Abstract
Previous studies have highlighted interactions between serotonergic systems and adverse early life experience as important gene x environment determinants of risk of stress-related psychiatric disorders. Evidence suggests that mice deficient in Tph2, the rate-limiting enzyme for brain serotonin synthesis, display disruptions in behavioral phenotypes relevant to stress-related psychiatric disorders. The aim of this study was to determine how maternal separation in wild-type, heterozygous, and Tph2 knockout mice affects mRNA expression of serotonin-related genes. Serotonergic genes studied included Tph2, the high-affinity, low-capacity, sodium-dependent serotonin transporter (Slc6a4), the serotonin type 1a receptor (Htr1a), and the corticosterone-sensitive, low-affinity, high-capacity sodium-independent serotonin transporter, organic cation transporter 3 (Slc22a3). Furthermore, we studied corticotropin-releasing hormone receptors 1 (Crhr1) and 2 (Crhr2), which play important roles in controlling serotonergic neuronal activity. For this study, offspring of Tph2 heterozygous dams were exposed to daily maternal separation for the first two weeks of life. Adult, male wild-type, heterozygous, and homozygous offspring were subsequently used for molecular analysis. Maternal separation differentially altered serotonergic gene expression in a genotype- and topographically-specific manner. For example, maternal separation increased Slc6a4 mRNA expression in the dorsal part of the dorsal raphe nucleus in Tph2 heterozygous mice, but not in wild-type or knockout mice. Overall, these data are consistent with the hypothesis that gene x environment interactions, including serotonergic genes and adverse early life experience, play an important role in vulnerability to stress-related psychiatric disorders.
Keywords: Crhr2, gene expression, Htr1a, maternal separation, Slc6a4, Slc22a3
Graphical Abstract
1. Introduction
Adverse early life experience is thought to increase vulnerability to trauma and stressor-related disorders, anxiety disorders, and affective disorders (Heim and Nemeroff, 2001; Caspi et al., 2003; Hettema et al., 2006; Teicher et al., 2006; Copeland et al., 2018). Maternal separation is one paradigm extensively used to model the effects of adverse early life experience on subsequent responses to stress in adulthood (Plotsky and Meaney, 1993; Gardner et al., 2009b; Vetulani, 2013). The model is based on separation of neonatal rat or mouse pups from their mother during a critical period of development (Parfitt et al., 2004; Gardner et al., 2009b). Rodents exposed to maternal separation (3 h) during the first two weeks of life display increased anxiety- and depressive-like states in adulthood (Wigger and Neumann, 1999; Kalinichev et al., 2002; Gardner et al., 2005; Slotten et al., 2006). The exact mechanisms involved in the behavioral effects of adverse early life experience are not well-defined; however, maternal separation has been shown to alter serotonergic systems within the brain (Gartside et al., 2003; Van Riel et al., 2004; Gardner et al., 2009a, 2009b; Wong et al., 2015), as well as the responsiveness of serotonergic systems to adverse experiences during adulthood (Gardner et al., 2005).
Dysregulation of serotonergic systems has been implicated in control of anxiety- and fear-related defensive behavioral responses (Graeff et al., 1996; Naughton et al., 2000; Lowry et al., 2009). Consistent with this hypothesis, allelic variation in the tryptophan hydroxylase 2 gene (Tph2), encoding Tph2, the rate-limiting enzyme in brain serotonin synthesis (Walther et al., 2003), is associated with disruption of behavioral phenotypes relevant to stress-related psychiatric disorders (Gutknecht et al., 2007; Kim et al., 2009; Waider et al., 2017). Within the brainstem dorsal raphe nucleus (DR) and median raphe nucleus (MnR), Tph2 is specifically expressed in serotonin (5-hydroxytryptamine; 5-HT) neurons (Gutknecht et al., 2009), and stress-induced changes in Tph2 mRNA expression are restricted to subregions of the DR (McEuen et al., 2008; Gardner et al., 2009b; Donner et al., 2012a; Donner et al., 2016; Donner et al., 2018). The DR and MnR are topographically organized, such that different regions of the raphe complex receive unique patterns of afferent information and give rise to distinct, topographically organized efferents to various structures in the forebrain and brainstem (Lowry et al., 2008; Paul and Lowry, 2013; Ren et al., 2018). Distinctively, the dorsal part of the DR (DRD; also referred to as the dorsomedial DR) and caudal part of the DR (DRC) innervate forebrain structures that are involved in emotional control and anxiety (Ottersen, 1981; Commons 2003; Okere and Waterhouse, 2006; Hale et al., 2008; Lowry et al., 2008a,b; McEuen et al., 2008; Spiacci et al.,2016; Ren et al., 2018). Additionally, the ventrolateral part of the DR (DRVL) / ventrolateral periaqueductal gray (VLPAG) give rise to axons that travel to the dorsal periaqueductal gray (Beitz, 1982; Stezhka and Lovick, 1997), a projection that has been implicated in anti-panic functions of 5-HT (Johnson et al., 2004, 2008; Gardner et al., 2005; Paul and Lowry, 2013; Gambeta et al., 2017; Waider et al., 2017). The frontal cortex-projecting interfascicular part of the DR (DRI) and the MnR may be involved in promoting tolerance and adaption to chronic stress (Deakin and Graeff, 1991; Graeff et al., 1996; Lowry, 2002; Forster et al., 2006, 2008; Hale and Lowry, 2011), but see (Andrade et al., 2013). Lastly, the ventral part of the DR (DRV) may be associated with, negative reward anticipation, losing outcome, and evaluation of wrong choices (Murphy et al., 1989; Stein et al., 2001; Holland and Gallagher, 2004; Mataix-Cols et al., 2004; Liu et al., 2007).
A number of previous studies using maternal separation (typically, postnatal day [PND] 1-14 or PND2-14) in C57BL/6 mice have found that C57BL/6 mice are resilient to maternal separation, with no increases in anxiety-like defensive behavioral responses and no alterations in stress-induced corticosterone secretion (Millstein and Holmes 2007; Murthy and Gould 2018; Own and Patel 2013;Savignac et al. 2011; Tan et al. 2017; Weidner et al. 2019). However, other studies of the effects of maternal separation on behavioral responses and stress-induced corticosterone in adult male C57BL/6 mice have found increased ethologically relevant anxiety-like defensive behavioral responses (e.g., stretch-attend postures in the open-field test) when taking into account a time-based analysis of behavior, with increased stretch-attend postures later in the test and increased stress-induced corticosterone secretion in mice previously exposed to maternal separation (van Heerden et al. 2010) or using measures of latency to explore the center of an open-field (Romeo et al. 2003). Other studies have found increased depressive-like behavioral responses in adult mice previously exposed to maternal separation (Varghese et al. 2006). It is now clear that the effects of maternal separation on anxiety-like defensive behavioral responses and stress-induced corticosterone secretion in adult C57BL/6 mice are also dependent on the gut microbiota (De Palma et al. 2015), which may explain some of the variability in previous studies. Furthermore, it is possible that the serotonin deficiency in Tph2+/−Tph2−/− mice may modify the physiological and behavioral consequences of maternal separation.
Previous studies investigating the effects of adverse early life experience on serotonergic gene expression in the dorsal raphe nucleus or brainstem, including Tph2 (Gardner et al. 2009b; Lukkes et al. 2013; Own et al. 2013; Wong et al. 2015), Htr1a (Bravo et al. 2014; Neumaier et al. 2002; Oreland et al. 2009), and Slc6a4 (Bravo et al. 2014; Gardner et al. 2009a; Lee et al. 2007; Oreland et al. 2009), have typically focused on one or two genes (see Table 1). To the best of our knowledge, no previous studies have investigated effects of adverse early-life experience on Slc22a3, Crhr1 or Crhr2 mRNA expression in adulthood. It may be the case that interactions between adverse early life experience and multiple serotonergic genes work together to produce physiological and behavioral stress responding. Of particular interest is Tph2, as maternal separation induces long-lasting dysregulation of hypothalamic-pituitary-adrenal (HPA) axis signaling (Ladd et al. 2000), and Tph2 mRNA expression, particularly in the dorsomedial dorsal raphe nucleus, is sensitive to chronic elevation of glucocorticoid hormones (Donner et al. 2012b; Donner et al. 2016), as well as acute (Donner et al. 2018), and chronic stress exposures (McEuen et al. 2008). Meanwhile, physiologic and behavioral outcomes of inescapable stressors are thought to be dependent on a functional desensitization of the 5-HT1A receptor, also in the dorsomedial dorsal raphe nucleus (Rozeske et al. 2011). Finally, stress-induced changes in Tph2 and Crhr1 mRNA expression in the dorsal raphe nucleus are dependent on Crhr2 genotype (McEuen et al. 2008). Together, these data suggest that interactions among serotonergic genes, such as Tph2, Htr1a, Slc6a4, and Slc22a3, as well as genes that control serotonergic signaling, such as Crhr1 and Crhr2, are likely to work together to produce physiological and behavioral stress responding following exposure to adverse early life experience.
Table 1.
Effects of maternal separation in wild type rodents on serotonergic gene expression in the dorsal raphe nucleus or brainstem during adulthood
| Species | Early-life experience | Genes studied | Age | Citation |
|---|---|---|---|---|
| Rat (SD) | Maternal separation, PND2-12 | ↓Htr1a, ↓Slc6a4 | 11-13 weeks (PND77-91) | (Bravo et al. 2014) |
| Rat (LE) | Maternal separation PND2-14 | ↑Tph2 (only after social defeat during adulthood; DRVL/VLPAG) | 10 weeks (PND70) | (Gardner et al. 2009b) |
| Rat (LE) | Maternal separation PND2-14 | ↑Slc6a4 (only after social defeat during adulthood) | 10 weeks (PND70) | (Gardner et al. 2009a) |
| Rat (SD) | Maternal separation PND 1-14 | ↓Slc6a4 | PND60 | (Lee et al. 2007) |
| Rat (LE) | Maternal separation PND2-14 | –Htr1a, –Htr1b | 23.5 weeks (PND 165) | (Neumaier et al. 2002) |
| Mouse (C57BL/6J) | Maternal separation PND2-14 | ↓Tph2, ↓Slc6a4 | PND60 | (Own et al. 2013) |
| Rat (W) | Maternal separation PND1-21 | Brainstem –Htr1a, –Htr2a, –Htr2c, –Htr3, –Slc6a4 | 3 weeks (PND21); 10 weeks (PND70) | (Oreland et al. 2009) |
| Mouse (C57BL/6J) | Maternal separation PND1-14 | Brainstem –Tph2, ↑Maoa | 12 weeks (PND84) | (Wong et al. 2015) |
Abbreviations: –, no effect; ↑, increase; ↓, decrease; DRVL/VLPAG, dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray; LE, Long Evans; SD, Sprague Dawley; W, Wistar.
This study aimed to investigate the interplay of gene x environment (G x E) interactions, including Tph2 deficiency and adverse early life experience, on brainstem serotonergic systems. We used wild-type, heterozygous, and Tph2 knockout mice as a model of Tph2 deficiency and decreased serotonin synthesis in the brain. We studied how Tph2 genotype and maternal separation, relative to animal facility-reared control conditions, affected mRNA expression of serotonin-related genes within subregions of the DR and in the MnR. Genes selected for analysis included: Tph2, encoding tryptophan hydroxylase 2 (Tph2; the rate-limiting enzyme in the biosynthesis of brain serotonin); Htr1a, encoding the 5-HT1A receptor, which can function as an inhibitory autoreceptor; Slc6a4, encoding the sodium-dependent, high-affinity, low-capacity serotonin transporter (Slc6a4; solute carrier family 6 [neurotransmitter transporter, serotonin], member 4); and Slc22a3, encoding the corticosterone-sensitive, sodium-independent, low-affinity, high-capacity serotonin transporter (Slc22a3; solute carrier family 22 [organic cation transporter], member 3). In addition, we studied corticotropin-releasing hormone type 1 and 2 receptors (Crhr1 and Crhr2), which have been shown to play an important role in control of serotonergic systems (for review, see Fox and Lowry, 2013). As the DR and MnR consist of anatomically and functionally distinct subregions, we analyzed mRNA expression within specific subregions of the DR and in the MnR.
2. Materials and Methods
2.1. Animals
All experiments were performed in accordance with the European Parliament and Council Directive (2010/63/EU) and were approved by local authorities (District Government of Lower Franconia, Würzburg, Germany: 55.2-2531.01-57/12). The generation and genotyping procedure of Tph2 knockout mice have been described in Gutknecht et al., 2008; see also, (Gutknecht et al., 2009, 2012b, 2015; Waider et al., 2017). Mice were backcrossed to C57BL/6N mice for more than ten generations. Female Tph2+/− mice were bred with male Tph2+/− mice (ZEMM, University of Würzburg, Germany) using a trio breeding scheme, with 2 females:1 male per cage. Prior to mating, mice were housed in groups of 2-7. The parental generation, as well as the study cohort, were housed under 14 h/10 h light-dark cycle with lights on at 7 a.m. and lights off at 9 p.m., at 21 ± 1 °C, with maximum humidity of 55%. Mice were housed in Macrolon Type II long cages (325 mm L x 170 mm W x 140 mm H), with bedding (ABEDD LTE E-001), an egg carton shelter, and unbleached pulp as nesting material (Fripa, EAN: 4000883749320). Food (SSNIFF, V1534-300, V1124-300, V1274-300) and water were available ad libitum.
2.2. Maternal separation
This experiment included a total of 18 litters. Upon weaning (P25), the pups were separated by genotype and condition, and the females were separated from the males and housed separately. Pups from five litters contributed to the Tph2+/+ HC treatment group (n = 8); seven litters contributed to the Tph2+/− HC treatment group (n = 8); four litters contributed to Tph2−/− HC treatment group (n = 6); five litters contributed to the Tph2+/+ MS treatment group (n = 7); seven litters contributed to the Tph2+/− MS treatment group (n = 8); and five litters contributed to Tph2−/− MS treatment group (n = 6).
The experimental timeline is illustrated in Figure 1. Maternal separation procedures have been previously described in detail (Gardner et al., 2005, 2009a, 2009b). The day of birth was declared P0. From P2 through P15, inclusive, half of the litters were randomly subjected to the maternal separation paradigm (MS; N = 21), while the other half were assigned to animal facility-reared control conditions (HC; N = 22). Among litters exposed to MS, all pups from the mixed-sex and mixed-genotype litters were taken out of the dam’s cage together with the respective nesting material, placed into a new cage (325 mm L x 170 mm W x 140 mm H) with fresh bedding material. Litters were then brought into an adjacent room, where they were kept under red light at 29 °C on average and humidity levels of about 65-70% for 3 h. The separation time point was randomly assigned in the light interval between 7 a.m. and 1 p.m. Control dams and litters were left undisturbed under animal facility-reared control conditions. On days P5, P10, and P15, litters and dams of both the control and the MS groups were weighed, while on days P5, P12, and P17, cages were changed. At P25, male pups (N = 43) of both control (N = 22) and maternal separation (N = 21) groups were weaned into groups of 4-7 animals per cage according to genotype and condition. Animals that showed repeated aggressive encounters with their cage mates were subsequently removed from the cage and single housed.. Females were used in a separate study to investigate the effects of Tph2 genotype and maternal separation on anxiety-like defensive behavioral responses and neural activity in brain regions controlling anxiety-like defensive behavioral responses (Auth et al. 2018). Following group housing, pups were left undisturbed except for weekly cage changes. At P100-P110, mice were euthanized by rapid decapitation. All mice were euthanized between 12:46 p.m. and 3:50 p.m. (mean 2:18 p.m.). Brain tissue was collected, frozen on dry ice, and stored at −80 °C for in situ hybridization histochemistry.
Figure 1.

Diagram of experimental design and timeline. Dams and litters were subjected to maternal separation (MS), a model of adverse early life experience, or animal facility-reared control conditions.
2.3. In situ hybridization histochemistry
Previously published methods were used for in situ hybridization histochemistry (Day and Akil, 1996; Fox et al., 2017). For a detailed description of the in situ hybridization histochemistry materials and methods, see Supplementary Material.
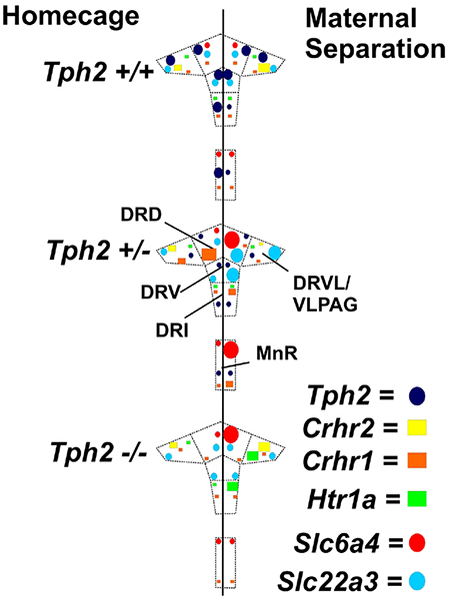
A rostrocaudal analysis atlas was prepared for Tph2, and this atlas was used for assigning rostrocaudal levels of analysis for Htr1a, Slc6a4, Slc22a3, Crhr1, or Crhr2 mRNA expression in the DR and MnR, according to a stereotaxic mouse brain atlas (Paxinos and Franklin, 2001). In other words, all rostrocaudal levels for all genes were assigned based on the rostrocaudal levels defined using Tph2 in situ hybridization histochemistry. According to Gardner et al. (Gardner et al., 2009b) and Tph immunostaining (Abrams et al., 2004), each rostrocaudal level was further divided into respective subregions of the DR (Fig. 2, S1–S5). Throughout all rostrocaudal levels, the mean gray value x area values for each subregion were then averaged, and all mean gray value x area values per treatment group were also summarized to display the overall expression in the DR or MnR. A total of 14 rostrocaudal levels were studied throughout the brainstem (Fig. 2, S1–S5). The subdivisions studied were summarized into the following anatomical subregions of the DR: dorsal raphe nucleus, caudal part (DRC), −4.832 mm to −5.252 mm from bregma; dorsal raphe nucleus, dorsal part (DRD), −4.160 mm to −4.748 mm from bregma; dorsal raphe nucleus, interfascicular part (DRI), −4.664 mm to −5.252 mm from bregma; dorsal raphe nucleus, ventral part (DRV), −4.160 mm to −4.664 mm from bregma; left and right dorsal raphe nucleus, ventrolateral part/ventrolateral periaqueductal gray region (left and right DRVL/VLPAG), −4.496 mm to −4.748 mm from bregma; and median raphe nucleus (MnR), −4.160 mm to −4.748 mm from bregma. For the DRC, DRD, DRI, DRV, and MnR, a single “grey value x area” value from each rostrocaudal level of each subregion from each animal was used for statistical analysis. In the case of the DRVL/VLPAG, “grey value x area” values from the left and right sides of the DRVL/VLPAG at each rostrocaudal level were averaged, and these average values from each animal were used for statistical analysis. During the in situ hybridization histochemistry, the investigator (MRA) was blind to the assignment of treatment groups. Image analysis of individual genes was conducted by different investigators as follows: Tph2 (MWL), Htr1a (MWL), Slc6a4 (KTN), Slc22a3 (KTN), Crhr1 (KSS), and Crhr2 (KTN). In each case, the investigator was blind to the assignment of treatment groups.
Figure 2.
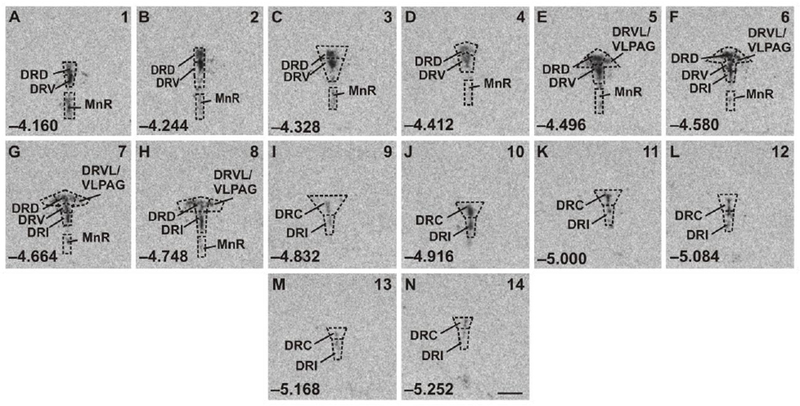
Atlas of mouse tryptophan hydroxylase 2 (Tph2) mRNA expression in the midbrain and pontine raphe complex (84 μm intervals) used for analysis of subregions of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR) with a high level of neuroanatomical resolution. Photographs are autoradiographic images of Tph2 mRNA expression from a representative adult male wild-type mouse in this study. The levels chosen for analysis ranged from (A) −4.160 mm bregma through (N) −5.252 mm bregma. Dashed lines delineate different subdivisions of the DR and MnR analyzed in this study, based on a stereotaxic atlas of the mouse brain (Paxinos and Franklin, 2001). Abbreviations: DRC, dorsal raphe nucleus, caudal part; DRD, dorsal raphe nucleus, dorsal part; DRI, dorsal raphe nucleus, interfascicular part; DRV, dorsal raphe nucleus, ventral part; DRVL, dorsal raphe nucleus, ventrolateral part; MnR, median raphe nucleus; VLPAG, ventrolateral periaqueductal gray. Numbers in the lower left of each panel indicate the rostrocaudal coordinates relative to bregma (in mm). Numbers in the upper right of each panel correspond to the x-axis values in graphical representation of the data (Figures 3–8; S6–S11). Scale bar, 1 mm.
2.4. Statistical analysis
A detailed description of our statistical approach can be found in the Supplementary Materials section. Briefly, statistical analyses were conducted using the software package IBM Statistical Package for the Social Sciences (version 24.0, SPSS Inc., Chicago, IL, USA). A linear mixed effects model (LMM) was used for the analysis of in situ hybridization histochemistry data, mean (corrected for background) gray value x area values for each rostrocaudal level of each DR and MnR subdivision for each rat were generated for Tph2, Htr1a, Slc6a4, Slc22a3, Crhr1, and Crhr2 gene expression. Two-tailed significance was set at p < 0.05. Post hoc pairwise comparisons were made with Fisher’s least significant difference (LSD) test. Again, two-tailed significance was set at p < 0.05.
3. Results
In situ hybridization histochemistry
In situ hybridization histochemistry was used to evaluate the effects of Tph2 genotype, i.e., wild-type (Tph2+/+), heterozygous (Tph2+/−), and homozygous (Tph2−/−) tryptophan hydroxylase knockout mice, with or without MS, on expression of a panel of serotonergic genes, i.e., Tph2, Htr1a, Slc6a4, Slc22a3, and genes important for control of serotonergic neurotransmission, Crhr1, and Crhr2.
3.1. Tph2 mRNA expression
Analysis of Tph2 mRNA expression using LMM analysis revealed a genotype x MS x rostrocaudal level interaction (F(24, 154.0) = 1.61, p < 0.05), as well as genotype x subregion x rostrocaudal level interaction (F(46, 178.9) = 1.91, p < 0.01; Fig. 3A–G, Fig. S6A–G, Table S1). The genotype effect on Tph2 mRNA expression was clearly evident throughout the DR and MnR, with the greatest expression of Tph2 mRNA in Tph2+/+ mice, intermediate expression in Tph2+/− mice, and no expression in Tph2−/− mice. Based on these findings, secondary linear mixed models were used to determine effects of MS and genotype within each subregion of the DR and in the MnR.
Figure 3.
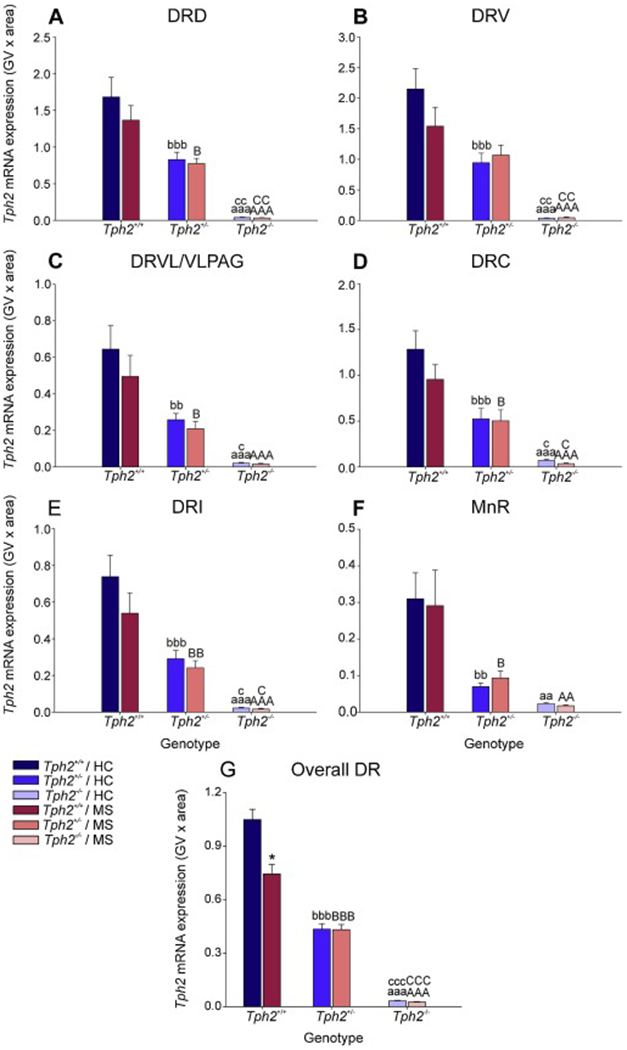
Effects of maternal separation in wild-type and Tph2-deficient mice on overall Tph2 mRNA expression within each subregion of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Graphs represent the mean ± SEM of Tph2 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) MnR, and (G) across all subregions in the DR and MnR. Male wild-type, heterozygous, and homozygous Tph2 knockout mice were exposed to either animal facility-reared control conditions (HC) or maternal separation (MS), consisting of daily separation (3 h) of mixed litters from the dam postnatal day 2 (P2) through P15, inclusive. Sample sizes per treatment group: Tph2+/+ HC (n = 8); Tph2 HC (n = 8); Tph2−/− HC (n = 6); Tph2+/+ MS (n = 7); Tph2+/− MS (n = 8); Tph2−/− MS (n = 6); (N = 43). Post hoc comparisons were made using Fisher’s least significant difference (LSD) tests; aap < 0.01, aaap < 0.001, Tph2+/+ HC versus Tph2+/− HC; bbp < 0.01, bbbp < 0.001, Tph2+/+ HC versus Tph2+/− HC; cp < 0.05, ccp < 0.01, cccp < 0.001, Tph2+/− HC versus Tph2−/− HC; AAp < 0.01, AAAp < 0.001, Tph2+/+ MS versus Tph2−/− MS; Bp < 0.05, BBp < 0.01, BBBp < 0.001, Tph2+/+ MS versus Tph2+/− MS; Cp < 0.05, CCp < 0.01, CCCp < 0.01, Tph2+/− MS versus Tph2−/− MS.
Secondary linear mixed model analysis of specific subregions highlighted genotype x rostrocaudal level interactions within the DRD (F(14, 35.7) = 3.70, p < 0.001), DRV (F(12, 32.0) = 3.72, p < 0.001), DRVL/VLPAG (F(6, 33.2) = 3.75, p < 0.01), DRC (F(10, 39.7) = 5.71, p < 0.001), DRI (F(16, 33.8) = 4.66, p < 0.001), and MnR (F(14, 26.5)= 2.57, p < 0.05; Fig. S6A–F; Table S1). For a detailed description of the post hoc comparisons for Tph2 mRNA expression within each rostrocaudal level of each subregion of the DR and in the MnR and globally within each rostrocaual level, see Supplementary Material. To further understand the effects of maternal separation and Tph2 genotype on subregional expression, data were averaged across rostrocaudal levels within each subregion (Fig. 3). Post hoc pairwise comparisons were conducted using Fisher’s least significant difference (LSD) test.
Within the DRD, Tph2 mRNA expression was decreased in Tph2+/− mice compared to Tph2+/+ mice, whether they underwent home-cage conditions (p < 0.001) or maternal separation (p < 0.05; Fig. 3A). Additionally, Tph2 mRNA expression was decreased in Tph2−/− mice compared to Tph2+/+ mice that underwent animal facility-reared control conditions (p < 0.001) as well as maternal separation (p < 0.001; Fig 3A). Likewise, Tph2 mRNA expression was decreased in Tph2−/− mice compared to Tph2+/− mice that underwent animal facility-reared control control conditions (p < 0.01) as well as maternal separation (p < 0.01; Fig 3A). A similar pattern of Tph2 mRNA expression was observed in all other subregions of the DR (DRV, Fig. 3B; DRVL/VLPAG, Fig. 3C; DRC, Fig. 3D; and DRI, Fig. 3E) and in the MnR (Fig. 3F).
Data from each treatment group were averaged, regardless of subregion and rostrocaudal level (Fig. 3G). Post hoc analysis was conducted to differentiate effects of MS and genotype globally across the brainstem raphe nuclei.
Again, Tph2 mRNA expression was decreased in Tph2+/− mice compared to Tph2+/+ mice, whether they underwent home-cage conditions (p < 0.001) or maternal separation (p < 0.05; Fig. 3G). Additionally, Tph2 mRNA expression was decreased in Tph2−/− mice compared to Tph2+/+ mice that underwent animal facility-reared control conditions (p < 0.001) as well as maternal separation (p < 0.001; Fig 3G). Likewise, Tph2 mRNA expression was decreased in Tph2−/− mice compared to Tph2+/− mice that underwent animal facility-reared control conditions (p < 0.001) as well as maternal separation (p < 0.001; Fig 3G).
Post hoc analysis also revealed an effect of MS, wherein mice that underwent MS had lower levels of Tph2 mRNA expression compared to animal facility-reared control mice, among Tph2+/+ mice (p < 0.001; Fig. 3G).
3.2. Htr1a mRNA expression
Analysis of Htr1a mRNA expression using LMM analysis revealed an interaction effect of genotype x MS x rostrocaudal level (F(25, 187.9) = 1.714, p < 0.05; Fig. 4A–G, Fig. S7A–G, Table S2). Based on this finding, secondary linear mixed models were used to determine effects of MS and genotype within each subregion of the DR and in the MnR.
Figure 4.
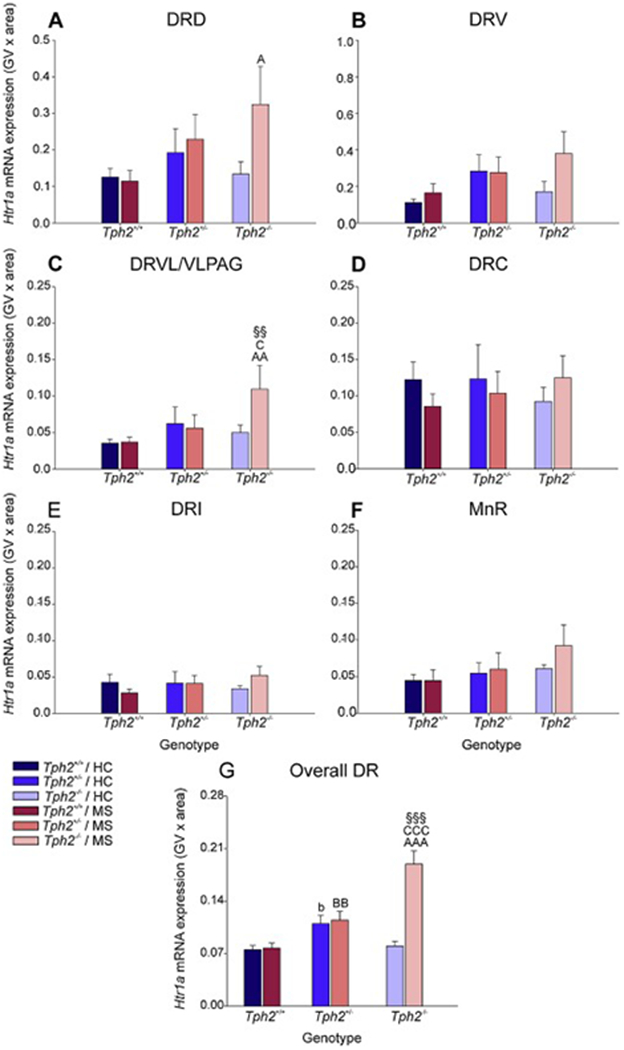
Effects of maternal separation in wild-type and Tph2-deficient mice on overall Htr1a mRNA expression within each subregion of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Graphs represent the mean ± SEM of Htr1a mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) MnR, and (G) across all subregions in the DR and MnR. Male wild-type, heterozygous, and homozygous Tph2 knockout mice were exposed to either animal facility-reared control conditions (HC) or maternal separation (MS), consisting of daily separation (3 h) of mixed litters from the dam postnatal day 2 (P2) through P15, inclusive. Sample sizes per treatment group: Tph2+/+ HC (n = 8); Tph2+/− HC (n = 8); Tph2−/− HC (n = 6); Tph2+/+ MS (n = 7); Tph2+/− MS (n = 8); Tph2−/− MS (n = 6); (N = 43). Post hoc comparisons were made using Fisher’s least significant difference (LSD) tests; §§p < 0.01, §§§p < 0.001, Tph2−/− MS versus Tph2−/− HC; bp < 0.05, Tph2+/+ HC versus Tph2+/− HC; AAp < 0.01, AAAp < 0.001, Tph2+/+ MS versus Tph2−/− MS; , BBp < 0.01, Tph2+/+ MS versus Tph2+/− MS; Cp < 0.05, CCCp < 0.001, Tph2+/− MS versus Tph2−/− MS.
Secondary linear mixed model analysis of specific subregions highlighted genotype x rostrocaudal level interactions within both the DRV (F(12, 38.1) = 2.2, p < 0.05) and DRVL/VLPAG (F(6, 32.6) = 2.80, p < 0.05), as well as genotype x MS x rostrocaudal level interactions within both DRI (F(16, 22.0) = 3.19, p < 0.01) and MnR (F(13, 56.5) = 3.78, p < 0.001; Fig. S7A–F; Table S2). For a detailed description of the post hoc comparisons for Htr1a mRNA expression within each rostrocaudal level of each subregion of the DR and in the MnR and globally within each rostrocaual level, see Supplementary Material.
To further understand the effects of maternal separation and Tph2 genotype on subregional expression, data were averaged across rostrocaudal levels within each subregion (Fig. 3). Post hoc pairwise comparisons were conducted using Fisher’s least significant difference (LSD) test.
Within the DRD, Htr1a mRNA expression was increased in Tph2−/− mice compared to Tph2+/+ mice that underwent maternal separation (p < 0.05; Fig 4A).
Within the DRVL/VLPAG, Htr1a mRNA expression was increased in Tph2−/− mice compared to Tph2+/+ mice that underwent animal facility-reared control conditions (p < 0.01) as well as maternal separation (p < 0.05; Fig 4C). Post hoc analysis also revealed an effect of MS, wherein mice that underwent MS had increased levels of Htr1a mRNA expression compared to animal facility-reared control mice, among Tph2−/− mice (p < 0.01; Fig. 4C).
There were no other effects of maternal separation or genotype in any other subregions of the DR or MnR (Fig 4B, Fig 4D–F).
Data from each treatment group were averaged, regardless of subregion and rostrocaudal level (Fig. 4G). Post hoc analysis was conducted to differentiate effects of genotype and MS on Tph2 mRNA expression globally across the raphe nuclei.
Among animal facility-reared control mice, there was an increase in Htr1a mRNA expression in Tph2+/− mice compared to Tph2+/+ mice (p < 0.05; Fig. 4G).
Furthermore, Tph2+/− mice that underwent MS had greater levels of Htr1a mRNA expression compared to Tph2+/+ mice (p < 0.01; Fig. 4G). A genotype effect was also found among mice that underwent MS, where Tph2−/− mice had greater levels of Htr1a mRNA expression compared to both Tph2+/+ mice and Tph2+/− mice (p < 0.001; Fig. 4G).
Post hoc analysis revealed an effect of MS in Tph2−/− mice, wherein mice that underwent MS had higher levels of Htr1a mRNA expression compared to animal facility-reared control mice (p < 0.001; Fig. 4G).
3.3. Slc6a4 mRNA expression
Analysis of Slc6a4 mRNA expression using LMM analysis revealed an interaction effect of genotype x MS x rostrocaudal level (F(26, 162.9) = 2.32, p < 0.001; Fig. 5A–G, Fig. S8A–G, Table S3). Based on this finding, secondary linear mixed models were used to determine effects of MS and genotype within each subregion of the DR and MnR serotonergic cell group.
Figure 5.

Effects of maternal separation in wild-type and Tph2-deficient mice on overall Slc6a4 mRNA expression within each subregion of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Graphs represent the mean ± SEM of Slc6a4 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) MnR, and (G) across all subregions in the DR and MnR. Male wild-type, heterozygous, and homozygous Tph2 knockout mice were exposed to either animal facility-reared control conditions (HC) or maternal separation (MS), consisting of daily separation (3 h) of mixed litters from the dam postnatal day 2 (P2) through P15, inclusive. Sample sizes per treatment group: Tph2+/+ HC (n = 8); Tph2+/− HC (n = 8); Tph2−/− HC (n = 6); Tph2+/+ MS (n = 7); Tph2+/− MS (n = 8); Tph2−/− MS (n = 6); (N = 43). Post hoc comparisons were made using Fisher’s least significant difference (LSD) tests; +p < 0.05, +++p < 0.001, Tph2+/− MS versus Tph2+/− HC; Ap < 0.05, AAp < 0.01, Tph2+/+ MS versus Tph2−/− MS;, Bp < 0.05, BBp < 0.01, Tph2+/+ MS versus Tph2+/− MS.
Interaction effects of genotype x MS x rostrocaudal level were evident in the DRD (F(14, 27.6) = 2.9, p < 0.01) and MnR (F(14, 19.7) = 2.7, p < 0.05; Fig. S8A,F, Table S3). For a detailed description of the post hoc comparisons for Slc6a4 mRNA expression within each subregion of the DR and in the MnR and globally within each rostrocaual level, see Supplementary Material.
To further understand the effects of maternal separation and Tph2 genotype on subregional expression, data were averaged across rostrocaudal levels within each subregion (Fig. 3). Post hoc pairwise comparisons were conducted using Fisher’s least significant difference (LSD) test.
Within the DRD, there was an effect of MS, wherein mice that underwent MS had increased levels of Slc6a4 mRNA expression compared to animal facility-reared control mice, among Tph2+/− mice (p < 0.05; Fig. 5A).
Within the DRV, Slc64 mRNA expression was increased in Tph2+/− mice compared to Tph2+/+ mice that underwent maternal separation (p < 0.05; Fig. 5B). Additionally, Slc6a4 mRNA expression was increased in Tph2−/− mice compared to Tph2+/+ mice that underwent maternal separation (p < 0.05; Fig 5B). Post hoc analysis also revealed an effect of MS, wherein mice that underwent MS had increased levels of Slc6a4 mRNA expression compared to animal facility-reared control mice, among Tph2+/− mice (p < 0.51; Fig. 5B).
There were no other effects of maternal separation or genotype in any other subregions of the DR or MnR (Fig 4C–F).
Data from each treatment group were averaged, regardless of subregion and rostrocaudal level (Fig. 5G). Post hoc analysis was conducted to differentiate effects of MS and genotype on Slc6a4 mRNA expression globally across the raphe nuclei.
A genotype effect was found among mice that underwent MS, where both Tph2+/− mice and Tph2−/− mice had greater levels of Slc6a4 mRNA expression compared to Tph2+/+ mice (p < 0.01; Fig. 5G).
Post hoc analysis revealed an effect of MS among Tph2+/− mice, wherein Tph2+/− mice that underwent MS had increased Slc6a4 mRNA expression compared to Tph2+/− mice exposed to animal facility-reared control conditions (p < 0.05; Fig. 5G).
3.4. Slc22a3 mRNA expression
Analysis of Slc22a3 mRNA expression using LMM analysis revealed a genotype x MS x rostrocaudal level interaction (F(26, 162.2) = 3.72, p < 0.01; Fig. 6A–G, Fig. S9A–G, Table S4). Based on this finding, secondary LMMs were used to determine effects of genotype and MS within each subregion of the DR and in the MnR.
Figure 6.
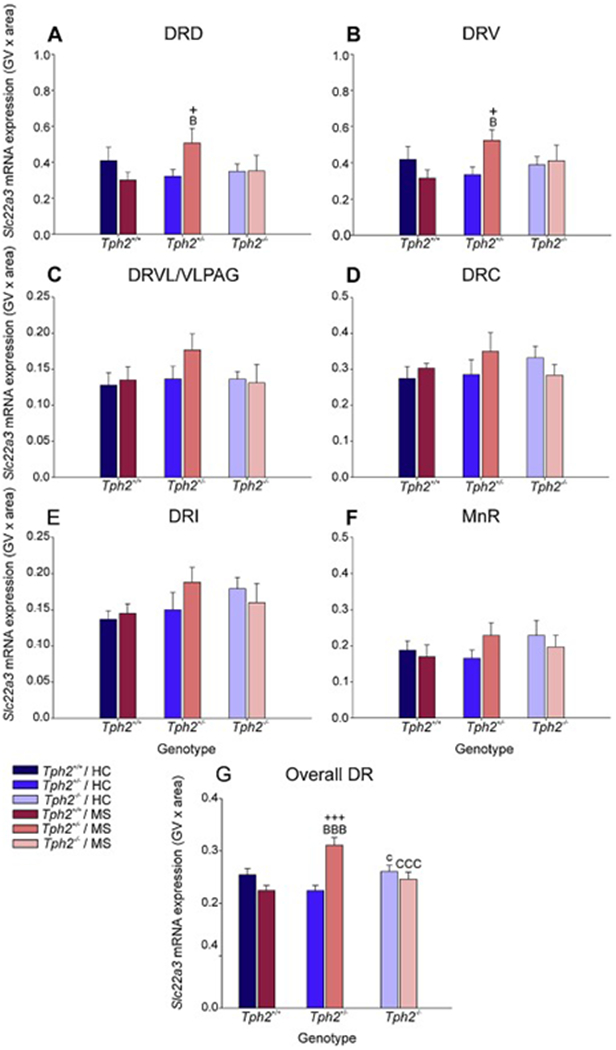
Effects of maternal separation in wild-type and Tph2-deficient mice on overall Slc22a3 mRNA expression within each subregion of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Graphs represent the mean ± SEM of Slc22a3 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) MnR, and (G) across all subregions in the DR and MnR. Male wild-type, heterozygous, and homozygous Tph2 knockout mice were exposed to either animal facility-reared control conditions (HC) or maternal separation (MS), consisting of daily separation (3 h) of mixed litters from the dam postnatal day 2 (P2) through P15, inclusive. Sample sizes per treatment group: Tph2+/+ HC (n = 8); Tph2+/− HC (n = 8); Tph2−/− HC (n = 6); Tph2+/+ MS (n = 7); Tph2+/− MS (n = 8); Tph2−/− MS (n = 6); (N = 43). Post hoc comparisons were made using Fisher’s least significant difference (LSD) tests; +p < 0.05, +++p < 0.001, Tph2+/− MS versus Tph2+/− HC; cp < 0.05, Tph2 HC versus Tph2−/− HC; Bp < 0.05, BBBp < 0.001, Tph2+/+ MS versus Tph2+/− MS; CCCp < 0.001, Tph2+/− MS versus Tph2−/− MS.
Secondary linear mixed model analysis of specific subregions highlighted MS x rostrocaudal level interactions within the DRD (F(7, 46.4) = 2.87, p < 0.05) as well as genotype x MS x rostrocaudal level interactions within the DRD (F(14, 48.5) = 2.16, p < 0.05), DRV (F(12, 45.7) = 3.63, p < 0.001), and DRVL/VLPAG (F(6, 39.8) = 2.39, p < 0.05; Fig. S9A,F, Table S3). Fora detailed description of the post hoc comparisons for Slc22a3 mRNA expression within each subregion of the DR and in the MnR and globally within each rostrocaudal level, see Supplementary Material.
To further understand the effects of maternal separation and Tph2 genotype on subregional expression, data were averaged across rostrocaudal levels within each subregion (Fig. 3). Post hoc pairwise comparisons were conducted using Fisher’s least significant difference (LSD) test.
Within the DRD, Slc22a3 mRNA expression was increased in Tph2+/− mice compared to Tph2+/+ mice that underwent maternal separation (p < 0.05; Fig. 6A). Post hoc analysis also revealed an effect of MS, wherein mice that underwent MS had increased levels of Slc22a3 mRNA expression compared to animal facility-reared control mice, among Tph2+/− mice (p < 0.05; Fig. 6A).
Similar results were also found within the DRV. Again, Slc22a3 mRNA expression was increased in Tph2+/− mice compared to Tph2+/+ mice that underwent maternal separation (p < 0.05; Fig. 6B), and Slc22a3 mRNA expression was increased in Tph2+/− mice that underwent maternal separation compared to animal facility-reared control mice (p < 0.05; Fig 6B).
There were no other effects of maternal separation or genotype in any other subregions of the DR or MnR (Fig 4C–F).
Data from each treatment group were averaged, regardless of subregion and rostrocaudal level (Fig. 6G). Post hoc analysis was conducted to differentiate effects of MS and genotype on Slc22a3 mRNA expression globally across the brainstem raphe nuclei.
Among animal facility-reared control mice, there was an increase in Slc22a3 mRNA expression in Tph2−/− mice compared to Tph2+/− mice (p < 0.05; Fig. 6G). A genotype effect was also found among mice that underwent MS, where Tph2−/− mice had greater levels of Slc22a3 mRNA expression compared to both Tph2+/+ mice and Tph2−/− mice (p < 0.001; Fig. 6G). Post hoc analysis revealed an effect of MS among Tph2+/− mice, wherein mice that underwent MS had increased Slc22a3 mRNA expression compared to animal facility-reared control mice (p < 0.001; Fig. 6G).
3.5. Crhr1 mRNA expression
Analysis of Crhr1 mRNA expression using LMM analysis revealed a main effect of genotype (F(2, 289.853) = 4.10, p < 0.05; Fig. 7A–G, Fig. S10A–G, 17, Table S5). Based on this finding, secondary LMMs were used to determine effects of genotype within each subregion of the DR and in the MnR.
Figure 7.
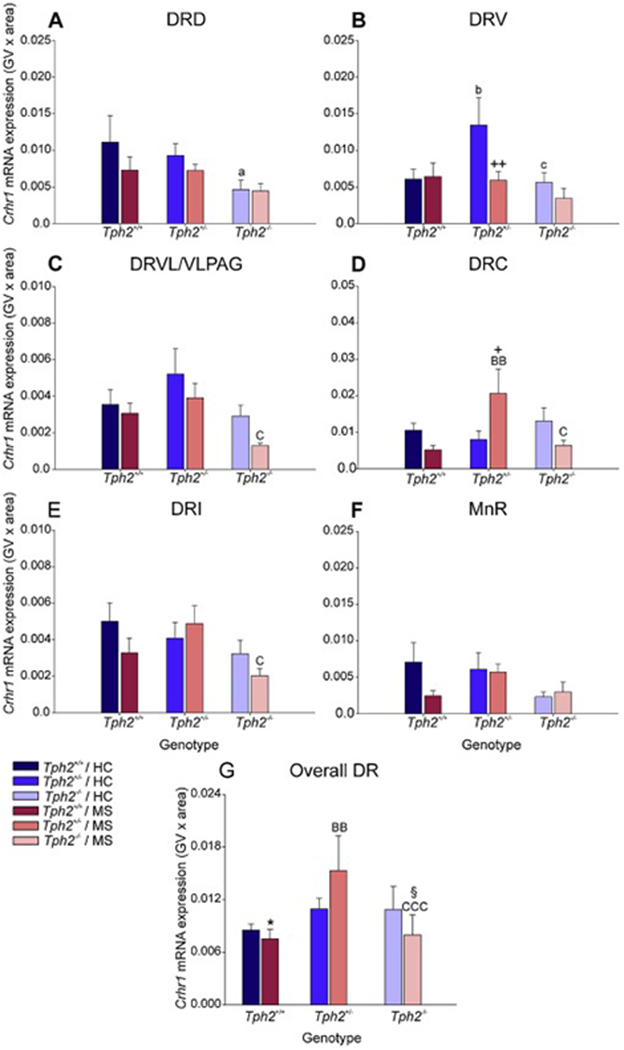
Effects of maternal separation in wild-type and Tph2-deficient mice on overall Crhr1 mRNA expression within each subregion of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Graphs represent the mean ± SEM of Crhr1 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) MnR, and (G) across all subregions in the DR and MnR. Male wild-type, heterozygous, and homozygous Tph2 knockout mice were exposed to either animal facility-reared control conditions (HC) or maternal separation (MS), consisting of daily separation (3 h) of mixed litters from the dam postnatal day 2 (P2) through P15, inclusive. Sample sizes per treatment group: Tph2+/+ HC (n = 8); Tph2 HC (n = 8); Tph2−/− HC (n = 6); Tph2+/+ MS (n = 7); Tph2+/− MS (n = 8); Tph2−/− MS (n = 6); (N = 43). Post hoc comparisons were made using Fisher’s least significant difference (LSD) tests; *p < 0.05, Tph2+/+ MS versus Tph2+/+ HC; +p < 0.05, ++p < 0.01, Tph2 MS versus Tph2 HC; §p < 0.05, Tph2−/− MS versus Tph2−/− HC; ap < 0.05, Tph2+/+ HC versus Tph2−/− HC; bp < 0.05, Tph2+/+ HC versus Tph2+/− HC; cp < 0.05, Tph2+/− HC versus Tph2−/− HC; BBp < 0.01, Tph2+/+ MS versus Tph2−/− MS; Cp < 0.05, Tph2+/− MS versus Tph2−/− MS.
Secondary linear mixed model analysis of specific subregions highlighted MS x rostrocaudal level interactions within the DRD (F(7, 18.4) = 3.28, p < 0.05), DRV (F(6, 25.8) = 7.89, p < 0.001), and MnR (F(7, 33.7) = 2.43, p < 0.05); genotype x rostrocaudal level interactions within the DRV (F(12, 25.8) = 4.46, p < 0.001), DRI (F(16, 34.8) = 3.25, p < 0.01), and MnR (F(14, 38.7) = 2.30, p < 0.05); and genotype x MS x rostrocaudal level interactions within the DRV (F(12, 25.8) = 3.18, p < 0.01). For a detailed description of the post hoc comparisons for Crhr1 mRNA expression within each rostrocaudal level of each subregion of the DR and in the MnR and globally within each rostrocaual level, see Supplementary Material.
To further understand the effects of maternal separation and Tph2 genotype on subregional Crhr1 mRNA expression, data were averaged across rostrocaudal levels within each subregion (Fig. 3). Post hoc pairwise comparisons were conducted using Fisher’s least significant difference (LSD) test.
Within the DRD, Crhr1 mRNA expression was decreased in Tph2−/− mice compared to Tph2+/+ mice under animal facility-reared control conditions (p < 0.05; Fig. 7A).
Within the DRV, Crhr1 mRNA expression was increased in Tph2+/− mice compared to Tph2+/+ mice under animal facility-reared control conditions (p < 0.05; Fig. 7B). Additionally, Crhr1 mRNA expression was decreased in Tph2−/− mice compared to Tph2+/− mice under animal facility-reared control conditions (p < 0.05; Fig. 7B). Post hoc analysis also revealed an effect of MS, wherein mice that underwent MS had decreased levels of Crhr1 mRNA expression compared to animal facility-reared control mice, among Tph2+/− mice (p < 0.01; Fig. 7B).
Within the DRVL/VLPAG, Crhr1 mRNA expression was decreased in Tph2−/− mice compared to Tph2+/− mice that underwent maternal separation (p < 0.05; Fig. 7C).
Within the DRC, Crhr1 mRNA expression was increased in Tph2+/− mice compared to Tph2+/+ mice that underwent maternal separation (p < 0.01; Fig. 7D). Additionally, Crhr1 mRNA expression was decreased in Tph2−/− mice compared to Tph2+/− mice that underwent maternal separation (p < 0.05; Fig. 7D). Post hoc analysis also revealed an effect of MS, wherein mice that underwent MS had increased levels of Crhr1 mRNA expression compared to animal facility-reared control mice, among Tph2+/− mice (p < 0.05; Fig. 7D).
Within the DRI, Crhr1 mRNA expression was decreased in Tph2−/− mice compared to Tph2+/− mice that underwent maternal separation (p < 0.05; Fig. 7E)
There were no effects of maternal separation or genotype in the MnR (Fig 4F).
Data from each treatment group were averaged, regardless of subregion and rostrocaudal level (Fig. 7G). Post hoc analysis was conducted to differentiate effects of MS and genotype on Crhr1 mRNA expression globally across the brainstem raphe nuclei.
An effect of genotype was observed among mice that underwent MS, in which Tph2+/− mice had increased Crhr1 mRNA expression compared to both Tph2+/+ mice and Tph2−/− mice (p < 0.01; Fig. 7G).
3.6. Crhr2 mRNA expression
Analysis of Crhr2 mRNA expression using LMM analysis revealed a genotype x MS x rostrocaudal level interaction (F(24, 62.1) = 2.28, p < 0.01, Fig. 8A–G, Fig. S11A–G, Table S6). Based on this finding, secondary linear mixed models were used to determine effects of treatment within each subregion of the DR and in the MnR.
Figure 8.
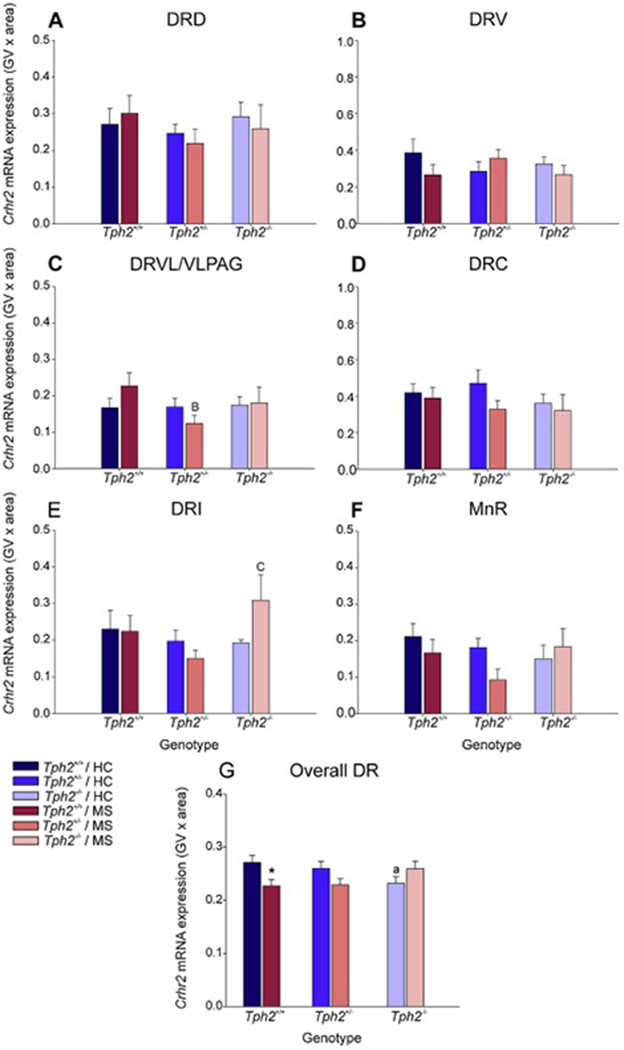
Effects of maternal separation in wild-type and Tph2-deficient mice on overall Crhr2 mRNA expression within each subregion of the dorsal raphe nucleus (DR) and median raphe nucleus (MnR). Graphs represent the mean ± SEM of Crhr2 mRNA expression in the (A) dorsal raphe nucleus, dorsal part (DRD), (B) dorsal raphe nucleus, ventral part (DRV), (C) dorsal raphe nucleus, ventrolateral part (DRVL), (D) dorsal raphe nucleus, caudal part (DRC), (E) dorsal raphe nucleus, interfascicular part (DRI), (F) MnR, and (G) across all subregions in the DR and MnR. Male wild-type, heterozygous, and homozygous Tph2 knockout mice were exposed to either animal facility-reared control conditions (HC) or maternal separation (MS), consisting of daily separation (3 h) of mixed litters from the dam postnatal day 2 (P2) through P15, inclusive. Sample sizes per treatment group: Tph2+/+ HC (n = 8); Tph2+/− HC (n = 8); Tph2−/− HC (n = 6); Tph2+/+ MS (n = 7); Tph2+/− MS (n = 8); Tph2−/− MS (n = 6); (N = 43). Post hoc comparisons were made using Fisher’s least significant difference (LSD) tests; *p < 0.05, Tph2+/+ MS versus Tph2+/+ HC; §p < 0.05, Tph2−/− MS versus Tph2−/− HC; Bp < 0.05, BBp < 0.05, Tph2+/+ MS versus Tph2+/− MS; Cp < 0.05, CCCp < 0.05, Tph2 MS versus Tph2−/− MS.
Secondary linear mixed model analysis of specific subregions highlighted MS x rostrocaudal level interactions within the DRV (F(6, 13.0) = 3.28, p < 0.05), genotype x rostrocaudal level interactions within the DRVL/VLPAG (F(6, 30.0) = 4.87, p < 0.001), and genotype x MS x rostrocaudal level interactions within the DRC (F(10, 17.4) = 7.78, p < 0.001). For a detailed description of the post hoc comparisons for Crhr2 mRNA expression within each rostrocaudal level of each subregion of the DR and in the MnR and globally within each rostrocaual level, see Supplementary Material.
Within the DRVL/VLPAG, Crhr2 mRNA expression was decreased in Tph2+/− mice compared to Tph2+/+ mice that underwent maternal separation (p < 0.05; Fig. 8C).
Within the DRI, Crhr2 mRNA expression was increased in Tph2+/− mice compared to Tph2+/− mice that underwent maternal separation (p < 0.05; Fig. 8E).
There were no other effects of maternal separation or genotype in any other subregions of the DR or MnR (Fig 4A–B, Fig 4D, Fig 4F).
Data from each treatment group were averaged, regardless of subregion and rostrocaudal level (Fig. 8G). Post hoc analysis was conducted to differentiate effects of MS and genotype on Crhr2 mRNA expression globally across the raphe.
Among animal facility-reared control mice, there was a decrease in Crhr2 mRNA expression in Tph2−/− mice in comparison to Tph2+/+ (p < 0.05; Fig. 8G). Post hoc analysis also revealed an effect of MS, wherein, among Tph2+/+ mice, mice that underwent MS had decreased Crhr2 mRNA expression compared to animal facility-reared control mice (p < 0.05; Fig. 8G).
4. Discussion
Here, we show that maternal separation differentially alters serotonergic gene expression, as well as genes that control serotonergic neuronal activity (i.e., Crhr1 and Crhr2), in the dorsal and median raphe nuclei of wild-type and Tph2-deficient mice. Wild-type mice responded to MS with a reduction of Tph2 mRNA expression in adulthood, an effect that was not detected in heterozygous Tph2 deficient mice, which already had reduced Tph2 mRNA expression. There were also interactions between Tph2 genotype and MS for other serotonergic genes; for example, MS increased Htr1a mRNA expression, but only in Tph2−/− mice, and increased Slc22a3 mRNA expression, but only in Tph2+/− mice. Finally, some interactions between Tph2 genotype and MS were subregion specific; for example, MS increased Slc6a4 mRNA expression, but only in Tph2+/− mice and only in the DRD. Finally, overall analysis of the data revealed that there was compensatory gene expression, discussed further below, in Tph2-deficient mice, relative to wild-type controls.
Overall, relative to wild-type control mice, Tph2+/− mice were estimated to have 47.7% of Tph2 mRNA expression levels, while Tph2−/− mice were estimated to have 3.4% of Tph2 mRNA expression, which is likely below the threshold for detection of Tph2. Previous studies have measured changes in Tph2 mRNA expression (Kriegebaum et al., 2010) and Tph2 protein (Gutknecht et al., 2008, 2012b) in Tph2 knockout mice. However, this is the first time Tph2 mRNA expression has been measured in the heterozygous Tph2 deficient mice alongside Tph2 wild-type and homozygous Tph2 deficient mice. This allowed us to observe a near-linear relationship with two Tph2 alleles displaying 100% Tph2 mRNA expression, one Tph2 allele displaying approximately 50% Tph2 mRNA expression, and zero Tph2 alleles displaying approximately 0%. The step-wise, gene dose-dependent reduction in Tph2 mRNA expression indicates biallelic expression of the Tph2 gene, rather than allelic exclusion of one of the chromosomes (Skipper, 2008). It should be noted, however, that the reduction of 5-HT concentrations in Tph2+/− mice is estimated to be only 10-20% (Mosienko et al., 2014), suggesting compensatory mechanisms to maintain brain 5-HT concentrations to near-normal levels in Tph2+/− mice.
Interactions described here between Tph2 mRNA expression and maternal separation provide an example of G x E interactions. Specifically, wild-type mice responded to MS with a reduction of Tph2 mRNA expression in adulthood, an effect that was not detected in heterozygous Tph2 deficient mice, which already had reduced Tph2 mRNA expression. The effect of MS on wild-type mice was evident in the overall DR and MnR, suggesting the mechanisms involved include mediators that affect all serotonergic neurons directly. One potential mediator is plasma glucocorticoid concentrations, which have been shown to drive diurnal variations in Tph2 mRNA expression in both the DR and MnR (Malek et al., 2004, 2005, 2007; Chen and Miller, 2012). In support of altered glucocorticoid signaling in Tph2-deficient mice, male Tph2−/− mice have reduced hippocampal glucocorticoid receptor expression relative to wild-type mice, while male Tph2−/− mice, but not wild-type mice, respond to chronic mild stress with increases in glucocorticoid receptor expression in the brainstem raphe nuclei (Gutknecht et al., 2015). Maternal separation increases activity of the hypothalamic-pituitary-adrenal (HPA) axis and plasma corticosterone concentrations in both rat (Vallée et al., 1997; Plotsky et al.,2005) and mouse (Parfitt et al., 2004; Kember et al., 2012) models. Interestingly, glucocorticoids increase Tph2 mRNA expression in rat models (Azmitia et al., 1993; Malek et al., 2007; Chen and Miller, 2012; Donner et al., 2016), but decrease Tph2 mRNA expression in mouse models (Clark et al., 2005, 2008, Heydendael and Jacobson, 2009, 2010; Chen and Miller, 2012; Vincent et al., 2017). This dichotomy may explain why MS increases Tph2 mRNA expression in the DR and MnR of rats (Gardner et al., 2009b), but decreases Tph2 mRNA expression in the DR and MnR of mice. The involvement of glucocorticoids in these responses to maternal separation in both rats and mice require further study.
We also observed interactions between Tph2 genotype and maternal separation for other serotonergic genes; for example, maternal separation increased Htr1a mRNA expression, but only in Tph2−/− mice. Spiacci Jr et al., 2016 previously demonstrated pharmacological activation of 5-HT1A receptors within the DRD and DRVL/VLPAG led to increased panic-like behavior. Serotonergic neurons in the DRD and DRVL/VLPAG project to the dorsal periaqueductal gray (DPAG), where 5-HT plays a role in inhibition of panic-like defensive behavioral responses (Stezhka and Lovick, 1997; Paul and Lowry, 2013; Gambeta et al., 2017). The observed increase in Htr1a mRNA expression in the DRD and DRVL/VLPAG in Tph2mice, as well as the previously reported increase in 5-HT1A receptor binding in the DR and functional coupling of 5-HT1A receptors in Tph2−/− mice (Gutknecht et al., 2012), would be consistent with dysregulation of the serotonergic neurons involved in inhibition of panic-like behavior, thus, leading to disinhibition of panic within the DPAG, corresponding with panic-like behavior found in Tph2−/− mice (Waider et al., 2017).
Additionally, there was an inverse relationship between the genotype effect on Tph2 mRNA and the genotype effect on Htr1a mRNA. In other words, among mice exposed to maternal separation, the Tph2+/− mice had increased Htr1a mRNA expression relative to wild-type mice, and Tph2−/− mice had even greater Htr1a mRNA expression. Interestingly, the increases in Htr1a mRNA expression among Tph2−/− mice were only found in the maternal separation condition, which could infer a compensatory mechanism of serotonin deficiency induced only by early life stress. In the absence of a stress-like condition, Boris Mlinar et al., 2017 found increased density and enhanced functioning of 5-HT1A receptors in the DR of Tph2−/− mice, but no change in the Htr1a mRNA expression. This may explain why there was no effect of the Tph2−/− genotype on Htr1a mRNA expression among animal facility-reared control mice, whereas maternal separation may induce alternative regulatory mechanisms, enhancing Htr1a mRNA expression. One possible mechanism is that increases in Htr1a mRNA expression in Tph2−/− mice exposed to maternal separation could be mediated by glucocorticoids induced by maternal separation, which would intertwine with the hypothesized effect of glucocorticoids on Tph2 mRNA expression. Previous studies have identified effects of glucocorticoids on 5-HT1A autoreceptor functioning (Fairchild et al., 2003; Bellido et al., 2004). Further investigation is required on the mechanisms that mediate early life stress-induced increase in Hrtla mRNA expression in the DR of Tph2−/− mice, specifically when interacting with glucocorticoids.
There were also interactions between Tph2 genotype and maternal separation for other important genes expressed in 5-HT neurons; for example, maternal separation increased Slc6a4 and Slc22a3 mRNA expression, but only in Tph2+/− mice. The Slc6a4 mRNA expression in this study correlates to that found by Waider et al., 2017, which depicts elevated Slc6a4 mRNA expression in the caudal part of the DRD and the rostral part of the DRV. For Slc22a3 mRNA expression, we also see this effect in the DRVL/VLPAG. Furthermore, it has previously been shown that maternal separation increases Slc6a4 mRNA expression in the DR (Gardner et al., 2009a), in association with decreased proactive coping behavior in rats (Gardner et al., 2005). Specifically, increased transporter expression, either Slc6a4 or Slc22a3, would lead to increased serotonergic clearance in the synapse and, thus, decreased serotonergic signaling on the postsynaptic cell. One of the primary projections from the DRVL/VPAG is to the DPAG (Stezhka and Lovick, 1997; Paul and Lowry, 2013). Decreased serotonin in the DPAG is associated with increased panic-like behavior (Gambeta et al., 2017). Additionally, chronic selective serotonin reuptake inhibitor treatment has been shown to alter serotonin signaling in the DPAG (Zangrossi and Graeff, 2014). Ultimately, increased Slc6a4 mRNA expression and Slc22a3 mRNA expression in the DRVL/VLPAG neurons of heterozygous Tph2 deficient mice could lead to increased serotonin clearance in the DPAG, resulting in increased panic-like behavior (Stezhka and Lovick, 1997; Gardner et al., 2005, 2009a; Paul and Lowry, 2013; Gambeta et al., 2017; Waider et al., 2017). Lastly, Slc6a4 and Slc22a3 display a similar pattern of mRNA expression, which may indicate that they are under the influence of the same regulatory mechanisms. This could be assessed with future experiments.
Crhr1 mRNA expression was low, relative to the other genes analyzed, in the DR and MnR across all treatment groups, consistent with previous studies in mice and rats (Van Pett et al., 2000; Day et al., 2004). In contrast, Crhr2 mRNA expression was higher across all treatment groups, consistent with previous studies in rats (Day et al., 2004). Decreases in Crhr2 mRNA expression in the brainstem raphe nuclei of wild-type mice exposed to maternal separation are consistent with previous studies showing that overexpression of Crh in the bed nucleus of the stria terminalis decreases Crhr2 in the dorsomedial DR (Sink et al., 2013). The maternal stress-induced decrease in Crhr2 in the brainstem raphe nuclei was absent in Tph2+/− and Tph2−/− mice, suggesting dysregulation of long-term adaptations following stressor exposure in Tph2-deficient mice.
5. Conclusions
Finally, overall analysis of the data revealed that there was compensatory gene expression in Tph2-deficient mice relative to wild-type controls. Maternal separation differentially altered serotonergic gene expression in wild-type, heterozygous, and homozygous Tph2-deficient mice, potentially in association with altered HPA axis activation. Altogether, these data are consistent with the hypothesis that G x E interactions, including serotonergic genes and adverse early life experience, play an important role in vulnerability to trauma- and stressor-related disorders, anxiety- (e.g., panic disorder) related disorders, as well as affective disorders during adulthood.
Supplementary Material
Highlights:
Maternal separation alters serotonergic gene expression in Tph2-deficient mice
G x E interactions altered gene expression in a topographically-specific manner
Maternal separation increased Slc6a4 mRNA expression, as observed previously in rats
Tph2 deficiency induced compensatory changes in serotonergic gene expression
Acknowledgements
We are grateful to Zachary D. Barger for proofreading the manuscript. Dr. Christopher A. Lowry is supported by the National Institute of Mental Health (grant number 1R21MH116263), Department of the Navy, Office of Naval Research Multidisciplinary University Research Initiative (MURI) Award (grant number N00014-15-1-2809), Department of Veterans Affairs Office of Research and Development (VA-ORD) RR&D Small Projects in Rehabilitation Research (SPiRE) (I21) (grant number 1 I21 RX002232-01), the Colorado Department of Public Health and Environment (CDPHE; grant number DCEED-3510), and the Alfred P. Sloan Foundation (grant number G-2016-7077). Christopher A. Lowry serves on the Scientific Advisory Board of Immodulon Therapeutics Ltd. Dr. Klaus-Peter Lesch is supported by Deutsche Forschungsgemeinschaft (DFG: SFB TRR 58/A5), the European Community (EC: AGGRESSOTYPE FP7/No. 602805; ERA-Net NEURON/RESPOND/No. 01EW1602B), Fritz Thyssen Foundation (Az. 10.13.1185) and 5-100 Russian Academic Excellence Project.
Abbreviations
- 5-HT
5-hydroxytryptamine (serotonin)
- 5-HT1A
serotonin type 1a receptor protein
- Crhr1
corticotropin-releasing hormone receptor 1 gene
- Crhr2
corticotropin-releasing hormone receptor 2 gene
- DPAG
dorsal periaqueductal gray
- DR
dorsal raphe nucleus
- DRC
dorsal raphe nucleus, caudal part
- DRI
dorsal raphe nucleus, interfascicular part
- DRV
dorsal raphe nucleus, ventral part
- DRVL
dorsal raphe nucleus, ventrolateral part
- G x E
gene by environment
- HC
home cage control
- HPA
hypothalamic-pituitary-adrenal
- Htr1a
serotonin type 1a receptor gene
- L
rostrocaudal level
- LMM
linear mixed effects model
- LSD
least significant difference
- MnR
median raphe nucleus
- mRNA
messenger ribonucleic acid
- MS
maternal separation
- P
postnatal day
- Slc22a3
solute carrier family 22 member 3 gene; organic cation transporter 3 gene
- Slc22a3
solute carrier family 22 member 3 protein; organic cation transporter 3 protein
- Slc6a4
solute carrier family 6 member 4 gene; serotonin transporter gene
- Slc6a4
solute carrier family 6 member 4 protein; serotonin transporter protein
- Tph2
tryptophan hydroxylase 2 gene
- Tph2
tryptophan hydroxylase 2 protein
- VLPAG
ventrolateral periaqueductal gray
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abrams JK, Johnson PL, Hollis JH, Lowry CA (2004) Anatomic and functional topography of the dorsal raphe nucleus. Annals of the New York Academy of Sciences 1018:46–57. [DOI] [PubMed] [Google Scholar]
- Andrade TG, Zangrossi H Jr, Graeff FG (2013) The median raphe nucleus in anxiety revisited. Journal of Psychopharmacology 27:1107–1115. [DOI] [PubMed] [Google Scholar]
- Auth CS, Weidner MT, Popp S, Strekalova T, Schmitt-Bohrer AG, van den Hove D, Lesch KP, Waider J (2018) Differential anxiety-related behaviours and brain activation in Tph2-deficient female mice exposed to adverse early environment. Eur Neuropsychopharmacology 28:1270–1283. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Liao B, Chen YS (1993) Increase of tryptophan hydroxylase enzyme protein by dexamethasone in adrenalectomized rat midbrain. Journal of Neuroscience 13:5041–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitz AJ (1982) The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience 7:133–159. [DOI] [PubMed] [Google Scholar]
- Bellido I, Hansson AC, Gomez-Luque AJ, Andbjer B, Agnati LF, Fuxe K (2004) Corticosterone strongly increases the affinity of dorsal raphe 5-HT1A receptors. Neuroreport 15:1457–1459. [DOI] [PubMed] [Google Scholar]
- Boris Mlinar, Montalbano A, Waider J, Lesch K-P, Corradetti R (2017) Increased functional coupling of 5-HT1A autoreceptors to GIRK channels in Tph2−/− mice. Eur NeuroPsychopharmacology 27:1258–1267. [DOI] [PubMed] [Google Scholar]
- Bravo JA, Dinan TG, Cryan JF (2014) Early-life stress induces persistent alterations in 5-HT1A receptor and serotonin transporter mRNA expression in the adult rat brain. Frontiers in Molecular Neuroscience 7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R (2003) Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301:386–389. [DOI] [PubMed] [Google Scholar]
- Chen G-L, Miller GM (2012) Advances in tryptophan hydroxylase-2 gene expression regulation new insights into serotonin-stress interaction and clinical implications. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 159B: 152–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JA, Pai L-Y, Flick RB, Rohrer SP (2005) Differential hormonal regulation of tryptophan hydroxylase-2 mRNA in the murine dorsal raphe nucleus. Biological Psychiatry 57:943–946. [DOI] [PubMed] [Google Scholar]
- Clark JA, Flick RB, Pai L-Y, Szalayova I, Key S, Conley RK, Deutch AY, Hutson PH, Mezey E (2008) Glucocorticoid modulation of tryptophan hydroxylase-2 protein in raphe nuclei and 5-hydroxytryptophan concentrations in frontal cortex of C57/B16 mice. Molecular Psychiatry 13:498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Connolley KR, Valentino RJ (2003) A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology 28:206–215. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Hinesley J, Chan RF, Aberg KA, Fairbank JA, van den Oord EJCG, Costello EJ (2018) Association of Childhood Trauma Exposure With Adult Psychiatric Disorders and Functional Outcomes. JAMANetw Open l:el84493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HEW, Akil H (1996) Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-beta: implications for mechanism of action. Neuroendocrinology 63:207–218. [DOI] [PubMed] [Google Scholar]
- Day HEW, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S (2004) Differential expression of 5HT-1A, a ib adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, γ-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. The Journal of Comparative Neurology 474:364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deakin JFW, Graeff FG (1991) 5-HT and mechanisms of defence. Journal of Psychopharmacology 5:305–315. [DOI] [PubMed] [Google Scholar]
- Donner NC, Johnson PL, Fitz SD, Kellen KE, Shekhar A, Lowry CA (2012a) Elevated lph2 mRNA expression in a rat model of chronic anxiety. Depression and Anxiety 29:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Montoya CD, Lukkes JL, Lowry CA (2012b) Chronic non-invasive corticosterone administration abolishes the diurnal pattern of tph2 expression. Psychoneuroendocrinology 37:645–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Siebler PH, Johnson DT, Villarreal MD, Mani S, Matti AJ, Lowry CA (2016) Serotonergic systems in the balance: CRHR1 and CRHR2 differentially control stress-induced serotonin synthesis. Psychoneuroendocrinology 63:178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner NC, Kubala KH, Hassell JE Jr., Lieb MW, Nguyen KT, Heinze JD, Drugan RC, Maier SF, Lowry CA (2018) Two models of inescapable stress increase tph2 mRNA expression in the anxiety-related dorsomedial part of the dorsal raphe nucleus. Neurobiology of Stress 8:68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duricki DA, Soleman S, Moon LDF (2016) Analysis of longitudinal data from animals with missing values using SPSS. Nature Protocols 11:1112–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckalbar WL, Lasku E, Infante CR, Elsey RM, Markov GJ, Allen AN, Comeveaux JJ, Losos JB, DeNardo DF, Huentelman MJ, Wilson-Rawls J, Rawls A and Kusumi K (2012) Somitogenesis in the anole lizard and alligator reveals evolutionary convergence and divergence in the amniote segmentation clock. Developmental Biology 363(1): 308–319. [DOI] [PubMed] [Google Scholar]
- Fairchild G, Leitch MM, Ingram CD (2003) Acute and chronic effects of corticosterone on 5-HT1A receptor-mediated autoinhibition in the rat dorsal raphe nucleus. Neuropharmacology 45:925–934. [DOI] [PubMed] [Google Scholar]
- Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, Renner KJ (2006) Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience 141:1047–1055. [DOI] [PubMed] [Google Scholar]
- Forster GL, Pringle RB, Mouw NJ, Vuong SM, Watt MJ, Burke AR, Lowry CA, Summers CH, Renner KJ (2008) Corticotropin-releasing factor in the dorsal raphe nucleus increases medial prefrontal cortical serotonin via type 2 receptors and median raphe nucleus activity. European Journal of Neuroscience 28:299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JH, Hassell JE Jr, Siebler PH, Arnold MR, Lamb AK, Smith DG, Day HEW, Smith TM, Simmerman EM, Outzen AA, Holmes KS, Brazell CJ, Lowry CA (2017) Preimmunization with a heat-killed preparation of Mycobacterium vaccae enhances fear extinction in the fear-potentiated startle paradigm. Brain, Behavior, and Immunity 66:70–84. [DOI] [PubMed] [Google Scholar]
- Fox JH, Lowry CA (2013) Corticotropin-releasing factor-related peptides, serotonergic systems, and emotional behavior. Frontiers in Neuroscience 7:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambeta E, Sestile CC, Fogaqa MV., Guimaraes FS, Audi EA, da Cunha JM, Zangrossi H Jr, Shimene de Melo Yamashita P, Zanoveli JM (2017) A serotonergic deficit in the dorsal periaqueductal gray matter may underpin enhanced panic-like behavior in diabetic rats. Behavioural Pharmacology 28:558–564. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA (2005) Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience 136:181–191. [DOI] [PubMed] [Google Scholar]
- Gardner KL, Hale MW, Lightman SL, Plotsky PM, Lowry CA (2009a) Adverse early life experience and social stress during adulthood interact to increase serotonin transporter mRNA expression. Brain Research 1305:47–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Hale MW, Oldfield S, Lightman SL, Plotsky PM, Lowry CA (2009b) Adverse experience during early life and adulthood interact to elevate tph2 mRNA expression in serotonergic neurons within the dorsal raphe nucleus. Neuroscience 163:991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside SE, Johnson DA, Leitch MM, Troakes C, Ingram CD (2003) Early life adversity programs changes in central 5-HT neuronal function in adulthood. European Journal of Neuroscience 17:2401–2408. [DOI] [PubMed] [Google Scholar]
- Graeff FG, Guimaraes FS, De Andrade TG, Deakin JF (1996) Role of 5-HT in stress, anxiety, and depression. Pharmacology Biochemistry and Behavior 54:129–141. [DOI] [PubMed] [Google Scholar]
- Grubbs FE (1969) Procedures for detecting outlying observations in samples. Technometrics 11:1–21. [Google Scholar]
- Gutknecht L, Jacob C, Strobel A, Kriegebaum C, Muller J, Zeng Y, Markert C, Escher A, Wendland J, Reif A, Mossner R, Gross C, Brocke B, Lesch K-P (2007) Tryptophan hydroxylase-2 gene variation influences personality traits and disorders related to emotional dysregulation. International Journal Neuropsychopharmacology 10:309. [DOI] [PubMed] [Google Scholar]
- Gutknecht L, Waider J, Kraft S, Kriegebaum C, Holtmann B, Reif A, Schmitt A, Lesch K-P (2008) Deficiency of brain 5-HT synthesis but serotonergic neuron formation in Tph2 knockout mice. Journal of Neural Transmission 115:1127–1132. [DOI] [PubMed] [Google Scholar]
- Gutknecht L, Kriegebaum C, Waider J, Schmitt A, Lesch K-P (2009) Spatio-temporal expression of tryptophan hydroxylase isoforms in murine and human brain : convergent data from Tph2 knockout mice. European Neuropsychopharmacology 19:266–282. [DOI] [PubMed] [Google Scholar]
- Gutknecht L, Araragi N, Merker S, Waider J, Sommerlandt FMJ, Mlinar B, Baccini G, Mayer U, Proft F, Ham on M, Schmitt AG, Corradetti R, Lanfumey L, Lesch K-P (2012) Impacts of brain serotonin deficiency following Tph2 inactivation on development and raphe neuron serotonergic specification. PLoS ONE 7:e43157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht L, Popp S, Waider J, Sommerlandt FMJ, Goppner C, Post A, Reif A, van den Hove D, Strekalova T, Schmitt A, ΟοHςο MB, Sommer C, Palme R, Lesch K-P (2015) Interaction of brain 5-HT synthesis deficiency, chronic stress and sex differentially impact emotional behavior in Tph2 knockout mice. Psychopharmacologyogy 232:2429–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Hay-Schmidt A, Mikkelsen JD, Poulsen B, Bouwknecht JA, Evans AK, Stamper CE, Shekhar A, Lowry CA (2008) Exposure to an open-field arena increases c-Fos expression in a subpopulation of neurons in the dorsal raphe nucleus, including neurons projecting to the basolateral amygdaloid complex. Neuroscience 157:733–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MW, Lowry CA (2011) Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology 213:243–264. [DOI] [PubMed] [Google Scholar]
- Heim CM, Nemeroff CB (2001) The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biological Psychiatry 49:1023–1039. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Kuhn JW, Prescott CA, Kendler KS (2006) The impact of generalized anxiety disorder and stressful life events on risk for major depressive episodes. Psychological Medicine 36:789–795. [DOI] [PubMed] [Google Scholar]
- Heydendael W, Jacobson L (2009) Glucocorticoid status affects antidepressant regulation of locus coeruleus tyrosine hydroxylase and dorsal raphe tryptophan hydroxylase gene expression. Brain Research 1288:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydendael W, Jacobson L (2010) Widespread hypothalamic-pituitary-adrenocortical axisrelevant and mood-relevant effects of chronic fluoxetine treatment on glucocorticoid receptor gene expression in mice. European Journal of Neuroscience 31:892–902. [DOI] [PubMed] [Google Scholar]
- Holland P, Gallagher M (2004) Amygdala-frontal interactions and reward expectancy. Current Opinion in Neurobiology 14:148–155. [DOI] [PubMed] [Google Scholar]
- Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM (2008) Redefining the serotonergic system by genetic lineage. Nature Neuroscience 11:417–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Lightman SL, Lowry CA (2004) A functional subset of serotonergic neurons in the rat ventrolateral periaqueductal gray implicated in the inhibition of sympathoexcitation and panic. Annals of the New York Academy of Sciences 1018:58–64. [DOI] [PubMed] [Google Scholar]
- Johnson P, Lowry C, Truitt W, Shekhar A (2008) Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. Journal of Psychopharmacology 22:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG (2002) Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacology Biochemistry and Behavior 73:131–140. [DOI] [PubMed] [Google Scholar]
- Kember RL, Dempster EL, Lee THA, Schalkwyk LC, Mill J, Fernandes C (2012) Maternal separation is associated with strain-specific responses to stress and epigenetic alterations to Nr3c1, Avp, and Nr4a1 in mouse. Brain and Behavior 2:455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-K, Lee H-J, Yang J-C, Hwang J-A, Yoon H-K (2009) A tryptophan hydroxylase 2 gene polymorphism is associated with panic disorder. Behav Genet 39:170–175. [DOI] [PubMed] [Google Scholar]
- Kriegebaum C, Song N-N, Gutknecht L, Huang Y, Schmitt A, Reif A, Ding Y-Q, Lesch K-P (2010) Brain-specific conditional and time-specific inducible Tph2 knockout mice possess normal serotonergic gene expression in the absence of serotonin during adult life. Neurochemistry International 57:512–517. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM (2000) Longterm behavioral and neuroendocrine adaptations to adverse early experience. Progress in Brain Research 122:81–103. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim HJ, Kim JG, Ryu V, Kim BT, Kang DW, Jahng JW (2007) Depressive behaviors and decreased expression of serotonin reuptake transporter in rats that experienced neonatal maternal separation. Neuroscience Research 58:32–39. [DOI] [PubMed] [Google Scholar]
- Liu X, Powell DK, Wang H, Gold BT, Corbly CR, Joseph JE (2007) Functional dissociation in frontal and striatal areas for processing of positive and negative reward information. Journal of Neuroscience 27:4587–4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry CA (2002) Functional subsets of serotonergic neurons: implications for control of the hypothalamic-pituitary-adrenal axis. Journal of Neuroendocrinology 14:911–923. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A (2008a) Serotonergic systems, anxiety, and affective disorder. Annals of the New York Academy of Sciences 1148:86–94. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Evans AK, Gasser PJ (2008b) Topographic organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus. Serotonin and Sleep: Molecular, Functional, and Clinical Aspects 25–67. [Google Scholar]
- Lowry CA, Lightman SL, Nutt DJ (2009) That warm fuzzy feeling: brain serotonergic neurons and the regulation of emotion. Journal of Psychopharmacology 23:394–400. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Kopelman JM, Donner NC, Hale MW, Lowry CA (2013) Development x environment interactions control tph2 mRNA expression. Neuroscience 237:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek ZS, Pevet P, Raison S (2004) Circadian change in tryptophan hydroxylase protein levels within the rat intergeniculate leaflets and raphe nuclei. Neuroscience 125:749–758. [DOI] [PubMed] [Google Scholar]
- Malek ZS, Dardente H, Pevet P, Raison S (2005) Tissue-specific expression of tryptophan hydroxylase mRNAs in the rat midbrain: anatomical evidence and daily profiles. European Journal of Neuroscience 22:895–901. [DOI] [PubMed] [Google Scholar]
- Malek ZS, Sage D, Pevet P, Raison S (2007) Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology 148:5165–5172. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Wooderson S, Lawrence N, Brammer MJ, Speckens A, Phillips ML (2004) Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Archives of General Psychiatry 61:564–576. [DOI] [PubMed] [Google Scholar]
- McEuen JG, Beck SG, Bale TL (2008) Failure to mount adaptive responses to stress results in dysregulation and cell death in the midbrain raphe. Journal of Neuroscience 28:8169–8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein RA, Holmes A (2007) Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neuroscience and Biobehavioral Reviews 31:3–17. [DOI] [PubMed] [Google Scholar]
- Mosienko V, Matthes S, Hirth N, Beis D, Flinders M, Bader M, Hansson AC, Alenina N (2014) Adaptive changes in serotonin metabolism preserve normal behavior in mice with reduced TPH2 activity. Neuropharmacology 85:73–80. [DOI] [PubMed] [Google Scholar]
- Mosienko V, Beis D, Pasqualetti M, Waider J, Matthes S, Qadri F, Bader M, Alenina N (2015) Life without brain serotonin: reevaluation of serotonin function with mice deficient in brain serotonin synthesis. Behavioral Brain Research 277:78–88. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Zohar J, Benkelfat C, Pato MT, Pigott TA, Insel TR (1989) Obsessive-compulsive disorder as a 5-HT subsystem-related behavioural disorder. Br J Psychiatry Suppl: 15–24. [PubMed] [Google Scholar]
- Murthy S, Gould E (2018) Early Life Stress in Rodents: Animal Models of Illness or Resilience? Front Behav Neurosci 12:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton M, Mulrooney JB, Leonard BE (2000) A review of the role of serotonin receptors in psychiatric disorders. Human Psychopharmacology 15:397–415. [DOI] [PubMed] [Google Scholar]
- Neumaier JF, Edwards E, Plotsky PM (2002) 5-HT(lB) mrna regulation in two animal models of altered stress reactivity. Biological Psychiatry 51:902–908. [DOI] [PubMed] [Google Scholar]
- Okere CO, Waterhouse BD (2006) Acute restraint increases NADPH-diaphorase staining in distinct subregions of the rat dorsal raphe nucleus: implications for raphe serotonergic and nitrergic transmission. Brain Research 1119:174–181. [DOI] [PubMed] [Google Scholar]
- Oreland S, Pickering C, Gokturk C, Oreland L, Arborelius L, Nylander I (2009) Two repeated maternal separation procedures differentially affect brain 5-hydroxytryptamine transporter and receptors in young and adult male and female rats. Brain Research 1305 Suppl:S37–S49. [DOI] [PubMed] [Google Scholar]
- Ottersen OP (1981) Afferent connections to the amygdaloid complex of the rat with some observations in the cat. III. Afferents from the lower brain stem. The Journal of Comparative Neurology 202:335–356. [DOI] [PubMed] [Google Scholar]
- Own LS, Iqbal R, Patel PD (2013) Maternal separation alters serotonergic and HPA axis gene expression independent of separation duration in mice. Brain Research 1515:29–38. [DOI] [PubMed] [Google Scholar]
- Own LS, Patel PD (2013) Maternal behavior and offspring resiliency to maternal separation in C57B1/6 mice. Hormones and Behavior 63:411–417. [DOI] [PubMed] [Google Scholar]
- Parfitt DB, Levin JK, Saltstein KP, Klayman AS, Greer LM, Helmreich DL (2004) Differential early rearing environments can accentuate or attenuate the responses to stress in male C57BL/6 mice. Brain Research 1016:111–118. [DOI] [PubMed] [Google Scholar]
- Paul ED, Lowry CA (2013) Functional topography of serotonergic systems supports the Deakin/Graeff hypothesis of anxiety and affective disorders. Journal of Psychopharmacology 27:1090–1106. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ (2001) Paxinos and Franklin’s The mouse brain in stereotaxic coordinates, 2nd ed Academic Press. [Google Scholar]
- Plotsky PM, Meaney MJ (1993) Early, postnatal experience alters hypothalamic corticotropinreleasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Molecular Brain Research 18:195–200. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ (2005) Longterm consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology 30:2192–2204. [DOI] [PubMed] [Google Scholar]
- Ren J, Friedmann D, Xiong J, Liu CD, Ferguson BR, Weerakkody T, DeLoach KE, Ran C, Pun A, Sun Y, Weissbourd B, Neve RL, Huguenard J, Horowitz MA, Luo L (2018) Anatomically Defined and Functionally Distinct Dorsal Raphe Serotonin Sub-systems. Cell 175:472–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG (2003) Anxiety and fear behaviors in adult male and female C57BL/6 mice are modulated by maternal separation. Hormones and Behavior 43:561–567. [DOI] [PubMed] [Google Scholar]
- Rozeske RR, Evans AK, Frank MG, Watkins LR, Lowry CA, Maier SF (2011) Uncontrollable, but not controllable, stress desensitizes 5-HT1A receptors in the dorsal raphe nucleus. Journal of Neuroscience 31:14107–14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignac HM, Dinan TG, Cryan JF (2011) Resistance to early-life stress in mice: effects of genetic background and stress duration. Frontiers in Behavioral Neuroscience 5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sink KS, Walker DL, Freeman SM, Flandreau El, Ressler KJ, Davis M (2013) Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Molecular Psychiatry 18(3):308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper M (2008) Gene expression: One allele or two? Nature Reviews Genetics 9:4–5. [Google Scholar]
- Slotten HA, Kalinichev M, Hagan JJ, Marsden CA, Fone KCF (2006) Long-lasting changes in behavioural and neuroendocrine indices in the rat following neonatal maternal separation: gender-dependent effects. Brain research 1097:123–132. [DOI] [PubMed] [Google Scholar]
- Spiacci A Jr, Luis Henschel Pobbe R, Matthiesen M, Zangrossi H Jr (2016) 5-HT1A receptors of the rat dorsal raphe lateral wings and dorsomedial subnuclei differentially control anxiety- and panic-related defensive responses. Neuropharmacology 107:471–479. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Liu Y, Shapira NA, Goodman WK (2001) The psychobiology of obsessive-compulsive disorder: how important is the role of disgust? Current Psychiatry Reports Rep 3:281–287. [DOI] [PubMed] [Google Scholar]
- Stezhka VV, Lovick TA (1997) Projections from dorsal raphe nucleus to the periaqueductal grey matter : studies in slices of rat midbrain maintained in vitro. Neuroscience Letters 230:57–60. [DOI] [PubMed] [Google Scholar]
- Tan S, Ho HS, Song AY, Low J, Je HS (2017) Maternal Separation Does Not Produce a Significant Behavioral Change in Mice. Experimental Neurobiology 26:390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Polcari A, McGreenery CE (2006) Sticks, stones, and hurtful words: relative effects of various forms of childhood maltreatment. The American Journal of Psychiatry 163:993–1000. [DOI] [PubMed] [Google Scholar]
- Vallée M, Mayo W, Dellu F, Le Moal M, Simon H, Maccari S (1997) Prenatal stress induces high anxiety and postnatal handling induces low anxiety in adult offspring: correlation with stress-induced corticosterone secretion. Journal of Neuroscience 17:2626–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heerden JH, Russell V, Korff A, Stein DJ, Illing N (2010) Evaluating the behavioural consequences of early maternal separation in adult C57BL/6 mice; the importance of time. Behavioral Brain Research 207:332–342. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RKW, Li H-Y, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE (2000) Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. The Journal of Comparative Neurology 428:191–212. [DOI] [PubMed] [Google Scholar]
- Van Riel E, Van Gemert NG, Meijer OC, Joels M (2004) Effect of early life stress on serotonin responses in the hippocampus of young adult rats. Synapse 53:11–19. [DOI] [PubMed] [Google Scholar]
- Varghese AK, Verdu EF, Bercik P, Khan WI, Blennerhassett PA, Szechtman H, Collins SM (2006) Antidepressants attenuate increased susceptibility to colitis in a murine model of depression. Gastroenterology 130:1743–1753. [DOI] [PubMed] [Google Scholar]
- Vetulani J (2013) Early maternal separation: a rodent model of depression and a prevailing human condition. Pharmacological Reports 65:1451–1461. [DOI] [PubMed] [Google Scholar]
- Vincent MY, Donner NC, Smith DG, Lowry CA, Jacobson L (2017) Dorsal raphe nucleus glucocorticoid receptors inhibit tph2 gene expression in male C57BL/6J mice. Neuroscience Letters 665:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waider J, Popp S, Lange MD, Kern R, Kolter JF, Kobler J, Donner NC, Lowe KR, Malzbender JH, Brazell CJ, Arnold MR, Aboagye B, Schmitt-Bohrer A, Lowry CA, Pape HC, Lesch K-P (2017) Genetically driven brain serotonin deficiency facilitates panic-like escape behavior in mice. Translational Psychiatry 7:el246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther DJ, Peter J-U, Bashammakh S, Hortnagl H, Voits M, Fink H, Bader M (2003) Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299:76–76. [DOI] [PubMed] [Google Scholar]
- Weidner MT, Lardenoije R, Eijssen L, Mogavero F, De Groodt LPMT, Popp S, Palme R, Forstner KU, Strekalova T, Steinbusch HWM, Schmitt-Bohrer AG, Glennon JC, Waider J, van den Hove DLA, Lesch K-P (2019) Identification of cholecystokinin by genome wide profiling as potential mediator of serotonin-dependent behavioral effects of maternal separation in the amygdala. Frontiers in Neuroscience 13:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigger A, Neumann ID (1999) Periodic maternal deprivation induces gender-dependent alterations in behavioral and neuroendocrine responses to emotional stress in adult rats. Physiology & Behavior 66:293–302. [DOI] [PubMed] [Google Scholar]
- Wong P, Sze Y, Gray LJ, Chang CCR, Cai S, Zhang X (2015) Early life environmental and pharmacological stressors result in persistent dysregulations of the serotonergic system. Frontiers in Behavioral Neuroscience 9:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangrossi H Jr, Graeff FG (2014) Serotonin in anxiety and panic: contributions of the elevated T-maze. Neuroscience & Biobehavioral Reviews Rev 46:397–406. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


