Abstract
Introduction
Stem cell-based therapies represent a valid approach to restore cardiac function due to their beneficial effect in reducing scar area formation and promoting angiogenesis. However, their translation into the clinic is limited by the poor differentiation and inability to secrete sufficient therapeutic factors. To address this issue, several strategies such as genetic modification and biophysical pre-conditioning have been used to enhance the efficacy of stem cells for cardiac tissue repair.
Methods
In this study, a biomimetic approach was used to mimic the natural mechanical stimulation of the myocardium tissue. Specifically, human adipose-derived stem cells (hASCs) were cultured on a thin gelatin methacrylamide (GelMA) hydrogel disc and placed on top of a beating cardiomyocyte layer. qPCR studies and metatranscriptomic analysis of hASCs gene expression were investigated to confirm the correlation between mechanical stimuli and cardiomyogenic differentiation. In vivo intramyocardial delivery of pre-conditioned hASCs was carried out to evaluate their efficacy to restore cardiac function in mice hearts post-myocardial infarction.
Results
The cyclic strain generated by cardiomyocytes significantly upregulated the expression of both mechanotransduction and cardiomyogenic genes in hASCs as compared to the static control group. The inherent angiogenic secretion profile of hASCs was not hindered by the mechanical stimulation provided by the designed biomimetic system. Finally, in vivo analysis confirmed the regenerative potential of the pre-conditioned hASCs by displaying a significant improvement in cardiac function and enhanced angiogenesis in the peri-infarct region.
Conclusion
Overall, these findings indicate that cyclic strain provided by the designed biomimetic system is an essential stimulant for hASCs cardiomyogenic differentiation, and therefore can be a potential solution to improve stem-cell based efficacy for cardiovascular repair.
Electronic supplementary material
The online version of this article (10.1007/s12195-018-0543-x) contains supplementary material, which is available to authorized users.
Keywords: Mechanical stimulation, Myogenic differentiation, Angiogenesis, Cardiac repair
Introduction
Myocardial infarction is one of the leading causes of mortality affecting people all over the world.16,76 This pathology occurs when there is a reduction of blood flow to the myocardial tissue caused by a partial or complete blockage of a coronary artery due to the formation of a cholesterol plaque.14 Traditional cardiac treatments such as stents, bypass surgery, and valve replacement aim to restore normal blood flow to the ischemic area but do not address the underlying cause of the disease.60 For this reason, there is an urgent need for alternative strategies that can boost the limited regenerative capacity of the myocardial tissue and reduce the risk of disease reoccurrence.
To this end, stem cell-based therapies represent a possible solution to overcome the limitations of current treatments aimed to promote myocardium regeneration. Among the variety of stem cell types, adult stem cells represent the ideal choice because they overcome ethical issues associated with the use of embryonic stem cells and possess a limited risk of immune rejection when harvested from the same patient. Additionally, they display other advantages including ease of harvest from different tissue sources (adipose, bone marrow, and umbilical cord, etc.), the possibility of in vitro expansion, and secretion of beneficial paracrine factors.20,60,62
Despite the aforementioned features and the efficacy demonstrated in pre-clinical studies, stem cell-based therapies present a limited translation into the clinic. One of the major reasons is that stem cells have a limited ability to function as competent myocytes and show poor differentiation and engraftment within the host tissue upon delivery to the myocardium. These limitations impact their survival rate and the long-term regenerative potential in vivo.60,62
Several in vitro approaches have been investigated to resolve these issues by augmenting the survival rate or the differentiation of adult stem cells before their transplantation. One possibility is to promote cardiomyogenic differentiation of stem cells prior to their delivery by genetic modification. For instance, expression of pro-survival markers such as proto-oncogene serine/threonine-protein kinase (Pim-1), B cell lymphoma-2 (Bcl2), Glycogen synthase kinase 3-beta (Gsk3-β) and GATA binding protein 4 (GATA4) have been shown to enhance stem cells proliferation and survival rate at the injured myocardium.7,13,32,33 Similarly, other studies have focused their attention on introducing insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), and angiopoietin-1 (Ang1) genes in the genome of stem cells to induce cardiac differentiation and angiogenesis.52,55,59,61 Gene transfection can be carried out by using both viral and non-viral carriers, however, several issues such as safety concerns, limited transfection efficiency and potential gene silencing in the host cell hinder the clinical translation of this strategy.73 Aside from gene delivery and genome editing, stem cells can be treated with growth factors such as TGF-β1, IGF-1, and FGF or small molecules and drugs including 5-azacytidine, ascorbic acid, retinoic acid and dynorphin B.19,25 In addition, adult stem cells can be co-cultured with neonatal rat cardiomyocytes in vitro. The direct cell-to-cell contact between the two different cell lines has been shown to induce a change in the stem cell phenotype in adult stem cells towards a cardiomyogenic lineage due to more efficient transduction of molecular signals.19,77 Moreover, other pre-conditioning treatments involve the culture of stem cell in hypoxic condition and serum starvation, which has been shown to increase their proliferation and differentiation due to upregulation of pro-survival and pro-angiogenic signaling pathways.21,57 Although promising, the mentioned pre-conditioned systems present a limited protective response due to down-regulation or desensitization of stem cell surface receptors.39 Another drawback is that all of the methods described above fail to consider the physiological environment of the myocardial tissue, which is a complex milieu of both biochemical and physical signals that dictate stem cell proliferation and differentiation.
A more efficient approach, which can closely mimic the intricate dynamics occurring in the native myocardium, considers the physical stimuli produced by the constant beating of cardiomyocytes as a cue to promote stem cell myogenic differentiation. Stem cells can sense these mechanical stimuli through a diverse group of membrane-anchored receptors such as stretch-activated ion channels, integrins and G-protein coupled receptors (GPCRs). The stimulation of these receptors leads to the activation of a multi-step signaling cascade, which can, in turn, upregulate selective transcription factors responsible for stem cell myogenic differentiation.9 Possible ways to recreate the mechanical environment of the beating heart consist of using bioreactors that emulate the cyclic strain produced by cardiomyocytes. Specifically, efforts in this direction have demonstrated a direct correlation between the application of a cyclic strain and cardiomyogenic differentiation of embryonic stem cells.17,22
Based on this concept, we aimed to develop a biomimetic system that can induce cardiomyogenic differentiation of stem cell upon exposure to cyclic strain generated by beating cardiomyocytes. Human adipose-derived stem cells (hASCs) were selected as the model stem cell line due to the ease of harvesting along with their inherent pro-angiogenic properties.8 hASCs were cultured on thin discs made of gelatin methacrylamide (GelMA), which were then placed on top of a monolayer of beating cardiomyocytes. GelMA is a well-known derivate of gelatin that has displayed excellent biocompatibility and can be processed into photocrosslinkable discs with tunable mechanical properties.27 The inclusion of the thin polymeric disc allowed the transmission of the mechanical stimuli to the hASCs monolayer that could be easily harvested at the end of the treatment. Furthermore, we tested the influence of cyclic strain on the genetic and angiogenic growth factor secretion profile of hASCs exposed to the physical stimuli in comparison to a static culture control. Finally, we delivered the pre-conditioned hASCs in an acute myocardial infarction (AMI) mice model and tested their ability to restore heart function by assessing ejection fraction, fractional shortening, scar area reduction and neovascularization in the peri-infarct region.
Materials and Methods
Gelatin Methacrylamide Synthesis
Gelatin methacrylamide (GelMA) was synthesized following a previously established protocol.71 Briefly, gelatin A (300 bloom grade, from porcine skin, Sigma-Aldrich, USA) was dissolved in phosphate buffered saline (PBS, 100 mL) at the concentration of 10% w/v while maintaining the temperature at 50 °C. 0.8 mL of methacrylic anhydride (Sigma-Aldrich, USA) per gram of gelatin was added dropwise, and the reaction mixture was stirred for 2 h at 50 °C. After the reaction was completed, the polymeric mixture was diluted with PBS (100 mL). Next, the undesired byproducts such as methacrylic acid were removed by dialyzing the solution (12–14 kDa molecular weight cut off) against distilled water for 7 days at 40 °C. The solution was then vacuum filtered (0.22 µm pore size), frozen in − 80 °C and lyophilized for 7 days. Finally, the freeze-dried polymer was used to fabricate GelMA discs.75
GelMA Disc Fabrication
GelMA discs hydrogels were obtained by solubilizing GelMA in PBS at the concentration of 7% w/v. The photoinitiator Irgacure 2959 (Sigma-Aldrich, USA) was mixed in the polymeric solution at the concentration of 0.1% w/w before UV irradiation. Photocrosslinked hydrogel discs were obtained by adding 150 µL of the polymer solution on a petri dish between two 100 μm thick spacers. A glass slide was gently placed on top of this pre-polymeric solution and exposed to UV irradiation (Omnicure S200, Lumen Dynamics, Canada) for 80 s at an intensity of 7 mW/cm2. The photo-crosslinked GelMA discs (100 μm thick) were then rinsed with PBS to remove any uncrosslinked polymer and treated with 1% penicillin/streptomycin (P/S) before using them for cell culture experiments.44
Frequency Sweep Analysis of GelMA Discs
The viscoelastic properties of the GelMA discs were studied by running a frequency sweep in the range of 0.01–10 Hz at 37 °C and 1% of strain using the AR 2000 Rheometer (TA Instruments, New Castle, DE). To define the range of viscoelastic region, preliminary strain sweep tests were carried out in the range of 0.1–100% of strain at the frequency of 1 Hz. The hydrogel discs (100 μm height, n = 3) were tested using a 20 mm smooth steel plate geometry, and frequency sweeps were performed on the swollen hydrogels discs. Specifically, GelMA discs were analyzed after soaking them for 1 h and 7 days in PBS 0.1 mM (pH 7.4), respectively. We also performed frequency sweep analysis on GelMA discs seeded with hASCs for 7 days to study whether the presence of hASCs had any effect on the mechanical properties of the GelMA discs.44
Swelling Study
Swelling studies were performed on freeze-dried hydrogel discs by first weighing them in the dried state and then soaking the gels in PBS at 37 °C. The swollen hydrogels were weighed at defined time intervals to assess the equilibrium of swelling. The swelling ratio (%) was measured using the following formula:
| 1 |
where Ws is the weight of the swollen hydrogel, and Wd represents the weight of the freeze-dried hydrogel.44,45
Human Adipose-Derived Stem Cells (hASCs) Culture
hASCs (Lot number 00061, donor: female 31–45 years old) were procured from RoosterBio (USA) and maintained in alpha-minimum essential medium (α-MEM, Invitrogen, USA) with 15% v/v fetal bovine serum (FBS) and 1% v/v penicillin-streptomycin (P/S) at 37 °C and 5% CO2. Passages 3–5 were used for all the studies. hASCs were seeded in 2D on the hydrogel discs at a cell density of 5 × 104 cells/mL and grown until 80% of confluency for 2 days.
Cardiomyocytes (CMs) Cell Culture
iPSC- derived human CMs (Lot number 103321) were purchased from cellular dynamics (Madison, WI, USA) and grown according to the manufacturer’s protocol. Briefly, 24 well plates were coated with a 0.1% w/v gelatin solution for 1 h, which was removed before adding the cell suspension. CMs were seeded on the treated well plates at a concentration of 1.2 × 105 cells/cm2 using the provided plating media. Cells were cultured at 37 °C and in a 7% CO2 environment. 48 h post-seeding, the plating media was replaced with an appropriate volume of maintenance media. Mechanically active cardiomyocytes were ready to be used 2 weeks post-culture. At this time point, cells formed a uniform monolayer with a synchronous beating.
Design of the Biomimetic Co-Culture System
The co-culture system was set up by first replacing the maintenance media for CMs with α-MEM media (15% FBS, 1% P/S). Next, the GelMA discs with hASCs seeded on them were gently placed onto the wells seeded with CMs without disturbing the cell layer.
Additionally, hASCs were cultured in a transwell, which was placed in the well containing CMs to study the effect of cardiomyogenic differentiation induced by the secretion of paracrine molecules. Both co-culture setups were maintained in α-MEM media (15% FBS, 1% P/S) at 37 °C and 7% CO2 for the entire duration of the experiments.
Confocal Microscopy
Immunofluorescence images of the co-culture were obtained by laser scanning confocal microscopy (LSCM). CMs were cultured on a 24-well glass-bottom tissue culture plate (IBDI, Germany) and hASCs were seeded on the GelMA discs as mentioned previously. Each cell monolayer was stained separately to visualize the distinct cell populations. Specifically, CMs were stained with phalloidin-AlexaFluor488 (Invitrogen, USA) to visualize actin fibers, while the actin component of hASCs’ cytoskeleton were stained with phalloidin-AlexaFluor594 (Invitrogen, USA). In both cases, the nuclei were stained with DAPI (Sigma-Aldrich, USA). All images were processed using the ImageJ software.
Immunoflourescence
To study the translocation of YAP1 and TAZ into the nucleus, we performed immunostaining of the hASCs seeded on GelMA discs. The hydrogel discs were retrieved after 7 days of co-culture (cyclic strain) and fixed with 4% paraformaldehyde. The cells were permeabilized with 0.3% Triton X-100 and blocked with 5% normal goat serum (45 min) following which they were stained with Purified Mouse anti-TAZ (BD Biosciences, USA) and anti-YAP1 (Abnova, Taiwan) monoclonal antibodies according to manufacturer’s protocols. Samples were then counterstained with Goat anti-mouse AlexaFluor 594 (Invitrogen, USA), which served as the fluorescent-labelled secondary antibody. Lastly, Diamidino-2-phenylindole dilactate (DAPI, Sigma-Aldrich, USA) was added to visualize the nuclei and images were obtained using fluorescent microscopy at 40× magnification.15
Beating Frequency Measurements
The following protocol was adopted to measure the beating frequency of CMs and corresponding displacement of hASCs layer on the GelMA discs. A suitable reference image of cells was taken at time t0 when the average motility is minimal. This image depicts a contraction-free state. The reference image was then compared to all the other images using particle image velocimetry (PIV) analysis. For each time point t and location x, the total movement (magnitude and directionality) relative to the reference image was calculated to obtain a series of displacement vector fields d(t,x). The average displacement (beat pattern) D(t) was measured at each time point t as the spatial average of the magnitudes|d(t,x)| for each displacement vector field. The D(t) displacement curves show a series of single peaks, which rise and decrease similar to an exponential function.56
qPCR Analysis of Mechanotransduction and Myogenic Differentiation Markers
Two different groups were investigated for qPCR analysis: (i) static control group (hASCs seeded on GelMA discs without exposure to cyclic strain of beating CMs) (ii) cyclic strain group (hASCs seeded on GelMA discs, co-cultured with beating cardiomyocytes). qPCR analysis of hASCs expression profile was carried out on Day 1 and Day 7 of treatment (n = 3). mRNA from each group was extracted at different time points using an RNeasy Mini Kit (Qiagen, Germany) and measured using a NanoDrop (Thermo Scientific, USA). Next, mRNA was converted to cDNA using the High-Capacity cDNA Conversion Kit (Applied Biosystems, USA). Finally, gene expression was evaluated using a mixture of predesigned primers and the KiCqStart SYBR Green Master Mix (Sigma-Aldrich, USA). All qPCR reactions were carried out with a Mastercycler Realplex (Eppendorf, Germany) and the fold expression levels were calculated using the relative ∆∆Ct method. GAPDH was employed as the housekeeping gene. Specifically, the effect of CMs beating over hASCs mechanotransduction response was studied by measuring the upregulation of ACTA2, TAZ, CDC42 and YAP1 markers. Myogenic differentiation was evaluated by assessing the expression of ACTC1 and TNNT2 genes.
Finally, to study the effect of cardiomyogenic differentiation induced by the secretion of paracrine molecules in the co-culture system, an additional group was investigated consisting of hASCs cultured on a transwell placed in a well seeded with CMs. The expression of ACTC1 and TNNT2 genes was assessed at 7 days and compared to the cyclic strain group.
RNA Extraction and cDNA Library Preparation
mRNA from each group was extracted at different time points using an RNeasy Mini Kit (Qiagen, Germany) and the quality of mRNA was measured using a 4200 TapeStation System (Agilent Technologies, Palo Alto, CA). The Metatranscriptome libraries were generated using TruSeq Stranded mRNA sample preparation kit (Illumina) on the Biomek FXP device following the manufacturer’s protocol. This automation method generates high quality stranded mRNA sequencing libraries compatible with Illumina sequencers. The enriched mRNA from the samples were polyadenylated using Escherichia coli poly(A) polymerase and converted to double-stranded complementary DNA (cDNA) via reverse transcription. The double-stranded cDNA from all samples were digested, purified and pooled together. The resulting library was quantified by qPCR and sequenced by Illumina MiSeq instrument using V3 reagents (Roche, Indianapolis, IN).
Metatranscriptomics Data Analysis
The quality of the sequence reads was verified with a FastQC software, which is a quality control tool for high throughput data (http://www.bioinformatics.babraham.ac.uk). Tophat (version 2.1.1) and Cufflinks (version 2.2.1) programs were used with default parameters to assemble de-novo transcriptomes.65 To understand the differential gene expression between the static control and cyclic strain groups, the relative expression of transcriptome was generated based on the Fragments Per Kilobase of transcript per Million mapped read (FPKM) in Cufflinks. The resulting FPKM values and fold change were visualized in the generated heatmaps using ggplot2, an open source R package (http://www.r-project.org/).72
Secretome Analysis
The conditioned media (secretome) was collected from static and cyclic strain groups at 7 days post-treatment, and an angiogenesis array was performed to detect the relative expression of several angiogenic growth factors (n = 3). A human angiogenesis array (RayBiotech) was used for this purpose according to the manufacturer’s protocol. The signals were visualized using a laser scanner equipped with a Cy3 wavelength (Axon GenePix).
In Vivo Acute Myocardial Infarction Surgery
Animal studies were performed according to the NIH Guide Care and Use of Laboratory Animals protocols (NIH publication 85–23, revised 1985) approved by the Institutional Animal Care and Use Committee (IACUC). In vivo experiments were carried out using an acute myocardial infarction (AMI) mice model consisting of 12-week-old female C57BL/6 mice. Cyclosporine (Sigma, 20 mg/kg body weight/day) was delivered through intraperitoneal injection. The drug was administered 2 days before the surgery and was injected for the entire duration of the experiment (21 days). Briefly, animals were anaesthetized with isoflurane gas, tracheally intubated and maintained on a Rodent ventilator (Minivent, Type 845 Germany) to assist their respiratory function. The body temperature of the mice was maintained at 37 °C for the duration of the surgery. The mice hearts were exposed by minimal left-sided thoracotomy, and myocardial infarction was induced by occlusion of the left anterior descending coronary artery following previously established protocols.47,50 Fifteen minutes post ligations of the arteries, the mice with AMI were subjected to different treatments (n = 4). Specifically, either PBS (sham control), Matrigel® (vehicle control), hASCs cultured in static conditions (hASC static) or hASCs pre-conditioned using the designed in vitro biomimetic system (hASC cyclic) were injected into the peri-infarct region of mice hearts 15 min post-infarction. hASCs for the last two groups were pre-cultured on the GelMA discs for 7 days and harvested using trypsin for 5 min and ultimately encapsulated in a Matrigel® hydrogel before injection into the injured myocardium. 5 × 104 cells/heart were delivered using 100 µL of Matrigel®, which was injected intramyocardially at multiple sites (3 sites/heart). Cardiac function was assessed by performing transthoracic echocardiography at day 3, and day 21 post surgery. At day 21, all animals were sacrificed. The excised hearts were either frozen or fixed with a 4% formalin solution for further studies.
In Vivo Assessment of Cardiac Function
Cardiac function was evaluated by carrying out transthoracic echocardiography at day 3, and day 21 post surgery using the Vevo®2100 Imaging System Visualsonics following a previously established procedure.51 After the mice were anesthetized using isoflurane gas, 2D images of hearts were obtained for M-modes through the anterior and posterior left ventricle (LV) walls. Indices of left ventricle (LV) systolic function including LV ejection fraction (EF), LV fractional shortening (FS), cardiac output (CO), and stroke volume (SV) were calculated using the following formulae2,47,51:
| 2 |
| 3 |
| 4 |
| 5 |
where, LVEDd corresponds to the end-diastolic diameter of the left ventricle and LVESd is the end-systolic diameter of the left ventricle measured from three consecutive cardiac cycles. EDV is the end-diastolic volume and ESV corresponds to the end-systolic volume.
Immunostaining of Heart Sections
Paraffin-embedded heart sections were deparaffinized by soaking the tissue sections alternatively in xylene and ethanol solutions (100%) for 5 mins each. Antigen retrieval was then performed using a citrate buffer solution and samples were then blocked using a CAS-block Histochemical Reagent (Thermo Fischer Scientific, USA) at room temperature for 2 h. This process was followed by three consecutive washes with PBS (5 min each). Samples were then incubated overnight at 4 °C with primary antibodies for von Willebrand Factor (vWF) (catalog # A0082, Dako, Agilent Pathology Solutions, USA) and smooth muscle actin (SMA) (catalog # A2547 SIGMA, Sigma-Aldrich, USA).52,53 Primary antibodies were used at a working dilution of 1:200 with CAS-Block. After washing samples with PBS three times (5 min each), they were incubated with the corresponding secondary antibodies (working dilution of 1:200 with CAS-Block) for 2 h at room temperature. DAPI (Sigma-Aldrich, USA) staining was performed to stain nuclei and images were obtained using fluorescent microscopy.18
Statistical Analysis
qPCR analysis, infarct size area, blood vessels density, CO, EF, FS and stroke volumes were assessed using four animals for each treatment group. Results were expressed as mean ± standard deviation. The differences among the groups were calculated using student’s t test and one-way ANOVA for repeated measurements with Tukey post hoc comparisons. Statistical significance for all tests should be p < 0.05 (GraphPad Prism Software). (*p < 0.05, **p < 0.01, and ***p < 0.001).
Results and Discussion
Design and Characterization of the Biomimetic Co-Culture System
Current cardiovascular treatments based on stem cell therapies suffer a series of limitations including lack of cell engraftment and limited differentiation at the injured site.20,60 These drawbacks are mainly due to a limited control over stem cell fate upon in vivo administration into the myocardial tissue. Therefore, there is a need to develop in vitro strategies aimed to modulate stem cells’ genetic profile as a preliminary step to increase their differentiation capabilities. Based on this concept, we have designed a strategy that can help achieve this goal without any drug treatment or genetic manipulation.
The proposed in vitro biomimetic approach provides a mechanical stimulus as a guiding factor to promote cardiomyogenic differentiation of stem cells. hASCs were seeded on a GelMA hydrogel disc, which was placed on top of a beating cardiomyocytes layer cultured on a well plate (Fig. 1a). GelMA was used in this study due to its biocompatibility and ease of fabrication into thin layers (100 µm) using UV crosslinking.67,75 The hydrogel can be washed after the network formation to remove unreacted photoreagents and can be customized in different sizes simply by varying the volume of polymer used before the process of crosslinking. The inclusion of a thin polymeric disc separating the two cell layers allows for easy harvesting of the hASCs from the GelMA disc after being exposed to the physical stimuli.
Figure 1.
Design and fabrication of the biomimetic system. (a) Schematic displays the overall design of the biomimetic system. hASCs were cultured on a thin GelMA disc placed on top of a layer of beating CMs. (b) Fluorescent confocal image of the 3D construct. Actin fibers of CMs were stained with Phalloidin tagged with Alexa Fluor 488 (Green) while the actin fibers of hASCs (top layer) were visualized using Alexa Fluor-594 (Red). Nuclei of both cell layers were stained using DAPI (blue). The middle section is represented by the GelMA disc. (c) Fluorescent images of the confluent monolayers of hASCs and CMs and the corresponding beating frequency graphs showing the displacement vs. time (sec). Scale bar = 100 µm (10× magnification). (d) Representative frequency sweeps test carried out at day 0 and day 7 in the range of frequencies from 0.01 up to 10 Hz. G’ storage modulus and G” viscous moduli are reported with solid and hollow circles, respectively. No significant change in the G’ values of GelMA discs was observed at the two different time points. (e) Equilibrium of swelling of GelMA discs in PBS (pH 7.4) carried out over a period of 7 days. The plateau was reached after 4 days. Data are reported as mean ± standard deviation (n = 6).
Confocal analysis was used to display the presence of the different cell layers in the biomimetic system (Fig. 1b, Video S1). The x and y axis were kept constant while changing the z coordinate during the imaging process. All the individual layers were stacked together to obtain a 3D image of the construct. The bottom layer is represented by CMs stained with Alexa Fluor 498 phalloidin to visualize actin fibers. Additionally, the top layer is formed by hASCs seeded on the hydrogel disc stained with Alexa Fluor 594 phalloidin to investigate actin organization. hASCs spread and displayed a spindle-like morphology on the surface of the hydrogel forming a confluent monolayer after 2 days post-seeding.
Previous studies have relied on the use of artificial bioreactors to simulate the cardiomyocyte beating, however these approaches present a limited ability to recreate the physiological heart function.17,22,58 On the contrary, our approach can better mimic the mechanical environment of the myocardial tissue since CMs are used to produce the mechanical stimulus.
The addition of a GelMA disc on the CMs did not affect their beating ability through the 7 days of the study (Video S2). CMs beating profile has been characterized by PIV analysis using image J. The beating generated by CMs was able to produce a corresponding displacement in the hASCs layer cultured on the hydrogel (Video S3). The average displacement D(t) produced on the hASCs monolayer on the GelMA disc was constant as reported (Fig. 1c). However, the two displacement graphs were not in sync with each other probably due to a time delay in the translation of the mechanical force from the bottom to the top layer.
The hydrogel could be removed after 7 days of culture while still maintaining its mechanical integrity as demonstrated by rheological studies. For instance, GelMA discs displayed similar G’ values at day 1 and day 7 as measured in the frequency sweep test after 7 days of contact in phosphate buffer saline (PBS, pH 7.4) (Fig. 1d). Similarly, GelMA discs seeded with hASCs and cultured for 7 days displayed the same value of G’ (Fig. S1). G’ or storage modulus is indicative of the degree of crosslinking of the GelMA network, and no significant change was observed in the range of frequencies tested. Freeze-dried GelMA discs were able to swell when in contact with PBS buffer and they reached an equilibrium of swelling after 4 days (Fig. 1e). Overall, these findings suggest that the proposed approach can successfully expose hASCs to a constant cyclic strain, which can be obtained without the use of any external mechanical device.
Effect of Cardiomyocytes Beating on hASCs Genetic Profile and Growth Factor Expression
The myocardial tissue is a mechanically dynamic environment where cardiomyocytes generate a constant cyclic strain determined by their rhythmic beating. Several studies have demonstrated the direct relation between this mechanical signal and the biological influence over stem cells fate.17,22 Based on this concept, the second part of the study was focused on defining whether mechanical stimulation generated by the beating of CMs could promote hASCs activation of mechano-transduction genes and the corresponding differentiation towards a cardiomyogenic lineage without the use of any external biochemical factors.
As the first step, we have determined the expression of several key genes that regulate the ability of hASCs to sense mechanical signals provided in the form of CMs beating. These genes are involved in an array of signaling cascades that allow the translation of a mechanical stimulus into a biological response.9 hASCs were exposed to CMs beating (cyclic strain) for 24 h, and the expression of Yes-associated protein 1 (YAP1), Tafazzin (TAZ), Cell division cycle-42 (CDC42) and alpha-actin-2 (ACTA2) was investigated. qPCR results were compared to the genetic expression of hASCs seeded on GelMA disc without any mechanical treatment (Static Control). A higher expression of the aforementioned mechano-transduction markers was observed within 24 h in hASCs subjected to cyclic strain when compared to the static control group (Fig. 2a).
Figure 2.
Activation of mechano-transduction genes, angiogenic and myogenic differentiation markers of hASCs post-exposure to cyclic strain stimuli. (a) qPCR analysis of the main mechano-transduction genes including ACTA2, TAZ, YAP1, and CDC42 after 1 day of exposure to cyclic strain induced by beating cardiomyocytes. The static control group consisted of hASCs cultured on GelMA disc without exposure to any physical stimulation (n = 3). (b) Schematic represents the YAP1-TAZ signaling mechano-transduction pathway, which was activated in hASCs post mechanical stimulation. (c) Metatranscriptomics analysis of different angiogenic and myogenic differentiation markers in hASCs cultured on GelMA disc with and without cyclic strain. Genes are subdivided into two groups based on the different range of gene expression among the groups. Data are reported as mean ± standard deviation and normalized to day 1 gene expression derived from hASCs cultured in static condition. *p < 0.05, **p < 0.01, and ***p < 0.001.
Specifically, YAP1 and TAZ genes encode for the corresponding proteins which are transcription coactivators of the Hippo pathway that controls cell growth, proliferation, and cell differentiation. The presence of a mechanical stimulus can induce the activation of both YAP1 and TAZ that are translocated into the nuclei and associate with the transcription enhancer factor 1 (TEF-1) also known as TEAD (Fig. 2b). Consequently, this binding step activates the transcription of other genes that can regulate stem cell differentiation into several lineages such as bone, adipose and cardiac tissue.6,11,34,38,46 We have proved the nuclear translocation of the YAP1-TAZ complex in the nucleus of hASCs by immunofluorescence staining after 7 days of co-culture using the designed biomimetic system (Fig. S2). Aside from the upregulation of YAP1 and TAZ genes, we observed the overexpression of two others key genes such as ACTA2 and CDC42 that are involved in the α-actin formation and actin polymerization respectively. The observed increase in CDC42 expression can be explained by the involvement of CDC42 protein as a regulator in the YAP1-TAZ mechano-transduction pathway as similarly reported in another study.34 These results indicate that the cardiomyocytes cyclic strain provided a sufficient mechanical stimulus capable of modulating the mechanobiological response in hASCs.
Subsequently, it was important to verify whether the biological modulation induced by CMs beating was responsible for modifying the genetic profile of hASCs towards a cardiomyogenic lineage. Particularly, recent studies have investigated the effect of cyclic strain on another type of stem cell as one of the possible factors directing their differentiation into cardiomyocytes.17,22 Hence, we focused our investigation on the expression of myogenic differentiation markers in hASCs post-treatment with cyclic strain.
qPCR analysis revealed a higher expression of alpha-cardiac actin (ACTC1) 5.12 ± 1.43 and Troponin T2 (TNNT2) 1.58 ± 0.30 in the cyclic strain group after 7 days of exposure in comparison with the static group (Fig. S3). ACTC1 encodes for α-actin, which is one the main protein forming the contractile apparatus of cardiac sarcomeres.3 Similarly, TNNT2 regulates the expression of cardiac troponin T2, which is located in the thin filament of the striated muscles and plays a major role in cardiac muscle contraction.64
Additionally, we performed a metatranscriptomic analysis of the main angiogenic and myogenic differentiation markers between the two groups. The generated heat maps indicate a higher fold change in the expression levels of key angiogenic and myogenic markers in the cyclic strain group (Fig. 2c). The list of the genes and their functions have been provided in Table 1. This data confirms the successful modulation of the genetic profile of hASCs towards a cardiomyocyte-like lineage. Hence, the cyclic strain provided by our designed biomimetic system is an efficient stimulant for cardiomyogenic differentiation of hASCs without the need of any additional biological factors.
Table 1.
Main cardiac and angiogenic markers found in the metatranscriptomic analysis.
| Gene acronym | Gene name | Main function | Ref. |
|---|---|---|---|
| ACTN2 | Alpha-Actinin-2 | Regulates actin-binding protein transcription | 63 |
| SMPX | Stretch-responsive skeletal muscle protein | Encodes for the SMPX protein which coordinates skeletal muscle functional state | 26 |
| MYH7 | Myosin heavy chain 7 | Controls the expression of β-heavy chain subunit of cardiac myosin | 68 |
| MYL7 | Myosin light chain 7 | Regulates the transcription of Myosin light chain 7 protein, which is expressed in cardiac muscle atria | 29 |
| MYL2 | Cardiac myosin light chain 2 | Encodes for the regulatory myosin light chain 2 protein that is commonly associated with cardiac myosin β-heavy chains | 24 |
| TNNC1 | Troponin C type 1, cardiac type | Encodes for troponin that is a central regulatory protein of striated muscles | 54 |
| SMYD1 | SET and MYND domain containing 1 | Regulates expression of proteins involved in heart development, cardiomyogenic differentiation, and cardiac morphogenesis | 31 |
| TNNT2 | Troponin T2, cardiac type | Regulates the transcription of tropomyosin binding subunit protein located in thin filament of striated muscles | 64 |
| NPPB | Natriuretic peptide B | Encodes for the cardiac natriuretic peptide B expression, which functions as a paracrine antifibrotic factor and maintains cardiovascular homeostasis | 30 |
| ACTA1 | Actin, alpha 1, skeletal muscle | Encodes for alpha actin which is a major constituent of the contractile apparatus | 23 |
| ANKRD1 | Ankyrin repeat domain 1 | Encodes for a protein that functions as a transcription factor and interacts with sarcomeric proteins | 40 |
| TNNI1 | Troponin I1, slow skeletal type | Regulates the expression of troponin I protein, which is the inhibitory subunit that mediates striated muscle relaxation | 69 |
| NKX 2.5 | Cardiac-specific homeobox 1 | This gene encodes for a homeobox-containing transcription factor that is involved in heart formation and development | 66 |
| MYH6 | Myosin heavy chain 6 | Encodes for the alpha heavy chain subunit of cardiac myosin that helps in cardiac muscle contraction | 12 |
| MYL4 | Myosin light chain 4 | Encodes for the expression of myosin light chain protein found in adult atria | 42 |
| FGF12 | Fibroblast growth factor 12 | Regulates the expression of fibroblast growth factors that possess mitogenic and cell survival properties | 28 |
| NCAM1 | Neural cell adhesion molecule 1 | Encodes for the transcription of the neural cell adhesion molecule which is a surface glycoprotein that mediates cell adhesion | 41 |
| VCAM1 | Vascular cell adhesion molecule 1 | Encodes for the expression of a cell surface sialoglycoprotein that controls adhesion of endothelial cells | 10 |
| IGF2 | Insulin-like growth factor 2 | Regulates the expression insulin-like growth factor 2 that regulates endothelial cell proliferation, differentiation, and survival | 5 |
Another important aspect to consider while designing a therapeutic stem cell-based strategy for cardiovascular regeneration is the need for a pro-angiogenic paracrine response that controls stem cell survival and cardiac remodeling. For instance, hASCs inherently possess the ability to secrete angiogenic growth factors such as Angiogenin, VEGF, bFGF, and HGF. These proteins can be beneficial for myocardial protection, cardiomyocytes proliferation and progenitor stem cell recruitment.35–37,48,78 For this reason, it was essential to verify whether the secretion profile of hASCs (secretome) was affected by the mechanical stimulation produced by the designed biomimetic system.
To assess this important factor, we performed an angiogenesis array on the secretome obtained from the static and the cyclic strain groups after 7 days of culture (Fig. S4). Angiogenin and VEGF were the most abundantly expressed growth factors but no significant difference in their concentration was found between the two groups. On the contrary, the secretion profile of HGF, Leptin, and PIGF was slightly increased in the cyclic strain group suggesting a possible effect of cyclic strain in enhancing the pro-angiogenic potential of hASCs. Overall, these findings indicate that the mechanical stimulation provided did not hamper hASCs’ ability to secrete a therapeutic secretome. Aside from their beneficial angiogenic properties, these paracrine factors can also regulate myogenic differentiation of mesenchymal stem cells as demonstrated in other studies.1,4,49,74 Therefore, we selected a co-culture setup where hASCs were exposed to cardiomyocytes paracrine factors without receiving any mechanical stimulation. Briefly, hASCs were cultured in a transwell and placed in co-culture with CMs without being exposed to CM beating (cyclic strain (-)) (Figure S5A). This additional study was important to demonstrate whether the co-culture secretome played a role in the upregulation of the cardiomyogenic markers observed in our study. qPCR analysis of ACTC1 and TNNT2 after 7 days of co-culture displayed no significant increase in both genes when compared to our biomimetic strategy (cyclic strain (+)) where hASCs were subjected to CM beating (Fig. S5B). Thus, the mechanical stimulation provided by our biomimetic approach can induce a suitable change in the genetic profile of hASCs towards a cardiomyocytes-like lineage, without affecting their angiogenic capability.
In Vivo Studies Using an Acute Myocardial Infarction Mice Model
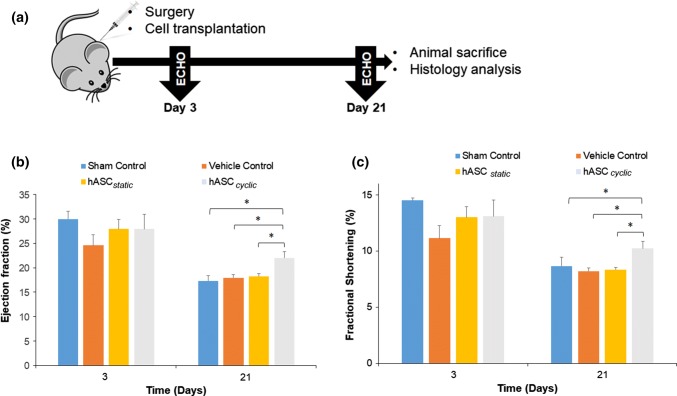
As the final step of our investigation, we evaluated the therapeutic efficacy of pre-conditioned hASCs in vivo in an immunodeficient AMI mice model. Our main hypothesis is that hASCs subjected to mechanical stimuli can significantly improve cardiac function when compared to hASCs cultured in a static environment. Specifically, the observed upregulation of cardiomyogenic markers, observed in vitro, should enhance the hASCs’ ability to act as competent myocytes in vivo leading to improved cardiovascular regeneration. To test this hypothesis, hASCs were cultured on the GelMA discs for 7 days in static and cyclic strain conditions as explained in the previous section. hASCs were then harvested from the GelMA discs and encapsulated in a Matrigel® hydrogel used as a carrier to deliver them into the injured myocardium of mice (Fig. 3a). We chose Matrigel® because it is a well-known biocompatible ECM-based matrix that has been widely used to deliver stem cell to the myocardium to ensure high viability, migration, and proliferation upon delivering.43
Figure 3.
In vivo assessment of cardiac function upon treatment with pre-conditioned hASCs. (a) Schematic showing the experimental design of the study. Four different groups were tested in an AMI mice immunodeficient model. Specifically, either PBS (sham control), Matrigel® (vehicle control), hASCs cultured in static conditions encapsulated in Matrigel® (hASC static) or hASCs pre-conditioned using the designed in vitro biomimetic system encapsulated in Matrigel® (hASC cyclic) were injected into the peri-infarct region of mice hearts 15 min post-infarction. (b) Calculated ejection fractions (EF%) at day 3 and day 21. A significant increase was observed in the cyclic strain group compared to the static group at day 21. (c) Assessment of fractional shortening (FS%) monitored at day 3 and day 21. An increase in this parameter was observed only in cyclic strain group at day 21. Data are expressed as mean ± standard deviation, *p < 0.05, **p < 0.01, and ***p < 0.001 (n = 4).
To assess the cardiac function, preliminary baseline echocardiographs (ECGs) were recorded for all the test groups and subsequently compared to ECGs collect at day 3 and 21. The two cardiac parameters obtained from the ECGs analysis were ejection fraction (EF%) and fractional shortening (FS%). The EF and FS are important systolic functional factors that are used to study the effect of AMI pathology on cardiac function. At day 3, no significant difference in cardiac function was found between the tested groups. However, on day 21 an increase in the two cardiac parameters was observed in the hASCs cyclic group (EF% 22.0 ± 2.6, FS% = 10.23 ± 1.28) with respect to the hASCs static group (EF% = 18.25 ± 1.12, FS% = 8.35 ± 0.39) (Figs. 3b and 3c).
On the contrary, no improvement in cardiac output (CO%) and stroke volume (SV) was observed at day 21 in the two groups (Fig. S6). These results confirmed a modest improvement in heart function post-AMI induced by the injection of pre-conditioned hASCs.
Aside from assessing cardiac function, it was also important to verify whether our strategy could have a beneficial effect in reversing cardiac remodeling, which involves cardiomyocyte necrosis and consequent scar area formation. Specifically, upon myocardial infarction, fibrous scar tissue replaces functional myocardium causing the thinning of the left ventricle (LV) wall. This change in myocardial tissue is irreversible and can ultimately lead to heart failure.
Histological analysis of heart sections performed on the different groups revealed the expected LV wall thinning due to myocardial infarction. A slight reduction in the infarct size area was observed in the hASC cyclic group (35.2 ± 1.9%) with respect to hASC static group (39.1 ± 9.2%). However, this difference was not statistically significant likely due to the limited number of animals tested (n = 3) (Figs. 4a and 4b).
Figure 4.
Evaluation of the in vivo pro-angiogenic properties and myocardial remodeling after treatment with pre-conditioned hASCs. Four different groups were tested as reported previously. (a) Masson’s trichrome stain cross-sections of mice hearts are indicating the scar formation in each group stained in blue. (b) Quantification of the percentage of myocardium replaced by infarcted tissue detected in each group. The infarct size was calculated based on at least three different hearts in each group (n = 3). (c) Representative micrographs of immunohistochemically stained sections of the peri-infarcted regions showing the presence of endothelial cells (vWF) in red, mural cells (SMA) green. Scale bar = 50 µm. (d) Quantification of blood vessels density obtained from a minimum of six fluorescent images per group. A higher blood vessels density was observed in the mice hearts that were treated with the mechanically pre-conditioned hASCs group (hASC cyclic) at day 21. Data are expressed as mean mean ± standard deviation, *p < 0.05, **p < 0.01, and ***p < 0.001 (n = 6).
Finally, an effective therapy for cardiac repair post-AMI should also promote neovascularization in the peri-infarct region aside from reducing the scar area formation. To evaluate the angiogenic potential of pre-conditioned hASCs, mice were sacrificed, and excised hearts were stained for vWF and SMA within the peri-infarct region (Fig. 4c) following established protocols.70 vWF staining is a useful strategy to detect the presence of endothelial cells which forms the inner lining of the lumen of blood vessels, while SMA protein is commonly found in vascular smooth muscle cells. A higher density of vWF and SMA positive blood vessels were detected in mice treated with hASC cyclic at day 21 (14.33 ± 1.60) in comparison to hASC static (10.66 ± 1.15) (Fig. 4d). This positive angiogenic response associated with improved cardiac function determined by the assessment of systolic functional parameters indicate the ability of our strategy to enhance the therapeutic efficacy of hASCs to treat myocardial infarction.
Conclusion
We have successfully designed a biomimetic system that closely mimics the native mechanical stimulation generated by CMs. The cyclic strain provided by the rhythmic beating allowed for the modulation of the genetic profile of hASCs. Specifically, we found an upregulation of mechano-transduction genes such as YAP1 and TAZ, as well as overexpression of cardiomyogenic markers as observed in the metatranscriptomic analysis. These findings are indicative of a direct correlation between this mechanical stimulus and cardiomyogenic differentiation of hASCs. Furthermore, the designed biomimetic system did not hinder the inherent ability of hASCs to secrete pro-angiogenic factors, which is beneficial for myocardial tissue repair. Finally, upon transplantation of the pre-conditioned hASCs into AMI mice model, we observed an improvement in cardiac function and increased angiogenesis in the peri-infarct region after 21 days. Overall, these data indicate that cyclic strain is an effective stimulant for hASCs cardiomyogenic differentiation, and represents a promising approach to generate more efficient stem-cell based therapies for cardiovascular repair.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (WMV 12,122 kb)
Supplementary material 3 (WMV 60,309 kb)
Supplementary material 4 (WMV 41,200 kb)
Acknowledgments
AP acknowledges an investigator grant provided by the Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (NIGMS) of the NIH Award Number P20GM103638 and Umbilical Cord Matrix Project fund from State of Kansas. RPHA acknowledges the support from National Institute of Health (NIH) Grant 1R01HL-10690. AC acknowledges support from AHA 16GRNT31030030 and NIH GM102801. Research reported in this publication was made possible by the services of Dr. Erik Lundquist and Ms. Jennifer Hackett at the KU Genome Sequencing Core. The authors also acknowledge the services provided by Dr. Stuart Macdonald and Ms. Boryana S Koseva at the KU-INBRE Bioinformatics Core. This core lab is supported by an Institutional Development Award (IDeA) from the NIGMS (P20GM103418) from the NIH. We also gratefully thank Ms. Heather Shinogle of the University of Kansas Microscopy and Analytical Imaging Laboratory for her assistance with confocal fluorescence microscopy. We further acknowledge Ms. Dona Gréta Isai from the University of Kansas Medical Center for her help in the non-invasive image analysis of cardiomyocytes contractility.
Conflict of interest
Aparna R. Chakravarti, Settimio Pacelli, Perwez Alam, Samik Bagchi, Saman Modaresi, Andras Czirok, Rafeeq P.H. Ahmed, and Arghya Paul declare no conflict of interests.
Ethical Standards
All animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23 revised 1985) and approved by IACUC. No human studies were carried out by the authors for this article. Only commercially obtained cells were used.
Footnotes
Arghya Paul, PhD, is an Assistant Professor at The University of Kansas (KU) in the Department of Chemical and Petroleum Engineering, and Bioengineering Graduate Program. He is also a member of The Center for Epigenetics and Stem Cell Biology, KU Medical Center. His Biointel Research Laboratory, funded by National Institute of General Medical Sciences and State of Kansas, focuses on developing new class of nano-bioactive hydrogels, biotransporters and engineered stem cells for cardiovascular and bone research. Broadly, his work aims to (1) innovate and study transformative technologies at the biomolecular and cellular level (2) exploit the cell-matrix interactions and mechanistic pathways, and (3) discover therapeutic strategies that can be translated to point-of-care patient applications. Before joining KU, Dr. Paul completed his postdoctoral fellowship at Harvard-MIT Division of Health Sciences and Technology and Wyss Institute for Biologically Inspired Engineering, working in the areas of nanomaterials, stem cells and regeneration therapy. He received his MS and PhD degrees in Biotechnology and Biomedical Engineering from McGill University, Canada, where his research was focused on developing gene-eluting vascular stents and microengineered stem cells for cardiovascular therapy. Dr. Paul has contributed to 75 + journal articles, 15 book chapters and holds multiple invention disclosures and patents. Based on his co-patented technologies, he is currently directing MangoGen Pharma Inc., a start-up company specialized in gene delivery technologies, as Chief Scientific Officer. His academic and research achievements have been acknowledged with multiple prestigious awards, fellowships and recognitions. To name a few: Fred Kurata Memorial Professorship at KU, Raymond Oenbring Award for Excellence in Teaching Chemical Engineering, Outstanding Young Scientist (talent category) by Macromolecular Chemistry Physics journal, Banting Postdoctoral Fellowship (Canada), Postdoctoral Training Fellowship (Le Fonds de Recherche du Quebec Nature et Technologies, FRQNT), Natural Sciences and Engineering Research Council of Canada (NSERC) Alexander Graham Bell Canada Graduate Scholarship, NSERC Michael Smith Foreign Study Supplement Award, McGill Medstar Award, Leslie A. Geddes Award for Best PhD Thesis.
This article is part of the 2018 CMBE Young Innovators special issue.
References
- 1.Aboalola D, Han VKM. Different effects of insulin-like growth factor-1 and insulin-like growth factor-2 on myogenic differentiation of human mesenchymal stem cells. Stem Cells Int. 2017 doi: 10.1155/2017/8286248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arif M, Pandey R, Alam P, Jiang S, Sadayappan S, Paul A, Ahmed RPH. MicroRNA-210-mediated proliferation, survival, and angiogenesis promote cardiac repair post myocardial infarction in rodents. J. Mol. Med. 2017;95:1369–1385. doi: 10.1007/s00109-017-1591-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augiere C, Megy S, El Malti R, Boland A, El Zein L, Verrier B, Mégarbané A, Deleuze J-F, Bouvagnet P. A novel alpha cardiac actin (ACTC1) mutation mapping to a domain in close contact with myosin heavy chain leads to a variety of congenital heart defects, arrhythmia and possibly midline defects. PLoS ONE. 2015;10:0127903. doi: 10.1371/journal.pone.0127903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beier JP, Bitto FF, Lange C, Klumpp D, Arkudas A, Bleiziffer O, Boos AM, Kneser U. Myogenic differentiation of mesenchymal stem cells co-cultured with primary myoblasts. Cell Biol. Int. 2011;35:397–406. doi: 10.1042/CBI20100417. [DOI] [PubMed] [Google Scholar]
- 5.Chao W, D’Amore PA. IGF2: epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev. 2008;19:111–120. doi: 10.1016/j.cytogfr.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen TH, Chen CY, Wen HC, Chang CC, Wang HD, Chuu CP, Chang CH. YAP promotes myogenic differentiation via the MEK5-ERK5 pathway. FASEB J.: Off. Publ. Fed. Soc. Exp. Biol. 2017;31:2963–2972. doi: 10.1096/fj.201601090R. [DOI] [PubMed] [Google Scholar]
- 7.Cho J, Zhai P, Maejima Y, Sadoshima J. Myocardial injection with GSK-3β-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circ. Res. 2011;108:478–489. doi: 10.1161/CIRCRESAHA.110.229658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai R, Wang Z, Samanipour R, Koo K-I, Kim K. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016;201:19. doi: 10.1155/2016/6737345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Angelo F, Tiribuzi R, Armentano I, Kenny JM, Martino S, Orlacchio A. Mechanotransduction: tuning stem cells fate. J. Funct. Biomater. 2011;2:67–87. doi: 10.3390/jfb2020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong A, Shen J, Zeng M, Campochiaro PA. Vascular cell-adhesion molecule-1 plays a central role in the proangiogenic effects of oxidative stress. Proc. Natl. Acad. Sci. 2011;108:14614–14619. doi: 10.1073/pnas.1012859108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 12.Epp TA, Dixon IM, Wang HY, Sole MJ, Liew CC. Structural organization of the human cardiac alpha-myosin heavy chain gene (MYH6) Genomics. 1993;18:505–509. doi: 10.1016/S0888-7543(11)80006-6. [DOI] [PubMed] [Google Scholar]
- 13.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitable D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase: Fischer: Pim-1 kinase enhances myocardial regeneration. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frangogiannis NG. Pathophysiology of myocardial infarction. Compr. Physiol. 2015;5:1841–1875. doi: 10.1002/cphy.c150006. [DOI] [PubMed] [Google Scholar]
- 15.Furukawa KT, Yamashita K, Sakurai N, Ohno S. The epithelial circumferential actin belt regulates YAP/TAZ through nucleocytoplasmic shuttling of merlin. Cell Rep. 2017;20(6):1435–1447. doi: 10.1016/j.celrep.2017.07.032. [DOI] [PubMed] [Google Scholar]
- 16.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB. Executive summary: heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 17.Gwak S-J, Bhang SH, Kim I-K, Kim S-S, Cho S-W, Jeon O. The effect of cyclic strain on embryonic stem cell-derived cardiomyocytes. Biomaterials. 2008;29:844–856. doi: 10.1016/j.biomaterials.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 18.Haider H, Lee YJ, Jiang S, Ahmed RP, Ryon M, Ashraf M. Phosphodiesterase inhibition with tadalafil provides longer and sustained protection of stem cells. Am. J. Physiol. 2010;299:H1395–H1404. doi: 10.1152/ajpheart.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heng BC, Haider HK, Sim EK-W, Cao T, Ng SC. Strategies for directing the differentiation of stem cells into the cardiomyogenic lineage in vitro. Cardiovasc. Res. 2004;62:34–42. doi: 10.1016/j.cardiores.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 20.Herberts CA, Kwa MSG, Hermsen HPH. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011;9:29. doi: 10.1186/1479-5876-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J. Thorac Cardiovasc. Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Zheng L, Gong X, Jia X, Song W, Liu M. Effect of cyclic strain on cardiomyogenic differentiation of rat bone marrow derived mesenchymal stem cells. PLoS ONE. 2012;7:e34960. doi: 10.1371/journal.pone.0034960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilkovski B, Clement S, Sewry C, North KN, Cooper ST. Defining alpha-skeletal and alpha-cardiac actin expression in human heart and skeletal muscle explains the absence of cardiac involvement in ACTA1 nemaline myopathy. Neuromuscular Disord. 2005;15:829–835. doi: 10.1016/j.nmd.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Kabaeva ZT, Perrot A, Wolter B, Dietz R, Cardim N, Correia JM, Schulte HD, Aldashev AA, Mirrakhimov MM, Osterziel KJ. Systematic analysis of the regulatory and essential myosin light chain genes: genetic variants and mutations in hypertrophic cardiomyopathy. Eur. J. Hum. Genet. 2002;10:741–748. doi: 10.1038/sj.ejhg.5200872. [DOI] [PubMed] [Google Scholar]
- 25.Kawai T, Takahashi T, Esaki M, Ushikoshi H, Nagano S, Fujiwara H. Efficient cardiomyogenic differentiation of embryonic stem cell by fibroblast growth factor 2 and bone morphogenetic protein 2. Circ. J. 2004;68:691–702. doi: 10.1253/circj.68.691. [DOI] [PubMed] [Google Scholar]
- 26.Kemp TJ, Sadusky TJ, Simon M, Brown R, Eastwood M, Sassoon DA, Coulton GR. Identification of a novel stretch-responsive skeletal muscle gene (Smpx) Genomics. 2001;72:260–271. doi: 10.1006/geno.2000.6461. [DOI] [PubMed] [Google Scholar]
- 27.Klotz BJ, Gawlitta D, Rosenberg AJ, Malda J, Melchels FP. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol. 2016;34:394–407. doi: 10.1016/j.tibtech.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kok LD, Tsui SK, Waye M, Liew CC, Lee CY, Fung KP. Cloning and characterization of a cDNA encoding a novel fibroblast growth factor preferentially expressed in human heart. Biochem. Biophys. Res. Commun. 1999;255:717–721. doi: 10.1006/bbrc.1999.0178. [DOI] [PubMed] [Google Scholar]
- 29.Kubalak SW, Miller-Hance WC, O’Brien TX, Dyson E, Chien KR. Chamber specification of atrial myosin light chain-2 expression precedes septation during murine cardiogenesis. J. Biol. Chem. 1994;269:16961–16970. [PubMed] [Google Scholar]
- 30.Lanfear DE. Genetic variation in the natriuretic peptide system and heart failure. Heart Failure Rev. 2010;15:219–228. doi: 10.1007/s10741-008-9113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li D, Niu Z, Yu W, Qian Y, Wang Q, Li Q, Yi Z, Luo J, Wu X, Wang Y, Schwartz RJ, Liu M. SMYD1, the myogenic activator, is a direct target of serum response factor and myogenin. Nucleic Acids Res. 2009;37:7059–7071. doi: 10.1093/nar/gkp773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, W., N. Ma, L.-L. Ong, C. Nesselmann, C. Klopsch, Y. Ladilov, D. Furlani, C. Piechaczek, J. M. Moebius, K. Lützow, A. Lendlein, C. Stamm, R. K. Li, G. Steinhoff. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem cells (Dayton, OH, U.S.), 25:2118-27, 2007. [DOI] [PubMed]
- 33.Li H, Zuo S, He Z, Yang Y, Pasha Z, Wang Y, Xu M. Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am. J. Physiol. 2010;299:H1772–H1781. doi: 10.1152/ajpheart.00557.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Low BC, Pan CQ, Shivashankar GV, Bershadsky A, Sudol M, Sheetz M. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588:2663–2670. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto R, Omura T, Yoshiyama M, Hayashi T, Inamoto S, Koh KR, Ohta K, Izumi Y, Nakamura Y, Akioka K, Takeuchi K, Yoshikawa J. Vascular endothelial growth factor-expressing mesenchymal stem cell transplantation for the treatment of acute myocardial infarction. Arterioscler. Thromb. Vasc. Biol. 2005;25:1168–1173. doi: 10.1161/01.ATV.0000165696.25680.ce. [DOI] [PubMed] [Google Scholar]
- 36.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nat. Clin. Pract. Cardiovasc. Med. Suppl. 2007;1:S21–S26. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 37.Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J. Mol. Cell. Cardiol. 2011;50:280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohri Z, Hernandez ADR, Krams R. The emerging role of YAP/TAZ in mechanotransduction. J. Thorac. Dis. 2017;9:E507–E509. doi: 10.21037/jtd.2017.03.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohsin S, Siddiqi S, Collins B, Sussman MA. Empowering adult stem cells for myocardial regeneration. Circ. Res. 2011;109:1415–1428. doi: 10.1161/CIRCRESAHA.111.243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moulik M, Vatta M, Witt SH, Arola AM, Murphy RT, McKenna WJ, Boriek AM, Oka K, Labeit S, Bowles NE, Arimura T, Kimura A, Towbin JA. ANKRD1, the gene encoding cardiac ankyrin repeat protein, is a novel dilated cardiomyopathy gene. J. Am. Coll. Cardiol. 2009;54:325–333. doi: 10.1016/j.jacc.2009.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagao K, Sowa N, Inoue K, Tokunaga M, Fukuchi K, Uchiyama K, Ito H, Hayashi F, Makita T, Inada T, Tanaka M, Kimura T, Ono K. Myocardial expression level of neural cell adhesion molecule correlates with reduced left ventricular function in human cardiomyopathy. Circ. Heart Failure. 2014;7:351–358. doi: 10.1161/CIRCHEARTFAILURE.113.000939. [DOI] [PubMed] [Google Scholar]
- 42.Orr N, Arnaout R, Gula LJ, Spears DA, Leong-Sit P, Li Q, Tarhuni W, Reischauer S, Chauhan VS, Borkovich M, Uppal S, Adler A, Coughlin SR, Stainier DY, Gollob MH. A mutation in the atrial-specific myosin light chain gene (MYL4) causes familial atrial fibrillation. Nat. Commun. 2016;7:11303. doi: 10.1038/ncomms11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ou L, Li W, Zhang Y, Wang W, Liu J, Sorg H, Furlani D, Gäbel R, Mark P, Klopsch C, Wang L, Lützow K, Lendlein A, Wagner K, Klee D, Liebold A, Li RK, Kong D, Steinhoff G, Ma N. Intracardiac injection of matrigel induces stem cell recruitment and improves cardiac functions in a rat myocardial infarction model. J. Cell. Mol. Med. 2011;15:1310–1318. doi: 10.1111/j.1582-4934.2010.01086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacelli S, Maloney R, Chakravarti AR, Whitlow J, Basu S, Modaresi S, Gehrke S, Paul A. Controlling adult stem cell behavior using nanodiamond-reinforced hydrogel: implication in bone regeneration therapy. Sci. Rep. 2017;7:6577. doi: 10.1038/s41598-017-06028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pacelli S, Paolicelli P, Dreesen I, Kobayashi S, Vitalone A, Casadei MA. Injectable and photocross-linkable gels based on gellan gum methacrylate: a new tool for biomedical application. Int. J. Biol. Macromol. 2015;72:1335–1342. doi: 10.1016/j.ijbiomac.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 46.Panciera T, Azzolin L, Cordenonsi M, Piccolo S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017;18:758. doi: 10.1038/nrm.2017.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandey R, Velasquez S, Durrani S, Jiang M, Neiman M, Crocker JS, Benoit JB, Rubinstein J, Paul A, Rafeeq A. MicroRNA-1825 induces proliferation of adult cardiomyocytes and promotes cardiac regeneration post ischemic injury. Am. J. Transl. Res. 2017;9:3120–3137. [PMC free article] [PubMed] [Google Scholar]
- 48.Pankajakshan D, Agrawal DK. Mesenchymal stem cell paracrine factors in vascular repair and regeneration. J. Biomed. Technol. Res. 2014 doi: 10.19104/jbtr.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pankajakshan D, Agrawal DK. Mesenchymal stem cell paracrine factors in vascular repair and regeneration. J. Biomed. Technol. Res. 2014 doi: 10.19104/jbtr.2014.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paul A, Binsalamah ZM, Khan AA, Abbasia S, Elias CB, Shum-Tim D, Prakash S. A nanobiohybrid complex of recombinant baculovirus and Tat/DNA nanoparticles for delivery of Ang-1 transgene in myocardial infarction therapy. Biomaterials. 2011;32:8304–8318. doi: 10.1016/j.biomaterials.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 51.Paul A, Hasan A, Kindi HA, Gaharwar AK, Rao VTS, Nikkhah M, Shin SR, Krafft D, Dokmeci MR, Shum-Tim D, Khademhosseini A. Injectable graphene oxide/hydrogel-based angiogenic gene delivery system for vasculogenesis and cardiac repair. ACS Nano. 2014;8:8050–8062. doi: 10.1021/nn5020787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paul A, Nayan M, Khan AA, Shum-Tim D, Prakash S. Angiopoietin-1-expressing adipose stem cells genetically modified with baculovirus nanocomplex: investigation in rat heart with acute infarction. Int. J. Nanomed. 2012;7:663–682. doi: 10.2217/nnm.11.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paul A, Srivastava S, Chen G, Shum-Tim D, Prakash S. Functional assessment of adipose stem cells for xenotransplantation using myocardial infarction immunocompetent models: comparison with bone marrow stem cells. Cell Biochem. Biophys. 2013;67:263–273. doi: 10.1007/s12013-011-9323-0. [DOI] [PubMed] [Google Scholar]
- 54.Pinto JR, Parvatiyar MS, Jones MA, Liang J, Ackerman MJ, Potter JD. A functional and structural study of troponin C mutations related to hypertrophic cardiomyopathy. J. Biol. Chem. 2009;284:19090–19100. doi: 10.1074/jbc.M109.007021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pons J, Huang Y, Arakawa-Hoyt J, Washko D, Takagawa J, Ye J, Grossman W, Hua S. VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem. Biophys. Res. Commun. 2008;376:419–422. doi: 10.1016/j.bbrc.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Rajasingh S, Thangavel J, Czirok A, Samanta S, Roby KF, Dawn B, Rajasingh J. Generation of functional cardiomyocytes from efficiently generated human iPSCs and a novel method of measuring contractility. PLoS ONE. 2015;10:0134093. doi: 10.1371/journal.pone.0134093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosova I, Dao M, Capoccia B, Link D, Nolta JA. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmelter M, Ateghang B, Helmig S, Wartenberg M, Sauer H. Embryonic stem cells utilize reactive oxygen species as transducers of mechanical strain-induced cardiovascular differentiation. FASEB J. 2006;20:1182–1184. doi: 10.1096/fj.05-4723fje. [DOI] [PubMed] [Google Scholar]
- 59.Sun L, Cui M, Wang Z, Feng X, Mao J, Chen P, Kangtao M, Chen F, Zhou C. Mesenchymal stem cells modified with angiopoietin-1 improve remodeling in a rat model of acute myocardial infarction. Biochem. Biophys. Res. Commun. 2007;357:779–784. doi: 10.1016/j.bbrc.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 60.Sun Q, Zhang Z, Sun Z. The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes Dis. 2014;1:113–119. doi: 10.1016/j.gendis.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang J-M, Wang J-N, Zhang L, Zheng F, Yang J-Y, Kong X, Guo LY, Chen L, Huang YZ, Wan Y, Chen SY. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc. Res. 2011;91:402–411. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thakker R, Yang P. Mesenchymal stem cell therapy for cardiac repair. Curr. Treat Options Cardiovasc. Med. 2014;16:323. doi: 10.1007/s11936-014-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tiso N, Majetti M, Stanchi F, Rampazzo A, Zimbello R, Nava A, Danieli GA. Fine mapping and genomic structure of ACTN2, the human gene coding for the sarcomeric isoform of alpha-actinin-2, expressed in skeletal and cardiac muscle. Biochem. Biophys. Res. Commun. 1999;265:256–259. doi: 10.1006/bbrc.1999.1661. [DOI] [PubMed] [Google Scholar]
- 64.Townsend PJ, Farza H, MacGeoch C, Spurr NK, Wade R, Gahlmann R, Yacoub MH, Barton PJ. Human cardiac troponin T: identification of fetal isoforms and assignment of the TNNT2 locus to chromosome 1q. Genomics. 1994;21:311–316. doi: 10.1006/geno.1994.1271. [DOI] [PubMed] [Google Scholar]
- 65.Trapnell, C., B. A. Williams, G. Pertea, A. Mortazavi, G. Kwan, M. J. van Baren, S. L. Salzberg, B. J. World, L. Pachter Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Turbay D, Wechsler SB, Blanchard KM, Izumo S. Molecular cloning, chromosomal mapping, and characterization of the human cardiac-specific homeobox gene hCsx. Mol. Med. 1996;2:86–96. [PMC free article] [PubMed] [Google Scholar]
- 67.Van Den Bulcke AI, Bogdanov B, De Rooze N, Schacht EH, Cornelissen M, Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1:31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 68.Villard E, Duboscq-Bidot L, Charron P, Benaiche A, Conraads V, Sylvius N, Komajda M. Mutation screening in dilated cardiomyopathy: prominent role of the beta myosin heavy chain gene. Eur Heart J. 2005;26:794–803. doi: 10.1093/eurheartj/ehi193. [DOI] [PubMed] [Google Scholar]
- 69.Wade R, Eddy R, Shows TB, Kedes L. cDNA sequence, tissue-specific expression, and chromosomal mapping of the human slow-twitch skeletal muscle isoform of troponin I. Genomics. 1990;7:346–357. doi: 10.1016/0888-7543(90)90168-T. [DOI] [PubMed] [Google Scholar]
- 70.Waters R, Alam P, Pacelli S, Chakravarti AR, Ahmed RPH, Paul A. Stem cell-inspired secretome-rich injectable hydrogel to repair injured cardiac tissue. Acta Biomater. 2017 doi: 10.1016/j.actbio.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waters R, Pacelli S, Maloney R, Medhi I, Ahmed RPH, Paul A. Stem cell secretome-rich nanoclay hydrogel: a dual action therapy for cardiovascular regeneration. Nanoscale. 2016;8:7371–7376. doi: 10.1039/C5NR07806G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wickham H. ggplot2. Wiley Interdiscip. Rev. 2011;3:180–185. doi: 10.1002/wics.147. [DOI] [Google Scholar]
- 73.Xia X, Zhang S-C. Genetic Modification of human embryonic stem cells. Biotechnol. Genet. Eng. Rev. 2007;24:297–309. doi: 10.1080/02648725.2007.10648105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Youssef A, Aboalola D, Han VKM. The roles of insulin-like growth factors in mesenchymal stem cell niche. Stem Cells Int. 2017;201:9453108. doi: 10.1155/2017/9453108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yue K, de Santiago GT, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zannad F, Agrinier N, Alla F. Heart failure burden and therapy. Europace. 2009;11(Suppl 5):v1–v9. doi: 10.1093/europace/eup304. [DOI] [PubMed] [Google Scholar]
- 77.Zeng J, Wang Y, Wei Y, Xie A, Lou Y, Zhang M. Co-culture with cardiomyocytes induces mesenchymal stem cells to differentiate into cardiomyocyte-like cells and express heart development-associated genes. Cell Res. 2008;18:S62. doi: 10.1038/cr.2008.152. [DOI] [Google Scholar]
- 78.Zhao L, Johnson T, Liu D. Therapeutic angiogenesis of adipose-derived stem cells for ischemic diseases. Stem Cell Res. Ther. 2017;8:125. doi: 10.1186/s13287-017-0578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 2 (WMV 12,122 kb)
Supplementary material 3 (WMV 60,309 kb)
Supplementary material 4 (WMV 41,200 kb)