Abstract
In the development of cancer therapeutics, no suitable replacements for the use of animals that are capable of modeling such complex disease processes are currently available. In orthotopic models, surgery is often required to access the target organ for tumor cell inoculation. Historically analgesics have been withheld in such models in light of potential effects on tumor development. The current study evaluated the effect of the opioid buprenorphine on tumor growth of a human ovarian cancer cell line (OVCAR5 OT luc2 mCherry). Female CB17 SCID mice (n = 150) underwent surgery for orthotopic inoculation and were assigned to 1 of 3 treatment groups: vehicle control, 1 dose of buprenorphine, or 2 doses of buprenorphine administered perioperatively. Bioluminescence imaging revealed no significant difference on tumor engraftment rate or growth between control and analgesia-treated groups. These data demonstrate that acute, perioperative analgesia with buprenorphine did not alter tumor growth. Although further research is needed to evaluate potential effects of buprenorphine in other cell lines and mouse strains, the justification for withholding analgesia and the potential influence of pain and stress due to insufficient analgesia in these models should be considered thoroughly.
The search for efficacious anticancer drugs is complex and involves measurement of several parameters that are available only by using a live animal model. Although experimental cancer compounds generally are first tested in one or more in vitro assays, live animals are often used to assess anticancer compound efficacy, because advanced surrogate in vitro assays that mirror in vivo therapeutic indices have not yet been developed. Currently, the only way to effectively study the intricacies of human tumor xenografts in vivo is through the use of immune-compromised animals, generally mice. An intact animal is necessary for evaluating local and distant metastases as well as for assessing compounds dependent on different routes of administration. These complex interactions require multiple organ systems to be in place and operational, and can only be achieved in living animals.
Orthotopic cancer models are commonly used to study tumor microenvironment interactions as well as distant metastases. Such models often involve making a small incision in the skin and muscle layers over the target organ where that tumor normally occurs, to achieve access for tumor cell inoculation. Although laparotomy has been suggested to induce postoperative pain and hyperalgesia,4,16-18,21,26,31 the potential effects of perioperative analgesic agents on preclinical models of cancer remain a concern. Numerous publications demonstrate that increased, repeated doses of NSAID33 and even opioids3,11 can affect tumor cell growth both in vitro and in vivo. The literature lacks scientific evidence regarding the evaluation of how a decreased analgesic dose, given once or twice perioperatively, might (or might not) alter tumor growth in animal models of cancer. We, therefore, designed an experiment to address this concern.
Potent centrally acting analgesic agents are used postoperatively in humans, and most pain-relieving agents in current use are natural opiates or synthetic morphine congeners. Opioid receptor expression is not limited to nervous tissue and has been found in multiple cell types, including tumor and tumor-associated cells.6 Documented effects of opioids on tumor cells include modulation of apoptosis,6,32 antiproliferative activity,25 invasion,12 adhesion,8,13 metastasis,13 the immune system,9,23 and angiogenesis11,29 across multiple indications and models. Opioids both inhibit22 and promote13,19 tumor activity at various stages of progression. At clinically relevant doses, opioids promote neovascularization in human breast tumor xenografts in mice, leading to progression.11 Although it is important to examine the influence of buprenorphine on tumor growth, the potential effects of unrelieved postoperative pain and surgical stress14 must also be considered. Surgical pain, stress, and anesthesia all influence immunosuppression, inflammation, and sympathetic stimulation and should be considered in preclinical cancer models, because they are clinically relevant factors.10,20,28,30
Traditionally, postoperative observations of an animal's appearance, posture, and behavior have served as reliable indicators of health status in rodents. As prey animals, mice instinctively mask overt signs of weakness, injury, or pain to avoid attracting the attention of predators,4 thus making postoperative pain and discomfort difficult to assess. Regardless of the challenges in assessing postoperative pain in rodents, the American College of Laboratory Animal Medicine's Guideline for the Assessment and Management of Pain in Rodents and Rabbits places laparotomy in the moderate to severe pain category, where it “requires the appropriate use of pain-relieving measures unless scientifically justified in an approved animal care and use protocol.”2 Furthermore, the Guide for the Care and Use of Laboratory Animals clearly highlights the following in its section on Pain and Distress: “The US Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training (see Appendix B) state that in general, unless the contrary is known or established, it should be considered that procedures that cause pain in humans may also cause pain in other animals (IRAC 1985).”14 According to the Guide, “pain is a stressor, and if not relieved, can lead to unacceptable levels of stress and distress in animals.”14 Clearly, the provision of analgesia supports an effort to focus on the refinement aspect of the 3Rs principles. From an ethical standpoint, there is a need for more research into the effects of analgesics in our scientific models, because it is becoming increasingly difficult to justify withholding of analgesia. In the current study, we sought to evaluate the effects of administering the potent opioid buprenorphine on tumor growth in an orthotopic setting using the OVCAR5 OT (luc2 mCherry, LMC) cell line derived from a human ovarian carcinoma.
Materials and Methods
Animals, husbandry, and welfare.
All animal studies were reviewed and approved by AbbVie's IACUC and were conducted in an AAALAC-accredited program; veterinary care was available to ensure appropriate animal care. Female CB17 SCID mice (age, 7 to 8 wk) were obtained from Charles River (Wilmington, MA) and housed at a maximum of 10 per cage on autoclaved Sani-Chips bedding (PJ Murphy Forest Products, Ladysmith, WI) in 20.5 cm ×43.2 cm ×15.2 cm polycarbonate microisolation caging. Autoclaved food (2018X, Envigo, Indianapolis, IN) and water were available without restriction. Room temperature and humidity were maintained according to the Guide standards (21 to 22 °C, humidity of 45% to 55%). Mice were provided an acclimation period of at least 1 wk prior to commencement of experiments and were housed in a room on a 12:12-h light:dark cycle.
Cell lines and culture conditions.
The human ovarian carcinoma cell line OVCAR5 OT LMC (derived from the parent cell line OVCAR5 [NCI] and authenticated by using short tandem repeat DNA profiling and harboring a dual-reporter fusion construct) has previously been described1 and was used in the current study. Cells were maintained at 37 °C and 5% CO2 in RPMI media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum (Hyclone, Logan, UT). A fusion construct of luc2 (Promega, Madison, WI) and mCherry (Clontech, Mountain View, CA) was cloned into the Lenti-X lentiviral vector (Clontech). Cells were transduced with lentiviral particles for 48 h, and a pool of cells stably expressing the fusion construct was selected by using 2 µg/mL puromycin for 2 wk.
Compounds, formulation, and administration.
Buprenorphine is a semisynthetic, highly lipophilic opioid derived from thebaine. Buprenorphine is approximately 25 to 50 times more potent than morphine.15 Compared with morphine, buprenorphine dissociates very slowly from μ-opioid receptors. The half-life for dissociation is approximately 166 min;8 therefore, clinical effects of buprenorphine usually do not parallel measured levels in plasma. Buprenorphine (Reckitt Benckiser Pharmaceuticals, Richmond, VA) was formulated in physiologic saline at a dose of 0.05 mg/kg and administered subcutaneously 30 min to 1 h before surgery (single-dose group) or at 30 min to 1 h before surgery and 24 h postoperatively (2-dose group).
Surgical orthotopic implantation of tumor cells and postoperative monitoring and care.
Mice were anesthetized with inhalation anesthesia (Sevoflurane, Patterson Veterinary Supply, Charlotte, NY) by using 2% to 3% for induction and 1% to 3% for maintenance. After loss of righting reflex, the abdomen of the mouse was shaved and prepped with povidone–iodine scrub (Betadine swabs and applicators, Emerson Healthcare, Wayne, PA), followed by an isopropyl alcohol wash. Lubricating ophthalmic ointment was applied to the eyes for protection. After a surgical plane of anesthesia was confirmed through absence of response to a toe pinch, a small incision was made through both the skin and muscle layers over the left renal area by using aseptic technique. The left ovary was located and exteriorized. While the ovary was supported, 20 μL of tumor cell suspension (25,000 cells/μL) was injected into the ovarian bursa by using a 0.3-mL insulin syringe with a 31-gauge needle (Becton Dickenson, Franklin Lakes, NJ). After inoculation, the muscle and skin layers were closed by using 4-0 polyglycolic acid suture (Ethicon, Somerville, NJ) in a continuous cross pattern. All surgeries were performed by the same surgeon. Mice were allowed to fully recover postoperatively after placement back in their home cage. The home cage was preplaced on a heated platform (Shor-line Thermal Pads, Kansas City, KS), where it remained for the 24-h recovery period. The heat was turned off before personnel left for the night, and mice remained in the postoperative recovery suite where they were closely monitored for the first 24 h after surgery. Overnight video recording of animals was performed by using a security camera DVR system (model DM-DV-2416P, DVRMaster, Northridge, CA) with analog cameras (model CMR601 480TVL, Sony, Tokyo, Japan), for monitoring of animals and to understand any postoperative deaths.
In vivo bioluminescence imaging.
Mice were randomly assigned to treatment groups and cages, and although treatment group was not identifiable on the cage cards, due to the experimental design of mice being implanted with or without buprenorphine on day 0, the study was not completely randomized or blinded. Imaging to determine tumor burden began 1 wk after inoculation. Groups of 10 mice were imaged concurrently, with data analysis at a later time. All mice were imaged once weekly thereafter to determine photon flux from each animal. Bioluminescence imaging studies were conducted by using an IVIS Spectrum Imaging System (Caliper Life Sciences, Mountain View, CA). Each mouse was first injected intraperitoneally with 0.2 mL of 15 mg/mL d-luciferin (Promega, Madison, WI) in sterile PBS according to the manufacturer's protocol and then was placed in the imaging system's anesthesia induction chamber, which contained 1.5% to 2.0% isoflurane (Baxter Healthcare, Deerfield, IL) in medical-grade oxygen, delivered at a rate of 2.0 L/min. Animals were then immediately transferred to the system's heated specimen stage to await imaging. Imaging began at 10 min after luciferin administration, when each mouse's average bioluminescence was previously found to reach maximal intensity. Postacquisition image analyses were performed by using Caliper Life Sciences’ Living Image software (version 4.3.1) and graphed to obtain absolute photon fluences (photons/s/steradian/cm2) in well-defined regions of interest.
Experimental design.
Female SCID mice were orthotopically inoculated with 0.5 × 106 OVCAR5 OT LMC cells per mouse. Each week for 5 wk, 30 mice were inoculated and assigned by injection order into 1 of 3 groups of 10 mice each. Each experimental group was designed to include a minimum of 50 tumor-bearing mice. The 3 main experimental groups consisted of 1) animals treated with vehicle control (saline, administered at the volume as the buprenorphine) 1 h prior to surgery, 2) animals treated with buprenorphine at 30 min to 1 h prior to surgery, and 3) animals treated with buprenorphine 30 min to 1 h prior to surgery and at 24 h after surgery. Mice were randomly assigned to cages before surgery. Due to deaths that occurred in the first group of treated mice, the timing of the buprenorphine (and vehicle) was changed to be given 30 min prior to surgery in an effort to increase survivability and improve outcome as the mice recovered from surgery.
Statistical methods and data analysis.
The assignment of 50 mice per group provided for at least 80% power by using a one-sided t-test to detect a 35% increase in comparing the tumor growth rate (under the log 10 scale) of treated compared with control groups at an a priori α of 0.05 (based on an estimated variance of 0.067). For each group of 50 mice, the following parameters were assessed at the end of the experiment: tumor ‘take rate,’ rate of tumor growth, and time to tumor endpoint. Take rate is defined as the percentage of animals retaining tumor compared with the total number of animals inoculated. Tumor growth (under a log10 scale) over time was analyzed and compared between treatments and the control group by using linear mixed-effect models. Endpoint was defined for each animal as reaching 3 × 109 photons/s by using the whole-body region-of-interest analysis or when animals demonstrated distress in overall health. This bioluminescent value was determined empirically in a previous study and precedes animal morbidity. Death was not an intended endpoint, and humane endpoints (for example, body weight loss of at least 20%, tumor size of 2 to 3 g [assuming a 20-g mouse]) were used and guided by the veterinary staff to minimize pain and distress. Animals were assessed daily for clinical signs of hunched posture, rough hair coat, decreased activity, weight loss, and ataxia. Tumor growth curves were plotted to day 28; this day corresponds to when approximately 50% of the mice in each group reached the endpoint of 3 × 109 photons/s.
Tumor growth (under a log10 scale) over time were analyzed and compared between treatments and the control group through fitting linear mixed-effect models with subjects as the random effect; treatment group, categorical time points, and the interaction between treatment group and time points as fixed effects; without baseline covariate and no cohort adjustment; and compound symmetry structure as the covariance matrix. The significance between treatment groups was tested according to the effect of the interaction between treatment group and time points. This analysis was performed by using PROC GLIMMIX in SAS version 9.4 (SAS Institute, Cary, NC).
Time-to-event analyses (GraphPad Software, La Jolla, CA) were performed to compare differences between treatment groups by using log-rank test on the endpoint of time to death over the course of the model and beyond the 72-h postoperative period.
Results
Tumor take rates, growth rates, and endpoint analysis.
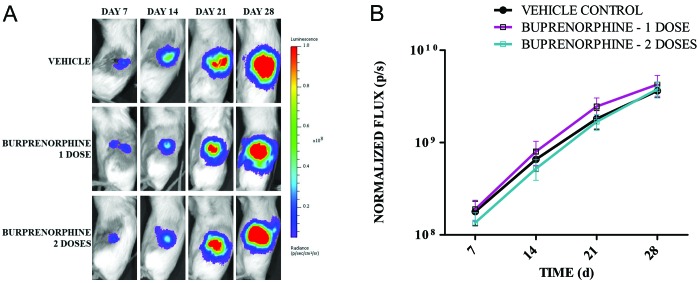
The tumor take rates were 100% for all experimental groups. As expected for this model, tumor growth rates increased over time in all groups (F3,400 = 644.21, P < 0.001; Figure 1), and there was no difference (F2,136 = 1.13, P = 0.36) in the tumor growth rate between control and analgesia-treated groups. There was no significant interaction of group and time (F6,400 = 0.27, P = 0.95).
Figure 1.
Buprenorphine does not significantly affect engraftment or growth of OVCAR-5 OT LMC cells implanted in ovary. A) Representative longitudinal bioluminescent images of an individual mouse across all 3 treatment groups. All image thresholds are set to the same scale to facilitate comparison. B) Bioluminescent images of each group (n = 50 mice per group) were acquired weekly after surgical implantation of OVCAR-5 OT LMC cells. Average normalized flux (photons/s) ± 95% CI is shown for each group.
Survival analysis.
Unexpected deaths occurred postoperatively 24 to 48 h after surgery. Using one-sided t-test to compare the proportion of surgical death between groups, we evaluated these deaths to gain insight into whether they might be related to analgesia treatment or to some aspect of perioperative or postoperative care. was used. Only 1 vehicle-treated mouse had a surgery-related death (Figure 2). By comparison, there were 4 deaths in mice treated with 1 dose of buprenorphine (1 h prior to surgery; P = 0.18), and 4 deaths in mice treated with 2 doses of buprenorphine (1 h prior to surgery and 24 h after surgery; P = 0.18), for a total of 8 deaths in animals treated with buprenorphine compared with only 1 death in vehicle-treated mice (P = 0.14). In addition, there were no differences between groups in survival over the course of the model or beyond the 72-h postoperative period (log-rank test, χ22 = 2.18; P = 0.34; Figure 2).
Figure 2.
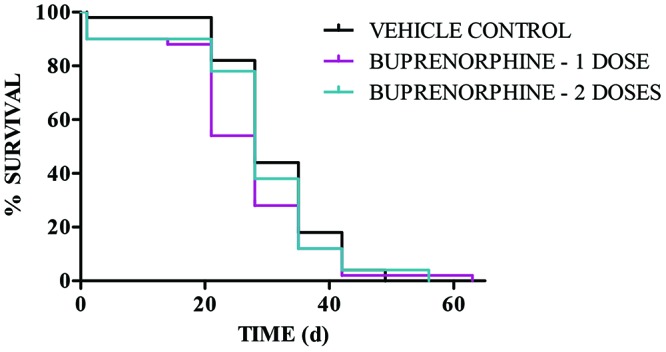
Buprenorphine does not significantly affect overall animal survival. This Kaplan–Meier plot shows percentage survival according to treatment group (n = 50 mice per group) compared with time. The endpoint for each animal was defined as reaching 3 × 109 photons/s or when animals demonstrated distress in animal health. Within 24 to 48 h after implantation, 1 vehicle-treated mouse had a surgery-related death, whereas there were 4 deaths in mice treated with 1 dose of buprenorphine and 4 deaths in mice treated with 2 doses of buprenorphine (yielding a total of 8 deaths in animals treated with buprenorphine compared with 1 death among vehicle-treated mice). Importantly, there is no statistical difference between groups.
Discussion
In our study, buprenorphine—when given perioperatively in 1 or 2 doses—had no effect on tumor growth rate. In fact, tumor growth rates increased over time in all groups, supporting the use of analgesia for postoperative pain in orthotopic models. However, we do suggest moving forward thoughtfully, because the use of analgesia in tumor models requiring surgery may involve performing studies to assess effects on the model (that is, various tumor cell lines and mouse strains may yield different results). However, we believe any influence will be minimal, because analgesics are provided for only short durations perioperatively.
The effects of buprenorphine on tumor growth and progression are proposed to be dependent on the expression of opioid receptors in the cell line being investigated. Endogenous opiates are expressed in tumor stroma and possibly exert a regulatory effect on adjacent tumor cells that express opioid receptors.7 The effect (or lack thereof) of the addition of an exogenous opioid on tumor cells, especially during the initial time period after tumor cell inoculation, is unknown. The protein expression and function of the opioid receptor subtypes in the OVCAR5 OT LMC cell line in vitro and in vivo are unknown as well.
Unfortunately, we experienced morbidity issues in mice treated with buprenorphine, all of which were presumed to center around maintaining body temperature. The aseptic scrub applications used during surgical prep27 can decrease body temperature in rodents. Fortunately, we quickly found solution-focused treatments and were able to improve outcome. In an effort to maintain mouse body temperature, everything that might cause hypothermia was minimized, such as reducing the body surface area that was washed, replacing warm saline for surgical prep, and using thermostatically controlled platforms to keep mice warm for at least 2 h after surgery. In addition, because of the deaths that occurred in the first group of treated mice, the administration of buprenorphine was changed to be given 30 min prior to surgery, rather than 1 h, in an effort to increase survivability as the mice recovered from surgery. Although postsurgical deaths still occurred after implementing these changes, they were more sporadic. Ultimately, although we had more total deaths in the buprenorphine-treated mice, statistical analysis revealed no significant difference for any treated group. There were 9 total deaths among 150 surgically implanted mice, which is a very low frequency, but the apparent differences between the vehicle- and buprenorphine-treated animals concerned us. After the current study, we also incorporated the use of heating blankets during surgery to further improve outcomes.
The American College of Laboratory Animal Medicine guideline categorizes minor laparotomy incisions in the mild to moderate pain category,2 and they recommend the administration of buprenorphine every 8 to 12 h at a dose of 0.05 to 0.1 mg/kg SC for surgeries like our orthotopic laparotomy. In addition, the guideline clarifies that buprenorphine should not be used alone for surgical procedures that are likely to cause more than moderate pain; multimodal analgesia should be used for surgeries associated with severe pain to ensure optimal pain relief.2 In light of these recommendations and in response to the current experiment, our IACUC now requires at least one dose of buprenorphine as perioperative analgesia in all mouse orthotopic tumor models, regardless of tumor cell line. More research is needed to determine the most appropriate analgesic regimen for this procedure in mice. In addition, another group found that one dose of the NSAID meloxicam, but not buprenorphine, significantly affected tumor seeding.20 Therefore, attention should be given to the analgesics used, the specific tumor type, and literature available, when considering multimodal analgesia.
In addition, the potential effects of unrelieved postoperative pain and surgical stress should weighed.5,9,24 Rather, although it is important to examine the influence of the analgesic on the model, it is also important to consider the effects of surgical stress and immunosuppression due to unrelieved pain and distress. Perhaps better understanding the variability introduced as a result of surgical stress (such as, unrelieved postoperative pain) would yield a more reliable model. Clinically, growth of both preexisting and new metastases has been associated with changes in both stress hormones and prostaglandins resulting from surgery, making surgery alone a risk factor.5 Both decreased NK cell activity and increased tumor metastasis have been associated with surgical stress in rats.8,9 Furthermore, surgery can increase the retention of tumor cells in rats, and both fentanyl and bupivacaine–morphine analgesia decreased this retention.24 In another study, surgery resulted in both increased mammary tumor growth and lung metastases in mice, with buprenorphine, administered preoperatively, reducing such growth.20 Furthermore, buprenorphine has been shown to prevent neuroendocrine activation, immunosuppression, and increased tumor growth induced by surgical stress.9 In light of seeking improved translation from preclinical to clinical modeling, we must consider the number of patients in clinical trials that are on some type of analgesic for cancer or surgery-related pain and the clinical relevance of provision of analgesia in our preclinical models. Such considerations highlight the need for better understanding of both treated and untreated postoperative pain as well as the effects of opioids, particularly as they relate to the quantitative and qualitative effects on tumor progression.
It would be of interest to follow the data after implementation of analgesia to assess whether variability in the model has changed. Just as the human response to pain is variable, we presume the same is true for animals, leading us to question whether analgesia would help to normalize the model with regard to pain and distress. Future studies should objectively evaluate the impact of perioperative analgesia in various surgical cancer models.
With the current data, we have demonstrated 1 or 2 doses of buprenorphine at 0.05 mg/kg had no effect on tumor growth by the OVCAR5 OT LMC cell line in female CB17 SCID mice. Recommendations for analgesia are clear, and we have insufficient justification for withholding analgesia. In general, perioperative analgesia should be considered, given that the animals are treated for such a short period of time. In addition, we must question the effects of withholding analgesia, specifically whether the potential influence and variability introduced by stress and pain due to insufficient analgesia may have greater ramification than the influence of 1 or 2 doses of an opioid analgesic. Given that more deaths occurred in mice treated with buprenorphine, the significance of maintaining body temperature during the perioperative period is particularly important. This study provides an exciting contribution to the area of the 3Rs as a refinement to analgesia, which could have broad applicability across many orthotopic models. Certainly more research is needed in this area, and we look forward to seeing more publications that contribute to this welfare issue at large.
Acknowledgments
We thank AbbVie's In Vivo Oncology Biology, Exploratory Statistics, and Comparative Medicine teams that supported this research.
Disclosures
All authors are employees of AbbVie. The design, study conduct, and financial support for this research was provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the publication.
References
- 1.Ackler S, Oleksijew A, Chen J, Chyla BJ, Clarin J, Foster K, McGonigal T, Mishra S, Schlessinger S, Smith M, Tahir S, Leverson J, Souers A, Boghaert E, Hickson J. 2015. Clearance of systemic hematologic tumors by venetoclax (ABT-199) and navitoclax. Pharmacol Res Perspect 3:1–16. 10.1002/prp2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.ACLAM Task Force Members. Kohn DF, Martin TE, Foley PL, Morris TH, Swindle MM, Vogler GA, Wixson SK. 2007. Public Statement guidelines for the assessment and management of pain in rodents and rabbits. J Am Assoc Lab Anim Sci 46:97–108. [PubMed] [Google Scholar]
- 3.Afsharimani B, Cabot P, Parat MO. 2011. Morphine and tumor growth and metastasis. Cancer Metastasis Rev 30:225–238. 10.1007/s10555-011-9285-0. [DOI] [PubMed] [Google Scholar]
- 4.Arras M, Rettich A, Cinelli P, Kasermann H, Burki K. 2007. Assessment of post-laparotomy pain in laboratory mice by telemetric recording or heart rate and heart rate variability. BMC Vet Res 3:1–10. 10.1186/1746-6148-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benish M, Ben-Eliyahu S. 2010. Surgery as a double-edged sword: a clinically feasible approach to overcome the metastatis-promoting effects of surgery by blunting stress and prostaglandin responses. Cancers (Basel) 2:1929–1951. 10.3390/cancers2041929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cecchi M, Capriles N, Watson SJ, Akil H. 2008. Differential responses to morphine-induced analgesia in the tail-flick test. Behav Brain Res 194:146–151. 10.1016/j.bbr.2008.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatikhine VA, Chevrier A, Chauzy C, Duval C, d'Anjou J, Girard N, Delpech B. 1994. Expression of opioid peptides in cells and stroma of human breast cancer and adenofibromas. Cancer Lett 77:51–56. 10.1016/0304-3835(94)90347-6. [DOI] [PubMed] [Google Scholar]
- 8.Debruyne DJ, Mareel MM, Bracke ME. 2010. Opioids affect focal contact-mediated cell—substrate adhesion. Eur J Cancer Prev 19:227–238. 10.1097/CEJ.0b013e328338770c. [DOI] [PubMed] [Google Scholar]
- 9.Franchi S, Panerai AE, Sacerdote P. 2007. Buprenorphine ameliorates the effect of surgery on hypothalamus—pituitary—adrenal axis, natural killer cell activity and metastatic colonization in rats in comparison with morphine or fentanyl treatment. Brain Behav Immun 21:767–774. 10.1016/j.bbi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Gottschalk A, Sharma S, Ford J, Durieux ME, Tiouririne M. 2010. The role of the perioperative period in recurrence after cancer surgery. Anesth Analg 110:1636–1643. 10.1213/ANE.0b013e3181de0ab6. [DOI] [PubMed] [Google Scholar]
- 11.Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. 2002. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res 62:4491–4498. [PubMed] [Google Scholar]
- 12.Harimaya Y, Koizumi K, Andoh T, Nojima H, Kuraishi Y, Saiki I. 2002. Potential ability of morphine to inhibit the adhesion, invasion and metastasis of metastatic colon 26-L5 carcinoma cells. Cancer Lett 187:121–127. 10.1016/S0304-3835(02)00360-9. [DOI] [PubMed] [Google Scholar]
- 13.Heaney A, Buggy DJ. 2012. Can anaesthetic and analgesic techniques affect cancer recurrence or metastasis? Br J Anaesth 109 Suppl 1:i17–i28. 10.1093/bja/aes421. [DOI] [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 15.Jasinski DR, Pevnick JS, Griffith JD. 1978. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry 35:501–516. 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- 16.Jirkof P, Cesarovic N, Rettich A, Nicholls F, Seifert B, Arras M. 2010. Burrowing behavior as an indicator of post-laparotomy pain in mice. Front Behav Neurosci 4:1–9. 10.3389/fnbeh.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liles JH, Flecknell PA. 1993. The influence of buprenorphine or bupivacaine on the postoperative effects of laparotomy and bile-duct ligation in rats. Lab Anim 27:374–380. 10.1258/002367793780745552. [DOI] [PubMed] [Google Scholar]
- 18.Liles JH, Flecknell PA, Roughan J, Cruz-Madorran I. 1998. Influence of oral buprenorphine, oral naltrexone or morphine on the effects of laparotomy in the rat. Lab Anim 32:149–161. 10.1258/002367798780600025. [DOI] [PubMed] [Google Scholar]
- 19.Lin X, Wang YJ, Li Q, Hou YY, Hong MH, Cao YL, Chi ZQ, Liu JG. 2009. Chronic high-dose morphine treatment promotes SH-SY5Y cell apoptosis via c-Jun N-terminal kinase-mediated activation of mitochondria-dependent pathway. FEBS J 276:2022–2036. 10.1111/j.1742-4658.2009.06938.x. [DOI] [PubMed] [Google Scholar]
- 20.Lofgren J, Miller AL, Lee CCS, Bradshaw C, Flecknell P, Roughan J. 2018. Analgesics promote welfare and sustain tumor growth in orthotopic 4T1 and B16 mouse cancer models. Lab Anim 52:351–364. 10.1177/0023677217739934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumiya LC, Sorge RE, Sotocinal SG, Tabaka JM, Wieskopf JS, Zaloum A, King OD, Mogil JS. 2012. Using the mouse grimace scale to reevaluate the efficacy of postoperative analgesics in laboratory mice. J Am Assoc Lab Anim Sci 51:42–49. [PMC free article] [PubMed] [Google Scholar]
- 22.Mathew B, Lennon FE, Siegler J, Mirzapoiazova T, Mambetsariev N, Sammani S, Gerhold LM, LaRiviere PJ, Chen CT, Garcia JG, Salgia R, Moss J, Singleton PA. 2011. The novel role of the mµ opioid receptor in lung cancer progression: a laboratory investigation. Anesth Analg 112:558–567. 10.1213/ANE.0b013e31820568af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mathews PM, Froelich CJ, Sibbitt WL, Jr, Bankhurst AD. 1983. Enhancement of natural cytotoxicity by β-endorphin. J Immunol 130:1658–1662. [PubMed] [Google Scholar]
- 24.Page GG, Blakely WP, Ben-Eliyahu S. 2001. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain 90:191–199. 10.1016/S0304-3959(00)00403-6. [DOI] [PubMed] [Google Scholar]
- 25.Panagiotou S, Bakogeorgou E, Papakonstanti E, Hatzoglou A, Wallet F, Dussert C, Stournaras C, Martin PM, Castanas E. 1999. Opioid agonists modify breast cancer cell proliferation by blocking cells to the G2/M phase of the cycle: involvement of cytoskeletal elements. J Cell Biochem 73:204–211. . [DOI] [PubMed] [Google Scholar]
- 26.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating postoperative pain in animals. Lab Anim 36:322–343. 10.1258/002367702320162423. [DOI] [PubMed] [Google Scholar]
- 27.Skorupski AM, Zhang J, Ferguson D, Lawrence F, Hankenson FC. 2017. Quantification of induced hypothermia from aseptic scrub applications during rodent surgery preparation. J Am Assoc Lab Anim Sci 56:562–569. [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder GL, Greenberg S. 2010. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth 105:106–115. 10.1093/bja/aeq164. [DOI] [PubMed] [Google Scholar]
- 29.Ustun F, Durmus-Altun G, Altaner S, Tuncbilek N, Uzal C, Berkarda S. 2010. Evaluation of morphine effect on tumor angiogenesis in mouse breast tumor model, EATC. Med Oncol 28:1264–1272. 10.1007/s12032-010-9573-5. [DOI] [PubMed] [Google Scholar]
- 30.Wallenstein SL, Kaiko RF, Rogers AG, Houde RW. 1986. Crossover trials in clinical analgesic assays: studies of buprenorphine and morphine. Pharmacotherapy 6:228–235. 10.1002/j.1875-9114.1986.tb03481.x. [DOI] [PubMed] [Google Scholar]
- 31.Wright-Williams SL, Courade JP, Richardson CA, Roughan JV, Flecknell PA. 2007. Effects of vasectomy surgery and meloxicam treatment on faecal corticosterone levels and behavior in 2 strains of laboratory mouse. Pain 130:108–118. 10.1016/j.pain.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Zhao M, Zhou G, Zhang Y, Chen T, Sun X, Stuart C, Hanley G, Li J, Zhang J, Yin D. 2009. β-arrestin 2 inhibits opioid-induced breast cancer cell death through Akt and caspase-8 pathways. Neoplasma 56:108–113. 10.4149/neo_2009_02_108. [DOI] [PubMed] [Google Scholar]
- 33.Xin B, Yokoyama Y, Shigeto T, Mizunuma H. 2007. Antitumor effect of nonsteroidal anti-inflammatory drugs on human ovarian cancers. Pathol Oncol Res 13:365–369. 10.1007/BF02940318. [DOI] [PubMed] [Google Scholar]