Two effective treatments for opioid addiction, methadone and buprenorphine-naloxone maintenance, should be widely implemented.
Abstract
Opioid use disorders (OUDs) are diseases of the brain with behavioral, psychological, neurobiological, and medical manifestations. Vulnerability to OUDs can be affected by factors such as genetic background, environment, stress, and prolonged exposure to μ-opioid agonists for analgesia. Two standard-of-care maintenance medications, methadone and buprenorphine-naloxone, have a long-term positive influence on health of persons with opioid addiction. Buprenorphine and another medication, naltrexone, have also been approved for administration as monthly depot injections. However, neither medication is used as widely as needed, due largely to stigma, insufficient medical education or training, inadequate resources, and inadequate access to treatment. Ongoing directions in the field include (i) personalized approaches leveraging genetic factors for prediction of OUD vulnerability and prognosis, or for targeted pharmacotherapy, and (ii) development of novel analgesic medicines with new neurobiological targets with reduced abuse potential, reduced toxicity, and improved effectiveness, especially for chronic pain states other than cancer pain.
ADDICTION TO μ-OPIOID AGONISTS SUCH AS HEROIN, PRESCRIPTION OPIOIDS, AND FENTANYL ANALOGS
Opioid use disorders (OUDs), including their most severe form (opioid addiction), are a major public health challenge, in both industrialized and developing countries (1, 2). Acute mortality from OUDs is related primarily to respiratory depression, modulated by μ-receptors in brainstem nuclei (3, 4). Opioid-induced mortality is also often observed in persons who are exposed to multiple other substances, including alcohol, cocaine, and benzodiazepines (5). There are other major sources of morbidity and comorbidity in OUDs, and these include increased prevalence of infectious diseases (e.g., HIV/AIDS and hepatitis C) (6).
Smoking and ingestion of dried opium wax isolated from the poppy bulbs of Papaver somniferum have been occurring for millennia, for the purposes of medical pain relief and of achieving altered states of consciousness (7). Physical dependence following daily chronic use of opium, defined in the context of withdrawal symptoms upon abstinence, has been historically documented as early as the 16th century (6). Two discoveries in the 19th century were crucial for the improvement of analgesic therapeutics as well as leading to devastating consequences in terms of opioid addiction and potential for fatal overdose as a consequence of respiratory depression: the extraction of morphine (Fig. 1A) as the primary active ingredient of opium and the development of the hypodermic needle for intravenous administration (7). Heroin (Fig. 1B) was later developed as a semisynthetic derivative of morphine, involving diacetylation of the hydroxyl groups that leads to a 10-fold increase in potency in vivo, due to enhanced delivery to the brain (8). Heroin itself has limited affinity for μ-opioid receptors, but following distribution to the brain undergoes biotransformation, initially yielding primarily 6-acetyl morphine and then morphine as active metabolites (Fig. 2A).
Fig. 1. Chemical structures of the most commonly abused opioids.
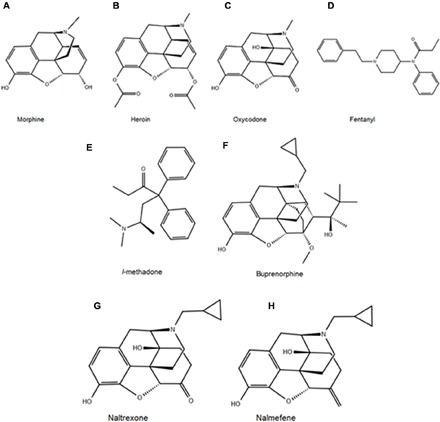
Structures of (A) morphine, (B) heroin, (C) oxycodone, and (D) fentanyl and of the opioidergic therapeutics methadone (E) and buprenorphine (F). In addition, shown are antagonists naltrexone (G) and nalmefene (H). The structurally similar morphinan derivatives (A, B, C, F, G, and H) derived from opium, or synthesized from thebaine obtained from opium, contrast sharply with the structures of the synthetic opioids methadone and fentanyl (E and D, respectively).
Fig. 2. Heroin addiction contrasted with methadone maintenance.
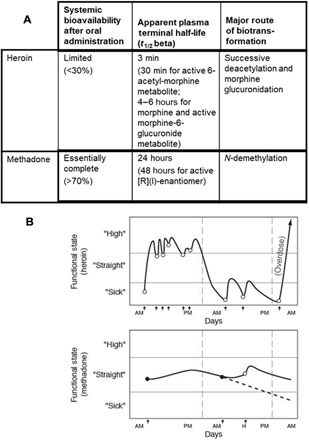
(A) Difference in plasma protein binding and metabolism results in substantially different pharmacokinetic profiles and bioavailability for heroin versus methadone (55). (B) Prototypic administration pattern and subjective state for heroin versus methadone. Multiple doses of heroin are self-administered daily to achieve a state of “high” (euphoria) or, in cases with a depleted supply, to avoid a feeling of “sick” (withdrawal). Methadone, at steady state with single daily administration, leads neither to subjective states of high nor sick (43).
Myriad derivatives of morphine have been synthesized in the quest for improved analgesics, beginning in the 19th century and continuing to this day. A prominent example of a drug developed as an improved analgesic is oxycodone (Fig. 1C). Oxycodone is synthesized from opium extracts of thebaine as a starting point (9). As can be seen from Fig. 1, oxycodone is structurally similar to morphine, sharing a common backbone structure, referred to as a morphinan. Although oxycodone has been in clinical use since shortly following its initial synthesis and characterization in the 1910s, its use increased vastly with the development of a patented 12-hour extended-release formulation, introduced in 1996, and enhanced marketing and prescriptions for pain therapy (10). Other common morphinans that are medically approved analgesics but are commonly misused include morphine (Fig. 1A), hydrocodone, and oxymorphone (11).
Synthetic compounds with considerable structural deviation from the classical morphinans also have μ-opioid agonist effects. Fentanyl (Fig. 1D), for instance, is a selective μ-opioid receptor agonist with high in vivo potency. Fentanyl was approved by the Food and Drug Administration (FDA) in 1968 and is available for perioperative intravenous or epidural/intrathecal administration, as well as in various other formulations for take-home use, including transdermal patches, buccal film, buccal spray, buccal tablet, nasal spray, and lozenges.
Various fentanyl analogs have also been approved for use in specific situations. Remifentanil was approved by the FDA in 1996 and is used in intravenous formulation during anesthesia. Sufentanil, approved initially in 1984, is similarly used perioperatively in conjunction with anesthesia and is also used epidurally for pain relief during labor and delivery. Very recently, a new sublingual tablet formulation of sufentanil was approved by the FDA for the treatment of acute pain, in medically supervised health care settings. Alfentanil was also approved for use in 1986 for perioperative intravenous administration in conjunction with anesthetics. Another analog, carfentanil, is used in veterinary medicine for large mammals and also as a positron emission tomography (PET) radiotracer (12). The potency of carfentanil makes it particularly dangerous, with a high potential for overdose, when abused by humans.
In recent years, there has been an increasing problem with production of fentanyl and its analogs in different countries including Mexico and China (13) and with these products entering the United States across borders and by mail (14–17). Fentanyl and its analogs have thus become “street” drugs, occasionally sold illicitly alone, and, more commonly, as additives to heroin (16) to increase the perceived potency of the latter. These synthetic compounds have been a major cause of recent increases in overdose deaths (see Fig. 3) (18).
Fig. 3. Opioid overdose deaths in the United States, 1999–2017.
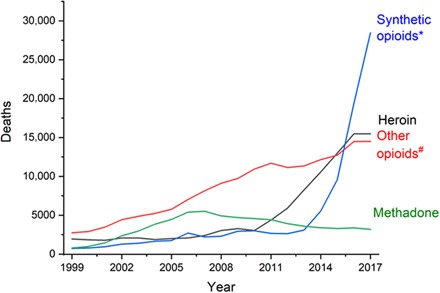
Data from Centers for Disease Control and Prevention, 2018 Annual Surveillance Report of Drug-Related Risks and Outcomes. Asterisk indicates synthetic opioids other than methadone, e.g., prescribed or illicit fentanyl, fentanyl analogs, and tramadol. Number sign indicates natural or semisynthetic opioids other than heroin, e.g., morphine, oxycodone, and hydrocodone.
The impact of excessive availability of prescription opioids
Medical and nonmedical use of prescription opioids, such as oxycodone, have been increasing markedly, especially in the United States (19), either in the patient to whom the medication was originally prescribed or frequently by someone else taking the unused medicine. This increase has occurred in part as a result of the World Health Organization’s reduced oversight of international sales and movement of opiates, beginning in the late 1980s. Further exacerbating this problem, recent changes in U.S. medical practice have encouraged physicians to prescribe “as much medication as any patient needs for relief of their pain” (a concept foreign to U.S. medical education until the mid-1990s), accompanied by extensive promotion and marketing of some opioid formulations during the same period. These factors have led to a marked change in physician prescription habits, from earlier prescriptions of 3 to 7 days of opioids for acute pain (such as surgical procedures and fractures) to current averages of pain medications up to 3 weeks or even longer (20). This increase has created a large excess of prescription opioids available for misuse, which can then progress to OUDs and use of illicit drugs such as heroin (21, 22).
EPIDEMIOLOGY
The most recent data from the federal government, primarily from the Substance Abuse and Mental Health Services Administration, the National Institute on Drug Abuse (NIDA), and the National Household Survey on Drug Abuse and Health, as well as Monitoring the Future, show that over 16 million people in the United States suffer from some addictive disease (see Table 1). The most common addiction is alcoholism, followed by addiction to cannabis, opioids, and cocaine.
Table 1. Epidemiology of drug use.
Prevalence of specific drug abuse and vulnerability to develop addictions. SAMHSA National Survey on Drug Use and Health, 2017; others 2007–2018.
| National household survey and related surveys (2007–2016) | |
| Heroin use—ever | ~5.2 million |
| Heroin addiction | ~652,000 |
| Illicit use of opiate medication—ever | ~37.1 million (i.e., 14.2% of the population 12 and over) |
| Dependence on such medication use | ~2.1 million |
| Opiate (heroin, fentanyl, and other) overdose deaths | ~72,3000 (in 2017)* |
| Cocaine use—ever | ~40.5 million |
| Cocaine addiction | ~966,000 |
| Alcohol use—ever | ~216 million |
| Alcoholism | ~14.5 million |
| Marijuana use—ever | ~123 million |
| Marijuana daily use | ~4 million |
| Development of addiction after self-exposure | |
| Opiate addiction | ~1 in 5 to 1 in 15 (20 to 6.5%) |
| Alcoholism, marijuana, and cocaine dependency | ~1 in 8 to 1 in 15 (12.5 to 6.5%) |
*National Center for Health Statistics (U.S. Centers for Disease Control and Prevention), 2019.
At least 1 million to 2 million persons in the United States suffer from addiction to heroin and other short-acting opioids (Table 1). It is estimated that over 37 million persons have misused short-acting opioids such as oxycodone and hydrocodone. The number of persons who have become addicted to these compounds has only been roughly calculated. Some epidemiological data show that approximately 20% of persons who self-administer a prescription opioid for nonmedical use will develop an OUD (23). In the past two decades, increasing numbers of people who started with misuse of prescription opioids (e.g., oxycodone) then commence the use of heroin because it is cheaper than illicit sales of these prescription opioids (24). In the past 5 years, the number of overdose deaths in the United States has risen to approximately 50,000/year. For example, in New York City alone, it has recently been estimated that there are approximately four overdose deaths each day (25). There have been substantial increases in opioid-induced overdose deaths in recent years, especially those involving heroin and fentanyl (18, 26). This statistic includes a widening “gender gap,” in which overdose vulnerability in males is increasing more than in females (18).
PUBLIC HEALTH NEED FOR INCREASE IN AVAILABILITY OF MEDICATION-BASED TREATMENT OF OPIOID ADDICTION
Because of the major stigma of drug abuse, there has been an almost complete absence in most medical schools of education about opioid addiction, its diagnosis, treatment of overdose, and chronic pharmacotherapy (27, 28). More broadly, most medical schools have only limited education about any other addictive disease as well.
The number of persons in methadone maintenance treatment programs (MMTPs) in the United States is approximately 382,000, while the number of persons in buprenorphine-naloxone treatment is approximately 112,000 (see Table 2). In the entire world, the number of people in MMTP is currently roughly 1.4 million (see table S1). For the less effective treatment with naltrexone (either as a daily oral medication or in depot injection formulation) (29), 23,000 persons are currently in treatment in the United States. Many of these persons entered naltrexone treatment due to the criminal justice system or due to regulations on physicians that exist in some (but not all) states.
Table 2. Status of methadone, buprenorphine, and extended-release naltrexone treatments for opioid addiction in the United States: Decrease and then increase in numbers in treatment 2015–2017 (SAMHSA, 2018).
| Treatment | U.S. patients in treatment | ||
| 2015 | 2016 | 2017 | |
|
Methadone maintenance |
356,843 | 345,443 (−11,400; −3.2%) |
382,867 (+37,424; +10.8%) |
|
Buprenorphine maintenance |
75,723 | 61,486 (−14,237; −18.8%) |
112,223 (+50,737; +82.5%) |
|
Extended-release naltrexone |
7035 | 10,128 (+3093; +44.0%) |
23,065 (+12,937; +128.7%) |
Studies have shown that fewer than 10% of persons with opioid addiction are able to achieve long-term abstinence without medication-assisted treatment with methadone or buprenorphine maintenance (30). No behavioral or cognitive treatments alone have been shown to be effective for patients with opioid addiction (or severe OUD).
It is disturbing that the number of persons in medication treatment overall remains very low, given the numbers afflicted with opioid addiction. However, in 2017, there appears to have been a modest increase in numbers of persons in both MMTP and buprenorphine-naloxone treatment, following a decrease in 2016 (see Table 2).
The major known factor contributing to the effectiveness of medication-assisted treatment is compliance in taking the medication daily. Compliance is not an issue with methadone maintenance treatment because federal regulations mandate that patients in treatment visit the clinic initially daily to receive their methadone dose, which can be reduced to weekly or monthly visits when a patient has been in successful long-term treatment (medical maintenance). That said, the strict federal regulations surrounding methadone maintenance have had the consequence of limiting the number of clinics and therefore reduce the availability of effective treatment to those in need. Buprenorphine must also be used daily but, under federal law, can be prescribed for up to 30 daily doses at a time, with the patients responsible for self-administering their daily dose.
Research over the past 50 years shows that the most critical need in the treatment of opioid addiction is the continued and expanded availability of treatment with a long-acting steady-state medication (μ-opioid receptor agonist or partial agonist). Research has documented that a relative “endorphin deficiency” develops in persons with long-term opioid addiction (31). Therefore, treatment with methadone or buprenorphine maintenance can be considered a long-term “replacement” therapy similar to thyroxin treatment for thyroid deficiency or insulin use for diabetes.
Criteria for OUD diagnosis
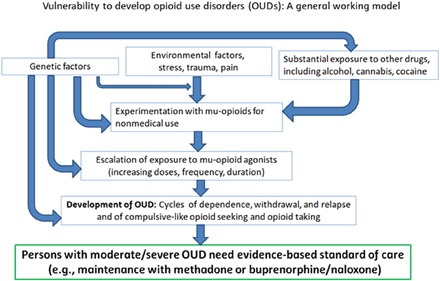
OUD is currently defined by the DSM-5 (fifth edition of the Diagnostic and Statistical Manual; www.DSM5.org), based on the number of clinical criteria that are met (32). Increasing numbers of criteria met can be used to qualify the diagnosis as mild, moderate, or severe. These criteria focus primarily on escalating self-exposure, tolerance, physical dependence, withdrawal, loss of control over intake, and proneness to relapse unless managed with chronic medication and related treatment. Several μ-receptor populations in brain, alone or in combination with other receptor systems, may mediate these different effects clinically and in translationally relevant models. Overall, the etiology of OUDs is multifactorial, and different types of mechanisms can contribute to vulnerability (see Fig. 4 and fig. S1).
Fig. 4. Model of the progression from misuse of opioids toward moderate or severe OUD (i.e., opioid addiction).
Development of physical dependence and withdrawal
After repeated exposure to μ-agonists, either in the context of medical prescription for analgesia or self-administration for nonmedical uses, a state of dependence develops. Withdrawal signs observed upon drug discontinuation include autonomic signs (e.g., piloerection, diarrhea, and changes in thermoregulation); sensory changes, including hyperalgesia; subjective anxiety-like effects; and neuroendocrine effects [e.g., increases in circulating levels of stress/hypothalamic-pituitary-adrenal axis hormones, adrenocorticotropic hormone (ACTH), and cortisol] (33). These diverse signs of withdrawal can be mediated by different neurobiological systems. Studies show that withdrawal can contribute to increased self-administration of μ-agonists (34), after the initial chronic exposure period.
The molecular and physiological underpinnings of μ-agonist dependence and withdrawal have been examined for decades. While several medications can be used to medically manage the severity of withdrawal (including the α2-adrenergic agonists clonidine or lofexidine) (35), the impact of the cycles of self-administration and withdrawal in OUDs remains a challenge and contributes to the continuation of the disease process. Some findings suggest that changes in μ-receptor signal transduction, as well as receptor cycling and internalization, occur after repeated exposure to μ-agonists (36). However, it is also clear that some withdrawal mechanisms develop on the basis of changes to neurobiological networks, which are downstream from μ-receptors (37, 38). Withdrawal signs (and other interoceptive signs) can function as triggers to drug-taking and changes in reward function (34, 39). The process of escalation of μ-agonist self-administration has also been examined in preclinical models (40).
Basic function of μ-opioid receptors
μ-opioid receptors are Gi/Go-coupled receptors [G protein (heterotrimeric GTP-binding protein)–coupled receptor], encoded by the gene OPRM1 (41), and their main endogenous ligands are β-endorphin and enkephalin-derived neuropeptides (encoded by POMC and PENK, respectively) (42, 43). μ-receptors are located in several areas of the central nervous system (CNS) and also the gastrointestinal tract, where they can modulate diverse biobehavioral functions including reward, mood, anxiety, neuroendocrine function, and also gastrointestinal motility (44). μ-receptor systems also interact with other major neurobiological systems, such as dopaminergic, glutamatergic, and neuropeptide systems, including the κ-opioid receptor/dynorphin system (encoded by OPRK1 and PDYN, respectively).
Molecular changes in brain after repeated exposure to short-acting μ-opioid agonists
Several studies have shown that repeated exposure to μ-agonists such as morphine, heroin, or oxycodone can cause changes to mRNA expression of numerous targets, including prodynorphin and κ-receptor genes (Pdyn and Oprk1, respectively) (45, 46). Using an mRNA array, it was found that several genes encoding neurotransmitter receptors (especially the γ-aminobutyric acid type A receptor β2 subunit; Gabrb2) were altered in the striatum after chronic oxycodone self-administration in adult mice (47). Other studies also show that molecular adaptations in the striatum and hippocampus differ between adult and adolescent mice, after chronic oxycodone self-administration (48, 49). For example, expression of some genes, such as monoamine oxidase a (Maoa), was up-regulated in the dorsal striatum of both adult and adolescent mice, after chronic oxycodone self-administration. However, other striatal genes, especially gastrin-releasing peptide receptor (Grpr), were differentially regulated after chronic oxycodone self-administration in adults and adolescents (48, 49).
Our laboratory has also recently reported RNA sequencing (RNA-seq) studies in the dorsal and ventral striatum (i.e., caudate-putamen and nucleus accumbens, respectively), which allowed an unbiased analysis of all targets affected after 14-day chronic oxycodone self-administration in adult mice (50–52). Focusing on neurotransmitter and neuropeptide systems, RNA-seq studies demonstrate that chronic oxycodone self-administration caused a change in pro-opiomelanocortin (Pomc), 5HT2a and 5HT7 receptors, galanin receptor, and glycine receptor. RNA-seq also shows that chronic oxycodone self-administration causes up-regulation of 54 and 126 genes involved in neuroinflammation/immunomodulation in the dorsal and ventral striatum, respectively (50). In addition, genes involved in axon guidance, in the integrin, semaphorin, and ephrin systems, were differentially altered in both the dorsal and ventral striatum, after chronic oxycodone self-administration (51). These RNA-seq data describe the complex gene regulation that occurs in the brain of subjects, which self-administered oxycodone over a relatively prolonged period, and indicates some of the brain processes that could be affected in persons with severe OUD.
Furthermore, some of the aforementioned molecular changes can persist or even emerge well after exposure to the μ-agonist is discontinued (46). The aforementioned studies show that repeated μ-agonist exposure results in complex and potentially long-lasting neuroadaptations that could underlie different aspects of opioid addiction and its relapsing features. Interventions on some of these molecular targets may be fruitful avenues for the development of mechanism-based prevention of opioid addiction or to minimize neural remodeling that may occur after iatrogenic exposure to μ-agonists.
CURRENT TREATMENT FOR OPIOID ADDICTION
μ-opioid agonist and partial agonist medications
Methadone
Research on developing a treatment for opiate addiction came to fruition at the Hospital of the Rockefeller Institute for Medical Research in 1964 by Dole et al. (53). The treatment developed was methadone maintenance treatment, approved by the FDA in 1972, which remains the most widely used effective therapeutic approach for opioid addiction (Table 3) (54–56).
Table 3. FDA-approved medications for OUD, with typical dosing paradigms for each of the approved formulations.
PO, per os (oral); SL, subligual; BUC, buccal; SQ, subcutaneous; IM, intramuscular.
| Treatment | Dose range | Considerations |
| Methadone (PO) | 80–150 mg/day (typical range) |
Maintenance dosing is determined during the early weeks of treatment following upward titration. Individual genetic and drug history differences may lead to requirement of higher doses than the typical range. FDA approved in 1972. |
| Buprenorphine- naloxone (SL or BUC) |
8–24 mg/day buprenorphine (1–6 mg/day naltrexone) (typical range) |
4:1 ratio (w/w) of buprenorphine- naloxone. Because of partial agonist nature of buprenorphine, no further treatment effect to be gained by doses greater than 24 mg/day. FDA approved in 2002. |
| Buprenorphine extended-release formulation (SQ) |
80–300 mg/monthly injection |
Two formulations available. FDA approved in 2016 and 2017. |
| Naltrexone tablets (PO)/extended- release formulation (IM) |
50 or 100 mg/day orally; 380 mg/ monthly IM injection |
Requires a patient to be opioid free for 7–10 days before administration. FDA approved in 1984 (tablets, no longer marketed); 2010 (extended release). |
In good-quality MMTPs, which provide adequate counseling, medical, and psychiatric care (which pertains in most local and national legislations and rules), 60 to 80% of persons can respond well, stay voluntarily in treatment for more than 1 year, and progressively decrease the use of illicit opioids over the first 3 to 6 months (57). However, approximately 20 to 40% of persons may drop out of treatment. In individuals receiving chronic oral methadone, intravenous or parenteral methadone does not cause euphoria (or high) because it rapidly binds to plasma proteins (53).
Methadone maintenance has greater retention than buprenorphine maintenance (see below), probably because the former is a full agonist at the μ-opioid receptor and also has modest N-methyl-d-aspartate receptor antagonist activity, which may further retard the development of tolerance (58, 59). Methadone needs to be used in moderate to high doses, usually 80 to 150 mg/day, to create sufficient cross-tolerance to “blockade” the euphoric effects by superimposed short-acting μ-agonists.
Methadone, when administered orally, has a slow onset and offset of action. When used to treat opioid addiction, moderate doses of methadone should be used initially (30 to 40 mg/day) and slowly increased, usually at the rate of 10 to 20 mg/week up to a daily dose that provides cross-tolerance to the effects of any superimposed short-acting μ-agonist, i.e., “narcotic blockade,” while preventing opioid withdrawal signs without causing euphoria (Fig. 2B) (53). With the increasing purity of heroin over the past two to three decades, the optimal treatment dose in most patients with opiate addiction is 80 to 150 mg/day, with higher doses needed in a small percentage of patients. These doses of methadone are markedly higher than those used to treat chronic pain, which usually range from 10 to 45 total mg/day, delivered in divided doses. Because of extensive binding to plasma protein, as well as to tissues, methadone enters the brain slowly and exits the brain slowly, allowing a steady state to develop (60). The half-life of racemic methadone (the usual form) in humans is approximately 24 hours (±4 hours). The half-life of the active enantiomer (l or R, Fig. 1E) is around 48 hours, and the half-life of the inactive enantiomer (d or S) is around 16 hours (61–63). Methadone is biotransformed to pyrolline and pyrrolidine metabolites, both inactive and excreted primarily not only in urine but also in feces. Methadone does not cause enhanced or inhibited microsomal activity. Therefore, doses of methadone can be kept constant for at least 10 years with little need for change. With a half-life for the racemic formulation of 24 hours, steady-state methadone can be achieved with once daily dosing of a specific dose within 1 week. PET investigations of formerly heroin-addicted individuals, maintained on steady-state methadone at an effective treatment dose, indicate that the occupancy of the μ-opioid receptor is not close to 100% but rather 30 to 40% (64).
Buprenorphine
Buprenorphine was originally developed in the 1970s as an analgesic (Fig. 1F) in the laboratory of J. Lewis, at Reckitt-Colman in the United Kingdom. When used as a maintenance medication for OUD, buprenorphine must be used by the sublingual, but not oral route, due to rapid liver biotransformation. Unfortunately, when used parenterally or injected intravenously, buprenorphine can have euphoric effects. However, it has also been shown that the addition of naloxone in the sublingual formulation of buprenorphine, if self-administered parenterally, prevents this euphoria for at least 30 min (i.e., the half-life of naloxone) (65). Very recently, sustained release implants of buprenorphine have been developed that last up to 30 days (Table 3) (66).
Buprenorphine and buprenorphine-naloxone are also effective for at least 6 months in at least 40 to 50% of unselected patients (67). The sublingual formulation with the largest buprenorphine dose (12 mg) is combined with 3 mg of naloxone. The FDA-approved package insert states that sublingual doses of buprenorphine larger than 24 mg have not been shown to have a further clinical advantage. As above for methadone, it is critical that buprenorphine maintenance doses should be sufficient to achieve blockade of short-acting μ-opioid agonists (68).
Buprenorphine administered by the sublingual route has an extended half-life compared to the half-life of intravenous buprenorphine, which is similar to short-acting opiates, and has also been shown to bind to the μ-opioid receptor with slow dissociation kinetics (69). Therefore, in treatment of addiction, buprenorphine has a sustained effect, as does methadone, but for different reasons. Rapid “on-off” effects of a μ-agonist affect signal transduction and result in adaptations including desensitization and tolerance (70). Maintenance with methadone orally or buprenorphine sublingually provides steady-state occupancy at μ-opioid receptors (12, 64), with limited tolerance, as shown by stable doses in the clinic, over prolonged periods. Since buprenorphine is a μ-receptor partial agonist with dissociation kinetics, it can block binding of other self-administered μ-opioid agonists. PET studies reveal that buprenorphine maintenance results in submaximal occupancy of brain μ-receptors (40 to 60%) (12). This maintenance treatment is able to block the effects of challenge with short-acting μ-agonists (68).
Opioid antagonist medications
As mentioned previously, naltrexone (Fig. 1G) has been approved as a treatment for opioid addiction, both with oral tablet administration and more recently intramuscular depot injections with sustained release for approximately 1 month (Table 3) (71). Naltrexone is primarily a μ-antagonist and is also a κ-opioid receptor partial agonist (72). Acute administration of naltrexone to a person who is actively dependent on μ-agonists results in precipitated withdrawal symptoms, which are aversive. Thus, prior to naltrexone induction, patients must be withdrawn from μ-agonists first, with several days of abstinence, initiated with or without tapering.
Daily oral naltrexone has been of only limited utility in the treatment of OUD-related morbidity (29). So far, only a few studies of limited duration have been performed on intramuscular depot naltrexone, designed to provide stable levels of naltrexone for approximately 1 month [for example, (73–75)]. The long-term clinical impact is still unclear (29). Depot naltrexone can block the effects of short-acting μ-agonists (76). However, to our knowledge, depot naltrexone has not been shown to normalize the persistent neurobiological changes that result from long-term exposure to illicit opioids.
Depot naltrexone could be potentially useful as a therapeutic modality before onset of multiple daily opioid use (a characteristic of dependence and addiction) before the development of persistent neurobiological disruptions. There is some concern that hepatotoxicity may result from chronic long-term naltrexone use, at least in a subset of patients. As an alternative, a similar compound, nalmefene (Fig. 1H), may also be useful in a depot formulation in the future. At this time, nalmefene is administered in oral formulation, as an “as-needed” medication for the treatment of alcohol use disorder, and approved for use in Europe and Japan. Nalmefene, similar to naltrexone, is a μ-opioid receptor antagonist and also has κ-partial agonist effects, primarily through G protein signaling (77, 78).
The most commonly used opioid antagonist against overdose is currently naloxone, which is of lower potency and shorter duration of action, compared to its congeners, naltrexone, and nalmefene. Naloxone has been of major importance for saving thousands of lives in overdose situations. With the recent availability of illicit fentanyl and its analogs, due to their enhanced potency and longer duration of action, single doses of naloxone are not always effective in rescuing opioid-induced respiratory depression (79). Multiple sequential injections of naloxone are sometimes necessary, particularly with fentanyl analogs. Naltrexone and nalmefene, which have longer durations of action, are therefore receiving current attention as anti-overdose medications against potent fentanyl analogs (80).
Long-acting medications such as methadone and buprenorphine-naloxone can be used on a long-term basis with little dose change. Long-acting medications allow normalization of functions in humans that are disrupted by short-acting μ-agonists, including stress responsivity and hormone-regulated reproductive function (specifically normalization of the hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes) (81, 82).
Human molecular genetics related to opioid use disorders
Variants of the μ-receptor gene, Oprm1
In 1998, we reported on an important and fairly common single-nucleotide polymorphism (SNP) of the μ-opioid receptor, the A118G variant, which changes an amino acid in the N terminus (83). In collaboration with Yu and colleagues (83), we showed that the A118G variant results in increased binding affinity of the endogenous neuropeptide, β-endorphin. We and others also showed that with this variant, there is greater signal transduction to the G protein–coupled inwardly rectifying potassium channel system.
In the initial clinical studies, we and others learned (83) that this A118G variant occurs in around 8 to 30% of European Caucasian populations and occurs in 40 to 60% of Asian populations. However, it is not present in African populations, unless admixture has occurred. Further work from our laboratory, carried out in collaboration with the Karolinska Institute (Stockholm, Sweden), showed that A118G is strongly associated with opioid addiction (84) and with alcoholism (85). Each of these findings was made in a European Caucasian population. Several other groups have also studied this variant in healthy humans and have found that one or two copies of the G nucleotide result in altered stress responsivity, including altered responsivity to a challenge with a μ-receptor antagonist, which normally activates this system (86, 87). Further, it has been shown that one or two copies of this variant markedly alter the response in normal volunteers to metyrapone, a neuroendocrine test compound that cuts off the production of cortisol by the adrenal cortex for about 8 hours, resulting usually in a surge of β-endorphin and ACTH (88). This effect arises because the normal negative feedback system by cortisol or other glucocorticoids is temporarily blocked. Thus, in persons with one or two copies of the A118G variant, a subnormal response to metyrapone testing was observed. This response is likely due to changes in β-endorphin binding to the μ-receptor in carriers of this variant, as this neuropeptide is part of the modulation of stress responsivity. Thus, with higher affinity binding of β-endorphin to the G variant–carrying μ-opioid receptor, one sees less activation of the stress axis, resulting in lower ACTH (and likely β-endorphin) levels after metyrapone challenge.
In addition, transgenic mice homozygous for the G variant self-administer over twice as much heroin (than the wild-type) (89). These findings show that even a single amino acid change in the coding region of the μ-opioid receptor can significantly increase the amount of self-administered short-acting μ-agonist.
Methadone and buprenorphine-naloxone maintenance treatment has been found to be effective in individuals with the Oprm1 A118G polymorphism and with several other polymorphisms in genes expressed in brain (90). The effectiveness of this treatment is probably due to the relatively high dose of both medications that are used in the treatment of opioid addiction. However, polymorphisms of genes involved in methadone pharmacokinetics are associated with differences in the dose required for effective maintenance (91, 92). Some studies have suggested that patients with one or two copies of the A118G variant may respond differently from those with the prototype both to pain and to analgesic treatment with a μ-opioid receptor agonist (93). Both methadone and buprenorphine can be effectively used in the treatment of pain at relatively low doses, compared to maintenance doses used in OUDs. To our knowledge, no study has shown a difference in analgesic effects of these two compounds in persons with one or two copies of the A118G variant.
Variants of the κ-opioid receptor (Oprk1) and prodynorphin (Pdyn) genes
A second group of gene variants that have been shown to be associated with different addictive diseases is the functional 68–base pair (bp) repeat present in one to four copies in the promoter of the prodynorphin (PDYN) gene, which encodes for the endogenous neuropeptide at κ-opioid receptors (94). Some studies have found an association of this polymorphism with aspects of opioid addiction (95, 96) in Caucasian populations, and similar findings of association of the number of 68-bp repeats have been reported in studies of the genetic determinants of cocaine addiction (97).
Variants of cannabinoid system genes
One laboratory has reported an association of fatty acid amide hydrolase gene variant 385C > A with opioid addiction (98). However, our laboratory was unable to confirm this finding in a larger sample of normal volunteer Caucasians, compared with those with opioid addiction, although we found several intriguing associations of polymorphisms of the cannabinoid receptor type 1 and opioid addiction (99). Across three different ethnicities studied (Caucasian Europeans, African-Americans, and Hispanics), a highly significant association was found of long repeats with heroin addiction (P = 0.009). Further, pointwise significant association of the allele 1359A (P = 0.006) and genotype 1359AA (P = 0.034) was associated with protection from heroin addiction in Caucasians.
Variants of nociceptin/orphanin FQ receptor genes
Our laboratory investigated the nociceptin/orphanin FQ receptor gene (OPRL1) with respect to genetic variants that might be associated with opioid addiction (100). In Caucasians, but not in African-Americans, we found that rs6090041 and rs6090043 variants of the OPRL1 gene were significantly associated pointwise with opioid addiction. Of the haplotypes formed by these two variants, one was associated with vulnerability to develop opioid addiction in Caucasians (pointwise P = 0.020), and another haplotype of these variants was associated with protection from developing opioid addiction in African-Americans (pointwise P = 0.04).
Recent human genetics of opioid addiction
Our laboratory conducted a very early (2010) genome-wide association study to identify gene variants that might contribute to the risk for developing heroin addiction (101). SNPs in several genes encoding for components of the endogenous opioid system, neurotransmitter systems, and the stress hormone system were associated with heroin addiction in multiple ethnicities (see table S2) (101, 102).
Current research areas that have clear translational potential for the development of new treatments or interventions
In addition to the aforementioned approved medications, some current research areas may have translational potential. Promising research areas include the development of novel analgesic moieties with decreased abuse potential or with reduced risk of toxicity or overdose. These developments are the product of decade-long research efforts in public and privately funded research in medicinal chemistry and pharmacology (both in vitro and in vivo, in rodents and nonhuman primates).
Current areas of development include the examination of “biased” μ-agonists, which may potentially have an improved profile (e.g., a relatively lower propensity to cause constipation, respiratory depression, or abuse potential) compared to classic μ-agonists such as fentanyl (103, 104). At this time, the superior characteristics of these agonists have not been demonstrated unequivocally (105). A second approach examined recently in preclinical models involves novel μ-agonists that would be active preferentially at the local site of injury or inflammation (e.g., at μ-receptors in the periphery) (106), thus diminishing risk of overdose and abuse potential, as the latter effects are mediated by receptors in the CNS.
A third approach has examined dual targeting of μ-opioid receptors and other receptors. One recent notable example, studied preclinically, is a dual μ-agonist/orphanin-agonist compound, which shows enhanced analgesia with a reduced burden of both respiratory depression and abuse potential (107).
More broadly, it has been shown that classic μ-agonists are not optimal for the chronic treatment of pain that is mediated by neuropathic or inflammatory mechanisms (108). Therefore, there has been a continued focus on novel pharmacological targets (i.e., not directed to the μ-receptors) for the chronic treatment of these kinds of pain.
Gaps in scientific knowledge and research directions that are likely critical for advancing the effectiveness in treatment and recovery
The goals and rationale for pharmacotherapy for opioid addiction (53) are for a pharmacotherapeutic agent (preferably used orally or sublingually) to prevent withdrawal symptoms, to reverse drug craving, and to normalize any functions that have been disrupted by chronic drug use, especially brain function (54). Further, the medication should be targeted to a specific site of action, a specific physiological system affected or deranged directly by the drug of abuse, and not simply symptomatically directed. Opioid addiction is often comorbid with other addictions (e.g., to cocaine and alcohol). Currently, we are also investigating development of medications to treat cocaine addiction and alcoholism, with the κ-opioid receptor as one major potential target (109–111).
One current goal would be the discovery of targets and approaches that may prevent the onset of OUDs after relatively brief exposure to μ-agonists (e.g., after short-term iatrogenic exposure for analgesia), with the aim of preventing the development of severe OUDs.
Studies have also shown that persons with OUD show persistently changed neuroendocrine stress–axis systems, which may contribute to continued risk of relapse (112). Recent work also shows that pain exposure per se (e.g., neuropathic or inflammatory pain) can result in neurobiological changes that could also increase the susceptibility of the individual to OUDs or to psychiatric comorbidities, such as anxiety or depression (113, 114).
Novel technologies such as RNA interference and CRISPR (for somatic, not germ cells) may be explored in the future, for prevention or therapeutic uses, both for analgesia and for the treatment of OUD. These approaches could include targeting of particular neuroanatomical areas and mechanisms that may underlie specific facets of analgesia and of OUD treatment. As with all “gene therapy”–based approaches for CNS disorders, the development of vectors that can be expressed in a human relatively noninvasively and effectively will be crucial, as it is the avoidance of “off-target” effects (115).
SUMMARY AND CONCLUSIONS
OUDs, including their most severe form, opioid addiction, are chronic relapsing diseases of the brain with multifactorial origins. Standard-of-care maintenance medications (methadone and buprenorphine-naloxone) are effective for the treatment of these diseases. However, the appropriate therapeutic use of these medications has been limited by stigma, insufficient medical education, and lack of resources. Ongoing research includes development of novel analgesic approaches that have greater effectiveness for chronic pain states (e.g., neuropathic and inflammatory pain), with a decreased burden of overdose risk and of abuse potential. Other approaches may also focus on mitigating the development of opioid addiction, before the emergence of substantial neurobiological changes and compulsive-like drug-taking behaviors.
Supplementary Material
Acknowledgments
We would like to thank O. Levran for consultation with the genetics summary, especially table S2. Further, we thank K. Lavoie and A. Sintim for administrative, illustration, and reference support. Funding: We acknowledge funding for our laboratory research from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (to M.J.K.), as well as the Robertson Therapeutic Discovery Fund, NIH NIDA grants DA018151 (subcontract to E.R.B.) and DA041730 (subcontract to B.R.). Author contributions: M.J.K. conceptualized the paper and wrote portions related to the history and pharmacology of methadone and buprenorphine, as well as the genetics work. E.R.B. also contributed to the conceptualization of the paper and wrote portions pertaining to preclinical research and the current opioid epidemic. B.R. wrote portions comprising the pharmacology and chemistry of the opioids. All authors put sustained effort into the illustrations, tables, references, and final composition. Competing interests: All authors are inventors on a patent related to this work filed by Rockefeller University (no. WO/2019/113419, published 13 June 2019). The authors declare that they have no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or in the references cited herein. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/10/eaax9140/DC1
Table S1. Methadone maintenance treatment for opiate (heroin) addiction.
Table S2. SNPs of genes related to endocrine stress responsivity that has been found to be associated with opioid addiction (101, 102, 116).
Fig. S1. Model for the contribution of pain states and pain treatment to the development of OUD.
REFERENCES AND NOTES
- 1.Scholl L., Seth P., Kariisa M., Wilson M., Baldwin G., Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb. Mortal. Wkly Rep. 67, 1419–1427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.N.O.D.C., United Nations Office of Drugs and Crime (2017).
- 3.Dahan A., Aarts L., Smith T. W., Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 112, 226–238 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Contet C., Kieffer B. L., Befort K., Mu opioid receptor: A gateway to drug addiction. Curr. Opin. Neurobiol. 14, 370–378 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Kandel D. B., Hu M. C., Griesler P., Wall M., Increases from 2002 to 2015 in prescription opioid overdose deaths in combination with other substances. Drug Alcohol Depend. 178, 501–511 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Des Jarlais D. C., Friedman S. R., Novick D. M., Sotheran J. L., Thomas P., Yancovitz S. R., Mildvan D., Weber J., Kreek M. J., Maslansky R., HIV-1 infection among intravenous drug users in Manhattan, New York City, from 1977 through 1987. JAMA 261, 1008–1012 (1989). [DOI] [PubMed] [Google Scholar]
- 7.Brownstein M. J., A brief history of opiates, opioid peptides, and opioid receptors. Proc. Natl. Acad. Sci. U.S.A. 90, 5391–5393 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawynok J., The therapeutic use of heroin: A review of the pharmacological literature. Can. J. Physiol. Pharmacol. 64, 1–6 (1986). [DOI] [PubMed] [Google Scholar]
- 9.Kalso E., Oxycodone. J. Pain Symptom Manage. 29, S47–S56 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Van Zee A., The promotion and marketing of oxycontin: Commercial triumph, public health tragedy. Am. J. Public Health 99, 221–227 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler S. F., Black R. A., Cassidy T. A., Dailey T. M., Budman S. H., Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct. J. 8, 29 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenwald M. K., Johanson C. E., Moody D. E., Woods J. H., Kilbourn M. R., Koeppe R. A., Schuster C. R., Zubieta J. K., Effects of buprenorphine maintenance dose on μ-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology 28, 2000–2009 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Drug Enforcement Administration, 2018 National Drug Threat Assessment (2018); www.dea.gov/sites/default/files/2018-11/DIR-032-18%202018%20NDTA%20final%20low%20resolution.pdf.
- 14.Rudd R. A., Aleshire N., Zibbell J. E., Gladden R. A., Increases in drug and opioid overdose deaths — United States, 2000–2014. Morb. Mortal. Wkly. Rep., 1378–1382 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Afshar M., Joyce C., Dligach D., Sharma B., Kania R., Xie M., Swope K., Salisbury-Afshar E., Karnik N. S., Subtypes in patients with opioid misuse: A prognostic enrichment strategy using electronic health record data in hospitalized patients. PLOS ONE 14, e0219717 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drug Enforcement Administration, Fentanyl (2017); www.dea.gov/sites/default/files/sites/getsmartaboutdrugs.com/files/publications/DoA_2017Ed_Updated_6.16.17.pdf#page=40.
- 17.Drug Enforcement Administration, Fentanyl (2019); www.dea.gov/factsheets/fentanyl.
- 18.Spencer M. R., Warner M., Bastian B. A., Trinidad J. P., Drug overdose deaths involving fentanyl, 2011–2016. Natl. Vital Stat. Rep. 68, 1–19 (2019). [PubMed] [Google Scholar]
- 19.Dart R. C., Surratt H. L., Cicero T. J., Parrino M. W., Severtson S. G., Bucher-Bartelson B., Green J. L., Trends in opioid analgesic abuse and mortality in the united states. N. Engl. J. Med. 372, 241–248 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Volkow N., Benveniste H., McLellan A. T., Use and misuse of opioids in chronic pain. Annu. Rev. Med. 69, 451–465 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Banerjee G., Edelman E. J., Barry D. T., Becker W. C., Cerda M., Crystal S., Gaither J. R., Gordon A. J., Gordon K. S., Kerns R. D., Martins S. S., Fiellin D. A., Marshall B. D., Non-medical use of prescription opioids is associated with heroin initiation among US veterans: A prospective cohort study. Addiction 111, 2021–2031 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brady K. T., McCauley J. L., Back S. E., Prescription opioid misuse, abuse, and treatment in the United States: An update. Am. J. Psychiatry 173, 18–26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha T. D., Kerridge B. T., Goldstein R. B., Chou S. P., Zhang H., Jung J., Pickering R. P., Ruan W. J., Smith S. M., Huang B., Hasin D. S., Grant B. F., Nonmedical prescription opioid use and DSM-5 nonmedical prescription opioid use disorder in the united states. J. Clin. Psychiatry 77, 772–780 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mars S. G., Bourgois P., Karandinos G., Montero F., Ciccarone D., “Every ‘never’ I ever said came true”: Transitions from opioid pills to heroin injecting. Int. J. Drug Policy 25, 257–266 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cerda M., Ransome Y., Keyes K. M., Koenen K. C., Tracy M., Tardiff K. J., Vlahov D., Galea S., Prescription opioid mortality trends in New York City, 1990-2006: Examining the emergence of an epidemic. Drug Alcohol Depend. 132, 53–62 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hedegaard H., Bastian B. A., Trinidad J. P., Spencer M., Warner M., Drugs most frequently involved in drug overdose deaths: United States, 2011-2016. Natl. Vital. Stat. Rep. 67, 1–15 (2018). [PubMed] [Google Scholar]
- 27.Wood E., Samet J. H., Volkow N. D., Physician education in addiction medicine. JAMA 310, 1673–1674 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surgeon General, Facing Addiction in America (2016); https://addiction.surgeongeneral.gov/.
- 29.Morgan J. R., Schackman B. R., Weinstein Z. M., Walley A. Y., Linas B. P., Overdose following initiation of naltrexone and buprenorphine medication treatment for opioid use disorder in a United States commercially insured cohort. Drug Alcohol Depend. 200, 34–39 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levran O., Peles E., Randesi M., da Rosa J. C., Adelson M., Kreek M. J., The μ-opioid receptor nonsynonymous variant 118A>G is associated with prolonged abstinence from heroin without agonist treatment. Pharmacogenomics 18, 1387–1391 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed B., Butelman E. R., Kreek M. J., Endogenous opioid system in addiction and addiction-related behaviors. Curr. Opin. Behav. Sci. 13, 196–202 (2017). [Google Scholar]
- 32.Compton W. M., Dawson D. A., Goldstein R. B., Grant B. F., Crosswalk between DSM-IV dependence and DSM-5 substance use disorders for opioids, cannabis, cocaine and alcohol. Drug Alcohol Depend. 132, 387–390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreek M. J., Ragunath J., Plevy S., Hamer D., Schneider B., Hartman N., ACTH, cortisol and β-endorphin response to metyrapone testing during chronic methadone maintenance treatment in humans. Neuropeptides 5, 277–278 (1984). [DOI] [PubMed] [Google Scholar]
- 34.Cooper Z. D., Shi Y. G., Woods J. H., Reinforcer-dependent enhancement of operant responding in opioid-withdrawn rats. Psychopharmacology 212, 369–378 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen M. I., McMahon T. J., Hameedi F. A., Pearsall H. R., Woods S. W., Kreek M. J., Kosten T. R., Effect of clonidine pretreatment on naloxone-precipitated opiate withdrawal. J. Pharmacol. Exp. Ther. 276, 1128–1135 (1996). [PubMed] [Google Scholar]
- 36.Williams J., Ingram S., Henderson G., Chavkin C., von Zastrow M., Schulz S., Koch T., Evans C., Christie M., Regulation of μ-opioid receptors: Desensitization, phosphorylation, internalization, and tolerance. Pharmacol. Rev. 65, 223–254 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Selley D. E., Nestler E. J., Breivogel C. S., Childers S. R., Opioid receptor-coupled G-proteins in rat locus coeruleus membranes: Decrease in activity after chronic morphine treatment. Brain Res. 746, 10–18 (1997). [DOI] [PubMed] [Google Scholar]
- 38.Akbarian S., Rios M., Liu R. J., Gold S. J., Fong H. F., Zeiler S., Coppola V., Tessarollo L., Jones K. R., Nestler E. J., Aghajanian G. K., Jaenisch R., Brain-derived neurotrophic factor is essential for opiate-induced plasticity of noradrenergic neurons. J. Neurosci. 22, 4153–4162 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altarifi A. A., Negus S. S., Some determinants of morphine effects on intracranial self-stimulation in rats: Dose, pretreatment time, repeated treatment, and rate dependence. Behav. Pharmacol. 22, 663–673 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Picetti R., Caccavo J. A., Ho A., Kreek M. J., Dose escalation and dose preference in extended-access heroin self-administration in Lewis and Fischer rats. Psychopharmacology 220, 163–172 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y., Mestek A., Liu J., Hurley J. A., Yu L., Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol. Pharmacol. 44, 8–12 (1993). [PubMed] [Google Scholar]
- 42.Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A., Cohen S., Numa S., Nucleotide sequence of cloned cDNA for bovine corticotropin-β-lipotropin precursor. Nature 278, 423–427 (1979). [DOI] [PubMed] [Google Scholar]
- 43.Gubler U., Seeburg P., Hoffman B. J., Gage L. P., Udenfriend S., Molecular cloning establishes proenkephalin as precursor of enkephalin-containing peptides. Nature 295, 206–208 (1982). [DOI] [PubMed] [Google Scholar]
- 44.Mansour A., Fox C. A., Thompson R. C., Akil H., Watson S. J., μ-Opioid receptor mRNA expression in the rat CNS: Comparison to mu-receptor binding. Brain Res. 643, 245–265 (1994). [DOI] [PubMed] [Google Scholar]
- 45.Wang X. M., Zhou Y., Spangler R., Ho A., Han J. S., Kreek M. J., Acute intermittent morphine increases preprodynorphin and kappa opioid receptor mRNA levels in the rat brain. Brain Res. Mol. Brain Res. 66, 184–187 (1999). [DOI] [PubMed] [Google Scholar]
- 46.Becker J. A., Kieffer B. L., Le Merrer J., Differential behavioral and molecular alterations upon protracted abstinence from cocaine versus morphine, nicotine, THC and alcohol. Addict. Biol. 22, 1205–1217 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Mayer-Blackwell B., Schlussman S. D., Randesi M., Butelman E. R., Ho A., Ott J., Kreek M. J., Extended access oxycodone self-administration and neurotransmitter receptor gene expression in the dorsal striatum of adult C57BL/6 J mice. Psychopharmacology 231, 1277–1287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayer-Blackwell B., Schlussman S. D., Butelman E. R., Ho A., Ott J., Kreek M. J., Zhang Y., Self administration of oxycodone by adolescent and adult mice affects striatal neurotransmitter receptor gene expression. Neuroscience 258, 280–291 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Brownstein A. J., Buonora M., Niikura K., Ho A., Correa da Rosa J., Kreek M. J., Ott J., Self administration of oxycodone alters synaptic plasticity gene expression in the hippocampus differentially in male adolescent and adult mice. Neuroscience 285, 34–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Liang Y., Levran O., Randesi M., Yuferov V., Zhao C., Kreek M. J., Alterations of expression of inflammation/immune-related genes in the dorsal and ventral striatum of adult C57BL/6J mice following chronic oxycodone self-administration: A RNA sequencing study. Psychopharmacology 234, 2259–2275 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuferov V., Zhang Y., Liang Y., Zhao C., Randesi M., Kreek M. J., Oxycodone self-administration induces alterations in expression of integrin, semaphorin and ephrin genes in the mouse striatum. Front. Psych. 9, 257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y., Liang Y., Randesi M., Yuferov V., Zhao C., Kreek M. J., Chronic oxycodone self-administration altered reward-related genes in the ventral and dorsal striatum of C57BL/6J mice: An RNA-seq analysis. Neuroscience 393, 333–349 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Dole V. P., Nyswander M. E., Kreek M. J., Narcotic blockade. Arch. Intern. Med. 118, 304–309 (1966). [PubMed] [Google Scholar]
- 54.Kreek M. J., Methadone-related opioid agonist pharmacotherapy for heroin addiction. History, recent molecular and neurochemical research and future in mainstream medicine. Ann. N. Y. Acad. Sci. 909, 186–216 (2000). [DOI] [PubMed] [Google Scholar]
- 55.M. J. Kreek, in Proceedings of the 61st Annual Scientific Meeting of the College on Problems of Drug Dependence, L. S. Harris, Ed. (NIDA Research Monographs, 2000), pp. 3–22. [PubMed] [Google Scholar]
- 56.Kreek M. J., Vocci F. J., History and current status of opioid maintenance treatments: Blending conference session. J. Subst. Abuse Treat. 23, 93–105 (2002). [DOI] [PubMed] [Google Scholar]
- 57.Adelson M., Linzy S., Peles E., Characteristics and outcome of male and female methadone maintenance patients: MMT in Tel Aviv and Las Vegas. Subst. Use Misuse 53, 230–238 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Trujillo K. A., Akil H., Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science 251, 85–87 (1991). [DOI] [PubMed] [Google Scholar]
- 59.Gorman A. L., Elliott K. J., Inturrisi C. E., The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-D-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci. Lett. 223, 5–8 (1997). [DOI] [PubMed] [Google Scholar]
- 60.Kreek M. J., Plasma and urine levels of methadone. N. Y. State J. Med. 73, 2773–2777 (1973). [PubMed] [Google Scholar]
- 61.Hachey D. L., Kreek M. J., Mattson D. H., Quantitative analysis of methadone in biological fluids using deuterium-labeled methadone and GLC-chemical-ionization mass spectrometry. J. Pharm. Sci. 66, 1579–1582 (1977). [DOI] [PubMed] [Google Scholar]
- 62.Kreek M. J., Hachey D. L., Klein P. D., Stereoselective disposition of methadone in man. Life Sci. 24, 925–932 (1979). [DOI] [PubMed] [Google Scholar]
- 63.Nakamura K., Hachey D. L., Kreek M. J., Irving C. S., Klein P. D., Quantitation of methadone enantiomers in humans using stable isotope-labeled [2H3]-, [2H5]-, and [2H8]Methadone. J. Pharm. Sci. 71, 40–43 (1982). [DOI] [PubMed] [Google Scholar]
- 64.Kling M. A., Carson R. E., Borg L., Zametkin A., Matochik J. A., Schluger J., Herscovitch P., Rice K. C., Ho A., Eckelman W. C., Kreek M. J., Opioid receptor imaging with positron emission tomography and [(18)F]cyclofoxy in long-term, methadone-treated former heroin addicts. J. Pharmacol. Exp. Ther. 295, 1070–1076 (2000). [PubMed] [Google Scholar]
- 65.Mendelson J., Jones R. T., Clinical and pharmacological evaluation of buprenorphine and naloxone combinations: Why the 4:1 ratio for treatment? Drug Alcohol Depend. 70, S29–S37 (2003). [DOI] [PubMed] [Google Scholar]
- 66.Haight B. R., Learned S. M., Laffont C. M., Fudala P. J., Zhao Y., Garofalo A. S., Greenwald M. K., Nadipelli V. R., Ling W., Heidbreder C., Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 393, 778–790 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Kakko J., Svanborg K. D., Kreek M. J., Heilig M., 1-year retention and social function after buprenorphine-assisted relapse prevention treatment for heroin dependence in Sweden: A randomised, placebo-controlled trial. Lancet 361, 662–668 (2003). [DOI] [PubMed] [Google Scholar]
- 68.Greenwald M. K., Comer S. D., Fiellin D. A., Buprenorphine maintenance and mu-opioid receptor availability in the treatment of opioid use disorder: Implications for clinical use and policy. Drug Alcohol Depend. 144, 1–11 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Englberger W., Kögel B., Friderichs E., Strassburger W., Germann T., Reversibility of opioid receptor occupancy of buprenorphine in vivo. Eur. J. Pharmacol. 534, 95–102 (2006). [DOI] [PubMed] [Google Scholar]
- 70.Williams J. T., Ingram S. L., Henderson G., Chavkin C., von Zastrow M., Schulz S., Koch T., Evans C. J., Christie M. J., Regulation ofμ-opioid receptors: Desensitization, phosphorylation, internalization, and tolerance. Pharmacol. Rev. 65, 223–254 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woody G. E., Metzger D. S., Injectable extended-release naltrexone for opioid dependence. Lancet 378, 664–665 (2011). [DOI] [PubMed] [Google Scholar]
- 72.Wentland M. P., Lou R., Lu Q., Bu Y., Denhardt C., Jin J., Ganorkar R., VanAlstine M. A., Guo C., Cohen D. J., Bidlack J. M., Syntheses of novel high affinity ligands for opioid receptors. Bioorg. Med. Chem. Lett. 19, 2289–2294 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee J. D., Nunes E. V. Jr., Novo P., Bachrach K., Bailey G. L., Bhatt S., Farkas S., Fishman M., Gauthier P., Hodgkins C. C., King J., Lindblad R., Liu D., Matthews A. G., May J., Peavy K. M., Ross S., Salazar D., Schkolnik P., Shmueli-Blumberg D., Stablein D., Subramaniam G., Rotrosen J., Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicentre, open-label, randomised controlled trial. Lancet 391, 309–318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krupitsky E., Zvartau E., Woody G., Use of naltrexone to treat opioid addiction in a country in which methadone and buprenorphine are not available. Curr. Psychiatry Rep. 12, 448–453 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Comer S. D., Sullivan M. A., Yu E., Rothenberg J. L., Kleber H. D., Kampman K., Dackis C., O'Brien C. P., Injectable, sustained-release naltrexone for the treatment of opioid dependence: A randomized, placebo-controlled trial. Arch. Gen. Psychiatry 63, 210–218 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Comer S. D., Collins E. D., Kleber H. D., Nuwayser E. S., Kerrigan J. H., Fischman M. W., Depot naltrexone: Long-lasting antagonism of the effects of heroin in humans. Psychopharmacology 159, 351–360 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Maillet E. L., Milon N., Heghinian M. D., Fishback J., Schurer S. C., Garamszegi N., Mash D. C., Noribogaine is a G-protein biased κ-opioid receptor agonist. Neuropharmacology 99, 675–688 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Bart G., Schluger J. H., Borg L., Ho A., Bidlack J. M., Kreek M. J., Nalmefene induced elevation in serum prolactin in normal human volunteers: Partial kappa opioid agonist activity? Neuropsychopharmacology 30, 2254–2262 (2005). [DOI] [PubMed] [Google Scholar]
- 79.Prekupec M. P., Mansky P. A., Baumann M. H., Misuse of novel synthetic opioids: A deadly new trend. J. Addict. Med. 11, 256–265 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krieter P., Gyaw S., Crystal R., Skolnick P., Fighting fire with fire: Development of intranasal nalmefene to treat synthetic opioid overdose. J. Pharmacol. Exp. Ther. jpet.118.256115, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kreek M. J., Wardlaw S. L., Hartman N., Raghunath J., Friedman J., Schneider B., Frantz A. G., Circadian rhythms and levels of β-endorphin, ACTH, and cortisol during chronic methadone maintenance treatment in humans. Life Sci. 33 (suppl. 1), 409–411 (1983). [DOI] [PubMed] [Google Scholar]
- 82.Cushman P. J., Kreek M. J., Methadone-maintained patients. Effect of methadone on plasma testosterone, FSH, LH, and prolactin. N. Y. State J. Med. 74, 1970–1973 (1974). [PubMed] [Google Scholar]
- 83.Bond C., LaForge K. S., Tian M., Melia D., Zhang S., Borg L., Gong J., Schluger J., Strong J. A., Leal S. M., Tischfield J. A., Kreek M. J., Yu L., Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: Possible implications for opiate addiction. Proc. Natl. Acad. Sci. U.S.A. 95, 9608–9613 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bart G., Heilig M., LaForge K. S., Pollak L., Leal S. M., Ott J., Kreek M. J., Substantial attributable risk related to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Mol. Psychiatry 9, 547–549 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bart G., Kreek M. J., Ott J., LaForge K. S., Proudnikov D., Pollak L., Heilig M., Increased attributable risk related to a functional μ-opioid receptor gene polymorphism in association with alcohol dependence in central Sweden. Neuropsychopharmacology 30, 417–422 (2005). [DOI] [PubMed] [Google Scholar]
- 86.Wand G. S., McCaul M., Yang X., Reynolds J., Gotjen D., Lee S., Ali A., The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology 26, 106–114 (2002). [DOI] [PubMed] [Google Scholar]
- 87.Hernandez-Avila C. A., Wand G., Luo X., Gelernter J., Kranzler H. R., Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the μ-opioid receptor locus (OPRM1). Am. J. Med. Genet. B Neuropsychiatr. Genet. 118B, 60–65 (2003). [DOI] [PubMed] [Google Scholar]
- 88.Ducat E., Ray B., Bart G., Umemura Y., Varon J., Ho A., Kreek M. J., Mu-opioid receptor A118G polymorphism in healthy volunteers affects hypothalamic-pituitary-adrenal axis adrenocorticotropic hormone stress response to metyrapone. Addict. Biol. 18, 325–331 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y., Picetti R., Butelman E. R., Ho A., Blendy J. A., Kreek M. J., Mouse model of the OPRM1 (A118G) polymorphism: Differential heroin self-administration behavior compared with wild-type mice. Neuropsychopharmacology 40, 1091–1100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crist R. C., Reiner B. C., Berrettini W. H., A review of opioid addiction genetics. Curr. Opin. Psychol. 27, 31–35 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levran O., Peles E., Hamon S., Randesi M., Adelson M., Kreek M. J., CYP2B6 SNPs are associated with methadone dose required for effective treatment of opioid addiction. Addict. Biol. 18, 709–716 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Levran O., O’Hara K., Peles E., Li D., Barral S., Ray B., Borg L., Ott J., Adelson M., Kreek M. J., ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence. Hum. Mol. Genet. 17, 2219–2227 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oertel B. G., Schmidt R., Schneider A., Geisslinger G., Lötsch J., The μ-opioid receptor gene polymorphism 118A>G depletes alfentanil-induced analgesia and protects against respiratory depression in homozygous carriers. Pharmacogenet. Genomics 16, 625–636 (2006). [DOI] [PubMed] [Google Scholar]
- 94.Rouault M., Nielsen D. A., Ho A., Kreek M. J., Yuferov V., Cell-specific effects of variants of the 68-base pair tandem repeat on prodynorphin gene promoter activity. Addict. Biol. 16, 334–346 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yuferov V., Randesi M., Butelman E. R., van den Brink W., Blanken P., van Ree J. M., Ott J., Kreek M. J., Association of variants of prodynorphin promoter 68-bp repeats in Caucasians with opioid dependence diagnosis: Effect on age trajectory of heroin use. Neurosci. Lett. 704, 100–105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ray R., Doyle G. A., Crowley J. J., Buono R. J., Oslin D. W., Patkar A. A., Mannelli P., DeMaria P. A. Jr., O’Brien C. P., Berrettini W. H., A functional prodynorphin promoter polymorphism and opioid dependence. Psychiatr. Genet. 15, 295–298 (2005). [DOI] [PubMed] [Google Scholar]
- 97.Williams T. J., LaForge K. S., Gordon D., Bart G., Kellogg S., Ott J., Kreek M. J., Prodynorphin gene promoter repeat associated with cocaine/alcohol codependence. Addict. Biol. 12, 496–502 (2007). [DOI] [PubMed] [Google Scholar]
- 98.Tyndale R. F., Payne J. I., Gerber A. L., Sipe J. C., The fatty acid amide hydrolase C385A (P129T) missense variant in cannabis users: Studies of drug use and dependence in Caucasians. Am. J. Med. Genet. B Neuropsychiatr. Genet. 144B, 660–666 (2007). [DOI] [PubMed] [Google Scholar]
- 99.Proudnikov D., Kroslak T., Sipe J. C., Randesi M., Li D., Hamon S., Ho A., Ott J., Kreek M. J., Association of polymorphisms of the cannabinoid receptor (CNR1) and fatty acid amide hydrolase (FAAH) genes with heroin addiction: Impact of long repeats of CNR1. Pharmacogenomics J. 10, 232–242 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Briant J. A., Nielsen D. A., Proudnikov D., Londono D., Ho A., Ott J., Kreek M. J., Evidence for association of two variants of the nociceptin/orphanin FQ receptor gene OPRL1 with vulnerability to develop opiate addiction in Caucasians. Psychiatr. Genet. 20, 65–72 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nielsen D. A., Ji F., Yuferov V., Ho A., He C., Ott J., Kreek M. J., Genome-wide association study identifies genes that may contribute to risk for developing heroin addiction. Psychiatr. Genet. 20, 207–214 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Levran O., Awolesi O., Linzy S., Adelson M., Kreek M. J., Haplotype block structure of the genomic region of the mu opioid receptor gene. J. Hum. Genet. 56, 147–155 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singla N., Minkowitz H. S., Soergel D. G., Burt D. A., Subach R. A., Salamea M. Y., Fossler M. J., Skobieranda F., A randomized, phase IIb study investigating oliceridine (TRV130), a novel μ-receptor G-protein pathway selective (μ-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty. J. Pain Res. 10, 2413–2424 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schmid C. L., Kennedy N. M., Ross N. C., Lovell K. M., Yue Z., Morgenweck J., Cameron M. D., Bannister T. D., Bohn L. M., Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171, 1165–1175.e13 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Negus S. S., Freeman K. B., Abuse potential of biased mu opioid receptor agonists. Trends Pharmacol. Sci. 39, 916–919 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spahn V., Del Vecchio G., Rodriguez-Gaztelumendi A., Temp J., Labuz D., Kloner M., Reidelbach M., Machelska H., Weber M., Stein C., Opioid receptor signaling, analgesic and side effects induced by a computationally designed pH-dependent agonist. Sci. Rep. 8, 8965 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ding H., Kiguchi N., Yasuda D., Daga P. R., Polgar W. E., Lu J. J., Czoty P. W., Kishioka S., Zaveri N. T., Ko M.-C., A bifunctional nociceptin and mu opioid receptor agonist is analgesic without opioid side effects in nonhuman primates. Sci. Transl. Med. 10, eaar3483 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clauw D. J., Essex M. N., Pitman V., Jones K. D., Reframing chronic pain as a disease, not a symptom: Rationale and implications for pain management. Postgrad. Med. 131, 185–198 (2019). [DOI] [PubMed] [Google Scholar]
- 109.Butelman E. R., Yuferov V., Kreek M. J., κ-opioid receptor/dynorphin system: Genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 35, 587–596 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dunn A. D., Reed B., Guariglia C., Dunn A. M., Hillman J. M., Kreek M. J., Structurally-related kappa opioid receptor agonists with substantial differential signaling bias: Neuroendocrine and behavioral effects in C57BL6 mice. Int. J. Neuropsychopharmacol. 21, 847–857 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Reed B., Butelman E. R., Fry R., Kimani R., Kreek M. J., Repeated administration of opra kappa (LY2456302), a novel, short-acting, selective KOP-r antagonist, in persons with and without cocaine dependence. Neuropsychopharmacology 43, 739–750 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Schluger J. H., Borg L., Ho A., Kreek M. J., Altered HPA axis responsivity to metyrapone testing in methadone maintained former heroin addicts with ongoing cocaine addiction. Neuropsychopharmacology 24, 568–575 (2001). [DOI] [PubMed] [Google Scholar]
- 113.Niikura K., Narita M., Butelman E. R., Kreek M. J., Suzuki T., Neuropathic and chronic pain stimuli downregulate central μ-opioid and dopaminergic transmission. Trends Pharmacol. Sci. 31, 299–305 (2010). [DOI] [PubMed] [Google Scholar]
- 114.Massaly N., Copits B. A., Wilson-Poe A. R., Hipólito L., Markovic T., Yoon H. J., Liu S., Walicki M. C., Bhatti D. L., Sirohi S., Klaas A., Walker B. M., Neve R., Cahill C. M., Shoghi K. I., Gereau R. W., McCall J. G., Al-Hasani R., Bruchas M. R., Morón J. A., Pain-induced negative affect is mediated via recruitment of the nucleus accumbens kappa opioid system. Neuron 102, 564–573 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y., Kong Q., Yue J., Gou X., Xu M., Wu X., Genome-edited skin epidermal stem cells protect mice from cocaine-seeking behaviour and cocaine overdose. Nat. Biomed. Eng. 3, 105–113 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Inturrisi C. E., Max M. B., Foley K. M., Schultz M., Shin S.-U., Houde R. W., The pharmacokinetics of heroin in patients with chronic pain. N. Engl. J. Med. 310, 1213–1217 (1984). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/10/eaax9140/DC1
Table S1. Methadone maintenance treatment for opiate (heroin) addiction.
Table S2. SNPs of genes related to endocrine stress responsivity that has been found to be associated with opioid addiction (101, 102, 116).
Fig. S1. Model for the contribution of pain states and pain treatment to the development of OUD.


