Abstract
Since 2001, more than 2.7 million U.S. military personnel have been deployed in support of operations in Southwest Asia and Afghanistan. Land-based personnel experienced elevated exposures to particulate matter and other inhalational exposures from multiple sources, including desert dust, burn pit combustion, and other industrial, mobile, or military sources. A workshop conducted at the 2018 American Thoracic Society International Conference had the goals of: 1) identifying key studies assessing postdeployment respiratory health, 2) describing emerging research, and 3) highlighting knowledge gaps. The workshop reviewed epidemiologic studies that demonstrated more frequent encounters for respiratory symptoms postdeployment compared with nondeployers and for airway disease, predominantly asthma, as well as case series describing postdeployment dyspnea, asthma, and a range of other respiratory tract findings. On the basis of particulate matter effects in other populations, it also is possible that deployers experienced reductions in pulmonary function as a result of such exposure. The workshop also gave particular attention to constrictive bronchiolitis, which has been reported in lung biopsies of selected deployers. Workshop participants had heterogeneous views regarding the definition and frequency of constrictive bronchiolitis and other small airway pathologic findings in deployed populations. The workshop concluded that the relationship of airway disease, including constrictive bronchiolitis, to exposures experienced during deployment remains to be better defined. Future clinical and epidemiologic research efforts should address better characterization of deployment exposures; carry out longitudinal assessment of potentially related adverse health conditions, including lung function and other physiologic changes; and use rigorous histologic, exposure, and clinical characterization of patients with respiratory tract abnormalities.
Keywords: deployment, particulate matter, constrictive bronchiolitis
Contents
Overview
Introduction
Methods
Exposure Overview
Exposure Assessment
Institute of Medicine Report
Effects of Desert Dust on Respiratory Illness
PM2.5 and Long-Term Pulmonary Health Effects in Nonmilitary Cohorts
Epidemiologic and Observational Studies in Previously Deployed Military Personnel
DoD Healthcare Encounters
VHA Healthcare Encounters
Prospective Cohort Studies
European Studies
Findings in Clinical Assessments of Previously Deployed Military Personnel and Veterans
Asthma and Airway Diseases
Reduction in Diffusing Capacity of the Lung for Carbon Monoxide
Constrictive Bronchiolitis and Other Small Airway Lung Biopsy Findings
Eosinophilic Pneumonia
Other Pulmonary Abnormalities
Airborne Hazards and Open Burn Pit Registry
New Approaches to Assessment of Deployment-related Exposures and Health Effects
Assessment of Deployment PM2.5 Exposures
DoD Serum Repository and Metabolomics
Mechanisms of Lung Epithelial Injury
Summary and Key Questions
Deployment-associated Exposures
Adverse Respiratory Health Effects
Key Questions
Overview
Land-based U.S. military personnel deployed to Afghanistan and Southwest Asia in support of military operations starting in 2001 experienced exposures to elevated levels of fine particulate matter (PM) as well as other, not well-characterized inhalational exposures, leading to concerns about potential adverse health effects (1). Workshop goals were to 1) summarize and assess studies evaluating postdeployment respiratory health, 2) update emerging research, and 3) identify conflicting findings and knowledge gaps.
Iraq, Afghanistan, and other deployment locations include large arid and semiarid regions where there is frequent exposure to geologic dust (2, 3). Additional sources of PM include various military operations such as burn pit emissions from open-air waste burning, vehicular exhaust, and poorly regulated industrial point sources. The workshop reviewed epidemiologic studies analyzing health encounters during military service that have demonstrated increased deployment-associated encounters for respiratory symptoms and selected airway disease diagnoses (4–6). We also considered reports based on Veterans Health Administration (VHA) encounters for health care, suggesting that former deployers may be more frequently diagnosed with obstructive lung disease, particularly asthma (7). In addition, we reviewed case series describing the clinical assessment of postdeployment dyspnea, and we considered Millennium Cohort Study data relevant to postdeployment asthma as well as other abnormalities (8–10). Of particular note, constrictive bronchiolitis and other small airway abnormalities have been reported in lung biopsies from case referral series (11). Based on a review of the established pathologic features of constrictive bronchiolitis (12–14), opinions among workshop participants differed regarding the interpretation of the prevalence of disease reported.
On the basis of a review of the adverse health effects of exposure to particulate matter with an aerodynamic diameter <2.5 μm (PM2.5) in general population studies (15, 16), the workshop concluded that it was plausible that deployers could have experienced particulate exposure–associated decrements in pulmonary function. It was recognized, however, that there have been no findings to date assessing long-term health effects in cohorts of U.S. military personnel exposed to high ambient PM2.5 concentrations. The workshop was updated on ongoing research projects. The Department of Veterans Affairs (VA) Cooperative Studies Program is using National Aeronautics and Space Administration (NASA) satellite data and airport visibility data (17, 18) to estimate historical PM2.5 concentrations and assess associations with pulmonary function and current asthma in a national cohort of veteran deployers. The Department of Defense (DoD) Serum Repository, which includes 60 million samples, may serve as a supplemental means to identify chemical compounds associated with combustion product–related environmental exposures (19). Prospective cohort studies (the Millennium Cohort Study [20] and a planned Comparative Health Assessment Interview Research Study) and follow-up of participants enrolled in the Airborne Hazards and Open Burn Pit Registry also may provide additional information regarding the development of deployment-related health conditions. Another study, based at National Jewish Health (NJH), assessing molecular effects of desert dust exposure in Southwest Asia may provide information regarding biologic mechanisms.
Key Conclusions
-
•
Deployed military personnel were exposed to ambient PM and other air pollutants, with contributions from a variety of sources, including geologic dust; mobile and stationary sources; industrial sites; and various military operations, including burn pits.
-
•
Studies of deployed military personnel reported to date have not included consistent assessment of self-reported or other estimated exposures potentially relevant to adverse respiratory effects.
-
•
Findings derived from epidemiologic studies conducted in nonmilitary populations raise concern about the potential adverse effects of PM exposure on pulmonary function in deployers.
-
•
On the basis of military health encounter data, returning military personnel had more frequent postdeployment health encounters than nondeployed personnel for respiratory symptoms and for airway disease, predominantly asthma.
-
•
Postdeployment asthma has been described in case series and among deployers assessed in the Millennium Cohort Study (U.S. DoD).
-
•
Other respiratory health conditions, including constrictive bronchiolitis and other small airway abnormalities, have been described in case series.
-
•
Workshop participants had heterogeneous views regarding the interpretation of histologic changes of constrictive bronchiolitis and other small airway findings derived from case series of veterans undergoing lung biopsy for postdeployment dyspnea.
-
•
There is a paucity of information regarding the long-term respiratory health status of postdeployment active military personnel and veterans.
-
•
There is a need for further research better characterizing PM and other deployment-related exposures, to better characterize the respiratory health of postdeployment military personnel and veterans, and to identify associations between such exposures and adverse respiratory health outcomes.
Introduction
Since October 2001, more than 2.7 million U.S. military personnel have been deployed to Central Asia (Afghanistan and Kyrgyzstan), Southwest Asia (Iraq, Kuwait, Qatar, and United Arab Emirates), and Africa (Djibouti) in support of Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), Operation New Dawn, and continuing operations (21). Land-based personnel deployed to these countries experienced potential exposures to elevated concentrations of fine PM and other pollutants, leading to concerns about possibly associated adverse health effects.
The American Thoracic Society (ATS) sponsored an all-day workshop, titled “Respiratory Health after Military Service in Southwest Asia and Afghanistan,” that took place at the 2018 ATS International Conference in San Diego, California. The workshop was initiated, in part, in response to reports of an increased prevalence of respiratory symptoms and lung disease among postdeployment veterans. In particular, reported findings of lung biopsy–defined constrictive bronchiolitis in symptomatic veterans have raised concern about a possible newly emerging pattern of lung injury in this population. Workshop goals were to 1) summarize and critically assess published data relevant to postdeployment respiratory health, 2) provide an update on emerging research on this question, and 3) identify knowledge gaps that could be addressed by future research.
Understanding the nature of deployment-associated inhalational exposures and their potential adverse respiratory health effects among veterans is relevant not only to VA providers but also to the wider healthcare community, because many veterans receive medical care outside the VHA. Approximately 40% of veterans are enrolled in VA healthcare programs, and only 65% of those access care in a given year (22). Moreover, the proportion of veterans who receive care outside the VHA is likely to increase with new VA policy initiatives encouraging community-based care. Thus, non-VHA providers are very likely to encounter patients with prior deployment-associated exposures who are experiencing potentially related adverse respiratory health effects.
Methods
A cross-disciplinary group of 25 experts representing a range of perspectives and viewpoints participated in the workshop. Participants represented the disciplines of pulmonary medicine, occupational and environmental medicine, medical pathology, epidemiology and biostatistics, respiratory physiology, and translational and laboratory-based research. Areas of specific expertise included air pollution exposure assessment, geographic information systems, lung pathology, exercise physiology, survey research methodology, and epidemiologic study design and analysis. Institutional backgrounds included academic medical centers, the VA (including VHA and VA medical centers), and the DoD.
There were 16 invited oral presentations. These presentations included the following:
-
1.
A review of surveys and epidemiologic studies performed among previously deployed military personnel, including non–U.S. military personnel;
-
2.
A summary of clinical data reported for previously deployed military personnel, including veterans treated in the VHA;
-
3.
A review of lung pathologic findings in postdeployment personnel, including biopsies interpreted as showing constrictive bronchiolitis;
-
4.
A review of airborne pollution exposures that might occur during deployment and their potential adverse respiratory health effects, including dust storms and other sources of PM such as burn pits and other military activities;
-
5.
An update on novel approaches to exposure assessment, including estimation of PM exposures using satellite technology and the use of biobanked DoD serum samples to assess biomarkers of exposure; and
-
6.
A description of other ongoing or planned relevant studies.
There were group discussions with all panelists after each presentation or main topic and a final overarching discussion of the workshop findings as a whole. After the workshop, each speaker provided their presentation materials (typically the set of slides shown), supplementing this with a written summary and references when appropriate. A writing committee composed of a subset of workshop participants reviewed a summary of the presentations. This summary underwent editing and was circulated to all participants for further comment. Potential conflicts of interest of all participants were disclosed before the workshop and updated before this publication. Conflict of interest disclosures were managed according to the policies and procedures of the ATS.
Exposure Overview
Exposure Assessment
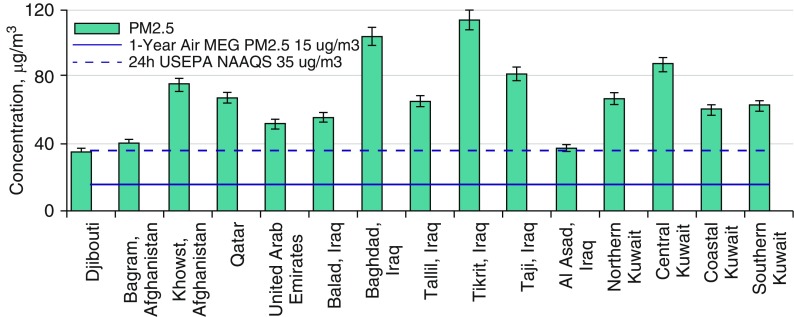
Iraq, Afghanistan, and other deployment locations include large arid or semiarid regions where there is frequent exposure to desert dust and sand, including from frequent dust storms (23–25). In addition to mineral particles, dust storm particles include airborne bacteria, fungal spores, plant and grass pollens, and other agricultural pollen grains (26, 27). Additional sources of PM include military operations, vehicle exhaust, and underregulated industrial sources. Open-air waste burning (burn pits) was the primary means of solid-waste management at military bases in Iraq, Afghanistan, and Djibouti that also contributed to potential exposures (1). Materials burned included plastics, metals, wood, and numerous other combustible materials. To assess exposure levels, the DoD conducted the Enhanced Particulate Matter Surveillance Program (EPMSP) to characterize airborne exposures at 15 sites, mainly in Iraq and Afghanistan and mainly during 2006 and 2007 (3, 28). These efforts were difficult in an active war zone, and only a small number of samples at each site were collected. For example, sampling occurred approximately four times per month at each location for PM2.5, raising concerns regarding the accurate assessment of exposure variability. In addition, the equipment was not designed to collect PM during the highest exposure days (including dust storms), a limitation that would have resulted in the overloading of the sampler particle impaction surface, leading to sampling error. Recognizing these limitations, the mean 24-hour PM2.5 values observed were consistently high at each site (∼40 μg/m3 to nearly 120 μg/m3) (Figure 1) with concentrations that considerably exceeded the current U.S. annual PM2.5 exposure standard (12 μg/m3), the 1-year military exposure guideline (solid blue line in Figure 1), and the 24-hour U.S. Environmental Protection Agency National Ambient Air Quality Standard (35 μg/m3; dashed blue line in Figure 1).
Figure 1.
Mean particulate matter with an aerodynamic diameter less than or equal to 2.5 μm (PM2.5) concentrations at 15 sites in Iraq, Afghanistan, Kuwait, and other sites in 2006 and 2007 (28). MEG = military exposure guideline; NAAQS = National Ambient Air Quality Standards; USEPA = U.S. Environmental Protection Agency.
Other exposure estimates indicated similarly elevated PM2.5 concentrations. For example, in daily sampling conducted in Kuwait City in 2004–2005 using equipment suitable for a desert environment, the annual average PM2.5 value was 53 μg/m3 (2, 29), within the range of mean PM2.5 concentrations found by EPMSP. As noted in this document (see below and Figure 2), the PM2.5 levels estimated based on military airport visibility data in Iraq, Afghanistan, Kuwait, and other countries in Southwest Asia (18) were also similar to the EPMSP concentrations.
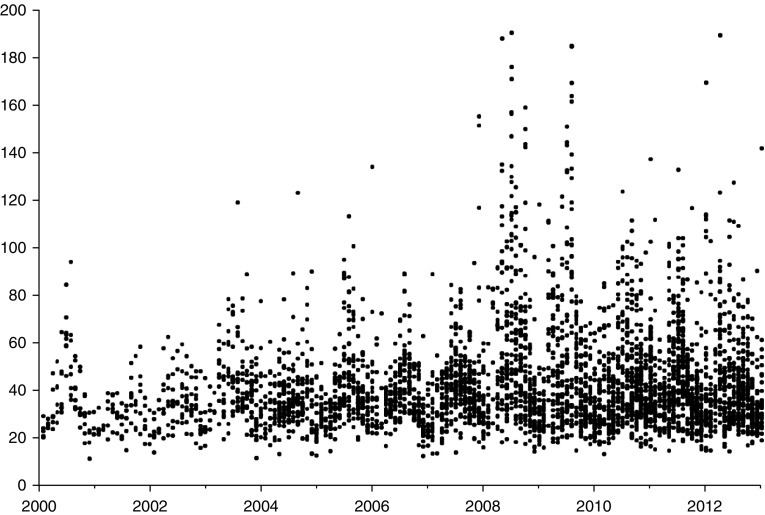
Figure 2.
Monthly predictions of particulate matter with an aerodynamic diameter less than or equal to 2.5 μm in μg/m3 (y-axis) by year (2000–2012) for sites in Afghanistan, Iraq, Kuwait, Kyrgyzstan, United Arab Emirates, Djibouti, and Qatar, based on military airport visibility data. Reprinted from Reference 18 by permission of Air & Waste Management Association, www.awma.org.
Institute of Medicine Report
There has been considerable discussion regarding the potential for adverse health effects of burn pit emissions, given the often-continuous combustion of military base waste. In 2006, 2007, and 2009, multiple air samples were collected by the U.S. Army at Joint Base Balad, the site of the largest burn pit in Iraq (30). PM2.5 concentrations upwind and downwind of the burn pit were similar, indicating that off-base sources (representing ambient PM2.5) significantly contributed to on-base exposures. Before the publication of subsequent studies reviewed at the workshop, the Institute of Medicine (IOM; currently the Health and Medicine Division of the National Academies of Sciences, Engineering, and Medicine) was charged by the VA with assessing whether burn pit–related exposures could contribute to long-term health effects after deployment. The 2011 IOM report titled “Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan,” noted that “a broader consideration of air pollution than exposure only to burn pit emissions―might be associated with long-term health effects, particularly in highly exposed populations (such as those who worked at the burn pit) or susceptible populations (for example, those who have asthma), mainly because of the high ambient concentrations of PM from both natural and anthropogenic, including military, sources” (1). The conclusions of this IOM report support the importance of considering the occurrence of health effects from all sources that jointly contribute to the high PM concentrations and also other pollutants. As an example of the contribution of multiple sources to ambient PM2.5, a source apportionment study from Kuwait estimated contributions of 54% from sand dust, 18% from oil combustion, 12% from the petrochemical industry, 11% from traffic, and 5% from other anthropogenic sources (2).
Findings derived from the EPMSP also indicate that multiple sources contribute to ambient PM exposures experienced during deployment (3). In addition to PM mass, the EPMSP characterized PM composition by conducting energy-dispersive X-ray fluorescence spectrometry for trace elements and scanning electron microscopy for particle filter samples. The major portion of PM was of geologic origin from local soils (31). Analysis also identified the contribution of industrial sources to PM that varied by location, such as lead, zinc, and other metals from nearby smelters and battery manufacturing, as well as from transportation activities, in particular due to the use of leaded gas. Carbon particles in samples were consistent with contributions from mobile and stationary combustion sources, including burn pits (1). At Joint Base Balad, polychlorinated dibenzo-para-dioxin/furans, polycyclic aromatic hydrocarbons, and volatile organic compounds were detected in both upwind and downwind air samples (1). The polycyclic aromatic hydrocarbon and volatile organic compound concentrations attributable to regional background sources, transportation emissions, and power generation were similar to concentrations noted in polluted environments outside the United States. The polychlorinated dibenzo-para-dioxin/furans concentrations were low and interpreted as most likely being attributable to burn pit emissions.
Effects of Desert Dust on Respiratory Illness
Despite the frequent occurrence of dust storms and the significant contribution of desert dust to ambient PM in the deployment region, a review of existing health studies presented at the workshop identified no large-scale epidemiologic studies assessing the cumulative effect of PM on pulmonary function or on risk of asthma or other chronic pulmonary diseases among exposed populations, although a few observational reports were available for review (P. Yiallouros, workshop presentation). Reports of adverse effects of dust storms on health have focused primarily on the description of short-term (acute and subacute) adverse respiratory effects. The first report of adverse effects of exposure to dust was reported in 1935 in response to a period of severe dust storms during the Dust Bowl period in the United States in Kansas, Colorado, New Mexico, Oklahoma, and Texas (32). These states experienced an increase in death rates attributable to acute respiratory infections, including bronchitis and pneumonia.
More recent studies have confirmed that desert dust exposures may have acute adverse health effects, both respiratory and nonrespiratory. There have been associations reported between desert dust events and increases in mortality of nonaccidental causes (33–35), cardiovascular mortality (36, 37), and respiratory mortality (38, 39). In Kuwait, dust storms also have been associated with emergency hospital admissions for asthma and for all respiratory causes; the association was strongest in children but also included adults (40). In studies that included younger adults of age comparable to that of deployed military personnel, days of desert dust exposure was associated with more emergency room visits for asthma and all respiratory causes in Athens (41); more asthma hospitalizations in El Paso, Texas (42); and an increase in asthma symptom scores in Japan resulting from transcontinental transport of dust (43). Desert dust also has been associated with more clinic visits for allergic rhinitis during dust storm events (44) and more allergic symptoms (45). Others have reported associations with respiratory infections, including hospital admissions for pneumonia (46–48) and chronic obstructive pulmonary disease (COPD) exacerbations (49, 50). Because dust storms include some quartz particles, it has been suggested that chronic exposure may result in nonoccupational silicosis on the basis of findings from surveys conducted in Himalayan villages (51, 52) and autopsy studies in Bedouins from the Negev region of Israel (53).
Surveys of military personnel that were conducted immediately after leaving deployment suggest that personnel could have experienced acute respiratory effects similar to those in the desert dust–exposed cohorts described above. Sanders and coworkers (54) surveyed 15,459 U.S. military personnel who had deployed to Iraq or Afghanistan during 2003–2004. Respiratory illnesses were reported by 69%. Of those with respiratory illness, 23% reported an allergy attack, 17% sought medical care, and 3.6% reported an asthma attack. In another survey conducted between April and July 2003, 95% of 1,250 consecutive service members returning from deployment completed a questionnaire assessing respiratory health retrospectively (55). The self-reported prevalence of wheezing in persons without a history of asthma was 19% during deployment compared with 6% before deployment, and it was 51% during deployment in persons with a history of asthma compared with 40% before deployment. Allergy symptoms, defined as sneezing, rhinitis, or eye irritation, were reported by 55% of those without asthma during deployment compared with 27% before deployment, and they were reported by 62% among those with asthma during deployment compared with 44% before deployment. Surveys of Polish troops serving in Afghanistan also demonstrated high rates of acute respiratory illness (56, 57). A study of Soviet troops during the 1979–1989 war in Afghanistan found that 43% of service personnel had bronchitis and/or pneumonia within the first year of service in Afghanistan (58).
PM2.5 and Long-Term Pulmonary Health Effects in Nonmilitary Cohorts
Population-based epidemiologic studies assessing exposure to ambient PM2.5 have demonstrated that chronic exposure (10–14 yr in duration) at concentrations lower than those experienced during deployment results in reduced pulmonary function assessed by spirometry. These studies include the Framingham Heart Study in the United States (15) and the Taiwan MJ Cohort Study (16), in which PM2.5 concentrations were estimated retrospectively at the participant’s home address using aerosol optical depth (AOD) data available from NASA satellites. AOD is a measure of light extinction by particles in the atmosphere that can be calibrated using ground stations to estimate ground-level PM2.5 (59–61). In the Taiwan MJ Cohort Study, there also was an increased risk of incident COPD attributable to greater exposure (16). Other cohort studies conducted in North America and Europe also suggest an association between ambient air pollution and the development of COPD (62–64). Small airway structural changes have also been described in association with ambient pollution in a comparison of lungs obtained at autopsy from nonsmoking Mexico City (high ambient air pollution) residents without lung disease and from Vancouver (low ambient air pollution) residents. The Mexico City residents had more fibrous tissue and smooth muscle in small airway walls (65).
The findings of these epidemiologic studies are consistent with the conclusions of the 2011 IOM report that considered the potential effects of exposure to burn pit emissions by reviewing effects in surrogate groups that included firefighters and incinerator workers (1). The IOM concluded that there was limited/suggestive evidence of an association between exposure to combustion products and long-term effects causing reduced pulmonary function. The IOM did not address the risk of asthma, but it considered those with preexisting asthma to be a susceptible subgroup. In contrast to studies demonstrating effects on pulmonary function, there is weaker evidence of an association between chronic PM exposure and new-onset asthma in adults (66–69).
An additional consideration that may impact health effects is the typical age of deployment at which military personnel are likely to be exposed to high concentrations of PM. As demonstrated by National Health and Nutrition Examination Survey data, lung growth as assessed by forced expiratory volume in 1 second (FEV1) occurs through the mid-20s (70). Exposure to particulate pollution during periods of lung growth in children reduces their maximally attained FEV1 (71). It is not known whether soldiers exposed to high concentrations of PM during their late teenage and early adult years are also more susceptible to adverse respiratory effects, given that they have not yet achieved their maximal FEV1. It has been recognized that a lower FEV1 in early adulthood is a risk factor for the development of future COPD (72). There is no comparable literature assessing pulmonary function in populations similar to previously deployed veterans who experienced high PM exposures over a relatively short duration based on total deployment time (typically lasting several months to several years among Southwest Asia and Afghanistan deployers, with an estimated mean [standard deviation (SD)] of 10.6 [6.6] mo) (E. Garshick, workshop presentation of unpublished data).
Epidemiologic and Observational Studies in Previously Deployed Military Personnel
DoD Healthcare Encounters
Asthma diagnosed at any age previously had been cause to be medically disqualified from enlistment in the U.S. military, although persons could apply for a medical waiver. Between 1997 and 2002, 15% of all U.S. Army medical waivers were for asthma. In 2004, the standards were relaxed to medically disqualify persons with asthma from military service only if diagnosed and symptomatic after age 13 (73, 74). Once in the military, persons are provided asthma medications if required, and fitness for duty is assessed on the basis of their ability to serve in their unit. It is likely that, since 2004, policy changes resulted in persons with a history of childhood asthma more likely to be inducted into the military and deployed.
Investigators from the U.S. Army Public Health Command reviewed clinical encounters using International Classification of Diseases, Ninth Revision (ICD-9), codes to categorize respiratory health–related conditions before and after deployment. Analysis of these data indicated a postdeployment increase in encounters for respiratory symptoms and obstructive lung diseases, predominantly asthma (4–6). In U.S. military personnel deployed as of December 31, 2005, pre- and postdeployment medical encounter rates 6 months before and 6 months after deployment were compared (4). Rates of encounters for asthma/COPD and allied conditions (ICD-9 codes 490–496) were significantly increased after deployment (rate ratio, 1.25; 95% confidence interval [CI], 1.13–1.39), although there was not a step-up in risk with multiple deployments. Respiratory symptom rates (ICD-9 code 786) also increased after deployment.
In another report (5), rates of respiratory encounters in U.S. Air Force and U.S. Army personnel deployed between 2005 and 2007 to two sites in Iraq with burn pits (Joint Base Balad and Taji) and two sites in Kuwait without burn pits were compared over up to 4 years, with further comparison with U.S.-based troops eligible for deployment but not deployed. Individuals with previous asthma or COPD encounters were excluded from the analysis. Adjusted for age, sex, and rank, rates for respiratory symptoms (ICD-9 code 786) and for asthma (ICD-9 code 493) were significantly elevated among the deployed compared with the nondeployed, with relative risks (RRs) of 1.25 (95% CI, 1.20–1.30) and 1.54 (95% CI, 1.33–1.78), for symptoms and asthma, respectively. There was no significant difference, however, in risk when individuals deployed to locations in Southwest Asia with and without burn pits were compared. In another study, personnel deployed to Kabul (Afghanistan) were followed for up to 12 years after deployment (6). Compared with nondeployed U.S. personnel, deployment to Kabul was associated with more encounters for respiratory symptoms (RR, 1.54; 95% CI, 1.43–1.62) and asthma (RR, 1.61; 95% CI, 1.22–2.12), adjusted for covariates including age, sex, race, and rank. These associations were not observed for deployment to Kyrgyzstan or Bagram Air Base (Afghanistan) for asthma, although Bagram deployers had a higher risk of respiratory symptom encounters than nondeployed U.S. personnel (RR, 1.12; 95% CI, 1.05–1.19). The findings of this study support an interpretation that the effects of deployment may vary on the basis of ambient PM and other related exposures, a concern raised in light of relatively high levels of air pollution in Kabul (6).
VHA Healthcare Encounters
In an assessment of asthma prevalence among returning troops, Szema and colleagues (75) reviewed the records of U.S. soldiers discharged from military service and examined between March 2004 through May 2007 at the Northport VA Medical Center. They found that 61 of 920 (6.6%) soldiers deployed to Iraq or Afghanistan had been diagnosed with asthma. This rate was higher than that of soldiers who remained in the United States (age and sex adjusted odds ratio [OR], 1.88; 95% CI, 1.38–2.56). In a follow-up study, record review through 2010 noted an asthma diagnosis in 6.2% of previous deployers compared with 0.7% of nondeployed personnel in an analysis unadjusted for other factors that may have influenced asthma rates (76). Among deployers receiving VHA health care nationally from October 2002 through September 2011, the prevalence of encounters for chronic lung disease increased (7). During that time, 760,621 previously deployed veterans received VHA care, and 4.5% (n = 34,228) had at least one diagnosis of chronic lung disease, including COPD (0.8%), asthma (3.4%), or interstitial lung disease (0.3%). In analyses adjusting for demographics, multiple deployments, surrogates of tobacco use (i.e., smoking cessation treatment), and traumatic brain injury diagnosis, there was a statistically significant increased prevalence of asthma and COPD through 2011. In contrast, interstitial lung disease prevalence remained stable during this period. Factors associated with any of the three lung disease diagnoses included proxies for both tobacco use and traumatic brain injury (as a surrogate for blast injury). In contradistinction to these studies among veterans, rates of asthma and chronic bronchitis among active duty personnel decreased over the same time period (2001–2013) (77).
In another VA-based study of respiratory diagnoses, in which VHA medical records between 2001 and 2010 were used, deployed U.S. veterans who sought VHA care within 1 year of their last deployment and who had at least encounters per year for a minimum of 2 years were analyzed (78). The respiratory diagnoses studied were similar to those examined by Pugh and colleagues (7), but they also included codes for nonspecific respiratory encounters. Overall, 182,338 Iraq/Afghanistan veterans met inclusion criteria, of whom 14% had a respiratory diagnosis. Approximately 77% of veterans with a respiratory diagnosis also had a comorbid mental health diagnosis. A mental health diagnosis within the first year after deployment was associated with report of a respiratory diagnosis over 5 years of follow-up, adjusting for multiple demographic factors, multiple deployments, and tobacco use proxies (OR, 1.41; 95% CI, 1.37–1.46). Specific diagnoses with a statistically significant association included acute and chronic bronchitis (OR, 1.39; 95% CI, 1.21–1.60) and asthma (OR, 1.16; 95% CI, 1.08–1.25).
Prospective Cohort Studies
The Millennium Cohort Study was established by the DoD to prospectively study the short- and long-term self-reported health effects of military service and has since become the largest and longest-running health study in military history (20). Compared with 46,077 participants from the first enrollment panel who completed baseline questionnaires in 2001–2003, subsequent deployed persons more frequently self-reported respiratory symptoms (surveyed 2004–2006) than did nondeployers (14% vs. 10%) (79). Symptoms were defined by self-reported persistent or recurring cough or shortness of breath. Increased symptom reporting was not associated with sea-based deployment (i.e., U.S. Navy personnel would have had lower PM exposures when at sea). Compared with nondeployers, deployed personnel from the U.S. Army (OR, 1.73; 95% CI, 1.57–1.91) and the U.S. Marine Corps (OR, 1.49; 95% CI, 1.06, 2.08) had increased odds of symptom reporting, adjusted for smoking, age, rank, sex, military occupation, and education. Among U.S. Army personnel, the odds of respiratory symptoms also increased with deployment length. In a subsequent analysis of U.S. Army and U.S. Air Force deployers participating in the Millennium Cohort Study, deployment to a location near a burn pit, defined as a 3- or 5-mile radius (both were examined) from a burn pit, at three locations in Iraq was not statistically associated with new-onset respiratory symptoms (80). There was also no statistical association in this analysis with self-reported incident asthma, chronic bronchitis, or emphysema. Subsequent and longer follow-up of a larger number of Millennium Cohort Study participants followed through 2013 (n = 77,770) revealed an increased risk of respondent-reported new onset of health professional–diagnosed asthma for those with deployment-related combat experience compared with those who did not deploy (9). The RR of new-onset asthma was similar for men (RR, 1.30; 95% CI, 1.14–1.47) and women (RR, 1.24; 95% CI, 1.05–1.46) after adjustment for multiple covariates that included smoking, body mass index (BMI), rank, branch, and post-traumatic stress disorder status. There was no association with multiple deployments or deployment duration, and there was no increase in asthma in persons deployed but not in combat. Explanatory factors for the observed differences associated with combat experience were not identified in the study. Additional analyses of Millennium Cohort data based on deployment proximity to burn pits are in progress, including updated assessments of postdeployment asthma, COPD, and respiratory symptoms (R. P. Rull, workshop presentation of unpublished data).
The VA Post Deployment Health Services Epidemiology Program has conducted the Health Study for a New Generation of US Veterans, reporting on 20,563 OEF/OIF veterans who replied to a 2009–2011 survey eliciting a history of self-reported, physician-diagnosed asthma, sinusitis, and bronchitis (81). Adjusted for birth year, sex, service branch, unit component, race/ethnicity, education, and smoking status, the prevalence of asthma and bronchitis diagnosed after 2001 (the period of U.S. involvement in Southwest Asia and Afghanistan) among the deployed veterans and nondeployed veterans was not significantly different. Deployed veterans, however, were more likely to report sinusitis (OR, 1.30; 95% CI, 1.13–1.49). The VA Post Deployment Health Services Epidemiology Program has completed data collection for a new epidemiologic study called the Comparative Health Assessment Interview Research Study. This study includes a population sample of 15,172 OEF/OIF/Operation New Dawn veterans, both deployed and nondeployed, and 4,654 civilians to assess a variety of health outcomes. The study plans to report on the prevalence of asthma and other lung diseases as well as to assess information on respiratory exposures from military, deployment, and occupational settings (A. I. Schneiderman, workshop presentation of unpublished data).
European Studies
A retrospective questionnaire-based study of Swedish military personnel who served primarily in Afghanistan (2008–2009) and who were surveyed 36 months to 5 years later found an increased prevalence of wheeze, wheeze without a cold, nocturnal coughing, and chronic bronchitis among soldiers compared with a referent group of civilians (82). A statistically significant relationship was found between months spent in a desert environment and wheeze, wheeze with breathlessness, and wheeze without a cold. Exposure to dust storms was also associated with report of nocturnal cough and chronic bronchitis. Studies of respiratory illness among Polish and Russian military personal soldiers while in service were cited previously (56–58), but there does not appear to be published data on postdeployment follow-up among these cohorts.
Findings in Clinical Assessments of Previously Deployed Military Personnel and Veterans
Four centers that regularly evaluate veterans and military personnel after deployment have published their clinical findings. These centers are the VA War Related Illness and Injury Study Center (WRIISC), NJH, Brooke Army Medical Center and other military medical facilities, and Vanderbilt University Medical Center. Their reported findings are summarized below.
Asthma and Airway Diseases
Assessment of active duty personnel and veterans at these four centers demonstrated that results of postdeployment spirometry were usually normal and that the most common specific diagnoses were asthma and nonspecific airway hyperresponsiveness. The VA WRIISC is a VHA national referral program (83) that provides comprehensive clinical evaluations of U.S. veterans with postdeployment health concerns. Among 124 veterans referred for evaluation to the New Jersey (NJ) WRIISC (both with and without respiratory symptoms), 26% had a positive bronchodilator response measured in spirometry. This was positively associated with deployment length, adjusted for smoking history (84). In a subsequent description of 138 veterans evaluated at the NJ WRIISC, 74.6% had normal pulmonary function, 19.6% had an obstructive deficit, and 5.8% had a restrictive deficit. These data, however, were not analyzed in regard to smoking history, BMI, clinical history, or the presence of respiratory symptoms (85).
At NJH, among 127 consecutive symptomatic military deployers referred for clinical evaluation, the most common symptoms were exertional dyspnea, 82%; cough, 77%; chest tightness, 74%; and wheezing, 67%. Only 2% were current smokers, and 36% were former smokers. Asthma was diagnosed in 31.5%, rhinitis/rhinosinusitis in 15%, and inducible laryngeal obstruction in 14.2% (S. D. Krefft, workshop presentation of unpublished data). Among 113 previously deployed, spirometry was normal in 70.8%, suggested restriction was present in 19.5%, obstruction was present in 5.3%, and a mixed pattern was present in 4.4%. At NJH, a pilot study exploring the utility of lung clearance index (LCI) testing as a marker of small airway dysfunction was conducted in 28 previously deployed veterans with respiratory symptoms evaluated at NJH. Compared with 24 referents without known lung disease, those previously deployed were found to have significantly higher mean LCI scores (86).
Brooke Army Medical Center investigators, in conjunction with other military treatment facilities, assessed the relationship between military deployment and chronic lung disease by electronic review of DoD medical records between 2005 and 2009. Additional direct clinical assessments were not performed as part of that study. There were 371 patients (52% previously deployed; 48% not deployed) with a minimum of three encounters for COPD/emphysema. There was no statistical difference in pulmonary function assessed by spirometry comparing deployers with nondeployers (87). Four hundred consecutive U.S. Army personnel undergoing medical discharge with a clinical diagnosis of asthma were also reviewed (previously deployed, 48.5%; nondeployers, 51.5%). Of 194 previously deployed with asthma, 52% had been diagnosed with asthma after deployment, whereas 48% had been diagnosed previously (10).
The STAMPEDE study (Study of Active Duty Military for Pulmonary Disease Related to Environmental Deployment Exposures) was conducted by the same investigators. STAMPEDE I prospectively evaluated 50 active duty military personnel with new-onset dyspnea during deployment (8). The primary findings in this study were that 42% had a nondiagnostic evaluation, whereas asthma and nonspecific bronchial hyperreactivity were the most common diagnoses, being present in 40%. There were frequent comorbid diagnoses, including sleep disorders (in 57%) and psychiatric conditions (in 68%). STAMPEDE II investigated the role of pre- and postdeployment spirometry in the clinical evaluation of postdeployment lung disease because spirometry is not routinely performed before deployment or as part of enlistment medical evaluations (88). In the predeployment phase, 1,693 soldiers from Fort Hood, Texas, participated. More than one-third of those surveyed reported a cigarette-smoking history, 73% had an elevated BMI, and 6.2% reported a history of asthma. Before deployment, abnormal spirometry was found in 22.3% of participants. Abnormal spirometry was more common in those with asthma (10.1% vs. 5.1%); those who failed physical fitness tests (9.0% vs. 4.6%); and those who had chronic respiratory symptoms, including wheezing, cough, or dyspnea (32.8% vs. 24.3%) (88). After deployment, 873 soldiers of the original cohort underwent repeat spirometry. A history of asthma and postdeployment wheezing was associated with airway obstruction after deployment, but an assessment of change in forced vital capacity and FEV1 before and after deployment using paired pulmonary function data was not conducted (89). In STAMPEDE III, an ongoing prospective study in which symptomatic military personnel undergo a comprehensive evaluation, the most common diagnoses in 310 participants included asthma (23%), nonspecific airway hyperresponsiveness (11%), gastroesophageal reflux (6%), and upper airway disorders (5%); 26% remained undiagnosed despite being symptomatic (M. J. Morris, workshop presentation of unpublished).
Reduction in Diffusing Capacity of the Lung for Carbon Monoxide
Among the four centers, the prevalence of a reduction in diffusing capacity of the lung for carbon monoxide (DlCO) varied, depending on center, referral population, and choice of predicted values. Among patients evaluated at the NJ WRIISC, 30% of 130 previously deployed veterans had a reduction in DlCO below the lower limit of normal, which was the only abnormality in 40.2% of patients with preserved spirometry and lung volumes (85). A reduction in DlCO was found in 23% of 48 symptomatic deployers assessed in STAMPEDE I (8). Among 108 consecutive postdeployment veterans assessed at NJH after referral for symptoms, 18.3% had an abnormally decreased DlCO. Among 38 active duty soldiers who were referred for assessment of dyspnea at Vanderbilt University Medical Center and who were reported to have constrictive bronchiolitis on lung biopsy, in 19 (50%), the only pulmonary function abnormality was an isolated reduction in DlCO (11). In 82 postdeployment service members with unexplained cough and dyspnea assessed at Walter Reed National Military Medical Center and at Fort Belvoir Community Hospital (in Virginia), DlCO, expressed as the mean percent predicted value, was 73.2% (SD, 12.1%), and 63.9% were below the fifth percentile using Crapo (90) predicted values (91). In contrast, only 9.8% had an abnormal diffusion value when Miller predicted values for DlCO were used (92). Of note, the prevalence of DlCO abnormalities from the VA WRIISC (30%) was based on Miller predicted values (85). The studies from Vanderbilt (11) and NJH used the Crapo reference equations (90), which predict higher normal values than Miller, potentially accounting for some of the differences among studies reporting DlCO abnormalities.
Constrictive Bronchiolitis and Other Lung Biopsy Findings
Pathologic criteria for constrictive bronchiolitis
As a result of case series of constrictive bronchiolitis (which has also been called “obliterative bronchiolitis”) reported after deployment, the workshop reviewed the standard pathologic criteria for assessing bronchiolitis in lung tissue samples (K. D. Jones, workshop presentation). The broad category of bronchiolitis refers to a range of disorders characterized by combinations of inflammation and fibrosis involving the small airways. The term “bronchiolitis obliterans” was noted to have a confusing history because of parallel definitions that existed until the ATS/European Respiratory Society classification agreed on a precise terminology (93, 94). Organizing pneumonia is the most common specific histologic finding to describe the entity previously referred to as “bronchiolitis obliterans organizing pneumonia.” In that condition, rounded branching polypoid plugs of granulation tissue form in the distal airways (a proliferative pattern) and extend down into alveolar ducts and alveolar spaces, and this has been associated with nitrogen dioxide exposure. In contrast to bronchiolitis obliterans organizing pneumonia, constrictive bronchiolitis is a pattern of injury characterized by subepithelial scarring resulting in narrowing or obliteration of the bronchioles, without the presence of luminal plugs. This pathology is commonly observed in chronic rejection among lung transplant recipients or in chronic graft-versus-host disease after bone marrow transplant. Other conditions in which constrictive bronchiolitis may be observed include autoimmune connective tissue disease (e.g., scleroderma, rheumatoid arthritis, systemic lupus erythematosus), other systemic disorders (e.g., inflammatory bowel disease), drug reactions (e.g., due to penicillamine), ingested toxins (e.g., Sauropus androgynus), and infection (e.g., adenovirus) (12–14, 94). Constrictive bronchiolitis has also been reported after inhalational injury, including cases after sulfur mustard (gas) used in the Iran–Iraq War (95), and in food-flavoring workers exposed to the ketone butter flavoring diacetyl (13, 96–98).
As noted by Epler (13, 14), the characteristic histopathologic finding of constrictive bronchiolitis is subepithelial scarring that leads to narrowing of the airway. There is widening of the space between basement membrane and elastica, and the segment of the bronchiole that is sclerotic and narrowed is short compared with its total length. Therefore, it is possible to miss regions of fibrotic scarring on initial sections unless there is clinical suspicion leading to review of additional sections. Overdiagnosis is also possible. In particular, narrowing of bronchioles due to ex vivo smooth muscle contraction is well described (99). This is due to normal airway smooth muscle contraction coupled with loss of tethering resulting in narrowing of the bronchiolar lumen, accompanied by scalloping or undulation of the respiratory epithelium. This artifact, which may simulate pathologic bronchiolar narrowing, is less prominent in lobectomy or pneumonectomy specimens (when perfusion fixation through the bronchi is performed) than in specimens obtained by video-assisted thoracoscopic surgical (VATS) biopsy. It was noted during the workshop that in some publications, such as one by Leslie (100), smooth muscle hypertrophy has been included in the description of constrictive bronchiolitis in addition to fibrotic changes. Most recent descriptions, however, emphasize that constrictive bronchiolitis is a fibrotic airway disorder (14, 101) and requires subepithelial fibrosis to be present (K. D. Jones, workshop presentation).
Results of lung biopsy in symptomatic deployers
Investigators from Vanderbilt University Medical Center published the first biopsy series selected from among 80 postdeployment military personnel referred from Ft. Campbell, Kentucky, with unexplained shortness of breath and exercise limitation, cough, or chest tightness after deployment (11). These personnel had evaluations including inspiratory and expiratory high-resolution computed tomography of the chest; pulmonary function testing; cardiopulmonary exercise testing; and in some cases, nonspecific bronchial challenge testing. Forty-nine underwent VATS lung biopsy, and 38 (median age, 33 yr; range, 23–44 yr) had pathology interpreted as findings of constrictive bronchiolitis. The predominant bronchiolar tissue changes were reported to include smooth muscle in 7 cases, fibrous tissue in 3 cases, and mixed smooth muscle and fibrous tissue in 28 cases. Other small airway biopsy findings included respiratory bronchiolitis (71.0% of biopsies), peribronchiolar inflammation (89.5%), pigment deposition (97.4%), polarizable material within pigment (94.7%), and bronchial-associated lymphoid tissue (50%). It has been suggested that past exposure to sulfur dioxide associated with the 2003 Mishraq Sulfur Mine fire (102) might explain these findings, although 10 of the 38 with constrictive bronchiolitis did not report this exposure history, and that condition has not been documented as associated with sulfur dioxide exposure. The 11 soldiers who underwent lung biopsy without findings of constrictive bronchiolitis did have other pathologic findings, including hypersensitivity pneumonitis, respiratory bronchiolitis, respiratory bronchiolitis–associated interstitial lung disease, and sarcoidosis. The original case series was updated to include 94 postdeployment personnel with dyspnea, of whom 75 had lung biopsies interpreted as findings of constrictive bronchiolitis (R. F. Miller, workshop presentation of unpublished data).
The other center with a similar clinical experience is NJH. In a series of 127 symptomatic military postdeployment personnel who underwent clinical evaluation at NJH, VATS lung biopsies from 52 were reported as having a range of overlapping abnormalities, including bronchiolitis (including constrictive bronchiolitis), emphysema with hyperinflation, and granulomatous pneumonitis (C. Rose, workshop presentation of unpublished data). At NJH, among 118 postdeployment referrals who underwent inspiratory and expiratory high-resolution computed tomography, air trapping was observed in approximately half and centrilobular nodularity in one-third, both of which suggest small airway disease but are not specifically diagnostic of constrictive bronchiolitis (S. D. Krefft, workshop presentation of unpublished data). There were differing views among workshop participants regarding the interpretation of the histologic changes considered as showing constrictive bronchiolitis.
In an ongoing study (C. Rose, workshop presentation of unpublished data), surgical lung biopsies from 50 deployers evaluated at either Vanderbilt or NJH were compared with 19 positive and 20 negative control lung tissue samples. Lung tissue samples were analyzed by a panel of four pulmonary pathologists blinded to sample status. Adjusting for smoking and age, and using subepithelial fibrosis as the primary histologic criterion, constrictive bronchiolitis was more common among deployers. Other abnormalities more prevalent among deployers included respiratory bronchiolitis, organizing pneumonia, hypersensitivity pneumonitis, and emphysema.
To investigate additional associations with nonmalignant pathologic pulmonary diagnoses, including small airway disease, DoD investigators at Brooke Army Medical Center and the Joint Pathology Center (JPC) conducted a retrospective review of 391 lung biopsy reports for nonneoplastic lung disease between 2005 and 2012 (137 in previous deployers). In that study, 45% of biopsies were obtained via transthoracic needle or via bronchoscopy (including some only with endobronchial biopsy samples), limiting the extent of tissue available for histologic review (103). Approximately 6% of deployers and nondeployers had small airway abnormalities, but none was interpreted as showing constrictive bronchiolitis. Deployed personnel were noted to have higher proportions of nonnecrotizing granulomas than nondeployed personnel (16.1% vs. 8.4%; P = 0.04). Findings were limited by the limited amount of lung tissue available in cases without VATS sampling and a paucity of information on clinical indications for biopsy. Future planned analyses at that center include a more in-depth histopathologic and radiologic review of surgical biopsies and lung resections in both deployed and nondeployed U.S. military personnel from 2002 to 2015 reviewed at the JPC. That study is planned to include additional cases from 2016 and 2017 and will have a subset of biopsy specimen cases analyzed by scanning electron microscopy and energy-dispersive X-ray spectroscopy (M. J. Morris, workshop presentation).
Eosinophilic Pneumonia
One of the earliest reported pulmonary diseases associated with deployment in Southwest Asia was acute eosinophilic pneumonia (AEP). This condition is usually idiopathic, but it has also been reported after inhalational exposures, including in smokers. First recognized by physicians at Landstuhl Regional Medical Center and based on bronchoalveolar lavage eosinophilia, the initial data on 18 patients was first reported in 2004 (104). An additional retrospective review of 43 patients with AEP (all deployed to Iraq, Afghanistan, Kuwait, or other locations, including the previous cases) between 2003 and 2010 was subsequently published. In that series, 91% were males; the mean age was 25.5 years; and cigarette smoking was reported in 91% of the patients (85% were recently new smokers) (105). AEP in deployed troops has not been associated with a particular exposure beyond cigarette smoking. In an observation potentially related to this syndrome, an elevated IgE (>100 kU/L) or peripheral eosinophilia (>2.8% or 300 cells/μl) was noted in 17 of 124 symptomatic deployers evaluated at NJH (Reference 86 and S. D. Krefft, workshop presentation of unpublished data).
Other Pulmonary Abnormalities
Diagnostic computed tomographic (CT) scans obtained in symptomatic deployers have generally been reported as normal or only subtly abnormal, showing mosaic attenuation consistent with air trapping and/or centrilobular nodules, consistent with small airway disease (Reference 86 and S. D. Krefft, workshop presentation of unpublished data). Among 49 postdeployers assessed during STAMPEDE I with CT scans, only 3 had air trapping on expiratory scans, and otherwise only minor nonspecific abnormalities were noted (8). In the lung biopsy series reported by Vanderbilt University Medical Center (11), CT scans were normal in 68%, and there was mild air trapping in 16%. Changes consistent with interstitial lung disease or other diffuse lung disease have not been observed, including in the review of biopsies from JPC (103).
Airborne Hazards and Open Burn Pit Registry
Public Law 112-260 Section 201, enacted in 2013, requires the VA to implement the Airborne Hazards and Open Burn Pit Registry to maintain a registry for those individuals who may have been exposed to burn pit emissions during their military service. The stated purpose is to monitor and ascertain adverse health effects from exposures and monitor the health care of veterans with concerns. There is an internet-based portal that allows veterans to self-enroll, complete an exposure and health questionnaire, and request a clinical assessment. As of May 2018, 140,691 had participated. A recent analysis of registry participants reported a statistically significant association between self-reported blast injury with self-reported dyspnea and/or decreased ability to exercise, adjusted for smoking and other exposures, including burn pit smoke (106). An earlier publication analyzed symptoms and reported health conditions in a subset of registry participants deployed either to a location with a burn pit or to a location without a burn pit, reporting an association between proximity to a burn pit and self-reported respiratory conditions (107). The National Academies of Sciences, Engineering, and Medicine reviewed the function of the registry and suggested that efforts be made to improve participation and focus the scope of information gathered (108). It also suggested that the data should be used to explore the potential to conduct epidemiologic studies. It is possible that a benefit of the registry will be to track the incidence of various pulmonary conditions that occur over time in an effort to assess patterns of disease in relation to health and exposure information provided at entry and in conjunction with other sources of information, including the VHA medical record (D. A. Helmer, workshop presentation).
New Approaches to Assessment of Deployment-related Exposures and Health Effects
Assessment of Deployment PM2.5 Exposures
A new study, VA Cooperative Studies Program #595, Pulmonary Health and Deployment to Southwest Asia and Afghanistan (NCT02825654; also called SHADE [Service and Health among Deployed Veterans]) has been designed to assess the respiratory health of previously deployed veterans. SHADE, which commenced recruitment in June 2018, is being conducted at six VA medical centers to enroll approximately 5,000 veterans with land-based deployments in Afghanistan and Southwest Asia. The primary objectives are to study whether greater cumulative exposure to PM2.5 experienced during deployment is associated with lower lung function assessed by pre- and post-bronchodilator spirometry and to examine associations with healthcare provider–diagnosed asthma. A strength of SHADE is an exposure assessment approach that does not rely on self-reported exposure. Historical NASA satellite and military airport visibility (visual range assessment) records will be used to reconstruct deployment-related PM2.5 at each veteran’s deployment locations (17, 18). This approach integrates ground-level PM2.5 data obtained from monitoring stations, airport visibility data in the countries where U.S. military personnel were deployed, and high-resolution AOD data available from NASA satellites (E. Garshick, workshop presentation).
On the basis of historical airport visibility data between 2000 and 2012 gathered from 104 military sites in Iraq, Afghanistan, Kuwait, Kyrgyzstan, United Arab Emirates, Djibouti, and Qatar calibrated with ground-level PM2.5 stations, estimated monthly average PM2.5 concentrations at these sites ranged from approximately 10 μg/m3 to 365 μg/m3, with values mainly between 50 μg/m3 and nearly 200 μg/m3 (Figure 2) (18). The feasibility of using land surface temperature determined by satellite-based remote sensing (109) to detect burn pit locations will also be assessed. A collaboration between the NASA Joint Propulsion Laboratory and the University of Southern California will use newly developed AOD data fractionated by particle characteristics. It is proposed that this information will be useful in determining the extent that various sources of pollution, such as burn pits or dust storms, contributed to the historical PM2.5 concentrations (E. Garshick, workshop presentation). A limitation of this approach is that exposures to relatively brief but high concentrations of PM, vapors, and gases from various military-related exposures (such as burning vehicles or blasts) may not be assessed.
DoD Serum Repository and Metabolomics
The DoD Serum Repository started in the mid-1980s to store serum samples remaining after required human immunodeficiency virus testing. Serum samples are obtained from active military personnel during periodic medical examinations, before overseas assignments, and before and after major deployments (19). As of June 30, 2016, the repository included 60 million samples stored at −30°C collected from about 10 million service members (110). These samples have been linked electronically to the Defense Medical Surveillance System. The Defense Medical Surveillance System can be used to select records for review on the basis of medical encounters and outcomes, demographics, deployment history, and military job codes. The repository is housed at the Defense Health Agency, Armed Forces Health Surveillance Branch, in Silver Spring, Maryland (111).
DoD and various university collaborators have conducted a series of pilot studies to assess the suitability of the sample repository for use in research. After confirmation that the samples were of sufficient quality to permit study (112), analysis of various chemicals believed to be associated with exposure was conducted using high-resolution mass spectrometry in deidentified samples. Serum samples collected from 200 persons before and after deployment were compared with those of nondeployed control subjects. Naphthalene was found to be elevated after deployment, and four dioxin/furan compounds were measurable in 38% of the samples and elevated after deployment (111, 113). Other exploratory analyses have found miRNAs differentially expressed in previous deployers, as have correlations with dioxin and dibenzofuran concentrations (114, 115). These analyses demonstrate that high-resolution metabolomic, gene expression, and other approaches may be used to identify associations between deployment, specific chemical compounds and/or altered gene expression, and eventually adverse health outcomes
Mechanisms of Lung Epithelial Injury
A 5-year DoD-funded study newly underway at NJH, titled “Mechanisms and Treatment of Deployment-Related Lung Injury: Repair of the Injured Epithelium,” will focus on understanding mechanisms by which exposure to PM may predispose the lung epithelium to injury after a second stimulus, with the long-term goal of developing strategies to promote lung repair. This investigation will test the hypothesis that exposure to respirable PM from Southwest Asia triggers inflammatory signaling pathways in respiratory epithelial cells, which, after a second stimulus (e.g., blast injury, tobacco smoke, allergens, viruses), leads to dysregulated production of proinflammatory mediators that drive lung epithelial cell injury. The project will apply transcriptomic and genetic analyses to in vivo and in vitro airway epithelial cells from postdeployment study participant patients and will include study of airway epithelial cytokines, growth factor expression, and markers of oxidative stress. It will also assess noninvasive clinical markers of small airway findings in symptomatic veterans, including computed tomography and LCI testing.
Summary and Key Questions
Deployment-associated Exposures
As described in the 2011 IOM report (1), military personnel deployed to Afghanistan and Southwest Asia (including Kuwait and Iraq) experienced a complex mixture of exposures. Most notable and widespread have been the ambient PM2.5 exposures known to be greater than U.S. concentrations. Sources contributing to PM2.5 included desert dust with a mix of organic and inorganic constituents, waste incineration from burn pits, and poorly regulated or unregulated local industrial and vehicular pollution. The quantitative assessment of PM2.5 over each deployment using airport visibility and NASA satellite AOD data, as proposed in the new SHADE study, represents an advance in exposure assessment that should reduce misclassification compared with self-reported exposure intensities. Future studies should include efforts to characterize more specific exposures that are based on job duties or deployment locations (using estimates of PM2.5 or other pollutants) rather than simply considering deployment status as a single exposure, because exposure may vary on the basis of job duties, location, and time in theater. The use of banked serum samples to characterize exposures using selected biomarkers related to burn pit or other military exposures may be feasible in the future to better characterize exposure status.
Adverse Respiratory Health Effects
Previous deployers with respiratory symptoms
Studies conducted by DoD analyzing military encounter data suggest that more encounters occur for respiratory symptoms and for obstructive lung disease, predominantly asthma, after deployment (4–6). A study in Swedish troops deployed to Afghanistan also documented the persistence of symptoms several years after deployment (82). Case series describing the evaluation of symptomatic military personnel do not note any single etiology, emphasizing the importance of a comprehensive clinical assessment, but asthma has been a common finding (References 8 and 75 and S. D. Krefft, workshop presentation of unpublished data). This is consistent with epidemiologic findings from the Millennium Cohort Study that demonstrate an increased risk of new-onset asthma (by self-report) related to combat exposure during deployment (9) and the observational findings in which approximately half of the deployed persons discharged with asthma after deployment did not have a previous known diagnosis (10). Nonetheless, a number of questions remain regarding the drivers of increased healthcare use for respiratory conditions and potential under- and overdiagnosis of specific conditions. The relationship of asthma after deployment with specific exposures, such as burn pit work, is not known, nor is the relationship with previous pulmonary function test abnormality or previous asthma diagnosis. A study underway at NJH designed to elucidate the molecular effects of desert dust PM exposures on airway epithelial cells may provide insights into the mechanisms and the potential for airway damage.
Constrictive bronchiolitis
Centers performing VATS lung biopsies in a selected group of previously deployed service members undergoing evaluation for dyspnea have reported constrictive bronchiolitis, other small airway abnormalities, and granulomas (Reference 11 and presentation of unpublished data, S. D. Krefft and C. Rose). Review of histologic criteria defining constrictive bronchiolitis indicated that these criteria have not been applied consistently across case series. Because the histologic criteria for diagnosis of constrictive bronchiolitis have varied, in particular the presence of bronchiolar subepithelial scarring, it has been difficult to compare results across centers. There was a heterogeneity of views among workshop participants regarding the interpretation of the findings of constrictive bronchiolitis and its potential relationship with dyspnea and related respiratory symptoms after deployment.
Future lung disease
The findings summarized in this workshop regarding estimated PM2.5 concentrations during deployment raise concerns about the future respiratory health of previously deployed military personnel (active duty and veterans), especially given that adverse respiratory health effects have been observed at PM concentrations much lower than those experienced during deployment (15, 16). Although multiple sources contributed to PM during deployment, the predominant contribution was desert dust. Epidemiologic studies have demonstrated an association between acute desert dust exposure and increased hospitalization for asthma, pneumonia, and other respiratory causes (40–42, 46–50). Desert dust exposure may also have contributed to the acute respiratory illness experienced during deployment, as reported retrospectively in postdeployment surveys (54, 55). There is little currently available data, however, to guide the assessment of long-term effects of repeated PM or other air pollutant exposures during deployment, nor is there prior literature quantifying chronic pulmonary health effects attributable to the substantially higher but much shorter exposures (usually months to up to several years).
Key Questions
Suggestions to further understanding of the health effects of PM and other potential exposures during deployment include the following:
-
1.
Better characterization of respiratory symptoms and clinical respiratory disease after deployment, including airway diseases in previous deployers presenting for health care and the potential exposures they experienced during deployment.
-
2.
Improved assessment of long-term clinical consequences and outcomes of former deployers with respiratory symptoms and with respiratory disease, because there is a lack published information regarding follow-up among such persons. Many do not receive a conclusive respiratory diagnosis when symptomatic. Population-based nonmilitary studies have demonstrated that chronic respiratory symptoms are associated with reduced pulmonary function and an increased risk of future pulmonary disease (116–118).
-
3.
More consistent characterization of specific histologic abnormalities, including the prevalence of constrictive bronchiolitis and other small airway abnormalities, established through review of biopsy material using standardized criteria comparable across studies and delineating the relationship of such pathologic abnormalities with PM and other exposures.
-
4.
Determination of the cross-sectional associations among cumulative exposures (in particular PM) and respiratory conditions (in particular asthma) and pulmonary function deficits, adjusted for smoking and other covariates.
-
5.
Evaluation of the long-term longitudinal effects of deployment-related exposures (such as self-reported exposures in the Airborne Hazards and Open Burn Pit Registry, externally estimated cumulative PM exposures in the SHADE study, or biomarkers in the DoD Serum Repository) on the incidence of adverse respiratory health conditions or pulmonary function decline over time.
Supplementary Material
Acknowledgments
This official workshop report was prepared by an ad hoc subcommittee of the ATS Assembly on Environmental, Occupational and Population Health and the ATS Environmental Health Policy Committee.
Members of the subcommittee are as follows:
Eric Garshick, M.D., M.O.H. (Co-Chair)1,2,3*
Susan P. Proctor, D.Sc. (Co-Chair)1,6
Paul D. Blanc, M.D., M.S.P.H. (Co-Chair)4,5
Joseph H. Abraham, Sc.D.7*
Coleen P. Baird, M.D., M.P.H.7‡
Paul Ciminera, M.D., M.P.H.8‡
Gregory P. Downey, M.D.9,10‡
Michael J. Falvo, Ph.D.11,12*
Jaime E. Hart, Sc.D.13,14*
Drew A. Helmer, M.D.11,12*
David A. Jackson, Ph.D.15‡
Michael Jerrett, Ph.D.16‡
Kirk D. Jones, M.D.5*
Ware Kuschner, M.D.17,18‡
Silpa D. Krefft, M.D., M.P.H.9,10,19*
Timothy Mallon, M.D., M.P.H.20*
Robert F. Miller, M.D.21*
Michael J. Morris, M.D.22*
Carrie A. Redlich, M.D., M.P.H.23‡
Cecile S. Rose, M.D., M.P.H.9,10*
Rudolph P. Rull, Ph.D., M.P.H.24*
Johannes Saers, M.D.25*
Aaron I. Schneiderman, Ph.D., M.P.H., R.N.26*
Nicholas L. Smith, Ph.D.27,28‡
Panayiotis Yiallouros, M.D., Ph.D.29*
1VA Boston Healthcare System, Boston, Massachusetts; 2Brigham and Women’s Hospital, Boston, Massachusetts; 3Harvard Medical School, Boston, Massachusetts; 4San Francisco VA Health Care System, San Francisco, California; 5University of California San Francisco School of Medicine, San Francisco, California; 6U.S. Army Research Institute of Environmental Medicine, Natick, Massachusetts; 7U.S. Army Public Health Center, Aberdeen Proving Ground, Maryland; 8Office of the Assistant Secretary of Defense for Health Affairs, Health Services Policy and Oversight, Washington, District of Columbia; 9National Jewish Health, Denver, Colorado; 10University of Colorado, Denver, Colorado; 11VA New Jersey Health Care System, East Orange, New Jersey; 12Rutgers New Jersey Medical School, Newark, New Jersey; 13Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts; 14Harvard T.H. Chan School of Public Health, Boston, Massachusetts; 15Walter Reed Army Institute of Research, Silver Spring, Maryland; 16Fielding School of Public Health, University of California, Los Angeles, Los Angeles, California; 17VA Palo Alto Health Care System, Palo Alto, California; 18Stanford University School of Medicine, Palo Alto, California; 19VA Eastern Colorado Health Care System, Aurora, Colorado; 20Uniformed Services University, Bethesda, Maryland; 21Vanderbilt University, Nashville, Tennessee; 22Brooke Army Medical Center, JBSA Fort Sam Houston, Texas; 23Yale University School of Medicine, New Haven, Connecticut; 24Deployment Health Research Department, Naval Health Research Center, San Diego, California; 25Swedish Army (Reserve), Örebro University Hospital, Örebro, Sweden; 26Veterans Health Administration, Washington, District of Columbia; 27Seattle Epidemiologic Research and Information Center, Department of Veterans Affairs Office of Research and Development Studies Program, Seattle, Washington; 28Department of Epidemiology, University of Washington, Seattle, Washington; and 29University of Cyprus Medical School, Nicosia, Cyprus
*Workshop presenters.
‡Workshop discussants.
Footnotes
This official workshop report of the American Thoracic Society was approved May 2019
An Executive Summary of this document is available at http://www.atsjournals.org/doi/suppl/10.1513/AnnalsATS.201904-344WS.
The views expressed in this article are those of the authors and the contents do not represent the views of the U.S. Department of Veterans Affairs, the U.S. Department of Defense, the United States Army, or the United States government.
NHRC Disclaimer: Dr. Rudolph Rull is an employee of the U.S. Government. This work was prepared as part of his official duties. Title 17, U.S.C. §105 provides that copyright protection under this title is not available for any work of the U.S. Government. Title 17, U.S.C. §101 defines a U.S. Government work as work prepared by a military service member or employee of the U.S. Government as part of that person’s official duties.
You may print one copy of this document at no charge. However, if you require more than one copy, you must place a reprint order. Domestic reprint orders: amy.schriver@sheridan.com; international reprint orders: louisa.mott@springer.com.
Author Disclosures: S.D.K. provided medicolegal consulting on exposure-related lung disease; developed podcast reviews of articles on occupational and exposure-related lung disease for Oakstone Medical Reviews; received support from the Sergeant Sullivan Foundation associated with the Center for Deployment-Related Lung Disease, National Jewish Health, for previous research on lung clearance index testing cited in this report. M.J.M. served as a speaker for Janssen Pharmaceuticals. E.G., J.H.A., C.P.B., P.C., G.P.D., M.J.F., J.E.H., D.A.J., M.J., W.K., D.A.H., K.D.J., T.M., R.F.M., S.P.P., C.A.R., C.S.R., R.P.R., J.S., A.I.S., N.L.S., P.Y., and P.D.B. reported no relevant commercial relationships.
Contributor Information
Collaborators: on behalf of the American Thoracic Society Assembly on Environmental, Occupational and Population Health and the American Thoracic Society Environmental Health Policy Committee
References
- 1.Institute of Medicine, Board on the Health of Select Populations, Committee on the Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan. Long-term health consequences of exposure to burn pits in Iraq and Afghanistan. Washington, DC: National Academies Press; 2011. [Google Scholar]
- 2.Alolayan MA, Brown KW, Evans JS, Bouhamra WS, Koutrakis P. Source apportionment of fine particles in Kuwait City. Sci Total Environ. 2013;448:14–25. doi: 10.1016/j.scitotenv.2012.11.090. [DOI] [PubMed] [Google Scholar]
- 3.Engelbrecht JP, McDonald EV, Gillies JA, Jayanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East—part 1: ambient sampling. Inhal Toxicol. 2009;21:297–326. doi: 10.1080/08958370802464273. [DOI] [PubMed] [Google Scholar]
- 4.Abraham JH, DeBakey SF, Reid L, Zhou J, Baird CP. Does deployment to Iraq and Afghanistan affect respiratory health of US military personnel? J Occup Environ Med. 2012;54:740–745. doi: 10.1097/JOM.0b013e318252969a. [DOI] [PubMed] [Google Scholar]
- 5.Abraham JH, Eick-Cost A, Clark LL, Hu Z, Baird CP, DeFraites R, et al. A retrospective cohort study of military deployment and postdeployment medical encounters for respiratory conditions. Mil Med. 2014;179:540–546. doi: 10.7205/MILMED-D-13-00443. [DOI] [PubMed] [Google Scholar]
- 6.Sharkey JM, Abraham JH, Clark LL, Rohrbeck P, Ludwig SL, Hu Z, et al. Postdeployment respiratory health care encounters following deployment to Kabul, Afghanistan: a retrospective cohort study. Mil Med. 2016;181:265–271. doi: 10.7205/MILMED-D-14-00690. [DOI] [PubMed] [Google Scholar]
- 7.Pugh MJ, Jaramillo CA, Leung KW, Faverio P, Fleming N, Mortensen E, et al. Increasing prevalence of chronic lung disease in veterans of the wars in Iraq and Afghanistan. Mil Med. 2016;181:476–481. doi: 10.7205/MILMED-D-15-00035. [DOI] [PubMed] [Google Scholar]
- 8.Morris MJ, Dodson DW, Lucero PF, Haislip GD, Gallup RA, Nicholson KL, et al. Study of Active Duty Military for Pulmonary Disease Related to Environmental Deployment Exposures (STAMPEDE) Am J Respir Crit Care Med. 2014;190:77–84. doi: 10.1164/rccm.201402-0372OC. [DOI] [PubMed] [Google Scholar]
- 9.Rivera AC, Powell TM, Boyko EJ, Lee RU, Faix DJ, Luxton DD, et al. Millennium Cohort Study Team. New-onset asthma and combat deployment: findings from the Millennium Cohort Study. Am J Epidemiol. 2018;187:2136–2144. doi: 10.1093/aje/kwy112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DelVecchio SP, Collen JF, Zacher LL, Morris MJ. The impact of combat deployment on asthma diagnosis and severity. J Asthma. 2015;52:363–369. doi: 10.3109/02770903.2014.973502. [DOI] [PubMed] [Google Scholar]
- 11.King MS, Eisenberg R, Newman JH, Tolle JJ, Harrell FE, Jr, Nian H, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365:222–230. doi: 10.1056/NEJMoa1101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewin-Smith MR, Morris MJ, Galvin JR, Harley RA, Franks TJ. The problems with constrictive bronchiolitis: histopathological and radiological perspectives. In: Baird CP, Harkins DK, editors. Airborne hazards related to deployment. Fort Sam Houston, TX: Borden Institute, U.S. Army Medical Department and School; 2015. pp. 153–161. [Google Scholar]
- 13.Epler GR.Diagnosis and treatment of constrictive bronchiolitis F1000 Med Rep 2010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epler GR. Constrictive bronchiolitis obliterans: the fibrotic airway disorder. Expert Rev Respir Med. 2007;1:139–147. doi: 10.1586/17476348.1.1.139. [DOI] [PubMed] [Google Scholar]
- 15.Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, et al. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham Heart Study. Am J Respir Crit Care Med. 2015;191:656–664. doi: 10.1164/rccm.201410-1875OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo C, Zhang Z, Lau AKH, Lin CQ, Chuang YC, Chan J, et al. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet Health. 2018;2:e114–e125. doi: 10.1016/S2542-5196(18)30028-7. [DOI] [PubMed] [Google Scholar]
- 17.Masri S, Garshick E, Coull BA, Koutrakis P. A novel calibration approach using satellite and visibility observations to estimate fine particulate matter exposures in Southwest Asia and Afghanistan. J Air Waste Manag Assoc. 2017;67:86–95. doi: 10.1080/10962247.2016.1230079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masri S, Garshick E, Hart J, Bouhamra W, Koutrakis P. Use of visual range measurements to predict fine particulate matter exposures in Southwest Asia and Afghanistan. J Air Waste Manag Assoc. 2017;67:75–85. doi: 10.1080/10962247.2016.1243169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perdue CL, Cost AA, Rubertone MV, Lindler LE, Ludwig SL. Description and utilization of the United States Department of Defense Serum Repository: a review of published studies, 1985-2012. PLoS One. 2015;10:e0114857. doi: 10.1371/journal.pone.0114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan MA, Smith TC, Smith B, Amoroso P, Boyko EJ, Gray GC, et al. Millennium cohort: enrollment begins a 21-year contribution to understanding the impact of military service. J Clin Epidemiol. 2007;60:181–191. doi: 10.1016/j.jclinepi.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Wenger JW, O’Connell C, Cottrell L. Examination of recent deployment experience across the services and components. Santa Monica, CA: Rand Corporation; 2018. [Google Scholar]
- 22.Bagalman E. The number of veterans that use VA health services: a fact sheet. Washington, DC: Congressional Research Service. 2014.
- 23.Sissakian V, Al-Ansari N, Knutsson S. Sand and dust storm events in Iraq. J Nat Sci. 2013;5:1084–1094. [Google Scholar]
- 24.Goudie AS. Desert dust and human health disorders. Environ Int. 2014;63:101–113. doi: 10.1016/j.envint.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Goudie AS. Dust storms: recent developments. J Environ Manage. 2009;90:89–94. doi: 10.1016/j.jenvman.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Al-Dabbas M, Abbas MA, Al-Khafaji R. The mineralogical and micro-organisms effects of regional dust storms over Middle East region. Int J Water Resour Arid Environ. 2011;1:129–141. [Google Scholar]
- 27.Polymenakou PN, Mandalakis M, Stephanou EG, Tselepides A. Particle size distribution of airborne microorganisms and pathogens during an intense African dust event in the eastern Mediterranean. Environ Health Perspect. 2008;116:292–296. doi: 10.1289/ehp.10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Research Council Review of the Department of Defense enhanced particulate matter surveillance program report. Appendix D - Final report of the Department of Defense enhanced particulate matter surveillance program. Washington, DC: National Academies Press; 2010. [PubMed]
- 29.Brown KW, Bouhamra W, Lamoureux DP, Evans JS, Koutrakis P. Characterization of particulate matter for three sites in Kuwait. J Air Waste Manag Assoc. 2008;58:994–1003. doi: 10.3155/1047-3289.58.8.994. [DOI] [PubMed] [Google Scholar]
- 30.U.S. Army Center for Health Promotion and Preventative Medicine Maryland; May 2008. USACHPPM Report No. 47-MA-08PV-08/AFIOH Report No. IOH-RS-BR-TR-2008-0001.
- 31.Engelbrecht JP, McDonald EV, Gillies JA, Jayanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East—part 2: grab samples and re-suspensions. Inhal Toxicol. 2009;21:327–336. doi: 10.1080/08958370802464299. [DOI] [PubMed] [Google Scholar]
- 32.Brown EG, Gottlieb S, Latbourn RL.Dust storms and their possible effect on health Public Health Rep 1935501369–1383.19315524 [Google Scholar]
- 33.Samoli E, Kougea E, Kassomenos P, Analitis A, Katsouyanni K. Does the presence of desert dust modify the effect of PM10 on mortality in Athens, Greece? Sci Total Environ. 2011;409:2049–2054. doi: 10.1016/j.scitotenv.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 34.Crooks JL, Cascio WE, Percy MS, Reyes J, Neas LM, Hilborn ED. The association between dust storms and daily non-accidental mortality in the United States, 1993-2005. Environ Health Perspect. 2016;124:1735–1743. doi: 10.1289/EHP216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Renzi M, Forastiere F, Calzolari R, Cernigliaro A, Madonia G, Michelozzi P, et al. Short-term effects of desert and non-desert PM10 on mortality in Sicily, Italy. Environ Int. 2018;120:472–479. doi: 10.1016/j.envint.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Stafoggia M, Zauli-Sajani S, Pey J, Samoli E, Alessandrini E, Basagaña X, et al. MED-PARTICLES Study Group. Desert dust outbreaks in Southern Europe: contribution to daily PM10 concentrations and short-term associations with mortality and hospital admissions. Environ Health Perspect. 2016;124:413–419. doi: 10.1289/ehp.1409164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neophytou AM, Yiallouros P, Coull BA, Kleanthous S, Pavlou P, Pashiardis S, et al. Particulate matter concentrations during desert dust outbreaks and daily mortality in Nicosia, Cyprus. J Expo Sci Environ Epidemiol. 2013;23:275–280. doi: 10.1038/jes.2013.10. [DOI] [PubMed] [Google Scholar]
- 38.Perez L, Tobias A, Querol X, Künzli N, Pey J, Alastuey A, et al. Coarse particles from Saharan dust and daily mortality. Epidemiology. 2008;19:800–807. doi: 10.1097/ede.0b013e31818131cf. [DOI] [PubMed] [Google Scholar]
- 39.Mallone S, Stafoggia M, Faustini A, Gobbi GP, Marconi A, Forastiere F. Saharan dust and associations between particulate matter and daily mortality in Rome, Italy. Environ Health Perspect. 2011;119:1409–1414. doi: 10.12989/ehp.1003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thalib L, Al-Taiar A. Dust storms and the risk of asthma admissions to hospitals in Kuwait. Sci Total Environ. 2012;433:347–351. doi: 10.1016/j.scitotenv.2012.06.082. [DOI] [PubMed] [Google Scholar]
- 41.Trianti SM, Samoli E, Rodopoulou S, Katsouyanni K, Papiris SA, Karakatsani A. Desert dust outbreaks and respiratory morbidity in Athens, Greece. Environ Health. 2017;16:72. doi: 10.1186/s12940-017-0281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grineski SE, Staniswalis JG, Bulathsinhala P, Peng Y, Gill TE. Hospital admissions for asthma and acute bronchitis in El Paso, Texas: do age, sex, and insurance status modify the effects of dust and low wind events? Environ Res. 2011;111:1148–1155. doi: 10.1016/j.envres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe M, Noma H, Kurai J, Shimizu A, Sano H, Kato K, et al. Association of sand dust particles with pulmonary function and respiratory symptoms in adult patients with asthma in western Japan using light detection and ranging: a panel study. Int J Environ Res Public Health. 2015;12:13038–13052. doi: 10.3390/ijerph121013038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang CC, Lee IM, Tsai SS, Yang CY. Correlation of Asian dust storm events with daily clinic visits for allergic rhinitis in Taipei, Taiwan. J Toxicol Environ Health A. 2006;69:229–235. doi: 10.1080/15287390500227415. [DOI] [PubMed] [Google Scholar]
- 45.Kanatani KT, Hamazaki K, Inadera H, Sugimoto N, Shimizu A, Noma H, et al. Japan Environment & Children’s Study Group Effect of desert dust exposure on allergic symptoms: a natural experiment in Japan Ann Allergy Asthma Immunol 2016116425–430.e7 [DOI] [PubMed] [Google Scholar]
- 46.Kang JH, Keller JJ, Chen CS, Lin HC. Asian dust storm events are associated with an acute increase in pneumonia hospitalization. Ann Epidemiol. 2012;22:257–263. doi: 10.1016/j.annepidem.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 47.Cheng MF, Ho SC, Chiu HF, Wu TN, Chen PS, Yang CY. Consequences of exposure to Asian dust storm events on daily pneumonia hospital admissions in Taipei, Taiwan. J Toxicol Environ Health A. 2008;71:1295–1299. doi: 10.1080/15287390802114808. [DOI] [PubMed] [Google Scholar]
- 48.Meng Z.Lu B. Dust events as a risk factor for daily hospitalization for respiratory and cardiovascular diseases in Minqin, China Atmos Environ 2007417038–7048. [Google Scholar]
- 49.Tam WW, Wong TW, Wong AH, Hui DS. Effect of dust storm events on daily emergency admissions for respiratory diseases. Respirology. 2012;17:143–148. doi: 10.1111/j.1440-1843.2011.02056.x. [DOI] [PubMed] [Google Scholar]
- 50.Vodonos A, Friger M, Katara I, Avnon L, Krasnov H, Koutrakis P, et al. The impact of desert dust exposures on hospitalizations due to exacerbation of chronic obstructive pulmonary disease. Air Qual Atmos Health. 2014;7:433–439. [Google Scholar]
- 51.Saiyed HN, Sharma YK, Sadhu HG, Norboo T, Patel PD, Patel TS, et al. Non-occupational pneumoconiosis at high altitude villages in central Ladakh. Br J Ind Med. 1991;48:825–829. doi: 10.1136/oem.48.12.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norboo T, Angchuk PT, Yahya M, Kamat SR, Pooley FD, Corrin B, et al. Silicosis in a Himalayan village population: role of environmental dust. Thorax. 1991;46:341–343. doi: 10.1136/thx.46.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bar-Ziv J, Goldberg GM. Simple siliceous pneumoconiosis in Negev Bedouins. Arch Environ Health. 1974;29:121–126. doi: 10.1080/00039896.1974.10666548. [DOI] [PubMed] [Google Scholar]
- 54.Sanders JW, Putnam SD, Frankart C, Frenck RW, Monteville MR, Riddle MS, et al. Impact of illness and non-combat injury during Operations Iraqi Freedom and Enduring Freedom (Afghanistan) Am J Trop Med Hyg. 2005;73:713–719. [PubMed] [Google Scholar]
- 55.Roop SA, Niven AS, Calvin BE, Bader J, Zacher LL. The prevalence and impact of respiratory symptoms in asthmatics and nonasthmatics during deployment. Mil Med. 2007;172:1264–1269. doi: 10.7205/milmed.172.12.1264. [DOI] [PubMed] [Google Scholar]
- 56.Korzeniewski K, Brzozowski R. Sickness profile among Polish troops deployed to Afghanistan in the years 2003-2005. Int Marit Health. 2011;62:63–70. [PubMed] [Google Scholar]
- 57.Korzeniewski K, Nitsch-Osuch A, Konarski M, Guzek A, Prokop E, Bieniuk K. Prevalence of acute respiratory tract diseases among soldiers deployed for military operations in Iraq and Afghanistan. Adv Exp Med Biol. 2013;788:117–124. doi: 10.1007/978-94-007-6627-3_18. [DOI] [PubMed] [Google Scholar]
- 58.Novozhenov VG, Gembitskiĭ EV. Pneumonia in young males in extreme conditions [in Russian] Klin Med (Mosk) 1998;76:18–20. [PubMed] [Google Scholar]
- 59.Kloog I, Koutrakis P, Coull BA, Lee HJ, Schwartz J. Assessing temporally and spatially resolved PM2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmos Environ. 2011;45:6267–6275. [Google Scholar]
- 60.Kloog I, Ridgway B, Koutrakis P, Coull BA, Schwartz JD. Long- and short-term exposure to PM2.5 and mortality: using novel exposure models. Epidemiology. 2013;24:555–561. doi: 10.1097/EDE.0b013e318294beaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee HJ, Coull BA, Bell ML, Koutrakis P. Use of satellite-based aerosol optical depth and spatial clustering to predict ambient PM2.5 concentrations. Environ Res. 2012;118:8–15. doi: 10.1016/j.envres.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adam M, Schikowski T, Carsin AE, Cai Y, Jacquemin B, Sanchez M, et al. Adult lung function and long-term air pollution exposure. ESCAPE: a multicentre cohort study and meta-analysis. Eur Respir J. 2015;45:38–50. doi: 10.1183/09031936.00130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garshick E. Effects of short- and long-term exposures to ambient air pollution on COPD. Eur Respir J. 2014;44:558–561. doi: 10.1183/09031936.00108814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schikowski T, Adam M, Marcon A, Cai Y, Vierkötter A, Carsin AE, et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur Respir J. 2014;44:614–626. doi: 10.1183/09031936.00132213. [DOI] [PubMed] [Google Scholar]
- 65.Churg A, Brauer M, del Carmen Avila-Casado M, Fortoul TI, Wright JL. Chronic exposure to high levels of particulate air pollution and small airway remodeling. Environ Health Perspect. 2003;111:714–718. doi: 10.1289/ehp.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Künzli N, Bridevaux PO, Liu LJ, Garcia-Esteban R, Schindler C, Gerbase MW, et al. Swiss Cohort Study on Air Pollution and Lung Diseases in Adults. Traffic-related air pollution correlates with adult-onset asthma among never-smokers. Thorax. 2009;64:664–670. doi: 10.1136/thx.2008.110031. [DOI] [PubMed] [Google Scholar]
- 67.Jacquemin B, Siroux V, Sanchez M, Carsin AE, Schikowski T, Adam M, et al. Ambient air pollution and adult asthma incidence in six European cohorts (ESCAPE) Environ Health Perspect. 2015;123:613–621. doi: 10.1289/ehp.1408206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Young MT, Sandler DP, DeRoo LA, Vedal S, Kaufman JD, London SJ. Ambient air pollution exposure and incident adult asthma in a nationwide cohort of U.S. women. Am J Respir Crit Care Med. 2014;190:914–921. doi: 10.1164/rccm.201403-0525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fisher JA, Puett RC, Hart JE, Camargo CA, Jr, Varraso R, Yanosky JD, et al. Particulate matter exposures and adult-onset asthma and COPD in the Nurses’ Health Study. Eur Respir J. 2016;48:921–924. doi: 10.1183/13993003.00845-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 71.Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369:571–577. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 72.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 73.Martin BL, Engler RJM, Klote MM, With CM, Krauss MR, Nelson MR. DeKoning BL, editor. Recruit medicine (Textbooks of Military Medicine series) Washington, DC: Department of the Army, Office of the Surgeon General, Borden Institute; 2006. Asthma and its implications for military recruits; pp. 89–108. [Google Scholar]
- 74.Millikan AM, Niebuhr DW, Brundage M, Powers TE, Krauss MR. Retention of mild asthmatics in the navy (REMAIN): a low-risk approach to giving mild asthmatics an opportunity for military service. Mil Med. 2008;173:381–387. doi: 10.7205/milmed.173.4.381. [DOI] [PubMed] [Google Scholar]
- 75.Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31:67–71. doi: 10.2500/aap.2010.31.3383. [DOI] [PubMed] [Google Scholar]
- 76.Szema AM, Salihi W, Savary K, Chen JJ. Respiratory symptoms necessitating spirometry among soldiers with Iraq/Afghanistan war lung injury. J Occup Environ Med. 2011;53:961–965. doi: 10.1097/JOM.0b013e31822c9f05. [DOI] [PubMed] [Google Scholar]
- 77.Abraham JH, Clark LL, Sharkey JM, Baird CP. Trends in rates of chronic obstructive respiratory conditions among US military personnel, 2001-2013. US Army Med Dep J. 2014 Jul-Sep:33–43. [PubMed] [Google Scholar]
- 78.Slatore CG, Falvo MJ, Nugent S, Carlson K. Afghanistan and Iraq war veterans: mental health diagnoses are associated with respiratory disease diagnoses. Mil Med. 2018;183:e249–e257. doi: 10.1093/milmed/usx108. [DOI] [PubMed] [Google Scholar]
- 79.Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD Ryan MAK; the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170:1433–1442. doi: 10.1093/aje/kwp287. [DOI] [PubMed] [Google Scholar]
- 80.Smith B, Wong CA, Boyko EJ, Phillips CJ, Gackstetter GD, Ryan MA, et al. Millennium Cohort Study Team. The effects of exposure to documented open-air burn pits on respiratory health among deployers of the Millennium Cohort Study. J Occup Environ Med. 2012;54:708–716. doi: 10.1097/JOM.0b013e31825107f9. [DOI] [PubMed] [Google Scholar]
- 81.Barth SK, Dursa EK, Peterson MR, Schneiderman A. Prevalence of respiratory diseases among veterans of Operation Enduring Freedom and Operation Iraqi Freedom: results from the National Health Study for a New Generation of U.S. Veterans. Mil Med. 2014;179:241–245. doi: 10.7205/MILMED-D-13-00338. [DOI] [PubMed] [Google Scholar]
- 82.Saers J, Ekerljung L, Forsberg B, Janson C. Respiratory symptoms among Swedish soldiers after military service abroad: association with time spent in a desert environment. Eur Clin Respir J. 2017;4:1327761. doi: 10.1080/20018525.2017.1327761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lincoln AE, Helmer DA, Schneiderman AI, Li M, Copeland HL, Prisco MK, et al. The war-related illness and injury study centers: a resource for deployment-related health concerns. Mil Med. 2006;171:577–585. doi: 10.7205/milmed.171.7.577. [DOI] [PubMed] [Google Scholar]
- 84.Falvo MJ, Abraham JH, Osinubi OY, Klein JC, Sotolongo AM, Ndirangu D, et al. Bronchodilator responsiveness and airflow limitation are associated with deployment length in Iraq and Afghanistan veterans. J Occup Environ Med. 2016;58:325–328. doi: 10.1097/JOM.0000000000000675. [DOI] [PubMed] [Google Scholar]
- 85.Falvo MJ, Helmer DA, Klein JC, Osinubi OY, Ndirangu D, Patrick-DeLuca LA, et al. Isolated diffusing capacity reduction is a common clinical presentation in deployed Iraq and Afghanistan veterans with deployment-related environmental exposures. Clin Respir J. 2018;12:795–798. doi: 10.1111/crj.12552. [DOI] [PubMed] [Google Scholar]
- 86.Krefft SD, Strand M, Smith J, Stroup C, Meehan R, Rose C. Utility of lung clearance index testing as a noninvasive marker of deployment-related lung disease. J Occup Environ Med. 2017;59:707–711. doi: 10.1097/JOM.0000000000001058. [DOI] [PubMed] [Google Scholar]
- 87.Matthews T, Abraham J, Zacher LL, Morris MJ. The impact of deployment on COPD in active duty military personnel. Mil Med. 2014;179:1273–1278. doi: 10.7205/MILMED-D-14-00066. [DOI] [PubMed] [Google Scholar]
- 88.Skabelund AJ, Rawlins FA, III, McCann ET, Lospinoso JA, Burroughs L, Gallup RA, et al. Pulmonary function and respiratory health of military personnel before Southwest Asia deployment. Respir Care. 2017;62:1148–1155. doi: 10.4187/respcare.05438. [DOI] [PubMed] [Google Scholar]
- 89.Morris MJ, Skabelund AJ, Rawlins FA, III, Gallup RA, Aden JK, Holley AB. Study of Active Duty Military Personnel for Environmental Deployment Exposures: Pre- and Post-Deployment Spirometry (STAMPEDE II) Respir Care. 2019;64:536–544. doi: 10.4187/respcare.06396. [DOI] [PubMed] [Google Scholar]
- 90.Crapo RO, Morris AH. Standardized single breath normal values for carbon monoxide diffusing capacity. Am Rev Respir Dis. 1981;123:185–189. doi: 10.1164/arrd.1981.123.2.185. [DOI] [PubMed] [Google Scholar]
- 91.Holley AB, Sobieszczyk M, Perkins M, Cohee BM, Costantoth CB, Mabe DL, et al. Lung function abnormalities among service members returning from Iraq or Afghanistan with respiratory complaints. Respir Med. 2016;118:84–87. doi: 10.1016/j.rmed.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 92.Miller A, Thornton JC, Warshaw R, Anderson H, Teirstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state: predicted values, lower limits of normal, and frequencies of abnormality by smoking history. Am Rev Respir Dis. 1983;127:270–277. doi: 10.1164/arrd.1983.127.3.270. [DOI] [PubMed] [Google Scholar]
- 93.Meyer KC, Raghu G, Verleden GM, Corris PA, Aurora P, Wilson KC, et al. ISHLT/ATS/ERS BOS Task Force Committee; ISHLT/ATS/ERS BOS Task Force Committee. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44:1479–1503. doi: 10.1183/09031936.00107514. [DOI] [PubMed] [Google Scholar]
- 94.Burgel PR, Bergeron A, de Blic J, Bonniaud P, Bourdin A, Chanez P, et al. Small airways diseases, excluding asthma and COPD: an overview. Eur Respir Rev. 2013;22:131–147. doi: 10.1183/09059180.00001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ghanei M, Tazelaar HD, Chilosi M, Harandi AA, Peyman M, Akbari HM, et al. An international collaborative pathologic study of surgical lung biopsies from mustard gas-exposed patients. Respir Med. 2008;102:825–830. doi: 10.1016/j.rmed.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 96.Akpinar-Elci M, Travis WD, Lynch DA, Kreiss K. Bronchiolitis obliterans syndrome in popcorn production plant workers. Eur Respir J. 2004;24:298–302. doi: 10.1183/09031936.04.00013903. [DOI] [PubMed] [Google Scholar]
- 97.Rose CS. Early detection, clinical diagnosis, and management of lung disease from exposure to diacetyl. Toxicology. 2017;388:9–14. doi: 10.1016/j.tox.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 98.Kreiss K. Occupational causes of constrictive bronchiolitis. Curr Opin Allergy Clin Immunol. 2013;13:167–172. doi: 10.1097/ACI.0b013e32835e0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thunnissen E, Blaauwgeers HJ, de Cuba EM, Yick CY, Flieder DB. Ex vivo artifacts and histopathologic pitfalls in the lung. Arch Pathol Lab Med. 2016;140:212–220. doi: 10.5858/arpa.2015-0292-OA. [DOI] [PubMed] [Google Scholar]
- 100.Leslie KO. My approach to interstitial lung disease using clinical, radiological and histopathological patterns. J Clin Pathol. 2009;62:387–401. doi: 10.1136/jcp.2008.059782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Allen TC. Pathology of small airways disease. Arch Pathol Lab Med. 2010;134:702–718. doi: 10.5858/134.5.702. [DOI] [PubMed] [Google Scholar]
- 102.Baird CP, DeBakey S, Reid L, Hauschild VD, Petruccelli B, Abraham JH. Respiratory health status of US Army personnel potentially exposed to smoke from 2003 Al-Mishraq sulfur plant fire. J Occup Environ Med. 2012;54:717–723. doi: 10.1097/JOM.0b013e3182572e37. [DOI] [PubMed] [Google Scholar]
- 103.Madar CS, Lewin-Smith MR, Franks TJ, Harley RA, Klaric JS, Morris MJ. Histological diagnoses of military personnel undergoing lung biopsy after deployment to Southwest Asia. Lung. 2017;195:507–515. doi: 10.1007/s00408-017-0009-2. [DOI] [PubMed] [Google Scholar]
- 104.Shorr AF, Scoville SL, Cersovsky SB, Shanks GD, Ockenhouse CF, Smoak BL, et al. Acute eosinophilic pneumonia among US military personnel deployed in or near Iraq. JAMA. 2004;292:2997–3005. doi: 10.1001/jama.292.24.2997. [DOI] [PubMed] [Google Scholar]
- 105.Sine CR, Hiles PD, Scoville SL, Haynes RL, Allan PF, Franks TJ, et al. Acute eosinophilic pneumonia in the deployed military setting. Respir Med. 2018;137:123–128. doi: 10.1016/j.rmed.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 106.Jani N, Falvo MJ, Sotolongo A, Osinubi OY, Tseng CL, Rowneki M, et al. Blast injury and cardiopulmonary symptoms in U.S. veterans: analysis of a national registry. Ann Intern Med. 2017;167:753–755. doi: 10.7326/M17-0711. [DOI] [PubMed] [Google Scholar]
- 107.Liu J, Lezama N, Gasper J, Kawata J, Morley S, Helmer D, et al. Burn pit emissions exposure and respiratory and cardiovascular conditions among airborne hazards and open burn pit registry participants. J Occup Environ Med. 2016;58:e249–e255. doi: 10.1097/JOM.0000000000000776. [DOI] [PubMed] [Google Scholar]
- 108.National Academies of Sciences, Engineering, and Medicine. Assessment of the Department of Veterans Affairs Airborne Hazards and Open Burn Pit Registry. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 109.Kloog I, Chudnovsky A, Koutrakis P, Schwartz J. Temporal and spatial assessments of minimum air temperature using satellite surface temperature measurements in Massachusetts, USA. Sci Total Environ. 2012;432:85–92. doi: 10.1016/j.scitotenv.2012.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lushniak B, Mallon CT, Gaydos JC, Smith DJ. Utility of the Department of Defense Serum Repository in assessing deployment exposure. J Occup Environ Med. 2016;58(8) Suppl 1:S1–S2. doi: 10.1097/JOM.0000000000000796. [DOI] [PubMed] [Google Scholar]
- 111.Mallon CT, Rohrbeck MP, Haines MK, Jones DP, Utell M, Hopke PK, et al. Introduction to Department of Defense research on burn pits, biomarkers, and health outcomes related to deployment in Iraq and Afghanistan. J Occup Environ Med. 2016;58(8) Suppl 1:S3–S11. doi: 10.1097/JOM.0000000000000775. [DOI] [PubMed] [Google Scholar]
- 112.Walker DI, Mallon CT, Hopke PK, Uppal K, Go YM, Rohrbeck P, et al. Deployment-associated exposure surveillance with high-resolution metabolomics. J Occup Environ Med. 2016;58(8) Suppl 1:S12–S21. doi: 10.1097/JOM.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Xia X, Carroll-Haddad A, Brown N, Utell MJ, Mallon CT, Hopke PK. Polycyclic aromatic hydrocarbons and polychlorinated dibenzo-p-dioxins/dibenzofurans in microliter samples of human serum as exposure indicators. J Occup Environ Med. 2016;58(8) Suppl 1:S72–S79. doi: 10.1097/JOM.0000000000000743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dalgard CL, Polston KF, Sukumar G, Mallon CT, Wilkerson MD, Pollard HB. MicroRNA expression profiling of the Armed Forces Health Surveillance Branch cohort for identification of “enviro-miRs” associated with deployment-based environmental exposure. J Occup Environ Med. 2016;58(8) Suppl 1:S97–S103. doi: 10.1097/JOM.0000000000000764. [DOI] [PubMed] [Google Scholar]
- 115.Woeller CF, Thatcher TH, Van Twisk D, Pollock SJ, Croasdell A, Hopke PK, et al. MicroRNAs as novel biomarkers of deployment status and exposure to polychlorinated dibenzo-p-dioxins/dibenzofurans. J Occup Environ Med. 2016;58(8) Suppl 1:S89–S96. doi: 10.1097/JOM.0000000000000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jaakkola MS, Jaakkola JJ, Ernst P, Becklake MR. Respiratory symptoms in young adults should not be overlooked. Am Rev Respir Dis. 1993;147:359–366. doi: 10.1164/ajrccm/147.2.359. [DOI] [PubMed] [Google Scholar]
- 117.Sunyer J, Basagaña X, Roca J, Urrutia I, Jaen A, Antó JM, et al. ECRHS study. Relations between respiratory symptoms and spirometric values in young adults: the European Community Respiratory Health Study. Respir Med. 2004;98:1025–1033. doi: 10.1016/j.rmed.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 118.Kalhan R, Dransfield MT, Colangelo LA, Cuttica MJ, Jacobs DR, Jr, Thyagarajan B, et al. Respiratory symptoms in young adults and future lung disease: the CARDIA Lung Study. Am J Respir Crit Care Med. 2018;197:1616–1624. doi: 10.1164/rccm.201710-2108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.