Abstract
Background:
Previous meta-analyses have estimated summary positive associations between clinical prostatitis and prostate cancer. However, none have accounted for detection bias, the possibility for increased prostate cancer screening and detection in men with clinical prostatitis, in their pooled estimates.
Methods:
We searched for studies that investigated the relation between clinical prostatitis and prostate cancer through November 2018. Random-effects meta-analysis was used to calculate summary odds ratios (ORs) among all studies and in strata defined by methods used to reduce detection bias.
Results:
Although an increased odds of prostate cancer was seen among men with a history of clinical prostatitis in all 38 eligible studies combined (OR=2.05, 95% CI: 1.64-2.57), this estimate attenuated to null among studies that performed the most rigorous analyses to limit detection bias (OR=1.16, 95% CI: 0.77-1.74).
Conclusions:
Our findings indicate that previously reported positive associations between clinical prostatitis and prostate cancer are likely due to detection bias.
Impact:
Studies using rigorous detection bias methods are warranted to replicate these findings, as well as to examine the possible relation between prostate inflammation and prostate cancer directly, rather than indirectly through the diagnosis of “prostatitis”, which includes a large proportion of men without evidence of prostate inflammation.
Keywords: prostatitis, inflammation, prostate cancer, epidemiology, detection bias
Introduction
As many as 20% of all cancers, including those of the colon, liver, stomach, esophagus, and pancreas, are estimated to be caused, at least in part, by chronic inflammation and inflammatory diseases 1,2. Chronic inflammatory processes have also been hypothesized to contribute to the development of other cancers, such as prostate cancer 3,4. One key piece of evidence supporting this hypothesis are findings from epidemiologic studies of clinical prostatitis (ostensibly “prostate inflammation”) and prostate cancer. Overall, meta-analyses of these studies have observed strong positive associations between clinical prostatitis and prostate cancer, supporting a role for prostate inflammation in prostate cancer development. 5-8. However, none of these meta-analyses took into consideration the potential for detection bias in their pooled estimates. This bias may occur if men with clinical prostatitis are more likely to be screened or investigated for prostate cancer than men without prostatitis. For instance, men with clinical prostatitis may be more likely to be screened for prostate cancer than men without symptoms to rule prostate cancer out as a cause of their symptoms or because of continued visits to a urologist following their prostatitis diagnosis. Clinical prostatitis may also cause men’s prostate-specific antigen (PSA) levels to rise 9, possibly contributing to an increased likelihood of prostate biopsy and occult prostate cancer detection. Although many of these possibilities have been recognized by previous authors and addressed in a few individual studies, none of the systematic reviews and meta-analyses performed to date have taken this bias into consideration.
To address this concern, we performed an updated systematic review and meta-analysis of associations between clinical prostatitis and prostate cancer, accounting for detection bias. Our updated meta-analysis also addressed additional methodological issues identified in previous meta-analyses, such as inclusion of duplicate studies 6-8, inclusion of estimates for exposures similar in spelling to, but distinct from, “prostatitis” (i.e., “prostitutes”) 6,7, and exclusion of cohort studies 7.
Materials and Methods
Study eligibility criteria
This study was completed in accordance with guidelines for the conduct of systematic reviews and meta-analyses of observational studies, as well as the PRISMA statement 10,11. All observational studies (cohort, nested case-control, case-control, and cross-sectional) investigating the association between clinical prostatitis and prostate cancer in humans were eligible. Studies with an exclusive focus on asymptomatic inflammation (NIH category IV prostatitis 12) in biopsy or radical prostatectomy specimens were not included.
Search strategy
The published literature was searched from January 1, 1946 to November 30, 2018 without language restrictions, using strategies created by a medical librarian. Search strategies were established and implemented in Ovid Medline, Embase, Scopus, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Cochrane Database of Systematic Reviews, Clinicaltrials.gov, and WHO International Clinical Trials Registry Platform. Standardized terms and key words were used including: prostatitis, prostatic inflammation, prostatic neoplasms, and prostate cancer (Supplementary Methods and Materials). The titles and abstracts of 2306 articles were screened to exclude reviews, case reports, commentaries or editorials, abstracts only, animal studies, and original articles without useable effect estimates (Supplementary Fig. S1). Following abstract review, a manual search was conducted for references cited in selected articles or previous reviews on this topic. In addition, the full text of articles examining genitourinary conditions (e.g., urethral stricture, cystitis, sexually transmitted infections) or procedures (e.g., vasectomy) in relation to prostate cancer were searched for prostatitis effect estimates because many older studies that investigated clinical prostatitis as part of a larger number of possible risk factors – typically other genitourinary conditions or procedures – did not always include references to clinical prostatitis in their abstract. When multiple articles from the same study population were found, the most recent or complete publication was included. One exception was two studies performed among health plan members at Kaiser Permanente 13,14. Although these studies overlapped by some years of enrollment, we retained both studies because of their likely minimal overlap in study participants as Weinmann et. al only included fatal prostate cancer cases. Two independent reviewers performed the literature review with disagreement settled by a third independent reviewer.
Data extraction and quality assessment
Data extraction was completed by two of three independent reviewers. Inconsistencies were re-reviewed by another independent reviewer and disagreement resolved by consensus among all reviewers. Any necessary information not found in the articles was obtained by contacting the corresponding author. Foreign articles were translated by reviewers with native language experience. When multiple estimates were reported in a study, we chose the most fully adjusted estimate. For studies reporting estimates from multiple control groups, we report only estimates derived from population-based and non-cancer hospital controls if possible 15-17. However, studies that did not separate non-cancer from cancer controls were retained 18-20. We assessed the quality of each study using a revised classification system based on the Newcastle-Ottawa Quality Assessment Scale 21. This score ranged from 0 to 8 and included items for: 1) type of controls (i.e., population-based, hospital-based, or with benign prostatic hyperplasia (BPH)), 2) characterization of cases (e.g., incident and histologically confirmed), 3) similarity of response rates between cases and controls, and 4) method of clinical prostatitis assessment (i.e., self-report or medical record-based).
Detection bias assessment
To address detection bias, we identified all studies that accounted for this bias, and reviewed their methods to develop three distinct groups of detection bias methods. These groups were developed without regard to the point estimates of each study and included in order of decreasing methodologic rigor (i.e., from most to least methodologic rigor): 1) Exclusion of prostatitis diagnoses in the short window before prostate cancer detection to reduce the possible influence of recent prostatitis diagnoses or symptoms on incidental prostate cancer detection. Studies within this category provided estimates excluding participants whose prostatitis diagnosis occurred less than 1 year, 2 years, or 5 years before prostate cancer diagnosis for cases or before the index date for controls. 2) Stratification/restriction based on prostate cancer screening to reduce the possible influence of a greater likelihood of routine prostate cancer screening among men diagnosed with prostatitis. Here, studies provided stratified or restricted estimates based on any recent PSA or digital rectal examination (DRE) screening for prostate cancer (1-2 years prior to diagnosis), any lifetime PSA or DRE screening for prostate cancer, number of recent PSA tests, or selection of study populations likely to engage in enhanced prostate cancer screening (i.e., men with BPH, men in the intervention arm of the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial). 3) Adjustment based on prostate cancer screening. Studies within this category adjusted statistical models for any of the previously mentioned screening methods or the frequency of physician visits. These three groups were compared to studies that did not take detection bias into account. Ideally, we would have also created a category for studies that met the criteria for both methods (1) and (2), but too few studies met the criteria for both of these methods to allow for separate analyses. Finally, we performed sensitivity analyses combining studies that used either method (1) or (2), recognizing that these methods could possibly be ranked interchangeably; and analyses combining all three methods.
Statistical analysis
We calculated summary odds ratios (ORs) to describe the association between clinical prostatitis and prostate cancer overall and within strata defined by detection bias methods. For our overall estimate, we performed the analysis in two different ways. First, we selected one estimate per study (typically the most adjusted estimate presented in either the abstract, main table, or conclusion of the manuscript, or in a previous meta-analysis using population-based controls when possible) and pooled these estimates using a traditional random effects model 22,23. This pooled OR was calculated for comparison with previous meta-analysis results, and heterogeneity was evaluated using the I2 statistic. For our second method, we included all maximally-adjusted estimates from each detection bias stratum per study and used a random effects model with robust variance estimation to account for inclusion of multiple estimates per study 24-26. A separate heterogeneity statistic was not calculated for this overall estimate because robust variance estimation incorporates between-study heterogeneity into pooled estimates and is robust to misspecification of the underlying covariance structure 24.
To address detection bias, we calculated separate pooled estimates within strata defined by detection bias methods. To evaluate the incremental influence of detection bias methods on the summary point estimates, we assigned a score based on the rigor of each method and modeled as a linear function in the meta-regression model (i.e., a test for linear trend 27). We also performed several subgroup and sensitivity analyses to assess the possible influence of other factors on our summary estimates. These included: 1) analyses stratified by study design; type of controls; prostatitis assessment method; study location and participant race/ethnicity; study quality; and age at prostate cancer diagnosis; 2) analyses restricted to studies assessing “chronic” prostatitis, as chronic exposures are typically more likely to contribute to cancer than acute exposures 8; 3) analyses excluding studies with control selection based on the presence or absence of benign prostatic conditions including BPH; 4) analyses limited to aggressive prostate cancer (SEER grade III-IV, or Gleason grade ≥7); and 5) analyses taking into consideration prostatitis treatment or regular anti-inflammatory drug use. Each of these analyses accounted for detection bias methodology employed (any versus none). Finally, to examine the influence of potentially unpublished studies on the summary OR estimates, funnel plots were created by regressing the log OR on the sample size and weighting by the inverse pooled variance 28. All analyses were performed in STATA version 15.
Results
A total of 38 studies were selected for inclusion in the final meta-analysis. Of these studies, 10 were prospective (3 cohort and 7 nested case-control), 26 were case-control (14 population-based, 6 hospital-based, 3 population and hospital-based, 2 family-based, and 1 BPH-based controls), and 2 were cross-sectional. Studies were derived from diverse populations in North America (n=24), Europe (n=3), Australia (n=1), the Middle East (n=2), and Asia (n=8). Ten included populations comprising at least 20% men of African descent (African American men and men of African descent from Barbados). Sample sizes for included studies ranged from 124 to 35,586 participants.
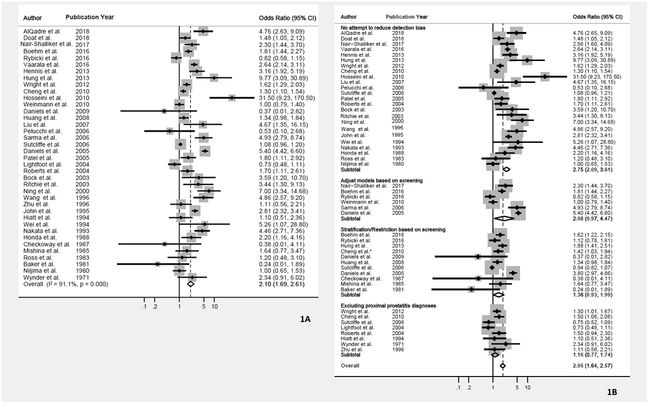
When estimates from all studies were combined irrespective of the potential influence of detection bias, a significant positive association was observed between clinical prostatitis and prostate cancer, using both a “main estimate” approach (OR=2.10; 95% CI: 1.69-2.61; I2=91.1, p≤0.001; Figure 1A), as well as an approach accounting for all 51 reported study estimates (OR=2.05; 95% CI: 1.64-2.57; Figure 1B). As these approaches produced estimates very similar in magnitude, we performed all further analyses using all available estimates per study.
Figure 1.
Forest plots showing the association between clinical prostatitis and prostate cancer (A) using only the primary estimate reported in the abstract or text for each study, and (B) stratified by method used to reduce detection bias. All available study estimates were pooled using robust variance estimation.
I2 =measure of heterogeneity; p=p-value; *=Pooled estimate for the subgroup contain additional estimates from this author.
Next, we conducted stratified analyses by detection bias methodology to evaluate the potential influence of detection bias on our pooled estimate (Table 2). Among studies that did not use methods to address detection bias, a strong positive association was observed (OR=2.75; 95% CI: 2.09-3.61) of even greater magnitude than our overall pooled estimate. However, in all other strata of studies that addressed detection bias, attenuated, non-significant associations were observed, with increasingly greater attenuation as the rigor of detection bias methodology improved (p=0.0002). Specifically, among studies that adjusted for prostate cancer screening or markers of screening, the least rigorous method for addressing detection bias, a weaker, non-significant, but still suggestive, positive association was observed (OR=2.08; 95% CI: 0.97-4.47). This association weakened among studies that provided estimates restricted to screened men (OR=1.36; 95% CI: 0.93-1.99) and weakened even further to the null among studies that excluded prostatitis diagnoses in the short window before prostate cancer diagnosis, the strongest method for addressing detection bias (OR=1.16; 95% CI: 0.77-1.74). Although the sample sizes for some subgroup analyses were small, even combining the two most rigorous categories (OR=1.26; 95% CI: 0.90-1.77) or grouping by any method of addressing detection bias resulted in non-significant, attenuated associations (OR=1.45; 95% CI: 0.98-2.15).
Table 2.
Pooled association between clinical prostatitis and prostate cancer by method used to reduce detection bias among 38 studies published from 1971-2018.
| Analysis | Studies | Estimates | Pooled Estimate1 | Adjusted Estimate2 | |||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | ||||
| No method used to reduce detection bias | 18 | 24 | 2.75 | 2.09-3.61 | 2.01 | 1.51-2.68 | |
| Detection bias method: | |||||||
| Adjustment by prostate cancer screening | 6 | 6 | 2.08 | 0.97-4.47 | - | ||
| Stratification or restriction based on prostate cancer screening | 11 | 13 | 1.36 | 0.93-1.99 | - | ||
| Excluding proximal prostatitis diagnoses (ie., near in time to prostate cancer diagnosis) | 8 | 8 | 1.16 | 0.77-1.74 | - | ||
| p-Trend3 | 0.0002 | ||||||
| Any method used to reduce detection bias | 20 | 27 | 1.45 | 0.98-2.15 | 1.19 | 0.78-1.82 | |
Abbreviations: OR-Odds Ratio, CI-Confidence Interval.
These values were obtained by random effects models using robust variance estimation.
Adjusted for susceptibility to recall bias (prospective studies or medical record prostatitis assessment vs. self-report prostatitis assessment).
Each detection bias method was assigned a score based on the rigor of each method and modeled as a linear function.
To explore additional factors that might explain some of the variation across study findings, we performed further stratified analyses, taking into account detection bias to the degree possible (any vs. no method). Although no significant differences were observed across strata defined by study methodology, non-significantly weaker associations were observed in studies less susceptible to recall bias (i.e., prospective studies, those that assessed prostatitis by medical record review, and higher quality studies), possibly suggesting a role for recall bias in explaining our overall findings (Table 3). To explore this possibility further, we adjusted our detection bias stratified results by susceptibility to recall bias and observed a slight attenuation of the estimates (OR=2.01; 95%CI 1.51-2.68 among studies that did not account for detection bias and OR=1.19; 95% CI: 0.78-1.82 among studies that employed any method to reduce detection bias; Table 2), suggesting an additional small influence of recall bias on previous prostatitis and prostate cancer findings.
Table 3.
Pooled association between clinical prostatitis and prostate cancer by study characteristics among 38 studies published from 1971-2018.
| Factor | Stratum | Studies | Estimates | Unadjusted Pooled Estimate 1 |
Accounting for Detection Bias 1 |
Differences between adjusted strata (P-value) |
||
|---|---|---|---|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | |||||
| Study Design2 | ||||||||
| Cohort and Nested Case-Control | 10 | 18 | 1.34 | 0.98-1.81 | 1.17 | 0.76-1.81 | ||
| Case-Control | 26 | 30 | 1.74 | 1.16-2.62 | 1.58 | 1.05-2.40 | 0.113 | |
| Type of controls among Case-Control Studies3 | ||||||||
| Population-based | 17 | 20 | 2.28 | 1.67-3.13 | 1.55 | 1.10-2.16 | ||
| Hospital | 9 | 9 | 2.85 | 1.43-5.71 | 1.70 | 0.84-3.44 | 0.458 | |
| Method of Prostatitis Assessment4 | ||||||||
| Medical Record | 8 | 11 | 1.51 | 0.94-2.44 | 1.24 | 0.70-2.22 | ||
| Interview | 17 | 19 | 2.50 | 1.41-4.44 | 1.77 | 0.98-3.20 | ||
| Self-Administered Questionnaire | 10 | 18 | 2.36 | 1.27-4.39 | 1.83 | 1.01-3.31 | 0.242 | |
| Population5 | ||||||||
| Western | 28 | 47 | 1.68 | 0.95-2.97 | 1.33 | 0.79-2.24 | ||
| European descent | 13 | 24 | 1.74 | 1.28-2.35 | 1.38 | 1.05-1.80 | ||
| African descent | 8 | 10 | 2.01 | 1.05-3.87 | 1.73 | 0.90-3.33 | ||
| Asian American6 | 2 | 3 | 3.02 | -- | 2.23 | -- | ||
| Eastern | 10 | 11 | 3.92 | 2.31-6.65 | 2.57 | 1.52-4.36 | ||
| East Asian (Taiwan, China, Japan) | 8 | 9 | 3.32 | 1.79-6.18 | 2.45 | 1.37-4.37 | ||
| Middle East6 | 2 | 2 | 8.94 | -- | 6.17 | -- | 0.026 | |
| Study Quality7 | ||||||||
| High | 9 | 12 | 1.52 | 0.97-2.36 | 1.18 | 0.75-1.87 | ||
| Moderate | 16 | 24 | 2.16 | 1.24-3.75 | 1.52 | 0.92-2.51 | ||
| Low | 13 | 15 | 2.47 | 1.36-4.50 | 1.76 | 0.93-3.33 | 0.234 | |
| Prostatitis Type | ||||||||
| Chronic Prostatitis8 | 4 | 5 | 1.02 | 0.69-1.49 | 0.84 | 0.52-1.36 | ||
| Prostatitis not further Specified | 34 | 39 | 2.24 | 1.77-2.84 | 1.66 | 1.26-2.18 | 0.016 | |
| Disease severity | ||||||||
| Aggressive prostate cancer6 | 3 | 5 | 1.23 | -- | 1.03 | -- | -- | |
| Study Period9 | ||||||||
| 1971-1987 | 6 | 6 | 1.74 | 1.28-2.37 | 1.53 | 1.16-2.01 | ||
| 1988-2006 | 15 | 25 | 1.37 | 0.80-2.37 | 1.15 | 0.68-1.97 | 0.345 | |
Abbreviations: OR-Odds Ratio, CI-Confidence Interval, BPH-Benign Prostatic Hyperplasia.
Values were calculated by random effects models, using robust variance estimation, and adjusted models account for utilization of any method to reduce detection bias (Yes/No).
Excluding two cross-sectional studies.
Excluding studies with family-based control groups and one study using BPH controls. Three studies included separate estimates for population-based and hospital control groups.
Excluding studies using multiple methods of prostatitis assessment.
Western: studies conducted in North America, Europe, and Australia; Eastern: studies conducted in the Middle East and Asia; European descent (European, Australian, or >90% Caucasian American); African descent (African American and men of African descent from Barbados); Middle East (Iran and Jordan) Studies without estimates stratified by ethnicity were excluded.
Small sample size precludes 95% CI estimation.
High quality: scores 6-8, Moderate quality: scores 4-5, Low quality: scores 1-3.
Chronic prostatitis as defined by NIH criteria or chronic symptoms (recurrent UTI or pelvic pain) lasting longer than a year.
United States-based studies only.
In analyses stratified by geography and race/ethnicity, we observed a stronger positive association among men of African descent (OR=1.73; 95% CI: 0.90-3.33; Table 3) than among those of European descent (OR=1.38; 95% CI: 1.05-1.80), even after accounting for detection bias. Some evidence of a stronger positive association was also seen among Asian American men (OR=2.23) and men residing in East Asia (OR=2.45; 95% CI: 1.37-4.37) and the Middle East (Iran and Jordan; OR=6.17), although the precision of several of these summary ORs was unreliable due to the limited number of estimates in each group. With respect to age at prostate cancer diagnosis, only two studies explored the association of clinical prostatitis and prostate cancer stratified by age and accounting for detection bias 29,30. Sutcliffe et. al., 29 observed a significant positive association between clinical prostatitis and prostate cancer among younger men (<59 years) screened for prostate cancer (OR=1.49; 95% CI: 1.08-2.06). However, this association was null among those aged 59-68 years and became protective among older men (≥69 years; OR=0.79 95% CI: 0.64-0.98). Similarly, Zhu et. al., 30 found evidence of a positive association among younger men (40-64 years; OR=1.78 95% CI: 0.82-3.85) that attenuated among older men (65-69 years; OR=1.24 CI: 0.67-2.29).
With respect to prostatitis type, null associations were observed among studies defining their exposure as chronic prostatitis, whereas positive associations (OR=1.66; 95% CI: 1.26-2.18) were observed when prostatitis subtype was not further specified. No differences in findings were observed between US studies conducted prior to 1987, the period before widespread PSA testing, and those conducted from 1987 onwards. In analyses limited to aggressive prostate cancer, some evidence of a null association was seen (OR=1.03), although small sample sizes rendered this estimate unreliable. Null associations were also found in studies investigating advanced stage (T3b or worse; OR=0.81; 95% CI: 0.50-1.30; 29) and fatal prostate cancer (OR=1.0; 95% CI: 0.79-1.414). No appreciable differences were observed in the overall pooled estimate when we excluded studies that either enriched 31,32 or depleted 13,15,16,18,20,33-35 their control populations of prostate conditions (e.g., BPH, high PSA, all benign prostate conditions). In the sole study using BPH controls, Checkoway et. al., 31 found some evidence of an inverse association (OR=0.38; 95% CI: 0.01-4.11). Finally, with respect to prostatitis treatment, only two studies assessed the possible influence of treatment or management of prostatitis either directly 29 or indirectly 36 on the association between clinical prostatitis and prostate cancer. Sutcliffe et. al., 29 found similar null results among those treated (RR=0.95; 95% CI: 0.83-1.09) and not treated (RR=1.10; 95% CI: 0.65-1.84) for prostatitis, whereas Doat et. al., 36 found that non‐steroidal anti‐inflammatory drug (NSAID) use modified the association of prostatitis and prostate cancer, with a potentially positive association among non-NSAID users (OR=2.11; 95% CI: 1.29-3.43) and a null association among ever users (OR=0.83; 95% CI: 0.44-1.58).
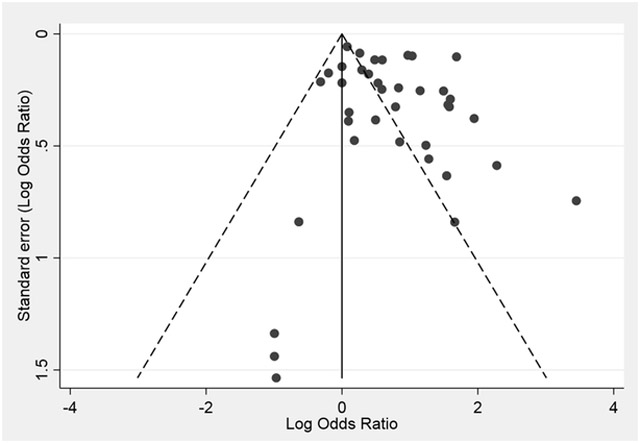
To evaluate the impact of publication bias on our meta-analysis conclusions, we used Begg’s funnel plot and statistical test, as well as Egger’s test. Visual inspection of Begg’s funnel plots suggested some funnel asymmetry (Figure 2), but statistical tests indicated a lack of significant publication bias (Begg’s test z=0.82, P=0.414; Egger’s test t=1.39, P=0.175).
Figure 2.
Funnel plot for publication bias analysis in the association between clinical prostatitis and prostate cancer
Discussion
In our systematic review and meta-analysis of 38 studies, we found that a history of clinical prostatitis was statistically associated with prostate cancer, but only when pooled estimates did not account for detection bias. When estimates did take detection bias into account, the overall pooled association was considerably weaker and non-significant, with a significant inverse linear trend between the degree of a priori-defined detection bias methodologic rigor and the strength of the observed association. Specifically, the association decreased from an approximate two-fold increased odds of prostate cancer in the stratum that did not address detection bias down to a null association in the stratum that used the most rigorous method to reduce detection bias. Although the sample size for this last analysis was small, inferences from this stratum are supported by the strong linear trend between the degree of detection bias methodologic rigor and the magnitude of the association, as well as the non-significant overall pooled association among studies that employed any method to reduce detection bias, regardless of the chosen method. Furthermore, our stratified findings suggest that much of the previously-observed positive associations between clinical prostatitis and prostate cancer may have been driven by recent prostatitis diagnoses, prompting increased prostate cancer screening and/or investigation.
To our knowledge, we are the first to examine the influence of detection bias on the pooled association between clinical prostatitis and prostate cancer, precluding a comparison of our detection bias findings to previous meta-analyses. However, our overall findings not taking detection bias into consideration are similar in direction and magnitude, if not stronger, than those from previous meta-analyses ((1.57 (95% CI: 1.01-2.45 5), 1.64 (95% CI: 1.36-1.98 7), and 1.68 (95% CI: 1.57, 1.79 6)), suggesting that similar results would have been observed in these meta-analyses had they taken detection bias into consideration and that our findings are not driven by differing eligibility criteria or inclusion of more recent studies. Likewise, our additional stratified findings are also consistent with those from previous meta-analyses. These include findings from analyses stratified by type of controls 5,6 and method of prostatitis assessment 6,7, many of which were lower in magnitude in studies that ascertained prostatitis exposure by medical record review. Together with our lower pooled estimates in prospective studies and those with higher methodologic quality, these findings support a possible role for differential recall bias – i.e., the probability that cases may have been more likely to recall or report a diagnosis of prostatitis than controls – in explaining some of our overall findings. Indeed, when we adjusted our detection bias stratified findings for susceptibility to recall bias, our pooled estimates attenuated even further. Nevertheless, this attenuation was considerably less than that observed after accounting for detection bias, suggesting that detection bias was the major contributor to previously observed positive findings.
Findings stratified by geography and race/ethnicity were also consistent with those from previous meta-analyses 6-8. Notably, our estimate for men of African descent was stronger than observed among Caucasian men, possibly because of lower background rates of prostate cancer screening in unexposed men (i.e., those without a history of prostatitis), giving exposed men a greater screening advantage and likelihood of prostate cancer detection. We also observed a stronger association among East Asian and Asian-American men, the latter of which we believe is the first such mention in the literature. Reasons for these stronger associations are unclear, but could include: 1) a similar screening advantage as described above for men of African descent, although the lower background rates of screening in this group would likely be driven by lower disease risk rather than lesser screening access; and 2) historical patient counseling patterns in some Asian countries in which prostate cancer patients were informed they had benign prostate conditions, such as prostatitis, rather than prostate cancer to avoid alarming them 37, a practice that may have been continued following migration. However, given the small number of studies conducted in Asian-American populations, additional studies are needed before any conclusions can be drawn about the magnitude of their association and its meaning. Similarly, the vast heterogeneity in estimates from the Middle Eastern studies precludes any conclusion based on their pooled estimate. Finally, in terms of tumor aggressiveness, only three studies provided estimates for aggressive prostate cancer 29,38,39, reducing the reliability of the standard error of the pooled estimate. However, the lower magnitude of this pooled estimate is consistent with our conclusion that previous findings were influenced by detection bias, as aggressive prostate tumors should be less susceptible to detection bias than non-aggressive tumors that may never have been diagnosed without prostate cancer screening or investigation 38.
Although our overall conclusion is that previous positive findings between clinical prostatitis and prostate cancer were driven by detection bias, we do not believe this conclusion rules out a role for prostate inflammation in prostate carcinogenesis because of the nature of the conditions captured by “clinical prostatitis”. This diagnosis represents a heterogeneous group of conditions, including acute bacterial prostatitis (NIH category I prostatitis), chronic bacterial prostatitis (category II), inflammatory chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS, category IIIa), and non-inflammatory CP/CPPS (category IIIb) 12, the latter of which may account for up to 45% of all prostatitis diagnoses 12,40. Therefore, clinical prostatitis broadly may not accurately reflect prostate inflammation or address our original hypothesis. While we saw a null association among studies assessing chronic prostatitis broadly (OR=0.84; 95% CI: 0.52-1.36), only two studies to date have distinguished clinical prostatitis subtypes more likely to reflect prostate inflammation, both of which accounted for detection bias and used diagnoses confirmed by laboratory evidence of infection/inflammation. The first of these studies observed positive associations for acute (OR=1.9; 95% CI: 0.9-3.8) and chronic bacterial prostatitis (OR=1.6; 95% CI: 0.8-3.1), but not for chronic nonbacterial prostatitis (OR=0.9; 95% CI: 0.4-1.8) 41, and the second observed generally null associations for acute (OR=0.92; 95% CI: 0.51-1.67) and chronic (OR=0.79; 95% CI: 0.53-1.17) bacterial prostatitis 32. These conflicting results highlight the importance of additional work in this area focused specifically on chronic bacterial prostatitis and inflammatory CP/CPPS, and accounting for detection bias.
Besides bacterial prostatitis and inflammatory CP/CPPS, inflammation may also be present in the prostate asymptomatically (NIH category IV prostatitis 12), a condition that was not studied in the present meta-analysis. Agreement between this common condition 42 and clinical prostatitis has been found to be low (e.g., 47.8% 32) 43, possibly suggesting distinct conditions. Interestingly, in a meta-analysis of 25 studies of asymptomatic prostatitis diagnosed based on thin needle biopsy, men with histologic prostate inflammation had a 55% overall decreased (rather than increased) odds of prostate cancer compared to those with no evidence of inflammation 44. This unexpected finding may be explained by several possible reasons. First, inflammatory cells (macrophages and lymphocytes) may potentially protect against prostate cancer by destroying “foreign” malignant cells (i.e., immunosurveillance) and preventing them from developing into a prostate tumor 45. Second, the study design/eligibility criteria of included studies may have led to a spurious protective association. Histologic studies depend on available prostate biopsy tissue for all participants, which typically requires a clinical indication for biopsy, such as an elevated PSA concentration. However, because PSA may rise for reasons other than prostate cancer, such as prostate inflammation, it is possible that previous studies may have observed a seemingly protective association because participants’ PSA was elevated for one, but not the other, reason – i.e., in the simplest scenario, if participants’ PSA was elevated because of prostate inflammation, then it was not elevated because of prostate cancer, leading to a protective association. Therefore, a better population in which to study asymptomatic prostatitis and prostate cancer may be men with prostate tissue available, but who were unselected for clinical indication for biopsy. One such unique study population with both of these features is the Prostate Cancer Prevention Trial (PCPT). In this study, the authors observed a positive association between asymptomatic inflammation and total and high-grade prostate cancer (OR=1.78; 95% CI: 1.04-3.06 and OR=2.24; 95% CI: 1.06-4.71, respectively 46) that persisted when restricted to those with low PSA concentration (<2ng/mL) and those without a clinical indication for biopsy. Further prospective evidence of this association was observed among PCPT participants who had not developed cancer by the conclusion of the original study, but who were followed subsequently for prostate cancer (OR=1.66; 95% CI: 0.70-3.96 47). Alternative study populations in which to investigate this challenging question include historical cohorts of BPH patients who underwent transurethral resection of the prostate and who were subsequently followed for prostate cancer incidence and mortality 48.
This meta-analysis represents the most expansive to date and the first to evaluate the association between clinical prostatitis and prostate cancer accounting for detection bias. However, in addition to the heterogeneity of diagnoses captured by “clinical prostatitis”, our meta-analysis has several further limitations. First, only eight studies to date have performed analyses excluding recent prostatitis diagnoses, reducing the precision of our least biased pooled estimate, and even fewer have performed analyses excluding recent prostatitis diagnoses and stratifying by prostate cancer screening. Nonetheless, although more studies are certainly warranted, our findings are still supported by the non-significant association observed when any method to reduce detection bias was used, and the highly significant inverse trend between the degree of methodologic rigor for addressing detection bias and the magnitude of the observed association. Second, we were not able to account for increased prostate cancer detection because of incidental prostate cancer diagnosis on biopsies triggered by prostatitis-mediated PSA elevation. However, residual detection bias would only be expected to attenuate our null results further. Third, we were unable to fully explore differences in the magnitude of association between sub-groups of interest (e.g. aggressive prostate cancer) independent of detection bias because of the small number of studies that addressed detection bias within these sub-groups. Small numbers also limited our meta-regression adjustment to “any” versus “no” method to reduce detection bias. Finally, we were not able to investigate possible differences in the association between clinical prostatitis and prostate cancer by age at prostate cancer diagnosis or a possible protective influence of therapy (e.g., antibiotics) or anti-inflammatory medications (e.g., NSAIDs) on this association because of the small number of studies that performed these types of analyses 29,30,36.
Overall, our findings indicate that previously reported associations between clinical prostatitis and prostate cancer were likely the result of detection bias. Additionally, even in the absence of detection bias, “clinical prostatitis” not further specified may not be an appropriate proxy for prostate inflammation. Future studies should address these concerns by focusing on inflammation-related types of clinical prostatitis (confirmed acute and chronic bacterial prostatitis, and inflammatory CP/CPPS), collecting detailed exposure data (e.g., age at onset, treatment, etc.) prospectively, and accounting for detection bias. Ultimately, some association between prostate inflammation and prostate cancer is biologically plausible; however, most epidemiologic studies conducted to date have not demonstrated this relation without serious concerns for bias. Correctly estimating this possible relation is important not only for etiologic purposes, but also for reassuring or properly counseling and screening men with prostatitis about their future risks of prostate cancer, given their documented concerns for developing this malignancy 49-51.
Supplementary Material
Table 1.
Characteristics of 38 studies that evaluated the association between clinical prostatitis and prostate cancer published from 1971-2018.
| First Author | Publication Year |
Study Location | Data Collection Period |
Participant Age Range at the Time of Prostatitis Assessment |
Method of Prostatitis Assessment |
Cases/Controls1 | Type of Controls |
OR (95% CI)2 | Methods used to Reduce Detection Bias3 |
Quality Score4 |
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort and Nested Case-Control (n=10) | ||||||||||
| Rybicki | 2016 | USA | 1990-2002 | -- | MR | 574/574 | NCC | 0.82 (0.58-1.15) | 2, 3 | 4 |
| Vaarala | 2016 | Finland | 1996-2012 | 20-59 | SAQ | 40/1,732 | -- | 2.64 (2.14-3.11)5 | -- | 2 |
| Hung | 2013 | Taiwan | 1996-2008 | 67 (mean) | MR | 1,184/4,736 | NCC | 9.77 (3.09-30.9) | 2 | 3 |
| Cheng | 2010 | USA | 2002-2006 | 45-69 | SAQ | 1,631/66,210 | -- | 1.30 (1.10-1.54) | 1, 2 | 5 |
| Weinmann | 2010 | USA | 1964-2000 | 45-84 | MR | 768/929 | NCC | 1.0 (0.79-1.4) | 3 | 5 |
| Daniels | 2009 | USA | 1996-2006 | 51-99 | MR | 65/195 | NCC | 0.37 (0.01-2.82)5 | 2 | 3 |
| Huang | 2008 | USA | 1993-2001 | 60-69 (IQR) | SAQ | 868/1,283 | NCC | 1.34 (0.98-1.84)5 | 2 | 2 |
| Sutcliffe | 2006 | USA | 1992-2002 | 46-81 | SAQ | 2,230/33,356 | -- | 1.08 (0.96-1.20) | 1, 2 | 7 |
| Zhu | 1996 | USA | 1989-1991 | 40-69 | MR/SAQ | 151/243 | NCC | 1.11 (0.56-2.21)5 | 1 | 4 |
| Hiatt | 1994 | USA | 1978-1985 | ≥30 | MR | 177/177 | NCC | 1.1 (0.5-2.3) | 1 | 6 |
| Retrospective Studies (n=28) | ||||||||||
| AlQadire | 2018 | Jordan | 2016 | 21-93 | MR | 165/177 | Hospital | 4.76 (2.63-9.09)6 | -- | 3 |
| Doat | 2017 | France | 2012-2013 | 40-75 | INT | 819/879 | POP | 1.48 (1.05-2.12)5 | -- | 4 |
| Nair-Shalliker | 2017 | Australia | 2006-2014 | 19-94 | SAQ | 1,180/862 | Other7 | 2.30 (1.44-3.70) | 3 | 4 |
| Boehm | 2016 | Canada | 2005-2012 | 59-70 | INT | 1,884/1,965 | POP | 1.81 (1.44-2.27) | 2, 3 | 6 |
| Hennis | 2013 | Barbados | 2002-2011 | 67 (mean) | INT | 963/941 | POP | 3.16 (1.92-5.19) | -- | 8 |
| Wright | 2012 | USA | 1993-2005 | 35-74 | INT | 1,752/1645 | POP | 1.62 (1.29-2.03)5 | 1 | 4 |
| Hosseini | 2010 | Iran | 2005-2008 | -- | INT | 137/137 | POP | 31.5 (9.2-170.5) | -- | 5 |
| Liu | 2007 | China | 2000-2006 | 40-86 (cases) | SAQ | 40/60 | POP | 4.67 (1.35-16.15)5 | -- | 3 |
| Hospital | 6.33 (1.62-24.77)5 | |||||||||
| Pelucchi | 2006 | Italy | 1985-1992 | <80 | INT | 280/689 | Hospital | 0.53 (0.10-2.68) | -- | 6 |
| Sarma | 2006 | USA | 1996-2001 | 40-79 | INT | 129/703 | POP | 4.93 (2.79-8.74) | 3 | 6 |
| Daniels | 2005 | USA | 2000-2002 | ≥65 | SAQ | 695/5125 | -- | 5.40 (4.42-6.60) | 2, 3 | 2 |
| Patel | 2005 | USA | 1996-1998 | 50-74 | INT | 669/596 | POP | 1.8 (1.1-2.9) | -- | 7 |
| Lightfoot | 2004 | Canada | 1995-1998 | 45-84 | INT/SAQ | 741/1,608 | POP | 0.73 (0.48-1.11) | 1 | 6 |
| Roberts | 2004 | USA | 1979-1997 | 70 (median) | MR | 409/803 | POP | 1.7 (1.1-2.6)8 | 1 | 5 |
| Bock | 2003 | USA | 1995-2002 | 31-84 | SAQ | 60/64 | --9 | 3.59 (1.20-10.70)5 | -- | 3 |
| Ritchie | 2003 | USA | 2000-2001 | 44-85 | SAQ | 58/99 | Hospital | 3.44 (1.30-9.13) | -- | 4 |
| Ning | 2000 | China | 1997-1999 | -- | INT | 96/96 | POP | 7.00 (3.34-14.68)5 | -- | 4 |
| Hospital | 10.27 (4.67-22.55)5 | |||||||||
| Wang | 1996 | China | 1989-1992 | 40 - 80 | INT | 117/296 | POP | 4.86 (2.57-9.20) | -- | 5 |
| Hospital | 6.57 (2.75-15.65) | |||||||||
| John | 1995 | USA/Canada | 1987-1991 | 70 (mean) | INT | 1,642/1,636 | POP | 2.81 (2.32-3.41)5 | -- | 4 |
| Wei | 1994 | China | -- | - | INT | 27/54 | Hospital | 5.26 (1.07-28.80) | -- | 3 |
| Nakata | 1993 | Japan | 1985-1990 | 53-93 | SAQ | 294/294 | POP | 4.46 (2.71-7.36) | -- | 4 |
| Honda | 1988 | USA | 1979-1982 | ≤60 | INT | 216/216 | POP | 2.2 (1.2-4.3) | -- | 5 |
| Checkoway | 1987 | USA | 1984-1985 | 50+ | INT | 40/64 | BPH | 0.38 (0.01-4.11)5 | 2 | 3 |
| Mishina | 1985 | Japan | -- | 47-86 | INT | 100/100 | POP | 1.64 (0.77-3.47)5,10 | 2 | 4 |
| Ross | 1983 | USA | 1972-1982 | <80 | INT | 110/110 | POP | 1.20 (0.48-3.10)5 | -- | 6 |
| Baker | 1981 | USA | 1977-1979 | 54-79 | INT/SAQ | 44/90 | --11 | 0.24 (0.01-1.89)5 | 2 | 3 |
| Niijma | 1980 | Japan | 1963-1978 | 40-90 | MR | 187/200 | Hospital | 1.00 (0.65-1.53)12 | -- | 1 |
| Wynder | 1971 | USA | 1965-1969 | 35-89 | INT | 300/400 | Hospital | 2.34 (0.91-6.02)5 | 1 | 2 |
Abbreviations: OR-Odds ratio, IQR-inter quartile range, SAQ-self-administered questionnaire, MR-medical record, INT-Interview, White-W, Black-B, Hispanic-H, Other-Other race, DK-Unknown race, NCC-Nested case-control, POP-population-based controls.
Values indicate non-missing prostatitis responses.
Primary OR estimate presented in either the abstract, main table, or conclusion of the manuscript, or in a previous meta-analysis.
Detection bias methodology: (1) Excluding proximal prostatitis diagnoses (ie., near in time to prostate cancer diagnosis); (2) Stratification or restriction based on prostate cancer screening; (3) Adjustment by prostate cancer screening; (--) No method used to reduce detection bias.
Quality scores: (1-3) Low Quality; (4-5) Moderate Quality; (6-10) High Quality.
Calculated from contingency table values within the article with confidence intervals estimated using Cornfield’s method or exact tests when expected cell frequencies < %5.
Based on the text and crude OR, assumed that the adjusted OR was presented in an inverse fashion.
Spouses of other cancer patients.
We selected this estimate for our “best estimate” analysis acknowledging that many estimates were provided within this study, but that this estimated reported in the abstract represented the summary effect estimate.
Entire study population was selected due to a prior family history of prostate cancer or prostate conditions.
Exposure defined as history of prostatitis or urethritis.
Entire study population was cross-sectionally selected due to an underlying BPH diagnosis.
Value presented as no association within the article, so OR=1.0 assumed and 95%CI estimated based on number of cases and controls and estimated prevalence of prostatitis in other early studies.
Acknowledgments
This work was supported by the Barnes-Jewish Hospital Foundation and Alvin J. Siteman Cancer Center. ML was additionally supported by the National Cancer Institute of the National Institutes of Health under award number T32CA190194. SK was additionally supported by DOD Prostate Cancer Research Program grant PC170173. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest disclosure: The authors declare no potential conflicts of interest.
Citations
- 1.Todoric J, Umemura A, Taniguchi K, Karin M. Inflammation and Cancer. Holland‐Frei Cancer Medicine. 2016:1–8. [Google Scholar]
- 2.Wang K, Karin M. Tumor-elicited inflammation and colorectal cancer In: Advances in cancer research. Vol 128 Elsevier; 2015:173–196. [DOI] [PubMed] [Google Scholar]
- 3.De Marzo AM, Platz EA, Sutcliffe S, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7(4):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakai YN, N. Inflammation and prostate carcinogenesis. Int J Urol. 2013;20(2):150–160. [DOI] [PubMed] [Google Scholar]
- 5.Dennis LK, Lynch CF, Torner JC. Epidemiologic association between prostatitis and prostate cancer. Urology. 2002;60(1):78–83. [DOI] [PubMed] [Google Scholar]
- 6.Ding H, Fan S, Zhang L, Hao Z, Liang C. Does prostatitis increase the risk of prostate cancer? A meta-analysis. Int J Clin Exp Med. 2017;10(3):4798–4808. [Google Scholar]
- 7.Jiang J, Li J, Yunxia Z, Zhu H, Liu J, Pumill C. The role of prostatitis in prostate cancer: meta-analysis. PLoS One. 2013;8(12):e85179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perletti G, Monti E, Magri V, et al. The association between prostatitis and prostate cancer. Systematic review and meta-analysis. Arch Ital Urol Androl. 2017;89(4):259–265. [DOI] [PubMed] [Google Scholar]
- 9.Bozeman CB, Carver BS, Eastham JA, Venable DD. Treatment of chronic prostatitis lowers serum prostate specific antigen. J Urol. 2002;167(4 I):1723–1726. [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012. [DOI] [PubMed] [Google Scholar]
- 11.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 12.Krieger JN, Nyberg L Jr., Nickel JC. NIH consensus definition and classification of prostatitis. JAMA. 1999;282(3):236–237. [DOI] [PubMed] [Google Scholar]
- 13.Hiatt RA, Armstrong MA, Klatsky AL, Sidney S. Alcohol consumption, smoking, and other risk factors and prostate cancer in a large health plan cohort in California (United States). Cancer Causes Control. 1994;5(1):66–72. [DOI] [PubMed] [Google Scholar]
- 14.Weinmann SS, J. A.:Rybicki BA:Enger SM:Van Den Eeden SK:Richert-Boe KE:Weiss NS Medical history, body size, and cigarette smoking in relation to fatal prostate cancer. Cancer Causes Control. 2010;21(1):117–125. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Yang Z, Li S. Prostate diseases, sexuality and prostate cancer: A case-control study. Medical Journal of Wuhan University. 2007;28(2):219–222. [Google Scholar]
- 16.Ning A Case control Study on the Relationship Between Prostate Cancer and History of Prostatic Diseases. Chinese journal of prevention and control of chronic non-communicable diseases. 2000;5:011. [Google Scholar]
- 17.Runtian W, Fangliu G, Hsing A. Sexual behavior, non-cancereous diseases and prostatic cancer: a case-control study [J]. Chinese Journal of Virology. 1996;8. [Google Scholar]
- 18.Niijima T, Koiso K. Incidence of prostatic cancer in Japan and Asia. Scand J Urol Nephrol Suppl. 1980;55:17–21. [PubMed] [Google Scholar]
- 19.Wei Q, Tang X, Yang Y, Zhan Y, Yin H. Risk factors of prostate cancer--a matched case-control study. Journal of West China University of Medical Sciences. 1994;25(1):87–90. [PubMed] [Google Scholar]
- 20.Wynder EL, Mabuchi K, Whitmore WF Jr. Epidemiology of cancer of the prostate. Cancer. 1971;28(2):344–360. [DOI] [PubMed] [Google Scholar]
- 21.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M. Newcastle-Ottawa quality assessment scale. Ottawa Hospital Research Institute; 2013. [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. [DOI] [PubMed] [Google Scholar]
- 23.Greenland S Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9(1):1–30. [DOI] [PubMed] [Google Scholar]
- 24.Hedges LV, Tipton E, Johnson MC. Robust variance estimation in meta-regression with dependent effect size estimates. Res Synth Methods. 2010;1(1):39–65. [DOI] [PubMed] [Google Scholar]
- 25.Tipton E Robust variance estimation in meta-regression with binary dependent effects. Res Synth Methods. 2013;4(2):169–187. [DOI] [PubMed] [Google Scholar]
- 26.Tipton E Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods. 2015;20(3):375. [DOI] [PubMed] [Google Scholar]
- 27.Tanner‐Smith EE, Tipton E. Robust variance estimation with dependent effect sizes: practical considerations including a software tutorial in Stata and SPSS. Res Synth Methods. 2014;5(1):13–30. [DOI] [PubMed] [Google Scholar]
- 28.Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta-analysis. Stat Med. 2001;20(4):641–654. [DOI] [PubMed] [Google Scholar]
- 29.Sutcliffe S, Giovannucci E, De Marzo AM, Leitzmann MF, Willett WC, Platz EA. Gonorrhea, syphilis, clinical prostatitis, and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2160–2166. [DOI] [PubMed] [Google Scholar]
- 30.Zhu KM, B.:Stergachis A:Daling JR:Levine RS Comparison of self-report data and medical records data: results from a case-control study on prostate cancer. Int J Epidemiol. 1999;28(3):409–417. [DOI] [PubMed] [Google Scholar]
- 31.Checkoway H, DiFerdinando G, Hulka BS, Mickey DD. Medical, life-style, and occupational risk factors for prostate cancer. Prostate. 1987;10(1):79–88. [DOI] [PubMed] [Google Scholar]
- 32.Rybicki BA, Kryvenko ON, Wang Y, et al. Racial differences in the relationship between clinical prostatitis, presence of inflammation in benign prostate and subsequent risk of prostate cancer. Prostate Cancer Prostatic Dis. 2016;19(2):145–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.John EM, Whittemore AS, Wu AH, et al. Vasectomy and prostate cancer: Results from a multiethnic case-control study. J Natl Cancer Inst. 1995;87(9):662–669. [DOI] [PubMed] [Google Scholar]
- 34.Mishina T, Watanabe H, Araki H, Nakao M. Epidemiological study of prostatic cancer by matched-pair analysis. Prostate. 1985;6(4):423–436. [DOI] [PubMed] [Google Scholar]
- 35.Pelucchi C, Talamini R, Negri E, Franceschi S, La Vecchia C. Genital and urinary tract diseases and prostate cancer risk. Eur J Cancer Prev. 2006;15(3):254–257. [DOI] [PubMed] [Google Scholar]
- 36.Doat S, Marous M, Rebillard X, et al. Prostatitis, other genitourinary infections and prostate cancer risk: Influence of non-steroidal anti-inflammatory drugs? Results from the EPICAP study. Int J Cancer. 2018. [DOI] [PubMed] [Google Scholar]
- 37.Nakata S, Imai K, Yamanaka H. Study of risk factors for prostatic cancer. Hinyokika Kiyo. 1993;39(11):1017–1024; discussion 1024-1025. [PubMed] [Google Scholar]
- 38.Boehm K, Valdivieso R, Meskawi M, et al. Prostatitis, other genitourinary infections and prostate cancer: results from a population-based case-control study. World J Urol. 2016;34(3):425–430. [DOI] [PubMed] [Google Scholar]
- 39.Cheng I, Witte JS, Jacobsen SJ, et al. Prostatitis, sexually transmitted diseases, and prostate cancer: The California men’s health study. PLoS One. 2010;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schaeffer AJ, Knauss JS, Landis JR, et al. Leukocyte and bacterial counts do not correlate with severity of symptoms in men with chronic prostatitis: the National Institutes of Health Chronic Prostatitis Cohort Study. J Urol. 2002;168(3):1048–1053. [DOI] [PubMed] [Google Scholar]
- 41.Roberts RO, Bergstralh EJ, Bass SE, Lieber MM, Jacobsen SJ. Prostatitis as a risk factor for prostate cancer. Epidemiology. 2004;15(1):93–99. [DOI] [PubMed] [Google Scholar]
- 42.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. 2008;54(6):1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nickel JC, Roehrborn CG, O’Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. Examination of the Relationship Between Symptoms of Prostatitis and Histological Inflammation: Baseline Data From the REDUCE Chemoprevention Trial. J Urol. 2007;178(3):896–901. [DOI] [PubMed] [Google Scholar]
- 44.Vasavada SR, Dobbs RW, Kajdacsy-Balla AA, Abern MR, Moreira DM. Inflammation on prostate needle biopsy is associated with lower prostate cancer risk: a meta-analysis. J Urol. 2018;199(5):1174–1181. [DOI] [PubMed] [Google Scholar]
- 45.Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704–2715. [DOI] [PubMed] [Google Scholar]
- 46.Gurel B, Lucia MS, Thompson IM Jr., et al. Chronic inflammation in benign prostate tissue is associated with high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2014;23(5):847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Platz EA, Kulac I, Barber JR, et al. A Prospective Study of Chronic Inflammation in Benign Prostate Tissue and Risk of Prostate Cancer: Linked PCPT and SELECT Cohorts. Cancer Epidemiol Biomarkers Prev. 2017;26(10):1549–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davidsson S, Fiorentino M, Andren O, et al. Inflammation, focal atrophic lesions, and prostatic intraepithelial neoplasia with respect to risk of lethal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(10):2280–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mehik A, Hellstrom P, Sarpola A, Lukkarinen O, Jarvelin MR. Fears, sexual disturbances and personality features in men with prostatitis: a population-based cross-sectional study in Finland. BJU Int. 2001;88(1):35–38. [DOI] [PubMed] [Google Scholar]
- 50.Turner JA, Ciol MA, Von Korff M, Berger R. Health concerns of patients with nonbacterial prostatitis/pelvic pain. Arch Intern Med. 2005;165(9):1054–1059. [DOI] [PubMed] [Google Scholar]
- 51.Collins MM, O’Leary MP, Barry MJ. Prevalence of bothersome genitourinary symptoms and diagnoses in younger men on routine primary care visits. Urology. 1998;52(3):422–427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.