Abstract
Aims
Ischaemic heart failure (IHF) patients have a poor prognosis even with current guideline‐derived therapy. Intramyocardial injections of autologous or allogeneic mesenchymal stromal cells might improve cardiac function leading to better clinical outcome.
Methods
The SCIENCE (Stem Cell therapy in IschEmic Non‐treatable Cardiac diseasE) consortium has initiated a Horizon 2020 funded multicentre phase II study in six European countries. It is a double‐blind, placebo‐controlled trial testing the safety and efficacy of allogeneic Cardiology Stem Cell Centre Adipose‐derived Stromal Cells (CSCC_ASC) from healthy donors or placebo in 138 symptomatic IHF patients. Main inclusion criteria are New York Heart Association class II–III, left ventricular ejection fraction < 45% and N‐terminal pro‐B‐type natriuretic peptide levels > 300 pg/mL. Patients are randomized in a 2:1 pattern to receive intramyocardial injections of either CSCC_ASC or placebo. CSCC_ASC and placebo treatments are prepared centralized at Rigshospitalet in 5 mL vials as an off‐the‐shelf product. Vials are distributed to all clinical partners and stored in nitrogen vapour tanks ready to be used directly after thawing. A total of 100 × 106 CSCC_ASC or placebo are injected directly into viable myocardium in the infarct border zone using the NOGA XP system (BDS, Cordis, Johnson & Johnson, USA). Primary endpoint is a centralized core‐laboratory assessed change in left ventricular end‐systolic volume at 6‐month follow‐up measured by echocardiography. The trial started in January 2017, 58 patients were included and treated until July 2018.
Conclusion
The SCIENCE trial will provide clinical data on efficacy and safety of intramyocardial cell therapy of allogeneic adipose‐derived stromal cells from healthy donors in patients with IHF.
Keywords: Heart failure, Adipose‐derived stromal cells, Ischaemic cardiomyopathy, Stem cells, Allogeneic therapy, Clinical trial
Introduction
Regenerative heart failure (HF) therapy might repair damaged heart muscle tissue.1 Several randomized clinical trials over recent years found the therapy to be safe but so far not effective, and clinical data remain conflicting. In particular, intramyocardial injection might reduce patient's HF symptoms.2, 3, 4, 5 Fluoroscopy‐guided intramyocardial injection of bone marrow (BM)‐derived progenitor cells (CD 133+ cells) in ischaemic refractory cardiomyopathy recently showed improved myocardial perfusion and improved angina symptoms.6
Mesenchymal stromal cells (MSC) have migratory abilities, can secrete protective factors, and act as a primary matrix for tissue regeneration.7 Furthermore, MSC possess immunosuppressive capacities, evade being recognized by a recipient immune system and can therefore be used as allogeneic therapy without eliciting an immune response.8, 9 The presence of MSC‐like cells in different tissues (adipose tissue, BM, peripheral blood, etc.) indicates their importance and pre‐clinical studies indicate that these cells have regenerative capacity regardless of tissue origin.7 Adipose‐derived stromal cells (ASC) are very promising since their tissue concentration is 100–300 times higher than the MSC concentration in the BM.10
Adipose‐derived stromal cells are capable of secreting a large number of angiogenesis‐related cytokines, including vascular endothelial growth factor, granulocyte/macrophage colony stimulating factor and stromal‐derived factor‐1α.11 They showed greater pro‐angiogenic action compared to BM‐MSC and can be an ideal source for therapeutic angiogenesis in ischaemic disease in terms of efficacy, accessibility and available tissue amounts.12
The production of allogeneic Cardiology Stem Cell Centre Adipose‐derived Stromal Cells (CSCC_ASC) from healthy donors was established at Rigshospitalet Copenhagen. CSCC_ASC are stored in nitrogen vapour containers as an off‐the‐shelf product in the hospital ready to be used without any delay. In a phase I study, this newly developed cryopreserved CSCC_ASC product from healthy donors was proven safe. Furthermore, feasibility of the treatment concept was demonstrated.13 The SCIENCE trial is a phase II, prospective, randomized, European multicentre, placebo‐controlled, double‐blind, investigator‐initiated clinical trial with NOGA‐guided intramyocardial injection of CSCC_ASC vs. placebo in patients with chronic ischaemic HF (IHF).
Study design
Study population
The SCIENCE trial has passed the Voluntary Harmonization process through Clinical Trials Facilitation Groups, European Medicines Agency. It was approved by the National Competent Authorities and Ethics Committees in Denmark, Germany, The Netherlands, Austria, Slovenia and Poland at the end of 2016. The study is conducted according to Good Clinical Practice and follows the latest version of the Helsinki Declaration adopted in 2013 at the 64th World Medical Association Assembly, Brazil. The study is registered in ClinicalTrials.gov (NCT02673164).
A total of 138 patients will be enrolled in the SCIENCE study. Key inclusion criteria are patients aged 30–80 years with chronic IHF, impaired left ventricular ejection fraction (LVEF < 45% measured by echocardiography), symptomatic HF [New York Heart Association (NYHA) class II–III], plasma N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) > 300 pg/mL (>35 pmol/L). All included patients should be on maximal tolerable standard HF medication and without further treatment options like valvular intervention/surgery or revascularization or implantation of a cardiac resynchronisation therapy (CRT) device. CRT device implantation between baseline and the 6‐month follow‐up exams will lead to study exclusion due to a possible effect of CRT on the primary endpoint of left ventricular end‐systolic volume (LVESV). All inclusion and exclusion criteria are listed in Table 1.
Table 1.
Inclusion and exclusion criteria of the SCIENCE trial
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy with defibrillation; CT, computed tomography; DMSO, dimethyl sulfoxide; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in 1 s; Hb, haemoglobin; HF, heart failure; HLA, human leucocyte antigen; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Overview of the study flow
Patients are screened for trial eligibility, treated and followed in six European HF centres. Patients with signed informed consent approved for the study are randomized 2:1 to receive intramyocardial injections of CSCC_ASC or placebo (isotonic saline) in a double‐blind design. Randomization is performed by personnel at the Cardiology Stem Cell Centre (CSCC) cell‐processing unit before shipment of treatment doses to clinical sites. To assure blinding of the treatment, the clinical teams, which are responsible for screening and follow‐up of the patients will not participate in the treatment procedure or be in the catheterization laboratory during the treatment. Follow‐up visits will be 1, 3, 6 and 12 months after treatment for safety and efficacy evaluation. The study algorithm and visit schedule are summarized in Figure 1.
Figure 1.
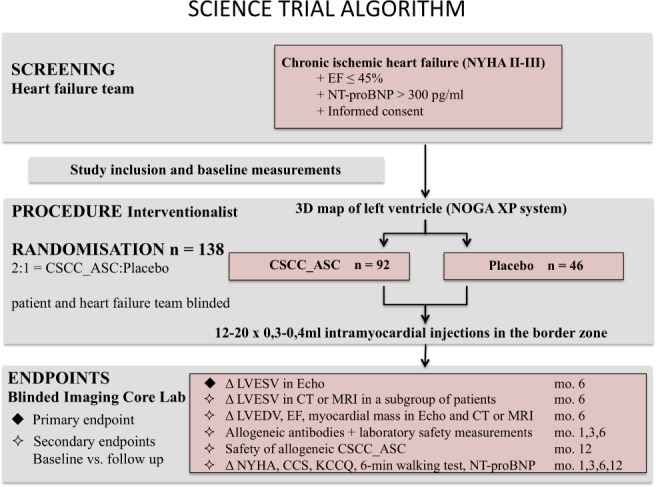
Algorithm of the SCIENCE trial. CCS, Canadian Cardiovascular Society; CSCC_ASC, Cardiology Stem Cell Centre Adipose‐derived Stromal Cells; CT, computed tomography; EF, ejection fraction; KCCQ, Kansas City Cardiomyopathy Questionnaire; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; MRI, magnetic resonance imaging; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association.
Cell production
CSCC_ASC and placebos (saline) are manufactured at CSCC, Rigshospitalet, University Hospital Copenhagen. The CSCC holds a manufacturing authorization (no. 23909) and a tissue establishment authorization (no. 32298) issued and inspected every second year by Danish Medicines Agency and Danish Patient Safety Authority, respectively. The manufacturing procedure is in compliance with the EU Guidelines for Good Manufacturing Practice (GMP) of Medicinal Products for Human Use (certificate of GMP compliance no. DK IMP 92217).
The cells are gained from abdominal adipose tissue after liposuction from healthy donors. A donor is eligible only if screening shows that the donor is healthy and free from risk factors and laboratory tests for relevant infectious disease agents are negative. Liposuction is performed according to CSCC Standard Operating Procedures. Approximately 100–150 mL lipoaspirate is obtained from each donor from the abdomen under local anaesthesia by a plastic surgeon. Animal‐free expansion is performed with human platelet lysate (Cook Regentec) in automated closed bioreactor systems (Quantum Cell Expansion System, Terumo BCT).14 The final cell product has had two passages in the bioreactor system. CSCC_ASC is cryopreserved in CellSeal vials (COOK Regentec) (110 million cells 5 mL) in CryoStor CS10 (BioLife Solutions). The concentration is a little higher than the intended 100 × 106 for the treatment. There is an estimated loss of 10 × 106 cells during handling of the cells. The cell doses 100 × 106 CSCC_ASC is based on the experience from our previous HF trial where we could see a dose titrating effect. Patients treated with > 84 × 206 cells had the highest improvement in LVEF and LVESV.4 CSCC_ASC is stored below −180°C in nitrogen vapour or dry‐storage until clinical use. Release criteria are sterility, viability (> 80%) and immunophenotype [stable positive markers CD90, CD105, CD73 > 80% and negative markers < 3% CD45, < 5% human leucocyte antigen (HLA)‐DR] and are listed in Table 2. The presence of mycoplasma is tested from all bioreactor expansions immediately prior to cell harvest, and the presence of bacteria, fungi and endotoxins is tested on the final product, immediately prior to cryopreservation.
Table 2.
Release criteria of the final Cardiology Stem Cell Centre Adipose‐derived Stromal Cells product
| Attribute | Acceptance criteria |
|---|---|
| Number of cells | 100–120 million cells |
| ASC viability | >80% |
| Donor serology |
Negative for anti‐antibody + Ag) Negative for anti‐HCV Negative for HBsAg Negative for anti‐HBc Negative for syphilis Negative for HTLV I/II |
| Sterility | |
| Bacteria/fungi | Negative/negative |
| Endotoxin level | < 70 EU/mL |
| Mycoplasma | Negative |
| Characterization (immunophenotype) |
CD90 > 80% CD105 > 80% CD73 > 80% CD45 < 3% HLA‐DR < 5% |
ASC, adipose‐derived stromal cells; HBsAg, hepatitis B surface antigen; HBc, hepatitis B core; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HLA, human leucocyte antigen; HTLV, human T‐lymphotropic virus.
Purity, differentiation capacity (adipose, bone, cartilage), and full phenotyping consistent with ISCT/IFATS standards for the ASC phenotype as well as genomic stability has been determined during manufacturing process development.14, 15, 16 Stability studies have been established, and currently 24‐month stability demonstrating sterility, viability, recovery, ASC immunophenotype, and cell potency as defined by in vitro cell adherence and proliferation, after thawing of the final product, has been documented and approved by competent authorities. Cell delivery is recommended within 1 h after thawing (5 min in sterile water and temperature set at 37°C). Samples from each batch of CSCC_ASC are stored at CSCC for future analyses of correlations between cell function and clinical efficacy as well as for statutory reference samples.
CSCC_ASC vials are shipped in a qualified portable nitrogen dry‐shipper to the trial participating HF units in Europe by World Courier, in accordance with European rules for Good Distribution Practices. The randomization code for each delivered vial is available in a sealed envelope at each site if there is an acute need for breaking the code in a case of an unexpected serious adverse event.
Safety
Allogeneic treatment
The final CSCC_ASC product is intended for allogeneic treatment. Each vial will only contain cells from one donor. A total of 6–8 donors will be used to produce the vials for the clinical trial. There will be no HLA tissue type matching between the donor and the patients. Allogeneic cell therapy generally poses a risk for graft‐versus‐host reaction or host‐versus‐graft reaction. A graft‐versus‐host reaction is considered insignificant from a safety perspective given the lack of immunologically active cells in the graft (< 3% CD45 positive cells, <5% HLA‐DR cells). MSC not only inhibit B‐cell proliferation, but also the cytokine‐induced proliferation of natural killer (NK) cells. Furthermore, they prevent cytotoxic activity and cytokine production due to a sharp down‐regulation of surface expression of the activating NK receptors.8 MSC are also able to suppress proliferation of stimulated peripheral blood mononuclear cells and to inhibit differentiation of monocyte‐derived dendritic cells. However, ASC show more potent immunomodulatory effects compared to BM‐MSC, which is related to higher levels of cytokine secretion.9 Furthermore, ASC express only low levels of major histocompatibility complex (MHC) class I (HLA‐ABC) and no MHC class II (HLA‐DR) or co‐stimulatory molecules, making them less likely to interact with recipient immune cells.8, 9 Although very low levels of antibody titres toward CSCC_ASC were detected in the phase I safety study with CSCC_ASC, these titres were not correlated with clinical events.13
Viral screening
Each donor is tested for human immunodeficiency virus, hepatitis B and C, syphilis and human T‐lymphotropic virus type I/II serology by serum analysis within 30 days prior to liposuction and on the day of donation. Donor testing is performed by the Virus Laboratory, The Blood Bank, Department of Clinical Immunology, Rigshospitalet, Copenhagen, as authorized by The Danish Patient Safety Authority.
Tissue typing and alloantibodies
Tissue typing (low HLA I and II genotyping) is performed of all donors for the purpose of alloantibody screening in patients after cell treatment; in The Netherlands it was requested by the Medical Research Ethics Committee (METC) to perform such analysis before randomization and allocate accordingly the correct donor samples at randomization. Blood samples of all patients in this trial will be stored for later centralized analyses of tissue antibodies and biomarkers.
NOGA‐guided injection
Three‐dimensional left ventricular (LV) mapping is performed using the NOGA XP® system (BDS, Cordis, Johnson & Johnson, USA). Intramyocardial injection of stem cells using the NOGA platform in patients with ischaemic disease has proven to be safe and feasible.4, 17, 18 It reduces the likelihood of systemic toxicity of the injected substance, resulting in minimal washout, limited exposure of non‐target organs and precise placement of the cells to peri‐ischaemic regions (border zone) of the myocardium.
Every patient receives an electromechanical three‐dimensional LV map by point‐by‐point measurement. Usually > 100 verified points are necessary to obtain a complete LV map. The system distinguishes between viable [unipolar voltage > 12 mV, bipolar voltage > 2.5 mV, local linear shortening (LLS) > 6%], non‐viable‐myocardium (unipolar voltage < 6 mV, bipolar voltage < 1.5 mV, LLS < 4%] and border zone (unipolar voltage 6–12 mV, bipolar voltage 1.5–2.5 mV, LLS 4–6%) around myocardial scar tissue. Cut‐off values may vary in the literature, because borders are not exactly defined.19 Mismatch between unipolar and bipolar signals is observed when the myocardial scar is not transmural. Border zones are defined after interpretation of all mapping data and 12–20 injections of 0.3–0.4 mL CSCC_ASC (in total 100 Mio cells) or placebo are performed with MYOSTAR® injection catheters (BDS, Cordis, Johnson & Johnson, USA) with 3D computer aided navigation precisely in this region. The number of injections may vary, depending on the scar size and the decision to split the injected volume to cover all areas of the border zone, with adequate distance between the injection points. Laboratory measurements of plasma creatine kinase‐MB and troponin will be made before treatment, 6 h and 1 day after to assess possible myocardial effects. Additionally, echocardiographic assessment is performed after the procedure to exclude pericardial effusion. Exemplary images of the NOGA procedure of a 69‐year‐old patient with IHF are shown in Figure 2.
Figure 2.
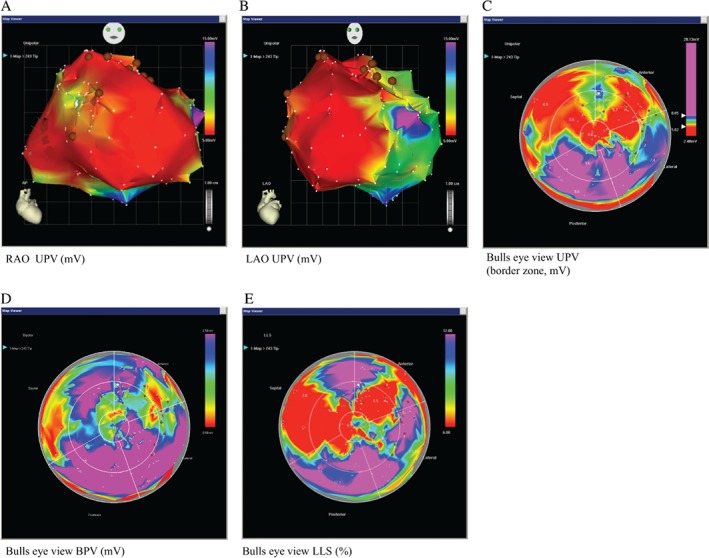
Exemplary images of the NOGA procedure of a 69‐year‐old man with ischaemic heart failure. Three‐dimensional left ventricular mapping performed using the NOGA XP® system. (A) Unipolar voltage (UPV) in right anterior oblique (RAO) (UPV 5–15 mV), (B) left anterior oblique (LAO) (UPV 5–15 mV) and (C) in bulls eye projections (UPV with focus on the border zone 5–8 mV) are displayed with margins manually set to standardized values. (D) Scar definition using bipolar voltage (BPV 0.5–2.5 mV) and (E) local linear shortening (LLS, 6–12%). Colour encoding is displayed at the right upper border of the pictures. White points indicate mapping points and brown dots indicate locations for injections in the infarction border zone. A mismatch between UPV, BPV and LLS is observed showing maintained electrical viability (UPV, BPV) in certain areas of the ventricle with no systolic movement (LLS), suggestive of hibernating myocardium; when scar is not transmural, a mismatch between UPV and BPV is seen, two injections were placed in this specific area; compare posterior area of map in the bulls eye perspective (viable and contractive) to the anterior‐septal region.
Endpoints
Primary endpoint is a centralized core‐laboratory assessed change in LVESV at 6‐month follow‐up measured in all patients by echocardiography.
Secondary endpoints are safety evaluated by development of donor‐specific de novo HLA antibodies and laboratory safety measurements 1, 3 and 6 months after treatment. Other endpoints are changes in LVEF, end‐diastolic volume and myocardial mass at 6‐month follow‐up measured by echocardiography. Computed tomography (CT) and magnetic resonance imaging (MRI) will be performed in a subgroup of patients at baseline and 6 months after treatment to evaluate further cardiac function and LVESV.
Other secondary endpoints are changes in NYHA class, Canadian Cardiovascular Society class, Kansas City Cardiomyopathy Questionnaire, Seattle Angina Questionnaire, 6‐minute walking test, additional echocardiographic measurements, and NT‐proBNP levels. In addition, safety of CSCC_ASC with respect to incidence and severity of serious adverse events and suspected unrelated serious adverse events will be evaluated at 12‐month follow‐up.
A combined endpoint will be assessed of:
Death, hospitalization for worsening HF, including insertion of a biventricular pacemaker, hospitalization due to ventricular tachycardia or fibrillation 1, 2 and 3 years after treatment.
Death, hospitalization for any cardiovascular reason, hospitalization for worsening HF, including insertion of a biventricular pacemaker, hospitalization due to ventricular tachycardia or fibrillation 1, 2 and 3 years after treatment.
Cardiac imaging and analysis
All patients will have echocardiographic assessment at baseline and in follow‐up visits 3, 6 and 12 months after treatment to evaluate cardiac parameters for primary and secondary endpoints. Patients with a plasma creatinine < 130 μmol/L may also undergo CT scan or MRI (only in patients without implantable cardioverter defibrillator or pacemaker) to determine the effect of regenerative therapy on myocardial function at baseline and 6‐month follow‐up.
Echocardiographic scans will be without contrast and assess LV end‐systolic and end‐diastolic volumes, myocardial mass, LVEF, radial and longitudinal global and segmental strain by two‐dimensional speckle‐tracking echocardiography, left atrial volume, valvular function, and tissue Doppler parameters (e', s'); this will be independently and blinded assessed using Philips Intellispace software. Regarding reproducibility for every individual patient, all exams will be performed using the same ultrasound machine preferably operated by the same technician. The inclusion scans will be investigated before treatment to ensure a proper quality of imaging before randomization. Endocardial and epicardial borders will be traced manually in end‐diastole and end‐systole and the mitral plane set to define the basal border of the left ventricle.
Study sites electronically transmit all imaging data to a central server at Netherlands Heart Institute & Lygature/TraIT, Amsterdam, The Netherlands. All image data are analysed blinded with the cvi post‐processing tool (for CT and MRI: Circle Cardiovascular Imaging, Calgary, Alberta, Canada). Also, NOGA data will be used to crosscheck and fuse with other imaging modalities to investigate its value in accurately pointing out the target border zone for treatment planning using the Cartbox‐2 software platform.20, 21
Power calculation and statistics
A total of 138 patients, randomized in a 2:1 pattern, with a maximum dropout rate of 15% before the 6‐month follow‐up, will be required to have a statistical power of > 90% to detect an absolute change in LVESV of 9.5 mL [estimated standard deviation (SD) 15 mL] and 12.7 mL (estimated higher SD of 20 mL). Change in LVEF of 3.2% (estimated SD 5%) and of 5.1% (estimated higher SD of 8%) results in a statistical power of > 90%. An alpha value of 5% was used in all calculations.
The power calculation of this trial is based on the results of the MSC‐HF trial, a placebo‐controlled comparison of intramyocardial injection of BM‐MSC (randomized in a 2:1 pattern) in patients with IHF.4 In this previous trial, the primary endpoint of LVESV measured with MRI or CT was significantly reduced in the BM‐MSC group (between‐group difference of 13.0 ± 12.9 mL, P = 0.001). There was also a significant improvement in LVEF measured with MRI or CT (between‐group difference of 6.2 ± 3.8%, P < 0.0001). SD in the MSC‐HF trial was 12.9% for LVESV and 3.8% for LVEF. Since the SD is higher if echocardiography is employed compared to CT or MRI, we did the power calculation of this trial with two SDs higher than in the MSC‐HF trial.
Statistical analyses will be performed using SAS 9·4 (SAS Institute Inc., Cary, NC, USA) and SPSS 22 (SPSS Inc., Chicago, IL, USA). For baseline characteristic comparison and follow‐up data between and within groups, an independent samples t‐test and paired t‐test for normally distributed continuous data will be used, respectively. Mann–Whitney U test will be used for between‐group comparisons of continuous non‐normally distributed data. For baseline categorical data, Pearson's chi‐square or Fisher's exact test will be used, as appropriate. For follow‐up data with more than two time‐points, repeated measures with autoregressive covariance structure will be used. For nominal repeated data, generalized estimating equations will be used. Fisher's exact test will be used for comparison of occurrences of serious adverse events between groups. A two sided P‐value of < 0.05 is considered statistically significant.
When data are ready for analysis, our statistician will evaluate missing data, whether the data are missing completely at random (MCAR) or missing at random (MAR). We will then decide about handling of missing values.
Current status
Patients will be enrolled during a recruitment phase of 24 months. The trial has been delayed due to different approval processes in the involved countries and implementation of new regulations for clinical use of human platelet lysate in the production of stem cell products. The trial started January 2017 and 58 patients have been enrolled until July 2018 without safety events. Since minimum study duration for one patient is 12 months, the overall study duration will be 4 years.
Discussion
The SCIENCE trial is the first European multicentre randomized phase II trial testing the safety and efficacy of allogeneic CSCC_ASC from healthy donors or placebo (saline) in 138 patients with symptomatic IHF.
Presently, there is no established and approved regenerative therapy for patients with heart disease. Our hypothesis is that cell therapy leads to myocardial regeneration and improvement of LV parameters. Therefore, the primary endpoint in this phase II trial is the centralized core‐laboratory assessed change in LVESV at 6‐month follow‐up. In the MSC‐HF trial, a significant improvement of LVESV and LVEF was observed after 6 months.4 LVESV is considered superior to LVEF when LVEF is low (< 50%) or when end‐systolic volume is high (> 100 mL).22 Furthermore, LVESV is a simple yet powerful echocardiographic marker of LV remodelling that can be measured easily and is an independent predictor of hospitalization for HF in patients with stable coronary heart disease.23 A limitation of the study is that not all patients can have measurements from echocardiography, CT and MRI. However, all patients receive measurements by echocardiography. Additionally, MRI or CT scans will be performed in a subgroup of patients without the possibility of establishing an equal distribution in the treatment and control group. Not all patients will undergo CT imaging due to different European radiation protection guidelines in the participating centres. Additionally, many HF patients have implantable cardioverter defibrillator or CRT devices and cannot undergo MRI imaging.
Cardiac cell therapy employing autologous BM cells applied via the intracoronary route was previously shown to be safe but not sufficiently effective.24 Intracoronary delivery may result in many cells directly lost to the systemic circulation. The transendocardial delivery route on the other hand, allows precise placement of cells in the border zone of ischaemic heart regions. Table 3 lists pertinent trials with cell treatment via the intramyocardial delivery route.2–5,17,18,25–30 Previous randomized clinical trials with different cell types suggest that cell‐based therapy via the intramyocardial route of acute and chronic cardiomyopathy is safe, feasible and possibly effective in improving various surrogate markers of LV function and symptoms.3, 4, 5, 17
Table 3.
Stem cell trials with intramyocardial delivery route in patients with ischaemic cardiomyopathy
| Author/acronym, publication dates, number of patients (trial design) | Disease and delivery route | Cell type and dose | Methods for efficacy measurement | Phase, results |
|---|---|---|---|---|
|
FOCUS‐HF27: 06/2011, 30 (randomized 2:1) |
CICM (EF < 40%), IM (NOGA) |
Autologous BMMC (30 × 106 ± 0 cells) vs. control |
Symptom reduction, Echo, SPECT |
Phase II, quality of life improved at 6 months (P = 0.009), no effect on cardiac function |
|
TABMMI5: 11/2011, 20 (non‐randomized) |
CICM (EF ≤ 40%), IM (TESI) |
Autologous BMMC, CD34+, CD133+ (96 ± 29 × 106 cells) |
Symptom reduction, Echo |
Phase II, exercise tolerance test improved (P < 0.01), EF improved (from 34.9 ± 4.3 to 41.9 ± 5.1 at12 months and 42.2 ± 7.1% at 24 months, P = 0.00005) |
|
ALSTER‐Stem Cell17: 10/2012, 23 (non‐randomized) |
3–4 weeks after acute myocardial infarction (EF < 45%), IM (NOGA) |
Autologous BMMC (220 ± 42 × 106 cells) | MRI | Phase II, EF (+7.9 ± 1.5%, P = 0.001) at 6 months, no effect on scar volume |
|
POSEIDON2: 12/2012, 30 (randomized, 5 patients each dose level and cell type) |
CICM (EF < 50%), IM (TESI) |
Autologous vs. allogeneic BM‐MSC (20, 100, 200 × 106 cells) |
Symptom reduction, ESP, CT |
Phase I/II, reduced infarct scar and end‐diastolic diameter (P = 0.001) at 13 months in both groups, no effect on EF. Symptom reduction in the autologous cell group. Inverse dose response to EF and LVESV |
| MSC‐HF4: 07/2015, 60 (randomized 2:1, double‐blind, placebo‐controlled) |
CICM (EF < 45%), IM (NOGA) |
Autologous BM‐MSC (77.5 ± 67.9 × 106 cells) vs. placebo | Symptom reduction, MRI, CT |
Phase II, improvement of LVESV –7.6 ± 13.2 mL (P = 0.001), EF +5.0 ± 3.8% and stroke volume + 15.6 ± 14.6 mL (P < 0.0001) at 6 months in the cell group, no significant symptom reduction |
|
Perin et al.3: 08/2015, 60 (20 randomized patients each dose, 2:1 vs. control) |
CICM (EF < 40%), IM (NOGA) |
Allogeneic BM‐MSC (25, 75, 150 × 106 cells) | Symptom reduction, Echo, SPECT | Phase II, HF‐MACE reduced at 36 months in 150 × 106 cell group (P = 0.025) |
|
MyStromalCell18: 11/2017, 10 (non‐randomized) |
CICM (EF ≤ 45%), IM (NOGA) |
Autologous ASC (100 × 106 cells) | Safety Labs, Echo, CT | Phase II, tendency towards efficacy |
| TRIDENT29: 11/2017, 30 (randomized 1:1, double‐blind) |
CICM (EF ≤ 50%), IM (TESI) |
Allogeneic BM‐MSC (20 or 100 × 106 cells) | Symptom reduction, CT, ESP | Phase II, reduced scar size in both groups (20 × 106 P = 0.001, 100 × 106 P = 0.0002), EF increased only in 100 × 106 cell group at 12 months (+3.7 U; IQR 1.1–6.1; P = 0.04) |
|
Kastrup et al.30: ongoing, 81 (randomized 2:1, double‐blind, placebo‐controlled) |
CICM (EF ≤ 45%), IM (NOGA) |
CSCC_ASC (110 × 106 cells) vs. placebo | Echo, MRI, CT | Phase II |
| IxCELL‐DCM25: 04/2016, 126 (randomized 1:1, double‐blind, placebo‐controlled) |
CICM (EF ≤ 35%), IM (NOGA) |
Autologous Ixmyelocel‐T cell product (CD90+ MSC and CD45+, CD14+ auto‐ fluorescent+ activated macrophages, cell number not applicable) | Symptom reduction, Echo | Primary endpoint (composite number of deaths, cardiovascular hospitalizations during 12‐month follow‐up) significantly reduced (P = 0.03), no effect on EF or LVESV |
| CHART‐128: 12/2016, 315 (randomized 1:1, double‐blind, sham‐controlled) |
CICM (EF ≤ 35%), IM (C‐Cathez™) |
Autologous BM‐derived cardiopoietic cells (>24 × 106 cells) | Symptom reduction, Echo | Hierarchical composite (mortality, worsening HF, Minnesota Living with Heart Failure Questionnaire score, 6‐minute walk test, LVESV, and EF) at 9 months neutral (P = 0.27) |
| ATHENA26: 02/2017, 31, (randomized 2:1, double‐blind, placebo‐controlled) |
CICM (EF 20–45%), IM (NOGA) |
Autologous ASC (n = 28: 40 × 106 and n = 3: 80 × 106 cells) | Symptom reduction, Echo, ESP | VO2 max favoured ASC, fewer hospitalizations and symptom reduction in the ASC arm, no difference in EF and LVESV |
ASC, adipose‐derived mesenchymal stromal cells; BM, bone marrow; BMMC, bone marrow mononuclear cells; BM‐MSC, bone marrow‐derived mesenchymal stem cells; CICM, chronic ischaemic cardiomyopathy; CSCC_ASC, Cardiology Stem Cell Centre Adipose‐derived Stromal Cells; CT, computed tomography; Echo, echocardiography; EF, ejection fraction; ESP, ergospirometry; HF, heart failure; IM, intramyocardial; IQR, interquartile range; LVESV, left ventricular end‐systolic volume; MACE, major adverse cardiac events; MRI, magnetic resonance imaging; VO2, oxygen uptake; SPECT, single photon emission computed tomography.
Discrepancies in previous trials were seen between LV function improvement and reduction in clinical endpoints. Improvement in LV function not always goes hand in hand with improvement of clinical outcome. In the recent IxCELL‐DCM trial, mortality and cardiovascular hospitalization rates were reduced, without significant effect on LV function.25 Patients in the ATHENA trials also had fewer hospitalizations and improvement in HF symptoms, but no improvement in LVEF.26 Additionally, age and medical co‐morbidities from HF may decrease the number, quality, and potency of autologous cells. Stratifying cell results by age in the FOCUS‐HF trial showed that younger patients (< 60 years) had more BM mononuclear cells than older patients (> 60 years), which may be one of the main reasons for contradictory results in autologous regenerative therapy.27 The largest trial so far (CHART‐1) with intramyocardial autologous BM cardiopoietic cells demonstrated safety but the primary endpoint (hierarchical composite of all‐cause mortality, worsening HF, LVESV and ejection fraction) was neutral.28
Based on the collected experience from previous clinical trials, the lack of an efficient homogeneous cell product prevents repeatability and dissemination of regenerative therapy. Therefore, a switch from autologous to allogeneic treatment is necessary to establish a standardized effective therapy option for patients with IHF. Allogeneic MSC and ASC have already been used in previous clinical trials without any side effects.3, 13, 29 The TRIDENT trial compared two doses of allogeneic MSC, directly injected intramyocardially in patients with ischaemic cardiomyopathy.29 Although both cell doses (20 and 100 × 106 cells) decreased scar size, only the 100 × 106 dose increased ejection fraction. Improvement of clinical outcomes and LV parameters following treatment with an allogeneic cell product of the BM is currently also investigated in a large phase III trial (DREAM HF‐1, NCT02032004).
The Cardiology Stem Cell Centre, Rigshospitalet, University Hospital Copenhagen, has established new cultivation protocols for the production of allogeneic CSCC_ASC from healthy donors, which can be stored in nitrogen vapour containers as an off‐the‐shelf product ready to be used. In a phase I study, the newly developed cryopreserved product CSCC_ASC was safe and demonstrated feasibility and efficacy in 10 patients with IHF.13 Ongoing is a phase II Danish multicentre study with CSCC_ASC in 81 patients with IHF parallel to the SCIENCE trial to provide further data on safety and regenerative efficacy. The cell product and eligibility criteria are similar to the SCIENCE trial.30 The SCIENCE trial will provide further clinical data from six large European sites on efficacy and safety of intramyocardial cell therapy of allogeneic treatment with this newly developed cell product.
To analyse the economic impact of clinical application of regenerative stem cell technology for myocardial function at the societal level and within the health organizations using the present technology, data related to social aspects of ASC therapy, relating to resource use and to health‐related quality of life, are collected in the trial, so as to create a solid base for more advanced economic analyses and approaches.
Acknowledgements
Joanna Ciosek, Sebastian Dworowy, Tomasz Jadczyk, Michal Kozłowski, Pawel Nadrowski, Ronja Sagalski, Esther Schlegel, Annette Schmidt, Anna Sikora, Dorota Skiba and Denise Traxler, Abbas Ali Qayyum, and Anders B. Mathiasen are all actively involved in the conduction of the SCIENCE trial.
Funding
This work was supported by an EU funding as part of the Horizon 2020 program to conduct this randomized multicentre clinical trial (SCIENCE grant no. 643478). The multinational SCIENCE consortium consists of heart failure units from Denmark, Germany, The Netherlands, Austria, Slovenia and Poland, coordinated by Prof. Jens Kastrup, Rigshospitalet, Copenhagen University Hospital, Denmark.
Conflict of interest: A.E., M.H‐S. and J.K. are inventors of a patent application [‘Stem Cell Therapy Based on Adipose‐Derived Stem Cells’ (20440PCT00 ‐ Publication WO 2017‐068140 ‐ 10051711 05501)] owned by the Capital Region of Denmark and Rigshospitalet, Copenhagen University Hospital. The other authors declare no conflicts of interest.
Contributor Information
Jens Kastrup, Email: jens.kastrup@regionh.dk.
on behalf of the SCIENCE Investigators:
Joanna Ciosek, Sebastian Dworowy, Tomasz Jadczyk, Michal Kozłowski, Pawel Nadrowski, Ronja Sagalski, Esther Schlegel, Annette Schmidt, Anna Sikora, Dorota Skiba, Denise Traxler, Abbas Ali Qayyum, and Anders B. Mathiasen
References
- 1. Burchfield JS, Dimmeler S. Role of paracrine factors in stem and progenitor cell mediated cardiac repair and tissue fibrosis. Fibrogenesis Tissue Repair 2008;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis‐Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow‐derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y, Willerson JT, Itescu S, Henry TD. A phase II dose‐escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res 2015;117:576–584. [DOI] [PubMed] [Google Scholar]
- 4. Mathiasen AB, Qayyum AA, Jorgensen E, Helqvist S, Fischer‐Nielsen A, Kofoed KF, Haack‐Sorensen M, Ekblond A, Kastrup J. Bone marrow‐derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo‐controlled trial (MSC‐HF trial). Eur Heart J 2015;36:1744–1753. [DOI] [PubMed] [Google Scholar]
- 5. de la Fuente LM, Stertzer SH, Argentieri J, Penaloza E, Koziner B, Rouy D, Altman PA. Transendocardial autologous bone marrow in myocardial infarction induced heart failure, two‐year follow‐up in an open‐label phase I safety study (the TABMMI study). EuroIntervention 2011;7:805–812. [DOI] [PubMed] [Google Scholar]
- 6. Bassetti B, Carbucicchio C, Catto V, Gambini E, Rurali E, Bestetti A, Gaipa G, Belotti D, Celeste F, Parma M, Righetti S, Biava L, Arosio M, Bonomi A, Agostoni P, Scacciatella P, Achilli F, Pompilio G. Linking cell function with perfusion: insights from the transcatheter delivery of bone marrow‐derived CD133(+) cells in ischemic refractory cardiomyopathy trial (RECARDIO). Stem Cell Res Ther 2018;9:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue‐derived MSC. Cell Commun Signal 2011;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer‐cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3‐dioxygenase and prostaglandin E2. Blood 2008;111:1327–1333. [DOI] [PubMed] [Google Scholar]
- 9. Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue‐derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow‐derived counterparts. Stem Cells Transl Med 2013;2:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Helder MN, Knippenberg M, Klein‐Nulend J, Wuisman PI. Stem cells from adipose tissue allow challenging new concepts for regenerative medicine. Tissue Eng 2007;13:1799–1808. [DOI] [PubMed] [Google Scholar]
- 11. Meliga E, Strem BM, Duckers HJ, Serruys PW. Adipose‐derived cells. Cell Transplant 2007;16:963–970. [DOI] [PubMed] [Google Scholar]
- 12. Kim Y, Kim H, Cho H, Bae Y, Suh K, Jung J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem 2007;20:867–876. [DOI] [PubMed] [Google Scholar]
- 13. Kastrup J, Haack‐Sorensen M, Juhl M, Harary Sondergaard R, Follin B, Drozd Lund L, Monsted Johansen E, Ali Qayyum A, Bruun Mathiasen A, Jorgensen E, Helqvist S, Jorgen Elberg J, Bruunsgaard H, Ekblond A. Cryopreserved off‐the‐shelf allogeneic adipose‐derived stromal cells for therapy in patients with ischemic heart disease and heart failure – a safety study. Stem Cells Transl Med 2017;6:1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haack‐Sorensen M, Juhl M, Follin B, Harary Sondergaard R, Kirchhoff M, Kastrup J, Ekblond A. Development of large‐scale manufacturing of adipose‐derived stromal cells for clinical applications using bioreactors and human platelet lysate. Scand J Clin Lab Invest 2018;17:1–8. [DOI] [PubMed] [Google Scholar]
- 15. Haack‐Sorensen M, Follin B, Juhl M, Brorsen SK, Sondergaard RH, Kastrup J, Ekblond A. Culture expansion of adipose derived stromal cells. A closed automated Quantum Cell Expansion System compared with manual flask‐based culture. J Transl Med 2016;14:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue‐derived stromal vascular fraction and culture expanded adipose tissue‐derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013;15:641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heeger CH, Jaquet K, Thiele H, Zulkarnaen Y, Cuneo A, Haller D, Kivelitz D, Schmidt T, Krause K, Metzner A, Schneider C, Kuck KH, Bergmann MW. Percutaneous, transendocardial injection of bone marrow‐derived mononuclear cells in heart failure patients following acute ST‐elevation myocardial infarction: ALSTER‐Stem Cell trial. EuroIntervention 2012;8:732–742. [DOI] [PubMed] [Google Scholar]
- 18. Qayyum AA, Mathiasen AB, Mygind ND, Kuhl JT, Jorgensen E, Helqvist S, Elberg JJ, Kofoed KF, Vejlstrup NG, Fischer‐Nielsen A, Haack‐Sorensen M, Ekblond A, Kastrup J. Adipose‐derived stromal cells for treatment of patients with chronic ischemic heart disease (MyStromalCell trial): a randomized placebo‐controlled study. Stem Cells Int 2017;2017:5237063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gyongyosi M, Dib N. Diagnostic and prognostic value of 3D NOGA mapping in ischemic heart disease. Nat Rev Cardiol 2011;8:393–404. [DOI] [PubMed] [Google Scholar]
- 20. Pavo N, Jakab A, Emmert MY, Strebinger G, Wolint P, Zimmermann M, Ankersmit HJ, Hoerstrup SP, Maurer G, Gyongyosi M. Comparison of NOGA endocardial mapping and cardiac magnetic resonance imaging for determining infarct size and infarct transmurality for intramyocardial injection therapy using experimental data. PLoS One 2014;9:e113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Slochteren FJ, van Es R, Gyongyosi M, van der Spoel TI, Koudstaal S, Leiner T, Doevendans PA, Chamuleau SA. Three dimensional fusion of electromechanical mapping and magnetic resonance imaging for real‐time navigation of intramyocardial cell injections in a porcine model of chronic myocardial infarction. Int J Cardiovasc Imaging 2016;32:833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end‐systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation 1987;76:44–51. [DOI] [PubMed] [Google Scholar]
- 23. McManus DD, Shah SJ, Fabi MR, Rosen A, Whooley MA, Schiller NB. Prognostic value of left ventricular end‐systolic volume index as a predictor of heart failure hospitalization in stable coronary artery disease: data from the Heart and Soul Study. J Am Soc Echocardiogr 2009;22:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gyongyosi M, Wojakowski W, Lemarchand P, Lunde K, Tendera M, Bartunek J, Marban E, Assmus B, Henry TD, Traverse JH, Moye LA, Surder D, Corti R, Huikuri H, Miettinen J, Wohrle J, Obradovic S, Roncalli J, Malliaras K, Pokushalov E, Romanov A, Kastrup J, Bergmann MW, Atsma DE, Diederichsen A, Edes I, Benedek I, Benedek T, Pejkov H, Nyolczas N, Pavo N, Bergler‐Klein J, Pavo IJ, Sylven C, Berti S, Navarese EP, Maurer G; ACCRUE Investigators . Meta‐Analysis of Cell‐based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ Res 2015;116:1346–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Povsic TJ, Zeiher AM. IxCELL‐DCM: rejuvenation for cardiac regenerative therapy? Lancet 2016;387:2362–2363. [DOI] [PubMed] [Google Scholar]
- 26. Henry TD, Pepine CJ, Lambert CR, Traverse JH, Schatz R, Costa M, Povsic TJ, David Anderson R, Willerson JT, Kesten S, Perin EC. The Athena trials: autologous adipose‐derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheter Cardiovasc Interv 2017;89:169–177. [DOI] [PubMed] [Google Scholar]
- 27. Perin EC, Silva GV, Henry TD, Cabreira‐Hansen MG, Moore WH, Coulter SA, Herlihy JP, Fernandes MR, Cheong BY, Flamm SD, Traverse JH, Zheng Y, Smith D, Shaw S, Westbrook L, Olson R, Patel D, Gahremanpour A, Canales J, Vaughn WK, Willerson JT. A randomized study of transendocardial injection of autologous bone marrow mononuclear cells and cell function analysis in ischemic heart failure (FOCUS‐HF). Am Heart J 2011;161:1078–1087 e3. [DOI] [PubMed] [Google Scholar]
- 28. Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, Merkely B, Musialek P, Wojakowski W, Andreka P, Horvath IG, Katz A, Dolatabadi D, El Nakadi B, Arandjelovic A, Edes I, Seferovic PM, Obradovic S, Vanderheyden M, Jagic N, Petrov I, Atar S, Halabi M, Gelev VL, Shochat MK, Kasprzak JD, Sanz‐Ruiz R, Heyndrickx GR, Nyolczas N, Legrand V, Guedes A, Heyse A, Moccetti T, Fernandez‐Aviles F, Jimenez‐Quevedo P, Bayes‐Genis A, Hernandez‐Garcia JM, Ribichini F, Gruchala M, Waldman SA, Teerlink JR, Gersh BJ, Povsic TJ, Henry TD, Metra M, Hajjar RJ, Tendera M, Behfar A, Alexandre B, Seron A, Stough WG, Sherman W, Cotter G, Wijns W; CHART Program . Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham‐controlled CHART‐1 clinical trial. Eur Heart J 2017;38:648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Florea V, Rieger AC, DiFede DL, El‐Khorazaty J, Natsumeda M, Banerjee MN, Tompkins BA, Khan A, Schulman IH, Landin AM, Mushtaq M, Golpanian S, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Valasaki K, Pujol MV, Ghersin E, Miki R, Delgado C, Abuzeid F, Vidro‐Casiano M, Saltzman RG, DaFonseca D, Caceres LV, Ramdas KN, Mendizabal A, Heldman AW, Mitrani RD, Hare JM. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (the TRIDENT study). Circ Res 2017;121:1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kastrup J, Schou M, Gustafsson I, Nielsen OW, Mogelvang R, Kofoed KF, Kragelund C, Hove JD, Fabricius‐Bjerre A, Heitman M, Haack‐Sorensen M, Lund LD, Johansen EM, Qayyum AA, Mathiasen AB, Ekblond A. Rationale and design of the first double‐blind, placebo‐controlled trial with allogeneic adipose tissue‐derived stromal cell therapy in patients with ischemic heart failure: a phase II Danish multicentre study. Stem Cells Int 2017;2017:8506370. [DOI] [PMC free article] [PubMed] [Google Scholar]


