Abstract
Cell-matrix interactions are fundamental to many developmental, homeostatic, immune and pathologic processes. Hyaluronan (HA), a critical component of the extracellular matrix (ECM) that regulates normal structural integrity and development, also regulates tissue responses during injury, repair, and regeneration. Though simple in its primary structure, HA regulates biological responses in a highly complex manner with balanced contributions from its molecular size and concentration, synthesis versus enzymatic and/or oxidative-nitrative fragmentation, interactions with key HA binding proteins and cell associated receptors, and its cell context-specific signaling. This review highlights the different, but inter-related factors that dictate the biological activity of HA and introduces the overarching themes that weave throughout this special issue of Matrix Biology on hyaluronan.
Keywords: Hyaluronan, Structure, Synthesis, Degradation, Receptors, Signaling
Introduction
The extracellular matrix (ECM) plays pivotal roles in cell self-renewal, fate, death, and signaling to regulate diverse functions including migration, proliferation, differentiation, tissue patterning, inflammation, and angiogenesis, among other homeostatic and pathological processes [1–3]. The complexity of ECM biology derives, in part, from the heterogeneity of its components, which participates in extraordinarily dynamic interactions with each other as well as with resident and migrating cells [4–6]. One of the most fascinating ECM components is hyaluronan (HA), a ubiquitously expressed glycosaminoglycan. While HA structure is deceptively simple with repeating disaccharide chains of N-acetyl-glucosamine and glucuronic acid, its biology is wonderfully complex [7–10]. This special issue will lend insights into the cornucopia of mechanisms of HA biology and its biological diversity.
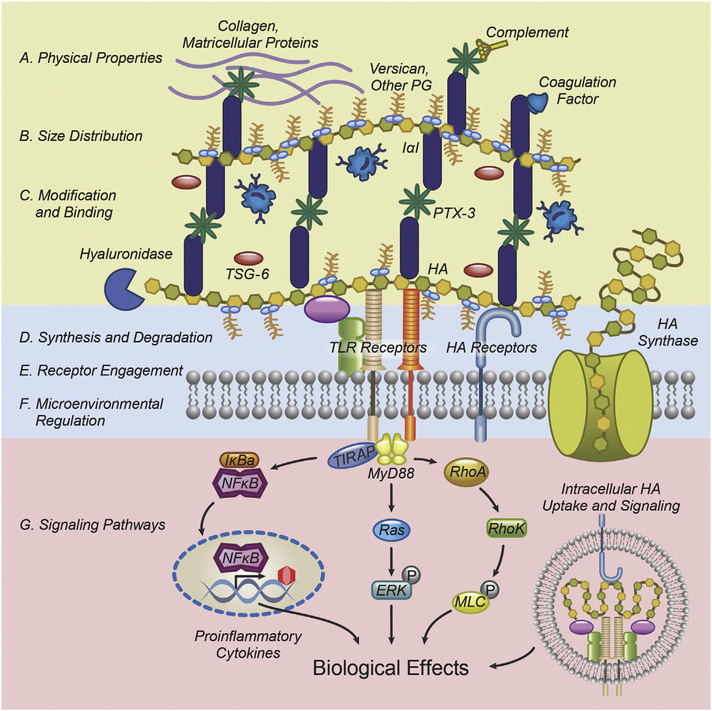
HA plays important roles in almost all areas of biology. Its interactions with cell receptors or other extracellular binding partners are important in cell and organ development, the response to tissue injury and inflammation, cell migration, cancer formation and resistance. A puzzling aspect of the existing literature is that, depending on context, HA has differing and sometimes contradictory effects on many biological functions; for example, pro- or anti-inflammatory, promoting and inhibiting either migration or cell proliferation. Research over the past several years has begun to shed light into the underlying mechanisms that explain these disparate effects. There are at least four key mechanisms by which HA function and activity are regulated (Fig. 1): 1) physical properties, size and molecular weight distribution; 2) chemical modifications, binding partners, crosslinking patterns, and macromolecular structure; 3) metabolism of HA synthesis and degradation, as well as regulation by the microenvironment; and 4) receptor engagement and downstream signaling. These mechanisms are not mutually exclusive. For example, HA metabolism alters size, distribution, and physicochemical properties, while microenvironmental regulation may affect crosslinking patterns and binding partner engagement. Thus, HA biology is best understood by determining how its individual effects contribute to a dynamic process that directs biology at the cellular, organ, and organismal levels.
Fig. 1.
HA function and activity depend on key properties: physicochemical parameters, size, distribution, functional modifications and binding partners in the ECM; synthesis and degradation, microenvironmental regulation and receptor engagement in the vicinity of the plasma membrane; and intracellular signaling pathways. The complex interaction of these mechanisms manifests as distinct biological effects at the cell, organ, and organismal level.
Physical properties, size, and molecular weight distribution
High molecular weight (HMW) HA is a hydroviscous substance, a characteristic that dictates many of its macromolecular effects. Given its extremely hydrophilic nature, HA can bind up to 1000× its weight in water, thus forming a very voluminous, expanded random-coil structure in aqueous physiological solutions. Interestingly, however, the structure of HMW HA varies with the context in which it is found. Thus, the glycocalyx of vascular endothelial cells consists of a brush-like border [11], the HA surrounding the cumulus cells around the oocyte is tightly bound and circular [12], and when surrounding chondrocytes, HA gives rise to a biomechanical structure that protects cartilage [13]. These variations of HMW HA likely arise from the macromolecular structure imparted by interacting HA-binding proteins and proteoglycans. Thus, HA most likely acts as an insulating coat or spatial buffer, permitting lowmolecular-weight molecules such as electrolytes and nutrients to diffuse, but blocking high-molecularweight proteins or even cells from reaching the cell surface, as elegantly demonstrated in the red blood cell exclusion assay [14]. Further, the endothelial HA glycocalyx prevents inflammatory cells from directly interacting with the endothelium by maintaining a nonadhesive surface and, in platelets, HA prevents adherence and degranulation [15]. Degradation of HA, however, reduces its barrier function thus making the HA matrix much more permeable and accessible to cell interactions.
HA size is one of the major determinants of its activity. Several studies have demonstrated that HA >1 MDa is anti-inflammatory and promotes epithelial cell homeostasis and survival. Thus, HA, at doses of 1 mg/ml or greater in its HMW form, inhibits inflammatory cell chemotaxis, phagocytosis, elastase release, and respiratory burst activity [16–19]. HMW HA also acts as an anti-inflammatory and anti-fibrotic agent in rheumatoid and osteoarthritis [20] and in the repair of tympanic membrane perforations [21]. Additionally, HMW HA accelerates cutaneous wound healing [22] and reduces adhesion formation after intra-abdominal surgery [23]. On the other hand, at lower concentrations and at lower molecular weights, fragmented HA (LMW HA) promotes monocyte maturation into macrophages as measured by production of insulin-like growth factor-1 [24] and IL-1β [25,26]. HA is also significantly increased during inflammatory conditions such as myocardial infarction, arthritis, and during transplant rejection [27–29]. Removal of HA by early treatment of myocardial infarction with hyaluronidase results in reduced myocardial fibrosis and infarct size [30]. HA fragments consist of LMW HA (<~500–700 kDa), and much smaller HA oligosaccharides (8–30-dimer lengths). Collectively, these fragments increase the expression of proinflammatory chemokines and iNOS in macrophage cell lines as well as in alveolar macrophages from injured lungs [25,31,32]. On the other hand, a hyaluronan tetrasaccharide blocks TLR2 activation and protects against ischemic brain injury [33]. The reasons for this pronounced sizedependency are not entirely clear. Several factors, including receptor clustering and engagement, cellular uptake, intra- vs extracellular signaling, and interactions with HA ligands such as heavy chains of inter-α-inhibitor (IαI), potentially related to linear or globular structures of different-sized HA chains, likely play a role.
HA modifications, binding partners, crosslinking patterns and macromolecular structure
The HA matrix can be best viewed as a canvas that is continually woven, unraveled, and decorated by dynamic patterns of hyaladherins which help shape HA-specific effects. Several ECM proteins interact directly with HA that significantly expand the repertoire of HA interactions with ECM components, and contribute to the diversity of HA responses. One of the best characterized examples is TSG-6. Heavy chain transfer from IαI to HA, as catalyzed by TSG-6, and further macromolecular stabilization by PTX3 change the conformation of HA [34]. The IαI heavy chains and PTX3 can also bind ECM proteins such as complement [35], and vitronectin [36], to indirectly attach these molecules onto the HA matrix complex. The decoration of HA with IαI heavy chains alters the adhesive properties of HA to invading cells, as do aggrecan and versican, which confers pro-and antiinflammatory activity. Importantly, the association of HA with these proteins changes dynamically during tissue injury, inflammation, and organ development, thus lending great plasticity and versatility to the biological properties of HA matrices. Importantly, these HA binding partners have significant functions beyond HA binding, thus vastly expanding the spectrum of HA matrix activity. These HA modifications can modulate their own interactions with these proteins. For example, the IαI heavy chain transfer onto HA chains is reversible with HMW HA, but irreversible when the heavy chains are transferred onto HA oligosaccharides [37]. Therefore, the evolution of HA sizes in response to injury may itself affect the interaction with proteins and may be a clearing mechanism for HA and HAassociated proteins.
HA metabolism (synthesis and degradation) and microenvironmental regulation
HA metabolism is intriguing in that one third of the body’s HA undergoes turnover daily [38]. The balance of HA synthesis and breakdown of HA either by hyaluronidases or oxidative and nitrative stresses define HA content and form. HA production is regulated at multiple levels including enzyme expression, post-transcriptional control by micro-RNAs and antisense HAS expression, and/or posttranslational modifications. HA is synthesized by at least three distinct, apparently functionally redundant synthases. However, the HASs appear to have different biological roles depending on specific contexts [39] The relative functions of each HAS have been investigated using knockout mice. Global ablation of Has2 is embryonic lethal and is associated with cardiac cushion defects. Conditional Has2 knockout mice demonstrate a wide variety of functions including activity in lung epithelia to support resilience after injury, in lung fibroblasts to support invasiveness and fibrosis [40], and in bone marrow to support hematopoiesis [41]. On the other hand, Has1 and Has3 knockout mice are phenotypically less dramatic. Has1 deficiency is associated with a failure to form the retrocalcaneal bursa [42], whereas Has3 loss results in a migratory defect in vascular smooth muscle cells that results in decreased neointimal formation following endothelial injury [43], as well as abnormal neuronal activity and seizures [44]. Interestingly, the combined deficiency of Has1 and Has3 deficiency is associated with increased inflammation and accelerated wound closure of full thickness wounds [45]. Of particular interest, the naked mole rat has a unique Has2 sequence, and concomitantly decreased hyaluronidase expression, resulting in HMW HA accumulation which is associated with longevity and greatly reduced tumorigenesis [46]. Pharmacologically, four-methylumbelliferone (4-MU) inhibits HA synthesis by depleting intracellular pools of UDP-glucuronic acid and decreasing HAS2 and HAS3 expression [47]. This tool has been used to determine the role of HA in tumor growth, inflammation, and autoimmunity [48].
HA catabolism is still incompletely understood. Several hyaluronidases have been identified; however, recent studies have cast doubt on the specificity or potency of the hyaluronidase activity of some of these proteins [49]. Among the most studied are Hyal1, Hyal2, and PH20. Hyal1 is an endoglycosidase that is active at an acidic pH and is found within lysosomes, serum, and extravascular space. A rare, genetically-induced deficiency in Hyal1 leads to the lysosomal storage disorder mucopolysaccharidosis IX and arthritis in children [50]. Conversely, in the skin, Hyal1 triggers adaptive immunity by generating HA oligosaccharides which activates TLR4 and CD44, the HA receptor, that leads to increased allergic inflammation via dendritic cell trafficking and improved clearance of bacterial skin infections [26,51–53]. Hyal2, a GPI-anchored protein with an acidic pH, hydrolyzes HMW HA into intermediate length HA that is internalized for further degradation by Hyal1. Hyal2 deficiency leads to high pre-weaning mortality as well as long-term cardiopulmonary dysfunction by 3 months of age. Additionally, Hyal2 is necessary for thrombopoiesis [15]. PH20 (or SPAM1), initially characterized as a hyaluronidase active at neutral and acidic pHs, is critical for fertilization of the oocyte by sperm. Subsequent studies demonstrated that this protein is also GPI-anchored and possesses signaling properties [54]. Pathologically, all three hyaluronidases are elevated in a variety of cancers and have been proposed as tumor biomarkers [55]. Indeed, the growing evidence of HA accumulation within the tumor stroma and its contribution to cancer progression has prompted the development of PEGylated PH20 as an anti-cancer therapeutic currently undergoing clinical trials [56]. Recent evidence demonstrates that HA degradation via hyaluronidases can be used therapeutically in inflammatory and infectious diseases as well, thus vastly expanding the spectrum of effects of HA metabolism [57]. More recently, HYaluronan Binding protein Involved In HA Depolymerization (HYBID), also known as KIAA1199, is a deafnessassociated gene established as a hyaladherin with endo-β-N-acetylglucosaminidase-dependent HA degradation activity [58]. Increased HA degradation in synovial fibroblasts was dependent on HYBID thereby implicating it in inflammatory arthritic synovium [58]. Subsequent studies have noted that HYBID is associated with the endoplasmic reticulum and regulates cancer cell migration [59], and is responsible for the generation of HA fragments in Crohn’s disease fibroblasts [60]. HYBID is highly expressed in the brain and HYBID knockout mice show memory dysfunction concurrent with an accumulation of HA in the hippocampus [61].
In addition to enzyme-mediated degradation, HA can be fragmented by reactive oxygen and nitrogen species. Oxygen radicals degrade HA in vitro [62–64], and activation of neutrophil myeloperoxidase can do the same in vivo [65]. Thus, HA fragments become part of the front-line defense to injury by activating innate and adaptive immunity.
Changes at the microenvironmental level also contribute to HA modification and activation of signaling pathways. For example, CD44, Hyal2 and the Na+/H+ exchanger (NHE1) are in close approximation in lipid rafts. Binding of HA to CD44 results in phosphorylation and activation of NHE1 to acidify the local environment which stimulates Hyal2 and Cathepsin B to modify HA and promote cell invasion [66].
HA receptor engagement and signaling
Several cell-associated HA receptors have been described and characterized in some detail. Differential expression of both content and timing, localization, functions, and unique activation pathways mediated by these receptors greatly expand the spectrum of HA action. CD44, the best characterized of all HA receptors, is a ubiquitous family of non-kinase, type 1 cellular hyaladherins with many described functions. CD44 mediates cell motility, inflammation, lymphocyte homing, and cell growth including tumorigenesis, but also participates in HA clearance and injury resolution. CD44 consists of 10 consistent exons and nine alternatively spliced exons resulting in a multitude of CD44 isoforms with distinct functions that remain incompletely elucidated. CD44 is promiscuous in its binding partners that include osteopontin, collagen, and fibronectin in addition to HA [67]. These properties result in the diverse function of CD44 in homeostasis and pathobiology.
The scavenging receptor Stabilin 2 or Hyaluronic Acid Receptor for Endocytosis (HARE) is a sinusoidal endothelial cell surface receptor in the liver, spleen, and lymph nodes that is responsible for the rapid clearance of HA, chondroitin sulfate and heparin from circulation [68,69]. Interestingly, ligand binding to Stabilin 2 activates intracellular signaling pathways including NFκB [70,71], and blocking Stabilin 2 is associated with a substantial increase in serum HA concentrations and an inhibition of tumor metastasis [72]. Most recently, Stabilin 2 has been implicated in the recycling of VWF and Factor VIII complex to regulate serum concentrations and modulate immune responses [73].
LYVE-1, structurally similar to CD44, is a marker for lymphatic versus blood endothelial cells, mediates dendritic and other inflammatory cell egress to the lymphatic lumen, and is necessary for antigen-specific T cell responses [74,75]. The unraveling of LYVE-1 functions reveals new horizons of HA-LYVE-1 interactions that may influence diverse actions including inflammation, transplantation, and drug delivery [76,77].
The Receptor for HA-Mediated Motility (RHAMM) or CD168 was first isolated as a 56–58 kDa protein from the supernatant of non-confluent embryonic chick heart fibroblasts and shown to regulate their ruffling and migration [78]. A number of critical functions, including the motility of thymocytes, lymphocytes, hematopoietic progenitor cells, malignant B lymphocytes, fibroblasts, smooth muscle cells, endothelial cells, and macrophages, as well as the regulation of MAP kinasemediated proliferative responses and cellular transformation have been ascribed to RHAMM [79–83]. Binding of HA to membrane RHAMM results in a transient burst of protein tyrosine phosphorylation, focal adhesion turnover [82], and regulation of the ERK kinase cascade through Ras [84]. A number of reports are consistent with a role for RHAMM in inflammatory and endothelial cell migration, thereby contributing to wound healing, in vivo angiogenesis and acute lung injury [79,85–87].
Layilin is a transmembrane, talin-binding protein homologous to C-type lectins that bind HA [88,89]. Layilin is key in maintaining gut epithelial integrity by regulating ZO-1, a tight junction protein, in response to a specific 35 kDa HA that is orally anti-inflammatory [90].
As noted above, HA interacts with CD44 and innate immune receptors such as TLR4 to activate the NLRP3 inflammasome [26,51–53,91]. HA signaling is thus dependent not only on the gene expression of specific receptors in a given cell, but also on receptor clustering and cell-specific intracellular signaling pathways activated downstream of the receptors. Many of these pathways remain to be mechanistically defined in more depth.
Organ development and injury responses
The ECM plays a pivotal role in organ development and cell differentiation [92,93]. A number of recent studies focused on the role of HA in organ and tissue differentiation, as well as in responses to tissue injury. These constitute a number of distinct processes that ultimately define the mechanisms of a disparate set of diseases that all appear to be influenced by HA. Indeed, HA regulates disease progression by regulating the inflammatory process and common themes are discernable that will likely lead to therapeutic approaches to ameliorate these conditions that carry high rates of morbidity and even mortality.
Therapeutic applications
The discovery that HMW HA had highly viscoelastic properties led to the development of clinical grade HMW HA as a therapeutic intervention for a wide array of therapeutic modalities including ophthalmic surgery, visco-supplementation for joint arthritis and pain control, wound healing, surgical adhesions, and tissue augmentation. HMW HA also serves as a scaffold for tissue engineering, as a drug delivery vector, and cosmetic uses as fillers for skin wrinkling [94–99].
Using the primary and secondary structures of HA-binding proteins and receptors, a variety of probes and therapeutic interventions have been developed. Employing a phage display library, Mummert and colleagues developed Pep1, a 12-mer HA-binding peptide for curtailing inflammatory responses to skin hypersensitivity [100] as well as a biotinylated Pep1 to localize HA in tissue sections [101]. In addition, peptides that bind to HA and block their interactions with CD44 reduce inflammation, neovascularization, and cancer metastasis [102–104]. These studies provide proof of concept that limiting HA function in injured tissues is a viable approach for restraining inflammation.
Various domains within RHAMM have been proposed. However, the best characterized are the two HA-binding domains near the carboxy terminus of the protein [105]. Site-directed mutagenesis of these HA-binding domains indicates that the minimum binding requirement for HA is represented by B (X7)B where B represents the basic amino acids arginine or lysine and X represents any non-acidic amino acid [106]. Identification of this HA-binding motif has allowed the creation of synthetic peptides with varying affinities for HA to competitively inhibit cell locomotion in vitro [106] and inflammation and fibrosis in vivo [107].
Knowledge gaps
There has been tremendous progress in HA biology over the past few years, as the contents of this Special Issue make abundantly clear. However, much remains to be elucidated. Below are some examples of “known unknowns” which may further explain the observed effects of hyaluronan:
What are the precise polymer-receptor interactions of HA? It is still unclear how HA fragment size influences receptor activation. Sophisticated experiments suggest that tertiary or quaternary interactions of HA receptors with the HA matrix may be invoked to explain these interactions [108–110]. These studies pave the way for future studies.
How are pro- or anti-inflammatory functions of LMW and HMW HA mediated and can contamination be safely ruled out as a contributing factor? Although in general LMW HA has pro-inflammatory properties, recent reports have questioned this, suggesting that contaminants such as LPS may, in fact, account for the previously discovered pro-inflammatory effects [111]. On the other hand, carefully conducted studies have demonstrated the pro-inflammatory functions of oligomeric HA [16]. The truth is likely somewhere in between. It is likely that some reports are confounded by contaminants, and it is equally possible that a lack of cell-specific receptors may account for negative results – ultimately, the effect of HA is likely to be the result of complex interactions between receptors, intracellular mediators, and the pathologic mechanisms being studied.
Analysis of HA interactions with its receptors and effects on signaling at the microdomain level. It is very likely that the divergent effects of HA are partly due to different receptor clusters with which it interacts, as well as their interactions with ECM binding partners of HA. Recent research is shedding light on these interactions, but much remains unknown.
Footnotes
Conflicts of interest
RCS is a co-founder of Eravon Therapeutics, Inc. that is focused on RHAMM-HA-based therapeutics. RCS also holds the William Buchanan Chair in Pediatrics at University of Texas Southwestern Medical Center. Neither author have any conflicts of interest related to this work.
References
- [1].Iozzo RV, Gubbiotti MA, Extracellular matrix: the driving force of mammalian diseases, Matrix Biol. 71-72 (2018) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Karamanos NK, Theocharis AD, Neill T, Iozzo RV, Matrix modeling and remodeling: a biological interplay regulating tissue homeostasis and diseases, Matrix Biol. 75-76 (2019) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Karamanos NK, Piperigkou Z, Theocharis AD, Watanabe H, Franchi M, Baud S, Brezillon S, Gotte M, Passi A, Vigetti D, Ricard-Blum S, Sanderson RD, Neill T, Iozzo RV, Proteoglycan chemical diversity drives multifunctional cell regulation and therapeutics, Chem. Rev 118 (18) (2018) 9152–9232. [DOI] [PubMed] [Google Scholar]
- [4].Mack M, Inflammation and fibrosis, Matrix Biol. 68–69 (2018) 106–121. [DOI] [PubMed] [Google Scholar]
- [5].Frevert CW, Felgenhauer J, Wygrecka M, Nastase MV, Schaefer L, Danger-associated molecular patterns derived from the extracellular matrix provide temporal control of innate immunity, J. Histochem. Cytochem 66 (4) (2018) 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wight TN, A role for proteoglycans in vascular disease, Matrix Biol. 71–72 (2018) 396–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wight TN, Provisional matrix: a role for versican and hyaluronan, Matrix Biol. 60–61 (2017) 38–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Karousou E, Misra S, Ghatak S, Dobra K, Gotte M, Vigetti D, Passi A, Karamanos NK, Skandalis SS, Roles and targeting of the HAS/hyaluronan/CD44 molecular system in cancer, Matrix Biol. 59 (2017) 3–22. [DOI] [PubMed] [Google Scholar]
- [9].Wight TN, Frevert CW, Debley JS, Reeves SR, Parks WC, Ziegler SF, Interplay of extracellular matrix and leukocytes in lung inflammation, Cell. Immunol 312 (2017) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Johnson P, Arif AA, Lee-Sayer SSM, Dong Y, Hyaluronan and its interactions with immune cells in the healthy and inflamed lung, Front. Immunol 9 (2018) 2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang X, Sun D, Song JW, Zullo J, Lipphardt M, Coneh-Gould L, Goligorsky MS, Endothelial cell dysfunction and glycocalyx - a vicious circle, Matrix Biol. 71–72 (2018) 421–431. [DOI] [PubMed] [Google Scholar]
- [12].Nagyova E, The biological role of hyaluronan-rich oocyte-cumulus extracellular matrix in female reproduction, Int J Mol Sci 19(1) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Huang Y, Askew EB, Knudson CB, Knudson W, CRISPR/Cas9 knockout of HAS2 in rat chondrosarcoma chondrocytes demonstrates the requirement of hyaluronan for aggrecan retention, Matrix Biol. 56 (2016) 74–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee GM, Johnstone B, Jacobson K, Caterson B, The dynamic structure of the pericellular matrix on living cells, J. Cell Biol 123 (6 Pt 2) (1993) 1899–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Petrey AC, Obery DR, Kessler SP, Flamion B, de la Motte CA, Hyaluronan depolymerization by megakaryocyte hyaluronidase-2 is required for thrombopoiesis, Am. J. Pathol 186 (9) (2016) 2390–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Foley JP, Lam D, Jiang H, Liao J, Cheong N, McDevitt TM, Zaman A, Wright JR, Savani RC, Toll-like receptor 2 (TLR2), transforming growth factor-beta, hyaluronan (HA), and receptor for HA-mediated motility (RHAMM) are required for surfactant protein A-stimulated macrophage chemotaxis, J. Biol. Chem 287 (44) (2012) 37406–37419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tamoto K, Nochi H, Tada M, Shimada S, Mori Y, Kataoka S, Suzuki Y, Nakamura T, High-molecular weight hyaluronic acids inhibit chemotaxis and phagocytosis but not lysosomal enzyme release induced by receptormediated stimulations in guinea pig phagocytes, Microbiol. Immunol 38 (1) (1994) 73–80. [DOI] [PubMed] [Google Scholar]
- [18].Suzuki Y, Yamaguchi T, Effects of hyaluronic acid on macrophage phagocytosis and active oxygen release, Agents Actions 38 (1993) 32–37. [DOI] [PubMed] [Google Scholar]
- [19].Akatsuka M, Yamamoto Y, Tobetto K, Yasui K, Ando T, Suppressive effects of hyaluronic acid on elastase release from rat peritoneal leucocytes, J. Pharm. Pharmacol 45 (1993) 110–114. [DOI] [PubMed] [Google Scholar]
- [20].Strachan RK, Smith P, Gardner DL, Hyaluronate in rheumatology and orthopedics: is there a role? Ann. Rheum. Dis 49 (1990) 949–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hellstrom S, Laurent C, Hyaluronan and healing of tympanic membrane perforations: an experimental study., Acta Otolaryngol. (Stockholm) 442 (Suppl.) (1987) 54–61. [DOI] [PubMed] [Google Scholar]
- [22].King SR, Hickerson WL, Proctor KG, Newsome AM, Beneficial actions of exogenous hyaluronic acid on wound healing, Surgery 109 (1991) 76–84. [PubMed] [Google Scholar]
- [23].Urman B, Gomel V, Jetha N, Effect of hyaluronic acid on postoperative intraperitoneal adhesion formation in the rat model, Fertil. Steril 56 (1991) 563–567. [PubMed] [Google Scholar]
- [24].Noble PW, Lake FR, Henson PM, Riches DW, Hyaluronate activation of CD44 induces insulin-like growth factor-1 expression by a tumor necrosis factor-alphadependent mechanism in murine macrophages, J. Clin. Invest 91 (6) (1993) 2368–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Osterholt HC, Dannevig I, Wyckoff MH, Liao J, Akgul Y, Ramgopal M, Mija DS, Cheong N, Longoria C, Mahendroo M, Nakstad B, Saugstad OD, Savani RC, Antioxidant protects against increases in low molecular weight hyaluronan and inflammation in asphyxiated newborn pigs resuscitated with 100% oxygen, PLoS One 7 (6) (2012), e38839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Yamasaki K, Muto J, Taylor KR, Cogen AL, Audish D, Bertin J, Grant EP, Coyle AJ, Misaghi A, Hoffman HM, Gallo RL, NLRP3/cryopyrin is necessary for interleukin-1 {beta} (IL-1{beta}) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury, J. Biol. Chem 284 (19) (2009) 12762–12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Waldenström A, Martinussen HJ, Gerdin B, Hällgren R, Accumulation of hyaluronan and tissue edema in experimental myocardial infarction, J. Clin. Invest 88 (1991) 1622–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wells AF, Klareskog L, Lindblad S, Laurent TC, Correlation between increased hyaluronan localized in arthritic synovium and the presence of proliferating cells. A role for macrophage-derived factors., Arthritis Rheum. 35 (4) (1992) 391–396. [DOI] [PubMed] [Google Scholar]
- [29].Wells AF, Larsson E, Tengblad A, Fellstrom B, Tufveson G, Klareskog L, Laurent TC, The localization of hyaluronan in normal and rejected human kidneys, Transplantation 50 (2) (1990) 240–243. [DOI] [PubMed] [Google Scholar]
- [30].Maclean D, Fishbein MC, Maroko PR, Braunwald E, Hyaluronidase-induced reductions in myocardial infarct size, Science 194 (1976) 199–200. [DOI] [PubMed] [Google Scholar]
- [31].McKee CM, Lowenstein CJ, Horton MR, Wu J, Bao C, Chin BY, Choi AMK, Noble PW, Hyaluronan fragments induce nitric oxide synthase in murine macrophages through a nuclear factor kB-dependent mechanism, J. Biol. Chem 272 (12) (1997) 8013–8018. [DOI] [PubMed] [Google Scholar]
- [32].McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW, Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages: the role of HA size and CD44, J. Clin. Invest 98 (10) (1996) 2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sunabori T, Koike M, Asari A, Oonuki Y, Uchiyama Y, Suppression of ischemia-induced hippocampal pyramidal neuron death by hyaluronan tetrasaccharide through inhibition of toll-like receptor 2 signaling pathway, Am. J. Pathol 186 (8) (2016) 2143–2151. [DOI] [PubMed] [Google Scholar]
- [34].Stober VP, Johnson CG, Majors A, Lauer ME, Cali V, Midura RJ, Wisniewski HG, Aronica MA, Garantziotis S, TNF-stimulated gene 6 promotes formation of hyaluronaninter-alpha-inhibitor heavy chain complexes necessary for ozone-induced airway hyperresponsiveness, J. Biol. Chem 292 (51) (2017) 20845–20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Garantziotis S, Hollingsworth JW, Ghanayem RB, Timberlake S, Zhuo L, Kimata K, Schwartz DA, Inter-alphatrypsin inhibitor attenuates complement activation and complement-induced lung injury, J. Immunol 179 (6) (2007) 4187–4192. [DOI] [PubMed] [Google Scholar]
- [36].Adair JE, Stober V, Sobhany M, Zhuo L, Roberts JD, Negishi M, Kimata K, Garantziotis S, Inter-alpha-trypsin inhibitor promotes bronchial epithelial repair after injury through vitronectin binding, J. Biol. Chem 284 (25) (2009) 16922–16930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW, Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice, J. Biol. Chem 284 (17) (2009) 11309–11317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [38].Triggs-Raine B, Natowicz MR, Biology of hyaluronan: insights from genetic disorders of hyaluronan metabolism, World J. Biol. Chem 6 (3) (2015) 110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Viola M, Karousou E, D’Angelo ML, Caon I, De Luca G, Passi A, Vigetti D, Regulated hyaluronan synthesis by vascular cells, Int J Cell Biol 2015 (2015) 208303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Li Y, Liang J, Yang T, Monterrosa Mena J, Huan C, Xie T, Kurkciyan A, Liu N, Jiang D, Noble PW, Hyaluronan synthase 2 regulates fibroblast senescence in pulmonary fibrosis, Matrix Biol. 55 (2016) 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Goncharova V, Serobyan N, Iizuka S, Schraufstatter I, de Ridder A, Povaliy T, Wacker V, Itano N, Kimata K, Orlovskaja IA, Yamaguchi Y, Khaldoyanidi S, Hyaluronan expressed by the hematopoietic microenvironment is required for bone marrow hematopoiesis, J. Biol. Chem 287 (30) (2012) 25419–25433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sikes KJ, Renner K, Li J, Grande-Allen KJ, Connell JP, Cali V, Midura RJ, Sandy JD, Plaas A, Wang VM, Knockout of hyaluronan synthase 1, but not 3, impairs formation of the retrocalcaneal bursa, J. Orthop. Res 36 (10) (2018) 2622–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kiene LS, Homann S, Suvorava T, Rabausch B, Muller J, Kojda G, Kretschmer I, Twarock S, Dai G, Deenen R, Hartwig S, Lehr S, Kohrer K, Savani RC, Grandoch M, Fischer JW, Deletion of hyaluronan synthase 3 inhibits neointimal hyperplasia in mice, Arterioscler. Thromb. Vasc. Biol 36 (2) (2016) e9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Arranz AM, Perkins KL, Irie F, Lewis DP, Hrabe J, Xiao F, Itano N, Kimata K, Hrabetova S, Yamaguchi Y, Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space, J. Neurosci 34 (18) (2014) 6164–6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mack JA, Feldman RJ, Itano N, Kimata K, Lauer M, Hascall VC, Maytin EV, Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3, J Invest Dermatol 132 (1) (2012) 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tian X, Azpurua J, Hine C, Vaidya A, Myakishev Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A, High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat, Nature 499 (7458) (2013) 346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kultti A, Pasonen-Seppanen S, Jauhiainen M, Rilla KJ, Karna R, Pyoria E, Tammi RH, Tammi MI, 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3, Exp. Cell Res 315 (11) (2009) 1914–1923. [DOI] [PubMed] [Google Scholar]
- [48].Nagy N, Kuipers HF, Frymoyer AR, Ishak HD, Bollyky JB, Wight TN, Bollyky PL, 4-Methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer, Front. Immunol 6 (2015) 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].McAtee CO, Barycki JJ, Simpson MA, Emerging roles for hyaluronidase in cancer metastasis and therapy, Adv. Cancer Res 123 (2014) 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Triggs-Raine B, Salo TJ, Zhang H, Wicklow BA, Natowicz MR, Mutations in HYAL1, a member of a tandemly distributed multigene family encoding disparate hyaluronidase activities, cause a newly described lysosomal disorder, mucopolysaccharidosis IX, Proc. Natl. Acad. Sci. U. S. A 96 (11) (1999) 6296–6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Taylor KR, Gallo RL, Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation, FASEB J. 20 (1) (2006) 9–22. [DOI] [PubMed] [Google Scholar]
- [52].Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL, Hyaluronan fragments stimulate endothelial recognition of injury through TLR4, J. Biol. Chem 279 (17) (2004) 17079–17084. [DOI] [PubMed] [Google Scholar]
- [53].Taylor KR, Yamasaki K, Radek KA, Di Nardo A, Goodarzi H, Golenbock D, Beutler B, Gallo RL, Recognition of hyaluronan released in sterile injury involves a unique receptor complex dependent on Tolllike receptor 4, CD44, and MD-2, J. Biol. Chem 282 (25) (2007) 18265–18275. [DOI] [PubMed] [Google Scholar]
- [54].Cherr GN, Yudin AI, Overstreet JW, The dual functions of GPI-anchored PH-20: hyaluronidase and intracellular signaling, Matrix Biol. 20 (8) (2001) 515–525. [DOI] [PubMed] [Google Scholar]
- [55].Lokeshwar VB, Selzer MG, Hyalurondiase: both a tumor promoter and suppressor, Semin. Cancer Biol 18 (4) (2008) 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Infante JR, Korn RL, Rosen LS, LoRusso P, Dychter SS, Zhu J, Maneval DC, Jiang P, Shepard HM, Frost G, Von Hoff DD, Borad MJ, Ramanathan RK, Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours, Br. J. Cancer 118 (2) (2018) 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bell TJ, Brand OJ, Morgan DJ, Salek-Ardakani S, Jagger C, Fujimori T, Cholewa L, Tilakaratna V, Ostling J, Thomas M, Day AJ, Snelgrove RJ, Hussell T, Defective lung function following influenza virus is due to prolonged, reversible hyaluronan synthesis, Matrix Biol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Yoshida H, Nagaoka A, Kusaka-Kikushima A, Tobiishi M, Kawabata K, Sayo T, Sakai S, Sugiyama Y, Enomoto H, Okada Y, Inoue S, KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization, Proc. Natl. Acad. Sci. U. S. A 110 (14) (2013) 5612–5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Evensen NA, Kuscu C, Nguyen HL, Zarrabi K, Dufour A, Kadam P, Hu YJ, Pulkoski-Gross A, Bahou WF, Zucker S, Cao J, Unraveling the role of KIAA1199, a novel endoplasmic reticulum protein, in cancer cell migration, J. Natl. Cancer Inst 105 (18) (2013) 1402–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Soroosh A, Albeiroti S, West GA, Willard B, Fiocchi C, de la Motte CA, Crohn’s disease fibroblasts overproduce the novel protein KIAA1199 to create proinflammatory hyaluronan fragments, Cell Mol Gastroenterol Hepatol 2 (3) (2016) 358–368 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yoshino Y, Ishisaka M, Tsuruma K, Shimazawa M, Yoshida H, Inoue S, Shimoda M, Okada Y, Hara H, Distribution and function of hyaluronan binding protein involved in hyaluronan depolymerization (HYBID, KIAA1199) in the mouse central nervous system, Neuroscience 347 (2017) 1–10. [DOI] [PubMed] [Google Scholar]
- [62].Kennett EC, Davies MJ, Degradation of matrix glycosaminoglycans by peroxynitrite/peroxynitrous acid: evidence for a hydroxyl-radical-like mechanism, Free Radic. Biol. Med 42 (8) (2007) 1278–1289. [DOI] [PubMed] [Google Scholar]
- [63].Kennett EC, Davies MJ, Glycosaminoglycans are fragmented by hydroxyl, carbonate, and nitrogen dioxide radicals in a site-selective manner: implications for peroxynitrite-mediated damage at sites of inflammation, Free Radic. Biol. Med 47 (4) (2009) 389–400. [DOI] [PubMed] [Google Scholar]
- [64].Li M, Rosenfeld L, Vilar RE, Cowman MK, Degradation of hyaluronan by peroxynitrite, Arch. Biochem. Biophys 341 (2) (1997) 245–250. [DOI] [PubMed] [Google Scholar]
- [65].Moseley R, Waddington RJ, Embery G, Degradation of glycosaminoglycans by reactive oxygen species derived from stimulated polymorphonuclear leukocytes, Biochim. Biophys. Acta 1362 (2–3) (1997) 221–231. [DOI] [PubMed] [Google Scholar]
- [66].Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E, CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase 2 and cathepsin B activation and breast tumor cell invasion, J. Biol. Chem 279 (26) (2004) 26991–27007. [DOI] [PubMed] [Google Scholar]
- [67].Chen C, Zhao S, Karnad A, Freeman JW, The biology and role of CD44 in cancer progression: therapeutic implications, J. Hematol. Oncol 11 (1) (2018) 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Harris EN, Kyosseva SV, Weigel JA, Weigel PH, Expression, processing, and glycosaminoglycan binding activity of the recombinant human 315-kDa hyaluronic acid receptor for endocytosis (HARE), J. Biol. Chem 282 (5) (2007) 2785–2797. [DOI] [PubMed] [Google Scholar]
- [69].Harris EN, Weigel JA, Weigel PH, The human hyaluronan receptor for endocytosis (HARE/Stabilin-2) is a systemic clearance receptor for heparin, J. Biol. Chem 283 (25) (2008) 17341–17350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Pandey MS, Baggenstoss BA, Washburn J, Harris EN, Weigel PH, The hyaluronan receptor for endocytosis (HARE) activates NF-kappaB-mediated gene expression in response to 40–400-kDa, but not smaller or larger, hyaluronans, J. Biol. Chem 288 (20) (2013) 14068–14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Pandey MS, Weigel PH, Hyaluronic acid receptor for endocytosis (HARE)-mediated endocytosis of hyaluronan, heparin, dermatan sulfate, and acetylated low density lipoprotein (AcLDL), but not chondroitin sulfate types A, C, D, or E, activates NF-kappaB-regulated gene expression, J Biol Chem 289 (3) (2014) 1756–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Hirose Y, Saijou E, Sugano Y, Takeshita F, Nishimura S, Nonaka H, Chen YR, Sekine K, Kido T, Nakamura T, Kato S, Kanke T, Nakamura K, Nagai R, Ochiya T, Miyajima A, Inhibition of Stabilin-2 elevates circulating hyaluronic acid levels and prevents tumor metastasis, Proc. Natl. Acad. Sci. U. S. A 109 (11) (2012) 4263–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Swystun LL, Lai JD, Notley C, Georgescu I, Paine AS, Mewburn J, Nesbitt K, Schledzewski K, Geraud C, Kzhyshkowska J, Goerdt S, Hopman W, Montgomery RR, James PD, Lillicrap D, The endothelial cell receptor stabilin-2 regulates VWF-FVIII complex half-life and immunogenicity, J. Clin. Invest 128 (9) (2018) 4057–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG, LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan, J. Cell Biol 144 (4) (1999) 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Johnson LA, Banerji S, Lawrance W, Gileadi U, Prota G, Holder KA, Roshorm YM, Hanke T, Cerundolo V, Gale NW, Jackson DG, Dendritic cells enter lymph vessels by hyaluronan-mediated docking to the endothelial receptor LYVE-1, Nat. Immunol 18 (7) (2017) 762–770. [DOI] [PubMed] [Google Scholar]
- [76].Banerji S, Hide BR, James JR, Noble ME, Jackson DG, Distinctive properties of the hyaluronan-binding domain in the lymphatic endothelial receptor Lyve-1 and their implications for receptor function, J. Biol. Chem 285 (14) (2010) 10724–10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Bauer J, Rothley M, Schmaus A, Quagliata L, Ehret M, Biskup M, Orian-Rousseau V, Jackson DG, Pettis RJ, Harvey A, Brase S, Thiele W, Sleeman JP, TGFbeta counteracts LYVE-1-mediated induction of lymphangiogenesis by small hyaluronan oligosaccharides, J Mol Med (Berl) 96 (2) (2018) 199–209. [DOI] [PubMed] [Google Scholar]
- [78].Turley EA, Purification of a hyaluronate-binding protein fraction that modifies cell social behaviour, Biochem. Biophys. Res. Commun 108 (3) (1982) 1016–1024. [DOI] [PubMed] [Google Scholar]
- [79].Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S, Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways, Matrix Biol. 26 (1) (2007) 58–68. [DOI] [PubMed] [Google Scholar]
- [80].Turley EA, Hyaluronan and cell locomotion, Cancer Met. Rev 11 (1992) 21–30. [DOI] [PubMed] [Google Scholar]
- [81].Turley EA, Noble PW, Bourguignon LY, Signaling properties of hyaluronan receptors, J. Biol. Chem 277 (7) (2002) 4589–4592. [DOI] [PubMed] [Google Scholar]
- [82].Hall CL, Wang C, Lange LA, Turley EA, Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase turnover, J. Cell Biol 126 (1994) 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hall CL, Yang B, Yang X, Zhang S, Turley M, Samuel S, Lange LA, Wang C, Curpen GD, Savani RC, Greenberg AH, Turley EA, Overexpression of the hyaluronan receptor RHAMM is transforming and is also required for H-ras transformation, Cell 82 (1) (1995) 19–26. [DOI] [PubMed] [Google Scholar]
- [84].Zhang S, Chang MC, Zylka D, Turley S, Harrison R, Turley EA, The hyaluronan receptor RHAMM regulates extracellular-regulated kinase, J. Biol. Chem 273 (18) (1998) 11342–11348. [DOI] [PubMed] [Google Scholar]
- [85].Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM, Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis, J. Biol. Chem 276 (39) (2001) 36770–36778. [DOI] [PubMed] [Google Scholar]
- [86].Savani RC, Wang C, Yang B, Zhang S, Kinsella MG, Wight TN, Stern R, Nance DM, Turley EA, Migration of bovine aortic smooth muscle cells after wounding injury, The role of hyaluronan and RHAMM, The Journal of clinical investigation 95 (3) (1995) 1158–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zaman A, Cui Z, Foley JP, Zhao H, Grimm PC, Delisser HM, Savani RC, Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury, Am. J. Respir. Cell Mol. Biol 33 (5) (2005) 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Borowsky ML, Hynes RO, Layilin, a novel talin-binding transmembrane protein homologous with C-type lectins, is localized in membrane ruffles, J. Cell Biol 143 (2) (1998) 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bono P, Rubin K, Higgins JM, Hynes RO, Layilin, a novel integral membrane protein, is a hyaluronan receptor, Mol. Biol. Cell 12 (4) (2001) 891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kim Y, West GA, Ray G, Kessler SP, Petrey AC, Fiocchi C, McDonald C, Longworth MS, Nagy LE, de la Motte CA, Layilin is critical for mediating hyaluronan 35kDa-induced intestinal epithelial tight junction protein ZO-1 in vitro and in vivo, Matrix Biol. 66 (2018) 93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Tighe RM, Garantziotis S, Hyaluronan interactions with innate immunity in lung biology, Matrix Biol. (2018), 10.1016/j.matbio.2018.01.027, pii: S0945–053X(17)30462–6 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Patel VN, Pineda DL, Hoffman MP, The function of heparan sulfate during branching morphogenesis, Matrix Biol. 57–58 (2017) 311–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ramos-Lewis W, Page-McCaw A, Basement membrane mechanics shape development: lessons from the fly, Matrix Biol. 75-76 (2019) 72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Balazs EA, Denlinger JL, Clinical uses of hyaluronan, Ciba Found Symp 143 (1989) 265–75; discussion 275–80, 281–5. [DOI] [PubMed] [Google Scholar]
- [95].Balazs EA, Viscosupplementation for treatment of osteoarthritis: from initial discovery to current status and results, Surg Technol Int 12 (2004) 278–289. [PubMed] [Google Scholar]
- [96].Price RD, Myers S, Leigh IM, Navsaria HA, The role of hyaluronic acid in wound healing: assessment of clinical evidence, Am. J. Clin. Dermatol 6 (6) (2005) 393–402. [DOI] [PubMed] [Google Scholar]
- [97].Volpi N, Schiller J, Stern R, Soltes L, Role, metabolism, chemical modifications and applications of hyaluronan, Curr. Med. Chem 16 (14) (2009) 1718–1745. [DOI] [PubMed] [Google Scholar]
- [98].Gaffney J, Matou-Nasri S, Grau-Olivares M, Slevin M, Therapeutic applications of hyaluronan, Mol. BioSyst 6 (3) (2010) 437–443. [DOI] [PubMed] [Google Scholar]
- [99].Prestwich GD, Hyaluronic acid-based clinical biomaterials derived for cell and molecule delivery in regenerative medicine, J. Control. Release 155 (2) (2011) 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Mummert ME, Mohamadzadeh M, Mummert DI, Mizumoto N, Takashima A, Development of a peptide inhibitor of hyaluronan-mediated leukocyte trafficking, J. Exp. Med 192 (6) (2000) 769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Zmolik JM, Mummert ME, Pep-1 as a novel probe for the in situ detection of hyaluronan, J. Histochem. Cytochem 53 (6) (2005) 745–751. [DOI] [PubMed] [Google Scholar]
- [102].Finlayson M, Modulation of CD44 activity by A6-peptide, Front. Immunol 6 (2015) 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Campo GM, Avenoso A, D’Ascola A, Nastasi G, Micali A, Puzzolo D, Pisani A, Prestipino V, Scuruchi M, Calatroni A, Campo S, Combined treatment with hyaluronan inhibitor Pep-1 and a selective adenosine A2 receptor agonist reduces inflammation in experimental arthritis, Innate Immun 19 (5) (2013) 462–478. [DOI] [PubMed] [Google Scholar]
- [104].Piotrowicz RS, Damaj BB, Hachicha M, Incardona F, Howell SB, Finlayson M, A6 peptide activates CD44 adhesive activity, induces FAK and MEK phosphorylation, and inhibits the migration and metastasis of CD44-expressing cells, Mol. Cancer Ther 10 (11) (2011) 2072–2082. [DOI] [PubMed] [Google Scholar]
- [105].Yang B, Zhang L, Turley EA, Identification of two hyaluronan-binding domains in the hyaluronan receptor RHAMM, J. Biol. Chem 268 (1993) 8617–8623. [PubMed] [Google Scholar]
- [106].Yang B, Yang BL, Savani RC, Turley EA, Identification of a common hyaluronan binding motif in the hyaluronan binding proteins RHAMM, CD44 and link protein, EMBO J. 13 (2) (1994) 286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Savani RC, Hou G, Liu P, Wang C, Simons E, Grimm PC, Stern R, Greenberg AH, DeLisser HM, Khalil N, A role for hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury, Am. J. Respir. Cell Mol. Biol 23 (4) (2000) 475–484. [DOI] [PubMed] [Google Scholar]
- [108].Bano F, Tammi MI, Kang DW, Harris EN, Richter RP, Single-molecule unbinding forces between the polysaccharide hyaluronan and its binding proteins, Biophys. J 114 (12) (2018) 2910–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Bano F, Banerji S, Howarth M, Jackson DG, Richter RP, A single molecule assay to probe monovalent and multivalent bonds between hyaluronan and its key leukocyte receptor CD44 under force, Sci. Rep 6 (2016) 34176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Dubacheva GV, Curk T, Mognetti BM, Auzely-Velty R, Frenkel D, Richter RP, Superselective targeting using multivalent polymers, J. Am. Chem. Soc 136 (5) (2014) 1722–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Dong Y, Arif A, Olsson M, Cali V, Hardman B, Dosanjh M, Lauer M, Midura RJ, Hascall VC, Brown KL, Johnson P, Endotoxin free hyaluronan and hyaluronan fragments do not stimulate TNF-alpha, interleukin-12 or upregulate co-stimulatory molecules in dendritic cells or macrophages, Sci. Rep 6 (2016) 36928. [DOI] [PMC free article] [PubMed] [Google Scholar]