Abstract
In the present report, we used highly elongated Drosophila bristle cells to dissect the role of dynein heavy chain (Dhc64C) in Golgi organization. We demonstrated that whereas in the bristle "somal" region Golgi units are composed of cis-, medial, and trans-Golgi compartments (“complete Golgi”), the bristle shaft contains Golgi satellites that lack the trans-Golgi compartment (hereafter referred to as “incomplete Golgi”) and which are static and localized at the base area. However, in Dhc64C mutants, the entire bristle shaft was filled with complete Golgi units containing ectopic trans-Golgi components. To further understand Golgi bristle organization, we tested the roles of microtubule (MT) polarity and the Dhc-opposing motor, kinesin heavy chain (Khc). For our surprise, we found that in Khc and Ik2Dominant-negative (DN) flies in which the polarized organization of MTs is affected, the bristle shaft was filled with complete Golgi, similarly to what is seen in Dhc64C flies. Thus, we demonstrated that MTs and the motor proteins Dhc and Khc are required for bristle Golgi organization. However, the fact that both Dhc64C and Khc flies showed similar Golgi defects calls for an additional work to elucidate the molecular mechanism describing why these factors are required for bristle Golgi organization.
Introduction
The Golgi complex possesses a highly ordered and characteristic morphology. In most mammal cells, the organelle appears as stacks of flattened cisternae that are linked by tubules to give rise to continuous structures known as ribbons [1, 2]. In most cell types, the Golgi ribbon is typically located in the perinuclear region near the centrosome. The exact localization is dependent on the microtubule (MT) and actin cytoskeleton, together with active transport or anchoring by motor proteins, such as dynein and kinesin [3]. It was, moreover, shown that MTs are closely associated with the cis-Golgi compartment [4]. In contrast, in non-neuronal Drosophila tissues, the Golgi apparatus appears as stacks dispersed in the cell cytoplasm, juxtaposed to the endoplasmic reticulum exit site (ERES), and does not form the continuous tubular organelle seen in mammalian cells [5].
Neurons, which are highly polarized cells, present a unique satellite secretory system that includes ER and Golgi outposts, preferentially localized to dendrites [6–8]. Although it is believed that the majority of protein synthesis, modification, and sorting takes place in the soma, it was shown that satellite Golgi at neuronal terminals is functional and may provide local membrane and protein supply [5, 6, 8, 9]. Moreover, it was demonstrated that dendritic Golgi outposts can serve as sites of de novo MT nucleation [10, 11], thus serving as microtubule-organizing centers (MTOCs). On the other hand, it was claimed that acentrosomal nucleation of MTs is only γ-tubulin-dependent and that Golgi outposts do not serve as sites for MT nucleation in neurons [12]. The appearance of distinct distal Golgi structures separated from the main Golgi apparatus and located near the nucleus mainly characterizes neuronal cells. However, discrete Golgi outposts are not only found in neurons. For instance, highly polarized mice astrocyte perivascular processes and end feet possess Golgi apparatus components [13], suggesting that the presence of Golgi outposts is not a unique feature of neurons, as was previously believed [14].
Similar to Drosophila dendrites, the bristle shaft contains acentrosomal stable and uniformly oriented MT arrays in which the minus-ends point distally (i.e., towards the growing edge of the bristle) [15]. Thus, the Drosophila bristle cell represents an excellent model for studying long-distance transport and the establishment of cellular polarity. The bristle shaft sprouts from the epithelial tissue for up to 450 μm, while the "soma", containing the polypoid nucleus, remains in the epithelial plane. During elongation, growth takes place at the tip area, which is the most dynamic part of the cell. Our lab and others have questioned the role of MT-dependent motors and asymmetrical MT organization in long-distance organelle transport [16–19]. Still, the organization of the Golgi in these unique polarized cells remains to be fully elucidated.
In the present study, we describe a previously uncharacterized Golgi apparatus in bristle cells. We also addressed the role of the MT array and its MT motor proteins, dynein and kinesin, in bristle Golgi organization. We found that in the somal region containing the nucleus, the Golgi contains cis-, medial, and trans-compartments juxtaposed to the ERES, while in the bristle shaft, the Golgi is composed of ERES, cis-, and medial compartments, yet completely lacks trans-Golgi components. We thus refer this complex as incomplete Golgi. These Golgi satellites were localized to the lower shaft area and appeared static. Relying on dynein heavy chain 64C (Dhc64C) and kinesin heavy chain (Khc) mutants to follow the contributions of these molecular motor to the positioning of Golgi satellites, we found that complete Golgi units containing trans-Golgi components were ectopically localized throughout the entire bristle shaft. Next, by affecting MT polarity through mutation of the Ik2 gene, we found that complete Golgi units were localized ectopically to the entire bristle shaft; similar to what was seen in the Dhc64C and Khc mutants. Thus, our results demonstrate that organized MT polarity and the MT motor proteins Dhc64C and Khc are required for bristle Golgi organization.
Materials and methods
Drosophila stocks
Oregon-R was used as a wild-type control. The following mutant and transgenic flies were used: Dhc64C4-19/TM6B, Dhc64C8-1/TM6B [20], FRT79D Dhc64C902/TM6B [21], UAS-Ik2DN [22], UAS-LVADN [7], Khc-IR (Vienna Drosophila RNAi Center; V44337), UAS-MnII-GFP, UAS-Gal-T-RFP [7, 23], UAS-GalNacT2-YFP/TagRFP [23], and UAS-GFP-Sten [24]. Bristle expression was induced under the control of the neur-Gal4 or sca-Gal4 driver.
Dissection and preparation of pupae for live imaging
Dissection of thorax tissue containing bristles for antibody staining and removal of the pupal case for live-imaging experiments was performed as previously described [15, 18].
Scanning electron microscopy
Adult Drosophila were fixed and gradually dehydrated by immersion in increasing concentrations of ethanol (25%, 50%, 75%, and 2×100%, each for 10 min). The samples were then completely dehydrated using increasing concentrations of hexamethyldisilazane in alcohol (50%, 75%, and 2×100%, each for 2 h), air-dried overnight, placed on stubs, coated with gold, and examined with a scanning electron microscope (SEM; JEOL model JSM-5610LV).
Bristle phalloidin and antibody staining
The procedures of tissue dissection used for fixation and staining were described previously [18, 25]. Confocal images were taken using an Olympus FV1000 laser scanning confocal microscope, appearing as Z-projections in a few optical frames that together covered the bristle cell. The primary antibodies used were mouse anti-acetylated tubulin monoclonal antibodies (1:250; Sigma), and anti-dGM130 (1:500; Abcam), anti-Sec16 antibodies [26]. Secondary antibodies included Cy3-conjugated goat anti-mouse (1:100; Jackson Immunoresearch) and Alexa Fluor 488-conjugated goat anti-rabbit (1:100; Molecular Probes) antibodies. For actin staining, we used Alexa Fluor 405-conjugated phalloidin (1:250; Molecular Probes).
Organelle tracking and statistical analysis
To measure the area and density of MnII-GFP and GalNacT2-YFP-positive particles, confocal Z-stack images of maximal quality of bristle cells were obtained. A Z-stack projection of the bristle shaft was divided into two halves exactly in the middle of the shaft length, resulting in two sections of even length, with one part being close to the base (soma) and the other being distal to the base, referred to as the tip. In the case of a dynein-mutated background, the bristle appeared as two independent parts because of an extremely high signal at the tip area, relative to the base. Images were analyzed using ImageJ software, and the area of each particle was measured automatically using the "analyze particles" tool. The density of MnII and GalNacT2-positive particles was measured as the number of particles in each half of the bristle divided by the overall area of that half-bristle (i.e., the number of particles/area of the base or tip half). Statistical analysis of both parameters, i.e., particle area and density, was performed using spilt-plot ANOVA with the cell part area being considered as the within-plot treatment and the genotype being considered as the whole-plot treatment. Repeats (random factors) were nested within a genotype. Statistical analyses were performed using STATISTICA, version 10.
Results
Golgi organization in the Drosophila bristle cell
To study the organization of the Golgi in polarized cells, we used the highly elongated Drosophila bristle cell as a model system, together with a set of ERES and Golgi markers. To identify the ERES, we used either Stenosis (Sten) tagged with GFP [24] or antibody staining of Sec16 [26]. As a medial Golgi compartment marker, we followed α-mannosidase II tagged with EGFP (MnII-GFP), while RFP/GFP-tagged N-acetylgalactosaminyltransferase 2 (GalNacT2-TagRFP/GFP) and RFP-tagged Gal-T [23] served as a trans-Golgi marker. For visualization of the cis-Golgi compartment, we followed anti-dGM130 antibody staining [23].
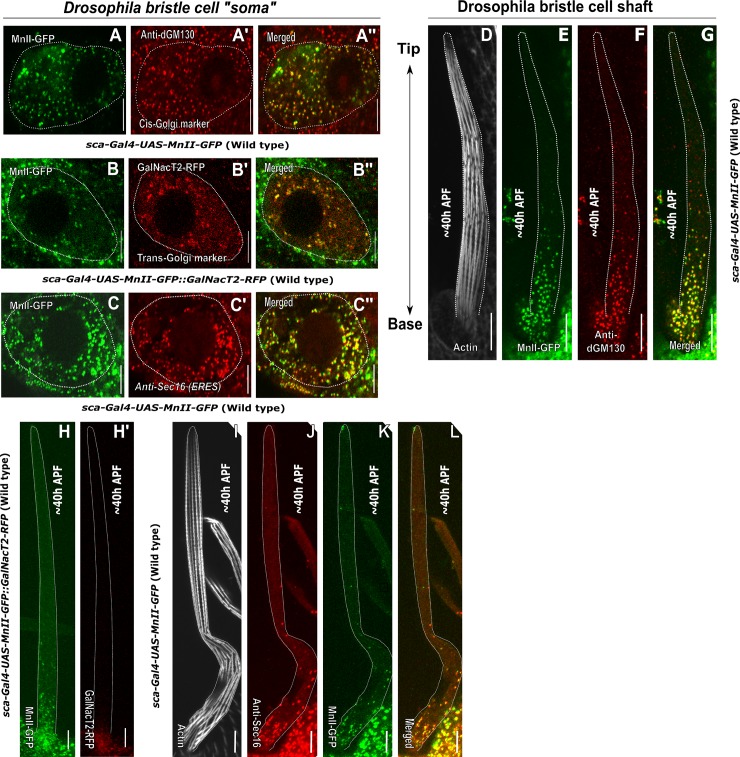
Examination of bristle cell Golgi organization revealed that the somal region contains Golgi stacks composed of cis- (Fig 1A' and 1A''), medial- (Fig 1A–1C, 1A'', 1B'' and 1C''), trans-Golgi compartments (Fig 1B and 1B') and ERES (Fig 1C' and 1C'') scattered throughout the cytoplasm. This type of Golgi is henceforth designated as a complete Golgi unit. Thus, similar to other Drosophila tissues [5, 27–29], the soma of the bristle cell contains Golgi mini-stacks and does not create higher-order structures, such as the ring-shaped organization observed in the soma of larval neurons [23]. In Drosophila larval dendritic shafts, the Golgi exists as a discrete "single compartment Golgi" or as a "Golgi mini-stack" composed of more than one compartment [23]. Examination of Golgi organization within the bristle shaft using MnII-GFP as a medial-Golgi marker revealed that the shaft contains MnII-positive particles, these particles are called hereafter Golgi satellites, (Fig 1D–1G, 1H, 1K and 1L) and are concentrated in the lower part of the bristle, with almost no such Golgi satellites being seen in the distal to soma tip area. We tested for differences in the density of Golgi satellites and the relative sizes of particles in the lower part (i.e., the half of the shaft close to the soma, designated as the base) and the upper part (i.e., the half of the bristle shaft distal to the soma, designated as the tip) of the bristle shaft (see Materials and Methods for details of the quantification process used). Such analysis of MnII-GFP -positive particles revealed that the density of Golgi satellites (i.e., the number of particles per μm2) at the base of the bristle shaft was significantly higher, as compared to the tip (For MnII-GFP, 0.18±0.05 and 0.04±0.06 particles/μm2, respectively; P<0.001, Table 1; 5 pupa, 15 bristles). In addition, the average area of each particle of both MnII-GFP was significantly different between the base and tip areas, (For MnII-GFP, 0.43±0.80 and 0.17±0.20 μm2, respectively; P<0.0015 Table 1; 5 pupa, 15 bristles)
Fig 1. Golgi organization in Drosophila bristles.
Confocal projections of representative WT “somal” and ~40 h APF bristle shaft areas. A-C''–Bristle “somal” region: A-A''–“Soma” of a WT bristle cell expressing sca-Gal4::UAS-MnII-GFP (a medial-Golgi marker) stained with anti-dGM130 antibodies (a cis-Golgi marker). A'–Anti-dGM130 antibody staining showing a cis-Golgi compartment localized throughout the bristle soma cytoplasm, A''–Merged image of green MnII-GFP and red anti-dGM130 antibody staining showing co-localization of medial- and cis-Golgi compartments. B-B''–Soma of a WT bristle cell expressing sca-Gal4::UAS-MnII-GFP (a medial-Golgi marker) and co-expressing GalNacT2-RFP (a trans-Golgi marker): B'–GalNacT2-RFP (a trans-Golgi marker) identifies trans-Golgi components localized throughout the bristle soma cytoplasm, B''–Merged image of green MnII-GFP and red GalNacT2-RFP showing co-localization of medial- and trans-Golgi compartments in the bristle cell soma. C-C'–Soma of a WT bristle cell expressing sca-Gal4::UAS-MnII-GFP (a medial-Golgi marker) stained with anti-Sec16-antibodies (to identify the ERES): C'–Anti-Sec16 antibody staining showing ERES localized throughout the bristle soma cytoplasm, C''–Merged image of green MnII-GFP and red anti-Sec16 antibody staining showing co-localization of medial-Golgi and ERES components in the bristle cell soma. D-L–Bristle external extension; shaft (the cell compartment emanating from soma out from the epithelial tissue plane). D-G–Bristle shaft expressing sca-Gal4::UAS-MnII-GFP co-stained with anti-dGM130 antibodies and phalloidin: D–Gray phalloidin-UV staining of actin bundles in a MnII-GFP expressing bristle, used here to highlight the cell perimeter, E–Green MnII-GFP, a medial-Golgi marker, is localized to the lower shaft area close to the somal region, F–Red anti-dGM130 antibody staining (cis-Golgi marker), G–Merged image of green MnII-GFP and red anti-dGM130 antibody staining showing co-localization of medial- and cis-Golgi compartments in the bristle shaft. H-H'–Bristle shaft co-expressing sca-Gal4::UAS-MnII-GFP (a medial-Golgi marker) and GalNacT2-RFP (a trans-Golgi marker): H–Green MnII-GFP, a medial-Golgi marker, is localized to the lower shaft area close to the somal region, H'–Red GalNacT2-RFP, indicating a trans-Golgi domain, does not enter the bristle shaft. I-L–Bristle shaft expressing sca-Gal4::UAS-MnII-GFP co-stained with anti-Sec16 (ERES) antibodies and phalloidin: I–Phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter, J–Red anti-Sec16 antibody staining localized to the lower shaft area close to the somal region, K–Green MnII-GFP localized to the lower shaft area close to the somal region, L–Merged image of green MnII-GFP and red anti-Sec-16-antibody staining showing co-localization of medial-Golgi and ERES components in the bristle shaft. APF-After prepupa formation. The scale bar represents 10 μm.
Table 1. Golgi outpost localization parameters in Dhc64C and khc mutant bristle shafts.
| Golgi outpost (MnII-GFP) localization parameters in Drosophila bristles | ||||||
|---|---|---|---|---|---|---|
| Genotype | Wild-type | Dhc64C8-1/Dhc64C4-19 | Khc- RNAi | |||
| Bristle cell area | tip | base | tip | base | tip | base |
| No. of pupae | 5 | 5 | 5 | |||
| No. of bristles | 15 | 16 | 15 | |||
| Particle area, avg (μm2) | 0.17±0.20 | 0.43±0.80* | 0.43±0.84*A | 0.32±0.52A | 0.20±0.52B | 0.40±0.19*B |
| Density (particle/μm2) | 0.04±0.06 | 0.18±0.05* | 0.30±0.03A | 0.43±0.16*A | 0.12±0.13A | 0.31±0.26*A |
*—represents a significant difference of the cell part (tip/base) within each genotype; letters represent a significant difference in corresponding cell parts (tip/tip; base/base) between genotypes.
A–Significantly different from WT
B–significantly different from Dhc64C mutant. The values reflect mean±s.d. (for a detailed description of the statistical analysis performed, see Materials and Methods).
We further found that these Golgi satellites to be composed of cis- (Fig 1D–1G) and medial-Golgi compartments (Fig 1E, 1H and 1K) juxtaposed to ERES (Fig 1I–1L) and completely lacking trans-Golgi domains (Fig 1H and 1H'). To confirm the complete absence of trans-Golgi components within the bristle shaft, as observed in GalNacT2-RFP-expressing bristles, we tested for the presence of an additional trans-Golgi marker, 1,4-galactosyltransferase (Gal-T)[23, 30]. We found no Gal-T-RFP in the wild type (WT) shaft (S1 Fig). Thus, our results show that whereas the Golgi apparatus of the bristle somatic region is composed of complete Golgi units (i.e, including cis-, medial- and trans-Golgi components), the bristle shaft contained Golgi satellites completely lacking trans-Golgi components.
Dhc64C is required for Golgi organization in the bristle shaft
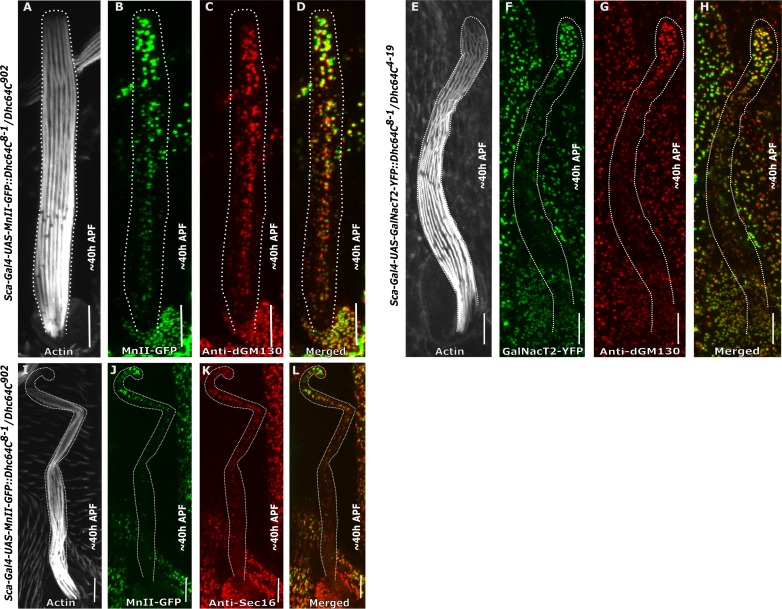
Given that in the Drosophila bristle, a stable MT array is organized with minus-end MT fibers pointing away from the soma [15] and with the notion that MTs and their motor proteins are required for Golgi organization [31–34], we studied the role of the minus-end MT motor protein dynein in bristle Golgi organization. First, we analyzed the role of Dhc64C using the "slow dynein" hypomorphic mutant allele Dhc64C8-1 [17, 20]. We found that in these mutants, Golgi satellites were no longer concentrated at the base of the bristle shaft as in the WT (Fig 1E–1G) but were instead found throughout the entire bristle shaft (Fig 2A–2L). These ectopically localized Golgi units contained cis- (Fig 2C, 2D, 2G and 2H), medial- (Fig 2B, 2D, 2J and 2L), and trans-Golgi compartments (Fig 2F and 2H) and ERES (Fig 2K and 2L), demonstrating that in Dhc64C mutants, the bristle shaft contains complete Golgi units rather than discrete Golgi satellites. Staining of MnII-GFP-expressing Dhc64C mutants with anti-dGM130 antibodies (a cis-Golgi marker) (Fig 2C) revealed co-localization of cis- and medial-Golgi markers (Fig 2D). Likewise, co-localization of GalNacT2-YFP (a trans-Golgi marker) expressed in Dhc64C mutants with anti-dGM130 antibody staining (Fig 2F–2H) reflected co-localization of the trans- and cis-markers (Fig 2H). Also, an additional trans-Golgi marker, 1,4-galactosyltransferase (Gal-T), was found throughout Dhc64C mutant bristle shafts (S1 Fig). We thus concluded that all three compartments are co-localized. Additionally, we showed that MnII-GFP was co-localized with ERES (Fig 2L). Thus, the Golgi particles found within the Dhc64C mutant bristle are complete Golgi units. Quantitative analysis revealed that the density of MnII-GFP-positive particles was slightly higher at the base than at the tip (For MnII-GFP, 0.43±0.16 and 0.30±0.03 particles/μm2, respectively; P<0.05; Table 1; 5 pupa, 16 bristles). For GalNacT2-YFP, 0.59±0.06 and 0.24±0.02 particles/μm2, respectively; P<0.05; Table 2; 5 pupa, 16 bristles. In addition, the density of MnII-GFP-positive particles was dramatically increased in both the tip and base, as compared to the WT (P<0.002 for both tip and base; Table 1; 5 pupa, 16 bristles). Such analysis was not possible for GalNacT2-YFP due to its absence from the WT bristle shaft. Also, the average area of both MnII-GFP- and GalNacT2-YFP-positive particles was significantly larger at the tip of the bristle shaft than at the base (For MnII-GFP, 0.46±0.63 and 0.29±0.39 μm2, respectively; P<0.001; Table 1; 5 pupa, 16 bristles). For GalNacT2-YFP, 0.53±0.03 and 0.26±0.20 μm2, respectively; P<0.003; Table 2; 5 pupa, 16 bristles. Moreover, these areas of MnII-GFP were significantly (P<0.001) different from what was seen in the corresponding cell parts in WT flies (Table 1; this analysis was not possible for GalNacT2-YFP since the WT bristle did not contain any trans-compartments in the shaft to analyze, Table 2). The defects in Golgi organization in the Dhc64C mutants bristle shaft led us to check whether the somal region of the bristle was affected. The closer examination revealed that in Dhc64C mutants, the Golgi apparatus is scattered throughout the somal region (S2 Fig), as in WT.
Fig 2. Golgi organization in Dhc64C mutant bristle cell shaft.
Confocal projections of Dynein heavy chain-mutated background bristle shafts from ~40 h APF. A-D–Dhc64C8-1/Dhc64C902 trans-heterozygote bristle shaft expressing sca-Gal4::MnII-GFP stained with anti-dGM130 (cis-Golgi marker) antibodies and phalloidin: A–Gray phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter: B–Green MnII-GFP, a medial-Golgi marker, is localized to the entire bristle shaft area, C–Red anti-dGM130 antibody staining (cis-Golgi marker), D–Merged image of green MnII-GFP and red anti-dGM130 antibody staining showing co-localization of medial- and cis-Golgi compartments in the bristle shaft. E-H–Dhc64C8-1/Dhc64C4-19 trans-heterozygote bristle shaft expressing sca-Gal4::GalNacT2-YFP (trans-Golgi marker) co-stained with anti-dGM130 antibodies and phalloidin: E–Gray phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter: F–Green GalNacT2-YFP trans-Golgi marker is ectopically localized to the entire bristle shaft area, G–Red anti-dGM130 antibody staining (cis-Golgi marker), H–Merged image of green GalNacT2-YFP and red anti-dGM130 antibody staining showing co-localization of trans- and cis-Golgi compartments in the mutant bristle shaft. I-L–Dhc64C8-1/Dhc64C902 trans-heterozygote bristle shaft expressing sca-Gal4::MnII-GFP, co-stained with anti-Sec16 (ERES) antibodies and phalloidin: I–Gray phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter: J—Green MnII-GFP, a medial-Golgi marker, is localized to the entire bristle shaft area, K–Red anti-Sec16 antibody staining localized to the entire shaft area, L–Merged image of green MnII-GFP and red anti-Sec-16-antibody staining showing co-localization of medial-Golgi and ERES components in the mutant bristle shaft. APF—After prepupa formation. Scale bar, 10 μm.
Table 2. Golgi outpost localization parameters in Dhc64C and khc mutant bristle shafts.
| Golgi outpost (GalNacT2-YFP) localization parameters in Drosophila bristles | ||||||
|---|---|---|---|---|---|---|
| Genotype | Wild-type | Dhc64C8-1/Dhc64C4-19 | Khc- RNAi | |||
| Bristle cell area | tip | base | tip | base | tip | base |
| No. of pupae | 5 | 5 | 5 | |||
| No. of bristles | 15 | 16 | 15 | |||
| Particle area, avg (μm2) | N/A | N/A | 0.53±0.03* | 0.26±0.20 | 0.23±0.18 A | 0.33±0.36*A |
| Density (particle/μm2) | N/A | N/A | 0.24±0.02 | 0.59±0.06* | 0.23±0.23 | 0.47±0.41* |
*—represents a significant difference of the cell part (tip/base) within each genotype; letters represent a significant difference in corresponding cell parts (tip/tip; base/base) between genotypes.
A–Significantly different from Dhc64C mutant. The values reflect mean±s.d. (for a detailed description of the statistical analysis performed, see Materials and Methods).
N/A- Not applicable.
Lava-lamp is not required for Golgi organization in the bristle shaft
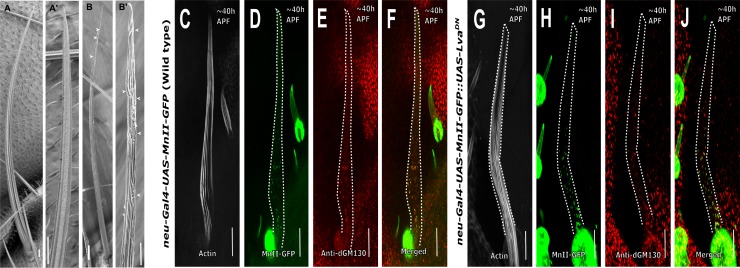
Previously it was shown that in Drosophila embryo cellularization, Golgi movement depends on cytoplasmic dynein [35]. Moreover, it was shown that the Golgin (Golgi-associated protein) Lava-lamp (Lva) mediates such dynein-based Golgi movements [35, 36]. Later, it was shown that Lva is also required for the dynamic profile of dendritic Golgi satellites [37]. Thus, we analyzed the role of Lva in bristle Golgi organization by disrupting Lva activity via over-expression of the Lva-dominant negative (LvaDN) protein [7]. We found that expression of LvaDN in bristles using neur-Gal4 but not sca-Gal4 affected bristle morphology, with the upper part of the bristle being twisted (90% of the bristle in 10 adults; Fig 3B and 3B'). On the other hand, using the same neur-Gal4, no defects in Golgi satellite organization were detected at the bristle shaft (Fig 3G–3J, S3 Fig) when compared to WT (Fig 3C–3F, S3 Fig), and no defects were found in the bristle somal region (S4 Fig).
Fig 3. Lava-lamp is not required for Golgi organization in the bristle shaft.
Scanning electron microscope images (A-B') of adult bristles from A–Wild type, A’–higher magnification image of the tip area showing characteristic parallel grooved surface morphology; B–neur-Gal4::UAS-LVADN fly bristle, B’—higher magnification image of the tip area of neur-Gal4::UAS-LVADN showing disrupted characteristic parallel grooved surface morphology highlighted by arrowheads. C-F- Confocal projections of neur-Gal4::UAS-MnII-GFP (Wild type) bristle shafts from ~40 h APF stained with anti-dGM130 (cis-Golgi marker) antibodies and phalloidin: C–Gray phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter: D–Green MnII-GFP, a medial-Golgi marker, is localized to the base of bristle shaft area, E–Red anti-dGM130 antibody staining (cis-Golgi marker), F–Merged image of green MnII-GFP and red anti-dGM130 antibody staining showing co-localization of medial- and cis-Golgi compartments in the bristle shaft. G-J- Confocal projections of Lava-lampDN background bristle shafts. G-J- neur-Gal4::UAS-Lava-lampDN bristle shafts from ~40 h APF co-expressing UAS-MnII-GFP and stained with anti-dGM130 (cis-Golgi marker) antibodies and phalloidin: G–Gray phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter: H–Green MnII-GFP, a medial-Golgi marker, is localized to the entire bristle shaft area, I–Red anti-dGM130 antibody staining (cis-Golgi marker), J–Merged image of green MnII-GFP and red anti-dGM130 antibody staining showing co-localization of medial- and cis-Golgi compartments in the bristle shaft. APF—After prepupa formation. Scale bar, 10 μm.
Kinesin heavy chain is required for Golgi organization in the bristle shaft
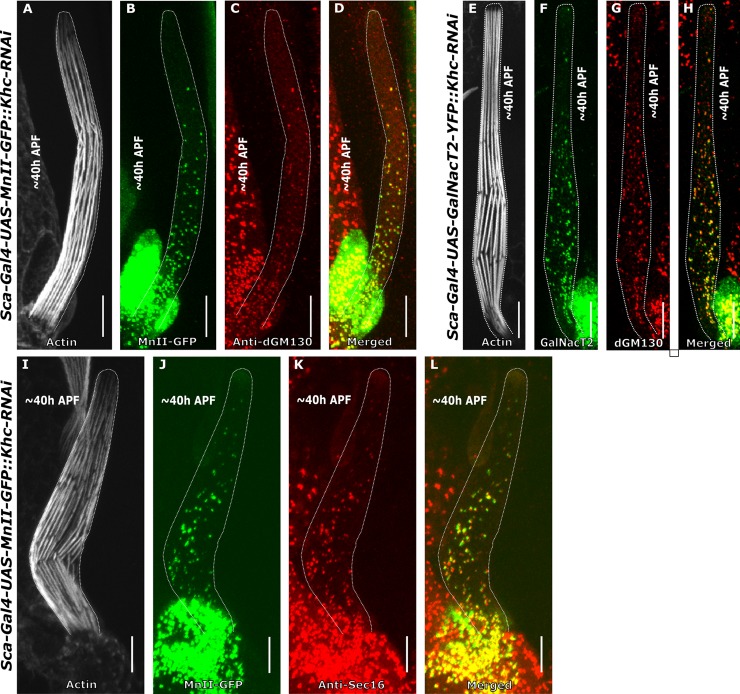
Next, we examined the role of the plus-end-directed MT motor kinesin, and more specifically, the conventional kinesin-41, kinesin heavy chain (Khc), in bristle Golgi organization. Although dynein is believed to be the primary motor in Golgi positioning, it was shown in vertebrate cells that kinesin-light chain is present in Golgi membranes [38]. Still, the role of kinesin in Golgi trafficking is not well understood. Accordingly, we down-regulated the Khc transcript specifically in the bristle using RNAi (for simplicity, these strains are henceforth termed Khc mutants). To our surprise, we found that similar to Dhc64C mutant flies, bristles in Khc mutants also contained complete Golgi units located throughout the entire bristle shaft (Fig 4) composed of co-localized cis- (Fig 4C and 4D), medial- (Fig 4B, 4D, 4J and 4L), and trans-Golgi components (Fig 4F and 4H) and ERES (Fig 4K and 4L). However, the distribution pattern of the Golgi units in the Khc mutant bristle shafts was different from that in the Dhc64C mutants. Similar to WT and Dhc64C mutant flies (Tables 1 and 2), the density of MnII-GFP was significantly higher at the base of the bristle than at the tip (For MnII-GFP, 0.31±0.26 particle/μm2 and 0.12±0.13 particle/μm2, respectively (P<0.001); Table 1; 5 pupa, 15 bristles). Moreover, the density of MnII-positive particles at both the tip and base was significantly higher than recorded for the WT (Table 1, P<0.0001) but similar to Dhc64C. However, in contrast to Dhc64C mutants, the average area of the MnII-GFP and GalNacT2-positive particles was significantly larger at the base of the bristle shaft than at the tip (For MnII-GFP, 0.40±0.19 μm2 and 0.20±0.52 μm2, respectively. For GalNacT2-YFP, 0.33±0.36 μm2 and 0.23±0.18 μm2, respectively, Table 2, 5 pupa, 15 bristles). Also, in the Khc mutant bristle somal region, no obvious defects in Golgi organization were detected (S5 Fig).
Fig 4. Golgi organization in kinesin heavy chain-mutated bristles.
Confocal projections of kinesin heavy chain-mutated background bristle shafts from ~40 h APF. A-D–Khc-RNAi bristle shaft expressing sca-Gal4::UAS-MnII-GFP co-stained with anti-dGM130 (cis-Golgi marker) antibodies and phalloidin: A–Gray phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter, B–Green MnII-GFP, a medial-Golgi marker, is localized to the entire bristle shaft area, C–Red anti-dGM130 antibody staining (cis-Golgi marker), D–Merged image of green MnII-GFP and red anti-dGM130 showing co-localization of medial- and cis-Golgi compartments in the bristle shaft. E-H–Khc-RNAi bristle shaft expressing sca-Gal4::UAS- GalNacT2-YFP (trans-Golgi marker) co-stained with anti-dGM130 antibodies and phalloidin: E–Gray phalloidin-UV staining of actin bundles in a GalNacT2-YFP-expressing bristle, used here to highlight the cell perimeter, F–Green GalNacT2-YFP, a trans-Golgi marker, is ectopically localized to the entire bristle shaft area, G–Red anti-dGM130 antibody staining (cis-Golgi marker), H–Merged image of green GalNacT2-YFP and red anti-dGM130 showing co-localization of trans- and cis-Golgi compartments in a mutated bristle shaft. I-L–Khc-RNAi bristle shaft expressing sca-Gal4::UAS-MnII-GFP co-stained with anti-Sec16 (ERES) antibodies and phalloidin: I–Gray phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter, J—green MnII-GFP, a medial-Golgi marker, is localized to the entire bristle shaft area, K–Red anti-Sec16 antibody staining localized to the entire shaft area, L–merged image of green MnII-GFP and red anti-Sec-16-antibody showing co-localization of medial- and ERES components in a mutated bristle shaft. APF—After prepupa formation. Scale bar, 10 μm.
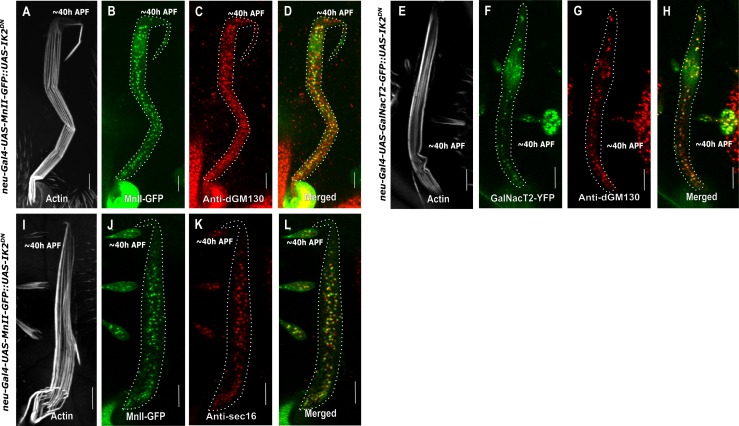
Mutation of Ik2 affects Golgi organization in the bristle shaft
We previously demonstrated that in the bristle, stable MTs are organized in an asymmetrical manner, with the minus-end pointing outwards [15]. Moreover, we found that the Spn-F/Ik2 complex is required for bristle MT polarity [39]. Thus, to test whether MT polarity is essential for bristle Golgi organization, we studied Golgi organization in flies expressing Ik2dominant-negative (Ik2DN) specifically in the bristle. Ik2DN is a defective kinase in which lysine at position 41 was changed to alanine (Ik2K41A). We and other have shown that expression of Ik2DN strongly affects bristle morphology and MT organization [15, 40, 41]. First, we found that expression with neur-Gal4 but not sca-Gal4 generated typical Ik2 bristle defects. Thus, we used the neur-Gal4 for further examinations. We found that similar to Dhc64C and Khc mutant flies, in Ik2DN mutants, the bristle contained complete Golgi units localized throughout the entire bristle shaft (Fig 5A–5L (and co-localized cis- (Fig 5C, 5D, 5G and 5H), medial- (Fig 5B, 5D, 5J and 5L) and trans-Golgi components (Fig 5F and 5H) and ERES (Fig 5K and 5L). Since we used different Gal4s for Golgi expression in Dhc64C and Khc-RNAi (sca-Gal4), in comparison to Ik2DN expression (neur-Gal4), we were not able to statistically compare Golgi parameter (area and density) between them. Instead, we compared the neur-Gal4::UAS-MnII-GFP and GalNacT2-positive particle densities in Ik2DN mutant bristle shafts versus their Gal4-UAS genetic background (neur-Gal4::UAS-MnII-GFP and GalNacT2-YFP).
Fig 5. Confocal projections of Ik2DN background bristle shafts from ~40 h APF.
A-D–neur-Gal4::UAS-Ik2DN bristle shaft co-expressing UAS-MnII-GFP and stained with anti-dGM130 (cis-Golgi marker) antibodies and phalloidin: A–Gray phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter: B–Green MnII-GFP, a medial-Golgi marker, is localized to the entire bristle shaft area, C–Red anti-dGM130 antibody staining (cis-Golgi marker), D–Merged image of green MnII-GFP and red anti-dGM130 antibody staining showing co-localization of medial- and cis-Golgi compartments in the bristle shaft. E-H–neur-Gal4::UAS-Ik2DN bristle shaft co-expressing UAS-GalNacT2-YFP (trans-Golgi marker) stained with anti-dGM130 antibodies and phalloidin: E–Gray phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter: F–Green GalNacT2-YFP, a trans-Golgi marker, is ectopically localized to the entire bristle shaft area, G–Red anti-dGM130 antibody staining (cis-Golgi marker), H–Merged image of green GalNacT2-YFP and red anti-dGM130 antibody staining showing co-localization of trans- and cis-Golgi compartments in the mutant bristle shaft. I-L–neur-Gal4::UAS-Ik2DN bristle shaft co-expressing UAS-MnII-GFP stained with anti-Sec16 (ERES) antibodies and phalloidin: I–Gray phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter: J—Green MnII-GFP, a medial-Golgi marker, is localized to the entire bristle shaft area, K–Red anti-Sec16 antibody staining is localized to the entire shaft area, L–Merged image of green MnII-GFP and red anti-Sec-16-antibody showing co-localization of medial-Golgi and ERES components in the mutant bristle shaft. APF—After prepupa formation. Scale bar, 10 μm.
First, we noticed that there was no difference in MnII-GFP-positive particle average area between the tip (0.32±0.23 μm2, Table 3) and base (0.32±0.28 μm2, Table 3) in the Ik2 mutant bristle shaft. The same pattern was also detected in GalNacT2-positive particle average area (tip: 0.24±0.04 μm2; base: 0.24±0.09 μm2, Table 3) from the Ik2 mutant. Interestingly, MnII-GFP-positive particles density was higher at the tip (0.26±0.10 particle/μm2, P<0.001; 5 pupa, 18 bristles, Table 3) but was similar at the base to what was noted in the WT (Table 3). In case of GalNacT2-YFP, this analysis was impossible because of its absence (Table 3). Moreover, in Ik2DN mutants no defects in Golgi organization at the somal region of the bristle could be detected (S6 Fig).
Table 3. Golgi outpost localization parameters in Ik2DN bristles.
| Golgi outpost localization parameters in Drosophila bristles | ||||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | Wild-type | Ik2DN | Wild-type | Ik2DN | ||||
| Golgi compartments | Medial- compartment (mnII) | Medial- compartment (mnII) |
Trans- compartment (GalNacT2) |
Trans- compartment (GalNacT2) |
||||
| Bristle cell area | Tip | Base | Tip | Base | Tip | Base | Tip | Base |
| No. of pupae | 5 | 5 | 5 | 5 | ||||
| No. of bristles | 15 | 18 | 18 | 18 | ||||
| Particle area avg (μm2) | 0.17±0.20 | 0.43±0.80* | 0.32±0.23A | 0.32±0.28 | N/A | N/A | 0.24±0.04 | 0.24±0.09 |
| Density (particle/μm2) | 0.04±0.06 | 0.18±0.05* | 0.26±0.10A | 0.20±0.32A | N/A | N/A | 0.30±0.03 | 0.23±0.07 |
*—represents a significant difference of the cell part (tip/base) within each genotype; letters represent a significant difference in corresponding cell parts (tip/tip; base/base) between genotypes.
A–Significantly different from WT. The values reflect mean±s.d. (for a detailed description of the statistical analysis performed, see Materials and Methods).
N/A- Not applicable.
Differential effects on Golgi organization among Dhc, Khc and Ik2 mutants
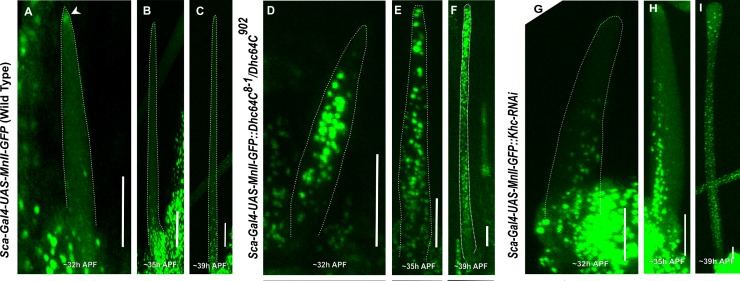
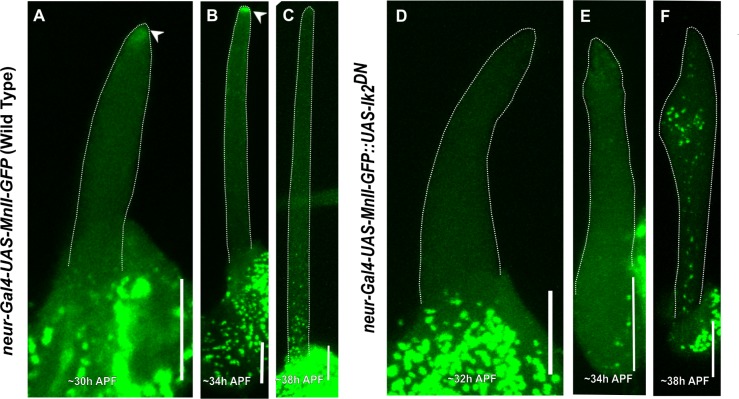
To understand the molecular mechanism by which Dhc64C, Khc and Ik2DN affect bristle Golgi organization; we followed the timing of Golgi organization in these mutants during bristle development. Since we used a different Gal4 for Ik2DN (neur-Gal4) than used with the Dhc64C and Khc mutants (sca-Gal4), we could only compare between Dhc64C and Khc mutants, with Ik2DN being compared to the control. We found that at early stages of WT bristle development, i.e. until 30–35 h APF (After prepupa formation), no Golgi satellites were detected in the bristle shaft in the WT (Fig 6A–6B). However, in both the Dhc64C (Fig 6D and 6E) and Khc (Fig 6G and 6H) mutants, even in the early stages of elongation, e.g., ~32 h APF, MnII-GFP positive particles could be detected throughout the bristle shaft. As mentioned above, later in the development of the Dhc64C and Khc mutants, the Golgi were spread all over the bristle shaft (Fig 6F and 6I). In Ik2DN, the timing of MnII-GFP entry into the bristle shaft was at 38 h APF in bristle development (Fig 7F).
Fig 6. Comparing developmental stages as a function of Golgi outpost localization.
Confocal projections of live WT developing bristle shafts expressing sca-Gal4::MnII-GFP (a medial-Golgi marker) at different elongation stages (A-C). A–~32 h APF, the developing bristle shaft shows no MnII-positive particles at this early stage of bristle development. Arrow points to the accumulation of MnII-GFP protein at the bristle tip, B–~35h APF, the developing bristle shaft shows no Golgi outposts localized to the bristle shaft. C–~39 h APF, the developing bristle shaft shows Golgi outposts beginning to accumulate at the base of the bristle shaft. D-F–Dynein heavy chain (Dhc64C8-1/Dhc64C902 trans-heterozygotes)-mutated developing bristle shafts expressing sca-Gal4::MnII-GFP: D–~32 h APF, the developing Dhc64C8-1/Dhc64C902 background bristle shaft shows MnII-positive particles filling the entire shaft area at this early stage of bristle development, E–~35 h APF, Golgi outposts are found throughout entire bristle shaft. The same is true for the following developmental stage of bristle shaft elongation: F– 39 h APF. G-I–developing bristle shafts expressing both sca-Gal4::MnII-GFP and UAS-Khc-RNAi; images show the corresponding developmental staging of Khc-depleted bristles, depicting the early appearance of Golgi outposts, G– 32 h APF, H– 35 h APF, I—At an advanced developmental stage (~39h APF), Golgi outposts localize evenly throughout the entire bristle shaft. APF—After prepupa formation. Scale bar, 10 μm.
Fig 7. Comparing developmental stages as a function of Golgi outpost localization in Ik2DN bristles.
Confocal projections of live WT developing bristle shafts expressing MnII-GFP by neur-Gal4 (a medial-Golgi marker) at different elongation stages (A-C). A–~32 h APF, the developing bristle shaft shows no MnII-positive particles at this early stage of bristle development. Arrow points to the accumulation of MnII-GFP protein at the bristle tip, B–~34h APF, the developing bristle shaft shows no Golgi outposts localized to the bristle shaft. Arrow points to the accumulation of MnII-GFP protein at the bristle tip, C–~38 h APF, the developing bristle shaft shows Golgi outposts beginning to accumulate at the base of the bristle shaft. D-F—neur-Gal4::UAS-Ik2DN-developing bristles co-expressing UAS-MnII-GFP, D—~32 h APF, the developing bristle shaft shows no Golgi outposts localized to the bristle shaft, E -~34 h APF, the developing bristle shaft shows no Golgi outpost accumulation, F—Developing bristle shaft at -~38 h showing Golgi outposts beginning to accumulate entire bristle shaft. APF—After prepupa formation. Scale bar, 10 μm.
Discussion
Golgi organization in Drosophila bristles
In different Drosophila tissues, the Golgi shows different organizational patterns, reflecting differences in cell type. In most Drosophila cells, the Golgi is organized as discontinuous stacks composed of ERES and cis-, medial-, and trans-Golgi compartments [5]. During spermatogenesis, the Golgi-based acroblast has a ring shape [42, 43]. In Drosophila larval neurons, the Golgi is differently organized. Here, the soma contains a high-ordered ring-shaped complete Golgi structure. In addition, similar to mammals, the dendrites contain either a discrete "single compartment Golgi" or "Golgi mini-stacks" composed of more than one Golgi compartment [23]. In our study, we showed that bristle cells contain complete Golgi stacks at the soma and incomplete Golgi composed of ERES, and cis-, and medial-Golgi compartments at the shaft. We show for the first time specifically in Drosophila that Golgi satellites can be found in cell types other than neurons. In the bristle, Golgi satellites are preferentially localized to the shaft base area. The role of these Golgi satellites remains, however, to be determined. In dendrites, Golgi outposts localize preferentially to branch points, where they are believed to serve as MTOCs (Microtubule organizing centers) [11, 44]. Thus, exact positioning of Golgi outposts is essential for establishing dendritic morphology. Additionally, in mammal neurons, Golgi outposts are more prominent during periods of intensive dendrite growth, while in mature neurons, their frequency decreases with time [14]. This points to not only tight spatial control of Golgi positioning but also to the developmental importance of the process. Here, we focused our analysis on the molecular machinery that regulates organelle positioning, although the function of Golgi satellites in the bristle cell remains to be elucidated.
The role of MTs in bristle Golgi organization
MTs are the main tracks for long-distance transport in the cell. Thus, MT array integrity and dynamics are essential for proper cellular elongation of both bristles and neurons. In highly polarized neurons, the dendrite and axon differ in terms of their MT array polarity. MTs in the axons of mammal and Drosophila neurons are oriented with their plus-ends pointed distally, while Drosophila dendrites possess MTs mainly oriented with their minus-ends pointed outwards. This difference in MT orientation is believed to provide directional cues for differential organelle transport [45–47]. To confirm this hypothesis, we decided to disrupt MT array polarity through expression of mutant Ik2 protein. We found that affecting MT polarity resulted in abnormal Golgi unit localization, together with ectopic trans-Golgi domain appearance. We thus showed that polarized MTs are essential for correct Golgi organization. Specifically, the presence of polarized MTs prevents the appearance of trans-Golgi components within the bristle shaft.
Possible roles of dynein and kinesin in bristle Golgi organization
Dynein is believed to be the major Golgi architecture effector [5, 48, 49]. In Drosophila dendrites, dynein was found to be responsible for dendrite-specific Golgi outpost localization and MT orientation [7, 37, 50]. In neurons, kinesin-1 plays both indirect and direct role in dendritic selective sorting of Golgi outposts [51]. Indirectly, kinesin depletion alters axon MT orientation, which leads to ectopic Golgi outposts’ localization to axons driven by a different motor on misleading MT tracks. Directly, control of kinesin-1 auto-inhibition prevents entry of Golgi outposts into the axon [51].
We found that in Dhc64C mutant flies, bristle shaft Golgi satellites contained an ectopic trans-Golgi compartment that co-localized with cis- and medial-Golgi components. This raises the question of the role of Dhc64C in bristle Golgi organization. One explanation is that Dhc64C is required for Golgi satellite anchoring/sorting. In this model, dynein may prevent the entry of Golgi units from the soma to the shaft, while in the shaft, dynein specifically anchors the organelle to the lower zone close to soma, preventing the localization of Golgi units to the distant growth area of the cell tip.
A second explanation for the defects in Golgi unit localization observed in Dhc64C mutants could be disrupted interactions with the oppositely-directed motor, kinesin. However, taking into consideration MT array organization (i.e., with the minus-end pointed towards the tip) in a fly expressing a dynein mutant, active kinesin would be expected to return Golgi back to the soma. Yet, exactly the opposite is true. Moreover, we demonstrate that, similarly to Dhc64C flies, the bristle shaft contained an ectopic trans-Golgi compartment co-localized with cis- and medial Golgi components. In summary, our results shed new light of Golgi organization in polarized cells, such as the Drosophila bristle. Still, further study is needed to understand the molecular mechanism by which the combination of a polarized MT network with MT motor protein function affects Golgi bristle organization.
Supporting information
Confocal projections of live developing bristle shafts expressing Gal-T-RFP (a trans-Golgi marker): A-B- Sca-Gal4-UAS-EB1-GFP::UAS-Gal-T-RFP (WT) developing bristle shaft showing no Gal-T-positive particles at an early stage of bristle development; A—Green EB1-GFP used to highlight the cell perimeter, B- Red—Gal-T-RFP. C-D—Confocal projections of dynein heavy chain-mutated background bristle shafts, Dhc64C8-1/Dhc64C4-19 trans-heterozygote bristle shaft expressing Gal-T-RFP stained with Oregon-green phalloidin; C–Red- The Gal-T-RFP (trans-Golgi) marker is localized to the entire bristle shaft area, D–Merged—green—phalloidin staining of actin bundles, used here to highlight the cell perimeter, red—Gal-T-RFP. Scale bar, 10 μm.
(EPS)
A-C'' -Confocal projections of representative of a WT bristle “somal” region from Dhc64C mutant background: A-A''–“Soma” of a dynein mutant bristle cell expressing sca-Gal4::MnII-GFP (a medial-Golgi marker) stained with anti-dGM130 antibodies (a cis-Golgi marker). A'–Anti-dGM130 antibody staining showing a cis-Golgi compartment localized throughout the bristle soma cytoplasm, A''–Merged image of green UAS-MnII-GFP and red anti-dGM130 antibody staining showing co-localization of medial- and cis-Golgi compartments. B-B''–Soma of a Dhc64C mutant bristle cell expressing sca-Gal4::GalNacT2-RFP (a Trans-Golgi marker), B’- stained with anti-dGM130 antibodies (a cis-Golgi marker), B''–Merged image of GalNacT2-YFP and red anti-dGM130 antibody staining showing co-localization of medial- and Trans-Golgi compartments. C-C''–Soma of a dynein mutant bristle cell expressing sca-Gal4::MnII-GFP (a medial-Golgi marker) stained with anti-Sec16-antibodies (to identify the ERES): C'–Anti-Sec16 antibody staining showing ERES localized throughout the bristle soma cytoplasm, C''–Merged image of green MnII-GFP and red anti-Sec16 antibody staining showing co-localization of medial-Golgi and ERES components in the bristle cell soma. The scale bar represents 10 μm.
(EPS)
A-D- A Wild type bristle shaft expressing neur-Gal4-UAS-GalNacT2-YFP (trans-Golgi marker) stained with anti-dGM130 (cis-Golgi marker) antibodies and phalloidin: A–Gray phalloidin-UV staining of actin bundles in a GalNacT2-YFP expressing bristle, used here to highlight the cell perimeter: B–Green GalNacT2-YFP, a trans-Golgi marker, is ectopically localized to the entire bristle shaft area, C–Red anti-dGM130 antibody staining (cis-Golgi marker), D–Merged image of green GalNacT2-YFP and red anti-dGM130 antibody staining showing co-localization of trans- and cis-Golgi compartments in the mutant bristle shaft. E-H- neur-UAS-Lava-lampDN bristle shaft co-expressing UAS-GalNacT2-YFP (trans-Golgi marker) and stained with anti-dGM130 (cis-Golgi marker) antibodies and phalloidin: E–Gray phalloidin-UV staining of actin bundles in a GalNacT2-YFP expressing bristle, used here to highlight the cell perimeter: F–Green GalNacT2-YFP, a trans-Golgi marker, is ectopically localized to the entire bristle shaft area, G–Red anti-dGM130 antibody staining (cis-Golgi marker), H–Merged image of green GalNacT2-YFP and red anti-dGM130 antibody staining showing co-localization of trans- and cis-Golgi compartments in the mutant bristle shaft. I-L–A wild type neur-Gal4::UAS-MnII-GFP bristle shaft expressing stained with anti-Sec16 (ERES) antibodies and phalloidin: I–Gray phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter: J—Green MnII-GFP, a medial-Golgi marker, is localized to the entire bristle shaft area, K–Red anti-Sec16 antibody staining is localized to the entire shaft area, L–Merged image of green MnII-GFP and red anti-Sec-16-antibody showing co-localization of medial-Golgi and ERES components in the mutant bristle shaft. M-P–neur-UAS-Lava-lampDN bristle shaft co-expressing UAS-MnII-GFP stained with anti-Sec16 (ERES) antibodies and phalloidin: M–Gray phalloidin-UV staining of actin bundles in a MnII-GFP-expressing bristle, used here to highlight the cell perimeter: N—Green MnII-GFP, a medial-Golgi marker, is localized to the entire bristle shaft area, O–Red anti-Sec16 antibody staining is localized to the entire shaft area, P–Merged image of green MnII-GFP and red anti-Sec-16-antibody showing co-localization of medial-Golgi and ERES components in the mutant bristle shaft. APF—After prepupa formation. The scale bar represents 10 μm.
(EPS)
Confocal projections of representative of bristle “somal” region from WT (A-A'', C-C'', E-E'') and LvaDN mutant background: A-A''–“Soma” of a WT mutant bristle cell expressing neur-Gal4::MnII-GFP (a medial-Golgi marker) stained with anti-dGM130 antibodies (a cis-Golgi marker). A'–Anti-dGM130 antibody staining showing a cis-Golgi compartment localized throughout the bristle soma cytoplasm, A''–Merged image of green MnII-GFP and red anti-dGM130 antibody staining showing co-localization of medial- and cis-Golgi compartments. B-B''–“Soma” of a LvaDN mutant bristle cell expressing neur-Gal4::MnII-GFP (a medial-Golgi marker) stained with anti-dGM130 antibodies (a cis-Golgi marker). B'–Anti-dGM130 antibody staining showing a cis-Golgi compartment localized throughout the bristle soma cytoplasm, B''–Merged image of green MnII-GFP and red anti-dGM130 antibody staining showing co-localization of medial- and cis-Golgi compartments. C-C''–Soma of a WT bristle cell expressing neur-Gal4::GalNacT2-YFP (a Trans-Golgi marker), C’- stained with anti-dGM130 antibodies (a cis-Golgi marker), C''–Merged image of GalNacT2-YFP and red anti-dGM130 antibody staining showing co-localization of medial- and Trans-Golgi compartments. D-D''–Soma of a LvaDN mutant bristle cell expressing GalNacT2-YFP (a Trans-Golgi marker), D’- stained with anti-dGM130 antibodies (a cis-Golgi marker), D''–Merged image of UAS-GalNacT2-YFP and red anti-dGM130 antibody staining showing co-localization of medial- and Trans-Golgi compartments. E-E''–Soma of a WT bristle cell expressing neur-Gal4::MnII-GFP (a medial-Golgi marker) stained with anti-Sec16-antibodies (to identify the ERES): E'–Anti-Sec16 antibody staining showing ERES localized throughout the bristle soma cytoplasm, E''–Merged image of green MnII-GFP and red anti-Sec16 antibody staining showing co-localization of medial-Golgi and ERES components in the bristle cell soma. F-F''–Soma of a LvaDN mutant bristle cell expressing MnII-GFP (a medial-Golgi marker) stained with anti-Sec16-antibodies (to identify the ERES): F'–Anti-Sec16 antibody staining showing ERES localized throughout the bristle soma cytoplasm, F''–Merged image of green MnII-GFP and red anti-Sec16 antibody staining showing co-localization of medial-Golgi and ERES components in the bristle cell soma. The scale bar represents 10 μm.
(EPS)
A-C'' -Confocal projections of representative of bristle “somal” region from kinesin heavy chain mutant background: A-A''–“Soma” of a bristle cell co-expressing sca-Gal4::UAS-MnII-GFP (a medial-Golgi marker) and UAS-Khc-RNAi stained with anti-dGM130 antibodies (a cis-Golgi marker). A'–Anti-dGM130 antibody staining showing a cis-Golgi compartment localized throughout the bristle soma cytoplasm, A''–Merged image of green MnII-GFP and red anti-dGM130 antibody staining showing co-localization of medial- and cis-Golgi compartments. B-B''–Soma of a bristle cell expressing sca-Gal4::UAS-GalNacT2-YFP (a Trans-Golgi marker) and UAS-Khc-RNAi, B’- stained with anti-dGM130 antibodies (a cis-Golgi marker), B''–Merged image of GalNacT2-YFP and red anti-dGM130 antibody staining showing co-localization of medial- and Trans-Golgi compartments. C-C''–Soma of a bristle cell expressing sca-Gal4::UAS-MnII-GFP (a medial-Golgi marker) and UAS-Khc-RNAi stained with anti-Sec16-antibodies (to identify the ERES): C'–Anti-Sec16 antibody staining showing ERES localized throughout the bristle soma cytoplasm, C''–Merged image of green MnII-GFP and red anti-Sec16 antibody staining showing co-localization of medial-Golgi and ERES components in the bristle cell soma. The scale bar represents 10 μm.
(EPS)
A-C'' -Confocal projections of representative of bristle “somal” region from Ik2DN mutant background: A-A''–“Soma” of a bristle cell expressing neur-Gal4::UAS-MnII-GFP (a medial-Golgi marker) and UAS- Ik2DN stained with anti-dGM130 antibodies (a cis-Golgi marker). A'–Anti-dGM130 antibody staining showing a cis-Golgi compartment localized throughout the bristle soma cytoplasm, A''–Merged image of green MnII-GFP and red anti-dGM130 antibody staining showing co-localization of medial- and cis-Golgi compartments. B-B''–Soma of a bristle cell expressing neur-Gal4::GalNacT2-YFP (a Trans-Golgi marker) and UAS-Ik2DN, B’- stained with anti-dGM130 antibodies (a cis-Golgi marker), B''–Merged image of GalNacT2-YFP and red anti-dGM130 antibody staining showing co-localization of medial- and Trans-Golgi compartments. C-C''–“Soma” of a bristle cell expressing neur-Gal4::UAS-MnII-GFP (a medial-Golgi marker) and UAS- Ik2DN stained with anti-Sec16-antibodies (to identify the ERES): C'–Anti-Sec16 antibody staining showing ERES localized throughout the bristle soma cytoplasm, C''–Merged image of green MnII-GFP and red anti-Sec16 antibody staining showing co-localization of medial-Golgi and ERES components in the bristle cell soma. The scale bar represents 10 μm.
(EPS)
Acknowledgments
We thank VDRC Austria and the Bloomington Stock Center for generously providing fly strains. We thank Catherine Rabouille for her enlightening comments and for providing the antibodies against Sec-16.
Data Availability
All relevant data are within the manuscript; DOI: https://doi.org/10.5061/dryad.6gt43qg.
Funding Statement
This work was supported by the Israel Science Foundation (ISF) (grant 278/16) to U.A.
References
- 1.Ayala I, Colanzi A. Alterations of Golgi organization in Alzheimer's disease: A cause or a consequence? Tissue Cell. 2017;49(2 Pt A):133–40. Epub 2016/11/30. 10.1016/j.tice.2016.11.007 . [DOI] [PubMed] [Google Scholar]
- 2.van Vliet C, Thomas EC, Merino-Trigo A, Teasdale RD, Gleeson PA. Intracellular sorting and transport of proteins. Prog Biophys Mol Biol. 2003;83(1):1–45. Epub 2003/05/22. . [DOI] [PubMed] [Google Scholar]
- 3.Klumperman J. Architecture of the mammalian Golgi. Cold Spring Harb Perspect Biol. 2011;3(7). Epub 2011/04/20. 10.1101/cshperspect.a005181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marsh BJ, Mastronarde DN, Buttle KF, Howell KE, McIntosh JR. Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc Natl Acad Sci U S A. 2001;98(5):2399–406. Epub 2001/02/28. 10.1073/pnas.051631998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kondylis V, Rabouille C. The Golgi apparatus: lessons from Drosophila. FEBS Lett. 2009;583(23):3827–38. Epub 2009/10/06. 10.1016/j.febslet.2009.09.048 . [DOI] [PubMed] [Google Scholar]
- 6.Aridor M, Guzik AK, Bielli A, Fish KN. Endoplasmic reticulum export site formation and function in dendrites. J Neurosci. 2004;24(15):3770–6. Epub 2004/04/16. 10.1523/JNEUROSCI.4775-03.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye B, Zhang Y, Song W, Younger SH, Jan LY, Jan YN. Growing dendrites and axons differ in their reliance on the secretory pathway. Cell. 2007;130(4):717–29. Epub 2007/08/28. 10.1016/j.cell.2007.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23(15):6188–99. Epub 2003/07/18. 10.1523/JNEUROSCI.23-15-06188.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanus C, Ehlers MD. Secretory outposts for the local processing of membrane cargo in neuronal dendrites. Traffic. 2008;9(9):1437–45. Epub 2008/06/06. 10.1111/j.1600-0854.2008.00775.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chabin-Brion K, Marceiller J, Perez F, Settegrana C, Drechou A, Durand G, et al. The Golgi complex is a microtubule-organizing organelle. Mol Biol Cell. 2001;12(7):2047–60. Epub 2001/07/14. 10.1091/mbc.12.7.2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ori-McKenney KM, Jan LY, Jan YN. Golgi outposts shape dendrite morphology by functioning as sites of acentrosomal microtubule nucleation in neurons. Neuron. 2012;76(5):921–30. Epub 2012/12/12. 10.1016/j.neuron.2012.10.008 PubMed Central PMCID: PMC3523279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen MM, McCracken CJ, Milner ES, Goetschius DJ, Weiner AT, Long MK, et al. Gamma-tubulin controls neuronal microtubule polarity independently of Golgi outposts. Mol Biol Cell. 2014;25(13):2039–50. Epub 2014/05/09. 10.1091/mbc.E13-09-0515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulay AC, Saubamea B, Adam N, Chasseigneaux S, Mazare N, Gilbert A, et al. Translation in astrocyte distal processes sets molecular heterogeneity at the gliovascular interface. Cell Discov. 2017;3:17005 Epub 2017/04/06. 10.1038/celldisc.2017.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanus C, Ehlers MD. Specialization of biosynthetic membrane trafficking for neuronal form and function. Curr Opin Neurobiol. 2016;39:8–16. Epub 2016/03/25. 10.1016/j.conb.2016.03.004 . [DOI] [PubMed] [Google Scholar]
- 15.Bitan A, Rosenbaum I, Abdu U. Stable and dynamic microtubules coordinately determine and maintain Drosophila bristle shape. Development. 2012;139(11):1987–96. Epub 2012/04/20. 10.1242/dev.076893 . [DOI] [PubMed] [Google Scholar]
- 16.Melkov A, Abdu U. Regulation of long-distance transport of mitochondria along microtubules. Cell Mol Life Sci. 2018;75(2):163–76. Epub 2017/07/14. 10.1007/s00018-017-2590-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melkov A, Baskar R, Alcalay Y, Abdu U. A new mode of mitochondrial transport and polarized sorting regulated by Dynein, Milton and Miro. Development. 2016;143(22):4203–13. Epub 2016/11/02. 10.1242/dev.138289 . [DOI] [PubMed] [Google Scholar]
- 18.Melkov A, Simchoni Y, Alcalay Y, Abdu U. Dynamic microtubule organization and mitochondrial transport are regulated by distinct Kinesin-1 pathways. Biol Open. 2015;4(12):1696–706. Epub 2015/11/20. 10.1242/bio.015206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaraj R, Adler PN. Dusky-like functions as a Rab11 effector for the deposition of cuticle during Drosophila bristle development. Development. 2012;139(5):906–16. Epub 2012/01/27. 10.1242/dev.074252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gepner J, Li M, Ludmann S, Kortas C, Boylan K, Iyadurai SJ, et al. Cytoplasmic dynein function is essential in Drosophila melanogaster. Genetics. 1996;142(3):865–78. Epub 1996/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Wang L, Hays TS, Cai Y. Dynein-mediated apical localization of crumbs transcripts is required for Crumbs activity in epithelial polarity. J Cell Biol. 2008;180(1):31–8. Epub 2008/01/16. 10.1083/jcb.200707007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oshima K, Takeda M, Kuranaga E, Ueda R, Aigaki T, Miura M, et al. IKK epsilon regulates F actin assembly and interacts with Drosophila IAP1 in cellular morphogenesis. Curr Biol. 2006;16(15):1531–7. Epub 2006/08/05. 10.1016/j.cub.2006.06.032 . [DOI] [PubMed] [Google Scholar]
- 23.Zhou W, Chang J, Wang X, Savelieff MG, Zhao Y, Ke S, et al. GM130 is required for compartmental organization of dendritic golgi outposts. Curr Biol. 2014;24(11):1227–33. Epub 2014/05/20. 10.1016/j.cub.2014.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forster D, Armbruster K, Luschnig S. Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila. Current biology: CB. 2010;20(1):62–8. 10.1016/j.cub.2009.11.062 . [DOI] [PubMed] [Google Scholar]
- 25.Guild GM, Connelly PS, Vranich KA, Shaw MK, Tilney LG. Actin filament turnover removes bundles from Drosophila bristle cells. J Cell Sci. 2002;115(Pt 3):641–53. Epub 2002/02/28. . [DOI] [PubMed] [Google Scholar]
- 26.Ivan V, de Voer G, Xanthakis D, Spoorendonk KM, Kondylis V, Rabouille C. Drosophila Sec16 mediates the biogenesis of tER sites upstream of Sar1 through an arginine-rich motif. Mol Biol Cell. 2008;19(10):4352–65. Epub 2008/07/11. 10.1091/mbc.E08-03-0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondylis V, Goulding SE, Dunne JC, Rabouille C. Biogenesis of Golgi stacks in imaginal discs of Drosophila melanogaster. Molecular biology of the cell. 2001;12(8):2308–27. 10.1091/mbc.12.8.2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yano H, Yamamoto-Hino M, Abe M, Kuwahara R, Haraguchi S, Kusaka I, et al. Distinct functional units of the Golgi complex in Drosophila cells. Proc Natl Acad Sci U S A. 2005;102(38):13467–72. Epub 2005/09/22. 10.1073/pnas.0506681102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rabouille C, Kuntz DA, Lockyer A, Watson R, Signorelli T, Rose DR, et al. The Drosophila GMII gene encodes a Golgi alpha-mannosidase II. J Cell Sci. 1999;112 (Pt 19):3319–30. Epub 1999/10/03. . [DOI] [PubMed] [Google Scholar]
- 30.Mackenzie JM, Jones MK, Westaway EG. Markers for trans-Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in flavivirus-infected cells. J Virol. 1999;73(11):9555–67. Epub 1999/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yadav S, Linstedt AD. Golgi positioning. Cold Spring Harb Perspect Biol. 2011;3(5). Epub 2011/04/21. 10.1101/cshperspect.a005322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harada A, Takei Y, Kanai Y, Tanaka Y, Nonaka S, Hirokawa N. Golgi vesiculation and lysosome dispersion in cells lacking cytoplasmic dynein. J Cell Biol. 1998;141(1):51–9. Epub 1998/05/16. 10.1083/jcb.141.1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corthesy-Theulaz I, Pauloin A, Pfeffer SR. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol. 1992;118(6):1333–45. Epub 1992/09/01. 10.1083/jcb.118.6.1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: An ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58(2):189–200. Epub 2004/01/06. 10.1002/neu.10314 . [DOI] [PubMed] [Google Scholar]
- 35.Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151(4):905–18. Epub 2000/11/15. 10.1083/jcb.151.4.905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papoulas O, Hays TS, Sisson JC. The golgin Lava lamp mediates dynein-based Golgi movements during Drosophila cellularization. Nat Cell Biol. 2005;7(6):612–8. Epub 2005/05/24. 10.1038/ncb1264 . [DOI] [PubMed] [Google Scholar]
- 37.Lin CH, Li H, Lee YN, Cheng YJ, Wu RM, Chien CT. Lrrk regulates the dynamic profile of dendritic Golgi outposts through the golgin Lava lamp. J Cell Biol. 2015;210(3):471–83. Epub 2015/07/29. 10.1083/jcb.201411033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gyoeva FK, Bybikova EM, Minin AA. An isoform of kinesin light chain specific for the Golgi complex. J Cell Sci. 2000;113 (Pt 11):2047–54. Epub 2000/05/12. . [DOI] [PubMed] [Google Scholar]
- 39.Dubin-Bar D, Bitan A, Bakhrat A, Kaiden-Hasson R, Etzion S, Shaanan B, et al. The Drosophila IKK-related kinase (Ik2) and Spindle-F proteins are part of a complex that regulates cytoskeleton organization during oogenesis. BMC Cell Biol. 2008;9:51 Epub 2008/09/18. 10.1186/1471-2121-9-51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shapiro RS, Anderson KV. Drosophila Ik2, a member of the I kappa B kinase family, is required for mRNA localization during oogenesis. Development. 2006;133(8):1467–75. Epub 2006/03/17. 10.1242/dev.02318 . [DOI] [PubMed] [Google Scholar]
- 41.Otani T, Oshima K, Onishi S, Takeda M, Shinmyozu K, Yonemura S, et al. IKKepsilon regulates cell elongation through recycling endosome shuttling. Dev Cell. 2011;20(2):219–32. Epub 2011/02/15. 10.1016/j.devcel.2011.02.001 . [DOI] [PubMed] [Google Scholar]
- 42.Farkas RM, Giansanti MG, Gatti M, Fuller MT. The Drosophila Cog5 homologue is required for cytokinesis, cell elongation, and assembly of specialized Golgi architecture during spermatogenesis. Mol Biol Cell. 2003;14(1):190–200. Epub 2003/01/17. 10.1091/mbc.E02-06-0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fari K, Takacs S, Ungar D, Sinka R. The role of acroblast formation during Drosophila spermatogenesis. Biol Open. 2016;5(8):1102–10. Epub 2016/08/03. 10.1242/bio.018275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horton AC, Racz B, Monson EE, Lin AL, Weinberg RJ, Ehlers MD. Polarized secretory trafficking directs cargo for asymmetric dendrite growth and morphogenesis. Neuron. 2005;48(5):757–71. Epub 2005/12/13. 10.1016/j.neuron.2005.11.005 . [DOI] [PubMed] [Google Scholar]
- 45.Baas PW, Deitch JS, Black MM, Banker GA. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A. 1988;85(21):8335–9. Epub 1988/11/01. 10.1073/pnas.85.21.8335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone MC, Roegiers F, Rolls MM. Microtubules have opposite orientation in axons and dendrites of Drosophila neurons. Mol Biol Cell. 2008;19(10):4122–9. Epub 2008/08/01. 10.1091/mbc.E07-10-1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakata T, Hirokawa N. Microtubules provide directional cues for polarized axonal transport through interaction with kinesin motor head. J Cell Biol. 2003;162(6):1045–55. Epub 2003/09/17. 10.1083/jcb.200302175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cole NB, Sciaky N, Marotta A, Song J, Lippincott-Schwartz J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol Biol Cell. 1996;7(4):631–50. Epub 1996/04/01. 10.1091/mbc.7.4.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho WC, Allan VJ, van Meer G, Berger EG, Kreis TE. Reclustering of scattered Golgi elements occurs along microtubules. Eur J Cell Biol. 1989;48(2):250–63. Epub 1989/04/01. . [PubMed] [Google Scholar]
- 50.Zheng Y, Wildonger J, Ye B, Zhang Y, Kita A, Younger SH, et al. Dynein is required for polarized dendritic transport and uniform microtubule orientation in axons. Nat Cell Biol. 2008;10(10):1172–80. Epub 2008/09/02. 10.1038/ncb1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelliher MT, Yue Y, Ng A, Kamiyama D, Huang B, Verhey KJ, et al. Autoinhibition of kinesin-1 is essential to the dendrite-specific localization of Golgi outposts. The Journal of cell biology. 2018. 10.1083/jcb.201708096 . [DOI] [PMC free article] [PubMed] [Google Scholar]