Abstract
Aims
Heart failure (HF) is associated with considerable symptom burden and impairment in physical functioning and quality of life. The sodium–glucose co‐transporter 2 inhibitor empagliflozin reduced the risk of HF hospitalisation and cardiovascular death in patients with type 2 diabetes and established cardiovascular disease in the EMPA‐REG OUTCOME trial, and could potentially improve congestion symptoms and exercise capacity in patients with HF. We describe the designs of the EMPERIAL‐Preserved and EMPERIAL‐Reduced trials of empagliflozin in patients with chronic stable HF, with or without type 2 diabetes.
Methods
EMPERIAL‐Preserved and EMPERIAL‐Reduced are randomised, placebo‐controlled trials designed to investigate the effects of empagliflozin on exercise capacity and patient‐reported outcomes in patients with chronic stable HF with preserved ejection fraction [HFpEF; left ventricular ejection fraction (LVEF) > 40%] and HF with reduced ejection fraction (HFrEF; LVEF ≤ 40%), respectively. In each trial, approximately 300 patients will be randomised 1:1 to receive empagliflozin 10 mg or placebo once daily for 12 weeks. In both trials, the primary endpoint is the change from baseline in 6‐min walk test distance at week 12. Key secondary endpoints are the change from baseline in Kansas City Cardiomyopathy Questionnaire total symptom score and change from baseline in dyspnoea score of the Chronic Heart Failure Questionnaire at week 12.
Conclusion
The EMPERIAL‐Preserved and EMPERIAL‐Reduced trials will determine the effects of empagliflozin on exercise capacity and patient‐reported outcomes in patients with HFpEF and HFrEF, respectively, and provide insight into the potential of empagliflozin in the treatment of patients with HF.
Clinical Trial Registration: ClinicalTrials.gov ID: NCT03448406 (EMPERIAL‐Preserved), NCT03448419 (EMPERIAL‐Reduced).
Keywords: Empagliflozin, Exercise capacity, Heart failure, Sodium–glucose co‐transporter 2 inhibitor
Introduction
Heart failure (HF) affects over 26 million people worldwide and is associated with high morbidity and mortality.1 Patients with HF are differentiated according to measurements of left ventricular ejection fraction (LVEF); patients with LVEF ≤ 40% are considered to have HF with reduced ejection fraction (HFrEF) and those with LVEF > 40% are considered to have HF with preserved ejection fraction (HFpEF). LVEF of > 40% to < 50% is considered to be HFpEF in many clinical trials2 and registries,3, 4 although more recently the term HF with mid‐range ejection fraction was introduced to categorise this group separately.5 HFrEF and HFpEF differ in several aspects including co‐morbidities and responses to treatment.5 However, exercise intolerance, which can manifest as dyspnoea or fatigue, is a cardinal symptom of HF that affects patients regardless of the underlying LVEF and leads to impairment in physical functioning and quality of life.5, 6, 7 Improving HF symptoms and improving the activity of daily living, such as exercise capacity, continue to be unmet medical needs in patients with HFrEF and HFpEF.
Empagliflozin is a selective sodium–glucose co‐transporter 2 (SGLT2) inhibitor used in the treatment of type 2 diabetes mellitus (T2DM). In the EMPA‐REG OUTCOME® trial in patients with T2DM and established cardiovascular disease, empagliflozin added to standard of care reduced the risk of cardiovascular death by 38%, hospitalisation for HF by 35% and all‐cause mortality by 32% compared to placebo.8 Patients with HF comprised 10% of the EMPA‐REG OUTCOME® trial population, and irrespective of the presence of HF at baseline in those patients, the reductions in risk of these outcomes were observed early and were consistent.9
The mechanisms responsible for the cardiovascular benefits of empagliflozin remain to be fully elucidated. Empagliflozin reduces renal glucose reabsorption, leading to increased urinary excretion of glucose, sodium and water.10, 11 This leads to a reduction in plasma volume,12 reflected by increases in haematocrit and haemoglobin13, 14 and reductions in arterial stiffness and vascular resistance15 in patients with T2DM. It has been suggested that treatment with empagliflozin may lead to a shift in metabolism from fat and glucose oxidation to a more energy‐efficient fuel such as ketones.16 In patients with T2DM, empagliflozin is associated with weight loss8, 17 and reductions in adiposity markers17 in addition to reductions in systolic and diastolic blood pressure without an increase in heart rate.8, 18
HFpEF is associated with obesity, hypertension, and anaemia, and consequently with ventricular and arterial stiffness, increased cardiac workload and oxygen consumption, and reduced cardiac oxygen supply, leading to reduced exercise tolerance.19 The combination of positive effects of empagliflozin on several of these factors could potentially improve exercise capacity and symptoms associated with congestion in patients with HFpEF, with or without diabetes. Patients with HFrEF are likely to benefit from the same combination of effects, particularly a reduction in congestion by osmodiuresis.
The EMPERIAL (Effect of EMPagliflozin on ExeRcise ability and heart failure symptoms In patients with chronic heArt faiLure)‐Preserved and EMPERIAL‐Reduced trials (with preserved and reduced ejection fractions, respectively) are randomised trials designed to evaluate the effect of empagliflozin on exercise capacity and patient‐reported outcomes in patients with HFpEF (defined as LVEF > 40%) and HFrEF (defined as LVEF ≤ 40%), respectively, with or without T2DM. Herein we describe the design of the EMPERIAL‐Preserved and EMPERIAL‐Reduced trials.
Study design
EMPERIAL‐Preserved and EMPERIAL‐Reduced are phase III, randomised, double‐blind, placebo‐controlled trials. The trials have been registered on ClinicalTrials.gov: NCT03448406 (EMPERIAL‐Preserved), NCT03448419 (EMPERIAL‐Reduced), and an independent ethics committee or institutional review board approved the clinical protocol at each participating centre. The trials will be conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines, and patients will provide written informed consent before study entry. It is planned that patients will be recruited from approximately 100 sites in eight countries: Australia, Canada, Germany, Greece, Italy, Norway, Poland, Portugal, Spain, Sweden, and the US. The first patient was enrolled in EMPERIAL‐Preserved on 5 April 2018 and in EMPERIAL‐Reduced on 26 March 2018.
Patients
To participate in EMPERIAL‐Preserved, patients must be ≥ 18 years of age with LVEF > 40% with HF diagnosed ≥ 3 months before screening and currently in New York Heart Association (NYHA) class II–IV, with a 6‐min walk test (6MWT) distance of ≥ 100 m at baseline and ≤ 350 m at screening and baseline. If oral diuretics are prescribed to control symptoms, the dose must have been stable for ≥ 2 weeks prior to study entry. Patients with estimated glomerular filtration rate [eGFR; according to the Chronic Kidney Disease Epidemiology Collaboration creatinine (CKD‐EPIcr) equation] < 20 mL/min/1.73 m2 or requiring dialysis and patients with type 1 diabetes are not eligible to participate. Key inclusion and exclusion criteria are summarised in Table 1. Detailed inclusion and exclusion criteria are listed in Appendix 1.
Table 1.
Key inclusion and exclusion criteria for the EMPERIAL‐Preserved trial
| Key inclusion criteria | Key exclusion criteria |
|---|---|
| • Heart failure diagnosed ≥ 3 months before screening, and currently in NYHA class II–IV. • Presence of ≥1 of the following: – Structural heart disease (left atrial enlargement and/or left ventricular hypertrophy) documented by echocardiogram at screening – Hospitalisation for heart failure within previous 12 months prior to screening. • Preserved ejection fraction, defined as LVEF > 40% (echocardiography) at screening per local reading and no prior measurement of LVEF ≤ 40% under stable conditions. • 6MWT distance of ≤ 350 m at screening and baseline. • Elevated NT‐proBNP (> 300 pg/mL for patients without atrial fibrillation; > 600 pg/mL for patients with atrial fibrillation). • If oral diuretics are prescribed to control symptoms, the dose must have been stable for ≥ 2 weeks prior to study entry. |
|
6MWT, 6‐min walk test; CKD‐EPIcr, Chronic Kidney Disease Epidemiology Collaboration creatinine equation; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
Increase in cardiac enzymes in combination with symptoms of ischaemia or newly developed ischaemic ECG changes.
To participate in EMPERIAL‐Reduced, patients must be ≥ 18 years of age with LVEF ≤ 40% with HF diagnosed ≥ 3 months before screening and currently in NYHA class II–IV, with a 6MWT distance of ≥ 100 to ≤ 350 m at screening and baseline. Patients should be on medical therapy for HFrEF consistent with prevailing cardiovascular guidelines at a stable dose for ≥ 4 weeks prior to screening, except for diuretics that must have been stable for ≥ 2 weeks prior to screening. Patients with eGFR (CKD‐EPIcr) < 20 mL/min/1.73 m2 or requiring dialysis and patients with type 1 diabetes are not eligible to participate. Key inclusion and exclusion criteria are summarised in Table 2. Detailed inclusion and exclusion criteria are listed in Appendix 2.
Table 2.
Key inclusion and exclusion criteria for the EMPERIAL‐Reduced trial
| Key inclusion criteria | Key exclusion criteria |
|---|---|
|
|
6MWT, 6‐min walk test; CKD‐EPIcr, Chronic Kidney Disease Epidemiology Collaboration creatinine equation; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
Increase in cardiac enzymes in combination with symptoms of ischaemia or newly developed ischaemic ECG changes.
Study plan
In both trials, following a screening period of 4 days to 3 weeks, eligible patients with chronic, stable HF will be randomised 1:1 to receive empagliflozin 10 mg or placebo once daily for 12 weeks (Figure 1). Randomisation will be performed in blocks using an Interactive Response Technology system without stratification. Patients will be required to return for study visits at week 6 and week 12, and for a follow‐up visit 7–14 days after the last dose of trial medication. Patients who prematurely discontinue trial medication will be asked to return as soon as possible for an early discontinuation visit and the follow‐up visit, and should return for their scheduled week 12 visit.
Figure 1.
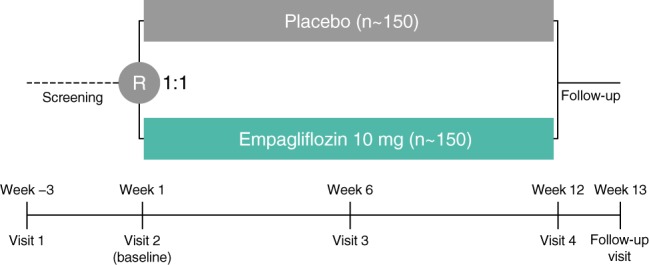
Design of the EMPERIAL‐Preserved and EMPERIAL‐Reduced trials. R, randomisation.
Measurements
Visits at baseline, week 6 and week 12 (and the early discontinuation visit, if applicable) will include measurements of the following: 6MWT distance, Kansas City Cardiomyopathy Questionnaire (KCCQ),20 Chronic Heart Failure Questionnaire Self‐Administered Standardised format (CHQ‐SAS),21 Clinical Congestion Score (based on a summary score of three items: orthopnoea, jugular venous distention, and oedema), Patient Global Impression of Severity of HF symptoms and dyspnoea, Patient Global Impression of Change in HF symptoms and dyspnoea, Clinician Global Impression of Severity of HF, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP). The 6MWT will be performed at screening, baseline, week 6, week 12, and the early discontinuation visit (if applicable). At baseline and at week 12, two 6MWTs will be performed at least 1 h apart to increase reliability and reduce variability. The distances from both tests will be recorded and the largest distance measured will be used for analysis. Screening and baseline measurements will be used to assess eligibility. The 6MWT visit should be preferably performed at same time of day at each visit to minimise intraday variability. The 6MWT protocol used in this trial is based on guidelines from the American Thoracic Society.22 A summary of study assessment timings is available in Appendix 3.
Endpoints
The primary endpoint in the EMPERIAL‐Preserved and EMPERIAL‐Reduced trials is the change from baseline in 6MWT distance at week 12. The key secondary endpoints in both trials are the change from baseline in KCCQ total symptom score at week 12 and the change from baseline in CHQ‐SAS dyspnoea score at week 12. Other secondary endpoints are: change from baseline in 6MWT distance at week 6; change from baseline in Clinical Congestion Score at week 12; change from baseline in Patient Global Impression of Severity of HF symptoms at week 12; change from baseline in Patient Global Impression of Severity of dyspnoea at week 12; Patient Global Impression of Change in HF symptoms at week 12; Patient Global Impression of Change in dyspnoea at week 12; and change from baseline in NT‐proBNP at week 12. A summary of study endpoints is shown in Appendix 4.
Safety will be assessed based on adverse events reported throughout the study and up to 7 days after the last dose of study medication [coded using the Medical Dictionary for Drug Regulatory Activities (MedDRA)], clinical laboratory tests, vital signs, 12‐lead electrocardiogram, and physical examination. Safety topics of special interest are hepatic injury [defined as an elevation of aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) > 3 fold the upper limit of normal (ULN) combined with an elevation of total bilirubin > 2 fold ULN measured in the same blood draw sample, and/or ALT and/or AST elevations ≥ 5 fold ULN], decreased renal function (defined as a ≥ 2 fold increase from baseline in creatinine to above the ULN), and ketoacidosis. Ketoacidosis events and hepatic events will be adjudicated by an independent Clinical Events Committee.
Study oversight
The trials were jointly designed by representatives of Boehringer Ingelheim, the sponsor of the trial, and the academic investigators who are members of the Executive Committee. The Executive Committee, which includes academic experts and sponsor representatives, supervised the trial designs and will supervise the trial operation. A Steering Committee consisting of the Executive Committee, National Coordinators and sponsor representatives will support the investigators in trial conduct, specifically recruitment and retention. An independent Data Monitoring Committee will evaluate safety data at regular intervals. The committee members are the same for both trials and are listed in Appendix 5.
Statistical considerations
Sample size
In each trial, a sample size of 150 patients per randomised treatment group is required to provide a 90% power to detect a 30 m treatment difference between empagliflozin and placebo in the change from baseline in 6MWT distance (considered to be clinically meaningful in patients with HF23), assuming a standard deviation of 72 m and worst‐case imputation of missing data for 5% of patients.
Statistical methodology
The null hypotheses of no effect of empagliflozin vs. placebo for the primary and key secondary endpoints will be tested in the following procedure to control the overall probability of a two‐sided type 1 error at 0.05. If the primary analysis of the primary endpoint reveals a significant result in favour of empagliflozin, both key secondary endpoints will be tested. For adjustment of multiplicity of the tests of the two key secondary endpoints, a Hochberg procedure will be followed. If the largest P‐value of the primary analysis of the two key secondary endpoints is below 5%, both null hypotheses of the key secondary endpoints will be rejected. Otherwise, if the smaller P‐value of the primary analysis of the two key secondary endpoints is below 2.5%, only the corresponding null hypothesis can be rejected. If the primary hypothesis is not rejected (i.e. if the primary analysis of change from baseline in 6MWT distance at week 12 is not met in favour of empagliflozin), the tests of the key secondary endpoints will be conducted in an exploratory fashion.
The primary analyses of primary and both key secondary endpoints will be a Wilcoxon rank test, including patients with missing data via a worst‐case imputation. Patients with an available week 12 measurement, regardless of whether on or off‐treatment, will be ranked based on change from baseline at week 12. In all other cases, a worst‐case imputation will be performed in the following manner: all imputed data due to missing data will be ranked lower than measured values. Patients who do not have a clinical event (death, serious adverse event or any adverse event leading to discontinuation) and have a value at week 6 will be ranked by their values. Patients who do not have a clinical event and have no valid post‐baseline value will be ranked below. Patients who have a clinical event will be given a lower rank and death is ranked below any other clinical event by time to death from randomization. This imputation strategy will be used for all confirmatory analyses (i.e. primary analyses of primary and key secondary endpoints). For estimation of effect, the non‐parametric Hodges–Lehmann estimate for the median difference will be calculated. Efficacy analyses will be conducted in all randomised patients. Sensitivity analyses for the primary and key secondary endpoints will include mixed model repeated measures (MMRM) analyses with the baseline value of the endpoint in question as a covariate, without imputation of missing values. Several exploratory subgroup analyses are pre‐specified in the statistical analysis plan including an analysis by diabetes status (diabetes vs. no diabetes).
Change from baseline in 6MWT distance at week 6 will be analysed using the same model as the primary analysis of the endpoint. The change from baseline to week 12 in Clinical Congestion Score will be evaluated using an MMRM analysis with treatment as a fixed effect and baseline Clinical Congestion Score as a linear covariate. The proportion of patients in every category of Global Impression of Change at week 12 and the proportions of patients categorised by the shift in Patient Global Impression of Severity scores between baseline and week 12 will be evaluated using Cochran–Mantel–Haenszel test. Change from baseline in NT‐proBNP at week 12 (after log‐transformation) will be evaluated using an MMRM with baseline log‐transformed NT‐proBNP as a covariate, without imputation of missing values. Safety will be analysed descriptively in patients treated with ≥ 1 dose of study drug. No interim analysis is planned.
Discussion
HFpEF and HFrEF have a significant impact on the physical functioning and well‐being of patients6, 7, 24 in addition to a substantial risk of mortality and hospitalisation.25, 26 Exercise capacity appears to correlate with quality of life outcomes in patients with HF, but to a greater extent in patients with HFpEF; in patients with HFrEF, raised left atrial pressure may play a greater role in determining exercise capacity. Recommendations for the treatment of HFrEF include use of angiotensin‐converting enzyme inhibitors, angiotensin receptor antagonists, an angiotensin receptor–neprilysin inhibitor, beta‐blockers, and mineralocorticoid receptor antagonists, all of which have been shown to reduce mortality in patients with HFrEF.5 However, HFrEF remains associated with symptom burden, reduced exercise capacity and impaired physical functioning.6, 24 Patients with HFpEF tend to be older, more often female, and more likely to have a history of hypertension and atrial fibrillation than patients with HFrEF.5 According to the current guidelines, no treatment has been shown to reduce mortality in patients with HFpEF.5 A recent meta‐analysis by Zheng et al.2 reported that beta‐blockers may reduce cardiovascular mortality, though meta‐analyses of drug interventions have shown no improvement in exercise capacity or functional outcomes in these patients, with only limited improvement in patient quality of life.2, 27 The management of HFpEF involves control of congestive symptoms, usually with diuretics, and the treatment of co‐morbidities.5 In the absence of effective treatments to reduce mortality, alleviating symptoms and improving patient well‐being may be important goals in the management of patients with HFpEF,5 and there is an unmet medical need for treatments that can improve quality of life in these patients.
Analyses have suggested that the effects of the SGLT2 inhibitor empagliflozin on mortality and HF hospitalisation in the EMPA‐REG OUTCOME trial were independent of its glucose‐lowering effects,9, 13, 28 and several of the other mechanisms proposed to explain the cardiovascular risk reduction with empagliflozin may be beneficial to patients with HF regardless of the presence of diabetes.29 Further, the effects of empagliflozin with the potential to improve exercise capacity in patients with HF are unlikely to differ by LVEF.29
The long‐term effects of empagliflozin on cardiovascular death and hospitalisation for HF across the spectrum of patients with HFpEF and HFrEF (with and without diabetes) are being investigated in the EMPEROR‐Preserved (NCT03057951) and EMPEROR‐Reduced trials (NCT03057977), respectively. Together, these trials will provide insight into the potential of empagliflozin in the treatment of patients with HFpEF and HFrEF.
Acknowledgements
Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Melanie Stephens and Elizabeth Ng of FleishmanHillard Fishburn, London, UK, during the preparation of this manuscript. The authors were fully responsible for all content and editorial decisions, were involved at all stages of manuscript development, and have approved the final version.
Funding
These studies are funded by the Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance.
Conflict of interest: W.T.A. receives consulting fees from Boehringer Ingelheim in his role as an Executive Committee Member for the EMPERIAL trials. P.P. receives consulting fees from Boehringer Ingelheim. J.L. has received consultancy fees from Abbott, Boehringer Ingelheim, Novartis, Relypsa, ResMed, VWave Medical, Cardiotronix Helath, and Edwards Lifesciences, and grants from AstraZeneca, and the National Institutes of Health. S.D.A. has received consultancy fees from AstraZeneca and Boehringer Ingelheim. W.J., C.Z., H.M., B.P., M. Brun, A.U., M. Brueckmann, and A.S. are employees of Boehringer Ingelheim.
Appendix 1. Inclusion and exclusion criteria for the EMPERIAL‐Preserved trial 1.
Inclusion criteria
All of the following criteria needed to be met:
≥ 18 years of age.
Written informed consent prior to admission to the trial.
Women of child‐bearing potential must agree to use birth control measures with a failure rate of < 1% per year during the treatment period of the study.
Heart failure diagnosed ≥ 3 months before screening, and currently in New York Heart Association class II–IV.
-
Presence of ≥ 1 of the following:
– Structural heart disease (left atrial enlargement and/or left ventricular hypertrophy) documented by echocardiogram at screening
– Hospitalisation for heart failure within previous 12 months prior to screening.
Preserved ejection fraction, defined as left ventricular ejection fraction (LVEF) > 40% (echocardiography) at screening per local reading and no prior measurement of LVEF ≤ 40% under stable conditions.
6‐min walk test (6MWT) distance of ≤ 350 m at screening and baseline.
Elevated N‐terminal pro‐brain natriuretic peptide (> 300 pg/mL for patients without atrial fibrillation; > 600 pg/mL for patients with atrial fibrillation).
If oral diuretics are prescribed to control symptoms, the dose must have been stable for ≥ 2 weeks prior to study entry.
Clinically stable at randomisation with no signs of heart failure decompensation (investigator's judgement).
Exclusion criteria
Myocardial infarction (increase in cardiac enzymes in combination with symptoms of ischaemia or newly developed ischaemic echocardiogram changes), coronary artery bypass graft, or other major cardiovascular surgery, stroke, or transient ischaemic attack within 90 days prior to screening.
Acute decompensated heart failure requiring i.v. diuretics, i.v. inotropes or i.v. vasodilators, or left ventricular assist device within 4 weeks prior to screening and up to baseline.
Previous or current randomisation in another empagliflozin heart failure trial.
Estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology creatinine equation) < 20 mL/min/1.73 m2 or requiring dialysis.
Type 1 diabetes.
Symptomatic hypotension or systolic blood pressure (SBP) < 100 mmHg at screening or baseline.
SBP ≥ 180 mmHg at screening or baseline, or SBP > 160 mmHg at both screening and baseline.
Atrial fibrillation or atrial flutter with a resting heart rate > 110 b.p.m. documented by echocardiogram at screening.
Unstable angina within 30 days prior to screening.
Largest 6MWT distance at baseline < 100 m.
Conditions that preclude exercise testing.
Patients in a structured (investigator's judgement) exercise training programme within 1 month prior to screening or planned to start one during the course of this trial.
Heart transplant recipient or listed for heart transplant.
Cardioverter‐defibrillator implantation within 1 month prior to screening or planned during the course of the trial.
Implanted cardiac resynchronisation therapy.
Cardiomyopathy based on infiltrative diseases (e.g. amyloidosis), accumulation diseases (e.g. haemochromatosis, Fabry disease), muscular dystrophies, cardiomyopathy with reversible causes (e.g. stress cardiomyopathy), hypertrophic obstructive cardiomyopathy, or known pericardial constriction.
Any severe (obstructive or regurgitant) valvular heart disease that either represents a risk for the conduct of the 6MWT or is expected to lead to surgery during the trial (investigator's opinion).
Chronic pulmonary disease (i.e. with known forced expiratory volume in 1 s < 50% requiring home oxygen, or oral steroid therapy or hospitalisation for exacerbation within 12 months, or significant chronic pulmonary disease (investigator's opinion), or primary pulmonary arterial hypertension).
Indication of liver disease, defined by serum levels of either alanine aminotransferase (SGPT), aspartate aminotransferase (SGOT), or alkaline phosphatase above 3 x upper limit of normal as determined at screening.
Haemoglobin < 9 g/dL at screening.
History of ketoacidosis.
Major surgery (major according to investigator's opinion) performed within 90 days prior to screening, or scheduled major elective surgery (e.g. hip or knee replacement) during the course of the trial.
Gastrointestinal surgery/disorder that could interfere with trial medication absorption in the investigator's opinion.
Any documented active or suspected malignancy or history of malignancy within 2 years prior to screening, except appropriately treated basal cell carcinoma of the skin or in situ carcinoma of uterine cervix or low‐risk prostate cancer.
Patients who must or wish to continue the intake of restricted medications or any drug considered likely to interfere with the safe conduct of the trial.
Current use or prior use of a sodium–glucose co‐transporter 2 (SGLT2) inhibitor or combined SGLT1 and 2 inhibitor within 12 weeks prior to screening or during screening period until randomisation. Discontinuation of an SGLT2 inhibitor or combined SGLT1 and 2 inhibitor for the purposes of study enrolment is not permitted.
Treatment with i.v. iron therapy or erythropoietin within 3 months prior to screening.
Currently enrolled in another investigational device or drug trial, or less than 30 days since ending another investigational device or drug trial(s), or receiving other investigational treatment(s). Patients participating in a purely observational trial will not be excluded.
Known allergy or hypersensitivity to empagliflozin or other SGLT2 inhibitors.
Chronic alcohol or drug abuse or any condition that, in the investigator's opinion, makes them an unreliable trial patient or unlikely to complete the trial.
Women who are pregnant, nursing, or who plan to become pregnant while in the trial.
Any other clinical condition that would jeopardise patients safety while participating in this trial, or may prevent the subject from adhering to the trial protocol.
Appendix 2. Inclusion and exclusion criteria for the EMPERIAL‐Reduced trial 1.
Inclusion criteria
All of the following criteria needed to be met:
≥ 18 years of age.
Written informed consent prior to admission to the trial.
Women of child‐bearing potential must agree to use birth control measures with a failure rate of < 1% per year during the treatment period of the study.
Heart failure diagnosed ≥ 3 months before screening, and currently in New York Heart Association class II–IV.
Reduced ejection fraction, defined as left ventricular ejection fraction ≤ 40% (echocardiography) at screening per local reading under stable conditions.
6‐min walk test (6MWT) distance of ≤ 350 m at screening and baseline.
Elevated N‐terminal pro‐brain natriuretic peptide (> 450 pg/mL for patients without atrial fibrillation; > 600 pg/mL for patients with atrial fibrillation) at screening.
On medical therapy for heart failure consistent with prevailing cardiovascular guidelines at a stable dose for ≥ 4 weeks prior to screening, except for diuretics which must have been stable for ≥ 2 weeks prior to screening.
Clinically stable at randomisation with no signs of heart failure decompensation (investigator's judgement).
Appropriate use of medical devices such as cardioverter‐defibrillator or a cardiac resynchronisation therapy consistent with prevailing local or international cardiovascular guidelines, and if a device is required, it must have been implanted for at least 3 months prior to screening for cardiac resynchronisation therapy and 1 month prior to screening for cardioverter‐defibrillator.
Exclusion criteria
Myocardial infarction (increase in cardiac enzymes in combination with symptoms of ischaemia or newly developed ischaemic echocardiogram changes), coronary artery bypass graft, or other major cardiovascular surgery, stroke, or transient ischaemic attack within 90 days prior to screening.
Acute decompensated heart failure requiring i.v. diuretics, i.v. inotropes or i.v. vasodilators, or left ventricular assist device within 4 weeks prior to screening and up to baseline.
Previous or current randomisation in another empagliflozin heart failure trial.
Estimated glomerular filtration rate (Chronic Kidney Disease Epidemiology creatinine equation) < 20 mL/min/1.73 m2 or requiring dialysis.
Type 1 diabetes.
Symptomatic hypotension or systolic blood pressure (SBP) < 100 mmHg at screening or baseline.
SBP ≥ 180 mmHg at screening or baseline, or SBP > 160 mmHg at both screening and baseline.
Unstable angina within 30 days prior to screening.
6MWT distance of < 100 m at baseline.
Conditions that preclude exercise testing.
Atrial fibrillation or atrial flutter with a resting heart rate > 110 b.p.m. documented by echocardiogram at screening.
Patients in a structured (investigator's judgement) exercise training programme within 1 month prior to screening or planned to start one during the course of this trial.
Heart transplant recipient or listed for heart transplant.
Currently implanted left ventricular assist device.
Cardiomyopathy based on infiltrative diseases (e.g. amyloidosis), accumulation diseases (e.g. haemochromatosis, Fabry disease), muscular dystrophies, cardiomyopathy with reversible causes (e.g. stress cardiomyopathy), hypertrophic obstructive cardiomyopathy, or known pericardial constriction.
Untreated ventricular arrhythmia with syncope in patients without cardioverter‐defibrillator documented within the 3 months prior to screening.
Planned implantation of cardioverter‐defibrillator or cardiac resynchronisation therapy during the course of the trial.
Diagnosis of cardiomyopathy induced by chemotherapy or peripartum within the 12 months prior to screening.
Symptomatic bradycardia or second or third degree heart block without a pacemaker after adjusting beta‐blocker therapy, if appropriate.
Chronic pulmonary disease (i.e. with known forced expiratory volume in 1 s < 50% requiring home oxygen, or oral steroid therapy or hospitalisation for exacerbation within 12 months, or significant chronic pulmonary disease (investigator's opinion), or primary pulmonary arterial hypertension.
Indication of liver disease, defined by serum levels of either alanine aminotransferase (SGPT), aspartate aminotransferase (SGOT), or alkaline phosphatase above 3 x upper limit of normal as determined at screening.
Haemoglobin < 9 g/dL at screening.
History of ketoacidosis.
Major surgery (major according to investigator's opinion) performed within 90 days prior to screening, or scheduled major elective surgery (e.g. hip or knee replacement) during the course of the trial.
Gastrointestinal surgery/disorder that could interfere with trial medication absorption in the investigator's opinion.
Any documented active or suspected malignancy or history of malignancy within 2 years prior to screening, except appropriately treated basal cell carcinoma of the skin or in situ carcinoma of uterine cervix or low‐risk prostate cancer.
Patients who must or wish to continue the intake of restricted medications or any drug considered likely to interfere with the safe conduct of the trial.
Current use or prior use of a sodium–glucose co‐transported 2 (SGLT2) inhibitor or combined SGLT1 and 2 inhibitor within 12 weeks prior to screening or during screening period until randomisation. Discontinuation of an SGLT2 inhibitor or combined SGLT1 and 2 inhibitor for the purposes of study enrolment is not permitted.
Treatment with i.v. iron therapy or erythropoietin within 3 months prior to screening.
Currently enrolled in another investigational device or drug trial, or less than 30 days since ending another investigational device or drug trial(s), or receiving other investigational treatment(s). Patients participating in a purely observational trial will not be excluded.
Known allergy or hypersensitivity to empagliflozin or other SGLT2 inhibitors.
Chronic alcohol or drug abuse or any condition that, in the investigator's opinion, makes them an unreliable trial patient or unlikely to complete the trial.
Women who are pregnant, nursing, or who plan to become pregnant while in the trial.
Any other clinical condition that would jeopardise patients safety while participating in this trial, or may prevent the subject from adhering to the trial protocol.
Appendix 3. Key study assessments in the EMPERIAL‐Preserved and EMPERIAL‐Reduced trials 1.
| Trial period | Screening | Randomized treatment period | FU period | |||
|---|---|---|---|---|---|---|
| Visit (day of treatment) | 1 (−21 to −4) | 2 (1) |
3
(43 ± 7) |
4/End of treatment (85 ± 7) | Early DC on visit | FU visit (±7) or DC + 7 days |
| Demographics | X | |||||
| Vital signs | X | X | X | X | X | X |
| HbA1c | X | X | X | |||
| eGFR (CKD‐EPIcr formula) | X | X | X | X | X | X |
| Safety lab | X | X | X | X | X | X |
| ECG | X | X | X | |||
| NT‐proBNP | X | X | X | X | X | |
| 6‐min walk test | X | X | X | X | X | |
| Adverse events | X | X | X | X | X | X |
| KCCQ | X | X | X | X | ||
| CHQ‐SAS | X | X | X | X | ||
| Clinical Congestion Scorea | X | X | X | X | ||
| Patient Global Impression of Severity of heart failure symptoms | X | X | X | X | ||
| Patient Global Impression of Severity of dyspnoea | X | X | X | X | ||
| Patient Global Impression of Change in heart failure symptoms | X | X | X | |||
| Patient Global Impression of Change in dyspnoea | X | X | X | |||
| Clinician Global Impression of Severity of CHF | X | X | X | X | ||
| Clinician Global Impression of Change in CHF severity | X | X | X | |||
| NYHA classification | X | X | X | X | X | |
| HCRU | X | X | X | X | ||
CHF, chronic heart failure; CHQ‐SAS, Chronic Heart Failure Questionnaire Self‐Administered Standardised format; CKD‐EPIcr, Chronic Kidney Disease‐Epidemiology Collaboration creatinine equation; DC, discontinuation; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; FU, follow‐up; HbA1c, glycated haemoglobin; HRCU, health care resource utilisation; KCCQ, Kansas City Cardiomyopathy Questionnaire; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association.
Patient's congestion will be assessed using a clinician‐based outcome assessment of orthopnoea, fatigue, jugular venous distention (as assessed by the investigator), and oedema. Each category will be assessed through a four‐measure questionnaire that will be further converted to a standardised 4‐point scale ranging from 0 to 3 as shown.
Clinical Congestion Score.
| Signs/symptoms | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Orthopnoea | None | Seldom | Frequent | Continuous |
| Jugular venous distention (cmH2O) | ≤6 | 6–9 | 10–15 | ≥ 15 |
| Oedema | Absent/trace | Slight | Moderate | Marked |
Appendix 4. Endpoints in the EMPERIAL‐Preserved and EMPERIAL‐Reduced trials 1.
| Primary endpoint |
|
| Key secondary endpoints |
|
| Other secondary endpoints |
Week 6
Week 12
|
Appendix 5. EMPERIAL‐Preserved and EMPERIAL‐Reduced Committee members 1.
Executive Committee
William Abraham, Stefan Anker, JoAnn Lindenfeld, Piotr Ponikowski, Martina Brueckmann, and Afshin Salsali.
Data Monitoring Committee
Francine Welty (USA, Chair), Tim Clayton (UK), Barry Greenberg (USA), Marvin Konstam (USA), Kennedy Lees (UK), Mike Palmer (UK), Klaus Parhofer (Germany), Terje Pedersen (Norway).
Clinical Event Committee
Peter Carson (USA, Chair), James Freston (USA), Neil Kaplowitz (USA), James Lewis (USA), Johannes Mann (Germany), John Petrie (UK).
National Coordinators
Piergiuseppe Agostoni (Italy), Javed Butler (USA), Akshay Desai (USA), Gerasimos Filippatos (Greece), Jonathan Howlett (Canada), Jerzy Wranicz (Poland), Josep Redón Mas (Spain), José Silva Cardoso (Portugal), Stefan Störk (Germany).
Contributor Information
William T. Abraham, Email: william.abraham@osumc.edu.
on behalf of the EMPERIAL Investigators and National Coordinators:
William Abraham, Stefan Anker, JoAnn Lindenfeld, Piotr Ponikowski, Martina Brueckmann, Afshin Salsali, Francine Welty, Tim Clayton, Barry Greenberg, Marvin Konstam, Kennedy Lees, Mike Palmer, Klaus Parhofer, Terje Pedersen, Peter Carson, James Freston, Neil Kaplowitz, James Lewis, Johannes Mann, John Petrie, Piergiuseppe Agostoni, Javed Butler, Akshay Desai, Gerasimos Filippatos, Jonathan Howlett, Jerzy Wranicz, Josep Redón Mas, José Silva Cardoso, and Stefan Störk
References
- 1. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–1133. [DOI] [PubMed] [Google Scholar]
- 2. Zheng SL, Chan FT, Nabeebaccus AA, Shah AM, McDonagh T, Okonko DO, Ayis S. Drug treatment effects on outcomes in heart failure with preserved ejection fraction: a systematic review and meta‐analysis. Heart 2018;104:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB; OPTIMIZE‐HF Investigators and Hospitals . Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF Registry. J Am Coll Cardiol 2007;50:768–777. [DOI] [PubMed] [Google Scholar]
- 4. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC; ADHERE Scientific Advisory Committee and Investigators . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 5. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891–975. [DOI] [PubMed] [Google Scholar]
- 6. Hoekstra T, Lesman‐Leegte I, van Veldhuisen DJ, Sanderman R, Jaarsma T. Quality of life is impaired similarly in heart failure patients with preserved and reduced ejection fraction. Eur J Heart Fail 2011;13:1013–1018. [DOI] [PubMed] [Google Scholar]
- 7. Smith GL, Masoudi FA, Vaccarino V, Radford MJ, Krumholz HM. Outcomes in heart failure patients with preserved ejection fraction: mortality, readmission, and functional decline. J Am Coll Cardiol 2003;41:1510–1518. [DOI] [PubMed] [Google Scholar]
- 8. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA‐REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128. [DOI] [PubMed] [Google Scholar]
- 9. Fitchett D, Zinman B, Wanner C, Lachin JM, Hantel S, Salsali A, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA‐REG OUTCOME® Investigators . Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA‐REG OUTCOME® trial. Eur Heart J 2016;37:1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heise T, Jordan J, Wanner C, Heer M, Macha S, Mattheus M, Lund SS, Woerle HJ, Broedl UC. Acute pharmacodynamic effects of empagliflozin with and without diuretic agents in patients with type 2 diabetes mellitus. Clin Ther 2016;38:2248–2264.e5. [DOI] [PubMed] [Google Scholar]
- 11. Heise T, Jordan J, Wanner C, Heer M, Macha S, Mattheus M, Lund SS, Woerle HJ, Broedl UC. Pharmacodynamic effects of single and multiple doses of empagliflozin in patients with type 2 diabetes. Clin Ther 2016;38:2265–2276. [DOI] [PubMed] [Google Scholar]
- 12. Schou MGL, Fitchett D, Zinman B, Inzucchi SE, Hehnke U, von Eynatten M, George J, Johansen OE, Wanner C. Empagliflozin exerts short‐ and long‐term effects on plasma volume in patients with type 2 diabetes: insight from EMPA‐REG OUTCOME. Circulation 2017;136:A15997 (abstr). [Google Scholar]
- 13. Inzucchi SE, Zinman B, Fitchett D, Wanner C, Ferrannini E, Schumacher M, Schmoor C, Ohneberg K, Johansen OE, George JT, Hantel S, Bluhmki E, Lachin JM. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA‐REG OUTCOME trial. Diabetes Care 2018;41:356–363. [DOI] [PubMed] [Google Scholar]
- 14. Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M, Zinman B; EMPA‐REG OUTCOME Investigators . Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation 2018;137:119–129. [DOI] [PubMed] [Google Scholar]
- 15. Chilton RGL, Park S, Inzucchi SE, Hehnke U, Woerle HJ, Johansen OE. Empagliflozin improves cardiovascular (CV) outcomes regardless of improvement in cardiac and vascular hemodynamic markers in type 2 diabetes patients at high CV risk in EMPA‐REG OUTCOME. Circulation 2017;136:A16626 (abstr). [Google Scholar]
- 16. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA‐REG OUTCOME study? A unifying hypothesis. Diabetes Care 2016;39:1115–1122. [DOI] [PubMed] [Google Scholar]
- 17. Neeland IJ, McGuire DK, Chilton R, Crowe S, Lund SS, Woerle HJ, Broedl UC, Johansen OE. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res 2016;13:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, Johansen OE. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 2015;17:1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hummel SL, Kitzman DW. Update on heart failure with preserved ejection fraction. Curr Cardiovasc Risk Rep 2013;7:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–1255. [DOI] [PubMed] [Google Scholar]
- 21. Evans RA, Singh SJ, Williams JE, Morgan MD. The development of a self‐reported version of the Chronic Heart Questionnaire. J Cardiopulm Rehabil Prev 2011;31:365–372. [DOI] [PubMed] [Google Scholar]
- 22. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 23. Shoemaker MJ, Curtis AB, Vangsnes E, Dickinson MG. Clinically meaningful change estimates for the six‐minute walk test and daily activity in individuals with chronic heart failure. Cardiopulm Phys Ther J 2013;24:21–29. [PMC free article] [PubMed] [Google Scholar]
- 24. Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 2002;288:2144–2150. [DOI] [PubMed] [Google Scholar]
- 25. Chan MM, Lam CS. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail 2013;15:604–613. [DOI] [PubMed] [Google Scholar]
- 26. Chioncel O, Lainscak M, Seferovic PM, Anker SD, Crespo‐Leiro MG, Harjola VP, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitzka F, Maggioni AP, Filippatos G. Epidemiology and one‐year outcomes in patients with chronic heart failure and preserved, mid‐range and reduced ejection fraction: an analysis of the ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017;19:1574–1585. [DOI] [PubMed] [Google Scholar]
- 27. Fukuta H, Goto T, Wakami K, Ohte N. Effects of drug and exercise intervention on functional capacity and quality of life in heart failure with preserved ejection fraction: a meta‐analysis of randomized controlled trials. Eur J Prev Cardiol 2016;23:78–85. [DOI] [PubMed] [Google Scholar]
- 28. Inzucchi SEKM, Fitchett D, Wanner C, Hehnke U, Kaspers S, George JT, Zinman B. Improvement in cardiovascular outcomes with empagliflozin is independent of glycemic control. Circulation 2018;138:1904–1907. [DOI] [PubMed] [Google Scholar]
- 29. Butler J, Hamo CE, Filippatos G, Pocock SJ, Bernstein RA, Brueckmann M, Cheung AK, George JT, Green JB, Januzzi JL, Kaul S, Lam CSP, Lip GYH, Marx N, McCullough PA, Mehta CR, Ponikowski P, Rosenstock J, Sattar N, Salsali A, Scirica BM, Shah SJ, Tsutsui H, Verma S, Wanner C, Woerle HJ, Zannad F, Anker SD; EMPEROR Trials Program . The potential role and rationale for treatment of heart failure with sodium–glucose co‐transporter 2 inhibitors. Eur J Heart Fail 2017;19:1390–1400. [DOI] [PubMed] [Google Scholar]


