Abstract
IMPORTANCE
Age-related cataract is a leading cause of visual impairment in the United States. The prevalence of age-related cataract is increasing, with an estimated 30.1 million Americans likely to be affected by 2020.
OBJECTIVE
To determine whether daily oral supplementation with lutein/zeaxanthin affects the risk for cataract surgery.
DESIGN, SETTING, AND PATIENTS
The Age-Related Eye Disease Study 2 (AREDS2), a multicenter, double-masked clinical trial, enrolled 4203 participants, aged 50 to 85 years, at risk for progression to advanced age-related macular degeneration.
INTERVENTIONS
Participants were randomly assigned to daily placebo; lutein/zeaxanthin, 10mg/2mg; omega-3 long-chain polyunsaturated fatty acids, 1 g; or a combination to evaluate the effects on the primary outcome of progression to advanced age-related macular degeneration.
MAIN OUTCOMES AND MEASURES
Cataract surgery was documented at annual study examination with the presence of pseudophakia or aphakia, or reported during telephone calls at 6-month intervals between study visits. Annual best-corrected visual acuity testing was performed. A secondary outcome of AREDS2 was to evaluate the effects of lutein/zeaxanthin on the subsequent need for cataract surgery.
RESULTS
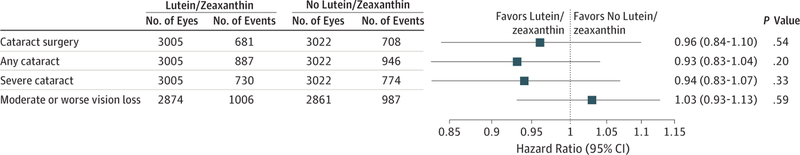
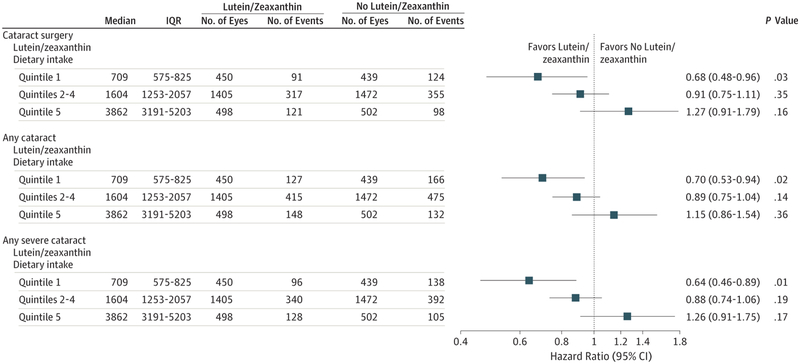
A total of 3159 AREDS2 participants were phakic in at least 1 eye and 1389 of 6027 study eyes underwent cataract surgery during the study, with median follow-up of 4.7 years. The 5-year probability of progression to cataract surgery in the no lutein/zeaxanthin group was 24%. For lutein/zeaxanthin vs no lutein/zeaxanthin, the hazard ratios for progression to cataract surgery was 0.96 (95% CI, 0.84–1.10; P = .54). For participants in the lowest quintile of dietary intake of lutein/zeaxanthin, the hazard ratio comparing lutein/zeaxanthin vs no lutein/zeaxanthin for progression to cataract surgery was 0.68 (95% CI, 0.48–0.96; P = .03). The hazard ratio for 3 or more lines of vision loss was 1.03 (95% CI, 0.93–1.13; P = .61 for lutein/zeaxanthin vs no lutein/zeaxanthin).
CONCLUSIONS AND RELEVANCE
Daily supplementation with lutein/zeaxanthin had no statistically significant overall effect on rates of cataract surgery or vision loss.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: .
Age-related cataract, the leading cause of blindness worldwide, is a leading cause of visual impairment in the United States.1–3 The prevalence of age-related cataract is increasing, with an estimated 30.1 million Americans likely to be affected by 2020, escalating the already large public health and economic burden of the disease.4 Numerous observational studies have reported inverse relationships between various dietary micronutrients and the development of age-related cataract or the occurrence of cataract surgery.5–10 Of greatest interest have been micronutrients with antioxidant capabilities because of the importance of oxidative damage in cataract formation. In the absence of any consensus about the importance of specific micronutrients, several controlled clinical trials have tested whether selected micronutrients with antioxidant characteristics or multivitamins affect cataract development.11–17 Because of variable results, no clear treatment recommendation has resulted from the trials conducted to date. This includes the Age-Related Eye Disease Study (AREDS), which tested a formulation containing vitamin C, 500 mg; vitamin E, 400 IU; and beta carotene, 15 mg; as well as the minerals zinc (as zinc oxide), 80 mg, and copper (as cupric oxide), 2 mg, for both age-related macular degeneration (AMD) and cataract.18 Although AREDS showed a 25% beneficial effect for reducing the risk for developing advanced AMD, it showed no statistically significant effect of the AREDS formulation on the progression of lens opacities.
The AREDS2 randomized clinical trial was designed to evaluate whether oral supplementation with lutein/zeaxanthin and/or omega-3 long-chain polyunsaturated fatty acids (LCPUFAs) might affect development of advanced AMD. A secondary goal was to evaluate the effect of lutein/zeaxanthin on the progression of age-related cataract. A rationale for examining the impact of lutein/zeaxanthin comes from observational data collected in AREDS, other epidemiologic studies, and animal studies.19 Lutein and zeaxanthin, xanthophylls with antioxidant capabilities, are the only carotenoids detected in the human lens.20 Observational studies have demonstrated an inverse association between dietary intake and/or blood levels of lutein/zeaxanthin and the progression of cataract, particularly the nuclear form and cataract surgery.21–24 AREDS2 provided an opportunity to examine the effect that daily dietary supplementation with lutein/zeaxanthin had on age-related cataracts in a randomized clinical trial. The primary cataract outcome in AREDS2 was cataract surgery, with the secondary outcomes of progression of lens opacities or change in visual acuity. Clinical trial results for the AMD component of AREDS2 are presented elsewhere.
Methods
Details of the AREDS2 study design described in a previous report25 are briefly summarized here. Eighty-two retinal specialty clinics enrolled 4203 participants, aged 50 to 85 years, from October 2006 through September 2008. Institutional review boards at the clinical sites approved the AREDS2 research protocol, and participants provided written informed consent. Recruitment of participants was based on retinal findings of bilateral large drusen or large drusen in 1 eye and advanced AMD in the fellow eye. At least 1 eye of each participant was free of advanced AMD. There were no specific inclusion criteria regarding lens opacity status other than the need for sufficiently clear media to allow quality fundus photographs. There was also no specific eligibility criterion for visual acuity.
A run-in phase using study placebo and the AREDS formulation tested the participants’ likely ability to adhere to the study regimen. Participants were eligible for enrollment at their randomization visit if they took at least 75% of the run-in medications and if they agreed to take the AREDS2 supplements and stop current use of supplements containing lutein, zeaxanthin, docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), vitamin C, vitamin E, beta carotene, zinc, or copper, unless supplied by AREDS2. They could not have other ocular diseases that might confound assessment of the ocular outcomes or other systemic diseases including lung cancer or other diseases associated with poor 5-year survival. Participants with previous cataract surgery were excluded only if the surgery was less than 3 months prior to enrollment.
Study Design
AREDS2 is a randomized, double-masked, placebo-controlled, 2 × 2 factorial trial evaluating the risks and benefits of adding lutein/zeaxanthin, 10 mg/2mg, and/or omega3 LCPUFAs, specifically DHA, 350 mg, and EPA, 650 mg, to the AREDS formulation to retard development of advanced AMD. Study participants were randomly assigned to take 1 of the following supplements daily: (1) placebo; (2) lutein/zeaxanthin; (3) DHA/EPA; or (4) lutein/zeaxanthin and DHA/EPA. This article evaluates the role of lutein/zeaxanthin for the treatment of age-related lens opacities, specifically for progression to cataract surgery. Components of the AREDS2 formulation, donated by DSM Nutritional Products, include lutein/zeaxanthin supplied as water soluble triglyceride compounds and omega-3 LCPUFA formulation supplied in ethyl ester form as Ropufa 75 n-33 EE.
A second randomization was conducted for the AMD component of the trial. Because of evidence that beta carotene may increase the risk for lung cancer in cigarette smokers and the AREDS formulation may contain more zinc than can be absorbed,26 we tested the effect of eliminating beta carotene and reducing the dosage of zinc in the AREDS formulation (not displayed in the consort diagram in Figure 1). These nutrients were considered only important for the treatment of AMD but they need to be considered for potential interaction with the primary randomization.
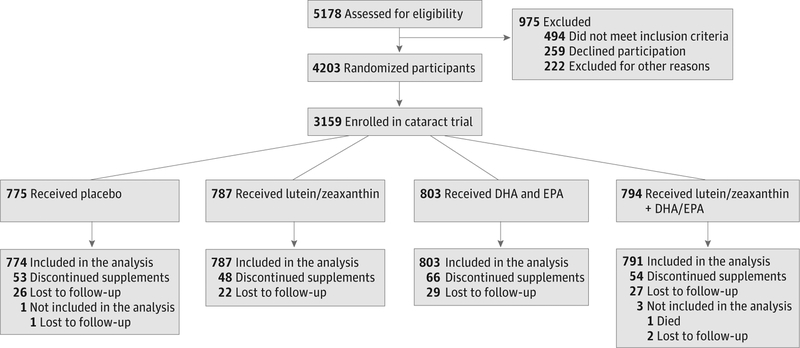
Figure 1.
Flow Diagram of Participants in the Age-Related Eye Disease Study 2 of Lutein/Zeaxanthin Treatment for Age-Related Cataract
DHA indicates docosahexaenoic acid; EPA, eicosapentaenoic acid.
Centrum Silver (Pfizer Inc) was offered to all study participants to standardize multivitamin intake. Participants and study personnel were masked to all treatment assignments.
Follow-up and Adherence
Follow-up study visits were conducted annually. Telephone contacts were scheduled 3 months after randomization and at 6 months between study visits to collect information about compliance with study medication, AMD treatment, cataract surgery, and occurrence of adverse events. Best-corrected visual acuity was obtained annually using a standardized protocol. Certified photographers obtained red reflex lens photographs at baseline and annually. The photographs were assessed by masked graders for the severity of cortical and posterior subcapsular cataract (PSC) lens opacities at the University of Wisconsin Fundus Photograph Reading Center, Madison, Wisconsin.
Adherence to the treatment regimen was assessed by pill count at each annual study visit. Serum levels of lipids, lutein/zeaxanthin, fat soluble vitamins, zinc, and copper were measured at baseline and years 1, 3, and 5 in 545 participants from a subset of clinics. Participants were followed up until October 2012. The median follow-up duration was 4.7 years (inter-quartile range, 4.4–5.1 years).
Outcome Measures
The study examined the effects of lutein/zeaxanthin on progression to cataract surgery with data collected during regular telephone contacts and the annual study visits. A study ophthalmologist examined the anterior segment using slitlamp biomicroscopy at the annual visit to diagnose or confirm the presence of pseudophakia or aphakia. The severity and progression of cortical and PSC opacities on the red reflex lens photographs and the presence of pseudophakia or aphakia were graded at the reading center.
The primary outcome of the cataract clinical trial was progression to cataract surgery. Other outcomes included (1) progression to cataract surgery or an absolute increase in opacity size (area) within the central 5 mm of the lens or 10% for cortical or 5% for PSC opacities; (2) progression to cataract surgery or a 20% absolute increase in the area of either opacity within the central 5 mm of the lens; and (3) a reduction in visual acuity of 15 or more letters from baseline. Safety outcomes included mortality.
Statistical Analyses
The unit of analysis for ophthalmic outcomes was by eye. The lens efficacy outcome, the time to progression to cataract surgery, was assessed with a Cox proportional hazards model using the Wei, Lin, and Weissfeld method for obtaining robust variance estimates, allowing for dependence among multiple event times (1 or 2 study eyes).27 The models were run with and without stratification by the secondary randomization. Patients lost to follow-up or who died during the course of the study were censored at the time of the last contact. Hazard ratios (HRs) and 95% CIs of the lutein main effect were computed. Additional analyses and subgroup analyses were analyzed in the same fashion as the primary lens efficacy outcome. All analyses were conducted following the intention-to-treat principle and using SAS software version 9.2 (SAS Institute Inc).
Results
AREDS2 enrolled 4203 people with a mean (SD) age of 73.1 (7.7) years. Bilateral pseudophakia was present in 1044 participants who were excluded from the cataract analyses, leaving 6027 study eyes (3159 participants). The study population had a mean (SD) age of 72 (7.7) years. Of this group, 2264 participants (72%) agreed to the secondary randomization evaluating modifications to the AREDS supplements. Nearly all of the remaining participants (98.2%) chose to take the commercial AREDS formulation (Figure 1). Baseline characteristics were comparable across the 4 treatment groups in the primary randomization (Table). Of the randomized participants, 96% were white and 55% were female. A large percentage of participants (89%) requested Centrum Silver at study entry. At baseline, 2878 participants were bilaterally phakic, while 281were pseudophakicin1 eye. At base-line, 1239 of 2943 participants (42%) had cortical cataract and 229 of 2941 (8%) had PSC cataract. Baseline cataract status was comparable across the 4 treatment groups.
Table.
Baseline Characteristics of Participants Enrolled in AREDS2 Cataract Study
| Baseline Characteristic | Primary Randomized Treatment, No. (%) | |||
|---|---|---|---|---|
| Placebo (n = 775) | Lutein/Zeaxanthin (n = 787) | DHA/EPA (n = 803) | Lutein/Zeaxanthin+DHA/EPA (n = 794) | |
| Race | ||||
| White | 741 (95.6) | 765 (97.2) | 765 (95.3) | 767 (96.6) |
| Black | 13(1.7) | 5 (0.6) | 17(2.1) | 12 (1.5) |
| Other mixed race | 21(2.7) | 17 (2.2) | 21 (2.6) | 15 (1.9) |
| Ethnicity | ||||
| Hispanic | 11(1.4) | 12 (1.5) | 19 (2.4) | 24 (3.0) |
| Age at randomization, median (IQR), y | 72 (66–77) | 72 (66–77) | 72 (66–77) | 72 (66–78) |
| Female | 414(53.4) | 435 (55.1) | 435 (54.2) | 455 (57.3) |
| Educationa | ||||
| Grade 11 or less | 44 (5.8) | 49 (6.3) | 53 (6.7) | 42 (5.4) |
| High school graduate | 196 (25.8) | 176 (22.8) | 198 (25.2) | 190 (24.3) |
| Some college or associate’s degree | 197 (25.9) | 216 (27.9) | 226 (28.8) | 215 (27.5) |
| Bachelor’s degree | 150 (19.7) | 165 (21.4) | 131 (16.7) | 171 (21.9) |
| Postgraduate degree | 174 (22.9) | 167 (21.6) | 178 (22.6) | 164 (21.0) |
| Diabetes mellitus status | 106 (13.7) | 84 (10.7) | 107 (13.3) | 103 (13.0) |
| Smoking status | ||||
| Never | 312 (40.3) | 356 (45.2) | 350 (43.6) | 362 (45.6) |
| Former | 412 (53.2) | 373 (47.4) | 400 (49.8) | 384 (48.4) |
| Current | 51 (6.6) | 58 (7.4) | 53 (6.6) | 48 (6.0) |
| Lens statusb | ||||
| Bilateral phakia | 716 (92.4) | 709 (90.1) | 730 (90.9) | 721 (90.8) |
| Unilateral pseudophakia | 59 (7.6) | 78 (9.9) | 73 (9.1) | 73 (9.2) |
| Cortical opacity present | 316 (43.5) | 288 (39.6) | 303 (40.5) | 332 (44.8) |
| Posterior subcapsular cataract present | 58 (8.0) | 57 (7.8) | 54(7.2) | 60 (8.1) |
| Dietary lutein/zeaxanthin, μg/d/1000 kcal | ||||
| Median | 2725 | 2590 | 2553 | 2572 |
| Quintile 1 range | 121–1466 | 245–1385 | 304–1467 | 43–1455 |
| Quintile 5 range | 4755–38 110 | 4825–34 398 | 4627–21 513 | 4489–39 790 |
Abbreviations: AREDS2, Age-Related Eye Disease Study 2; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; IQR, interquartile range.
Fifty-seven participants refused to answer.
Missing lens gradings: cortical, 216 participants; posterior subcapsular cataract, 219 participants.
About 7% of the primary study cohort permanently stopped their study medications but were still followed up. About 3% of participants began taking lutein/zeaxanthin in both treatment arms; approximately 19% of these participants stopped their study medications permanently. Approximately 80% of the participants took the study medications at least 75% of the time, as assessed by pill count. There were no differences in adherence among the treatment groups.
Follow-up
Of the 3159 randomized participants, 104 (3%) were lost to follow-up and 204 (6%) died. Distributions were similar across the 4 treatment groups.
Data Quality
Masked duplicate photographic gradings were conducted every month on 5% of eyes randomly selected from the previous month’s evaluations, resulting in 1293 eyes that had repeat gradings between 2009 and 2012. Of these, 777 eyes were phakic. Duplicate gradings of the red reflex lens photographs demonstrated 93% agreement for the presence of cortical opacities and 97% agreement for the presence of PSC. The mean difference between the 2 grades was 0.03 mm2 (95% CI, −5.31 to 5.25) for cortical and 0.03 mm2 (95% CI, −0.7 to 0.81) for PSC opacities.
Dietary and Serum Levels of Lutein/Zeaxanthin
Baseline dietary intake of the study nutrients, excluding the supplements, such as Centrum Silver, was balanced across treatment groups. Dietary intake of lutein/zeaxanthin in AREDS2 participants was similar to that of participants in the Women’s Health Study of female health professionals, a highly educated and well-nourished group.27 Both the AREDS2 and the Women’s Health Study evaluated dietary intake with the Harvard Semi-Quantitative Assessment Food Frequency Questionnaire.28
Serum Levels
Serum levels of the study nutrients were balanced across treatment groups at baseline. Median baseline serum levels of lutein in participants randomized to lutein increased by 191% to 215% at years 1, 3, and 5, while those randomized to placebo showed minimal changes. Although not statistically significant, mean percentage increase in serum levels of lutein/zeaxanthin in those randomized to lutein/zeaxanthin and beta carotene were lower than those randomized to lutein/zeaxanthin alone at year 5 (171% vs 270%, respectively; P = .08).
Outcomes
Progression to Cataract Surgery
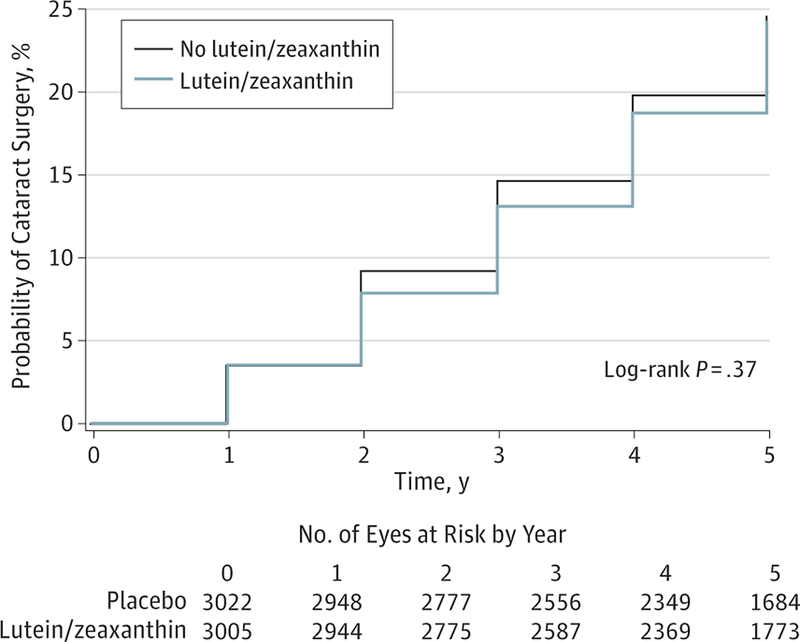
Of the 6027 study eyes, 1389 study eyes (23%; 876 participants) underwent cataract surgery during the study. The number of eyes and Kaplan-Meier probabilities (N[%]) of progression to cataract surgery by 5 years for study eyes randomized to lutein/zeaxanthin or no lutein/zeaxanthin were 681 eyes (24%) and 708 eyes (24%), respectively (Figure 2). A comparison of lutein/zeaxanthin to no lutein/zeaxanthin for progression to cataract surgery resulted in a HR of 0.96 (95% CI, 0.84–1.10; P = .54) (Figure 3). Subgroup analyses restricted to quintiles of dietary lutein/zeaxanthin intake resulted in HRs for the first and fifth quintiles of 0.68 (95% CI, 0.48–0.96; P = .03) and 1.27 (95% CI, 0.91–1.79; P = .16), respectively (Figure 4). Results for quintiles 2 through 4, also displayed in Figure 4, were greater than quintile 1 and less than quintile 5. A test of interaction between treatment and the quintile groups showed a P value of .006.
Figure 2.
Progression to Cataract Surgery in Age-Related Eye Disease Study 2 Participants by Treatment (Lutein/Zeaxanthin vs No Lutein/Zeaxanthin)
Figure 3.
Effect of Lutein/Zeaxanthin Supplementation on the Progression of Cataract Stratified by Dietary Intake of Lutein/Zeaxanthin
Figure 4.
Effect of Lutein/Zeaxanthin Supplementation on Moderate Vision Loss (3 or More Lines) From Baseline Stratified by Dietary Intake of Lutein/Zeaxanthin
IQR indicates interquartile range.
Although there was no scientific rationale to consider omega-3 LCPUFAs to be important in reducing the progression to cataract surgery, this analysis was conducted for completeness. Comparison of omega-3 LCPUFAs vs no omega-3 LCPUFAs resulted in a nonstatistically significant HR of 1.01 (95% CI, 0.88–1.15; P = .93).
Development of Any Cataract
Of the 6027 study eyes, 1833 (30%) had an outcome defined as any cataract. For this outcome, the comparison of lutein/zeaxanthin vs no lutein/zeaxanthin yielded a HR of 0.93 (95% CI, 0.83–1.04; P = .20) (Figure 3). In subgroup analyses restricted to quintiles of dietary lutein/zeaxanthin intake, HRs for the first and fifth quintiles were 0.70 (95% CI, 0.53–0.94; P = .02) and 1.15 (95% CI, 0.86–1.54; P = .36), respectively, for the lutein/zeaxanthin vs no lutein/zeaxanthin comparison (Figure 4). The results for quintiles 2 through 4 were in between the values for quintiles 1 and 5. A test of interaction between treatment and the quintile groups showed a P value of .01.
Development of Any Severe Cataract
Of the 6027 eligible eyes, 1504 (25%) had an outcome defined as severe cataract. For lutein/zeaxanthin vs no lutein/zeaxanthin, the HR for progression to severe cataract was 0.94 (95% CI, 0.83–1.07; P = .33). For the same outcome, in subgroup analyses by quintiles of lutein/zeaxanthin intake, a comparison of lutein/zeaxanthin vs no lutein/zeaxanthin resulted in HRs of 0.64 (95% CI, 0.46–0.89; P = .008) and 1.26 (95% CI, 0.91–1.75; P = .17) for the lowest and highest quintiles of dietary lutein/zeaxanthin, respectively. A test of interaction between treatment and the quintile groups had a P value of .003.
Visual Acuity
None of the nutrients studied affected rates of moderate or worse vision loss, defined as a loss of 15 or more letters from baseline. The HR for the development of moderate vision loss was 1.03 (95% CI, 0.93–1.13; P = .59; Figure 3) for the lutein/zeaxanthin vs no lutein/zeaxanthin comparison. Subgroup analyses restricted to quintiles of dietary lutein/zeaxanthin in-take resulted in nonstatistically significant HRs from the first through fifth quintiles (data not shown).
Safety Outcomes
No clinically or statistically significant serious adverse effect was associated with the treatments. Oral supplementation daily with lutein/zeaxanthin, DHA/EPA, or the modifications of the AREDS formulation had no effect on mortality. The HR for lutein/zeaxanthin vs no lutein/zeaxanthin for mortality was 0.92 (95% CI, 0.70–1.21; P = .54).
Discussion
AREDS reported that use of oral supplements containing vitamin C, vitamin E, and beta carotene, as well as the minerals zinc and copper, did not affect the progression of lens opacities.18 In AREDS2, we found neither beneficial nor harmful effects on the rates of cataract surgery or moderate vision loss when lutein/zeaxanthin was added to the AREDS formulation. No statistically significant effect was noted for any or severe cataract, defined as cataract surgery or specific levels of progression of cortical or PSC opacities.
Interpreting the cataract findings, particularly those for lutein/zeaxanthin, requires consideration of the AREDS2 study design and study population. AREDS2 volunteers were generally better educated and better nourished than the general population, with higher dietary intake levels of lutein/zeaxanthin and omega-3 LCPUFAs. AREDS2 participants in the lowest quintile of dietary intake of lutein/zeaxanthin showed some evidence of a beneficial effect of lutein/zeaxanthin supplementation on the progression to the cataract outcomes. Vitamin supplementation in a relatively undernourished population in China reduced the risk for progression of lens opacities.13 Whether there is a subgroup of persons who are relatively less well nourished that would benefit from lutein/zeaxanthin supplementation to slow the progression of lens opacities remains uncertain.
A second consideration is that a large proportion of AREDS2 participants (89%) elected to take Centrum Silver supplements, which contain a small amount of lutein, 250 μg, along with other carotenes and other antioxidants that may affect cataract development. AREDS2 was designed to test large doses of lutein/zeaxanthin, 10mg/2mg, against near dietary levels of intake. However, a previous randomized clinical trial17 and observational data from AREDS29,30 suggested that use of multi-vitamins, such as Centrum, might retard cataract development. The effect of Centrum use on our results cannot be determined because nearly all participants took the supplement.
The possible effect of competitive absorption of carotenoids, which has been demonstrated in other human studies31,32 and in animal studies,33 also needs consideration. About half of the AREDS2 cohort who consented to the secondary study randomization were assigned to take high doses of beta carotene and the other half to no beta carotene. Some participants received both beta carotene and lutein/zeaxanthin, while others received lutein/zeaxanthin alone. The reduction in the serum levels of lutein/zeaxanthin in those participants who received both carotenoids may be the result of the apparent systemic competitive absorption of carotenoids.
An additional consideration in interpreting the results is the timing and duration of the use of the supplements. The mean age of AREDS2 participants at enrollment into the follow-up study was 72 years. At that time, 42% already had some cortical opacities and 8% had some PSC opacities. Given the advanced age of participants, cataracts may have already begun to develop in many who had no apparent opacities at the start of the study. Perhaps the intervention was too late or of insufficient duration to affect the outcomes.
AREDS2 was conducted at 82 retinal specialty clinics and the primary focus was on the retinal outcomes. Although the optimal approach to assessing the lens outcome, using specialized lens photography, was beyond the scope possible for AREDS2 whose investigators were all retinal specialists without such equipment, we were able to incorporate red reflex lens photographs for grading the severity of cortical and PSC lens opacities. Assessing nuclear opacities requires specialized slit-lamp photography, which was not readily available in a retinal practice, so that information on nuclear cataract was incomplete. Because of the missing information on all subtypes, we used a clinically important outcome, cataract surgery, as our primary outcome. The absence of an effect of the interventions on the rates of cataract surgery may indicate an absence of an effect on nuclear and/or PSC opacities, which are the subtypes most likely to affect vision and by far the most common causes of the need for cataract surgery.34–36 Nuclear and PSC opacities have been reported in 65% and 61% of surgical cases, respectively.36 Cortical opacities alone are an uncommon cause of cataract surgery.
Strengths of the study include 5-year follow-up, low losses to follow-up, standardized data collection, and good compliance with the treatment regimen. The adherence data are further supported by data showing that serum levels of nutrients were elevated throughout the study, on average, in participants assigned to the medications. The primary outcome, cataract surgery, is a clinically important and an easily verified outcome.
While observational studies have suggested that higher dietary intake or higher blood levels of lutein and zeaxanthin may have a protective effect on the development of cataract, this randomized, placebo-controlled trial did not find an effect of supplementation with lutein/zeaxanthin on cataract surgery, cortical or PSC lens opacity progression, or vision loss. Whether supplementation would be beneficial for less well-nourished populations requires further study.
Supplementary Material
Funding/Support:
This study was supported by the intramural program funds and contracts from the National Eye Institute/National Institutes of Health, the Department of Health and Human Services, Bethesda, MD (contract No. HHS-N-260–2005-00007-C and ADB contract No. N01-EY-5–0007). Funds were contributed to these contracts by the following National Institutes of Health institutes: Office of Dietary Supplements; National Center for Complementary and Alternative Medicine; National Institute on Aging; National Heart, Lung and Blood Institute; and National Institute of Neurological Disorders and Stroke. The study medications and raw materials were provided by Alcon, Bausch and Lomb, DSM, and Pfizer.
AREDS2 Research Group Authors/Writing Team:
The following investigators of the American Lung Association Asthma Clinical Research Centers take authorship responsibility for the study results: Emily Y. Chew, MD; John Paul SanGiovanni, ScD; Frederick L. Ferris, MD; Wai T. Wong, MD, PhD; Elvira Agron, MA; Traci E. Clemons, PhD; Robert Sperduto, MD; Ronald Danis, MD; Suresh R. Chandra, MD; Barbara A. Blodi, MD; Amitha Domalpally, MD; Michael J. Elman, MD; Andrew N. Antoszyk, MD; Alan J. Ruby, MD; David Orth, MD; Susan B. Bressler, MD; Gary E. Fish, MD; George B. Hubbard, M; Michael L. Klein, MD; Thomas R. Friberg, MD; Philip J. Rosenfeld, MD, PhD; Cynthia A. Toth, MD; Paul Bernstein, MD, PhD.
AREDS2 Research Group Authors/Writing Team Affiliations:
National Eye Institute/National Institutes of Health, Bethesda, Maryland (Chew, SanGiovanni, Ferris, Wong, Agron); EMMES Corp, Rockville, Maryland (Clemons, Sperduto); University of Wisconsin, Madison, Wisconsin (Danis, Chandra, Blodi, Domalpally); Elman Retina Group PA, Baltimore, Maryland (Elman); Charlotte Eye, Ear, Nose and Throat Associates, Charlotte, North Carolina (Antoszyk); Vision Research Foundation, Royal Oak, Michigan (Ruby); Ingalls Memorial Hospital, Harvey, Illinois (Orth); Retina Division at Wilmer Eye Institute, Baltimore, Maryland (Bressler); Texas Retina Associates, Dallas, Texas (Fish); Emory University Eye Center, Atlanta, Georgia (Hubbard); Devers Eye Institute, Portland, Oregon (Klein); University of Pittsburgh Medical Center Eye Center, Pittsburgh, Pennsylvania (Friberg); Bascom Palmer Eye Institute, Miami, Florida (Rosenfeld); Duke University, Durham, North Carolina (Toth); University of Utah (Moran Eye Center), Salt Lake City, Utah (Bernstein).
Footnotes
AREDS2 Research Group Writing Team: The members of the writing team and their affiliations are found at the end of this article.
Conflict of Interest Disclosures: None reported.
Group Information: The AREDS2 Research Group is found online in the Supplement (eAppendix).
REFERENCES
- 1.Pascolini D, Mariotti SP, Pokharel GP, et al. 2002 global update of available data on visual impairment: a compilation of population-based prevalence studies. Ophthalmic Epidemiol. 2004;11(2):67–115. [DOI] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. [PMC free article] [PubMed] [Google Scholar]
- 3.Congdon N, O’Colmain B, Klaver CC, et al. ; Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. [DOI] [PubMed] [Google Scholar]
- 4.Congdon N, Vingerling JR, Klein BE, et al. ; Eye Diseases Prevalence Research Group. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122(4):487–494. [DOI] [PubMed] [Google Scholar]
- 5.Leske MC, Chylack LT Jr, Wu SY. The Lens Opacities Case-Control Study: risk factors for cataract. Arch Ophthalmol. 1991;109(2):244–251. [DOI] [PubMed] [Google Scholar]
- 6.Seddon JM, Christen WG, Manson JE, et al. The use of vitamin supplements and the risk of cataract among US male physicians. Am J Public Health. 1994;84(5):788–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leske MC, Chylack LT Jr, He Q, et al. Antioxidant vitamins and nuclear opacities: the longitudinal study of cataract. Ophthalmology. 1998;105(5):831–836. [DOI] [PubMed] [Google Scholar]
- 8.Mares-Perlman JA, Lyle BJ, Klein R, et al. Vitamin supplement use and incident cataracts in a population-based study. Arch Ophthalmol. 2000;118(11):1556–1563. [DOI] [PubMed] [Google Scholar]
- 9.Jacques PF, Chylack LT Jr, Hankinson SE, et al. Long-term nutrient intake and early age-related nuclear lens opacities. Arch Ophthalmol. 2001;119(7):1009–1019. [DOI] [PubMed] [Google Scholar]
- 10.Kuzniarz M, Mitchell P, Cumming RG, Flood VM. Use of vitamin supplements and cataract: the Blue Mountains Eye Study. Am J Ophthalmol. 2001;132(1):19–26. [DOI] [PubMed] [Google Scholar]
- 11.Teikari JM, Virtamo J, Rautalahti M, Palmgren J, Liesto K, Heinonen OP. Long-term supplementation with alpha-tocopherol and beta-carotene and age-related cataract. Acta Ophthalmol Scand. 1997;75(6):634–640. [DOI] [PubMed] [Google Scholar]
- 12.Christen WG, Manson JE, Glynn RJ, et al. A randomized trial of beta carotene and age-related cataract in US physicians. Arch Ophthalmol. 2003;121(3):372–378. [DOI] [PubMed] [Google Scholar]
- 13.Sperduto RD, Hu TS, Milton RC, et al. The Linxian cataract studies: two nutrition intervention trials. Arch Ophthalmol. 1993;111(9):1246–1253. [DOI] [PubMed] [Google Scholar]
- 14.McNeil JJ, Robman L, Tikellis G, Sinclair MI, McCarty CA, Taylor HR. Vitamin E supplementation and cataract: randomized controlled trial. Ophthalmology. 2004;111(1):75–84. [DOI] [PubMed] [Google Scholar]
- 15.Christen W, Glynn R, Sperduto R, Chew E, Buring J. Age-related cataract in a randomized trial of beta-carotene in women. Ophthalmic Epidemiol. 2004;11(5):401–412. [DOI] [PubMed] [Google Scholar]
- 16.Gritz DC, Srinivasan M, Smith SD, et al. The Antioxidants in Prevention of Cataracts Study: effects of antioxidant supplements on cataract progression in South India. Br J Ophthalmol. 2006;90(7):847–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maraini G, Williams SL, Sperduto RD, et al. ; Clinical Trial of Nutritional Supplements and Age-Related Cataract Study Group. A randomized, double-masked, placebo-controlled clinical trial of multivitamin supplementation for age-related lens opacities: clinical trial of nutritional supplements and age-related cataract report no. 3. Ophthalmology. 2008;115(4):599–607. [DOI] [PubMed] [Google Scholar]
- 18.Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119(10):1439–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorey CK, Granata L, Nichols CR, Cheng KM, Craft NE. Dietary modulation of lens zeaxanthin in quail. Exp Eye Res. 2005;81(4):464–477. [DOI] [PubMed] [Google Scholar]
- 20.Yeum KJ, Taylor A, Tang G, Russell RM. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Invest Ophthalmol Vis Sci. 1995;36(13):2756–2761. [PubMed] [Google Scholar]
- 21.Delcourt C, Carrière I, Delage M, Barberger-Gateau P, Schalch W; POLA Study Group. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sci. 2006;47(6):2329–2335. [DOI] [PubMed] [Google Scholar]
- 22.Moeller SM, Voland R, Tinker L, et al. ; CAREDS Study Group; Women’s Health Initiative. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study, an ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2008;126(3):354–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vu HT, Robman L, Hodge A, McCarty CA, Taylor HR. Lutein and zeaxanthin and the risk of cataract: the Melbourne Visual Impairment Project. Invest Ophthalmol Vis Sci. 2006;47(9):3783–3786. [DOI] [PubMed] [Google Scholar]
- 24.Brown L, Rimm EB, Seddon JM, et al. A prospective study of carotenoid intake and risk of cataract extraction in US men. Am J Clin Nutr. 1999;70(4):517–524. [DOI] [PubMed] [Google Scholar]
- 25.Chew EY, Clemons T, SanGiovanni JP, et al. ; The AREDS2 Research Group. The Age-Related Eye Disease Study 2 (AREDS2): study design and baseline characteristics (AREDS2 report number 1). Ophthalmology. 2012;119(11):2282–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hambidge M Underwood Memorial Lecture: human zinc homeostasis: good but not perfect. J Nutr. 2003;133(5, supp 1):1438S–1442S. [DOI] [PubMed] [Google Scholar]
- 27.Wei LJ, Jin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84(408):1065. doi: 10.2307/2290084. [DOI] [Google Scholar]
- 28.Christen WG, Liu S, Glynn RJ, Gaziano JM, Buring JE. Dietary carotenoids, vitamins C and E, and risk of cataract in women: a prospective study. Arch Ophthalmol. 2008;126(1):102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–199. [DOI] [PubMed] [Google Scholar]
- 30.Milton RC, Sperduto RD, Clemons TE, Ferris FL III; Age-Related Eye Disease Study Research Group. Centrum use and progression of age-related cataract in the Age-Related Eye Disease Study: a propensity score approach: AREDS report No. 21. Ophthalmology. 2006;113(8):1264–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeum KJ, Russell RM. Carotenoid bioavailability and bioconversion. Annu Rev Nutr. 2002;22:483–504. [DOI] [PubMed] [Google Scholar]
- 32.Kostic D, White WS, Olson JA. Intestinal absorption, serum clearance, and interactions between lutein and beta-carotene when administered to human adults in separate or combined oral doses. Am J Clin Nutr. 1995;62(3):604–610. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Roger Illingworth D, Connor SL, Barton Duell P, Connor WE. Competitive inhibition of carotenoid transport and tissue concentrations by high dose supplements of lutein, zeaxanthin and beta-carotene. Eur J Nutr. 2010;49(6):327–336. [DOI] [PubMed] [Google Scholar]
- 34.Adamsons I, Muñoz B, Enger C, Taylor HR. Prevalence of lens opacities in surgical and general populations. Arch Ophthalmol. 1991;109(7):993–997. [DOI] [PubMed] [Google Scholar]
- 35.Lewis A, Congdon N, Munoz B, et al. Cataract surgery and subtype in a defined, older population: the SEECAT Project. Br J Ophthalmol. 2004;88(12):1512–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor A Cataract: relationship between nutrition and oxidation. J Am Coll Nutr. 1993;12(2):138–146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.