Abstract
A significant concern in laboratory animal medicine is contamination due to pathogen outbreaks and how to adequately decontaminate small equipment. Many factors play a role in the selection of the decontamination method including cost, efficacy, personnel time and safety. Chlorine dioxide (ClO2) gas is an effective method, but decontamination often requires a ClO2 gas generator with a specialized air-tight exposure chamber. Although this method works well for large-scale decontamination, the use of a gas generator may be impractical and too costly for smaller-scale decontamination. The goal of this study was to create and validate an effective, small-scale decontamination method that uses ClO2 gas and which is an affordable, efficient, safe, and reproducible. First, we identified a product that generates ClO2 gas after the combination of 2 dry reagents. To find an affordable exposure chamber, we evaluated the ability of 4 household totes with gasket-seal lid systems to retain ClO2 gas and relative humidity (RH). The efficacy of decontamination was validated by concurrently using 2 different biologic indicators (BI), Bacillus atrophaeus (B.a.) and Geobacillus stearothermophilus (G.s.). All household totes evaluated held sufficient gas and RH for a 15-h cycle, providing adequate contact time to inactivate both BI evaluated. Our results suggest that a total exposure dose of 71 ± 42 ppm-h of ClO2 gas over 15 h at 90% or greater RH is adequate to inactivate both B.a. and G.s. There was no statistical significance between the 2 BI as indicators for decontamination; 65 of 230 (28.3%) B.a. and 75 of 230 (32.6%) G.s spore strips were positive for growth (P = 0.36). In conclusion, we successfully combined a variety of low-cost materials to establish an effective, small-scale method to decontaminate laboratory equipment. Depending on the size of the tote and whether BI are used, the cost of our method is roughly 1% that of large-scale ClO2 gas generators used with specialized air-tight exposure chambers.
Abbreviations: Ba., Bacillus atrophaeus; BI, biologic indicator; EMS, environmental monitoring system; G.s., Geobacillus stearothermophilus; RH, relative humidity; SLE, small lab equipment
After a rodent pathogen outbreak, laboratory equipment must be decontaminated or discarded to prevent reintroduction of the pathogenic organism. Because discarding a variety of research equipment and supplies can be costly, decontamination is preferred. Due to the amount of material that often is involved, manual cleaning of these small items by using a liquid disinfectant is labor-intensive and can be inconsistent between personnel.3,12 As an adjunct to manual cleaning or as the sole method, gaseous decontamination has proven to be very effective against a broad range of microorganisms.6,11,15,30,31,33,44 Common gases used for decontamination include ClO2, ethylene oxide, paraformaldehyde, hydrogen peroxide, glutaraldehyde, and peracetic acid. Comparisons and the limitations of these methods have previously been published.5,6,12
ClO2 gas was registered as a disinfectant by the US Environmental Protection Agency in 198840 and has since become popular in the healthcare industry and biomedical research.1,2,4,6,13,17,18,36,37 ClO2 is a potent oxidizer and disrupts bacterial cell walls and damages viral capsid proteins and viral RNA.33,36 However, several factors markedly influence the efficacy of ClO2 gas. The effectiveness of ClO2 gas as a decontaminant is directly correlated to relative humidity (RH) and contact time.14,19,25,35,42,44 In studies assessing the inactivation of biologic indicators (BI) at low humidity (that is, less than 50%) compared with high humidity (that is, greater than 50%), high humidity enhanced the antimicrobial effect of ClO2 gas.25,27,29,35,42,44 A 2016 study demonstrated a synergistic effect in the inactivation of spores regarding ClO2 gas concentration with increased RH and contact time.42 Therefore, these 2 factors of RH and contact time can be manipulated to improve the efficacy of ClO2 gas decontamination.
ClO2 gas decontamination typically is performed by using large generators in areas such as office buildings, vehicles, and hospital rooms.17,18,20,22,23,35 These commercially available generators can also be used for the decontamination of biologic safety cabinets and— with specifically designed airtight chambers—even smaller research equipment.4-6,23 Because many of these areas are essential to daily operations, the decontamination process is ideally completed rapidly, with most reports ranging from 15 s to 9 h.2,6,20,33 To achieve rapid decontamination, high concentrations of ClO2 gas, such as 5 to 30 mg/L (1800 to 10,800 ppm), are often necessary.4,6,18,20,22,23 However, for small-scale applications, the use of large ClO2 gas generators and specialized airtight exposure chambers is not always practical, and prolonged or overnight turnaround times often can be acceptable. Furthermore, although using high concentrations of ClO2 gas can be very effective, it also can be an occupational health concern.5,38,40
The objective of the current study was to establish and validate a decontamination method using ClO2 gas that is affordable, efficient, safe, and reproducible. We hypothesized that household totes with gasket-seal lid systems would maintain ClO2 gas concentrations and RH sufficient for use as decontamination exposure chambers. Knowing that ClO2 gas efficacy is directly correlated to higher RH and longer contact time, we established a standard RH greater than 90% and an overnight (15 h) contact (cycle) time to maximize effectiveness. To validate decontamination, we used 2 endospore BI, Bacillus atrophaeus (B.a.) or Geobacillus stearothermophilus (G.s.) which have been used in previous studies for decontamination validation with ClO2 gas. 21-23To minimize the potential for personnel exposure, we examined ClO2 gas decontamination efficacy and BI reliability at low doses. However, because questions still remain regarding the consistency and variability of inactivation when these 2 BI are tested congruently,21,23 we hypothesized that B.a. would be inactivated more consistently than G.s. for validation of decontamination.
Materials and Methods
ClO2 gas generation.
In this study, we used Stayfresh Wipeout (ICA, TriNova, Newnan, GA), which consists of 2 powdered reagents (A and B) that—when mixed together—immediately begin to generate ClO2 gas (5 NaClO2 + 4 HCl → 4 ClO2 + 5 NaCl + 2 H2O). Equal quantities of both reagents were weighed on an analytical scale and placed into separate 50-mL conical tubes. The reagents were then combined in a single 50-mL tube and shaken vigorously for 30 s. The mixed reagents were poured into a Tyvek sachet, which was placed in a tote. According to information supplied by the vendor, the ClO2 gas concentration in the tote should peak at 4 to 7 h and then decrease over the 15-h cycle. Because reagents A and B were always combined in equal quantities, all references to quantities refer to total product weight (that is, 0.50 g of reagent A + 0.50 g of reagent B = 1.0 g of total product weight). All test replicates of ClO2 gas decontamination were performed in a laboratory fume hood (Protector, Labconco, Kansas City, MO) which is certified annually and has a face-level air velocity of 121 ft/min.
ClO2 exposure chambers and gas detection.
For exposure chambers, commercially available household totes with gasket-seal lid systems were purchased at common retail stores. The totes investigated were a 30-L Gasket Box (model 1933, Sterilite, Townsend, MA), 43-L Weathertight Tote (model UCB-SD, IRIS, Pleasant Prairie, WI), 56-L Weather Shield Box (model WSB-LD, Ziploc, SC Johnson and Sons, Racine, WI), and the 75-L Weathertight Trunk (model SIA-760D, IRIS). Of these, only the 43-L Weathertight Tote was advertised as airtight.
The ClorDiSys Environmental Monitoring System (EMS, Clordisys, Somerville, NJ) datalogger was used to quantify and record ClO2 gas concentration (in mg/L) and exposure dose (in ppm-h; 1 mg/L is equal to 360 ppm), temperature (in °C), and RH (in %). The ClO2 detection range of the EMS is 0.004 to 7 mg/L and remained within the calibration range during the study. According to the EMS measurement of ClO2 gas concentration within the tote, a gas concentration of 0.35 mg/L became undetectable in less than 2 min in the fume hood once the tote lid was removed. For monitoring, a hole was drilled in the lid of each tote through which the temperature and RH probe was placed, and another 2 holes were drilled into one side of each tote through which tubing was passed for gas sample collection and return. Adhesive putty (HandiTak, Super Glue, Ontario, CA) was used to create a seal at the junction of the probe, sample tubing, and tote. To passively generate humidity inside each tote, 15 mL of tap water was poured onto a dry kitchen sponge (Scotch-Brite, 3M, St Paul, MN), which then was placed in the tote. This volume of water was previously determined to ensure the tote would achieve and maintain a RH of greater than 90% (data not shown). After an overnight (15-h) cycle, the lid of the tote was removed while it was in the fume hood, and the tote was allowed to aerate for 2 min prior to removal from the hood. A constant temperature of 22 ± 2 °C was maintained within the fume hood.
BI.
Filter-paper spore strips each containing 6 log10 G.s. spores (6 mm × 30 mm; catalog no. TCDS-06, Crosstex International, Hauppauge, NY) in a Tyvek pouch with culture vials (catalog no. GMBCP-100, Crosstex) and 6 log10 B.a. (6 mm × 19 mm, catalog no. ACD/6, Mesa Labs, Lakewood, CO) in a Tyvek pouch with culture vials (catalog no. RM/100, Mesa Labs) were used according to the manufacturers’ instructions. After a 15-h cycle of ClO2 exposure, spore strips were removed aseptically from their Tyvek pouches by using flamed forceps and placed into the manufacturers’ modified soybean casein digest broth growth medium. Vials were incubated for 48 h at 37 °C for B.a. and 58 °C for G.s., with observations for growth at 24 and 48 h. To rule out contamination, BI with growth that was unanticipated were gram-stained and evaluated microscopically to confirm the identification of a single bacterial colony that morphologically matched positive controls.
Determining ClO2 gas concentration, RH, and tote retention.
The 43-L tote was the only tote evaluated that was marketed as having an airtight seal and therefore was considered the reference standard for this study. For the production of a ClO2 gas standard curve with high RH, the 43-L tote was used to measure the ClO2 gas concentration (mg/L) and exposure dose (ppm-h) that was generated at a total product weight of 0.5, 1.0, 2.0, or 4.0 g (n = 5 cycles/weight/15 h). Concurrently, both types of BI were taped into the tote at 5 locations: bottom left, bottom right, top left, top right, and middle. A positive control was placed outside of the fume hood. Each of the remaining totes (30, 56, and 75 L) was evaluated for ClO2 gas and RH retention by using total product weights of 2.0 and 4.0 g (n = 3 to 5 cycles/weight/15 h) without BI. Concentration (mg/L) and exposure dose (ppm-h) were recorded by using the EMS every 5 min for 15 h.
Placement of lab equipment.
Items of small lab equipment (SLE; mouse restrainer, rat restrainer, gas anesthesia induction chamber, digital calculator, analog calipers, scissors, thumb forceps, test tube holder with 3 test tubes, and 4 pens) were placed into the 43-L tote. One of each type of BI was taped to the equipment in 5 different, yet consistent, locations (Figure 1). Three cycles with a total product weight of 2.0 and 4.0 g (n = 3 cycles/weight/15 h) were used to determine whether the ClO2 gas inactivated both types of BI which were intermingled within the equipment. These cycles were repeated under these same conditions but with the addition of a small battery-powered fan at low speed (product no. Lileng-831, efluky, Santa Rosa, CA) within the tote to facilitate gas movement around equipment (n = 3 cycles/weight/15 h). In a subset experiment, SLE and each type of BI were placed into the 75-L tote, with a total product weight of 8 g (n = 3 cycles/weight/15 h). For the entire study, the same SLE and BI were used and placed in the same positions.
Figure 1.

Image of BI (B.a. and G.s.) placement in SLE in the 43 L tote.
Statistical analysis.
By using R version 3.5.0 (The R Foundation, www.r-project.org), linear regression and Pearson correlation were performed regarding the maximal amount of ClO2 gas generated relative to the total product weight placed into totes. All other graphs and statistical analyses were performed by using SigmaPlot 11.2 (Systat Software, Point Richmond, CA). One-way ANOVA followed by Holm–Sidak pairwise multiple comparison was performed to assess the amount of ClO2 gas and RH remaining after 15 h in each of the 4 totes as compared with the reference standard 43-L tote. In addition, χ2 analysis was performed for the total number of positive and negative BI of each species by using a 2 × 2 contingency table, given that both types of BI were exposed to ClO2 gas under the same conditions.
Results
Establishing a standard curve for ClO2 gas and inactivation of BI.
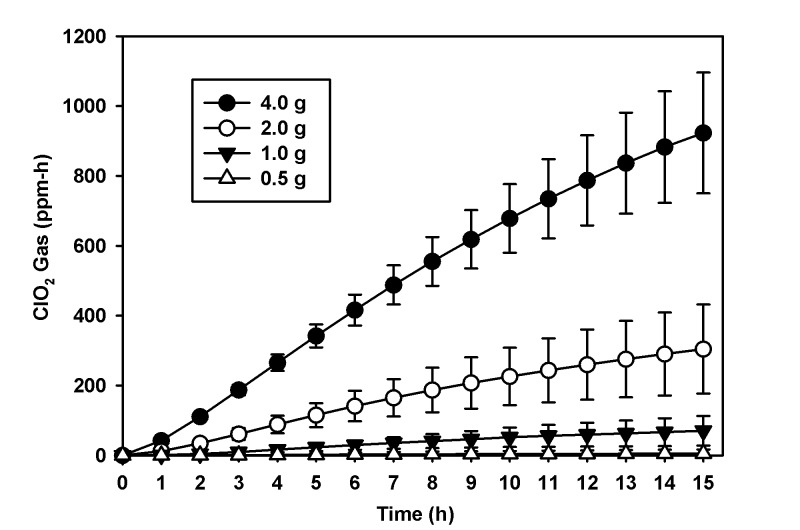
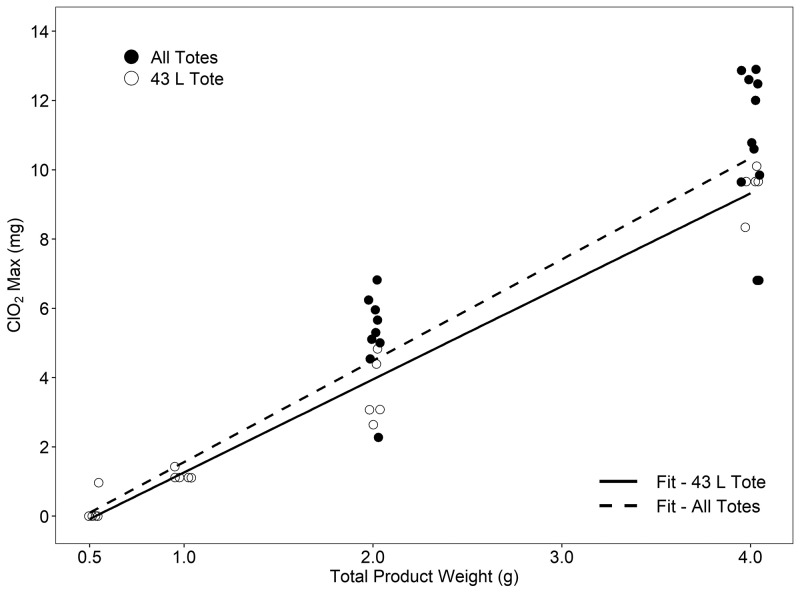
To establish a standard curve of ClO2 gas production according to the total product weight of reagents A and B, we used the 43-L tote. When reagents were combined to obtain 0.5, 1.0, 2.0, and 4.0 g, the mean ClO2 exposure dose was 5 ± 11, 71 ± 42, 304 ± 127, and 923 ± 172 ppm-h, respectively at 90% RH or greater (n = 5 cycles/weight/15 h; Figure 2). Four of the 5 cycles performed at 0.5 g were below the detection limit of the EMS. Using Pearson correlation, we found a significant (P < 0.001) positive relationship between the mass (in grams) of reagent and the maximal release of ClO2 in the 43-L tote (R2 = 0.97). For the 43-L tote, the equation for the line of best fit was y = 2.686x – 1.424, where x equals the mass (in grams) of product and y is an estimate of the maximal mass (in milligrams) of ClO2 produced (Figure 3). During the generation of the ClO2 gas standard curve, the inactivation of both BI was evaluated; 18 of 100 B.a. and 23 of 100 G.s. spore strips were positive for growth (P = 0.48, Table 1).
Figure 2.
ClO2 gas exposure dose (ppm-h mean and SD) per total product weight (g) over 15 h. This figure represents the scaling of ClO2 gas exposure dose (ppm-h) achieved with increasing quantities in total product weight over 15 h in the 43 L tote.
Figure 3.
ClO2 max (mg mean) per total product weight (g) for the 43 L tote and all additional totes combined. The figure represents the ability of StayfreshTM Wipeout to produce ClO2 gas in a linear fashion. The solid fit line (R2 = 0.97, P < 0.001) with open circles represents the ability of the product to constantly produce ClO2 gas at each total product weight (0.5, 1.0, 2.0, and 4.0g) in the 43 L tote. The black circles represent the data from the 30, 56, 75 L totes. The dashed line (R2 = 0.88, P < 0.001) is the best fit for data from all totes. See text for the equation of each line.
Table 1.
Number of positive and total BI (B.a. and G.s.) used for each total product weight (0.5, 1.0, 2.0 and 4.0 g, n = 5 cycles/weight/15 h) in 43 L tote. *(P = 0.48)
| Positive Biologic Indicators (+/total) | |||||||
| B. atrophaeus Amounta (g) | ppm-h | BL | BR | TL | TR | M | Total |
| 0.5 | 5.25 ± 12 | 3 | 3 | 4 | 3 | 4 | 17/25 |
| 1.0 | 71 ± 42 | 0 | 0 | 0 | 0 | 0 | 0/25 |
| 2.0 | 304 ± 127 | 0 | 0 | 0 | 1 | 0 | 1/25 |
| 4.0 | 923 ± 173 | 0 | 0 | 0 | 0 | 0 | 0/25 |
| Total | 3/20 | 3/20 | 4/20 | 4/20 | 4/20 | 18/100* | |
| G. stearothermophilus Amounta (g) | ppm-h | BL | BR | TL | TR | M | Total |
| 0.5 | 5.25 ± 12 | 4 | 3 | 4 | 4 | 4 | 19/25 |
| 1.0 | 71 ± 42 | 0 | 0 | 0 | 0 | 0 | 0/25 |
| 2.0 | 304 ± 127 | 1 | 0 | 0 | 0 | 1 | 2/25 |
| 4.0 | 923 ± 173 | 0 | 1 | 0 | 0 | 1 | 2/25 |
| Total | 5/20 | 4/20 | 4/20 | 4/20 | 6/20 | 23/100* | |
Total product weight (g)
BL, bottom left; BR, bottom right; TL, top left; TR, top right; M, middle
Evaluation of commercial totes.
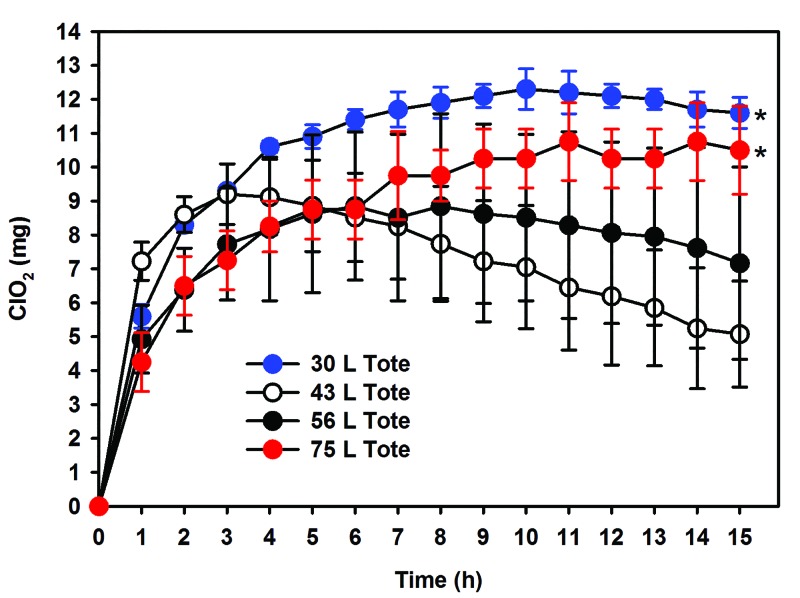
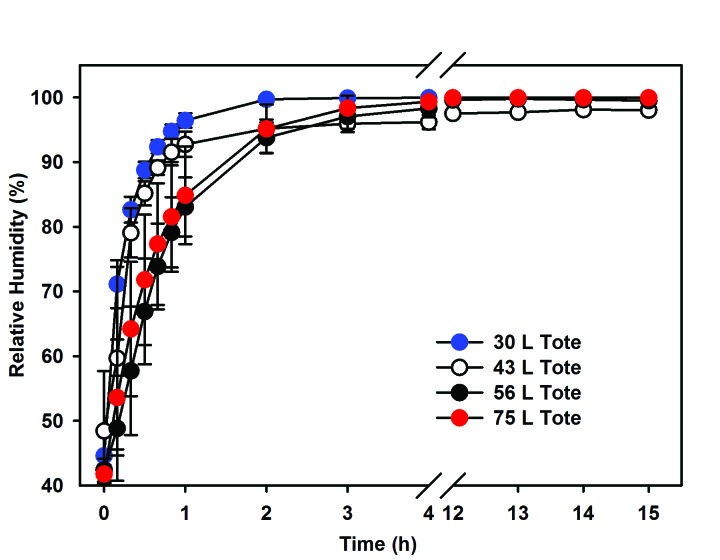
Similar to the 43-L tote, each additional tote (30, 56, and 75 L) was evaluated to confirm the ability to retain ClO2 gas by measuring the maximal amount of ClO2 gas released when using a total product weight of 2.0 and 4.0 g at greater than 90% RH (n = 3 to 5 cycles/weight/15 h). Our data demonstrate that the 30-, 56-, and 75-L totes were consistent with or exceeded the maximum ClO2 release achieved within the 43-L tote. All data sets were combined to estimate the maximum ClO2 release by reagent weight, independent of the tote. Repeating the Pearson correlation we again found a significant (P < 0.001) positive relationship between mass of reagent and the maximal release of ClO2 within all totes (R2 = 0.88). For all totes combined, the equation for the line of best fit was y = 2.924x – 1.363 (Figure 3). Table 2 displays the estimated maximal release of ClO2 for the line using only the 43-L data and that of the data from all totes combined. With the combined data, the estimates increased slightly but, in practice, remained a conservative estimate for maximal ClO2 release independent of the model of tote. In addition, the ability of all totes to retain ClO2 gas for 15 h was assessed by using total product weights of 2.0 and 4.0 g (n = 3 to 5 cycles/weight/15 h). For ClO2 gas to be effective, increased humidity must be obtained and was retained for 15 h through the addition of a moist sponge. All totes retained ClO2 gas approximately as well or better than the 43-L reference standard tote (Figure 4), exceeded 90% RH within 2 h, and maintained RH in excess of 90% for 15 h (Figure 5). When compared with the reference standard 43-L tote, both the 30- and 75-L totes retained more (P < 0.05) ClO2 gas after 15 h. However, RH did not differ between the totes after 15 h.
Table 2.
Estimated maximum of CIO2 released over 15 h, according to the various line fits
| mg CIO2 | ||
| Total mass (g) of product | 43-L tote | All totes |
| 1 | 1.26 | 1.56 |
| 2 | 3.95 | 4.49 |
| 3 | 6.63 | 7.41 |
| 4 | 9.32 | 10.33 |
The equation used for the airtight tote was y = 2.686x – 1.424, and the equation for the combined data was y = 2.924x – 1.363.
Figure 4.
ClO2 (mg mean and SD) over 15 h for all 4 totes. The figure represents retention of ClO2 gas at a total product weight of 4.0g for all 4 totes (30, 43, 56 and 75 L). This data shows that the 3 totes (30, 56, and 75 L) hold gas adequately or better than the 43 L tote. *P < 0.05 as compared with the reference standard 43 L tote.
Figure 5.
Relative humidity (RH mean and SD) for all 4 totes (30, 43, 56, 75 L) over 15 h. This figure represents the ability for all 4 totes to hold and maintain ≥ 90% RH for 15 h.
Placement of SLE.
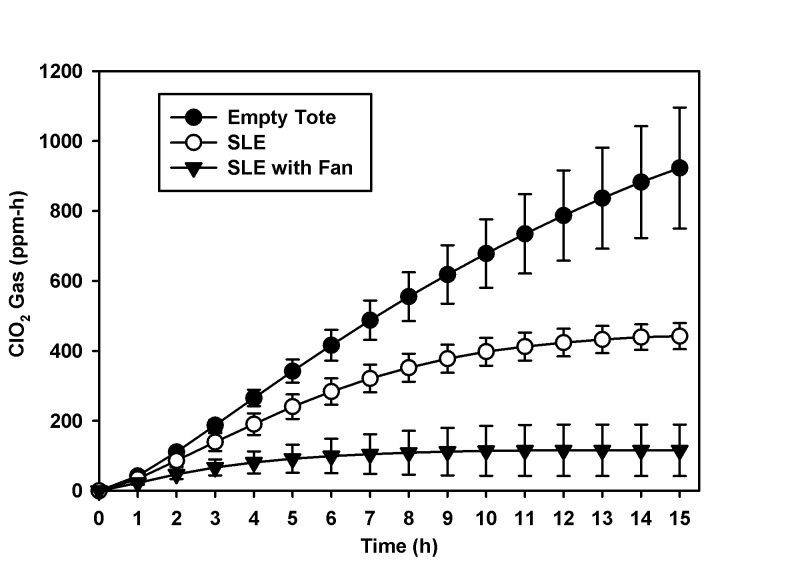
When SLE and BI were placed in the 43-L tote, the ClO2 gas exposure doses for 2.0 g and 4.0 g were 120 ± 35 and 442 ± 37 ppm-h, respectively (n = 3 cycles/weight/15 h, Figure 6). Combining the BI results from all of these cycles, a total of 4 of 30 B.a. and 6 of 30 G.s. spore strips were positive for growth (Table 3). When the experiment was repeated with the addition of a small battery-powered fan to aid in gas distribution around SLE within the tote, combined reagent amounts of 2.0 and 4.0 g generated exposure doses of 18 ± 22 and 115 ± 73 ppm-h, respectively (n = 3 cycles/weight/15 h, Figure 6). With the addition of the battery-powered fan, the combined BI data from these cycles revealed that 16 of 30 B.a. and 18 of 30 G.s. spore strips were positive for growth. In a subset experiment, the same numbers of SLE and BI were placed in the 75-L tote with a total product weight of 8.0 g and generating a ClO2 gas exposure dose of 1225 ± 102 ppm-h (n = 3 cycles/weight/15 h). All 15 BI of B.a. and G.s. were negative for growth (Table 3).
Figure 6.
ClO2 gas exposure dose (ppm-h mean and SD) generated by total product weight of 4.0 g over 15 h. This figure represents the decrease in ClO2 gas exposure dose (ppm-h) with the addition of SLE and SLE with fan in the 43 L tote. SLE = small lab equipment.
Table 3.
Number of BI (B.a. and G.s.) that tested positive after placement of SLE and fan
| Positive Biologic Indicators (+/total) | |||||||||||||||
| G. stearothermophilus | B. atrophaeus | ||||||||||||||
| BC | MR | RR | IIC | ICL | Total | BC | MR | RR | IIC | ICL | Total | ||||
| 43-L tote, 2.0 ga | ppm-h | ||||||||||||||
| No fan, no SLEb | 304 ± 127 | 2/25 | 1/25 | ||||||||||||
| SLE only | 120 ± 35 | 2 | 0 | 0 | 0 | 2 | 4/15 | 2 | 0 | 0 | 0 | 1 | 3/15 | ||
| SLE + Fanc | 18 ± 22 | 3 | 3 | 3 | 3 | 3 | 15/15 | 3 | 3 | 2 | 3 | 3 | 14/15 | ||
| Total | 5/6 | 3/6 | 3/6 | 3/6 | 5/6 | 19/30 | 5/6 | 3/6 | 2/6 | 3/6 | 4/6 | 17/30 | |||
| 43 L, 4.0 ga | ppm-h | ||||||||||||||
| No Fan, No SLEb | 923 ± 173 | 2/25 | 0/25 | ||||||||||||
| SLE | 442 ± 37 | 1 | 0 | 0 | 0 | 1 | 2/15 | 1 | 0 | 0 | 0 | 0 | 1/15 | ||
| SLE + Fanc | 115 ± 73 | 2 | 0 | 0 | 0 | 1 | 3/15 | 1 | 0 | 1 | 0 | 0 | 2/15 | ||
| Total | 3/6 | 0/6 | 0/6 | 0/6 | 2/6 | 5/30 | 3/6 | 0/6 | 0/6 | 0/6 | 0/6 | 3/30 | |||
| 75 L, 8.0 ga | ppm-h | ||||||||||||||
| SLE | 1225 ± 102 | 0 | 0 | 0 | 0 | 0 | 0/15 | 0 | 0 | 0 | 0 | 0 | 0/15 | ||
| Total | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/15 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/15 | |||
Total product weight (g)
BI used but not associated with SLE, data is represented in Table 1
Small battery-powered fan
SLE, small lab equipment; BC, bottom of the calculator; MR, inside mouse restrainer; RR, inside rat restrainer; IIC, inside induction chamber; ICL, outside of induction chamber lid
Discussion
In this study, we successfully identified several economical products that can be used to create an inexpensive and efficient method for decontaminating SLE by using ClO2 gas. An essential component to the success of this method is a product that predictably creates ClO2 gas by simply combining 2 dry reagents. For ClO2 gas to be effective, an air-tight chamber and sufficient RH and contact time are required. Another essential component is an inexpensive, commercially available household tote that can be used as an airtight exposure chamber. All 4 commercially available totes that we evaluated, spanning 3 different brands and varying in volume, had gasket-seal lid systems that held ClO2 gas and maintained RH at 90% or greater for 15 h. The scalable nature of ClO2 gas production combined with the variety of gasket-seal totes makes our method customizable to meet the needs of the end user.
When using this method, it is first important to determine the type of decontamination intended (that is, sterilization compared with disinfection). The vast majority of known laboratory rodent pathogens and opportunists do not form endospores and do not demonstrate noteworthy resistance to disinfection. The most notable exceptions are the spore-former Clostridium piliforme, the causative agent of Tyzzer disease,10 and Syphacia spp. eggs24 which required a ClO2 gas dose of 1440 ppm-h at 52% to 67% RH to achieve complete inactivation.8 Although murine norovirus has outstanding environmental stability, a greater than 5 log10 reduction has been achieved by using a ClO2 gas dose of 24 ppm-h at 85% RH.44 In contrast, vegetative bacteria such as Escherichia coli and Staphylococcus aureus showed a greater than 5 log10 and greater than 2 log10 reduction, respectively, with a total ClO2 gas dose of 0.25 ppm-h at 52% RH.26 A direct comparison of the exposure doses mentioned above is not possible because the RH varied in each report; the general trend is consistent with the principle of a hierarchy in chemical germicidal resistance.39
According to this principle, sterilization of endospores well exceeds the ClO2 gas dose required for the inactivation of nonenveloped viruses, vegetative bacteria, and finally enveloped viruses. However, we are unaware of a low-cost indicator that can be used to quantify ClO2 gas exposure to confirm intermediate (inactivation of vegetative microorganisms, fungi, and most viruses) to high-level (inactivation of vegetative microorganisms, viruses, and some endospores)39 disinfection other than BI containing fewer than 6 log10 spores. Therefore, we are constrained to using BI due to their commercial availability, low cost, ease of use, and broad acceptance for the assessment of disinfection and sterilization.21,23 We did not evaluate the efficacy of this method on specific lab animal pathogens because doing so was beyond the scope of our study. We encourage institutions to perform their own validations.
Because sterilization of a BI would be excessive for eliminating most rodent pathogens, we attempted to determine the lowest dose of ClO2 gas that would successfully sterilize BI that contains either B.a. or G.s. Using the method of fractionation, we performed an experiment of stepwise reductions in ClO2 gas dose and assessed both BI species for a breakpoint at which inactivation was no longer achieved.21 From our results, we determined that a minimal dose of 71 ± 42 ppm-h of ClO2 gas at greater than 90% RH for 15 h inactivates both B.a. and G.s. BI. Attempts to investigate a lower ClO2 dose was at the lower limits of our ClO2 gas detection abilities. From these findings, we conclude that B.a. and G.s. demonstrate similar sensitivity to ClO2 gas exposure. Over the course of the entire project, 230 of each B.a. and G.s. BI were tested concurrently in each cycle; 65 of 230 (28.3%) B.a. and 75 of 230 (32.6%) G.s. BI tested positive for growth under the same exposure conditions. Although the difference between B.a. and G.s that tested positive for growth is not statistically significant (P = 0.36), this difference may be relevant to the end user if this method is used to validate sterilization. Several articles report similiar inconsistency when using these two BI for ClO2 gas decontamination.7,14,21 Many factors may play a role in this variability, as discussed later in this paper. While it may see logical to use the BI that is more resistant to the ClO2 gas exposure, this does not represent “real-world” application and is an “unrealistic goal” as other more resistant species are likely to be found. The literature acknowledges that although G.s. has more variability in inactivation the two species B.a. and G.s. are comparable in resistance to ClO2 gas.7,14,21 However, for reliable validation of decontamination, the National Sanitation Associaton chose to use B.a. BI with ClO2 gas decontamination of biological safety cabinets.21,23 The results from our study using these 2 BI, low dose ClO2 gas, and a long exposure time support the aforementioned findings.
Over the course of the study, several cycles yielded an exposure dose that exceeded 71 ± 42 ppm-h at which neither BI B.a. or G.s. was inactivated. When only BI (that is, no SLE) were in the tote, 5% of BI (5 of 100) tested positive for growth at an exposure dose range of 304 to 923 ppm-h (Table 1). The literature suggests that variability of inactivation can occur among BI of different manufacturers and different lot numbers of the same manufacturer.21,34 In addition, the mechanism of 3D ‘islanding’ (that is, clumping) of the endospores on the substrate carrier can cause variability of inactivation.21,34 Regarding the organism itself, all genotypes of an organism may not have the same order of resistance when exposed to the same decontaminate.34 These potential variables whether from the manufacturer or the endospore themselves hold a possible explanation for these findings. However, with the addition of SLE, BI position also played a large role in failed inactivation. With the placement of BI in fixed locations among SLE, 16.6% of BI (15 of 90) failed to be inactivated at an exposure dose range of 115 to 385 ppm-h (Table 3). Of the 15 BI that failed to be inactivated, 9 BI were positioned under the calculator, meaning between the calculator and the floor of the tote. Similarly, 5 of 15 BI were on the induction chamber lid, where the BI were positioned between the lid and the side wall of the tote (Figure 1). The findings suggest that ClO2 gas inside the tote can achieve the minimal required dose for inactivation of the BI but that the placement of SLE within the tote may only disinfect an area of the SLE as compared with sterilize. As a result, a refinement to this method may include using a rack to lift materials off the bottom of the tote and taking care to not overfill the tote but to position items loosely away from the side walls and other materials to improve gas exposure. When using a total product amount of 8 g in the 75-L tote that contained SLE, all BI—regardless of location—were inactivated (Table 3). Complete inactivation of BI can be due to a combination of the 75-L tote's increased ability to hold gas (Figure 4) and an increase in the amount of ClO2 gas produced (mg/L) within the tote (Table 3).
As with most low-cost alternatives, some limitations to our system must be considered. When using a ClO2 gas generator, a constant gas concentration can be maintained within the exposure chamber. In contrast, by using the method we described, a finite quantity of ClO2 gas is produced that depends on the amount of the gas-producing material that is used. In addition, during the 15-h cycle, the concentration of ClO2 gas will decrease gradually. With the placement of SLE in the tote, the ClO2 gas concentration decreased by 59% on average as compared with an empty tote (Figure 6 and Table 3). An explanation for the reduction of the detectable ClO2 gas may be due to the adsorption or consumption of the gas by the materials which make up the SLE.41 Alternatively, an important feature of ClO2 gas that may explain the decrease in exposure dose is that ClO2 gas is highly water-soluble.16,28,29,40 We hypothesize that with the addition of SLE to the tote, the surface area on which condensation could form at high RH increased. The solubility of the ClO2 gas within the water molecules16,35 on SLE surfaces renders ClO2 gas undetectable by the EMS. Independent of the mechanism, we suspect the surface area of the equipment and the materials of which the items are made contribute to a decrease in the ClO2 gas exposure dose. As a result, calculating the amount of ClO2 gas required to achieve BI sterilization is difficult, given the variability in the amount of laboratory equipment that could be placed in the tote.
Regarding ClO2 for decontamination, several articles mentioned the use of a fan to help distribute the gas within the exposure chamber.22,32,35,42 With these reports in mind, we performed 3 identical cycles with and without the addition of a small battery-powered fan. However, the addition of the fan paradoxically led to an average 91% decrease in the amount of ClO2 gas, resulting in the majority of BI testing positive for growth (Figure 6 and Table 3). Because the commercially available totes are not a completely sealed system, we considered that the fan may be forcing ClO2 gas out the gasket-seal. However, because the fan is not producing positive pressure within the tote, we do not believe this explanation accounts for the loss or consumption of ClO2 gas. Unfortunately, we are unable to adequately explain this observation despite the abundance of studies that used a fan during ClO2 gas decontamination. Nevertheless, because of our findings, we do not recommend using a fan with the method that we described here. When using shorter cycle times, the desire to use a fan is understandable, as a means to rapidly distribute the gas within the chamber to achieve adequate exposure. However, our data demonstrate that over a 15-h exposure cycle, ClO2 gas is able to inactivate BI in all quadrants of the tote without using an internal fan.
Taking into consideration these factors that decrease ClO2 gas doses when using a fixed source, it is tempting to simply inundate the system with excess ClO2 gas to ensure sterilization. Although this practice will achieve sterilization under most circumstances, there is potential for complications. Due to the oxidizing properties of ClO2 gas, corrosion on metal and electronic equipment has been reported after exposure to high concentrations of ClO2 gas with concurrent high RH.9,43 Although assessment of corrosion was beyond the scope of our current project, it is important to note that the handheld calculator that we used in this study still operates after 20 cycles and a cumulative exposure dose of 5797 ppm-h. In contrast, the stainless-steel scissors and forceps that received the same dose as the calculator have noticeable surface corrosion which was not present at the beginning of the study.
If confirmation of sterilization is required, using a BI will extend the turnaround time from 15 h to an additional 48 h to confirm inactivation of the BI. In addition, because there is no scavenging system with this method, the use of a fume hood is necessary. Unlike expensive airtight exposure chambers made of rigid materials, the sides and walls of the low-cost totes are flexible. During our study, we found that manipulations of the totes, such as lifting and pressing on the sides or lid, resulted in the release of ClO2 gas from the gasket-seal. Therefore, if our described method is used, it is important not to manipulate the totes, because they are not a completely sealed system.
Because ClO2 gas is a health hazard at high concentrations, we recommend working closely with the appropriate institutional safety officials to establish a standard operating procedure. It is important to ensure adequate ventilation not only in the immediate area where this method is performed (for example, in a fume hood) but also in adjacent spaces. Exposure to ClO2 gas at high concentrations can cause irritation to the eyes, nose, and throat and may result in bronchitis and pulmonary edema.5,40 If engineering controls such as increased ventilation are not available, a risk assessment should be performed to determine whether the space is safe for the use of this method or to identify appropriate personal protective equipment, such as air purifying respirators. If possible, exposure monitoring should be performed to ensure that ClO2 gas is not released from the tote at concentrations that would pose a health hazard. The Occupational Safety and Health Administration permissible exposure limit for ClO2 gas is 0.1 ppm for an 8-h time-weighted average, and the National Institute for Occupational Safety and Health short-term exposure limit is 0.3 ppm for periods not to exceed 15 min.5,40 For our study, all tests were performed within a fume hood. According to EMS measurement of ClO2 gas concentration within the tote, gas concentrations reached undetectable levels in approximately 2 min after the tote lid was opened in the fume hood. Therefore, we recommend opening the lid to the tote for a minimum of 2 min prior to the retrieval of contents, thereby allowing gas exhaust and preventing direct exposure of personnel to high gas concentrations while removing materials from within the tote. Adhering to these safety measures will ensure that this method can be performed with minimal safety risks.
The goal of this study was to create and validate an effective, small-scale method of ClO2 gas decontamination that is affordable, efficient, safe, and reproducible. By using inexpensive but effective products, we demonstrated that sterilization or disinfection can occur at a cost that is approximately 100-fold less than that of large-scale commercial ClO2 gas generators with specialized airtight chambers. When using our method, the greatest cost is the initial investment, which includes the purchase of the tote, kitchen sponge, ClO2 gas-producing product, and the BI spore strips and culture vials. Depending on whether BI are used, the cost per cycle can range from approximately $0.10 to $7.00. With a conservative amount of SLE within the tote, our data suggest that a concentration of 0.21 to 0.27 mg/L of ClO2 gas at greater than 90% RH over 15 h can be used for disinfection of common vegetative bacteria and viral laboratory animal pathogens and may even provide sterilization. However, the limitations of this method should be understood, and end users should validate these processes within their own institutions. With appropriate safety measures and a clear understanding of the end goal of decontamination (sterilization compared with disinfection), this method can be established quickly and easily at any institution for everyday use.
Acknowledgments
This project was funded by the University of Colorado Denver, Office of Laboratory Animal Resources. We thank Derek Smith from the University of Colorado Cancer Center Biostatistics and Bioinformatics Shared Resource for his skilled statistical support of this project. The manufacturers of all the products used had no input or influence on study design or interpretation of results.
References
- 1.Akamatsu A, Lee C, Morino H, Miura T, Ogata N, Shibata T. 2012. Six-month low level chlorine dioxide gas inhalation toxicity study with 2-wk recovery period in rats. J Occup Med Toxicol 7:1–8. 10.1186/1745-6673-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhawana JM, Stubblefield AL, Newsome A, Cahoon B. 2014. Surface decontamination of plant tissue explants with chlorine dioxide gas. In Vitro Cell Dev Biol Plant 51:214–219. [Google Scholar]
- 3.Boyce JM. 2016. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control 5:1–10. 10.1186/s13756-016-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buttner MP, Cruz P, Stetzenbach LD, Klima-Comba AK, Stevens VL, Cronin TD. 2004. Determination of the efficacy of 2 building decontamination strategies by surface sampling with culture and quantitative PCR analysis. Appl Environ Microbiol 70:4740–4747. 10.1128/AEM.70.8.4740-4747.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC). [Internet]. 2008. Guideline for disinfection and sterilization in healthcare facilities. [Cited 18 August 2018]. Available at: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/index.html
- 6.Czarneski MA, Lorcheim K. 2011. A discussion of biological safety cabinet decontamination methods: formaldehyde, chlorine dioxide, and vapor phase hydrogen peroxide. Appl Biosaf 16:26–33. 10.1177/153567601101600104. [DOI] [Google Scholar]
- 7.Czarneski MA, Lorcheim P. 2008. Validation of chlorine dioxide sterilization, p 263–268. In: Agalloco J, Carleton FJ, editors. Validation of pharmaceutical processes, 3rd ed New York (NY): Informa Healthcare. [Google Scholar]
- 8.Czarra JA, Adams JK, Carter CL, Hill WA, Coan PN. 2014. Exposure to chlorine dioxide gas for 4 hours renders syphacia ova nonviable. J Am Assoc Lab Anim Sci 53:364–367. [PMC free article] [PubMed] [Google Scholar]
- 9.Derkits G, Mandich M, Reents W, Franey J, Xu C, Fleming D, Kopf R, Ryan S. 2010. Reliability of electronic equipment exposed to chlorine dioxide used for biological decontamination. IEEE International Reliability Physics Symposium (IRPS) Anaheim, California, 2–6 May 2010. 2010 IEEE International Reliability Physics Symposium. 879–880. 10.1109/IRPS.2010.5488715 [DOI] [Google Scholar]
- 10.Duncan AJ, Carman RJ, Olsen GJ, Wilson KH. 1993. Assignment of the agent of Tyzzer's disease to Clostridium piliforme comb. nov. on the basis of 16S rRNA sequence analysis. Int J Syst Bacteriol 43:314–318. [DOI] [PubMed] [Google Scholar]
- 11.Golkowski M, Golkowski C, Leszczynski J, Plimpton SR, Maslowski P, Foltynowicz A, Jun Y, McCollister B. 2012. Hydrogen-peroxide-enhanced nonthermal plasma effluent for biomedical applications. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc 40:1984–1991. 10.1109/TPS.2012.2200910. [DOI] [Google Scholar]
- 12.Gordon D, Carruthers BA, Theriault S. 2012. Gaseous decontamination methods in high-containment laboratories. Appl Biosaf 17:31–39. 10.1177/153567601201700107. [DOI] [Google Scholar]
- 13.Han Y, Applegate B, Linton RH, Nelson PE. 2003. Decontamination of Bacillus thuringiensis spores on selected surfaces by chlorine dioxide gas. J Environ Health 66:16–20. [PubMed] [Google Scholar]
- 14.Jeng DK, Woodworth AG. 1990. Chlorine dioxide gas sterilization under square-wave conditions. Appl Environ Microbiol 56:514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahnert A, Seiler P, Stein M, Aze B, McDonnell G, Kaufmann SHE. 2005. Decontamination with vaporized hydrogen peroxide is effective against Mycobacterium tuberculosis. Lett Appl Microbiol 40:448–452. 10.1111/j.1472-765X.2005.01683.x. [DOI] [PubMed] [Google Scholar]
- 16.Linton RH, Han Y, Selby TL, Nelson PE. 2006. Gas-/vapor-phase sanitation (decontamination) treatments, p 401–436. Chapter 18. In: Sapers GM, Gorny JR, Yousef AE, editors. Microbiology of fruits and vegetables 1st ed Boca Raton (FL): CRC Press. [Google Scholar]
- 17.Lowe JJ, Gibbs SG, Iwen PC, Smith PW. 2012. A case study on decontamination of a biosafety level-3 laboratory and associated ductwork within an operational building using gaseous chlorine dioxide. J Occup Environ Hyg 9:D196–D205. 10.1080/15459624.2012.733592. [DOI] [PubMed] [Google Scholar]
- 18.Lowe JJ, Gibbs SG, Iwen PC, Smith PW, Hewlett AL. 2013. Decontamination of a hospital room using gaseous chlorine dioxide: Bacillus anthracis, Francisella tularensis, and Yersinia pestis. J Occup Environ Hyg 10:533–539. 10.1080/15459624.2013.818241. [DOI] [PubMed] [Google Scholar]
- 19.Lowe JJ, Gibbs SG, Iwen PC, Smith PW, Hewlett AL. 2013. Impact of chlorine dioxide gas sterilization on nosocomial organism viability in a hospital room. Int J Environ Res Public Health 10:2596–2605. 10.3390/ijerph10062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowe JJ, Hewlett AL, Iwen PC, Smith PW, Gibbs SG. 2013. Evaluation of ambulance decontamination using gaseous chlorine dioxide. Prehosp Emerg Care 17:401–408. 10.3109/10903127.2013.792889. [DOI] [PubMed] [Google Scholar]
- 21.Luftman HS, Regits MA. 2008. B. atrophaeus and G. stearothermophilus biological indicators for chlorine dioxide gas decontamination. Appl Biosaf 13:143–157. 10.1177/153567600801300304. [DOI] [Google Scholar]
- 22.Luftman HS, Regits MA, Lorcheim P, Czarneski MA, Boyle T, Aceto H, Dallap B, Munro D, Faylor K. 2006. Chlorine dioxide gas decontamination of large animal hospital intensive and neonatal care units. Appl Biosaf 11:144–154. 10.1177/153567600601100306. [DOI] [Google Scholar]
- 23.Luftman HS, Regits MA, Lorcheim P, Lorcheim K, Paznek D. 2008. Validation study for the use of chlorine dioxide gas as a decontaminant for biological safety cabinets. Appl Biosaf 13:199–212. 10.1177/153567600801300403. [DOI] [Google Scholar]
- 24.Meade TM, Watson J. 2014. Characterization of rat pinworm (Syphacia muris) epidemiology as a means to increase detection and elimination. J Am Assoc Lab Anim Sci 53:661–667. [PMC free article] [PubMed] [Google Scholar]
- 25.Morino H, Fukuda T, Miura T, Lee C, Shibata T, Sanekata T. 2009. Inactivation of feline calicivirus, a norovirus surrogate, by chlorine dioxide gas. Biocontrol Sci 14:147–153. 10.4265/bio.14.147. [DOI] [PubMed] [Google Scholar]
- 26.Morino H, Fukuda T, Miura T, Shibata T. 2011. Effect of low-concentration chlorine dioxide gas against bacteria and viruses on a glass surface in wet environments. Lett Appl Microbiol 53:628–634. 10.1111/j.1472-765X.2011.03156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SH, Kang DH. 2015. Antimicrobial effect of chlorine dioxide gas against foodborne pathogens under differing conditions of relative humidity. Lebensm Wiss Technol 60:186–191. 10.1016/j.lwt.2014.09.031. [DOI] [Google Scholar]
- 28.Park SH, Kang DH. 2018. Effect of temperature on chlorine dioxide inactivation of Escherichia coli O157:H7, Salmonella typhimurium, and Listeria monocytogenes on spinach, tomatoes, stainless steel, and glass surfaces. Int J Food Microbiol 275:39–45. 10.1016/j.ijfoodmicro.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Park SH, Kim WJ, Kang DH. 2018. Effect of relative humidity on inactivation of foodborne pathogens using chlorine dioxide gas and its residues on tomatoes. Lett Appl Microbiol 67:154–160. [DOI] [PubMed] [Google Scholar]
- 30.Pottage T, Macken S, Giri K, Walker JT, Bennett AM. 2012. Low-temperature decontamination with hydrogen peroxide or chlorine dioxide for space applications. Appl Environ Microbiol 78:4169–4174. 10.1128/AEM.07948-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ragland NH, Miedel EL, Gomez JM, Engelman RW. 2017. Staphylococcus xylosus PCR-validated decontamination of murine individually ventilated cage racks and air handling units by using ‘active-closed’ exposure to vaporized hydrogen peroxide. J Am Assoc Lab Anim Sci 56:742–751. [PMC free article] [PubMed] [Google Scholar]
- 32.Rastogi VK, Ryan SP, Wallace L, Smith LS, Shah SS, Martin GB. 2010. Systematic evaluation of the efficacy of chlorine dioxide in decontamination of building interior surfaces contaminated with anthrax spores. Appl Environ Microbiol 76:3343–3351. 10.1128/AEM.02668-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanekata T, Fukuda T, Miura T, Morino H, Lee C, Maeda KEN, Araki K, Otake T, Kawahata T, Shibata T. 2010. Evaluation of the antiviral activity of chlorine dioxide and sodium hypochlorite against feline calicivirus, human influenza virus, measles virus, canine distemper virus, human herpesvirus, human adenovirus, canine adenovirus and canine parvovirus. Biocontrol Sci 15:45–49. 10.4265/bio.15.45. [DOI] [PubMed] [Google Scholar]
- 34.Shintani H, Akers JE. 2000. On the cause of performance variation of biological indicator used for sterility assurance. PDA J Pharm Sci Technol 54:332–342. [PubMed] [Google Scholar]
- 35.Shirasaki Y, Matsuura A, Uekusa M, Ito Y, Hayashi T. 2016. A study of the properties of chlorine dioxide gas as a fumigant. Exp Anim 65:303–310. 10.1538/expanim.15-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith DJ, Ernst W, Herges GR. 2015. Chloroxyanion residues in cantaloupe and tomatoes after chlorine dioxide gas sanitation. J Agric Food Chem 63:9640–9649. 10.1021/acs.jafc.5b04153. [DOI] [PubMed] [Google Scholar]
- 37.Stratilo CW, Crichton MKF, Sawyer TW. 2015. Decontamination efficacy and skin toxicity of two decontaminants against Bacillus anthracis. PLoS One 10:1–11. 10.1371/journal.pone.0138491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.US Department of Health and Human Services. [Internet]. 2004. Toxicological profile for chlorine dioxide and chlorite. [Cited 18 August 2018]. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp160.pdf
- 39.US Department of Health and Human Services. 2009. Biosafety in microbiological and biomedical laboratories (BMBL), 5th ed. Bethesda (MD): HHS Publication. [Google Scholar]
- 40.US Environmental Protection Agency. [Internet].2006. Reregistration eligibility decision (RED) for chlorine dioxide and sodium chlorite (case 4023). [Cited 18 August 2018]. Available at: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/red_PC-020503_3-Aug-06.pdf
- 41.US Environmental Protection Agency. [Internet]. 2008. Material demand studies: Interaction of chlorine dioxide gas with building materials. [Cited 18 August 2018]. Available at: https://nepis.epa.gov/Exe/ZyPDF.cgi/P1005VFM.PDF?Dockey=P1005VFM.PDF
- 42.Wang T, Wu J, Qi J, Hao L, Yi Y, Zhang Z. 2016. Kinetics of inactivation of Bacillus subtilis subsp. niger spores and Staphylococcus albus on paper by chlorine dioxide gas in an enclosed space. Appl Environ Microbiol 82:3061–3069. 10.1128/AEM.03940-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu C, Fleming D, Mandich ML, Reents WD, Derkits GE, Franey JP, Kopf R, Ryan S. 2010. Interconnection reliability assessment for electronic equipment exposed to chlorine dioxide used for biological decontamination. In Proceedings, 56th IEEE Holm Conference on Electrical Contacts, Charleston, South Carolina, 04–07 October, 2010. Institute of Electrical and Electronics Engineers Incorporated (IEEE).1–12. [Google Scholar]
- 44.Yeap JW, Kaur S, Lou F, DiCaprio E, Morgan M, Linton R, Li J. 2015. Inactivation kinetics and mechanism of a human norovirus surrogate on stainless steel coupons via chlorine dioxide gas. Appl Environ Microbiol 82:116–123. 10.1128/AEM.02489-15. [DOI] [PMC free article] [PubMed] [Google Scholar]