Introduction
For medically intractable temporal lobe epilepsy (TLE), Level I evidence has established the superiority of anterior temporal lobectomy (ATL) over either continued medical management alone or stereotactic radiosurgery (SRS) in achieving durable seizure freedom (1-2). Recent work has indicated that the piriform cortex, a structure not included in traditional operative nor radiosurgical volumes, plays a key role in TLE and that inclusion of this structure in operative resection can increase the likelihood of seizure freedom (3). For TLE patients who are not operative candidates, this structure provides a potential SRS target. Presently, there exists no methodology for radiation treatment planning delineation of the piriform cortex.
Methods
Using noncontrast head computed tomography (CT) and brain magnetic resonance imaging (MRI), a standardized methodology for delineating the piriform cortex was created. To simulate patients undergoing linear accelerator (LINAC) SRS, contours were created from images of previously treated patients having undergone LINAC SRS whose lesions did not anatomically distort the primary olfactory cortex (4-5). Contours for LINAC SRS were designed from fusion of noncontrast CT and T1-weighted noncontrast MRI with 0.1 centimeter (cm) axial slice thickness. To simulate patients undergoing Gamma Knife stereotactic radiosurgery (GKRS), contours were created from the Leksell stereotactic frame-based 0.1 cm axial slice thickness T1 and T2 3D fast spoiled gradient echo (FSPGR) images of patients having undergone previous GKRS for pathology not distorting the primary olfactory cortex.
Contouring
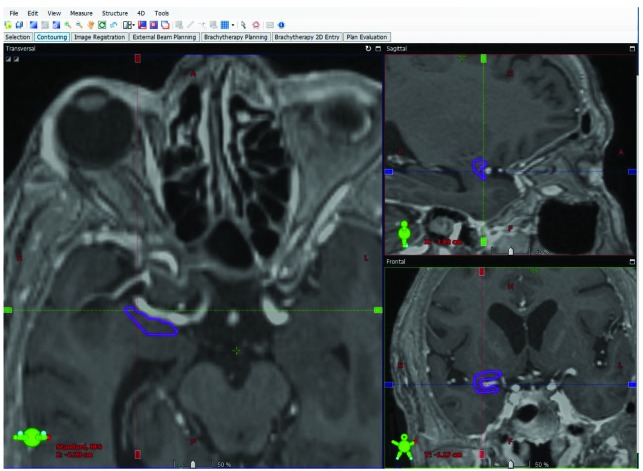
The piriform cortex was contoured eight separate times from four patients (two LINAC SRS, two GKRS). Contouring for LINAC SRS originated within one cm anterior to the anterior commissure, rostromedial to the amygdala, inferior to the putamen, inferolateral to the globus pallidus, and inferomedial to the claustrum, terminating posteriorly at the level of the anterior commissure (Figure 1). The frontal lobe and temporal lobe portions of the piriform cortex (lateral to the olfactory tubercle and periamygdaloid complex, respectively) encompassing the lateral aspect of the middle cerebral artery were reliably contoured, best visualized on coronal imaging (Figure 1). These same anatomic landmarks were successfully used for GKRS contouring. The slightly greater targeting accuracy of GKRS, obviating the need for placing the 0.2 cm margin typically administered to account for LINAC SRS geometric errors (6), may potentially be of clinical significance in treating TLE.
Figure 1.
Contour from fused noncontrast head CT/T1-weighted brain MRI for simulated linear accelerator stereotactic radiosurgery demonstrating the right piriform cortex on axial, sagittal and coronal images.
Further investigation is warranted to determine the safety and efficacy of piriform cortex SRS for TLE, particularly since SRS underdosing of the traditional TLE target (parahippocampal gyrus, amydgala, and anterior 2 cm of the hippocampus) has resulted in fatal consequences from failure to achieve seizure control (7). Given the established 52% three-year Engel Class I outcome (seizure-freedom without medication) rate of SRS for TLE from Level I evidence, it is plausible that adding the piriform cortex to the traditional TLE SRS volume increases the rate of Engel Class I outcomes towards the 78% three-year rate associated with ATL, or can be used in patients previously having undergone ATL who have not achieved Engel Class I outcomes and are no longer surgical candidates (2).
Acknowledgments
The authors would like to thank Sook Kien Ng, PhD and Sarah Masick, RT for invaluable assistance.
Footnotes
Source of financial support/funding statement
This manuscript received no funding.
Authors’ disclosure of potential conflicts of interest
The authors have nothing to disclose.
Author contributions
Study concept and design: Shearwood McClelland III
Acquisition, analysis, or interpretation of data: Shearwood McClelland III, Gordon A Watson
Drafting of the manuscript: Shearwood McClelland III
Critical revision of the manuscript for important intellectual content: Shearwood McClelland III, Gordon A Watson
Administrative, technical, or material support: Shearwood McClelland III, Gordon A Watson
Study supervision: Shearwood McClelland III, Gordon A Watson
References
- 1. Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and efficiency of surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345(5):311-318. [DOI] [PubMed] [Google Scholar]
- 2. Barbaro NM, Quigg M, Ward MM, Chang EF, Broshek DK, Langfitt JT, Yan G, Laxer KD, Cole AJ, Sneed PK, Hess CP, Yu W, Tripathi M, Heck CN, Miller JW, Garcia PA, McEvoy A, Fountain NB, Salanova V, Knowlton RC, Bagić A, Henry T, Kapoor S, McKhann G, Palade AE, Reuber M, Tecoma E. Radiosurgery versus open surgery for mesial temporal lobe epilepsy: The randomized, controlled ROSE trial. Epilepsia. 2018;59(6):1198-1207. [DOI] [PubMed] [Google Scholar]
- 3. Galovic M, Baudracco I, Wright-Goff E, Pillajo G, Nachev P, Wandschneider B, Woermann F, Thompson P, Baxendale S, McEvoy AW, Nowell M, Mancini M, Vos SB, Winston GP, Sparks R, Prados F, Miserocchi A, de Tisi J, van Graan LA, Rodionov R, Wu C, Alizadeh M, Kozlowski L, Sharan AD, Kini LG, Davis KA, Litt B, Ourselin S, Moshé SL, Sander JWA, Löscher W, Duncan JS, Koepp MJ. Association of piriform cortex resection with surgical outcomes in patients with temporal lobe epilepsy. JAMA Neurol. 2019. Mar 11. doi: 10.1001/jamaneurol.2019.0204. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Silveira-Moriyama L, Glass P, Rajan S, Carvalho R, Reis F, Penatti CA, Muio V. The hitchhiker’s guide to the rhinencephalon. Arq Neuropsiquiatr. 2016;74(4):329-336. [DOI] [PubMed] [Google Scholar]
- 5. Gonçalves Pereira PM, Insausti R, Artacho-Pérula E, Salmenperä T, Kälviäinen R, Pitkänen A. MR volumetric analysis of the piriform cortex and cortical amygdala in drug-refractory temporal lobe epilepsy. AJNR J Am Neuroradiol. 2005;26(2):319-332. [PMC free article] [PubMed] [Google Scholar]
- 6. McClelland S, 3rd, Watson GA. Impact MRI of timing on accuracy of stereotactic radiosurgical planning: visualizing the forest from the trees. Int J Radiat Oncol Biol Phys. 2019;103(4):1012-1013. [DOI] [PubMed] [Google Scholar]
- 7. Srikijvilaikul T, Najm I, Foldvary-Schaefer N, Lineweaver T, Suh JH, Bingaman WE. Failure of Gamma Knife radiosurgery for mesial temporal lobe epilepsy: report of five cases. Neurosurgery. 2004;54(6):1395-1404. [DOI] [PubMed] [Google Scholar]