Abstract
The foreign body response is an immunological process that leads to the rejection of implanted devices and presents a fundamental challenge to their performance, durability, and therapeutic utility. Recent advances in materials development and device design are now providing strategies to overcome this immune-mediated reaction. Here, we briefly review our current mechanistic understanding of the foreign body response and highlight new anti-FBR technologies from this decade that have been applied successfully in biomedical applications relevant to implants, devices, and cell-based therapies. Further development of these important technologies promises to enable new therapies, diagnostics, and revolutionize the management of patient care for many intractable diseases.
The foreign body response (FBR) is an immune-mediated reaction to implanted materials where a cascade of inflammatory events and wound-healing processes result in fibrosis, or the cellular and collagenous deposition that encapsulates implants [1–3]. These events can compromise the performance and durability of implantable devices that require use over extended periods. Implant isolation by fibrosis often interferes with function, as a thick fibrotic layer can disrupt biosensing functions, cause pain, cut off the nourishment for cell-based implants, and ultimately lead to device failure [1,4].
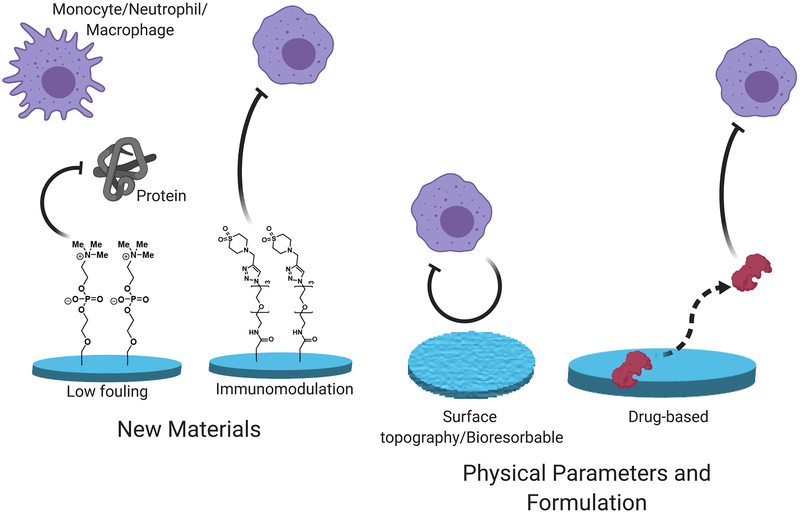
Overcoming the FBR could pave the way for implementing new therapeutic modalities, such as cell-based therapies, and improving the performance and durability of medical devices. A wide range of materials from naturally occurring polymers to synthetic materials [4–6] have been described as generating fibrotic responses, and there is a critical need for materials and formulations that possess both anti-inflammatory and anti-fibrotic properties [1,7,8]. Recent approaches have adopted novel strategies and materials that can successfully mitigate the FBR in a variety of animal models, including non-human primates (Figure 1). Combining new materials with low-fouling or immunomodulatory properties with optimized physical parameters for anti-fibrotic device design of drug-eluting formulations can be an effective strategy to achieve long-term mitigation of the FBR.
Figure 1.
An overview of the approaches taken to mitigate the immunological processes responsible for the foreign body response (FBR).
There are a number of review articles which provide a comprehensive account of the FBR and biomaterials development [1,9–15]. Rather than reiterate the content of these reviews, our aim is to highlight the new anti-FBR technologies from this decade that have been applied successfully in biomedical applications relevant to implants, devices, and cell-based therapies. In this perspective, we will briefly summarize our current understanding of the underlying biological mechanisms of the FBR, the recent advances that have been made to mitigate these fibrotic responses, and the future implications for the application of these new technologies for therapies and diagnostic devices.
1. Review of the Foreign Body Response (FBR)
The FBR is a complicated interplay between the innate and adaptive immune system that is not yet fully understood [1]. Improved immunological models of this response are helping guide improved design criteria for implantable materials. Here, we will briefly summarize the current understanding of the complex orchestration of immune events that directs the FBR (Figure 2).
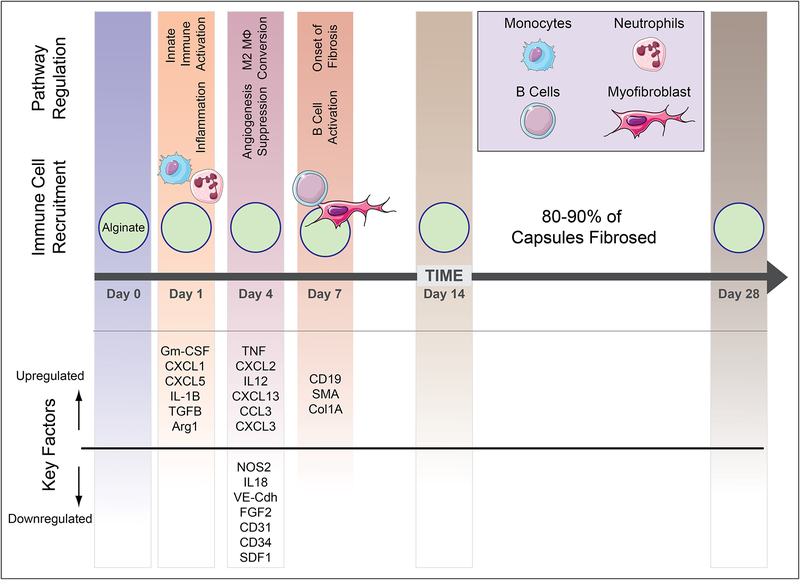
Figure 2.
Kinetic profile of host immune response to implanted biomaterials based on interpretation of data presented references [24,25].
Non-specific protein adsorption on the surface of implanted devices is an early-stage event that is associated with subsequent inflammation and wound healing processes [16]. A number of proteins that adsorb to biomaterial surfaces have been identified, and their relative adsorption ratio is dependent on the properties of the biomaterial itself. The composition and structure of the absorbed proteins can direct immune cell recognition and activation [17,18]. Since the composition of adsorbed proteins depends on the properties of the implanted material, the profile of these proteins might initiate immune-mediated processes that promote the FBR [16]. For example fibrinogen, a prominent adsorbed protein, has been shown to promote inflammation after surface deposition by interaction with integrin Mac-1 [19]. Pioneering work by Ratner, Horbett, and colleagues decades ago have highlighted the critical influence of the composition and unfolding pattern of absorbed proteins on biomaterials surfaces and foreign body response [9,20]. Following this early-stage protein adsorption, neutrophils begin to appear within a few hours at the implantation site and are the primary cell type for the first 2 days after implantation, at which point macrophages that differentiated from infiltrating monocytes become predominant [21]. The onset of inflammation triggers neutrophil degranulation, leading to the secretion of proteolytic enzymes and reactive oxygen species aimed at killing any pathogen infiltration but also damage the biomedical implant itself. This response can cause corrosion of certain materials susceptible to damage under oxidative environments such a polyurethane implants [22]. Materials are also susceptible to fouling by neutrophils by their secretion of extracellular fibers called as Neutrophil Extracellular Traps (NETs) [23].
Monocytic infiltration into the implant site leads to the secretion of cytokines, Interleukin-1 (IL-1), IL-8, and chemokines, monocyte chemotactic protein-1 (MCP-1), CXCL13, and macrophage inflammatory protein (MIP) [26]. These chemokines attract and stimulate more monocytic infiltration and macrophage activation. The infiltrating monocytes can differentiate into macrophages with varied polarization states and phenotypes [24,27]. The activated macrophages stay at the implantation site for multiple days and attempt to phagocytose the foreign material. The continuous presence of the implant leads to further macrophage differentiation and eventually to the fusion into foreign body giant cells (FBGC), containing up to 100 nuclei. Transforming Growth factor β (TGF-β), IL-1, and TNF-α seems to be important factors in the formation of FBGC and can be found in high concentrations around biomedical implants [28,29]. TGF-β has been shown to enhance the transformation of fibroblasts to myofibroblasts, promote the formation of extracellular matrix, and activate the contractile state of fibroblasts [30]. Fibroblasts appear in relevant numbers around day 7 after implantation and increase to until they represent the majority of cells in the fibrotic capsule on day 28 [31]. In addition, macrophages within the tissue capsule can begin to transdifferentiate into myofibroblasts [32–34]. The percentage of α-smooth muscle actin (α-SM) positive cells, a fibroblast marker, increases over time and their numbers peak on day 28. In the latter stages of the encapsulation, the formation of new blood vessels is induced by VEGF secretion from myeloid and giant cells [35]. However, there are not only myeloid cells found in the forming capsule, but other cells including lymphocytes and B cells have been shown to be involved in the FBR [29,36]. CXCL13 is produced by macrophages that attract B cells around day 7. The B cells have been shown to assist in the formation of the foreign body capsule [24].
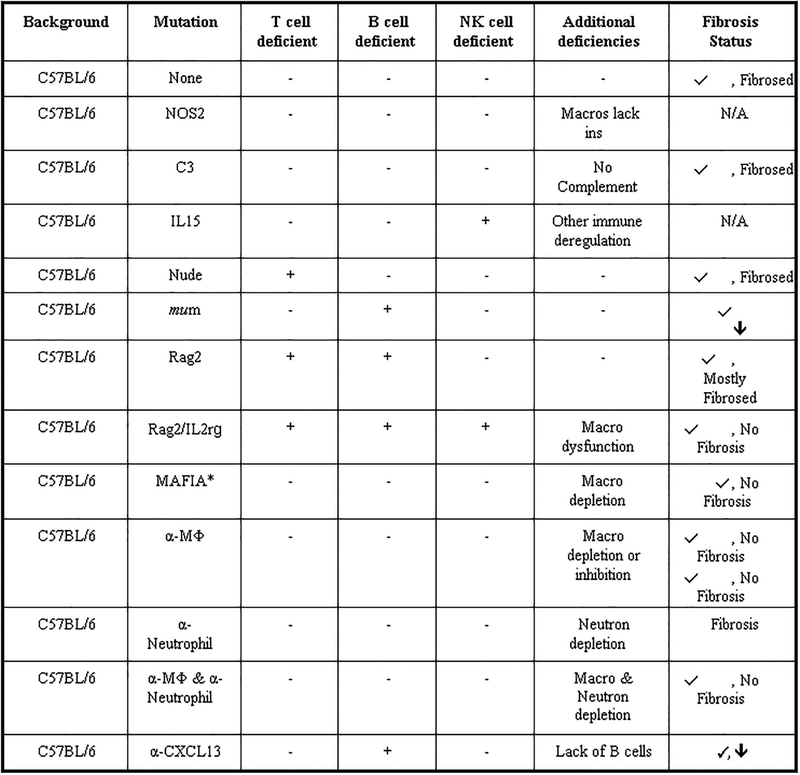
Valuable knowledge about the mechanism and hence hints on how to prevent or reduce the FBR can be gained from systematic profiling of mice with varying degrees of immune perturbations (i.e. genetic knockouts, chemical and/or antibody guided immune cell depletions) [24,31,37]. The most dramatic effect comes from the deletion of macrophages that eliminates the formation of an FBR completely. Campbell and coworkers showed that clodronate liposome-induced macrophage deletion prevents the FBR. Clodronate-induced deletion of phagocytes blocked monocyte infiltration, FBGC formation, neovascularization, and fibrosis [31,37]. In a systematic investigation, we looked at the effect of deleting different immune cell populations on the FBR (Figure 3) [24]. In accordance to the published literature, deletion of macrophages prevented fibrosis to subcutaneous and intraperitoneal alginate sphere implants (0.5 mm in diameter) in immune competent C57BL/6 mice after one month. B cell deletion resulted in a thinner capsule while the deletion of T cells did not affect the capsule thickness, and neutrophil deletion even lead to increased fibrosis of the alginate spheres. This increased response may result from the loss of the Ly6g-granulocyte myeloid-derived suppressor cell subset, that prevents excessive immune responses [38].
Figure 3.
Systems profiling of immune cell perturbations and its influence on Foreign Body Reactions and fibrosis. Using a combination of genetic knockouts and targeted immune depletions, macrophages were the only immune cell population required for fibrotic encapsulation of implanted alginate spheres. Right column: representative summary responses based on phase contrast images showing fibrosis levels on 500 μm alginate spheres retrieved from wild type C57BL/6 mice (n = 5/group), after 14-day intraperitoneal implantations. * = as reported. N/A = while available, not tested due to not being essential players. Figure and caption adapted with permission from reference [24].
Considering the important role that macrophages play in the FBR, it doesn’t come as a surprise that macrophage receptors that sample their environment also play an important role in the FBR. Integrins are a cell surface receptor family that facilitates the initial adhesion of the cell to the extracellular matrix. Fibrinogen is a main component of plasma and is well known protein found adsorbed to implanted biomaterials. Fibronectin and vitronectin, among others, are also important proteins commonly found on implanted biomaterials that share a high abundance in the tri-peptide sequence Arg-Gly-ASP (RGD) that is recognized by several integrins, known as RGD-binding integrins [39]. Macrophages bind and adhere to the RGD domain of these adsorbed proteins via the αMβ2 integrin, the Mac-1 receptor (CD11b/CD18) [40]. Mac-1 knock-out mice develop a 27% thinner fibrotic capsule around polyethylene terephthalate disks (PET) 14 days after subcutaneous implantation while blocking RGD ligands resulted in a 45% thinner capsule [41]. Selectins are a group of cell adhesion molecules that are activated by cytokines and play an important role in inflammation, and feature a carbohydrate recognition domain that is expressed by lymphocytes (L-selectin) and endothelial cells (E-selectin and P-selectin) [42]. C57BL/6 mice deficient in both P and E-selectin have a reduced fibrotic response to implanted PET disks after two weeks [43], consistent with the observed reduction of macrophages around the implants 16 h after the implantation. Toll-like receptors (TLR) also play an essential role in inflammation by recognition of exogenous and endogenous structures released from damaged tissue. TLR4 deletion showed a significant (>90 %) reduction in both numbers of blood vessels, polynuclear cells, and a 50% reduction in fibrotic capsule thickness to subcutaneously implanted silicon disks 14 days after the implantation [44].
Matricellular proteins such as SPARC (secreted protein, acidic and rich in cysteine), hevin, thrombospondins 1 and 2, osteopontin, and tenascin-C are part of the extracellular matrix. These proteins are involved in the modulation of cell-matrix interactions and cell proliferation and apoptosis and also play roles in propagating the FBR [42,45–47]. SPARC, also known as osteonectin, is a secreted calcium-binding glycoprotein that controls the production of numerous ECM proteins [48]. SPARC-null mice produce a significantly thinner fibrotic capsule to subcutaneously implanted polydimethylsiloxane disks and cellulose Millipore filters after 4 weeks [49]. Hevin, a homologue from the SPARC family, has no effect on the FBR when deleted [50], however, a double null variant of SPARC and hevin decreased capsule thickness more than just deletion of SPARC alone. Furthermore, the vascularization of the capsule is also increased in the double null variant, highlighting the importance for matricellular proteins for the regulation of angiogenesis and collagen deposition. Deletion of thrombospondin 2 (TSP2), an angiogenesis inhibitor, in TSP2-null mice yielded polydimethylsiloxane (PDMS) disks with a thicker but highly vascularized collagen capsule after a 4-week implantation period [51]. While this result lacked successful FBR reduction, the vascularized structure might allow the exchange with body fluids and still be enabling for biosensor applications. This finding was later confirmed when plasmids encoding TSP2 were co-delivered with implants to TSP2-null mice and resulted in a reduction of implant vascularization [52]. There has been much discourse about the role of neovascularization in the FBR and whether it contributes to the FBR or helps mitigate it [4,53]. In an interesting experiment Dondossola et al. showed FBR reduction using a VEGF trap in a subcutaneous implant model in C57BL/6 mice [37]. The VEGF block reduced collagen deposition, the number of giant cells around the implant, and stopped blood vessel formation. In this study inhibition of VEGF positively impacted the foreign body response, however, for many applications where live cells are combined with biomaterials the optimum design would produce minimal FBR but is supportive of vascularization [54]. This goal remains a major quest for current research efforts by the bioengineering community.
2. Optimizing Physical Properties and Formulation Approaches
2.1. Surface Topography: Size, Shape, and Texture
Physical parameters such as the shape, size, and texture of the biomedical implant are intrinsic properties that are also important factors that modulate the FBR [1,4,9,13,15]. The surface of a biomedical implant profoundly affects the behavior of macrophages and other immune cells, with implants lacking sharp edges and a smooth surface generally being more biocompatible and inducing less inflammation [13,55,56]. Indeed, even changes in surface roughness at the nanoscale have been associated with increased protein adsorption [57–61], and different nanostructured topographies can affect cellular interactions [62,63]. Varied immunological responses have also been characterized against a wide range of different biomaterial shapes [25,56,64–71]. A key study by Ward et al. implanted cylinders, made from different materials, subcutaneously in Sprague-Dawley rat for seven weeks and compared the capsule thickness of different cylinder diameters, materials. They found that the FBR capsule thickness depends on the material and that a porous surface results in a reduced capsule thickness. Thin cylinders (0.3 mm) compared to thick cylinders (2.0 mm) elicited a thinner capsule [72].
In a systematic study, we looked at the effect of the size of spherical implants by comparing alginate spheres with diameters around 0.3mm, 0.4 mm, 0.5mm, 0.7mm, 1mm, 1.5mm, and 1.9mm that were implanted into the peritoneal cavity of C57BL6 mice [25]. The spheres were retrieved after two weeks and showed a significant decrease in the level of cellular overgrowths with increased sphere diameter. This effect was repeated with other materials, including stainless steel, glass, polycaprolactone, and polystyrene. This size-dependent fibrotic effect also translated to larger rodent models: 0.5mm and 2.0mm glass spheres were transplanted into the peritoneal cavity of Sprague-Dawley rats for two weeks. The larger glass spheres came out clean while the smaller clumped together and were embedded in a thick fibrotic capsule. The same result was obtained with SLG20 alginate hydrogel spheres that were subcutaneously implanted in non-human primates.
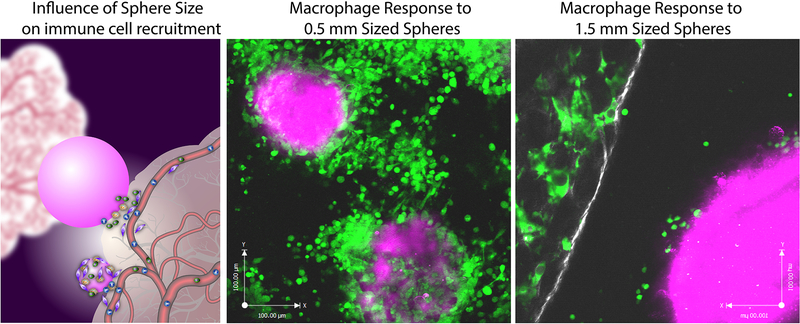
We further performed a functional test of encapsulated pancreatic islet cells in diabetic mice. The transplantation of free islets to diabetic patients started in 1989 [73]. These patients need lifelong immunosuppression to prevent immediate rejection by the host immune system. Various immune isolation strategies have been designed and utilized since, without yielding a clinical product [74]. The size hypothesis was further tested by encapsulating Islets of Langerhans in alginate spheres with diameters of 0.5 mm and 1.5 mm that were implanted in STZ-treated diabetic C57BL/6 mice. All mice receiving 0.5 mm capsule lost blood glucose control after 30 days while the mice implanted with 1.5 mm capsules controlled the blood glucose for more than 180 days [25]. We are still exploring the underlying mechanism behind these observations, and we currently hypothesize that increased sphere size reduces imbedding of implants into microgrooves of peripheral tissue. This effect creates additional barriers to the kinetics of immune cell extravasation out of peripheral tissue and on to the implant surface (Figure 4).
Figure 4.
Extravasation of macrophage cells from peripheral tissue on to spherical materials of 0.5 mm and 1.5 mm spheres. (a) schematic describing how smaller spheres better conform to peripheral adjacent tissue cervices compared to larger spheres and this influence on the ability of immune cells to extravasate on to implants from vasculature. In vivo intravital imaging of macrophage behavior and accumulation at 7 days post-implantation on to (b) 0.5 mm and (c) 1.5 mm diameter sized Ba+ crosslinked alginate spheres. (macrophages depicted in green, peripheral tissue in white and implanted spheres in magenta). Figure and caption adapted with permission from reference [25].
Macrophages are essential for capsule formation in the foreign body reaction and are therefore an important target for FBR prevention. They constantly attach, probe, and respond to cues of their environment. Based on cues from their environment they polarize into different subtypes: proinflammatory M1, or anti-inflammatory M2a, immune-regulating M2b, or tissue remodeling M2c [75]. Increasing the ratio of M2 to M1 macrophages is one strategy to improve tissue remodeling outcomes [76]. Ratner and coworkers showed that porous pHEMA-co-MAA scaffolds with a defined pore size, around 30 μm diameter that mimick decellularized extracellular matrices, polarized more macrophages to a M2 phenotype and resulted in reduced capsule thickness and enhanced neovascularization after 4 weeks [77].
Recent studies have investigated the association of reduced capsule thickness with the M2:M1 macrophage polarization ratio. pHEMA scaffolds with no pores, 34 μm and 160 μm pores were implanted into subcutaneous mouse tissue [78], and the results indicated a more complicated relationship than just the M2:M1 polarization ratio. Macrophages in the 34 μm pores displayed increased M1 markers and decreased M2 markers, and macrophages right next to the implant possessed increased M2 markers. This effect has also been observed in other materials, including silicone elastomer and polyurethane [79].
The hierarchal architecture and surface roughness of implanted devices can influence foreign body reactions [80]. Clinical data with breast prosthetic implants has shown varying foreign body responses based on implant surface architectures. Microsized textures have been introduced on the surface of silicone breast implants, and the textured breast implants produced lowered rates of capsule formation in short-term clinical follow-up compared non textured implants [81]. However, the long-term effects are not fully understood and careful investigation is needed to better elucidate the mechanisms responsible for altered immune responses through surface texturing. Importantly, the United States FDA recently warned that patients with textured silicone breast implants may have a higher risk of developing anaplastic large-cell lymphoma (ALCL) compared to smooth ones. These cases of ALCL appear to be linked to aggressively textured surfaces with roughness of >300 μm [82]. Researchers have proposed and explored tuned biomimetic designs of nano/micro architectures to produce desired biocompatibility and safety. [83]. Human clinical data from breast implants suggest that surface topography can affect host immune reactions, however more studies are needed to identify the most impactful and safe design for chronic implants.
An interesting approach is to “hide” the biomedical implant in the extracellular matrix as a form of immune isolation [27,84]. Sandor et al. coated tissue expanders with decellularized extracellular matrices and implant them in SC space for nine weeks into non-human primate model [85]. They reported minimal capsule formation implants coated with non-irradiated freeze-dried human matrix, while gamma-irradiated matrix coated expanders where mostly encapsulated, like non-encapsulated expanders. This is an interesting approach, however, not practical as the harvesting of a human extracellular matrix is preventatively difficult and expensive.
2.2. Drug-Based Approaches
Suppression of the inflammatory response was tried to with the co-delivery of nonsteroidal anti-inflammatory drugs (NSAIDs) with the implants. These strategies proved effective in inhibiting the early stages of the FBR but could not provide durable, long-term protection [53,86]. Corticosteroids and tyrosine kinase inhibitors have had some effect on the lifetime of implanted biosensors [4,87]. This anti-fibrotic effect is supported by the finding that corticosteroids reduce the levels of TGFβ and hence, reduce the number of myofibroblasts and inhibits angiogenesis [88]. Dexamethasone, a potent anti-inflammatory, has been used in drug-releasing devices engineered to release over extended periods of time (3 months) and reduce device fibrosis [89,90]. However, when the release stopped in these devices, an inflammatory response could be observed, and the devices were encapsulated. Anti-inflammatory drug-eluting devices for preventing the FBR and has also been reviewed elsewhere [91].
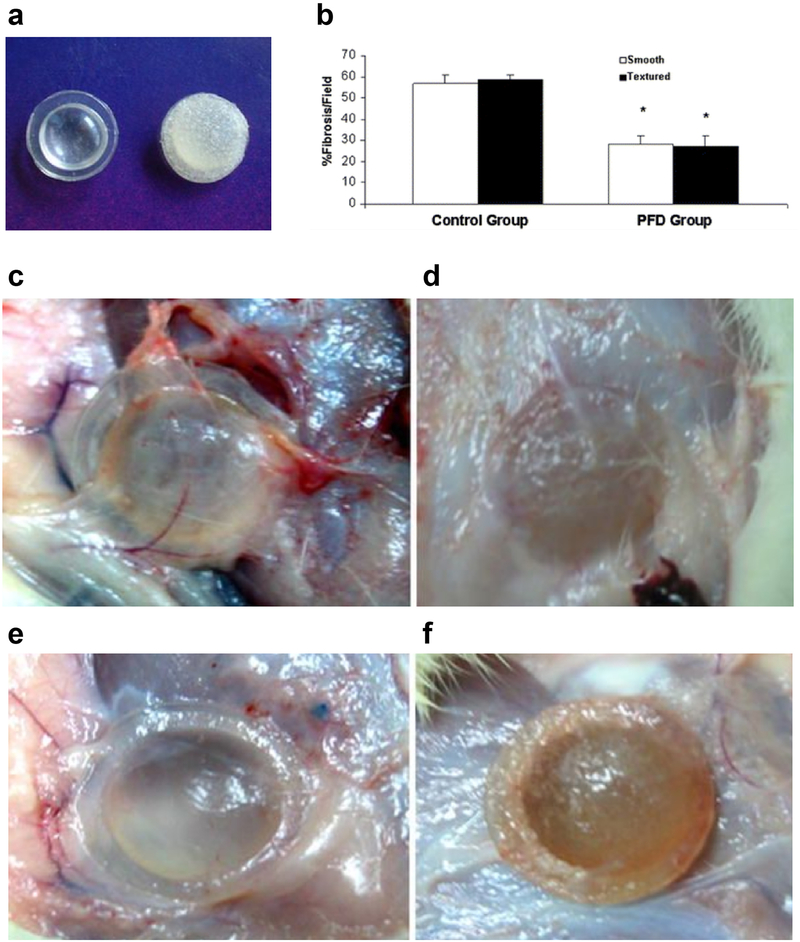
Antifibrotic agents used to treat fibrosis of lung, liver, and kidney, have been used to reduce the FBR, as there are many mechanistic similarities between the FBR and fibrosis of vital organs [92]. Submammary implantation of silicone gel implants with Pirfenidone resulted in a 50% reduction of collagen content around the implants (Figure 5) [93]. Pirfenidone is an approved drug for curing the idiopathic pulmonary fibrosis by downregulating growth factors and procollagens I and II. An alternative approach utilizing RNA interference technology (RNAi) has also been reported [94]. Small-interfering RNA (siRNA) targeting collagen type I (COL1A1), the major component of fibrous tissue, was delivered from electrospun nanofibers. Reduced collagen production and consequently a reduced capsule thickness around the subcutaneously implanted nanofibers was observed in Sprague-Dawley rats after 2 and 4 weeks. However this approach is not always successful, as a previous attempt delivering siRNA targeting mTOR, which in vitro significantly inhibited fibroblast proliferation and type I collagen expression, from PEG based hydrogels coating subcutaneous implants in C57BL/6 mice proved ineffective in reducing the thickness of the fibrous surrounding the capsules in vivo [95].
Figure 5.
Silicone breast implant embedded with Pirfenidone. (a) Both smooth and textured implants were tested in rats. (b) Fibrosis analysis of the implants based on Masson’s trichrome staining of immunohistological sections. Drug-treated mice showed reduced fibrosis to control implants. Images of (c) smooth, (d) textured, (e) drug-treated smooth, and (f) drug-treated textured implants. Figure and caption adapted with permission from reference [93].
The elution of chemokines is another attractive strategy for modulating the immune responses to implants. In a study aimed at improving outcome for islet transplantation, alginate microspheres formulated with sufficient amounts of the chemokine CXCL12 have resisted fibrosis and promoted the survival of the encapsulated islets [96]. The chemokine is proposed to recruit Treg cells, reduce the number of Teff cells to the site of implantation, and reduce inflammatory signaling. More recently, the authors demonstrated the value of this approach in supporting the long-term survival of human stem cell-derived SC-β cells in an immunocompetent diabetic mouse model [97]. These results further highlight the utility of promoting an anti-inflammatory environment at the implantation site to mitigate the FBR.
For many applications it will be necessary to combine various design elements to develop macro-scaled devices that can provide spatiotemporal control over delivery of a wide range of bioactive molecules [74]. For example, in the field of encapsulated cell therapies for the treatment of type 1 diabetes combinations of drug elution and improved materials designs have yielded improved performance of transplanted grafts [98]. For example Anderson and colleagues demonstrated that formulations of anti-inflammatory drug crystals prepared as compact lattice structures combined with larger alginate spherical capsules loaded with insulin producing cells could achieve extended durable cures in rodent and non-human primates [99]. Stabler and coworkers demonstrated the utility of crystallization as an approach to improve oxygen delivery to the encapsulated cells by incorporating calcium oxide crystals within encapsulated cell constructs [100].
2.3. Bioresorbable Device Formulations
Real-time in vivo monitoring is an attractive capability for understanding dynamic changes in physiological states. While there are numerous techniques for monitoring physiological processes, such as functional magnetic resonance imaging, near-infrared spectroscopy, magnetoencephalography and positron emission tomography, these methods do not provide continuous measurements over timeframes (days to months) that enable dynamic physiological monitoring. Implantable electronic implants are an attractive solution, but traditional non-degradable devices suffer from limitations to their utility and durability due to increased infection risk, immunological processes such as the FBR, and the need for subsequent surgical extraction to reduce associated health risks to the patient [101–103]. Bioresorbable device technologies, or implants that fully degrade after a defined period, is a way to avoid the FBR to implantable devices by providing an alternative pathway for immune processes to resolve inflammatory responses, and offer unique opportunities where diagnosis and therapy can occur at specific sites inside the body.
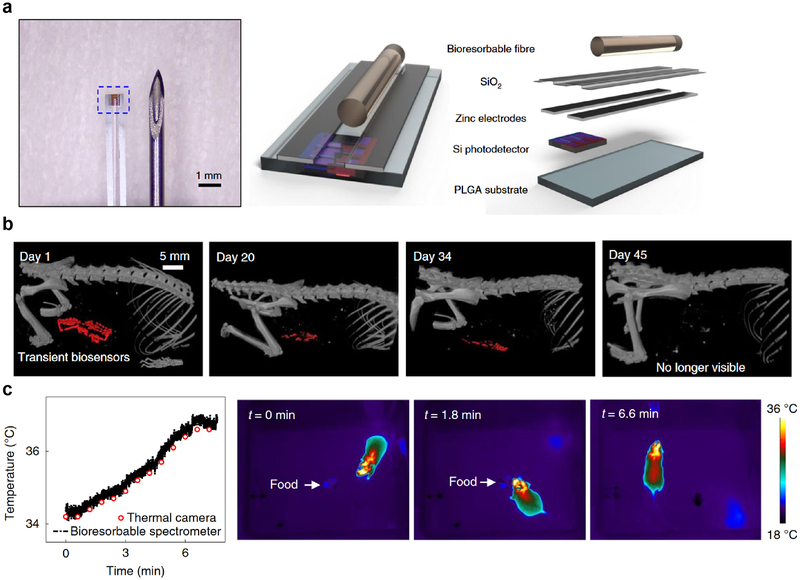
Complete bioresorption leads to benign end products that are cleared naturally and eliminate the hardware without the need for implant extraction. Recently there have been numerous studies that have successfully developed these degradable sensors and evaluated their in vivo utility. Common to many of these remotely-controlled devices are their meso-scaled dimensions (mm) and micron thickness, with active elements fabricated from inorganic materials (silicon, zinc, magnesium, etc.) encapsulated in a biodegradable polymeric material. The degradation rate of these devices can be modulated by adjusting the thickness of the active element and encapsulating material, making devices that last days, weeks, or months in vivo. Rogers and coworkers have designed a number of these bioresorbable electronic systems and have successfully monitored in mice a variety of physiological metrics, such as: intracranial pressure, spatiotemporally mapping of electrical activity from the cerebral cortex and cardiac muscle tissue, and cerebral temperature and oxygenation (Figure 6) [104–106]. Analogously, Son et al. fabricated larger resorbable electronic stents that can apply controlled therapy for endovascular diseases [107]. Tao et al. encapsulated their active elements in silk to make implantable devices that can affect infection abatement [108]. In addition to electronic sensing modalities, resorbable devices that facilitate spectroscopic measurements in the visible and near-infrared wavelengths have also been applied mice, thereby providing a complementary strategy for monitoring and sensing key physiological parameters [109,110].
Figure 6.
A bioresorbable fibre optic probe for making physiological measurements. (a) The Si nanomembrane photodetector is encapsulated in a PLGA-based fibre optic fibre. (b) Computed tomography imaging in mice showing the gradual resorption of the probe over a period of 7 weeks. (c) In vivo function of the bioresorbable spectrometer implanted in mice, showing measurement of brain temperature during food intake tests. Figure and caption adapted with permission from reference [106].
The recent emergence of these bioresorbable implant technologies offer an attractive approach to avoid the FBR altogether. These approaches are well suited for biomedical applications where implant functions are only necessary for a limited period and where physiological monitoring is key. While other biomedical applications, such as cell-based therapy, may be more difficult to envision benefitting from this approach it nonetheless is a viable strategy for implant technologies where function is only required for specific periods after implantation.
3. New Materials that Mitigate the FBR
3.1. Zwitterionic materials
Jiang and colleagues investigated if the ultra-low fouling properties that have been described for zwitterionic materials would be a viable strategy to mitigate foreign body responses to implants in mice [5,111–113]. The investigators focused their efforts specifically on carboxybetaine chemotype due to its low protein absorption, its biomimetic nature, and the ease with which these moieties can be incorporated into implantable materials. Previously the authors had demonstrated that methacrylate analogs of this zwitterionic moiety could be polymerized to form hydrogels with superior anti-fouling properties when compared to pHEMA. To evaluate the ability of these zwitterionic materials to resist fibrosis, PCBMA hydrogels were implanted subcutaneously in the C57BL/6 mice and then characterized for their inflammatory and fibrotic responses at 1 week, 4 weeks, and 3 months. By immunohistochemistry, the PCBMA gels showed few inflammatory cells at the hydrogel-tissue interface compared to pHEMA, and minimal collagen encapsulation of the implant even 3 months post-implantation. The authors also noted the formation of blood vessels adjacent to PCBMA hydrogels, supporting the notion of a more “normal” extracellular matrix rather than the collagenous deposition resulting from a foreign body response. In contrast, pHEMA gels were markedly encapsulated by 4 weeks. Since the measured mechanical properties of the gels were the comparable, the authors conclude that the low-fouling property of the PCBMA hydrogel must be responsible for the anti-fibrotic effect. To investigate a plausible mechanism for this effect, the authors characterized the macrophages found at the hydrogel-tissue interface. By triple-label immunofluorescence a larger number of macrophages expressing anti-inflammatory markers were measured at the interface with PCBMA gels when compared to pHEMA. Taken together with increased angiogenesis at the implantation site, this data lead the authors to conclude that polarization of macrophages at the hydrogel-tissue interface to an anti-inflammatory/wound-healing phenotype enabled the zwitterionic gel to resist fibrosis.
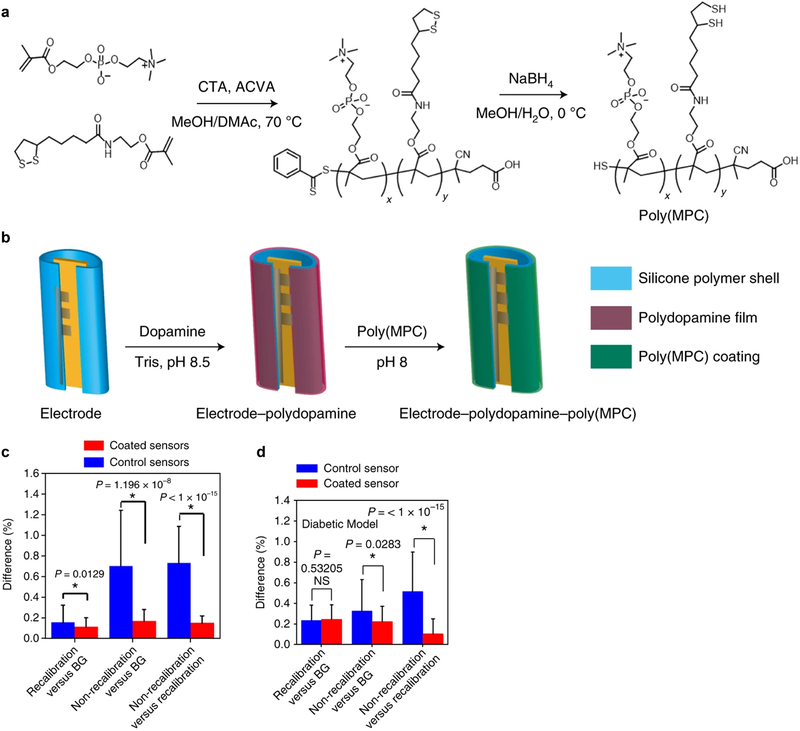
Seeking to incorporate the low-fouling and anti-fibrotic properties of zwitterionic materials into a platform suitable for cell encapsulation, the Anderson lab coated alginate microspheres with a zwitterionic poly(methacryloyloxyethyl) phosphorylcholine (pMPC) containing copolymer [114]. A dopamine-based conjugation strategy was implemented, where oxidative self-polymerization of dopamine to an alginate microcapsule surface is followed by attachment to the thiol-containing zwitterionic copolymers. Implantation of these coated microcapsules into the IP space of C57BL/6 mice showed lower levels of fibrotic deposition after 14 days in vivo complemented by lower levels of macrophages and neutrophils from the retrieved capsules. Further demonstrating the utility of the dopamine conjugation strategy for biomedical devices, Anderson and coworkers applied this strategy to improve the function of continuous blood glucose monitors (CGM) (Figure 7) [115]. Traditional CGMs suffer from reliability and short-term noise issues that have limited their use for only 6 days post-implantation of the sensor and require constant recalibration [116]. The authors hypothesized that FBRs against the implanted sensor were responsible for the observed reliability and noise issues. To test if FBR mitigation could improve the CGM function and signal noise, sensors were coated with zwitterionic PEG polymers using the dopamine conjugation strategy discussed above. To determine which zwitterionic moiety produced the lowest inflammation subcutaneously, the researchers measured the inflammatory levels of a library of 64 zwitterionic hydrogels 7 days post-implantation. Poly(MPC) yielded the lowest levels of inflammation, and the CGM sensor electrodes were coated utilizing the same dopamine conjugation strategy reported earlier. The performance of these sensors was not disrupted by the coating process, and their in vivo performance was measured by utilizing glucose challenges over a three day period. Uncoated sensors showed deviations in their signal calibrations within this period, while the coated sensors maintained their linear calibrations and displayed significantly higher accuracy levels without any need for recalibration. The improved performance of the coated sensors was justified by the lower inflammatory and foreign-body response associated processes that were measured relative to uncoated electrodes. The improved performance and accuracy of the coated CGM electrodes even extended into diabetic NHPs, further supporting the translational potential of this approach.
Figure 7.
Low-fouling polymeric coatings improve CGM performance. (a) Zwitterionic Poly(MPC) polymers were copolymerized with thiol-containing monomers. (b) A dopamine-based strategy was used to coat CGM electrodes. Glucose-sensing performance of the coated electrodes was superior in both (c) murine and (d) NHP models of diabetes. Figure and caption adapted with permission from reference [115].
3.2. Modified Alginates
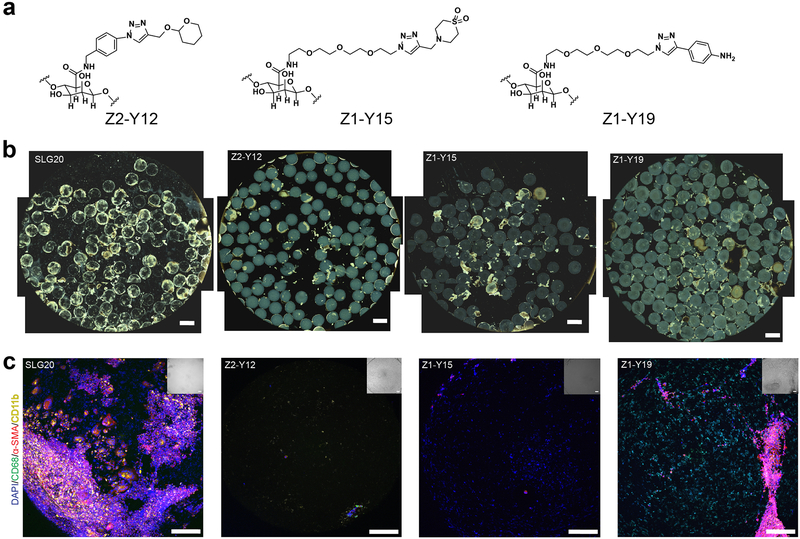
A separate strategy we undertook was a chemical modification approach to optimize the biological performance of alginate (Figure 8) [117]. Rather than alter the bulk properties of the polymer which are already quite effective for a cell-encapsulating material, we hypothesized that making alginate analogs with sufficient small molecule modification of the polymer backbone could modulate immune-recognition events that trigger the foreign body response. Since understanding the molecular nature of these recognition events is still an active area of research, we made a diverse set of 774 alginate analogs bearing drug-like structures in a combinatorial manner. To evaluate this collection, we monitored early inflammation in vivo as a general indicator of immune recognition by injecting the hydrogels subcutaneously and measuring cathepsin activity (a marker for degranulation) at the injection sites seven days later. This inflammatory screening narrowed our focus to 200 alginate analogs that showed a significantly reduced cathepsin activity (20–80%) compared to unmodified alginate. We were able to narrow this down further by selecting a 69 lead analogs from this pool of 200 and retested them in the subcutaneous inflammation assay formulated as microcapsules to control for implant geometry, and selected our lead analogs as the top ten polymers with the lowest inflammation levels. Since the intraperitoneal (IP) space is a leading implantation site for cell-based implants, microcapsules fabricated from these lead materials were then implanted IP and the level of fibrosis on the capsules were characterized upon removal after 14 days. Phase contrast and immunofluorescence staining for cellular, macrophage, and myofibroblast deposition supported three lead materials with minimal collagenous and cellular deposition. These observations were supported by significant reductions in quantified levels of α-smooth muscle actin, collagen, macrophages, and neutrophils extracted from the explanted capsules. Intra vital imaging in mice of fluorescent microcapsules further corroborated these results, with a marked decrease of macrophages in the IP space of a live anesthesized animal and no macrophage aggregation observed at the implant surface. To further establish the translational potential of these materials into higher-order animal species, 1.5 mm spheres of the three lead materials and an unmodified alginate control were implanted into the IP space of non-human primates (NHP, cynomolgus macaques). Retrieval of the spheres 4 weeks after implantation mirrored the rodent results, and even after a six-month period spheres could be retrieved with minimal fibrotic deposition.
Figure 8.
Small-molecule modifications of the alginate backbone can mitigate the FBR. (a) The lead small-molecule modifications that were identified from a combinatorial modified alginate library. These lead modified alginates successfully mitigated fibrosis in the IP space of both immunocompetent murine and NHP animal models. (b) Representative phase contrast imaging of retrieved capsules for the control (SLG20) and modified alginate formulations after 4 weeks in NHP are shown. (c) Immunofluorescence imaging of the retrieved capsules in (b) showed reduced markers of general cellular material (DAPI), myofibroblasts (α-SMA), and macrophages (CD11b). Figure and caption adapted with permission from reference [117].
Interestingly, the mechanism by which these lead materials resist fibrosis is still to be determined. All three lead materials contained triazole-containing scaffolds for their chemical modifications, consistent with a common mechanism of action [117]. Measurements taken of the mechanical strength, surface roughness, and protein adsorption of the modified, lead alginate microcapsules revealed comparable values to that of unmodified alginate microcapsules and unable to account for the biological performance. The lead alginate microcapsules also displayed distinct surface topographies from each other (and control capsules) by cryo-SEM, precluding any justification based on surface features. Confocal raman spectroscopy of the microcapsules showed that two of the three modified alginate capsules showed a concentrated localization of their chemical modifications at the surface of the capsule, while the third showed a more uniform distribution. Interestingly, these same two lead materials performed better compared than the third, more uniformly distributed, alginate modification in NHPs. Taken together, these results lead us to conclude that the chemical modifications themselves were modulating macrophage behavior at the molecular level to inhibit macrophage activation and disrupt fibrotic processes, as opposed to an optimization of the bulk properties of the material as a whole. Related studies examining the effect of chemically-modified alginates on macrophages have reached similar conclusions [118]. In summary, taking a combinatorial approach to material development combined with an in vivo focused evaluation strategy enabled the discovery of alginates capable of resisting the foreign body response, even in translationally relevant NHP models.
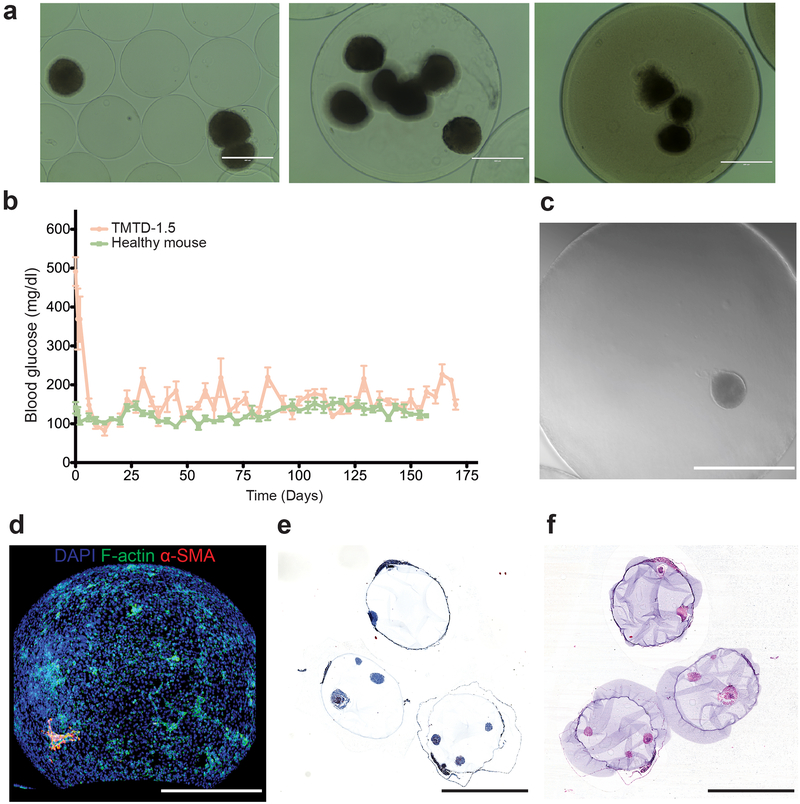
We then investigated whether anti-fibrotic materials had the potential to improve the functional, therapeutic performance of cell-based implants (Figure 9) [119]. Melton and coworkers had previously established a differentiation protocol to generate at Harvard, we encapsulated human stem cell-derived β cells (SC-β) in one of our lead materials and evaluated their ability to restore normoglycemia in an immunocompetent diabetic mouse model of type 1 diabetes [119]. Utilizing a human-derived cell transplant in a mouse recipient model provided a high-bar for success, since immune processes for tissue rejection are aggressive in xenogeneic settings. Over the course of three months, only diabetic mice implanted with SC-β cells encapsulated in our lead alginate formulated as large spheres (>1.5 mm diameter) were able to maintain normoglycemia over the entire study period. Unencapsulated cells were rejected quickly, and cells encapsulated in control, unmodified alginate spheres were unable to maintain normoglycemia for more than 15–30 days. As anticipated, lower levels of cellular, collagenous, and macrophage deposition were measured on retrieved capsules of the lead formulation compared to the controls. Immunostaining of the cells before implantation and after retrieval showed that the SC-β cells retained their differentiation state throughout the study. Mirroring the fibrosis results from our early studies, the encapsulated SC-β implants were able to maintain normoglycemia as far out as six months with their function intact. C-peptide levels throughout the six-month period were comparable to wild-type mice, and an intravenous glucose-tolerance test at the end of the study period showed dynamic glucose correction comparable to that of a healthy mouse. The capability of these modified alginate materials to resist foreign body responses successfully translated to the improved performance of a therapeutic cell therapy in an immunocompetent disease model of diabetes. Finally, these results also extended into an allogeneic islet transplantation in cynomolgus macaques, where encapsulated donor islets from were implanted into recipient NHPs [120]. Islets encapsulated in a leading chemically-modified alginate (Z1-Y15) as 1.5 mm spheres successfully protected viable and glucose-responsive islets for 4 months in the bursa omentalis of the NHP recipients, further establishing the long-term transplantation potential of this technology for cell-based therapies.
Figure 9.
Modified alginates enable successful long-term transplantation of human-derived SC-β cells in diabetic, immunocompetent mice. (a) Encapsulated SC- β cells in TMTD-alginate spheres with 1.5mm diameters. (b) The modified alginate spheres supported long-term (6 month) glycemic correction. The encapsulated cells showed signs of reduced fibrosis even at the end of the 6-month period. (c) Brightfield imaging of a retrieved sphere with encapsulated SC-β cell cluster seen inside. (d) Immunofluorescence straining, (e) Masson’s trichrome, and (f) H&E histology of the retrieved spheres. Figure and caption adapted with permission from reference [119].
4. Conclusion and Future Perspective
The FBR is a fundamental obstacle to the performance and durability of many implantation technologies. Major advances in the development of new anti-fibrotic materials, optimal physical device parameters, formulations, and in our understanding of this complex immunological process have enabled solutions to this long-standing biomedical challenge. From both the literature and our own experience, choosing the right animal models to study the FBR is key for developing technologies that translate into higher-order species. For example, not all murine strains display robust FBRs and an implant optimized in the wrong strain may fail when tested in NHPs and even humans. Implants in Balb/c versus C57BL/6 mice are representative of these strain-dependent effects, where C57BL/6 mice have responses in line with FBR observed in NHPs and humans [73,120–123]. As our understanding of the underlying immunology has advanced there are new drug-based approaches for modulating the FBR, and understandably there is a focus to make these formulation-based approaches achieve long-term durability by extending the drug-release period. Fully bioresorbable and functional devices have now been described owing to more advanced fabrication methods and offer a new way to avoid the complications of the FBR altogether when function is only required for short periods. The development of new materials and optimal physical parameters that mitigate the FBR provide both a new starting point for creating implants with inherent anti-fibrotic properties and also a useful tool to uncovering new mechanistic targets for future design.
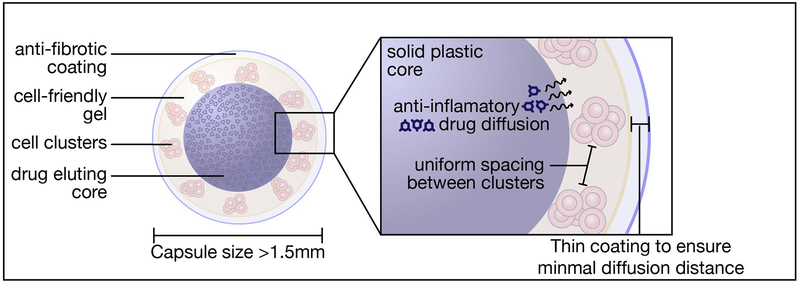
In this perspective, we have highlighted biomedical modalities that can immediately benefit from these advances, from existing CGM devices, bioresorbable functional implants, to emerging cell-based therapies. The application of anti-fibrotic technologies to cell-based therapeutics is particularly important, since this new modality has potential to revolutionize the management of patient care for many intractable diseases. The unique ability of cells to precisely sense their environment, process signals, and biologically respond enables new therapeutic opportunities, and the field of synthetic biology is enabling the design of engineered cell lines that can deliver a wide range of biologic therapies. These new cell products still require immunoprotective technologies that shield them from transplant rejection, and the innovations outlined here that mitigate the FBR will play a role in producing high-performing and durable clinical products. To this end, it is likely that durability will require a combination of the innovations in new anti-fibrotic materials, optimal physical parameters of the implant, and anti-inflammatory/anti-fibrotic drug-eluting capability (Figure 10). Finally, the insights from these advances will catalyze next generation technologies and provide tools for deeper probing of FBR mechanisms, furthering the development of truly biocompatible and immunologically tolerated biomedical devices.
Figure 10.
Schematic of a macroscaled device design that combines various approaches to addressing foreign body response to achieve improved in vivo performance for cell-based therapies.
Acknowledgements
This work was supported by grants from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIH NIDDK RFA-DK-15–030, NIH NIDDK 5R01DK120459), CPRIT (RR160047), JDRF (PNF-2018–662-S-B), and a Sigilon Therapeutics SRA. Figure 1 was created in Biorender (https://biorender.io).
Footnotes
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by Advanced Drug Delivery Reviews. O.V. and A.J.V. are scientific founders of Sigilon Therapeutics.
References
- 1.Anderson JM, Rodriguez A, Chang DT, Foreign body reaction to biomaterials, Seminars in Immunology. 20 (2008) 86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wick G, Grundtman C, Mayerl C, Wimpissinger T-F, Feichtinger J, Zelger B, Sgonc R, Wolfram D, The Immunology of Fibrosis, Annual Review of Immunology. 31 (2013) 107–135. doi: 10.1146/annurev-immunol-032712-095937. [DOI] [PubMed] [Google Scholar]
- 3.Wynn TA, Ramalingam TR, Mechanisms of fibrosis: therapeutic translation for fibrotic disease, Nat Med. 18 (2012) 1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ward WK, A Review of the Foreign-body Response to Subcutaneously-implanted Devices: The Role of Macrophages and Cytokines in Biofouling and Fibrosis, J Diabetes Sci Technol. 2 (2008) 768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Cao Z, Bai T, Carr L, Ella-Menye J-R, Irvin C, Ratner BD, Jiang S, Zwitterionic hydrogels implanted in mice resist the foreign-body reaction, Nat Biotech. 31 (2013) 553–556. doi: 10.1038/nbt.2580. [DOI] [PubMed] [Google Scholar]
- 6.Ratner BD, Reducing capsular thickness and enhancing angiogenesis around implant drug release systems, Journal of Controlled Release. 78 (2002) 211–218. doi: 10.1016/S0168-3659(01)00502-8. [DOI] [PubMed] [Google Scholar]
- 7.Langer R, Perspectives and Challenges in Tissue Engineering and Regenerative Medicine, Advanced Materials. 21 (2009) 3235–3236. doi: 10.1002/adma.200902589. [DOI] [PubMed] [Google Scholar]
- 8.Harding JL, Reynolds MM, Combating medical device fouling, Trends in Biotechnology. 32 (2014) 140–146. doi: 10.1016/j.tibtech.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Ratner BD, Biomaterials: Been There, Done That, and Evolving into the Future, Annu. Rev. Biomed. Eng 21 (2019) 171–191. doi: 10.1146/annurev-bioeng-062117-120940. [DOI] [PubMed] [Google Scholar]
- 10.Boddupalli A, Zhu L, Bratlie KM, Methods for Implant Acceptance and Wound Healing: Material Selection and Implant Location Modulate Macrophage and Fibroblast Phenotypes, Advanced Healthcare Materials. 5 (2016) 2575–2594. doi: 10.1002/adhm.201600532. [DOI] [PubMed] [Google Scholar]
- 11.Shayanti Mukherjee, Saeedeh Darzi, Kallyanashis Paul, Werkmeister Jerome A., Gargett Caroline E., Mesenchymal stem cell-based bioengineered constructs: foreign body response, cross-talk with macrophages and impact of biomaterial design strategies for pelvic floor disorders, Interface Focus. 9 (2019) 20180089. doi: 10.1098/rsfs.2018.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams DF, Biocompatibility Pathways: Biomaterials-Induced Sterile Inflammation, Mechanotransduction, and Principles of Biocompatibility Control, ACS Biomater. Sci. Eng 3 (2017) 2–35. doi: 10.1021/acsbiomaterials.6b00607. [DOI] [PubMed] [Google Scholar]
- 13.Mariani E, Lisignoli G, Borzì RM, Pulsatelli L, Biomaterials: Foreign Bodies or Tuners for the Immune Response?, Int J Mol Sci. 20 (2019). doi: 10.3390/ijms20030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung L, Maestas DR, Housseau F, Elisseeff JH, Key players in the immune response to biomaterial scaffolds for regenerative medicine, Adv. Drug Deliv. Rev 114 (2017) 184–192. doi: 10.1016/j.addr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Chandorkar Y, K R, Basu B, The Foreign Body Response Demystified, ACS Biomater. Sci. Eng 5 (2019) 19–44. doi: 10.1021/acsbiomaterials.8b00252. [DOI] [PubMed] [Google Scholar]
- 16.Wells LA, Guo H, Emili A, Sefton MV, The profile of adsorbed plasma and serum proteins on methacrylic acid copolymer beads: Effect on complement activation, Biomaterials. 118 (2017) 74–83. [DOI] [PubMed] [Google Scholar]
- 17.Swartzlander MD, Barnes CA, Blakney AK, Kaar JL, Kyriakides TR, Bryant SJ, Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics, Biomaterials. 41 (2015) 26–36. doi: 10.1016/j.biomaterials.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vu VP, Gifford GB, Chen F, Benasutti H, Wang G, Groman EV, Scheinman R, Saba L, Moghimi SM, Simberg D, Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles, Nature Nanotechnology. 14 (2019) 260. doi: 10.1038/s41565-018-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang L, Ugarova TP, Plow EF, Eaton JW, Molecular determinants of acute inflammatory responses to biomaterials, Journal of Clinical Investigation. 97 (1996) 1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proteins at Interfaces III State of the Art, (n.d.). doi: 10.1021/bk-2012-1120.ch014. [DOI] [Google Scholar]
- 21.Kastellorizios M, Tipnis N, Burgess DJ, Immune Responses to Biosurfaces. 865 (2015) 93–108. [DOI] [PubMed] [Google Scholar]
- 22.Labow RS, Meek E, Santerre JP, Neutrophil‐mediated biodegradation of medical implant materials, Journal of Cellular Physiology. 186 (2001) 95–103. [DOI] [PubMed] [Google Scholar]
- 23.Jhunjhunwala S, Aresta-DaSilva S, Tang K, Alvarez D, Webber MJ, Tang BC, Lavin DM, Veiseh O, Doloff JC, Bose S, Vegas A, Ma M, Sahay G, Chiu A, Bader A, Langan E, Siebert S, Li J, Greiner DL, Newburger PE, von Andrian UH, Langer R, Anderson DG, Neutrophil Responses to Sterile Implant Materials, PLoS One. 10 (2015) e0137550. doi: 10.1371/journal.pone.0137550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doloff JC, Veiseh O, Vegas AJ, Tam HH, Farah S, Ma M, Li J, Bader A, Chiu A, Sadraei A, Colony stimulating factor-1 receptor is a central component of the foreign body response to biomaterial implants in rodents and non-human primates, Nature Materials. 16 (2017) 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, Jhunjhunwala S, Chiu A, Siebert S, Tang K, Hollister-Lock J, Aresta-Dasilva S, Bochenek M, Mendoza-Elias J, Wang Y, Qi M, Lavin DM, Chen M, Dholakia N, Thakrar R, Lacík I, Weir GC, Oberholzer J, Greiner DL, Langer R, Anderson DG, Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates, Nature Materials. 14 (2015) 643–651. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamashiro S, Kamohara H, Wang J-M, Yang D, Gong W-H, Yoshimura T, Phenotypic and functional change of cytokine-activated neutrophils: inflammatory neutrophils are heterogeneous and enhance adaptive immune responses, Journal of Leukocyte Biology. 69 (2001) 698–704. [PubMed] [Google Scholar]
- 27.Wolf MT, Dearth CL, Ranallo CA, LoPresti ST, Carey LE, Daly KA, Brown BN, Badylak SF, Macrophage polarization in response to ECM coated polypropylene mesh, Biomaterials. 35 (2014) 6838–6849. doi: 10.1016/j.biomaterials.2014.04.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li AG, Quinn MJ, Siddiqui Y, Wood MD, Federiuk IF, Duman HM, Ward WK, Elevation of transforming growth factor beta (TGFβ) and its downstream mediators in subcutaneous foreign body capsule tissue, Journal of Biomedical Materials Research Part A. 82 (2007) 498–508. [DOI] [PubMed] [Google Scholar]
- 29.Robitaille R, Dusseault J, Henley N, Desbiens K, Labrecque N, Hallé J-P, Inflammatory response to peritoneal implantation of alginate–poly-l-lysine microcapsules, Biomaterials. 26 (2005) 4119–4127. doi: 10.1016/j.biomaterials.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 30.Meckmongkol TT, Harmon R, McKeown-Longo P, Van De Water L, The fibronectin synergy site modulates TGF-β-dependent fibroblast contraction, Biochemical and Biophysical Research Communications. 360 (2007) 709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mooney JE, Rolfe BE, Osborne GW, Sester DP, van Rooijen N, Campbell GR, Hume DA, Campbell JH, Cellular plasticity of inflammatory myeloid cells in the peritoneal foreign body response, The American Journal of Pathology. 176 (2010) 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell JH, Efendy JL, Han C-L, Girjes AA, Campbell GR, Haemopoietic origin of myofibroblasts formed in the peritoneal cavity in response to a foreign body, Journal of Vascular Research. 37 (2000) 364–371. [DOI] [PubMed] [Google Scholar]
- 33.Campbell GR, Ryan GB, Origin of myofibroblasts in the avascular capsule around free-floating intraperitoneal blood clots, Pathology. 15 (1983) 253–264. [DOI] [PubMed] [Google Scholar]
- 34.Franz S, Rammelt S, Scharnweber D, Simon JC, Immune responses to implants – A review of the implications for the design of immunomodulatory biomaterials, Biomaterials. 32 (2011) 6692–6709. doi: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 35.Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G, The role of macrophage phenotype in vascularization of tissue engineering scaffolds, Biomaterials. 35 (2014) 4477–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraser CC, Nanoparticle therapy for allergic and inflammatory disease, Anti-Inflammatory & Anti-Allergy Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Inflammatory and Anti-Allergy Agents). 9 (2010) 54–70. [Google Scholar]
- 37.Dondossola E, Holzapfel BM, Alexander S, Filippini S, Hutmacher DW, Friedl P, Examination of the foreign body response to biomaterials by nonlinear intravital microscopy, Nature Biomedical Engineering. 1 (2016) 0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wood KJ, Bushell A, Hester J, Regulatory immune cells in transplantation, Nature Reviews Immunology. 12 (2012) 417–430. [DOI] [PubMed] [Google Scholar]
- 39.Ruoslahti E, RGD and other recognition sequences for integrins, Annual Review of Cell and Developmental Biology. 12 (1996) 697–715. [DOI] [PubMed] [Google Scholar]
- 40.Altieri DC, Mannucci PM, Capitanio AM, Binding of fibrinogen to human monocytes, Journal of Clinical Investigation. 78 (1986) 968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaveri TD, Lewis JS, Dolgova NV, Clare-Salzler MJ, Keselowsky BG, Integrin-directed modulation of macrophage responses to biomaterials, Biomaterials. 35 (2014) 3504–3515. doi: 10.1016/j.biomaterials.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bornstein P, Sage EH, Matricellular proteins: extracellular modulators of cell function, Current Opinion in Cell Biology. 14 (2002) 608–616. [DOI] [PubMed] [Google Scholar]
- 43.Tang L, Jiang W, Welty SE, The participation of P‐and E‐selectins on biomaterial‐mediated tissue responses, Journal of Biomedical Materials Research. 62 (2002) 471–477. [DOI] [PubMed] [Google Scholar]
- 44.Auquit-Auckbur I, Caillot F, Arnoult C, Menard J-F, Drouot L, Courville P, Tron F, Musette P, Role of toll-like receptor 4 in the inflammation reaction surrounding silicone prosthesis, Acta Biomaterialia. 7 (2011) 2047–2052. [DOI] [PubMed] [Google Scholar]
- 45.Murphy-Ullrich JE, Sage EH, Revisiting the matricellular concept, Matrix Biology. 37 (2014) 1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris AH, Kyriakides TR, Matricellular proteins and biomaterials, Matrix Biology. 37 (2014) 183–191. doi: 10.1016/j.matbio.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aamodt JM, Grainger DW, Extracellular matrix-based biomaterial scaffolds and the host response, Biomaterials. 86 (2016) 68–82. doi: 10.1016/j.biomaterials.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradshaw AD, Sage EH, SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury, The Journal of Clinical Investigation. 107 (2001) 1049–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Puolakkainen P, Bradshaw AD, Kyriakides TR, Reed M, Brekken R, Wight T, Bornstein P, Ratner B, Sage EH, Compromised production of extracellular matrix in mice lacking secreted protein, acidic and rich in cysteine (SPARC) leads to a reduced foreign body reaction to implanted biomaterials, The American Journal of Pathology. 162 (2003) 627–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barker TH, Framson P, Puolakkainen PA, Reed M, Funk SE, Sage EH, Matricellular homologs in the foreign body response: hevin suppresses inflammation, but hevin and SPARC together diminish angiogenesis, The American Journal of Pathology. 166 (2005) 923–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kyriakides TR, Leach KJ, Hoffman AS, Ratner BD, Bornstein P, Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity, Proceedings of the National Academy of Sciences. 96 (1999) 4449–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kyriakides TR, Hartzel T, Huynh G, Bornstein P, Regulation of angiogenesis and matrix remodeling by localized, matrix-mediated antisense gene delivery, Molecular Therapy. 3 (2001) 842–849. [DOI] [PubMed] [Google Scholar]
- 53.Kastellorizios M, Papadimitrakopoulos F, Burgess DJ, Multiple tissue response modifiers to promote angiogenesis and prevent the foreign body reaction around subcutaneous implants, Journal of Controlled Release. 214 (2015) 103–111. [DOI] [PubMed] [Google Scholar]
- 54.Vishwakarma A, Bhise NS, Evangelista MB, Rouwkema J, Dokmeci MR, Ghaemmaghami AM, Vrana NE, Khademhosseini A, Engineering Immunomodulatory Biomaterials To Tune the Inflammatory Response, Trends in Biotechnology. 34 (2016) 470–482. doi: 10.1016/j.tibtech.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Salthouse TN, Some aspects of macrophage behavior at the implant interface, Journal of Biomedical Materials Research Part A. 18 (1984) 395–401. [DOI] [PubMed] [Google Scholar]
- 56.Matlaga BF, Yasenchak LP, Salthouse TN, Tissue response to implanted polymers: the significance of sample shape, Journal of Biomedical Materials Research Part A. 10 (1976) 391–397. [DOI] [PubMed] [Google Scholar]
- 57.Hulander M, Lundgren A, Berglin M, Ohrlander M, Lausmaa J, Elwing H, Immune complement activation is attenuated by surface nanotopography, Int J Nanomedicine. 6 (2011) 2653–2666. doi: 10.2147/IJN.S24578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roach P, Eglin D, Rohde K, Perry CC, Modern biomaterials: a review—bulk properties and implications of surface modifications, J Mater Sci: Mater Med. 18 (2007) 1263–1277. doi: 10.1007/s10856-006-0064-3. [DOI] [PubMed] [Google Scholar]
- 59.Scopelliti PE, Borgonovo A, Indrieri M, Giorgetti L, Bongiorno G, Carbone R, Podestà A, Milani P, The Effect of Surface Nanometre-Scale Morphology on Protein Adsorption, PLOS ONE. 5 (2010) e11862. doi: 10.1371/journal.pone.0011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rechendorff K, Hovgaard MB, Foss M, Zhdanov VP, Besenbacher F, Enhancement of Protein Adsorption Induced by Surface Roughness, Langmuir. 22 (2006) 10885–10888. doi: 10.1021/la0621923. [DOI] [PubMed] [Google Scholar]
- 61.Hovgaard MB, Rechendorff K, Chevallier J, Foss M, Besenbacher F, Fibronectin Adsorption on Tantalum: The Influence of Nanoroughness, J. Phys. Chem. B 112 (2008) 8241–8249. doi: 10.1021/jp801103n. [DOI] [PubMed] [Google Scholar]
- 62.Baker DW, Liu X, Weng H, Luo C, Tang L, Fibroblast/Fibrocyte: Surface Interaction Dictates Tissue Reactions to Micropillar Implants, Biomacromolecules. 12 (2011) 997–1005. doi: 10.1021/bm1013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jahed Z, Molladavoodi S, Seo BB, Gorbet M, Tsui TY, Mofrad MR, Cell responses to metallic nanostructure arrays with complex geometries., Biomaterials. 35 (2014) 9363–9371. doi: 10.1016/j.biomaterials.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 64.Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, Elisseeff JH, Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells, Science. 352 (2016) 366–370. doi: 10.1126/science.aad9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E, Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization, Nat. Immunol 9 (2008) 847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y, Kawaguchi A, Hasegawa H, Kajino K, Ninomiya T, Ijiro K, Sawa H, Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in Vitro and in Vivo, ACS Nano. 7 (2013) 3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 67.Padmore T, Stark C, Turkevich LA, Champion JA, Quantitative analysis of the role of fiber length on phagocytosis and inflammatory response by alveolar macrophages, Biochim Biophys Acta Gen Subj. 1861 (2017) 58–67. doi: 10.1016/j.bbagen.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schanen BC, Karakoti AS, Seal S, Drake III DR, Warren WL, Self WT, Exposure to Titanium Dioxide Nanomaterials Provokes Inflammation of an in Vitro Human Immune Construct, ACS Nano. 3 (2009) 2523–2532. doi: 10.1021/nn900403h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sunshine JC, Perica K, Schneck JP, Green JJ, Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells, Biomaterials. 35 (2014) 269–277. doi: 10.1016/j.biomaterials.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vaine CA, Patel MK, Zhu J, Lee E, Finberg RW, Hayward RC, Kurt-Jones EA, Tuning Innate Immune Activation by Surface Texturing of Polymer Microparticles: The Role of Shape in Inflammasome Activation, The Journal of Immunology. 190 (2013) 3525–3532. doi: 10.4049/jimmunol.1200492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bartneck M, Keul HA, Singh S, Czaja K, Bornemann J, Bockstaller M, Moeller M, Zwadlo-Klarwasser G, Groll J, Rapid Uptake of Gold Nanorods by Primary Human Blood Phagocytes and Immunomodulatory Effects of Surface Chemistry, ACS Nano. 4 (2010) 3073–3086. doi: 10.1021/nn100262h. [DOI] [PubMed] [Google Scholar]
- 72.Ward WK, Slobodzian EP, Tiekotter KL, Wood MD, The effect of microgeometry, implant thickness and polyurethane chemistry on the foreign body response to subcutaneous implants, Biomaterials. 23 (2002) 4185–4192. [DOI] [PubMed] [Google Scholar]
- 73.Scharp DW, Marchetti P, Encapsulated islets for diabetes therapy: History, current progress, and critical issues requiring solution, Advanced Drug Delivery Reviews. 67–68 (2014) 35–73. doi: 10.1016/j.addr.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 74.Kearney CJ, Mooney DJ, Macroscale delivery systems for molecular and cellular payloads, Nat Mater. 12 (2013) 1004–1017. doi: 10.1038/nmat3758. [DOI] [PubMed] [Google Scholar]
- 75.Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ, Biomaterial based modulation of macrophage polarization: a review and suggested design principles, Materials Today. 18 (2015) 313–325. [Google Scholar]
- 76.Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, Daly KA, Reing JE, Badylak SF, Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials, Acta Biomaterialia. 8 (2012) 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD, Proangiogenic scaffolds as functional templates for cardiac tissue engineering, Proc. Natl. Acad. Sci. U.S.A 107 (2010) 15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD, Porous Implants Modulate Healing and Induce Shifts in Local Macrophage Polarization in the Foreign Body Reaction, Ann Biomed Eng. (2013) 1–9. doi: 10.1007/s10439-013-0933-0. [DOI] [PubMed] [Google Scholar]
- 79.Ratner BD, The biocompatibility manifesto: biocompatibility for the twenty-first century, Journal of Cardiovascular Translational Research. 4 (2011) 523–527. [DOI] [PubMed] [Google Scholar]
- 80.Barr S, Hill E, Bayat A, Current implant surface technology: an examination of their nanostructure and their influence on fibroblast alignment and biocompatibility, Eplasty. 9 (2009) e22. [PMC free article] [PubMed] [Google Scholar]
- 81.Barnsley GP, Sigurdson LJ, Barnsley SE, Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials, Plast. Reconstr. Surg 117 (2006) 2182–2190. doi: 10.1097/01.prs.0000218184.47372.d5. [DOI] [PubMed] [Google Scholar]
- 82.Loch-Wilkinson A, Beath KJ, Knight RJW, Wessels WLF, Magnusson M, Papadopoulos T, Connell T, Lofts J, Locke M, Hopper I, Cooter R, Vickery K, Joshi PA, Prince HM, Deva AK, Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia and New Zealand: High-Surface-Area Textured Implants Are Associated with Increased Risk, Plast. Reconstr. Surg 140 (2017) 645–654. doi: 10.1097/PRS.0000000000003654. [DOI] [PubMed] [Google Scholar]
- 83.Kyle DJT, Oikonomou A, Hill E, Bayat A, Development and functional evaluation of biomimetic silicone surfaces with hierarchical micro/nano-topographical features demonstrates favourable in vitro foreign body response of breast-derived fibroblasts, Biomaterials. 52 (2015) 88–102. doi: 10.1016/j.biomaterials.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Bracaglia LG, Fisher JP, Extracellular Matrix-Based Biohybrid Materials for Engineering Compliant, Matrix-Dense Tissues, Advanced Healthcare Materials. 4 (2015) 2475–2487. doi: 10.1002/adhm.201500236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sandor M, Singh D, Silverman RP, Xu H, De Deyne PG, Comparative host response of 2 human acellular dermal matrices in a primate implant model, Eplasty. 14 (2014). [PMC free article] [PubMed] [Google Scholar]
- 86.Chandorkar Y, Bhaskar N, Madras G, Basu B, Long-Term Sustained Release of Salicylic Acid from Cross-Linked Biodegradable Polyester Induces a Reduced Foreign Body Response in Mice, Biomacromolecules. 16 (2015) 636–649. doi: 10.1021/bm5017282. [DOI] [PubMed] [Google Scholar]
- 87.Avula MN, Rao AN, McGill LD, Grainger DW, Solzbacher F, Foreign body response to subcutaneous biomaterial implants in a mast cell-deficient Kitw-Sh murine model, Acta Biomaterialia. 10 (2014) 1856–1863. doi: 10.1016/j.actbio.2013.12.056. [DOI] [PubMed] [Google Scholar]
- 88.Miller M, Cho JY, McElwain K, McElwain S, Shim JY, Manni M, Baek JS, Broide DH, Corticosteroids prevent myofibroblast accumulation and airway remodeling in mice, American Journal of Physiology-Lung Cellular and Molecular Physiology. 290 (2006) L162–L169. [DOI] [PubMed] [Google Scholar]
- 89.Bhardwaj U, Sura R, Papadimitrakopoulos F, Burgess DJ, PLGA/PVA hydrogel composites for long-term inflammation control following sc implantation, International Journal of Pharmaceutics. 384 (2010) 78–86. [DOI] [PubMed] [Google Scholar]
- 90.Bhardwaj U, Papadimitrakopoulos F, Burgess DJ, A review of the development of a vehicle for localized and controlled drug delivery for implantable biosensors, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morais JM, Papadimitrakopoulos F, Burgess DJ, Biomaterials/tissue interactions: possible solutions to overcome foreign body response, The AAPS Journal. 12 (2010) 188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Love RJ, Jones KS, Biomaterials, fibrosis, and the use of drug delivery systems in future antifibrotic strategies, Critical Reviews™ in Biomedical Engineering. 37 (2009). [DOI] [PubMed] [Google Scholar]
- 93.Gancedo M, Ruiz-Corro L, Salazar-Montes A, Rincón AR, Armendáriz-Borunda J, Pirfenidone prevents capsular contracture after mammary implantation, Aesthetic Plastic Surgery. 32 (2008) 32–40. [DOI] [PubMed] [Google Scholar]
- 94.Rujitanaroj P, Jao B, Yang J, Wang F, Anderson JM, Wang J, Chew SY, Controlling fibrous capsule formation through long-term down-regulation of collagen type I (COL1A1) expression by nanofiber-mediated siRNA gene silencing, Acta Biomaterialia. 9 (2013) 4513–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takahashi H, Wang Y, Grainger DW, Device-based local delivery of siRNA against mammalian target of rapamycin (mTOR) in a murine subcutaneous implant model to inhibit fibrous encapsulation, Journal of Controlled Release. 147 (2010) 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen T, Yuan J, Duncanson S, Hibert ML, Kodish BC, Mylavaganam G, Maker M, Li H, Sremac M, Santosuosso M, Forbes B, Kashiwagi S, Cao J, Lei J, Thomas M, Hartono C, Sachs D, Markmann J, Sambanis A, Poznansky MC, Alginate Encapsulant Incorporating CXCL12 Supports Long-Term Allo- and Xenoislet Transplantation Without Systemic Immune Suppression, American Journal of Transplantation. 15 (2015) 618–627. doi: 10.1111/ajt.13049. [DOI] [PubMed] [Google Scholar]
- 97.Alagpulinsa DA, Cao JJL, Driscoll RK, Sîrbulescu RF, Penson MFE, Sremac M, Engquist EN, Brauns TA, Markmann JF, Melton DA, Poznansky MC, Alginate-microencapsulation of human stem cell–derived β cells with CXCL12 prolongs their survival and function in immunocompetent mice without systemic immunosuppression, American Journal of Transplantation. 0 (n.d.). doi: 10.1111/ajt.15308. [DOI] [PubMed] [Google Scholar]
- 98.Desai T, Shea LD, Advances in islet encapsulation technologies, Nature Reviews Drug Discovery. 16 (2017) 338–350. doi: 10.1038/nrd.2016.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Farah S, Doloff JC, Müller P, Sadraei A, Han HJ, Olafson K, Vyas K, Tam HH, Hollister-Lock J, Kowalski PS, Griffin M, Meng A, McAvoy M, Graham AC, McGarrigle J, Oberholzer J, Weir GC, Greiner DL, Langer R, Anderson DG, Long-term implant fibrosis prevention in rodents and non-human primates using crystallized drug formulations, Nat Mater. (2019). doi: 10.1038/s41563-019-0377-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL, Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials, PNAS. 109 (2012) 4245–4250. doi: 10.1073/pnas.1113560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takafumi Hamaoka, McCully Kevin K., Niwayama Masatsugu, Chance Britton, The use of muscle near-infrared spectroscopy in sport, health and medical sciences: recent developments, Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 369 (2011) 4591–4604. doi: 10.1098/rsta.2011.0298. [DOI] [PubMed] [Google Scholar]
- 102.Ferrari M, Quaresima V, A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application, NeuroImage. 63 (2012) 921–935. doi: 10.1016/j.neuroimage.2012.03.049. [DOI] [PubMed] [Google Scholar]
- 103.Lloyd-Fox S, Blasi A, Elwell CE, Illuminating the developing brain: The past, present and future of functional near infrared spectroscopy, Neuroscience & Biobehavioral Reviews. 34 (2010) 269–284. doi: 10.1016/j.neubiorev.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 104.Kang S-K, Murphy RKJ, Hwang S-W, Lee SM, Harburg DV, Krueger NA, Shin J, Gamble P, Cheng H, Yu S, Liu Z, McCall JG, Stephen M, Ying H, Kim J, Park G, Webb RC, Lee CH, Chung S, Wie DS, Gujar AD, Vemulapalli B, Kim AH, Lee K-M, Cheng J, Huang Y, Lee SH, Braun PV, Ray WZ, Rogers JA, Bioresorbable silicon electronic sensors for the brain, Nature. 530 (2016) 71–76. doi: 10.1038/nature16492. [DOI] [PubMed] [Google Scholar]
- 105.Yu KJ, Kuzum D, Hwang S-W, Kim BH, Juul H, Kim NH, Won SM, Chiang K, Trumpis M, Richardson AG, Cheng H, Fang H, Thompson M, Bink H, Talos D, Seo KJ, Lee HN, Kang S-K, Kim J-H, Lee JY, Huang Y, Jensen FE, Dichter MA, Lucas TH, Viventi J, Litt B, Rogers JA, Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex, Nature Materials. 15 (2016) 782–791. doi: 10.1038/nmat4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bai W, Shin J, Fu R, Kandela I, Lu D, Ni X, Park Y, Liu Z, Hang T, Wu D, Liu Y, Haney CR, Stepien I, Yang Q, Zhao J, Nandoliya KR, Zhang H, Sheng X, Yin L, MacRenaris K, Brikha A, Aird F, Pezhouh M, Hornick J, Zhou W, Rogers JA, Bioresorbable photonic devices for the spectroscopic characterization of physiological status and neural activity, Nat Biomed Eng. 3 (2019) 644–654. doi: 10.1038/s41551-019-0435-y. [DOI] [PubMed] [Google Scholar]
- 107.Son D, Lee J, Lee DJ, Ghaffari R, Yun S, Kim SJ, Lee JE, Cho HR, Yoon S, Yang S, Lee S, Qiao S, Ling D, Shin S, Song J-K, Kim J, Kim T, Lee H, Kim J, Soh M, Lee N, Hwang CS, Nam S, Lu N, Hyeon T, Choi SH, Kim D-H, Bioresorbable Electronic Stent Integrated with Therapeutic Nanoparticles for Endovascular Diseases, ACS Nano. 9 (2015) 5937–5946. doi: 10.1021/acsnano.5b00651. [DOI] [PubMed] [Google Scholar]
- 108.Tao H, Hwang S-W, Marelli B, An B, Moreau JE, Yang M, Brenckle MA, Kim S, Kaplan DL, Rogers JA, Omenetto FG, Silk-based resorbable electronic devices for remotely controlled therapy and in vivo infection abatement, Proceedings of the National Academy of Sciences. 111 (2014) 17385–17389. doi: 10.1073/pnas.1407743111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu L, Yang Z, Meacham K, Cvetkovic C, Corbin EA, Vázquez-Guardado A, Xue M, Yin L, Boroumand J, Pakeltis G, Sang T, Yu KJ, Chanda D, Bashir R, Gereau RW, Sheng X, Rogers JA, Biodegradable Monocrystalline Silicon Photovoltaic Microcells as Power Supplies for Transient Biomedical Implants, Advanced Energy Materials. 8 (2018) 1703035. doi: 10.1002/aenm.201703035. [DOI] [Google Scholar]
- 110.Bai W, Yang H, Ma Y, Chen H, Shin J, Liu Y, Yang Q, Kandela I, Liu Z, Kang S-K, Wei C, Haney CR, Brikha A, Ge X, Feng X, Braun PV, Huang Y, Zhou W, Rogers JA, Flexible Transient Optical Waveguides and Surface-Wave Biosensors Constructed from Monocrystalline Silicon, Advanced Materials. 30 (2018) 1801584. doi: 10.1002/adma.201801584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang S, Cao Z, Ultralow-fouling, functionalizable, and hydrolyzable zwitterionic materials and their derivatives for biological applications, Adv. Mater. Weinheim 22 (2010) 920–932. doi: 10.1002/adma.200901407. [DOI] [PubMed] [Google Scholar]
- 112.Cao B, Tang Q, Cheng G, Recent advances of zwitterionic carboxybetaine materials and their derivatives, J Biomater Sci Polym Ed. 25 (2014) 1502–1513. doi: 10.1080/09205063.2014.927300. [DOI] [PubMed] [Google Scholar]
- 113.Carr LR, Zhou Y, Krause JE, Xue H, Jiang S, Uniform zwitterionic polymer hydrogels with a nonfouling and functionalizable crosslinker using photopolymerization, Biomaterials. 32 (2011) 6893–6899. doi: 10.1016/j.biomaterials.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 114.Yesilyurt V, Veiseh O, Doloff JC, Li J, Bose S, Xie X, Bader AR, Chen M, Webber MJ, Vegas AJ, Langer R, Anderson DG, A Facile and Versatile Method to Endow Biomaterial Devices with Zwitterionic Surface Coatings, Advanced Healthcare Materials. 6 (2017) 1601091. doi: 10.1002/adhm.201601091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xie X, Doloff JC, Yesilyurt V, Sadraei A, McGarrigle JJ, Omami M, Veiseh O, Farah S, Isa D, Ghani S, Joshi I, Vegas A, Li J, Wang W, Bader A, Tam HH, Tao J, Chen H, Yang B, Williamson KA, Oberholzer J, Langer R, Anderson DG, Reduction of measurement noise in a continuous glucose monitor by coating the sensor with a zwitterionic polymer, Nature Biomedical Engineering. 2 (2018) 894. doi: 10.1038/s41551-018-0273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang Y, Vaddiraju S, Gu B, Papadimitrakopoulos F, Burgess DJ, Foreign Body Reaction to Implantable Biosensors, J Diabetes Sci Technol. 9 (2015) 966–977. doi: 10.1177/1932296815601869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, Fenton P, Kang JW, Hollister-Locke J, Bochenek MA, Chiu A, Siebert S, Tang K, Jhunjhunwala S, Aresta-Dasilva S, Dholakia N, Thakrar R, Vietti T, Chen M, Cohen J, Siniakowicz K, Qi M, McGarrigle J, Lyle S, Harlan DM, Greiner DL, Oberholzer J, Weir GC, Langer R, Anderson DG, Combinatorial hydrogel library enables identification of materials that mitigate the foreign body response in primates, Nat. Biotechnol 34 (2016) 345–352. doi: 10.1038/nbt.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bygd HC, Bratlie KM, The effect of chemically modified alginates on macrophage phenotype and biomolecule transport, Journal of Biomedical Materials Research Part A. 104 (2016) 1707–1719. doi: 10.1002/jbm.a.35700. [DOI] [PubMed] [Google Scholar]
- 119.Vegas AJ, Veiseh O, Gürtler M, Millman JR, Pagliuca FW, Bader AR, Doloff JC, Li J, Chen M, Olejnik K, Tam HH, Jhunjhunwala S, Langan E, Aresta-Dasilva S, Gandham S, McGarrigle JJ, Bochenek MA, Hollister-Lock J, Oberholzer J, Greiner DL, Weir GC, Melton DA, Langer R, Anderson DG, Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice, Nat. Med 22 (2016) 306–311. doi: 10.1038/nm.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bochenek MA, Veiseh O, Vegas AJ, McGarrigle JJ, Qi M, Marchese E, Omami M, Doloff JC, Mendoza-Elias J, Nourmohammadzadeh M, Khan A, Yeh C-C, Xing Y, Isa D, Ghani S, Li J, Landry C, Bader AR, Olejnik K, Chen M, Hollister-Lock J, Wang Y, Greiner DL, Weir GC, Løkensgard Strand B, Rokstad AMA, Lacik I, Langer R, Anderson DG, Oberholzer J, Alginate encapsulation as long-term immune protection of allogeneic pancreatic islet cells transplanted into the omental bursa of macaques, Nat Biomed Eng. 2 (2018) 810–821. doi: 10.1038/s41551-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.King A, Sandler S, Andersson A, The effect of host factors and capsule composition on the cellular overgrowth on implanted alginate capsules, J. Biomed. Mater. Res 57 (2001) 374–383. doi:. [DOI] [PubMed] [Google Scholar]
- 122.Manoury B, Caulet-Maugendre S, Guénon I, Lagente V, Boichot E, TIMP-1 is a key factor of fibrogenic response to bleomycin in mouse lung, Int J Immunopathol Pharmacol. 19 (2006) 471–487. [DOI] [PubMed] [Google Scholar]
- 123.Jacobs-Tulleneers-Thevissen D, Chintinne M, Ling Z, Gillard P, Schoonjans L, Delvaux G, Strand BL, Gorus F, Keymeulen B, Pipeleers D, Sustained function of alginate-encapsulated human islet cell implants in the peritoneal cavity of mice leading to a pilot study in a type 1 diabetic patient, Diabetologia. 56 (2013) 1605–1614. doi: 10.1007/s00125-013-2906-0. [DOI] [PubMed] [Google Scholar]