Abstract
We report on the detection of two series of harmonically related doublets in IRC +10216. From the observed frequencies, the rotational constant of the first series is B = 1380.888 MHz and that of the second series is B = 1381.512 MHz. The two series correspond to two species with a 2Σ electronic ground state. After considering all possible candidates, and based on quantum chemical calculations, the first series is assigned to MgC3N and the second to MgC4H. For the latter species, optical spectroscopy measurements support its identification. Unlike diatomic metal-containing molecules, the line profiles of the two new molecules indicate that they are formed in the outer layers of the envelope, as occurs for MgNC and other polyatomic metal-cyanides. We also confirm the detection of MgCCH that was previously reported from the observation of two doublets. The relative abundance of MgC3N with respect to MgNC is close to one while that of MgC4H relative to MgCCH is about ten. The synthesis of these magnesium cyanides and acetylides in IRC +10216 can be explained in terms of a two-step process initiated by the radiative association of Mg+ with large cyanopolyynes and polyynes followed by the dissociative recombination of the ionic complexes.
Keywords: molecular data, line: identification, stars: carbon, circumstellar matter, stars: individual (IRC +10216), astrochemistry
1. Introduction
Metal-containing molecules are rarely observed in the interstellar medium, but are widely detected in circumstellar envelopes around evolved stars. Stable closed-shell diatomic molecules containing Al, Na, or K and a halogen atom have been detected in the prototypical carbon star envelope IRC +10216 (Cernicharo & Guélin 1987). These species are mostly formed in the hot inner parts of the envelope, close to the star. Metals like Na, K, Ca, Cr, and Fe are found to survive in the gas phase as neutral and/or ionized atoms in the outer layers of IRC +10216 (Mauron & Huggins 2010), which points to a very rich metal chemistry in the cool outer envelope. Various triatomic metal cyanides (M−NC or/and M−CN) containing Mg, Na, Al, Si, K, Fe, and Ca (most of which are open-shell radicals) have been detected in IRC +10216 (Kawaguchi et al. 1993; Turner et al. 1994; Ziurys et al. 1995, 2002; Guélin et al. 2000, 2004; Pulliam et al. 2010; Zack et al. 2011; Cernicharo et al. 2019). Guélin et al. (1993) studied the spatial distribution of MgNC and concluded that this radical arises from a shell located at 15″ from the star.
The only tetratomic metal-bearing species detected so far in IRC +10216 is HMgNC (Cabezas et al. 2013). A pentatomic species, MgC3N, was postulated by Petrie et al. (2003) as a potential abundant species in IRC +10216. They suggested that a series of doublets with rotational constant around 1395 MHz, although initially assigned to an electronic excited state of C6H by Aoki (2000), were in fact produced by MgC3N. However, Cernicharo et al. (2008) have shown that these doublets certainly belong to the 2Σ state of the ν11 bending mode of C6H in its 2Π ground electronic state. Nevertheless, MgC3N, together with MgC4H, which could be formed in the cold outer layers of IRC +10216, are interesting molecules to be searched for in this object.
Sensitive line surveys can be used as the magic tool to reveal the molecular content of astronomical sources and to search for new molecules. Once we are able to identify the lines coming from the isotopologues and vibrationally excited states of known molecules, then a forest of unidentified lines appear that provide the opportunity to discover new molecules and insights into the chemistry and chemical evolution of the observed object.
In this Letter we report the discovery of two new series of doublets with very close rotational constants (1380.9 MHz and 1381.5 MHz) produced by two new molecular species having a 2Σ ground electronic state. On the basis of precise quantum chemical calculations and an exploration of all possible candidates, we assign these lines to MgC3N and MgC4H.
2. Observations
The observations at 3 mm presented in this paper were carried out with the IRAM 30m radio telescope and have been described in detail by Cernicharo et al. (2019). Briefly, the data in the 3 mm window correspond to observations acquired during the last 35 years and cover the 70-116 GHz domain with very high sensitivity (1-3 mK). Examples of these data can be found in Cernicharo et al. (2004, 2007, 2008, 2019) and Agúndez et al. (2008, 2014). The observing mode, where we wobbled the secondary mirror by ±90” at a rate of 0.5 Hz, ensured flat baselines. This observing method provides reference data free from emission from all molecules but CO (see Cernicharo et al. 2015). The emission of all other molecular species is restricted to a region ≤ 15-20″ from the star (see e.g. Guélin et al. 1993; Agúndez et al. 2015, 2017; Velilla Prieto et al. 2015; Quintana-Lacaci et al. 2016).
The observations in the Q band (32-50 GHz) were performed in May and June 2019 with the 40m radiotelescope of the Centro Astronómico de Yebes (IGN, Spain). New receivers built for the European Research Council synergy project Nanocosmos were installed at the telescospe and used for these observations during its commissioning phase. The experimental set-up will be described in detail by Tercero et al. (2019). Briefly, the Q-band receiver consists of two HEMT cold amplifiers covering the 32-50 GHz band with horizontal and perpendicular polarizations. Receiver temperatures vary from 22 K at 32 GHz up to 42 K at 50 GHz. The spectrometers are 16 × 2.5 GHz FFTs with a spectral resolution of 38.1 kHz providing the whole coverage of the Q-band in both polarizations. The total observing time on-source for the observations presented here corresponds to ~ 24 h. The observing mode was position switching with an off position at 300″ in azimuth.
Pointing corrections were obtained by observing strong nearby quasars and the SiO masers of R Leo. Pointing errors were always within 2-3″. The intensity scale, antenna temperature (), was corrected for atmospheric absorption using the ATM package (Cernicharo 1985; Pardo et al. 2001). The beam size of the IRAM 30m telescope in the 3mm domain is 21-30″, while for the 40m telescope the beam size in the Q band is in the range 36-56″. The main beam efficiency of the 40m telescope varies between 60 % at 32 GHz and 50 % at 50 GHz. Calibration uncertainties of 10% were adopted for data covering such a long observing period in the 3 mm domain. A similar uncertainty was assumed for the data in the Q band. Additional uncertainties could arise from the line intensity fluctuation with time induced by the variation of the stellar infrared flux (Cernicharo et al. 2014; Pardo et al. 2018). All data were analysed using the GILDAS package1.
3. Results
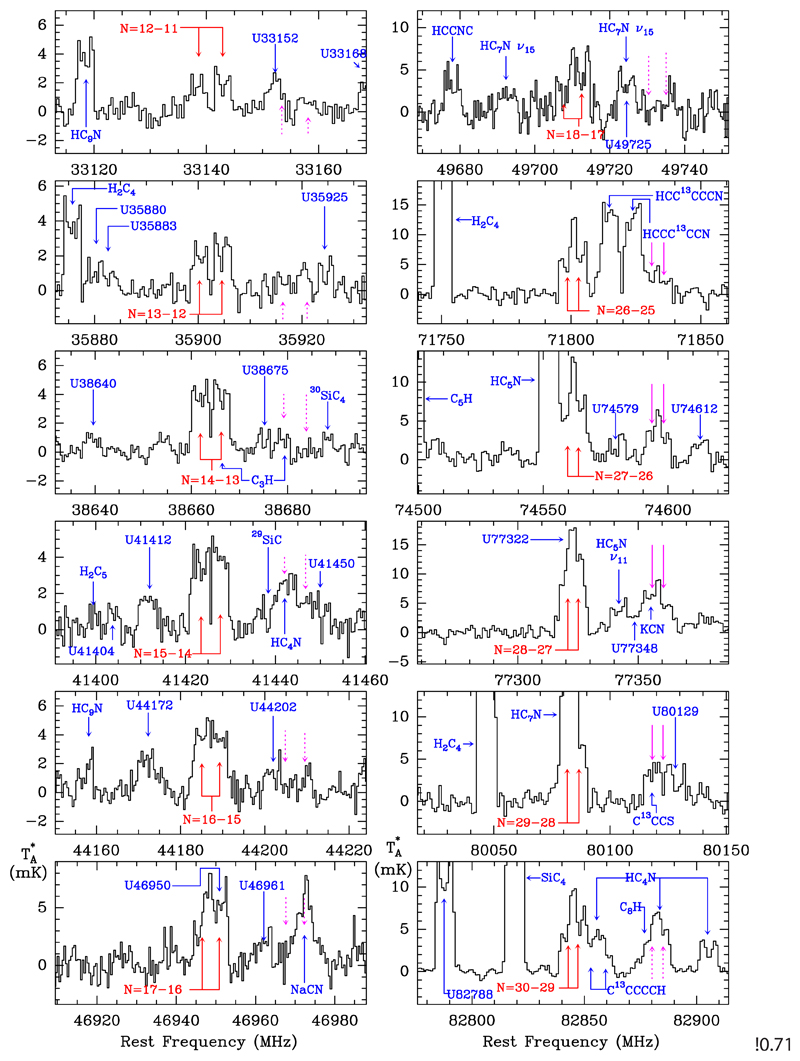
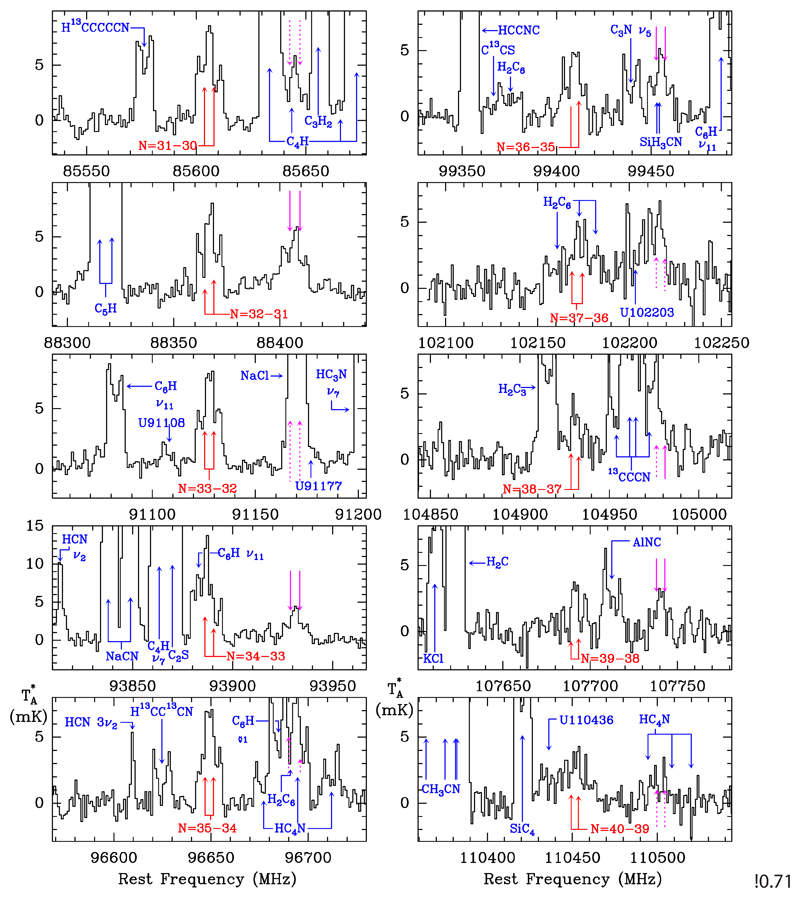
In the IRAM 30m data we found a series of doublets (S 1) whose components are separated by ~4.3 MHz. The doublets are in harmonic relation with integer quantum numbers from Nup=26 to Nup=40. Some of these doublets show some overlap with other features, as shown in Figs. 1 and 2. As a first step we verified that no other harmonic relation can apply and the lines cannot be due to any known species. Once we were sure that the doublets belong to a new molecular species, we searched for the corresponding doublets in the Q band, which correspond to quantum numbers from Nup=12 to Nup=18. All them were detected and are shown in Fig. 1. Interestingly, none of these doublets are present in the previous survey of IRC +10216 in the Q band performed with the Nobeyama radiotelescope (Kawaguchi et al. 1995). This is due to the higher sensitivity of our data, less than 1 mK (see Fig. 1) versus 5 mK in the Nobeyama data. Finally, a total of 22 doublets (see Figs. 1 and 2) were found between the Q and the 3 mm bands. Their frequencies are given in Table A.1 of Appendix A. From a fit to the observed frequencies, we obtain the following rotational, distortion, and spin-rotation constants:
Fig. 1.
Observed doublets towards IRC+10216 (black histogram). The vertical scale is the antenna temperature in mK and the horizontal scale is the rest frequency in MHz. Shown are the lines of the series of doublets S 1 (labelled in red) and those of series S 2, with the same quantum numbers as S 1 (in magenta); unidentified (U) lines and lines arising from other species are also shown (in blue). When lines of the S 2 series are blended with other features, or are too weak to be detected (those below 50 GHz), their positions are indicated by dashed arrows.
Fig. 2.
Same as in Figure 1, but for the doublets in the 3 mm domain observed with the IRAM 30m radio telescope.
B = 1380.888 ± 0.001 MHz
D = 0.0760 ± 0.0005 kHz
γ = 4.35 ± 0.04 MHz
After a careful examination of the data we realized that some unidentified features located on the right side of the S 1 doublets are in fact harmonically related doublets with integer quantum numbers (S 2). They are indicated by magenta arrows in Figs. 1 and 2. The S 2 doublets have the same quantum numbers as S 1 and correspond to a 2Σ molecule. The frequencies of S 2, given in Table A.2 of Appendix A, are fitted with the following constants:
B = 1381.512 ± 0.004 MHz
D = 0.074 ± 0.002 kHz
γ = 4.7 ± 0.1 MHz
A detailed analysis of possible carriers for S 1 and S 2 is given in Appendix B. After discarding all plausible molecular species, we conclude that our best candidates are metal-bearing species attached to a C3N or C4H group. Species containing Na or Al attached to C3N or C4H groups have singlet ground electronic states and therefore cannot be the carriers of S 1 and S 2. In fact, AlC3N and NaC3N have been observed in the laboratory (Cabezas et al. 2014, 2019) and their rotational constants are too low with respect to those quoted above for S 1 and S 2. In contrast, molecular species containing Mg or Ca present 2Σ electronic states and they are postulated as the candidates responsible for the S 1 and S 2 rotational transitions. In a previous paper, Cabezas et al. (2019) reported ab initio calculations for both MgC3N and CaC3N species. The calculated rotational constant for MgC3N indicates that this molecule could be one of the carriers of S 1 or S 2. On the other hand, the rotational constant of CaC3N is much smaller than those of S 1 and S 2, discarding Ca as a candidate. Hence, we performed high-level quantum chemical calculations for both MgC4H and MgC3N (see Appendix C). For MgC3N we obtain B = 1376.5 MHz, D = 0.065 kHz, and γ = 4.1 MHz. For MgC4H we obtained a slightly higher value of B, 1377.4 MHz, D = 0.061 kHz, and γ = 4.4 MHz. All these results are given in Appendix C.
High-resolution optical spectroscopy of the A2Π − X2Σ electronic transition of MgC4H were performed by Ding et al. (2008) and Chasovskikh et al. (2008). They obtain a rotational constant of B = 1384.7 ± 6 MHz. Subsequent observations of the same electronic transition of MgC4H by Forthomme et al. (2010) provide a much more accurate value for the rotational constant, B = 1380.9 ± 0.2 MHz. The spectral resolution of these observations is 0.002 cm−1 (~60 MHz) and near 250 transitions have been observed with upper quantum numbers up to N = 42. Hence, the derived rotational constant and its uncertainty are highly confident. Using the relation D = B3/ω2, these authors estimated the distortion constant to be 0.077 kHz. Hence, either of the two series S 1 or S 2 may be assigned to MgC4H. The calculated dipole moment of MgC3N is 6.3 D and that of MgC4H 2.1 D (see Appendix C). Consequently, both species will harbour a different line intensity distribution with quantum number N. We consider that MgC4H is responsible for the S 2 series as its dipole moment of 2.1 D produces rotational transitions in IRC +10216 peaking in the 3 mm domain with weak emission below 50 GHz. Consequently, the S 1 series is assigned to MgC3N because with its high dipole moment its rotational transitions are strong in the Q band and will start to decline in intensity for high values of N in the 3mm band (similar to the behaviour of HC5N). Moreover, all quantum chemical calculations indicate that the rotational constant of MgC4H is slightly larger than that of MgC3N, as observed for S 2 and S 1. Consequently, we conclude that we have detected, and fully characterized spectroscopically in space, the magnesium-bearing species MgC3N (S 1) and MgC4H (S 2). The definitive confirmation will require laboratory rotational spectroscopy. We also present new observations (see Appendix E) that confirm the tentative detection of MgCCH in IRC +10216 reported by Agúndez et al. (2014).
4. Discussion
The observed line intensities of MgC3N can be fit to two components, a cold one with a rotational temperature of 15 ± 2 K and a column density of (5.7 ± 1.0) × 1012 cm−2, and a warm one with Trot = 34 ± 6 K and N = (3.6 ± 0.6) × 1012 cm−2. For MgC4H we derive a relatively high rotational temperature (36±6 K), which is consistent with the line intensity distribution quoted above, and a column density of (2.2 ± 0.5) × 1013 cm−2. The determination of column densities can be quite uncertain if infrared pumping (IR) plays a role in the excitation of the rotational levels (see Appendix D). These effects could be particularly important for molecules having low-frequency bending modes such as HC3N, HC5N, C4H, MgC3N, and MgC4H (see Appendix D). Nevertheless, our two magnesium-bearing species have similar low-frequency bending modes (see Tables C.3 and C.4) and we could expect to have similar effects in the infrared pumping of their rotational levels. Therefore, the ratio of column densities MgC4H/MgC3N ~2.5 should not be greatly affected by IR pumping effects. On the other hand, the abundance ratio MgC4H/MgCCH ~10 may be overestimated if IR pumping is important for MgC4H (see Appendices D and E). It is true that it seems counterintuitive to have a higher abundance for MgC4H than for MgCCH, but in the chemical model discussed below everything depends on the branching ratios of the different fragmentation channels in the dissociative recombination of the complexes, which are difficult to anticipate (see below). Usually, within a given chemical family, larger molecules tend to be less abundant in IRC+10216. However, the anions CnH−, for which their synthesis also relies on the radiative association of CnH radicals and electrons, show an increase in abundance with the number of carbon atoms.
In order to investigate the formation of the various magnesium cyanides and acetylides detected in IRC +10216 (MgNC, MgCN, HMgNC, MgC3N, MgCCH, and MgC4H) we carried out chemical model calculations based on the model of the outer envelope by Agúndez et al. (2017). As in previous models dealing with the formation of metal-bearing molecules in the outer shells of IRC +10216 (Millar 2008; Cabezas et al. 2013; Cernicharo et al. 2019), the synthesis relies on a two-step mechanism starting with the radiative association of Mg+ with large cyanopolyynes and polyynes and the subsequent dissociative recombination of the corresponding Mg+/NC2n+1H and Mg+/C2nH2 complexes with electrons (Petrie 1996).
The rate coefficients for the radiative associations of Mg+ with polyynes and cyanopolyynes were taken from the calculations by Dunbar & Petrie (2002). We consider that open-shell metal-containing species (MgNC, MgCN, MgC3N, MgCCH, and MgC4H) react with electrons and H atoms, while closed-shell molecules (HMgNC) do not. The branching ratios of the different fragmentation channels in the dissociative recombination of the Mg+/NC2n+1H and Mg+/C2nH2 ions are not known. We have therefore adjusted them to obtain column density ratios in agreement with those derived from observations. We find that in order to reproduce the relative column densities of the magnesium cyanides, the branching ratios of the channels yielding HMgNC, MgCN, and MgC3N should respectively be 0.01, 0.05, and 0.7 of that yielding MgNC. In the case of the magnesium acetylides, the branching ratio of the channel producing MgCCH should be 0.1 of that yielding MgC4H. It is curious that while MgNC is slightly more abundant than MgC3N, in the case of magnesium acetylides the larger molecule MgC4H is significantly more abundant than MgCCH. If the conclusion of MgC4H/MgCCH > 1 is not in error due to IR pumping effects, this should be a consequence of the different behaviour in the fragmentation of Mg+/C2nH2 complexes with respect to Mg+/NC2n+1H.
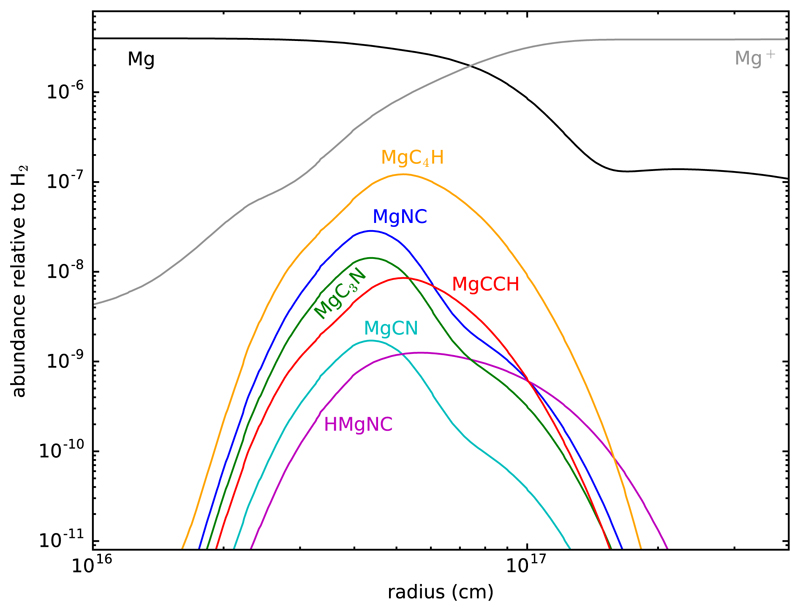
The absolute column densities of the Mg-bearing molecules are approximately reproduced (calculated column densities for MgC3N and MgC4H are 5.4 × 1012 and 3.6 × 1013 cm−2, respectively) adopting an initial abundance of Mg relative to H of 1.5 × 10−6, which is about 4 % of the cosmic abundance of Mg. This value is higher than that used by Cabezas et al. (2013), mainly because in this model we include more metals (Mg, Na, Al, and Ca) and the new and abundant detected molecule MgC3N must be accounted for. Unfortunately, Mauron & Huggins (2010) did not observe atomic Mg in the outer layers of IRC +10216, and therefore we do not have constraints on the abundance of atomic Mg. The results from the chemical model are shown in Fig. 3. In general, the two-step mechanism of Petrie (1996) can account for the formation of Mg cyanides and acetylides in IRC +10216. The main uncertainty is related to the branching ratios of the different fragmentation channels in the dissociative recombination of the cationic metal complexes, something that would be worth investigating in detail in the future.
Fig. 3.
Abundances calculated with the chemical model for the different Mg-bearing molecules detected in IRC +10216.
Supplementary Material
Acknowledgements
The Spanish authors thank the Ministerio de Ciencia Innovación y Universidades for funding support from the CONSOLIDER-Ingenio program ‘ASTROMOL’ CSD 2009-00038, AYA2012-32032, AYA2016-75066-C2-1-P. We also thank ERC for funding through grant ERC-2013-Syg-610256-NANOCOSMOS. MA thanks the Ministerio de Ciencia Innovación y Universidades for the Ramón y Cajal grant RyC-2014-16277. CB thanks the Ministerio de Ciencia Innovación y Universidades for the Juan de la Cierva grant FJCI-2016-27983. LVP acknowledges support from the Swedish Research Council and from ERC consolidator grant 614264.
Footnotes
Based on observations carried out with the IRAM 30m telescope and the Yebes 40m telescope. IRAM is supported by INSU/CNRS (France), MPG (Germany) and IGN (Spain). The 40m radiotelescope at Yebes Observatory is operated by the Spanish Geographic Institute (IGN, Ministerio de Fomento).
References
- Adler TB, Knizia G, Werner H-J. J Chem Phys. 2007;127:221106. doi: 10.1063/1.2817618. [DOI] [PubMed] [Google Scholar]
- Agúndez M, Cernicharo J. ApJ. 2006;650:374. [Google Scholar]
- Agúndez M, Fonfría JP, Cernicharo J, et al. A&A. 2008;479:493. [Google Scholar]
- Agúndez M, Cernicharo J, Guélin M. A&A. 2014;570:45. [Google Scholar]
- Agúndez M, Cernicharo J, Quintana-Lacaci G, et al. ApJ. 2015;814:143. doi: 10.1088/0004-637X/814/2/143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agúndez M, Cernicharo J, Quintana-Lacaci G, et al. A&A. 2017;601:A4. doi: 10.1051/0004-6361/201630274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki K. Chem Phys Lett. 2000;323:55. [Google Scholar]
- Bacalla X, Salumbides EJ, Linnartz HH, et al. J Phys Chem A. 2016;120:6402. doi: 10.1021/acs.jpca.6b06647. [DOI] [PubMed] [Google Scholar]
- Bjorvatten T. J Mol Structure. 1974;20:75. [Google Scholar]
- Brewster MA, Apponi AJ, Xin J, Ziurys LM. Chem Phys Lett. 1999;310:411. [Google Scholar]
- Cabezas C, Cernicharo J, Alonso JL, et al. ApJ. 2013;775:133. [Google Scholar]
- Cabezas C, Barrientos C, Largo A, et al. J Chem Phys. 2014;141:104305. doi: 10.1063/1.4894501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabezas C, Barrientos C, Largo A, et al. J Chem Phys. 2019 doi: 10.1063/1.5110670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castor JI. MNRAS. 1970;149:111. [Google Scholar]
- Cernicharo J. Internal IRAM report. Granada: IRAM; 1985. [Google Scholar]
- Cernicharo J, Guélin M, Kahane C, et al. A&A. 1991;246:213. [Google Scholar]
- Cernicharo J, Guélin M. A&A. 1987;183:L10. [Google Scholar]
- Cernicharo J, Guélin M, Kahane C. A&AS. 2000;142:181. [Google Scholar]
- Cernicharo J, Guélin M, Pardo JR. ApJ. 2004;615:L145. [Google Scholar]
- Cernicharo J, Guélin M, Agúndez M, et al. A&A. 2007;467:L37. [Google Scholar]
- Cernicharo J, Guélin M, Agúndez M, et al. ApJ. 2008;688:L83. [Google Scholar]
- Cernicharo J. In: Stehl C, Joblin C, d’Hendecourt L, editors. ECLA 2011; Proc of the European Conference on Laboratory Astrophysics, EAS Publications Series, 2012; Cambridge: Cambridge Univ Press; 2012. https://nanocosmos.iff.csic.es/?page_id=1619. [Google Scholar]
- Cernicharo J, Teyssier D, Quintana-Lacaci G, et al. ApJ. 2014;796:L21. doi: 10.1088/2041-8205/796/1/L21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Marcelino N, Agúndez M, Guélin M. A&A. 2015;575:A91. doi: 10.1051/0004-6361/201424565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cernicharo J, Guélin M, Agúndez M, et al. A&A. 618:A4. [Google Scholar]
- Cernicharo J, Velilla-Prieto L, Agúndez M, et al. A&A. 2019;627:L4. doi: 10.1051/0004-6361/201936040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasovskikh E, Jochnowitz EB, Maier JP. J Phys Chem A. 2008;112:8686. doi: 10.1021/jp803969a. [DOI] [PubMed] [Google Scholar]
- Ding H, Apetrei C, Chacaga L, Maier JP. ApJ. 2008;677:348. [Google Scholar]
- Dunbar RC, Petrie S. ApJ. 2002;564:792. [Google Scholar]
- Frisch MJ, Trucks GW, Schlegel HB, et al. Gaussian 16 rev A. 2016:03. [Google Scholar]
- Forthomme D, Linton C, Tokaryk DW, et al. Chem Phys Lett. 2010;488:116. [Google Scholar]
- Gou X, Zhang J, Li J, et al. Chem Phys. 2009;360:27. [Google Scholar]
- Guélin M, Lucas R, Cernicharo J. A&A. 1993;280:L19. [Google Scholar]
- Guélin M, Muller S, Cernicharo J, et al. A&A. 2000;363:L9. [Google Scholar]
- Guélin M, Muller S, Cernicharo J, et al. A&A. 2004;426:L49. [Google Scholar]
- Hill JG, Mazumder S, Peterson KA. J Chem Phys. 2010a;132:054108. doi: 10.1063/1.3308483. [DOI] [PubMed] [Google Scholar]
- Hill JG, Peterson KA. Phys Chem Chem Phys. 2010b;12:10460. doi: 10.1039/c0cp00020e. [DOI] [PubMed] [Google Scholar]
- Jerosimić S, Wester R, Gianturco FA. PCCP. 2019;21:11405. doi: 10.1039/c9cp00877b. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K, Kasai Y, Ishikawa S, Kaifu N. Pub Astron Soc Japan. 1995;47:853. [Google Scholar]
- Kawaguchi K, Kagi E, Hirano T, et al. ApJ. 1993;406:L39. [Google Scholar]
- Knizia G, Adler TB, Werner H-J. J Chem Phys. 2009;130:054104. doi: 10.1063/1.3054300. [DOI] [PubMed] [Google Scholar]
- McCarthy MC, Apponi AJ, Gottlieb CA, et al. J Chem Phys. 2001;115:870. [Google Scholar]
- Millar TJ. Ap&SS. 2008;313:223. [Google Scholar]
- Mauron N, Huggins PJ. A&A. 2010;513:A31. [Google Scholar]
- Pardo JR, Cernicharo J, Serabyn E. IEEE Trans Antennas and Propagation. 2001;49:12. [Google Scholar]
- Pardo JR, Cernicharo J, Velilla-Prieto L, et al. A&A. 615:L4. [Google Scholar]
- Petrie S. MNRAS. 1996;282:807. [Google Scholar]
- Petrie S. MNRAS. 1999;302:482. [Google Scholar]
- Petrie S, Kagi E, Kawaguchi K. MNRAS. 2003;343:209. [Google Scholar]
- Pulliam RL, Savage C, Agúndez M, et al. ApJ. 2010;725:L181. [Google Scholar]
- Quintana-Lacaci G, Agúndez M, Cernicharo J, et al. A&A. 2016;592:A51. doi: 10.1051/0004-6361/201527688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolev VV. Cambridge: Harvard University Press; 1960. [Google Scholar]
- Sumiyoshi Y, Katoh K, Endo Y. Chem Phys Lett. 2005;414:82. [Google Scholar]
- Tercero F, et al. 2019 in preparation. [Google Scholar]
- Turner B, Steimle TC, Meerts L. ApJ. 1994;426:L97. [Google Scholar]
- Umeki H, Nakajima M, Endo Y. J Chem Phys. 2014;141:184303. doi: 10.1063/1.4900740. [DOI] [PubMed] [Google Scholar]
- Velilla Prieto L, Cernicharo J, Quintana-Lacaci G, et al. ApJ. 2015;805:L13. doi: 10.1088/2041-8205/805/2/L13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner H-J, Knowles PJ, Knizia G, et al. MOLPRO version 2018. 2018;1 [Google Scholar]
- Zack LN, Halfen DT, Ziurys LM. ApJ. 2011;733:L36. [Google Scholar]
- Ziurys LM, Apponi AJ, Guélin M, Cernicharo J. ApJ. 1995;445:L47. [Google Scholar]
- Ziurys LM, Savage C, Highberger JL, et al. ApJ. 2002;564:L45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.