Abstract
Recent evidence from genetics, animal model systems and biochemical studies suggests that defects in membrane trafficking play an important part in the pathophysiology of Parkinson’s disease (PD). Mutations in leucine-rich repeat kinase 2 (LRRK2) constitute the most frequent genetic cause of both familial and sporadic PD, and LRRK2 has been suggested as a druggable target for PD. Although the precise physiological function of LRRK2 remains largely unknown, mounting evidence suggests that LRRK2 controls membrane trafficking by interacting with key regulators of the endosomal-lysosomal pathway and synaptic recycling. In this review, we discuss the genetic, biochemical and functional links between LRRK2 and membrane trafficking. Understanding the mechanism by which LRRK2 influences such processes may contribute to the development of disease-modifying therapies for PD.
Keywords: LRRK2, Parkinson’s disease, Rab, Synaptic vesicle, Vesicle trafficking
INTRODUCTION
Neurons are long-lived cells that are not replaced during the entire lifespan of the organism, and continuous exchange of materials is essential for neuronal survival and function throughout the lifespan. In addition, neurons are the most morphologically complex cells and are highly polarized. Thus, neurons can be extremely vulnerable to disruption of intracellular trafficking, and it is no coincidence that defects in vesicle or membrane trafficking have emerged as hallmarks of most, or perhaps all, neurodegenerative diseases. The link between impaired trafficking and disease progression is more evident in Parkinson’s disease (PD). A number of PD-related genes, both familial and sporadic, such as SNCA (a gene encoding α-synuclein), LRRK2, VPS35, ATP13A2, ATP6AP2, GBA, PINK1, RAB29, RAB39B, and SYNJ1 can be mapped to the membrane and/or synaptic vesicle trafficking pathway (1). The motor symptoms of PD are primarily attributed to the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNc). Anatomical analysis of individual dopaminergic neurons in SNc has revealed that a single dopaminergic neuron exhibits an extremely high level of arborization (average total length of an axon from a single neuron is ∼50 cm in rats) and forms a massive number of synapses (100,000–250,000 synapses per neuron in rats), which are orders of magnitude greater than those in other neurons (2, 3). The exceptional cellular morphology of dopaminergic neurons imposes a phenomenal burden on the molecular machinery that controls intracellular trafficking. Herein, we review recent genetic, biochemical, and functional studies that suggest a role of leucine-rich repeat kinase 2 (LRRK2) in intracellular vesicle trafficking. LRRK2 has also been implicated in extracellular or transcellular mechanisms, such as inflammation and disease propagation between cells, but here the focus will be on intracellular mechanisms.
LRRK2
Mutations in LRRK2 (also known as PARK8) gene constitute the most frequent cause of familial PD (4–6), and genome-wide association studies (GWAS) have linked common genetic variants in the LRRK2 locus to sporadic PD (7). Autosomal dominant missense mutations in LRRK2 account for 1% to 2% of all cases of PD and reach 30% to 40% in certain ethnic groups (8, 9). Familial PD cases carrying mutations in LRRK2 manifest clinical symptoms and neuropathological features that are indistinguishable from idiopathic PD cases (10, 11). Therefore, understanding the physiological and pathological roles of LRRK2 might provide critical molecular insights into the mechanism of neurodegeneration and help the development of therapeutic strategies for both inherited and sporadic PD cases.
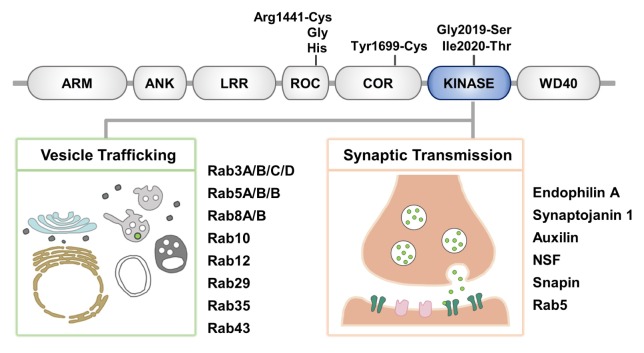
The LRRK2 gene encodes a large 280 kDa protein consisting of a serine/threonine kinase domain, Ras of complex (ROC) GTPase domain, and the C-terminal of ROC (COR) domain surrounded by multiple protein-protein interaction domains (Fig. 1). The N-terminal region contains armadillo, ankyrin, and leucine-rich repeat motifs, and the C-terminal region contains WD40 repeat domains. The presence of both the kinase and the GTPase enzymatic domains and multiple protein-protein interaction motifs suggests that LRRK2 plays diverse roles and may also act as a scaffold for the assembly of protein complexes to integrate and coordinate a wide range of cellular signaling pathways. Indeed, various studies have suggested that LRRK2 plays a role in multiple fundamental cellular processes, including mitochondrial function, autophagy, cytoskeletal remodeling, and protein synthesis (12–15).
Fig. 1.
Overview of LRRK2 and its substrates in vesicle or membrane trafficking. (Top) Schematic representation of LRRK2 domains, including armadillo repeats (ARM), ankyrin repeats (ANK), leucine-rich repeats (LRR), a ROC domain, a COR domain, a kinase domain, and a C-terminal WD40 domain. Pathogenic mutations are indicated above. (Bottom) Two categories of LRRK2 substrates discussed in this review. LRRK2 phosphorylates a subset of Rab GTPases, master regulators of vesicle trafficking (left), and key molecules of synaptic transmission (right).
The role of LRRK2 in the onset and progression of PD and the molecular mechanism that leads to neurodegeneration are not fully understood; however, PD-causing mutations cluster within the ROC GTPase, COR, and the kinase domains of LRRK2. G2019S mutation in the kinase domain is by far the most prevalent, known genetic cause of neurodegeneration (16). The G2019S mutation is located in the kinase activation loop and augments the kinase activity towards generic kinase substrates (17). Importantly, neurotoxicity induced by LRRK2-G2019S in culture and in vivo critically depends on the kinase activity (18–21). These lines of evidence suggest that the kinase activity of LRRK2 might be central to PD pathogenesis and the progression of neurodegeneration (22).
LOCATION OF LRRK2
Endogenous LRRK2 is largely a cytosolic protein associated with membranous structures but is generally excluded from the nucleus. Notably, LRRK2 was particularly enriched in membrane-associated fractions (23–25). At the ultrastructural level, LRRK2-positive immunolabeling was localized to Golgi apparatus, endoplasmic reticulum, mitochondria, multivesicular bodies (MVBs), and lysosomal and endosomal vesicles (23). Expression of a GFP-reporter construct in cells also located LRRK2 to a variety of different membranous compartments, including the neck of caveolae, microvilli, MVBs and autophagic vacuoles, suggesting a possible role in endolysosomal-autophagic pathway (26). A bioinformatic approach using manually-curated protein-protein interaction datasets reiterated the role of LRRK2 and LRRK2 interactome in intracellular transport and vesicular trafficking (27).
It is not clear how LRRK2 manages to associate with many different types of membranous structures. The possibility that LRRK2 binds directly to each of the membranous structures through an intrinsic affinity for lipids has not been formally excluded. However, the lack of a known membrane-spanning or membrane-targeting signal within LRRK2 together with the localization of LRRK2 in diverse vesicular or membranous structures with different lipid compositions suggest that LRRK2 might be recruited to each target membrane by interacting with specific membrane-bound proteins. This possibility is supported by the presence of several protein-protein interaction motifs in LRRK2 and is further strengthened by the identification of a growing list of membrane-associated, LRRK2-interacting proteins.
THE ROLE OF LRRK2 IN VESICLE TRAFFICKING
In addition to the localization of LRRK2 to multiple membranous compartments (23), mounting evidence suggests that regulation of vesicular transport is a proximal event in LRRK2 signaling. LRRK2 has been implicated in the regulation of autophagy, lysosomal maturation and trafficking, and the endosomal pathway (28, 29).
Rab GTPases are the largest subgroup of the Ras-like GTPase superfamily and comprise more than 60 members in the human genome. Rab proteins reside in specific membranous compartments and are master regulators of vesicle trafficking, controlling virtually all aspects of vesicle or membrane traffic (30–32). By cycling between GTP-bound and GDP-bound states, Rab proteins function as molecular switches and orchestrate the formation, maturation, transport, tethering, and fusion of vesicles. Once the Rab protein is inserted into its respective target membrane, a guanine nucleotide exchange factor (GEF) mediates the conversion from the GDP- to the GTP-bound form. The active, GTP-bound Rab recruits effector proteins that specifically control certain aspects of trafficking and is hydrolyzed back to its inactive state, a process which is facilitated by a GTPase-activating protein (GAP). GDP-bound Rab becomes a substrate of GDP dissociation inhibitor (GDI), which extracts Rab in its GDP-bound state from the membrane. Rab bound to GDI can then be reinserted into a membrane to start the cycle again.
Many lines of evidence have suggested physical, genetic and functional interactions between LRRK2 and several members of the Rab GTPases (29, 33–36). In yeast-two-hybrid screening, LRRK2 has been shown to interact with Rab5, Rab32 and Rab38 (34, 37). The Drosophila homolog of LRRK1 and LRRK2, dLRRK interacts with Rab7 and a mutation in dLrrk corresponding to the G2019S mutation disturbs Rab7-dependent perinuclear localization of the lysosome (29).
Recently, we and others have identified a subset of Rab GTPases as authentic substrates of LRRK2 (38–40). Steger et al. performed large-scale phosphoproteomic screens using fibroblasts from knock-in mice carrying either the LRRK2-G2019S mutation or the kinase inhibitor-resistant LRRK2-A2016T mutation, which led to the identification of a single substrate of LRRK2, Rab10. Further analysis in HEK293FT cells with group I Rabs showed that LRRK2 also phosphorylated Rab1b and Rab8a (39). In a following study, Rab3a/b/c/d, Rab5a/b/c, Rab8a/b, Rab10, Rab12, Rab29, Rab35 and Rab43 were phosphorylated by LRRK2 (41). By performing in vitro LRRK2 kinase assays with forty-five human Rab GTPases, we also found that Rab1a, Rab3c, Rab8a, and Rab35 were phosphorylated by LRRK2 at a conserved Thr residue in the switch II motif, which is known to mediate the binding with interactors responsible for vesicle transport (38). Moreover, we showed that the phos-phorylation of Rab1a, Rab3c, Rab8a, and Rab35 was enhanced by LRRK2-G2019S compared with wild-type LRRK2, while it was decreased in Lrrk2 knockout mice and in cells treated with LRRK2 kinase inhibitors (38). Importantly, dysregulation of Rab phosphorylation in the LRRK2 site induced neurotoxicity in primary neuronal cultures. Furthermore, intracranial injection of the adeno-associated virus (AAV) expressing either nonphosphorylatable (AAV-Rab35-T72A) or phosphomimetic (AAV-Rab35-T72D) mutant of Rab35 into the SN induced substantial degeneration of dopaminergic neurons in vivo (38).
Among LRRK2 substrates, Rab29 (also known as Rab7L1) is one of the five genes located in the PARK16 locus linked to PD (42). Previously, an unbiased search for interactors of LRRK2 and a brain transcriptomics approach found that LRRK2 interacted with Rab29, and dysfunction of LRRK2-Rab29 signaling caused dysregulation of the autophagy-lysosome system (26, 33, 35, 43). More recent studies have suggested that Rab29 recruits LRRK2 to the trans-Golgi network (TGN) and enhances its kinase activity, leading to the phosphorylation of other LRRK2 substrates, Rab8 and Rab10 (40, 44). Rab29 has also been shown to recruit LRRK2 to stressed lysosomes, which, in turn, induced lysosomal accumulation of other LRRK2 substrate Rabs, such as Rab3, Rab8, Rab10, and Rab35 (45). It will be interesting to investigate if recruitment of LRRK2 to discrete membranous compartments by specific Rab proteins applies more generally to tether and activate LRRK2 at distinct subcellular locations and enable phosphorylation of a specific subset of substrates.
The first gene identified in familial PD was SNCA, which encodes a synaptic vesicle-associated protein, α-synuclein. Aggregates of α-synuclein are major constituents of Lewy bodies and Lewy neurites, which are cardinal pathological features of PD. Several Rab GTPases have been identified as modulators of α-synuclein toxicity, and notably, the same Rab proteins are also implicated in LRRK2 signaling. Aggregation of α-synuclein in long-term cultures of human induced pluripotent stem cell (iPSC)-derived dopamine neurons resulted in lysosomal dysfunction, and upregulation of Rab1a, a substrate of LRRK2, could restore protein trafficking and lysosomal function (46). These results are reminiscent of the ability of Ypt1 (the yeast homolog of Rab1) to alleviate impaired ER-to-Golgi transport caused by overexpression of α-synuclein in yeast (47). Moreover, Rab1, Rab3a and Rab8a, all of which are LRRK2 substrates, prevented α-synuclein-induced degeneration of dopaminergic neurons in a nematode model of PD and in rat primary midbrain cultures (48). Furthermore, a previous study showed that α-synuclein from A30P α-synuclein transgenic mice (Tg5093) interacted with Rab3a, Rab5, and Rab8 (49). Vesicular compartments and trafficking events regulated by these Rab proteins may represent intracellular locations or pathways where the interplay between α-synuclein and LRRK2 take place.
THE ROLE OF LRRK2 IN SYNAPTIC VESICLE RECYCLING
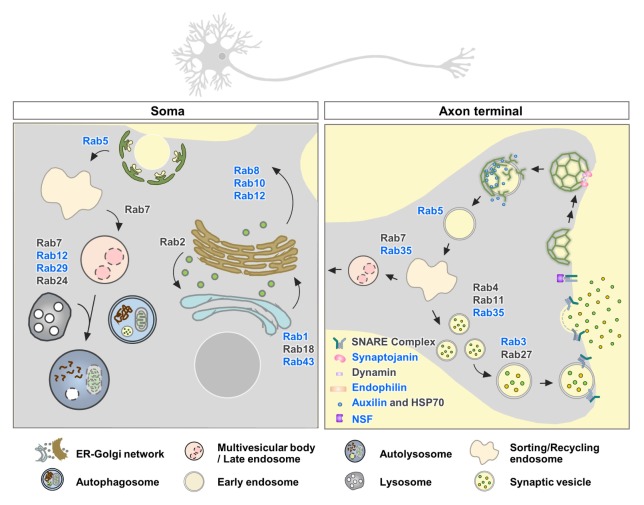
Neurons undergo high rates of synaptic transmission, which requires efficient membrane trafficking at the presynaptic axon terminal to recover and recycle membranes that have fused with the plasma membrane during neurotransmitter release. Postmortem studies of PD brains support the notion that synaptic dysfunction is an early event in PD (1), and increasing lines of evidence suggest that LRRK2 plays a role in clathrin-dependent endocytosis and recycling of synaptic vesicles (Fig. 2). Clathrin-dependent synaptic vesicle endocytosis (SVE) is initiated by recruiting clathrin at the plasma membrane via adaptor protein 2 (AP-2). Clathrin-coated vesicle (CCV) then invaginates from the plasma membrane by the actions of proteins that sense and induce membrane curvature, such as endophilin. CCV then physically separates from the plasma membrane by the action of the GTPase dynamin. Shedding of the adaptors depends on phosphatidyl inositol hydrolysis by synaptojanin, and the ATPase Hsp70 and its cofactor auxilin remove the clathrin lattice after fission, and thereby, produce synaptic vesicles that can be reused (50). LRRK2 has been suggested to play a role in synaptic vesicle recycling via phosphorylation of endophilin A1, an essential molecule involved in clathrin-mediated SVE (51). Endophilin contains an N-terminal amphipathic α-helices followed by the Bin/amphiphysin/Rvs (BAR) domain and a C-terminal SH3 domain. Endophilin promotes early steps of SVE by generating membrane curvature at the plasma membrane and facilitates later steps via clathrin-uncoating (52–55). Membrane curvature can be generated by (i) the BAR domain, which forms a crescent-shaped dimer that interacts with and forces membranes to conform to their own intrinsic protein shape (scaffolding mechanism) and (ii) the amphipathic α-helices, which embed in the lipid bilayer and thereby generate a wedging force (wedging mechanism) (56). It has been suggested that the generation of vesicles and tubes requires different structures and mechanisms of endophilin A1 and the transition between the two membranous structures can be regulated via LRRK2-mediated phosphorylation (56). The LRRK2 phosphorylation site S75 is shallowly inserted on vesicles but is deeply embedded upon tubulation. In contrast to wild-type and the nonphosphorylatable mutant (S75A) of endophilin A1 that increased tubulation, the phosphomimetic mutant (S75D) destabilized tubulation and favored vesiculation by preventing deep insertion of the helical wedges (56). Therefore, it is possible that LRRK2-mediated phosphorylation of endophilin A1 regulates how endophilin structurally interacts with and curves the membrane. This function might play a role at the endocytic neck, a structure which is transiently generated and rapidly destabilized. It remains to be determined whether pathogenic LRRK2 mutants via its aberrant kinase activity disrupt the balance of different types of membrane curvature.
Fig. 2.
LRRK2 substrates involved in vesicle trafficking and synaptic transmission. LRRK2 phosphorylates a subset of Rab GTPases that control the endolysosomal-autophagy pathway and key molecules involved in synaptic vesicle endocytosis and recycling. Proteins that are suggested as direct substrates of LRRK2 are depicted in blue.
In addition to generating membrane curvature at early stages of SVE, endophilin recruits dynamin and synaptojanin (57, 58). Synaptojanin-1 has been identified as a substrate of LRRK2 via phosphoproteomic analysis of Drosophila brains over-expressing human LRRK2-R1441C (59). Synaptojanin-1 is a lipid phosphatase, dephosphorylating phosphatidylinositol 4,5-bisphosphate (PIP2) to phosphatidylinositol 4-phosphate and to phosphatidylinositol via its dual phosphatase activity. Activation of synaptojanin-1 releases AP-2 from CCVs and promotes synaptic vesicle uncoating during endocytosis. The LRRK2 phosphorylation sites, T1131 and S1142 in synaptojanin are located within the C-terminal proline-rich domain, which is known to mediate the binding with the SH3 domain of endophilin A. Therefore, LRRK2-dependent phosphorylation of synaptojanin-1 may modulate the interaction with endophilin, and accordingly endophilin-dependent SVE. It is also possible that the enzymatic activity of synaptojanin-1 is regulated by phosphorylation. A functional consequence of synaptojanin-1 phosphorylation by LRRK2 has yet to be determined.
After fission, auxilin induces clathrin uncoating of the CCVs by recruiting the ATPase Hsc70. The pathological linkage between DNAJC6 (auxilin) and PD has been suggested through GWAS studies in patients with juvenile and early-onset atypical parkinsonism (60, 61). In iPSC-derived dopaminergic neurons obtained from reprogrammed PD patient fibroblasts harboring the LRRK2-R1441C mutation, the levels of several SVE proteins, such as auxilin, AP-2, endophilin A1, dynamin 1, and synaptojanin were reduced, and activity-dependent endocytic capacity was also decreased when compared with those derived from healthy controls (62). In addition, LRRK2 physically interacted with auxilin and induced the phosphorylation of auxilin at S627 residue located in the clathrin-binding domain. LRRK2-mediated phosphorylation of auxilin has thus been suggested to control auxilin and clathrin binding and the CCV uncoating process during SVE. In the LRRK2-R1441G patient-derived dopaminergic neurons, synaptic vesicle density was reduced and cytosolic dopamine levels were elevated (62). In the synaptic terminal, dopamine should be rapidly packaged into synaptic vesicles. Accumulation of cytosolic dopamine can be oxidized and reach toxic levels. The accumulation of oxidized dopamine, increased α-synuclein levels, and decreased lysosomal glucocerebrosidase activity in the LRRK2-R1441G patient-derived dopaminergic neurons could all be suppressed by overexpressing wild-type auxilin, suggesting that LRRK2-mediated auxilin dysfunction disrupts SVE and generates toxic, oxidized dopamine, which might contribute to dopaminergic neurodegeneration in LRRK2-linked PD (62).
In addition to SVE, LRRK2 is involved in synaptic vesicle fusion by regulating the SNARE complex dissociation via phosphorylation of NSF (63). Vesicular SNARE (v-SNARE) located on the synaptic vesicle membrane and the target SNARE (t-SNARE) present on the plasma membrane assemble into a highly stable alpha-helical SNARE complex that juxtaposes the two membranes together to catalyze membrane fusion. After synaptic vesicle fusion, the SNARE complex is dissociated for reuse. At the transition period of exocytosis and endocytosis, the disassembly of SNARE complex is initiated by the formation of SNARE-SNAP-NSF complex, followed by NSF-catalyzed ATP hydrolysis and release of individual SNAREs (64). LRRK2 phosphorylates NSF at T645 located within the D2 domain of NSF. T645 is predicted to be a key residue for protein oligomerization, which regulates the ability of the D1 domain to hydrolyze ATP (65). LRRK2 phosphorylation of NSF enhanced the ATPase activity of NSF and increased the rate of SNARE complex disassembly in vitro (63).
Snapin, known as SNAP-25 (synaptosomal-associated protein 25) binding protein, is another LRRK2 kinase substrate (66). SNAP-25 is a core component of the SNARE complex. Inhibition of the interaction between snapin and SNAP-25 blocks the association of SNARE complex with synaptotagmin. Snapin interacts with multiple vesicle trafficking-related proteins, and calcium-dependent exocytosis is impaired in snapin null mice (67). It has been shown that LRRK2 phosphorylates snapin at T117. The phosphomimetic snapin-T117D decreases its interaction with SNAP-25 and prevents the association of synaptotagmin with SNARE complex and reduces the number of readily releasable pool of synaptic vesicles. These results suggest that LRRK2 may be involved in neurotransmitter release.
CONCLUSION
Analysis of postmortem human brain tissue from PD brain donors using correlative light and electron microscopy (CLEM), serial block-free scanning electron microscopy (SEFSEM), and stimulated emission depletion (STED)-based super resolution microscopy showed that α-synuclein immunopositive inclusions were crowded with fragmented membranes, organelles and vesicular structures (68), supporting the notion that defects in membrane trafficking are a potential driver of pathogenesis of PD. Here we have discussed that intracellular localization of LRRK2, the effects of LRRK2 mutations, and the newly identified substrates of LRRK2 together strengthen the possibility that vesicular trafficking is a proximal event of LRRK2 signaling. Although our understanding of LRRK2 function in membrane trafficking has greatly advanced in recent years, much remains to be learned. Understanding of how LRRK2 and PD-linked gene mutations affect membrane trafficking and how defects in vesicular or synaptic trafficking cause dopaminergic dysfunction will not only advance the field of membrane trafficking but also may have strong implications for developing disease-modifying treatments for PD.
ACKNOWLEDGEMENTS
This work was supported by the Research Resettlement Fund for the new faculty of Seoul National University (550-20180037 to EMH), the Research Institute for Veterinary Science, Seoul National University and the Brain Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2016M3C7A1905386, 2017M3C7A1043848).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–216. doi: 10.1038/nature20414. [DOI] [PubMed] [Google Scholar]
- 2.Moss J, Bolam JP. A dopaminergic axon lattice in the striatum and its relationship with cortical and thalamic terminals. J Neurosci. 2008;28:11221–11230. doi: 10.1523/JNEUROSCI.2780-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuda W, Furuta T, Nakamura KC, et al. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J Neurosci. 2009;29:444–453. doi: 10.1523/JNEUROSCI.4029-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paisan-Ruiz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Funayama M, Hasegawa K, Ohta E, et al. An LRRK2 mutation as a cause for the parkinsonism in the original PARK8 family. Ann Neurol. 2005;57:918–921. doi: 10.1002/ana.20484. [DOI] [PubMed] [Google Scholar]
- 7.Nalls MA, Pankratz N, Lill CM, et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesage S, Ibanez P, Lohmann E, et al. G2019S LRRK2 mutation in french and north african families with Parkinson’s disease. Ann Neurol. 2005;58:784–787. doi: 10.1002/ana.20636. [DOI] [PubMed] [Google Scholar]
- 9.Benamer HT, de Silva R. LRRK2 G2019S in the North African population: a review. Eur Neurol. 2010;63:321–325. doi: 10.1159/000279653. [DOI] [PubMed] [Google Scholar]
- 10.Cookson MR. The role of leucine-rich repeat kinase 2 (LRRK2) in Parkinson’s disease. Nat Rev Neurosci. 2010;11:791–797. doi: 10.1038/nrn2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paisan-Ruiz C, Lewis PA, Singleton AB. LRRK2: cause, risk, and mechanism. J Parkinsons Dis. 2013;3:85–103. doi: 10.3233/JPD-130192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Yan MH, Fujioka H, et al. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet. 2012;21:1931–1944. doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orenstein SJ, Kuo SH, Tasset I, et al. Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci. 2013;16:394–406. doi: 10.1038/nn.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parisiadou L, Xie C, Cho HJ, et al. Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. J Neurosci. 2009;29:13971–13980. doi: 10.1523/JNEUROSCI.3799-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin I, Kim JW, Lee BD, et al. Ribosomal protein s15 phosphorylation mediates LRRK2 neurodegeneration in Parkinson’s disease. Cell. 2014;157:472–485. doi: 10.1016/j.cell.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trinh J, Guella I, Farrer MJ. Disease penetrance of late-onset parkinsonism: a meta-analysis. JAMA Neurol. 2014;71:1535–1539. doi: 10.1001/jamaneurol.2014.1909. [DOI] [PubMed] [Google Scholar]
- 17.West AB, Moore DJ, Biskup S, et al. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greggio E, Jain S, Kingsbury A, et al. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis. 2006;23:329–341. doi: 10.1016/j.nbd.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Lee BD, Shin JH, VanKampen J, et al. Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson’s disease. Nat Med. 2010;16:998–1000. doi: 10.1038/nm.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith WW, Pei Z, Jiang H, Dawson VL, Dawson TM, Ross CA. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci. 2006;9:1231–1233. doi: 10.1038/nn1776. [DOI] [PubMed] [Google Scholar]
- 21.West AB, Moore DJ, Choi C, et al. Parkinson’s disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet. 2007;16:223–232. doi: 10.1093/hmg/ddl471. [DOI] [PubMed] [Google Scholar]
- 22.Lee BD, Dawson VL, Dawson TM. Leucine-rich repeat kinase 2 (LRRK2) as a potential therapeutic target in Parkinson’s disease. Trends Pharmacol Sci. 2012;33:365–373. doi: 10.1016/j.tips.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biskup S, Moore DJ, Celsi F, et al. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol. 2006;60:557–569. doi: 10.1002/ana.21019. [DOI] [PubMed] [Google Scholar]
- 24.Shin N, Jeong H, Kwon J, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res. 2008;314:2055–2065. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Piccoli G, Condliffe SB, Bauer M, et al. LRRK2 controls synaptic vesicle storage and mobilization within the recycling pool. J Neurosci. 2011;31:2225–2237. doi: 10.1523/JNEUROSCI.3730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alegre-Abarrategui J, Christian H, Lufino MM, et al. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18:4022–4034. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manzoni C, Denny P, Lovering RC, Lewis PA. Computational analysis of the LRRK2 interactome. PeerJ. 2015;3:e778. doi: 10.7717/peerj.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong Y, Yamaguchi H, Giaime E, et al. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A. 2010;107:9879–9884. doi: 10.1073/pnas.1004676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dodson MW, Zhang T, Jiang C, Chen S, Guo M. Roles of the Drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Hum Mol Genet. 2012;21:1350–1363. doi: 10.1093/hmg/ddr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 31.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivero-Rios P, Gomez-Suaga P, Fernandez B, et al. Alterations in late endocytic trafficking related to the pathobiology of LRRK2-linked Parkinson’s disease. Biochem Soc Trans. 2015;43:390–395. doi: 10.1042/BST20140301. [DOI] [PubMed] [Google Scholar]
- 33.MacLeod DA, Rhinn H, Kuwahara T, et al. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron. 2013;77:425–439. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waschbusch D, Michels H, Strassheim S, et al. LRRK2 transport is regulated by its novel interacting partner Rab32. PLoS One. 2014;9:e111632. doi: 10.1371/journal.pone.0111632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beilina A, Rudenko IN, Kaganovich A, et al. Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc Natl Acad Sci U S A. 2014;111:2626–2631. doi: 10.1073/pnas.1318306111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez-Suaga P, Rivero-Rios P, Fdez E, et al. LRRK2 delays degradative receptor trafficking by impeding late endosomal budding through decreasing Rab7 activity. Hum Mol Genet. 2014;23:6779–6796. doi: 10.1093/hmg/ddu395. [DOI] [PubMed] [Google Scholar]
- 37.Heo HY, Kim KS, Seol W. Coordinate regulation of neurite outgrowth by LRRK2 and Its interactor, Rab5. Exp Neurobiol. 2010;19:97–105. doi: 10.5607/en.2010.19.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong GR, Jang EH, Bae JR, et al. Dysregulated phosphorylation of Rab GTPases by LRRK2 induces neurodegeneration. Mol Neurodegener. 2018;13:8. doi: 10.1186/s13024-018-0240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steger M, Tonelli F, Ito G, et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. Elife. 2016;5:12813. doi: 10.7554/eLife.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z, Bryant N, Kumaran R, et al. LRRK2 phosphorylates membrane-bound Rabs and is activated by GTP-bound Rab7L1 to promote recruitment to the trans-Golgi network. Hum Mol Genet. 2018;27:385–395. doi: 10.1093/hmg/ddx410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steger M, Diez F, Dhekne HS, et al. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife. 2017;6:31012. doi: 10.7554/eLife.31012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simon-Sanchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41:1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pihlstrom L, Rengmark A, Bjornara KA, et al. Fine mapping and resequencing of the PARK16 locus in Parkinson’s disease. J Hum Genet. 2015;60:357–362. doi: 10.1038/jhg.2015.34. [DOI] [PubMed] [Google Scholar]
- 44.Purlyte E, Dhekne HS, Sarhan AR, et al. Rab29 activation of the Parkinson’s disease-associated LRRK2 kinase. EMBO J. 2018;37:1–18. doi: 10.15252/embj.201798099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eguchi T, Kuwahara T, Sakurai M, et al. LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc Natl Acad Sci U S A. 2018;115:E9115–E9124. doi: 10.1073/pnas.1812196115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D. alpha-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci U S A. 2016;113:1931–1936. doi: 10.1073/pnas.1520335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooper AA, Gitler AD, Cashikar A, et al. Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gitler AD, Bevis BJ, Shorter J, et al. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci U S A. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalfo E, Gomez-Isla T, Rosa JL, et al. Abnormal alpha-synuclein interactions with Rab proteins in alpha-synuclein A30P transgenic mice. J Neuropathol Exp Neurol. 2004;63:302–313. doi: 10.1093/jnen/63.4.302. [DOI] [PubMed] [Google Scholar]
- 50.Saheki Y, De Camilli P. Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol. 2012;4:a005645. doi: 10.1101/cshperspect.a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matta S, Van Kolen K, da Cunha R, et al. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron. 2012;75:1008–1021. doi: 10.1016/j.neuron.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 52.Gallop JL, Jao CC, Kent HM, et al. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J. 2006;25:2898–2910. doi: 10.1038/sj.emboj.7601174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masuda M, Takeda S, Sone M, et al. Endophilin BAR domain drives membrane curvature by two newly identified structure-based mechanisms. EMBO J. 2006;25:2889–2897. doi: 10.1038/sj.emboj.7601176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai J, Hu Z, Dittman JS, Pym EC, Kaplan JM. Endophilin functions as a membrane-bending molecule and is delivered to endocytic zones by exocytosis. Cell. 2010;143:430–441. doi: 10.1016/j.cell.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milosevic I, Giovedi S, Lou X, et al. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron. 2011;72:587–601. doi: 10.1016/j.neuron.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ambroso MR, Hegde BG, Langen R. Endophilin A1 induces different membrane shapes using a conformational switch that is regulated by phosphorylation. Proc Natl Acad Sci U S A. 2014;111:6982–6987. doi: 10.1073/pnas.1402233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hill E, van Der Kaay J, Downes CP, Smythe E. The role of dynamin and its binding partners in coated pit invagination and scission. J Cell Biol. 2001;152:309–323. doi: 10.1083/jcb.152.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verstreken P, Koh TW, Schulze KL, et al. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/S0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- 59.Islam MS, Nolte H, Jacob W, et al. Human R1441C LRRK2 regulates the synaptic vesicle proteome and phosphoproteome in a Drosophila model of Parkinson’s disease. Hum Mol Genet. 2016;25:5365–5382. doi: 10.1093/hmg/ddw352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Edvardson S, Cinnamon Y, Ta-Shma A, et al. A deleterious mutation in DNAJC6 encoding the neuronalspecific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS One. 2012;7:e36458. doi: 10.1371/journal.pone.0036458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koroglu C, Baysal L, Cetinkaya M, Karasoy H, Tolun A. DNAJC6 is responsible for juvenile parkinsonism with phenotypic variability. Parkinsonism Relat Disord. 2013;19:320–324. doi: 10.1016/j.parkreldis.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Nguyen M, Krainc D. LRRK2 phosphorylation of auxilin mediates synaptic defects in dopaminergic neurons from patients with Parkinson’s disease. Proc Natl Acad Sci U S A. 2018;115:5576–5581. doi: 10.1073/pnas.1717590115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belluzzi E, Gonnelli A, Cirnaru MD, et al. LRRK2 phosphorylates pre-synaptic N-ethylmaleimide sensitive fusion (NSF) protein enhancing its ATPase activity and SNARE complex disassembling rate. Mol Neurodegener. 2016;11:1. doi: 10.1186/s13024-015-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ryu JK, Jahn R, Yoon TY. Review: Progresses in understanding N-ethylmaleimide sensitive factor (NSF) mediated disassembly of SNARE complexes. Biopolymers. 2016;105:518–531. doi: 10.1002/bip.22854. [DOI] [PubMed] [Google Scholar]
- 65.Yu RC, Hanson PI, Jahn R, Brunger AT. Structure of the ATP-dependent oligomerization domain of N-ethylmaleimide sensitive factor complexed with ATP. Nat Struct Biol. 1998;5:803–811. doi: 10.1038/1843. [DOI] [PubMed] [Google Scholar]
- 66.Yun HJ, Park J, Ho DH, et al. LRRK2 phosphorylates Snapin and inhibits interaction of Snapin with SNAP-25. Exp Mol Med. 2013;45:e36. doi: 10.1038/emm.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian JH, Wu ZX, Unzicker M, et al. The role of Snapin in neurosecretion: snapin knock-out mice exhibit impaired calcium-dependent exocytosis of large dense-core vesicles in chromaffin cells. J Neurosci. 2005;25:10546–10555. doi: 10.1523/JNEUROSCI.3275-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shahmoradian SH, Lewis AJ, Genoud C, et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat Neurosci. 2019;22:1099–1109. doi: 10.1038/s41593-019-0423-2. [DOI] [PubMed] [Google Scholar]