Abstract
Bisphosphonates are the mainstay of therapy worldwide for osteoporosis. However, bisphosphonates also have limitations. The objective of this study was to determine the role of miR-101-3p/Rap1b signal pathway in osteoclast differentiation after treatment with bisphosphonates. Our results revealed that miR-101-3p was an important regulator in bisphosphonates treated-osteoclasts. When miR-101-3p was down-regulated in bone marrow-derived macrophage-like cells (BMMs), the development of mature osteoclasts was promoted, and vice versa. However, alendronate decreased multinucleated cell number regardless of whether miR-101-3p was knocked down or over-expressed. TRAP activity assay confirmed the above results. Luciferase assay indicated that miR-101-3p was a negative regulator of Rap1b. Western blot analysis revealed that protein expression level of Rap1b in BMMs transfected with OV-miR-101-3p was lower than that in BMMs transfected with an empty vector. Rap1b overexpression increased TRAP-positive multinucleated cells, while Rap1b inhibition decreased the cell numbers. In vivo data showed that miR-101-3p inhibited osteoclast differentiation in ovariectomized mice while overexpressed of Rap1b blocked the differentiation. Taken together, our data demonstrate that miR-101-3p/Rap1b signal pathway plays a key role in osteoclast differentiation after treatment with bisphosphonates.
Keywords: Bisphosphonates, miR-101-3p, Osteoclast, Osteoporosis, Rap1b
INTRODUCTION
Osteoporosis is a complex bone disease that causes loss of bone density associated with mineral composition and bone strength. This disease affects millions of individuals worldwide and increases the risk of fractures. It is especially prevalent in menopausal women (1). Bone resorption and bone formation are well-coupled and processesed. Inhibition of bone resorption affects bone formation and the efficacy of anti-bone-resorption drugs. Previous studies have indicated that the key factor of keeping normal function of osteoblast is osteoclast number while abnormal formation and resorption of osteoclast play a significant role in osteoporosis pathogenesis (2).
Bisphosphonates are the mainstay of therapy worldwide for osteoporosis. They inhibit bone resorption by acting on osteoclasts to reduce their activity or increase the rate of apoptosis. Although bisphosphonates can markedly ameliorate osteoporosis, their side-effects such as osteonecrosis of the jaw, gastrointestinal side-effects, and atypical subtrochanteric fracture largely limit their clinical application for osteoporosis treatment. Bisphosphonates are known to exert anti-resorptive action by blocking farnesyl pyrophsophate synthase (3, 4). However, the detailed mechanism remains unclear. Based on the current situation, examining the change in expression profile before and after bisphosphonate treatment in osteoclasts might shed some light on biological pathways that are perturbed by biphosphonate.
MicroRNAs (miRNAs) are double-stranded non-coding RNA of ∼22-nucleotide (nt) in length. Non-coding RNA molecules play crucial roles as regulators of gene expression in different organisms. miRNAs have been identified in the variety of pathological and biological processes, including proliferation, apoptosis, and differentiation of osteoclast, bone resorption, and bone remodeling (5–9). However, the relationship between miRNA and effect of bisphosphonates treatment on osteoclastogenesis remains unclear. Among various bisphosphonates, 4-amino-lhydroxybutylidene-l,l-bisphosphonate (alendronate, Alen) has been widely used (10).
In this study, we chose alendronate as the representative bisphosphonate to determine differential expression of miRNAs and potential target genes related to bioactive effects of bisphosphonates on osteoclastogenesis. We also investigated potential drug targets for osteoporosis based on bisphosphonates treatment.
RESULTS
miR-101-3p is a key mediator in biological pathways after bisphosphonates treatment
We located and manually curated one gene expression data set GSE63009 related to expression profile of osteoclasts treated with bisphosphonates. Gene set enrichment analysis (GSEA) was performed for all expression profiles of microRNA targets motif gene sets(MIR) from its molecular signatures database (11). We focused on up-regulated miRNA target genes of osteoclasts treated with bisphosphonates. GSEA enrichment plots and Venn diagram results demonstrated that target genes of miR-504, miR-101, miR-142, miR-431, and miR-202 were enriched in alendronate or risedronate treated cells (Fig. 1A–1C). To confirmed hub genes of these five microRNAs, ClueGO and CluePedia tool kits were used to determine which biological functions were statistically over-represented in the lists. Cytoscape software was used to construct miRNA-mRNA network (12–14). The functional network implied that miR-101-3p could be the hub gene of these five microRNAs. In addition, miR-101-3p was up-regulated in bisphosphonates treated osteoclasts (Fig. 1D and 2A), suggesting that miR-101-3p might be a key mediator in bisphosphonates treated-osteoclasts. Thus, it was selected for further study.
Fig. 1.
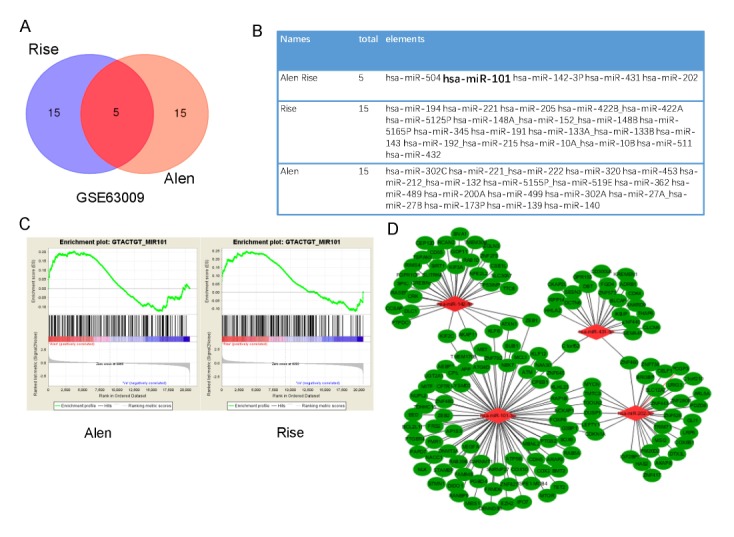
miR-101-3p is a key mediator of biological pathways induced by bisphosphonates treatment. GSEA results displaying clustering of transcriptions within aimed list of miRNAs (miR-504, miR-101, miR-142, miR-431, and miR-202) were both enriched in alendronate-treated cells and risedronate-treated cells. (A) Venn diagrams, (B) Candidate miRNAs lists of Risedronate (Rise)-treated, (C) Alendronate (Alen) treated, (D) GSEA enrichment plots. The node that has the most links (miR-101-3p) is defined as the hub gene of the module.
Fig. 2.
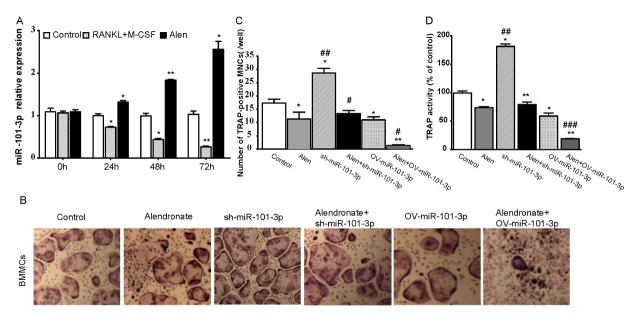
miR-101-3p is essential in bisphosphonates regulated osteoclast differentiation. (A) BMMs were cultured with RANKL (50 ng/ml) and M-CSF(30 ng/ml) for 0, 24, 48, or 72 h. Cells were also treated with Alen. qRT-PCR was used to detect the relevant expression of miR-101-3p. (B) BMMS isolated from mice were cultured with RANKL and M-CSF for 72 h and transfected with sh-miR-101-3p or OV-miR-101-3p plasmid with or without Alen treatment. TRAP-positive MNCs with more than three nuclei were counted as mature osteoclasts (400X). (C) Number of TRAP-positive MNCs (/well). (D) TRAP activity. *P < 0.05 and **P < 0.01 versus the control group, #P < 0.05, ##P < 0.01 and ###P < 0.001 versus Alen treated group.
miR-101-3p is essential in bisphosphonate regulated osteoclast differentiation
First, qRT-PCR was used to detect miR-101-3p expression in osteoclast differentiation after bisphosphonate treatment. Results showed that miR-101-3p expression was decreased during osteoclast differentiation in BMMs induced by RANKL and M-CSF cytokines as compared with controls, while it was increased in cells treated with alendronate (Fig. 2A). However, no obvious alteration in miR-101-3p expression was identified in BMMs without treatment with M-CSF or RANKL. miR-101-3p was one of the most strongly down-regulated miRNAs in osteoclastogenesis among the five miRNAs (data not shown), consistent with the previous GSEA results.
BMMs cells were then cultured with RANKL and M-CSF and transfected with sh-miR-101-3p, OV-miR-101-3p plasmid, or NC. When cells were transfected with sh-miR-101-3p plasmid, the development of mature osteoclasts characterized as TRAP-positive multinucleated cells was promoted, while it was inhibited by miR-101-3p overexpression and alendronate (Fig. 2B, 2C). Simultaneously, alendronate decreased TRAP-positive cells regardless whether miR-101-3p was down- or up-regulated, although TRAP-positive multinucleated cells from sh-miR-101-3p transfected cells were more than OV-miR-101-3p transfected cells. Moreover, TRAP activity results (Fig. 2D) induced by both stimulators were consistent with previous studies. These data demonstrate that miR-101-3p is an important mediator during inhibition of osteoclast differentiation by bisphosphonate.
miR-101-3p inhibits osteoclast differentiation by directly targeting Rap1b
Next, we attempted to discover how miR-101-3p regulated osteoclast activity and bone resorption during bisphosphonate treatment. miR-101-3p target genes were searched using TargetScan7.2 (http://www.targetscan.org/), microRNA.org (http://www.microRNA.org/), and miRwalk2 (http://zmf.umm.uniheidelberg.de/apps/zmf/mirwalk2/) micoRNA databases. Candidates were analyzed together with GSEA miRNA target genes acquired from data set GSE63009. We identified Rap1b as a candidate target for controlling miR-101-3p expression (Fig. 3A, 3B). Results showed that miR-101-3p targeted at Rap1b gene at positions 309–316 and 965–971 (Fig. 3C). To analyze the relationship between miR-101-3p and Rap1b, a luciferase reporter assay containing wild-type (wt) or mutant (mut) miR-101-3p target sites in the Rap1b 3′-UTR was performed. Results indicated that overexpression of miR-101-3p decreased the activity of Rap1b wt-3′-UTRs (965–971) luciferase compared to control transfected cells (Fig. 3D), whereas it had no apparently effect on Rap1b wt-3′-UTRs (309–316) (Not presented), indicating that miR-101-3p suppressed the expression of Rap1b by binding to target sites in their 3′-UTRs (965–971). These results suggest that miR-101-3p can negatively regulate protein level of Rap1b.
Fig. 3.
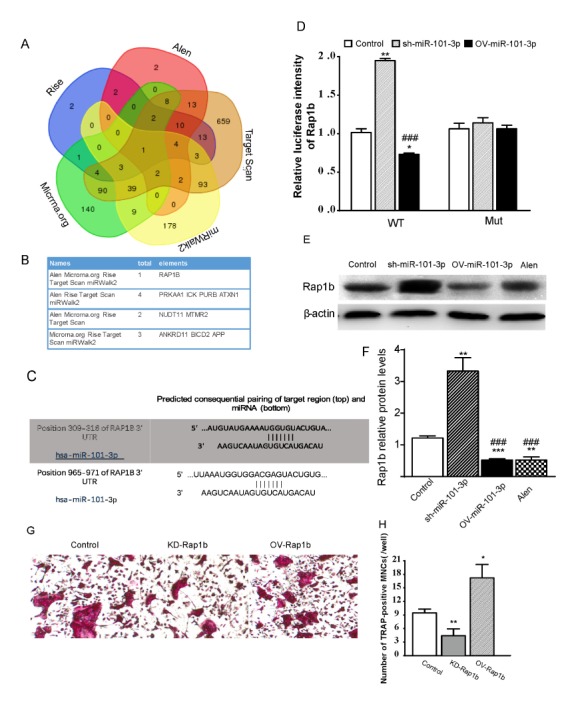
miR-101-3p inhibits osteoclast differentiation by directly targeting Rap1b. (A) Venn diagrams of likely targets determined by GSEA pre-ranked function and predicted targets by MiRanda, miRWalk2, and Target scan. (B) Differentially expressed genes. (C) Candidate targeted genes of miR-101-3p. (D) Putative miR-101-3p binding sequence on Rap1b RNA. In HEK293T cells, Rap1b wild-type-3′-UTR containing miR-101-3p binding sites or mutated counterpart was cloned into a luciferase reporter vector and co-transfected with OV-miR-101-3p, sh-miR-101-3p, and control plasmid. Relevant luciferase activity was then determined. (E) Protein expression of Rap1b in BMMs cells. (F) Rap1b relative protein expression. (G) TRAP-positive MNCs (400X). (H) Number of TRAP-positive MNCs (/well). *P < 0.05; ***P < 0.001 vs. controls. ###P < 0.001 versus sh-miR-101-3p group.
To address whether miR-101-3p-regulated Rap1b expression was a key factor for bisphosphonates inhibiting osteoclast differentiation, BMMs were transfected with plasmids to overexpress miR-101-3p or inhibit miR-101-3p and treated with alendronate. Cells were then differentiated into osteoclasts in osteoclastogenesis condition for 24 h. Western blot analysis showed that Rap1b protein expression level in BMMs transfected with OV-miR-101-3p plasmid or treated with alendronate was both lower than that in BMMs transfected with empty vector, while level of Rap1b in BMMs transfected with sh-miR-101-3p was increased (Fig. 3E, 3F). TRAP staining showed that Rap1b overexpression increased multinucleated cell numbers, while Rap1b inhibition decreased the cell numbers. Taken together, our results suggest that miR-101-3p can inhibit osteoclast differentiation by targeting Rap1b with bisphosphonate treatment.
miR-101-3p inhibits osteoclast differentiation by targeting Rap1b with bisphosphonates treatment in vivo
To detect the function of miR-101-3p in the process of bisphosphonates-inhibited osteoclast differentiation, OV-miR-101-3p/atelocollagen complex was injected via tail veins into ovariectomized (OVX) mice. To clarify the role of Rap1b in miR-101-3p-regulated osteoclast differentiation, Rap1b plasmid was also injected into OVX mice with OV-miR-101-3p/ atelocollagen complex. Four weeks after injection, higher bone density and bone volume fraction (BV/TV) were observed in tibiae in miR-101-3p overexpressed and Alen treated group by X radiograph and Micro-CT examination (Fig. 4A–4D). TRAP assay indicated that the percentage of bone surface covered by osteoclasts (Oc.S/B.S, %) after miR-101-3p injection was decreased in OVX-induced osteoclast formation. Meanwhile, expression level of receptor activator of nuclear factor-kappa B ligand (RANKL) gene and the ratio of RANKL/osteoprotegerin (OPG) were decreased in OVX mice after miR-101-3p injection compared to those in OVX mice (Fig. 4E–4G). Western blot analysis also showed that Rap1b protein expression level was increased in OVX mice, while it was down-regulated in mice when OV-miR-101-3p plasmid was injected or when alendronate was used for treatment (Fig. 4H and 4I). There was no significant difference in above expression level between alendronate treated and miR-101-3p injected OVX mice. Howerver, when OV-Rap1b plasmid was co-injected with OV-miR-101-3p or Alen, all osteogenesis changes were blocked. Serum ELISA results showed that miR-101-3p could decrease both CTX (carboxy-terminal telopeptides oftypeIcollagen) and PINP (amino-terminal propeptide of type I procollagen) activity in OVX mice, while Alen only decreased CTX activity without affecting PINP activity compared to Sham controls. On the other hand, both decreased effects were largely prevented in OVX mice treated with OV-Rap1b plasmid (Fig. 4J and 4K). These results suggest that bisphosphonates might be able to inhibit osteoclast differentiation and promote osteogenesis of OVX mice through up-regulation of miR-101-3p by targeting Rap1b.
Fig. 4.
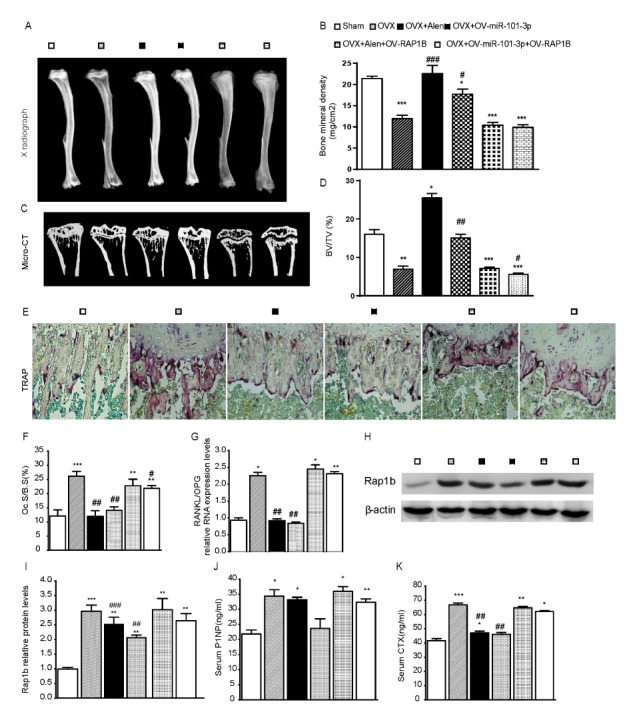
miR-101-3p inhibits osteoclast differentiation by targeting Rap1b with bisphosphonates treatment in vivo. (A) Representative contact radiographs and (C) micro-CT scanned sections of tibiae from different groups. (B) Bone Mineral Density (BMD). (D) BV/TV of the tibiae. (E) TRAP staining (400X). (F) Oc.S/B.S (%). (G) qRT-PCR was used to assess RANKL and OPG RNA expression and the relative ratio of RANKL/OPG mRNA level was calculated. (H) Protein expression of Rap1b in different groups of mice. (I) Rap1b relative protein expression. All data are presented as mean ± SD. Serum ELISA results of OV-miR-101-3p or Alen effect on CTX (J) and PINP (K) activity in OVX mice. *P < 0.05, **P < 0.01, ***P < 0.01 versus the Sham group, #P < 0.05, ##P < 0.01, ###P < 0.001 versus the OVX group.
DISCUSSION
In this study, we found that miR-101-3p was a key regulator in the process of bisphosphonates-inhibited osteoporosis. We also showed that miR-101-3p was a negative regulator of osteoclast differentiation of BMMs, consistent with the effect of bisphosphonates. Furthermore, our results suggest that bisphosphonates can inhibit bone resorption through miR-101-3p/Rap1b signal pathway. Thus, therapeutic overexpression of miR-101-3p in osteoclasts could be a potential strategy to improve effect of bisphosphonates treatment on osteoporosis in estrogen deficiency women.
Previous reports have shown that under pathological settings, osteoclastic bone resorption can be regulated by miRNAs. Osteoclastic miR-214 contributes to osteolytic bone metastasis and promotes osteoclastogenesis (15, 16). Overexpression of miR-335-5p in the osteoblast lineage induces osteogenic differentiation and bone formation in mice (17). The role of miRNAs in osteoclast differentiation after bisphosphonates treatment remains unclear. miR-101-3p has been reported to play an important role in tumorigenesis (18–20). However, whether it plays a critical role in osteoclast regulation is unclear. In the present study, we found that miR-101-3p was down-regulated during M-CSF and RANKL induced osteoclastogenesis from BMMs. In addition, it was up-regulated in cells treated with bisphosphonates. We further revealed that miR-101-3p could target Rap1b to inhibit osteoclastic bone resorption during differentiation of BMMs. Ras-related protein 1 (Rap1) is a universally expressed small GTPase that has two isoforms: Rap1a and Rap1b. A previous study has demonstrated that disruption of Rap1b in mice not only enhances osteoblast differentiation, but also impairs chondrocyte differentiation (21). Mice with Rap1-deficient osteoclasts are osteopetrotic. Disruption of Rap1/talin activity arrests integrin function in osteoclasts and inhibits their resorptive activity both in vitro and in vivo (22). In our study, effects of Rap1b overexpression or knockdown in BMMs on osteoclast differentiation were confirmed. We also observed that the expression of Rap1b was gradually up-regulated during the process of osteoclast differentiation induction. In addition, down-regulation of Rap1b could inhibit osteoclast differentiation, demonstrating that Rap1b is required for the negative effect of miR-101-3p in regulating osteoclast differentiation.
Estrogen deficiency is associated with rapid bone loss in women, particularly in trabecular bone. We used ovariectomy (OVX) model to evaluate bone phenotype after bisphosphonates treatment and transfection with OV-miR-101-3p in OVX mice and Sham littermates followed by X radiograph and Micro-CT analysis. All parameters evaluated revealed that bisphosphonates treated mice and OV-miR-101-3p injected mice had higher bone density and bone mass than Sham mice. More importantly, the percentage of bone surface covered by osteoclasts (Oc.S/BS, %) shown by TRAP-staining and the ratio of RANKL/OPG were significantly different between miR-101-3p injected and Sham mice. Bisphosphonate treatment decreased TRAP positive area in OVX mice compared to Sham mice. However, when OV-Rap1b plasmid was co-treated with OV-miR-101-3p or Alen, all osteogenesis changes were blocked. Serum ELISA results also confirmed that miR-101-3p decreased bone formation marker P1NP and bone resorption marker CTX-1 serum levels, although bisphosphonate did not affect P1NP levels. On the other hand, OV-Rap1b plasmid increased both P1NP and CTX-1 serum levels in OVX mice. These results confirmed that miR-101-3p could inhibit bone resorption in OVX mice.
Previous study has demonstrated that miR-101 can promote osteogenic differentiation by targeting the EZH2/Wnt/beta-Catenin signaling pathway (23). Thus, our study did not focus on the role of miR-101-3p in osteogenesis, although bisphosphonates can interact with osteoblasts and enhance their proliferation and maturation (24). Our results validated the role of miR-101-3p in bone resorption, clarifying other roles it played in bone formation.
Overall, our data demonstrate that bisphosphonates can promote osteogenesis by regulating miR-101-3p/Rap1b signal pathway to inhibit osteoclast differentiation. Our findings clarified the molecular mechanisms underlying complex functions of bisphosphonates. These results indicate that miR-101-3p possesses great potential as a novel class of therapeutic targets for treating osteoclast-related diseases.
MATERIALS AND METHODS
Detailed materials and methods are included in the Supplemental Material.
Supplementary Information
ACKNOWLEDGEMENTS
This work was supported by grants from the National Natural Science Foundation of China (No. 81572149 and No. 81600697) and from the National Basic Research Program of China (2014CB942903).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 2.Ji X, Chen X, Yu X. MicroRNAs in Osteoclastogenesis and Function: Potential Therapeutic Targets for Osteoporosis. Int J Mol Sci. 2016;17:349. doi: 10.3390/ijms17030349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuen T, Stachnik A, Iqbal J, et al. Bisphosphonates inactivate human EGFRs to exert antitumor actions. Proc Natl Acad Sci U S A. 2014;111:17989–17994. doi: 10.1073/pnas.1421410111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soares AP, do Espírito Santo RF, Line SR, et al. Bisphosphonates: Pharmacokinetics, bioavailability, mechanisms of action, clinical applications in children, and effects on tooth development. Environ Toxicol Pharmacol. 2016;42:212–217. doi: 10.1016/j.etap.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Fordham JB, Guilfoyle K, Naqvi AR, Nares S. MiR-142-3p is a RANKL-dependent inducer of cell death in osteoclasts. Sci Rep. 2016;6:24980. doi: 10.1038/srep24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu CH, Sui BD, Du FY, et al. miR-21 deficiency inhibits osteoclast function and prevents bone loss in mice. Sci Rep. 2017;7:43191. doi: 10.1038/srep43191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ke K, Sul OJ, Rajasekaran M, Choi HS. MicroRNA-183 increases osteoclastogenesis by repressing heme oxygenase-1. Bone. 2015;81:237–246. doi: 10.1016/j.bone.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Sang S, Zhang Z, Qin S, Li C, Dong Y. MicroRNA-16-5p Inhibits Osteoclastogenesis in Giant Cell Tumor of Bone. Biomed Res Int. 20172017:3173547. doi: 10.1155/2017/3173547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang XH, Geng GL, Su B, Liang CP, Wang F, Bao JC. MicroRNA-338-3p inhibits glucocorticoid-induced osteoclast formation through RANKL targeting. Genet Mol Res. 2016;15 doi: 10.4238/gmr.15037674. [DOI] [PubMed] [Google Scholar]
- 10.Lee D, Heo DN, Kim HJ, et al. Inhibition of Osteoclast Differentiation and Bone Resorption by Bisphosphonate-conjugated Gold Nanoparticles. Sci Rep. 2016;6:27336. doi: 10.1038/srep27336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bindea G, Galon J, Mlecnik B. CluePedia Cytoscape plugin: pathway insights using integrated experimental and in silico data. Bioinformatics. 2013;29:661–663. doi: 10.1093/bioinformatics/btt019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bindea G, Mlecnik B, Hackl H, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091–1093. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohl M, Wiese S, Warscheid B. Cytoscape: software for visualization and analysis of biological networks. Methods Mol Biol. 2011;696:291–303. doi: 10.1007/978-1-60761-987-1_18. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Li D, Dang L, et al. Osteoclastic miR-214 targets TRAF3 to contribute to osteolytic bone metastasis of breast cancer. Sci Rep. 2017;7:40487. doi: 10.1038/srep40487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao C, Sun W, Zhang P, et al. miR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol. 2015;12:343–353. doi: 10.1080/15476286.2015.1017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Tang Y, Zhu X, et al. Overexpression of MiR-335-5p Promotes Bone Formation and Regeneration in Mice. J Bone Miner Res. 2017;12:2466–2475. doi: 10.1002/jbmr.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng Y, Ding S, Chen K, et al. Functional analysis of miR-101-3p and Rap1b involved in hepatitis B virus-related hepatocellular carcinoma pathogenesis. Biochem Cell Biol. 2014;92:152–162. doi: 10.1139/bcb-2013-0128. [DOI] [PubMed] [Google Scholar]
- 19.Gong J, Chu Y, Xu M, et al. Esophageal squamous cell carcinoma cell proliferation induced by exposure to low concentration of cigarette smoke extract is mediated via targeting miR-101-3p/COX-2 pathway. Oncol Rep. 2016;35:463–471. doi: 10.3892/or.2015.4379. [DOI] [PubMed] [Google Scholar]
- 20.Liu P, Ye F, Xie X, et al. mir-101-3p is a key regulator of tumor metabolism in triple negative breast cancer targeting AMPK. Oncotarget. 2016;7:35188–35198. doi: 10.18632/oncotarget.9072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maruyama T, Jiang M, Abbott A, et al. Rap1b Is an Effector of Axin2 Regulating Crosstalk of Signaling Pathways During Skeletal Development. J Bone Miner Res. 2017;32:1816–1828. doi: 10.1002/jbmr.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou W, Izawa T, Zhu T, et al. Talin1 and Rap1 are critical for osteoclast function. Mol Cell Biol. 2013;33:830–844. doi: 10.1128/MCB.00790-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Meng Y, Cui Q, et al. MiR-101 Targets the EZH2/Wnt/beta-Catenin the Pathway to Promote the Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. Sci Rep. 2016;6:36988. doi: 10.1038/srep36988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS. Osteoblast proliferation and maturation by bisphosphonates. Biomaterials. 2004;25:4105–4115. doi: 10.1016/j.biomaterials.2003.11.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.