Abstract
Myeloid-derived suppressor cells (MDSC) represent a primary mechanism of immune evasion in tumors and have emerged as a major obstacle for cancer immunotherapy. The immunoinhibitory activity of MDSC is tightly regulated by the tumor microenvironment (TME) and occurs through mechanistic mediators that remain unclear. Here, we elucidated the intrinsic interaction between the expression of AMP-activated protein kinase alpha (AMPKα) and the immunoregulatory activity of MDSC in tumors. AMPKα signaling was increased in tumor-MDSC from tumor-bearing mice and ovarian cancer patients. Transcription of the Ampkα1-coding gene, Prkaa1, in tumor-MDSC was induced by cancer cell-derived granulocyte-monocyte colony-stimulating factor (GM-CSF) and occurred in a Stat-5-dependent manner. Conditional deletion of Prkaa1 in myeloid cells, or therapeutic inhibition of Ampkα in tumor-bearing mice, delayed tumor growth, inhibited the immunosuppressive potential of MDSC, triggered anti-tumor CD8+ T-cell immunity, and boosted the efficacy of T-cell immunotherapy. Complementarily, therapeutic stimulation of AMPKα signaling intrinsically promoted MDSC immunoregulatory activity. In addition, Prkaa1 deletion antagonized the differentiation of monocytic-MDSC (M-MDSC) to macrophages, and re-routed M-MDSC, but not granulocytic-MDSC (PMN-MDSC), into cells that elicited direct anti-tumor cytotoxic effects through nitric oxide synthase 2 (Nos2)-mediated actions. Thus, our results demonstrate the primary role of AMPKα1 in the immunosuppressive effects induced by tumor-MDSC, and support the therapeutic use of AMPK-inhibitors to overcome MDSC-induced T-cell dysfunction in cancer.
Keywords: MDSC, AMPKα1, Tumor immunity, Nos2, nitric oxide
Introduction
“Emergency” myelopoiesis is characterized by an elevated production of myeloid precursors in the bone marrow as a response to acute infection or injury (1). During this process, monocytic and granulocytic cells actively participate in the elimination of agents through direct cytotoxic events and activation of T, B, and NK cells. However, this physiological process is de-railed in cancer, resulting in a chronic production of myeloid precursors that inhibit the development of protective anti-tumor immunity and support the formation, growth, and metastasis of tumors (2). These immunoregulatory immature myeloid cell populations are referred as myeloid-derived suppressor cells (MDSC) and are divided into “early stage” (e-MDSC), monocytic (M-MDSC) and granulocytic (PMN-MDSC) subsets (3). MDSC represent a key mechanism for the evasion of protective anti-tumor T-cell immunity and a significant obstacle for the development of effective therapies against cancer (4). Despite their undeniable relevance, there are no effective clinical therapies to permanently overcome the immunoregulatory effects of MDSC in cancer patients (5). This can be explained in part by the exaggerated myelopoiesis, the redundancy of the regulatory pathways, and the high plasticity of MDSC in individuals with tumors (5).
The exposure of MDSC to the tumor microenvironment (TME) potentiates their ability to thwart protective anti-tumor immunity through the induction of multiple pathways, including the expression of Arginase I and Nitric oxide synthase 2 (Nos2); and the release of reactive oxygen species (ROS) and peroxynitrite (PNT) (6). Also, the TME drives differentiation of M-MDSC into tumor-associated macrophages (TAM) (7-9). Production of several TME-related factors such as GM-CSF, G-CSF, and IL-6, activate interconnected signaling pathways that control MDSC survival, immunosuppression, and differentiation (4). However, the precise signaling mediators whereby the TME controls MDSC-related immune suppression remain unclear.
AMP-activated protein kinase (AMPK) is a heterotrimeric complex highly conserved from yeast to animals and comprises a catalytic subunit (AMPKα1 or 2) and two regulatory subunits (AMPKβ and γ) that function as a metabolic sensor to maintain energy homeostasis in stressed cells (10). Myeloid cells preferentially express AMPKα1 rather than AMPKα2 (11, 12). AMPK activity is regulated by an elevated AMP/ATP ratio and other stress mediators in the TME, and is highly dependent on its expression and phosphorylation (13, 14). Upon activation, AMPK promotes metabolic plasticity through multiple processes including the promotion of fatty acid oxidation and mitochondrial homeostasis (15-17), which have been recently reported as major drivers of the immunoregulatory activity of myeloid cells in tumors (18). Although the role of AMPK is well established in cancer cells, the intrinsic effect of AMPK in the modulation of MDSC in tumors remains controversial. Initial reports showed that pharmacological activation of AMPK blocked MDSC function in tumors (19-25), while additional investigations indicated that AMPK promoted MDSC activity (26-28). Development of conditional AMPK-deficient models will enable precise elucidation of the actions of AMPK signaling in tumor-associated MDSC.
In this study, we sought to dissect the interaction between the expression of AMPKα1 and the immunoregulatory effects triggered by MDSC in tumors. Conditional deletion of the Ampkα1-coding gene, Prkaa1 in myeloid cells, or inhibition of Ampkα in tumor-bearing mice, impaired MDSC suppressive activity, blunted M-MDSC-to-macrophage differentiation, and de-railed M-MDSC into anti-tumor cytotoxic cells by Nos2-dependent pathways. These results demonstrate the major role of AMPKα1 in the immunosuppressive activity of MDSC in tumors and provide new strategies for the therapeutic inhibition of MDSC-driven T-cell dysfunction in cancer.
Material and Methods
Cell Lines and Animals
Cell lines Lewis lung carcinoma (LLC), EL4 thymoma, B16-F10 melanoma (ATCC), MCA-38 colon carcinoma (Kerafast), B16-GM-CSF melanoma (Dr. Esteban Celis, Augusta University), and ID8-Defb29/Vegf-a ovarian carcinoma (29, 30) (Dr. Conejo-Garcia, Moffitt) were cultured in RPMI-1640 (Lonza) supplemented with 10% fetal calf serum (Gemini), 25 mM Hepes, 4 mM L-glutamine, and 100 U/ml of penicillin-streptomycin (Invitrogen). B16 cells were transduced with lentivirus coding for non-targeting shRNA control or Csf2-targeting shRNA (Dharmacon, RHS6848 and RMM3981–200805373) and selected in 2 μg/ml puromycin-containing medium. Tumor cell lines were authenticated on May 2018 and validated to be mycoplasma-free using an ATCC detection kit in October 2018. All studies were conducted with cells within the first 5 passages. C57BL/6J mice (6 to 8-wk-old) were from Envigo. Myeloid cell-conditional Prkaa1-deficient (Prkaa1KO) mice were developed after breeding Prkaa1 loxP/loxP (Prkaa1flox) mice with those carrying Lysozyme promoter-driven Cre recombinase (both from the Jackson Laboratories). Pmel-1 mice and Nos2-deficient mice were from the Jackson Laboratories. Mice were s.c. injected with LLC, EL4, MCA-38, B16, or B16-GM-CSF cells, as reported (31). MMTV-PyMT breast tumor cells from transgenic animals (Dr. Ruffell, Moffitt) were implanted orthotopically in the mammary fat pads, and ID8-Defb29/Vegf-a tumor cells were injected i.p. and mice evaluated until they reached a weight gain greater than 30% (29). Tumor volume was tested using calipers and calculated using the formula [(small diameter)2 × (large diameter) × 0.5]. All studies using animals were approved by the Moffitt-IACUC and followed Moffitt’s Comparative Medicine facility guidelines.
Patient Population
A tissue microarray (TMA, Moffitt Cancer Center) was available for 79 de-identified and pathologically confirmed high-grade advanced serous epithelial ovarian carcinoma tumors and 10 healthy ovary or fallopian tube tissues. Also, peripheral blood from de-identified patients with advanced ovarian carcinoma and healthy donors was obtained from a tissue repository established by Dr. Conejo-Garcia (Moffitt Cancer Center). Moreover, we obtained de-identified mobilized peripheral blood stem cells (PBSC) from healthy donors for hematopoietic stem cell transplantation (HSCT) (Georgia Cancer Center Biorepository). Additionally, T-cells were isolated from de-identified buffy coats from healthy blood donors (One-Blood). Studies using de-identified human samples were covered through an exempt-approved Institutional Review Board (IRB) protocol and were developed following the Regulatory Affairs Committee guidelines at Moffitt Cancer Center. Investigators and biorepository facilities obtained informed written consent forms from the de-identified subjects.
Reagents
For modulation of AMPKα activity, MDSC were treated with 5-Aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (Aica-R, 200 μM, Millipore), Metformin (10 mM, Millipore), or Dorsomorphin-Compound C (CC, 5 μM, Cayman). Moreover, LLC-bearing mice received CC (15 mg/kg, i.t.), Metformin (150 mg/kg, i.p.), or Aica-R (0.5 mg/kg, i.p.) 9 days post-tumor injection and continued to be treated daily until tumor endpoint. For in vitro studies inhibiting Nos2, we used L-NG-Monomethylarginine (L-NMMA, 500 μM, Cayman) and Lysine-dihydrochloride (L-NIL, 300 μM, Cayman); whereas for in vivo assays, LLC-bearing mice were treated daily starting at day 0 of tumor injection with 20 mg/kg L-NIL (Cayman, i.p.). Human IL-6, mouse granulocyte-monocyte colony stimulating factor (GM-CSF) and mouse granulocyte-colony stimulating factor (G-CSF) were from Gemini. Human GM-CSF was from eBioscience. Thioglycolate broth from Sigma-Aldrich was prepared at 4% in water, autoclaved, and stored in dark for 2 weeks before i.p. injection. To test the role of GM-CSF in TES-treated MDSC, we utilized blocking antibodies against mouse-GM-CSF (5 μg/ml, Clone MP1022E9) and/or mouse-GM-CSF receptor α (1 μg/ml, Clone 698423, R&D systems). Rat IgG2a isotype (Clone 2A3, BioXcell) was used as control.
Flow cytometry
Surface staining was performed followed labeling with viability Zombie dyes (Biolegend) and purified anti-mouse CD16/CD32 antibodies (Clone 2.4G2, BD Biosciences). The following antibodies were used: CD45-BV785 or BV421 (Clone 30-F11, Biolegend), CD11b-FITC or BV421 (Clone M1/70, Biolegend), Gr1-PE-Cy5 or PE/Dazzle594 (Clone RB6–8C5, Biolegend), F4/80-APC-AF700 (Clone BM8, Biolegend), Ly6G-APC (Clone 1A8, Tonbo), Ly6C-PE or FITC (Clone AL-21, BD Biosciences). For intracellular detection of Nos2 (Clone CXNFT, eBioscience), AMPKα (Clone F6, Cell Signaling Technologies), or phospho-AMPKα (Thr172) (Rabbit polyclonal 40H9, Cell Signaling Technologies), tumor cell suspensions or MDSC were cultured for 6 hours in the presence of GolgiStop (0.8 μl/ml, BD Biosciences) plus LPS (1 μg/ml, Sigma-Aldrich) for Nos2; or GolgiStop (0.8 μl/ml), phorbol myristate acetate (PMA, 750 ng/mL, Sigma-Aldrich) and ionomycin (50 μg/mL, Sigma-Aldrich) for total and phospho AMPKα. Intracellular staining was completed using Cytofix/ Cytoperm and Perm-Wash buffers (BD Biosciences). T-cell proliferation was detected using Carboxyfluorescein succinimidyl ester (CFSE) (Molecular Probes). T-cells were labeled with 1 μM CFSE at 37°C for 15 minutes. For T-cell:MDSC co-cultures (1:0.5 ratio, unless specified), T-cells were primed with plate-bound anti-CD3 plus anti-CD28 (1 μg/ml each, Clones 145–2C11 and 37.51 from BD Biosciences) and cultured with MDSC or TAM for 72 hours. For human T-cell:MDSC (1:2 ratio) co-cultures, T-cells were primed with soluble anti-CD3 (1 μg/ml, clone OKT3, Thermo-Fisher) and anti-CD28 (0.5 μg/ml, clone L293, BD Biosciences) in 96 well-plates bound with 10 μg/ml goat anti-mouse (KPL). Results are expressed as percentage of proliferating T-cells, determined by the dilution of CFSE fluorescence compared with non-stimulated T-cells. Murine MDSC were identified by flow cytometry as: MDSC (CD45+ CD11b+ Gr1+ F4/80neg), PMN-MDSC (CD45+ CD11b+ Gr1+ Ly6Clow Ly6G+), and M-MDSC (CD45+ CD11b+ Gr1+ Ly6Chigh Ly6Gneg) (3); while TAM were recognized as CD45+ CD11b+ Gr1neg F4/80+. Cells resembling human MDSC were identified by flow cytometry as e-MDSC (CD45+ CD33+ HLA-DRneg/low CD14neg CD15neg), PMN-MDSC (CD45+ CD33+ HLA-DRneg/low CD15+ CD14neg), and M-MDSC (CD45+ CD33+ HLA-DRneg/low CD14+ CD15neg) (3). Cells were collected using a CytoFlex flow cytometer (Beckman Coulter). Analyses were performed using the FlowJo V10 (FlowJo).
Western Blot
Whole cellular lysates were electrophoresed in 8 or 10% Tris-Glycine gels (Novex-Invitrogen), transferred into nitrocellulose membranes (Bio-Rad), and immunoblotted with antibodies against phospho-Ampkα (Thr172) (Rabbit polyclonal 40H9, Cell Signaling Technologies), Ampkα (Clone F6, Cell Signaling Technologies), Nos2 (Clone 54/iNOS, BD Biosciences), Arginase I (Goat polyclonal N20, Santa Cruz Biotechnologies), Phospho-Stat-5 (Tyr694) (Rabbit polyclonal D47E7, and Clone 14H2, Cell Signaling Technologies), Stat-5 (Rabbit polyclonal D2O6Y, Cell Signaling Technologies), Stat-5a (Rabbit monoclonal E289, Abcam), Stat-5b (Rabbit polyclonal AF1584, R&D systems), or Vinculin (Clone hVIN-1, Sigma-Aldrich) (All at 1:1000). Horseradish peroxidase linked anti-mouse IgG, anti-rabbit IgG (both from GE Healthcare), or anti-goat IgG (Santa Cruz Biotechnologies) were used as secondary antibodies and used at 1:5000. Membrane-bound immune complexes were detected using ECL-Western Blot Substrate Reagent (Thermo-Fisher) and images acquired using a Chemidoc Imaging System and analyzed using the Image-Lab software (Bio-Rad).
Isolation of cells and development of MDSC
CD3+ T-cells were isolated from spleen and lymph nodes of C57BL/6 mice, or from purchased human buffy-coat units (One-Blood) using T-cell negative selection kits (MagniSort, Invitrogen). Purity ranged between 95% and 99% as tested by flow cytometry. MDSC were isolated from cellular suspensions of spleen or tumors digested with DNase I and Liberase (Roche) (31). Also, MDSC, M-MDSC, PMN-MDSC, and TAM were isolated by fluorescence-activated cell sorting. Splenic-MDSC were cultured in the presence of 20 ng/ml GM-CSF or 20% LLC-tumor explants (TES), prepared from filtered supernatants of primary LLC tumors cultured overnight (1×107/ml). Also, splenic-MDSC (2×106) from CD45.1+ mice bearing EL4 tumors (~2000 mm3) were transferred into the peritoneal cavity of CD45.2+ mice: 1) bearing established i.p. EL4 tumors (CD45.2+); 2) undergoing thioglycolate-induced peritonitis for 12 hours; or 3) naïve controls. Peritoneal CD45.1+ cells were sorted 18 hours later. In vitro developed human MDSC were generated from CD34+ CD33+ PBSC of healthy donors for HSCT. Precursors were cultured for 7 days with GM-CSF and IL-6 (20 ng/ml each) and different AMPKα modulating agents or 30% tumor-conditioned medium from renal cell carcinoma 786–0 cells (hTES) (31).
Depletion of CD8+ T-cells or MDSC and Adoptive Transfer
To eliminate CD8+ T-cells or MDSC-like cells, LLC-bearing mice were treated with 400 μg anti-CD8 (clone 53.6.72, BioXcell) or 250 μg anti-Gr1 (clone RB6–8C5, BioXcell), respectively. Maintenance i.p. doses of the depleting antibodies were given every 3rd day until tumor endpoint. In MDSC co-injection studies, 1×106 tumor-MDSC, PMN-MDSC, or M-MDSC from Prkaa1flox and Prkaa1KO mice bearing LLC tumors were co-injected s.c. with 1×106 LLC cells. For adoptive T-cell therapy (ACT), mice bearing B16 tumors for 6 days, received ACT with 1×106 negatively sorted CD8+ pmel T-cells pre-activated for 48 hours with gp10025–33 (AnaSpec). Ten days after pmel transfer, lymph nodes were activated for 24 hours with 1 μg/ml gp10025–33 and tested for IFNγ expression using EliSpot (R&D systems).
Immunofluorescence
Formalin fixed paraffin embedded baked TMA sections were transferred to a BOND RX (Leica Biosystems) and staining performed using an automated OPAL-IHC system (PerkinElmer). Briefly, slides were treated with the PerkinElmer blocking buffer for 10 min and incubated with the specific primary antibodies, followed by OPAL-HRP polymer and one OPAL fluorophore. Individual antibody complexes were stripped after each round of detection and DAPI applied as the last staining. Auto-fluorescence slides (negative control) included primary and secondary antibodies, omitting the OPAL fluorophores. Slides were imaged with a Vectra®3 Automated Quantitative Pathology Imaging System. Cells resembling MDSC were identified by imaging as M-MDSC (Pan-cytokeratinneg HLA-DRneg/low CD14+ CD15neg) or PMN-MDSC (Pan-cytokeratinneg HLA-DRneg/low CD15+ CD14neg). Multi-layer TIFF images were exported from InForm (PerkinElmer) into HALO (Indica Labs) for quantitative analysis. Each fluorophore was assigned to a dye color and positivity thresholds determined visually per marker based on intensity thresholds normalized for exposure (counts/2 bit depth x exposure time x gain x binning area). Cell segmentation results from each core were analyzed using FCS Express 6 Image Cytometry (De Novo software).
Cytotoxicity assay
CFSE-labeled EL4 cells were co-cultured with M-MDSC or PMN-MDSC (ratio 1:5) (31), and in the presence or the absence of L-NMMA or L-NIL. EL4 cells were also co-cultured with M-MDSC from tumor-bearing mice treated with L-NIL, or from Nos2KO or wildtype mice treated with CC. After 24 hours of co-culture, EL4 cells were stained with Violet Zombie dye and then fixed with Cytofix buffer (BD Biosciences). Percentage of cell-dead within the EL4-CFSE+ cells was measured by flow cytometry.
Quantitative real time-PCR (RT-PCR) and Multiplex arrays
Total RNA was extracted using TRIzol (Life Technologies), according to the manufacturer’s instructions. RNA was reversely transcribed using the Verso cDNA synthesis Kit (Invitrogen). RT-PCR analyses were performed with SYBGreen (Bio-Rad) using primers synthetized by Integrated DNA Technologies (IDT, Coralville, IA). The following primers were used: Gapdh: forward 5’-CTGCCCAGAACATCATCCCT-3’, reverse 5’-ACTTGGCAGGTTCTCCAGG-3’; Prkaa1: forward 5’-GTCAAAGCCGACCCAATGATA-3’, reverse 5’-CGTACACGCAAATAATAGGGGTT-3’. Fold-change expression was calculated by comparing the RNA values from experimental samples relative to the endogenous Gapdh control, compared with the results obtained from a pooled sample. Thus, fold change =2−Δ(ΔCT), where ΔCT = CT (Prkaa1) – CT (Gapdh); and Δ(ΔCT) = ΔCT (Prkaa1) – ΔCT (Gapdh pool of all samples). RT2 profiler cancer inflammation and immunity crosstalk PCR array (Qiagen) was done in tumor-MDSC from LLC-bearing Prkaalflox and Prkaa1KO mice, following the vendor’s suggestions.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assays were done using a SimpleChip kit (Cell Signaling Technologies), following the vendor’s recommendations. Briefly, digested and cross-linked chromatin was prepared from 4×106 tumor-MDSC or splenic-MDSC treated or not with GM-CSF or TES for 72 hours, followed by immunoprecipitation with antibodies against phospho-Stat-5, Histone H3, or rabbit IgG (Cell Signaling Technologies). Eluted and purified DNA was analyzed by qPCR with the following primers targeting the Prkaa1 promoter: forward 5’-AGCTTTCTTCCCCCTGAATACTTT-3’; reverse 5’-CTCCTAGTTCCATATTCTGGCT-3’. Primers against Rpl30 promoter provided in the kit were used as housekeeping control.
Statistical Analysis
Two-tailed unpaired Student’s t-test was used for most of the statistical analyses using the Prism software (Graph-Pad). Also, analyses of survival were assessed through Mantel-Cox test. P value of <0.05 was considered statistically significant. Specific statistical test results are indicated in each figure: *, p<0.05; **, p<0.01 ***, p<0.001.
Results
AMPKα induction in tumor-exposed MDSC.
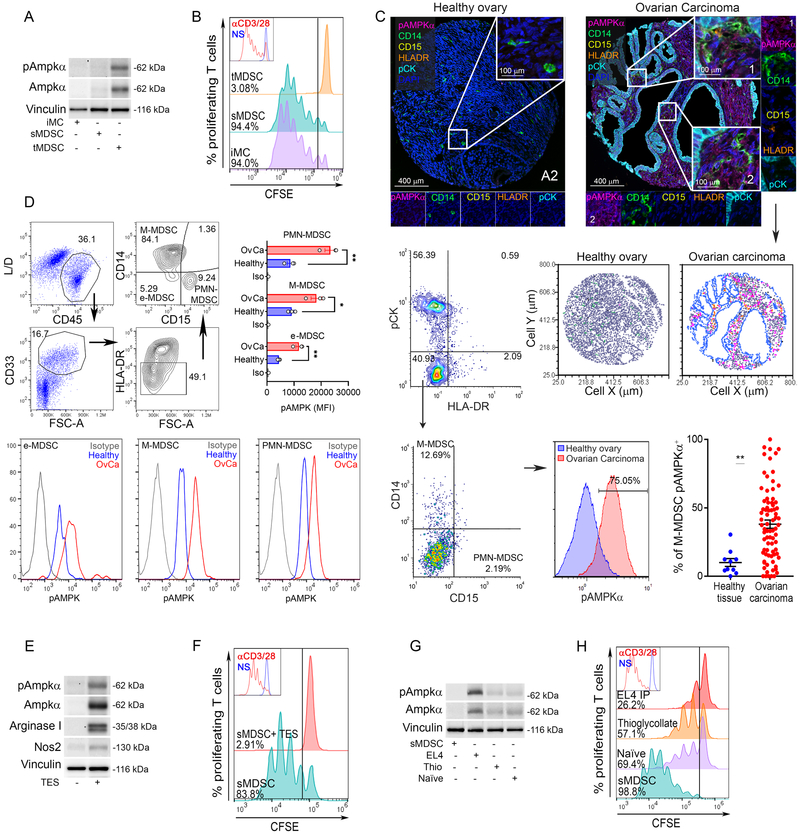
To understand the potential interaction between AMPKα signaling and the regulatory function of tumor-associated MDSC, we monitored the expression of Ampkα and the immunosuppressive activity of tumor and splenic-MDSC (CD11b+ Gr1+) from mice bearing LLC tumors; and splenic PMN, monocytes, and immature myeloid cells (CD11b+ Gr1+, iMC) from mice without tumors. An increased expression of total and phospho-Ampkα and an augmented ability to impair T-cell proliferation were found in tumor-MDSC, compared to splenic-MDSC from LLC-bearing mice or iMC from tumor-free mice (Figure 1A-B). Also, an elevation of total and phospho-Ampkα was noted in tumor-MDSC from B16, EL4, or MMTV-PyMT-bearing mice, compared to splenic controls (Suppl. Figure 1A). Notably, the Ampkα signaling activation in tumor-MDSC occurred in PMN-MDSC and M-MDSC (Suppl. Figure 1B). Next, we studied whether AMPKα signaling was also heightened in human-MDSC infiltrating tumors. Using high-resolution automated multispectral imaging, we assessed the expression of phospho-AMPKα in a TMA made from 79 patients with advanced high-grade serous ovarian tumors and 10 healthy ovary controls. Higher levels of phospho-AMPKα were noticed in cells resembling M-MDSC (Pan-cytokeratinneg HLA-DRneg/low CD14+ CD15neg) from ovarian tumors, compared to cellular counterparts from healthy ovaries (Figure 1C). However, analysis of phospho-AMPKα in tumor PMN-MDSC could not be completed as limited numbers of Pan-cytokeratinneg HLA-DRneg/low CD14neg CD15+ cells were found in ovarian tumors or healthy tissues (Figure 1C, Suppl. Figure 1C). To further explore the activation of AMPKα in MDSC subsets, we then examined the expression of phospho-AMPKα in peripheral blood from ovarian cancer patients and healthy controls by flow cytometry. Higher phospho-AMPKα levels were found in cells resembling e-MDSC, PMN-MDSC, and M-MDSC (3) from advanced ovarian cancer patients, compared to controls (Figure 1D), indicating the active AMPKα signaling in circulating MDSC from ovarian cancer patients.
Figure 1. TME induces AMPKα in MDSC and boosts their immunoregulatory activity.
(A). Total and phospho-Ampkα in tumor-MDSC (tMDSC) and splenic-MDSC (sMDSC) from mice bearing LLC tumors, and in CD11b+ Gr1+ cells (iMC) from non-tumor bearing mice. Illustrative Immunoblot result from 5 mice.
(B) Representative result from 5 repeats showing the regulatory effect of tMDSC, sMDSC, and iMC on the proliferation of CFSE-labeled T-cells primed with plate-bound anti-CD3/CD28. T-cell proliferation was assessed 72 hours post-activation by flow cytometry. N=5.
(C). Top: Representative images (20x resolution and further 10x digital magnification) showing phospho-AMPKα (Magenta), CD14 (Green), CD15 (Yellow), HLA-DR (Orange), pan-Cytokeratin (pCK, Cyan), and DAPI (Blue) in a TMA containing healthy ovarian tissues (N=10, left) and ovarian carcinoma tumors (N=79, right) by Vectra Automated Multispectral Imaging. Middle right: Representative cellular phenotype map showing the location of cells resembling M-MDSC (pCKneg HLA-DRneg CD14+ CD15neg, green dots), phospho-AMPKα+ M-MDSC (pink dots), monocytes-Dcs (pCKneg HLA-DR+ CD14+ CD15neg) (orange dots), pCK+ tumor cells (blue dots), and undefined stromal cells pCKneg (grey dots). pCKneg HLA-DRneg cells from ovarian tumors or healthy tissues (Middle left) were gated and plotted for CD14 (M-MDSC) or CD15 (PMN-MDSC) (Bottom left), and M-MDSC compared for phospho-AMPKα expression (Bottom center) and frequencies of phospho-AMPKα+ M-MDSC (Bottom right). Mean ± s.e.m.**p < 0.01 by unpaired Student’s t-test.
(D) Top-left: Representative gating strategy for identification of e-MDSC, M-MDSC, and PMN-MDSC from peripheral blood of ovarian cancer patients and healthy controls. Merged (Top-right) and representative (bottom) phospho-AMPKα levels in MDSC subsets (N= 3/group).
(E). Total and phospho-Ampkα, Arginase I, and Nos2 in splenic-MDSC treated for 72 hours with 20% TES from primary LLC tumors. Representative immunoblot from 3 repeats.
(F). Anti-proliferative effect of MDSC from (E) on CFSE-labeled T-cells primed as in (B). N=3.
(G-H). CD45.1+ sMDSC were transferred into: naïve CD45.2+ mice, CD45.2+ mice bearing i.p. CD45.2+ EL4 tumors, or CD45.2+ mice previously injected with thioglycolate. CD45.1+ sMDSC were sorted 18 hours later. Representative results of total and phospho-Ampkα expression (G) and immunosuppressive activity on T-cells (H). N=4
Next, we investigated the contribution of the TME in the induction of Ampkα in MDSC by treating splenic-MDSC with LLC tumor explants (TES). Upregulation of total and phospho-Ampkα, higher levels of the MDSC-inhibitory factors Arginase I and Nos2, and elevated T-cell immunoregulatory activity, were noticed in TES-treated MDSC, compared to controls (Figure 1E-F). To elucidate the specific effect of the TME vs. overall inflammation in the induction of Ampkα in MDSC, we transferred CD45.1+ splenic-MDSC into the peritoneum of CD45.2+ mice previously injected with peritoneal EL4 tumors or the inflammatory agent thioglycolate. CD45.1+ cells were sorted 18 hours later and tested for the expression of Ampkα and the ability to impair T-cell proliferation. Higher levels of total and phospho-Ampkα and an augmented capacity to block T-cell proliferation were noted in MDSC previously transferred into EL4 tumors, compared to those from chemically-induced peritonitis or untreated mice (Figure 1G-H). Thus, results indicate the driving effect of the TME in the induction of AMPKα in MDSC and the potential interaction between AMPKα and MDSC activity.
TME-associated GM-CSF triggers Ampkα expression in MDSC through Stat-5.
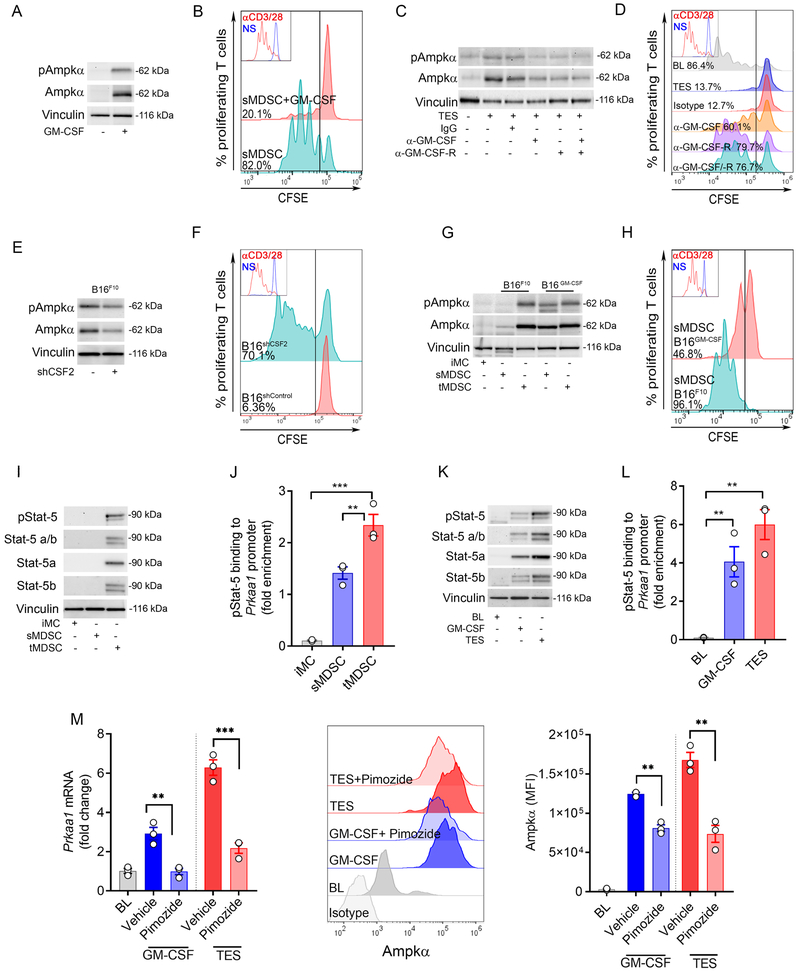
We aimed to identify the TME factors driving Ampkα expression in tumor-MDSC. We focused on the role of GM-CSF as its production in the TME plays a primary role in MDSC activity (32, 33). Treatment of splenic-MDSC with GM-CSF induced the expression of total and phospho-Ampkα and enhanced their ability to blunt T-cell proliferation (Figure 2A-B, Suppl. Figure 2A-C). Moreover, blockade of GM-CSF and GM-CSF receptor α partially blunted the upregulation of Ampkα and the immunosuppressive activity of TES-treated MDSC (Figure 2C-D, Suppl. Figure 2D), suggesting the effect of the TME-associated GM-CSF in the induction of Ampkα in MDSC. Next, we tested the role of the cancer cell-derived GM-CSF in the induction of Ampkα in MDSC. Lower expression of total and phospho-Ampkα and reduced immunosuppressive activity were found in tumor-MDSC from mice bearing GM-CSF-silenced B16 tumors compared to controls (Figure 2E-F, Suppl. Figure 2E-F). In agreement, higher levels of total and phospho-Ampkα and augmented ability to impair T-cell proliferation were found in splenic-MDSC from mice carrying B16 cells overexpressing GM-CSF (B16-GM-CSF) (Figure 2G-H, Suppl. Figure 2G), indicating the role of cancer cell-derived GM-CSF in the induction of AMPKα in MDSC.
Figure 2. TME-associated GM-CSF triggers Ampkα expression in MDSC through Stat-5.
(A). Splenic-MDSC were isolated from LLC-bearing mice and treated for 72 hours with GM-CSF (20 ng/ml). Next, the expression of total and phospho-Ampkα was monitored. Results are a representative result from 3 independent experiments.
(B). MDSC from (A) were co-cultured with CFSE-labeled T-cells primed with anti-CD3/CD28. T-cell proliferation was tested 72 hours later by flow cytometry. Representative result from N=3.
(C-D). Splenic-MDSC from LLC-bearing mice were treated for 72 hours with 20% TES and in the presence of IgG isotype, anti-GM-CSF, and/or anti-GM-CSF-Rα. Total and phospho-Ampkα expression (C) and capacity to block T-cell proliferation (D) were then assessed. Results are a representative illustration from 3 independent repeats.
(E-F). Tumor-MDSC from mice bearing B16 tumors previously transduced to express control or GM-CSF (Csf2)-targeting shRNA were tested for the expression of total and phospho-Ampkα and for the capacity to block T-cell proliferation. Results are representative from N=3.
(G). Representative result showing expression of total and phospho-Ampkα in iMC, or splenic-MDSC (sMDSC) and tumor-MDSC (tMDSC) from mice bearing s.c. B16-F10 or B16-GM-CSF tumors for 15 days. N=6 from 3 independent repeats.
(H). Splenic-MDSC from mice bearing similar sized B16-F10 or B16-GM-CSF tumors were tested for their ability to impair T-cell proliferation as in (B). Result is similar to 3 repeats.
(I). Expression of total Stat-5, Stat-5a, Stat-5b and phospho-Stat-5 in sMDSC and tMDSC from mice bearing s.c. LLC tumors, and iMC from spleens of tumor-free mice. N=3
(J). ChIP assay to detect endogenous binding of phospho-Stat-5 to Prkaa1 promoter in cells from (I). Bars are Mean ± s.e.m. of 3 independent experiments. ***p < 0.001; **p < 0.01.
(K). Total Stat-5, Stat-5a, Stat-5b and phospho-Stat-5 in splenic-MDSC (BL) from LLC-bearing mice and treated for 72 hours with GM-CSF or 20% TES. Representative data from N=3.
(L). Endogenous binding of phospho-Stat-5 to Prkaa1 promoter by ChIP assay in cells from (K). Bars are represented as Mean ± s.e.m. of 3 repeats. **p < 0.01.
(M). Prkaa1 mRNA and AMPKα protein levels in baseline splenic-MDSC (BL) from LLC-bearing mice treated for 72 hours with GM-CSF or 20% TES in the presence of Stat-5 inhibitor, Pimozide (10 μM) or vehicle. Mean ± s.e.m. or representative of N=3. ***p < 0.001; **p < 0.01.
Next, we sought to identify the intracellular mediators by which GM-CSF promoted Prkaa1 transcription. Our recent report showed the interaction between GM-CSF production in the TME and the phosphorylation of Stat-5 in tumor-MDSC (18). Elevated levels of total Stat-5, Stat-5a, Stat-5b; and augmented expression and endogenous binding of phospho-Stat-5 to the Prkaa1 promoter were found in tumor-infiltrating MDSC compared to splenic-MDSC or iMC (Figure 2I-J, Suppl. Figure 2H), and in splenic-MDSC treated with TES or GM-CSF (Figure 2K-L, Suppl. Figure 2I). Notably, the inhibition of Stat-5 through Pimozide impaired the induction of Prkaa1 mRNA and Ampkα protein in GM-CSF or TES-treated splenic-MDSC (Figure 2M). Thus, our results suggest the primary role of TME-associated GM-CSF and intrinsic signaling through Stat-5 in the induction of Ampkα in tumor-MDSC.
AMPKα modulation regulates the immunosuppressive activity of tumor-MDSC.
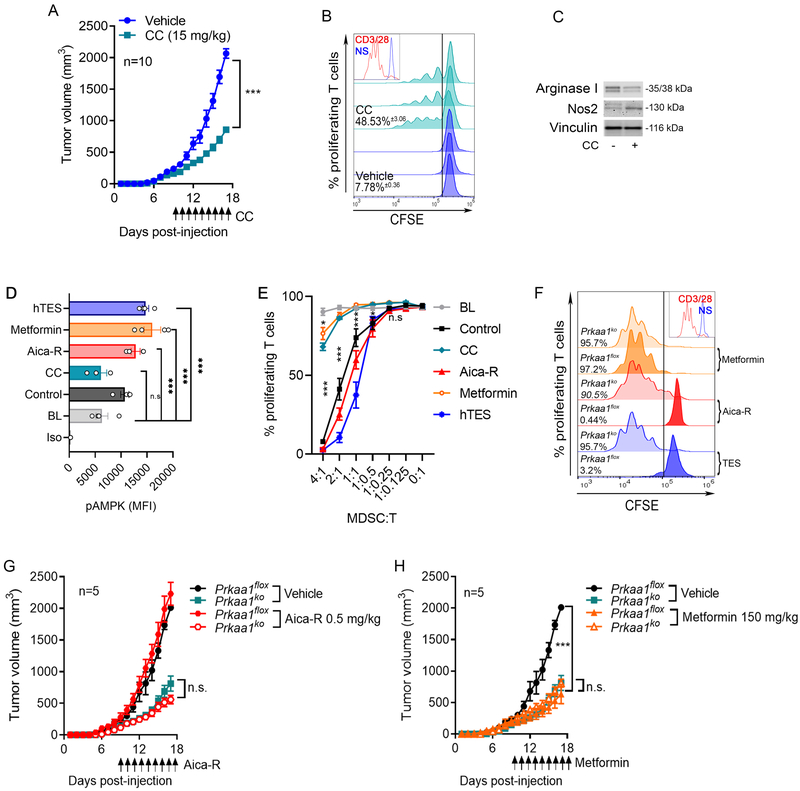
We tested the therapeutic effects of the inhibition of AMPKα in tumor-bearing mice. Delayed tumor growth and alterations in tumor-MDSC, including impaired immunosuppressive activity, diminished expression of the immune inhibitory factor Arginase I, and increased Nos2 levels, were found LLC-bearing mice treated with the AMPK inhibitor, CC, compared to controls (Figure 3A-C). Because MDSC activity depends on tumor burden, we further tested the intrinsic effects of the modulation of AMPKα in the activity of human MDSC developed from myeloid precursors (Suppl. Figure 3) (18, 34), after exposure to effective doses of the AMPK agonists, Aica-R and Metformin, or the AMPK inhibitor, CC (Figure 3D). In agreement with the immune suppressive role of AMPK in MDSC, we observed a higher immunoregulatory activity in Aica-R pre-treated MDSC and reduced immunoinhibitory potential in CC-conditioned MDSC, compared to controls, which correlated with corresponding changes in phospho-AMPKα levels (Figure 3D-E). Also, despite the elevation of phospho-AMPKα in Metformin-treated MDSC, we found a decrease in their immunosuppressive potential (Figure 3D-E), suggesting opposite effects of the AMPK agonists, Aica-R and Metformin, in human MDSC. To further evaluate possible off-target effects of the AMPK agonists, we repeated the treatments in splenic-MDSC from myeloid cell-conditional Ampkα1-null (Prkaa1KO) mice, created after crossing Prkaalflox mice with those carrying Lysozyme-driven Cre recombinase. Prkaa1 deletion antagonized the increased immune regulatory activity observed in TES or Aica-R-exposed MDSC, without impacting Metformin-treated MDSC (Figure 3F), suggesting that TES and Aica-R, but not Metformin, modulate MDSC regulatory activity in an Ampkα1-dependent manner. Furthermore, we tested the role of myeloid cell-Ampkα1 in the tumor effects induced by Aica-R or Metformin treatments in mice. Prkaa1 deletion overcame the slight potentiation of tumor growth induced by Aica-R treatment in mice (Figure 3G). Conversely, the delayed tumor growth noticed in LLC-bearing mice treated with Metformin was not altered by deletion of Prkaa1 in myeloid cells (Figure 3H), showing that Aica-R, but not Metformin, induced anti-tumor effects in a myeloid cell Ampkα-related manner.
Figure 3. Ampk modulation regulates the immunosuppressive activity of tumor-MDSC.
(A). Tumor growth in LLC-bearing mice treated daily with CC, starting at day 9 post-tumor injection. Mean kinetics ± s.e.m of 7 mice/group in 2 repeats. ***p < 0.001
(B). Tumor-MDSC from LLC-bearing mice treated with CC or vehicle (day 17 post-tumor inoculation) were monitored for ability to blunt proliferation of CFSE-labeled T-cells primed with anti-CD3/CD28. Histograms show 3 repeats and percentages are Mean ± s.e.m.
(C). Arginase I and Nos2 levels in tumor-MDSC from (B). Representative result from N=3.
(D-E). Human MDSC developed after treatment of myeloid precursors with GM-CSF plus IL-6, and CC, Aica-R, Metformin, or 30% hTES for 7 days were tested for phospho-AMPKα levels by flow cytometry (D) and for their ability to block proliferation of CFSE-labeled T-cells (anti-CD3/CD28) (E). N=3
(F). Splenic-MDSC from LLC-bearing flox and Prkaa1KO mice were treated for 72 hours with 20% TES, Aica-R or Metformin and tested for the ability to block anti-CD3/CD28-induced T-cell proliferation. Representative finding from 3 repeats.
(G-H). Flox and Prkaa1KO mice were injected with LLC tumors and 9 days later received daily treatments with Aica-R or Metformin. Tumor volume was tested until endpoint. N=10/group.
Prkaa1 deletion impairs MDSC suppressive activity and differentiation into TAM.
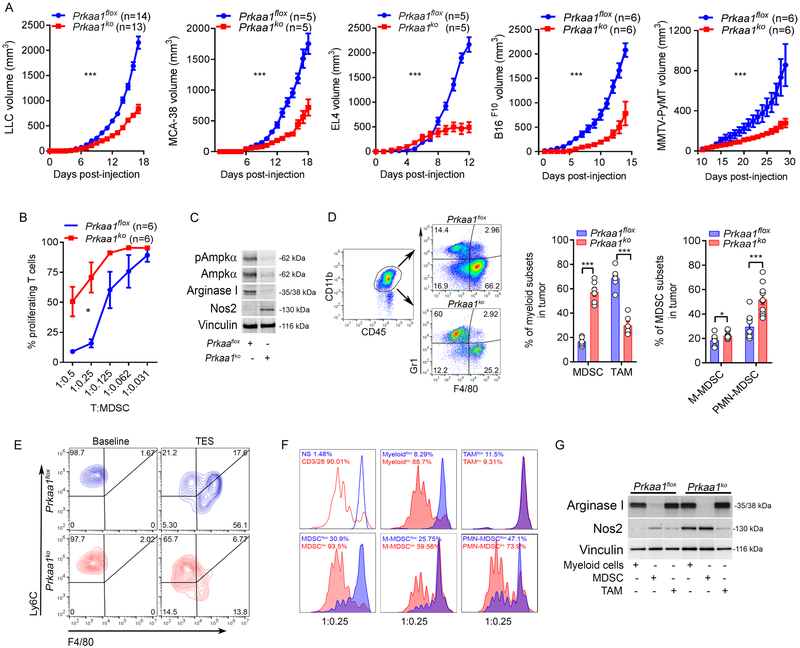
To determine the specific contribution of Ampkα1 in myeloid cells in tumor growth, we used myeloid cell-conditional Prkaa1KO mice. A delay in the growth of several tumors, including LLC, MCA-38, EL4, B16, and MMTV-PyMT was noticed in Prkaa1KO mice, compared to Prkaalflox controls (Figure 4A). Also, extended survival was found in LLC, EL4, B16, and ID8-Defb29/Vegf-a bearing Prkaa1KO mice, compared to controls (Suppl. Figure 4A). Furthermore, an evident decrease in the T-cell suppressive potential was noticed in tumor-MDSC from Prkaa1KO mice, compared to those from control mice (Figure 4B), which correlated with a lower expression of Arginase I and a surprising upregulation of Nos2 (Figure 4C). Additionally, gene transcript assessment showed increased levels of Nos2, Il12a, Tlr4, Cxcl1, and Myd88; and lower Aicda, Tnfsf10, and Cxcl10 mRNA expression in tumor-MDSC from Prkaa1KO mice compared to controls (Suppl. Figure 4B-C). Because gene targeting using Lysozyme-Cre recombinase does not specifically affect MDSC, we then distinguished the effect of the deletion of Prkaa1 on the two most frequent myeloid subsets in LLC tumors, MDSC and TAM. Increased accumulation of cells resembling MDSC, PMN-MDSC, and M-MDSC; and lower frequency of TAM were found in tumors from Prkaa1KO mice compared to controls (Figure 4D). Because M-MDSC represent a primary source for TAM expansion in the TME (7-9), we tested the effect of Ampkα in the M-MDSC-to-TAM differentiation. In accordance with the lower frequency of TAM in tumors from Prkaa1KO mice, a diminished differentiation of TES-conditioned M-MDSC to macrophages was observed upon Prkaa1 deletion (Figure 4E). Also, we compared the role of Ampkα1 in the T-cell-suppressive activity of MDSC vs. TAM. Prkaa1 deletion impacted the immunoregulatory activity and the levels of Arginase I in tumor-MDSC, while promoting Nos2 expression. However, these effects were not observed in TAM (Figure 4F-G). Interestingly, the low immunosuppressive activity of Prkaa1KO MDSC from tumors was observed both in PMN-MDSC and M-MDSC (Figure 4F). Thus, results show the role of Prkaa1 in the differentiation and immunoinhibitory function of tumor-MDSC, but not in TAM suppressive activity.
Figure 4. Prkaa1 intrinsically regulates MDSC activity and differentiation.
(A). Flox and Prkaa1KO mice were injected in 2 independent repeats with LLC (N=14), MCA-38 (N=5), EL4 (N=5), B16 (N=6), or MMTV-PyMT (N=6) tumors. Average kinetics ± s.e.m. ***p < 0.001.
(B-C). Tumor-MDSC were isolated from flox and Prkaa1KO mice bearing LLC tumors for 17 days and tested for their ability to impair proliferation of CFSE-labeled T-cells primed with anti-CD3/CD28 (B), and for the expression of total and phospho-AMPKα, Arginase I, Nos2, and vinculin (C). Results are a representative finding from 6 mice/group and 2 independent repeats. *p < 0.05 by two-tailed unpaired Student’s t-test.
(D). Left: strategy for evaluation of MDSC and TAM in tumors. Myeloid cells were gated based on CD11b+, followed by discrimination of MDSC (CD45+ CD11b+ Gr1+ F4/80neg) and TAM (CD45+ CD11b+ Gr1neg F4/80+). Center: MDSC and TAM frequency in LLC tumors from flox and Prkaa1KO mice. N=6 from 2 independent studies. Right: PMN-MDSC (CD45+ CD11b+ Gr1+ Ly6Clow Ly6G+) and M-MDSC (CD45+ CD11b+ Gr1+ Ly6Chigh Ly6Gneg) in LLC tumors from flox and Prkaa1KO mice. N=10 from 2 repeats. Bars are Mean ± s.e.m. ***p < 0.001, *p < 0.05.
(E). Flow cytometry sorted splenic-M-MDSC from flox and Prkaa1KO mice bearing LLC tumors for 17 days were treated with 20% TES for 72 hours, after which their differentiation into TAM was evaluated by flow cytometry. N=3.
(F). Tumor-associated myeloid cells (CD11b+), tumor-MDSC subsets and TAM were sorted by flow cytometry from flox and Prkaa1KO mice bearing LLC tumors (17 days) and tested for ability to block T-cell proliferation as in (B). N=3 repeats.
(G). Representative expression of Arginase I and Nos2 by western blot in sorted cells from (F). Results are from 3 independent repeats.
Conditional deletion of Prkaa1 in MDSC primes anti-tumor T-cell responses.
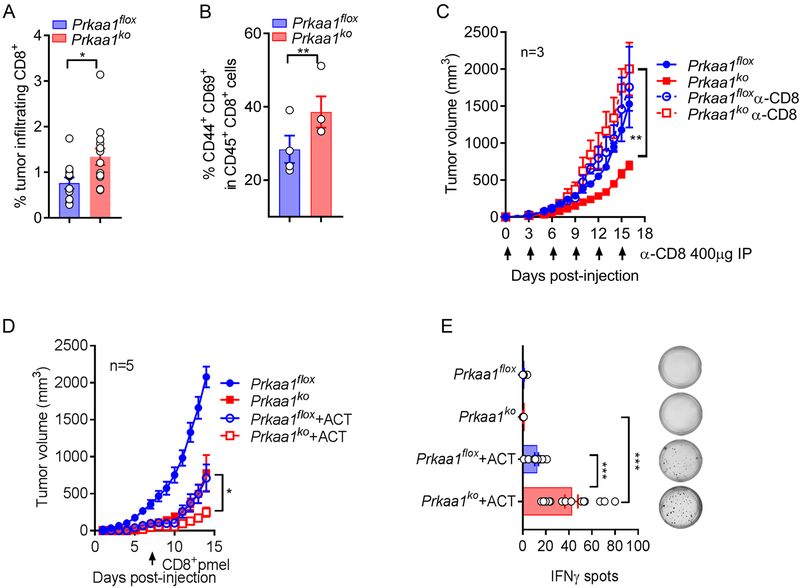
We next sought to elucidate the role of T-cell immunity in the anti-tumor effects induced by the conditional deletion of Prkaa1 in myeloid cells. In agreement with the role of Ampkα1 in the promotion of MDSC function, elevated frequency of CD8+ T-lymphocytes and CD8+ CD44+ CD69+ antigen-experienced T-cells was observed in tumors from Prkaa1KO mice, compared to controls (Figure 5A-B). Moreover, depletion of CD8+ T-cells restored tumor growth in Prkaa1KO mice (Figure 5C). Next, we tested the impact of the MDSC-Ampkα1 in tumor-induced T-cell tolerance using an ACT model against the tumor-antigen gp100, in which activated anti-gp10025–33 transgenic pmel T-cells were transferred into mice bearing B16 tumors. A significant delay in tumor growth and a higher frequency of IFNγ-expressing pmel T-cells were found in B16-bearing Prkaa1KO mice undergoing ACT, compared to flox controls receiving the same amount of pmel T-cells (Figure 5D-E). Thus, results suggest the key role of MDSC-related Ampkα1 in tumor-induced T-cell dysfunction.
Figure 5. Prkaa1 deletion in myeloid cells promotes anti-tumor T-cell immunity.
(A-B). Frequency of CD45+CD8+ (A) and antigen-experienced CD44+ CD69+ effector CD8+ (B) T-cells was evaluated in LLC tumors from Prkaa1flox or Prkaa1KO mice. (N=13 tumors/group). Bar graphs are Mean ± s.e.m. and *p < 0.05, **p < 0.01 by two-tailed unpaired Student’s t-test.
(C). LLC tumor growth in Prkaa1flox (closed blue circle) or Prkaa1KO (closed red square) mice, with or without 400 μg α-CD8 antibody injection on every 3rd day (open blue circle or open red square, respectively). Average tumor volume ± s.e.m in 3 mice/group. **p < 0.01 using unpaired Student’s t-test.
(D). Flox and Prkaa1KO mice were injected s.c. with B16 tumors and 6 days later, mice received 1X106 CD8+ Pmel T-cells primed with gp10025–33 for 48 hours. Tumor volume was then monitored. Mean tumor volume ± s.e.m in 5 mice/group. *p < 0.05 by unpaired Student’s t-test.
(E). Lymph nodes from flox or Prkaa1KO mice bearing B16 tumors were collected 5 days after pmel T-cell transfer and evaluated for IFNγ production by EliSpot upon activation with gp10025–33 for 24 hours. (N=3 repeats of 4 lymph nodes/group). Mean ± s.e.m. ***p < 0.001 using unpaired Student’s t-test.
Prkaa1-deficient M-MDSC eliminate tumor cells in a Nos2-mediated manner.
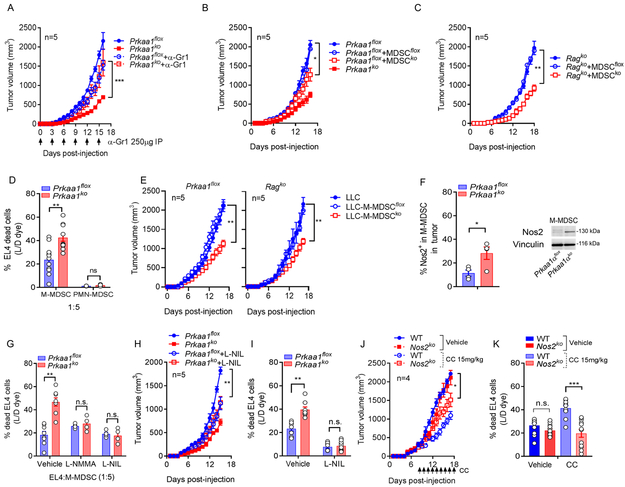
To determine whether Prkaa1 deletion transforms MDSC into cells that elicit anti-tumor actions, we eliminated MDSC using anti-Gr1 antibodies; and co-injected Prkaa1flox mice with LLC cells and tumor-MDSC from Prkaa1KO or flox mice (31). MDSC depletion restored tumor growth in Prkaa1KO mice and delayed tumor progression in control mice (Figure 6A). Also, in agreement with the anti-tumor actions of Prkaa1KO MDSC, delayed tumor growth was found after transfer of Prkaa1KO-MDSC, compared to mice receiving flox-MDSC (Figure 6B). Notably, Prkaa1KO-MDSC triggered similar anti-tumor effects after transfer into immunodeficient RagKO mice (Figure 6C), indicating that Prkaa1 deletion transforms MDSC into cells that potentially elicit direct tumor cytotoxicity. To further explore this possibility, we assessed the cytotoxic potential of tumor-MDSC subsets from flox and Prkaa1KO mice on cultured EL4 tumor cells. Prkaa1KO-M-MDSC showed enhanced ability to eliminate EL4 tumor cells, compared to Prkaa1KO-PMN-MDSC or tumor-MDSC subsets from Prkaa1flox mice (Figure 6D). Moreover, co-injection of LLC cells with Prkaa1KO M-MDSC, but not with Prkaa1KO PMN-MDSC, resulted in delayed tumor growth in both wildtype and RagKO mice (Figure 6E, Suppl. Figure 5), confirming the ability of Prkaa1KO M-MDSC to induce direct anti-tumor effects. Interestingly, the increased anti-tumor cytotoxic activity of Prkaa1KO-M-MDSC correlated with a higher expression of Nos2 (Figure 6F) and was completely inhibited after treatment with the Nos2 inhibitors, L-NMMA or L-NIL (Figure 6G), suggesting the impact of Nos2 in the anti-tumor cytotoxic actions induced by Prkaa1KO-M-MDSC. Next, we elucidated whether the inhibition of Nos2 overcame the effects observed in Prkaa1KO mice. Treatment of LLC-bearing Prkaa1KO mice with the Nos2 inhibitor, L-NIL, partially restored tumor growth and ablated the ability of tumor-M-MDSC to kill tumor cells (Figure 6H-I). Accordingly, knockdown of Nos2 in LLC-bearing mice partially prevented the anti-tumor effects and M-MDSC cytotoxic activity induced after treatment with CC (Figure 6J-K). Thus, the overall results indicate that knockdown of Prkaa1 induced M-MDSC-like cells with the ability to elicit direct anti-tumor effects through Nos2-dependent pathways.
Figure 6. Prkaa1-deficient M-MDSC eliminate tumor cells in a Nos2-mediated manner.
(A). Tumor volume in flox or Prkaa1KO mice bearing LLC tumors and treated or not with 250 μg anti-anti-Gr1 every 3rd day until tumor endpoint. N=5 per group. ***p < 0.001 by Student’s t-test.
(B-C). Tumor growth in C57BL/6 (B) and immunodeficient RagKO (C) mice injected with LLC cells alone or co-injected at a 1:1 ratio with tumor-MDSC harvested from flox or Prkaa1KO mice bearing LLC tumors. Mean ± s.e.m from 5 mice/group for B; and 3 mice/group for C. *p < 0.05; **p < 0.01 by Student’s t-test.
(D). MDSC subsets were sorted from flox or Prkaa1KO mice carrying s.c. LLC tumors for 17 days, and evaluated for their ability to kill EL4 tumors, as described in Methods. Bar graphs are Mean ± s.e.m of triplicates from 4 independent repeats. Ns > 0.05, **p < 0.01 by Student’s t-test.
(E). Sorted tumor-M-MDSC from flox and Prkaa1KO mice bearing LLC tumors for 17 days were co-injected at a 1:1 ratio with LLC cells into control (left) and RagKO (right) mice as in (B-C). Tumor volume was then assessed. N=5/group. **p < 0.01 by Student’s t-test.
(F). Nos2 expression by flow cytometry (left) and western blot (right) in control and Prkaa1KO M-MDSC sorted from mice bearing LLC-tumors. N=4. *p < 0.05.
(G) Cytotoxicity assay of M-MDSC on EL4 cells (D) was tested in the presence of the Nos2 inhibitors L-NMMA or L-NIL. Mean ± s.e.m of N=4. Ns > 0.05, **p < 0.01 by Student’s t-test.
(H) Tumor growth in flox and Prkaa1KO mice bearing LLC tumors treated daily with L-NIL (20 mg/kg, i.p.) or vehicle starting the day of tumor injection. Average tumor volume ± s.e.m in 5 mice/group. **p < 0.01 using unpaired Student’s t-test.
(I). Tumor-M-MDSC were sorted from mice from (H) and evaluated for the ability to eliminate EL4 tumor cells in vitro. Mean ± s.e.m of triplicates of N=3. Ns > 0.05, **p < 0.01 by unpaired Student’s t-test.
(J) Wildtype (WT) and Nos2KO mice were injected with s.c. LLC tumors and treated 9 days later with CC (15 mg/kg, i.t.) or vehicle, as described in the Methods. Average tumor volume ± s.e.m in 4 mice/group. *p < 0.05 using unpaired Student’s t-test.
(K). Tumor-M-MDSC sorted from mice from (I) were tested for the ability to kill EL4 tumor cells in vitro. Mean ± s.e.m of triplicates of N=4. Ns > 0.05, ***p < 0.001 by unpaired Student’s t-test.
Discussion
Our study reveals a new role of AMPKα as a direct mediator of the immunosuppressive activity and differentiation of MDSC in tumors and suggests the therapeutic potential of inhibiting AMPK signaling as a strategy to restore protective myelopoiesis in cancer.
Upregulation and phosphorylation of AMPKα are key steps in the cellular adaptation to various stress conditions, including nutrient deprivation, elevation of intracellular AMP-ADP/ATP ratio, accumulation of reactive species, and hypoxia (10). Priming of AMPKα1 occurs through its phosphorylation by the liver-kinase-B1 (LKB1) and the calcium/calmodulin-dependent kinase kinase 2 (CAMKK2) (13, 14); and aims to restore energy homeostasis in stressed cells by inhibiting anabolic processes consuming ATP and by activating catabolic signals that generate ATP (15-17). Interestingly, stimulation of AMPK signaling in cancer cells has emerged as a key regulator of anti-tumor immune responses. Activation of AMPK by aerobic glycolysis in breast tumors promoted MDSC expansion through the production of G-CSF and GM-CSF (35). Also, rapidly proliferating cancer cells imposed nutrient competition and accumulation of multiple reactive metabolites, such as adenosine, that activated AMPK in tumor-associated myeloid cells (36). In contrast to the immunosuppressive effects of cancer cell-expressed AMPK, stimulation of AMPK in breast cancer tumors through the AMPK agonist, Metformin, endogenously induced phosphorylation and degradation of PD-L1, thereby promoting protective T-cell immunity (37). Thus, priming of AMPK signaling in malignant cells can activate both anti-tumor and pro-tumor responses.
Our results show that treatment of tumor-bearing mice with CC or conditional deletion of Prkaa1 in myeloid cells blunted the immunoregulatory function of MDSC and improved protective T-cell immunity. Similarly, inhibition of AMPK by CC thwarted the expression of the immune inhibitory factor Arginase I in bone marrow-derived MDSC treated with GM-CSF and IL-6 (28). The immunoregulatory effects of AMPK are not restricted to MDSC. Indeed, previous reports described the role of AMPK in the activation of immunosuppressive phenotypes in macrophages and in the restriction of effector T-cell expansion in tumors (12, 38). In contrast to our argument that AMPK drives the tolerogenic actions of MDSC, treatment of tumor-bearing mice with the AMPK agonists Metformin, Phenformin, or OSU-53 exerted anti-tumor activities that correlated with a lower immunosuppressive function of MDSC (19-25). Conversely, additional studies showed that treatment of M-MDSC with Metformin promoted their immunosuppressive activity in allogeneic skin grafts (26); and enhanced MDSC response to the immunoinhibitory factor prostaglandin E2 in models of doxorubicin-resistant tumors (27). The paradoxical effects induced by Metformin, and other AMPK activators, on the activity of MDSC could be explained by several possibilities, including the potential combined effects of the modulation of AMPK in different cellular populations in the TME, the levels of the nutritional stress and AMPK signaling in different tumor models, and potential off-target effects (39). In agreement with the AMPKα-independent effects of Metformin (39-41), our results showed that the intrinsic effects induced by Metformin in MDSC were not responsive to the deletion of Prkaa1. Similarly, the anti-tumor effects induced by Metformin in LLC-bearing mice were not impacted by the elimination of Prkaa1 in myeloid cells. Because Metformin has been reported to modulate additional targets, including mTORC1, Protein Kinase A and mitochondrial Glycerophosphate Dehydrogenase (39-41), development of therapeutic models in specific deficient mice for these targets will enable to establish the precise mechanism of action of Metformin in MDSC.
Previous results demonstrated the driving effect of the TME in the differentiation of M-MDSC into TAM (7-9). Our findings show that deletion of Prkaa1 antagonized M-MDSC-to-TAM differentiation, which agrees with a recent report highlighting the role of Ampkα1 in the differentiation of atherosclerosis-linked monocytes-to-macrophages (42). Notably, although AMPK has been previously shown to control anti-inflammation signals in macrophages (11, 12), we did not observe alterations in the immunosuppressive activity or changes in the levels of Arginase 1 or Nos2 in Prkaa1KO TAM, indicating that Prkaa1 deletion impairs differentiation of M-MDSC-to-TAM, but not TAM immunoregulatory activity. Mechanistic mediators for the M-MDSC-to-TAM differentiation process include the induction of the hypoxia-inducible factor-1 alpha (HIF-1α), the decreased transcriptional activity of Stat-3, the activation of the RAR-related orphan receptor C (RORC1), Notch, or Aryl hydrocarbon receptor (AhR), and the signaling mediated by M-CSF and GM-CSF receptors (8, 9, 43-45). Although the mechanisms by which AMPKα1 regulates M-MDSC-to-TAM differentiation remain to be elucidated, it is conceivable that the effect of TME-derived GM-CSF in the transcriptional induction of Prkaa1, combined with relevant stress mediators in the TME such as hypoxia or nutrient deprivation, could induce activation of Ampkα1 and promote M-MDSC-to-TAM differentiation. Moreover, accumulation of anti-inflammatory cytokines, IL-10 and TGF-β in the TME could modulate AMPK and regulate M-MDSC-to-TAM differentiation (36).
The immunosuppressive activity of MDSC is regulated in part through an increased co-expression of Arginase I and Nos2 and the production of peroxynitrite (6). Paradoxically, Nos2-dependent tumoricidal and immunogenic anti-tumor effects were induced in TIP-DCs following T-cell ACT (46). In addition, treatment of tumor-bearing mice with the synthetic double-stranded RNA analog Poly-I:C transformed MDSC into cells that produced higher levels of nitric oxide and exhibited cytotoxic activity against cancer cells (47). Similarly, our results demonstrate that Prkaa1KO M-MDSC triggered direct anti-tumor cytotoxic actions in a Nos2-dependent manner. Also, the upregulation of Nos2 in Prkaa1KO M-MDSC complements previous reports showing the inhibitory effect of AMPK on the expression of Nos2 in macrophages, myocytes, and adipocytes (48). An important question unaddressed by our results is how the expression of Nos2 in MDSC could elicit paradoxical immunosuppressive or cytotoxic anti-tumor effects. Although the mechanisms mediating these opposite processes remain unknown, it is conceivable that the availability of the amino acid L-arginine could play a role. In fact, the expression of Arginase I in tumor-MDSC decreases the availability of the amino acid L-Arginine (49), which uncouples Nos2 to generate peroxynitrite rather than nitric oxide (50). Because we observed a dramatic decrease in the expression of Arginase I correlating with higher levels of Nos2 in Prkaa1KO MDSC, it is possible that the production of immunosuppressive peroxynitrite in M-MDSC is replaced for tumor-cytotoxic nitric oxide. This prediction remains to be tested.
In summary, our results demonstrate the primary role of AMPKα1 in the immunosuppressive activities induced by tumor-MDSC; and provide strategies to overcome MDSC-driven T-cell dysfunction in tumors, which could enhance the effects of different forms of immunotherapy.
Supplementary Material
Statement of Significance. AMPK alpha-1 regulates the immunosuppressive activity and differentiation of tumor-MDSC, suggesting AMPK inhibition as a potential therapeutic strategy to restore protective myelopoiesis in cancer.
Acknowledgments
Authors would like to thank J. Kroger from the Flow Cytometry core; S. McCarthy from the CLIA Tissue Imaging core, and Janis De la Iglesia, PhD for their insightful advice. This work was partially supported by R01-CA184185 and R01-CA233512 to P.C.R.; and R01-CA157664, R01-CA124515, R01-CA178687, R01-CA211913 and U01-CA232758 to JRCG. Support for shared resources was provided by Cancer Center Support Grant (CCSG) CA076292 to H. Lee Moffitt Cancer Center.
Financial support: This work was partially supported by R01-CA184185 and R01-CA233512 to P.C.R.; and R01-CA157664, R01-CA124515, R01-CA178687, R01-CA211913 and U01-CA232758 to JRCG. Support for shared resources was provided by Cancer Center Support Grant (CCSG) CA076292 to H. Lee Moffitt Cancer Center.
Abbreviations:
- ACT
Adoptive T-cell therapy
- Aica-R
5-Aminoimidazole-4-carboxamide 1-β-D-ribofuranoside
- Ampkα
AMP-activated protein kinase α
- CAMKK2
calcium/calmodulin-dependent kinase kinase 2
- CC
compound C
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte-monocyte colony-stimulating factor
- LLC
Lewis lung carcinoma
- LKB1
liver-kinase-B1
- TES
LLC tumor explants
- L-NIL
Lysine dihydrochloride
- L-NMMA
L-NG-Monomethylarginine
- MDSC
Myeloid-derived suppressor cells
- PMN-MDSC
granulocytic
- M-MDSC
monocytic
- Nos2
nitric oxide synthase 2
- PNT
peroxynitrite
- ROS
reactive oxygen species
- Stat-5
signal transduction and activator of transcription 5
- TAM
tumor-associated macrophages
- TME
tumor microenvironment
Footnotes
Disclosure of conflict of interest: The authors declare no potential conflicts of interest.
References
- 1.Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119:2991–3002. [DOI] [PubMed] [Google Scholar]
- 2.Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming V, Hu X, Weber R, Nagibin V, Groth C, Altevogt P, et al. Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Front Immunol 2018;9:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 2010;70:5728–39. [DOI] [PubMed] [Google Scholar]
- 8.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia D, Shaw RJ. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol Cell. 2017;66:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaibhav K, Braun M, Khan MB, Fatima S, Saad N, Shankar A, et al. Remote ischemic post-conditioning promotes hematoma resolution via AMPK-dependent immune regulation. J Exp Med. 2018;215:2636–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawley SA, Fullerton MD, Ross FA, Schertzer JD, Chevtzoff C, Walker KJ, et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012;336:918–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. [DOI] [PubMed] [Google Scholar]
- 15.Kim SJ, Tang T, Abbott M, Viscarra JA, Wang Y, Sul HS. AMPK Phosphorylates Desnutrin/ATGL and Hormone-Sensitive Lipase To Regulate Lipolysis and Fatty Acid Oxidation within Adipose Tissue. Mol Cell Biol. 2016;36:1961–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeon SM, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabinovitch RC, Samborska B, Faubert B, Ma EH, Gravel SP, Andrzejewski S, et al. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell Rep. 2017;21:1–9. [DOI] [PubMed] [Google Scholar]
- 18.Al-Khami AA, Zheng L, Del Valle L, Hossain F, Wyczechowska D, Zabaleta J, et al. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Oncoimmunology. 2017;6:e1344804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, et al. Metformin-Induced Reduction of CD39 and CD73 Blocks Myeloid-Derived Suppressor Cell Activity in Patients with Ovarian Cancer. Cancer Res. 2018;78:1779–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin G, Lian J, Huang L, Zhao Q, Liu S, Zhang Z, et al. Metformin blocks myeloid-derived suppressor cell accumulation through AMPK-DACH1-CXCL1 axis. Oncoimmunology. 2018;7:e1442167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Li M, Trousil S, Zhang Y, Pasca di Magliano M, Swanson KD, et al. Phenformin Inhibits Myeloid-Derived Suppressor Cells and Enhances the Anti-Tumor Activity of PD-1 Blockade in Melanoma. J Invest Dermatol. 2017;137:1740–8. [DOI] [PubMed] [Google Scholar]
- 22.Uehara T, Eikawa S, Nishida M, Kunisada Y, Yoshida A, Fujiwara T, et al. Metformin induces CD11b+ cell-mediated growth inhibition of an osteosarcoma: implications for metabolic reprogramming of myeloid cells and antitumor effects. Int Immunol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishii N, Matsumura T, Kinoshita H, Motoshima H, Kojima K, Tsutsumi A, et al. Activation of AMP-activated protein kinase suppresses oxidized low-density lipoprotein-induced macrophage proliferation. J Biol Chem. 2009;284:34561–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trikha P, Plews RL, Stiff A, Gautam S, Hsu V, Abood D, et al. Targeting myeloid-derived suppressor cells using a novel adenosine monophosphate-activated protein kinase (AMPK) activator. Oncoimmunology. 2016;5:e1214787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Veirman K, Menu E, Maes K, De Beule N, De Smedt E, Maes A, et al. Myeloid-derived suppressor cells induce multiple myeloma cell survival by activating the AMPK pathway. Cancer Lett. 2019;442:233–41. [DOI] [PubMed] [Google Scholar]
- 26.Wu T, Zhao Y, Wang H, Li Y, Shao L, Wang R, et al. mTOR masters monocytic myeloid-derived suppressor cells in mice with allografts or tumors. Sci Rep. 2016;6:20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rong Y, Yuan CH, Qu Z, Zhou H, Guan Q, Yang N, et al. Doxorubicin resistant cancer cells activate myeloid-derived suppressor cells by releasing PGE2. Sci Rep. 2016;6:23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammami I, Chen J, Murschel F, Bronte V, De Crescenzo G, Jolicoeur M. Immunosuppressive activity enhances central carbon metabolism and bioenergetics in myeloid-derived suppressor cells in vitro models. BMC Cell Biol. 2012;13:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svoronos N, Perales-Puchalt A, Allegrezza MJ, Rutkowski MR, Payne KK, Tesone AJ, et al. Tumor Cell-Independent Estrogen Signaling Drives Disease Progression through Mobilization of Myeloid-Derived Suppressor Cells. Cancer Discov. 2017;7:72–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–91. [DOI] [PubMed] [Google Scholar]
- 31.Sierra RA, Trillo-Tinoco J, Mohamed E, Yu L, Achyut BR, Arbab A, et al. Anti-Jagged Immunotherapy Inhibits MDSCs and Overcomes Tumor-Induced Tolerance. Cancer Res. 2017;77:5628–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–37. [PMC free article] [PubMed] [Google Scholar]
- 33.Morales JK, Kmieciak M, Knutson KL, Bear HD, Manjili MH. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1- bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res Treat. 2010;123:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casacuberta-Serra S, Pares M, Golbano A, Coves E, Espejo C, Barquinero J. Myeloid-derived suppressor cells can be efficiently generated from human hematopoietic progenitors and peripheral blood monocytes. Immunol Cell Biol. 2017;95:538–48. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Tanikawa T, Kryczek I, Xia H, Li G, Wu K, et al. Aerobic Glycolysis Controls Myeloid-Derived Suppressor Cells and Tumor Immunity via a Specific CEBPB Isoform in Triple-Negative Breast Cancer. Cell Metab. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biswas SK. Metabolic Reprogramming of Immune Cells in Cancer Progression. Immunity. 2015;43:435–49. [DOI] [PubMed] [Google Scholar]
- 37.Cha JH, Yang WH, Xia W, Wei Y, Chan LC, Lim SO, et al. Metformin Promotes Antitumor Immunity via Endoplasmic-Reticulum-Associated Degradation of PD-L1. Mol Cell. 2018;71:606–20 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu YP, Brown JR, Sag D, Zhang L, Suttles J. Adenosine 5’-monophosphate-activated protein kinase regulates IL-10-mediated anti-inflammatory signaling pathways in macrophages. J Immunol. 2015;194:584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saeedi R, Parsons HL, Wambolt RB, Paulson K, Sharma V, Dyck JR, et al. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am J Physiol Heart Circ Physiol. 2008;294:H2497–506. [DOI] [PubMed] [Google Scholar]
- 40.Foretz M, Hebrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56:1898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Zhu H, Ding Y, Liu Z, Cai Z, Zou MH. AMP-activated protein kinase alpha1 promotes atherogenesis by increasing monocyte-to-macrophage differentiation. J Biol Chem. 2017;292:7888–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strauss L, Sangaletti S, Consonni FM, Szebeni G, Morlacchi S, Totaro MG, et al. RORC1 Regulates Tumor-Promoting “Emergency” Granulo-Monocytopoiesis. Cancer Cell. 2015;28:253–69. [DOI] [PubMed] [Google Scholar]
- 44.Kumar V, Cheng P, Condamine T, Mony S, Languino LR, McCaffrey JC, et al. CD45 Phosphatase Inhibits STAT3 Transcription Factor Activity in Myeloid Cells and Promotes Tumor-Associated Macrophage Differentiation. Immunity. 2016;44:303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Overmeire E, Stijlemans B, Heymann F, Keirsse J, Morias Y, Elkrim Y, et al. M-CSF and GM-CSF Receptor Signaling Differentially Regulate Monocyte Maturation and Macrophage Polarization in the Tumor Microenvironment. Cancer Res. 2016;76:35–42. [DOI] [PubMed] [Google Scholar]
- 46.Marigo I, Zilio S, Desantis G, Mlecnik B, Agnellini AHR, Ugel S, et al. T Cell Cancer Therapy Requires CD40-CD40L Activation of Tumor Necrosis Factor and Inducible Nitric-Oxide-Synthase-Producing Dendritic Cells. Cancer Cell. 2016;30:377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shime H, Matsumoto M, Seya T. Double-stranded RNA promotes CTL-independent tumor cytolysis mediated by CD11b(+)Ly6G(+) intratumor myeloid cells through the TICAM-1 signaling pathway. Cell Death Differ. 2017;24:385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pilon G, Dallaire P, Marette A. Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase: a new mechanism of action of insulin-sensitizing drugs. J Biol Chem. 2004;279:20767–74. [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, et al. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–49. [DOI] [PubMed] [Google Scholar]
- 50.Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proc Natl Acad Sci U S A. 1997;94:6954–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.