Abstract
Microbial nitrogen use efficiency (NUE) is the efficiency by which microbes allocate organic N acquired to biomass formation relative to the N in excess of microbial demand released through N mineralization. Microbial NUE thus is critical to estimate the capacity of soil microbes to retain N in soils and thereby affects inorganic N availability to plants and ecosystem N losses. However, how soil temperature and soil moisture/O2 affect microbial NUE to date is not clear. Therefore, two independent incubation experiments were conducted with soils from three land uses (cropland, grassland and forest) on two bedrocks (silicate and limestone). Soils were exposed to 5, 15 and 25 °C overnight at 60% water holding capacity (WHC) or acclimated to 30 and 60% WHC at 21% O2 and to 90% WHC at 1% O2 over one week at 20 °C. Microbial NUE was measured as microbial growth over microbial organic N uptake (the sum of growth N demand and gross N mineralization). Microbial NUE responded positively to temperature increases with Q10 values ranging from 1.30 ± 0.11 to 2.48 ± 0.67. This was due to exponentially increasing microbial growth rates with incubation temperature while gross N mineralization rates were relatively insensitive to temperature increases (Q10 values 0.66 ± 0.30 to 1.63 ± 0.15). Under oxic conditions (21% O2), microbial NUE as well as gross N mineralization were not stimulated by the increase in soil moisture from 30 to 60% WHC. Under suboxic conditions (90% WHC and 1% O2), microbial NUE markedly declined as microbial growth rates were strongly negatively affected due to increasing microbial energy limitation. In contrast, gross N mineralization rates increased strongly as organic N uptake became in excess of microbial growth N demand. Therefore, in the moisture/O2 experiment microbial NUE was mainly regulated by the shift in O2 status (to suboxic conditions) and less affected by increasing water availability per se. These temperature and moisture/O2 effects on microbial organic N metabolism were consistent across the soils differing in bedrock and land use. Overall it has been demonstrated that microbial NUE was controlled by microbial growth, and that NUE controlled gross N mineralization as an overflow metabolism when energy (C) became limiting or N in excess in soils. This study thereby greatly contributes to the understanding of short-term environmental responses of microbial community N metabolism and the regulation of microbial organic-inorganic N transformations in soils.
Keywords: Temperature, Soil moisture, Microbial NUE, Microbial growth, Gross N mineralization
1. Introduction
Microbes break down soil organic matter by enzymes which enables them to assimilate organic N compounds directly such as oligopeptides, free amino acids and amino sugars (Barraclough, 1997; Jones et al., 2005; Hu et al., 2018). However, due to widespread resource imbalances and C limitation in soils (Mooshammer et al., 2014b), microbes tend to take up organic N in excess of what they require, resulting in a partitioning of organic N acquired between biomass formation and excretion of excess N through N mineralization. The proportion of organic N allocated to biosynthesis (mainly growth) relative to acquired N (organic N uptake) is termed microbial nitrogen use efficiency (NUE) (Mooshammer et al., 2014a). On the one hand, microbial NUE reflects the capacity of organic N retention in microbial biomass. It has been demonstrated that under N limitation, most acquired organic N is allocated to growth and microbial biomass, resulting in a high microbial NUE. In contrast, under conditions of N excess or C limitation a smaller fraction of organic N is used for biomass formation and more is used to meet the C demand while excess N is mineralized, resulting in low microbial NUE (Mooshammer et al., 2014a). When microbes allocate organic N to produce new biomass this improves the stabilization of N in soils. On the other hand, microbial NUE regulates the gross production of ammonium from organic N. High gross N mineralization rates are observed in soil microbes with low microbial NUE, providing inorganic N to plant growth as well as fostering N losses via nitrification coupled to nitrate leaching and denitrification. Therefore, microbial NUE determines the balance between anabolic and catabolic N processes and controls the fluxes at the intersection of organic and inorganic soil N cycles.
Despite the importance of microbial NUE, fundamental mechanisms and controls of microbial NUE are still unclear. Microbial NUE is likely affected by soil physicochemical and biological properties, such as substrate quality and quantity, microbial community composition, soil pH and clay content, which are strongly shaped by types of land use and bedrock. Forest soils are characterized by abundant C and high C/N ratios compared to grasslands, while cropland soils are managed with applications of inorganic fertilizers and receive less organic C inputs from litter and roots (Booth et al., 2005). Soil pH is strongly affected by bedrock and liming and was suggested as a prime factor influencing microbial community composition and microbial growth rates (Bååth and Arnebrant, 1994; Rousk et al., 2009, 2010). Since microbial NUE is regulated by substrate availability and microbial nutrient imbalances (Mooshammer et al., 2014a), microbial NUE might therefore be affected by land use and bedrock.
Soil temperature and moisture are among the most important environmental factors to potentially alter the balance between microbial biomass synthesis and N mineralization. It has been widely accepted that enhanced temperatures tend to raise microbial activity and accelerate turnover rates of soil N (Dalias et al., 2002; Guntiñas et al., 2012; Wang et al., 2017). Growth rates of both fungi and bacteria increased as temperature increased from 0 to 45 °C (Pietikäinen et al., 2005) and from 5 to 25 °C (Zheng et al., 2019), causing increasing allocation of N to anabolic processes fueling microbial growth. Differently, soil moisture regulates microbial metabolism and N cycling in two opposite ways (Sierra, 1997). At low soil water content microbial NUE might increase with moisture due to slow substrate diffusion and water limitation (Borken and Matzner, 2009). Microbial activity and N processes are thought to be promoted in moister soils and gross N mineralization was observed to increase in the range of 30%–90% WHC (Zaman et al., 1999; Zaman and Chang, 2004; Cheng et al., 2014), indicating a decreasing efficiency of microbial biomass formation and of microbial NUE. However, O2 becomes limiting in soils as they become water-logged (Grable and Siemer, 1968; Hollesen and Matthiesen, 2015) and obligate aerobic microbes as well as their oxidative metabolic processes are then inhibited in soils and sediments - when turning anoxic/suboxic – as indicated as microbial growth decreased markedly while respiration showed only little response (Bastviken et al., 2003; Zheng et al., 2019). This indicates that microbial NUE may decline under oxygen deficiency due to depression on microbial growth rates caused by C (energy) limitation.
To our knowledge, no published studies tested the effects of land use, bedrock, temperature and moisture/O2 and their interactions on microbial NUE, which largely impedes our understanding of microbial N metabolism and of soil and ecosystem N sequestration. In this study, microbial NUE was measured in soils from three major land uses (cropland, grassland and forest) and from two contrasting bedrocks (silicate and limestone), in an attempt to unravel major drivers of soil microbial NUE. At the same time three different temperature (5, 15 and 25 °C) and moisture/O2 (30 and 60% WHC at 21% O2 content, 90% WHC at 1% O2 content) treatments were applied to the soils in the short-term, aiming to explore the influence of temperature and moisture/O2 on microbial NUE. Considering the strong decline in O2 contents in soils close to water saturation (Grable and Siemer, 1968; Hollesen and Matthiesen, 2015), the 90% WHC treatment with suboxic conditions (1% O2 content) were combined. This allowed a better representation of natural conditions and at the same time to reduce the workload. All experiments were conducted over a short preincubation period (1 day in the temperature experiment, 7 days in the moisture/O2 experiment) to reduce effects of changes in microbial community structure. The experiments therefore targeted the short-term effects of the environmental drivers as well as bedrock and land use effects on soil microbial N metabolism. The hypotheses of this study were:
-
(I)
Microbial NUE is impacted by land use and bedrock. Microbial NUE is expected to be high in forest soils being high in organic C but relatively low in available N. Microbial NUE is expected to be low in cropland soils with less plant C input but strong N fertilization. Moreover, microbial NUE is expected to be higher in limestone than in the silicate soils, resulting from more favorable conditions for microbial growth in soils with higher pH.
-
(II)
Temperature and moisture/O2 changes play a prominent role for microbial NUE in the short-term, therefore causing consistent responses of microbes (microbial NUE and growth) across all soils.
-
(III)
Microbial growth and gross N mineralization rates are accelerated by temperature in the range of 5–25 °C. Microbial NUE is promoted as a result of greater increases in anabolic processes (growth rates) than in catabolism at higher temperatures.
-
(IV)
Microbial NUE increases from 30 to 60% WHC as a consequence of elevated substrate diffusion while microbial NUE declines dramatically at 90% WHC and 1% O2 due to energy limitation of microbial growth caused by O2 depletion.
2. Methods and materials
2.1. Sampling sites
Cropland, grassland and forest soils were sampled at two adjacent sites differing in bedrock (silicate and limestone) in June 2016. The two sampling sites were located in the central Enns valley, Styria, Austria and soils developed on silicate (LFZ Raumberg-Gumpenstein 47° 29′ N, 14° 6′ E, 690 m a.s.l.) and limestone (Moarhof in Trautenfels-Pürgg 47° 30′ N, 14° 4′ E, 708 m a.s.l). The soils were classified as Cambisols on silicate, and Luvisols on limestone. Mean annual temperature (MAT) is 7.2 °C and mean annual precipitation (MAP) is 980 mm (period 1980–2016). The forest was dominated by spruce (Picea abies L.) on silicate and by spruce and ash (Fraxinus excelsior L.) on limestone. Both grasslands were fertilized and regularly grazed by livestock, being sheep on silicate and cattle on limestone. Cabbage, onion, potato and beans were cultivated on cropland soils on silicate; barley, wheat and oat were grown on limestone soils. Mineral soils were collected to a depth of 15 cm in four replicates at each site using a root corer (diameter 8 cm) the replicates being sampled across major differences in topography and processed independently. Plants, litter and organic layers on the surface were removed by hand prior to soil sampling. All soil cores were collected within two days, and passed through 4 mm sieves on the day of sampling. Soils were transported back to the laboratory in Vienna and sieved to 2 mm within a day.
2.2. Soil physical and chemical properties
Soil water content (SWC) was measured gravimetrically after oven drying fresh soils in aluminum dishes for three days at 85 °C. Water holding capacity (WHC) was determined by repeated saturation of fresh soil (10 g) in a funnel with a filter paper and drainage for 2.5 h to compute the water retained in soils at field capacity on a dry matter basis. Soil pH was measured in water with a soil to solution ratio of 1:2.5 using an ISFET electrode (Sentron, Austria). Prior to measurements of soil organic C and total N, carbonate was removed from limestone soils by 2 M HCl. The acid-treated soils were oven dried and finely ground using a ball mill (MM2000, Retsch, Germany). Soil organic C (SOC) and N (TN) contents were subsequently quantified by an elemental analyzer (Carlo Erba 1110, CE Instruments) coupled to a Delta Plus isotope ratio mass spectrometer (Finnigan MAT) via a Conflo III (Thermo Fisher).
Dissolved C and N contents were measured in 1M KCl extracts (1:7.5 (w: v)). Soil total dissolved nitrogen (TDN) and dissolved organic carbon (DOC) contents were measured with a DOC/TN analyzer (TOC-VCPH/CPN/TNM-1, Shimadzu, Austria). Free amino acid (FAA) concentrations were determined fluorimetrically following the modified method from Jones et al. (Jones et al., 2002; Prommer et al., 2014). NH4+ and NO3− concentrations were quantified by colorimetric methods as described by Hood-Nowotny et al. (2010). Dissolved organic nitrogen (DON) was calculated as the difference between TDN and inorganic N in the KCl extracts. Microbial biomass C and N (Cmic, Nmic) were estimated by the chloroform fumigation extraction method (Vance et al., 1987) and corrected by an extraction factor of 0.45. Soil texture, exchangeable cations (K+, Na+, Mg2+, Ca2+, Fe3+, Al3+), base saturation (BS) and effective cation-exchange capacity (CEC) were assayed by the Institute of Sustainable Plant Production, Federal Agency for Food Security (AGES, Vienna, Austria), according to standard procedures (ÖNORM). Acid ammonium oxalate- and dithionite/citrate-extractable Fe and Al were measured as proxies of humusbound, amorphous and crystalline metal (hydr)oxides (Courchesne and Turmel, 2007) by ICP-MS at the Institute of Soil Sciences, University of Natural Resources and Life Sciences, Vienna, Austria.
2.3. Incubation experiments
To investigate the responses of microbial NUE and gross N transformation processes (N mineralization, NH4+ immobilization and nitrification) to soil temperature and soil moisture/O2 in the short term, we set up two separate laboratory incubation experiments with microcosms.
Before the temperature experiment, 250 g sieved soil of each sample was transferred into polyethylene Ziploc bags and amended with Milli-Q water to achieve 60% WHC. The amended soils were kept in a thermal incubator at 15 °C for seven days to achieve moisture equilibration. Soil bags were opened regularly for aeration and water lost was added. These soils were then weighed into 50 ml polypropylene vials in several replicates one day before starting the isotope pool dilution (IPD) experiments and exposed to 5, 15 and 25 °C, respectively, allowing for estimations of labile N pools (Nmic, FAA, NH4+ and NO3−), microbial N processes (microbial N growth, gross N mineralization, gross NH4+ immobilization, and gross nitrification) and potential enzyme activities (β-glucosidase, leucine-amino peptidase and acid phosphatase) at three levels of temperature.
The moisture/O2 experiment was performed two weeks later. Soils were prepared in triplicate in 50 ml polypropylene vials and the water contents were adjusted to 30, 60 and 90% WHC respectively by gentle drying in ambient air or by water addition. The adjusted soils were incubated at 20 °C at two O2 levels: soils with 30 and 60% WHC were exposed to normal air at 21% O2 while soils with 90% WHC were incubated in a suboxic chamber at 1% O2. The aliquots were then kept under the respective conditions for one week before starting measurements and aerated every second day. Soil N pools, gross N processes, and soil enzymes were measured consecutively.
2.4. Isotope pool dilution assays
Gross N mineralization, NH4+ immobilization and nitrification were estimated by isotope pool dilution techniques (15N-IPD) as modified by Wanek et al. (2010). In principle, the assays were conducted by labeling the NH4+ and NO3− pool respectively with enriched 15N tracers and the gross rates of influx to and efflux from the target pool were calculated based on the differences in isotopic composition and pool size between two time points. Considering the stimulatory effects caused by addition of the 15N tracer on immobilization rates, low amounts of inorganic 15N were applied which amounted to less than 20% of the initial pool sizes. Initial pool sizes of NH4+ and NO3− were measured the day before starting the 15N-IPD experiments by colorimetric methods (Hood-Nowotny et al., 2010).
To determine gross N mineralization, aliquots of 4 g fresh soils were weighed into 50 ml polypropylene vials in duplicate and labelled dropwise with 200 μl of (15NH4)2SO4 (98 atom% 15N, Spectra and Cambridge Isotope Laboratories, Europe, Radeberg, Germany). The concentrations of tracer solutions were adjusted according to preceding measurements of soil NH4+ to increase isotopic enrichment to<20 at %. Labelled soils were shaken vigorously to distribute the tracer solution homogeneously and then incubated under the specific conditions of temperature and moisture/O2. The soil incubations were terminated after 4 h (t1) and 24 h (t2) by extraction with 20 ml of 1M KCl (60 min at room temperature, filtration through ash-free filter paper). Soil NH4+ contents were quantified directly after extraction (Hood-Nowotny et al., 2010). Prior to 15N isotopic analyses, NH4+ in the extracts was isolated using a micro-diffusion method (Brooks et al., 1989; Zhang et al., 2015). For this, soil extracts were pipetted into 20 ml polypropylene scintillation vials and diluted with 1M KCl if necessary to reach a final volume of 10 ml with NH4+ concentrations less than 50 μM. The vials were capped after addition of each an acid trap (4mm diameter of cellulose filter paper disks soaked with 4 μl 2.5M KHSO4, wrapped in Teflon tape) and MgO powder (100 mg, to raise the pH > 9.5) and shaken slowly at room temperature for three days. The acidified cellulose disks with the collected NH4+ were picked out from the acid traps (i.e. the Teflon tapes) after drying and dissolved in 2 ml of Milli-Q water by shaking for 30 min. The NH4+ concentrations were then diluted with Milli-Q water to reach 20 μM and the isotopic composition of NH4+ was measured by a chemical conversion procedure (Zhang et al., 2015). Briefly, aliquots of 1 ml of the acid trap (Milli-Q water) extract were added to 12 ml screw cap exetainers. NH4+ was oxidized to nitrite (NO2−) by addition of 0.1 ml of sodium hypobromite (BrO−) solution and the remaining BrO− was deactivated by adding 50 μl sodium arsenate (0.51 g NaAsO2 in 10 ml of Milli-Q water) after reacting for 30 min. The BrO− reagent was prepared as described in Zhang et al. (2007). Then, 0.15 ml of buffered sodium azide (1M NaN3 in 50% acetic acid) was injected into the tightly sealed exetainers to reduce the produced NO2− to nitrous oxide (N2O) at room temperature, and 0.1 ml of 10M NaOH solution was injected to terminate the reaction after 60 min.
To determine gross nitrification rates, aliquots of 4 g fresh soils were weighed into 50 ml polypropylene centrifuge tubes and labelled dropwise with 200 μl of K15NO3 (98 atom%, Isotec-Sigma Aldrich, Vienna, Austria). The concentrations of the tracer solutions were adjusted according to preceding measurements of soil NO3− to increase isotopic enrichment to < 20 at%. A similar workflow was carried out as mentioned for gross N mineralization, with two time points for assay termination (4 and 24 h). NO3− contents were measured directly after extraction with 1 M KCl (Hood-Nowotny et al., 2010). The isotopic ratios and concentrations of NO3− were then measured in 1 ml aliquots of each soil extract by chemical conversion of NO3− to NO2− and further to N2O by injections of buffered NaN3 and acidic VCl3 (McIlvin and Altabet, 2005; Lachouani et al., 2010). For this, first 200 μl buffered sodium azide (1M NaN3 in 10% acetic acid) and then 1 ml of acidic VCl3 (50mM VCl3 in 1M HCl) were added with gas tight syringes and allowed to react for 18 h at 37 °C, and then stopped by injecting 300 μl 6M NaOH.
The produced N2O from both, NH4+ and NO3−, was isotopically characterized by a purge-and-trap IRMS (PT-IRMS) consisting of a cryofocusing unit on a Gasbench II headspace analyzer (Thermo Fisher, Germany) coupled to a Finnigan Delta V Advantage IRMS (Thermo Fisher, Germany) (Lachouani et al., 2010). A series of natural abundance and 15N-labelled standards of NH4+ and NO3− were prepared in the same matrix (1M KCl) and run with each batch of samples to allow determination of concentrations and isotopic ratios of NH4+ and NO3−.
2.5. Determination of microbial growth rate and microbial NUE
Here, a new approach to measure microbial NUE is proposed based on concurrent measurements of microbial growth and gross N mineralization rates. Microbial NUE is calculated as the ratio of microbial growth N rate (Ngrowth) over microbial organic N uptake rate (Nuptake). Microbial organic N uptake represents the sum of microbial growth and gross N mineralization (MN). Microbial NUE is dimensionless and ranges between 0 and 1.
Microbial growth N rates were determined by 18O–H2O incorporation from soil water into double-stranded DNA (dsDNA) of dividing soil microorganisms (Spohn et al., 2016; Zheng et al., 2019). In this method both growth and mineralization rates are measured over 24 h, thus providing the same time integral for anabolic and catabolic processes, and allowing for more robust representations of microbial organic N uptake (the sum of microbial growth N and N mineralization rates) and growth allocation.
For this method aliquots of 0.4 g fresh soil were weighed into 2 ml plastic tubes with caps in duplicates. Half of the soil aliquots were labelled with 18O–H2O (97.0 at%, Campro Scientific) reaching a final 18O enrichment of 20.0 at% in soil water. In parallel, the other half was amended with the same amount of natural abundance H2O serving as controls. All tubes were transferred into serum bottles (35 ml glass vials with crimp caps) and incubated after capping for 24 h. Then the soil samples were retrieved, frozen in liquid nitrogen and stored at −80 °C before further analysis. Microbial dsDNA was extracted from the frozen soils with a kit (FastDNA™ SPIN Kit for Soil, MP Biomedicals, Germany) and quantified by the Picogreen fluorescence assay (Quant-iT™ PicoGreen® dsDNA Reagent, Thermo Fisher, Germany) following the manufacturers’ instructions. The oxygen content and oxygen isotope composition (18O/16O) of dsDNA was analyzed after drying aliquots of the DNA extract in silver capsules by a Thermochemical Elemental Analyzer coupled to an Isotope Ratio Mass Spectrometer (TC/EA-IRMS, Delta V Advantage, Thermo Fisher, Germany). Microbial biomass N was determined at the same time as microbial growth rates were measured, to allow calculations of the ratios of microbial biomass N to soil DNA content (fDNA-N factor). Calculation of microbial growth was described shortly below and in detail in Zheng et al. (2019), and microbial N growth (Ngrowth) was computed by multiplying DNA produced by fDNA-N. In this study, microbial growth therefore represents the microbial growth rate on a biomass N basis.
Total dsDNA produced (μg) during the 24 h incubation period was calculated according to differences in 18O measurements between labelled and unlabelled DNA samples.
where Ototal is the total O content of the dried DNA extract (μg O), at %excess is the at% 18O of the labelled sample minus the mean at% 18O of control samples. The average weight% of O in DNA is 31.21, according to the average formula (C39H44O24N15P4). The conversion factor (fDNA-N) was calculated as the ratio of soil Nmic (mg N kg−1) to soil DNA content (mg N kg−1), which was measured and calculated for each individual soil sample. The specific fDNA-N values were then applied to each soil replicate individually which multiplied by the DNA production rate, enabled calculating microbial growth rates based on dry soil mass (Ngrowth, mg N kg−1 d−1).
where DW is the soil dry mass and t is the incubation time in days.
The initial approach to quantify microbial NUE is constrained by an indirect assessment of microbial growth using free amino acid consumption rates (AAuptake; as a proxy of organic N uptake) minus gross N mineralization rates (MN) as a proxy of growth rates (Wanek et al., 2010; Mooshammer et al., 2014a).
The method quantifies the contribution of amino acids to N assimilation, but does not include other potential N source such as amino sugars that become available to microbes by microbial necromass decomposition in soils (Hu et al., 2017, 2018). By this the initial approach might underestimate organic N uptake and therefore microbial NUE, and better is termed microbial amino acid use efficiency (Andresen et al., 2015). Moreover, amino acid consumption can potentially be stimulated by adding 15N-labelled substrates to label the free amino acid pool, though addition levels are usually restricted to ∼20% of the native pool size therefore having little impact on rate measurements. Finally, amino acid uptake rates are determined over a time period of 1 h only while gross N mineralization rates are measured over 24 h, causing the process rates contributing to calculations of microbial NUE to be out of balance in terms of time integral. Despite the few but important studies on microbial NUE conducted using the isotope pool dilution technique, alternative methods are therefore clearly required to obtain more robust estimations of microbial NUE across a large range of soils.
2.6. Potential enzyme activities
β-Glucosidase (BG), leucine amino peptidase (LAP) and acid phosphatase (PHO) were determined fluorimetrically with artificial substrate additions (Kaiser et al., 2010). 4-Methylumbelliferyl (MUF) based substrates were used to assay the enzyme activities of BG and PHO. An L-Leucine-7-amido-4-methylcoumarin (AMC) based substrate was used to measure LAP. Soil slurries (1:100 (w:v)) were prepared in 50 mM sodium acetate buffer (pH = 5) and appropriate substrates were pipetted into wells of black microplates in triplicates and incubated in the dark for 30–180 min with repeated measurements of sample fluorescence. Microplates were read by a TECAN Infinite® M200 spectrophotometer at an excitation/emission wavelength of 365/450 nm. The fluorescence of samples was corrected for quenching and the concentrations of released MUF and AMC by enzymatic cleavage were calibrated by respective standards.
2.7. Calculations and statistics
Gross production and immobilization rates of NH4+ and NO3− were calculated using the 15N-IPD equations developed by Kirkham and Bartholomew (1954). NO3− immobilization rates were negative in some soils due to low NO3− assimilation processes or due to the heterogeneity between replicates and thus were not further considered.
The temperature sensitivity (Q10) of a specific process is its response to an increase in temperature of 10 °C (Kirschbaum, 1995). In our study, Q10 values of microbial N processes, microbial NUE and soil enzymes were calculated with a linear regression model after logarithmic transformations (Hu et al., 2018) across all three incubation temperatures.
where R is the measured process including gross N mineralization, NH4+ immobilization, gross nitrification, microbial growth, microbial N uptake, and potential enzyme activities, Q10 is the temperature sensitivity of the parameters mentioned above, T is the incubation temperature (°C) and b is a fitted coefficient.
All statistical analyses were performed using R 3.1.3 (R Development Core Team, 2015). Normal distribution and homoscedasticity were tested with Shapiro-Wilk test and Levene test. Data were transformed if necessary. Two-way ANOVA was conducted to test the effects of bedrock and land use followed by Tukey's HSD post-hoc test. Pearson correlations were used to assess the relations between soil properties with microbial N processes as well as with microbial NUE. Path analyses were run to discriminate the direct and indirect factors influencing microbial NUE at 15 °C and 60% WHC. The function of sem () was performed in the R language package of “lavaan”. Three-way ANOVA was conducted to check for the main effects of bedrock, land use, and temperature or soil moisture/O2, and their interactions. The relative importance of the different main factors and their interactions was further calculated as the percentage of variance (sum of squares (SS)) contributed by each factor and their interactions to the total variance in three-way ANOVAs. In addition, nonlinear models were performed to investigate general relationship between microbial growth and microbial NUE using the nls() function in the “lme4” package of R language, and applied linear regression models to explore the effect of microbial NUE on gross N mineralization with temperature and moisture/O2 treatments. Significance levels were set to P < 0.05 and all values are presented as mean ± 1 standard error of four replicates.
3. Results
3.1. Bedrock and land use effects on basic soil properties
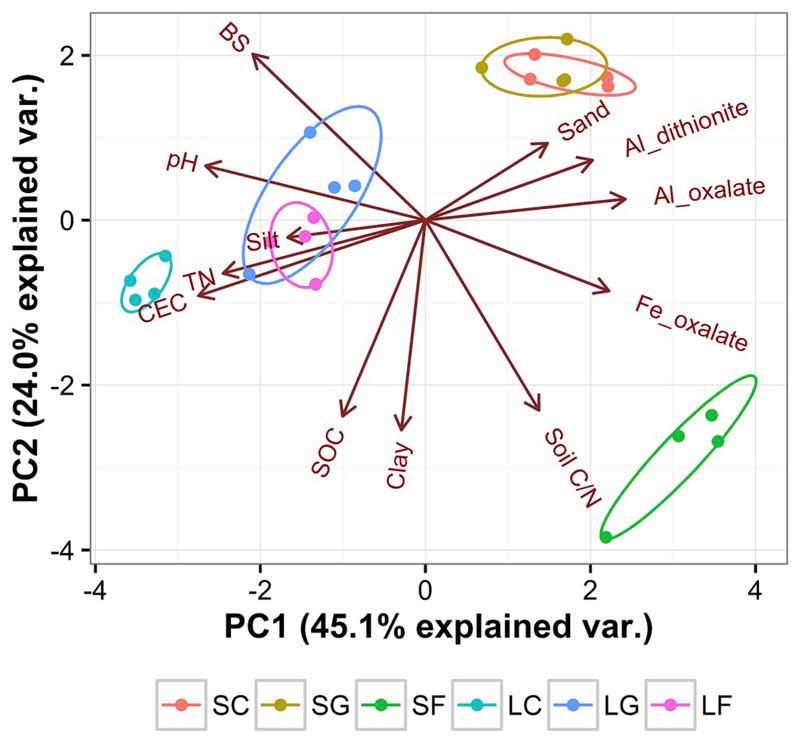
Soil physicochemical properties were strongly affected by land use and bedrock (Table 1, Fig. 1). Soil pH was lower in silicate soils (pH 4.2 to 5.9) than in limestone soils (pH 6.1 to 8.2), and across both bedrocks decreased in the order cropland > grassland > forest. Fine soil particle contents (clay and silt) were higher in limestone than in silicate soils while sand contents behaved inversely. Oxalate and dithionite extractable Al and Fe oxides (except dithionite-extractable Fe) differed between bedrocks, with higher contents in silicate soils, but were not affected by land use. Soil TN and NO3− were higher in limestone soils, while DOC and free amino acids were higher in silicate soils. Microbial biomass (Cmic and Nmic) was higher in limestone soils than in silicate soils, and the maximum values were found in grassland soils on both bedrocks.
Table 1.
Basic properties of initial soils from three land uses (cropland, grassland, forest) on two distinct bedrocks (silicate, limestone), with indications of significance of bedrock (B), land use (L), and their interaction (B × L). Significance levels are represented as asterisks: *P < 0.05, **P < 0.01, ***P < 0.001; not significant, n.s. Data presented are means ± 1SE (n = 4).
| Unit | Silicate | Limestone | B | L | B × L | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cropland | Grassland | Forest | Cropland | Grassland | Forest | |||||
| pH (water) | 5.9 ± 0.4 | 5.3 ± 0.2 | 4.2 ± 0.1 | 8.2 ± 0.2 | 6.4 ± 0.2 | 6.1 ± 0.1 | *** | *** | * | |
| Sand | % | 44 ± 0.6 | 61 ± 2.5 | 47 ± 1.0 | 29 ± 1.5 | 47 ± 13.2 | 22 ± 1.6 | ** | ** | n.s. |
| Silt | % | 47 ± 0.6 | 33 ± 2.3 | 35 ± 0.9 | 56 ± 0.6 | 44 ± 11.1 | 62 ± 1.8 | *** | * | n.s. |
| Clay | % | 8 ± 0.3 | 5 ± 0.2 | 17 ± 0.2 | 14 ± 1.0 | 8 ± 2.2 | 14 ± 1.5 | * | *** | ** |
| exch Ca | cmolc kg−1 | 6.8 ± 1.9 | 4.0 ± 0.3 | 0.2 ± 0.0 | 32.0 ± 0.3 | 20.8 ± 3.7 | 21.0 ± 0.5 | *** | *** | * |
| exch Al | cmolc kg−1 | 0.3 ± 0.2 | 0.8 ± 0.1 | 8.7 ± 0.6 | 0 ± 0 | 0 ± 0 | 0.0 ± 0 | – | – | – |
| CEC | cmolc kg−1 | 8.4 ± 2.0 | 5.4 ± 0.3 | 9.8 ± 0.8 | 33.7 ± 0.4 | 22.7 ± 3.2 | 22.8 ± 0.5 | *** | ** | ** |
| BS | % | 93.4 ± 3.5 | 82.7 ± 3.0 | 5.2 ± 0.2 | 100.0 ± 0.0 | 99.5 ± 0.2 | 99.3 ± 0.1 | *** | *** | *** |
| SOC | g C kg−1 | 21.8 ± 1.1 | 26.7 ± 0.9 | 49.9 ± 7.6 | 47.0 ± 0.9 | 43.9 ± 5.4 | 36.8 ± 2.4 | ** | n.s. | *** |
| TN | g N kg−1 | 2.2 ± 0.1 | 2.8 ± 0.1 | 2.5 ± 0.4 | 4.8 ± 0.1 | 4.6 ± 0.6 | 3.3 ± 0.1 | *** | * | * |
| Soil C/N | 10.0 ± 0.2 | 9.5 ± 0.1 | 19.6 ± 0.2 | 9.9 ± 0.2 | 9.7 ± 1.0 | 11.3 ± 0.4 | ** | *** | *** | |
| Cmic | mg C kg−1 | 72.1 ± 7.2 | 158.6 ± 7.6 | 96.9 ± 17.3 | 317.4 ± 11.0 | 357.1 ± 39.7 | 203.7 ± 6.4 | *** | *** | *** |
| Nmic | mg N kg−1 | 48.7 ± 7.1 | 34.8 ± 2.8 | 13.2 ± 5.0 | 63.6 ± 6.8 | 63.6 ± 8.6 | 23.8 ± 2.6 | *** | *** | ** |
| Cmic/Nmic | 4.1 ± 0.3 | 4.1 ± 0.1 | 6.1 ± 0.3 | 4.5 ± 0.1 | 4.9 ± 0.4 | 4.4 ± 0.2 | n.s. | ** | *** | |
| DOC | mg C kg−1 | 64.6 ± 3.6 | 85.6 ± 4.3 | 160.9 ± 13.1 | 52.5 ± 1.8 | 53.7 ± 12.6 | 53.4 ± 6.9 | *** | *** | *** |
| TDN | mg N kg−1 | 28.0 ± 3.0 | 35.2 ± 3.0 | 24.4 ± 1.6 | 38.7 ± 0.9 | 35.6 ± 3.5 | 32.0 ± 3.3 | * | * | n.s. |
| DON | mg N kg- | 15.7 ± 1.0 | 32.4 ± 2.3 | 13.6 ± 1.9 | 16.9 ± 1.2 | 16.0 ± 1.4 | 15.2 ± 1.9 | ** | *** | *** |
| FAA | mg N kg−1 | 4.1 ± 1.0 | 7.0 ± 0.6 | 4.1 ± 0.7 | 1.5 ± 0.1 | 2.2 ± 0.5 | 2.4 ± 0.5 | *** | n.s. | n.s. |
| NH4+ | mg N kg−1 | 1.3 ± 0.7 | 0.7 ± 0.1 | 6.3 ± 0.7 | 1.8 ± 0.8 | 0.7 ± 0.2 | 1.2 ± 0.6 | ** | *** | *** |
| NO3− | mg N kg−1 | 11.0 ± 1.8 | 2.1 ± 1.1 | 4.5 ± 0.9 | 20.0 ± 0.8 | 18.9 ± 2.1 | 15.7 ± 1.6 | *** | ** | * |
| Al_oxalate | g kg−1 | 3.7 ± 0.1 | 2.9 ± 0.2 | 3.3 ± 0.0 | 1.6 ± 0.1 | 1.7 ± 0.4 | 2.1 ± 0.1 | *** | n.s. | * |
| Fe_oxalate | g kg−1 | 8.5 ± 0.4 | 7.1 ± 0.5 | 11.2 ± 1.0 | 4.8 ± 0.2 | 7.9 ± 1.5 | 4.9 ± 0.0 | *** | n.s. | *** |
| Al_dithionite | g kg−1 | 5.5 ± 0.3 | 4.1 ± 0.5 | 4.3 ± 0.3 | 2.7 ± 0.1 | 3.2 ± 0.8 | 3.5 ± 0.2 | *** | n.s. | * |
| Fe_dithionite | g kg−1 | 24.2 ± 1.3 | 18.8 ± 1.8 | 26.2 ± 1.8 | 24.9 ± 1.1 | 39.7 ± 10.3 | 26.3 ± 1.7 | n.s. | n.s. | n.s. |
Fig. 1.
Principal component analysis of soil characteristics from three land uses (C, cropland; G, grassland; F, forest) on two distinct bedrocks (S, silicate; L, limestone).
3.2. Bedrock and land use effects on soil N processes and microbial NUE
Here land use and bedrock effects were tested on microbial processes measured under standard conditions in the temperature experiment (15 °C and 60% WHC). Microbial growth rates were in the range of 0.67–3.59 mg N kg−1 d−1 and were not affected by land use and bedrock (Table 2). Microbial NUE varied between 0.22 and 0.84, and was neither affected by land use or bedrock, but strongly by their interaction. This became clearly evident from the inverse land use effects in silicate (cropland < grassland < forest) and in limestone soils (cropland > grassland > forest). Gross N mineralization rates ranged from 0.53 to 2.62 mg N kg−1 d−1 but did not show a consistent pattern across land uses and bedrocks. Gross nitrification rates were much lower than gross N mineralization rates. Gross nitrification was lowest in silicate forest soils and highest in grassland soils.
Table 2.
Microbial processes and microbial NUE at 15 °C and 60% WHC in soils from three land uses (cropland, grassland, forest) on two bedrocks (silicate, limestone). The effects of bedrock (B), land use (L) and their interaction (B × L) are presented as asterisks: *P < 0.05, **P < 0.01, ***P < 0.001; not significant, n.s. Data presented are means ± 1SE (n = 4).
| Unit | Silicate | Limestone | B | L | B × L | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cropland | Grassland | Forest | Cropland | Grassland | Forest | |||||
| Microbial growth | mg N kg−1 d−1 | 0.67 ± 0.24 | 3.59 ± 2.29 | 2.00 ± 1.02 | 2.66 ± 0.54 | 3.21 ± 3.06 | 1.37 ± 0.42 | n.s. | n.s. | n.s. |
| Gross N mineralization | mg N kg−1 d−1 | 2.32 ± 0.26 | 2.62 ± 0.14 | 0.66 ± 0.14 | 0.53 ± 0.10 | 2.06 ± 0.27 | 2.52 ± 0.45 | n.s. | *** | *** |
| Microbial uptake | mg N kg−1 d−1 | 2.99 ± 0.64 | 6.21 ± 2.19 | 2.65 ± 0.99 | 3.19 ± 0.68 | 5.27 ± 2.83 | 3.89 ± 1.16 | n.s. | ** | n.s. |
| Microbial NUE | 0.22 ± 0.03 | 0.54 ± 0.07 | 0.73 ± 0.07 | 0.84 ± 0.03 | 0.53 ± 0.10 | 0.36 ± 0.04 | n.s. | n.s. | *** | |
| NH4+ immobilization | mg N kg−1 d−1 | 2.77 ± 0.27 | 3.14 ± 0.21 | 1.51 ± 0.16 | 0.61 ± 0.15 | 1.06 ± 0.20 | 2.38 ± 0.25 | *** | n.s. | *** |
| Gross nitrification | mg N kg−1 d−1 | 0.70 ± 0.21 | 0.96 ± 0.36 | 0.04 ± 0.02 | 0.35 ± 0.18 | 1.83 ± 0.54 | 1.05 ± 0.30 | * | * | ** |
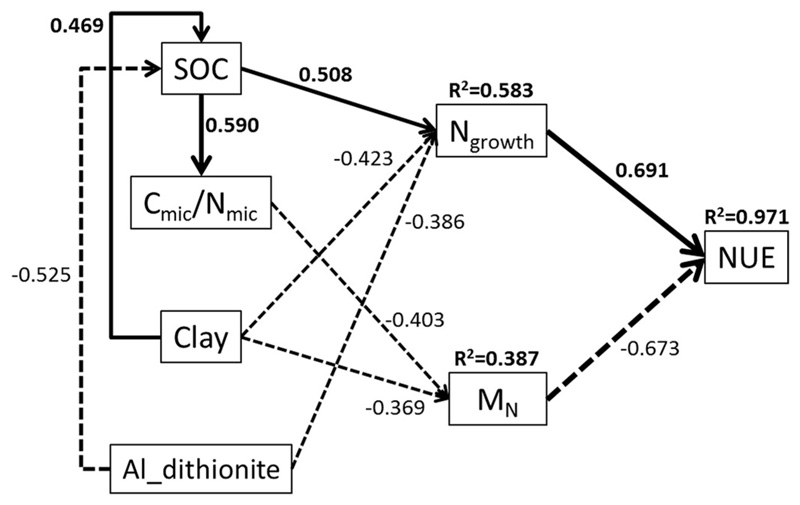
Soil N processes and microbial NUE were related to several soil physicochemical properties (Table 3). Microbial growth rates were related to microbial biomass (Cmic and Nmic), DON, soil texture (sand and silt) and oxalate- and dithionite-extractable Al contents. Gross N mineralization rates were negatively related to clay, SOC, soil C/N and microbial C/N. Microbial NUE increased with SOC and Cmic, as well as with soil C/N and microbial C/N. No significant correlations were found between microbial NUE and any of the measured soil N pools. The result of path analysis (Fig. 2) indicated that SOC, microbial C/N, clay content and dithionite-extractable Al content were major factors influencing microbial growth, gross N mineralization and thereby microbial NUE.
Table 3.
Correlation analysis of soil N transformation processes and microbial NUE with soil physicochemical properties. Significance code: *P < 0.05, **P < 0.01, ***P < 0.001.
| Microbial growth | Gross N mineralization | Microbial NUE | Gross nitrification | |
|---|---|---|---|---|
| pH | 0.087 | −0.173 | 0.179 | 0.119 |
| Sand | 0.594** | 0.096 | 0.190 | 0.157 |
| Silt | −0.577** | 0.071 | −0.320 | −0.016 |
| Clay | −0.381 | −0.520** | 0.254 | −0.483* |
| CEC | 0.024 | −0.341 | 0.334 | 0.032 |
| BS | 0.104 | 0.421* | −0.295 | 0.493* |
| SOC | 0.293 | −0.555** | 0.676** | −0.156 |
| TN | 0.232 | −0.219 | 0.401 | 0.155 |
| Soil C/N | 0.092 | −0.540** | 0.442* | −0.372 |
| Cmic | 0.493* | −0.184 | 0.453* | 0.408* |
| Nmic | 0.424* | −0.132 | 0.403 | 0.410* |
| Cmic/Nmic | 0.177 | −0.527** | 0.472* | −0.135 |
| DOC | 0.183 | −0.369 | 0.397 | −0.380 |
| TDN | 0.447* | 0.055 | 0.294 | 0.305 |
| DON | 0.411* | 0.372 | 0.083 | 0.209 |
| FAA | 0.270 | 0.349 | −0.087 | −0.072 |
| NH4+ | −0.090 | −0.574** | 0.393 | −0.511* |
| NO3− | 0.046 | −0.134 | 0.074 | 0.236 |
| Al_oxalate | −0.457* | 0.190 | −0.470* | −0.400 |
| Fe_oxalate | −0.333 | −0.128 | −0.150 | −0.281 |
| Al_dithionite | −0.562** | 0.302 | −0.608** | −0.220 |
| Fe_dithionite | −0.332 | −0.003 | −0.191 | 0.162 |
Fig. 2.
Path analysis of the effect of soil properties on microbial growth (Ngrowth), gross N mineralization (MN) and microbial NUE under standard conditions (15 °C, 60% WHC). R2 values provided boxes represent the variance explained by relevant factors. Solid and dashed arrows represent positive and negative effects, respectively. Line width indicates the strength of the effects of variables on each other. The path analysis model had a χ2 = 7.0, P = 0.722, df = 10, RMSEA < 0.001.
3.3. Temperature effects on soil N processes and microbial NUE
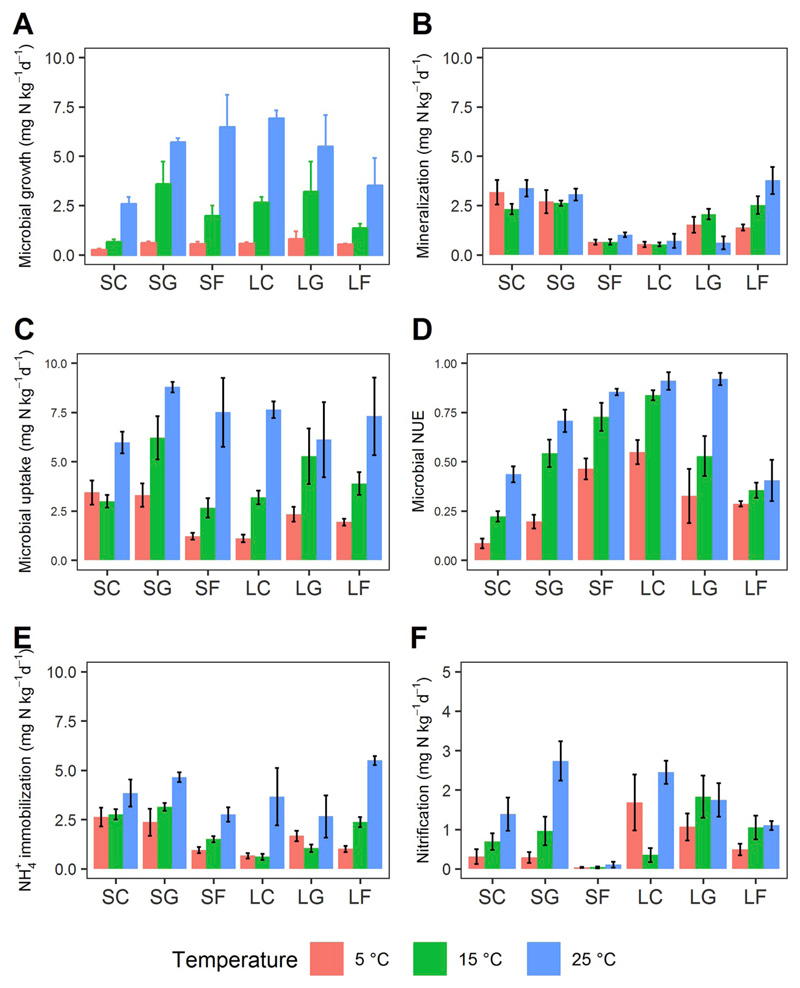
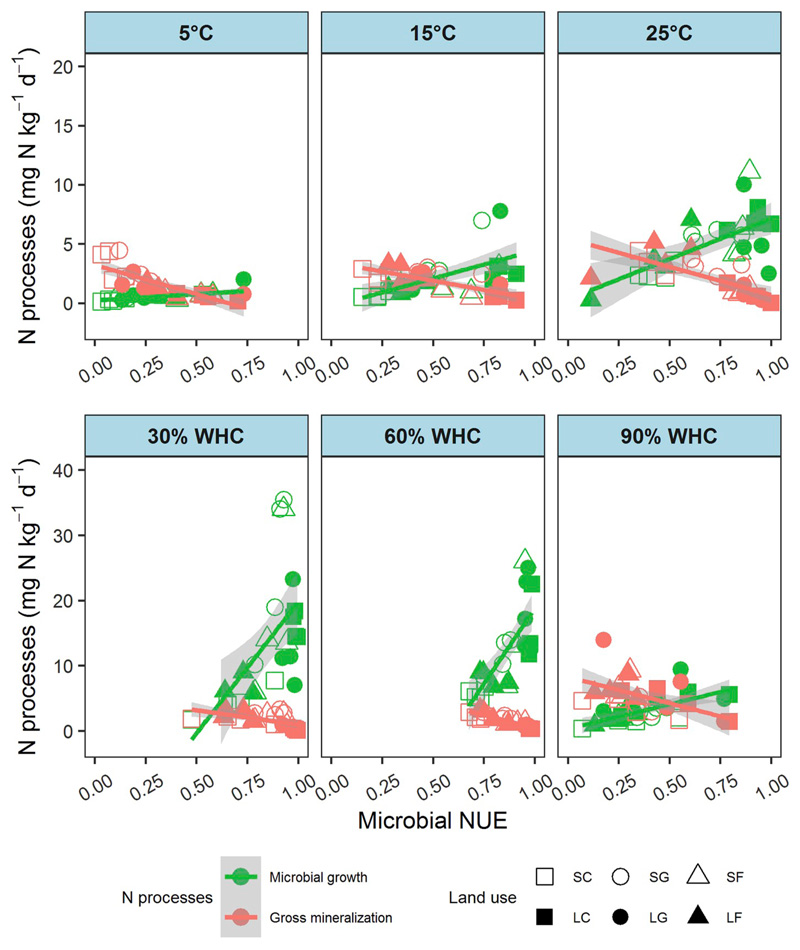
Microbial growth rates and enzyme activities were accelerated by increasing temperature from 5 to 25 °C (Fig. 3A, Fig. S1 A-C), while gross N mineralization rates remained almost constant with temperature (Fig. 3B, Table 4). Both microbial growth and gross N mineralization rates were low at 5 °C while microbial growth rates increased rapidly at higher temperatures in contrast with gross mineralization rates (Fig. 5). Microbial growth became predominant over organic N uptake and microbial NUE increased with temperature in all soils (Fig. 3D), with a greater contribution of microbial growth than gross N mineralization to explain this response (Fig. S3 A and B).
Fig. 3.
Effects of soil temperature on soil microbial N processes: (A) microbial growth, (B) gross N mineralization, (C) microbial organic N uptake, (D) microbial NUE, (E) NH4 + immobilization, and (F) gross nitrification, in soils from three land uses (C, cropland; G, grassland; F, forest) on two distinct bedrocks (S, silicate; L, limestone). Process rates were measured at 5, 15 and 25 °C. Data presented are means ± 1SE (n = 4).
Table 4.
Relative effects (% of total variation) of temperature (T) or soil moisture (M), bedrock (B), land use (L) and their interactions on microbial growth, N mineralization, microbial organic N uptake, microbial NUE, NH4+ immobilization and nitrification with indications of significance as asterisks. Significance levels: *P < 0.05, **P < 0.01, ***P < 0.001.
| Microbial growth | Gross N mineralization | Microbial uptake | Microbial NUE | NH4+ immobilization | Gross nitrification | |
|---|---|---|---|---|---|---|
| Temperature | ||||||
| B | 0.0 | 7.3** | 1.1 | 3.2** | 10.2*** | 15.8*** |
| L | 4.6* | 2.9 | 6.1* | 0.2 | 1.5 | 17.8*** |
| T | 44.3*** | 1.1 | 43.9*** | 33.9*** | 29.0*** | 9.6** |
| B × T | 0.5 | 4.3 | 1.0 | 0.3 | 3.1 | 1.3 |
| L × T | 1.8 | 4.7 | 1.3 | 3.5* | 6.0* | 3.3 |
| B × L | 6.4* | 26.5*** | 3.9 | 40.5*** | 18.1*** | 12.1*** |
| B × L × T | 5.2 | 2.7 | 5.0 | 2.7 | 5.6* | 5.6 |
| Residuals | 37.2 | 50.4 | 37.8 | 15.6 | 26.6 | 34.6 |
| Moisture | ||||||
| B | 2.4* | 8.2** | 2.1 | 0.9 | 4.7** | 3.9** |
| L | 6.5*** | 7.2* | 10.2*** | 2.1 | 8.4** | 10.6*** |
| M | 53.3*** | 9.8** | 19.9*** | 36.4*** | 33.0*** | 25.0*** |
| B × M | 1.7 | 7.3* | 3.3 | 0.8 | 4.7* | 17.1*** |
| L × M | 0.4 | 5.6 | 2.3 | 2.5 | 1.5 | 9.8** |
| B × L | 14.8*** | 7.6* | 19.2*** | 4.6 | 0.8 | 1.7 |
| B × L × M | 2.3 | 5.8 | 7.8* | 4.2 | 17.4*** | 6.3* |
| Residuals | 18.5 | 48.5 | 35.0 | 48.5 | 29.6 | 25.6 |
Fig. 5.
The relationships between microbial growth and gross N mineralization rates with microbial NUE across different treatments of temperature (5, 15 and 25 °C) and soil moisture (30%, 60% and 90% WHC).
Temperature sensitivities (5−25 °C) of soil N processes and microbial NUE differed between soils (Table 5). Microbial growth showed the highest temperature sensitivity (Q10) among all studied processes, ranging from 2.28 ± 0.56 to 3.53 ± 0.22. Microbial NUE was sensitive to temperature changes with Q10 values ranging from 1.30 ± 0.05 to 2.48 ± 0.34. Q10 values of NUE were lower at higher SOC and clay content (Table S2). NH4+ immobilization and nitrification exhibited a higher temperature sensitivity compared to gross N mineralization.
Table 5.
Temperature sensitivities (Q10) of soil N transformation processes and microbial NUE, with indications of significant effects of bedrock (B), land use (L) and their interaction (B × L). Significance levels are presented as asterisks: *P < 0.05, **P < 0.01, ***P < 0.001; not significant, n.s. Data presented are means ± 1SE (n = 4).
| Q10 values | Silicate | Limestone | B | L | B × L | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cropland | Grassland | Forest | Cropland | Grassland | Forest | ||||
| Microbial growth | 3.30 ± 0.38 | 3.12 ± 0.16 | 3.41 ± 0.37 | 3.53 ± 0.22 | 2.92 ± 0.25 | 2.28 ± 0.56 | n.s | n.s | n.s |
| Gross N mineralization | 1.06 ± 0.10 | 1.11 ± 0.13 | 1.28 ± 0.09 | 1.07 ± 0.40 | 0.66 ± 0.30 | 1.63 ± 0.15 | n.s | n.s | n.s |
| Microbial uptake | 1.35 ± 0.10 | 1.68 ± 0.13 | 2.47 ± 0.24 | 2.75 ± 0.31 | 1.58 ± 0.21 | 1.86 ± 0.24 | n.s | n.s | *** |
| Microbial NUE | 2.48 ± 0.34 | 1.98 ± 0.25 | 1.38 ± 0.08 | 1.30 ± 0.05 | 2.24 ± 0.17 | 1.33 ± 0.08 | * | *** | ** |
| NH4+ immobilization | 1.23 ± 0.12 | 1.66 ± 0.05 | 1.75 ± 0.24 | 2.23 ± 0.29 | 1.44 ± 0.18 | 2.39 ± 0.20 | ** | * | * |
| Gross nitrification | 2.79 ± 0.57 | 2.75 ± 0.19 | 1.52 ± 0.39 | 1.70 ± 0.52 | 1.83 ± 0.66 | 1.89 ± 0.60 | n.s | n.s | n.s |
3.4. Moisture/O2 content effects on soil N processes and microbial NUE
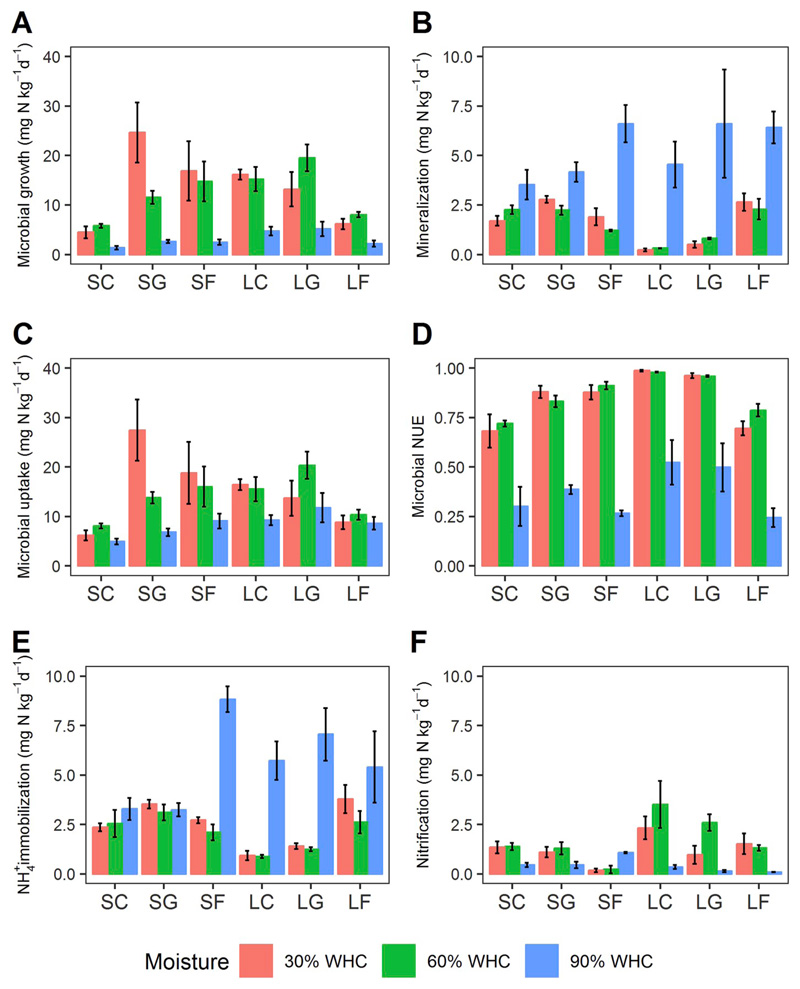
No significant difference was detected in any measured soil N process as well as in microbial NUE between 30 and 60% WHC under oxic conditions (Fig. 4, Table S3). Microbial growth rates were apparently higher than gross N mineralization rates, and microbial NUE was more than 0.5 in most soils (Fig. 5). In contrast, changing moisture/O2 conditions to 90% WHC and 1% O2 content dramatically decreased microbial growth and organic N uptake rates (Fig. 4 A and C), while gross N mineralization rates increased markedly (Fig. 4B). Microbial NUE declined under suboxic conditions caused by the depression of microbial growth rates (Fig. 5). Across overall moisture/O2 treatments, microbial NUE was found largely driven by microbial growth while gross N mineralization responded to a smaller extent (Fig. S3 C and D). NH4+ immobilization increased, particularly in silicate forest soils and in all limestone soils (Fig. 4E). Nitrification was severely depressed in most soils, except in acidic forest soils, under suboxic conditions (Fig. 4F). Concurrently, NH4+ concentrations increased by an order of magnitude, whereas NO3− concentrations declined to levels close to the detection limit (Fig. S2 H and I).
Fig. 4.
Effects of soil moisture on soil microbial N processes: (A) microbial growth, (B) gross N mineralization, (C) microbial organic N uptake, (D) microbial NUE, (E) NH4+ immobilization, and (F) gross nitrification, in soils from three land uses (C, cropland; G, grassland; F, forest) on two distinct bedrocks (S, silicate; L, limestone). Process rates were measured at 30 and 60% WHC (21% O2) and 90% WHC (1% O2). Data presented are means ± 1SE (n = 4).
4. Discussion
In this study we for the first time demonstrate that microbial NUE responds highly sensitively to short-term fluctuations in soil temperature and soil moisture/O2 regime, and therefore strongly affects inorganic N cycling and microbial N retention. In contrast bedrock and land use effects were mediated by changes in soil physicochemistry and soil biology affecting C and N availability. We moreover demonstrated that NUE is mainly driven by factors promoting microbial growth, and that changes in microbial NUE adversely impacted microbial N mineralization. Our view of gross N mineralization has greatly changed in the recent years from an extracellular process controlling soil N cycling to an intracellular process driven by the balance between microbial N demand and supply (Mooshammer et al., 2014a). Microbial N metabolism is central to the metabolic functioning of microbes and therefore was shown to be strongly controlled (Kingsbury et al., 2006; Shimizu, 2013). The balance between anabolic processes (such as nucleic acid and protein biosynthesis, and growth) and catabolic processes (that eventually trigger exudation of catabolic products in excess of microbial N demand) in microbes is therefore highly regulated (Mooshammer et al., 2014a), and microbial NUE is regulated independent of microbial C metabolism, though some cross-talk between microbial C and N metabolism is expected (Kingsbury et al., 2006; Shimizu, 2013).
4.1. Land use and bedrock effects on microbial NUE and soil N processes
As an important component of microbial NUE, microbial growth triggers the assimilation of organic N and thus promotes the efficiency of retaining N in microbial biomass. Microbial growth is assumed to be highly dependent on substrate quantity and quality (Rousk and Bååth, 2011), while it was not directly correlated with available C, N and soil C/N (except DON) in this study. In contrast, microbial growth was strongly affected by soil texture and Al (hydr)oxides through changing substrate availability. Soil organic matter (SOM) can be stabilized by organo-mineral interactions with clays and silts (Jensen et al., 1989; Lützow et al., 2006) and Fe- and Al (hydr)oxides (Wiseman and Püttmann, 2005), further reinforced by high Ca2+, CEC, BS, and calcium carbonate (Six et al., 2004; Doetterl et al., 2015; Minick et al., 2017; Rowley et al., 2018). However, this organic matter is likely more resistant against decomposition due to stronger mineral-organic matter binding, causing eventual nutrient limitation. In addition, microbial growth was found to be positively linked to microbial biomass (Cmic and Nmic), indicating the co-dependency of microbial biomass and growth during nutrient utilization. It is interesting to note that microbial growth was not affected by soil pH though soil reaction is a major driver of microbial community composition (Fierer and Jackson, 2006; Lauber et al., 2009) and nutrient availability (Bleasing, 2012; Augusto et al., 2017) and differentially affects bacterial and fungal growth (Rousk et al., 2009; Rousk and Bååth, 2011).
Compared to microbial growth, gross N mineralization seems less informative when interpreting the allocation of organic N to biosynthesis and growth as it is regulated by microbial NUE releasing organic N in excess. However, changes in gross N mineralization indicate a nutrient imbalance to microbial growth, which is useful to investigate the decoupling of organic N uptake and actual N demand decreasing microbial NUE. In this study, gross N mineralization was found to increase with decreasing SOC and microbial C/N as well as with clay content, as a consequence of enhancement in relative C limitations. Under C limiting conditions organic compounds containing C and N such as amino acids and other DON compounds are utilized by microbes for growth and for energy metabolism (Schimel and Bennett, 2004; Geisseler et al., 2010), and with increasing C limitation a larger fraction of organic N taken up is not retained or allocated to growth but mineralized and exuded as NH4+, causing increases in gross N mineralization. Therefore we found that with increasing C availability microbial NUE increased and gross N mineralization decreased. In their data synthesis Booth et al. (2005) also found that gross N mineralization was negatively related to soil C/N, after correcting for effects of SOC quantity. Although microbial biomass N (Wang et al., 2018) and soil pH (Cheng et al., 2013) were reported also as important effectors of gross N mineralization, these were not confirmed by our dataset. Overall, microbial growth and gross N mineralization are crucial drivers and outputs of microbial NUE but they correlate with microbial NUE in opposite directions (Table S1). According to the result of path analysis, microbial NUE was primarily affected by enhanced N limitation (higher SOC and wider microbial C/N) and greater physical protection of SOC (higher clay content and Al (hydr)oxides). Furthermore, microbial NUE was almost equally related to microbial growth and gross N mineralization, but growth and N mineralization were independent and not correlated (Table S1). This means that factors promoting anabolic processes and growth did not or negatively affect catabolic processes such as N mineralization. Again this highlights the central role that microbial NUE plays in regulating the partitioning of organic N between anabolic and catabolic processes.
4.2. Temperature effects on microbial NUE and soil N processes
As expected, microbes became active and turned to grow as temperatures increased. The activities of enzymes functioning in soil organic C, N and P mobilization were also promoted across all soils (Fig. S1 A-C), likely increasing the substrate availability for microbial assimilation. Consistent with previous studies (Pietikäinen et al., 2005; Barcenas-Moreno et al., 2009), microbial growth rates were stimulated by increasing temperatures, and growth increased exponentially from 5 to 25 °C in all studied soils. Contrasting to our hypothesis, gross N mineralization rates remained invariant to temperature which also was different from the findings of most other related studies (Schütt et al., 2014; Cheng et al., 2015). Microbial N uptake rates increased dramatically with temperature at the same time, but N requirements for growth were more strongly enhanced causing an increase in microbial NUE and a decline in the proportion of organic N released as NH4+. Therefore, microbial growth rates and gross N mineralization rates exhibited different responses to increasing temperature in all soils, while microbial NUE increased obviously regulated by enhanced growth N demands (Fig. S3 A and B).
In contrast, microbial growth was more strongly limited than N mineralization at low temperatures (5 °C). Though microbial NUE varied in a wide range from 0.05 to 0.75 in soils, catabolic processes were more strongly negatively related to microbial NUE at low temperatures, with a relatively flat growth-NUE relationship. This indicates that growth (proliferation, cell division) is more cold sensitive than catabolic processes, i.e. gross N mineralization. In contrast, at higher temperatures the relationship between NUE and N mineralization changed little while the slope of the relationship between growth and NUE increased markedly, indicating a greater control of anabolic processes at intermediate to high soil temperatures and therefore increasing microbial N stabilization in warmer soils.
Microbial NUE regulates the proportion of organic N routed to mineralization, providing the substrate to nitrification. Nitrification was found to increase with temperature (Van Schöll et al., 1997; Lang et al., 2010), however, it was not related to microbial NUE here (data not shown) due to other factors more strongly affecting nitrification, for example low soil pH in silicate forests. Likewise, gross NH4+ immobilization responded positively to temperature increases, while nitrification accounted only for a minor fraction of NH4+ immobilization in most soils.
4.3. Soil moisture and oxygen effects on N processes and microbial NUE
Despite the wealth of studies on gross N transformation processes in unsaturated soils much less is known of their response to soil water saturation and O2 limitation. Microbial activity was high under oxic conditions (21% O2), conditions that prevailed at 30 and 60% WHC. This indicates that most soil N processes catalyzed by aerobic microbes were not stimulated by increases in soil water content from suboptimal to optimal levels. Substrate accessibility and diffusivity are supposed to increase with soil water content i.e. also from 30 to 60% WHC, due to faster diffusion rates of substrates and extracellular enzymes along thicker and better connected water films coating the soil particles (Linn and Doran, 1984; Or et al., 2007; Manzoni et al., 2012). Furthermore, more labile substrates might become dissolved into the growing soil water films as well as be released from weak interactions with mineral surfaces, further stimulating microbial activity. One possible explanation for this unresponsiveness is the wide optimal moisture range of soil microbial metabolism. This is supported by maximum bacterial growth rates being reached at relatively low soil moisture levels, i.e. at around 20% water content on a dry matter basis which translates to approximately 33% WHC (Iovieno and Bååth, 2008). Extrapolating the (absent) response of microbial N processes to soil moisture levels between 30 and 60% WHC to 90% WHC implies that one would also not expect that changes induced at highest WHC would affect substrate/enzyme diffusion to an extent that triggers major changes in soil N processes. However, the highest WHC level also switched the microcosms to a suboxic regime (1% O2), causing microbial O2 limitation reinforced by slowed O2 transport through the water filled pores.
Potential enzyme activities declined or remained constant (Fig. S2 A - C), implying a slowing down of microbial metabolic activity (Zaman et al., 1999). Microbial growth declined remarkably under suboxic conditions, compared to rates measured at 30 and 60% WHC at 21% O2. The slow growth rates can either be attributed to substrate limitation or to energy limitation of the aerobic microbial community. Substrate availability seemed not to have become limiting after one week of incubation under suboxic conditions, as the concentrations of free amino acids as labile energy-rich substrates increased at 90% WHC (Figs. S2 and G). In contrast, soil microbes were severely inhibited by O2 limitation, with indications of a switch from aerobic respiration to anaerobic respiration based on the use of alternative electron acceptors such as NO3− by denitrification (Figs. S2 and I). Nitrate levels declined to levels below the limit of quantification after one-week suboxia, high-lighting denitrification as alternative to aerobic respiration for energy production. While nitrification decreased due to O2 limitation, as also reported by Pett-Ridge et al. (2006), gross N mineralization was promoted dramatically in all soils. This implies that N was not the limiting element under suboxic conditions, but that microorganisms were restricted by C (energy) and therefore took up and utilized free amino acids for energy production rather than for N. Other studies also found that under anoxic conditions soil microbial metabolism produces energy with a lower efficiency by anaerobic respiration than under oxic conditions, also negatively affecting organic matter decomposition rates and microbial growth (Boyd, 1995; Bastviken et al., 2001; Schädel et al., 2016). Moreover, microbial NUE was found to decline strongly at 90% WHC, supporting microbial limitation by energy (C), and henceforth gross N mineralization as an N overflow metabolism increased as organic N uptake became in excess of microbial N demand for growth. Therefore, shifting the soil O2 status from oxic to suboxic was likely to reverse microbial N limitation to C limitation, thus turning the balance from predominant anabolic processes to predominant catabolic processes.
5. Conclusions
In this study microbial NUE was driven by microbial growth but in turn regulated microbial N mineralization. Across the studied soils differing in land use and bedrock, microbial NUE was primarily affected by C availability, microbial C/N relations, and organic matter sorption (clay content and Al (hydr) oxides), implying the important regulation by C–N imbalances. Consistent across all soils microbial NUE increased with temperature, and decreased with O2 depletion. Future experiments shall expand on these findings and explore the long-term effects of environmental factors on microbial growth and NUE. This will greatly advance the understanding of the soil inorganic N cycle and has strong ecosystem repercussions such as for soil N conservation versus soil N leakage by gaseous or hydrological pathways.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.soilbio.2019.05.019.
Acknowledgements
We thank Margarete Watzka for technical assistance and anyone who provided help in the lab experiments. We thank Annika Retzmann, Johanna Irrgeher and Thomas Prohaska for determination of oxalate- and dithionite-extractable Fe and Al contents. We further thank the funding support from the Austrian Science Fund FWF (P 28037-B22).
References
- Andresen L, Bode S, Tietema A, Boeckx P, Rütting T. Amino acid and N mineralization dynamics in heathland soil after long-term warming and repetitive drought. Soils. 2015;1:341–349. [Google Scholar]
- Augusto L, Achat DL, Jonard M, Vidal D, Ringeval B. Soil parent material—a major driver of plant nutrient limitations in terrestrial ecosystems. Global Change Biology. 2017;23:3808–3824. doi: 10.1111/gcb.13691. [DOI] [PubMed] [Google Scholar]
- Bååth E, Arnebrant K. Growth rate and response of bacterial communities to pH in limed and ash treated forest soils. Soil Biology and Biochemistry. 1994;26:995–1001. [Google Scholar]
- Barcenas-Moreno G, Gomez-Brandon M, Rousk J, Baath E. Adaptation of soil microbial communities to temperature: comparison of fungi and bacteria in a laboratory experiment. Global Change Biology. 2009;15:2950–2957. [Google Scholar]
- Barraclough D. The direct or MIT route for nitrogen immobilization: a 15N mirror image study with leucine and glycine. Soil Biology and Biochemistry. 1997;29:101–108. [Google Scholar]
- Bastviken D, Ejlertsson J, Tranvik L. Similar bacterial growth on dissolved organic matter in anoxic and oxic lake water. Aquatic Microbial Ecology. 2001;24:41–49. [Google Scholar]
- Bastviken D, Olsson M, Tranvik L. Simultaneous measurements of organic carbon mineralization and bacterial production in oxic and anoxic lake sediments. Microbial Ecology. 2003;46:73–82. doi: 10.1007/s00248-002-1061-9. [DOI] [PubMed] [Google Scholar]
- Bleasing D. Marschner's mineral nutrition of higher plants. Agricultural Science. 2012;24:17. [Book Review] [Google Scholar]
- Booth MS, Stark JM, Rastetter E. Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecological Monographs. 2005;75:139–157. [Google Scholar]
- Borken W, Matzner E. Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Global Change Biology. 2009;15:808–824. [Google Scholar]
- Boyd CE. Bottom Soils, Sediment, and Pond Aquaculture. Springer; Boston, MA, USA: 1995. Soil organic matter, anaerobic respiration, and oxidation—reduction; pp. 194–218. [Google Scholar]
- Brooks P, Stark JM, McInteer B, Preston T. Diffusion method to prepare soil extracts for automated Nitrogen-15 analysis. Soil Science Society of America Proceedings. 1989;53:1707–1711. [Google Scholar]
- Cheng Y, Wang J, Mary B, Zhang J-B, Cai Z-C, Chang SX. Soil pH has contrasting effects on gross and net nitrogen mineralizations in adjacent forest and grassland soils in central Alberta, Canada. Soil Biology and Biochemistry. 2013;57:848–857. [Google Scholar]
- Cheng Y, Wang J, Wang S-Q, Zhang J-B, Cai Z-C. Effects of soil moisture on gross N transformations and N2O emission in acid subtropical forest soils. Biology and Fertility of Soils. 2014;50:1099–1108. [Google Scholar]
- Cheng Y, Wang J, Zhang J-B, Wang S-Q, Cai Z-C. The different temperature sensitivity of gross N transformations between the coniferous and broad-leaved forests in subtropical China. Soil Science & Plant Nutrition. 2015;61:506–515. [Google Scholar]
- Courchesne F, Turmel MC. Extractable Al, Fe, Mn, and Si. In: Carter MR, Gregorich EG, editors. Soil Sampling and Methods of Analysis. second ed. CRC Press; Boca Raton, FL, USA: 2007. pp. 307–315. [Google Scholar]
- Dalias P, Anderson JM, Bottner P, Coûteaux M-M. Temperature responses of net nitrogen mineralization and nitrification in conifer forest soils incubated under standard laboratory conditions. Soil Biology and Biochemistry. 2002;34:691–701. [Google Scholar]
- Doetterl S, Stevens A, Six J, Merckx R, Van Oost K, Pinto MC, Casanova-Katny A, Muñoz C, Boudin M, Venegas EZ. Soil carbon storage controlled by interactions between geochemistry and climate. Nature Geoscience. 2015;8:780. [Google Scholar]
- Fierer N, Jackson RB. The diversity and biogeography of soil bacterial communities. Proceedings of the National Academy of Sciences. 2006;103:626–631. doi: 10.1073/pnas.0507535103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisseler D, Horwath WR, Joergensen RG, Ludwig B. Pathways of nitrogen utilization by soil microorganisms – a review. Soil Biology and Biochemistry. 2010;42:2058–2067. [Google Scholar]
- Grable AR, Siemer E. Effects of bulk density, aggregate size, and soil water suction on oxygen diffusion, redox potentials, and elongation of corn roots 1. Soil Science Society of America Journal. 1968;32:180–186. [Google Scholar]
- Guntiñas ME, Leirós MC, Trasar-Cepeda C, Gil-Sotres F. Effects of moisture and temperature on net soil nitrogen mineralization: a laboratory study. European Journal of Soil Biology. 2012;48:73–80. [Google Scholar]
- Hollesen J, Matthiesen H. The influence of soil moisture, temperature and oxygen on the oxic decay of organic archaeological deposits. Archaeometry. 2015;57:362–377. [Google Scholar]
- Hood-Nowotny R, Umana NH-N, Inselbacher E, Oswald-Lachouani P, Wanek W. Alternative methods for measuring inorganic, organic, and total dissolved nitrogen in soil. Soil Science Society of America Journal. 2010;74:1018–1027. [Google Scholar]
- Hu Y, Zheng Q, Wanek W. Flux analysis of free amino sugars and amino acids in soils by isotope tracing with a novel liquid chromatography/high resolution mass spectrometry platform. Analytical Chemistry. 2017;89:9192–9200. doi: 10.1021/acs.analchem.7b01938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Zheng Q, Zhang S, Noll L, Wanek W. Significant release and microbial utilization of amino sugars and d-amino acid enantiomers from microbial cell wall decomposition in soils. Soil Biology and Biochemistry. 2018;123:115–125. doi: 10.1016/j.soilbio.2018.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovieno P, Bååth E. Effect of drying and rewetting on bacterial growth rates in soil. FEMS Microbiology Ecology. 2008;65:400–407. doi: 10.1111/j.1574-6941.2008.00524.x. [DOI] [PubMed] [Google Scholar]
- Jensen E, Christensen BT, Sørensen L. Mineral-fixed ammonium in clay-and silt-size fractions of soils incubated with 15 N-ammonium sulphate for five years. Biology and Fertility of Soils. 1989;8:298–302. [Google Scholar]
- Jones DL, Kemmitt SJ, Wright D, Cuttle SP, Bol R, Edwards AC. Rapid intrinsic rates of amino acid biodegradation in soils are unaffected by agricultural management strategy. Soil Biology and Biochemistry. 2005;37:1267–1275. [Google Scholar]
- Jones DL, Owen AG, Farrar JF. Simple method to enable the high resolution determination of total free amino acids in soil solutions and soil extracts. Soil Biology and Biochemistry. 2002;34:1893–1902. [Google Scholar]
- Kaiser C, Koranda M, Kitzler B, Fuchslueger L, Schnecker J, Schweiger P, Rasche F, Zechmeister-Boltenstern S, Sessitsch A, Richter A. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytologist. 2010;187:843–858. doi: 10.1111/j.1469-8137.2010.03321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury JM, Goldstein AL, McCusker JH. Role of nitrogen and carbon transport, regulation, and metabolism genes for Saccharomyces cerevisiae survival in vivo. Eukaryotic Cell. 2006;5:816–824. doi: 10.1128/EC.5.5.816-824.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham D, Bartholomew W. Equations for following nutrient transformations in soil, utilizing tracer data. Soil Science Society of America Journal. 1954;18:33–34. [Google Scholar]
- Kirschbaum MU. The temperature dependence of soil organic matter decomposition, and the effect of global warming on soil organic C storage. Soil Biology and Biochemistry. 1995;27:753–760. [Google Scholar]
- Lachouani P, Frank AH, Wanek W. A suite of sensitive chemical methods to determine the δ15N of ammonium, nitrate and total dissolved N in soil extracts. Rapid Communications in Mass Spectrometry. 2010;24:3615–3623. doi: 10.1002/rcm.4798. [DOI] [PubMed] [Google Scholar]
- Lang M, Cai Z-C, Mary B, Hao X, Chang SX. Land-use type and temperature affect gross nitrogen transformation rates in Chinese and Canadian soils. Plant and Soil. 2010;334:377–389. [Google Scholar]
- Lauber CL, Hamady M, Knight R, Fierer N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Applied and Environmental Microbiology. 2009;75:5111–5120. doi: 10.1128/AEM.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn DM, Doran JW. Effect of water-filled pore space on carbon dioxide and nitrous oxide production in tilled and nontilled soils 1. Soil Science Society of America Journal. 1984;48:1267–1272. [Google Scholar]
- Lützow Mv, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions–a review. European Journal of Soil Science. 2006;57:426–445. [Google Scholar]
- Manzoni S, Taylor P, Richter A, Porporato A, Ågren GI. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytologist. 2012;196:79–91. doi: 10.1111/j.1469-8137.2012.04225.x. [DOI] [PubMed] [Google Scholar]
- McIlvin MR, Altabet MA. Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater. Analytical Chemistry. 2005;77:5589–5595. doi: 10.1021/ac050528s. [DOI] [PubMed] [Google Scholar]
- Minick KJ, Fisk MC, Groffman PM. Soil Ca alters processes contributing to C and N retention in the Oa/A horizon of a northern hardwood forest. Biogeochemistry. 2017;132:343–357. [Google Scholar]
- Mooshammer M, Wanek W, Hämmerle I, Fuchslueger L, Hofhansl F, Knoltsch A, Schnecker J, Takriti M, Watzka M, Wild B. Adjustment of microbial nitrogen use efficiency to carbon: nitrogen imbalances regulates soil nitrogen cycling. Nature Communications. 2014a;5 doi: 10.1038/ncomms4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooshammer M, Wanek W, Zechmeister-Boltenstern S, Richter A. Stoichiometric imbalances between terrestrial decomposer communities and their resources: mechanisms and implications of microbial adaptations to their resources. Frontiers in Microbiology. 2014b;5:22. doi: 10.3389/fmicb.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Or D, Smets BF, Wraith J, Dechesne A, Friedman S. Physical constraints affecting bacterial habitats and activity in unsaturated porous media–a review. Advances in Water Resources. 2007;30:1505–1527. [Google Scholar]
- Pett-Ridge J, Silver WL, Firestone MK. Redox fluctuations frame microbial community impacts on N-cycling rates in a humid tropical forest soil. Biogeochemistry. 2006;81:95–110. [Google Scholar]
- Pietikäinen J, Pettersson M, Bååth E. Comparison of temperature effects on soil respiration and bacterial and fungal growth rates. FEMS Microbiology Ecology. 2005;52:49–58. doi: 10.1016/j.femsec.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Prommer J, Wanek W, Hofhansl F, Trojan D, Offre P, Urich T, Schleper C, Sassmann S, Kitzler B, Soja G. Biochar decelerates soil organic nitrogen cycling but stimulates soil nitrification in a temperate arable field trial. PLoS One. 2014;9:e86388. doi: 10.1371/journal.pone.0086388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousk J, Bååth E. Growth of saprotrophic fungi and bacteria in soil. FEMS Microbiology Ecology. 2011;78:17–30. doi: 10.1111/j.1574-6941.2011.01106.x. [DOI] [PubMed] [Google Scholar]
- Rousk J, Brookes PC, Bååth E. Contrasting soil pH effects on fungal and bacterial growth suggest functional redundancy in carbon mineralization. Applied and Environmental Microbiology. 2009;75:1589–1596. doi: 10.1128/AEM.02775-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousk J, Brookes PC, Bååth E. The microbial PLFA composition as affected by pH in an arable soil. Soil Biology and Biochemistry. 2010;42:516–520. [Google Scholar]
- Rowley MC, Grand S, Verrecchia ÉP. Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry. 2018;137:27–49. [Google Scholar]
- Schädel C, Bader MKF, Schuur EAG, Biasi C, Bracho R, Čapek P, De Baets S, Diáková K, Ernakovich J, Estop-Aragones C, Graham DE, et al. Potential carbon emissions dominated by carbon dioxide from thawed permafrost soils. Nature Climate Change. 2016;6:950. [Google Scholar]
- Schimel JP, Bennett J. Nitrogen mineralization: challenges of a changing paradigm. Ecology. 2004;85:591–602. [Google Scholar]
- Schütt M, Borken W, Spott O, Stange CF, Matzner E. Temperature sensitivity of C and N mineralization in temperate forest soils at low temperatures. Soil Biology and Biochemistry. 2014;69:320–327. [Google Scholar]
- Shimizu K. Metabolic regulation of a bacterial cell system with emphasis on Escherichia coli metabolism. ISRN Biochemistry. 2013;2013 doi: 10.1155/2013/645983. 645983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra J. Temperature and soil moisture dependence of N mineralization in intact soil cores. Soil Biology and Biochemistry. 1997;29:1557–1563. [Google Scholar]
- Six J, Bossuyt H, Degryze S, Denef K. A history of research on the link between (micro) aggregates, soil biota, and soil organic matter dynamics. Soil and Tillage Research. 2004;79:7–31. [Google Scholar]
- Spohn M, Klaus K, Wanek W, Richter A. Microbial carbon use efficiency and biomass turnover times depending on soil depth–Implications for carbon cycling. Soil Biology and Biochemistry. 2016;96:74–81. [Google Scholar]
- Van Schöll L, Van Dam A, Leffelaar P. Mineralisation of nitrogen from an incorporated catch crop at low temperatures: experiment and simulation. Plant and Soil. 1997;188:211–219. [Google Scholar]
- Vance ED, Brookes PC, Jenkinson DS. An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry. 1987;19:703–707. [Google Scholar]
- Wanek W, Mooshammer M, Blöchl A, Hanreich A, Richter A. Determination of gross rates of amino acid production and immobilization in decomposing leaf litter by a novel 15N isotope pool dilution technique. Soil Biology and Biochemistry. 2010;42:1293–1302. [Google Scholar]
- Wang C, Wang N, Zhu J, Liu Y, Xu X, Niu S, Yu G, Han X, He N. Soil gross N ammonification and nitrification from tropical to temperate forests in eastern China. Functional Ecology. 2018;32:83–94. [Google Scholar]
- Wang J, Zhang J, Müller C, Cai Z. Temperature sensitivity of gross N transformation rates in an alpine meadow on the Qinghai-Tibetan Plateau. Journal of Soils and Sediments. 2017;17:423–431. [Google Scholar]
- Wiseman CLS, Püttmann W. Soil organic carbon and its sorptive preservation in central Germany. European Journal of Soil Science. 2005;56:65–76. [Google Scholar]
- Zaman M, Chang S. Substrate type, temperature, and moisture content affect gross and net N mineralization and nitrification rates in agroforestry systems. Biology and Fertility of Soils. 2004;39:269–279. [Google Scholar]
- Zaman M, Di JH, Cameron CK, Frampton MC. Gross nitrogen mineralization and nitrification rates and their relationships to enzyme activities and the soil microbial biomass in soils treated with dairy shed effluent and ammonium fertilizer at different water potentials. Biology and Fertility of Soils. 1999;29:178–186. [Google Scholar]
- Zhang L, Altabet MA, Wu T, Hadas O. Sensitive measurement of NH4+ 15N/14N (δ15NH4+) at natural abundance levels in fresh and saltwaters. Analytical Chemistry. 2007;79:5297–5303. doi: 10.1021/ac070106d. [DOI] [PubMed] [Google Scholar]
- Zhang S, Fang Y, Xi D. Adaptation of micro-diffusion method for the analysis of 15N natural abundance of ammonium in samples with small volume. Rapid Communications in Mass Spectrometry. 2015;29:1297–1306. doi: 10.1002/rcm.7224. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Hu YT, Zhang SS, Noll L, Bockle T, Richter A, Wanek W. Growth explains microbial carbon use efficiency across soils differing in land use and geology. Soil Biology and Biochemistry. 2019;128:45–55. doi: 10.1016/j.soilbio.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.