Abstract
Objectives:
To evaluate pharmacokinetics and pharmacogenetics of contraceptive implant progestin concentrations in HIV-positive women initiating efavirenz- or nevirapine-containing antiretroviral therapy (ART).
Design:
We analyzed stored samples from women self-reporting implant use in the Partners PrEP Study.
Methods:
Plasma samples collected every six months were analyzed for levonorgestrel and etonogestrel concentrations. Progestin concentrations from samples collected after ART initiation were compared to pre-ART concentrations for intraindividual comparisons. We used adjusted linear mixed models to compare hormone concentrations between individuals on efavirenz and nevirapine to a no ART group. We then evaluated whether possessing certain alleles with known or possible influences on efavirenz, nevirapine, or progestin metabolism were associated with changes in progestin concentrations or modified the association between ART use and progestin concentrations.
Results:
Our analysis included 11 women who initiated efavirenz, 13 who initiated nevirapine, and 36 who remained ART-naïve. In the efavirenz group, the adjusted geometric mean ratio (aGMR) of levonorgestrel was 0.39 (90% confidence intervals (0.31, 0.49); p<0.001) and the etonogestrel aGMR was 0.51 (0.34, 0.76); p=0.006) compared to the control group. No difference was observed in the nevirapine group compared to controls (levonorgestrel 0.93 (0.74, 1.18); p=0.64; etonogestrel 1.07 (0.77, 1.50); p=0.73). Possession of four allele variants were found to result in further reductions in progestin concentrations among those receiving efavirenz.
Conclusions:
Concomitant use of efavirenz significantly reduces levonorgestrel or etonogestrel concentrations by 61% and 49%, respectively, compared to no ART use. We also report allelic variants in hepatic enzymes that influenced the extent of the observed drug-interaction between progestins and efavirenz.
Keywords: antiretroviral therapy, hormonal contraception, efavirenz, implants, etonogestrel, levonorgestrel, pharmacokinetic, pharmacogenetics
INTRODUCTION
The majority of people living with HIV are women, with an estimated 13 million HIV-positive women of reproductive age living in sub-Saharan Africa.[1] Nearly two-thirds of pregnancies among HIV-positive women in sub-Saharan Africa are unintended,[2] and effective contraception can prevent unintended pregnancies, reducing maternal mortality and perinatal HIV transmission.[3, 4] Contraceptive implants, containing the progestins, levonorgestrel or etonogestrel, are the most effective contraceptives available and are increasingly being used by HIV-positive women in sub-Saharan Africa.[5]
The World Health Organization (WHO) recommends initiation of antiretroviral therapy (ART) for HIV-positive individuals, and dolutegravir-containing ART is recommended as a first-line regimen.[6] Enthusiasm for dolutegravir’s wide-spread rollout in resource-limited settings has been hampered by its possible link with neural tube defects.[7] Therefore, efavirenz-containing ART remains the primary regimen for women of reproductive age in resource-limited settings, including those on contraceptive implants. However, due to drug-drug interactions resulting from induction of hepatic cytochrome P450 (CYP450) enzymes, the concomitant use of efavirenz reduces the effectiveness of contraceptive implants. In a prior study among Kenyan women, those using implants and efavirenz-containing ART faced three-fold higher rates of pregnancies than those using nevirapine-containing ART,[8] a result supported by pharmacokinetic (PK) data showing reduced implant progestin concentrations with concomitant efavirenz use.[9–12] The existing PK evaluations are limited by large inter-subject variability. Furthermore, though both levonorgestrel and etonogestrel are believed to be metabolized primarily by CYP3A4, the mechanisms that underlie metabolism of progestins are poorly understood. Genetic polymorphisms in hepatic enzymes and transporters, which often vary by ethnic group, may be an important factor influencing progestin metabolism, extent of drug-drug interactions, and subsequent contraceptive efficacy.[13, 14]
PK studies that address inter-subject variability by including within-subject sampling and PG analyses with ethnic groups traditionally less represented in such analyses are needed to fill knowledge gaps regarding concomitant implant and efavirenz use. Our first objective was to conduct a PK evaluation studying the effect of initiating efavirenz- or nevirapine-containing ART on levonorgestrel and etonogestrel plasma concentrations, comparing concentrations pre- and post-ART initiation within each woman (intra-subject comparisons) as well as comparing the changes in concentrations across groups by ART category (inter-subject comparisons). Our second objective was to characterize pharmacogenetic (PG) polymorphisms associated with progestin concentration changes among these women, and whether any PG polymorphisms modify the effect of ART on hormone concentrations.
METHODS
Study population
We used data from HIV-positive women less than 50 years of age enrolled in the Partners PrEP Study in Kenya and Uganda from 2008–2010 who self-reported implant use, some of whom went on to initiate ART later. Detailed enrollment and follow-up procedures for participants are described elsewhere.[15–17] Women not eligible for ART initiation at the time of Partners PrEP Study enrollment had their clinical and immunological status monitored at study visits. If participants became eligible to initiate ART according to national guidelines during follow-up, they were referred to HIV facilities for ART initiation. Contraception was not provided by the study; instead, women were referred to routine clinical care if desiring contraception. Women self-reported implant use and the implant insertion dates were not available. Women self-reporting the use of other hormonal methods, intrauterine device, or surgical methods, or those without documented ART status were excluded.
We analyzed six-monthly plasma samples, which were collected regardless of menstrual cycles, for each woman. For women initiating ART, we included women with at least one sample available for analysis pre- or post-ART. Five women in the efavirenz group had one sample either pre- or post-ART initiation and the remainder of the women had at least two samples either pre- or post-ART. To be included in this analysis, we required that the plasma samples were within six months of each other and the date of ART initiation, when applicable. To help account for the expected decreases in progestin concentrations over time,[14, 18] we included samples from women who did not initiate ART but had samples available from ≥6 study visits to represent levonorgestrel or etonogestrel exposure over time in the absence of ART.
One woman in the Partners PrEP Study became pregnant while concomitantly using an implant and efavirenz-containing ART. Because she initiated the implant after efavirenz, we could not include her in the PK or PG evaluations. We analyzed all progestin plasma samples collected for this woman and descriptively present these findings.
ART classification
We classified ART regimen use at the time of sample collection into three categories: efavirenz-containing ART, nevirapine-containing ART, or no ART. The no ART group included all samples from ART-naïve controls as well as pre-ART initiation samples for women who initiated ART. The efavirenz- and nevirapine-containing ART groups consisted of samples collected after initiation of the respective ART regimens. We defined an ART regimen as at least a three-drug combination of antiretrovirals, and monotherapy, for instance, for prevention of mother-to-child transmission was not considered “initiation of ART.” ART initiation dates were self-reported and not verified against their clinical care records. Date of ART initiation was imputed as the midpoint of the preceding interval before ART use was reported or, when available, using the self-reported number of days of ART use. For the purposes of characterizing intra-subject comparisons in the no ART group, the time of the median sample collection for each ART-unexposed woman was used to split her observations into “pre-ART” and “post-ART” periods for comparison.
Candidate gene selection
For the PG evaluation, the following 18 alleles were chosen for analysis based on known or possible influences on efavirenz, nevirapine, or implant progestin metabolism[19, 20]: CYP2B6: rs4803419 (15582C>T), rs3745274 (516G>T); ABCB1: rs3842 (4046A>G), rs1045642 (3435C>T), rs2032582 (2677G>A/T); CYP2B6: rs28399499 (983T>C); CYP3A5: rs776746 (*3 allele, 6986A>G), rs10264272 (*6 allele, 14690G>A), rs41303343 (*7 27131insT); CYP3A4: rs2740574 (−392A>G), rs35599367 (*22 allele, 15497C>T); CYP2A6: rs8192726 (*9B allele, 1836G>T), rs28399433 (−48A>C); NR1I2: rs1523130 (44477A>G), rs2472677 (63396C>T); NR1I3 rs2307424 (540C>T), and rs3003596 (1089T>C).
Single nucleotide polymorphism (SNP) genotyping
We extracted genomic DNA from 100 µL of whole blood using a Qiagen (Germantown, MD) DNAeasy blood and tissue kit. We then genotyped the DNA samples using Applied Biosystems (Foster City, CA) Taqman™ genotyping assays according to the manufacturer’s instructions. PCR amplification was done using a BioRad (Hercules, CA) CFX Connect™ Real-Time PCR Detection System and allelic discrimination determined by post-run analysis using CFX Manager 3.1 software. For quality control, we ran samples from the UW School of Pharmacy human liver bank of known genotypes for each assay.
Primary outcomes: Progestin concentrations in plasma
We simultaneously quantified plasma concentrations of levonorgestrel, etonogestrel, and endogenous progesterone using a validated, high-performance liquid chromatography-heated electrospray ionization-tandem triple quadrupole mass spectrometry (LC-MS/MS) assay.[21] The lower limit of quantification (LLQ) for levonorgestrel and etonogestrel were both 0.02 ng/mL and endogenous progesterone was 0.01 ng/mL. When both levonorgestrel and etonogestrel concentrations above the LLQ were detected for a sample, and the levonorgestrel concentrations were markedly higher than concentrations consistent with implant use (possibly due to oral levonorgestrel-containing contraceptive use),[14] the levonorgestrel values were excluded (16 samples from 10 women). One woman had several consecutive levonorgestrel concentrations quantified and then a single etonogestrel concentration detected; the etonogestrel observation was excluded from analysis. One woman had both levonorgestrel and etonogestrel concentrations detected in the same samples consistent with either implant use; all observations were excluded from analysis since it was not possible to determine which implant was being used. For endogenous progesterone concentrations, we quantified the number of samples with concentrations above both a lower cutoff of 3 ng/mL and a higher cutoff of 5 ng/mL; endogenous progesterone concentrations above 3 ng/mL are associated with recent ovulation but controversy remains regarding the exact cutoff most predictive of ovulation.[22]
Statistical analysis
We used frequencies (and percentages) and median (and interquartile ranges (IQR)) to describe categorical and continuous variables, respectively. Etonogestrel and levonorgestrel concentrations were log-transformed for analyses. For the PK evaluations, we used the observed geometric means (GM) and ratios (GMR) to summarize progestin concentrations pre- vs. post-ART (intra-subject comparisons). We used linear mixed models to compare hormone concentrations for observations post-ART initiation in the efavirenz and nevirapine groups to the no ART group (inter-subject comparisons). Beta coefficients were exponentiated to obtain GMRs of hormone levels and 90% confidence intervals (CIs) for the post-ART vs. no ART observations. All models were adjusted for days from ART initiation, and multivariate models were also adjusted for a priori selected covariates of age, body mass index (BMI), nationality (Kenyan or Uganda) and HIV viral load measured closest to analyzed sample, as a marker for antiretroviral adherence.
For the PG evaluations, of our 18 candidate alleles, two alleles were excluded from the analysis. One allele (s35599367) was monomorphic with all participants with C/C expression. Two alleles in CYP2A6 (rs28399433 and rs8192726) were in perfect linkage disequilibrium (r2=1), so rs8192726 was excluded from analysis. One allele was not in Hardy-Weinberg equilibrium at a threshold of p<0.0001 (rs3745274, p=0.0001); however, since this allele is one of the best characterized alleles regarding progestin and ART drug-drug interactions, we chose to report the findings. We calculated minor allele frequencies (MAF) by nationality (Kenya vs. Uganda), and used a chi-square test to assess for differences in the MAFs. All p-values were greater than 0.05, indicating that any potential bias arising from population structure for these variants by nationality would not be significant, so we did not conduct stratified analysis by nationality (data not shown). We ran separate, additive linear mixed models for each of the 16 alleles in our PG evaluations. Due to reduced sample sizes when restricting analyzes by implant type and allelic variants, we combined women using either implant by conducting a z-score transformation of their hormone concentrations, so that the values were “normalized” for the distribution of concentrations for each hormone type. We then tested for an association of number of allele variant copies (0,1,2) with changes in hormone concentrations, adjusting for ART category (main effects model). Next, we tested whether each allelic variant modified the association between ART use and hormone concentration changes by adding an interaction term for number of variant allele copies and ART category (interaction model). In addition to the adjustment variables described for the PK models, we also adjusted PG models for implant type. We used a Bonferroni adjustment to determine the significance threshold of 0.05/16=0.003 for the multiple comparisons in the PG analysis. For the main effects PG models, we report the GMRs of progestin concentrations among women with each additional variant copy of the allele, obtained by exponentiating the beta coefficient for the allele variant variable, and the corresponding p-values. For the interaction PG models, we report the fold change in progestin GMRs between women on ART and women not on ART with each additional copy of the variant allele, obtained by exponentiating the beta coefficient of the interaction term, and the corresponding p-values.
For PK and PG linear mixed models, we selected a spatial autoregression correlation structure based on Akaike information criterion. The spatial correlation structure accounts for correlation between women’s repeated measures and is ideal for data like ours where spacing of repeated measures differs between individuals. We conducted all analyses with SAS version 9.4 statistical software (SAS Institute Inc., Cary, NC, USA).
RESULTS
This analysis included 11 women who initiated efavirenz, 13 who initiated nevirapine, and 36 who remained ART-naïve during follow-up (Table 1). In the efavirenz group, two women had only one pre-ART initiation sample, one woman had only one post-ART initiation sample, and one woman had only one pre- and one post-ART initiation sample available for analysis. All women in the nevirapine and the ART-naïve groups had at least two pre- and post-ART initiation samples available for analysis. The median age of all included women was 33 (IQR 30–36.5). Parity, marital status, years of formal schooling, nationality, BMI, and follow-up time in this analysis were comparable between the two ART groups and varied somewhat from the ART-naïve group.
Table 1:
Baseline characteristics of HIV-infected women on contraceptive implants by ART category
| Characteristic | Efavirenz (n=11) |
Nevirapine (n=13) |
ART-naïve (n=36) |
|||
|---|---|---|---|---|---|---|
| n (%) or median (IQR) | ||||||
| Age, years | 34 (31, 36) | 32 (31, 35) | 33 (30, 37) | |||
| Parity | 3 (2, 3) | 4 (1, 4) | 4 (3, 5) | |||
| Married | 11 (100%) | 12 (92%) | 36 (100%) | |||
| Educational status, years in school | 7 (3, 12) | 7 (4, 8) | 4 (3, 7) | |||
| Ethnicity | ||||||
| Kikuyo | 3 (27%) | 1 (8%) | 5 (14%) | |||
| Iteso | 2 (18%) | 5 (38%) | 14 (39%) | |||
| Luo | 1 (9%) | 3 (23%) | 4 (11%) | |||
| Other | 5 (45%) | 4 (31%) | 13 (36%) | |||
| Country | ||||||
| Kenya | 7(63%) | 7 (56%) | 13 (37%) | |||
| Uganda | 4 (37%) | 6 (44%) | 23 (63%) | |||
| BMI kg/m2 | 22.8 (21.8, 25.7) | 22.5 (21.5, 24.0) | 22.2 (21.5, 24.5) | |||
| Samples | Pre-ART | Post-ART | Pre-ART | Post-ART | Pre-ART1 | Post-ART1 |
| Total number of samples contributing to analysis | 27 | 22 | 34 | 37 | 121 | 104 |
| Number of days since ART initiation, median (min, max) | −239 (−630, −36) | 209 (40, 658) | −210 (−630, −42) | 236 (41, 799) | −252 (−732, −38) | 252 (0, 566) |
| Number of samples with viral suppression (<1000 copies/mL) | 11/27 (41%) | 17/22 (77%) | 8/34 (24%) | 36/37 (97%) | 30/121 (25%) | 34/104 (33%) |
BMI=body mass index; ART=antiretroviral therapy
The median time for each woman in the no ART group was used to split the observations into “pre-ART” and “post-ART” so as to facilitate characterizing changes over time (i.e. intra-subject comparisons) in this group
PK evaluation
The GM for levonorgestrel and etonogestrel concentrations in the pre- and post-ART samples per woman (i.e. intra-subject comparisons) are described in Table 2. The adjusted progestin GMRs comparing efavirenz and the nevirapine groups to the no ART group (i.e. inter-subject comparisons) were 0.39 ((0.31, 0.49); p<0.001) and 0.93 ((0.74, 1.18); p=0.64), respectively. The adjusted progestin GMRs of etonogestrel comparing the efavirenz and nevirapine groups to the no ART group (i.e. inter-subject comparisons) were 0.51 ((0.34, 0.76); p=0.006) and 1.07 ((0.77, 1.50); p=0.73), respectively.
Table 2:
Pharmacokinetic results by ART category and implant type
| Unadjusted1 | Adjusted2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ART category | # women (samples) | Pre-ART GM hormone concentrations (pg/mL) 3 | Post-ART GM hormone concentrations (pg/mL) 3 | Observed Pre-ART: Post-ART GMR4 | Predicted ART: No ART GMR5 (90% CI) | p-value | Predicted ART: No ART GMR5 (90% CI) | p-value | |
| Levonorgestrel | ART-naïve | 14 (85) | 441.95 | 402.40 | 0.91 | Ref | -- | Ref | -- |
| EFV | 8 (33) | 333.40 | 89.91 | 0.27 | 0.33 (0.27, 0.41) | <0.001 | 0.39 (0.31, 0.49) | <0.001 | |
| NVP | 6 (36) | 460.01 | 330.23 | 0.72 | 0.84 (0.67, 1.04) | 0.17 | 0.93 (0.74. 1,18) | 0.64 | |
| Etonogestrel | ART-naïve | 22 (140) | 255.74 | 204.79 | 0.80 | Ref | -- | Ref | -- |
| EFV | 3 (16) | 218.38 | 132.91 | 0.61 | 0.57 (0.38, 0.84) | 0.02 | 0.51 (0.34, 0.76) | 0.006 | |
| NVP | 7 (36) | 305.05 | 218.82 | 0.72 | 0.88 (0.67, 1.15) | 0.42 | 1.07 (0.77, 1.50) | 0.73 | |
ART=antiretroviral therapy; GM=geometric mean; GMR=geometric mean ratio; EFV=efavirenz; NVP=nevirapine
Adjusted only for days from ART initiation
Adjusted for days from ART initiation, age, BMI, nationality, and closest viral load
The median time for each ART-naïve woman was used to split the observations into “pre-ART” and “post-ART” so as to facilitate intra-subject comparisons in this group
The observed GMR is intra-subject, calculated as the ratio between geometric means of observed pre- and post-ART initiation samples
The no ART (reference) category GMRs from models include all samples from ART-naïve women as well as pre-ART samples from those who initiated ART during study, in order to better estimate changes due to initiating ART while accounting for background change in concentrations over time. Thus, these models incorporate information from within women and well as between women.
PG evaluation
Of the allele variants we evaluated for changes in levonorgestrel or etonogestrel concentrations in the main effects models, no alleles met our significance threshold but the number of variant copies for one allele, CYP2B6 15582C>T, was associated with a 48% increase in progestin concentrations after adjusting for ART use (p=0.03; Table 3). In our interaction models, only one allele, ABCB1 3435C>T (p<0.001), met our significance threshold and was associated with 88% greater reduction in progestin GMRs in the efavirenz group relative to the no ART group. Nonetheless, the following variants were associated with 64–85% greater reductions in progestin GMRs in the efavirenz group relative to the no ART group at p<0.05: CYP2B6 983T>C, CYP3A5*3 6986A>G, and CYP3A5*7 27131insT. No allele variants were significantly associated with differences in the progestin GMRs in the nevirapine group relative to the no ART group.
Table 3:
Effect modification of the association between ART use and progestin concentrations by possession of variant alleles
| Main effect PG models1 | Interaction PG models2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Variant SNP (rsID) | All women (n=59 women) | No ART (n=59 women) | Efavirenz (n=11 women) | Nevirapine (n=12 women) | ||||||||||
| # women (samples) with variant | MA (n=59) |
Progestin GMR (per additional variant copy) (90% CI) |
p-value4 | # women (samples) with variant | MAF (n=59) |
# women (samples) with variant | MAF (n=11) |
Progestin GMR fold difference per additional variant copy (90% CI) |
Interaction p-value4 | # women (samples) with variant | MAF (n=12) |
Progestin GMR fold difference per additional variant copy (90% CI) |
Interaction p-value4 | ||
| CYP2B6 | 15582C>T (rs4803419) | 9 (53) | 0.08 | 1.48 (1.11, 1.99) | 0.03 | 9 (42) | 0.08 | 2 (6) | 0.14 | 1.47 (0.85, 2.52) | 0.24 | 1 (5) | 0.04 | 0.68 (0.23, 2.01) | 0.56 |
| 983T>C (rs28399499) | 7 (43) | 0.07 | 1.14 (0.81, 1.60) | 0.52 | 7 (40) | 0.07 | 1 (3) | 0.05 | 0.15 (0.05, 0.43) | 0.004 | 0 (0) | 0.00 | -- | -- | |
| 516G>T (rs3745274)5 | 39 (226) | 0.33 | 0.80 (0.61, 1.06) | 0.06 | 39 (185) | 0.33 | 8 (17) | 0.36 | 0.65 (0.30, 1.42) | 0.36 | 8 (24) | 0.33 | 0.88 (0.45, 1.71) | 0.75 | |
| ABCB1 | 4046A>G (rs3842) | 24 (131) | 0.25 | 1.19 (0.96, 1.47) | 0.19 | 24 (112) | 0.25 | 4 (6) | 0.18 | 0.78 (0.37, 1.61) | 0.57 | 6 (13) | 0.29 | 0.86 (0.53, 1.39) | 0.60 |
| 3435C>T (rs1045642) | 7 (39) | 0.06 | 1.12 (0.74, 1.69) | 0.65 | 7 (31) | 0.06 | 2 (4) | 0.09 | 0.12 (0.05, 0.29) | <0.001 | 2 (4) | 0.08 | 1.12 (0.50, 2.52) | 0.81 | |
| 2677G>A (rs2032582a) | 0 (0) | 0.00 | -- | -- | 0 (0) | 0.00 | 0 (0) | 0.00 | -- | -- | 0 (0) | 0.00 | -- | -- | |
| 2677G>T (rs2032582t) | 6 (33) | 0.05 | 1.03 (0.66, 1.59) | 0.92 | 6 (25) | 0.05 | 1 (4) | 0.05 | 2.72 (0.85, 8.75) | 0.16 | 2 (4) | 0.08 | 1.34 (0.58, 3.10) | 0.57 | |
| CYP2A6 | 1836G>T (rs8192726) | 8 (47) | 0.08 | 0.98 (0.71, 1.35) | 0.90 | 8 (40) | 0.08 | 2 (6) | 0.14 | 0.61 (0.36, 1.03) | 0.12 | 1 (1) | 0.04 | 0.62 (0.17, 2.20) | 0.53 |
| CYP3A4 | -392A>G (rs2740574) | 56 (323) | 0.77 | 1.11 (0.89, 1.38) | 0.45 | 56 (268) | 0.77 | 10 (20) | 0.77 | 0.59 (0.33, 1.04) | 0.12 | 12 (35) | 0.71 | 0.68 (0.36, 1.27) | 0.30 |
| CYP3A5 | 6986A>G (rs776746) | 16 (88) | 0.15 | 1.26 (0.97, 1.63) | 0.14 | 16 (73) | 0.15 | 3 (6) | 0.14 | 0.36 (0.17, 0.76) | 0.02 | 4 (9) | 0.17 | 1.70 (0.91, 3.20) | 0.16 |
| 14690G>A (rs10264272) | 26 (154) | 0.28 | 1.00 (0.83, 1.20) | 0.99 | 26 (119) | 0.28 | 5 (11) | 0.32 | 1.63 (1.05, 2.53) | 0.07 | 7 (24) | 0.33 | 0.63 (0.39, 1.02) | 0.11 | |
| 27131insT (rs41303343) | 16 (100) | 0.14 | 0.82 (0.61, 1.10) | 0.26 | 16 (95) | 0.14 | 2 (5) | 0.09 | 0.26 (0.12, 0.59) | 0.007 | 0 (0) | 0.00 | -- | -- | |
| NR1I2 (PXR) | 44477A>G (rs1523130) | 14 (78) | 0.12 | 0.83 (0.61, 1.12) | 0.30 | 14 (63) | 0.12 | 5 (8) | 0.23 | 1.79 (0.88, 3.62) | 0.18 | 2 (7) | 0.08 | 0.94 (0.42, 2.14) | 0.91 |
| 63396C>T (rs2472677) | 44 (245) | 0.42 | 1.08 (0.86, 1.36) | 0.59 | 44 (199) | 0.42 | 9 (18) | 0.45 | 0.61 (0.36, 1.03) | 0.12 | 10 (28) | 0.46 | 0.62 (0.17, 2.20) | 0.53 | |
| NR1I3 | 540C>T (rs2307424) | 13 (76) | 0.11 | 1.18 (0.86, 1.62) | 0.39 | 13 (56) | 0.11 | 2 (5) | 0.09 | 2.49 (1.06, 5.86) | 0.08 | 4 (15) | 0.17 | 1.15 (0.59, 2.24) | 0.73 |
| 1089T>C (rs3003596) | 46 (267) | 0.52 | 0.93 (0.77, 1.13) | 0.55 | 46 (221) | 0.52 | 11 (16) | 0.55 | 0.76 (0.49, 1.18) | 0.30 | 12 (30) | 0.50 | 0.97 (0.62, 1.51) | 0.90 | |
ART=antiretroviral therapy; MAF=minor allele frequency; GMR=geometric mean ratio; SNP=single nucleotide polymorphism; rsID=reference SNP ID
Models adjusted for ART category, days from ART initiation, implant type, BMI, log of closest viral load measure, and country
Assesses the association of the variant allele with progestin concentrations independent of ART category; model estimates indicate progestin GMR with each additional variant allele copy
Assesses effect modification of the association between ART category and progestin concentrations by variant allele; model estimates indicate fold change in progestin GMR of efavirenz/nevirapine vs. no ART groups with each additional variant copy
Includes all samples from women who did not initiate ART and pre-ART samples from women who initiated regimens with efavirenz or nevirapine; therefore, all women from the sample are represented
We used a Bonferroni adjustment of significance threshold of p=0.003, but highlight here all p-values <0.05
Allele exceeds Hardy-Weinberg equilibrium threshold of p<0.001 (p=0.0001)
Endogenous progesterone evaluation
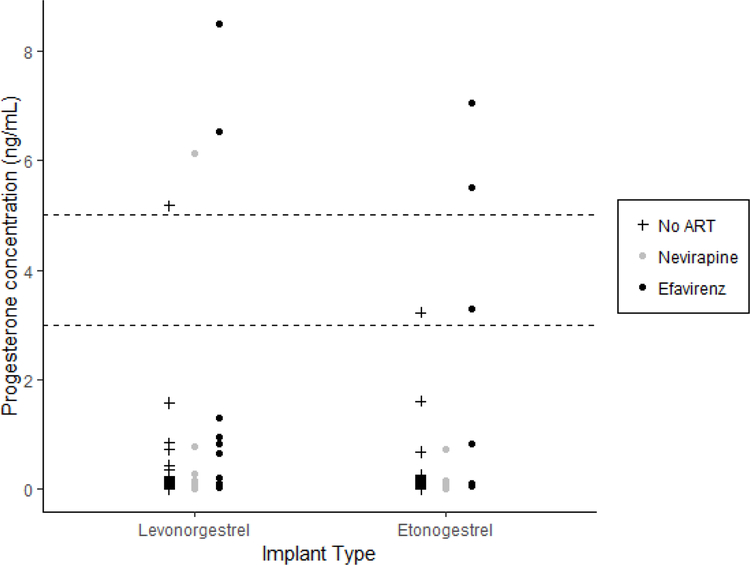
Among women using levonorgestrel implants, using either cutoff of 3 ng/mL or 5 ng/mL, high endogenous progesterone concentrations were detected in two (14%), one (5%), and one (1%) samples in the efavirenz, nevirapine, and no ART groups, respectively (Figure 1). Among women using etonogestrel implants, using a lower cutoff of 3ng/mL, high endogenous progesterone concentrations were detected in the three (38%), zero, and one (1%) samples in the efavirenz, nevirapine, and no ART groups, respectively; and using a higher cutoff of 5ng/mL, high endogenous progesterone concentrations were detected in two (25%) samples in the efavirenz group only.
Figure 1: Endogenous progesterone concentrations by ART category and implant type.
For each implant type, progesterone concentrations are displayed for the no ART (left, plus symbol), nevirapine (middle, grey circle), and efavirenz (right, black circle) groups.
Pregnancy and concentrations
The one woman who became pregnant while concomitantly using an implant and efavirenz-containing ART had the following timeline. She first reported initiation of efavirenz-containing ART on March 2, 2011, then first reported implant use on June 30, 2011. One sample was available for analysis from September 22, 2011, approximately three months after her first report of implant use, and showed a levonorgestrel concentration of 140 pg/mL and an endogenous progesterone concentration of >10.0 ng/mL; her second sample available from March 14, 2012, showed a levonorgestrel concentration of 180 pg/mL and an endogenous progesterone concentration of 3.3 ng/mL. On April 16, 2012, her urine test was positive for a pregnancy and on April 24, 2012, she experienced a pregnancy loss.
DISCUSSION
Utilizing pre- and post-ART plasma samples among women on contraceptive implants, we demonstrate that concomitant use of efavirenz significantly reduces levonorgestrel and etonogestrel concentrations by 61% and 49%, respectively, as compared to women not yet on ART. We also report on a new finding of a greater array of allelic variants in hepatic enzymes associated with these reduced concentrations.
Our PK findings demonstrating a significant reduction of hormone concentrations with efavirenz initiation are similar to prospective, parallel group comparisons. Scarsi and colleagues reported a 57% reduction in levonorgestrel concentrations among women on efavirenz vs. no ART at 48 weeks after levonorgestrel implant insertion.[10] Three of twenty (15%) women became pregnant between 36 and 48 weeks after implant insertion. Among women already on efavirenz-containing ART and initiating etonogestrel implants, Vieira and colleagues noted a 2.9 fold reduction in the etonogestrel area under the concentration curve over the first 24 weeks of implant use compared to the no ART group.[9] Collectively, these studies and our PK data support the findings of the largest cohort study to date examining the clinical outcome of pregnancies, which demonstrated that women concomitantly using efavirenz and implants faced nearly 3 times higher rates of pregnancies than women using nevirapine with implants.[8] However, given the overall effectiveness of the implants (<1% failure rate),[23] the absolute rates of pregnancy with efavirenz plus implants were still lower than those faced by women using efavirenz and most other forms of contraception (e.g. 3.3 pregnancies/100 women-years of implant use vs. 5.4 of depot medroxyprogesterone acetate use, both reported with concomitant efavirenz use).[8] Current HIV and contraceptive guidelines recommend caution regarding implant use with efavirenz. However, with multiple PK and clinical studies consistently demonstrating reduced concentrations or effectiveness of implant hormones when used with efavirenz, national and international authorities should consider recommending offering an alternative ART regimen—where available—to these women.
We report on novel interactions showing greater reduction in progestin concentrations with efavirenz use associated with allelic variations in ABCB1, CYP2B6, CYP3A5*3, and CYP3A5*7. While prior studies have identified genetic polymorphisms in CYP450 enzymes that may contribute to differences in contraceptive PK,[19, 20] ours is the first study to identify an association between SNPs in ABCB1-encoding genes and larger reductions in progestin concentrations among efavirenz compared to ART-naïve implant users. This finding is surprising given that ABCB1 encodes the P-glycoprotein drug efflux pump, an ATP-binding cassette (ABC) membrane protein that plays a role in pumping drugs out of the cell, of which efavirenz is not a known substrate.[26] A previous PG study evaluated the effects of the ABCB1 3435C>T variant on ART drug concentrations and clinical outcomes among white individuals living with HIV and found that patients who possessed the TT genotype had lower efavirenz plasma concentrations relative to those with TC and CC genotypes, which does not align with our findings given established PK interactions between efavirenz and implant progestins.[27] Another study showed an association between possession of the ABCB1 3435C>T variant and slower recovery of CD4 count after efavirenz initiation among 50 individuals living with HIV in Belgium, but no association was observed between the SNP and efavirenz pharmacokinetic parameters. This study, however, did observe an association between another ABCB1 polymorphism (3842T>C) and a higher EFV accumulation ratio.[28] Thus, the implications of ABCB1 polymorphism, antiretroviral metabolism, and any drug-drug interactions requires further exploration. As for CYP enzymes, Neary et al. have previously reported that Ugandan women who possessed the variants for CYP2B6 516G>T, resulting in slow efavirenz metabolism, had lower levonorgestrel concentrations when combined with efavirenz than those without these polymorphisms.[19] We did not find a significant interaction between CYP2B6 516G>T and ART use in our analysis, but did see reduced plasma progestin concentrations with efavirenz use among women possessing another CYP2B6 variant (983T>C). Ultimately, given our small sample size, caution is advised in interpreting these PG findings as definitive. Of note, we find varying results in the main effects and the interaction models, which is anticipated given that the main effects model examines the relationship between allele variations and hormone concentrations after adjustment for ART use, while the effect modifier model examines whether a variant allele modifies the association between ART use and hormone concentrations. Further studies with a more diverse and larger population could allow us to determine whether women with these specific polymorphisms are indeed at higher risk of drug-drug interactions between implants and ART.
Implants are believed to prevent pregnancies by gradually releasing progestins into the serum, thereby suppressing ovulation, increasing cervical mucus viscosity, and possibly altering the endometrium. Although our numbers are small, that higher proportions of women in the efavirenz group had high endogenous progesterone concentrations, associated with luteal activity, suggests that the efficacy of the implants via ovulation suppression may be compromised with concomitant efavirenz use. A similar finding was reported in another PK study of implants and efavirenz.[9] Our finding of endogenous progesterone concentrations consistent with luteal activity further supports the hypothesis that implant efficacy is reduced with concomitant efavirenz use due to lower circulating progestin concentrations. The one case we analyzed where a pregnancy occurred with concomitant implant and efavirenz use supports this hypothesis; the woman had high endogenous progesterone concentrations consistent with luteal activity, coupled with relatively low levonorgestrel concentrations, one to six months before she became pregnant.
HIV treatment guidelines in resource-rich settings now include integrase inhibitor-containing ART, particularly with dolutegravir, as first-line ART regimens.[29, 30] Similarly, dolutegravir-containing ART is now recommended as first-line globally, but with the significant exception for women of reproductive age due to the potential risk of neural tube defects.[6] Limited data suggest that dolutegravir does not significantly influence contraceptive progestin concentrations,[24] and dolutegravir’s concomitant use with hormonal contraceptives is supported by relevant guidelines.[6, 25] While the rollout for dolutegravir has been paused for women of reproductive age, offering women who are already on implants plus efavirenz the choice to switch to dolutegravir-containing ART is a potential opportunity to avoid unintended pregnancies with efavirenz. Similarly, women on hormonal contraception should not be denied access to dolutegravir, as these women are highly unlikely to become pregnant due to the implants’ high effectiveness.
While our PK and PG evaluations made opportune use of existing samples and is strengthened by longitudinal intrasubject comparisons pre- and post-ART for each woman, several limitations exist to our work. First, implant insertion dates are not known for the women limiting our inter-subject comparisons, although the comparisons pre- and post- ART initiation are valid within each woman. Second, the number of samples and the total duration of time each woman contributes varies both within and across ART groups; our adjusting for days from ART initiation and use of a correlation structure that accounts for spatial autocorrelation should help mitigate any marked differences between the groups. Inter- and intra-subject variability can be better characterized by future studies with consistent sampling. Third, sporadic plasma collections, irrespective of menstrual cycles, pose marked challenges in interpretation of endogenous progesterone concentrations; however, more resource-intensive approaches to detecting ovulation, such as frequent ultrasounds, are less feasible, particularly in Sub-Saharan Africa. Finally, the multiple testing in our PG analyses may increase the likelihood of Type I error, and while we made a Bonferroni adjustment, a significance threshold of p-value of 0.003 may be too conservative.
CONCLUSION
Our study demonstrates that among women already on contraceptive implants, concomitant use of efavirenz significantly reduces levonorgestrel or etonogestrel concentrations and could increase the risk of ovulation and subsequent pregnancy. We also discovered a greater array of allelic variants in hepatic enzymes and membrane transporters that modify the association between ART use and serum progestin concentrations. With multiple PK and clinical studies consistently demonstrating reduced hormone concentrations or effectiveness of implants when used concomitantly with efavirenz, national and international authorities could recommend offering alternative but superior ART regimens, such as dolutegravir-containing ART, to women living with HIV already using or desiring contraceptive implants.
ACKNOWLEDGEMENTS
We thank all the women and the clinical trial site teams who participated in the Partners PrEP Study.
Partners PrEP Study Team
University of Washington Coordinating Center and Central Laboratories, Seattle, USA:
Connie Celum (principal investigator, protocol co-chair), Jared M. Baeten (medical director, protocol co-chair), Deborah Donnell (protocol statistician), Robert W. Coombs, Jairam R. Lingappa, M. Juliana McElrath.
Study sites and site principal investigators: Eldoret, Kenya (Moi University, Indiana University): Kenneth H. Fife, Edwin Were; Kabwohe, Uganda (Kabwohe Clinical Research Center): Elioda Tumwesigye; Jinja, Uganda (Makerere University, University of Washington): Patrick Ndase, Elly Katabira; Kampala, Uganda (Makerere University): Elly Katabira, Allan Ronald; Kisumu, Kenya (Kenya Medical Research Institute, University of California San Francisco): Elizabeth Bukusi, Craig R. Cohen; Mbale, Uganda (The AIDS Support Organization, CDC-Uganda): Jonathan Wangisi, James D. Campbell, Jordan W. Tappero; Nairobi, Kenya (University of Nairobi, University of Washington): James Kiarie, Carey Farquhar, Grace John-Stewart; Thika, Kenya (University of Nairobi, Kenya Medical Research Institute, University of Washington, Jomo Kenyatta University): Nelly R. Mugo, Kenneth Ngure; Tororo, Uganda (CDC-Uganda, The AIDS Support Organization): James D. Campbell, Jordan W. Tappero, Jonathan Wangisi.
Data management was provided by DF/Net Research, Inc. (Seattle, USA) and site laboratory oversight was provided by Contract Lab Services (University of the Witwatersrand, Johannesburg, South Africa).
Sources of funding:
US Centers for Disease Control and Prevention (CDC, U48 DP 005013 SIP 14–023), the US National Institutes of Health (NIH, NICHD R21HD074439 and NIAID P30-AI-027757 and UM1-AI-106701, OD011092), and the Bill & Melinda Gates Foundation (OPP47674) supported the study. Dr. Patel was supported by the NIH National Institute of Allergy and Infectious Diseases (K23AI120855). Dr. Scarsi was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD085887). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the funding agencies or the United States Government.
Footnotes
Members of the study team listed after the Acknowledgements
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References:
- 1.UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2013 In. Geneva, Switzerland: UNAIDS; 2013. [Google Scholar]
- 2.Desgrees-du-Lou A, Msellati P, Viho I, Yao A, Yapi D, Kassi P, et al. Contraceptive use, protected sexual intercourse and incidence of pregnancies among African HIV-infected women. DITRAME ANRS 049 Project, Abidjan 1995–2000. International Journal of STD & AIDS 2002; 13(7):462–468. [DOI] [PubMed] [Google Scholar]
- 3.Halperin DT, Stover J, Reynolds HW. Benefits and costs of expanding access to family planning programs to women living with HIV. AIDS 2009; 23(Supp 1):S123–S130. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. Global plan towards the elimination of new HIV infections In. Geneva, Switzerland; 2011. [Google Scholar]
- 5.Khu NH, Vwalika B, Karita E, Kilembe W, Bayingana RA, Sitrin D, et al. Fertility goal-based counseling increases contraceptive implant and IUD use in HIV-discordant couples in Rwanda and Zambia. Contraception 2013; 88(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidance In. Geneva: World Health Organization (WHO); July 2018. [Google Scholar]
- 7.WHO. Statement on dolutegravir: Potential safety issue affecting women living with HIV using dolutegravir at the time of conception In. Geneva, Switzerland: World Health Organization (WHO); May 18, 2018. [Google Scholar]
- 8.Patel RC, Onono M, Gandhi M, Blat C, Hagey J, Shade SB, et al. Pregnancy rates in HIV-positive women using contraceptives and efavirenz-based or nevirapine-based antiretroviral therapy in Kenya: a retrospective cohort study. The Lancet HIV 2015; 2(11):e474–e482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vieira CS BM, de Souza RM, Brito MB, Rocha Prandini TR, Amaral E, Bahmondes L, Duarte G, Quintana SM, Scaranari C, Ferriani RA. Effect of antiretroviral therapy including lopinavir/ritonavir or efavirenz on etonogestrel-releasing implant pharmacokinetics in HIV-positive women. J Acquir Immune Defic Syndr 2014; 66(4):378–385. [DOI] [PubMed] [Google Scholar]
- 10.Scarsi KK, Darin KM, Nakalema S, Back DJ, Byakika-Kibwika P, Else LJ, et al. Unintended Pregnancies Observed With Combined Use of the Levonorgestrel Contraceptive Implant and Efavirenz-based Antiretroviral Therapy: A Three-Arm Pharmacokinetic Evaluation Over 48 Weeks. Clin Infect Dis 2016; 62(6):675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chappell CA, Lamorde M, Nakalema S, Chen B, Mackline H, Riddler S, et al. Efavirenz decreases etonogestrel exposure: a pharmacokinetic evaluation of implantable contraception with antiretroviral therapy. AIDS 2017. [DOI] [PMC free article] [PubMed]
- 12.Kreitchmann R, Stek A, Best B, Capparelli E, Wang J, Shapiro DE, et al. Interaction between etonogestrel-releaseing implant and three antiretroviral regimens. In: Conference on Retroviruses and Opportunistic Infections (CROI). Seatte, WA; February 13–17, 2017. [Google Scholar]
- 13.Coukell AJ, Balfour JA. Levonorgestrel subdermal implants. A review of contraceptive efficacy and acceptability. Drugs 1998; 55(6):861–887. [DOI] [PubMed] [Google Scholar]
- 14.Sivin I, Wan L, Ranta S, Alvarez F, Brache V, Mishell DR Jr., et al. Levonorgestrel concentrations during 7 years of continuous use of Jadelle contraceptive implants. Contraception 2001; 64(1):43–49. [DOI] [PubMed] [Google Scholar]
- 15.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mujugira A, Baeten JM, Donnell D, Ndase P, Mugo NR, Barnes L, et al. Characteristics of HIV-1 serodiscordant couples enrolled in a clinical trial of antiretroviral pre-exposure prophylaxis for HIV-1 prevention. PLoS One 2011; 6(10):e25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pyra M, Heffron R, Mugo NR, Nanda K, Thomas KK, Celum C, et al. Effectiveness of hormonal contraception in HIV-infected women using antiretroviral therapy. AIDS 2015; 29(17):2353–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wenzl R, van Beek A, Schnabel P, Huber J. Pharmacokinetics of etonogestrel released from the contraceptive implant Implanon. Contraception 1998; 58(5):283–288. [DOI] [PubMed] [Google Scholar]
- 19.Neary M, Lamorde M, Olagunju A, Darin KM, Merry C, Byakika-Kibwika P, et al. The Effect of Gene Variants on Levonorgestrel Pharmacokinetics when Combined with Antiretroviral Therapy containing Efavirenz or Nevirapine. Clin Pharmacol Ther 2017. [DOI] [PMC free article] [PubMed]
- 20.Moreno I, Quinones L, Catalan J, Miranda C, Roco A, Sasso J, et al. [Influence of CYP3A4/5 polymorphisms in the pharmacokinetics of levonorgestrel: a pilot study]. Biomedica 2012; 32(4):570–577. [DOI] [PubMed] [Google Scholar]
- 21.Blue SW, Winchell AJ, Kaucher AV, Lieberman RA, Gilles CT, Pyra MN, et al. Simultaneous quantitation of multiple contraceptive hormones in human serum by LC-MS/MS. Contraception 2018; 97(4):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Practice Committee of the American Society for Reproductive M. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril 2015; 103(6):e44–50. [DOI] [PubMed] [Google Scholar]
- 23.Trussell J Contraceptive failure in the United States. Contraception 2011; 83(5):397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song IH, Borland J, Chen S, Wajima T, Peppercorn AF, Piscitelli SC. Dolutegravir Has No Effect on the Pharmacokinetics of Oral Contraceptives With Norgestimate and Ethinyl Estradiol. Ann Pharmacother 2015; 49(7):784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep 2016; 65(3):1–103. [DOI] [PubMed] [Google Scholar]
- 26.Stormer E, von Moltke LL, Perloff MD, Greenblatt DJ. Differential modulation of P-glycoprotein expression and activity by non-nucleoside HIV-1 reverse transcriptase inhibitors in cell culture. Pharm Res 2002; 19(7):1038–1045. [DOI] [PubMed] [Google Scholar]
- 27.Fellay J, Marzolini C, Meaden ER, Back DJ, Buclin T, Chave JP, et al. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet 2002; 359(9300):30–36. [DOI] [PubMed] [Google Scholar]
- 28.Elens L, Vandercam B, Yombi JC, Lison D, Wallemacq P, Haufroid V. Influence of host genetic factors on efavirenz plasma and intracellular pharmacokinetics in HIV-1-infected patients. Pharmacogenomics 2010; 11(9):1223–1234. [DOI] [PubMed] [Google Scholar]
- 29.Adolescents. PoAGfAa. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV In. Edited by Services DoHaH; May 30, 2018. [Google Scholar]
- 30.Society EAC. Guidelines for treatment of HIV-positive adults In; October 2017.