Abstract
The prevalence of nasopharyngeal carcinoma is characterized by an unbalanced distribution: the disease is particularly prevalent in East and Southeast Asia. In this article, we review the evolution of the International Union Against Cancer/American Joint Committee on Cancer staging system for nasopharyngeal carcinoma. With the increasing using of newer imaging methods, more advanced radiotherapy techniques and systemic chemotherapy, we also discuss newer clinical features that might affect staging. Finally, we propose the future direction of staging and potential prognostic factors that have a major influence on the treatment outcomes of this disease.
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is a squamous cell carcinoma arising from the epithelial lining of the nasopharynx. According to the International Agency for Research on Cancer, 129,079 new cases of NPC were reported in 2018.1 Compared to other cancer types, NPC is uncommon, with a very unique geographical distribution, whereby more than 70% of new cases are reported in East and Southeast Asia.1
The current International Union Against Cancer (UICC) and the American Joint Committee on Cancer (AJCC) TNM Classification for NPC remains the most accurate prognostic tool to stratify patients for treatment and evaluation of treatment outcomes.2–4 The system is based purely on the following three anatomical criteria: the local extent of the cancer within the site of origin (T), the degree of metastatic involvement of the regional lymph nodes (N), and the presence or absence of distant metastatic disease (M). The introduction of the TNM Staging System fifth edition jointly adopted by AJCC and the UICC in 1997 was a milestone.5 This consensus on a unified NPC staging and classification system was reached based on the results of retrospective studies worldwide.6–9 The seventh edition of this staging system was released in 2009, and it was recently updated (eighth edition) in 2016,10 further refining the staging of primary and nodal disease and the stage groups.11,12
In the past two decades, there have been significant advancements in imaging technology, radiotherapy (RT) delivery, and systemic chemotherapy for NPC patients.13–17 With these changes in diagnosis and treatment, the TNM classification systems have also been significantly refined. This article presents in detail the evolution of the TNM classification from the fifth to the eighth edition.5,10,18,19
THE EIGHth EDITION OF THE TNM CLASSIFICATION: – A SUMMARY OF THE CHANGES (Table 1)
Table 1. .
Comparison between the seventh and eighth editions of the UICC/AJCC staging system for nasopharyngeal carcinoma
| Seventh edition | Eighth edition | ||
| Primary tumor (T) | |||
| TX | Primary tumor cannot be assessed | TX | Primary tumor cannot be assessed |
| T0 | No tumor identified, but EBV-positive cervical node(s) involvement | ||
| T1 | Nasopharynx, oropharynx or nasal cavity without parapharyngeal extension | T1 | Nasopharynx, oropharynx or nasal cavity without parapharyngeal extension |
| T2 | Parapharyngeal extension | T2 | Parapharyngeal extension, adjacent soft tissue involvement (medial pterygoid, lateral pterygoid, prevertebral muscles) |
| T3 | Bony structures of skull base and/or paranasal sinuses | T3 | Bony structures (skull base, cervical vertebra) and/or paranasal sinuses |
| T4 | Intracranial, cranial nerves, hypopharynx, orbit, infratemporal fossa/masticator space | T4 | Intracranial extension, cranial nerve, hypopharynx, orbit, extensive soft tissue involvement (beyond the lateral surface of the lateral pterygoid muscle), parotid gland |
| Regional lymph nodes (N) | |||
| NX | Regional lymph nodes cannot be assessed | NX | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis | N0 | No regional lymph node metastasis |
| N1 | Unilateral cervical, unilateral or bilateral retropharyngeal lymph nodes, above the supraclavicular fossa;≤6 cm | N1 | Unilateral cervical, unilateral or bilateral retropharyngeal lymph nodes, above the caudal border of cricoid cartilage;≤6 cm |
| N2 | Bilateral metastasis in lymph node(s), 6 cm or less in greatest dimension, above the supraclavicular fossa | N2 | Bilateral metastasis in lymph node(s), 6 cm or less in greatest dimension, above the caudal border of cricoid cartilage |
| N3a | Greater than 6 cm in dimension | N3 | >6 cm and/or below caudal border of cricoid cartilage (regardless of laterality) |
| N3b | Supraclavicular fossa | ||
| Distant metastasis (M) | |||
| M0 | No distant metastasis | M0 | No distant metastasis |
| M1 | Distant metastasis | M1 | Distant metastasis |
| Stage group | |||
| I | T1 N0 M0 | I | T1 N0 M0 |
| II | T2 N0-1 M0, T1 N1 M0, | II | T2 N0-1 M0, T0-1 N1 M0 |
| III | T1-3 N2 M0, T3 N0–1 M0 | III | T3N0-2 M0, T0-2 N2 M0 |
| IVA | T4 N0–2 M0 | IVA | T4 or N3 M0 |
| IVB | Any T N3 M0 | IVB | Any T, any N M1 |
| IVC | Any T, any N M1 | ||
| Summary of changes | |||
| T category | |||
| T0 is added for EBV-positive cervical node(s) involvement despite unidentified primary tumor | |||
| |||
| |||
| N category | |||
| |||
| |||
| |||
EBV, Epstein-Barr virus; UICC/AJCC, International Union Against Cancer/American Joint Committee on Cancer.
One of the revisions in the current edition is the adjustment in the T0 stages defined in the previous edition.10 The T0 stage was added for Epstein-Barr virus (EBV)-positive unknown primary tumors with cervical lymph node (CLN) involvement. The second revision is the specific description of T2, from parapharyngeal extension to tumor involvement of the medial pterygoid, lateral pterygoid, and prevertebral muscles.20 The third revision is that the previous T4 criteria “masticator space” and “infratemporal fossa” are now replaced by a specific description of soft-tissue involvement to avoid ambiguity. The fourth revision is that the previous N3b criterion for the supraclavicular fossa (SCF) has been changed to the lower neck (as defined by nodal extension below the caudal border of the cricoid cartilage)21,22 : N3a and N3b are merged into a single N3 category, which is now defined as unilateral or bilateral metastasis in any direction, with 6 cm being the largest dimension in CLN(s) in the cricoid cartilage.
IMAGING STUDIES
Significant stage migration has been observed with the increasing use of advanced imaging technology. With the advantages of excellent tissue contrast, multiplanar capacity, and a lack of radiation and bone beam-hardening artefacts, MRI allows for a more accurate evaluation of local disease extension, especially parapharyngeal tumor extension, oropharyngeal extension, and ethmoid sinus.23–25 These resulted in changes in 49.8% of T stage cases, 10.7% of N stage cases, and 38.6% of clinical stage cases according to the sixth AJCC/UICC staging system,26 and enabled a more accurate delineation of tumor margins in the nasopharynx.26–29 On the other hand, 18F-FDG-positron emission tomography (PET)/CT affords more sensitivity than does the conventional work-up (i.e. chest radiography, abdominal ultrasound, and skeletal scintigraphy) in detecting distant metastasis,30 small CLN metastases, and local residual/recurrent disease.27,31–33 Peng et al showed PET/CT examination resulted in modification of N categories and overall stage for 135 (28.7%) and 46 (9.8%) patients, respectively.32 In a study by Law et al, PET-CT was valuable in terms of impacting management in 33% of patients (major impact in detecting M1 disease in 8% and medium impact by upstaging the N category or showing the exact lymph node in 25%).34
UNSETTLED ANATOMIC ISSUES
Classification of T0
In the eighth edition of the AJCC staging manual for NPC, the definition of T0 is added for EBV-positive unknown primary tumors with CLN involvement.10 This assignment is based on evidence level III, which means the supporting evidence is somewhat problematic. In fact, EBV is not only related to NPC but also lymphoepithelioma-like carcinoma, which arises from mucosal sites outside the nasopharynx, including the salivary gland, oropharynx, sinonasal tract, and non-head and neck regions.35–38 Lymphoepithelioma-like carcinoma is morphologically similar to undifferentiated NPC with a high frequency of concurrent CLN metastases but could occur in multiple sites. Therefore, it is an arbitrary assumption that all occult primary tumors with EBV-related CLN metastases originate from the nasopharynx. In patients with EBV-positive CLNs, Mao et al showed the most common primary sites after the nasopharynx (52.3%) were the salivary gland (24.8%), lung (7.9%), oropharynx (3.4%), nasal cavity/maxillary (3.4%), oral cavity (1.1%), orbit (1.1%), and liver (0.4%). The origins of EBV-positive CLNs may not be restricted to the nasopharynx alone, and are likely to involve the head and neck or non-head and neck regions. The NPC T0 classification should be cautiously assigned to such tumors.39
PROGNOSTIC SIGNIFICANCE OF PREVERTEBRAL SPACE INVOLVEMENT
In the current eighth edition of the staging system, prevertebral muscle involvement was added as a T2 criterion. Several retrospective studies have shown that prevertebral space involvement (PSI) is an independent prognostic factor.40–42 For patients with treatment using two-dimensional RT, PSI was associated with a poor prognosis on local and distant control.41 In Lee”s study, distant metastasis-free survival (DMFS) increased from 72 to 100% after adjuvant chemotherapy in the patients with PSI. However, this effect was not observed in the group without PSI.42 Zhou et al retrospectively reviewed 506 patients in the intensity modulated radiotherapy (IMRT) era and found that PSI was an independent prognostic factor for both overall survival (OS) and DMFS, and suggested a classification of T4.40 In a study by Pan et al, in which prevertebral muscle involvement was added as a T2 criterion, the OS of patients with PSI was similar to that of those with parapharyngealspace involvement.20
CERVICAL VERTEBRAE AND PAROTID GLAND INVOLVEMENT
The eighth TNM staging system added the cervical vertebrae and parotid gland, which were not classified in the previous versions of the staging system. The incidence rate of concurrent tumor invasion into the cervical vertebrae and parotid gland observed by MRI was <5%.43 In the study by Pan et al, the subgroup of patients with parotid gland infiltration showed poor OS, similar to that of the subgroup with intracranial extension and/or cranial nerve palsy. Moreover, there was no statistically significant difference in OS between those showing involvement of pterygoid structures alone and those presenting with erosion of the skull base and/or cervical vertebra (86% vs 79%).20 However, there is no separate list covering the prognosis in cases with involvement of the cervical vertebra and parotid gland. At present, there is no research on the prognosis of cervical vertebra and parotid gland invasion, and further investigation is needed on the staging of cervical vertebra and parotid gland invasion.
STAGING: NODAL METASTASIS
Supraclavicular fossa
The SCF, originally described by Ho,44,45 is the triangular region defined by the superior margin of the sternal end of the clavicle, the superior margin of the lateral end of the clavicle, and the point where the neck meets the shoulder. This definition of SCF involvement is based primarily on clinical examination, and there was no reliable method to define the SCF radiologically by using the above clinical landmarks before CT cross-sectional imaging was widely employed for N classification in NPC.46,47 Ng et al suggested replacing the SCF with the radiological nodal levels IV and Vb, and found that this substitution was predictive for both distant control and overall survival.48 The study by Mao et al on 924 patients staged by MRI also supported the use of levels as defining criteria.49 For NPCs treated with IMRT, Li et al21 also reported that the involvement of levels IV and Vb and the SCF as defined by the International Consensus Guidelines47,50 was an independent prognostic factor for distant failure and disease failure. Compared to the seventh UICC/AJCC, Yue et al showed that N-categories based on the lower cervical levels (extension below the caudal edge of the cricoid cartilage) provided more satisfactory distinction between hazard ratios for distant and disease failure for each N category. N3a and N3b as defined by the seventh UICC/AJCC showed similar DMFS (p = 0.31) and Disease-free survival (DFS) (p = 0.21) rates.22 Hence, one modification brought along with the eighth edition will be changing the definition of lower neck extension to lymphatic spread below the caudal edge of the cricoid cartilage as N3, and merging N3a and N3b into a single N3 category.
MEASUREMENTS OF THE MAXIMAL NODAL DIAMETER ON MRI SLICES
A study from Hong Kong that included almost 5000 NPC cases without distant metastasis found that, when adjusted for other meaningful parameters, palpation-determined LN size (greatest diameter ≤6 cm vs. >6 cm) was a significant prognostic factor.51 On the basis of that study, cervical LN size (greatest diameter >6 cm) has been included in the UICC/AJCC NPC staging system N3 subset since 1992 (i.e. the fourth edition). Subsequent staging systems have also adopted this criterion, including the eighth edition of the UICC/AJCC staging system. However, the criterion is based on the evaluation of palpable LNs, which would differ between clinicians. Furthermore, the widespread use of CT, MRI, PET-CT, and other advanced techniques in NPC can allow detection of LN metastases at a higher rate of 77–88.1%,52,53 compared to only about 60% by palpation.54 Both the LN levels and laterality are assessed based on MRI, but in the eighth staging system, the nodal size is defined according to the largest dimension without specifying the plane to be used. Mao et al found that the MRI-determined longest CLN diameter is a significant prognostic factor for both disease failure and distant failure in NPC in univariate analyses.55 However, only CLN level and laterality and extranodal neoplastic spread (ENS) had such significant prognostic value for distant failure and disease failure in multivariate analyses. Li et al showed that maximal axial diameter (MAD) values of >4 cm were associated with a higher risk of nodal failure (p = 0.05). Multivariate analysis showed level was the only independent prognostic factor for distant failure (p < 0.01) and disease failure (p < 0.01).21 There is a need to investigate the significant cut-off values of MRI-determined LN size, the measurement (axial plane MAD or the longest diameter of all planes) for the N classification in the future.
Parotid area lymph node metastases
The pattern of nodal metastasis in NPC follows an orderly spread down the neck with infrequent node skipping; the most commonly involved regions include the retropharyngeal space, levels II, III, IV, and V, followed by level Ib and the supraclavicular nodes.52,56–60 The parotid lymph nodes (PLNs), classified as level VIII in the latest International Consensus Guidelines for nodal levels,61 are also at risk of harboring metastasis from the nasopharynx. However, the classification of PLN metastasis was not identified until the eighth edition of the AJCC/UICC staging system. Zhang et al reported that the incidence of PLN metastasis was 1.2% (21/1811), and PLN involvement was associated with significantly poorer progression-free survival (PFS), OS, and DMFS, with a significantly higher hazard ratio for distant failure than N2 disease and similar to N3 disease.62 In the study by Xu et al, the results also showed that the 5 year DMFS and regional recurrence-free survival (RRFS) curves for PLN metastasis were significantly separated from that for N2 disease but crossed that for N3 disease.63 Although the rate of parotid lymph node metastasis is actually low, the inconsistent classification may affect treatment strategies for patients with PLN metastasis. Therefore, the staging of parotid lymph node metastasis needs to be further clarified.
Future direction
The following improvements should be considered: regrouping of T and N categories for better distinction of hazard between stages and incorporation of other prognostic factors beyond anatomic staging.
Simplification of T staging
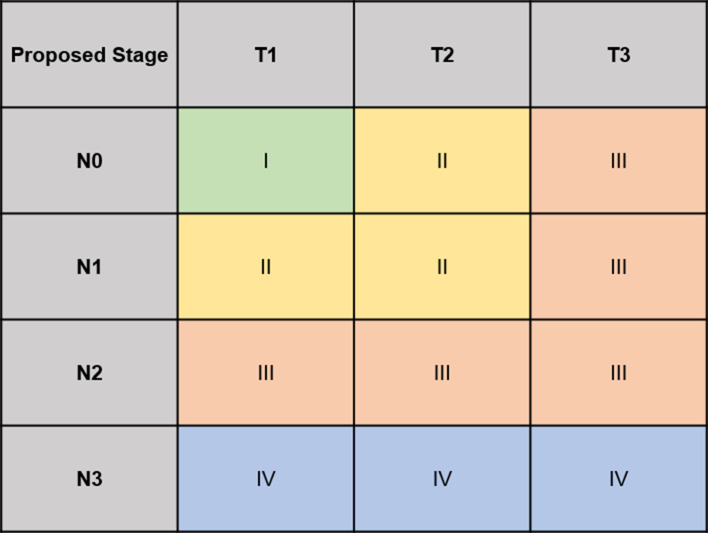
The eighth edition of the AJCC NPC staging system has been proven to provide more accurate prediction of treatment outcomes than the seventh edition.64 The local control rate for NPC is currently 90–95% for patients treated using modern techniques.65–67 However, these advances have altered the prognostic value of staging parameters for local failure,68 and the prognostic value of the T category may have become weaker. The survival curves for T2 and T3 almost overlap, with no significant differences in locoregional relapse-free survival and DFS.64 Considering the improved local control provided by modern techniques, Tang et al re-evaluated the prognostic value of the eighth edition T categories with an independent, external validation cohort. The results showed that in the IMRT era, merging T2 and T3 to a proposed T2 provided significant differences in local recurrence-free survival, DFS, and OS between the proposed T categories.This article also suggested a new regrouping of T and N classification, and defining proT1N0 as Stage I; proT1N1/proT2N0-1 as Stage II; proT3N0-2/proT1-2N2 as Stage III; and proT1-3N3 as stage IVa generated orderly, significant differences in DFS and OS between stages in the training set and external validation cohort69 (Figure 1).
Figure 1.
The proposed T classification for the study of the tang et al. The proposed T2 was combination of T2 and T3 for the 7th AJCC/UICC staging system
The role of other prognostic factors beyond anatomic staging
Many studies have been conducted to evaluate whether the incorporation of other clinical factors and molecular biomarkers into the staging system would better predict survival. Larger tumors are related to an increased number of clonogenic tumor cells, as well as tumor hypoxia, which is associated with increased radioresistance and possibly altered levels of intercellular communication factors.70,71 Several studies have shown a positive correlation between the primary tumor volume and various survival outcomes in NPC.72–82 However, NPC is a highly infiltrative tumor with a propensity to spread along the adjacent soft tissue as well as to the skull base and foramina. Therefore, the traditional measurement of tumor size in NPC is time-consuming, labor-intensive, or limited in availability. In recent years, IMRT has gradually replaced two-dimensional-CRT as the primary RT technique. With the increased use of computerized treatment planning systems for the delineation of three-dimensional tumor volume in radiation therapy, the calculation of NPC tumor volume has become easier. Among these studies, the series by Guo et al is the largest (n = 694), and their results suggest an improved predictive ability of T-classifications when the primary tumor volume is incorporated.76 Furthermore, cell-free EBV DNA was correlated with the tumor burden, TNM stage, and survival of NPC patients.83–87 Analysis of plasma/serum EBV DNA using real-time polymerase chain reaction is now widely used under clinical conditions for primary diagnosis, prognostication, and monitoring relapse and response to treatment, as well as population screening.88 The addition of pretreatment plasma EBV DNA into the eighth edition staging system has been shown to significantly improve its prognostic performance.89,90 Most importantly, the cutoff levels vary widely between different studies and international efforts are still ongoing to standardize the quantitative assay. Other biomarkers such as LDH, DNA methylation, miRNAs, and mRNAs also have demonstrated their prognostic value and potential clinical applications in nasopharyngeal carcinoma. A six-hypermethylated gene panel91 and a five-miRNA signature92 were found to be associated with survival in nasopharyngeal carcinoma patients, respectively.
Important changes have been introduced in the 8th AJCC/UICC staging system. However, new parameters which are prognostic and practical for future staging updates would be needed. More individualized treatment strategy would continue to be altered with the modified staging system.
Many studies have been conducted to evaluate whether the incorporation of other clinical factors and molecular biomarkers into the staging system would better predict survival. Larger tumors are related to an increased number of clonogenic tumor cells, as well as tumor hypoxia, which is associated with increased radioresistance and possibly altered levels of intercellular communication factors.70,71 Several studies have shown a positive correlation between the primary tumor volume and various survival outcomes in NPC.72–82 However, NPC is a highly infiltrative tumor with a propensity to spread along the adjacent soft tissue as well as to the skull base and foramina. Therefore, the traditional measurement of tumor size in NPC is time-consuming, labor-intensive, or limited in availability. In recent years, IMRT has gradually replaced two-dimensional-CRT as the primary RT technique. With the increased use of computerized treatment planning systems for the delineation of three-dimensional tumor volume in radiation therapy, the calculation of NPC tumor volume has become easier. Among these studies, the series by Guo et al is the largest (n = 694), and their results suggest an improved predictive ability of T-classifications when the primary tumor volume is incorporated.76 Furthermore, cell-free EBV DNA was correlated with the tumor burden, TNM stage, and survival of NPC patients.83–87 Analysis of plasma/serum EBV DNA using real-time polymerase chain reaction is now widely used under clinical conditions for primary diagnosis, prognostication, and monitoring relapse and response to treatment, as well as population screening.88 The addition of pretreatment plasma EBV DNA into the eighth edition staging system has been shown to significantly improve its prognostic performance.89,90 Most importantly, the cutoff levels vary widely between different studies and international efforts are still ongoing to standardize the quantitative assay. Other biomarkers such as LDH, DNA methylation, miRNAs, and mRNAs also have demonstrated their prognostic value and potential clinical applications in nasopharyngeal carcinoma. A six-hypermethylated gene panel91 and a five-miRNA signature92 were found to be associated with survival in nasopharyngeal carcinoma patients, respectively.
Contributor Information
Rui Guo, Email: guorui@sysucc.org.cn.
Yan-Ping Mao, Email: maoyp@sysucc.org.cn.
Ling-Long Tang, Email: tangll@sysucc.org.cn.
Lei Chen, Email: chenlei@sysucc.org.cn.
Ying Sun, Email: sunying@sysucc.org.cn.
Jun Ma, Email: majun2@mail.sysu.edu.cn.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Kattan MW, Hess KR, Amin MB, Lu Y, Moons KGM, Gershenwald JE, et al. American Joint Committee on Cancer acceptance criteria for inclusion of risk models for individualized prognosis in the practice of precision medicine. CA Cancer J Clin 2016; 66: 370–4. doi: 10.3322/caac.21339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee AWM, Ng WT, Chan LK, Chan OSH, Hung WM, Chan CC, et al. The strength/weakness of the AJCC/UICC staging system (7th edition) for nasopharyngeal cancer and suggestions for future improvement. Oral Oncol 2012; 48: 1007–13. doi: 10.1016/j.oraloncology.2012.03.022 [DOI] [PubMed] [Google Scholar]
- 4.WT N, Yuen KT, KH A, et al. Staging of nasopharyngeal carcinoma - The past, the present and the future. Oral Oncol 2013;. [DOI] [PubMed] [Google Scholar]
- 5.Sobin LH WC. International Union Against Cancer (UICC): TNM classification of malignant tumours. In: Wiley-Liss New York:, ed. 5th; 1997. [Google Scholar]
- 6.Cooper JS, Cohen R, Stevens RE. A comparison of staging systems for nasopharyngeal carcinoma. Cancer 1998; 83: 213–9. doi: [DOI] [PubMed] [Google Scholar]
- 7.Lee AW, Foo W, Law SC, Poon YF, O SK, Tung SY, et al. Staging of nasopharyngeal carcinoma: from Ho's to the new UICC system. Int J Cancer 1999; 84: 179–87. doi: [DOI] [PubMed] [Google Scholar]
- 8.Ozyar E, Yildiz F, Akyol FH, Atahan IL. Comparison of AJCC 1988 and 1997 classifications for nasopharyngeal carcinoma. American Joint Committee on Cancer. Int J Radiat Oncol Biol Phys 1999; 44: 1079–87. [DOI] [PubMed] [Google Scholar]
- 9.Hong MH, Mai HQ, Min HQ, Ma J, Zhang EP, Cui NJ, et al. A comparison of the Chinese 1992 and fifth-edition International Union Against Cancer staging systems for staging nasopharyngeal carcinoma. Cancer 2000; 89: 242–7. doi: [DOI] [PubMed] [Google Scholar]
- 10.Amin MB ES, Greene F.et al. AJCC Cancer Staging Manual, 8th. New York, NY: Springer; 2016. [Google Scholar]
- 11.Amin MB. American Joint Committee on Cancer. AJCC cancer staging manual. New York: Springer; 2017. [Google Scholar]
- 12.Tang L-L, Chen Y-P, Mao Y-P, Wang Z-X, Guo R, Chen L, et al. Validation of the 8th Edition of the UICC/AJCC Staging System for Nasopharyngeal Carcinoma From Endemic Areas in the Intensity-Modulated Radiotherapy Era. J Natl Compr Canc Netw 2017; 15: 913–9. doi: 10.6004/jnccn.2017.0121 [DOI] [PubMed] [Google Scholar]
- 13.Chan ATC. Nasopharyngeal carcinoma. Ann Oncol 2010; 21 Suppl 7(Suppl 7): vii308–12vii. doi: 10.1093/annonc/mdq277 [DOI] [PubMed] [Google Scholar]
- 14.Chua MLK, JTS W, Hui EP, Chan ATC. Nasopharyngeal carcinoma. The Lancet 2015;. [DOI] [PubMed] [Google Scholar]
- 15.Wei WI, Sham JST. Nasopharyngeal carcinoma. The Lancet 2005; 365: 2041–54. doi: 10.1016/S0140-6736(05)66698-6 [DOI] [PubMed] [Google Scholar]
- 16.Lee AWM, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin Radiat Oncol 2012; 22: 233–44. doi: 10.1016/j.semradonc.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 17.Kamran SC, Riaz N, Lee N. Nasopharyngeal carcinoma. Surg Oncol Clin N Am 2015; 24: 547–61. doi: 10.1016/j.soc.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 18.Edge SB BD, Compton CC.et al. AJCC cancer staging manual. : 7th. Philadelphia: Lippincott-Raven; 2009. [Google Scholar]
- 19.Greene FL PD, Fleming ID.et al. AJCC cancer staging manual. In: Lippincott- Raven, ed. 6th; 2002. [Google Scholar]
- 20.Pan JJ, WT N, Zong JF, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 2015;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W-F, Sun Y, Mao Y-P, Chen L, Chen Y-Y, Chen M, et al. Proposed lymph node staging system using the International Consensus Guidelines for lymph node levels is predictive for nasopharyngeal carcinoma patients from endemic areas treated with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2013; 86: 249–56. doi: 10.1016/j.ijrobp.2012.09.003 [DOI] [PubMed] [Google Scholar]
- 22.Yue D, Xu Y-F, Zhang F, Lin L, Mao Y-P, Li W-F, et al. Is replacement of the supraclavicular fossa with the lower level classification based on magnetic resonance imaging beneficial in nasopharyngeal carcinoma? Radiother Oncol 2014; 113: 108–14. doi: 10.1016/j.radonc.2014.08.036 [DOI] [PubMed] [Google Scholar]
- 23.Poon PY, Tsang VH, Munk PL. Tumour extent and T stage of nasopharyngeal carcinoma: a comparison of magnetic resonance imaging and computed tomographic findings. Can Assoc Radiol J 2000; 51: 287–95quiz 286. [PubMed] [Google Scholar]
- 24.Ng SH, Chang TC, Ko SF, Yen PS, Wan YL, Tang LM, SH N, SF K, et al. Nasopharyngeal carcinoma: MRI and CT assessment. Neuroradiology 1997; 39: 741–6. [DOI] [PubMed] [Google Scholar]
- 25.King AD, Teo P, Lam WWM, Leung SF, Metreweli C, et al. Paranasopharyngeal Space Involvement in Nasopharyngeal Cancer: Dectection by CT and MRI. Clin Oncol 2000; 12: 397–402. doi: 10.1053/clon.2000.9199 [DOI] [PubMed] [Google Scholar]
- 26.Liao X-B, Mao Y-P, Liu L-Z, Tang L-L, Sun Y, Wang Y, et al. How does magnetic resonance imaging influence staging according to AJCC staging system for nasopharyngeal carcinoma compared with computed tomography? Int J Radiat Oncol Biol Phys 2008; 72: 1368–77. doi: 10.1016/j.ijrobp.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 27.Chen W-S, Li J-J, Hong L, Xing Z-B, Wang F, Li C-Q, et al. Comparison of MRI, CT and 18F-FDG PET/CT in the diagnosis of local and metastatic of nasopharyngeal carcinomas: an updated meta analysis of clinical studies. Am J Transl Res 2016; 8: 4532–47. [PMC free article] [PubMed] [Google Scholar]
- 28.Chung N-N, Ting L-L, Hsu W-C, Lui LT, Wang P-M. Impact of magnetic resonance imaging versus CT on nasopharyngeal carcinoma: primary tumor target delineation for radiotherapy. Head Neck 2004; 26: 241–6. doi: 10.1002/hed.10378 [DOI] [PubMed] [Google Scholar]
- 29.King AD, Lam WW, Leung SF, Chan YL, Teo P, Metreweli C, et al. MRI of local disease in nasopharyngeal carcinoma: tumour extent vs tumour stage. Br J Radiol 1999; 72: 734–41. doi: 10.1259/bjr.72.860.10624338 [DOI] [PubMed] [Google Scholar]
- 30.Ng S-H, Chan S-C, Yen T-C, Chang JT-C, Liao C-T, Ko S-F, et al. Staging of untreated nasopharyngeal carcinoma with PET/CT: comparison with conventional imaging work-up. Eur J Nucl Med Mol Imaging 2009; 36: 12–22. doi: 10.1007/s00259-008-0918-7 [DOI] [PubMed] [Google Scholar]
- 31.Chua MLK, Ong SC, Wee JTS, Ng DCE, Gao F, Tan TWK, et al. Comparison of 4 modalities for distant metastasis staging in endemic nasopharyngeal carcinoma. Head Neck 2009; 31: 346–54. doi: 10.1002/hed.20974 [DOI] [PubMed] [Google Scholar]
- 32.Peng H, Chen L, Tang L-L, Li W-F, Mao Y-P, Guo R, et al. Significant value of 18F-FDG-PET/CT in diagnosing small cervical lymph node metastases in patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Chin J Cancer 2017; 36: 95. doi: 10.1186/s40880-017-0265-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei J, Pei S, Zhu X. Comparison of 18F-FDG PET/CT, MRI and SPECT in the diagnosis of local residual/recurrent nasopharyngeal carcinoma: A meta-analysis. Oral Oncol 2016; 52: 11–17. doi: 10.1016/j.oraloncology.2015.10.010 [DOI] [PubMed] [Google Scholar]
- 34.Law A, Peters LJ, Dutu G, Rischin D, Lau E, Drummond E, et al. The utility of PET/CT in staging and assessment of treatment response of nasopharyngeal cancer. J Med Imaging Radiat Oncol 2011; 55: 199–205. doi: 10.1111/j.1754-9485.2011.02252.x [DOI] [PubMed] [Google Scholar]
- 35.Chan JYK, Wong EWY, Ng S-K, Vlantis AC. Non-nasopharyngeal head and neck lymphoepithelioma-like carcinoma in the United States: A population-based study. Head Neck 2016; 38 Suppl 1(Suppl 1): E1294–E1300. doi: 10.1002/hed.24215 [DOI] [PubMed] [Google Scholar]
- 36.Weiss LM, Movahed LA, Butler AE, Swanson SA, Frierson HF, Cooper PH, et al. Analysis of lymphoepithelioma and lymphoepithelioma-like carcinomas for Epstein-Barr viral genomes by in situ hybridization. Am J Surg Pathol 1989; 13: 625–31. doi: 10.1097/00000478-198908000-00001 [DOI] [PubMed] [Google Scholar]
- 37.Wenig BM. Lymphoepithelial-like carcinomas of the head and neck. Semin Diagn Pathol 2015; 32: 74–86. doi: 10.1053/j.semdp.2014.12.004 [DOI] [PubMed] [Google Scholar]
- 38.Zhan KY, Nicolli EA, Khaja SF, Day TA. Lymphoepithelial carcinoma of the major salivary glands: Predictors of survival in a non-endemic region. Oral Oncol 2016; 52: 24–9. doi: 10.1016/j.oraloncology.2015.10.019 [DOI] [PubMed] [Google Scholar]
- 39.Luo W-J, Feng Y-F, Guo R, Tang L-L, Chen L, Zhou G-Q, Luo Wei-Jie FY-F, Rui G, Ling-Long T, Lei C, Guan-Qun Z, Wen-Fei L, et al. Patterns of EBV-positive cervical lymph node involvement in head and neck cancer and implications for the management of nasopharyngeal carcinoma T0 classification. Oral Oncol 2019; 91: 7–12. doi: 10.1016/j.oraloncology.2019.01.012 [DOI] [PubMed] [Google Scholar]
- 40.Zhou G-qun, Mao Y-P, Chen L, Li W-F, Liu L-Z, Sun Y, et al. Prognostic value of prevertebral space involvement in nasopharyngeal carcinoma based on intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2012; 82: 1090–7. doi: 10.1016/j.ijrobp.2010.11.063 [DOI] [PubMed] [Google Scholar]
- 41.Feng A-C, Wu M-C, Tsai SYC, Chan K-Y, Cheng SH, Wang A, et al. Prevertebral muscle involvement in nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2006; 65: 1026–35. doi: 10.1016/j.ijrobp.2006.02.012 [DOI] [PubMed] [Google Scholar]
- 42.Lee C-C, Chu S-T, Chou P, Lee C-C, Chen L-F. The prognostic influence of prevertebral space involvement in nasopharyngeal carcinoma. Clin Otolaryngol 2008; 33: 442–9. doi: 10.1111/j.1749-4486.2008.01770.x [DOI] [PubMed] [Google Scholar]
- 43.Liang S-B, Sun Y, Liu L-Z, Chen Y, Chen L, Mao Y-P, et al. Extension of local disease in nasopharyngeal carcinoma detected by magnetic resonance imaging: improvement of clinical target volume delineation. Int J Radiat Oncol Biol Phys 2009; 75: 742–50. doi: 10.1016/j.ijrobp.2008.11.053 [DOI] [PubMed] [Google Scholar]
- 44.JHC H.Halnan KE B. J, Crowther D, Nasopharynx Treatment of cancer. London: Chapman and Hall; 1982. 249–67. [Google Scholar]
- 45.Fleming ID CJ, Henson DE.et al. American Joint Committee on Cancer: AJCC Cancer Staging Manual. 5th ed Philadelphia: Lippincott-Raven; 1997. [Google Scholar]
- 46.Butman JA, Lee P. A criterion for distinguishing level V nodes from clavicular nodes. Arch Otolaryngol Head Neck Surg 2000; 126: 806–7. [PubMed] [Google Scholar]
- 47.Grégoire V, Eisbruch A, Hamoir M, Levendag P. Proposal for the delineation of the nodal CTV in the node-positive and the post-operative neck. Radiother Oncol 2006; 79: 15–20. doi: 10.1016/j.radonc.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 48.Ng WT, Lee AWM, Kan WK, Chan J, Pang ESY, Yau TK, et al. N-staging by magnetic resonance imaging for patients with nasopharyngeal carcinoma: pattern of nodal involvement by radiological levels. Radiother Oncol 2007; 82: 70–5. doi: 10.1016/j.radonc.2006.11.010 [DOI] [PubMed] [Google Scholar]
- 49.Mao Y-P, Liang S-B, Liu L-Z, Chen Y, Sun Y, Tang L-L, et al. The N staging system in nasopharyngeal carcinoma with radiation therapy oncology group guidelines for lymph node levels based on magnetic resonance imaging. Clin Cancer Res 2008; 14: 7497–503. doi: 10.1158/1078-0432.CCR-08-0271 [DOI] [PubMed] [Google Scholar]
- 50.Grégoire V, Levendag P, Ang KK, Bernier J, Braaksma M, Budach V, et al. CT-based delineation of lymph node levels and related CTVs in the node-negative neck: DAHANCA, EORTC, GORTEC, NCIC,RTOG consensus guidelines. Radiother Oncol 2003; 69: 227–36. doi: 10.1016/j.radonc.2003.09.011 [DOI] [PubMed] [Google Scholar]
- 51.Lee AW, Foo W, Law CK, et al. N-staging of nasopharyngeal carcinoma: discrepancy between UICC/AJCC and Ho systems. Union Internationale Contre le Cancer. American Joint Committee for Cancer. Clin Oncol 1996; 8: 155–9. [DOI] [PubMed] [Google Scholar]
- 52.Ng S-H, Chang JT-C, Chan S-C, Ko S-F, Wang H-M, Liao C-T, et al. Nodal metastases of nasopharyngeal carcinoma: patterns of disease on MRI and FDG PET. Eur J Nucl Med Mol Imaging 2004; 31: 1073–80. doi: 10.1007/s00259-004-1498-9 [DOI] [PubMed] [Google Scholar]
- 53.Liu L-Z, Zhang G-Y, Xie C-M, Liu X-W, Cui C-Y, Li L, et al. Magnetic resonance imaging of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: patterns of spread. Int J Radiat Oncol Biol Phys 2006; 66: 721–30. doi: 10.1016/j.ijrobp.2006.05.054 [DOI] [PubMed] [Google Scholar]
- 54.van den Brekel MW, Castelijns JA, Croll GA, Stel HV, Valk J, van der Waal I, Brekel MW, Van Den CJA, et al. Magnetic resonance imaging vs palpation of cervical lymph node metastasis. Arch Otolaryngol Head Neck Surg 1991; 117: 663–73. [DOI] [PubMed] [Google Scholar]
- 55.Mao Y-P, Tang L-L, Chen L, Sun Y, Qi Z-Y, Zhou G-Q, et al. Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin J Cancer 2016; 35: 103. doi: 10.1186/s40880-016-0167-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ho FCH, Tham IWK, Earnest A, Lee KM, Lu JJ. Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: a meta-analysis of clinical evidence. BMC Cancer 2012; 12: 98. doi: 10.1186/1471-2407-12-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li W-F, Sun Y, Chen M, Tang L-L, Liu L-Z, Mao Y-P, et al. Locoregional extension patterns of nasopharyngeal carcinoma and suggestions for clinical target volume delineation. Chin J Cancer 2012; 31: 579–87. doi: 10.5732/cjc.012.10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu L-Z, Zhang G-Y, Xie C-M, Liu X-W, Cui C-Y, Li L, et al. Magnetic resonance imaging of retropharyngeal lymph node metastasis in nasopharyngeal carcinoma: patterns of spread. Int J Radiat Oncol Biol Phys 2006; 66: 721–30. doi: 10.1016/j.ijrobp.2006.05.054 [DOI] [PubMed] [Google Scholar]
- 59.Sham JST, Choy D, Wei WI. Nasopharyngeal carcinoma: Orderly neck node spread. Int J Radiat Oncol Biol Phys 1990; 19: 929–33. doi: 10.1016/0360-3016(90)90014-B [DOI] [PubMed] [Google Scholar]
- 60.Tang L, Mao Y, Liu L, Liang S, Chen Y, Sun Y, et al. The volume to be irradiated during selective neck irradiation in nasopharyngeal carcinoma: analysis of the spread patterns in lymph nodes by magnetic resonance imaging. Cancer 2009; 115: 680–8. doi: 10.1002/cncr.24049 [DOI] [PubMed] [Google Scholar]
- 61.Grégoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, et al. Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol 2014; 110: 172–81. doi: 10.1016/j.radonc.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Li W-F, Chen L, Mao Y-P, Guo R, Zhang F, et al. Prognostic value of parotid lymph node metastasis in patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy. Sci Rep 2015; 5: 13919. doi: 10.1038/srep13919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xu Y, Chen X, Zhang M, Xiao Y, Zong J, Guo Q, et al. Prognostic effect of parotid area lymph node metastases after preliminary diagnosis of nasopharyngeal carcinoma: a propensity score matching study. Cancer Med 2017; 6: 2213–21. doi: 10.1002/cam4.1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amin MB. American Joint Committee on Cancer. AJCC cancer staging manual. New York: Springer; 2016. [Google Scholar]
- 65.Lai S-Z, Li W-F, Chen L, Luo W, Chen Y-Y, Liu L-Z, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys 2011; 80: 661–8. doi: 10.1016/j.ijrobp.2010.03.024 [DOI] [PubMed] [Google Scholar]
- 66.Lee AWM, Sze WM, Au JSK, Leung SF, Leung TW, Chua DTT, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys 2005; 61: 1107–16. doi: 10.1016/j.ijrobp.2004.07.702 [DOI] [PubMed] [Google Scholar]
- 67.Haberer-Guillerm S, Touboul E, Huguet F. Intensity modulated radiation therapy in nasopharyngeal carcinoma. Eur Ann Otorhinolaryngol Head Neck Dis 2015; 132: 147–51. doi: 10.1016/j.anorl.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 68.Mao Y-P, Tang L-L, Chen L, Sun Y, Qi Z-Y, Zhou G-Q, et al. Prognostic factors and failure patterns in non-metastatic nasopharyngeal carcinoma after intensity-modulated radiotherapy. Chin J Cancer 2016; 35: 103. doi: 10.1186/s40880-016-0167-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tang L-L, Liang S-B, Huang C-L, Zhang F, Xu C, Mao Y-P, et al. The development and external validation of simplified T category classification for nasopharyngeal carcinoma to improve the prognostic value in the intensity-modulated radiotherapy era. Cancer Med 2019; 8: 2213–22. doi: 10.1002/cam4.2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson CR, Thames HD, Huang DT, Schmidt-Ullrich RK. The tumor volume and clonogen number relationship: tumor control predictions based upon tumor volume estimates derived from computed tomography. Int J Radiat Oncol Biol Phys 1995; 33: 281–7. doi: 10.1016/0360-3016(95)00119-J [DOI] [PubMed] [Google Scholar]
- 71.Lartigau E, Le Ridant AM, Lambin P, Weeger P, Martin L, Sigal R, et al. Oxygenation of head and neck tumors. Cancer 1993; 71: 2319–25. doi: [DOI] [PubMed] [Google Scholar]
- 72.Chang C-C, Chen M-K, Liu M-T, Wu H-K. The effect of primary tumor volumes in advanced T-staged nasopharyngeal tumors. Head Neck 2002; 24: 940–6. doi: 10.1002/hed.10151 [DOI] [PubMed] [Google Scholar]
- 73.Chen M-K, Chen C-M, Lee M-C, et al. Primary Tumor Volume Is an Independent Predictor of Outcome Within pT4a-Staged Tongue Carcinoma. Annals of Surgical Oncology 2010;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chong VFH, Zhou J-Y, Khoo JBK, Chan K-L, Huang J. Correlation between MR imaging-derived nasopharyngeal carcinoma tumor volume and TNM system. Int J Radiat Oncol Biol Phys 2006; 64: 72–6. doi: 10.1016/j.ijrobp.2005.06.027 [DOI] [PubMed] [Google Scholar]
- 75.Chua DTT, Sham JST, Leung LHT, Tai KS, Au GKH, et al. Tumor volume is not an independent prognostic factor in early-stage nasopharyngeal carcinoma treated by radiotherapy alone. Int J Radiat Oncol Biol Phys 2004; 58: 1437–44. doi: 10.1016/j.ijrobp.2003.09.075 [DOI] [PubMed] [Google Scholar]
- 76.Guo R, Sun Y, Yu X-L, Yin W-J, Li W-F, Chen Y-Y, et al. Is primary tumor volume still a prognostic factor in intensity modulated radiation therapy for nasopharyngeal carcinoma? Radiother Oncol 2012; 104: 294–9. doi: 10.1016/j.radonc.2012.09.001 [DOI] [PubMed] [Google Scholar]
- 77.Lee C-C, Chu S-T, Ho H-C, Lee C-C, Hung S-K. Primary tumor volume calculation as a predictive factor of prognosis in nasopharyngeal carcinoma. Acta Otolaryngol 2008; 128: 93–7. doi: 10.1080/00016480701361921 [DOI] [PubMed] [Google Scholar]
- 78.Lee C-C, Huang T-T, Lee M-S, Hsiao S-H, Lin H-Y, Su Y-C, et al. Clinical application of tumor volume in advanced nasopharyngeal carcinoma to predict outcome. Radiat Oncol 2010; 5: 20. doi: 10.1186/1748-717X-5-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sarisahin M, Cila A, Ozyar E, Yıldız F, Turen S. Prognostic significance of tumor volume in nasopharyngeal carcinoma. Auris Nasus Larynx 2011; 38: 250–4. doi: 10.1016/j.anl.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 80.Shen C, Lu JJ, Gu Y, Zhu G, Hu C, He S, et al. Prognostic impact of primary tumor volume in patients with nasopharyngeal carcinoma treated by definitive radiation therapy. Laryngoscope 2008; 118: 1206–10. doi: 10.1097/MLG.0b013e31816ed587 [DOI] [PubMed] [Google Scholar]
- 81.Sze W-M, Lee AWM, Yau T-K, Yeung RMW, Lau K-Y, Leung SKC, et al. Primary tumor volume of nasopharyngeal carcinoma: prognostic significance for local control. Int J Radiat Oncol Biol Phys 2004; 59: 21–7. doi: 10.1016/j.ijrobp.2003.10.027 [DOI] [PubMed] [Google Scholar]
- 82.Zhou J-Y, Chong VFH, Khoo JBK, Chan K-L, Huang J. The relationship between nasopharyngeal carcinoma tumor volume and TNM T-classification: a quantitative analysis. Eur Arch Otorhinolaryngol 2007; 264: 169–74. doi: 10.1007/s00405-006-0163-2 [DOI] [PubMed] [Google Scholar]
- 83.Leung SF, Chan KCA, Ma BB, Hui EP, Mo F, Chow KCK, et al. Plasma Epstein-Barr viral DNA load at midpoint of radiotherapy course predicts outcome in advanced-stage nasopharyngeal carcinoma. Ann Oncol 2014; 25: 1204–8. doi: 10.1093/annonc/mdu117 [DOI] [PubMed] [Google Scholar]
- 84.Lo YM, Chan AT, Chan LY, Leung SF, Lam CW, Huang DP, et al. Molecular prognostication of nasopharyngeal carcinoma by quantitative analysis of circulating Epstein-Barr virus DNA. Cancer Res 2000; 60: 6878–81. [PubMed] [Google Scholar]
- 85.Lo YM, Chan LY, Chan AT, Leung SF, Lo KW, Zhang J, et al. Quantitative and temporal correlation between circulating cell-free Epstein-Barr virus DNA and tumor recurrence in nasopharyngeal carcinoma. Cancer Res 1999; 59: 5452–5. [PubMed] [Google Scholar]
- 86.Lo YM, Leung SF, Chan LY, Lo KW, Zhang J, Chan AT, et al. Plasma cell-free Epstein-Barr virus DNA quantitation in patients with nasopharyngeal carcinoma. Correlation with clinical staging. Ann N Y Acad Sci 2000; 906: 99–101. doi: 10.1111/j.1749-6632.2000.tb06597.x [DOI] [PubMed] [Google Scholar]
- 87.Ma BBY, King A, Lo YMD, Yau YY, Zee B, Hui EP, BB M, YM L, et al. Relationship between pretreatment level of plasma Epstein-Barr virus DNA, tumor burden, and metabolic activity in advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2006; 66: 714–20. doi: 10.1016/j.ijrobp.2006.05.064 [DOI] [PubMed] [Google Scholar]
- 88.Yip TTC, Ngan RKC, Fong AHW, Law SCK. Application of circulating plasma/serum EBV DNA in the clinical management of nasopharyngeal carcinoma. Oral Oncol 2014; 50: 527–38. doi: 10.1016/j.oraloncology.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 89.Lee VH, Kwong DL, Leung TW, et al. The addition of pretreatment plasma Epstein-Barr virus DNA into the 8th edition of nasopharyngeal cancer TNM stage classification. Int J Cancer 2018;. [DOI] [PubMed] [Google Scholar]
- 90.Guo R, Tang LL, Mao YP, et al. Proposed modifications and incorporation of plasma Epstein-Barr virus DNA improve the TNM staging system for Epstein-Barr virus-related nasopharyngeal carcinoma. Cancer 2018;. [DOI] [PubMed] [Google Scholar]
- 91.Jiang W, Liu N, Chen X-Z, Sun Y, Li B, Ren X-Y, et al. Genome-Wide Identification of a Methylation Gene Panel as a Prognostic Biomarker in Nasopharyngeal Carcinoma. Mol Cancer Ther 2015; 14: 2864–73. doi: 10.1158/1535-7163.MCT-15-0260 [DOI] [PubMed] [Google Scholar]
- 92.Liu N, Chen N-Y, Cui R-X, Li W-F, Li Y, Wei R-R, et al. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: a microRNA expression analysis. Lancet Oncol 2012; 13: 633–41. doi: 10.1016/S1470-2045(12)70102-X [DOI] [PubMed] [Google Scholar]