Abstract
Purpose
To perform dosimetric validation of the plan adaptation and high-definition motion management (HDMM) system of Gamma Knife® IconTM in various clinical scenarios.
Methods and materials
We built an assembly for a pitch-adjustable anthropomorphic head phantom. We then used films to measure dosimetric and positional accuracy in 13 clinical scenarios, including movement near HDMM thresholds, multiple plan adaptations, frequent coughing, and initial setup error.
Results
The dose for the superiorly located 4-mm shot was decreased up to 7-13% near 2- to 3-mm HDMM thresholds in the chin-down position. Dosimetric deviation was within ±3.5% for initial pitch angles of up to 20°. Multiple treatment interruption and frequent coughing did not cause substantial dosimetric deviation (<2%).
Conclusion
Our results indicated that dosimetric accuracy of the Gamma Knife® IconTM system is reliable even in extreme treatment conditions. However, the user should exercise caution for superiorly located small lesions with an HDMM threshold ≥2 mm or in the scenario of large initial setup error.
Keywords: Gamma Knife Icon, plan adaptation, HDMM
Introduction
The Gamma Knife (GK) Icon (Elekta, Stockholm, Sweden) has a novel image guidance and motion tracking system consisting of cone beam computed tomography (CBCT) for pretreatment patient setup and tracking of a patient’s nose marker for the high-definition motion management (HDMM) system.[1-4] During a mask-based treatment, if a patient moves more than a pre-defined HDMM alarm threshold (e.g., 1.5 mm), gating is activated, which suspends dose delivery. If the displacement of the marker on the patient’s nose does not return to within the pre-defined HDMM alarm threshold, a new CBCT image is acquired and co-registered with a reference CBCT image (CBCTref). During plan adaptation, the planned shot coordinates are automatically updated to maintain their relative locations to anatomical structures so that the planned dosimetry is maintained. This procedure is repeated whenever a CBCT image is re-acquired.[5]
The GK Icon system has been evaluated in various studies. In addition to GK Icon commissioning experiences described by AlDahlawi et al[6] and Zeverino et al[4], Li et al measured inter- and intrafractional target motion relative to the head motion of six patients with CBCT and infrared optical tracking and found that the largest intrafractional motion occurred in the cranial-caudal direction (pitch) and target motion was smaller than patient’s head motion detected by CBCT and infrared tracking.[1] Wright et al studied the relationship between the nose tip marker and intracranial rotational motion using manually introduced rotation of a phantom. They found that overall intracranial anatomy displacements were 43% lower than the nose displacements.[7]
Using an assembly of a Lucy phantom (Standard Imaging, Middleton, WI, USA), an offset neck connector, and a motor, Burton et al concluded that superiorly located tumors have greater movement during the treatment than that observed by HDMM, and the location of the tumor in relation to the patient’s nose and pivot point in the neck should be carefully considered.[8] In other studies the accuracy and reproducibility of HDMM were studied with either a three-dimensionally printed phantom[9] or a movable phantom[10]. However, the impact of pitch rotation on dosimetry and position for various clinical treatment scenarios has not been thoroughly studied. It is important for users to understand how head rotation at various HDMM alarm thresholds can affect dosimetry relative to an uninterrupted treatment. For example, a patient’s movement could approach and remain near the HDMM alarm threshold during the entire treatment, or multiple plan adaptations may be needed because a patient’s movement could surpass the HDMM alarm threshold several times, leading to treatment pauses, re-imaging, and necessitating multiple plan adaptations. Alternatively, a patient could require frequent restroom breaks (necessitating multiple treatment interruptions), or a patient could cough frequently during treatment. Lastly, it is still unclear how much initial setup rotational error could impact the daily adapted plan. Understanding the dosimetric impact of these scenarios is critical for accurate and safe radiation treatment to patients.
Evaluating these scenarios as part of end-to-end testing of the GK Icon system requires an anthropomorphic phantom that can move automatically in a remote-controlled manner during the treatment. The purpose of the current study was to perform dosimetric and positional validation of the plan adaptation and HDMM system of GK Icon for various clinical scenarios using a remote-controlled pitch-adjustable phantom. First, we designed and built the assembly of a pitch-adjustable anthropomorphic head phantom. Second, 13 conceptual representations of treatment scenarios were selected for end-to-end testing of the GK Icon workflow. These scenarios included treatments with patient displacement near various HDMM alarm thresholds, multiple plan adaptations, simulated patient coughing, and initial pitch angle setup errors. Lastly, dosimetric and positional accuracies were measured using EBT film and compared with those with no interruption or movement.
Materials and Methods
Remote-controlled pitch-adjustable head phantom
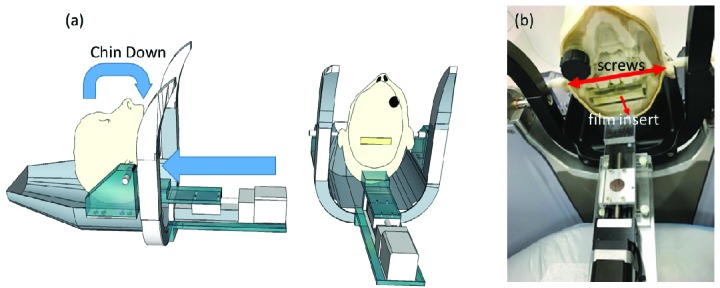
We used an anthropomorphic head phantom (RT-safe, Artotinis, Greece) with bony anatomy and bone-equivalent density representing the human skull (Figure 1). Because the largest intrafractional head movement during a mask-based treatment is pitch rotation[1], we constructed an assembly that rotates the phantom on this rotation axis, which is defined by the two rods inserted in both ears of the head phantom. The head phantom, suspended by the two rods, is rotated by pushing a linear slider attached to a stepper motor (XSlide, Velmex, Bloomfield, NY) sitting on a custom-made base platform, which is securely attached to the GK Icon mask adaptor. The stepper motor controller (Velmex, Bloomfield, NY) is connected to a long cable and controlled by a laptop computer located in the treatment console area. This assembly is capable of rotating the head phantom up to a 20° pitch angle within ±0.2°. With our configuration, 74 motor steps are needed to displace a reflective nose marker tracked by HDMM by 1 mm, which is equivalent to 0.4° of pitch motion verified by the CBCT co-registration.
Figure 1.
(a) Graphical description of assembly. (b) Photograph of the actual assembly mounted to an Icon mask adapter.
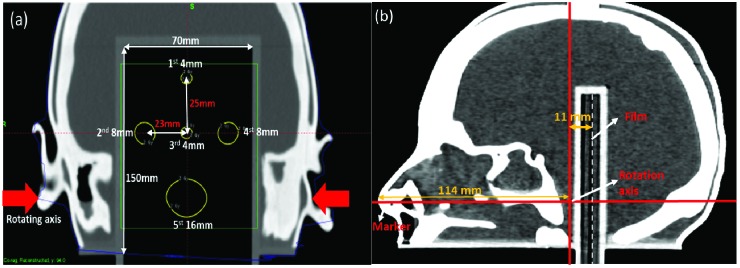
The head phantom had a 7-cm (width) × 15-cm (length) film insert. As shown in Figure 2, the film insert was placed in the phantom’s mid-coronal plane spanning mid-brain to base of skull, and the film plane was located slightly posterior to the axis of the head rotation. The space within the skull was filled with water.
Figure 2.
Computed tomography images of the head phantom used to generate the plan. (a) Coronal view of the locations of the five planned shots. (b) Sagittal view of the nose marker, rotation axis, and film.
Plan for dosimetric and positional verification using an EBT film insert
A CT scan of the head phantom was acquired using 1-mm slice thickness. A plan was generated as shown in Figure 2. The plan consisted of five shots: two with 4-mm collimator shots, two with 8-mm shots, and one with a 16-mm shot. The prescribed dose for each shot was 2 Gy per fraction to the 50% isodose lines.
EBT3 Gafchromic film (Radiation Products Design, Inc., Albertville, MN) was used for measurement. The films were calibrated on the same day of each measurement by delivering calibrated doses, ranging from 0 to 10 Gy, to the calibration films using a stack of solid water and a 6-MV flattening filtered beam with a Truebeam STx Linac system (Varian, Palo Alto, CA). The calibration was performed by measuring the delivered doses with a field size of 10 × 10 cm2 at a calibration depth of 1.5 cm with source-to-surface distance of 100 cm. After 24 hours, the films were scanned on a flatbed scanner (Epson Expression 11000XL, Long Beach, CA) to 48-bit images at a resolution of 72 dots per inch. Dosimetric film analysis was performed using Film QA Pro software (Ashland, Bridgewater, NJ, USA) and positional analysis was performed using OmniPro software (IBA dosimetry, Schwarzenbruck, Germany) by analyzing two film profiles. Each treatment scenario was repeated three time to evaluate the measurement repeatability. Because each CBCT scan contributed no more than 2.5 mGy to the total dose, CBCT doses were not subtracted from the film measurements.[4]
Dosimetric deviation was measured using dose maps generated with the Film QA Pro software and values of the maximum dose in each shot were recorded. Each film was scanned three times and the maximum dose values per shot were averaged. The percent relative dose was calculated by dividing the maximum dose by that of the measurement without interruption or movement. Finally, the average and standard deviation of the relative doses per shot were calculated from three repeated measurements.
After the films were aligned to the reference film in OmniPro software using four pre-pinpricked marks, the maximum X- or Z-profile distance between two profiles at full width at half maximum was recorded for each scenario. The measurement uncertainty of the profile comparison was estimated to be 0.3 mm owing to the size of the pinpricks and the scanning resolution. Gamma analysis used a 2%/1 mm distance-to-agreement criteria.[3, 4]
Dosimetric and positional deviation for head displacement near HDMM alarm thresholds
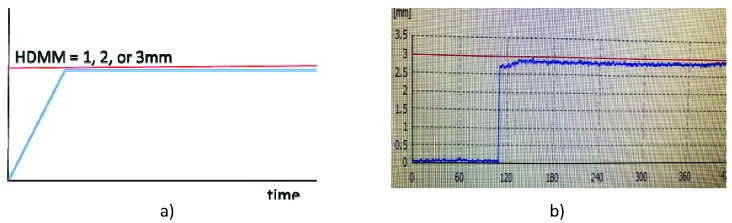
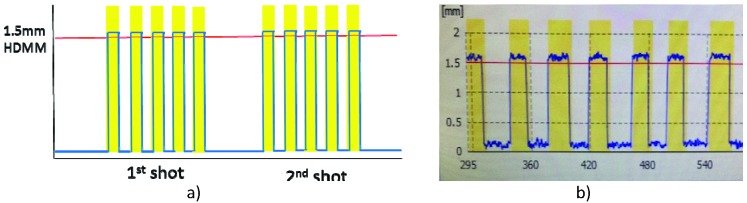
To investigate dosimetric and positional deviation at 1-, 2-, and 3-mm HDMM alarm thresholds, we rotated the phantom head after each treatment began in either the chin-up or chin-down direction to just below the HDMM alarm threshold. The phantom head remained in this position during the entire treatment, as shown Figure 3. This represents a scenario that would not require plan adaptation. The irradiated film was compared with one generated without any head rotation. For 1-, 2-, and 3-mm HDMM displacements, the phantom head was remotely rotated by 0.4°, 0.8°, and 1.2° pitch angles, respectively, while the beam was on. The amount of pitch angle change was verified by acquiring a CBCT image after the treatment was completed.
Figure 3.
a) Conceptual representation of the treatment scenario of head phantom displacement near the high-definition motion monitoring (HDMM) alarm threshold. The displacement measured by HDMM (blue line) remained just below the 1-, 2-, or 3-mm HDMM alarm threshold (red line). b) Actual screen capture for this scenario.
Dosimetric and positional deviation for multiple interruptions during treatment with plan adaptation
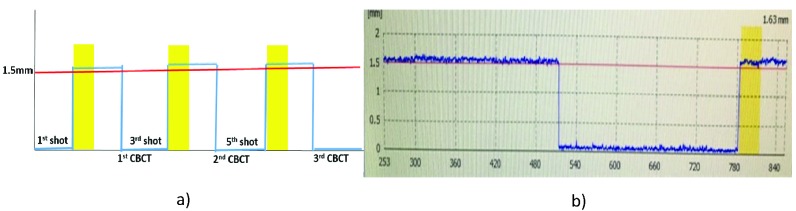
The purpose of this scenario was to investigate dosimetric and positional deviation for multiple plan adaptations following re-imaging caused by patient movement above the HDMM alarm threshold. This scenario could apply to a patient’s movement during treatment or resuming treatment after a restroom break. With a pre-defined HDMM alarm threshold of 1.5 mm or 3 mm, three interruptions were caused by remote-controlled pitch motions to above the threshold levels during the first, third, and fifth planned shots (Figure 2). After a 30-second beam hold, the treatment was paused in the middle of the first shot, and a second CBCT scan was acquired and automatically co-registered to CBCTref. Each new CBCT image acquisition reset the HDMM system’s reference baseline. After online review of the first adapted plan, the treatment was continued until the middle of the third shot. This interruption and plan adaptation procedure was repeated for the third and fifth shots (Figure 4) and the same amount of head rotation was accumulated with every interruption.
Figure 4.
a) Conceptual representation of the treatment scenario of multiple interruptions at the 1.5-mm high-definition motion monitoring (HDMM) alarm threshold. The yellow-colored line show gated beams due to the phantom movements out of HDMM alarm threshold in the middle of the first, third, and fifth shots. CBCT, cone beam computed tomography. b) Actual screen capture for this scenario.
Dosimetric and positional deviation for initial setup error at a pitch angle of up to 20°
Dosimetric and positional deviation was investigated in terms of plan adaptation caused by initial pitch angle difference. The same plan was delivered with initial pitch angles of 0°, 5°, 10°, 15°, or 20° rotated by the remote-controlled stepper motor before daily CBCT1, and each angle was verified within ±0.2° by co-registering CBCTref and CBCT1. The adapted plan was then reviewed, and treatment was delivered.
Dosimetric and positional deviation for patient coughing (short excursion above and return to below HDMM alarm thresholds)
The purpose of this scenario was to investigate dosimetric and positional deviations when a patient coughs frequently during a mask-based treatment, causing multiple gated beams. If the patient’s displacement returns to below the HDMM alarm threshold, then dose delivery is automatically re-initiated after 3 seconds. If the gating is activated for more than 30 seconds or more than five times during the same shot, then the treatment is paused, and the couch is retracted. Using the 1.5-mm HDMM alarm level setting, which is our default clinical setting, we rotated the head five times for a short period (5 seconds) during each shot (Figure 5). This scenario did not require re-imaging or plan adaptation because the head displacement returned to a level below the HDMM alarm threshold each time.
Figure 5.
a) Conceptual treatment scenario representing frequent patient coughing. Each shot was suspended five times (yellow areas), resulting in temporary suspension of beam delivery until the displacement returned to a level below the high-definition motion monitoring (HDMM) alarm threshold. b) Actual screen capture for this scenario.
Results
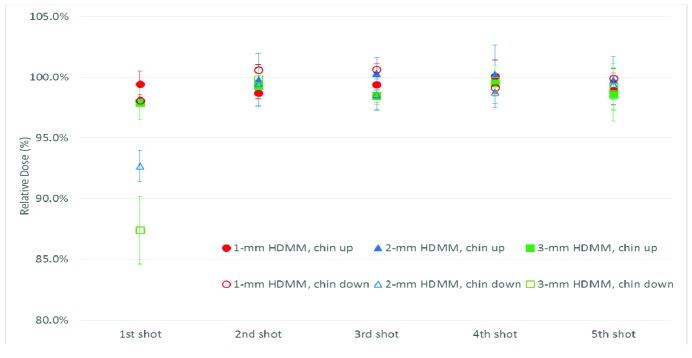
Dosimetric deviation for displacement near the HDMM alarm threshold
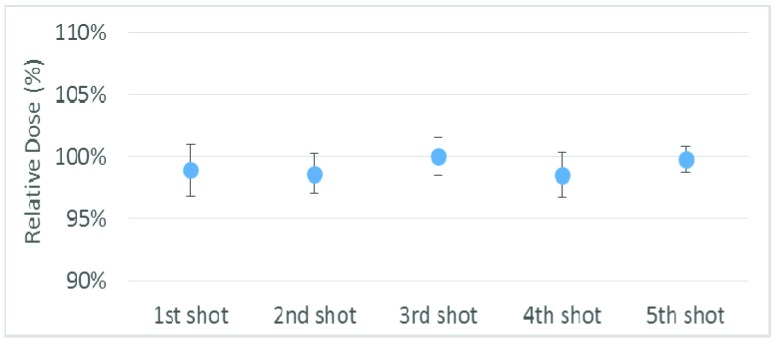
Figure 6 shows the dosimetric deviation for head rotation in either the chin-up or chin-down direction remaining near the HDMM alarm threshold during beam-on time. The x-axis shows the individual shot corresponding to Figure 2a and the y-axis represents a relative maximum dose to the measurement without movement. The error bars in all figures represent the standard deviation of each shot from three measurements. Dosimetric deviation was within the uncertainty of film measurement (±3%)[11-13] compared with the plan with no head rotation, except that the dose decreased by up to 7% and 13% for the first shot in the chin-down position at the 2- and 3 mm HDMM alarm thresholds, respectively. There are two possible reasons for this: 1) the first shot is located superiorly the farthest from the head rotation axis, and 2) the positional sensitivity was highest at the 4-mm shot because it has the sharpest dose drop-off.
Figure 6.
Dosimetric deviations for chin-up or chin-down displacement near the limit of the high-definition motion monitoring (HDMM) alarm threshold (1, 2, or 3 mm). The x-axis represents the shot number and the y-axis the relative maximum dose of each shot. Error bars represent the standard deviation of each shot from three measurements.
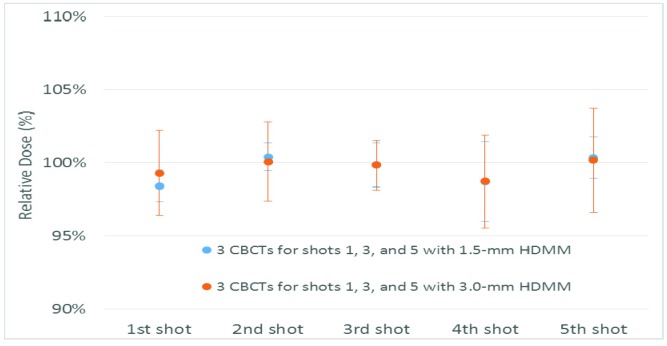
Dosimetric deviation for a treatment with multiple interruptions
Figure 7 shows dosimetric deviation due to treatment interruptions in the middle of the first, third, and fifth shots, causing multiple plan adaptations. The relative dose showed less than 1.5% deviation for all shots regardless of HDMM alarm threshold (1.5-mm or 3.0-mm).
Dosimetric deviation for initial setup error at a pitch angle of up to 20°
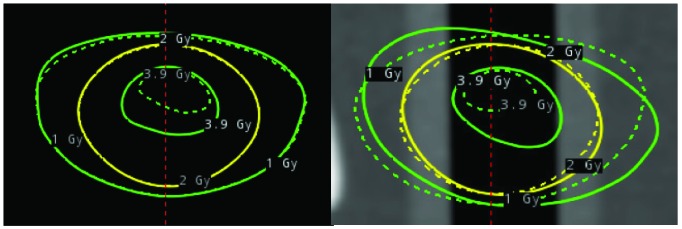
Figure 8 shows the dosimetric deviation when the initial setup pitch angle was set to 5°, 10°, 15°, or 20° compared with CBCTref. The dose deviated as the angle increased by more than 10° and the dose of the fifth shot increased up to 2.9% and that of the first shot decreased up to 3.5% at a pitch angle of 20°. One possible reason for dose deviation is that although the planned shot coordinates are updated to maintain their location relative to anatomic structures during plan adaptation, there are no other changes, including re-normalization or changes to shot times to account for the effective depth for each shot. The dose increase was also observed within the treatment plan adaptation, which showed deviation of the adapted isodose lines relative to the original plan (Figure 9).
Figure 9.
Overlay of the planned isodose lines (dotted lines) for 1.0 Gy (green), 2.0 Gy (yellow), and 3.9 Gy (green) and the adapted doses for delivery (solid lines) according to daily cone beam computed tomography studies for dose evaluation of the fifth shot. The film plane is shown in coronal view (left) and sagittal view (right).
Dosimetric deviation for patient coughing at the 1.5-mm HDMM alarm threshold
Figure 10 shows the dosimetric deviation for a patient coughing (short excursion above and return to below the 1.5-mm threshold) five times during each shot. The dose deviation was within 1.0%, indicating that dosimetric deviation was not clinically significant.
Positional accuracy in the coronal plane and gamma analysis of the 13 clinical treatment scenarios with various HDMM alarm thresholds
With consideration of uncertainty of 0.3 mm from placement of the pin-pricks and scan resolution, the positional accuracy of most clinical treatment scenarios remained within measurement uncertainty (Table 1). Gamma analysis showed a passing rate of >97% for all scenarios, including multiple interruptions at the 3-mm HDMM alarm threshold and an initial head rotation of 20o.
Table 1.
Positional accuracy and gamma analysis of the 13 clinical treatment scenarios with various high-definition motion monitoring (HDMM) alarm thresholds.
| Treatment scenario | Deviation, mm | Gamma (2%/1 mm) |
| 1-mm HDMM within threshold, chin up | 0.1 | 99.2 |
| 2-mm HDMM within threshold, chin up | 0.3 | 99.7 |
| 3-mm HDMM within threshold, chin up | 0.4 | 99.2 |
| 1-mm HDMM within threshold, chin down | 0.2 | 99.4 |
| 2-mm HDMM within threshold, chin down | 0.3 | 99.2 |
| 3-mm HDMM within threshold, chin down | 0.4 | 99.5 |
| 3 interruptions with 1.5-mm HDMM | 0.2 | 98.8 |
| 3 interruptions with 3.0-mm HDMM | 0.2 | 98.7 |
| Patient coughing | 0.1 | 99.0 |
| Initial setup error of 5° pitch angle | 0.2 | 99.4 |
| Initial setup error of 10° pitch angle | 0.3 | 99.1 |
| Initial setup error of 15° pitch angle | 0.2 | 98.8 |
| Initial setup error of 20° pitch angle | 0.3 | 97.0 |
Figure 7.
Dosimetric deviations for multiple interruptions during treatment that required plan adaptation. Error bars represent the standard deviation of each shot from three measurements. CBCT, cone beam computed tomography; HDMM, high-definition motion management.
Figure 8.
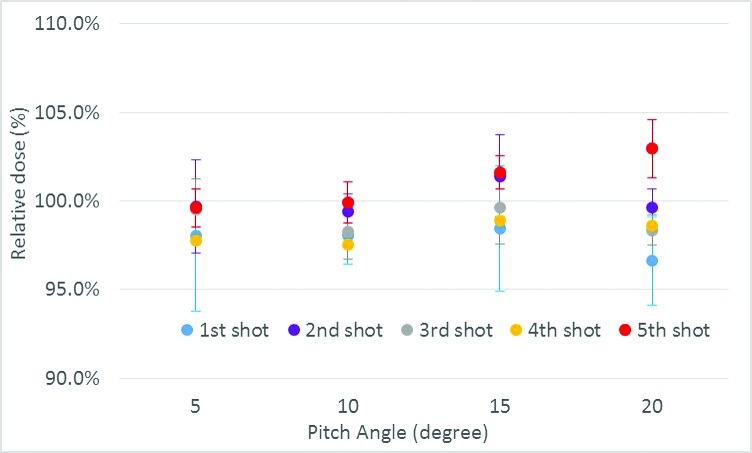
Dosimetric deviations for displacement of pitch angle up to 20° during initial setup. Error bars represent the standard deviation of each shot from three measurements.
Figure 10.
Dosimetric deviation for patient coughing at the 1.5-mm high-definition motion monitoring alarm threshold. Error bars represent the standard deviation of each shot from three measurements.
Discussion
The current study shows the importance of end-to-end testing of realistic clinical scenarios using a pitch angle-adjustable phantom. For example, although dosimetric deviation in the chin-up position without plan adaptation was within 2% for HDMM alarm thresholds of up to 3 mm, dosimetric deviation for the chin-down position showed a dramatic decrease of up to 7-13% for the 2- and 3-mm HDMM alarm thresholds in the most superiorly located shot. This result shows the importance of using plan adaptation and selecting a proper HDMM alarm setting. Dosimetric deviation in the superior target in the pitch direction was consistent with that reported by Burton et al.[8]
Multiple adapted plans showed dose deviations of less than 1.5% even at the 3-mm HDMM alarm threshold. We observed dose deviations with the first and fifth shots at an initial setup pitch angle of 20°, revealing the possible limitation in plan adaptation after a large head rotation. Even though this is an extreme scenario, it suggests that repositioning a patient is advisable if the daily co-registration shows a head pitch rotation of more than 10°.
Frequent short interruptions, such as coughing, did not cause substantial dosimetric deviation. Positional deviation measured in the coronal film plane was not clinically significant in any clinical scenarios. Therefore, we concluded that the plan adaptation and HDMM system of the GK Icon were validated with 13 scenarios.
Wright et al commented that pitch movement of the head generates uncertainty in the relationship between nose tip movement and intracranial movement.[7] It is crucial to validate dosimetric and positional accuracy with HDMM systems for rotational head movement, much more so than for translational movement. To the best of our knowledge, the current study is novel in that the pitch angle-adjustable phantom system was designed and built with an anthropomorphic phantom driven by a high-precision stepper motor. Using this phantom, we were able to analyze and verify dosimetric and positional uncertainty for realistic clinical treatment scenarios.
The results of our study are limited by the specific geometry of the phantom assembly, especially the location of the rotating axis in relation to that of the film (in the coronal plane instead of the sagittal plane) and shot configuration. The film was posteriorly located from the center of the rotation axis by 11 mm, whereas the nose marker was anteriorly placed at 114 mm from the axis. The film plane was closer to the rotation axis than to the nose marker. However, the pivot point in the current study is realistic in the sense that the patient’s pivot point is most likely near the foramen magnum connected to the cervical spine. As Burton et al noted, it is important to consider the location of a lesion in relation to the nose tip and pivot point.[8] EBT3 film also has larger inherent measurement uncertainties, such as dosimetric accuracy (±3%), compared with an ionization chamber.
The experiments described in this work could be used for annual or monthly quality assurance. Other GK Icon users could use such tests or end-to-end tests of plan adaptation and the HDMM system for commissioning. Our findings confirm the reliability of the GK Icon system but also caution against treating a superiorly located small lesion while using ≥2-mm HDMM alarm threshold or applying plan adaptation at a large initial setup pitch angle. In addition, we tested a simple treatment plan instead of a more complicated clinical plan that would include targets and organs-at-risk. To increase clinical relevance, further studies should include a realistic patient plan that includes critical structures.
In conclusion, the GK Icon system is a reliable tool for plan adaptation and motion management for patients undergoing frameless radiosurgery. Dosimetric and positional deviation from the head rotation related to clinical scenarios was successfully presented using a motorized anthropomorphic head phantom in the current study. Our results indicate that the dosimetric and positional accuracy of the GK Icon system is acceptable even in extreme clinical treatment conditions. However, caution needs to be taken for dosimetric uncertainty when treating small lesions located superiorly from the rotational axis with an HDMM alarm threshold of more than 2 mm or when applying plan adaptation with a large initial setup rotational error.
Acknowledgments
The authors thank Erica Goodoff in Scientific Publications at The University of Texas MD Anderson Cancer Center for editing this manuscript.
Footnotes
Authors disclosure of potential conflicts of interest
The authors have nothing to declare.
Author contributions
Conception and design: Eun Young Han, Zhifei Wen, Kelly Tharp
Data collection: Eun Young Han, Luo Dershan, Zhifei Wen
Data analysis and interpretation: Eun Young Han, Zhifei Wen, Jong Oh Kim
Manuscript writing: Eun Young Han
Manuscript review: Luo Dershan, Jong Oh Kim, Zhifei Wen, Tina Briere
Final approval of manuscript: Zhifei Wen, Tina Briere
References
- 1. W Li, Bootsma G, Von Schultz O, Carlsson P, Laperriere N, Millar BA, Jaffray D, Chung C. Preliminary evaluation of a novel thermoplastic mask system with intra-fraction motion monitoring for future use with image-guided Gamma Knife. Cureus. 2016;8(3):e531. Epub 2016/04/16. doi: 10.7759/cureus.531. PubMed PMID: 27081592; PubMed Central PMCID: PMCPMC4829406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. AlDahlawi I, Prasad D, Podgorsak MB. Evaluation of stability of stereotactic space defined by cone-beam CT for the Leksell Gamma Knife Icon. J Appl Clin Med Phys. 2017;18(3):67-72. Epub 2017/04/19. doi: 10.1002/acm2.12073. PubMed PMID: 28419781; PubMed Central PMCID: PMCPMC5689865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dorenlot A, Champoudry J. End-to-end tests of the new Elekta Gamma Knife ICON. Phys Med. 2016;32:355. doi: 10.1016/j.ejmp.2016.11.081. [DOI] [Google Scholar]
- 4. Zeverino M, Jaccard M, Patin D, Ryckx N, Marguet M, Tuleasca C, Schiappacasse L, Bourhis J, Levivier M, Bochud FO, Moeckli R. Commissioning of the Leksell Gamma Knife® Icon. Med Phys. 2017;44(2):355-63. Epub 2017/01/31. doi: 10.1002/mp.12052. PubMed PMID: 28133748. [DOI] [PubMed] [Google Scholar]
- 5. Elekta. Leksell GammaPlan Online Reference Manual. 2017.
- 6. AlDahlawi I, Prasad D, Podgorsak MB. Quality assurance tests for the Gamma Knife® Icon image guidance system. J Appl Clin Med Phys. 2018;19(5):573-9. Epub 2018/08/05. doi: 10.1002/acm2.12417. PubMed PMID: 30076672; PubMed Central PMCID: PMCPMC6123129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wright G, Harrold N, Hatfield P, Bownes P. Validity of the use of nose tip motion as a surrogate for intracranial motion in mask-fixated frameless Gamma Knife® Icon therapy. J Radiosurg SBRT. 2017;4(4):289-301. Epub 2018/01/04. PubMed PMID: 29296453; PubMed Central PMCID: PMCPMC5658824. [PMC free article] [PubMed] [Google Scholar]
- 8. Burton K. End-To-End testing of the Leksell Gamma Knife ICON system. Georgia Institute of Technology. 2017;MS Thesis.
- 9. Wu C, Radevic MB, Glass JS, Skubic SE. Technical note: A 3D-printed phantom for routine accuracy check of Gamma Knife Icon HDMM system. J Appl Clin Med Phys. 2018;19(4):299-301. Epub 2018/05/26. doi: 10.1002/acm2.12339. PubMed PMID: 29797396; PubMed Central PMCID: PMCPMC6036381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blake SW, Winch L, Appleby H. EP-1942: Initial experience with the Elekta Leksell Gamma Knife Icon system: Commissioning, QA and workflow. Radiother Oncol. 2016;119:S921. doi: 10.1016/S0167-8140(16)33193-0. [DOI] [Google Scholar]
- 11. Wołowiec P, Kukołowicz PF. The analysis of the measurement uncertainty with application of small detectors made of Gafchromic EBT films for the range of doses typical for in vivo dosimetry in teleradiotherapy. Radiat Measurements. 2016;92:72-9. doi: 10.1016/j.radmeas.2016.08.001. [DOI] [Google Scholar]
- 12. Marroquin EYL, Herrera González JA, Camacho López MA, Barajas JEV, García-Garduño OA. Evaluation of the uncertainty in an EBT3 film dosimetry system utilizing net optical density. J Appl Clin Med Phys. 2016;17(5):466-81. doi: 10.1120/jacmp.v17i5.6262. PubMed PMID: 27685125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sorriaux J, Kacperek A, Rossomme S, Lee JA, Bertrand D, Vynckier S, Sterpin E. Evaluation of Gafchromic EBT3 films characteristics in therapy photon, electron and proton beams. Phys Med: Eur J Med Phys. 2013;29(6):599-606. doi: 10.1016/j.ejmp.2012.10.001.. [DOI] [PubMed] [Google Scholar]