Abstract
Stereotactic body radiation therapy (SBRT) is a relatively new technology, and its use among patients with benign spinal tumors has limited prospective data. Similar to intracranial benign tumors treated successfully with SBRT, benign spinal tumors of the same histology can also develop, and SBRT may be an effective treatment alternative in inoperable or recurrent cases. Outcomes in patients with neurofibromatosis type 1, neurofibromatosis type 2, or schwannomatosis treated with SBRT have also been reported. Single institution reports have shown local control rates over 90% and improvement in clinical symptoms. The optimum dose and fractionation to maximize local control and minimize toxicity is unknown, with few incidences of radiation treatment-related toxicities. Given the location and benign nature of these tumors, careful management of dose to critical organs is essential. With continued follow-up, the optimum use of SBRT in patients with benign spinal tumors can be better defined.
Keywords: SBRT, radiosurgery, spinal meningioma, neurofibroma, schwannoma, benign tumor
Introduction
Benign spinal tumors (BST) account for nearly 70% of all spinal tumors [1]. Primary surgical resection remains the standard treatment, demonstrating excellent tumor control and low morbidity [2-7]. Previous attempts to use external beam radiotherapy (EBRT) for spinal tumors have been limited by spinal cord tolerance [8, 9]. However, with development of stereotactic body radiation therapy (SBRT), high doses of conformal radiation can be delivered to a target while sparing the spinal cord. The use of SBRT for the treatment of benign spinal tumors has been emerging.
Types of Benign Spinal Tumors
The most common benign tumors include meningiomas and nerve sheath tumors (NST) (schwannomas and neurofibromas), which comprise 25% and 25-30% of spinal tumors, respectively [1, 3, 6, 7, 10, 11]. These tumors are commonly intradural and extramedullary in location [12].
Radiographically, meningiomas and NSTs are typically iso-intense on T1 weighted, and hyperintense on T2 weighted MR imaging [10, 12-15]. Meningiomas show moderate, homogenous post-gadolinium enhancement, whereas schwannomas demonstrate more avid enhancement that may be heterogeneous [13]. Neurofibromas tend to have a more homogeneous contrast enhancement compared to schwannomas (Table 1).
Table 1.
Imaging findings of benign spine tumors by histology
| Histology | CT findings | MRI - T1 | MRI - T2 | MR Contrast Enhancement | Other |
| Meningioma | -Frequent calcifications -May have bone erosions, sclerosis or mixed osseous changes -May enlarge adjacent paranasal sinuses |
Iso/hypo-intense | Hyper/iso-intense, | Homogeneous enhancement (except calcifications) | -Relatively more common in thoracic location -More common in females -Dural tail sign -Displacement of spinal cord (widening of the ipsilateral subarachnoid space) |
| Schwannoma | -Iso/hypo-dense -Bone remodeling -Scalloping of larger lesions -Rounded -Cystic changes |
Iso-intense | -Hyper-intense, heterogeneous -Fluid signal intensity in cystic components -May have target sign (peripheral T2 hyperintensity with central hypointensity) |
-Avid, irregular/heterogeneous enhancement | -Often in cervical/lumbar dorsal nerve roots with foraminal extension (dumbbell shape) -Peripherally located within nerve root -Fluid-fluid level may be present -Well circumscribed Multiple lesions in NF2 and schannomatosis |
| Neurofibroma | -Bone remodeling -Scalloping of larger lesions -Round or fusiform |
Iso-intense | -Hyper-intense -T2 hyperintense rim with central hypointensity (target sign) |
-Typically homogeneous enhancement | -Infiltrative lesion within nerve roots -Difficult to distinguish from schwannoma, particularly in the setting of NF2 -Commonly smaller than schwannomas -Multiple lesions may be seen in NF1 |
Patient Selection
Microsurgical resection is the standard treatment for BSTs. However, patients with multifocal disease, advanced age, poor performance status, or comorbidities may be poor surgical candidates [16]. Patients with inoperable, incompletely resected, or recurrent disease after prior surgical therapy may also be candidates for alternate treatment approaches. Although histopathologic diagnosis is generally preferred prior to SBRT, some patients are treated without pathologic confirmation when the risk of biopsy is considered too high and/or radiographic and clinical characteristics are deemed sufficient.
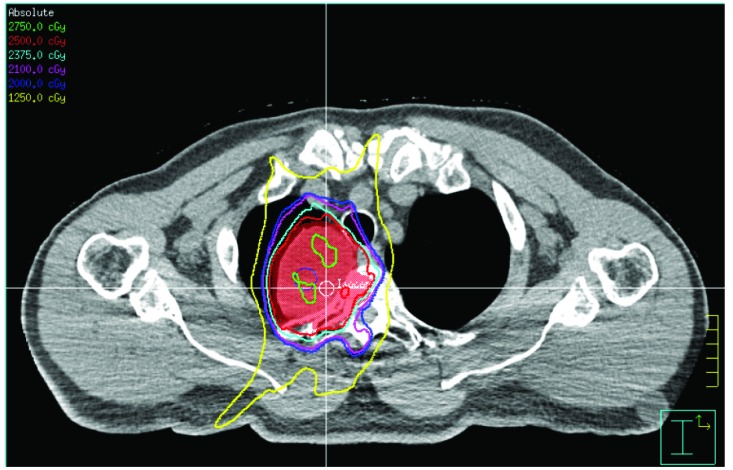
Patients who undergo spinal SBRT for BSTs are typically symptomatic with pain, sensory disturbances, extremity weakness, and/or incontinence. However, asymptomatic patients may also be considered for SBRT, particularly if further growth may cause neurologic compromise. SBRT is typically considered to be appropriate for well circumscribed lesions, with a spatial relationship to the spinal cord that allows differential dosing to the spinal cord and tumor (Figure 1) [17]. SBRT is contraindicated in patients with spinal instability and/or osseous cord compression or myelopathy. Reirradiation with SBRT can be feasible depending on the previous dose to the spinal cord at the index spinal segment [16].
Figure 1.
A T4 neurofibroma treated with SBRT to a dose of 25 Gy in 5 fractions (red isodose line).
When treating benign tumors, the risks must be carefully evaluated against benefits. The endpoints commonly evaluated with BSTs include pain relief and local control, however this must be weighed against late toxicities such as radiation myelopathy.
Radiation Planning and Delivery
Treatment Device
Several systems are available, including robotic-based (CyberKnife) and linear accelerator (LINAC) based systems, while newer MRI based platforms are on the horizon. Image guidance with intrafraction orthogonal X-rays (CyberKnife X-Sight and BrainLab ExacTrac) or cone beam CT in conjunction with robust immobilization devices (e.g. Elekta BodyFIX) are an essential aspect of the precision of SBRT [18-20]. Emerging technologies for SBRT include ring-mounted LINAC with rotational capabilities and MRI-based image guidance [21].
CT Simulation
Patients are simulated and treated in supine position with appropriate immobilization. Thermoplastic masks extending to the chest can be used for cervical and upper thoracic spine lesions (down to T4), while semi-rigid vacuum body fixation can be used in lower thoracic and lumbosacral lesions [17, 22-25]. CT based simulation is performed with ≤1.25 mm slice thickness within the range of target volumes and organs at risk (OAR) that may be within the beam path.
Delineation of Targets and Organs-at-Risk and Radiation Treatment Planning
Fusion of treatment planning CT and MRI facilitates delineation of gross tumor volume (GTV) (T1 with gadolinium) and spinal cord (T2). As BSTs are typically well demarcated on MRI, a clinical target volume (CTV) expansion is unnecessary. Planning target volume (PTV) expansions vary among different institutions (range 0-3 mm). Some institutions add 1.5-2 mm margins to the spinal cord contour to generate a planning-at-risk volume (PRV) which accounts for independent physiologic spinal cord and intra-fraction patient bulk motion, and potential variations in spinal cord contouring [26]. When a PTV expansion is performed, the portion extending into the spinal cord or spinal cord PRV should be trimmed to avoid overdosing OARs.
Dose regimens vary throughout the literature and range from 11.6 Gy to 30 Gy in 1-6 fractions. Compared to spinal metastases, lower biological effective doses (BED) are typically needed to yield durable local tumor control. Dr. Sahgal has published the spinal cord tolerance guidelines for radiation naïve patients, and interested readers can refer to the paper for more details [27]. In addition, Dr. Kalash from the University of Pittsburgh Medical Center published the results of their retrospective series on dose de-escalation with no difference in local control of BSTs between high dose (BED10Gy >30) versus low dose SBRT [28].
Additionally, inverse treatment planning algorithms allow for maximum tumor coverage while protecting the spinal cord and other critical organs. The importance of an optimal treatment planning algorithm cannot be overemphasized. In a study from Case Western Reserve University when Ray Tracing algorithm was used instead of Monte Carlo calculation for planning CyberKnife-based SBRT in the thoracic region, the actual PTV coverage was decreased and the spinal cord dose was significantly underestimated in some patients [29]. The Imaging and Radiation Oncology Core (IROC) Houston, in cooperation with the RTOG, has tested several treatment planning systems and provided a list of acceptable systems for dose calculation within a medium of heterogeneity, as is often seen with thoracic spine lesions [30].
Spinal Meningiomas
Spinal meningiomas comprise 10% of all meningiomas and 25% of all spinal tumors [31, 28]. Most spinal meningiomas occur in the thoracic region, posterolateral to the spinal cord [10, 14].
In the Stanford University series, 32 spinal meningiomas with a mean volume of 3.03 cc (0.14-11.05cc) were treated to a median dose of 20.57 Gy (16–30 Gy) over a median of 2 fractions [25]. Approximately 50% of lesions had been previously resected, and the remaining were diagnosed by imaging. At median follow-up of 29 months, all treated meningiomas were either stable (47%) or decreased (53%) in size. Overall, 91% of meningiomas had stable or improved neurologic symptoms, with 57% of patients reporting pain improvement and 43% reporting minimal change. One case of late onset transient myelitis was observed 9 months after treatment for a 7.6 cc recurrent meningioma treated to 24 Gy over 3 fractions.
Colleagues from the University of Pittsburgh treated 13 spinal meningiomas (11 with pathologic diagnosis) to a median dose of 21.25 Gy (range 17.5-25 Gy) with mostly single fraction SBRT [17]. At a median follow-up of 14 months, all lesions were radiographically controlled, and a single event of spinal cord toxicity was observed. Similar excellent rates of radiographic control have also been noted by others at a median follow up of 18-25 months, without spinal cord toxicities (Table 2).
Table 2.
Published studies reviewed.
| Author, Publication Date | Patients (no.) | Patients with NF (no.) | Total Lesions (no.) | Lesions by Histology (no.) | Radiation Delivery | Prescription Dose, % Isodose Line | Fractionation | Median Follow-up (months) | Outcomes | Radiation Toxicities (no. patients) |
| Gerszten P, 2003 [16] | 15 | NR | 15 | 2 Meningiomas 25 Schwannomas 5 Neurofibromas |
CyberKnife | 1200 - 2000 cGy, 80% | 1 | mean 12 | 100% LC | None |
| Dodd R, 2006 [42] | 51 | 17 | 55 | 16 Meningiomas 30 Schwannomas 9 Neurofibromas |
CyberKnife | 1600 - 3000 cGy, 80% | 1 - 5 | 23 | 95% LC | 1 Radiation Myelopathy |
| Sahgal A, 2007 [35] | 16 | 3 | 19 | 2 Meningiomas 0 Schwannomas 11 Neurofibromas |
CyberKnife | Median 2100 cGy, 80% | 3 | 25 | 12 month FFP**: 89% 3-year FFP**: 71% |
1 Nausea |
| Gerszten P, 2008 [17] | 73 | 30 | 73 | 13 Meningiomas 35 Schwannomas 25 Neurofibromas |
CyberKnife | 1500 - 2500 cGy, 80% | 1 - 3 | 37 | 100% LC 73% PC |
3 Brown-Sequard syndrome |
| Murovic J, 2009 [66] | 15 | 3 | 17 | 0 Meningiomas 10 Schwannomas 7 Neurofibroma |
CyberKnife | 1600 - 2400 cGy | 1 - 3 | 6 - 72 | 76% LC 85% PC 86% NC |
None |
| Selch M, 2009 [36] | 20 | 8 | 25 | 0 Meningiomas 8 Schwannomas 8 Neurofibromas |
LINAC | 1200 - 1500 cGy, ≥90% | 1 | 18 | 100% LC 100% NC |
None |
| Sachdev S, 2011 [25] | 87 | 25 | 103 | 32 Meningiomas 47 Schwannomas 24 Neurofibromas |
CyberKnife | 1400 - 3000 cGy |
1 - 5 | 29 | 98% LC 67-100% PC 67-91% NC |
1 Transient Myelitis |
| Gerszten P, 2012 [69] | 45 | 9 | 45 | 10 Meningiomas 16 Schwannomas 14 Neurofibromas | LINAC (Synergy S) | 1600 cGy | 1 - 3 | 32 | 100% LC 79% PC*** 93% NC |
None |
| Kufeld M, 2012 [34] | 36 | 3 | 36 | 11 Meningiomas 25 Schwannomas |
CyberKnife | 1340 - 1400 cGy, 70% | 1 | 18 | 100% LC 97-100% PC 100% NC |
1 Transient Myelitis |
| Niazi T, 2012 [67] | 1 | 0 | 1 | 0 Meningiomas 1 Schwannomas 0 Neurofibromas |
LINAC | 1500 cGy, 90% | 1 | 15 | Stable Disease | None |
| Marchetti M, 2013 [23] | 18 | 5 | 21 | 11 Meningiomas 9 Schwannomas 1 Neurofibroma |
CyberKnife | 1160 - 2270 cGy, 95% | 1 - 6 | 38 | 100% LC 79% PC 79% NC |
1 Sciatalgia 1 Nausea |
| Lee M, 2015 [37] | 11 | NR | 11 | 11 Meningiomas | Median 1500 cGy - 2600 cGy | 1 - 3 | 46.9 | 100% LC | None | |
| Shin DW, 2015 [49] | 58 | 7 | 110 | 47 Schwannomas | LINAC | Median 1300 cGy | 1 | 44 | 95% LC 100% PC 100% NC |
None |
| Kalash R, 2018 [28] | 38 | NR | 47 | 15 Meningiomas 13 Schwannomas |
CyberKnife LINAC |
900 - 2100 cGy | 1 - 3 | 54 | 76% LC | None |
| Elibe R, 2018 [33] | 9 | NR | 9 | 4 Meningiomas 3 Schwannomas |
LINAC | 1000 - 2400 cGy, 90% | 1 | 13 | 77% LC^ 88% PC^ 65% NC^ |
No toxicity after treatment of benign lesions |
Key: NF = Neurofibromatosis, M = Meningioma, S = Schwannoma, N = Neurofibroma, LC = Local Control, PC = Pain Control, NC = Neurologic Control, NR = Not Reported
Range,
Actuarial Kaplan Meier (excluding previously irradiated lesions),
Pain Improvement, ^ mixed results of treated benign and malignant lesions
After treatment, patients with spinal meningiomas may be monitored radiographically. In a Korean study, 11 patients treated with SRS (7 patients; median dose 15 Gy) or SBRT (4 patients; median dose 26 Gy in 3 fractions) were followed for a median of 46.9 months [37]. All lesions were controlled locally, with an average volume reduction of 29.7%. No statistically significant changes in enhancement patterns or T2 signal intensity were found.
Overall, spinal meningiomas treated with SBRT to doses ranging from 16 Gy in one fraction to 30 Gy in 5 fractions achieved excellent local control rates comparable to surgical outcomes after gross total resection [28, 38, 39]. The results also parallel findings seen with fractionated radiotherapy and single fraction radiosurgery for intracranial meningiomas [40, 41]. SBRT also appears to provide pain improvement in up to 30% of patients over the initial weeks to months, however motor deficits rarely improve [25, 34, 42]. The comparable outcomes of spinal SBRT and both surgery and intracranial stereotactic radiosurgery suggest that SBRT for spinal meningiomas is feasible, although longer follow up is necessary to better define its therapeutic role.
Spinal Schwannomas
Spinal schwannomas comprise a third of spinal neoplasms [7, 42]. Spinal schwannomas have varied growth rates, similar to acoustic schwannomas, and only a minority of tumors will manifest with symptoms [7, 42-44]. They are typically located posterior to the spinal cord, and thus surgical resection is preferred [2, 7, 39]. However, these tumors may be associated with neurofibromatosis type 2 (NF2) and schwannomatosis and can present with multiple lesions [44-48]. NF2-associated lesions are clinically more aggressive, tend to grow faster, cause neurological deficits sooner, recur more frequently, and are less responsive to treatment [7, 11, 16].
In the largest published series from Dr. Sachdev at the Stanford University Cancer Center, 47 spinal schwannomas were treated with Cyberknife-based SBRT, for which 11%, 21%, and 7% of lesions from the entire cohort (103 spinal lesions) had associated diagnoses of neurofibromatosis type 1 (NF1), NF2, and schwannomatosis, respectively [25]. The mean tumor volume was 6.18 cc (0.05-54.52 cc) and mean dose delivered was 18.74 Gy (14-24 Gy) over a median of 1 fraction (range: 1-4 fractions). At a median follow up of 29 months (range: 6-87 months), a single lesion progressed 73 months after treatment after receiving 18 Gy in 3 fractions, resulting in a crude control rate of 98%. Radiographic regression was noted in 47% of patients, half of which decreased to less than half the original tumor size. Pain was improved in 54% of patients and progressed in only 14%. Salvage surgery was attempted in four patients (only one of which experienced radiographic progression), with subsequent symptomatic improvement in three of four patients and no change in symptoms in one patient. No late spinal cord toxicities were noted.
A Korean series reported outcomes of 54 patients with benign tumors (47 spinal schwannomas), who presented with pain (63%) or neurologic symptoms (24%) [49]. Most were treated with single fraction SBRT (72%) to a median of 13 Gy as primary therapy, and the remaining patients received a combination of surgery and SBRT. At a mean follow up of 43.2 months [range: 12-136.8 months], the crude radiographic control rate was 95%, with 55% of lesions showing regression. Transient swelling was noted in 20% of lesions at a median time from SRS of 8 months (range: 5.1-44.3), and tumoral enhancement suggestive of necrosis was noted in 69%; neither finding was significantly associated with local control (p=0.253 and p=0.067, respectively). Overall, significant improvements in pain scores were noted at a median of 8.1 months, and all patients with neurological symptoms improved after combined surgery and SBRT.
Drs. Gerszten and Burton reported 35 spinal schwannomas treated with Cyberknife-based SBRT, with overall rates of NF1 and NF2 being 29% and 12%, respectively [16]. The mean tumor volume was 11.0 cc (1.0 – 47.7 cc), and 59% of lesions were located in the cervical spine. Prescription doses ranged from 17.5-25 Gy in one fraction. At a median follow up of 37 months (8-71 months; all patients), the radiographic control rate was 100%. Among initially symptomatic patients, 82.4% noted improvement in pain, and 80% had improvement (60%) or stabilization (20%) of neurologic symptoms. Three patients ultimately underwent salvage surgery for progressive symptoms. Two patients experienced transient myelopathy, with subsequent return of strength.
Published series demonstrate excellent crude local control rates after single fraction SBRT for schwannomas, ranging above 90-95% [16, 23, 25, 29, 33, 34, 36, 49], similar to published surgical studies [2, 7, 11, 50], and intracranial schwannomas treated with SRS [50-54]. Excellent symptomatic pain improvement has also been demonstrated over the course of months, with only a minority of patients experiencing persistent symptoms requiring surgical salvage therapy. Outcomes may be influenced by patients with NF, as surgical series have demonstrated adverse outcomes in this patient subset [7, 11]. Rates of NF among SBRT series ranges up to 41% of the total population [16], with potential for higher rates relative to surgical series due to the multiplicative and refractory nature of these lesions. Although no clear association between NF and local control for schwannomas has been evident thus far, this association may be limited due to the low patient numbers and limited follow up. Although encouraging, continued follow up is necessary to confirm outcomes.
Spinal Neurofibromas
Neurofibromas comprise about 3.5% of all spinal tumors [6, 42]. Surgical resection is still preferred for pathologic confirmation in non-NF1/NF2 patients and given transformation risk on NF1/NF2 patients. However, lesions are more commonly found on the ventral nerve root and require a more complex surgical approach [5, 49, 55]. Therefore, non-surgical approaches are often considered given the burden of disease seen in many patients.
A series from University of Pittsburgh reported outcomes of 25 patients with neurofibromas treated with CyberKnife-based SBRT [16]. Most (80%) were associated with NF1 and presented with pain (52%) or neurological deficits (16%). Patients were treated to a mean dose of 21.3 Gy in a single fraction. At a median follow up of 37 months, the tumor control rate was 100%. Among symptomatic patients, 77% achieved improved or stable pain, while 50% of patients with motor deficits showed improved function. No patients experienced late spinal cord toxicity or malignant transformation.
In the Stanford University series, 24 spinal neurofibromas were treated with CyberKnife-based SBRT to a median dose of 20 Gy over a median of 2 fractions [25]. After a median follow up of 29 months, all lesions were controlled locally despite only 18% demonstrating any significant radiographic regression. A minority of patients (17%) experienced pain relief, while 33% experienced worsening pain. Two patients required surgical resection due to refractory symptoms.
Other series have also shown neurofibromas to be relatively difficult to control. In a UCSF series, at a median follow up of 25 months, Dr. Sahgal noted one radiographic failure and one symptomatic failure among 11 patients treated for spinal neurofibromas to doses of 21 Gy in 3 fractions and 30 Gy in 3 fractions, respectively [35]. Dr. Selch’s group also reported worsening symptoms in 2 of 8 patients treated with SBRT despite radiographic control [36].
Among benign tumors treated with SBRT, neurofibromas appear to be the most symptomatic, with the poorest clinical response after treatment. The infiltrative nature of the lesions causes the tumor margins to be less distinct, making them more prone to marginal recurrences. Additionally, neurofibromas are associated with NF1, where multiple lesions may be present and treatments may be less effective due to failure to treat the appropriate symptomatic lesion [25, 42, 46, 56]. Similar poor response rates have been seen in surgical series [6, 61]. Despite these findings, radiographic control remained high, and there were no significant late toxicities, although further follow up is necessary to confirm these findings.
Radiation Toxicities
The most feared potential toxicity of SBRT for BSTs is radiation myelopathy. Traditionally, radiation myelopathy is diagnosed by the presence of neurological symptoms corresponding to an irradiated spinal cord segment, with correlative radiographic findings such as edema and necrosis, without evidence of other etiologies [58]. The main dosimetric risk factors for myelopathy include total dose, fraction size, length of spinal cord irradiation, and total duration of treatment [42, 59, 60]. The accepted spinal cord dose with conventional fractionation is 45-50 Gy at 1.8-2 Gy per fraction, resulting in a 0.03-0.1% risk of myelopathy between 6 and 24 months after treatment [42, 59-62]. With hypofractionated regimens, early estimates of single fraction spinal cord tolerance had been quoted as 8-10 Gy [63, 64]. Recently, robust retrospective analyses have estimated the risk of myelopathy after partial volume spinal irradiation of 13 Gy in 1 fraction or 20 Gy in 3 fractions to be <1% [65]. Dr. Sahgal reported a multi-institutional dosimetric analysis, based on 9 cases of grade 4 spinal cord toxicity compared against 66 controls [27]. After normalizing the fractionation schemes by the equivalent dose in 2 Gy fractions (EQD2), a linear regression model showed significant differences in dose to the thecal sac among patients with and without myelopathy to volumes ranging from point doses to 0.8 cc. The most significant differences were seen for maximum point doses, suggesting even small volumes of spinal cord must be accounted for to practice SBRT safely. In the final analysis, investigators estimated the doses the thecal sac required to limit the risk of myelitis to < 5% were less than 12.4 Gy in 1 fraction, 17 Gy in 2 fractions, 20.3 Gy in 3 fractions, 23 Gy in 4 fractions, or 25.4 Gy in 5 fractions. With comparable local control in a report of low-dose SBRT (BED10Gy <30) versus high-dose, and continued follow-up of long term toxicities, dose-deescalated SBRT to BSTs may also be an area of future investigation in select patients at increased risk of radiation toxicity.
In the selected series, four patients presented with radiation-induced myelopathy (Table 2). The first patient was treated for a recurrent, 7.6 cc C7-T2 meningioma, to a total prescription dose of 24 Gy in 3 fractions. The dose volume histogram (DVH) analysis showed a 4.7 cc volume of spinal cord receiving over 8 Gy, 0.1 cc volume receiving 27 Gy, and a max point dose 29.9 Gy. Approximately 9 months after treatment, the patient developed posterior column dysfunction with associated edema on imaging. After treatment with steroids, the patient’s symptoms resolved [42, 25].
Three patients in the University of Pittsburgh series experienced transient spinal cord compression after treatment for a meningioma and two schwannomas. All patients were treated for cervical lesions, two of which received previous subtotal resections. All received marginal prescription radiation doses of 20 Gy in a single fraction and received 8 Gy to less than 0.02 cc of the spinal cord. At 5, 12, and 13 months from SBRT, each patient developed symptoms of Brown Sequard syndrome, including posterior column motor dysfunction to a strength of 4+/5 and associated T2 weighted changes on MRI.
After treatment with steroids, vitamin E, and gabapentin, with or without hyperbaric oxygen, two patients’ symptoms resolved completely, while one patient had 5-/5 hemibody strength. Given the low doses delivered, authors postulated the previous surgical resection and/or cervical treatment location may be risk factors for radiation injury.
Transient tumor swelling has also been demonstrated in a subset of patients. In two series from a South Korean group, 37% and 56.9% spinal foraminal nerve sheath tumors and benign spinal neurogenic tumors, respectively, treated with single or multifraction SBRT showed transient swelling without worsening of neurologic function [69]. As such, transient spinal cord compression requiring surgical intervention can occur and has to be kept in mind after SBRT for benign spine tumors if new neurologic symptoms develop.
Conclusion
SBRT for BSTs appears to be effective in terms of radiographic local control, regardless of histology, with rates approaching 100% at a median follow up of 18-43 months. Symptomatic control remains less predictable. Good outcomes have been shown with pain control in meningiomas and schwannomas, but there’s room for improvement of motor deficits, patients with neurofibromas, and those with NF1. Overall, SBRT of BSTs appears to be safe and effective at tumor control and symptom management in nonsurgical patients. More outcome data from larger patient databases with longer follow-up intervals are necessary to better define the role of SBRT in benign spinal tumors. Further research in this area is desperately needed.
Acknowledgments
Authors’ disclosure of potential conflicts of interest
Dr. Samuel Chao reports receiving an honorarium from Varian Medical Systems, Inc. Dr. Sahgal reports other from Abbvie, grants and other from Elekta/Elekta AB, other from Accuray Inc., other from Varian medical systems, other from BrainLAB, other from Merck, other from Roche, other from International Stereotactic Radiosurgery Society (ISRS), other from Medtronic Kyphon, outside the submitted work. Dr. Redmond reports grants and other from Accuray, grants from Elekta AB, personal fees from Medtronic, outside the submitted work. Dr. Lo reports other disclosure from Elekta AB, outside the submitted work.
Other authors have nothing to disclose.
Author Contributions
Conception and design: Simon Lo, Lindsay Hwang, Christian Okoye, Ravi Patel
Data collection: Lindsay Hwang, Christian Okoye, Ravi Patel
Manuscript writing: Lindsay Hwang, Christian Okoye
Final approval of manuscript: Simon Lo, Arjun Sahgal, Matthew Foote, Kristin J. Redmond, Christoph Hofstetter, Rajiv Saigal, Mahmud Mossa-Basha, William Yuh, Nina A. Mayr, Samuel T. Chao, MD, Eric L. Chang
Symbols and Abbreviations
Stereotactic body radiation therapy (SBRT)
Benign spinal tumors (BST)
External beam radiotherapy (EBRT)
Nerve sheath tumors (NST)
Linear accelerator (LINAC)
Gross tumor volume (GTV)
Clinical target volume (CTV)
Planning target volume (PTV)
Planning-at-risk volume (PRV)
Organs at risk (OAR)
Biological effective doses (BED)
Imaging and Radiation Oncology Core (IROC)
Neurofibromatosis type 1 (NF1)
Neurofibromatosis type 2 (NF2)
Equivalent dose in 2 Gy fractions (EQD2)
Dose volume histogram (DVH)
References
- 1. Schellinger KA, Propp JM, Villano JL, McCarthy BJ. Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. 2008;87(2):173-179. [DOI] [PubMed] [Google Scholar]
- 2. Conti P, Pansini G, Mouchaty H, Capuano C, Conti R. Spinal neurinomas: retrospective analysis and long-term outcome of 179 consecutively operated cases and review of the literature. Surg Neurol. 2004;61(1):34-4. [DOI] [PubMed] [Google Scholar]
- 3. Mccormick PC. Surgical management of dumbbell and paraspinal tumors of the thoracic and lumbar spine. Neurosurgery. 1996;38(1):67-74; discussion 74-65. [DOI] [PubMed] [Google Scholar]
- 4. Mccormick PC. Surgical management of dumbbell tumors of the cervical spine. Neurosurgery. 1996;38(2):294-300. [DOI] [PubMed] [Google Scholar]
- 5. Parsa AT, Lee J, Parney IF, Weinstein P, Mccormick PC, Ames C. Spinal cord and intradural-extraparenchymal spinal tumors: current best care practices and strategies. J Neurooncol. 2004;69(1-3):291-318. [DOI] [PubMed] [Google Scholar]
- 6. Seppälä MT, Haltia MJ, Sankila RJ, Jääskeläinen JE, Heiskanen O. Long-term outcome after removal of spinal neurofibroma. J Neurosurg. 1995;82(4):572-577. [DOI] [PubMed] [Google Scholar]
- 7. Seppälä MT, Haltia MJ, Sankila RJ, Jääskeläinen JE, Heiskanen O. Long-term outcome after removal of spinal schwannoma: a clinicopathological study of 187 cases. J Neurosurg. 1995;83(4):621-626. [DOI] [PubMed] [Google Scholar]
- 8. Faul CM, Flickinger JC. The use of radiation in the management of spinal metastases. J Neurooncol. 1995; 23(2):149-16. [DOI] [PubMed] [Google Scholar]
- 9. Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32(2):193-199. [DOI] [PubMed] [Google Scholar]
- 10. Abul-Kasim K, Thurnher MM, Mckeever P, Sundgren PC. Intradural spinal tumors: current classification MRI features. Neuroradiology. 2008;50(4):301-314. [DOI] [PubMed] [Google Scholar]
- 11. Klekamp J, Samii M. Surgery of spinal nerve sheath tumors with special reference to neurofibromatosis. Neurosurgery. 1998;42(2):279-289. [DOI] [PubMed] [Google Scholar]
- 12. Chung JY, Lee JJ, Kim HJ, Seo HY. Characterization of magnetic resonance images for spinal cord tumors. Asian Spine J. 2008;2(1):15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Verdelhan O, Haegelen C, Carsin-Nicol B, Riffaud L, Amlashi SFA, Brassier G, Carsin M, Morandi X. MR imaging features of spinal schwannomas and meningiomas. J Neuroradiol. 2005;32(1):42-49. [DOI] [PubMed] [Google Scholar]
- 14. Liu WC, Choi G, Lee S-H, Han H, Lee JY, Jeon YH, Park HS, Park JY, Paeng SS. Radiological findings of spinal schwannomas and meningiomas: focus on discrimination of two disease entities. Eur Radiol. 2009;19(11):2707-2715. [DOI] [PubMed] [Google Scholar]
- 15. Matsumoto S, Hasuo K, Uchino A, Mizushima A, Furukawa T, Matsuura Y, Fukui M, Masuda K. MRI of intradural-extramedullary spinal neurinomas and meningiomas. Clin Imaging. 1993;17(1):46-52. [DOI] [PubMed] [Google Scholar]
- 16. Gerszten PC, Burton SA. Clinical assessment of stereotactic IGRT: spinal radiosurgery. Med Dosim. 2008;33(2):107-116. [DOI] [PubMed] [Google Scholar]
- 17. Gerszten PC, Burton SA, Ozhasoglu C, Mccue KJ, Quinn AE. Radiosurgery for benign intradural spinal tumors. Neurosurgery. 2008;62(4):887-895; discussion 895-886. [DOI] [PubMed] [Google Scholar]
- 18. Foote M, Letourneau D, Hyde D, Massicotte E, Raja R, Fehlings M, Fisher C, Lewis S, La Macchia N, Yu E, Laperriere NJ, Sahgal A. Technique for stereotactic body radiotherapy for spinal metastases. J Clin Neurosci. 2011;14(2):276-279. [DOI] [PubMed] [Google Scholar]
- 19. Nalichowski A, Kaufman I, Gallo J, Bossenberger T, Solberg T, Ramirez E, Yan Y, Fredrick J, Bichay T, Mayville A, Burmeister J. Single fraction radiosurgery/stereotactic body radiation therapy (SBRT) for spine metastasis: A dosimetric comparison of multiple delivery platforms. J Appl Clin Med Phys. 2017;18(1):164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rubio C, Morera R, Hernando O, Leroy T, Lartigau SE. Extracranial stereotactic body radiotherapy. Review of main SBRT features and indications in primary tumors. Rep Pract Oncol Radiother. 2013;18(6):387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stanescu T, Jans HS, Pervez N, Stavrev P, Fallon BG. A study on the magnetic resonance imaging (MRI)-based radiation treatment planning of intracranial lesions. Phys Med Biol. 2008;53(13):3579-93. [DOI] [PubMed] [Google Scholar]
- 22. Gerszten PC, Ozhasoglu C, Burton SA, Vogel WJ, Atkins BA, Kalnicki S, Welch WC. CyberKnife frameless single-fraction stereotactic radiosurgery for benign tumors of the spine. Neurosurg Focus. 2003;14(5):e16. [DOI] [PubMed] [Google Scholar]
- 23. Marchetti M, De Martin E, Milanesi I, Fariselli L. Intradural extramedullary benign spinal lesions radiosurgery. Medium- to long-term results from a single institution experience. Acta Neurochir (Wien). 2013;155(7):1215-1222. [DOI] [PubMed] [Google Scholar]
- 24. Ryu SI, Chang SD, Kim DH, Murphy MJ, Le QT, Martin DP, Adler JR. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49(4):838-846. [DOI] [PubMed] [Google Scholar]
- 25. Sachdev S, Dodd RL, Chang SD, Soltys SG, Adler JR, Luxton G, Choi CYH, Tupper L, Gibbs IC. Stereotactic radiosurgery yields long-term control for benign intradural, extramedullary spinal tumors. Neurosurgery. 2011;69(3):533-539. [DOI] [PubMed] [Google Scholar]
- 26. Tseng CL, Sussman MS, Atenafu EG, Letourneau D, Ma L, Soliman H, Thibault I, Cho BC, Simeonov A, Yu E, Fehlings MG, Sahgal A. Magnetic resonance imaging assessment of spinal cord and cauda equina motion in supine patients with spinal metastases planned for spine stereotactic body radiation therapy. International journal of radiation oncology, biology, physics. 2015;91(5):995-1002. [DOI] [PubMed] [Google Scholar]
- 27. Sahgal A, Weinberg V, Ma L, Chang E, Chao S, Muacevic A, Gorgulho A, Soltys S, Gerszten PC, Ryu S, Angelov L, Gibbs I, Wong CS, Larson DA. Probabilities of radiation myelopathy specific to stereotactic body radiation therapy to guide safe practice. Int J Radiat Oncol Biol Phys. 2013;85(2):341-347. [DOI] [PubMed] [Google Scholar]
- 28. Kalash R, Glaser SM, Flickinger JC, Burton S, Heron DE, Gerszten PC, Engh JA, Amankulor NM, Vargo JA. Stereotactic body radiation therapy for benign spine tumors: is dose de-escalation appropriate?. J Neurosurg Spine, 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okoye CC, Patel RB, Hasan S, Podder T, Khouri A, Fabien J, Zhang Y, Dobbins D, Sohn JW, Yuan J, Yao M, Machtay M, Sloan AE, Miller J, Lo SS. Comparison of Ray Tracing and Monte Carlo Calculation Algorithms for Thoracic Spine Lesions Treated With CyberKnife-Based Stereotactic Body Radiation Therapy. Technol Cancer Res Treat, 2015. [DOI] [PubMed] [Google Scholar]
- 30. Imaging and Radiation Oncology Core (2015) Treatment Planning System - Algorithm List Updated. Available online at: http://rpc.mdanderson.org/rpc/services/Anthropomorphic_%20Phantoms/TPS%20-%20algorithm%20list%20updated.pdf (accessed 11 Apr 2016). [Google Scholar]
- 31. Helseth A, Mørk SJ. Primary intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg. 1989;71(6):842-845. [DOI] [PubMed] [Google Scholar]
- 32. Solero CL, Fornari M, Giombini S, Lasio G, Oliveri G, Cimino C, Pluchino F. Spinal meningiomas: review of 174 operated cases. Neurosurgery. 1989;25(2):153-160. [PubMed] [Google Scholar]
- 33. Elibe E, Boyce-Fappiano D, Ryu S, Siddiqui MS, Lee I, Rock J, Siddiqui F. Stereotactic radiosurgery for primary tumors of the spine and spinal cord. J Radiosurg SBRT. 2018; 5(2):107-113. [PMC free article] [PubMed] [Google Scholar]
- 34. Kufeld M, Wowra B, Muacevic A, Zausinger S, Tonn JC. Radiosurgery of spinal meningiomas and schwannomas. Technol Cancer Res Treat. 2012;11(1):27-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sahgal A, Chou D, Ames C, Ma L, Lamborn K, Huang K, Chuang C, Aiken A, Petti P, Weinstein P, Larson D. Image-guided robotic stereotactic body radiotherapy for benign spinal tumors: theUniversity of California San Francisco preliminary experience. Technol Cancer Res Treat. 2007;6(6):595-604. [DOI] [PubMed] [Google Scholar]
- 36. Selch MT, Lin K, Agazaryan N, Tenn S, Gorgulho A, Demarco JJ, Desalles AA. Initial clinical experience with image-guided linear accelerator-based spinal radiosurgery for treatment of benign nerve sheath tumors. Surg Neurol. 2009;72(6):668-674. [DOI] [PubMed] [Google Scholar]
- 37. Lee ME, Hwang YJ, Sohn MJ, Lee BH, Kim SY. Assessment of the treatment response of spinal meningiomas after radiosurgery focusing on serial MRI findings. Jpn J Radiol. 2015;33(9):547-558. [DOI] [PubMed] [Google Scholar]
- 38. King AT, Sharr MM, Gullan RW, Bartlett JR. Spinal meningiomas: a 20-year review. British journal of neurosurgery. 1998;12(6):521-526. [DOI] [PubMed] [Google Scholar]
- 39. Schick U, Marquardt G, Lorenz R. Recurrence of benign spinal neoplasms. Neurosurg Rev. 2001;24(1):20-25. [DOI] [PubMed] [Google Scholar]
- 40. SS Lo, Cho KH, Hall WA, Kossow RJ, Hernandez WL, Mccollow KK, Gerbi BJ, Higgins PD, Lee CK, Dusenbery KE. Single dose versus fractionated stereotactic radiotherapy for meningiomas. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2002;29(3):240-248. [DOI] [PubMed] [Google Scholar]
- 41. Pollock BE, Stafford SL, Link MJ, Garces YI, Foote RL. Single-fraction radiosurgery for presumed intracranial meningiomas: efficacy and complications from a 22-year experience. International journal of radiation oncology, biology, physics. 2012;83(5):1414-1418. [DOI] [PubMed] [Google Scholar]
- 42. Dodd RL, Ryu MR, Kamnerdsupaphon P, Gibbs IC, Chang SD, Jr., Adler JR., Jr CyberKnife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery. 2006;58(4):674-685. [DOI] [PubMed] [Google Scholar]
- 43. Kameyama S, Tanaka R, Honda Y, Hasegawa A, Yamazaki H, Kawaguchi T. The long-term growth rate of residual acoustic neurinomas. Acta Neurochir (Wien). 1994;129(3-4):127-130. [DOI] [PubMed] [Google Scholar]
- 44. Mautner VF, Tatagiba M, Lindenau M, Fünsterer C, Pulst SM, Baser ME, Kluwe L, Zanella FE. Spinal tumors in patients with neurofibromatosis type 2: MR imaging study of frequency, multiplicity, and variety. AJR J Am Roentgenol. 1995;165(4):951-955. [DOI] [PubMed] [Google Scholar]
- 45. Evans DG, Trueman L, Wallace A, Collins S, Strachan T. Genotype/phenotype correlations in type 2 neurofibromatosis (NF2): evidence for more severe disease associated with truncating mutations. J Med Genet. 1998;35(6):450-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Halliday AL, Sobel RA, Martuza RL. Benign spinal nerve sheath tumors: their occurrence sporadically and in neurofibromatosis types 1 and 2. J Neurosurg. 1991;74(2):248-253. [DOI] [PubMed] [Google Scholar]
- 47. Maccollin M, Chiocca EA, Evans DG, Friedman JM, Horvitz R, Jaramillo D, Lev M, Mautner VF, Niimura M, Plotkin SR, Sang CN, Stemmer-Rachamimov A, Roach ES. Diagnostic criteria for schwannomatosis. Neurology. 2005;64(11):1838-1845. [DOI] [PubMed] [Google Scholar]
- 48. Seppälä MT, Sainio MA, Haltia MJ, Kinnunen JJ, Setälä KH, Jääskeläinen JE. Multiple schwannomas: schwannomatosis or neurofibromatosis type 2? J Neurosurg. 1998;89(1):36-41. [DOI] [PubMed] [Google Scholar]
- 49. Shin D-W, Sohn M-J, Kim H-S, Lee D-J, Jeon SR, Hwang YJ, Jho E-H. Clinical analysis of spinal stereotactic radiosurgery in the treatment of neurogenic tumors. J Neurosurg Spine. 2015;1-9. [DOI] [PubMed] [Google Scholar]
- 50. Safavi-Abbasi S, Senoglu M, Theodore N, Workman RK, Gharabaghi A, Feiz-Erfan I, Spetzler RF, Sonntag VK. Microsurgical management of spinal schwannomas: evaluation of 128 cases. Journal of neurosurgery. Spine. 2008;9(1):40-47. [DOI] [PubMed] [Google Scholar]
- 51. Lo WL, Yang KY, Huang YJ, Chen WF, Liao CC, Huang YH. Experience with Novalis stereotactic radiosurgery for vestibular schwannomas. Clin Neurol Neurosurg. 2014;121:30-34. [DOI] [PubMed] [Google Scholar]
- 52. Morimoto M, Yoshioka Y, Kotsuma T, Adachi K, Shiomi H, Suzuki O, Seo Y, Koizumi M, Kagawa N, Kinoshita M, Hashimoto N, Ogawa K. Hypofractionated stereotactic radiation therapy in three to five fractions for vestibular schwannoma. Japanese journal of clinical oncology. 2013;43(8):805-812. [DOI] [PubMed] [Google Scholar]
- 53. Prasad D, Steiner M, Steiner L. Gamma surgery for vestibular schwannoma. J Neurosurg. 2000;92(5):745-759. [DOI] [PubMed] [Google Scholar]
- 54. Sheehan J, Yen CP, Arkha Y, Schlesinger D, Steiner L. Gamma knife surgery for trigeminal schwannoma. J Neurosurg. 2007;106(5):839-845. [DOI] [PubMed] [Google Scholar]
- 55. Gambardella G, Gervasio O, Zaccone C. “Approaches and surgical results in the treatment of ventral thoracic meningiomas. Review of our experience with a postero-lateral combined transpedicular-transarticular approach.”. Acta Neurochir (Wien). 2003;145(5):385-392. [DOI] [PubMed] [Google Scholar]
- 56. Thakkar SD, Feigen U, Mautner VF. Spinal tumours in neurofibromatosis type 1: MRI an study of frequency, multiplicity and variety. Neuroradiology. 1999;41(9):625-629. [DOI] [PubMed] [Google Scholar]
- 57. Taleb FS, Guha A, Arnold PM, Fehlings MG, Massicotte EM. Surgical management of cervical spine manifestations of neurofibromatosis Type 1: long-term clinical and radiological follow-up in 22 cases. Journal of neurosurgery. Spine. 2011;14(3):356-366. [DOI] [PubMed] [Google Scholar]
- 58. Wong CS, van Dyk J, Milosevic M, Laperriere NJ. Radiation myelopathy following single courses of radiotherapy and retreatment. International journal of radiation oncology, biology, physics. 1994;30(3):575-581. [DOI] [PubMed] [Google Scholar]
- 59. Rampling R, Symonds P. Radiation myelopathy. Current opinion in neurology. 1998;11(6):627-632. [DOI] [PubMed] [Google Scholar]
- 60. Schultheiss TE, Kun LE, Ang KK, Stephens LC. Radiation response of the central nervous system. International journal of radiation oncology, biology, physics. 1995;31(5):1093-1112. [DOI] [PubMed] [Google Scholar]
- 61. Marcus RB, Jr., Million RR. The incidence of myelitis after irradiation of the cervical spinal cord. International journal of radiation oncology, biology, physics. 1990;19(1):3-8. [DOI] [PubMed] [Google Scholar]
- 62. Schultheiss TE. The radiation dose-response of the human spinal cord. International journal of radiation oncology, biology, physics. 2008;71(5):1455-1459. [DOI] [PubMed] [Google Scholar]
- 63. Ryu S, Jin JY, Jin R, Rock J, Ajlouni M, Movsas B, Rosenblum M, Kim JH. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109(3):628-636. [DOI] [PubMed] [Google Scholar]
- 64. Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18(4):215-222. [DOI] [PubMed] [Google Scholar]
- 65. Kirkpatrick JP, Van Der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. International journal of radiation oncology, biology, physics. 2010;76(3 Suppl):S42-49. [DOI] [PubMed] [Google Scholar]
- 66. Murovic JA, Gibbs IC, Chang SD, Mobley BC, Park J, Adler JR. Foraminal nerve sheath tumors: intermediate follow-up after CyberKnife radiosurgery. Neurosurgery. 2009;64(2):A33–A43. [DOI] [PubMed] [Google Scholar]
- 67. Niazi TN, Bowers CA, Schmidt MH. Role of adjuvant radiosurgery after thoracoscopic microsurgical resection of a spinal schwannoma. Case Rep Neurol Med 2012;2012:345830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kang HJ, Hwang YJ, Kim YH, Kim SY, Lee BH, Sohn MJ. Follow-up MR findings of spinal foraminal nerve sheath tumors after stereotactic irradiation. Jpn J Radiol. 2013;31(3):192-196. [DOI] [PubMed] [Google Scholar]
- 69. Gerszten PC, Chen S, Quader M, Xu Y, Novotny J, Jr, Flickinger JC. Radiosurgery for benign tumors of the spine using the Synergy S with cone-beam computed tomography image guidance. J Neurosurg. 2012. December;117 Suppl:197-202. [DOI] [PubMed] [Google Scholar]