Abstract
Nitric oxide (NO) represents a potential wound therapeutic agent due to its ability to regulate inflammation and eradicate bacterial infections. Two broad strategies exist to utilize NO for wound healing: liberating NO from endogenous reservoirs and supplementing NO from exogenous sources. This progress report examines the efficacy of a variety of NO-based methods to improve wound outcomes, with particular attention given to diabetes-associated chronic wounds.
Keywords: chronic wounds, diabetes, nitric oxide, wound healing
Graphical Abstract
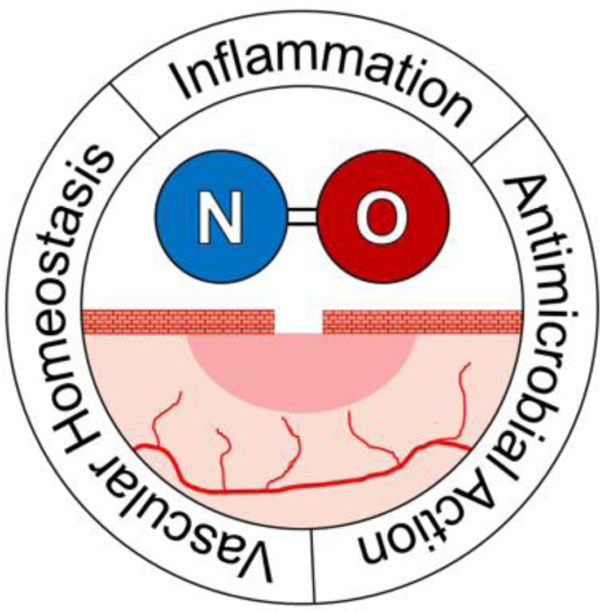
Nitric oxide (NO)-based therapies for chronic wound healing are explored in this progress report. The use of NO to accelerate wound healing is motivated by its role as an endogenous regulator of inflammation and antibacterial agent. A number of NO-based wound healing strategies have been developed, including those that liberate NO from endogenous reservoirs and those that dose exogenous NO.
1. Introduction
Chronic wounds are wounds that have entered a state of pathological inflammation instead of following a healthy healing timeline. While the typical progression of a healthy wound from injury to complete wound closure occurs within thirty days, chronic wounds stall and can remain unresolved indefinitely.[1,2] In total, chronic wounds (e.g., pressure ulcers, vascular ulcers, and diabetic ulcers) affect between 2.4 and 4.5 million people in the United States causing a significant humanistic and financial burden.[3] Such chronicity can have severe ramifications for diabetic patients as chronic wounds are the leading cause of diabetes-associated amputations. Due to the threat of chronic wounds to diabetic health and the increasing prevalence of diabetes, many researchers have focused on developing more effective strategies for wound treatment.
The difficulties associated with designing an appropriate treatment are potentiated by the systemic complications of diabetes (e.g., tissue hypoxia, impaired inflammatory response, decreased collagen production).[4] The typical protocol for a chronic wound treatment includes debridement of necrotic tissue, use of topical antibiotics to control infection, and application of a wound dressing (e.g., films, fibers, hydrogels). Additional therapeutic intervention may take the form of negative pressure therapy, hyperbaric oxygen therapy (HBOT), or application of growth factors.[3,5,6] A major cause of stalled wound healing stems from unresolved infection.[2] Inflamed wounds provide a suitable environment for bacterial colonization, with infection causing tissue damage and triggering further inflammation. The increasing prevalence of antibiotic-resistant bacteria renders treatment with antibiotics less effective, motivating the use of a topical antimicrobial agent that does not foster resistance.
Nitric oxide (NO), the endogenous regulator of inflammation and an antibacterial agent with no demonstrated resistance, has become an attractive candidate for wound healing therapy.[7] The ability of NO to serve as a single agent wound therapeutic or in conjunction with established wound therapy protocols (e.g., wound dressings, HBOT) underlines its versatility and merits further research. Herein, we review the recent developments of NO-based therapies for wound healing, with an emphasis on the role of these therapies regarding diabetic wound healing.
1.1. Biology of Wound Healing:
Normal wound healing consists of four interconnected and overlapping phases: hemostasis, inflammation, proliferation, and tissue remodeling, with each phase involving different cell populations (Figure 1).[1,6] Acute wounds occur after a sudden insult to the skin, often in the form of a cut or burn. Immediately following injury, re-establishment of hemostasis to stop bleeding begins through the formation of a clot by local vasculature constriction, platelet degranulation, and fibrin activation. Once bleeding is under control, the inflammatory stage commences. Dead and damaged cells and invading microbes in the injured tissue are removed through metalloprotease (MMP)-mediated phagocytosis and release of reactive oxygen species (ROS) by infiltrating neutrophils, macrophages, and lymphocytes.[8] Upon clearance of pathogens and detritus, the proliferation phase of healing begins. Growth factors stimulate the generation of new tissue, including the formation of blood vessels by angiogenesis, granulation tissue by fibroblasts, and a provisional extracellular matrix (ECM) by MMPs. During this phase, the open surface area of the wound shrinks as myofibroblasts at the wound edges undergo contraction.[9] The final phase of wound healing is tissue remodeling; new tissue formed in the proliferation phase matures and the vascular density of the wound decreases from high proliferation-phase levels.
Figure 1.
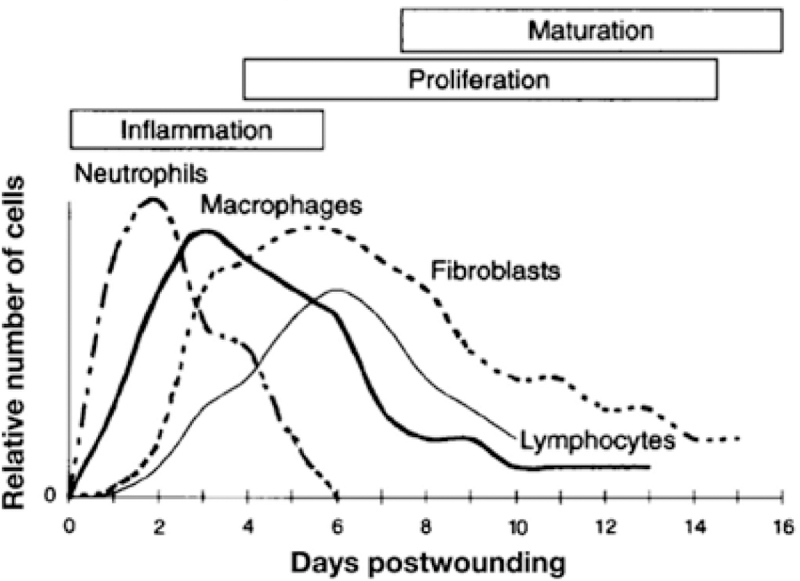
Time course of different cells populations in the wound during the healing process. Reproduced with permission.[50] Copyright 1997, Elsevier.
1.2. Impaired Wound Healing in Diabetes:
Not all wounds proceed according to a normal healing timeline. Some injuries result in the development of chronic wounds, wherein injured tissue enters a state of pathologic inflammation that results in protracted and incomplete healing. In acute wounds, the four stages of healing typically take place within a month, and the healed tissue closely resembles pre-wound tissue.[10] However, the chronic wound healing timeline can extend for months or even stall in the inflammation phase indefinitely. As opposed to the sudden injury often responsible for acute wounds, chronic wounds are more likely to be caused by persistent stimuli (i.e., repeated tissue trauma, pressure points, or ischemia). In these cases, cutaneous tissue breaks down and the damaged tissue can become a medium for bacterial growth.[11] Infection and insufficient tissue oxygenation are two major factors that contribute to chronic wound formation.[1] Incomplete microbial clearance elongates the inflammatory stage of healing. If inflammation is left unchecked, biofilms may form, resulting in the self-secretion of an exopolysaccharide matrix (EPS) that protects bacteria from the host immune response and antibiotic treatment.[1,2] Normoxic conditions are also crucial because oxygen is required for various processes in wound healing, including epithelization, angiogenesis, and collagen deposition.[12] Of note, hypoxia (<20 mm Hg) is a reliable indicator of chronic wounds.[13]
Therefore, systemic factors or diseases that induce tissue hypoxia and/or leave a patient vulnerable to infection often lead to chronic wounds. Conditions that correspond with poor wound healing outcomes include malnutrition, senescence, stress, obesity, alcoholism, cancer, immunodeficiency, and ischemia. However, diabetes is the disease that contributes to the greatest number of diagnosed chronic wounds. Collectively, diabetes is a group of metabolic diseases typified by dysregulation of blood glucose levels that often lead to chronic wound formation through complex pathophysiological mechanisms (Figure 2).[1,14] First, frequent hyperglycemia (i.e., increased blood glucose levels) results in increased levels of advanced glycation end-products (AGEs) in the blood. Glycation end-products directly contribute to the formation of high concentrations of reactive oxygen (ROS) and reactive nitrogen (RNS) species. When ROS/RNS levels exceed the antioxidant capacity of a given tissue, damage to the host may tissue occur.[15] In the inflammation stage of wound healing, ROS/RNS species are essential to wound healing in that they accelerate the clearance of dead tissue and pathogens. At excessive concentrations, these reactive species can prevent transition from the inflammatory to the proliferative stage and thus hinder the formation of new, healthy tissue.[9,13]
Figure 2.
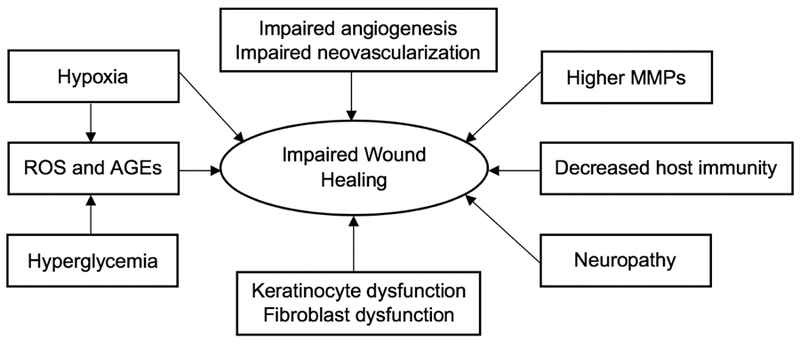
The potential effects of diabetes on wound healing.
Adapted with permission.[1] Copyright 2010, SAGE Publications.
The relative levels of signaling molecules (e.g., pro-inflammatory cytokines, proteases, and growth factors) in chronic wounds are often imbalanced, contrary to strict regulation and coordination found in acute wounds. Chronic wounds experience a dramatic increase in the concentration of pro-inflammatory cytokines directly after injury. Elevated cytokine concentrations result in a prolonged inflammatory stage and overproduction of metalloproteases (MMPs).[16] Upregulation of MMPs causes the continuous breakdown of ECM components and further degradation of already undersupplied growth factors.[8,16,17] As such, dysregulated cytokine expression is highly detrimental to regrowth of healthy tissue.
Lower levels of neuropeptides (e.g., substance P and nerve growth factor) attributed to diabetic neuropathy are also responsible for decreased cell chemotaxis and growth factor production.[18] Decreased levels of growth factors, particularly vascular endothelial growth factor (VEGF), disrupt the homing and mobilization of endothelial progenitor cells (EPCs), which are responsible for restoration of the vasculature. Diminished angiogenesis results in hypoxia caused by insufficient tissue perfusion.[19] Diabetic people are more likely to have chronic wounds with co-infections because defective leukocyte chemotaxis and phagocytosis lead to poorer bacterial load clearance and the formation of intractable biofilms.[20] Endotoxins from bacteria increase tissue damage, further impairing wound healing. A schematic of the differences between normal and diabetic wounds is summarized in Figure 3.[21]
Figure 3.
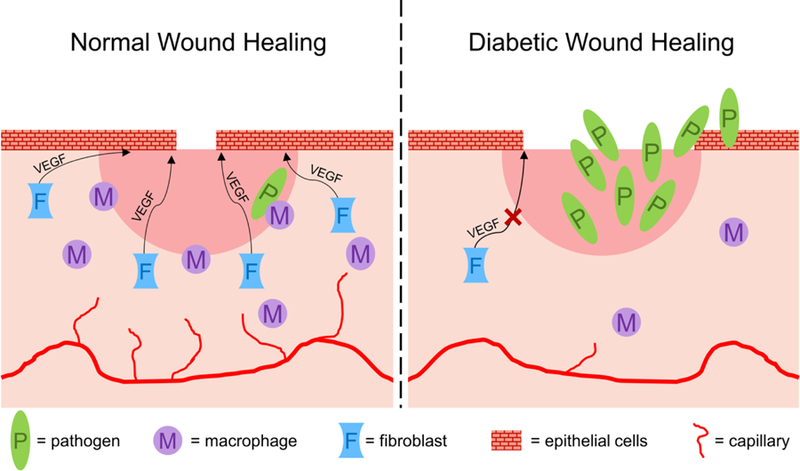
Mechanisms of normal versus diabetic wound healing. In a normal wound, macrophages combat wound bed pathogens, while fibroblasts deliver VEGF to initiate regrowth of epithelial cells and close the open wound. In diabetic wounds, insufficient angiogenesis and macrophage function result in significant pathogen invasion, while lower fibroblast presence delays re-epitheliazation and wound closure.
Adapted with permission.[153] Copyright 2017, The Royal Society of Chemistry.
While diabetes is a systemic pressure that may cause any wound a patient endures to become chronic, diabetics are especially prone to develop a certain type of chronic wound known as a diabetic foot ulcer (DFU). With a lifetime incidence in 25% of diabetic patients, DFUs account for almost half of all diabetes-related hospitalizations and directly precede more than 80% of diabetes-related lower leg amputations.[22] Like many chronic wounds, DFUs are often the result of repeated tissue trauma. This trauma is compounded by the oxidative stress associated with chronic hyperglycemia, which serves to damage the structural integrity of blood vessels, causing them to become enlarged. These enlarged veins prevent blood flow upwards and out of lower extremities, leading to increased pressure and decreased circulation in the legs and feet.[23] The chronic wound is infiltrated by neutrophils and macrophages in response to the release of inflammatory cytokines by damaged and infected tissue. Concentrations of the inflammatory cytokines TNF-α and interleukin have been reported to be up to 100-fold greater in DFUs than in acute wounds.[8] A prolonged inflammatory phase effectively bypasses the release of growth factors that would signal the healing cascade to begin in a healthy wound.[24,25] Diabetic patients also have an impaired ability to fight infection, resulting from an impaired immune response to invading pathogens.[20] Wounds that occur in the lower extremities of diabetic patients thus result in hypoxic, infected tissue, which is a potent combination that often compromises wound healing. As a result, many studies focus on DFUs as both a model for chronic wounds and a critical therapeutic target.
In summary, acute wounds have an environment with suitable mitogenic activity, low concentrations of inflammatory cytokines and MMPs, high levels of growth factors, sufficient bacterial clearance, and functioning fibroblasts. This environment promotes wounds that heal quickly and completely without the need for significant therapeutic intervention. In contrast, chronic wounds have low mitogenic activity, high levels of both inflammatory cytokines and MMPs, low levels of growth factors, biofilm formation, and fibroblasts that are senescent. These wounds experience elongated or even completely arrested healing timelines, which often result in severe patient morbidity.[8] The process of wound healing is best understood as a sequential shift in predominant cell populations as progression is made through the four phases.[26] As the population and activity of a given cell type changes through the different stages of healing, any proposed therapeutic for a wound must be effectively timed to changing cell populations.[1]
1.3. Role of Endogenous Nitric Oxide in Wound Healing:
Nitric oxide (NO) is an endogenous gasotransmitter that plays a central role in wound healing. It is generated from the terminal guanidine moiety of l-arginine through a five electron oxidation process catalyzed by a group of three isozymes, called nitric oxide synthases (NOS).[27] This group of isozymes includes endothelial (eNOS), neuronal (nNOS), and inducible nitric oxide synthase (iNOS). Two of the isozymes, nNOS and eNOS, are constitutively expressed and catalyze low level NO generation through cyclic guanosine triphosphate (GMP) in vascular endothelial cells and neurons, respectively.[28] The third isozyme, iNOS, is an inducible form only produced in response to acute inflammatory stimuli.[29] These stimuli include invading pathogens and pro-inflammatory cytokines (e.g., IFN-α, TNF) released after wounding events by neutrophils and macrophages. Once fully expressed, the concentration of iNOS greatly exceeds that of the two constitutively expressed forms (i.e., eNOS and nNOS).[30] Nitric oxide has concentration-dependent characteristics, with pM-nM concentrations generated by eNOS and nNOS corresponding with anti-inflammatory processes, and nM-µM concentrations generated by iNOS responsible for pro-inflammatory and antimicrobial effects.[30,31] Thus, iNOS is the most impactful isozyme that regulates pathogen clearance and acute inflammation in wound healing. Temporally, NO is produced at higher levels by iNOS during the inflammatory phase; lower eNOS-derived NO levels become relevant during the proliferative and maturation phases (Figure 4).[32]
Figure 4.
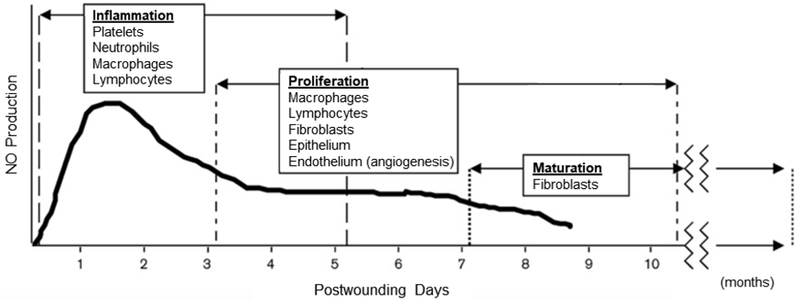
Temporal production of NO within the healing wound juxtaposed with the phases of healing and the cell populations that predominate each phase.
Reproduced with permission.[32] Copyright 2000, Wolters Kluwer Health, LWW.
Nitric oxide plays a central role in the regulation of three major parts of the wound healing process: vascular homeostasis, inflammation, and antimicrobial action. Nitric oxide impacts vascular homeostasis, which is disrupted in the event of an injury. Upon tissue injury, a healthy healing process depends upon platelet aggregation and blood clot formation to stop further blood loss. Nitric oxide generated by eNOS serves to prevent platelet adhesion to the vessel walls. Reactive oxygen species released by the damaged vasculature react with and inactivate NO, with the resulting NO deficiency leading to platelet aggregation and thrombus formation.[33,34] Nitric oxide has been shown to regulate cytokines that initiate inflammation (e.g. interleukins, monocytes, and neutrophils) with downstream effects on the recruitment of keratinocytes.[35,36] A detailed summary of NO’s impact on a panel of inflammatory cytokines has previously been reported by Kobayashi et al.[35] Nitric oxide has also been shown to upregulate MMP concentrations,[37] modulate the migration and attachment of neutrophils to the endothelium,[36,38] and coordinate leukocyte recruitment in the wound-healing timeline.[39]
Lastly, NO plays an essential role in antimicrobial mechanisms that decrease bacterial loads at wound sites. Upon injury to the skin, a portal is created for the invasion of the injured tissue by microbial pathogens.[40] The tissue damage caused by microbe-released toxins can delay or prevent proper healing mechanisms. Nitric oxide has antimicrobial properties resulting from a combination of nitrosative and oxidative mechanisms.[41] Upon reaction with oxygen and superoxide, nitric oxide forms dinitrogen trioxide and peroxynitrite, respectively. Dinitrogen trioxide causes DNA deamination; peroxynitrite results in lipid peroxidation and membrane damage.[42,43] While eukaryotic cells have evolved mechanisms to scavenge these reactive species and thus mitigate their destruction, bacteria have not and will be killed.[44] In addition, NO has been shown to exhibit dose-dependent antibacterial activity against biofilms.[45] With additional protection afforded by an exopolysaccharide matrix, these communities of bacteria are highly resistant to traditional antibiotics.[46] Though the exact mechanism of NO’s anti-biofilm action remains unclear, it is known that NO facilitates biofilm dispersal at low NO concentrations (10−12-10−9 M)[47] and complete biofilm eradication at higher NO concentrations (10−6-10−3 M).[45,48] Large concentrations of NO from macrophage-generated iNOS, therefore, act as a broad-spectrum antimicrobial against both planktonic and biofilm-based bacteria, reducing bacterial loads to a level at which normal wound healing processes are able to proceed.[49]
Abnormal production of NO, which is a characteristic of diabetes, has been linked to impaired wound healing and the development of chronic wounds.[31] Current evidence suggests that diabetic wound fluid has significantly lower levels of NO than healthy wound fluid, due to downregulated eNOS.[50,51] Macrophages, keratinocytes, and fibroblasts all express elevated levels of iNOS at a healthy wound site, but that expression is suppressed in diabetes. In the absence of the recruiting effect of NO, macrophages are found in lower densities in chronic wounds, diminishing pathogen clearance.[52,53] Inhibited NO production strongly reduces the number of keratinocytes during the process of re-epithelization and further antagonizes keratinocyte proliferation and differentiation.[54–56] Fibroblasts in diabetic chronic wounds express lower levels of both iNOS and eNOS, become senescent, and do not produce collagen or form ECM. Thus, decreased NO in extracellular wound fluid corresponds with decreased collagen content and weaker wound breaking strength.[57] Levels of NO may also be used as prognostic markers, with abnormal NO concentrations serving as reliable predictors of poor healing outcomes in septic burn patients and chronic DFUs.[58,59]
1.4. Nitric Oxide as a Wound Healing Therapeutic:
Since the discovery of the roles of endogenous NO in wound healing processes in the late 1990s, many have pointed to the potential utility of NO as a therapeutic agent for the treatment of chronic wounds.[32,50] As described above, levels of NO have been implicated in the failure of the healing process. Insufficient NO in diabetic wounds impedes migration and action of wound healing cell types, while excessive NO, associated with infected or highly inflamed wounds, results in further tissue damage. Therefore, modulation of NO has been seen as an attractive solution to impaired wound healing. The use of NO as a wound healing therapeutic may be categorized by two broad strategies: generating NO from endogenous reservoirs and dosing with NO from exogenous sources.
Generation of NO from endogenous sources may be accomplished by two mechanisms: l-arginine supplementation as a substrate for NOS and inhibition of NOSs, specifically iNOS. Dosing exogenous NO can be accomplished by a number of methods. Simple techniques include treating wounds using formulations of nitrite under acidified conditions or gaseous NO. Further, small molecule NO-releasing donors can be applied directly to wounds encapsulated via their encapsulation polymers, gels, and dressings. Nitric oxide donors can also be chemically attached to macromolecular scaffolds, providing potentially greater stability to labile NO donors and enabling targeted, tunable delivery of NO. The goal of both endogenous and exogenous therapeutic NO dosage is to regulate inflammation and tissue remodeling in chronic wounds.
2. Endogenous Nitric Oxide for Chronic Wound Therapy
2.1. l-arginine Supplementation:
l-arginine is the sole substrate for nitric oxide synthases and the immediate precursor to NO production. Nitric oxide is generated on the terminal guanidine residue of l-arginine, which also generates citrulline as an end product.[60] The body does not provide sufficient amounts of arginine during the times of stress associated with a wounding event. Thus, arginine supplementation of endogenous stores has been proposed as a way to normalize or enhance wound healing. While arginine plays an important role in other physiological processes (e.g., regulation of growth hormones, role in the urea cycle, and antioxidant generation), for the purposes of this progress report, attention will be given to the explicit effect arginine has on NO levels in wound healing.
Dietary supplementation has been the primary route of arginine dosage employed. A typical diet allows for up to 5 grams of arginine to be absorbed per day, with the rest passed through the excretory system.[61,62] Nearly 40% of absorbed arginine is degraded in the intestines, with the remainder portioned into the circulatory system via the portal vein.[61,63] Circulating arginine is available for NO production and is typically present in concentrations of 75–100 µM. Of note, protein-bound arginine is not available for oxidation by nitric oxide synthases, as the isozymes only react with free arginine.[61] Given the absorption mechanism of arginine, diet supplementation would maximize available free arginine in circulation, and thus increase NO production.
Supplemental arginine therapy has been studied clinically since 1978, even before the establishment of arginine’s role as a NOS substrate. In an initial study, Seifter et al. compared wound characteristics in rats with an arginine-free diet to those in rats with an arginine-supplemented diet (i.e., additional 1.8% arginine). The authors found that collagen precursor deposition and wound breaking strength increased in the arginine-supplemented rats.[64] While this study motivated decades of subsequent dietary arginine research, it was entirely based on rats with non-chronic wounds, leaving it unclear whether the arginine supplementation would really alter the healing processes of chronic wounds. The first study that considered diabetes-associated chronic wounds was published by Witte et al. in which diabetes was induced using streptozotocin prior to creating incisional wounds.[50] A subset of the diabetic rats were fed with 1 g kg−1 arginine by gavage, whereas control rats were dosed with saline. Concentrations of nitrite and nitrate were correlated to wound breaking strength. Wound fluid concentrations of NO and its stable byproducts were depressed in the diabetic rats but returned to normal in arginine-fed rats. The wound breaking strength of arginine-supplemented diabetic rats was significantly improved compared to saline-dosed controls. This study was the first to suggest that arginine supplementation restores NO-production pathways that are typically deficient in diabetes. Shi et al. reported subsequently that wound breaking strength increases corresponded to enhanced hydroxyproline deposition, a collagen precursor.[65] Diabetic rats that were fed a combination arginine/proline-supplemented diet had improved angiogenesis and wound closure rates compared to isonitrogenous control-fed rats. Macrophage-expression of iNOS was also reduced in the treated rats, an indication of reduced inflammation favoring expedited wound closure. The study elucidated the daily progression of wound healing over the first 5 d after wounding. At day 5, significant improvements in angiogenesis and nitrogen balance begin to present.[66] An interesting result of this work was that epithelization appeared to be unaffected by arginine supplementation after five days of treatment, suggesting that dietary supplementation of arginine may have insufficient therapeutic power in this important aspect of wound healing.
More recently, the impact of dietary supplementation of arginine was examined in human trials. As with all human studies, a large number of subjects and blinded experiments are essential for overcoming bias in sampling and clinical assessment. One such double-blind study assessed the effect of an arginine-containing oral nutritional supplement on wound healing of diabetic foot ulcers (DFUs) in 270 diabetic patients with chronic wounds.[67] A subset of the subjects (n=130) ingested a drink containing arginine twice daily for 16 weeks. No significant overall difference was observed between the nutrient-supplemented and control groups in terms of time to heal or wound closure. However, an interesting subpopulation result was discovered. Diabetic patients treated with arginine who had decreased limb perfusion were 1.66 times more likely to heal than those with normal limb perfusion. The authors attributed this data to the positive influence of NO on microvascular hemodynamic changes, as the addition of arginine was speculated to impact vascular deficiency. A conclusion about the direct impact NO had on wound characteristics from this study remains speculative, as biomarkers for NO activity were not analyzed. While DFUs without concomitant impaired blood flow may not benefit from supplemental arginine, patients with impaired perfusion may experience better healing outcome. This result points to a mechanism wherein nutrient deficiencies associated with poor circulation may be overcome by dosing extra nutrients, such as arginine to promote wound healing.
Dietary supplementation is not the only way that arginine has been used as a wound healing therapeutic. Administration of arginine directly to the wound site has also been shown to improve wound healing outcomes. Shearer et al. reported that systemic administration of arginine can lead wound-site macrophages to release high levels of arginase, suppressing the amount NO produced through substrate limitation.[68] In this manner, local administration of arginine to a wound site may more effectively and selectively modulate wound healing. This strategy was explored by Varedi et al., with a particular interest in how topical administration of arginine might impact the rate of wound closure.[69] Three and five days after creating excisional dorsum wounds in diabetic rats, l-arginine in saline (200 or 400 µM) was applied to the wound. The rate of wound closure following this treatment and was profoundly accelerated, with less than 15% of arginine-supplemented wounds still open 12 d post-wounding compared to more than 40% of the control wounds. In order to understand the change in wound closure rates, tissue morphology was monitored by post-terminal tissue explantation and staining. Arginine-supplemented wounds contained basal keratinocytes that assumed a flatter, more cuboidal morphology as well as a thinner epidermis. Together, these morphological changes suggest that the proliferation and differentiation of keratinocytes is significantly impacted by the local application of arginine.
Therapeutic release of arginine from a traditional wound dressing has also been investigated as means for accelerating wound healing. While topical application of aqueous arginine represents a simple method of local delivery, other research has focused on the controlled delivery of l-arginine from hydrocolloid-based wound dressings. Encapsulation of l-arginine in a nanofibrous hydrocolloid was studied by Reesi et al. using a rodent wound-healing model.[70] Lignin nanofibers were surface modified with arginine via electrostatic interactions, which resulting in sustained release of arginine from the dressing. Nanofibers have become an attractive wound dressing material due to their high aspect ratio and three-dimensional structure that stimulate cell proliferation and skin regeneration, promote hemostasis, and absorb wound exudate.[71] The authors reported controlled release of arginine for >24 h after application. Arginine-releasing nanofibrous dressings were compared to both dressings without arginine and to aqueous solutions of arginine. The direct comparison to aqueous arginine was used to elucidate the impact of controlled release via the dressing. Therapeutics applied to incisional wounds were analyzed based on apparent re-epithelialization, collagen deposition, vascular formation, and wound closure rates. Arginine-releasing dressings were characterized as having increased re-epithelization, more extensive collagen deposition, and a greater number of new vessels than any of the control therapeutics. The most dramatic result came from the comparison of wound closure rates between arginine dressings, arginine solutions, and controls. While the control- and solution-treated wounds achieved closure levels of only 57.3±4.9 and 49.1±4.2%, respectively, treatment with sustained arginine release resulted in 93.2±3.7% closure as compared to day 0. This dramatic improvement in closure rates constitutes compelling evidence for the benefits of arginine release from a polymer dressing into a wound.
In summary, the use of arginine represents a potential therapy for treating chronic wounds due to its ability to increase NO concentrations in the wound area. Therapeutic arginine can be dosed through dietary supplementation, topical application, or controlled release from a wound dressing. As local, controlled delivery of any therapeutic is desired to achieve selective and effective treatment, wound dressings that controllably release arginine represent are a promising future direction for the field.
2.2. NOS Isozymes Regulation
Nitric oxide synthase isozymes are responsible for NO production in the body. In this manner, direct regulation of these enzymes may prove useful for therapeutic purposes. Schwentker et al. suggested that iNOS is the most efficacious choice among the nitric oxide synthase isozymes for use as a wound healing therapeutic.[72] Both eNOS and iNOS play important roles in the wound healing process: eNOS is expressed constitutively and iNOS synthesis is upregulated in times of tissue stress. Though eNOS and iNOS are both necessary and active in wound healing mechanisms, the moderate levels of NO generated by eNOS regulate inflammation whereas iNOS provides levels of NO that are large enough to eradicate acute infection-causing pathogens. Previous reports have described wound healing in iNOS-knockout mice and have found that cutaneous wound healing is severely impaired in the total absence of iNOS.[73] Other studies have conversely found that inhibition of nitric oxide synthase improves ischemia and reperfusion injuries.[74] These opposing results, which point to the concentration-dependent impact of NO, motivate the regulation of NOS enzymes as a potential therapy.
The possibility of delivering NOS to the wound site using a gene vector technique was reported by Yamasaki et al.[75] The severely compromised wound healing response in iNOS knockout mice was restored following a single application of an adenovirus gene vector containing human iNOS cDNA. In comparison to control wounds, wounds treated with the iNOS vector had increased nitrite concentrations and less time to complete wound closure (15 vs. 25 d). The authors chose iNOS over its constitutive isoforms because high-level NO generation catalyzed by iNOS allowed for application of a single topical dose rather than periodic reapplication. Despite the promising results of this study, no additional reports have appeared furthering the delivery of NOS genes for wound healing applications. In light of the considerable improvement in gene delivery methods in those intervening years, this iNOS delivery strategy merits careful reconsideration.
The utility of regulating iNOS expression in response to infections present in chronic wounds was explored by Karamercan et al.[76] The authors of this study were especially interested about large NO levels produced by iNOS during sepsis, whereby NO acts as an endotoxin. In order to reduce potential tissue damage at high NO concentrations, two iNOS inhibitors (N-nitro-l-arginine methyl ester or l-NAME and N5-(1-imino-methyl)-l-ornithine or l-NIO) were employed to reverse the impairment of wound healing by endotoxemia. Escherichia coli endotoxin was injected into the wounds, which was subsequently treated with containing 10 mg kg−1 l-NAME, l-NIO, or saline control. The extent of collagen and wound breaking strength were studied as a function of the specific treatment given. Endotoxin-infected wounds treated with both inhibitors displayed much higher collagen content than wounds treated with saline control. Further, mean tensile strength of the wounds also improved with the addition of the iNOS inhibitors, with the irreversible action of l-NIO actually displaying tensile strength that was not distinguishable from a non-infected healed wound, suggesting that iNOS inhibition, especially by l-NIO, restores the production of collagen in impaired wounds to normal levels.
However, Stallmeyer et al. reported an important caveat to the Karamercan sepsis study.[56] They found that l-NIO treatment in rats that were not septic experienced a severely impaired re-epithelization. In particular, the epithelia at the edges of the wound displayed an atrophied morphology, with lessened keratinocyte proliferation. It was concluded that iNOS is essential to re-epithelization and therefore critical to healthy wound healing. With respect to wound healing, underexpression of iNOS was determined to be as detrimental as uninhibited iNOS.
The ratio of eNOS to iNOS in a wound environment appears to be essential to the prognosis of wound healing. While inhibition of iNOS may improve healing outcomes in severely infected wounds, it is antagonistic to healing of non-infected chronic wounds. NOS regulation as a therapeutic for wound healing does not appear to be as broadly applicable as other NO-based therapeutic strategies.
3. Exogenous Nitric Oxide for Chronic Wound Therapy
3.1. Gaseous NO
A straightforward way to dose NO to a chronic wound is in its gaseous form. Applying high pressures of therapeutic gases, particularly oxygen, is commonly employed through hyperbaric therapy.[6] Hyperbaric oxygen therapy (HBOT) is a recommended therapy for treating chronic wounds because it counteracts tissue hypoxia. Nitric oxide can also be employed in hyperbaric therapy and has even been included in traditional HBOT as a mediator of the toxic effects of high pressures of oxygen.[6] As a gas, NO can be administered directly to a wound as the primary therapeutic agent without the need for any additional carrier species. The dose and duration of therapy is controllable through manipulation of the concentration and/or flow rate of the therapeutic gas.
Miller et al. reported the use of gaseous NO to address a chronic DFU that had persisted for more than 2 years.[77] For 14 consecutive days, the foot of the diabetic patient was continuously exposed to 200 ppm of NO (balance air) using a hyperbaric boot device. While the wound was initially badly infected and malodorous, steady and positive healing was quickly noted. At day 3, healthy granulation tissue was forming, and by day 14, the ulcer surface area was reduced by 70%. Six weeks after treatment, the wound was 90% healed, with 100% healing achieved at 26 weeks. While the healing timeline of this wound was still extended, the gaseous NO initiated healing of the wound that heretofore had not advanced for two years. Importantly, side effects or discomfort from this treatment were not reported.
This promising preliminary case study was supplemented by the analysis of the efficacy of gaseous NO in more statistically robust studies, at the cost of moving from a human subject to an animal model. Due to NO’s role as an antimicrobial agent, gaseous NO administration may reduce the influence that bacterial infection has on impaired healing. In terms of safety, Rezakhanlou et al. reported that gaseous NO could be safely dosed for 8 h at 5, 25, 75, and 200 ppm using a mouse lymphocyte model.[78] None of the studied concentrations of gaseous NO damaged the ECM and some even increased lymphocyte proliferation. Pressures greater than 200 ppm resulted in decreased cell viability and immune cell proliferation. These results imply that wound infections can be treated with NO at concentrations of 5–200 ppm. With particular interest in NO’s antibacterial action, Ghaffari et al. tested 8-h exposures of 200 ppm on rabbits with severe Staphylococcus aureus infected wounds.[79] While this treatment protocol did not impact re-epithelization or angiogenesis, it improved collagen deposition and wound breaking strength. Most promisingly, the bacterial loads of the wounds were reduced by one log after just 3 daily rounds of treatment. The reduced infection corresponded with a less severe immune response, an essential step in a healthy wound timeline progression. This study serves as evidence for gaseous NO as a potent antibacterial agent and its potential utility for treating chronic wounds.
Shekhter et al. employed higher concentrations of NO (up to 500 ppm) but for only 60-s once-daily for 6 d to mitigate toxicity concerns.[80] Even with an intermittent dosing mechanism, rats with both infected and clean wounds were characterized as having reduced tissue hypoxia and microbial infection, increased angiogenesis, significantly reduced recovery time, and improved tissue morphology. Analysis of the wound fluid suggested a mechanism for gaseous NO’s impact on wound healing. Specifically, the authors noted that exposure to gaseous NO increased the levels of peroxynitrite in the wound tissue. Peroxynitrite, a cytotoxic free radical, initiates mobilization of protective mechanisms, including NO production.[81,82] The authors established that treatment by gaseous NO is accompanied by enhanced levels of NO in tissue, that regulates the healing processes of impaired chronic wounds.
Gaseous NO as a therapeutic for non-healing wounds has also been explored in terms of antimicrobial action. Many chronic wounds enter into an impaired healing state, in part, because of concomitant infections. While NO has been shown to reduce the bacterial load in infected wounds, the direct impact of gaseous NO on inflammatory processes requires further elucidation. Currently, such impact has not been separated from the effect of lowered bacterial load. Understanding the role of gaseous NO therapy in the inflammatory response might allow for treatment of chronic wounds that are not simultaneously infected.
The therapeutic potential of gaseous NO is often limited to a hospital setting due to the hazards associated with pressurized NO cylinders and need for continuous oversight.[49,83] Additional concerns regarding for gaseous NO delivery include NO’s high reactivity, particularly with oxygen in the air to form harmful byproducts, such as nitrogen dioxide (NO2).[84] This reactivity often necessitates that gaseous NO therapy occur in anoxic environments, limiting the potential for therapeutic use outside of a hospital setting.[83] Both NO and NO2 pose serious systemic toxicity concerns when inhaled, starting at concentrations as low as 40 and 1.5 ppm, respectively.[85] Thus, significant safety protocols and monitoring are required for gaseous NO treatments of chronic wounds
3.2. Generation of Nitric Oxide by Acidified Nitrite
The reaction of nitrite with acid also results in NO production exogenously through a three-step reaction. First, nitrite reacts with free hydrogen cations to form nitrous acid (HNO2). Nitrous acid then decomposes to yield dinitrogen trioxide (N2O3), with water as a byproduct. Subsequently, N2O3 dissociates in aqueous environments to form equimolar amounts of nitrogen dioxide (NO2) and NO.[86] This facile generation of NO allows for localized treatment, with salts like sodium nitrite easily incorporated into creams and ointments. While nitrite applied by itself to a wound would interact with basal acidic species to produce NO, it is most effective to provide acidic co-reactants to assure complete conversion of nitrite to NO.[87] Two creams combined on the skin, one incorporating sodium nitrite and the other an acidic agent, usually citric or ascorbic acid, will immediately generate NO. The NO payloads are tuned by varying the concentration of sodium nitrite and acid in the respective creams.
In two studies, Zhu et al. demonstrated the use of acidified nitrite solutions to treat full thickness burn wounds in rats and mice.[88,89] In both cases, the wounds were non-chronic, but the experiments proved useful in understanding the effects of NO on general wound healing. For example, wound closure rates and re-epithelization were assessed upon applying two-part hydroxyethylcellulose-based gel was applied to burn wounds. The authors reported at least a 50% increase in the wound closure rate for all nitrite-treated wounds relative to controls. The concentration of NO in the wound fluid was measured electrochemically to couple results to therapeutic mechanism. Higher concentrations of NO (>5 mM) resulted in more beneficial outcomes. Furthermore, suspension of the treatment after 3 days resulted in loss of healing improvements. The authors claimed that NO-promoted growth factors (i.e., TGF-β1 and IL-8) stimulated inflammatory cell infiltration, which is essential for the removal of damaged tissue and pathogens. Together, the studies underlined the importance of quantifying NO in the wound to ensure proper therapeutic timeline and doses.
Of importance, the physiology of healthy wounds and chronic wounds is different. Chronic diabetic wounds are often so impaired that they have stopped progressing along a healing timeline. In these cases, any healing enhancement is considered a positive outcome. Diabetes-associated chronic wounds in mice were used by Weller et al. to study the effects of an acidified nitrite cream on wound closure.[90] Specifically, mice treated daily with an acidified nitrite cream 18 d post-wounding had greater than 90% closure of incisional wounds versus controls, which were only 60% healed (i.e., closed). The timing of the treatment protocol was found to be critical with the outcome, with cream applied on the day of wounding significantly inhibiting wound closure rates when compared to a placebo cream (51.9 vs. 80.6%, respectively). Creams applied beginning the third day after wounding achieved full wound closure over the same timeline, indicating that supplemental NO in diabetic models in the early stages of healing, hemostasis and early inflammation, may not be as helpful as in later stages, when NO deficiency can cause healing processes to stall.
Numerous examples have appeared in the literature describing the application of acidified nitrite creams to human wounds. The first such study commenced in 2004 and monitored 37 patients with Buruli ulcers (chronic ulcers contaminated with a severe mycobacterial infection).[91] Sodium nitrite/citric acid gels at two doses (6 and 9 wt% in aqueous cream) were applied daily to the Buruli ulcers for 6 weeks. Patients treated with the nitrite-based cream had dramatic improvement in healing outcomes. Ulcer size decreased by almost 56% when treated with the acidified nitrite gel, while the placebo group saw no change or even increases in ulcer size. This study produced preliminary evidence that treatment with acidified nitrite creams is both well tolerated and effective. Nevertheless, further work was needed to broaden the applicability of the therapeutic technique to other chronic wound types. Ormerod et al. used a similar nitrite cream to treat clinical wounds contaminated with methicillin-resistant S. aureus (MRSA).[92] Antibiotic-resistant bacteria that colonize a wound represent a formidable obstacle to wound healing, as traditional antibiotics fail to eradicate the entrenched bacteria. Wounds infected with MRSA are exceedingly unlikely to spontaneously heal or be impacted by existing pharmaceutical intervention.[93] Weller et al. reports that S. aureus is susceptible to the antimicrobial action of NO.[94] Additionally, initial NO resistance susceptibility testing indicate that the likelihood for resistance is low.[7,95–97] Ormerod et al. reported full eradiation of MRSA colonies with corresponding improvement in wound closure rates for 15 wounds across 8 patients receiving daily applications of 4.5 wt% nitrite cream over a 5-d period.[92] Though lower concentrations of nitrite were used in this study relative to that described in the aforementioned Buruli ulcer study (i.e., 6 wt% vs. 4.5 wt%), both found that acidified nitrite creams were effective in treating infected, non-healing wounds in humans. Nevertheless, this discrepancy suggests that the required NO dose using acidified nitrite may vary depending on the condition of the chronic wound.
While acidified nitrite is often applied suspended in ointment, Friedman and coworkers reported on a nitrite-containing hydrogel/glass composite nanoparticle (NO-np) system.[98] The particle backbone was composed of tetramethylorthosilicate (TMOS), polyethylene glycol (PEG), and chitosan. Nitric oxide, generated in the particle pores from the thermal reduction of nitrite by glucose, is released through hydration-mediated uncapping of the pores. The overall duration of release from the NO-np was roughly 24 h.[98] The authors reported the efficacy of such particles as a wound healing platform through a number of preclinical studies.[95,96,99,100] The biocidal action of the NO-np system was initially examined against MRSA- and Acinetobacter baumannii-infected wounds using mouse models.[95,96] In both instances, accelerated wound closure rates were reported with NO treatment. Mihu et al. reported complete closure of full-thickness infected wounds within 7–8 d as compared to control wounds, which healed only after 10–12 d.[96] Further analysis determined that the bacterial levels in each set of wounds was significantly reduced by the addition of NO-np. Increased collagen content was also reported for mice treated with NO-np, an early indicator of tissue remodeling (Figure 5).[95,96]
Figure 5.
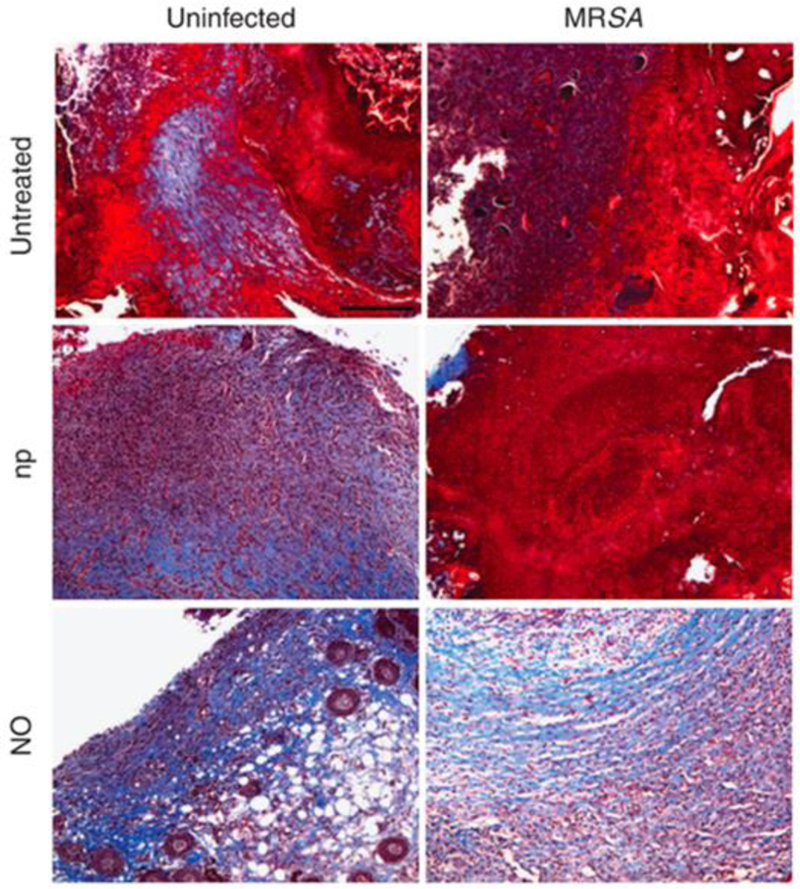
NO-nps decrease collagen degradation in skin lesions of BALB/c mice. Histological analysis of mice uninfected and untreated, uninfected treated with nanoparticles without nitric oxide (np), uninfected treated with NO-nps (NO), untreated MRSA-infected, np-treated MRSA-infected, and MRSA-infected treated with NO, after 7 d of treatment. Mice were infected with 107 bacterial cells. The blue stain indicates collagen.
Reproduced with permission.[95] Copyright 2009, Elsevier.
To explore the behavior of fibroblasts in response to NO-np therapy, an in vitro scratch assay was performed using human-sourced dermal fibroblasts.[98] Fibroblast migration was observed to accelerate when treated with NO-np relative to controls (non-NO-releasing nanoparticles) or those wounds not treated. More rapid migration of fibroblasts is expected to increase collagen formation, a primary function of this cell type. This in vitro assay potentially points to a biological mechanism for the increased of collagen content observed for NO-treated tissue. An additional study evaluated NO’s role in wound healing by applying the NO-np to non-infected mouse wounds.[100] Again, increased collagen content noted in the NO-np-treated mice. More macrophage-like cells were also observed in response to NO treatment, supporting the theory that NO promotes infiltration of inflammatory cells to the wound site. Treatment with NO-np also enhanced wound vascularization, resulting in greater tissue oxygenation, a factor in promoting wound healing.[100] By increasing fibroblast/macrophage migration and angiogenesis, wounds treated with acidified nitrite should heal faster than untreated wounds.
These studies show that nitrite-derived NO treatment is beneficial to both healthy and infected wounds. Research was further to determine whether these benefits also apply to a healing-impaired model using non-obese, diabetic, severe combined immunodeficiency (NOD-SCID) mice.[99] After 7 days of once daily treatment, the NO-np materials displayed led to wound healing. The NO-treated wounds had ~57% closure versus ~15% for controls. The authors concluded that treatment with the acidified nitrite-based NO-releasing nanoparticles was beneficial for healing of healthy, infected, and immunocompromised chronic wounds. Of note, these nanoparticles could also be used in combination with traditional wound treatments (e.g., wound dressings) for individuals struggling with non-healing diabetic wounds.
Certain side effects must be considered when using acidified nitrite-based treatments, as both sodium nitrite and acids can cause skin irritation. A dose-dependent relationship regarding adverse reactions (e.g., itching, pain), was reported by Ormerod et al. for acidified nitrite treatments.[101] Careful consideration should be made when developing nitrite creams in order to accelerate wound healing status without invoking undue skin irritation. Identifying the lowest effective doses of nitrite and acid will ensure maximal healing efficacy with minimal side effects. Finally, an inability to tune the NO-release kinetics of acidified nitrite systems undermines their therapeutic utility, as one cannot control the rate of NO delivery to the wound. Nevertheless, acidified nitrite, whether delivered through a two-part cream or a nanoparticle system, represents a simple, potentially clinically-viable method to improve the healing of impaired wounds.
3.3. Low Molecular Weight and Macromolecular NO Donors
A multitude of NO donor systems have been developed to store and spontaneously release NO. The vast majority of current wound treatment strategies employ one of two classes of donors: S-nitrosothiols and N-diazeniumdiolates. While both types of donors release NO under physiological conditions, each donor category has unique advantages with regards to the therapeutic release of NO.
S-nitrosothiols, referred to as RSNOs, are an endogenous class of molecules and also easily formed exogenously via nitrosation of primary thiol groups.[102] Nitric oxide stored on RSNOs is released by photothermal degradation via homolytic cleavage of the S-N bond, which liberates one mole of NO per thiol group. Additionally, copper(I) can cause the catalytic decomposition of RSNOs to release NO.[102,103] Due to low endogenous copper levels, photothermal decomposition is the primary trigger for NO release in biological systems.[104–106] These release triggers make it possible for release of NO under physiological conditions. Diverse NO-release kinetics are possible depending on the parent structure of the RSNO and the degree of exposure to external triggers.[102,103] Instability due to heat and light exposure may result in premature NO release, leading to concerns regarding storage of RSNO-based materials. Often, instability may be circumvented by storing the dry samples in dark, cold environments, though this obviously may reduce the viability of RSNO-based dressings in a clinical setting.
A second class of NO donors, diazeniumdiolates (NONOates), are often exploited to address RSNO storage concerns. Diazeniumdiolate NO donors can form on either carbon or nitrogen atoms, but N-diazeniumdiolates are the far more common system due to spontaneous release of NO under physiological conditions.[97,107] Briefly, N-diazeniumdiolates form on secondary amines through reaction with high pressures of gaseous NO under basic conditions. Proton-initiated release of NO occurs spontaneously in aqueous media at physiological pH.[107,108] The NO-release kinetics of NONOates are also controlled by environmental factors (e.g., pH, temperature) and the structure of the NO donor precursor (e.g., polyamines). For instance, the presence of protonated primary amines on the NO donor stabilize the negative charge of the NONOate, and the ensuing, more stable NONOate structure does not break down to NO as quickly, prolonging NO release.[108–110] Due to a unique NO-release mechanism, the storage of NONOates requires an anhydrous environment in order to preserve NO payloads.
While low molecular weight NO donors (<1 kDa) are capable of tunable NO storage and release, certain limitations have slowed their clinical use. Nitric oxide release from low molecular weight donors is often not as well controlled (i.e., rapid release of NO from precursor structure resulting in undesirable donor breakdown) and the precursors can be cytotoxic, particularly in the case of the amine-containing donors for N-diazeniumdiolates.[111,112] These consequences may be mitigated through the use of macromolecular scaffolds for NO delivery. Low molecular weight donors may be encapsulated within a scaffold (e.g., liposomes) or covalently bound to the scaffold structure (e.g., biopolymers, dendrimers, silica nanoparticles).[112] Modifying the scaffold (e.g., molecular weight, size/shape of nanoparticles) alters the NO-release properties (i.e., NO payload, half-life, and release duration) as well as the therapeutic utility (e.g., antibacterial efficacy, cytotoxicity). Utilization of macromolecular scaffolds thus allows for enhanced control over NO-release kinetics, with promising utility for enhancing wound healing. In addition to control over NO-release kinetics through the choice of NO donor or scaffold, encapsulation within a polymer matrix allows for precise control over the properties of the wound dressings. Factors such as hydrophobicity or porosity of the polymer may impact the kinetics of NO release and achievable NO payloads, affecting both antibacterial efficacy and wound healing. Balancing these factors, along with specific physical properties of the films (e.g., flexibility, wound exudate absorption), allows for the fine-tuned development of wound dressings. To date, NO-based treatment strategies for both diabetic and non-diabetic chronic wounds are being developed using low molecular weight donors (e.g., N-diazeniumdiolates and S-nitrosothiols) and macromolecular scaffolds.
3.3.1. S-Nitrosothiols NO Donors
S-nitrosothiols are the endogenous sink of NO in the body. The biocompatibility associated with this role motivates the use of RSNOs for therapeutic action. Li and Lee synthesized poly(vinyl pyrrolidone) (PVP) and poly(vinyl methyl ether-co-maleic anhydride) (PVMMA) co-polymers that gelled from the hydrogen bonding between the polymers. The PVMMA was directly modified with thiol-containing glutathione (GSH) or S-nitroso(Ɣ-Glu-Cys)5-Gly (PC5), precursors for RSNOs.[113] This PVP-PVMMA complex released NO under ambient light conditions for 10 d (total NO payload of ~45–80 nmol mg−1 polymer). A diabetic rat model was used to assess the wound healing properties of the dry powder. Transparent wound dressings were used to cover the wound and provide a physical barrier. Statistically significant improvements in wound closure rates were observed in rats treated on days 4, 7, and 10 with GSNO-PVMMA/PVP. Wound closure rates were less significant after day 10, coinciding with exhaustion of the NO supply and motivating the development of systems capable of extended NO release.[113] This approach is attractive because it is simple and easily incorporated with traditional wound dressings.
Covalent modification of a polymer with RSNO donors has also proved useful in controlling NO-release kinetics. Vogt et al. found that S-nitroso-N-acetylpenicillamine (SNAP), a small molecule NO donor, released NO in an extended fashion when covalently affixed to gelatin nanofibers.[114] As the primary mechanism of NO release from this RSNO was photolysis, the NO release was tunable by light exposure. Nitric oxide totals were tuned from 24–59 nmol mg−1 fibers upon illumination with 16–54 candela light. In vitro antibacterial assays with Staphylococcus aureus using the zone of inhibition assay indicated increased inhibition zones for SNAP-gelatin fibers relative to controls. Indeed, illumination of the SNAP-gelatin fibers with 54 candela facilitated complete bacterial inhibition. The light-dependent bacterial inhibition indicates that NO concentration is an essential consideration for NO-releasing wound dressings, as higher NO payloads demonstrate enhanced antibacterial efficacy. Of note, high NO payloads have been shown to be cytotoxic to healthy mammalian cells,[115] and thus additional studies are required to determine whether the illuminated NO-releasing SNAP-gelatin fibers are cytotoxic to fully ascertain their potential as wound dressings.
Small molecule RSNOs have also been encapsulated within polymeric films. Kim et al. reported on NO-releasing chitosan films doped with NO-modified glutathione (GSNO).[116] As chitosan itself has been described as an antibacterial material,[117,118] NO-releasing chitosan may prove superior for wound healing applications. In vitro analysis showed that treatment of S. aureus and Pseudomonas aeruginosa colonies with the GSNO-containing chitosan led to a one- and two-log reduction in bacterial load, respectively, versus chitosan control films.[116] A full-thickness (i.e., damage inflicted through the epidermis into subcutaneous tissue) rat wound model was used to evaluate the wound healing potential of the novel dressing. While wound size decreased with time with the control chitosan films, the use of GSNO-doped chitosan films accelerated wound healing to achieve nearly complete closure by day 15 (Figure 6).[116] Analysis of fully healed wounds revealed an enhanced formation of granulation tissue and increased collagen fiber content. In addition to bacterial inhibition, the results of this study indicate the benefits of NO-releasing chitosan films on tissue remodeling.
Figure 6.
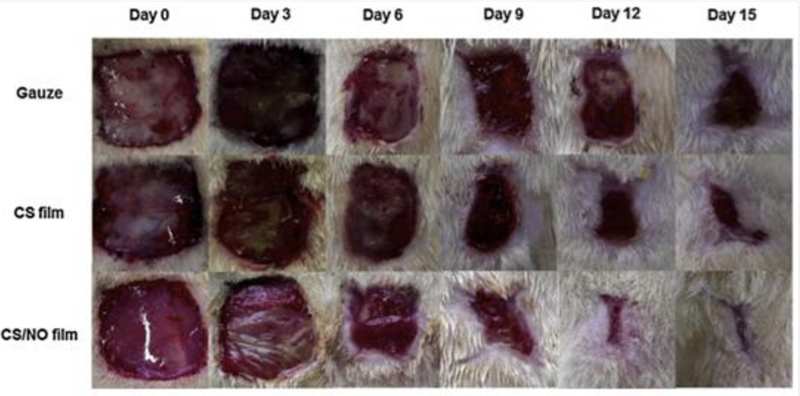
Representative photographs of Sprague-Dawley rat wounds treated with gauze controls, chitosan (CS) films, and NO-releasing chitosan (CS/NO) films for 15 d.
Reproduced with permission.[116] Copyright 2015, Elsevier.
Hydrogel-based wound treatments provide many benefits for wound healing, primarily in terms of wound hydration.[119] Due to this advantage, a number of small molecule RSNOs encapsulated in hydrogels have been developed and evaluated for wound healing benefits. De Oliveira and coworkers prepared Pluronic-F127 hydrogels that encapsulated two low molecular weight RSNOs: S-nitrosoglutathione (GSNO) and S-nitroso-N-acetylcysteine (SNAC).[120] Nitric oxide release through photothermal decomposition was stabilized by the hydrogel matrix, resulting in extended NO release and a half-life of greater than 3 h. Initial in vivo studies in which the hydrogels were applied to human forearms confirmed that elevated dermal blood flow persisted for at least 3 h after hydrogel application.[121] In this manner, topical application of NO can is useful for increasing blood flow near the wound site and providing a potential benefit towards wound healing. As complications of diabetes include poor blood flow, the authors subsequently focused on diabetic wounds in streptozotocin-induced diabetic rats.[122] Two doses of GSNO (23 and 230 mM) loaded hydrogels were applied to the skin of healthy and diabetic rats. The hydrogel of greater GSNO concentration (230 mM) doubled the perfusion in both normal and diabetic rats. In contrast, the hydrogel containing the lower GSNO dose (23 mM) did not impact blood flow, suggesting a concentration threshold for NO to promote perfusion. Of importance, no systemic effects (e.g., changes in systolic blood pressure or heart rate) were reported, suggesting the safety and tolerability of this treatment. Increased localized tissue perfusion with the larger NO dose suggests high potential utility, but further work is required to determine appropriate dosing protocols. In another study, the NO-releasing wound dressings were applied to excisional wounds using a healthy rat model.[123] The GSNO-loaded hydrogels were applied over varying dosage schedules for 8 d following wounding. Wounded rats had the best healing outcomes when treated with NO-releasing hydrogels over a period corresponding with both the inflammatory and proliferative phases rather than during either of those phases alone. At day 14, only 16.6% of the wounds treated during just one phase were completely healed, whereas 50% of the wounds treated over both phases healed fully. Application of NO through both inflammatory and proliferative phases appears more likely to benefit overall wound healing in terms of enhanced re-epithelialization, collagen fiber maturation, and tissue granulation.
More recently, de Oliveira and coworkers reported on the combined use of NO-releasing hydrogels and NO-releasing film dressings on mouse excisional wounds during the initial stages of healing.[124] Following their previous study, they applied either control or GSNO-doped Pluronic-F127 hydrogel to a wound bed. A polymer film containing RSNO-functionalized poly(vinyl alcohol) (PVA) was applied on top of the hydrogel as a wound dressing. Using real-time NO measurements, the authors determined that the underlying hydrogel served to modulate the NO release from the PVA film, lessening the burst release and concomitantly extending the overall NO-release duration (>24 h). Together, the combination of the hydrogel and the film outperformed either material on its own in terms of the enhanced wound contraction, increased collagen deposition, and an accelerated inflammatory stage. The authors attributed the increased wound-healing activity to the controlled manner in which the combined materials released NO. A slow, prolonged NO-release profile appeared to be most advantageous for improving wound-healing outcomes.
3.3.2. N-Diazeniumdiolates NO Donors
Relative to their S-nitrosothiol counterparts, N-diazeniumdiolates (NONOates) are more often utilized for NO-based wound healing treatments because of their ability to spontaneously release NO in aqueous media. Both low molecular weight NONOates and NONOate-modified macromolecular scaffolds have been investigated through a direct application and in conjunction with current wound treatments, such as wound dressings. For example, Dashti et al. described the in vivo wound healing efficacy of low molecular weight donor NONOate-modified diethylenetriamine (DETA/NO). As a powder, DETA/NO was applied to full-thickness wounds in streptozotocin-induced diabetic rats to monitor any effects over a 21-d period.[26] Wound closure rates served as the primary measurement of wound healing. The application of DETA/NO led to accelerated wound closure rates in the diabetic rats.[26] Increased concentrations of nitrate in the rat’s urine provided evidence, albeit indirect, that DETA/NO delivered NO to the wound site. Due to the indirect nature of this method, however, the influence of NO-release kinetics remains nebulous. Follow-on studies examining whether direct application of the NO donor is cytotoxic to surrounding tissue remain necessary, as small molecule NO donors have been found to be cytotoxic due to their potential decomposition to carcinogenic nitrosamines.[83,111,125] Macromolecular scaffolds tend to be less toxic as the propensity to be phagocytosed is lower. In addition, such materials facilitate additional chemical modifications that may promote tissue integration. The cytotoxicity concerns for low molecular weight NONOate-based therapeutics can be mitigated by covalently binding the N-diazeniumdiolate NO donors to the macromolecular scaffold. Hetrick et al. first compared the antibacterial efficacy of low molecular weight NONOates (e.g., PROLI/NO) to macromolecular scaffolds (e.g., silica nanoparticles).[111] The authors confirmed that at the respective bactericidal doses, the NO-releasing silica nanoparticles were significantly less toxic than PROLI/NO, indicating the benefits of macromolecular NO release for mitigating cytotoxicity.
Hetrick et al. also reported on the role of NO-release kinetics on bactericidal action by comparing N-methylaminopropyltrimethoxysilane (MAP3)- and N-(6-aminohexyl)aminopropyltrimethoxysilane (AHAP3)-modified silica nanoparticles against bacteria biofilms common to wounds (i.e., P. aeruginosa, S. aureus, Escherichia coli, Staphylococcus epidermidis, and Candida albicans). The NO-release half-lives of the two particle systems were ~6 and 18 min, respectively. Faster NO-releasing MAP3-modified particles were ~1000 times more efficacious than AHAP3-modified particles with respect to biocidal efficacy. With MAP3-modified silica nanoparticles, biofilms from the five tested pathogens were reduced by at least 99%, with Gram-negative E. coli and P. aeruginosa reduced by 99.999%.[126] However, even with the use of a nontoxic macromolecular scaffold, considerable cytotoxicity toward L929 mouse fibroblasts exposed to the NO-releasing nanoparticles was observed, with toxicity comparable with commercial antiseptics (i.e., povidone iodine and chlorhexidine). These materials will only represent a viable chronic wound treatment option when their toxicity towards healthy mammalian cells has been addressed.
Shabani et al. reported on a NO-releasing polymer whereby the NONOate group was incorporated within a polyethyleneimine cellulose backbone (PEIC/NO) to demonstrate the utility of covalently binding the NONOates to macromolecular scaffolds.[127] Repeated application of the PEIC/NO to full-thickness dermal models (rat model) enhanced wound closure rates relative to controls. The authors further analyzed the impact of these donors on systemic blood pressure. A two NO donor comparison study of PEIC/NO and DETA/NO (half-lives of ~16 and 20 h, respectively) demonstrated a more severe decrease in systolic blood pressure for DETA/NO compared to PEIC/NO treatments. However, the mildly depressed systolic pressure when using PEIC/NO returned to normal levels within approximately 3 h. In contrast, the topical application of DETA/NO led to a significant decrease in systolic pressure that lasted for >6 h.[127] The authors attributed this prolonged hypotension to the greater NO storage characteristic of DETA/NO, implying that the NO payload delivered to the wound plays a large role in the efficacy and impact of an NO-based wound healing treatment. A follow-on study by the same group investigated the effects of a similar polymer backbone, linear polyethyleneimine (l-PEI/NO), on wound repair in aged rats with impaired wound healing.[128] Surprisingly, neither l-PEI nor l-PEI/NO promoted wound healing. In addition to l-PEI’s inherent toxicity at high doses, the addition of NO-release led to clotting and impaired collagen organization. The prolonged, high concentrations of NO delivered from this system at the studied doses also led to adverse tissue responses,[128] reaffirming the need to tune NO payload for a given application.
Different NO-release kinetics from polyethyleneimine (PEI) were reported by Nurhasni et al. via poly(lactic-co-glycolic acid) (PLGA)-coated NONOate-functionalized PEI nanoparticles.[129] Sustained NO release (>6 d) was observed and attributed to less water-induced breakdown of the NONOate (to NO). The NO-releasing PLGA/PEI particles demonstated potent antibacterial action against both MRSA and P. aeruginosa (~99.99% killing), with low cytotoxicity to mammalian cells (>80% cell viability) at particle concentrations as low as 5 mg mL−1. This wound healing therapeutic was studied in vivo using a full-thickness mouse wound model following application of a single treatment 1 d post-wounding. Compared to controls, the PLGA/PEI particles significantly reduced wound area by day 7 (75% smaller), promoting skin structure resembling a healthy epidermis. Together, the studies give evidence that sustained, low doses of NO may be more effective at marshalling wound healing than a high NO flux via burst release.
Though direct application of NO-releasing materials to a wound clearly affects wound healing, NO-based wound treatment strategies must translate to clinical scenarios in order to be relevant.[130] In this regard, both low molecular weight NO donors and NO-donor-modified macromolecular scaffolds have been incorporated within polymeric wound dressings to exploit the potential therapeutic utility of NO release. For example, polymeric films doped with low molecular weight NO donors were reported by Brisbois et al. in a study that evaluated the wound healing potential of polyurethane films doped with dibutylhexanediamine (DBHD/N2O2) and PLGA.[131] The two-layer arrangement consisted of an active polyurethane layer containing DBHD/N2O2 and PLGA, surrounded by a non-NO-releasing topcoat prepared with the same polyurethane composition. Nitric oxide-release kinetics were dependent on the hydrophobicity of the polyurethane (i.e., water uptake of 6.2 ± 0.7 versus 22.5 ± 1.1 wt% for polyurethanes SG-80A and SP-60D-20, respectively), as increasing hydrophilicity resulted in greater water diffusion into the film to liberate NO. SG-80A polyurethane, with an NO flux of 12.8–14.5 × 10−10 mol cm−2 min−1 for ~24 h, was chosen for in vivo testing. The infection model consisted of exposing mouse burn wounds to Acinetobacter baumannii. Infected wounds treated with the NO-releasing polyurethane exhibited a ~4 log reduction in bacterial counts compared to controls.[131] The benefit of encapsulating a low molecular weight NO donor within a polyurethane matrix lies in the direct applicability to a nearly any commercially available wound dressing.
While polymeric films represent a diverse class of wound dressings, the use of fibers has additional advantages, particularly for wound healing applications. Polymeric fibers are characterized by increased surface area per volume compared to films. Furthermore, the nanofibrous matrix of the fibers mimics the ECM, providing a three-dimensional scaffold for the migration of inflammatory cells.[132,133] An increase in wound dressing porosity has been shown to facilitate more oxygen and moisture permeation.[134] It has also been reported that the hydration (i.e., water absorption) of electrospun fibers is 10–100 times greater than analogue film dressings.[132,133,135] Drawbacks to using fibers as NO-releasing wound dressings include potential undesirable leaching of the NO-modified materials, hastened NO-release kinetics due to increased water absorption, and a lower volume capacity, leading to decreased NO payloads.[136]
The design of NO-releasing electrospun polymer fibers containing both low molecular weight NO donors and NO donor-modified macromolecular scaffolds was first investigated by Coneski et al.[137] Briefly, PROLI/NO, a fast-releasing low molecular weight NO donor with a half-life of ~2 s, was doped into polyurethane and poly(vinyl chloride) fibers doped. The encapsulation of PROLI/NO within the polymer matrix slowed the rate of NO release, increasing the NO release half-lives by up to 200 times depending on fiber composition and resulting hydrophilicity. Koh et al. synthesized amine-functionalized NO-releasing silica nanoparticles doped into electrospun polyurethane fibers for application to wounds dressings. The resulting NO payloads for the silica-doped fibers ranged from 7.5–120 nmol mg−1.[136] While tunable NO-release kinetics were achieved by varying NO donor type, polyurethane hydrophobicity, and dopant concentrations, the overall NO payloads were low relative to fiber mass. Additionally, particles modified with AHAP3 NO donor-modified particles leached high levels of silica, limiting the therapeutic utility of the NO-releasing fibers. Worley et al. subsequently developed dual-action antibacterial polyurethane fibers containing biocidal quaternary ammonium and NO-releasing poly(amidoamine) (PAMAM) dendrimers. Using two biocides improved the antibacterial efficacy and reduced the risk of resistance by way of multiple mechanistic bacterial eradication pathways.[138] The authors reported a 4-log reduction in bacterial viability for both Gram-negative (E. coli and P. aeruginosa) and Gram-positive (S. aureus and methicillin-resistant S. aureus) bacteria. Minimal cytotoxicity (<20% reduction in viability) to L929 mouse fibroblasts was observed, further supporting the therapeutic utility of these wound dressings. Of note, none of these materials have been evaluated preclinically to date, and thus their therapeutic utility has not yet been determined. Nevertheless, the promising nature of their controllable NO-release kinetics and the prior literature demonstrating the benefits of NO suggest they could improve healing outcomes, especially given their efficient NO-delivery and minimal toxicity.
Nitric oxide-releasing electrospun polymer fibers can also be prepared using acrylonitrile (AN)-based terpolymers covalently modified with NONOates.[139] Lowe et al. reported a similar extended release duration (~7–14 d) of similar NO payloads to that achieved by Koh et al. (i.e., 79 nmol mg−1 fibers).[136,139] While low NO loading is still prevalent in these fibers, covalent bonding of the NONOates to the polymer backbone mitigates the concerns associated with NO donor or macromolecular scaffold leaching. These polymer fibers were evaluated using an in vivo excisional wound mouse model to monitor the effects of NO on wound closure and regulation of endogenous NO-inducible genes.[139] On day 14, the wounds treated with NO displayed more significant wound closure relative to controls. In addition, 60% of the NO-treated wounds displayed a greater capillary density than controls (Figure 7).[139] Upregulation of NO-induced gene expression was also observed for three genes (i.e., JunB, cFOS, and eNOS) within 30 min of treatment. The analysis of wound healing by monitoring NO-induced gene expression and angiogenesis provides further quantitative evidence of the benefits NO release may have on wound healing outcomes.
Figure 7.
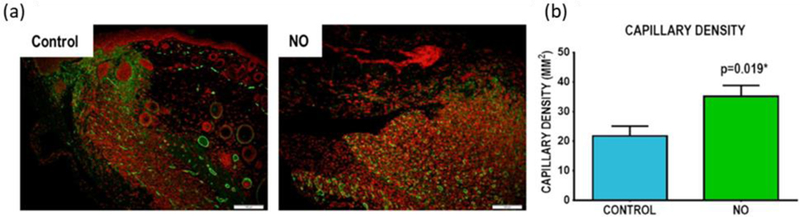
Histological analyses of SKH-1 mouse wound beds with daily administration of NO-releasing dressings. (a) Representative histology images of α-SMA IHC staining (capillaries stained red). (b) Average capillary density for control and NO-treated wounds.
Reproduced with permission.[139] Copyright 2015, Elsevier.
Beyond films and fibers, NONOate-based wound therapies have also employed hydrogels to create ointments and dressings. Kang et al. described an NO-releasing ointment that combined NONOate-functionalized PEI with Pluronic-F127 in a PEG hydrogel matrix.[140] While the NONOates released NO for >4 h, the PEG matrix maintained a moist environment necessary for wound healing. When applied daily to a full-thickness mouse wound model for 13 d, this NO-releasing ointment resulted in advanced re-epithelization, formation of granulation tissue, improved angiogenesis, and near complete wound closure. By incorporating the NO-releasing polymer within the moisture-retaining hydrogel matrix, the authors were able to demonstrate accelerated wound-healing activity, especially during the proliferative phase of healing.
Masters et al. reported the benefits of covalent coupling a NO donor to a poly(vinyl alcohol) (PVA) hydrogels.[130] The authors modified the PVA hydrogels with amines to facilitate subsequent NO donor loading, with two formulations giving total NO payloads of 0.06 and 0.48 µmol g−1 hydrogel. In vitro cytotoxicity studies using fibroblasts revealed that the hydrogels were non-toxic (>90% cell viability). The utility of the dressings was then tested in vivo using a genetically-induced diabetic mouse model. The application of NO to the wounds led to a significant increase in collagen; however, this study also noted a concentration dependence. The increased NO concentration provided by the greater payload system facilitated an increase in collagen production. Additionally, granulation tissue was found to be thicker in NO-treated wounds, indicative of increased mechanical stability. As impaired diabetic wound healing is characterized by decreased fibroblast proliferation, the increase in collagen production and wound breaking strength upon NO treatment suggest high clinical potential to improve wound healing.
3.3.3. Zeolites
While the vast majority of NO-donor-based wound treatment strategies employ the use of N-diazeniumdiolates or S-nitrosothiols, alternative chemistries do exist. For example, Neidrauer et al. developed a topical ointment containing NO-loaded zeolites.[141] Rather than relying on covalent binding of NO to a donor, zeolites are porous structures that retain gaseous NO through chemisorption. Displacement of the gas by water leads to the spontaneous release of NO in aqueous conditions. The antibacterial action of a NO-releasing zeolite-based ointment was exceptional, yielding at least 5-log reductions of E. coli, A. baumannii, S. epidermidis, and MRSA and a 3-log reduction against a fungal species C. albicans after exposure an 8-h test period. Unfortunately, cytotoxicity studies indicated that only ~33% of fibroblast cells were viable after treatment for 24 h. While similar results are observed with commercial antiseptics (e.g., povidone, iodine), the cytotoxicity elicited by the zeolites suggest poor utility for wound healing applications. Nevertheless, the ointment was tested using obese rats with full-thickness wounds. Healing rates were determined to be faster for NO-treated wounds compared to wounds treated with a control ointment (15.1 and 11.7% d−1, respectively). Despite the earlier noted cytotoxicity, adverse side-effects were not observed in the in vivo study.
4. Perspectives on NO Therapy for Wound Healing
4.1. Kinetic Dependence of Nitric Oxide
The field’s knowledge of the biological activity of NO related to wound healing is still growing. The healing result of any NO-based wound therapy appears to be highly dependent on NO payload and delivery duration stemming from the concentration-dependent nature of NO on physiological processes. While micromolar concentrations of NO are associated with pathogen clearance and pro-inflammatory signaling, picomolar concentrations of NO stimulate tissue regeneration and the resolution of the healing process. The kinetic dependence of NO’s antimicrobial action has been demonstrated in several studies,[142,143] albeit this body of work is contradictory. Additionally, both increased[88,89,116] and decreased[123,144] inflammatory cell counts have been reported for materials with similar NO-release properties. A more definitive evaluation of NO flux on inflammatory cell count and other markers of wound healing progression is needed to understand whether these opposing results are a result NO release kinetics or other factors (e.g., NO donor precursor and/or the polymeric backbone).
Accurate measurement and reporting of relevant NO kinetic parameters is also essential for truly understanding both results and how best to implement a given NO-based therapeutic. While measurement of NO is difficult, even in vitro, because it is a highly reactive species that rapidly degrades to nitrite in oxygenated media (i.e., half-life of NO is <2 s)[37], accurate methods for measuring NO quickly do exist, including chemiluminescence, colorimetric assays, and electrochemistry.[145] Further work is required to enable the use of these analytical tools in more complex settings (i.e., in vivo models), where NO release could be measured alongside of assessment of wound healing efficacy.[88,89] Methods that facilitate kinetic profiling of NO release, both in vitro and in vivo, would enable defining important kinetic parameters, including normalized NO payload, half-life, and total duration of release. Such ideas are not new; Hunter et al. reported the need for accurate and normalized NO measurement in complex media.[146] Standard reporting of kinetic parameters would facilitate easier comparison of NO donor systems/materials developed in different labs, but also more definitive conclusions about the ideal NO-release kinetic profile for a given indication (i.e., wound healing). As our understanding of the biological role of NO in wound healing continues to expand, more accurate quantitation of NO is absolutely necessary to fully harness it as a therapeutic.
4.2. Choice of Chronic Wound Model
In addition to careful monitoring and reporting of NO-release kinetics, the use of appropriate and established preclinical models is essential for assessing the therapeutic to ensure translation to the desired host (i.e., humans). Wound healing is a complex process consisting of overlapping physiological phases. The extent and nature of healing, therefore, cannot be properly evaluated by a single assay or test. Rather, a diverse set of assays should be performed using a given test material, and then evaluated using an in vivo model. Nearly all of the materials/strategies described in this review were tested using only rodent models with acute wounds. Of course, the healing processes between mice/rats and humans differ in significant ways. While mice and rat wounds undergo healing by contracture of the wound edges, human wounds heal by re-epithelization.[147] Such a difference calls into question the conclusions made about wound healing efficacy for materials only assessed in a rodent model. Swine represent the gold standard animal model for predicting human wound healing.[148] Like humans, pigs heal through re-epithelization and have similar responses to growth factors, distinct from small mammals (Figure 8).[148] In a review of animal studies, Sullivan et al. reported the efficacy of materials tested on pigs had a 78% percent concordance with human studies, while small mammal studies had only 53% concordance.[148] In vitro studies were characterized by 57% concordance, which suggests, surprisingly, that studies done in vitro have an equal, or even slightly greater, propensity to predict response in humans as in vivo work carried out in rodent models. Indeed, no animal model will ever fully replicate human wound healing. Thus, it is important to select models that come the closest to doing so prior to clinical trials. Evidence suggests that a shift to pigs as the animal model of choice for wound healing studies would yield more convincing and translatable results.
Figure 8.
Comparison of histological features from swine and human skin. H&E stained section taken through comparable portions of the dermis in both tissues.
Reproduced with permission.[148] Copyright 2001, Wiley-VCH.
Upon selecting an appropriate animal model to evaluate a potential wound healing therapy, additional steps must be taken to replicate a chronic wound environment. As discussed in this paper (Section 1.3), chronic and healthy wounds differ in important ways. Indeed, chronic wounds have been characterized as having lower levels of oxygen and NO, prolonged inflammatory response, fewer growth factors, and increased susceptibility to infection.[8] Testing a chronic wound therapeutic in a healthy wound model fails to take into account these vital differences. Many models have been developed to mimic the conditions of a chronic wound in animals. Most commonly, type 1 and type 2 diabetes are genetically induced in rodents.[149] Type 1 diabetes is also modeled in swine by dosing with alloxan or streptozocin, resulting in pancreatic β-cell destruction.[150] Additional casual factors for chronic wounds (e.g., obesity, infection) can also be replicated in pre-clinical wound models, depending on the therapeutic target of a given wound healing therapy.[151,152] Again, the complexity of a chronic wound is difficult to fully recapitulate in a single animal model, but careful consideration must be taken in the choice of model for a given wound healing therapeutic.
Acknowledgements
Financial support was provided by the National Institutes of Health (DK108318).
Author Biographies

Maggie J. Malone-Povolny is a graduate student in the Department of Chemistry at the University of North Carolina at Chapel Hill under the direction of Prof. Mark H. Schoenfisch. Her research concerns the use of nitric oxide to extend the lifetime of continuous glucose biosensors. She holds a B.S. in Chemistry from the University of St. Thomas.

Sara E. Maloney received her B.S. in Chemistry from Towson University. She is working toward her Ph.D. in Chemistry under the guidance of Prof. Mark H. Schoenfisch at the University of North Carolina at Chapel Hill. Her research interests include developing nitric oxide-releasing biopolymers for wound healing applications.

Mark H. Schoenfisch is the Peter A. Ornstein Distinguished Professor of Chemistry in the Department of Chemistry and Eshelman School of Pharmacy, Division of Pharmacoengineering and Molecular Pharmaceutics. His research intersts include analytical sensors, biomaterials, and the nitric oxide-release therapeutics.
Footnotes
Conflicts of Interest:
The authors declare the following competing financial interest(s): Mark H. Schoenfisch is a co-founder and maintains a financial interest in Novan, Inc. and Vast Therapeutics, Inc. Both companies commercialize macromolecular nitric oxide storage and release vehicles for clinical indications.
References:
- [1].Guo S, DiPietro LA, J. Dent. Res. 2010, 89, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bjarnsholt T, Kirketerp-Møller K, Jensen PØ, Madsen KG, Phipps R, Krogfelt K, Høiby N, Givskov M, Wound Repair Regen. 2008, 16, 2. [DOI] [PubMed] [Google Scholar]
- [3].Richmond N, Maderal A, Vivas A, Dermatol. Ther. 2013, 26, 187. [DOI] [PubMed] [Google Scholar]
- [4].Blakytny R, Jude E, Diabet. Med. 2006, 23, 594. [DOI] [PubMed] [Google Scholar]
- [5].Powers JG, Higham C, Broussard K, Phillips TJ, J. Am. Acad. Dermatol. 2016, 74, 607. [DOI] [PubMed] [Google Scholar]
- [6].Mathieu D, Handbook on Hyperbaric Medicine, Springer, 2006. [Google Scholar]
- [7].Smith AW, Adv. Drug Deliv. Rev. 2005, 57, 1539. [DOI] [PubMed] [Google Scholar]
- [8].Mast BA, Schultz GS, Wound Repair Regen. 1996, 4, 411. [DOI] [PubMed] [Google Scholar]
- [9].Campos ACL, Groth AK, Branco AB, Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 281. [DOI] [PubMed] [Google Scholar]
- [10].Han G, Ceilley R, Adv. Ther. 2017, 34, 599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stechmiller JK, Childress B, Cowan L, Nutr. Clin. Pract. 2005, 20, 52. [DOI] [PubMed] [Google Scholar]
- [12].Castilla DM, Liu Z-J, Velazquez OC, Adv. Wound Care 2012, 1, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tandara AA, Mustoe TA, World J. Surg. 2004, 28, 294. [DOI] [PubMed] [Google Scholar]
- [14].Olokoba AB, Obateru OA, Olokoba LB, Oman Med. J. 2012, 27, 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bansal S, Siddarth M, Chawla D, Banerjee BD, Madhu SV, Tripathi AK, Mol. Cell. Biochem. 2012, 361, 289. [DOI] [PubMed] [Google Scholar]
- [16].Trengove MK, Stacey MC, McCauley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G, Wound Repair Regen. 1999, 7, 442. [DOI] [PubMed] [Google Scholar]
- [17].Wysocki AB, Staiano-Coico L, Grinnell F, J. Invest. Dermatol. 1993, 101, 64. [DOI] [PubMed] [Google Scholar]
- [18].Galkowska H, Wojewodzka U, Olszewski WL, Wound Repair Regen. 2006, 14, 558. [DOI] [PubMed] [Google Scholar]
- [19].Tonnesen MG, Feng X, Clark RAF, Investig J. Dermatology Symp. Proc. 2000, 5, 40. [DOI] [PubMed] [Google Scholar]
- [20].Hirsch T, Spielmann M, Zuhaili B, Koehler T, Fossum M, Steinau HU, Yao F, Steinstraesser L, Onderdonk AB, Eriksson E, BMC Surg. 2008, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brem H, Tomic-Canic M, J. Clin. Invest. 2007, 117, 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Reiber GE, Boyko EJ, Smith DG, in Evid. Base Diabetes Care, 1995, pp. 409–428.
- [23].Valencia IC, Falabella A, Kirsner RS, Eaglstein WH, J. Am. Acad. Dermatol. 2001, 44, 401. [DOI] [PubMed] [Google Scholar]
- [24].Bryant RA, Acute and Chronic Wounds: Nursing Management, 2000.
- [25].Fahey TJ, Sadaty A, Jones WG, Barber A, Smoller B, Shires GT, J. Surg. Res. 1991, 50, 308. [DOI] [PubMed] [Google Scholar]
- [26].Dashti N, Ansari M, Shabani M, Vardasti S, Mirsalehian A, Noori Mughehi MH, Hatmi ZN, Iran. J. Public Health 2003, 32, 59. [Google Scholar]
- [27].Bredt DS, Snyder SH, Proc. Natl. Acad. Sci. U. S. A. 1990, 87, 682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Knowles RG, Moncada S, Biochem. J. 1994, 298, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Förstermann U, Sessa WC, Eur. Heart J. 2012, 33, 829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Donnini S, Ziche M, Antioxidants Redox Signal. 2002, 4, 817. [DOI] [PubMed] [Google Scholar]
- [31].Nathan CF, Hibbs JB, Curr. Opin. Immunol. 1991, 3, 65. [DOI] [PubMed] [Google Scholar]
- [32].Efron DT, Most D, Barbul A, Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 197. [DOI] [PubMed] [Google Scholar]
- [33].Loscalzo J, Freedman J, Inbal A, Keaney JF, Michelson AD, Vita JA, Trans. Am. Clin. Climatol. Assoc. 2000, 111, 158. [PMC free article] [PubMed] [Google Scholar]
- [34].Freedman JE, Loscalzo J, J. Thromb. Haemost. 2003, 1, 1183. [DOI] [PubMed] [Google Scholar]
- [35].Kobayashi Y, J. Leukoc. Biol. 2010, 88, 1157. [DOI] [PubMed] [Google Scholar]
- [36].Ajuebor MN, Virág L, Flower RJ, Perretti M, Szabó C, Immunology 1998, 95, 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thomas DD, Ridnour LA, Isenberg JS, Flores-Santana W, Switzer CH, Donzelli S, Hussain P, Vecoli C, Paolocci N, Ambs S, et al. , Free Radic. Biol. Med. 2008, 45, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nolan S, Dixon R, Norman K, Hellewell P, Ridger V, Am. J. Pathol. 2008, 172, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Andonegui G, Kubes P, in Nitric Oxide Inflamm., 2001, pp. 117–118.
- [40].Vinik AI, Maser RE, Mitchell BD, Freeman R, Diabetes Care 2003, 26, 1553. [DOI] [PubMed] [Google Scholar]
- [41].Wink DA, Mitchell JB, Free Radic. Biol. Med. 1998, 25, 434. [DOI] [PubMed] [Google Scholar]
- [42].Fang FC, Nat. Rev. Microbiol. 2004, 2, 820. [DOI] [PubMed] [Google Scholar]
- [43].Möller MN, Li Q, Lancaster JR, Denicola A, IUBMB Life 2007, 59, 243. [DOI] [PubMed] [Google Scholar]
- [44].Fang FC, MBio 2011, 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Barraud N, Kelso M, Rice S, Kjelleberg S, Curr. Pharm. Des. 2014, 21, 31. [DOI] [PubMed] [Google Scholar]
- [46].Römling U, Balsalobre C, J. Intern. Med. 2012, 272, 541. [DOI] [PubMed] [Google Scholar]
- [47].Hossain S, Nisbett L-M, Boon EM, Acc. Chem. Res. 2017, 50, 1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Neufeld BH, Reynolds MM, Biointerphases 2016, 11, 1. [DOI] [PubMed] [Google Scholar]
- [49].Schairer DO, Chouake JS, Nosanchuk JD, Friedman AJ, Virulence 2012, 3, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Witte MB, Kiyama T, Barbul A, Br. J. Surg. 2002, 89, 1594. [DOI] [PubMed] [Google Scholar]
- [51].Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M, J. Clin. Invest. 2001, 108, 1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shimizu K, Naito S, Urata Y, Sekine I, Kondo T, Katayama I, Br. J. Dermatol. 1998, 138, 769. [DOI] [PubMed] [Google Scholar]
- [53].Kreuger MR, Tames DR, Mariano M, Immunology 1998, 95, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Arany I, Brysk MM, Brysk H, Tyring SK, Biochem. Biophys. Res. Commun. 1996, 220, 618. [DOI] [PubMed] [Google Scholar]
- [55].Krischel V, Bruch-Gerharz D, Suschek C, Kröncke KD, Ruzicka T, Kolb-Bachofen V, J. Invest. Dermatol. 1998, 111, 286. [DOI] [PubMed] [Google Scholar]
- [56].Stallmeyer B, Kämpfer H, Kolb N, Pfeilschifter J, Frank S, J. Invest. Dermatol. 1999, 113, 1090. [DOI] [PubMed] [Google Scholar]
- [57].Schäffer MR, Tantry U, Efron PA, Ahrendt GM, Thornton FJ, Barbul A, Surgery 1997, 121, 513. [DOI] [PubMed] [Google Scholar]
- [58].Boykin JV, Wound J, Ostomy Cont. Nurs. 2010, 37, 25. [DOI] [PubMed] [Google Scholar]
- [59].Preiser J-C, Reper P, Vlasselaer D, Vray B, Zhang H, Metz G, Vanderkelen A, Vincent J-L, J. Trauma Inj. Infect. Crit. Care 1996, 40, 368. [DOI] [PubMed] [Google Scholar]
- [60].Hibbs JB, Taintor RR, Vavrin Z, Science (80-. ). 1987, 235, 473. [DOI] [PubMed] [Google Scholar]
- [61].Zaloga GP, Siddiqui R, Terry C, Marik PE, Nutr. Clin. Pract. 2004, 19, 201. [DOI] [PubMed] [Google Scholar]
- [62].Nieves C, Langkamp-Henken B, Biomed. Pharmacother. 2002, 56, 471. [DOI] [PubMed] [Google Scholar]
- [63].Sitren HS, Fisher H, Br. J. Nutr. 1977, 37, 195. [DOI] [PubMed] [Google Scholar]
- [64].Seitfer E, Rettura G, Barbul A, Levenson SM, Surgery 1978, 84, 224. [PubMed] [Google Scholar]
- [65].Shi HP, Fishel RS, Efron DT, Williams JZ, Fishel MH, Barbul A, J. Surg. Res. 2002, 106, 299. [DOI] [PubMed] [Google Scholar]
- [66].Raynaud-Simon A, Belabed L, Le Naour G, Marc J, Capron F, Cynober L, Darquy S, Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, 1053. [DOI] [PubMed] [Google Scholar]
- [67].Armstrong DG, Hanft JR, Driver VR, Smith APS, Lazaro-Martinez JL, Reyzelman AM, Furst GJ, Vayser DJ, Cervantes HL, Snyder RJ, et al. , Diabet. Med. 2014, 31, 1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Shearer JD, Richards JR, Mills CD, Caldwell MD, Am. J. Physiol. 1997, 272, 181. [DOI] [PubMed] [Google Scholar]
- [69].Varedi M, Akbari Z, Dehghani GA, Tabei SZ, Iran. J. Basic Med. Sci. 2009, 12, 173. [Google Scholar]
- [70].Reesi F, Minaiyan M, Taheri A, Drug Deliv. Transl. Res. 2018, 8, 111. [DOI] [PubMed] [Google Scholar]
- [71].Liu Y, Zhou S, Gao Y, Zhai Y, Asian J. Pharm. Sci. 2018, 4, 1. [Google Scholar]
- [72].Schwentker A, Vodovotz Y, Weller R, Billiar TR, Nitric Oxide 2002, 7, 1. [DOI] [PubMed] [Google Scholar]
- [73].Kitano T, Yamada H, Kida M, Okada Y, Saika S, Yoshida M, Dermatol. Res. Pract. 2017, 2017, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Viñas JL, Sola A, Genescà M, Alfaro V, Pí F, Hotter G, Free Radic. Biol. Med. 2006, 40, 992. [DOI] [PubMed] [Google Scholar]
- [75].Yamasaki K, Edington HDJ, McClosky C, Tzeng E, Lizonova A, Kovesdi I, Steed DL, Billiar TR, J. Clin. Invest. 1998, 101, 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Karamercan A, Ercan S, Bozkurt S, Curr. Ther. Res. 2006, 67, 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Miller CC, Miller MK, Ghaffari A, Kunimoto B, J. Cutan. Med. Surg. 2004, 8, 233. [DOI] [PubMed] [Google Scholar]
- [78].Rezakhanlou A. Moeen, Miller C, McMullin B, Ghaffari A, Garcia R, Ghahary A, Cell Biol. Int. 2011, 35, 407. [DOI] [PubMed] [Google Scholar]
- [79].Ghaffari A, Jalili R, Ghaffari M, Miller CC, Ghahary A, Wound Repair Regen. 2007, 15, 368. [DOI] [PubMed] [Google Scholar]
- [80].Shekhter AB, Serezhenkov VA, Rudenko TG, Pekshev AV, Vanin AF, Nitric Oxide 2005, 12, 210. [DOI] [PubMed] [Google Scholar]
- [81].Laude K, Thuillez C, Richard V, Am. J. Physiol. Hear. Circ. Physiol. 2002, 283, 1418. [DOI] [PubMed] [Google Scholar]
- [82].García-Nogales P, Almeida A, Bolaños JP, J. Biol. Chem. 2003, 278, 864. [DOI] [PubMed] [Google Scholar]
- [83].Yang Y, Qi PK, Yang ZL, Huang N, Biosurface and Biotribology 2015, 1, 177. [Google Scholar]
- [84].Miller OI, Celermajer DS, Deanfield JE, Macrae DJ, Arch. Dis. Child. 1994, 70, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Weinberger B, Laskin DL, Heck DE, Laskin JD, Toxilogical Sci. 2001, 59, 5. [DOI] [PubMed] [Google Scholar]
- [86].Brasted RC, Anal. Chem. 1952, 24, 1111. [Google Scholar]
- [87].Dave RN, Joshi HM, Venugopalan VP, J. Mater. Sci. Mater. Med. 2012, 23, 3097. [DOI] [PubMed] [Google Scholar]
- [88].Zhu H, Ka B, Murad F, World J. Surg. 2007, 31, 624. [DOI] [PubMed] [Google Scholar]
- [89].Zhu H, Wei X, Bian K, Murad F, J. Burn Care Res. 2008, 29, 804. [DOI] [PubMed] [Google Scholar]
- [90].Weller R, Finnen MJ, Nitric Oxide 2006, 15, 395. [DOI] [PubMed] [Google Scholar]
- [91].Phillips R, Adjei O, Lucas S, Benjamin N, Society 2004, 48, 2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ormerod AD, Shah AAJ, Li H, Benjamin NB, Ferguson GP, Leifert C, BMC Res. Notes 2011, 4, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Tentolouris N, Petrikkos G, Vallianou N, Zachos C, Daikos GL, Tsapogas P, Markou G, Katsilambros N, Clin. Microbiol. Infect. 2006, 12, 186. [DOI] [PubMed] [Google Scholar]
- [94].Weller R, Price RJ, Ormerod AD, Benjamin N, J. Appl. Microbiol. 2001, 90, 648. [DOI] [PubMed] [Google Scholar]
- [95].Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, Friedman AJ, Nosanchuk JD, Friedman JM, J. Invest. Dermatol. 2009, 129, 2463. [DOI] [PubMed] [Google Scholar]
- [96].Mihu MR, Sandkovsky U, Han G, Friedman JM, Nosanchuk JD, Martinez LR, Virulence 2010, 1, 62. [DOI] [PubMed] [Google Scholar]
- [97].Yang L, Feura ES, Ahonen MJR, Schoenfisch MH, Adv. Healthc. Mater. 2018, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Friedman AJ, Han G, Navati MS, Chacko M, Gunther L, Alfieri A, Friedman JM, Nitric Oxide 2008, 19, 12. [DOI] [PubMed] [Google Scholar]
- [99].Blecher K, Martinez LR, Tuckman-Vernon C, Nacharaju P, Schairer D, Chouake J, Friedman JM, Alfieri A, Guha C, Nosanchuk JD, et al. , Nanomedicine Nanotechnology, Biol. Med. 2012, 8, 1364. [DOI] [PubMed] [Google Scholar]
- [100].Han G, Nguyen LN, MacHerla C, Chi Y, Friedman JM, Nosanchuk JD, Martinez LR, Am. J. Pathol. 2012, 180, 1465. [DOI] [PubMed] [Google Scholar]
- [101].Ormerod AD, Van Voorst Vader PC, Majewski S, Vanscheidt W, Benjamin N, Van Der Meijden W, JAMA Dermatology 2015, 151, 854. [DOI] [PubMed] [Google Scholar]
- [102].Wang PG, Xian M, Tang X, Wu X, Wen Z, Cai T, Janczuk AJ, Chem. Rev. 2002, 102, 1091. [DOI] [PubMed] [Google Scholar]
- [103].Singh RJ, Hogg N, Joseph J, Kalyanaraman B, J. Biol. Chem. 1996, 271, 18596. [DOI] [PubMed] [Google Scholar]
- [104].Williams DLH, Acc. Chem. Res. 1999, 32, 869. [Google Scholar]
- [105].Dicks AP, Williams DLH, Chem. Biol. 1996, 3, 655. [DOI] [PubMed] [Google Scholar]
- [106].Catalani S, Paganelli M, Gilberti ME, Rozzini L, Lanfranchi F, Padovani A, Apostoli P, Trace Elem J. Med. Biol. 2018, 45, 176. [DOI] [PubMed] [Google Scholar]
- [107].Hrabie JA, Keefer LK, Chem. Rev. 2002, 102, 1135. [DOI] [PubMed] [Google Scholar]
- [108].Keefer LK, ACS Chem. Biol. 2011, 6, 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Hrabie JA, Klose JR, Wink DA, Keefer LK, J. Org. Chem. 1993, 58, 1472. [Google Scholar]
- [110].Ahonen MJR, Suchyta DJ, Zhu H, Schoenfisch MH, Biomacromolecules 2018, 19, 1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Hetrick EM, Shin JH, Stasko NA, Johnson CB, Wespe DA, Holmuhamedov E, Schoenfisch MH, ACS Nano 2008, 2, 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Riccio DA, Schoenfisch MH, Chem. Soc. Rev. 2012, 41, 3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Li Y, Lee PI, Mol. Pharm. 2010, 7, 254. [DOI] [PubMed] [Google Scholar]
- [114].Vogt C, Xing Q, He W, Li B, Frost MC, Zhao F, Biomacromolecules 2013, 14, 2521. [DOI] [PubMed] [Google Scholar]
- [115].Murphy MP, Biochim. Biophys. Acta 1999, 1411, 401. [DOI] [PubMed] [Google Scholar]
- [116].Kim JHJO, Noh JK, Thapa RK, Hasan N, Choi M, Kim JHJO, Lee JH, Ku SK, Yoo JW, Int. J. Biol. Macromol. 2015, 79, 217. [DOI] [PubMed] [Google Scholar]
- [117].Sudarshan NR, Hoover DG, Knorr D, Food Biotechnol. 1992, 6, 257. [Google Scholar]
- [118].Singh R, Shitiz K, Singh A, Int. Wound J. 2017, 14, 1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Koehler J, Brandl FP, Goepferich AM, Eur. Polym. J. 2018, 100, 1. [Google Scholar]
- [120].Shishido SM, Seabra AB, Loh W, De Oliveira MG, Biomaterials 2003, 24, 3543. [DOI] [PubMed] [Google Scholar]
- [121].Seabra AB, Fitzpatrick A, Paul J, De Oliveira MG, Weller R, Br. J. Dermatol. 2004, 151, 977. [DOI] [PubMed] [Google Scholar]
- [122].Seabra AB, Pankotai E, Fehér M, Somlai Á, Kiss L, Bíró L, Szabó C, Kollai M, De Oliveira MG, Lacza Z, Br. J. Dermatol. 2007, 156, 814. [DOI] [PubMed] [Google Scholar]
- [123].Amadeu TP, Seabra AB, de Oliveira MG, Monte-Alto-Costa A, J. Surg. Res. 2008, 149, 84. [DOI] [PubMed] [Google Scholar]
- [124].Schanuel FS, Raggio Santos KS, Monte-Alto-Costa A, de Oliveira MG, Colloids Surfaces B Biointerfaces 2015, 130, 182. [DOI] [PubMed] [Google Scholar]
- [125].Annich GM, Meinhardt JP, Mowery KA, Ashton BA, Merz SI, Hirschl RB, Meyerhoff ME, Bartlett RH, Crit. Care Med. 2000, 28, 915. [DOI] [PubMed] [Google Scholar]
- [126].Hetrick EM, Shin JH, Paul HS, Schoenfisch MH, Biomaterials 2009, 30, 2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Shabani M, Pulfer SK, Bulgrin JP, Smith DJ, Wound Repair Regen. 1996, 4, 353. [DOI] [PubMed] [Google Scholar]
- [128].Bauer JA, Rao W, Smith DJ, Wound Repair Regen. 1998, 6, 569. [DOI] [PubMed] [Google Scholar]
- [129].Nurhasni H, Cao J, Choi M, Kim I, Lee BL, Jung Y, Yoo J-W, Int. J. Nanomedicine 2015, 10, 3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Masters KSB, Leibovich SJ, Belem P, West JL, Poole-Warren LA, Wound Repair Regen. 2002, 10, 286. [DOI] [PubMed] [Google Scholar]
- [131].Brisbois EJ, Bayliss J, Wu J, Major TC, Xi C, Wang SC, Bartlett RH, Handa H, Meyerhoff ME, Acta Biomater. 2014, 10, 4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Liu M, Duan X-P, Li Y-M, Yang D-P, Long Y-Z, Mater. Sci. Eng. C 2017, 76, 1413. [DOI] [PubMed] [Google Scholar]
- [133].Bhardwaj N, Kundu SC, Biotechnol. Adv. 2010, 28, 325. [DOI] [PubMed] [Google Scholar]
- [134].Doillon CJ, J. Biomater. Appl. 1988, 2, 562. [DOI] [PubMed] [Google Scholar]
- [135].Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P, Polym. Adv. Technol. 2010, 21, 77. [Google Scholar]
- [136].Koh A, Carpenter AW, Slomberg DL, Schoenfisch MH, ACS Appl. Mater. Interfaces 2013, 5, 7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Coneski PN, Nash JA, Schoenfisch MH, ACS Appl. Mater. Interfaces 2011, 3, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Worley BV, Soto RJ, Kinsley PC, Schoenfisch MH, ACS Biomater. Sci. Eng. 2016, 2, 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Lowe A, Bills J, Verma R, Lavery L, Davis K, Balkus KJ, Acta Biomater. 2015, 13, 121. [DOI] [PubMed] [Google Scholar]
- [140].Kang Y, Kim J, Lee YM, Im S, Park H, Kim WJ, Control J. Release 2015, 220, 624. [DOI] [PubMed] [Google Scholar]
- [141].Neidrauer M, Ercan UK, Bhattacharyya A, Samuels J, Sedlak J, Trikha R, Barbee KA, Weingarten MS, Joshi SG, J. Med. Microbiol. 2014, 63, 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Lu Y, Slomberg DL, Sun B, Schoenfisch MH, Small 2013, 9, 2189. [DOI] [PubMed] [Google Scholar]
- [143].Backlund CJ, Worley BV, Sergesketter AR, Schoenfisch MH, J. Dent. Res. 2015, 94, 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Amadeu TP, Seabra AB, de Oliveira MG, Costa AMA, J. Eur. Acad. Dermatology Venereol. 2007, 21, 629. [DOI] [PubMed] [Google Scholar]
- [145].Hetrick EM, Schoenfisch MH, Annu. Rev. Anal. Chem. 2009, 2, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Hunter RA, Privett BJ, Henley WH, Breed ER, Liang Z, Mittal R, Yoseph BP, McDunn JE, Burd EM, Coopersmith CM, et al. , Anal. Chem. 2013, 85, 6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Zomer HD, Trentin AG, J. Dermatol. Sci. 2018, 90, 3. [DOI] [PubMed] [Google Scholar]
- [148].Sullivan TP, Eaglstein WH, Davis SC, Mertz P, Wound Repair Regen. 2001, 9, 66. [DOI] [PubMed] [Google Scholar]
- [149].King AJF, Br. J. Pharmacol. 2012, 166, 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Bellinger DA, Merricks EP, Nichols TC, ILAR J. 2006, 47, 243. [DOI] [PubMed] [Google Scholar]
- [151].Spurlock ME, Gabler NK, J. Nutr. 2008, 138, 397. [DOI] [PubMed] [Google Scholar]
- [152].Rich J, Lee JC, Diabetes 2005, 54, 2904. [DOI] [PubMed] [Google Scholar]
- [153].Kasiewicz LN, Whitehead KA, Biomater. Sci. 2017, 5, 1962. [DOI] [PubMed] [Google Scholar]


