Abstract
Objective:
We evaluated the risk factors for massive bleeding based on angiographic findings in patients with placenta previa and accreta who underwent balloon occlusion of the internal iliac artery (BOIA) during cesarean section.
Methods:
We performed a retrospective analysis using the clinical records of 42 patients with placenta previa and accreta who underwent BOIA during cesarean section between 2006 and 2017 in Gunma university hospital. We reviewed incidence of collateral arteries to the uterus on the initial aortography. We evaluated the visualization of the ovarian artery arising directly from the abdominal aorta, round ligament artery arising from the external iliac artery/inferior epigastric artery, and the iliolumbar artery. In addition, the clinical characteristics were reviewed. Patients with an estimated blood loss during delivery of >2500 ml, >4 packed red blood cell transfusions, uterine artery embolization after delivery, or hysterectomy were defined as the massive bleeding group. We compared between the massive and non-massive bleeding groups.
Results:
20 patients (48%) had a massive bleeding. No procedure-related severe complications were observed. The massive and non-massive bleeding groups differed in terms of operation time (p < 0.001), hysterectomy (p < 0.001), post-operative hospital stay (p < 0.05), and visualization of round ligament arteries to the uterus [15/20 (75%) patients, p < 0.01].
Conclusion:
The incidence of collateral blood supply from a round ligament artery to the uterus may be a risk factor for massive bleeding in patients with placenta previa and accreta who have undergone BOIA during cesarean section.
Advances in knowledge:
Angiographic visualization of collateral circulation from the round ligament artery to the uterus may be a risk factor for massive bleeding in patients with placenta previa and accreta who have undergone BOIA during cesarean section.
Introduction
Placenta previa is an abnormal complete or partial placental implantation in the lower uterine segment. The incidence rate of placenta previa is approximately 0.5–1% of all deliveries, which has been increasing with the increasing rate of cesarean section worldwide.1,2 Placenta accreta is a condition where the placenta firmly adheres to the myometrium because of partial or total absence of the decidua basalis. Its variants include accreta, increta, and percreta, which are classified according to the depth of trophoblastic invasion.3 In this study, the general term placenta accrete will refer to all three grades of abnormal trophoblastic invasion unless otherwise specified. Placenta accrete and previa sometimes occur a life-threatening complication during cesarean section. Therefore, pre-operative diagnosis and perioperative management are essential. Abnormal invasive placenta can be diagnosed before delivery by ultrasonography and MRI. However, more than expected, massive perioperative hemorrhage occurs in high-risk pregnancy cases.4–6 Management for prenatally diagnosed placenta previa usually consists of a scheduled cesarean section. The conventional management of placenta previa with accreta is cesarean hysterectomy with the placenta left in situ. However, to preserve the uterus and fertility, alternative management of hysterectomy is needed.7 Prophylactic balloon occlusion of the internal iliac artery (BOIA) is sometimes performed to control massive hemorrhage.2,3 However, prophylactic BOIA is controversial because insufficient bleeding control is sometimes reported.4,5 It is well established that the incidence of placenta previa increases with advancing age and higher parity. Surgical history, especially if a previous cesarean section was performed for placenta previa, is related to the recurrent development of placenta previa and, more importantly, placenta accreta.8,9 However, the patient group for whom use of prophylactic BOIA is effective for the management of placenta previa with accrete is not well investigated. We evaluated the efficacy of prophylactic BOIA on the basis of angiography findings in patients with placenta previa and accrete. In the English literature, no report exists on the prediction of outcome based on angiography findings.
methods and materials
We performed this retrospective analysis using the clinical records of 42 patients diagnosed as having placenta previa with accreta who underwent perioperative placement of an internal iliac occlusion balloon during cesarean section between September 2006 and October 2017. This study was approved by the ethics committee of Gunma university hospital. All the patients were examined using ultrasonography and/or MRI during the prenatal period, and the diagnosis of placenta previa with accreta was confirmed. Placenta previa was diagnosed using sonography, as internal cervical os was found to be covered completely or partially by the placenta. The criterion for the diagnosis of placenta previa with accreta is the presence of at least one of the following sonographic findings: (a) obliteration of the normal hypoechoic or subplacental zone; (b) extensive intra- or subplacental lacunae (dilated venous spaces); (c) tortuous vessels or lacunae in the myometrium; and (d) disruption or thinning of the uterine serosa-bladder interface10. If ultrasonography diagnosis alone was not enough, MRI was additionally performed. Prophylactic BOIA was performed for all hemodynamically stable patients. Under local anesthesia, bilateral femoral arterial punctures and insertion of 6-French vascular sheaths were performed. First, the initial abdominal aortography was performed with a 4-French pigtail catheter (Terumo Clinical Supply Co. Ltd., Gifu, Japan) to identify the internal iliac artery and collateral arteries to the uterus (Figure 1). When we performed aortography with a pigtail catheter, 15–20 ml of contrast agent (300 mg iodine/mL) was injected at a flow rate of 10 ml s−1. Imaging was performed in the anteroposterior plane at three frames per second. A 6-French occlusion balloon catheter (Boston Scientific, Watertown, MA) was positioned with its tip into the anterior division of the contralateral internal iliac artery (Figure 2). Pulsed low-dose fluoroscopic guidance (between 1 and 8 pulses per second) was used to minimize radiation exposure to the mother and fetus. Once positioning was satisfactory, the catheters were securely taped to the skin.
Figure 1. .
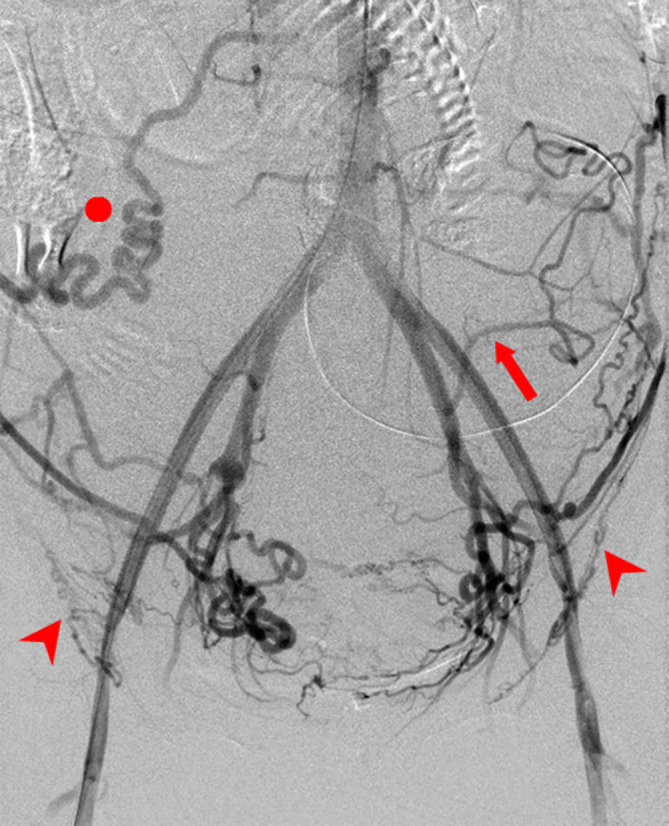
A 41-year-old female with placenta previa and accreta. The initial abdominal aortography image shows collateral arteries to the uterus. Prophylactic balloon occlusion of the internal iliac artery was performed during cesarean section. However, the estimated blood loss was 4753 ml. To control massive bleeding, hysterectomy and 8 units of packed red blood cell transfusion were needed. Arrowheads: bilateral round ligament artery, arrow: left iliolumbar artery, circle: right ovarian artery
Figure 2. .
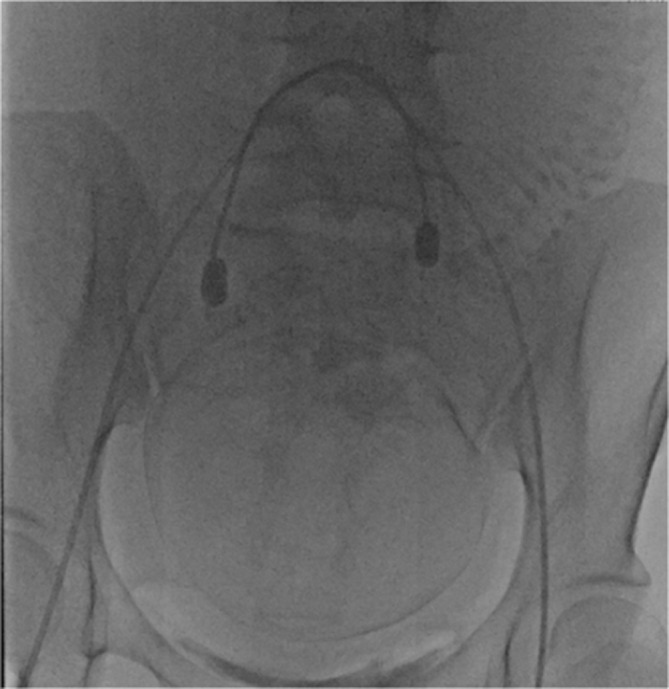
Angiographic image showing occlusion balloons placed within both internal iliac arteries.
The patients underwent a cesarean section under spinal anesthesia in the operating room immediately after balloon placement. When the baby was delivered, and the umbilical cord was clamped, the occlusion balloons were inflated immediately. The obstetrician then surgically excised as much of the placenta as possible along with any myometrium and reconstructed the uterus under general anesthesia associated with tracheal intubation. After the patient underwent hysterectomy or delivered the placentas, the balloons were deflated just before skin closure when hemostasis was secured in the pelvic cavity. We reviewed incidence of collateral arteries to the uterus on the initial aortography. We especially evaluated the incidence of angiographic visualization of the ovarian artery arising directly from the abdominal aorta, round ligament artery arising from the external iliac artery/inferior epigastric artery, and the iliolumbar artery. Blinded image interpretations were performed in random order by two investigators. The investigators evaluated for the presence of collateral arteries. The clinical characteristics from the patients’ medical records, including obstetric history, maternal background, the course of delivery, management, and pathological findings regarding the degree of placental adhesion, were reviewed. These characteristics included patient age, body mass index, parity, number of previous cesarean sections, gestational days at cesarean section, Apgar score (<1 min after delivery), estimated blood loss, blood transfusion, postoperative hospital stay, and clinical findings. The estimated blood loss was quantified using the volume of suction containers, weight of swabs, and visual estimation of vaginal blood loss.
Cases with estimated blood loss during delivery (>2500 ml), packed red blood cell (RBC) transfusion (>4 units), transcatheter arterial embolization after delivery, or hysterectomy for uncontrolled hemorrhage were defined as the massive bleeding group. We compared between the massive and non-massive bleeding groups. Fluoroscopy time and radiation dose (in mGy) were obtained from the fluoroscopy performed at the end of the procedure. The entrance skin radiation dose in the area of the irradiated field was considered an approximation for the fetal radiation dose.5 Descriptive data are presented as mean ± standard deviation. The variables were compared between the two groups using the Student t test and Mann–Whitney U test, or the Fisher exact test was used to compare potential risk factors for massive bleeding. We used the SPSS v. 22 software (IBM Corp., Tokyo, Japan), and p values of <0.05 were defined as statistically significant.
Results
All 42 patients with placenta previa and accreta underwent BOIA during cesarean section. Table 1 shows all 42 patients’ characteristics. In 27 patients (64%), the uterus was preserved, and the patients resumed normal menstruation within a year. No severe complications related to the balloon occlusion procedures were observed. No maternal or neonatal death occurred. In one patient, transcatheter arterial embolization was performed 2 days after hysterectomy because of continuous bleeding. 20 patients (48%) had a massive bleeding. Table 2 shows the clinical characteristics of the massive and non-massive bleeding groups. The groups differed in operation time (p < 0.001), hysterectomy (p < 0.001), post-operative hospital stay (p < 0.05), and visualization of round ligament arteries to the uterus [15/20 (75%) patients, p < 0.01]. Visualization of ovarian arteries [9/20 (45%)] and iliolumber arteries [4/20 (20%)] to the uterus were not a risk factor of massive bleeding. Table 3 shows the clinical characteristics of the visualization and non-visualization of round ligament artery to the uterus groups. The groups differed in estimated blood loss (p < 0.01), packed RBC transfusion (p < 0.05) and postoperative hospital stay (p < 0.05). No early or delayed maternal complications of endovascular balloon placement occurred and no damage to the adjacent pelvic organs during the operation was observed. All the mothers and babies were healthy at the time of discharge. The fluoroscopy time and fetal radiation dose were 6.1 ± 2.5 min and 25.5 ± 8.2 mGys, respectively.
Table 1. .
All 42 patients’ characteristics
| Age (y) | 33.6 ± 4.9 |
| BMI | 25.3 ± 2.8 |
| Parity (n) | 1.1 ± 0.9 |
| Mean number of previous cesarean sections | 1.1 ± 0.7 |
| Number of previous cesarean sections | (n) |
| 0 | 7 |
| 1 | 25 |
| 2 | 9 |
| 3 | 1 |
| Degree of placental adhesion | (n) |
| Accreta | 33 |
| Increta | 6 |
| Percreta | 3 |
| Mean gestational age (d) | 253.3 ± 13.4 |
| Mean birth weight (g) | 2619 ± 511 |
| Mean operation time (min) | 151 ± 85 |
| Estimated blood loss (mL) | 3706 ± 3852 |
| Hysterectomy (n) | 15 |
| Number of pRBC transfusions (units) | 5.7 ± 8.0 |
| Post-operative hospital stay (d) | 9.3 ± 3.2 |
BMI, body mass index; RBC, red blood cell.
Table 2. .
Clinical characteristics of the massive and non-massive bleeding groups
| Non-massive bleeding group (n = 22) |
Massive bleeding group (n = 20) |
p-value | |
| Age (y) | 34.0 ± 5 | 33.2 ± 4.7 | 0.61 |
| BMI | 25.2 ± 3.1 | 25.3 ± 2.4 | 0.95 |
| Parity | 1.3 ± 0.5 | 1.6 ± 0.8 | 0.18 |
| Number of previous cesarean sections | 0.9 ± 0.6 | 1.3 ± 0.7 | 0.079 |
| Degree of placental adhesion | |||
| Accreta | 18 | 15 | 0.71 |
| Increta | 2 | 4 | 0.40 |
| Percreta | 2 | 1 | 1.0 |
| Gestational age (d) | 254.0 ± 12 | 252.6 ± 15 | 0.73 |
| Apgar score (1 min after delivery) | 5.7 ± 2.4 | 6.4 ± 1.85 | 0.57 |
| Birth weight of infant (g) | 2519.5 ± 484.9 | 2728.7 ± 517.1 | 0.14 |
| Operation time (min) | 94.0 ± 34.2 | 214.1 ± 80.6 | <0.001*** |
| Estimated blood loss (mL) | 1475.5 ± 520.2 | 6161.3 ± 4400.2 | <0.001*** |
| Hysterectomy | 0 | 15 | <0.001*** |
| Number of packed RBC transfusions (units) | 10.8 ± 9.3 | 1.1 ± 0.7 | <0.001*** |
| Post-operative hospital stay (d) | 8.5 ± 2.7 | 10.1 ± 3.5 | 0.039a |
| Visualization of collateral artery to the uterus | |||
| Ovarian artery | 7 (32%) | 9 (45%) | 0.53 |
| Round ligament artery | 7 (32%) | 15 (75%) | 0.0068** |
| Iliolumber artery | 2 (9%) | 4 (20%) | 0.40 |
BMI, body mass index; RBC, red blood cell.
p < 0.05, **p < 0.01, ***p < 0.001
Table 3. .
Clinical characteristics of the visualization and non-visualization of round ligament artery to the uterus groups
| Visualization of round ligament artery to the uterus group (n = 22) | Non-visualization of round ligament artery to the uterus group (n = 20) | p-value | |
| Age (y) | 34 ± 5.5 | 33.1 ± 4.1 | 0.43 |
| BMI | 25.4 ± 2.7 | 25.2 ± 2.9 | 0.83 |
| Parity | 1.4 ± 0.7 | 1.2 ± 0.7 | 0.54 |
| Number of previous cesarean sections | 1.2 ± 0.7 | 1.0 ± 0.7 | 0.48 |
| Degree of placental adhesion | |||
| Accreta | 16 | 17 | |
| Increta | 5 | 1 | |
| Percreta | 1 | 2 | |
| Gestational age (d) | 147 ± 5.0 | 144.7 ± 10 | 0.95 |
| Apgar score (1 min after delivery) | 6.2 ± 2.1 | 5.8 ± 2.3 | 0.44 |
| Birth weight of infant (g) | 2693.2 ± 367 | 2537.6 ± 623 | 0.72 |
| Operation time (min) | 169.9 ± 82.3 | 130.6 ± 84.0 | 0.11 |
| Estimated blood loss (mL) | 4818.8 ± 4542.2 | 2483.7 ± 2368.4 | 0.0025** |
| Hysterectomy | 11 | 4 | 0.058 |
| Number of packed RBC transfusions (units) | 6.7 ± 8.2 | 3.0 ± 6.5 | 0.043a |
| Post-operative hospital stay (d) | 10.0 ± 3.4 | 8.5 ± 2.8 | 0.025a |
BMI, body mass index; RBC, red blood cell.
p < 0.05, **p < 0.01
Discussion
The results of our study indicated that prophylactic placement of an internal iliac arterial balloon in patients with placenta previa and accreta during cesarean section was safe. However, in cases of visualized collateral blood supply from the round ligament artery to the uterus, prophylactic placement of an internal iliac arterial balloon did not significantly contribute to hemostasis during the cesarean section.
Morbidly adherent placenta is a major cause of massive postpartum hemorrhage and is associated with significant maternal morbidity, mortality, and even perinatal death. Early diagnosis and intervention in these conditions can more readily enable physicians to minimize the risks to the mother and fetus. Unfortunately, the incidence of morbidly adherent placenta is increasing worldwide and believed to be associated with the increasing prevalence of cesarean section.2 Uterine contraction and hemostatic surgical methods were used to reduce the risk of intraoperative hemorrhage and preserve the uterus. When all other methods failed in controlling hemorrhage, hysterectomy was sometimes performed. However, most pregnant females do not easily accept hysterectomy because it deprives them of the chance of pregnancy. The use of balloon occlusion for bleeding control was first reported in surgical procedures performed during the Korean War.11 Prophylactic BOIAs as an adjunct was first described by Dubois and has resulted in mixed outcomes since then.12 Prophylactic arterial balloon occlusion is a contentious problem, and various treatment efficacies have been reported in past studies. A retrospective study of intra aortic balloon occlusion reported post-operative complications were about 4.4%, including arterial thrombosis and femoral nerve ischemic injury.13 A Japanese study supported that prophylactic balloon occlusion was more effective for the common iliac artery than for the internal iliac artery owing to the rich collateral uterine blood supply.14 Some studies15 have reported that prophylactic BOIA could reduce intraoperative blood loss and improve the operative field owing to the decreased pulse pressure distal to the occlusion site. However, no randomized controlled clinical trials have been performed and no reports have been accumulated regarding which arteries are more effective for prophylactic balloon occlusion (the internal iliac artery, common iliac artery, or aorta).7 Even if BOIAs is used, whole blood supply to the pelvic organs is unavoidable because of the presence of several collateral arteries in the pelvis. While internal iliac artery branch vessels (uterine, internal pudendal, and vesical) are often the dominant supply vessels, the uterus and placenta obtain extensive collateral supply from the aortic (ovarian and iliolumbar) and external iliac (lateral circumflex iliac, inferior epigastric, iliolumbar, obturator, and external pudendal) vascular territories.16 The uterine arteries account for most of the blood supply to the uterus, but the extensive collateral supply from the ovarian arteries is not affected.16 Our study shows that the round ligament artery arising from the external iliac artery/inferior epigastric artery may play an important role in the blood supply to the uterus when the internal iliac arteries are occluded by balloon catheters. Only a few studies have described the clinical significance of the round ligament arteries. Some studies have reported that the round ligament artery is an anastomotic supplier to the uterine artery in patients who have undergone uterine artery embolization.16–19 If angiographic visualization of collateral blood supply from the round ligament arteries to the uterus is observed in patients with placenta previa and accreta, management of massive bleeding with prophylactic BOIAs may not be appropriate. Temporary occlusion of the round ligament artery with bioabsorbable embolization materials may be effective before cesarean section.
Previous papers have documented a significant maternal complication rate of up to 15% due to balloon placement that include the puncture site, hematoma dissection of the femoral arteries, and air in pressurized lines.20,21 However, in our study, no complications were found. We believe this is attributable to the participation of an experienced interventional radiology team from the beginning to the end of the procedure. If balloons are managed by clinicians who are not familiar with interventional radiology, complications such as balloon over- or underinflation and bursting/insufficient balloon bulging could easily occur and radiation exposure during balloon placement might not be minimized.
Adverse pregnancy outcomes can be caused by radiation exposure, including intrauterine growth restriction, prenatal death, small head size, mental retardation, organ malformation, and childhood cancer.22 Fetal radiation exposure is of great concern in all the patients, and limiting the fluoroscopy time is the most important step to reduce the radiation dose. If the radiation dose is <100 mGy, exposure to radiation is not an indication of gestational termination. Neither fetal anomalies nor pregnancy loss is associated with radiation doses of <50 mGy.22 Malformation risks are significant when the radiation dose is >150 mGy.23 The overall radiation dose in our patients was approximately 20 mGy, which is sufficiently less than 100 mGy. We observed no anomalies in the neonates during the follow-up. However, the long-term effect is unknown, so further follow-up is needed.
Limitations
The potential limitations of this study should be acknowledged. First, this was a small retrospective cohort study with a small sample size. A prospective study with a large number of patients should be performed in the near future. Second, on the initial abdominal aortography, we did not adjust the volume and flow rate of the contrast agent injection in accordance with the physical constitution of the patients. Arterial blood flow measurements indicated that vasoconstriction and vasospasm could affect the arterial blood flow to the uterus. Clinical decision-making was based on input from both obstetricians and patients. Many collateral arteries to the uterus were found, but we did not evaluate all collateral arteries. Because the initial abdominal aortography alone was not enough to evaluate all collateral arteries. Selective angiography of the anastomotic branches (ovarian, round ligament, and iliolumbar arteries) was not performed to confirm perfusion to the uterus. We did not evaluate the hemodynamics to the uterus under the prophylactic placement of an internal iliac arterial balloon during cesarean section.
Conclusion
Although numbers of patient were limited, prophylactic placement of an internal iliac arterial balloon in patients with placenta previa and accreta during cesarean section was safe. Angiographic visualization of collateral circulation from the round ligament artery to the uterus may be a risk factor for massive bleeding in patients with placenta previa and accreta who have undergone BOIA during cesarean section.
Footnotes
Consent for publication: Written informed consent for publication was obtained from all patients and residents participating in the study.
Ethics approval and consent to participate: Written informed consent was obtained from all patients and residents participating in the study, as approved by the ethics committee of Gunma university hospital.
Contributor Information
Hiroyuki Tokue, Email: tokue@s2.dion.ne.jp.
Azusa Tokue, Email: azusa45@yahoo.co.jp.
Yoshito Tsushima, Email: yyoshitottsushima@yahoo.co.jp.
Takeshi Kameda, Email: patho9719537@yahoo.co.jp.
REFERENCES
- 1.Bowman ZS, Eller AG, Bardsley TR, Greene T, Varner MW, Silver RM. Risk factors for placenta accreta: a large prospective cohort. Am J Perinatol 2014; 31: 799–804. [DOI] [PubMed] [Google Scholar]
- 2.Picel AC, Wolford B, Cochran RL, Ramos GA, Roberts AC. Prophylactic internal iliac artery occlusion balloon placement to reduce operative blood loss in patients with invasive placenta. Journal of Vascular and Interventional Radiology 2018; 29: 219–24. doi: 10.1016/j.jvir.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 3.Fan Y, Gong X, Wang N, Mu K, Feng L, Qiao F, et al. A prospective observational study evaluating the efficacy of prophylactic internal iliac artery balloon catheterization in the management of placenta previa–accreta. Medicine 2017; 96: e8276. doi: 10.1097/MD.0000000000008276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ono Y, Murayama Y, Era S, Matsunaga S, Nagai T, Osada H, et al. Study of the utility and problems of common iliac artery balloon occlusion for placenta previa with accreta. J. Obstet. Gynaecol. Res. 2018; 44: 456–62. doi: 10.1111/jog.13550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y-L, Duan X-H, Han X-W, Wang L, Zhao X-L, Chen Z-M, et al. Comparison of temporary abdominal aortic occlusion with internal iliac artery occlusion for patients with placenta accreta – a non-randomised prospective study. Vasa 2017; 46: 53–7. doi: 10.1024/0301-1526/a000577 [DOI] [PubMed] [Google Scholar]
- 6.Tan YL, Suharjono H, Lau NL, Voon HY. Prophylactic bilateral internal iliac artery balloon occlusion in the management of placenta accreta: a 36-month review. Med J Malaysia 2016; 71: 111–6. [PubMed] [Google Scholar]
- 7.Dilauro MD, Dason S, Athreya S. Prophylactic balloon occlusion of internal iliac arteries in women with placenta accreta: literature review and analysis. Clin Radiol 2012; 67: 515–20. doi: 10.1016/j.crad.2011.10.031 [DOI] [PubMed] [Google Scholar]
- 8.Chattopadhyay SK, Kharif H, Sherbeeni MM. Placenta praevia and accreta after previous caesarean section. Eur J Obstet Gynecol Reprod Biol 1993; 52;):: 151–652. doi: 10.1016/0028-2243(93)90064-J [DOI] [PubMed] [Google Scholar]
- 9.Clark SL, Koonings PP, Phelan JP. Placenta previa/accreta and prior cesarean section. Obstet Gynecol 1985; 66: 89–92. [PubMed] [Google Scholar]
- 10.Levine AB, Kuhlman K, Bonn J. Placenta accreta: comparison of cases managed with and without pelvic artery balloon catheters. J Matern Fetal Med 1999; 8: 173–6. [DOI] [PubMed] [Google Scholar]
- 11.Edwards WS, Salter PP, Carnaggio VA. Intraluminal aortic occlusion as a possible mechanism for controlling massive intra-abdominal hemorrhage. Surg Forum 1953; 4: 496–9. [PubMed] [Google Scholar]
- 12.Dubois J, Garel L, Grignon A, Lemay M, Leduc L. Placenta percreta: balloon occlusion and embolization of the internal iliac arteries to reduce intraoperative blood losses. Am J Obstet Gynecol 1997; 176: 723–6. doi: 10.1016/S0002-9378(97)70582-9 [DOI] [PubMed] [Google Scholar]
- 13.Wei X, Zhang J, Chu Q, Du Y, Xing N, Xu X, et al. Prophylactic abdominal aorta balloon occlusion during caesarean section: a retrospective case series. Int J Obstet Anesth 2016; 27: 3–8. doi: 10.1016/j.ijoa.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 14.Minas V, Gul N, Shaw E, Mwenenchanya S, et al. Prophylactic balloon occlusion of the common iliac arteries for the management of suspected placenta accreta/percreta: conclusions from a short case series. Arch Gynecol Obstet 2015; 291: 461–5. doi: 10.1007/s00404-014-3436-9 [DOI] [PubMed] [Google Scholar]
- 15.Mok M, Heidemann B, Dundas K, Gillespie I, Clark V, et al. Interventional radiology in women with suspected placenta accreta undergoing caesarean section. Int J Obstet Anesth 2008; 17: 255–61. doi: 10.1016/j.ijoa.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 16.Pelage JP, Le Dref O, Soyer P, Jacob D, Kardache M, Dahan H, et al. Arterial anatomy of the female genital tract: variations and relevance to transcatheter embolization of the uterus. American Journal of Roentgenology 1999; 172: 989–94. doi: 10.2214/ajr.172.4.10587133 [DOI] [PubMed] [Google Scholar]
- 17.Saraiya PV, Chang TC, Pelage J-P, Spies JB. Uterine artery replacement by the round ligament artery: an anatomic variant discovered during uterine artery embolization for leiomyomata. Journal of Vascular and Interventional Radiology 2002; 13: 939–41. doi: 10.1016/S1051-0443(07)61779-5 [DOI] [PubMed] [Google Scholar]
- 18.JY W, Kim HC, Chung JW, Jun JK, Jae HJ, Park JH. Importance of angiographic visualization of round ligament arteries in women evaluated for intractable vaginal bleeding after uterine artery embolization. J Vasc Interv Radiol 2009; 20: 1031–5. [DOI] [PubMed] [Google Scholar]
- 19.Leleup G, Fohlen A, Dohan A, Bryan-Rest L, Le Pennec V, Limot O, et al. Value of round ligament artery embolization in the management of postpartum hemorrhage. Journal of Vascular and Interventional Radiology 2017; 28: 696–701. doi: 10.1016/j.jvir.2017.01.016 [DOI] [PubMed] [Google Scholar]
- 20.Nicholson PJ, O’Connor O, Buckley J, Spence LD, Greene RA, Tuite DJ. Prophylactic placement of internal iliac balloons in patients with abnormal placental implantation: maternal and foetal outcomes. Cardiovasc Intervent Radiol 2018; 41: 1488–93. doi: 10.1007/s00270-018-1983-3 [DOI] [PubMed] [Google Scholar]
- 21.Shrivastava V, Nageotte M, Major C, Haydon M, Wing D. Case-Control comparison of cesarean hysterectomy with and without prophylactic placement of intravascular balloon catheters for placenta accreta. Am J Obstet Gynecol 2007; 197: 402.e1-–402.e5e1. doi: 10.1016/j.ajog.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 22.McCollough CH, Schueler BA, Atwell TD, Braun NN, Regner DM, Brown DL, et al. Radiation exposure and pregnancy: when should we be concerned? RadioGraphics 2007; 27: 909–17. doi: 10.1148/rg.274065149 [DOI] [PubMed] [Google Scholar]
- 23. American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice. Committee Opinion No. 656: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol 2016; 127: e75–80. [DOI] [PubMed] [Google Scholar]


