Abstract
Gnotobiotic animal research has expanded markedly over the past decade. Although germ-free animals were first described more than 100 y ago, little evidence-based guidance is available on best operational procedures. A key aspect of gnotobiotic technology is the sterilization of animal enclosures, most commonly flexible vinyl film isolators. The objective of this study was to determine the most effective methods for chemical sterilization of gnotobiotic isolators and associated equipment. As test microbes, we used bacteria from 4 different accidental isolator contaminations that occurred in a gnotobiotic core facility. Identification by 16S ribotyping revealed facultative anaerobic firmicutes, including several Paenibacillus and Bacillus species, and obligate aerobic actinobacteria, namely Micrococcus luteus, among the contaminants. We selected 6 products commonly used for disinfecting hospital rooms, kitchens, and veterinary facilities to represent chlorine-oxide– and peroxide-based disinfectants and tested the hypothesis that these 2 classes are equally effective. However, evaluation of bactericidal and sporicidal activity in liquid cultures revealed that chlorine oxide-based disinfectants were more effective than peroxide-based disinfectants. In both groups, various products effectively sterilized gnotobiotic isolators by fogging in field tests, although bactericidal concentrations were markedly higher than those in suspension cultures, and effectiveness was contact-time–dependent. In addition, in both groups, some disinfectants were excessively corrosive to ferrous metals and acrylic. These results demonstrate that no single disinfectant has all desirable properties and that the different characteristics of disinfectants must be balanced during their selection. However, chlorine oxide-based disinfectants were generally more effective and less corrosive than peroxide-based products.
The resident microbiota of mammals has evolved to colonize and thrive in various regions of the body, most notably in the gut, skin, and genital tract but also in the airways and even placenta.1,13 The human microbiome consists of more than 1000 species and 100 trillion bacteria, indicating that the number of bacteria greatly exceeds the number of host cells.29 Critical new insights into the composition and structure of the microbiota under health and disease conditions have emerged over the last decade, with the advent of high-throughput sequencing technologies. The physiologic functions of the microbiome in vitamin production, nutrient absorption, immune development, bone growth, brain development, and other processes remain incompletely understood and are an urgent topic of ongoing research.2
Studies of microbiome–host interactions are challenging because the contributions of individual bacterial species to physiologic processes in the body have proven remarkably difficult to dissect. One of the most powerful approaches to this challenge is the use of gnotobiotic animals, whose microbiomes are fully defined and can be controlled. Such animals require as a starting point the establishment of the germ-free state, in which an animal is free of all microbial colonization. Colonization (association) of such animals with known microbes can then be used to manipulate microbial composition and investigate the ensuing host responses.
Germ-free animals were first reported at the end of the 19th century,18 but it was not until the 1930s and 1940s that techniques were sufficiently reliable to generate thriving breeding colonies of germ-free guinea pigs and rats.33,35,36 Although gnotobiotic animals have been used regularly since that time, their use has expanded markedly only relatively recently (since the 2000s), in parallel with the advancements in microbiomics. Numerous technologic challenges to conducting gnotobiotic animal research exist. Specifically, personnel must be highly trained to avoid contamination of gnotobiotic environments. Animals and equipment must be handled inside isolators by using thick gloves that limit dexterity. Most importantly, all food, water, bedding, caging, enrichment items, and all other materials to be placed inside of isolators must be sterilized to prevent contamination. Accidental contaminations—which occur infrequently but regularly in most gnotobiotic facilities—lead to losses of valuable animals, time, personnel efforts, and research opportunities.
Meticulous technique and personnel training are clearly important for successful maintenance of gnotobiotic animals, yet the best approaches and practices for routine husbandry of such animals remain largely dependent on anecdotal evidence and personal experience.15,28,42,45 To improve the performance of gnotobiotic core facilities, we sought to take an evidence-based approach to evaluate key processes in gnotobiotic husbandry. A major aspect of gnotobiotic technology is the sterilization of animal enclosures to eliminate all microorganisms, including bacterial spores.37 Consequently, we set out to determine the most effective methods to chemically sterilize gnotobiotic isolators and associated equipment. Given the common use of both chlorine-oxide– and peroxide-based disinfectants in hospital rooms, kitchens, and veterinary facilities, we tested the hypothesis that these 2 classes of disinfectants are equally effective in gnotobiotic applications, by using liquid culture systems for vegetative and spore forms of relevant bacteria, fogging of gnotobiotic isolators in field tests, and corrosion testing of the relevant materials.
Materials and Methods
Gnotobiotic animal husbandry.
Germ-free C57BL/6NTac (GF-B6) mice initially were obtained from Taconic Biosciences (Rensselaer, NY) and housed in standard polycarbonate mouse cages with wire lids (7.5 × 11.5 × 5.0 in.) inside 2 × 2 × 4 ft flexible-film isolators (Class Biologically Clean, Madison, WI) at 21 to 23 °C, 30% to 70% relative humidity, and a 12:12-h light:dark cycle. Mice were group-housed on autoclaved Aspen chip bedding (Northeastern Products, Nepco, Madison, WI), fed autoclaved 19% protein extruded rodent diet (2019S Teklad, Envigo, Somerset, NJ) without restriction, and had unrestricted access to water that was purified inhouse by reverse osmosis and sterilized by autoclaving. Mice were provided with autoclaved 2-in. squares of nesting material (Nes3600, Ancare, Bellmore, NY). All materials and supplies were autoclaved at 121 °C for a minimum of 1 h inside a stainless steel supply cylinder (Class Biologically Clean). Sterility of bedding, water, and feed was confirmed by plating samples or extracts onto Luria–Bertani agar and Columbia blood agar, followed by incubation under aerobic and anaerobic conditions, as described later. Autoclaved supply cylinders were coupled to the entry ports (diameter, 18 in. depth, 9 in.) of the gnotobiotic isolators for transfer of the contents. In light of the outcome of the present study, we used MB-10 (Quip Laboratories, Wilmington, DE; hereafter disinfectant A) at the manufacturer-recommended full strength and an atomizing sprayer (Spray System, Wheaton, IL) at 12 psi of pressure for routine sterilization of ports and isolators. Ports were disinfected by using a single cycle of 50 mL of disinfectant over 12 min (split into 3 min of active fogging and 9 min of passive incubation), followed by 5 min of exhaust before the port was opened to the inside of an isolator. Isolators were disinfected through 5 cycles of 50 mL of disinfectant each for 3 min of active fogging and 1 min of passive incubation, for a total of 20 min, followed by overnight exhaust and drying. All procedures were reviewed and approved by the IACUC of the University of California, San Diego.
Identification of bacterial contaminants.
Fecal samples were routinely obtained from animals in gnotobiotic isolators for sterility testing by collecting multiple fresh pellets from several mouse cages. Sterile PBS was added to fecal pellets, and samples were vortexed and immediately plated onto Luria–Bertani agar and Columbia blood agar. Plates were incubated for as long as 72 h at 37 °C under both aerobic (room air) and anaerobic (AnaeroPack System, Mitsubishi, New York, NY) conditions. If any growth was detected, a minimum of 5 morphologically distinct colonies was collected from each plate, replated, and grown on the appropriate agar to obtain well-defined, single colonies. Each clonal isolate was stored at –80 °C in brain-heart infusion broth with 50% glycerol. For bacterial identification, an aliquot of each colony was directly added to a PCR reaction mixture containing Q5 Hot Start High-fidelity Master Mix and Taq polymerase (New England Biolabs, Ipswich, MA) and the universal 16S rRNA primers 5′ AGA GTT TGA TCC TGG CTC AG 3′ (27F) and 5′ CAG GAA CCG CGG CTG CTG GC 3′ (520R). PCR reaction conditions were: 95 °C for 2 min for initial cell lysis and denaturation; 35 cycles of 94 °C denaturation for 30 s, 56 °C annealing for 30 s, and 72 °C extension for 2 min; followed by a final extension step at 72 °C for 5 min. PCR products were run on a 1% agarose gel and stained with ethidium bromide to confirm amplification of the expected single 468- to 505-bp products, which were then purified by using the Zymogen PCR Purification Kit (Zymo Research, Irvine, CA) and submitted for sequencing (Eton Biosciences, San Diego, CA). Sequences were compared by using the Basic Local Alignment Search Tool (BLASTN; National Center for Biotechnology Information), and the closest matches in GenBank were used to assign the taxonomic level.
Disinfectants.
A wide range of chemical disinfectants and sterilants have been developed for different applications in the food industry and for medical and veterinary applications,5 including for gnotobiotic rodent facilities, but 2 major groups are most practical in terms of safety and cost: chlorine-oxide–based disinfectants and peroxide-based disinfectants. We selected 6 representative disinfectants for this study (Table 1). For chlorine-oxide products, we chose commercial bleach (Germicidal Ultra Bleach, Waxie Sanitary Supply, San Diego, CA), Clidox (at the recommended 1:3:1 v/v/v dilution of Clidox-S base:tap water:Clidox-S activator; Pharmacal Research Laboratories, Waterbury, CT; hereafter disinfectant B), and MB-10 (disinfectant A; 1.5-g tablet dissolved in 125 mL tap water; Quip Laboratories, Wilmington, DE). For peroxide-containing products, we used the following ready-to-use products: concentrated (30% v/v) hydrogen peroxide solution (Fischer Chemical, Fair Lawn, NJ),23 Spor-klenz (Steris, St Louis, MO; hereafter disinfectant C),24 and Rescue (Virox Animal Health, Oakville, Ontario, Canada; hereafter disinfectant D). All products are registered with the Environmental Protection Agency, with the exceptions of concentrated hydrogen peroxide and bleach. Publicly available information on acute toxicity of the active ingredients in the disinfectants is listed in Table 1. We note that the selected products are commonly termed disinfectants, although our study objectives were to determine their properties as sterilants. In contrast, conventional chemical sterilants such as ethylene oxide and glutaraldehyde were not considered here because of their highly toxic properties and considerable difficulties in using them safely for gnotobiotic equipment.
Table 1.
Disinfectants used in this study
| Active ingredients (concentration in undiluted stock solution) | Oral rat LD50 of active ingredients (mg/kg) | |
| Bleach | Sodium hypochlorite (6% w/v) | 8910 |
| Disinfectant A | Sodium chlorite (0.25% w/v) | 105–177 |
| Sodium dichloroisocyanurate dihydrate (0.084% w/v) | 1420–1670 | |
| Disinfectant B | Sodium chlorite (0.17% w/v) | 105–177 |
| Hydroxyacetic acid (2% w/v) | 1600–3200 (20% aqueous solution) | |
| Hydrogen peroxide | Hydrogen peroxide (30% w/v) | 1193 at 35% |
| Disinfectant C | Hydrogen peroxide (1.0% w/v) | 1193 at 35% |
| Peracetic acid (0.08% w/v) | 1540 | |
| Disinfectant D | Hydrogen peroxide, accelerated (0.5% w/v) | 1193 |
Listed concentrations are of the active ingredients in the ready-to-use disinfectant stock solutions (which are considered full-strength or 100% stocks for the purpose of this study); see Methods for information regarding manufacturers. In addition, several of the agents (for example, disinfectants B and C) are registered with the Environmental Protection Agency as chemical sterilants, but for simplicity, all agents are referred to as disinfectants throughout the document. Acute oral LD50 values for rats are from the manufacturers’ Safety Data Sheets or the TOXNET Toxicology Data Network of the US National Library of Medicine.
Disinfectant activity testing.
Four representative bacteria isolated from contaminated gnotobiotic isolators were used in suspension assays: Bacillus licheniformis, Paenibacillus macerans, Paenibacillus thermophilus, and Micrococcus luteus. In addition, a laboratory strain of Escherichia coli (DH5α) was used as a universal bacterial indicator strain, and Geobacillus stearothermophilus was used for paired testing of spores and vegetative bacteria. Spores of G. stearothermophilus were obtained from a spore-based biologic test assay (Traditional Self-Contained Biologic Indicators, Crosstex, Hauppauge, NY). Each glass test vial was opened aseptically, and the spore-infiltrated paper discs were removed and suspended in PBS to release the spores. To obtain vegetative G. stearothermophilus bacteria, the spore suspension was plated onto Luria–Bertani agar and incubated at 55 °C for 24 h.
Stock cultures of B. licheniformis, P. macerans, and P. thermophilus were grown in brain–heart infusion broth, whereas those of M. luteus, E. coli, and G. stearothermophilus were grown overnight in Luria–Bertani broth with shaking under aerobic conditions at 37 °C, with the exception of G. stearothermophilus, which was grown at 55 °C. Bacteria were washed in PBS solution, and bacterial densities were estimated by optical density at 600 nm and adjusted to 107 cfu/mL in PBS. Numbers of spores were determined by direct microscopic counting. For suspension culture testing, disinfectants were serially diluted in PBS in 96-well plates, and vegetative bacteria were added to a final density of 107 cfu/mL, whereas spores were added at a final density of 105 cfu/mL. After 15 min of contact time at room temperature, suspensions were diluted at least 1:10 in PBS to minimize further disinfectant action. Using quantitative bacterial growth assays at multiple disinfectant concentrations, we confirmed that bacterial growth was not inhibited at disinfectant concentrations more than 10-fold below their minimal lethal disinfectant strength. After dilution with PBS, samples were immediately plated onto Luria–Bertani agar and incubated at 37 °C or 55 °C (G. stearothermophilus) for 24 to 72 h. Growth was recorded as a positive result indicative of insufficient sterility, and the lowest disinfectant concentration (relative to the respective full-strength stock solutions) with no growth was recorded as the minimal lethal disinfectant strength needed to kill all bacteria. For these calculations, we did not correct for any differences in the concentration of active ingredients (Table 1), given that the goal of the study was to evaluate and compare commercially available disinfectant products rather than specific ingredients.
Corrosion tests.
Corrosion testing was done by using zinc-plated flat steel washers (diameter, 0.75 in.; thickness 0.06 in. thickness metal), 2 × 2-cm pieces of the same vinyl that is used for the gnotobiotic isolators, and acrylic discs (diameter, 0.75 in.; thickness, 0.25 in.), which were cut with a circular saw from unused isolator ports. Metal washers and acrylic discs were placed into 4 mL and 7 mL, respectively, of 100% stock disinfectant in 6-well plates in a fume hood. Wells were allowed to dry and were refilled every 48 to 72 h over a period of 12 d to mimic an accelerated exposure cycle for isolator parts regularly exposed to disinfectants by fogging or other forms of contact, followed by subsequent drying through evaporation. After disinfectant exposure, washers and discs were rinsed with distilled water and dried, and photographs were taken. For quantification of corrosion in the metal washers, color photographs were converted to grayscale, and the total signal intensity of the washers, a measure of light absorption related to corrosive surface changes, was determined by using Image J software.38 The background intensity of new washers was subtracted, and intensity was expressed relative to black (100% light absorption).
Fogging field tests of disinfectants.
Agar plates were prepared with minimal bacterial growth media,8 inoculated evenly with 108 E. coli in 100 µL, and dried briefly before use. Ten plates were placed in different locations on the floor, walls, and ceiling inside a clean gnotobiotic isolator. Plates on the sides and ceiling had sterilized metal washers incorporated into the agar and were held in place by magnets. The isolator was closed and inflated as done for routine maintenance. Various dilutions of disinfectants were prepared and used to fog the isolators continuously for 1.5, 3, or 6 min using an atomizing sprayer (Spray System) at 12 psi of air pressure. After fogging, the isolator was left for 5 min without disturbance and then exhausted for an additional 5 min to remove the fog before opening. Agar plates were closed by using lids inside the isolator to avoid contamination, removed from the isolator, and incubated at 37 °C for as long as 72 h; any growth on the plates was considered to be positive indication of insufficient sterility.
Statistics.
Nonparametric (Mann–Whitney U test, Kruskal–Wallis test) or parametric (ANOVA, Dunnett) tests were performed in Prism (GraphPad Software, La Jolla, CA) as appropriate to determine statistically significant differences between disinfectants and treatment groups. A P value less than 0.05 was considered as statistically significant.
Results
Identification of bacterial contaminants in gnotobiotic isolators.
Over a 2-y operation period of a gnotobiotic mouse core facility using flexible film isolators, routine microbiologic testing of fecal samples cultured by using different growth media under aerobic and anaerobic conditions revealed several accidental and independent contaminations. To identify the bacterial contaminants, we isolated random colonies from agar plates that had shown the most bacterial growth and subjected them to 16S ribotyping with universal PCR primers. Sequence analysis of the resulting PCR products revealed a total of 8 different strains from 6 different bacterial species during 4 independent contamination events (Table 2). Three of the bacteria were identified in 2 independent contaminations of different isolators, whereas the other 5 bacteria were unique to particular contaminations, suggesting that they originated from independent sources. Furthermore, 3 of the contaminations yielded multiple bacterial species, although only a single species were identified among several bacterial colonies in the fourth contamination. The notable paucity of bacterial diversity suggests that only a few bacterial species contaminated the isolators and that some contaminations may even be monoclonal.
Table 2.
Identification of bacterial contaminants in gnotobiotic isolators
| No. of identifications in isolator |
||||||
| Species | Closest matched strain (similarity) | Phylum | A | B | C | D |
| Micrococcus luteus | JGTA-S5 (99%) | Actinobacteria | 1 | |||
| Bacillus licheniformis | ST3 (99%) | Firmicutes | 1 | 1 | ||
| CCM28B (99%) | Firmicutes | 7 | ||||
| Paenibacillus thermophilus | WP-1 (98%) | Firmicutes | 1 | 4 | ||
| Paenibacillus macerans | ZY18 (97%) | Firmicutes | 1 | |||
| IB-I4 (96%) | Firmicutes | 1 | 4 | |||
| Paenibacillus dendritiformis | RRLKE4 (99%) | Firmicutes | 8 | |||
| Paenibacillus motobuensis | GE10-1 (97%) | Firmicutes | 8 | |||
Bacterial contaminants were discovered during routine screens of fecal samples of germ-free mice maintained in gnotobiotic isolators and were identified by using 16S rRNA ribotyping and sequencing. The closest matches of the sequences to 16S rRNA in GenBank and the percentage of similarity are given. Four independent contaminations (isolators A through D) and at least 5 individual bacterial colonies were analyzed for each contamination. The numbers represent the various colony identifications among the contaminations of each of the isolators.
The 6 identified bacterial species belonged to only 2 phyla, Actinobacteria and Firmicutes (Table 2), and all are known to be obligate aerobes or facultative anaerobes but not strict anaerobes. Consistent with these findings, we detected bacterial growth under aerobic or both aerobic and anaerobic conditions but not under anaerobic conditions only.
Activities of various disinfectants against bacterial contaminants in culture.
Because our sterilization procedures apparently had failed to kill the bacteria that were identified in the contaminated isolators, we began to explore the effectiveness of various disinfectants against the contaminants. We selected a range of chlorine-oxide– and peroxide-based disinfectants that were readily commercially available, either in the form of products formulated and sold as medical or veterinary disinfectants or as chemicals with known disinfectant or sterilant properties (Table 1). Four representative bacterial strains from the contaminations, as well as E. coli as a commonly used bacterial indicator strain, were exposed for a brief period (15 min) to various concentrations of disinfectants in PBS at room temperature, diluted to minimize further disinfection, plated on growth agar, and cultured overnight. As a measure of killing potency, we determined the lowest disinfectant concentration —analogous to the minimal lethal concentration in antibiotics testing and expressed as a percentage of the full-strength disinfectant stock concentration—that prevented all bacterial growth.
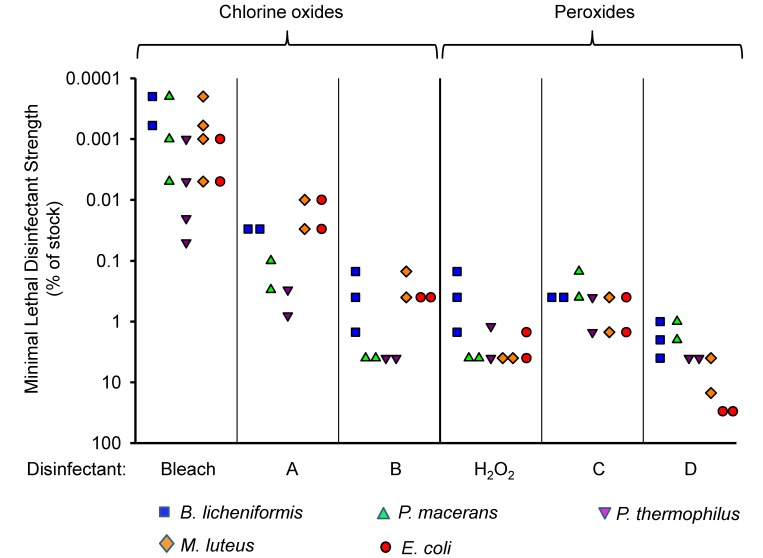
All 6 tested disinfectants killed the 5 test bacterial strains at their respective full-strength (that is, 100%) stock concentrations, but the killing potency of the 2 main groups of disinfectants and the specific formulations differed (Figure 1). The activity of the chlorine-oxide–based disinfectants was, on average, greater than that of the peroxide-based disinfectants (medians of minimal lethal disinfectant strengths across all bacteria and experiments were 0.05% for chlorine oxides and 2% for peroxides; P < 0.0001 by Mann-Whitney U test). For example, bleach and disinfectant A were bactericidal for M. luteus and E. coli at greater than 1000-fold dilution, whereas hydrogen peroxide and disinfectant D lost activity against the same 2 bacteria at less than 100-fold dilution. In addition, we noted 50- to 500-fold variation in the killing activity of the same disinfectant against different bacteria, although similar variations occurred for both chlorine-oxide– and peroxide-based disinfectants, and no bacterial strain was consistently more resistant to all or even most of the disinfectants (Figure 1). These results demonstrate that different disinfectants differ in their killing activity against different target bacteria, leading to variable and wide margins of safety (that is, the ratio of disinfectant stock concentration to its minimal lethal disinfectant strength), although the chlorine-oxide–based disinfectants were generally more potent than the peroxides.
Figure 1.
Disinfectant activity against bacterial contaminants in suspension cultures. The indicated bacteria derived from independent isolator contaminations and E. coli were exposed to various dilutions of disinfectants for 15 min in a buffered saline solution at room temperature. After dilution to minimize further disinfectant action, bacteria were plated onto growth agar and incubated overnight at 37 °C. Disinfectant strength is shown as the highest dilution (that is, strength) relative to the respective stock concentrations that prevented all bacterial growth. Each data point represents the result from a separate experiment.
Disinfectant activity against bacterial spores.
Most of the contaminating bacteria we identified are known or presumed to form spores,20,26,49 raising the possibility that the bacteria existed as spores during decontamination. Because spores can be more resistant to disinfection and sterilization than vegetative forms,44 we sought to compare the killing activity of the different disinfectants against matched pairs of spores and vegetative forms. Initial attempts to generate spores of the isolated contaminant bacteria under several previously reported induction conditions were unsuccessful.20,26,49 Instead, we resorted to a well-established spore former, G. stearothermophilus, which is widely used as a biologic indicator for testing autoclave function.19 Spores and vegetative forms of G. stearothermophilus were exposed briefly (15 min) to various concentrations of each disinfectant in PBS and tested for survivors by growth on agar plates.
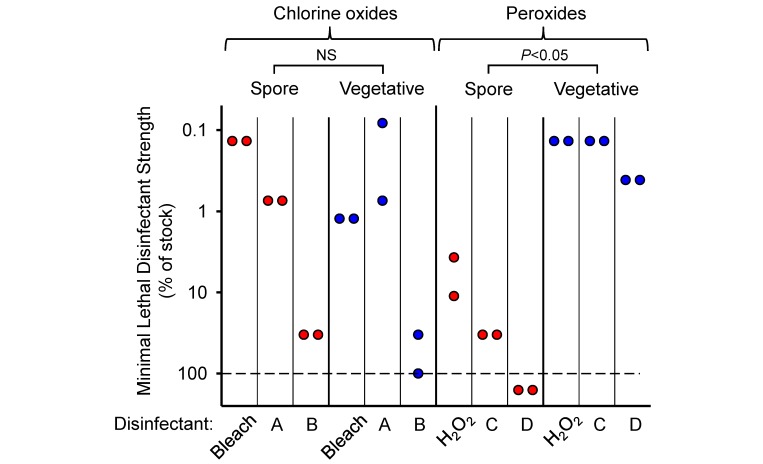
Five of the 6 disinfectants were effective in killing both spores and vegetative bacteria at their respective full-strength stock concentrations, but both relative and absolute killing activities clearly differed (Figure 2). Specifically, all 3 chlorine oxide-based disinfectants were at least as, if not more, active against spores as they were against vegetative forms (geometric means of 3.7% compared with 20.2%, respectively), with bleach and disinfectant A showing a greater than 100-fold safety margin for spore killing relative to the undiluted stock solutions. In contrast, all 3 peroxide-based disinfectants were significantly less effective (by greater than 100-fold) against spores relative to the vegetative forms (geometric means of 73.3% compared with 0.2%), and one of them, disinfectant D, had no activity at all against spores even at the recommended stock concentration (Figure 2). These findings indicate that disinfectants differ in their activity against spores in ways that cannot be readily predicted from their killing capacity against the vegetative forms. The results also show that the 2 chlorine-oxide–based disinfectants, bleach and disinfectant A, are particularly potent against spores.
Figure 2.
Comparison of disinfectant activity against spores and vegetative bacterial forms. Spores and vegetative forms of Geobacillus stearothermophilus were incubated with different dilutions of disinfectants for 15 min in a buffered saline solution at room temperature. Subsequently, bacteria were diluted, plated onto growth agar, and incubated overnight at 55 °C. Disinfectant strength is shown as the highest dilution (that is, strength) relative to the respective stock concentrations that prevented all bacterial growth. Each data point represents the result from a separate experiment. The combined killing data for spores compared with vegetative forms for each class of disinfectant (that is, chlorine oxides and peroxides) were evaluated by using the Mann–Whitney U test for the (*, P < 0.05; NS, not significant). The dashed line represents the highest tested disinfectant strength; data points below the line indicate lack of killing at 100% strength.
Corrosion testing of disinfectants.
Routine use of disinfectants in a gnotobiotic animal facility requires not only excellent killing activity with good safety margins against a wide range of potential microbial contaminants but also compatibility with the gnotobiotic equipment, particularly acrylic (used in transfer ports), vinyl (used in flexible film isolators) and ferrous metals (steel used in port holders and autoclaving cylinders). These materials come into regular contact with disinfectants, so corrosion or other damage caused by repeated use could eventually require costly and time-consuming replacement. To assess the corrosive potential of each disinfectant, we exposed ferrous metal (in form of zinc-plated steel washers) and acrylic (in form of small discs) to each of the 6 disinfectants. To mimic the real-life situation of routine isolator use characterized by repeated exposure to disinfectants, the test items underwent multiple intense cycles of day-long disinfectant submersion followed by evaporative drying for a total of 12 d.
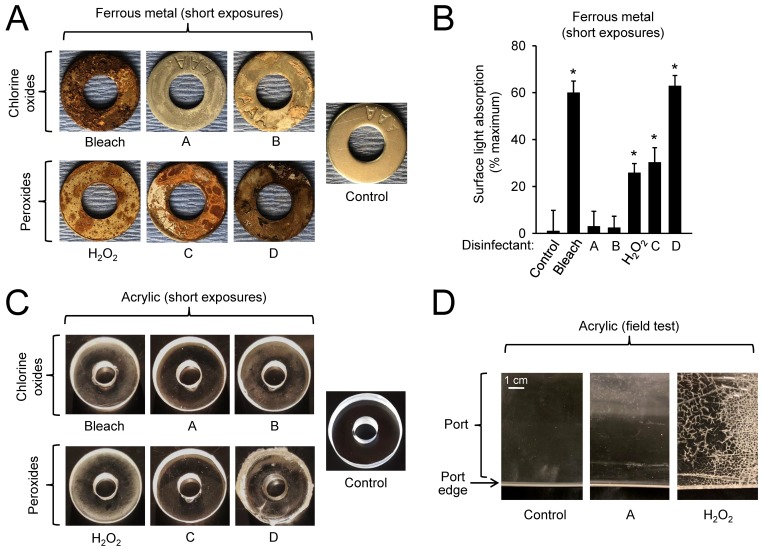
For the ferrous metal (steel), the most corrosive disinfectants were bleach, disinfectant D, hydrogen peroxide, and disinfectant C, whereas the least corrosive were disinfectants A and B (Figure 3 A and B). For acrylic, the most corrosive disinfectants were hydrogen peroxide, bleach, and disinfectant B, each causing pitting and fissuring of the acrylic surface, whereas disinfectants A and C had little visible effect on the material (Figure 3 C). Disinfectant D produced a thick clear substance on the surface that was difficult to remove, but the acrylic underneath appeared to be unaffected. For vinyl, only disinfectant D caused visible alterations in form of a thick liquid with precipitates on the material that could not be wiped off without apparent damage to the material. None of the other 5 disinfectants had noteworthy destructive effects, suggesting that vinyl is probably the least problematic in terms of disinfectant exposure of all the standard materials used in isolator and port construction.
Figure 3.
Corrosion testing of disinfectants. (A and B) Metal washers and (C) acrylic discs were exposed to several short-exposure cycles of soaking in disinfectants and drying over a 12-d period, washed in water, and dried. (A and C) Photographs were taken at equal magnification and light exposure and are representative of 1 of 3 experiments. New metal washers and acrylic discs were used as controls. (B) For the metal washers, corrosion was quantified by determining light absorption as a measure of corrosive surface changes. The background intensity of new washers was subtracted, and intensity (mean ± SE) was expressed relative to black (100% light absorption); *, P < 0.05 compared with nonexposed control (n = 4; ANOVA with Dunnett posthoc test). (D) Acrylic ports of gnotobiotic isolators were used routinely in field tests for 3 mo with regular (typically weekly) disinfectant fogging, as required for standard husbandry, by using 1 of the 2 indicated disinfectants, and representative photographs of the port edges were taken at equal magnifications. A new acrylic port was used as a control.
Field tests of 2 of the disinfectants, disinfectant A and hydrogen peroxide, by using acrylic isolator ports over a 3-mo period in cycles of exposure and drying as part of regular gnotobiotic animal husbandry confirmed that hydrogen peroxide caused deep pitting, fissuring, and cracking of the acrylic surface, whereas disinfectant A did not cause any visible surface damage (Figure 3 D). Taken together, these findings indicate that ferrous metal was affected to the greatest extent by disinfectants, particularly by bleach and disinfectant D; that acrylic was damaged by hydrogen peroxide, bleach, and disinfectant B; and that vinyl was largely resistant to the tested disinfectants.
Field testing of disinfectant activity by fogging in gnotobiotic isolators.
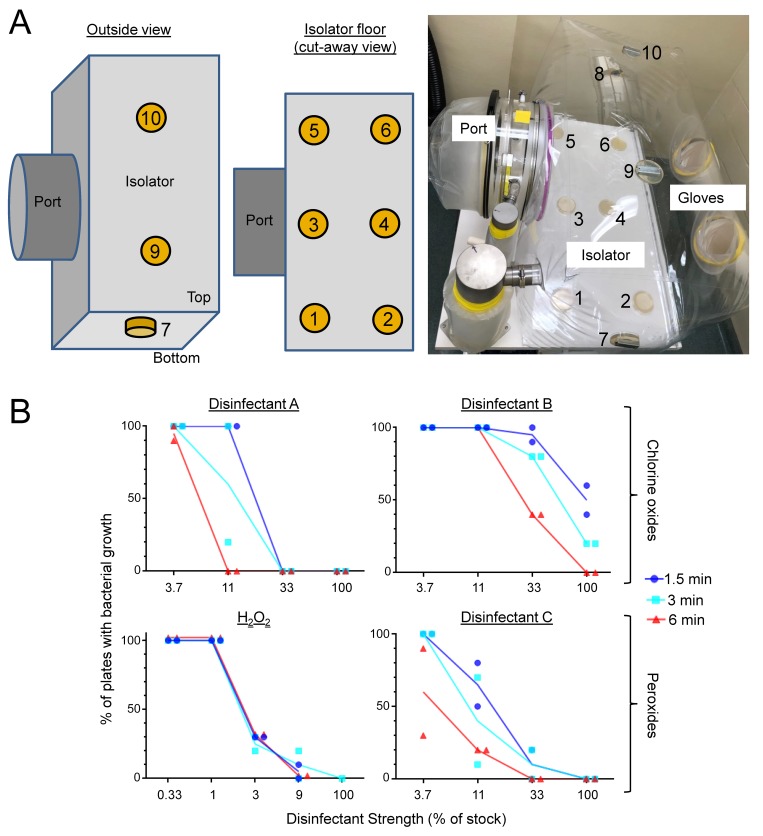
The initial bacterial killing tests were done in suspension assays to minimize confounding factors, but use in gnotobiotic applications requires comprehensive surface sterilization in flexible-film isolators. Although such chemical sterilization may be achievable by diligent wiping with a disinfectant-soaked cloth, a more reliable approach is aerosol spraying (‘fogging’).3 To test disinfectant activity in a fogging regimen, we used agar test plates (diameter, 10 cm), onto which 108 test bacteria (E. coli) were freshly plated evenly across the surface and which we placed in 10 locations in a test isolator, including on the floor and ceiling (Figure 4 A). The plate-loaded test isolators were closed and inflated as usual and then fogged with different concentrations of disinfectants for various times (1.5, 3 and 6 min; longer times led to unacceptable accumulation of liquid in ports and isolators). After a 5-min incubation period without fogging (to take advantage of the hang times of the fog droplets for further disinfection without adding more disinfectant) and a subsequent 5-min exhaust period (to evacuate the fog from the isolator for safe opening), the plates were removed and incubated overnight to promote growth of any remaining live bacteria. Results were expressed as the percentage of plates within the test isolator that showed any discernible bacterial growth. Untreated isolators served as controls and consistently showed growth on all test plates (that is, 100% positive).
Figure 4.
Field testing of disinfectants in gnotobiotic isolators. (A) Ten agar plates freshly inoculated with E. coli were placed inside a gnotobiotic isolator in different locations: 6 on the floor (nos. 1 through 6), 2 on the sides (nos. 7 and 8), and 2 on the ceiling (nos. 9 and 10). The plates on the sides and ceiling contained sterilized metal washers in the agar and were held in place by magnets applied from the outside of the isolator. (B) Isolators were closed, inflated, and fogged for various times (1.5 to 6 min) by using different concentrations of the indicated disinfectants. After a 5-min incubation period without fogging and an additional 5-min exhaust period, plates were removed from the isolators and incubated overnight to promote growth of any remaining live bacteria. Disinfectant effectiveness is shown as the percentage of the plates in an isolator that showed bacterial growth. Each data point represents 1 experiment. Lines indicate the mean of each respective condition and disinfectant.
For field testing, we selected 2 of the chlorine oxide-based disinfectants (products A and B) and 2 of the peroxide-based disinfectants (hydrogen peroxide and product C). Three of the 4 disinfectants were 100% effective at the full-strength stock concentrations for all 3 fogging periods, whereas disinfectant B was only partly effective at 1.5 and 3 min (Figure 4 B). Further analysis of the conditions that yielded only partial effectiveness (that is, neither growth nor sterility on all 10 plates in an isolator) showed that, irrespective of the disinfectant, test plates located on the isolator ceiling (whose surfaces were facing down) were harder to sterilize than those located on the sides or the floor (87% ± 4%, 51% ± 11%, and 18% ± 6% [mean ± SE] of the plates on the ceiling, sides, and floor, respectively, had growth under conditions of partial overall effectiveness; n = 9 experiments, P < 0.001 by Kruskal–Wallis test). However, longer fogging times generally increased disinfectant effectiveness, such that sterilization was achieved with lower concentrations of disinfectants A, B, and C at 6 min compared with 1.5 min, although longer times had little effect on the activity of hydrogen peroxide (Figure 4 B).
These results demonstrate that fogging is highly effective in sterilizing bacterial test cultures in gnotobiotic isolators in a contact time–dependent manner. However, optimal activity requires disinfectant concentrations that are markedly higher than those required for bacterial killing in suspensions tests.
Discussion
Gnotobiotic animal technology is critically dependent on reliable and effective chemical sterilization regimens for materials and equipment that cannot be sterilized by heat. A single contamination event of a gnotobiotic isolator renders the entire animal population in that isolator highly suspect or outright unsuitable for further experimentation,2,10 leading to waste of animals, resources, and personnel time. No antibiotic regimen can rescue contaminated animals and return them to a gnotobiotic state. For these reasons, systematic evaluation of best practices for preparing and maintaining sterile equipment is important for improving laboratory animal care and research integrity, yet evidence-based approaches remain the exception rather than the rule in gnotobiotic animal care. Our study makes a significant contribution in this area through the systematic analysis of different disinfectants for their effectiveness against potential microbial contaminants and utility in routine gnotobiotic animal care.
A key question for our study was which microbes to use for disinfectant testing. We reasoned that bacteria from actual contaminations would be most suitable, given that our sterilization protocol at the time apparently had failed to prevent contamination, indicating that an improved approach would have to be effective against those contaminants. In analyzing several independent contaminations, we identified a number of bacterial contaminants by using 16S ribotyping. Most were facultatively anaerobic Firmicutes, including several Paenibacillus and Bacillus species. Consistent with this observation, another report similarly discovered 2 Paenibacillus and Bacillus species in gnotobiotic isolator contaminations.32 Most of these bacteria are found in soil and on plants and are known or presumed to form endospores.12,16,20,21,26,40,49 In one contamination, we also identified M. luteus, which belongs to the phylum Actinobacteria and is an obligate aerobe associated with soil, plants, and the human skin microbiome.17 The characteristics of the contaminants do not directly allow reconstruction of the contamination routes, but they suggest that contamination occurred either via surfaces contaminated by contact with human skin (for example, hands) or unclean air or through the introduction of contaminated feed or bedding. We did not identify any anaerobes among contaminants, and no growth was ever detected under anaerobic conditions only, consistent with the assumption that contaminants presumably exist in viable form for extended periods with access to air, as might be expected for surface contaminations. However, we did not optimize the collection conditions for highly oxygen-sensitive obligate anaerobes, so it remains possible that our studies missed such anaerobes, which are common in the normal mammalian gut microbiota.6,25 Furthermore, we did not use a wide range of specialized bacterial growth media, which might be required to detect fastidious microorganisms, so such organisms might also have been overlooked in the routine sterility testing. PCR analysis of bacterial 16S rRNA might have helped in this situation, although this approach is generally less sensitive than culture methods for the detection of gnotobiotic contaminations.10
The tested disinfectants showed large (greater than 105-fold) differences in antimicrobial activity (or strength). Some of these differences can be explained by the different concentrations of the active ingredients in the disinfectant stocks. For example, concentrated hydrogen peroxide stocks contain 30% H2O2, whereas disinfectant D has only 0.5% H2O2, albeit in a proprietary accelerated form. This 60-fold difference approximately corresponds to the increased disinfectant strength and safety margin of hydrogen peroxide over disinfectant D. Similarly, disinfectant A contains 20% sodium chlorite compared with 0.85% in disinfectant B, a difference that can probably account for some of the greater than 100-fold difference in average antimicrobial activity between these disinfectants. However, additional factors likely are involved. For example, disinfectant A also contains sodium dichloroisocyanurate, which can act as a slow-release source of chlorine oxides34 and might synergize with sodium chlorite, whereas disinfectant B has no additional active ingredients listed.
Our findings indicate that chlorine-oxide–based disinfectants typically were more potent than peroxide-containing products against the vegetative forms of the test bacteria. The underlying reasons are unclear, mostly because the mechanisms of bacterial killing by chlorine oxides (such as chlorite and hypochlorite) are not well understood, although they are thought to involve a combination of cell wall and membrane disruption and macromolecular inactivation.30 By comparison, peroxides act by causing lethal oxidative damage to critical macromolecules.43 Neither class of disinfectants showed consistent differences in killing potency against the various test bacteria, suggesting that their mechanisms of bacterial killing are universal and that they therefore are likely to be similarly effective against other potential bacterial contaminants.
The chlorine-oxide– and peroxide-based disinfectants showed striking differences in regard to sporicidal activity, in that all 3 peroxides displayed greater than 80-fold loss of activity against test spores, whereas none of the chlorine oxides showed diminished activity against spores compared with the vegetative forms of the bacterial test strain. These findings are consistent with prior reports in which spores of another bacterium, Clostridium difficile, were effectively killed by sodium hypochlorite but relatively resistant to hydrogen peroxide;8 however, contrasting results, in which hydrogen peroxide was more effective than sodium hypochlorite for spore killing, have also been reported.4 These apparent discrepancies may reflect the use of different test regimens and endpoints. In addition, the prior studies had different objectives and did not directly assess disinfectant activity against spores compared with vegetative forms of the same test bacteria, as we did in the current study. In any case, it is clear from our results that potency against the vegetative forms is not predictive for sporicidal activity, underlining that spore killing likely differs mechanistically from the killing of vegetative forms.27,39
Field testing of disinfectants by aerosol fogging of gnotobiotic isolators revealed that disinfectant strength was not closely correlated with activity in suspension test cultures. The 2 chlorine-oxide–based disinfectants required more than 200-fold higher concentrations for sterilization of the agar-plated test bacteria than their minimal lethal strength against the same test bacteria in suspension cultures. This difference was less notable for the 2 peroxide-based disinfectants, but even those needed 5- to 30-fold higher disinfectant strengths for sterilization of the plated test bacteria compared with liquid cultures. A key reason for these differences may be contact time, given that bacteria in suspension cultures were exposed to disinfectants for 15 min, whereas isolator fogging times could not readily be increased beyond 6 min without generating excessive liquid on the isolator floor. The importance of contact time for disinfectant activity is well documented in the literature3,47,48 and is underlined by our observation of time-dependent increases in killing activity for fogging with the 2 chlorine oxide-based disinfectants, suggesting that a trade-off exists between maximal disinfectant activity and minimal accumulation of residual disinfectant in isolators after fogging. Other factors such as droplet size of the aerosol, extent of evaporation and gaseous diffusion, humidity, temperature, and presence of any interfering substances are likely to be additional determinants of aerosol effectiveness.14,31,41
An ideal disinfectant for gnotobiotic animal care would be maximally active against both the vegetative and spore forms of a broad range of potential contaminants with good safety margins, fast-acting, minimally corrosive for metals and plastics, ready-to-use and odor-free, and easily available at low costs. Our current results suggest that no single disinfectant has all these desirable properties and that these various characteristics must be balanced during disinfectant selection. Moreover, different gnotobiotic facilities may emphasize different features when selecting the most suitable disinfectants according to available equipment and infrastructure. In our situation, we placed emphasis on high activity against spores (which eliminated one of the peroxide-based disinfectants [disinfectant D]), minimal damage to metal and acrylic (which argued against bleach and hydrogen peroxide), and effective aerosol-mediated microbial killing. Consequently, we ordered the tested disinfectants from most to least suitable for gnotobiotic applications, with the combination of sodium chlorite and sodium dichloroisocyanurate (disinfectant A) topping the list, followed by the peroxide disinfectant product C, and sodium chlorite alone (disinfectant B). Our studies further suggest that surface disinfection alone will not prevent all microbial contaminations and that systematic exploration of sterilization procedures for other potential contamination sources, particularly food and bedding, is warranted. However, our current findings emphasize that optimal surface disinfection is an important element of gnotobiotic animal care.
For all disinfectants, human and animal exposure can be a health concern.6,11,22 A survey of publicly available toxicity information suggests that acute toxicity of the active disinfectant ingredients varies dramatically (Table 1), but a comprehensive evaluation of the occupational exposure hazards, particularly for inhalation, is not readily available. Such an evaluation would have to consider not only the various concentrations of the active ingredients in the full-strength disinfectant solutions but also exposure frequency and relevant health endpoints. For example, contact with high-level disinfectants can cause dermatitis, respiratory symptoms, irritation of ocular and nasal mucous membranes, and aversive reactions to strong odors, although their relative incidence is unclear.46 It would be advisable to minimize all exposure to disinfectants, preferably through engineering controls, such as the removal of aerosols and gases directly from the gnotobiotic isolators and ports through targeted exhaust systems. Direct exhaust can minimize or even avoid room air contamination, which would otherwise require elaborate personal respirators to filter chemical agents from contaminated room air for adequate protection of veterinary personnel.
Acknowledgments
We thank the animal care staff for their patience and cooperation, which enabled us to conduct these studies during routine animal care operations, and Dr Kent Osborn for his support and encouragement. This work was supported by NIH R25 Training Grant OD016569 and NIH grant DK120515.
References
- 1.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. 2014. The placenta harbors a unique microbiome. Sci Transl Med 6:1–22. 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Asmakh M, Zadjali F. 2015. Use of germ-free animal models in microbiota-related research. J Microbiol Biotechnol 25:1583–1588. 10.4014/jmb.1501.01039. [DOI] [PubMed] [Google Scholar]
- 3.Andersen BM, Rasch M, Hochlin K, Jensen FH, Wismar P, Fredriksen JE. 2006. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J Hosp Infect 62:149–155. 10.1016/j.jhin.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbut F, Menuet D, Verachten M, Girou E. 2009. Comparison of the efficacy of a hydrogen peroxide dry-mist disinfection system and sodium hypochlorite solution for eradication of Clostridium difficile spores. Infect Control Hosp Epidemiol 30:507–514. 10.1086/597232. [DOI] [PubMed] [Google Scholar]
- 5.Campagna MV, Faure-Kumar E, Treger JA, Cushman JD, Grogan TR, Kasahara N, Lawson GW. 2016. Factors in the selection of surface disinfectants for use in a laboratory animal setting. J Am Assoc Lab Anim Sci 55:175–188. [PMC free article] [PubMed] [Google Scholar]
- 6.Desselberger U. 2018. The mammalian intestinal microbiome: composition, interaction with the immune system, significance for vaccine effacy, and potential for disease therapy. Pathogens 7:1–13. 10.3390/pathogens7030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dumas O, Wiley AS, Quinot C, Varraso R, Zock JP, Henneberger PK, Speizer FE, Le Moual N, Camargo CA., Jr 2017. Occupational exposure to disinfectants and asthma control in US nurses. Eur Respir J 50:1–18. 10.1183/13993003.00237-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards AN, Karim ST, Pascual RA, Jowhar LM, Anderson SE, McBride SM. 2016. Chemical and stress resistances of Clostridium difficile spores and vegetative cells. Front Microbiol 7:1–13. 10.3389/fmicb.2016.01698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elbing KL, Brent R. 2018. Recipes and tools for culture of Escherichia coli. Curr Protoc Mol Biol 125:1–14. 10.1002/cpmb.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontaine CA, Skorupski AM, Vowles CJ, Anderson NE, Poe SA, Eaton KA. 2015. How free of germs is germ-free? Detection of bacterial contamination in a germ-free mouse unit. Gut Microbes 6:225–233. 10.1080/19490976.2015.1054596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu TY, Gent P, Kumar V. 2012. Efficacy, efficiency, and safety aspects of hydrogen peroxide vapour and aerosolized hydrogen peroxide room disinfection systems. J Hosp Infect 80:199–205. 10.1016/j.jhin.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Gkorezis P, Van Hamme J, Bottos E, Thijs S, Balseiro-Romero M, Monterroso C, Kidd PS, Rineau F, Weyens N, Sillen W, Vangronsveld J. 2016. Draft genome sequence of Bacillus licheniformis strain GB2, a hydrocarbon-degrading and plant growth-promoting soil bacterium. Genome Announc 4:1–2. 10.1128/genomeA.00608-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, NISC Comparative Sequencing Program. Bouffard GG, Blakesley RW, Murray PR, Green ED, Turner ML, Segre JA. 2009. Topographical and temporal diversity of the human skin microbiome. Science 324:1190–1192. 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan J, Chan M, Brooks BW, Rohonczy L. 2013. Influence of temperature and organic load on chemical disinfection of Geobacillus steareothermophilus spores, a surrogate for Bacillus anthracis. Can J Vet Res 77:100–104. [PMC free article] [PubMed] [Google Scholar]
- 15.Gustafsson BE. 1959. Lightweight stainless steel systems for rearing germfree animals. Ann N Y Acad Sci 78:17–28. 10.1111/j.1749-6632.1959.tb53092.x. [DOI] [PubMed] [Google Scholar]
- 16.Hamasaki Y, Watanabe Y, Kotoura S, Fuchu H, Sugiyama M, Hashizume K, Morita H. 2005. Paenibacillus macerans possesses 2 types of 16S rDNA copies in a genome with a length difference of 12 base-pairs. Biosci Biotechnol Biochem 69:1995–1998. 10.1271/bbb.69.1995. [DOI] [PubMed] [Google Scholar]
- 17.Hanafy RA, Couger MB, Baker K, Murphy C, O'Kane SD, Budd C, French DP, Hoff WD, Youssef N. 2016. Draft genome sequence of Micrococcus luteus strain O'Kane implicates metabolic versatility and the potential to degrade polyhydroxybutyrates. Genom Data 9:148–153. 10.1016/j.gdata.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinecke H. 1990. [[Origins of the gnotobiotic technique—Kuster, Nuttall, Schottelius, and Thierfelder]] Z Versuchstierkd 33:19–22. [Article in German].2181795 [Google Scholar]
- 19.Huesca-Espitia LC, Suvira M, Rosenbeck K, Korza G, Setlow B, Li W, Wang S, Li YQ, Setlow P. 2016. Effects of steam autoclave treatment on Geobacillus stearothermophilus spores. J Appl Microbiol 121:1300–1311. 10.1111/jam.13257. [DOI] [PubMed] [Google Scholar]
- 20.Iida K, Amako K, Takade A, Ueda Y, Yoshida S. 2007. Electron microscopic examination of the dormant spore and the sporulation of Paenibacillus motobuensis strain MC10. Microbiol Immunol 51:643–648. 10.1111/j.1348-0421.2007.tb03952.x. [DOI] [PubMed] [Google Scholar]
- 21.Iida K, Ueda Y, Kawamura Y, Ezaki T, Takade A, Yoshida S, Amako K. 2005. Paenibacillus motobuensis sp. nov., isolated from a composting machine utilizing soil from Motobu-town, Okinawa, Japan. Int J Syst Evol Microbiol 55:1811–1816. 10.1099/ijs.0.63636-0. [DOI] [PubMed] [Google Scholar]
- 22.Kaelin RM, Kapanci Y, Tschopp JM. 1988. Diffuse interstitial lung disease associated with hydrogen peroxide inhalation in a dairy worker. Am Rev Respir Dis 137:1233–1235. 10.1164/ajrccm/137.5.1233. [DOI] [PubMed] [Google Scholar]
- 23.Krause J, McDonnell G, Riedesel H. 2001. Biodecontamination of animal rooms and heat-sensitive equipment with vaporized hydrogen peroxide. Contemp Top Lab Anim Sci 40:18–21. [PubMed] [Google Scholar]
- 24.Leggett MJ, Schwarz JS, Burke PA, McDonnell G, Denyer SP, Maillard JY. 2015. Mechanism of sporicidal activity for the synergistic combination of peracetic acid and hydrogen peroxide. Appl Environ Microbiol 82:1035–1039. 10.1128/AEM.03010-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch SV, Pedersen O. 2016. The human intestinal microbiome in health and disease. N Engl J Med 375:2369–2379. 10.1056/NEJMra1600266. [DOI] [PubMed] [Google Scholar]
- 26.Madslien EH, Granum PE, Blatny JM, Lindback T. 2014. L-alanine-induced germination in Bacillus licheniformis—the impact of native gerA sequences. BMC Microbiol 14:1–10. 10.1186/1471-2180-14-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melly E, Cowan AE, Setlow P. 2002. Studies on the mechanism of killing of Bacillus subtilis spores by hydrogen peroxide. J Appl Microbiol 93:316–325. 10.1046/j.1365-2672.2002.01687.x. [DOI] [PubMed] [Google Scholar]
- 28.Nicklas W, Keubler L, Bleich A. 2015. Maintaining and monitoring the defined microbiota status of gnotobiotic rodents. ILAR J 56:241–249. 10.1093/ilar/ilv029. [DOI] [PubMed] [Google Scholar]
- 29.NIH HMP Working Group. Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, Bonazzi V, McEwen JE, Wetterstrand KA, Deal C, Baker CC, Di Francesco V, Howcroft TK, Karp RW, Lunsford RD, Wellington CR, Belachew T, Wright M, Giblin C, David H, Mills M, Salomon R, Mullins C, Akolkar B, Begg L, Davis C, Grandison L, Humble M, Khalsa J, Little AR, Peavy H, Pontzer C, Portnoy M, Sayre MH, Starke-Reed P, Zakhari S, Read J, Watson B, Guyer M. 2009. The NIH Human Microbiome Project. Genome Res 19:2317–2323. 10.1101/gr.096651.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ofori I, Maddila S, Lin J, Jonnalagadda SB. 2017. Chlorine dioxide oxidation of Escherichia coli in water—a study of the disinfection kinetics and mechanism. J Environ Sci Health A Tox Hazard Subst Environ Eng 52:598–606. 10.1080/10934529.2017.1293993 [DOI] [PubMed] [Google Scholar]
- 31.Oh SW, Gray PM, Dougherty RH, Kang DH. 2005. Aerosolization as novel sanitizer delivery system to reduce foodborne pathogens. Lett Appl Microbiol 41:56–60. 10.1111/j.1472-765X.2005.01711.x. [DOI] [PubMed] [Google Scholar]
- 32.Packey CD, Shanahan MT, Manick S, Bower MA, Ellermann M, Tonkonogy SL, Carroll IM, Sartor RB. 2013. Molecular detection of bacterial contamination in gnotobiotic rodent units. Gut Microbes 4:361–370. 10.4161/gmic.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips BP, Wolfe PA, Rees CW, Gordon HA, Wright WH, Reyniers JA. 1955. Studies on the ameba–bacteria relationship in amebiasis; comparative results of the intracecal inoculation of germfree, monocontaminated, and conventional guinea pigs with Entamoeba histolytica. Am J Trop Med Hyg 4:675–692. 10.4269/ajtmh.1955.4.675. [DOI] [PubMed] [Google Scholar]
- 34.Proto A, Zarrella I, Cucciniello R, Pironti C, De Caro F, Motta O. 2016. Bactericidal and fungicidal activity in the gas phase of sodium dichloroisocyanurate (NaDCC). Curr Microbiol 73:287–291. 10.1007/s00284-016-1040-x. [DOI] [PubMed] [Google Scholar]
- 35.Reyniers JA. 1932. The use of germfree guinea pigs in bacteriology. Proc Indiana Acad Sci 42:35–37. [Google Scholar]
- 36.Reyniers JA, Trexler PC, Ervin RF. 1946. Rearing germ-free albino rats. Lobund Rep Nov: 1–84. [PubMed] [Google Scholar]
- 37.Rutala WA, Weber DJ. 2013. Disinfection and sterilization: an overview. Am J Infect Control 41:S2–S5. 10.1016/j.ajic.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Schneider CA, Rasband WS, Eliceiri KW. 2013. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Setlow P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol 101:514–525. 10.1111/j.1365-2672.2005.02736.x. [DOI] [PubMed] [Google Scholar]
- 40.Tcherpakov M, Ben-Jacob E, Gutnick DL. 1999. Paenibacillus dendritiformis sp. nov., proposal for a new pattern-forming species and its localization within a phylogenetic cluster. Int J Syst Bacteriol 49:239–246. 10.1099/00207713-49-1-239. [DOI] [PubMed] [Google Scholar]
- 41.Teng Z, Luo Y, Alborzi S, Zhou B, Chen L, Zhang J, Zhang B, Millner P, Wang Q. 2018. Investigation on chlorine-based sanitization under stabilized conditions in the presence of organic load. Int J Food Microbiol 266:150–157. 10.1016/j.ijfoodmicro.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 42.Trexler PC, Reynolds LI. 1957. Flexible film apparatus for the rearing and use of germfree animals. Appl Microbiol 5:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vatansever F, de Melo WC, Avci P, Vecchio D, Sadasivam M, Gupta A, Chandran R, Karimi M, Parizotto NA, Yin R, Tegos GP, Hamblin MR. 2013. Antimicrobial strategies centered around reactive oxygen species—bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol Rev 37:955–989. 10.1111/1574-6976.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vohra P, Poxton IR. 2011. Efficacy of decontaminants and disinfectants against Clostridium difficile. J Med Microbiol 60:1218–1224. 10.1099/jmm.0.030288-0. [DOI] [PubMed] [Google Scholar]
- 45.Vowles CJ, Anderson NE, Eaton KA. 2016. Gnotobiotic mouse technology: an illustrated guide. Boca Raton (FL): CRC Press. [Google Scholar]
- 46.Weber DJ, Consoli SA, Rutala WA. 2016. Occupational health risks associated with the use of germicides in health care. Am J Infect Control 44:e85–e89. 10.1016/j.ajic.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 47.West AM, Teska PJ, Lineback CB, Oliver HF. 2018. Strain, disinfectant, concentration, and contact time quantitatively impact disinfectant efficacy. Antimicrob Resist Infect Control 7:1–8. 10.1186/s13756-018-0340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams DE, Worley SD, Wheatley WB, Swango LJ. 1985. Bactericidal properties of a new water disinfectant. Appl Environ Microbiol 49:637–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, Gao S, Wei DQ, Yang LL, Huang X, He J, Zhang YJ, Tang SK, Li WJ. 2012. Paenibacillus thermophilus sp. nov., a novel bacterium isolated from a sediment of hot spring in Fujian province, China. Antonie van Leeuwenhoek 102:601–609. 10.1007/s10482-012-9755-6. [DOI] [PubMed] [Google Scholar]