Abstract
Heterotetrameric clathrin Adaptor Protein complexes (APs) orchestrate the formation of coated vesicles for transport among organelles of the cell periphery. AP1 binds membranes enriched for PI4P, such as the TGN, while AP2 associates with PIP2 of the plasma membrane. At their respective membranes, AP1 and AP2 bind the cytoplasmic tails of transmembrane protein cargo and clathrin triskelions, thereby coupling cargo recruitment to coat polymerization. Structural, biochemical, and genetic studies have revealed that APs undergo conformational rearrangements and reversible phosphorylation to cycle between different activity states. While membrane, cargo and clathrin have been demonstrated to promote AP activation, growing evidence supports that membrane-associated proteins such as Arf1 and FCHo also stimulate this transition. APs may be returned to the inactive state via a regulated process involving phosphorylation and a protein called NECAP. Finally, because antiviral mechanisms often rely on appropriate trafficking of membrane proteins, viruses have evolved novel strategies to evade host defenses by influencing the conformation of APs. This review will cover recent advances in our understanding of the molecular inputs that stimulate AP1 and AP2 to adopt structurally and functionally distinct configurations.
Keywords: Clathrin adaptors, AP1, AP2, endocytosis, Arf1, muniscins, FCHo, tetherin, NECAP
Graphical Abstract

Introduction
The inside of a eukaryotic cell is organized into membrane-bound organelles to allow for compartmentalization of cellular processes. To shuttle transmembrane proteins (cargo) from one compartment to another, a region of the donor membrane containing cargo is encased by a ‘coat’ of regulatory and scaffolding proteins that facilitate cargo packaging and vesicle formation. Once the coated vesicle forms, it is released into the cytosol where it is uncoated and trafficked to an acceptor membrane for fusion and cargo delivery. Most transport vesicles in the periphery of the cell, including those originating from the plasma membrane, utilize a coat containing the three-legged triskelion scaffold protein called clathrin (Figure 1A).1
Figure 1. Structure and function of heterotetrameric clathrin adaptor proteins.
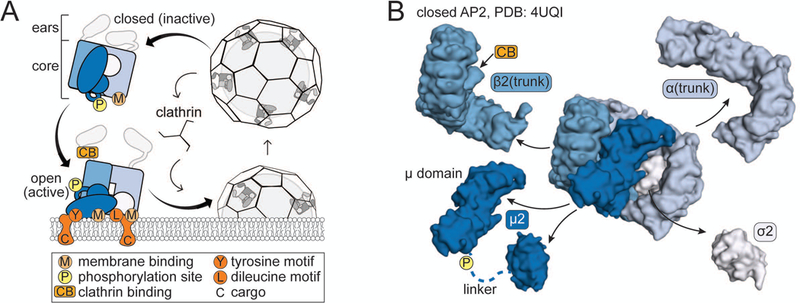
A. Schematic of the Adaptor Protein (AP) cycle. B. Structure of the AP2 core, split apart to reveal subunit composition of APs. Same key as in A. The μ2 subunit comprises two subdomains connected by a flexible linker (dashed line), which contains the phosphorylation site. In AP1, γ is the equivalent of AP2 α. Note: the β2(trunk) depicted here also includes the linker sequence containing the clathrin-binding box (29).
Clathrin does not appear to bind membrane nor cargo. Instead, early work unveiled that an ‘assembly factor’ present in the coat was required to couple them together.2,3 Later, this assembly factor was purified as a heterogeneous mix that was separable into two hydroxylapatite-binding fractions, HA-I and HA-II4 and later named the Assembly Polypeptides or Adaptor Proteins (APs), AP1 and AP2.5 Subsequent studies revealed that AP1 and AP2 mediate trafficking at different membranes6–8. Clathrin-mediated trafficking from the plasma membrane to endosomes depends on AP2, while trafficking from the Golgi to endosomes9,10 and from the endosomal system to other destinations11,12 relies on AP1. This review will highlight the recent structural advances that have yielded a better understanding of how APs are pivotal regulators of clathrin-coated vesicle trafficking.
Molecular Composition of APs
Biochemical analysis of APs purified from bovine brains determined they are composed of two ~100 kD large adaptin subunits (β1 and γ for AP1, β2 and α for AP2), one ~50 kD medium subunit (μ, specifically μ1 for AP1 and μ2 for AP2) which has two folded domains connected by a linker, and one ~18 kD small subunit (σ, specifically σ1 for AP1 and σ2 for AP2).5,13 Freeze-etch electron microscopy and proteolysis experiments revealed that APs have a central ‘core,’ which is now known to be composed of μ, σ and the helical solenoid (trunk) domains of two large adaptins that are connected via flexible, protease-sensitive linkers to folded appendage (ear) domains (Figure 1).14–16 AP1 and AP2 each have patches of positively charged amino acids believed to target the complex to the appropriate membrane by binding to either phosphatidylinositol 4-phosphate (PI4P) in the case of AP117 or phosphatidylinositol 4,5-bisphosphate (PIP2) for AP2.18 AP1 and AP2 also have binding pockets for specific amino acid sequences (motifs) on the cytoplasmic tails of cargo. On the μ subunit is a binding site for tyrosine motifs (YxxΦ) where Y is a tryrosine, x is any amino acid, and Φ is a bulky, hydrophobic amino acid.19,20 On the σ subunit is a binding site for dileucine motifs (D/ExxxLL/I) where D is aspartate, E is glutamate, L is leucine, and I is isoleucine.21 The ears are thought to act as binding platforms for numerous clathrin accessory proteins.22,23 Clathrin itself binds AP1 and AP2 via a clathrin-binding box sequence in the linker of the β subunit (Figure 1A).24 In this manner, APs lie at the heart of vesicle formation and serve as gatekeepers for clathrin-mediated trafficking.
Conformational Rearrangements
It is now appreciated that AP complexes undergo conformational rearrangements and that these different states may enable spatiotemporal control of AP activity. The initial evidence of distinct conformations was a difference in the protease-sensitivity of μ in soluble APs versus in coat-associated APs.14,25,26 High-resolution structures of AP cores have unveiled a host of molecular snapshots and enabled us to attribute functional significance to these different conformational states. The first crystal structure of the AP2 core revealed what is now accepted to be an inactive, or ‘closed,’ conformation (Figure 2, closed).27 Only a single membrane-binding pocket, that of α28, is surface exposed (Figure 1A), and the nearby dileucine motif-binding site on σ2 is plugged by the N-terminus of β2 (Figure 3A). The other membrane-binding pocket, that of μ2, is situated on a different face of the complex and partially occluded, as is the nearby tyrosine motif-binding pocket.27 A more recent structure revealed that the clathrin-binding box on β2 is also pressed against the closed AP2 core, where it is unlikely to bind clathrin (Figure 3B, C).29 On the basis of these observations it was predicted that AP2 must undergo a large conformational change in order to fully engage membrane, cargo and clathrin.27 The crystal structure of the AP1 core is strikingly similar to that of AP2.30 Indeed, the protease site on μ that is sensitive to digestion when APs are clathrin-associated appears to be inaccessible in this structure, further suggesting a similar requirement for conformational activation.30 It is possible the closed form of APs represents a soluble, cytosolic state that serves to prevent premature association with cargo and clathrin.
Figure 2. Conformational rearrangement of adaptor protein complexes.

A. Color key for AP subunits. B. Four different conformations of APs (PDB ID above) oriented such that the membrane would be horizontal and below the complex. C and D. The same complexes as in (B), rotated as indicated at left.
Figure 3. Functional consequence of adaptor protein reorganization.
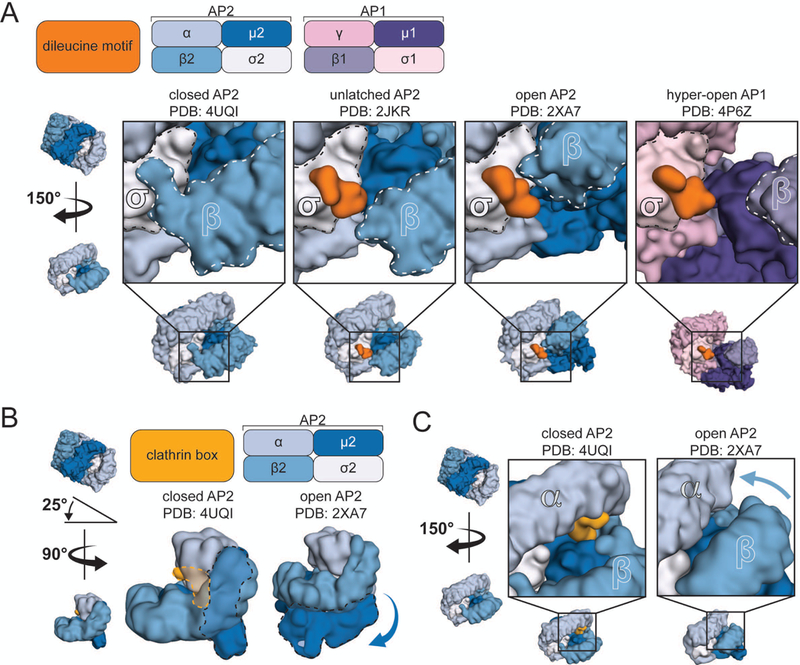
A. Close-up view of the dileucine motif binding pocket in four different conformations (indicated above). B and C. Interaction of the clathrin-binding box with the closed AP2 core. Positions of the μ2 subunit and clathrin-binding box within two configurations of the core (B, dashed lines). In the open conformation, the μ2 subunit has pivoted around the core (B, arrow) and the β2 solenoid has approached that of α (C, arrow), potentially excluding the clathrin-binding box.
High resolution crystal structures of the AP2 core bound to synthetic cargo peptides were solved, revealing that high concentrations of cargo are sufficient to induce a conformational change in the adaptor complex in vitro.31,32 Addition of peptide representing a dileucine motif resulted in a crystal structure in which the binding pocket on σ2 is now occupied by cargo, thereby displacing the N-terminus of β2 (Figure 3A) and inducing a 20˚ flexion of the helical solenoids of α (Figure 2C, unlatched). However, this ‘unlatched’ AP2 is thought to represent only a partially active form of the complex because the cargo- and membrane-binding pockets of μ2 remain blocked.31
A crystal structure of what is believed to be the active, open form of AP2 was ultimately obtained via co-crystallization with a tyrosine motif peptide (Figure 2, open). The binding pocket on μ2 is now occupied by the peptide, while the σ2 site is engaged by a cryptic dileucine motif of a myc tag on an adjacent AP2 core (Figure 3A).32 In this conformation, the C-terminal domain of μ2 has become dislodged and rotated out from the center of the complex, exposing the protease-sensitive surface loop of μ2. Importantly, all cargo and membrane binding sites are now co-planar (Figure 1A), and although the β2 linker was not included in these expression constructs, the clathrin-binding box site is now hidden (Figure 3B, C). These features indicate that this form of AP2 is competent to engage both membrane and cargo, and potentially clathrin as well.32 Later, a crystal structure of the AP1 core in this same active conformation was obtained (see AP Activation below) confirming that both APs can adopt active conformations with similar attributes (Figure 4A).33 Not only do these structures validate previous predictions, they offer an elegant example of how molecular rearrangement is intimately coupled to biological function.
Figure 4. Activation of adaptor protein complexes.

A. Two views of the Arf1-activated AP1 dimer. Two Arf1 proteins stabilize two hyper-open AP1 cores. B. Model for AP2 activation by the muniscin protein FCHo. The dimerized F-BAR domain is modeled onto a membrane and the μHD is bound to DPF motifs from Eps15. These terminal domains are connected by a central linker (dashed line). Hypothetical contact between the APA and AP2 is depicted.
There appears to be more than one active conformation of APs. Through studying viral hijacking of APs, a unique ‘hyper-open’ form of AP1 has been visualized (Figure 2, hyper-open).34 Because APs regulate trafficking, they are co-opted by viruses in order to subvert hosts’ antiviral mechanisms. The HIV membrane protein, Viral protein U (Vpu), diverts trafficking of the host membrane protein tetherin (also called BST2) that normally binds newly-budded viral progeny to restrict their release from the cell surface.35 The cytosolic domain of Vpu appears to stabilize the binding of tetherin’s cytosolic domain to AP1. Co-crystallization of a fusion construct of these domains with AP1 revealed that the Vpu fragment bound AP1 using its diluecine motif, ExxxLV, (Figure 3A) while tetherin engaged AP1 via an unusual double tyrosine motif, YxY.34 Importantly, the complex appears to have rotated open further than in previous structures, such that a new major contact has formed between μ1 and γ (the AP2 α equivalent; Figure 2, hyper-open).33,34 Further investigation will be necessary to understand what dictates the open forms of APs and whether the complexes can adopt additional conformational states (see Maybe It’s Not So Simple section below).
AP Activation
How are APs converted to the active conformation at the appropriate time and place? Even though the presence of cargo peptides was sufficient to generate unlatched,31 open32 and hyper-open34 structures of APs, it is not clear how cargo would initially interact with binding sites on APs that are inaccessible in the closed structures.27,30 Likewise, there is evidence that clathrin coats will stimulate AP2 to bind synthetic cargo peptides,26 but the clathrin-binding site appears to be inaccessible in closed AP2 as well (Figure 3B, C).29 Instead of inducing activation of adaptors, cargo and clathrin might secondarily stabilize the active form of APs to promote the growth of nascent pits. This step might represent an endocytic checkpoint to abort nonproductive pits.36–38
Membranes appear to stimulate conformational rearrangement of APs. The initial localization of soluble APs to the appropriate membrane is likely mediated by the phospholipid binding site that is surface exposed in the closed structure (Figure 1A).30,39,40 For AP2, PIP2-containing liposomes enhance cargo binding,40 as does poly-anionic heparin, presumably by mimicking membranes.32 Inclusion of cargo in PIP2 liposomes will cooperatively stimulate AP2 to bind clathrin.29 These data suggest that activation of AP2 follows a hierarchical progression with membrane phospholipids at the top, followed by cargo and clathrin39 but it remains unclear whether these inputs are sufficient to fully commit AP2 to endocytic pit formation.
By contrast, the small GTPase, ADP ribosylation factor 1 (Arf1), fulfills many requirements of a bona fide allosteric activator of AP1. Early studies indicated that membrane-associated, GTP-bound Arf1 is required for AP1 localization and function at the Golgi,41–43 potentially synergizing membrane and cargo binding.44 More recently, co-crystallization of the AP1 core with a soluble, GTP-locked form of Arf1 revealed that Arf1 is capable of promoting the active form of AP1 in the absence of both membrane and cargo (Figure 4A).33 In this crystal structure, two AP1 cores are connected by two Arf1 proteins forming a dimeric complex. Each Arf1 simultaneously engages each AP1 via two interfaces: the central region of one AP1’s γ subunit, and the N-terminus the other AP1’s β1 subunit. This second interface buries the switch I and II regions of Arf1 that are known to engage effectors upon GTP binding.45 Modeling of the dimeric AP1-Arf1 structure on a virtual membrane suggests that all of the membrane and cargo binding sites could simultaneously engage a single surface (Figure 4A). This structure further suggests that initiation of clathrin-coated structures on intracellular membranes may require two membrane-associated, GTP-bound Arf1 proteins that cooperate to convert two AP1 complexes to the active state.33 Intriguingly, this model is also consistent with single molecule imaging data suggesting that endocytic pits initiate when two AP2 complexes become stabilized on the membrane.38 Future experiments are needed to determine whether dimerization of clathrin adaptors is a common initiation mechanism.
Recent work has suggested that members of a protein family called ‘muniscins’46 are allosteric activators of AP2. This family includes the Fer/CIP4 Homology domain only proteins, FCHo1 and FCHo2 47 hereafter collectively referred to as FCHo. These membrane-associated proteins appear at endocytic sites on the plasma membrane prior to,48 or coincident with,49 AP2. Depletion of FCHo in tissue culture cells reduces AP2 nucleation events48 and disrupts clathrin structures on the plasma membrane.50,51 Careful analysis reveals that FCHo sustains, rather than initiates, endocytic pit formation: knockdown of FCHo increases the number of short-lived, abortive, endocytic structures without affecting the total number of initiation events.38 These data suggest that FCHo causes AP2 to establish a higher affinity interaction with the membrane.
Mechanistic insight into FCHo activity was revealed by a genetic screen in C. elegans. Nematodes lacking their sole muniscin protein, FCHO-1, phenocopy loss of AP2 subunits, indicating that AP2 is inactive in fcho-1 mutants. A suppressor screen for mutations that bypass the requirement for FCHO-1 isolated dominant missense mutations in AP2 subunits.52 When mapped onto the crystal structures of AP227,31,32 these mutations appear to specifically disrupt the inactive conformation of AP2, thereby promoting the active form of the complex.52 This screen demonstrates that the closed structure of AP227 exists in cells and is biologically equivalent to inactive AP2. Moreover, FCHo may be what normally promotes AP2’s transition to the active state, perhaps via allostery.
Allosteric modulation of AP2 by FCHo would require that a domain of FCHo directly stimulates AP2. The terminal domains of muniscins have well characterized interaction profiles, and neither are known to bind AP2. FCHo has an N-terminal membrane-binding domain typified by the crescent-shaped (meniscus) F-BAR domain (Figure 4B).53 The F-BAR domain binds to and tubulates PIP2-containing membranes in vitro54 but is not conserved in all muniscin proteins.46 For example, SH3-containing GRB2-like protein 3-Interacting Protein (SGIP) has an alternative membrane phospholipid-binding domain at its N-terminus.55 Thus, the N-terminus of FCHo likely mediates localization to the plasma membrane, but this domain does not appear to be required for AP2 activation in vivo.50,52
The C-terminal domain of FCHo is structurally46 and evolutionarily56,57 related to the C-terminal domain of the AP μ subunit (the μ domain) (Figure 4B). This μ Homology Domain (μHD) of FCHo binds endocytic pioneer proteins such as Epidermal growth factor Pathway Substrate 15 (Eps15) or Eps15 Related (EpsR), and intersectin.46,48 This three-protein ‘FEI complex’ is needed for efficient internalization of some cargos and recruits the ESCRT-0 complex to the plasma membrane to pre-engage ubiquitinated cargo destined for the lysosome.58 However, it does not interact directly with AP2.48
Structure-function analyses by independent groups determined that the central linker of FCHo contains a domain conserved across muniscins46 that appears to activate AP2. This AP2 Activator (APA) domain,52 both immunoprecipitates AP2 from cell lysates and directly binds the recombinant AP2 core in vitro.50,52,59 Expression of the APA in vivo restores AP2 activity both in tissue culture cells depleted of FCHo proteins50 and in fcho-1 mutant worms.52 Thus, AP2 activation correlates with APA binding, supporting the model that FCHo is an allosteric activator of AP2.
What positions the APA at sites of endocytosis? The μHD of FCHo, which nucleates the FEI complex with Eps15,48 is required for efficient endocytosis of cargo58 and for proper localization of FCHo to clathrin-coated pits.51 Eps15, in turn, binds to the AP2 ears.22,60,61 Thus, these interactions may position the APA close to AP2 which would increase the efficiency of its activation (Figure 4B).62,63 To understand how Eps15 engages binding partners using DPF motifs, structures of μHDs from FCHo1 and SGIP in complex with fragments of Eps15 were solved.62,63 The DPF motifs of Eps15 nestle into a hydrophobic groove on the μHD that complements the spacing of phenylalanines in the repeated motif. Mislocalization of the μHD to the Golgi in FCHo knockout cells recruits Eps15 away from the plasma membrane and reduces cargo uptake.62 These data highlight the importance of the FEI complex for efficient endocytosis.
While there are strong data suggesting that the APA domain may be important for AP2 activity, additional work is needed to determine whether the APA is a bona fide allosteric activator. For example, is the APA alone sufficient to drive AP2 into the active conformation or does it cooperate with membrane, cargo, and clathrin, or perhaps with other endocytic proteins? Is dimerization of AP2 important for activation, as observed for AP1?33 Indeed, FCHo appears to dimerize53 and initiation of coat formation at the plasma membrane is coincident with the arrival of two AP2 complexes (see model Figure 4B).38 These questions may be answered in future structural and biochemical studies.
AP μ Phosphorylation
APs are known to be phosphorylated at multiple sites64,65 and most studies have focused on phosphorylation of a conserved threonine (T156 in AP2, or T154 in AP1) in the linker connecting the N-terminal and C-terminal domains of μ (Figure 1B).66 The most likely kinases are the AP2-Associated Kinase (AAK1)67 and the cyclin-G-Associated Kinase (GAK).68–70 AAK1 co-purifies with AP2, binds the α ear, and directly phosphorylates μ.67 GAK, which was originally isolated as a cyclin G-binding protein,68 is the non-neuronal homolog of auxilin, a cofactor that stimulates the heat shock protein Hsc70 to uncoat clathrin from vesicles.71,72 Additionally, GAK co-purifies with clathrin-coated vesicles,69 binds to the AP2 α and AP1 γ ears, and phosphorylates the μ subunit of both complexes.70 While the kinases and targets are clear, the physiological consequence of μ phosphorylation has been difficult to determine.
Some data suggest that phosphorylation exerts a stimulatory effect on APs. Phosphorylation of the μ subunits strengthens AP association with membranes and cargo.25,73,74 In tissue culture cells, expression of phosphorylation-defective AP2 (T156A) or treatment with kinase inhibitors both result in cargo internalization defects.39,75 These data suggest that phosphorylation promotes the active form of APs. Consistent with this model, the μ linker is unstructured and potentially accessible to kinases in the closed conformation (Figure 5A).27,30
Figure 5. Negative regulation of adaptor proteins.
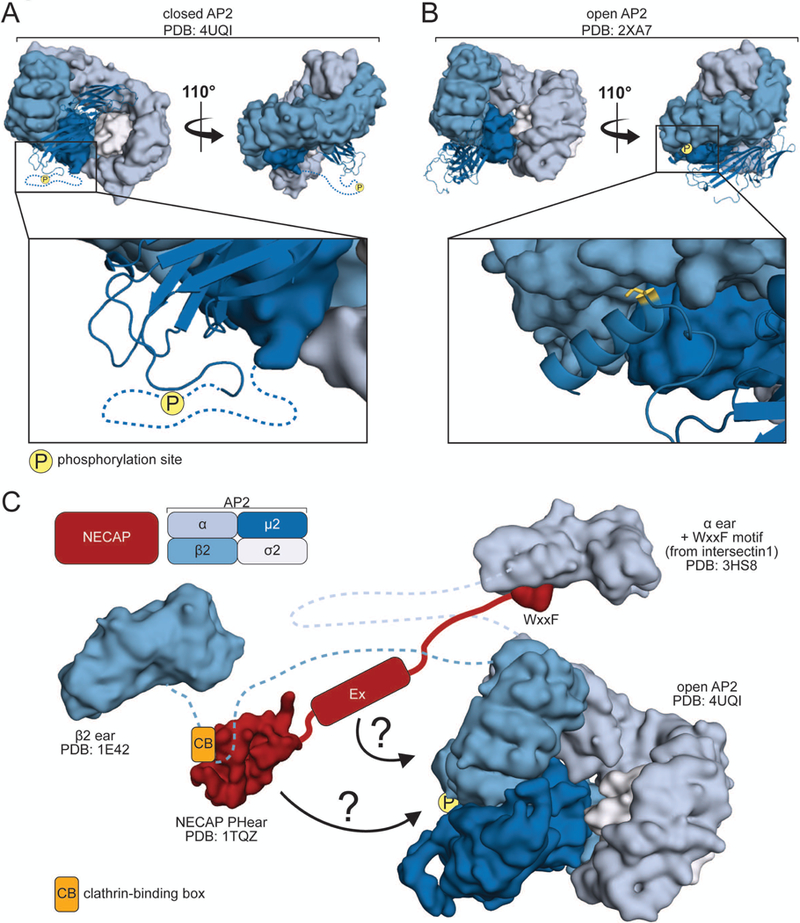
A. View of closed AP2 depicting the hypothetical location of T156 in the μ2 linker, the site of phosphorylation. B. View of open AP2 showing the location of μ2 T156 (yellow), packed against the β2 subunit. C. Model for mechanism of NECAP action. NECAP binds the clathrin-binding box of the β2 linker via the PHear domain, and the ear of α via a WxxF motif (Note: the motif in the structure is from intersectin 1, not NECAP). Additionally, NECAP binds the open, phosphorylated AP2 core. This interaction may be mediated by the PHear and Ex domains (arrows).
There is also data to suggest μ phosphorylation might stabilize, rather than induce, the open conformation of APs. The μ2 linker is hypo-phosphorylated in worms with inactive AP2 (fcho-1 mutants) and becomes hyper-phosphorylated in worms with mutations in AP2 that promote the active conformation.52 Thus, the substrate for the kinase appears to be open AP2, not closed AP2. Indeed, clathrin precedes the arrival of GAK at endocytic sites,49 AP kinases are incorporated in vesicle coats76 and clathrin stimulates AAK1 activity.77,78 This further suggests phosphorylation may occur during, or even after, coat formation on AP complexes that have already adopted the open conformation. One potential problem with this model is that in all open structures of APs, a region of the μ linker that includes the threonine forms a helical structure that is inserted into a trough formed by β and μ (Figure 5B).32,33 It is not entirely clear if kinases could access the threonine in this configuration.
Curiously, some data even hints that phosphorylation may inhibit AP2 activity. When AAK1 was first identified, initial analysis showed that recombinant AAK1 exerted an inhibitory effect on an in vitro assay of endocytosis.67 Additionally, overexpression of AAK1 also inhibited transferrin uptake in tissue culture.79 Currently, the physiological significance of μ phosphorylation remains unsettled and is worthy of further investigation (see AP Inactivation section below).
AP Inactivation
While much attention has focused on mechanisms to activate APs, it is unclear whether there are also mechanisms to close (inactivate) APs even though this transition is likely to occur in some cellular contexts. Inactivation of APs may prevent aberrant vesicle formation when adaptors do not fully engage cargo.36,37 Inactivation may also facilitate removal of vesicle coats to expose machinery required for fusion with target membranes.7 It is unclear whether these events occur stochastically, or whether there are negative regulators that shuttle APs back to the closed state.
Potential candidates for negative regulators of APs are a family of proteins called adaptiN Ear-binding Coat-Associated Proteins (NECAPs). NECAPs were originally identified as components of clathrin coats and shown to bind the adaptin ears of AP2 α and AP1 γ.80 Vertebrates express two isoforms, brain-specific NECAP1 and ubiquitous NECAP2. Initial characterization revealed that NECAP1 may act early in endocytosis to regulate clathrin binding and accessory protein recruitment to AP2,81 whereas NECAP2 appears to regulate an AP1-dependent recycling pathway from endosomes.82 Outside of vertebrates, most multicellular eukaryotes express a single NECAP.83 In C. elegans, loss of NECAP bypasses the requirement for FCHO-1.84 Similar to the mutations in AP2 that promote the open conformation, loss of NECAP causes AP2 to accumulate in a protease-sensitive, phosphorylated active state. Heterologous expression of NECAPs (both vertebrate isoforms and one fungal) complement loss of worm NECAP and restore the inactive state of AP2.84 This genetic evidence implies that NECAPs can act as ‘brakes’ to limit AP2 activity.
How might NECAPs negatively regulate AP2? NECAPs are believed to bind the adaptin ears via a WxxF motif at the end of the NECAP disordered C-terminal tail (Figure 5C).80 However, a fungal NECAP lacking this motif can inactivate AP2 when expressed in worms.84 The well-conserved Pleckstrin Homology-like (PHear) domain (Figure 5C)85 at the N-terminus can interact with the clathrin-binding box on the β linker, which could limit the binding of clathrin to active APs.81 In the absence of this inhibitory effect, APs might more readily progress to coat formation. This is consistent with the observations that in the absence of NECAP, endocytic structures on the membrane become enlarged81 and AP2 accumulates in an open, phosphorylated state.84 Additionally, it was discovered that NECAPs directly bind the AP2 core, specifically when the complex is in a phosphorylated, open conformation.84 Taken together, these data suggest that active AP2 represents the substrate for NECAP binding, and that NECAPs somehow shuttle these complexes to a closed, dephosphorylated state. This model is consistent with the observations that NECAPs are recruited to the plasma membrane at the same time as clathrin,49 presumably after AP2 is already open, and that dephosphorylation of AP1 promotes vesicle uncoating.25
Many questions remain regarding NECAPs’ mechanism of action. Do NECAPs directly convert AP2 to the closed state or do they cooperate with other factors? What is the nature of the interface between NECAP and AP2 that mediates adaptor inactivation? Because missense mutations in the PHear domain specifically disrupt NECAP binding to phosphorylated AP2, the PHear domain may either bind to the site of phosphorylation or to a conformation induced by phosphorylation (see model Figure 5C). Additionally, PHear mutants retain binding to open, non-phosphorylated AP2, suggesting that a region outside of the PHear domain specifically interacts with open complexes (see model Figure 5C). Indeed, the domain of conservation that extends beyond the PHear domain, known as the Extended (Ex) domain, appears to bind AP2 at an undetermined location.81 NECAPs are also thought to bind and regulate AP180,82 and the AP1 μ1 linker is known to be phosphorylated.25 Perhaps NECAPs serve as negative regulators of AP1 as well. Future biochemical and structural studies are required to reveal the mechanisms by which NECAPs inactivate APs.
Maybe It’s Not So Simple
While the structural data presented thus far generate a compelling and informative model for AP activation and inactivation, there are many proteins coordinating clathrin-mediated trafficking,1,86 making it easy to imagine this model may be over-simplified.87 Numerous interactions between APs and the multitude of clathrin-coat accessory proteins likely remain to be characterized, and the AP appendage domains, which are thought to interact with many of these proteins,22,23,88,89 have been absent from all AP core structures thus far. In addition, APs may adopt conformations that have yet to be visualized. Cryo-EM tomographic structures of the coatomer Coat Protein complex I (COPI) reveal that the AP-like subcomplex within these coats adopts an extended conformation that appears to be even more open than the ‘hyper-open’ AP1 structure.90–92 There is also evidence that APs may be capable of functioning as two separate hemicomplexes (β/μ, and either α/σ2 or γ/σ1), as each half retains some level of activity in the absence of the other.21,93 Are there instances when APs split and the hemicomplexes act independently from each other?
The recent renaissance in EM techniques has enabled visualization of multi-molecular trafficking complexes. Cryo-EM was recently used to visualize a novel trimeric complex composed of AP1, Arf1 and tetherin fused to the HIV ‘negative factor’ (Nef), another viral hijacker of APs.94,95 Each monomer of the trimer consists of one AP1, two Arf1s, and one tetherin:Nef. Additionally, AP1 has adopted the hyper-open conformation34 and Arf1 forms the interface between the monomers.94,95 Interestingly, there exist two conformations of the trimer: a ‘closed’ trimer where the membrane binding sites on the hyper-open AP1 are hidden, and an ‘open’ trimer where these binding sites are exposed.95 When the open trimer structure is iteratively docked onto the crystal structure of the AP1-Arf1 dimer,33 a hexagonal ring is formed. The dimensions of this hexagon align with the structure of the clathrin cage,96 generating the intriguing possibility that the hexagon could template clathrin lattice formation.95 Because the closed trimer cannot form this hexagon to template clathrin, there may exist mechanisms to regulate trafficking through structural changes at the level of the coat, not just at the level of the adaptor.94 The Arf1 and AP1 binding sites are conserved prior to HIV evolution, indicating the hexagonal lattice framework of Arf1 and AP1 is likely a more ancient feature of coated vesicles and might have implications beyond viral biology.95
As techniques such as cryoEM and tomography are advanced, we will no doubt be able to explore avenues that have historically been out of our reach. What do APs look like when bound simultaneously to membranes and cargo, or when incorporated into the clathrin coat? How do the appendage domains influence AP structure and regulation? The next decade of studying adaptor protein regulation is likely to be exciting as technological advances make the visualization of these more complex and multi-molecular structures possible.
Synopsis:
The adaptor complexes AP1 and AP2 are the molecular ‘linchpins’ of clathrin-mediated vesicle trafficking. They are believed to exist as inactive heterotetramers in the cytosol, but upon association with phospholipids of the appropriate membrane undergo a series of conformational rearrangements to expose binding sites for membrane, cargo and clathrin.
This review will highlight recent structural, biochemical and genetic studies that have yielded insight into cellular proteins that govern these transitions and mechanisms viruses have evolved to subvert them.
Acknowledgements:
G.M.B. and E.A.P. were supported by NIH training grant GM007273-43. G.M.B. is supported by NSF graduate research fellowship DGE-1650441. G.H. is supported by NIH grant R01 GM127548-01A1.
Footnotes
The authors declare no competing interests.
References
- 1.Mettlen M, Chen PH, Srinivasan S, Danuser G, Schmid SL. Regulation of Clathrin-Mediated Endocytosis. Annu Rev Biochem. 2018;87:871–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keen JH, Willingham MC, Pastan IH. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979;16(2):303–312. [DOI] [PubMed] [Google Scholar]
- 3.Zaremba S, Keen JH. Assembly polypeptides from coated vesicles mediate reassembly of unique clathrin coats. J Cell Biol. 1983;97(5 Pt 1):1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearse BM, Robinson MS. Purification and properties of 100-kd proteins from coated vesicles and their reconstitution with clathrin. EMBO J. 1984;3(9):1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keen JH. Clathrin assembly proteins: affinity purification and a model for coat assembly. J Cell Biol. 1987;105(5):1989–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson MS. 100-kD coated vesicle proteins: molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J Cell Biol. 1987;104(4):887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson MS, Pearse BM. Immunofluorescent localization of 100K coated vesicle proteins. J Cell Biol. 1986;102(1):48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahle S, Mann A, Eichelsbacher U, Ungewickell E. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988;7(4):919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klumperman J, Hille A, Veenendaal T, et al. Differences in the endosomal distributions of the two mannose 6-phosphate receptors. J Cell Biol. 1993;121(5):997–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4(3):202–212. [DOI] [PubMed] [Google Scholar]
- 11.Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol. 2002;4(8):605–609. [DOI] [PubMed] [Google Scholar]
- 12.Meyer C, Zizioli D, Lausmann S, et al. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19(10):2193–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virshup DM, Bennett V. Clathrin-coated vesicle assembly polypeptides: physical properties and reconstitution studies with brain membranes. J Cell Biol. 1988;106(1):39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsui W, Kirchhausen T. Stabilization of clathrin coats by the core of the clathrin-associated protein complex AP-2. Biochemistry. 1990;29(48):10791–10798. [DOI] [PubMed] [Google Scholar]
- 15.Heuser JE, Keen J. Deep-etch visualization of proteins involved in clathrin assembly. J Cell Biol. 1988;107(3):877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaremba S, Keen JH. Limited proteolytic digestion of coated vesicle assembly polypeptides abolishes reassembly activity. J Cell Biochem. 1985;28(1):47–58. [DOI] [PubMed] [Google Scholar]
- 17.Wang YJ, Wang J, Sun HQ, et al. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114(3):299–310. [DOI] [PubMed] [Google Scholar]
- 18.Gaidarov I, Keen JH. Phosphoinositide-AP-2 interactions required for targeting to plasma membrane clathrin-coated pits. J Cell Biol. 1999;146(4):755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohno H, Stewart J, Fournier MC, et al. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269(5232):1872–1875. [DOI] [PubMed] [Google Scholar]
- 20.Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282(5392):1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doray B, Lee I, Knisely J, Bu G, Kornfeld S. The gamma/sigma1 and alpha/sigma2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol Biol Cell. 2007;18(5):1887–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmid EM, Ford MG, Burtey A, et al. Role of the AP2 beta-appendage hub in recruiting partners for clathrin-coated vesicle assembly. PLoS Biol. 2006;4(9):e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Praefcke GJ, Ford MG, Schmid EM, et al. Evolving nature of the AP2 alpha-appendage hub during clathrin-coated vesicle endocytosis. EMBO J. 2004;23(22):4371–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shih W, Gallusser A, Kirchhausen T. A clathrin-binding site in the hinge of the beta 2 chain of mammalian AP-2 complexes. J Biol Chem. 1995;270(52):31083–31090. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh P, Kornfeld S. AP-1 binding to sorting signals and release from clathrin-coated vesicles is regulated by phosphorylation. J Cell Biol. 2003;160(5):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapoport I, Miyazaki M, Boll W, et al. Regulatory interactions in the recognition of endocytic sorting signals by AP-2 complexes. EMBO J. 1997;16(9):2240–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ. Molecular architecture and functional model of the endocytic AP2 complex. Cell. 2002;109(4):523–535. [DOI] [PubMed] [Google Scholar]
- 28.Gaidarov I, Chen Q, Falck JR, Reddy KK, Keen JH. A functional phosphatidylinositol 3,4,5-trisphosphate/phosphoinositide binding domain in the clathrin adaptor AP-2 alpha subunit. Implications for the endocytic pathway. J Biol Chem. 1996;271(34):20922–20929. [DOI] [PubMed] [Google Scholar]
- 29.Kelly BT, Graham SC, Liska N, et al. Clathrin adaptors. AP2 controls clathrin polymerization with a membrane-activated switch. Science. 2014;345(6195):459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heldwein EE, Macia E, Wang J, Yin HL, Kirchhausen T, Harrison SC. Crystal structure of the clathrin adaptor protein 1 core. Proc Natl Acad Sci U S A. 2004;101(39):14108–14113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly BT, McCoy AJ, Spate K, et al. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456(7224):976–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson LP, Kelly BT, McCoy AJ, et al. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell. 2010;141(7):1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren X, Farias GG, Canagarajah BJ, Bonifacino JS, Hurley JH. Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1. Cell. 2013;152(4):755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia X, Weber E, Tokarev A, et al. Structural basis of HIV-1 Vpu-mediated BST2 antagonism via hijacking of the clathrin adaptor protein complex 1. Elife. 2014;3:e02362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425–430. [DOI] [PubMed] [Google Scholar]
- 36.Ehrlich M, Boll W, Van Oijen A, et al. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118(5):591–605. [DOI] [PubMed] [Google Scholar]
- 37.Loerke D, Mettlen M, Yarar D, et al. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 2009;7(3):e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cocucci E, Aguet F, Boulant S, Kirchhausen T. The first five seconds in the life of a clathrin-coated pit. Cell. 2012;150(3):495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kadlecova Z, Spielman SJ, Loerke D, Mohanakrishnan A, Reed DK, Schmid SL. Regulation of clathrin-mediated endocytosis by hierarchical allosteric activation of AP2. J Cell Biol. 2017;216(1):167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Honing S, Ricotta D, Krauss M, et al. Phosphatidylinositol-(4,5)-bisphosphate regulates sorting signal recognition by the clathrin-associated adaptor complex AP2. Mol Cell. 2005;18(5):519–531. [DOI] [PubMed] [Google Scholar]
- 41.Stamnes MA, Rothman JE. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 1993;73(5):999–1005. [DOI] [PubMed] [Google Scholar]
- 42.Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993;123(3):561–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seaman MN, Sowerby PJ, Robinson MS. Cytosolic and membrane-associated proteins involved in the recruitment of AP-1 adaptors onto the trans-Golgi network. J Biol Chem. 1996;271(41):25446–25451. [DOI] [PubMed] [Google Scholar]
- 44.Le Borgne R, Griffiths G, Hoflack B. Mannose 6-phosphate receptors and ADP-ribosylation factors cooperate for high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271(4):2162–2170. [DOI] [PubMed] [Google Scholar]
- 45.Kumawat A, Chakrabarty S, Kulkarni K. Nucleotide Dependent Switching in Rho GTPase: Conformational Heterogeneity and Competing Molecular Interactions. Sci Rep. 2017;7:45829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reider A, Barker SL, Mishra SK, et al. Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation. EMBO J. 2009;28(20):3103–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uezu A, Umeda K, Tsujita K, Suetsugu S, Takenawa T, Nakanishi H. Characterization of the EFC/F-BAR domain protein, FCHO2. Genes Cells. 2011;16(8):868–878. [DOI] [PubMed] [Google Scholar]
- 48.Henne WM, Boucrot E, Meinecke M, et al. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science. 2010;328(5983):1281–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS Biol. 2011;9(3):e1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Umasankar PK, Ma L, Thieman JR, et al. A clathrin coat assembly role for the muniscin protein central linker revealed by TALEN-mediated gene editing. Elife. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mulkearns EE, Cooper JA. FCH domain only-2 organizes clathrin-coated structures and interacts with Disabled-2 for low-density lipoprotein receptor endocytosis. Mol Biol Cell. 2012;23(7):1330–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hollopeter G, Lange JJ, Zhang Y, et al. The membrane-associated proteins FCHo and SGIP are allosteric activators of the AP2 clathrin adaptor complex. Elife. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henne WM, Kent HM, Ford MG, et al. Structure and analysis of FCHo2 F-BAR domain: a dimerizing and membrane recruitment module that effects membrane curvature. Structure. 2007;15(7):839–852. [DOI] [PubMed] [Google Scholar]
- 54.Frost A, Perera R, Roux A, et al. Structural basis of membrane invagination by F-BAR domains. Cell. 2008;132(5):807–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uezu A, Horiuchi A, Kanda K, et al. SGIP1alpha is an endocytic protein that directly interacts with phospholipids and Eps15. J Biol Chem. 2007;282(36):26481–26489. [DOI] [PubMed] [Google Scholar]
- 56.Gadeyne A, Sanchez-Rodriguez C, Vanneste S, et al. The TPLATE adaptor complex drives clathrin-mediated endocytosis in plants. Cell. 2014;156(4):691–704. [DOI] [PubMed] [Google Scholar]
- 57.Hirst J, Schlacht A, Norcott JP, et al. Characterization of TSET, an ancient and widespread membrane trafficking complex. Elife. 2014;3:e02866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mayers JR, Wang L, Pramanik J, et al. Regulation of ubiquitin-dependent cargo sorting by multiple endocytic adaptors at the plasma membrane. Proc Natl Acad Sci U S A. 2013;110(29):11857–11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Umasankar PK, Sanker S, Thieman JR, et al. Distinct and separable activities of the endocytic clathrin-coat components Fcho1/2 and AP-2 in developmental patterning. Nat Cell Biol. 2012;14(5):488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Benmerah A, Gagnon J, Begue B, Megarbane B, Dautry-Varsat A, Cerf-Bensussan N. The tyrosine kinase substrate eps15 is constitutively associated with the plasma membrane adaptor AP-2. J Cell Biol. 1995;131(6 Pt 2):1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iannolo G, Salcini AE, Gaidarov I, et al. Mapping of the molecular determinants involved in the interaction between eps15 and AP-2. Cancer Res. 1997;57(2):240–245. [PubMed] [Google Scholar]
- 62.Ma L, Umasankar PK, Wrobel AG, et al. Transient Fcho1/2Eps15/RAP-2 Nanoclusters Prime the AP-2 Clathrin Adaptor for Cargo Binding. Dev Cell. 2016;37(5):428–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimada A, Yamaguchi A, Kohda D. Structural basis for the recognition of two consecutive mutually interacting DPF motifs by the SGIP1 mu homology domain. Sci Rep. 2016;6:19565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilde A, Brodsky FM. In vivo phosphorylation of adaptors regulates their interaction with clathrin. J Cell Biol. 1996;135(3):635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bar-Zvi D, Mosley ST, Branton D. In vivo phosphorylation of clathrin-coated vesicle proteins from rat reticulocytes. J Biol Chem. 1988;263(9):4408–4415. [PubMed] [Google Scholar]
- 66.Pauloin A, Thurieau C. The 50 kDa protein subunit of assembly polypeptide (AP) AP-2 adaptor from clathrin-coated vesicles is phosphorylated on threonine-156 by AP-1 and a soluble AP50 kinase which co-purifies with the assembly polypeptides. Biochem J. 1993;296 ( Pt 2):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conner SD, Schmid SL. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J Cell Biol. 2002;156(5):921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kanaoka Y, Kimura SH, Okazaki I, Ikeda M, Nojima H. GAK: a cyclin G associated kinase contains a tensin/auxilin-like domain. FEBS Lett. 1997;402(1):73–80. [DOI] [PubMed] [Google Scholar]
- 69.Korolchuk VI, Banting G. CK2 and GAK/auxilin2 are major protein kinases in clathrin-coated vesicles. Traffic. 2002;3(6):428–439. [DOI] [PubMed] [Google Scholar]
- 70.Umeda A Identification of the universal cofactor (auxilin 2) in clathrin coat dissociation. European Journal of Cell Biology. 2000;79(5):336–342. [DOI] [PubMed] [Google Scholar]
- 71.Greener T, Zhao X, Nojima H, Eisenberg E, Greene LE. Role of cyclin G-associated kinase in uncoating clathrin-coated vesicles from non-neuronal cells. J Biol Chem. 2000;275(2):1365–1370. [DOI] [PubMed] [Google Scholar]
- 72.Ungewickell E, Ungewickell H, Holstein SE, et al. Role of auxilin in uncoating clathrin-coated vesicles. Nature. 1995;378(6557):632–635. [DOI] [PubMed] [Google Scholar]
- 73.Fingerhut A, von Figura K, Honing S. Binding of AP2 to sorting signals is modulated by AP2 phosphorylation. J Biol Chem. 2001;276(8):5476–5482. [DOI] [PubMed] [Google Scholar]
- 74.Ricotta D, Conner SD, Schmid SL, von Figura K, Honing S. Phosphorylation of the AP2 mu subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J Cell Biol. 2002;156(5):791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olusanya O, Andrews PD, Swedlow JR, Smythe E. Phosphorylation of threonine 156 of the mu2 subunit of the AP2 complex is essential for endocytosis in vitro and in vivo. Curr Biol. 2001;11(11):896–900. [DOI] [PubMed] [Google Scholar]
- 76.Pauloin A, Bernier I, eJolles P. Presence of cyclic nucleotide-Ca2+ independent protein kinase in bovine brain coated vesicles. Nature. 1982;298(5874):574–576. [DOI] [PubMed] [Google Scholar]
- 77.Jackson AP, Flett A, Smythe C, Hufton L, Wettey FR, Smythe E. Clathrin promotes incorporation of cargo into coated pits by activation of the AP2 adaptor micro2 kinase. J Cell Biol. 2003;163(2):231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Conner SD, Schroter T, Schmid SL. AAK1-mediated micro2 phosphorylation is stimulated by assembled clathrin. Traffic. 2003;4(12):885–890. [DOI] [PubMed] [Google Scholar]
- 79.Conner SD, Schmid SL. Differential requirements for AP-2 in clathrin-mediated endocytosis. J Cell Biol. 2003;162(5):773–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ritter B, Philie J, Girard M, Tung EC, Blondeau F, McPherson PS. Identification of a family of endocytic proteins that define a new α-adaptin ear-binding motif. EMBO reports. 2003;4(11):1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ritter B, Murphy S, Dokainish H, et al. NECAP 1 regulates AP-2 interactions to control vesicle size, number, and cargo during clathrin-mediated endocytosis. PLoS Biol. 2013;11(10):e1001670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chamberland JP, Antonow LT, Dias Santos M, Ritter B. NECAP2 controls clathrin coat recruitment to early endosomes for fast endocytic recycling. J Cell Sci. 2016;129(13):2625–2637. [DOI] [PubMed] [Google Scholar]
- 83.Dergai M, Iershov A, Novokhatska O, Pankivskyi S, Rynditch A. Evolutionary Changes on the Way to Clathrin-Mediated Endocytosis in Animals. Genome Biol Evol. 2016;8(3):588–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Beacham GM, Partlow EA, Lange JJ, Hollopeter G. NECAPs are negative regulators of the AP2 clathrin adaptor complex. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ritter B, Denisov AY, Philie J, et al. The NECAP PHear domain increases clathrin accessory protein binding potential. EMBO J. 2007;26(18):4066–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaksonen M, Roux A. Mechanisms of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2018;19(5):313–326. [DOI] [PubMed] [Google Scholar]
- 87.Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448(7156):883–888. [DOI] [PubMed] [Google Scholar]
- 88.Brett TJ, Traub LM, Fremont DH. Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure. 2002;10(6):797–809. [DOI] [PubMed] [Google Scholar]
- 89.Owen DJ, Vallis Y, Noble ME, et al. A structural explanation for the binding of multiple ligands by the alpha-adaptin appendage domain. Cell. 1999;97(6):805–815. [DOI] [PubMed] [Google Scholar]
- 90.Bykov YS, Schaffer M, Dodonova SO, et al. The structure of the COPI coat determined within the cell. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dodonova SO, Aderhold P, Kopp J, et al. 9A structure of the COPI coat reveals that the Arf1 GTPase occupies two contrasting molecular environments. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dodonova SO, Diestelkoetter-Bachert P, von Appen A, et al. VESICULAR TRANSPORT. A structure of the COPI coat and the role of coat proteins in membrane vesicle assembly. Science. 2015;349(6244):195–198. [DOI] [PubMed] [Google Scholar]
- 93.Gu M, Liu Q, Watanabe S, et al. AP2 hemicomplexes contribute independently to synaptic vesicle endocytosis. Elife. 2013;2:e00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morris KL, Buffalo CZ, Sturzel CM, et al. HIV-1 Nefs Are Cargo-Sensitive AP-1 Trimerization Switches in Tetherin Downregulation. Cell. 2018;174(3):659–671 e614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shen QT, Ren X, Zhang R, Lee IH, Hurley JH. HIV-1 Nef hijacks clathrin coats by stabilizing AP-1:Arf1 polygons. Science. 2015;350(6259):aac5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fotin A, Cheng Y, Sliz P, et al. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature. 2004;432(7017):573–579. [DOI] [PubMed] [Google Scholar]


