming a two-tailed test with significance level, α = 0.05, a modest correlation between trials for the repeated measures (ρ = 0.20) to detect a moderately large effect size (i.e., ES = 0.60 in standard deviation units) for the CVA algorithm score. A priori power analysis suggested 10 participants in each cell is sufficient to achieve target power (60 participants randomly assigned to visualization group by level of training). However, because only two participants reported any exposure to the supraclavicular approach, the level of training was not a factor and post hoc power analysis based on the visualization factor alone suggests that 28 participants in each group is sufficient to attain target power (N = 56).
RESULTS
Results are based on participants with signed informed consent and complete data (N = 69). The algorithm score range is from 0 to 600. The scores of three anesthesiologist co-authors (AR, JS, and WS), with a combined clinical experience of 25 years in central venous access and all comfortable with the supraclavicular approach to the subclavian vein prior to the initiation of the study, for three attempts each at the supraclavicular approach without visualization yielded a mean of 578 and a median of 572. Although scores were reduced by errors and near misses as described above, most participants lost points in the scoring algorithm due to the time penalty alone. As a result, score distributions were negatively skewed. Reported in Table 2 are descriptive statistics (medians, interquartile range, and 95% confidence intervals for the medians), as well as the results of our hypothesis tests (Wilcoxon rank sum statistic, S, P values, the Hodges-Lehman estimator for the difference in medians, and 95% confidence intervals for the median difference). Supplemental Figure 1 shows a graphical representation of all the scores, which shows very little variation unless participants have a complication.
Table 2.
Algorithm Scores for the Supraclavicular Approach Descriptive Statistics by Group (RTVG vs DVG) and Statistical Comparisons
| Trial/Group | Real-time visualization (RTV) | Delayed visualization (DV) | ||
|---|---|---|---|---|
| Median (95% CI) | IQRa | Median (95% CI) | IQR | |
| Trial 1 | 584.8 (577.1, 587.2) | 17.4 | 565.6 (559.9, 581.6) | 34.3 |
| Trial 2 | 590.2 (583.0, 593.6) | 24.4 | 576.7 (563.4, 581.9) | 30.7 |
| Trial 3 | 584.1 (569.0, 587.8) | 28.0 | ||
| Between-group comparisonsb | Wc | P | Hodges-Lehman estimator difference in medians (95% CI) | |
| RTV1 − DV1 | 1146.0 | 0.01 | 10.20 (2.2, 23.1) | |
| RTV2 − DV2 | 1163.5 | 0.007 | 10.75 (3.4, 20.9) | |
| RTV2 − DV3 | 1069.0 | 0.13 | 4.2 (−0.8, 11.4) | |
| (RTV1 − RTV2) vs. (DV1 − DV2) | 976.5 | 0.70 | ||
| (RTV1 − RTV2) vs. (DV1 − DV3) | 876.5 | 0.41 | ||
| Within-group comparisons | Md | P | Median for the difference variables (95% CI) | |
| RTV2 − RTV1 | 5.5 | 0.05 | 5.8 (0.9, 10.3) | |
| DV2 − DV1 | 6.0 | 0.09 | 3.1 (−0.1, 9.1) | |
| DV3 − DV1 | 14.0 | <0.0001 | 5.6 (2.85, 15.4) | |
| DV3 − DV2 | 4.0 | 0.28 | 1.2 (−0.9, 6.3) | |
Interquartile Range, the difference between upper and lower quartiles (Q1 − Q3, where Q2 is the median).
RTV1 = Real Time Visualization Group, Trial 1; DV1 = Delayed Visualization Group, Trial 1; DV2 = Delayed Visualization Group, Trial 2; DV3 = Delayed Visualization Group, Trial 3.
W is the Wilcoxon Rank Sum test statistic; the p value is based on the normal approximation with continuity correction, an adjustment that is made when a discrete distribution is approximated by a continuous distribution.
M is the Wilcoxon Sign test statistic.
There was no difference in performance gains (trial 1 – trial 2) between the RTVG and the DVG (P = 0.70). The difference between the first trial (trial 1 for both groups) and the final trial (trial 2 for the RTVG and trial 3 for the DVG) was also not dependent on group (P = 0.41) as seen in Table 2.
Since the real time-visualization group had 3D visualization while the delayed visualization group did not, there was a significant difference in performance between the groups at trial 1 (P = 0.01). The Hodges-Lehman estimator for the median difference with 95% a confidence interval is 10.20 (2.2, 23.1). As hypothesized, there was also a significant difference in performance observed at trial 2 (P = 0.007). The group that received real-time visualization during trial 1 performing better than the group which would receive delayed visualization before trial 3. The estimated median difference and 95% CI is 10.75 (3.4, 20.9). There was no difference in performance between the two groups on their final trial (trial 2 for RTVG; trial 3 for DVG), P = 0.13. The estimated median difference and 95% CI is 4.2 (−0.8, 11.4).
Within groups comparisons show that for the real-time visualization group, given the score range with a high score of 600, performance is extremely high for both attempts (medians for trial 1 = 584.8 vs. trial 2 = 590.2). The difference in performance between trial 1 and trial 2 is not significant (P = 0.08). This suggests that high scores were produced on trial 1, with real-time 3D visualization available, and that the scores remained high on the second trial when visualization was not available to participants. For the delayed visualization group, there was no difference between trial 1 vs trial 2 (P = 0.24), both without visualization. Thus, there was no improvement in performance after a second attempt without 3D visualization. After viewing the delayed 3D visualization of their performance on trial 2, performance was significantly better on trial 3 when compared to trial 1 (P = 0.0007), but not for trial 3 versus trial 2 (P = 0.17).
Only 2 of the 69 participants reported that they had ever used the supraclavicular approach for central line placement. Despite this lack of familiarity, only one participant (DVG group) failed to access the subclavian vein on the first trial. All participants, regardless of group assignment (with or without real-time visualization), successfully accessed the subclavian vein on their final trial. Three participants required more than one attempt to access the vein (two, six, and eight attempts) on trial 1; only one participant needed more than one attempt on their last trial. Table 3 reports overall complications and scoring penalties. In addition to the participants who caused a pneumothorax, three additional participants received a penalty in the scoring algorithm for coming “close” (<1 cm from the lung) to causing a pneumothorax on trial 1 and three on their final trial. There was no overlap in these errors (the participants causing pneumothorax were not the same).
Table 3.
Errors and Potential Complications by Approach Using the Mixed Reality Simulator
| Approach | |
|---|---|
| Supraclavicular | |
| Backwall | |
| Baseline | 8 |
| Final simulation trial | 4 |
| Pneumothorax | |
| Baseline | 3 |
| Final simulation trial | 1 |
| Arterial puncture | |
| Baseline | 1 |
| Final simulation trial | 0 |
| Extra attempts | |
| Baseline | 3 |
| Final simulation trial | 1 |
Study participants also completed surveys prior to and immediately following the simulator session; results reported in Table 4 show relevant items. Participants were overwhelmingly positive about simulator training for the supraclavicular approach to subclavian vein access both as an educational tool for trainees and as an aid to improve their own technical proficiency. Participants were also asked to rate their confidence in performing the supraclavicular approach for subclavian vein central venous access pre- and post-training on a 10-point scale where 1 = “not at all confident” and 10 = “completely confident.” Not surprisingly, given the fact that only two participants reported any previous experience with this technique, on average, the confidence for all participants prior to training was 1.6 (SD = 1.1). Mean post-training confidence rating was 7.2 (SD = 1.8). The median for the confidence gain scores, 6.0 (IQR = 3.0), was significantly greater than 0 (Wilcoxon signed rank test, S = 11.39, P < 0.0001). Although confidence gains in the RTVG (median = 6.0) were greater than in the DVG (median = 5.0), the Wilcoxon rank sum test shows this difference was not significant, S = 876.5, P = 0.08.
Table 4.
Participants’ Reactions to Simulator Training for the Supraclavicular Approach to the Subclavian Vein
| Real-time visualization | Delayed visualization | All participants | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Survey item | Agreea | Strongly agree | Mean (SD) | Agree | Strongly agree | Mean (SD) | Agree | Strongly agree | Mean (SD) |
| Experience on this simulator could be useful in clinical practice for supraclavicular approach to obtaining subclavian central venous access. | 33.3% | 66.7% | 4.67 (0.48) | 12.0% | 88.0% | 4.88 (0.33) | 25.4% | 74.6% | 4.75 (0.44) |
| The simulator should be used as a training/education tool to teach residents the supraclavicular technique of subclavian central venous access. | 29.3% | 70.7% | 4.71 (0.46) | 4.0% | 96.0% | 4.96 (0.20) | 19.7% | 80.3% | 4.80 (0.40) |
| The training received with the simulator improved my technical proficiency in supraclavicular technique of subclavian central venous access. | 28.6% | 71.4% | 4.71 (0.46) | 8.0% | 92.0% | 4.92 (0.28) | 20.9% | 79.1% | 4.79 (0.41) |
| Yes | No | Yes | No | Yes | No | ||||
| Do you think that this experience with the simulator will help you perform supraclavicular technique for subclavian central venous access. | 95.2% | 4.8% | 96.0% | 4.0% | 95.5% | 4.5% | |||
Response options for these questions evaluating the simulator training used a scale with five response options:
1 = Strongly disagree, 2 = Disagree 3 = Neutral 4 = Agree 5 = Strongly agree
For all questions in Table 2, there were no responses that weren’t 4 or 5.
DISCUSSION
The original motivation for this study was to evaluate the learning outcomes and efficacy of a new mixed reality procedural simulator. This research was part of a larger study to investigate the efficacy of a mixed reality simulator with and without visual augmentation (real-time, 3D, and color visualization) for teaching various approaches to CVA. No statistically significant differences in performance between the two groups due to real-time 3D visualization were identified when comparing the gain scores between the first and last trials for each group (Hypothesis 2). Despite not finding a significant benefit afforded by real-time 3D visualization compared to delayed 3D visualization, on average, participants improved significantly in their ability to successfully access the subclavian vein via the supraclavicular approach after interacting with the simulator. This finding is similar to the improvement found in other task trainers.5–9 The availability of real-time 3D visualization was associated with significantly higher scores for RTVG on Attempt 1 compared to the DVG Attempt 1. Although the comparison of the two groups on the gain score between trial 1 and trial 2 was not significant (P = 0.70), the algorithm scores for the RTVG remained significantly higher than DVG on trial 2 (even though RTVG no longer had access to visualization), P = 0.007. These results indicate that the benefits of exposure to the real-time visualization (possibly in forming a 3D mental model of the relevant anatomy for the supraclavicular approach) may have been retained and that a second attempt alone is not enough to optimize performance.
Our findings indicate that 3D visualization is helpful in improving performance as measured by the scores, irrespective of whether the visualization was in real-time or delayed, supporting our first hypothesis.
Users of the CVA procedural simulator reported a significant improvement in their self-rated confidence in supraclavicular access to the subclavian vein. This is very encouraging given the fact that only two participants reported any prior experience with the supraclavicular technique. This study is also, for most participants, their only exposure to the supraclavicular approach to the subclavian vein. With only two to three attempts by each participant, the CVA simulator, in combination with scripted video instructions, was able to show subjective evidence of the improvement in confidence, as well as a low complication rate (most penalties were from a time delay). This would suggest that the supraclavicular approach to the subclavian vein is a relatively easy procedure to learn and that simulation improved the participant’s performance and their confidence in attempting it.
The findings of the study show a floor and ceiling effect for the algorithm scores. Providers may be uncomfortable accessing the subclavian using the supraclavicular approach because they never trained with it, but US imaging may increase a provider’s confidence. Because US was not used in the referenced literature, it was not used in this study. However, using US guidance may help further improve confidence and decrease the potential for complications with the supraclavicular approach.
The safety and teachability of the supraclavicular approach were not the premises of this study, and as such, further study would be necessary to evaluate the supraclavicular approach compared to the infraclavicular approach. This approach may be advantageous in patients with a cervical collar in place and concomitant thoracic injuries and those undergoing CPR. Although the reason the infraclavicular approach is relatively more popular is not known, it may be because it was described earlier in the literature.2 This is despite its high complication rate and the longer distance that needs to be traversed percutaneously to actually access the subclavian vein via the subclavian approach.
The Kirkpatrick evaluation model has four levels where each level builds upon the previous one (Kirkpatrick 1998). Assessment of adoption of training into daily clinical practice (Kirkpatrick 3) as well as the directly attributable effect of the training on patient outcomes (Kirkpatrick 4) are eventual goals of our research, but are beyond the scope of this article. The study we describe is at Kirkpatrick level 2 (learning outcomes). In a methodical process where each step builds upon the previous Kirkpatrick level in a crawl, walk, run progression, we purposely limited our initial approach to a learning outcome study. The learning outcome study is needed to establish that training with the simulator improves learning to enable us to approach our hospital administration (and other educators) with hard evidence that may be of help in convincing our management that the 3D visualization-based training should be provided to all relevant clinical personnel.
CONCLUSIONS
The CVA simulator was efficacious for training clinicians to access the subclavian vein using the supraclavicular approach that was, for most participants, unfamiliar. The use of real-time 3D visualization during an access attempt showed significantly improved scores compared to the group without 3D visualization. Training with the CVA simulator did not prevent errors in technique, nor were significant differences found in using real-time 3D visual augmentation (visualization) while performing access attempts versus playing back prior attempts using delayed 3D visualization. More studies are necessary to determine whether the improved scores resulting from visual augmentation are clinically significant and will translate into better patient outcomes. Additional studies are needed to establish if the use of US imaging can decrease the complication rate with the supraclavicular approach in both simulated and actual clinical settings.
Supplementary Material
Supplemental Figure 1. Graphical representation of the participants’ scores during the study.
Key Points Summary.
Does visual augmentation (3D color visualization) enhance learning and performance during central venous access in a mixed reality procedural simulator?
Automatically generated performance scores improved significantly with real-time or delayed visualization.
Visualization is efficacious whether in real time or delayed in a mixed reality simulator that has both physical and virtual (visualization) components.
Funding:
Research reported in this publication was partly supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award numbers UL1TR000064 and UL1TR001427 and by U.S. Army Medical Research Acquisition Activity under Award No. W81XWH-14-1-0113. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, MD 21702–5014. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Footnotes
Conflict of interest: No conflicts of interest.
REFERENCES
- 1.Yoffa D. Supraclavicular subclavian venepuncture and catheterisation. Lancet. 1965;2:614–617. [DOI] [PubMed] [Google Scholar]
- 2.Patrick SP, Tijunelis MA, Johnson S, Herbert ME. Supraclavicular subclavian vein catheterization: the forgotten central line. West J Emerg Med. 2009;10:110–114. [PMC free article] [PubMed] [Google Scholar]
- 3.Conroy JM, Rajagopalan PR, Baker JD, Bailey MK. A modification of the supraclavicular approach to the central circulation. South Med J. 1990;83:1178–1181. [DOI] [PubMed] [Google Scholar]
- 4.Dronen S, Thompson B, Nowak R, Tomlanovich M. Subclavian vein catheterization during cardiopulmonary resuscitation. A prospective comparison of the supraclavicular and infraclavicular percutaneous approaches. JAMA. 1982;247:3227–3230. [PubMed] [Google Scholar]
- 5.Robinson AR III, Gravenstein N, Cooper LA, Lizdas DE, Luria I, Lampotang S. A mixed-reality part-task trainer for subclavian venous access. Simul Healthc. 2014;9:56–64. doi: 10.1097/SIH.0b013e31829b3fb3. [DOI] [PubMed] [Google Scholar]
- 6.Barsuk JH, Cohen ER, McGaghie WC, Wayne DB. Long-term retention of central venous catheter insertion skills after simulation-based mastery learning. Acad Med. 2010;85:S9–S12. doi: 10.1097/ACM.0b013e3181ed436c. [DOI] [PubMed] [Google Scholar]
- 7.Barsuk JH, Cohen ER, Feinglass J, McGaghie WC, Wayne DB. Use of simulation-based education to reduce catheter-related bloodstream infections. Arch Intern Med. 2009;169:1420–1423. [DOI] [PubMed] [Google Scholar]
- 8.Barsuk JH, McGaghie WC, Cohen ER, O’Leary KJ, Wayne DB. Simulation-based mastery learning reduces complications during central venous catheter insertion in a medical intensive care unit. Crit Care Med. 2009;37:2697–2701. [PubMed] [Google Scholar]
- 9.Barsuk JH, McGaghie WC, Cohen ER, Balachandran JS, Wayne DB. Use of simulation-based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit. J Hosp Med. 2009;4:397–403. [DOI] [PubMed] [Google Scholar]
- 10.Lampotang S, Lizdas D, Rajon D, Luria I. Mixed simulators: augmented physical simulators with virtual underlays. 2013 IEEE Virtual Reality. 2013;7–10. [Google Scholar]
- 11.Lampotang S, Bova FJ, Lizdas DE, et al. A subset of mixed simulations: augmented physical simulations with virtual underlays. In Proceedings of the 2012 Interservice/Industry Training, Simulation, and Education Conference: Paper No. 12077, 2012. [Google Scholar]
- 12.Czarnik T, Gawda R, Perkowski T, Weron R. Supraclavicular approach is an easy and safe method of subclavian vein catheterization even in mechanically ventilated patients: analysis of 370 attempts. Anesthesiology. 2009;111:334–339. [DOI] [PubMed] [Google Scholar]
- 13.Hollander M, Wolfe D. Nonparametric Statistical Methods (2nd Ed). New York: John Wiley & Sons. [Google Scholar]
- 14.Decker C. Calculating a Nonparametric Estimate and Confidence Interval Using SAS® Software, Glaxo Wellcome Inc., Research Triangle Park, NC. Available online: http://www.lexjansen.com/pharmasug/2000/Coders/cc01.pdf. Accessed January 25, 2017. [Google Scholar]
- 15.Lanham B, Maxson-Cooper P. Is six sigma the answer for nursing to reduce medical errors and enhance patient safety? Nursing Economics. 2003;211:39–41. [PubMed] [Google Scholar]
- 16.Tepper OM, Small K, Rudolph L, Choi M, Karp N. Virtual 3-dimensional modeling as a valuable adjunct to aesthetic and reconstructive breast surgery. Am J Surg. 2006;192:548–51. [DOI] [PubMed] [Google Scholar]
- 17.Kirkpatrick DL. Evaluating training programs: The four levels. San Francisco: Berrett-Koehler, 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Graphical representation of the participants’ scores during the study.