Abstract
Objectives:
Zidovudine (ZDV) is a nucleoside reverse transcriptase inhibitor that could cause telomere shortening through inhibition of telomerase. We examined the association between in utero exposure to ZDV and telomere length (TL) at birth in HIV-exposed uninfected (HEU) newborns.
Methods:
We selected 94 ZDV-exposed HEU children and 85 antiretroviral therapy (ART)-unexposed HEU children from the Surveillance Monitoring for ART Toxicities Study and the Women and Infants Transmission Study. We assessed relative TL in stored peripheral blood mononuclear cells taken in the first 7 days of life using quantitative polymerase chain reaction. We used linear regression to compare relative TL between ZDV-exposed and ART-unexposed children. We additionally evaluated relative TL according to maternal and infant characteristics.
Results:
Relative TL was longer in ZDV-exposed children compared to ART-unexposed individuals (adjusted mean ratio difference 0.21, 95%CI 0.15–0.28, p<0.001). We found an inverse correlation between maternal HIV RNA levels and infant relative TL (-0.06 per log10 copies, 95%CI −0.08 to −0.03, p<0.001). Relative TL was not associated with maternal CD4 count, maternal age, gestational age, sex, sample storage time, or maternal substance use (p>0.05).
Conclusion:
Relative TL was longer in ZDV-exposed infants. This difference may reflect beneficial health effects of antiretroviral therapy during pregnancy, since we observed an inverse association with maternal HIV RNA levels.
Keywords: HIV-exposed uninfected, pregnancy, zidovudine, nucleoside reverse transcriptase inhibitor, telomere length
INTRODUCTION
Antiretroviral therapy (ART) has dramatically reduced AIDS morbidity and mortality as well as mother-to-child transmission of HIV. Zidovudine (ZDV) was introduced in 1994 to prevent vertical transmission and has been widely used [1], usually with other antiretrovirals. In the US as of 2009, 73% of HIV-exposed but uninfected (HEU) children (born to HIV-infected mothers) were exposed in utero to ZDV [2].
ZDV is a nucleoside reverse transcriptase inhibitor (NRTI) with antiretroviral effects that include competitive inhibition of nucleoside binding to HIV reverse transcriptase and DNA chain-termination. Carcinogenic properties of ZDV have been suggested based on in vitro and animal studies (summarized in [3]). In response to existing evidence, the International Agency for Research on Cancer classified ZDV as possibly carcinogenic to humans [3].
Telomeres are nucleoprotein complexes located at chromosome ends that protect chromosome integrity. Telomeres shorten with each cell division due to incomplete replication of the 3’ DNA end. Telomerase is a reverse transcriptase that extends the TTTAGG(n) nucleotide repeats at chromosome ends. Since NRTIs including ZDV are known to inhibit telomerase activity in vitro [4, 5], it has been postulated that telomere length (TL) in individuals taking ZDV could be shorter than normal. Shorter TL after ZDV exposure has been observed in vitro and in animal studies [6, 7].
It is important to determine whether NRTIs affect TL, because abnormal TL is implicated in diseases including both cancer [8] and cardiovascular disease [9]. In HIV infected individuals, ART including NRTIs has been shown to increase mean blood TL [10, 11]. However, data on HEU children after in utero NRTI exposure are limited. A prior epidemiologic study of HEU children found no association between leukocyte TL and in utero ART exposure (predominately ZDV-based regimens) [12], while another study showed longer leukocyte TL in HEU children exposed to ZDV, lamivudine, and nevirapine or nelfinavir than in HIV-unexposed uninfected children [13]. However, these studies were limited by a small number of ART-unexposed controls (n=39) [12] or lack of comparison with ART-unexposed HEU children [13]. In the current study, we examine the association between in utero exposure to ZDV and peripheral blood mononuclear cell TL in newborn HEU children.
METHODS
This study included data and specimens from 179 HEU children; 94 were exposed to ZDV in utero (most of whom were also exposed to other ART medications), and 85 were unexposed to ZDV or other ART medications. ZDV-exposed children were born during 2007–2017 (except one in 1995), and ART-unexposed children during 1990–1996 (except one in 2017). ZDV-exposed children were selected from the Surveillance Monitoring for ART Toxicities (SMARTT) Study as part of the Pediatric HIV/AIDS Cohort Study network, which has enrolled children with detailed in utero ART exposure data into two cohorts from 22 sites in the US and Puerto Rico; its Static cohort includes HEU children born as early as 1995 and the Dynamic cohort includes HEU children born in 2007 through the present [14, 15]. ART-unexposed children were mostly from the Women and Infants Transmission Study (WITS, n=84; n=1 from SMARTT), which enrolled pregnant women living with HIV and their children from 6 US sites during 1989–2003 [16]. Adopting a previously applied method [17], we used a historical comparison group of ART-unexposed children, because a contemporaneous unexposed group in the current ART era in the US would oversample children whose mothers were not receiving proper medical care. Children were frequency-matched on sex, race, and maternal substance use.
We retrieved information on maternal ART during pregnancy and participant characteristics at birth including maternal age, gestational age, birthweight, and latest maternal HIV RNA levels and CD4 cell counts before delivery. Information on women’s race/ethnicity, smoking, alcohol consumption, and illicit substance use was obtained by self-report. All mothers provided written informed consent for enrollment into SMARTT and/or WITS. The present study used deidentified samples and was exempted from human subjects review by the Office of Human Subjects Research Protections at the National Institutes of Health.
Peripheral blood mononuclear cells (PBMCs) were obtained in the first 7 days of life and frozen. DNA was extracted in a single batch using the same method. TL was measured using quantitative polymerase chain reaction (qPCR), adapted from [18] and described in [19]. Briefly, the ratio of amplified signals for telomere (T) and an autosomal single copy gene 36B4 (S) was normalized to internal quality control samples to yield a standardized T/S ratio (hereafter referred to as “relative TL”). All samples were assayed in triplicate and average values were used for statistical analyses. The coefficient of variation for control samples across the three plates was 4.9%.
We compared demographic and clinical characteristics between ZDV-exposed and ART-unexposed children using chi-square, Fisher exact, and Wilcoxon rank sum tests. Linear regression was used to evaluate the association between ZDV and relative TL and a stepwise method for covariate selection was used in adjusted models (p=0.15 for entry and exit). Statistical analyses were performed using SAS version 9.4 or R version 3.4.4. Statistical significance was defined using two-sided p<0.05.
RESULTS
Compared with ART-unexposed children, ZDV-exposed children had lower gestational age at birth (median, 38.2 versus 39 weeks, p<0.001). Mothers of ZDV-exposed children were older at delivery (median, 28.5 versus 26.0 years, p=0.003) and were more likely to have suppressed HIV RNA < 400 copies/ml (88% versus 12%, p<0.001) than mothers of ART-unexposed children. Maternal tobacco, alcohol and marijuana use (no mothers used illicit drugs other than marijuana) during pregnancy did not differ (Table 1). Among ZDV-exposed children, 78% were exposed to ZDV during the first trimester, and all were exposed during the second and third trimesters (median duration 29.4 weeks). About 88% of the ZDV-exposed children were also exposed to other antiretrovirals (8.5% non-nucleoside reverse transcriptase inhibitors [NNRTIs] but not protease inhibitors [PIs], 63.8% PIs but not NNRTIs, 10.6% to both NNRTIs and PIs). About 98% of ZDV-exposed children were also exposed to other NRITs (Supplemental Table 1). The most frequent concomitant NRTI used was lamivudine (90.4%), followed by tenofovir (33.0%), emtricitabine (28.7%) and abacavir (18.1%).
Table 1.
Participant characteristics
| Variable | ZDV-exposed (N=94) | ART-unexposed (N=85) | p | ||
|---|---|---|---|---|---|
| Birth weight (kg), median (range) | 3.0 | (1.7, 4.4) | 3.1 | (1.9, 4.6) | 0.0261 |
| Birth weight, n (%) | 0.242 | ||||
| 1,500–2,500 gm | 13 | (14%) | 7 | (8%) | |
| ≥ 2,500 gm | 81 | (86%) | 78 | (92%) | |
| Gestational age (weeks), median (range) | 38.2 | (32.6, 42.1) | 39.0 | (32.0, 43.0) | <0.0011 |
| Gestational age, n (%) | 0.313 | ||||
| 32 to <34 weeks | 1 | (1%) | 1 | (1%) | |
| 34 to <37 weeks | 16 | (17%) | 8 | (9%) | |
| 37 weeks or more | 77 | (82%) | 76 | (89%) | |
| Sex, n (%) | 0.852 | ||||
| Male | 50 | (53%) | 44 | (52%) | |
| Female | 44 | (47%) | 41 | (48%) | |
| Group, n (%) | <0.0013 | ||||
| Public WITS | 0 | (0%) | 84 | (99%) | |
| SMARTT WITS | 1 | (1%) | 0 | (0%) | |
| SMARTT w/o WITS | 93 | (99%) | 1 | (1%) | |
| Year of birth, n (%) | <0.0012 | ||||
| 1990–1994 | 0 | (0%) | 82 | (96%) | |
| 1995–2017 | 94 | (100%) | 3 | (4%) | |
| Mothers age at delivery (years), median (range) | 28.5 | (16.9, 45.0) | 26.0 | (15.0, 40.0) | 0.0031 |
| Participant’s race/ethnicity, n (%) | 0.013 | ||||
| White | 6 | (6%) | 13 | (15%) | |
| African American | 33 | (35%) | 37 | (44%) | |
| Hispanic | 53 | (56%) | 29 | (34%) | |
| Other | 2 | (2%) | 6 | (7%) | |
| Days from birth to sample draw, median (range) | 1.0 | (0, 7.0) | 1.0 | (0, 6.9) | 0.761 |
| Last CD4 count during pregnancy (cells/mm3), median (range) | 502.0 | (34.0, 1198.0) | 595.0 | (74.0, 2330.0) | 0.071 |
| Last RNA level during pregnancy (copies/ml), median (range) | 48 | (0, 64361) | 8079 | (0, 672005) | <0.0011 |
| <400 | 82 | (88%) | 10 | (12%) | <0.0012 |
| ≥400 | 11 | (12%) | 75 | (88%) | |
| Mother ever used tobacco, n (%) | 0.272 | ||||
| Yes | 26 | (28%) | 30 | (35%) | |
| No | 68 | (72%) | 55 | (65%) | |
| Mother ever used alcohol, n (%) | 0.112 | ||||
| Yes | 24 | (26%) | 31 | (36%) | |
| No | 70 | (74%) | 54 | (64%) | |
| Mother ever used marijuana/hashish, n (%) | 0.192 | ||||
| Yes | 15 | (16%) | 8 | (9%) | |
| No | 79 | (84%) | 77 | (91%) | |
| NNRTIs use during pregnancy, n (%) | <0.0012 | ||||
| Yes | 18 | (19%) | 0 | (0%) | |
| No | 76 | (81%) | 85 | (100%) | |
| PIs use during pregnancy, n (%) | <0.0012 | ||||
| Yes | 70 | (74%) | 0 | (0%) | |
| No | 24 | (26%) | 85 | (100%) | |
Wilcoxon rank-sum test
Chi-square test
Fisher’s exact test
Abbreviations: ZDV: zidovudine; ART: antiretroviral therapy; WITS: The Women and Infants Transmission Study; SMARTT: The Surveillance Monitoring for ART Toxicities Study; NNRTIs: non-nucleoside reverse transcriptase inhibitors; PIs: protease inhibitors
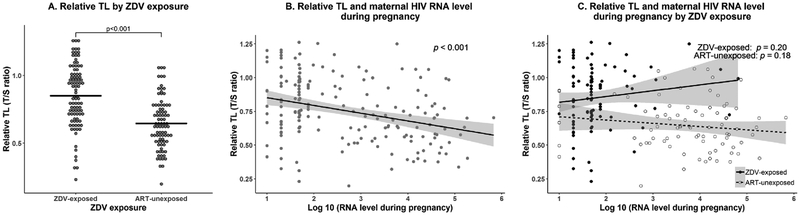
ZDV exposure was associated with longer relative TL at birth (mean±standard deviation T/S, 0.85±0.23 versus 0.65±0.19, p<0.001) (Figure 1A). After adjusting for race, ZDV exposure remained statistically significantly associated with longer relative TL (adjusted mean T/S difference, 0.21, 95%CI 0.15–0.28, p<0.001). Further adjustment for gestational age and birth weight did not change the result (adjusted mean T/S difference, 0.21, 95%CI 0.14–0.27, p<0.001). Among ZDV-exposed children, the duration of ZDV exposure was not associated with relative TL (p=0.45, Supplemental Figure 1). In addition, no statistically significant differences were noted between children exposed to ZDV and PIs and those exposed to ZDV without PIs (p=0.08), or between children exposed to ZDV and NNRTIs and those exposed to ZDV without NNRTIs (p=0.33) (Supplemental Figure 2A–B).
Figure 1. Association of relative telomere length at birth with zidovudine exposure in utero and mother’s HIV RNA level during pregnancy.
a. Associations between relative telomere length at birth and zidovudine exposure in utero. Horizontal bars denote mean relative telomere length values.
b. Associations between relative telomere length at birth and mother’s last HIV RNA level during pregnancy for all subjects. RNA level data were log10 transformed.
c. Associations between relative telomere length at birth and mother’s last HIV RNA level during pregnancy according to in utero zidovudine exposure status. RNA level data were log10 transformed. Closed circle/solid line: ZDV-exposed, open circle/dotted line: ART-unexposed.
Relative TL was inversely associated with maternal HIV RNA (-0.06 per log10 copies, 95%CI −0.08 to −0.03, p<0.001, Figure 1B). In ZDV exposure stratified analyses, relative TL was not associated with maternal HIV RNA levels in ZDV-exposed (0.04 per log10 copies, p=0.20, Figure 1C) or ART-unexposed children (-0.02 per log10 copies, p=0.18). Relative TL was not associated with maternal CD4 count or other characteristics including maternal age, gestational age, sex, sample storage time, or maternal substance use (Supplemental Figure 3A–H).
DISCUSSION
In this study of 179 newborn HEU children, in utero ZDV exposure was associated with longer relative TL in PBMC. Our results differ from a previous study that found no association between in utero ART exposure and TL [12]. However, that study included few ART-unexposed children. Moreover, ART exposure was shorter in that study (median 20 versus 29.4 weeks in our study) with higher maternal viral loads close to delivery among ART-exposed children (median 1455 versus 48 copies/mL in our study) [12], suggesting that suboptimal viral suppression or a shorter duration of reverse transcriptase inhibition may have influenced the study results.
We found that maternal HIV RNA levels were inversely correlated with infant TL, suggesting effective treatment of HIV contributes to the longer TL in ZDV-exposed children. Longer TL in ZDV-exposed children could be related to better maternal health and more favorable gestational growth environment. Given our modest sample size, we cannot rule out that the longer TLs were present only for a subgroup of ZDV-exposed children related to use of certain other antiretroviral medications as combination ART was common in the ZDV-exposed group (Table 1). It is also possible that ZDV could have had an adverse effect on telomerase and infant TL that was masked by other beneficial effects of ART. Maternal HIV viral load has been associated with changes in CD8 T-cells subsets in HEU children [20]. However, it is unclear whether alterations in T-cell subpopulations could explain our results, and we did not have TL data on leukocyte subtypes to evaluate this hypothesis. Considering the short exposure period (≤7 days) prior to our assessment of TL, we do not expect that ZDV prophylaxis during or after birth to impact TL in HEU children in our study. Not surprisingly, it has been shown that leukocyte TL in HEU newborns is not affected by ZDV prophylaxis [13].
A strength of our study is the use of a control group comprising HEU children without any ART exposure (including NRTIs) in utero. Secondly, matching children on potential confounders reduced the possibility that those influenced our results. Our study also has limitations. Although we selected children matching on important confounders, unmeasured factors may have influenced our findings. Given that ART-unexposed children were born earlier than ZDV-exposed children, differences in sample storage time or advances in prenatal care over time could have played a role in the observed TL differences. There has also been growing use of other NRTIs over time, and these may have different in utero effects on TL. Additionally, qPCR measurement of relative TL is influenced by laboratory factors [21]. However, DNA in this study was extracted from frozen PBMCs in a single batch using the same method. Lastly, due to the small sample size of our study, we were limited in our ability to examine subgroups.
In conclusion, we observed that ZDV use during pregnancy was associated with longer relative TL at birth relative to ART-unexposed HEU children. Future studies are needed to elucidate biological mechanisms linking in utero antiretroviral exposure to PBMC TL in newborns.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the children and families for their participation in WITS and PHACS, and the individuals and institutions involved in the conduct of WITS and PHACS. Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson). The following institutions, clinical site investigators and staff participated in conducting PHACS SMARTT in 2017, in alphabetical order: Ann & Robert H. Lurie Children’s Hospital of Chicago: Ellen Chadwick, Margaret Ann Sanders, Lynn Heald, Ruthellen Williams; Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynnette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Emma Stuard, Mahboobullah Mirza Baig, Alma Villegas; Children’s Diagnostic & Treatment Center: Ana Puga, Dia Cooley, Patricia A. Garvie, James Blood; New York University School of Medicine: William Borkowsky, Sandra Deygoo, Marsha Vasserman; Rutgers - New Jersey Medical School: Arry Dieudonne, Linda Bettica, Juliette Johnson; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins, Jamie Russell-Bell; San Juan Hospital/Department of Pediatrics: Nicolas Rosario, Lourdes Angeli-Nieves, Vivian Olivera; SUNY Downstate Medical Center: Stephan Kohlhoff, Ava Dennie, Ady Ben-Israel, Jean Kaye; Tulane University School of Medicine: Russell Van Dyke, Karen Craig, Patricia Sirois; University of Alabama, Birmingham: Marilyn Crain, Paige Hickman, Dan Marullo; University of California, San Diego: Stephen A. Spector, Kim Norris, Sharon Nichols; University of Colorado, Denver: Elizabeth McFarland, Emily Barr, Christine Kwon, Carrie Chambers; University of Florida, Center for HIV/AIDS Research, Education and Service: Mobeen Rathore, Kristi Stowers, Saniyyah Mahmoudi, Nizar Maraqa, Laurie Kirkland; University of Illinois, Chicago: Karen Hayani, Lourdes Richardson, Renee Smith, Alina Miller; University of Miami: Gwendolyn Scott, Sady Dominguez, Jenniffer Jimenez, Anai Cuadra; Keck Medicine of the University of Southern California: Toni Frederick, Mariam Davtyan, Guadalupe Morales-Avendano, Janielle Jackson-Alvarez; University of Puerto Rico School of Medicine, Medical Science Campus: Zoe M. Rodriguez, Ibet Heyer, Nydia Scalley Trifilio.
FUNDING
This work was supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health. The Pediatric HIV/AIDS Cohort Study (PHACS), the parental study of the Surveillance Monitoring for ART Toxicities (SMARTT) Study, was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) (Principal Investigator: George Seage; Program Director: Julie Alperen) and the Tulane University School of Medicine (HD052104) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Ellen Chadwick; Project Director: Patrick Davis).
Footnotes
Publisher's Disclaimer: DISCLAIMER
The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
POTENTIAL CONFLICTS OF INTEREST
All authors: No potential conflicts of interest
REFERENCES
- 1.PHACS Data and Operations Center. Pediatric HIV/AIDS Cohort Study (PHACS) SMARTT Annual Administrative Report. May 27, 2016. Boston, MA: Accessed from https://phacsstudy.org/. Accessed on Apr 5, 2019 [Google Scholar]
- 2.Griner R, Williams PL, Read JS, Seage GR 3rd, Crain M, Yogev R, et al. In utero and postnatal exposure to antiretrovirals among HIV-exposed but uninfected children in the United States. AIDS patient care and STDs 2011; 25(7):385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans., International Agency for Research on Cancer Some antiviral and antineoplastic drugs, and other pharmaceutical agents. Lyon, France: IARC; 2000. [Google Scholar]
- 4.Leeansyah E, Cameron PU, Solomon A, Tennakoon S, Velayudham P, Gouillou M, et al. Inhibition of telomerase activity by human immunodeficiency virus (HIV) nucleos(t)ide reverse transcriptase inhibitors: a potential factor contributing to HIV-associated accelerated aging. The Journal of infectious diseases 2013; 207(7):1157–1165. [DOI] [PubMed] [Google Scholar]
- 5.Hukezalie KR, Thumati NR, Côté HCF, Wong JMY. In Vitro and Ex Vivo Inhibition of Human Telomerase by Anti-HIV Nucleoside Reverse Transcriptase Inhibitors (NRTIs) but Not by Non-NRTIs. PLOS ONE 2012; 7(11):e47505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olivero OA, Anderson LM, Diwan BA, Haines DC, Harbaugh SW, Moskal TJ, et al. Transplacental effects of 3’-azido-2’,3’-dideoxythymidine (AZT): tumorigenicity in mice and genotoxicity in mice and monkeys. Journal of the National Cancer Institute 1997; 89(21):1602–1608. [DOI] [PubMed] [Google Scholar]
- 7.Gomez DE, Tejera AM, Olivero OA. Irreversible telomere shortening by 3’-azido-2’,3’-dideoxythymidine (AZT) treatment. Biochemical and biophysical research communications 1998; 246(1):107–110. [DOI] [PubMed] [Google Scholar]
- 8.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nature reviews Genetics 2005; 6(8):611–622. [DOI] [PubMed] [Google Scholar]
- 9.Yeh JK, Wang CY. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016; 7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montejano R, Stella-Ascariz N, Monge S, Bernardino JI, Perez-Valero I, Montes ML, et al. Impact of Nucleos(t)ide Reverse Transcriptase Inhibitors on Blood Telomere Length Changes in a Prospective Cohort of Aviremic HIV-Infected Adults. The Journal of infectious diseases 2018; 218(10):1531–1540. [DOI] [PubMed] [Google Scholar]
- 11.Stella-Ascariz N, Montejano R, Rodriguez-Centeno J, Alejos B, Schwimmer C, Bernardino JI, et al. Blood Telomere Length Changes After Ritonavir-Boosted Darunavir Combined With Raltegravir or Tenofovir-Emtricitabine in Antiretroviral-Naive Adults Infected With HIV-1. The Journal of infectious diseases 2018; 218(10):1523–1530. [DOI] [PubMed] [Google Scholar]
- 12.Imam T, Jitratkosol MH, Soudeyns H, Sattha B, Gadawski I, Maan E, et al. Leukocyte telomere length in HIV-infected pregnant women treated with antiretroviral drugs during pregnancy and their uninfected infants. Journal of acquired immune deficiency syndromes (1999) 2012; 60(5):495–502. [DOI] [PubMed] [Google Scholar]
- 13.Ajaykumar A, Soudeyns H, Kakkar F, Brophy J, Bitnun A, Alimenti A, et al. Leukocyte Telomere Length at Birth and During the Early Life of Children Exposed to but Uninfected With HIV After In Utero Exposure to Antiretrovirals. The Journal of infectious diseases 2018; 217(5):710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams PL, Seage GR 3rd, Van Dyke RB, Siberry GK, Griner R, Tassiopoulos K, et al. A trigger-based design for evaluating the safety of in utero antiretroviral exposure in uninfected children of human immunodeficiency virus-infected mothers. American journal of epidemiology 2012; 175(9):950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams PL, Crain MJ, Yildirim C, Hazra R, Van Dyke RB, Rich K, et al. Congenital anomalies and in utero antiretroviral exposure in human immunodeficiency virus-exposed uninfected infants. JAMA pediatrics 2015; 169(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez EM, Mendez H, Rich K, Sheon A, Fox H, Green K, et al. Maternal drug use in perinatal HIV studies. The Women and Infants Transmission Study. Ann N Y Acad Sci 1993; 693:245–248. [DOI] [PubMed] [Google Scholar]
- 17.Marsit CJ, Brummel SS, Kacanek D, Seage GR, Spector SA, Armstrong DA, et al. Infant peripheral blood repetitive element hypomethylation associated with antiretroviral therapy in utero. Epigenetics 2015; 10(8):708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res 2002; 30(10):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gadalla SM, Wang T, Dagnall C, Haagenson M, Spellman SR, Hicks B, et al. Effect of Recipient Age and Stem Cell Source on the Association between Donor Telomere Length and Survival after Allogeneic Unrelated Hematopoietic Cell Transplantation for Severe Aplastic Anemia. Biology of Blood and Marrow Transplantation 2016; 22(12):2276–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Deus N, Moraleda C, Serna-Bolea C, Renom M, Menendez C, Naniche D. Impact of elevated maternal HIV viral load at delivery on T-cell populations in HIV exposed uninfected infants in Mozambique. BMC infectious diseases 2015; 15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dagnall CL, Hicks B, Teshome K, Hutchinson AA, Gadalla SM, Khincha PP, et al. Effect of pre-analytic variables on the reproducibility of qPCR relative telomere length measurement. PLOS ONE 2017; 12(9):e0184098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.